Abstract
Antimicrobial resistance is a global health problem with strong social and economic im-pacts. The development of new antimicrobial agents is considered an urgent challenge. In this regard, Antimicrobial Peptides (AMPs) appear to be novel candidates to overcome this problem. The mecha-nism of action of AMPs involves intracellular targets and membrane disruption. Although the exact mechanism of action of AMPs remains controversial, most AMPs act through membrane disruption of the target cell. Several strategies have been used to improve AMP activity, such as peptide dimeri-zation. In this review, we focus on AMP dimerization, showing many examples of dimerized peptides and their effects on biological activity. Although more studies are necessary to elucidate the relation-ship between peptide properties and the dimerization effect on antimicrobial activity, dimerization constitutes a promising strategy to improve the effectiveness of AMPs.
Keywords: Peptide, antimicrobial, dimerization, mechanism of action, membrane, Antimicrobial Peptides (AMPs)
1. INTRODUCTION: ANTIMICROBIAL PEPTIDES (AMPs)
The occurrence of pathogenic microorganisms resistant to antibiotics has been increasing, while few new antibiotics have been discovered and approved for commercialization [1]. This situation has led to a global health problem, with strong social and economic impacts [2]. Therefore, there is an urgent need for new molecules to control diseases caused by these resistant microorganisms. Among these molecules, Antimicrobial Peptides (AMPs) appear to be an interesting alternative since they act through mechanisms in which the pathogens rarely develop resistance [3]. In contrast to conventional antibiotics, which exert their toxic activity by binding to specific targets, most AMPs have been considered membrane-destabilizing molecules.
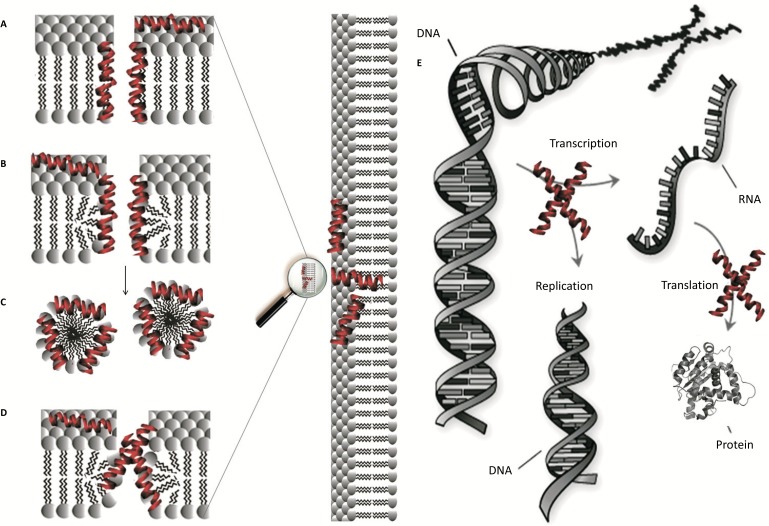
Antimicrobial peptides usually show a broad spectrum of action against gram-negative and gram-positive bacteria [4-8], fungi [9], viruses [10] and tumor cells [11, 12]. Although the main mechanism of action is membrane disruption [13], other mechanisms include inhibiting intracellular targets, such as DNA and RNA, and inhibiting protein synthesis and microbial enzymes (Figure. 1). The exact mechanism of action of AMPs in the membrane of the target cell remains controversial and is dependent on the peptide concentration and the lipid composition [14, 15].
Figure 1.
Mechanism of action proposed for AMPs: “barrel-stave” pore (a); “toroidal pore” (b); “detergent-like” (c); “disordered toroidal pore” (d) and intracellular targets (e).
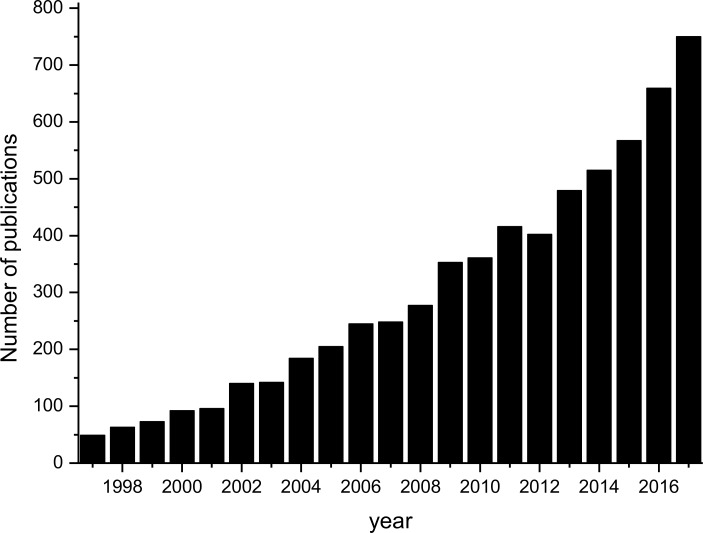
The broad spectrum of action, the rapid microbicide activity and the ability to be used in combination with other antibiotics make studies with AMPs an increasing line of research, showing the high potential of these molecules [16, 17]. The number of publications related to AMPs is growing exponentially, indicating that research on this topic is a current and important subject (Figure. 2).
Figure 2.
Evolution of the number of publications about AMPs (Source: PubMed, 2018. Search criteria: “antimicrobial peptides” in title/abstract).
1.1. AMPs Properties
Despite the remarkable biological diversity from which the AMPs were discovered, these molecules have certain characteristics in common. Membrane-active AMPs exhibit common physicochemical properties that characterize them as a special group of biomolecules: 1) normally consist of 12 to 50 amino acid residues, 2) have a net positive charge, 3) have approximately 50% hydrophobic residues, and 4) form an amphipathic α-helix in contact with the membrane. Cationicity is due to the presence of basic amino acid residues such as arginine and lysine. Many models show that this character is crucial for the initial attraction of AMPs on the membrane surface. Amphipathicity is characterized by the separation of the α-helix in a hydrophobic and a hydrophilic face. The amphipathic character is formed by a periodic sequence of polar and apolar residues in a range of three to four amino acid residues. Thus, the polar and apolar side chains of amino acid residues are positioned appropriately for their segregation between opposite faces [18-21].
1.2. Mechanism of Action of AMPs
The molecular understanding of the mechanism of action of AMPs is still not entirely clear [22, 23]. The model known as “barrel-stave” pores (Figure. 1A) describes the formation of barrel-shaped pores, where each stave can be represented by a peptide chain. In this model, the hydrophobic face of the peptide interacts with the hydrophobic chain of the phospholipids, while the hydrophilic surface remains oriented inside the pore. According to the “toroidal pore” (Figure. 1B), the peptides remain associated with the head groups of the phospholipids, inducing a curvature in the lipid bilayer. This model differs from the “barrel-stave” model because in addition to the peptides, the polar head groups of the lipids also form part of the pore. An alternative to the classic “toroidal pore” is the so-called “disordered toroidal pore”, which predicts that the inside of the pore would not be well structured, showing higher entropy (Figure. 1D). Finally, the mechanism called “carpet-like” or “detergent-like” model (Figure. 1C) proposes membrane permeabilization by the means of the detergent action of the peptides without the formation of pores. Thus, when a “threshold concentration” of the peptide molecules on the surface of the membrane is reached, micellar aggregates occur, starting the process of solubilization. It has been suggested that this mechanism can be considered an extreme form of the “toroidal pore” mechanism [24-27].
