Abstract
Introduction
Severity of dementia and neuropsychiatric symptoms contribute to increasing informal care costs. We examined which neuropsychiatric symptoms subdomains (NPS-SD) were associated with informal costs in a population-based sample.
Methods
Dementia progression and informal costs (2015 dollars) were estimated from the Cache County Dementia Progression Study. Overall NPS and specific NPS-SD were assessed with the Neuropsychiatric Inventory. Generalized Estimating Equations (GEE with gamma-distribution/log-link) modeled the relationship between NPS-SDs and informal cost trajectories.
Results
Two hundred eighty participants (52.1% female; age M = 85.67, SD = 5.60) exhibited an adjusted cost increase of 5.6% (P = .005), 6.4% (P < .001), 7.6% (P = .030), and 13% (P = .024) for every increasing Neuropsychiatric Inventory unit in psychosis-SD, affective-SD, agitation/aggression-SD, and apathy-SD, respectively. An increase in each unit of apathy was associated with a 2% annual decrease in costs (P = .040).
Discussion
We extend our prior work on informal costs and dementia severity by identifying NPS-SD associated with informal costs. Interventions targeting NPS-SD may lower informal costs.
Keywords: Informal costs of dementia, Neuropsychiatric symptoms, Dementia, Alzheimer's disease
Highlights
-
•
Specific neuropsychiatric symptoms/areas affect informal costs of dementia care differently over time; some have little to no impact.
-
•
Agitation/aggression, affective, and psychosis increase persons with dementia informal costs of dementia care over time.
-
•
Apathy increases rate of change in informal costs of dementia care over time.
1. Introduction
Anticipated high levels of dementia prevalence [1] and associated costs of dementia care [1], [2], [3] intensify the need to identify modifiable factors that can lower these costs. Costs related to dementia care are comprised of both formal and informal costs. Caregiver time used for the provision of care for persons with dementia (PWD) is a significant unpaid informal cost, estimated to contribute between 31% and 49% of the total cost of dementia care [2]. Disease management strategies that decrease the time spent by caregivers in providing care to their care-recipients with dementia are key to reducing informal costs of dementia care and caregiver burden.
Increased informal costs of dementia care are associated with increased disease severity as well as the presence of neuropsychiatric symptoms (NPS) [3]. Moreover, NPS in PWD are associated with increased nursing home placement/institutionalization [4], which constitutes a sizable component of formal dementia care costs [1], [2]. In the United States, these costs are paid predominantly through Medicare/Medicaid payments. Keeping PWD dwelling in their communities rather than in long-term care facilities is desirable for both the caregiver and the care recipient [5], [6].
NPS span a wide variety of behavioral symptoms ranging from agitation/aggression to apathy as well as sleep and appetite problems [7]. Given their heterogeneity, NPS are likely to differ in underlying causes [8] and approaches for effective interventions. NPS overall have been found to increase informal costs of dementia care [3], [9]. In prior work, we found that NPS increased daily informal costs of care by 2% per point increase in total Neuropsychiatric Inventory (NPI) score [3]. In the present study, we build on our previous finding that NPS are associated with increased informal costs of dementia care, independent of disease severity, by identifying NPS subdomains (NPS-SD) that affect informal costs of care in a population-based sample [3].
2. Methods
This study used extant data from the Dementia Progression Study (DPS), a longitudinal, population-based study of risk factors and outcomes among cognitive, functional, and NPS domains in dementia. Participants of the DPS were incident (newly identified) cases of dementia from the Cache County (Utah) Study on Memory in Aging (CCSMA). A full description of these studies can be found in previous publications [10], [11]. The identification of persons with dementia occurred in four triennial waves of dementia screening and evaluation beginning in 1995 when 90% of the residents of Cache County aged 65 years and older were enrolled in CCSMA. At each wave, participants underwent a multistaged dementia screening and evaluation protocol. Dementia diagnoses were based on an evaluation completed at the clinical assessment that included neuropsychological testing, neurological, and physical examination of the participant, and a clinical history (including NPS) completed by informant report. Brain MRI, standard laboratory tests for dementia [10], and a physician examination, were requested in cases of suspected dementia or its prodrome. An expert panel of neurologists, neuropsychologists, geriatric psychiatrists, and a cognitive neuroscientist reviewed all available clinical data and assigned a diagnosis of dementia according to criteria of the Diagnostic and Statistical Manual, 3rd Edition Revised [12]. Age of dementia onset was assigned as the age at which the participant unambiguously met Diagnostic and Statistical Manual, 3rd Edition Revised criteria. Underlying illness causing dementia was assigned according to standard research criteria. For example, Alzheimer's disease followed criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association [13] and vascular dementia followed the National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherche et l'Enseignement en Neurosciences [14]. Those diagnosed with dementia, along with their caregivers, were invited to participate in the DPS.