Even though widely studied for the last three decades, the mechanism of action of AMPs remains elusive [28, 29]. The knowledge of the mechanism of action of a particular bioactive molecule is always an essential issue to support its activity and is important in the design of new molecules. In this regard, various techniques have been used to evaluate the mechanism of action, especially nuclear magnetic resonance,
molecular dynamics, isothermal titration calorimetry, optical microscopy, leakage of carboxyfluorescein, fluorescence spectroscopy and circular dichroism, and others [30-33].
2. RATIONAL DESIGN OF AMPs: DIMERIZATION
Innumerable synthetic variants of AMPs have been produced, but few are able to reach clinical application. The main reasons are the low stability in physiological conditions and the lack of selectivity to prokaryotic cells [34, 35]. To increase the stability of AMPs against proteases, different strategies have been addressed, highlighting peptide dimerization. Many studies have shown that regardless of the mechanism of action, the aggregation/oligomerization of AMP molecules is a prerequisite for its action. Therefore, in addition to the factors described above, it is currently suggested that peptide oligomerization also contributes to its activity and selectivity [25, 36-40]. Considering these studies, several AMPs were dimerized to increase their antimicrobial activity and selectivity [41-47]. In addition, dimeric versions have the potential to be more resistant to proteases compared to monomers. The peptide A3-APO is an example; it retains full antibacterial activity in the presence of mouse serum [48].
2.1. Strategies for AMPs Dimerization
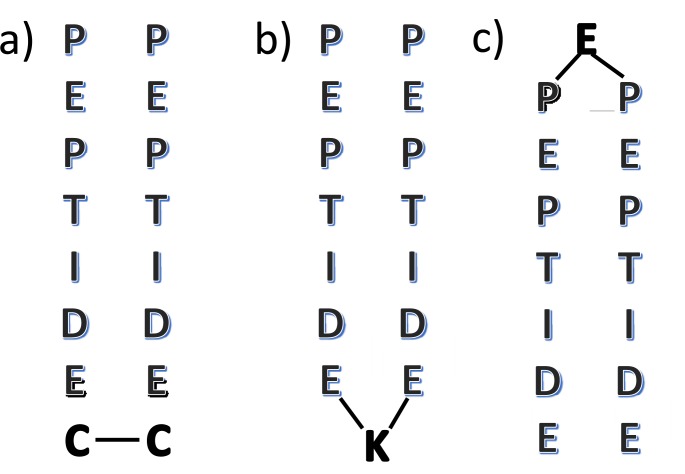
Different strategies have been applied for the synthesis of dimeric peptides. Dimers have been synthesized mainly by disulfide bonds, by incorporating cysteine residues in any position of peptide, and by amide bonds, by incorporating lysine or glutamate residues in the C- or N-terminal position, respectively (Figure. 3) [37, 41, 43, 49-56].
Figure 3.
Common dimerization strategies. Peptides linked by a disulfide bond. The figure shows cysteine residues at the C-terminal position but residues could be at any position (a). C-terminal lysine-linked peptides (b). N-terminal glutamate-linked peptides (c).
In the case of disulfide bond-linked peptides, cysteine residue can be incorporated as an extra residue (if not present as a constituent residue of the peptide) or by substituting a particular residue (generally a serine residue, if present, due to the similar side chain structure). The substitution/incorporation can be in any position of the peptide sequence, given the possibility of testing several dimer analogs. However, it is important to note that disulfide dimer synthesis involves an additional step after peptide chain elongation. Cysteine residues must be oxidized to produce the dimeric molecule. This procedure is generally achieved by air oxidation and significantly reduces the yield of peptide synthesis [55, 57, 58]. On the other hand, amide bond linked peptides can be produced by the incorporation of a lysine residue for C-terminal dimerization or glutamate residue for N-terminal dimerization. The first approach uses the alpha and epsilon amino groups of a lysine residue to produce amide bonds with the alpha amine groups of the first residue of the two-peptide chains. Fmoc-Lys(Fmoc)-OH can be used for this purpose by attaching it to the resin, and after alpha and epsilon-Fmoc group deprotection, the two peptide chains are simultaneously elongated. The second approach requires, before the cleavage from resin, the use of Fmoc-Glu-OH with its alpha and delta carboxylic group without the protection group, to link two elongated peptide chains [37, 52, 59]. It must be considered that C-terminal dimerization produces a dimeric molecule with two free N-terminal amines (positively charged at biological pH) from the first residue. On the other hand, N-terminal dimerization produces only one free N-terminal amines when an amino acid like glutamate is used as a linker. This difference could be responsible for the different antimicrobial activities of these analogs. It is well established that N-terminal modifications could affect the activity of AMPs [60-62]. We have showed that the charge of the N-terminus plays an important role in driving the selectivity of the AMP peptides. By modification of N-terminus, the peptide was active only against Gram-positive bacteria [61]. In this manner, dimerization on N-terminus is not a good strategy to improve biological activity.
2.2. Linker and Spacers
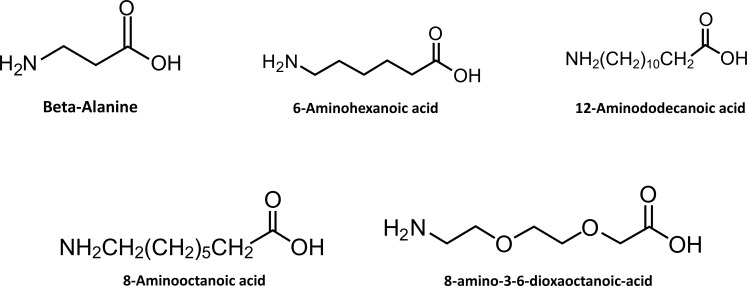
Linkers and spacers are molecules used to link and separate other molecules to avoid interactions among them, once biological activity can be affected by the distance between the molecules. In nature, they are used to connect and separate many protein domains without interfering in their functions [63]. They are also employed to link peptides to form multimeric molecules such as peptide dimers, trimers, tetramers and oligomers [64, 65]. For the dimerization of peptides, linkers are used to attach two molecules, which can be identical or different, resulting in homodimers and heterodimers, respectively. As an example, in Figure. 3, lysine and glutamic acid are used as linkers for the formation of a homodimer peptide. The linkers possess functional groups as carboxyl or amine groups, which are responsible for attaching to the C or N terminus of peptides. In addition, spacers can be used, but they are not essential in the dimerization of peptides; nevertheless, they are very useful, especially when the molecules require space between them to conserve or improve its biological activity (Figure. 4).