2.1. Procedures of the DPS
From 2002–2007, surviving PWD from the CCSMA and their caregivers were invited to join the DPS [11]. The care dyad were visited semiannually for follow-up neuropsychological assessments and interviews. PWD completed a brief neurological and physical examination and a 30-minute core neuropsychological test battery at each visit, with supplemental assessments at alternating visits. Among the cognitive assessments completed was the Mini-Mental State Examination (MMSE) [15]. Interviews with the caregivers consisted of updated information about the participant: medical history (i.e., medical conditions and medications) and subjective health status, NPS (see measures for description), and functional status. Additionally, caregivers completed a caregiver activity survey (see measures below) annually regarding the amount of time the PWD received assistance in daily tasks across all caregivers. This estimation was the basis for calculating informal caregiver cost. Following each visit, the study team completed the Clinical Dementia Rating Scale (CDR) [16] as a measure of dementia severity and General Medical Health Rating (GMHR) [17] for a measure of overall health. The CDR assesses the domains of memory, orientation, judgment and problem-solving, community affairs, home and hobbies, and personal care using the following scale: 0 (normal), 0.5 (questionable), 1 (mild), 2 (moderate), 3 (severe), 4 (profound), and 5 (terminal). A global CDR score (max = 5) was calculated using a standard algorithm. The GMHR serves as a brief index of global health and medical comorbidity of PWD by using the following rating system: 1 = poor, 2 = fair, 3 = good, and 4 = excellent. All procedures of the CCSMA and DPS were approved by the Institutional Review Boards of Utah State University, the Johns Hopkins University, and Duke University. Current analyses were restricted to noninstitutionalized individuals with follow-ups for up to 10 years. The latter was selected to facilitate convergence of statistical models and due to the sparse number of observations beyond 10 years of follow-up time (n = 28).
2.2. Measures
2.2.1. Outcome variable: Informal costs of care
Estimates for the informal costs of care were based on a caregiver activity survey, which required the caregiver to estimate the amount of time spent assisting the PWD across several activities over the previous 24 hours. These activities included the following: answering questions, leaving reminders, transportation needs, dressing, grooming, meals and eating, and supervising the participant's activities. As with previous cost analyses in the DPS [3], [18] and as indicated by previous studies [19], [20], total caregiving time was calculated by summing across categories and capped at a maximum of 16 hours.
Informal cost of care was calculated using the Utah median hourly wage as reported in the U.S. Bureau of Labor's Occupational Employment Statistics [21] for the year of the visit (spanning from 2002 to 2012). Informal costs were represented in 2015 dollars, adjusting for inflation by employing a derived Medical Consumer Price Index multiplier based on the “medical care services” values from the annual average Urban Consumer Price Index [3].
2.2.2. Predictor variables
NPS were assessed using the 12-domain NPI [22], a clinical informant interview surveying the following behavioral disturbances: delusions, hallucinations, agitation/aggression, irritability, depression, anxiety, euphoria, disinhibition, aberrant motor behavior, apathy, sleep, and appetite. With a maximum of 144 points, the NPI delivers a total symptom score based on frequency and severity of each subdomain. Subdomains including affective (i.e., depression, anxiety, and irritability) and psychotic (i.e., delusions and hallucinations) clusters, along with single domain items of apathy, agitation/aggression, sleep, and appetite were analyzed separately. While each subdomain has a maximum of 12 points, the affective and psychotic clusters have maximum values of 36 and 24 points, respectively. The NPI has demonstrated internal consistency (Cronbach's α = 0.88), interrater reliability (i.e., between rater agreement for frequency = 93%–100% and severity = 89.4%–100%) and test-retest reliability (i.e., frequency r = 0.79 and severity r = 0.86), although reliability varies by symptom domain. For example, test-retest reliabilities across subdomains range from r = 0.51-0.98 in frequency ratings and r = 0.51–0.87 in severity ratings [22].
In the present analyses, we investigated the individual contributions of the following variables in predicting costs of informal care in 2015 dollars: the total 12-item NPI and the following subdomains: apathy, agitation-aggression, sleep, and appetite, as well as affective and psychotic symptom clusters. Covariates tested in adjusted models included time-varying medical status (GMHR), type of dementia (i.e., vascular dementia versus other), age of dementia onset, baseline education, and cognitive status with time-varying MMSE. The MMSE was used as a covariate as an objective indicator of dementia severity. The CDR was not used due to the significant correlation with MMSE [3]. Only significant (P < .05) items were retained in adjusted models. Time was characterized in years from the baseline visit.
2.3. Analytic approach
Descriptive statistics were conducted to characterize the sample at the baseline. We used separate generalized estimating equations (GEE) to examine the association between the predictor variables (along with covariates) and the informal costs of dementia care over time. Informal costs were modeled with NPI total score and NPI subdomains (NPI-SD) independently and as interacting with time to determine rate of change. Variables with significant Wald values were retained (α = .05). As cost distributions are highly positively skewed, a gamma distribution with a logarithmic link function was applied to transform the dependent variable (cost + $.01, to address values of 0). This approach was previously applied to the DPS data when estimating costs [3]. To facilitate interpretation, parameter estimates from log-link models were exponentiated. Thus, the exponentiated model coefficients (exp β) presented here are interpreted as having a multiplicative effect, rather than additive in traditional linear regression. Statistical modeling was performed using SPSS version 24 software.
3. Results
Two hundred eighty persons (52.1% female) from the DPS cohort met the inclusion criteria for this study. The maximum number of observations ranged from n = 576 to n = 583, depending on the completion of NPI-SD. Most participants were diagnosed with Alzheimer's dementia (AD) (72.1%) and had attained at least a high school education (85.7%). The mean (SD) age of dementia onset was 82.37 (5.84) years and 81.1% of the sample was in excellent or good health at baseline. The median cost of dementia care at baseline was $9.70/day in 2015 dollars. Table 1 provides follow-up information and further description of the baseline characteristics of our sample.
Table 1.
Dementia Progression Study subject cohort characteristics at baseline (N = 280).
| Age, years, mean (SD) | 85.67 (5.60) |
| Age of dementia onset, years, mean (SD) | 82.37 (5.84) |
| Female (%) | 52.1 |
| Dementia type (%) | |
| Alzheimer's disease | 72.1 |
| Vascular dementia, no AD | 13.2 |
| Other dementia | 14.6 |
| General Medical Health Rating (%) | |
| Fair/Poor | 18.6 |
| Good/Excellent | 81.4 |
| Died during study (%) | 85.0 |
| Less than high school education (%) | 14.3 |
| MMSE (median, max 30) | 23.0 |
| CDR (median, max 5) | 1.0 |
| NPI (median, 0 = none, min = 0; max = 48) | 8.0 |
| Dementia duration (median, years) | 2.84 |
| Costs of dementia care (median, $2015/day) | $9.70 |
| Costs of dementia care [mean, $2015/day, (SD)] | $43.33 (82.52) |
# Assessments per person:
# Follow-up years per person (for those with ≥2 assessments)
|
Abbreviations: MMSE, Mini-Mental State Examination; CDR, Clinical Dementia Rating Scale; NPI, Neuropsychiatric Inventory.
3.1. Cost of informal dementia care with time-varying NPS
Increasing severity of NPS was associated with increased informal costs. In unadjusted GEE models, the NPI-12 total score (expβ = 1.026; P < .001), agitation-aggression (expβ = 1.095; P < .001), and the affective symptoms (expβ = 1.072; P < .001) were associated with higher costs. Note that the GEE model for psychosis did not converge without the inclusion of covariates (see following paragraph). Increasing time of follow-up was associated with increases in daily informal costs, ranging from approximately 18% to 22% per year (expβ ranging from 1.184 to 1.220; all P < .05). Sleep and appetite were not significantly associated with costs over time (P = .494 and .958, respectively). Table 2 displays parameter estimates of unadjusted GEE models.
Table 2.
Association of NPI-12 total and NPI subdomains with informal costs of dementia care since dementia onset [Cost = β0 + β1time (dementia duration) + β2NPI-SD + ε]
| Model/Variable | exp β | 95% CI | P Value |
|---|---|---|---|
| Model 1 NPI-12 Total | |||
| Intercept | 12.794 | 8.058–20.315 | <.001 |
| Time (y) | 1.184 | 1.100–1.275 | <.001 |
| NPI Total | 1.026 | 1.013–1.039 | <.001 |
| Model 2 NPI Apathy Subdomain | |||
| Intercept | 14.524 | 9.883–21.342 | <.001 |
| Time (y) | 1.217 | 1.136–1.304 | <.001 |
| Apathy | 1.040 | 0.998–1.083 | .063 |
| Model 3 NPI Agitation–Aggression Subdomain | |||
| Intercept | 16.153 | 11.181–23.338 | <.001 |
| Time (y) | 1.191 | 1.115–1.273 | <.001 |
| Agitation–Aggression | 1.095 | 1.038–1.156 | <.001 |
| Model 4 NPI Sleep Subdomain | |||
| Intercept | 15.670 | 10.789–22.758 | <.001 |
| Time (y) | 1.220 | 1.140–1.305 | <.001 |
| Sleep | 1.015 | 0.973–1.058 | .494 |
| Model 5 NPI Appetite Subdomain | |||
| Intercept | 16.234 | 11.154–23.629 | <.001 |
| Time (y) | 1.218 | 1.138–1.302 | <.001 |
| Appetite | 0.999 | 0.954–1.045 | .958 |
| Model 6 NPI Affective Subdomain | |||
| Intercept | 12.639 | 8.209–19.459 | <.001 |
| Time (y) | 1.216 | 1.130–1.308 | <.001 |
| Affective | 1.072 | 1.039–1.106 | <.001 |
| Model 7 NPI Psychosis Subdomain∗ | |||
Model did not converge.