Figure 4.
Examples of spacers with different lengths and polarities that can affect the biological activity of a peptide.
Lorenzón and coworkers reported the effects of different spacers used for Ctx-Ha peptide dimerization (sequence: Gly-Trp-Leu-Asp-Val-Ala-Lys-Lys-Ile-Gly-Lys-Ala-Ala-Phe-Asn-Val-Ala-Lys-Asn-Phe-Leu-CONH2). The dimeric forms were obtained by the addition of a Lys residue at the C-terminus of the peptide Ctx-Ha. The Fmoc-8-amino-3,6-dioxaoctanoic acid contains an ethoxy group and was used as a polar spacer, while the Fmoc-8-amino-octanoic acid contains methyl groups and was used as an apolar spacer; however, both spacers are the same size. The antimicrobial activity was better for the peptide containing the polar spacer, which was due to the additional interaction with the head groups of the membrane phospholipids or cell wall. The apolar group decreased the initial interaction and the biological activity [49]. Thus far, the results demonstrate that linker flexibility and polarity play key roles in hemolytic and biological activities in antimicrobial peptide dimers [49, 56, 64]. Linkers and spacers can be used to synthesize dimeric forms of peptides efficiently; however, they need to be carefully designed, with consideration of the polarity, length and position, to assure that they do not decrease the biological activity of the peptides.
3. DIMER VS MONOMER
3.1. Magainin 2 Dimers
The AMP Magainin 2 (MG2) was one of the first AMPs discovered and might be one of the most studied since its discovery by Michael Zasloff in 1987. MG2 belongs to the magainin family, which are AMPs isolated from the skin of Xenopus laevis [14, 66, 67]. In addition to most AMPs, MG2 lacks a defined secondary structure in water but adopts an amphipathic helical structure in the presence of membrane mimetics or secondary structure-inducing solvents. In terms of biological activity, the peptide has a wide spectrum of action against gram-positive and gram-negative bacteria, fungi, protozoa, and even cancer cells [68-70]. This multifunctional activity makes MG2 a very interesting molecule to study, with great potential as a new drug. Since its discovery, a large number of MG2 analogs have been synthesized in an attempt to increase its biological activity and improve its pharmacotechnical properties [71-74]. MG2 dimerization has led to an increase in its antimicrobial activity. Table 1 shows the effects of dimerization on the antimicrobial activity of MG2 dimers. The molecules are slightly different, although the three versions correspond to molecules dimerized by the extreme C-terminus
Table 1. Antimicrobial activity of MG2 dimers.
Lysine-linked MG2 dimers showed an increased antibacterial activity of 8-16 times when compared to the monomeric MG2 peptide. This C-terminal lysine-linked increased the proximity and orientation of peptide chains. On the other hand, both cysteine-linked dimers showed an increased antibacterial activity of 2-4 times. It is important to note that the linkage of chains by a lysine comprises four aliphatic carbons, while a disulfide bond comprises just one. This difference in length could affect the interactions and flexibility of the chains. Specific modifications of the sequence (F12W, N22C, and the addition of βA) might also be responsible for the differential increase in antimicrobial activity of the dimeric versions. N-terminal dimerization using glutamic acid-linked did not promote the increase of antimicrobial activities of MG2 [52]. This result showed that the best linkage position to produce a dimeric molecule is the C-terminus position. The C-terminal dimerization preserve two free N-terminal amines charged positively while N-terminal dimerization could affect the charge and the initial interaction with the membrane. The dimerization of (MG2)2K did not change the peptide structure and initial interaction and/or mechanism of action, but promote the proximity of the peptide chains and decrease the number of molecules required to pore formation and increase the biological activity.
3.2. Aurein 1.2 Dimers
Several AMPs from the aurein family, which were originally isolated from the Australian frogs Litoria aurea and Litoria raniformis, have been extensively studied. One of the most active peptides of the aurein family is aurein 1.2 (AU), a short 13-residue peptide with a molecular mass of 1480 g mol-1. Aurein 1.2 is active against microorganisms and tumor cells and possesses low toxicity against red blood cells [52, 75]. Lorenzón and coworkers synthesized two AU dimers: (AU)2K and E(AU)2, with lysine and glutamic acid residues used as linkers, respectively. Circular dichroism spectra indicated that these AU dimers have a “coiled coil” structure in water, while AU displayed a typical spectrum for disordered structures. Hemolytic and vesicle permeabilization assays showed that AU has a concentration-dependent activity, while this effect was less evident for the dimeric versions. In addition, carboxyfluorescein release experiments with LUVs showed that both dimer and monomeric peptides were able to permeabilize vesicles, although the ratio of leakage response to increases in peptide concentration were different. Optical microscopy experiments showed that both versions induced pore opening and promoted the burst of the vesicles. In addition, isothermal titration calorimetry on the LUVs also showed significant differences in peptide membrane interactions. Together, these data clearly demonstrated that dimerization changes the mechanism of action of AU [52].
As shown in Table 2, dimerization of aurein 1.2 decreases the ability of the peptide to inhibit the growth of bacteria and fungi. (AU)2K was unable to inhibit the growth of C. albicans but promoted the aggregation of cells, which was elucidated as an interaction of the peptides with yeast cell wall carbohydrates called mannans [76]. In addition, its ability to aggregate yeast cells makes the dimeric versions of AU a promising future drug candidate for preventing C. albicans adhesion to biological targets and medical devices, such as prostheses and catheters, preventing diseases caused by this fungus [75, 76].
Table 2. Antimicrobial activity of AU dimers.
| Peptide | MIC (µmol/L) | ||
|---|---|---|---|
| E. coli | S. aureus | C. albicans | |
| AU | 16 | 8 | 32 |
| (AU)2K | 128 | >128 | >128 |
| E(AU)2 | 128 | >128 | >128 |
These results indicated that the effect of the dimerization on biological activity of peptides could change the AMP mechanism of action and their biological activity. The change of the structure in solution support different initial interactions with the cell wall and change the mechanism of action of aurein 1.2. Melittin peptide also exhibited the same behavior (as mentioned below).