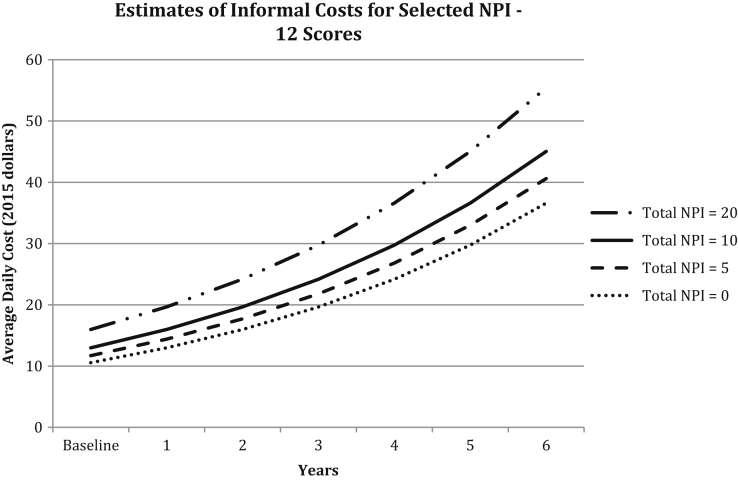
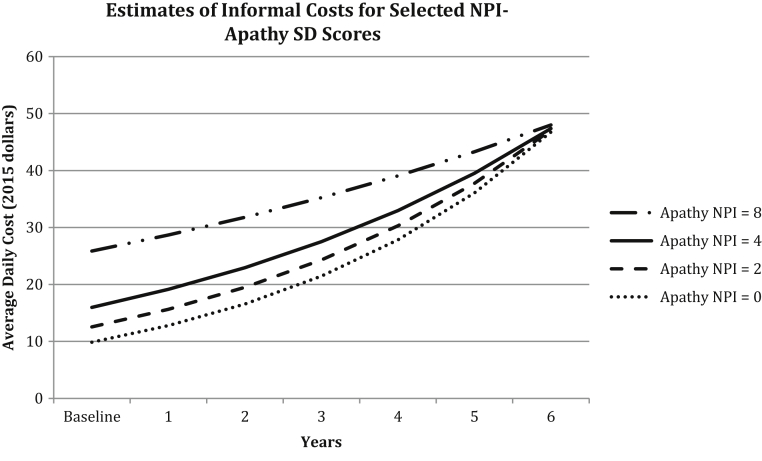
With the inclusion of covariates, all predictors were statistically significant as main effects or in interactions with other variables (see Table 3) and model convergence was achieved for psychosis NPS-SD. NPI-12 total score was associated with an approximate 2% (expβ = 1.021; P = .004) increase in daily informal costs for each unit increase in NPI-12 total score (see Fig. 1). Informal costs increased approximately 7.6% (expβ = 1.076; P = .030) for each increasing NPI-unit in agitation/aggression-SD but did not increase over time (P = .601). Similar increases were found for affective-SD (expβ = 1.064; P < .001; time interaction P = .769); and psychosis-SD (expβ = 1.056; P = .005; time interaction P = .389). Apathy-SD was associated with an approximate 13% increase in informal costs at the baseline per unit increase in apathy score (expβ = 1.128; P = .024). However, there was a 2% decrease in informal costs per year per unit change in apathy score (time interaction expβ = 0.981; P = .040). Fig. 2 illustrates the rate of change in apathy-SD for selected values at baseline and with increasing dementia severity (decreasing MMSE score by 1.67 points per year). While informal costs were higher for those with more severe apathy symptoms during earlier years of dementia, because of the 2% decrease in costs per year, estimated daily costs by apathy score converged over time. The relationship between apathy score and change in daily informal costs is depicted in the figure, which shows an overall increase in cost over time that accompanies increasing dementia severity, but with a slower rate of increase among those with higher apathy scores.
Table 3.