3.3. Other Peptides
Several research groups have studied the effects of dimerization on the biological activity of AMPs. In addition to the increase in antimicrobial activity and its velocity achieved, researchers were also attracted by the potential increase in other properties promoted by dimerization. Table 3 and 4 shows various AMPs that have been used as a template for studying the effects of dimerization. Specifically, we showed some peptides properties and the effects of dimerization on antimicrobial and hemolytic activities. The effects of dimerization on the biological activity of AMPs showed that the increase in biological activity is not a general rule. It is clear that dimerization affects the biological activity of the peptides, sometimes by increasing the antimicrobial activity, sometimes decreasing. In addition, some dimeric versions are hemolytic. According to some authors, the improvement in antimicrobial activity is correlated with an increase in hydrophobicity and net positive charge of its surface area, which enhances LPS binding and neutralization [77-79]. Furthermore, the ability to aggregate and to adopt a well-defined structure could be important to enhance the antimicrobial activity of dimers [54], since dimerized peptides have a lower concentration dependence for reaching the permeabilization threshold compared with the monomers. The “preassembling state” of peptide dimers leads to a reduced number of molecules necessary to form effective pore structures in membrane bilayers. Additionally, the peptide
Table 3. Dimeric antimicrobial peptides with increased activity.
| Peptide | Linker Unit/Position | Sequence | Length | Charge |
Charge/
Length |
MON and DIM
structure (Aqueous/ Membrane Mimetic) |
Antimicro-
bial Activity |
Hemolytic Activity |
Other
Effects |
Referen-
ces |
|---|---|---|---|---|---|---|---|---|---|---|
| DH (histatin) | K/C-term | KRKFHEKHHSHRGY | 14 | +8 | 0.67 | No information | Increased against S. aureus |
Not determined | Same “in vivo” activity | [41] |
| Ctx-Ha | K-AEEAc/C-term | GWLDVAKKIGKAAFNVAKNFL | 21 | +4 | 0.19 | MON: random coil/helicoidal DIM: random coil/helicoidal |
Increased against gram- |
Increased | Higher velocity and percentage of membrane permeabilization |
[49] |
| Magainin 2 | βAC/C-term | GIGKFLHSAKKFGKAFVGEIMNSAC | 25 | +4 | 0.16 | No information | Increased against gram- and gram+ |
Increased | Higher membrane permeabilization |
[50] |
| Magainin 2 | C/C-term | GIGKFLHSAKKFGKAFVGEIMCS | 23 | +4 | 0.17 | MON: random coil/helicoidal DIM: random coil/helicoidal |
Increased against gram- |
Increased | Higher membrane permeabilization |
[51] |
| Magainin 2 | K/C-term | GIGKFLHSAKKFGKAFVGEIMNS | 23 | +4 | 0.17 | MON: random coil/helicoidal DIM: random coil/helicoidal |
Increased against gram- and gram+ |
Increased | Higher velocity and percentage of membrane permeabilization |
[59] |
| di-K18Hc | C/C-term | WLNALLKKGLNCAKGVLA | 18 | +4 | 0.22 | MON: random coil/helicoidal DIM: random coil/helicoidal |
Increased against antibiotic- resistant bacteria |
Decreased | Active in elevated concentrations of NaCl or MgCl2 |
[53] |
| V2-dimer | K/C-term | RGRKVVRRKK | 10 | +7 | 0.7 | No information | Increased against gram- |
Not determined | Broader spectrum of antimicrobial activity |
[54] |
| p-BthTX-I | C/C-term | KKYRYHLKPFCKK | 13 | 6+ | 0.46 | MON: random coil/random coil DIM: random coil/random coil |
Increased against gram- and gram+ |
Similar | None | [55] |
| J-AA/ J-RR | Htrz (triazole) /N-term | GLLKRIKTLL / RRWWRF | 10/6 | 4+/4+ | 0.4/0.67 | MON: random coil/helicoidal DIM: random coil/helicoidal |
Increased | Similar | None | [82] |
| cys-pep1037 | C/N-term | KRFRIRVRV | 9 | 6+ | 0.67 | No information | Increased | Not determined | None | [81] |
Table 4. Dimeric antimicrobial peptides with decreased activity.
| Peptide | Linker Unit/Position | Sequence | Length | Charge |
Charge/
Length |
MON and DIM structure
(Aqueous/Membrane Mimetic) |
Antimicrobial Activity | Hemolytic Activity |
Other
Effects |
References |
|---|---|---|---|---|---|---|---|---|---|---|
| Ctx-Ha | K/C-term | GWLDVAKKIGKAAFNVAKNFL | 21 | +4 | 0.19 | MON: random coil/helicoidal DIM: random coil/helicoidal |
Decreased against gram+ | Increased | Higher velocity and percentage of membrane permeabilization | [49] |
| Ctx-Ha | K-Aoc/C-term | GWLDVAKKIGKAAFNVAKNFL | 21 | +4 | 0.19 | MON: random coil/helicoidal DIM: random coil/helicoidal |
Decreased against gram+, gram- and yeast | Increased | Higher velocity and percentage of membrane permeabilization | [49] |
| Aurein 1.2 | K/C-term | GLFDIIKKIAESF | 13 | +1 | 0.08 | MON: random coil/helicoidal DIM: coiled-coil/helicoidal |
Decreased against gram+, gram- and yeast | Increased | Dimerization changes the mechanism of action | [83] |
| Aurein 1.2 | E/N-term | GLFDIIKKIAESF | 13 | +1 | 0.08 | MON: random coil/helicoidal DIM: coiled-coil/helicoidal |
Decreased against gram+, gram- and yeast | Similar | Dimerization changes the mechanism of action | [83] |
| PST13-RK | C/C-term | KKKFPWWWPFKKK | 13 | +7 | 0.54 | MON: ND/β-turn DIM: ND/β-turn |
Decreased against gram+ and gram- | Not determined | Mammalian cell toxicity | [37] |
| PST13-RK | K/C-term | KKKFPWWWPFKKK | 13 | +7 | 0.54 | MON: ND/β-turn DIM: ND/β-turn |
Decreased against gram+ and gram- | Not determined | Mammalian cell toxicity | [37] |
| Melittin | C/middle of polypeptide chain | GIGAVLKVLTTGLCALISWIKRKRQQ | 26 | +5 | 0.19 | MON: random coil/helicoidal DIM: helicoidal/helicoidal |
Decreased against gram+ and gram- | Increased | - | [56] |
ND: Not Determined
chain proximity imposed by dimerization could also reduce the time required to form those pore structures. However, some studies have shown that dimeric peptides could lose their microbial properties. An acceptable explanation is that the dimers could be inhibited from passing through the cell walls of microbial cells [49, 80]. Moreover, the interaction with cell wall components, the conformational changes or the peptide aggregation prior to membrane binding could explain the lower capacity of some dimeric AMPs reaching the membrane of microorganisms [75]. These controversial studies show that the effects of dimerization of AMPs need to be better studied. Then, further work is needed to determine the parameters that must be taken into account when choosing an AMP to be dimerized.
The data presented in Tables 3 and 4 showed that most of the peptides that have an increased antimicrobial activity have been dimerized at the C-terminus. As discussed for Magainin 2, C-terminal dimerization preserve two free N-terminal amines charged positively, while N-terminal dimerization decrease the positive charge, affecting the initial interaction with the membrane. Dimeric peptides derived from 1037 and anapolin(J-AA)/RW are exceptions because those peptides were dimerized by N-terminus and have an increased activity. In the case of peptide 1037, N-terminus dimerization were achieve by the use of a cysteine (not glutamate) preserving two amines charged positively [81]. J-AA/RW dimers have lost N-terminus positive charges, but the overall charge/length ratio is high enough to minimize the reduced charge due to dimerization [82]. In our understanding, there is not a clear pattern of the effect of the charge/length relationship. However, the higher charge/size ratio may favor the activity of dimeric versions by the electrostatic repulsion of the peptide chains, preventing aggregation. The peptide secondary structure changes imposed by dimerization also affect biological activity. Dimeric peptide with increased antimicrobial activity has in common the same structure in solution and in membrane mimetics than monomer. The proximity of the peptides chains and decrease the number of molecules required to pore formation may explain the increase in activity. In this case, we believe that the mechanism of action is the same for both peptides.
It is interesting to note that dimerization of Ctx-Ha, aurein 1.2 and melittin peptides decreased the antimicrobial activity but increased the hemolytic activity and the percentage of membrane permeabilization. The red blood cells and vesicle have only lipids on the surface, lacking the components of bacterial cell walls, as peptidoglycan. The change of the structure in solution promote different initial interactions with the cell wall, as polysaccharide, and change the mechanism of action [76]. Peptide di-K18Hc is the only one with reduced toxicity, plus retained antimicrobial activity in elevated concentrations of NaCl or MgCl2 [53].
CONCLUSION
Several AMPs were linked together as dimers to improve the antimicrobial activity, although, for some AMPs, dimeriza-tion results in a decrease of activity. It appears that the assembled-state of dimers contributes to the proximity and orientation of peptide chains, enhancing pore formation and antimicrobial
activity. The factors that lead a monomeric AMP to become a more active dimeric molecule are not well established. However, several factors must be considered for the design of a new dimeric antimicrobial peptide. The examples analyzed in this work showed that peptide N-terminus charge, linker position, structure, and interaction with cell wall components could affect the biological activity of dimeric peptides. In summary, dimerization constitutes a promising strategy to improve the effectiveness of some AMPs, although more studies are necessary to elucidate the relationship between peptide properties and the dimerization effect on antimicrobial and hemolytic activity. To date, hundreds of synthetic AMPs are in clinical development, and even more are in advanced stages of preclinical development. These AMPs, and new AMPs isolated from natural organisms or designed by computational methods, could have their antimicrobial activity optimized by dimerization.
ACKNOWLEDGEMENTS
The authors are grateful to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Fundação Amparo a Pesquisa do Estado de São Paulo (FAPESP - 13/07600-3, 12/15346-7) and the Coordenação de Aperfeiçoamento de Nível Superior (CAPES) for financial support.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.WHO. Antimicrobial resistance: Global report on surveillance. Geneva: World Health Organization; 2014. p. 256. [Google Scholar]
- 2.Shlaes D.M., Sahm D., Opiela C., Spellberg B. The FDA reboot of antibiotic development. Antimicrob. Agents Chemother. 2013;57(10):4605–4607. doi: 10.1128/AAC.01277-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marr A.K., Gooderham W.J., Hancock R.E. Antibacterial peptides for therapeutic use: Obstacles and realistic outlook. Curr. Opin. Pharmacol. 2006;6(5):468–472. doi: 10.1016/j.coph.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 4.de Freitas L.M., Lorenzón E.N., Santos-Filho N.A., de Paula Zago L.H., Uliana M.P., de Oliveira K.T., Cilli E.M., Fontana C.R. Antimicrobial photodynamic therapy enhanced by the peptide aurein 1.2. Sci. Rep. 2018;8(1):4212. doi: 10.1038/s41598-018-22687-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aida K.L., Kreling P.F., Caiaffa K.S., Calixto G.M.F., Chorilli M., Spolidorio D.M., Santos-Filho N.A., Cilli E.M., Duque C. Antimicrobial peptide-loaded liquid crystalline precursor bioadhesive system for the prevention of dental caries. Int. J. Nanomedicine. 2018;13:3081. doi: 10.2147/IJN.S155245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carretero G.P., Vicente E.F., Cilli E.M., Alvarez C.M., Jenssen H., Schreier S. Dissecting the mechanism of action of actinoporins. Role of the N-terminal amphipathic α-helix in membrane binding and pore activity of sticholysins I and II. PLoS One. 2018;13(8):e0202981. doi: 10.1371/journal.pone.0202981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kishi R.N.I., Stach-Machado D., de Lacorte Singulani J., dos Santos C.T., Fusco-Almeida A.M., Cilli E.M., Freitas-Astúa J., Picchi S.C., Machado M.A. Evaluation of cytotoxicity features of antimicrobial peptides with potential to control bacterial diseases of citrus. PLoS One. 2018;13(9):e0203451. doi: 10.1371/journal.pone.0203451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masias E., Dupuy F.G., da Silva Sanches P.R., Farizano J.V., Cilli E., Bellomio A., Saavedra L., Minahk C. Impairment of the class IIa bacteriocin receptor function and membrane structural changes are associated to enterocin CRL35 high resistance in Listeria monocytogenes. Biochim. Biophys. Acta, Gen. Subj. 2017;1861(7):1770–1776. doi: 10.1016/j.bbagen.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Zhao J., Zhao C., Liang G., Zhang M., Zheng J. Engineering antimicrobial peptides with improved antimicrobial and hemolytic activities. J. Chem. Inf. Model. 2013;53(12):3280–3296. doi: 10.1021/ci400477e. [DOI] [PubMed] [Google Scholar]