Adjusted models of association of NPI-12 total and NPS subdomains with informal costs of dementia care since dementia onset
| Model 1. Neuropsychiatric Inventory∗ total 12 (observations = 556) | |||
|---|---|---|---|
| Variable |
exp β | 95% CI | P Value |
| Intercept | 23.732 | 10.149–55.494 | <.001 |
| Time (y) | 1.122 | 1.043–1.208 | .002 |
| NPI Total 12 | 1.021 | 1.007–1.035 | .004 |
| Time-varying MMSE | 0.946 | 0.922–0.971 | <.001 |
| No vascular dementia (vs. any) | 1.993 | 1.363–2.913 | <.001 |
| General medical health rating [fair/poor vs. good/excellent (ref.)] |
1.643 |
1.060–2.546 |
.026 |
| Model 2. Agitation/aggression NPS-SD (observations = 583) | |||
| Intercept | 38.777 | 17.677–85.061 | <.001 |
| Time (y) | 1.094 | 1.020–1.173 | .012 |
| Agitation–aggression NPS-SD | 1.076 | 1.007–1.149 | .030 |
| Time-varying MMSE | 0.936 | 0.913–0.959 | <.001 |
| No vascular dementia [vs. any (ref.)] | 2.072 | 1.454–2.953 | <.001 |
| General medical health rating [fair/poor vs. good/excellent (ref.)] |
1.605 |
1.075–2.396 |
.021 |
| Model 3. Affective cluster (observations = 576) | |||
| Intercept | 29.160 | 13.462–63.167 | <.001 |
| Time (y) | 1.112 | 1.034–1.195 | .004 |
| Affective NPS-SD | 1.064 | 1.031–1.097 | <.001 |
| Time-varying MMSE | 0.939 | 0.917–0.963 | <.001 |
| No vascular dementia [vs. any (ref.)] | 2.020 | 1.399–2.918 | <.001 |
| General medical health rating [fair/poor vs. good/excellent (ref.)] |
1.682 |
1.118–2.531 |
.013 |
| Model 4. Psychosis cluster (observations = 578) | |||
| Intercept | 33.413 | 14.591–76.515 | <.001 |
| Time (y) | 1.095 | 1.020–1.175 | .013 |
| Psychosis NPS-SD | 1.056 | 1.017–1.097 | .005 |
| Time-varying MMSE | 0.944 | 0.919–0.969 | <.001 |
| No vascular dementia [vs. any (ref.)] | 1.964 | 1.362–2.832 | <.001 |
| General medical health rating [fair/poor vs. good/excellent (ref.)] |
1.568 |
1.052–2.335 |
.027 |
| Model 5. Apathy NPS-SD (observations = 582) | |||
| Intercept | 33.107 | 14.604–75.053 | <.001 |
| Time (y) | 1.152 | 1.061–1.250 | <.001 |
| Apathy NPS-SD | 1.128 | 1.016–1.253 | .024 |
| Time X Apathy NPS-SD | 0.981 | 0.963–0.999 | .040 |
| Time-varying MMSE | 0.932 | 0.910–0.954 | <.001 |
| No vascular dementia [vs. any (ref.)] | 2.053 | 1.435–2.938 | <.001 |
| General medical health rating [fair/poor vs. good/excellent (ref.)] | 1.645 | 1.074–2.518 | .022 |
Abbreviations: NPS-SD, Neuropsychiatric symptoms-subdomains; MMSE, Mini-Mental State Examination.
Neuropsychiatric Inventory (NPI): a 12-domain clinical informant interview surveying NPS (neuropsychiatric symptoms). The NPI-12 total assesses delusions, hallucinations, agitation/aggression, irritability, depression, anxiety, euphoria, disinhibition, aberrant motor behavior, apathy, sleep, and appetite. Specific subdomains, including affective and psychotic, along with single domain items of apathy, agitation/aggression, sleep, and appetite were analyzed separately.
Fig. 1.
Cost of informal dementia care with NPI-12 total score. This figure displays increasing costs over time and with increasing dementia severity (decreasing Mini-Mental State Examination [MMSE] score each year), holding total NPI score constant at values 0, 5, 10, and 20 for a hypothetical participant with a nonvascular form of dementia and in good/excellent general health. Note: MMSE scores at each time point were estimated from a linear mixed effects model from these data, controlling for age. Estimated MMSE for an 82-year-old participant at intercept was 27.23 points and average MMSE score decline was estimated at 1.67 points per year.
Fig. 2.
Change in the cost of informal dementia care with varying apathy NPI-SD scores. This figure displays increasing costs over time and with increasing dementia severity (decreasing Mini-Mental State Examination [MMSE] score each year), holding apathy NPI-SD score constant at values 0, 2, 4, and 8 for a hypothetical participant with a nonvascular form of dementia and in good/excellent general health. Note: MMSE scores at each time point were estimated from a linear mixed effects model from these data, controlling for age. Estimated MMSE score for an 82-year-old participant at intercept was 27.23 points and average MMSE score decline was estimated at 1.67 points per year.
Covariates that significantly predicted daily informal costs included dementia severity (MMSE), dementia type, and general medical health (GMHR). Higher MMSE score was associated with lower daily informal costs (expβ = 0.932–0.946; P < .001). Compared with those with vascular dementia, those with AD or other dementias had increased informal costs (expβ = 1.964–2.072; P < .001). Furthermore, persons in fair/poor health had higher costs (expβ = 1.568–1.682; P < .05) compared with those in good/excellent health.