- 10.Huang Y.W., Lee C.T., Wang T.C., Kao Y.C., Yang C.H., Lin Y.M., Huang K.S. The development of peptide-based antimicrobial agents against dengue virus. Curr. Protein Pept. Sci. 2018;19(10):998–1010. doi: 10.2174/1389203719666180531122724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Libério M.S., Joanitti G.A., Azevedo R.B., Cilli E.M., Zanotta L.C., Nascimento A.C., Sousa M.V., Júnior O.R.P., Fontes W., Castro M.S. Anti-proliferative and cytotoxic activity of pentadactylin isolated from Leptodactylus labyrinthicus on melanoma cells. Amino Acids. 2011;40(1):51–59. doi: 10.1007/s00726-009-0384-y. [DOI] [PubMed] [Google Scholar]
- 12.Pinto M.E.F., Najas J.Z., Magalhães L.G., Bobey A.F., Mendonça J.N., Lopes N.P., Leme F.V.M., Teixeira S.P., Trovó M., Andricopulo A.D. Inhibition of breast cancer cell migration by cyclotides isolated from Pombalia calceolaria. J. Nat. Prod. 2018;81(5):1203–1208. doi: 10.1021/acs.jnatprod.7b00969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato H., Feix J.B. Peptide-membrane interactions and mechanisms of membrane destruction by amphipathic α-helical antimicrobial peptides. Biochim. Biophys. Acta (BBA)-. Biomembr. 2006;1758(9):1245–1256. doi: 10.1016/j.bbamem.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 14.Gregory S.M., Pokorny A., Almeida P.F. Magainin 2 revisited: A test of the quantitative model for the all-or-none permeabilization of phospholipid vesicles. Biophys. J. 2009;96(1):116–131. doi: 10.1016/j.bpj.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang K.F., Nagarajan R., Mello C.M., Camesano T.A. Characterization of supported lipid bilayer disruption by chrysophsin-3 using QCM-D. J. Phys. Chem. B. 2011;115(51):15228–15235. doi: 10.1021/jp209658y. [DOI] [PubMed] [Google Scholar]
- 16.Peters B.M., Shirtliff M.E., Jabra-Rizk M.A. Antimicrobial peptides: Primeval molecules or future drugs? PLoS Pathog. 2010;6(10) doi: 10.1371/journal.ppat.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mechkarska M., Meetani M., Michalak P., Vaksman Z., Takada K., Conlon J.M. Hybridization between the African clawed frogs Xenopus laevis and Xenopus muelleri (Pipidae) increases the multiplicity of antimicrobial peptides in skin secretions of female offspring. Comp. Biochem. Physiol. Part D Genomics Proteomics. 2012;7(3):285–291. doi: 10.1016/j.cbd.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Matsuzaki K. Control of cell selectivity of antimicrobial peptides. BBA: Biomembranes. 2009;1788(8):1687–1692. doi: 10.1016/j.bbamem.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Li Y., Xiang Q., Zhang Q., Huang Y., Su Z. Overview on the recent study of antimicrobial peptides: Origins, functions, relative mechanisms and application. Peptides. 2012;37(2):207–215. doi: 10.1016/j.peptides.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Wang K., Yan J., Dang W., Liu X., Chen R., Zhang J., Zhang B., Zhang W., Kai M., Yan W. Membrane active antimicrobial activity and molecular dynamics study of a novel cationic antimicrobial peptide polybia-MPI, from the venom of Polybia paulista. Peptides. 2013;39:80–88. doi: 10.1016/j.peptides.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Tan J., Huang J., Huang Y., Chen Y. Effects of single amino acid substitution on the biophysical properties and biological activities of an amphipathic α-helical antibacterial peptide against gram-negative bacteria. Molecules. 2014;19(8):10803–10817. doi: 10.3390/molecules190810803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gofman Y., Linser S., Rzeszutek A., Shental-Bechor D., Funari S.S., Ben-Tal N., Willumeit R. Interaction of an antimicrobial peptide with membranes: Experiments and simulations with NKCS. J. Phys. Chem. B. 2010;114(12):4230–4237. doi: 10.1021/jp909154y. [DOI] [PubMed] [Google Scholar]
- 23.Wimley W.C. Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem. Biol. 2010;5(10):905–917. doi: 10.1021/cb1001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melo M.N., Ferre R., Castanho M.A.R.B. Antimicrobial peptides: Linking partition, activity and high membrane-bound concentrations. Nat. Rev. Microbiol. 2009;7(3):245–250. doi: 10.1038/nrmicro2095. [DOI] [PubMed] [Google Scholar]
- 25.Sengupta D., Leontiadou H., Mark A.E., Marrink S.J. Toroidal pores formed by antimicrobial peptides show significant disorder. BBA: Biomembranes. 2008;1778(10):2308–2317. doi: 10.1016/j.bbamem.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Brogden K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005;3(3):238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 27.Mechler A., Praporski S., Atmuri K., Boland M., Separovic F., Martin L.L. Specific and selective peptide-membrane interactions revealed using quartz crystal microbalance. Biophys. J. 2007;93(11):3907–3916. doi: 10.1529/biophysj.107.116525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y., Chen C.H., Hu D., Ulmschneider M.B., Ulmschneider J.P. Spontaneous formation of structurally diverse membrane channel architectures from a single antimicrobial peptide. Nat. Commun. 2016;7:13535. doi: 10.1038/ncomms13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wimley W.C., Hristova K. Antimicrobial peptides: Successes, challenges and unanswered questions. J. Membr. Biol. 2011;239(1-2):27–34. doi: 10.1007/s00232-011-9343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galdiero S., Falanga A., Cantisani M., Vitiello M., Morelli G., Galdiero M. Peptide-lipid interactions: Experiments and applications. Int. J. Mol. Sci. 2013;14(9):18758–18789. doi: 10.3390/ijms140918758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riske K.A. Chapter four-optical microscopy of giant vesicles as a tool to reveal the mechanism of action of antimicrobial peptides and the specific case of gomesin. Adv. Planar Lipid Bilayers Liposomes. 2015;21:99–129. [Google Scholar]
- 32.Berglund N.A., Piggot T.J., Jefferies D., Sessions R.B., Bond P.J., Khalid S. Interaction of the antimicrobial peptide polymyxin B1 with both membranes of E. coli: A molecular dynamics study. PLOS Comput. Biol. 2015;11(4):e1004180. doi: 10.1371/journal.pcbi.1004180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arias M., Prenner E.J., Vogel H.J. Calorimetry methods to study membrane interactions and perturbations induced by antimicrobial host defense peptides. Antimicrob. Peptide Method Protocol. 2017;1548:119–140. doi: 10.1007/978-1-4939-6737-7_9. [DOI] [PubMed] [Google Scholar]