4. Discussion
Extending our previous findings [3], we estimated the association of various neuropsychiatric symptom subdomains (aggression/agitation, affective, appetite, sleep, psychosis, and apathy) with informal costs of dementia care using the population-based community cohort from the Cache County Dementia Progression Study. Building on our previous work where overall NPS (NPI-Total) increased daily informal costs of dementia care by 2% for each point increase in NPI score, we found that the occurrence of agitation/aggression-SD, affective-SD, and psychosis-SD increased daily informal costs of dementia care by 8%, 6%, and 6%, respectively, for each point increase in NPS-SD score. In addition, we found that NPS in the apathy-SD initially (at the baseline) resulted in higher daily informal costs of dementia care by 13% for each point increase in apathy-SD score and that this was offset over time with approximately a 2% decrease for each point increase in apathy-SD score per year. Finally, we found that the appetite and sleep NPS-SD did not impact daily costs of informal dementia care.
Based on these more granular findings of the varying contribution of different NPS-SD to informal costs of dementia care, tailored intervention disease management targets could be explored to both lessen these costs/caregiver time spent in care and to improve individual PWD lives. Specifically, patients manifesting NPS in one NPS-SD at a given time may benefit from interventions found to be effective in managing that particular symptom subdomain. Some examples of efficacious interventions include music therapy for apathy [23], behavior therapy with pleasant events and relaxation for depression and anxiety [24], [25], [26], and environmental modifications to eliminate triggers [27], sensory stimulation [23], [28], [29], and bright-light therapy [30] for agitation and aggression.
To our knowledge, this is one of the first studies in the United States to identify the impact of specific NPS-SD on the informal costs of care in a community-dwelling population-based cohort of PWD. Largely, our findings are in line with previous studies on overall increases in informal costs of dementia care over time [31], [32], although studies where NPS were available were very limited. Among the few studies examining the impact of NPS on costs of dementia care was Rapp et. al. [9], which used a French community-dwelling dementia clinical trial cohort drawn from 50 French memory clinics with 2 years of follow-up. Rapp et al. found that cognitive and functional decline, as well as presence of NPS, was indicative of increased overall costs of care. Despite an inability to make a direct comparison of our results to Rapp et. al. [9], we too found that cognitive decline, as well as presence of NPS, was indicative of increased overall informal costs of dementia care. More recently, Costa et al. examined cost of dementia care specifically attributed to agitation among 1997 patients with dementia from eight European countries in the “Right Time Place Care Study” [33]. In their analyses, restricted to baseline data, they reported a 17% increase in monthly informal care costs in the home setting with increasing agitation (defined as NPI cluster score combining agitation, irritability, disinhibition, and aberrant motor behavior). Although agitation was defined as a cluster of symptoms in their study, the results are similar to our results with respect to an increase in informal costs; as well we did not find an association between agitation and rate of change in costs over time.
Our study has some limitations including both population homogeneity as well as small sample size. In addition, we developed our cost data from the caregiver hours acquired from an activity survey in which the primary caregiver was interviewed; this is subject to recall bias. We also do not report on dementia care received in nursing homes or other institutions (39 PWD were excluded from our analyses due to residence in nursing homes). Furthermore, for cohort participants residing in assisted living facilities, caregiver hours and thus informal care costs are likely to be underestimated. Finally, the large number of statistical analyses increased the possibility of a type 1 error. However, given the number of outcomes modeled (7), a Bonferroni correction would be alpha less than .007; all of the neuropsychiatric symptoms and subdomain scores were significant at an alpha less than the .007 with the exception of agitation/aggression and time interaction with apathy domain. We presented these data, however, in this exploratory work.
Our study also has a variety of strengths, including the distinctiveness of the measures and information available in a longitudinal population-based community-dwelling cohort with dementia, as well as high population participation rates and frequent visits over a long follow-up period [11] up to 10 years. The data available in this cohort afforded examination over time of both occurrence of various NPS symptoms in different domains and caregiving hours; this enabled establishing the impact of various NPS-SD on “real cost” estimates of informal care in this community-dwelling PWD population. Also, our caregiver activity survey included the estimation of caregiver time spent from multiple caregivers. Finally, in our study, we quantified changes in informal costs of dementia care associated with the presence of various NPS in different NPS-SD using a commonly used clinical scale, the NPI; this can allow for assessing the value of different NPS management strategies and interventions by changes in these NPS-SD measures and on the NPI-Total.
In conclusion, our main findings are that daily costs of informal dementia care increase substantially with the presence of NPS in the following NPS-SD: (1) agitation/aggression-SD, affective-SD, and psychosis-SD and (2) apathy-SD over time, although in the latter domain with prolonged duration, the rate of increase in informal costs diminished. Identifying and targeting interventions to manage modifiable factors such as reduction of NPS in community dwellers with dementia is a key element to reducing annual costs of dementia care, given that informal caregiving and institutionalization are universally recognized as costly elements of caring for those with dementia [1].