- 34.Vlieghe P., Lisowski V., Martinez J., Khrestchatisky M. Synthetic therapeutic peptides: Science and market. Drug Discov. Today. 2010;15(1-2):40–56. doi: 10.1016/j.drudis.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Steckbeck J.D., Deslouches B., Montelaro R.C. Antimicrobial peptides: New drugs for bad bugs? Expert Opin. Biol. Ther. 2014;14(1):11–14. doi: 10.1517/14712598.2013.844227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu W.L., Shin S.Y. Effects of dimerization of the cell-penetrating peptide Tat analog on antimicrobial activity and mechanism of bactericidal action. J. Pept. Sci. 2009;15(5):345–352. doi: 10.1002/psc.1120. [DOI] [PubMed] [Google Scholar]
- 37.Yang S.T., Kim J.I., Shin S.Y. Effect of dimerization of a beta-turn antimicrobial peptide, PST13-RK, on antimicrobial activity and mammalian cell toxicity. Biotechnol. Lett. 2009;31(2):233–237. doi: 10.1007/s10529-008-9848-5. [DOI] [PubMed] [Google Scholar]
- 38.Glukhov E., Stark M., Burrows L.L., Deber C.M. Basis for selectivity of cationic antimicrobial peptides for bacterial versus mammalian membranes. J. Biol. Chem. 2005;280(40):33960–33967. doi: 10.1074/jbc.M507042200. [DOI] [PubMed] [Google Scholar]
- 39.Sal-Man N., Oren Z., Shai Y. Preassembly of membrane-active peptides is an important factor in their selectivity toward target cells. Biochemistry. 2002;41(39):11921–11930. doi: 10.1021/bi0260482. [DOI] [PubMed] [Google Scholar]
- 40.Sengupta J., Khan M.A., Huppertz B., Ghosh D. In-vitro effects of the antimicrobial peptide Ala8,13,18-magainin II amide on isolated human first trimester villous trophoblast cells. Reprod. Biol. Endocrinol. 2011;9:49. doi: 10.1186/1477-7827-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Welling M.M., Brouwer C.P.J.M., Hof W., Veerman E.C.I., Amerongen A.V.N. Histatin-derived monomeric and dimeric synthetic peptides show strong bactericidal activity towards multidrug-resistant Staphylococcus aureus in vivo. Antimicrob. Agents Chemother. 2007;51(9):3416–3419. doi: 10.1128/AAC.00196-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lakshminarayanan R., Liu S., Li J., Nandhakumar M., Aung T.T., Goh E., Chang J.Y., Saraswathi P., Tang C., Safie S.R., Lin L.Y., Riezman H., Lei Z., Verma C.S., Beuerman R.W. Synthetic multivalent antifungal peptides effective against fungi. PLoS One. 2014;9(2):e87730. doi: 10.1371/journal.pone.0087730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee J.Y., Yang S.T., Lee S.K., Jung H.H., Shin S.Y., Hahm K.S., Kim J.I. Salt-resistant homodimeric bactenecin, a cathelicidin-derived antimicrobial peptide. FEBS J. 2008;275(15):3911–3920. doi: 10.1111/j.1742-4658.2008.06536.x. [DOI] [PubMed] [Google Scholar]
- 44.Taylor K., McCullough B., Clarke D.J., Langley R.J., Pechenick T., Hill A., Campopiano D.J., Barr P.E., Dorin J.R., Govan J.R.W. Covalent dimer species of beta-defensin Defr1 display potent antimicrobial activity against multidrug-resistant bacterial pathogens. Antimicrob. Agents Chemother. 2007;51(5):1719–1724. doi: 10.1128/AAC.01531-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Güell I., Ferre R., Sørensen K.K., Badosa E., Ng-Choi I., Montesinos E., Bardají E., Feliu L., Jensen K.J., Planas M. Multivalent display of the antimicrobial peptides BP100 and BP143. Beilstein J. Org. Chem. 2012;8:2106–2117. doi: 10.3762/bjoc.8.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hernandez-Gordillo V., Geisler I., Chmielewski J. Dimeric unnatural polyproline-rich peptides with enhanced antibacterial activity. Bioorg. Med. Chem. Lett. 2014;24(2):556–559. doi: 10.1016/j.bmcl.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dewan P.C., Anantharaman A., Chauhan V.S., Sahal D. Antimicrobial action of prototypic amphipathic cationic decapeptides and their branched dimers. Biochemistry. 2009;48(24):5642–5657. doi: 10.1021/bi900272r. [DOI] [PubMed] [Google Scholar]
- 48.Otvos L., Wade J.D., Lin F., Condie B.A., Hanrieder J., Hoffmann R. Designer antibacterial peptides kill fluoroquinolone-resistant clinical isolates. J. Med. Chem. 2005;48(16):5349–5359. doi: 10.1021/jm050347i. [DOI] [PubMed] [Google Scholar]
- 49.Lorenzón E.N., Cespedes G.F., Vicente E.F., Nogueira L.G., Bauab T.M., Castro M.S., Cilli E.M. Effects of dimerization on the structure and biological activity of antimicrobial peptide Ctx-Ha. Antimicrob. Agents Chemother. 2012;56:3004–3010. doi: 10.1128/AAC.06262-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mukai Y., Matsushita Y., Niidome T., Hatekeyama T., Aoyag H. Parallel and antiparallel dimers of magainin 2: Their interaction with phospholipid membrane and antibacterial activity. J. Pept. Sci. 2002;8(10):570–577. doi: 10.1002/psc.416. [DOI] [PubMed] [Google Scholar]
- 51.Dempsey C.E., Ueno S., Avison M.B. Enhanced membrane permeabilization and antibacterial activity of a disulfide-dimerized magainin analogue. Biochemistry. 2003;42(2):402–409. doi: 10.1021/bi026328h. [DOI] [PubMed] [Google Scholar]
- 52.Lorenzón E., Riske K., Troiano G., Da Hora G., Soares T., Cilli E. Effect of dimerization on the mechanism of action of aurein 1.2. Biochim. Biophys. Acta (BBA)-. Biomembr. 2016;1858(6):1129–1138. doi: 10.1016/j.bbamem.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 53.Jang W.S., Kim C.H., Kim K.N., Park S.Y., Lee J.H., Son S.M., Lee I.H. Biological activities of synthetic analogs of halocidin, an antimicrobial peptide from the tunicate Halocynthia aurantium. Antimicrob. Agents Chemother. 2003;47(8):2481–2486. doi: 10.1128/AAC.47.8.2481-2486.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou L., Liu S., Chen L., Li J., Ong L., Guo L., Wohland T., Tang C., Lakshminarayanan R., Mavinahalli J. The structural parameters for antimicrobial activity, human epithelial cell cytotoxicity and killing mechanism of synthetic monomer and dimer analogues derived from hBD3 C-terminal region. Amino Acids. 2011;40(1):123–133. doi: 10.1007/s00726-010-0565-8. [DOI] [PubMed] [Google Scholar]
- 55.Santos-Filho N.A., Lorenzon E.N., Ramos M.A., Santos C.T., Piccoli J.P., Bauab T.M., Fusco-Almeida A.M., Cilli E.M. Synthesis and characterization of an antibacterial and non-toxic dimeric peptide derived from the C-terminal region of Bothropstoxin-I. Toxicon. 2015;103:160–168. doi: 10.1016/j.toxicon.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 56.Jamasbi E., Batinovic S., Sharples R.A., Sani M.A., Robins-Browne R.M., Wade J.D., Separovic F., Hossain M.A. Melittin peptides exhibit different activity on different cells and model membranes. Amino Acids. 2014 doi: 10.1007/s00726-014-1833-9. [DOI] [PubMed] [Google Scholar]