Research in Context.
-
1.
Systematic Review: In our literature review, we sought studies of informal, longitudinal costs of dementia care with measures of behavioral symptoms and dementia severity conducted among community-dwellers with dementia. Few such studies were identified; information regarding caregiving costs, disease severity, and behavioral symptoms was lacking. Although Hurd's study (NEJM 2013) had informal care costs among community dwellers with dementia, it was cross-sectional without clinical measures of dementia.
-
2.
Interpretation: In our population-based cohort with dementia, we extended our prior work on informal costs of care by identifying specific behaviors associated with these costs. Daily informal costs of dementia care increase with specific behavioral symptoms predicting higher costs. Targeting interventions to manage these symptoms in dementia is important to decreasing annual costs of dementia care.
-
3.
Future Directions: Studying larger, more sociodemographically diverse, community dwelling dementia cohorts will provide results concerning generalizability of these findings and help prioritize appropriate disease management strategies.
Acknowledgments
This study was supported by National Institute on Aging grants R01AG21136, R01AG11380, and P50AG005146 (Johns Hopkins ADRC). The authors are indebted to the Cache County Memory Study Investigators and staff and the participants and caregivers of the Cache County Studies.
Footnotes
Presented in preliminary form at the Annual Meeting of the Gerontological Society of America, November, 2016.
References
- 1.2016 Alzheimer's disease facts and figures. Alzheimers Demen. 2016;12:459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Hurd M.D., Martorell P., Delavande A., Mullen K.J., Langa K.M. Monetary costs of dementia in the United States. New Engl J Med. 2013;368:1326–1334. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rattinger G.B., Schwartz S., Mullins C.D., Corcoran C.D., Zuckerman I.H., Sanders C. Dementia severity and the longitudinal costs of informal care in the Cache County population. Alzheimers Dement. 2015;11:946–954. doi: 10.1016/j.jalz.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaffe K., Fox P., Newcomer R., Sands L., Lindquist K., Dane K. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 2002;287:2090–2097. doi: 10.1001/jama.287.16.2090. [DOI] [PubMed] [Google Scholar]
- 5.Martyr A., Nelis S.M., Quinn C., Wu Y.-T., Lamont R.A., Henderson C. Living well with dementia: A systematic review and correlational meta-analysis of factors associated with quality of life, well-being and life satisfaction in people with dementia. Psychol Med. 2018;48:1–10. doi: 10.1017/S0033291718000405. [DOI] [PubMed] [Google Scholar]
- 6.Sampson E.L. Palliative care for people with dementia. Br Med Bull. 2010;96:159–174. doi: 10.1093/bmb/ldq024. [DOI] [PubMed] [Google Scholar]
- 7.Cummings J.L., Mackell J., Kaufer D. Behavioral effects of current Alzheimer's disease treatments: A descriptive review. Alzheimers Dement. 2008;4:49–60. doi: 10.1016/j.jalz.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Tascone L.D.S., Payne M.E., MacFall J., Azevedo D., de Castro C.C., Steffens D.C. Cortical brain volume abnormalities associated with few or multiple neuropsychiatric symptoms in Alzheimer's disease. PLoS One. 2017;12:e0177169. doi: 10.1371/journal.pone.0177169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rapp T., Andrieu S., Molinier L., Grand A., Cantet C., Mullins C.D. Exploring the relationship between Alzheimer's disease severity and longitudinal costs. Value Health. 2012;15:412–419. doi: 10.1016/j.jval.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Breitner J.C., Wyse B.W., Anthony J.C., Welsh-Bohmer K.A., Steffens D.C., Norton M.C. APOE-epsilon4 count predicts age when prevalence of AD increases, then declines: the Cache County Study. Neurology. 1999;53:321–331. doi: 10.1212/wnl.53.2.321. [DOI] [PubMed] [Google Scholar]
- 11.Tschanz J.T., Corcoran C.D., Schwartz S., Treiber K., Green R.C., Norton M.C. Progression of cognitive, functional, and neuropsychiatric symptom domains in a population cohort with Alzheimer dementia: The Cache County Dementia Progression Study. Am J Geriatr Psychiatry. 2011;19:532–542. doi: 10.1097/JGP.0b013e3181faec23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diagnostic and statistical manual of mental disorders. In: Association AP, editor. 3rd edition, revised ed1987.
- 13.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 14.Roman G.C., Tatemichi T.K., Erkinjuntti T., Cummings J.L., Masdeu J.C., Garcia J.H. Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 15.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-Mental State” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Hughes C.P., Berg L., Danziger W.L., Coben L.A., Martin R.L. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 17.Lyketsos C.G., Galik E., Steele C., Steinberg M., Rosenblatt A., Warren A. The general medical health rating: A bedside global rating of medical comorbidity in patients with dementia. J Am Geriatr Soc. 1999;47:487–491. doi: 10.1111/j.1532-5415.1999.tb07245.x. [DOI] [PubMed] [Google Scholar]
- 18.Rattinger G.B., Fauth E.B., Behrens S., Sanders C., Schwartz S., Norton M.C. Closer caregiver and care-recipient relationships predict lower informal costs of dementia care: The Cache County Dementia Progression Study. Alzheimers Dement. 2016;12:917–924. doi: 10.1016/j.jalz.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu C.W., Scarmeas N., Torgan R., Albert M., Brandt J., Blacker D. Clinical characteristics and longitudinal changes of informal cost of Alzheimer's disease in the community. J Am Geriatr Soc. 2006;54:1596–1602. doi: 10.1111/j.1532-5415.2006.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Penrod J.D., Kane R.L., Finch M.D., Kane R.A. Effects of post-hospital Medicare home health and informal care on patient functional status. Health Serv Res. 1998;33:513–529. [PMC free article] [PubMed] [Google Scholar]
- 21.Occupational Employment and Wage Estimates.
- 22.Cummings J.L., Mega M., Gray K., Rosenberg-Thompson S., Carusi D.A., Gornbein J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 23.Holmes C., Hopkins V., Hensford C., MacLaughlin V., Wilkinson D., Rosenvinge H. Lavender oil as a treatment for agitated behaviour in severe dementia: A placebo controlled study. Int J Geriatr Psychiatry. 2002;17:305–308. doi: 10.1002/gps.593. [DOI] [PubMed] [Google Scholar]
- 24.Paukert A.L., Calleo J., Snow L., Wilson N., Petersen N.J., Kunik M.E. Peaceful mind: An open trial of cognitive-behavioral therapy for anxiety in persons with dementia. Int Psychogeriatrics. 2010;22:1012–1021. doi: 10.1017/S1041610210000694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orgeta V., Qazi A., Spector A., Orrell M. Psychological treatments for depression and anxiety in dementia and mild cognitive impairment: Systematic review and meta-analysis. Br J Psychiatry. 2015;207:293–298. doi: 10.1192/bjp.bp.114.148130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spector A., Charlesworth G., King M., Lattimer M., Sadek S., Marston L. Cognitive–behavioural therapy for anxiety in dementia: Pilot randomised controlled trial. Br J Psychiatry. 2015;206:509–516. doi: 10.1192/bjp.bp.113.140087. [DOI] [PubMed] [Google Scholar]
- 27.Kverno K.S., Black B.S., Nolan M.T., Rabins P.V. Research on treating neuropsychiatric symptoms of advanced dementia with non-pharmacological strategies, 1998-2008: A systematic literature review. Int Psychogeriatrics. 2009;21:825–843. doi: 10.1017/S1041610209990196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burns A., Perry E., Holmes C., Francis P., Morris J., Howes M.-J.R. A double-blind placebo-controlled randomized trial of Melissa officinalis oil and donepezil for the treatment of agitation in Alzheimer's disease. Demen Geriatr Cogn Disord. 2011;31:158–164. doi: 10.1159/000324438. [DOI] [PubMed] [Google Scholar]
- 29.Lin P.W., Chan W.C., Ng B.F., Lam L.C. Efficacy of aromatherapy (Lavandula angustifolia) as an intervention for agitated behaviours in Chinese older persons with dementia: A cross-over randomized trial. Int J Geriatr Psychiatry. 2007;22:405–410. doi: 10.1002/gps.1688. [DOI] [PubMed] [Google Scholar]
- 30.Burns A., Allen H., Tomenson B., Duignan D., Byrne J. Bright light therapy for agitation in dementia: A randomized controlled trial. Int Psychogeriatrics. 2009;21:711–721. doi: 10.1017/S1041610209008886. [DOI] [PubMed] [Google Scholar]
- 31.Zhu C.W., Leibman C., McLaughlin T., Scarmeas N., Albert M., Brandt J. The effects of patient function and dependence on costs of care in Alzheimer's disease. J Am Geriatr Soc. 2008;56:1497–1503. doi: 10.1111/j.1532-5415.2008.01798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu C.W., Leibman C., McLaughlin T., Zbrozek A.S., Scarmeas N., Albert M. Patient dependence and longitudinal changes in costs of care in Alzheimer's disease. Demen Geriatr Cogn Disord. 2008;26:416–423. doi: 10.1159/000164797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costa N., Wubker A., De Mauleon A., Zwakhalen S.M.G., Challis D., Leino-Kilpi H. Costs of care of agitation associated with dementia in 8 European countries: Results from the RightTimePlaceCare Study. J Am Med Directors Assoc. 2018;19:95.e1–95.e10. doi: 10.1016/j.jamda.2017.10.013. [DOI] [PubMed] [Google Scholar]