- 57.Andreu D., Albericio F., Solé N.A., Munson M.C., Ferrer M., Barany G. Formation of disulfide bonds in synthetic peptides and proteins. Pept. Synth. Protocol; 1995. pp. 91–169. [DOI] [PubMed] [Google Scholar]
- 58.Postma T.M., Albericio F. Disulfide formation strategies in peptide synthesis. Eur. J. Org. Chem. 2014;2014(17):3519–3530. [Google Scholar]
- 59.Lorenzón E., Santos-Filho N., Ramos M., Bauab T., Camargo I., Cilli E. C-terminal lysine-linked magainin 2 with increased activity against multidrug-resistant bacteria. Prot. Pept. Lett. 2016 doi: 10.2174/0929866523666160511150907. [DOI] [PubMed] [Google Scholar]
- 60.Cilli E.M., Pigossi F.T., Crusca E., Ros U., Martinez D., Lanio M.E., Alvarez C., Schreier S. Correlations between differences in amino-terminal sequences and different hemolytic activity of sticholysins. Toxicon. 2007;50(8):1201–1204. doi: 10.1016/j.toxicon.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 61.Crusca E., Rezende A., Marchetto R., Mendes-Giannini M., Fontes W., Castro M., Cilli E. Influence of N-terminus modifications on the biological activity, membrane interaction, and secondary structure of the antimicrobial peptide Hylin-a1. Biopolymers. 2011;96(1):41–48. doi: 10.1002/bip.21454. [DOI] [PubMed] [Google Scholar]
- 62.Jahnsen R.O., Sandberg-Schaal A., Frimodt-Møller N., Nielsen H.M., Franzyk H. End group modification: Efficient tool for improving activity of antimicrobial peptide analogues towards gram-positive bacteria. Eur. J. Pharm. Biopharm. 2015;95(Pt A):40–46. doi: 10.1016/j.ejpb.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 63.Karolak-Wojciechowska J., Fruziński A., Czylkowski R., Paluchowska M., Mokrosz M. Spacer conformation in biologically active molecules. Part 2. Structure and conformation of 4-[2-(diphenylmethylamino) ethyl]-1-(2-methoxyphenyl) piperazine and its diphenylmethoxy analog-potential 5-HT 1A receptor ligands. J. Mol. Struct. 2003;657(1):7–17. [Google Scholar]
- 64.Reddy Chichili V.P., Kumar V., Sivaraman J. Linkers in the structural biology of protein-protein interactions. Protein Sci. 2013;22(2):153–167. doi: 10.1002/pro.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Larsen A.N., Sørensen K.K., Johansen N.T., Martel A., Kirkensgaard J.J., Jensen K.J., Arleth L., Midtgaard S.R. Dimeric peptides with three different linkers self-assemble with phospholipids to form peptide nanodiscs that stabilize membrane proteins. Soft Matter. 2016;12(27):5937–5949. doi: 10.1039/c6sm00495d. [DOI] [PubMed] [Google Scholar]
- 66.Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: Isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. USA. 1987;84(15):5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee W., Lee D.G. Magainin 2 induces bacterial cell death showing apoptotic properties. Curr. Microbiol. 2014;69(6):794–801. doi: 10.1007/s00284-014-0657-x. [DOI] [PubMed] [Google Scholar]
- 68.Lehmann J., Retz M., Sidhu S.S., Suttmann H., Sell M., Paulsen F., Harder J., Unteregger G., Stöckle M. Antitumor activity of the antimicrobial peptide magainin II against bladder cancer cell lines. Eur. Urol. 2006;50(1):141–147. doi: 10.1016/j.eururo.2005.12.043. [DOI] [PubMed] [Google Scholar]
- 69.Westerhoff H.V., Zasloff M., Rosner J.L., Hendler R.W., De Waal A., Vaz Gomes A., Jongsma P.M., Riethorst A., Juretić D. Functional synergism of the magainins PGLa and magainin-2 in Escherichia coli, tumor cells and liposomes. Eur. J. Biochem. 1995;228(2):257–264. [PubMed] [Google Scholar]
- 70.Aboudy Y., Mendelson E., Shalit I., Bessalle R., Fridkin M. Activity of two synthetic amphiphilic peptides and magainin-2 against herpes simplex virus types 1 and 2. Int. J. Pept. Protein Res. 1994;43(6):573–582. doi: 10.1111/j.1399-3011.1994.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 71.Zasloff M., Martin B., Chen H.C. Antimicrobial activity of synthetic magainin peptides and several analogues. Proc. Natl. Acad. Sci. USA. 1988;85(3):910–913. doi: 10.1073/pnas.85.3.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Han E., Lee H. Effects of PEGylation on the binding interaction of magainin 2 and tachyplesin I with lipid bilayer surface. Langmuir. 2013;29(46):14214–14221. doi: 10.1021/la4036985. [DOI] [PubMed] [Google Scholar]
- 73.Unger T., Oren Z., Shai Y. The effect of cyclization of magainin 2 and melittin analogues on structure, function, and model membrane interactions: Implication to their mode of action. Biochemistry. 2001;40(21):6388–6397. doi: 10.1021/bi0026066. [DOI] [PubMed] [Google Scholar]
- 74.Bessalle R., Kapitkovsky A., Gorea A., Shalit I., Fridkin M. All-D-magainin: Chirality, antimicrobial activity and proteolytic resistance. FEBS Lett. 1990;274(1/2):151–155. doi: 10.1016/0014-5793(90)81351-n. [DOI] [PubMed] [Google Scholar]
- 75.Lorenzón E.N., Sanches P.R., Nogueira L.G., Bauab T.M., Cilli E.M. Dimerization of aurein 1.2: Effects in structure, antimicrobial activity and aggregation of Cândida albicans cells. Amino Acids. 2013;44(6):1521–1528. doi: 10.1007/s00726-013-1475-3. [DOI] [PubMed] [Google Scholar]
- 76.Lorenzón E.N., Piccoli J.P., Cilli E.M. Interaction between the antimicrobial peptide Aurein 1.2 dimer and mannans. Amino Acids. 2014;46(11):2627–2631. doi: 10.1007/s00726-014-1832-x. [DOI] [PubMed] [Google Scholar]
- 77.Liu S., Zhou L., Lakshminarayanan R., Beuerman R. Multivalent antimicrobial peptides as therapeutics: Design principles and structural diversities. Int. J. Pept. Protein Res. 2010;16(3):199–213. doi: 10.1007/s10989-010-9230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen Y.X., Guarnieri M.T., Vasil A.I., Vasil M.L., Mant C.T., Hodges R.S. Role of peptide hydrophobicity in the mechanism of action of alpha-helical antimicrobial peptides. Antimicrob. Agents Chemother. 2007;51(4):1398–1406. doi: 10.1128/AAC.00925-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim S., Kim S.S., Lee B.J. Correlation between the activities of α-helical antimicrobial peptides and hydrophobicities represented as RP HPLC retention times. Peptides. 2005;26(11):2050–2056. doi: 10.1016/j.peptides.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 80.Jiang Z.Q., Vasil A.I., Gera L., Vasil M.L., Hodges R.S. Rational design of alpha-helical antimicrobial peptides to target gram-negative pathogens, Acinetobacter baumannii and Pseudomonas aeruginosa: Utilization of charge, ‘specificity determinants,’ total hydrophobicity, hydrophobe type and location as design parameters to improve the therapeutic ratio. Chem. Biol. Drug Des. 2011;77(4):225–240. doi: 10.1111/j.1747-0285.2011.01086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thamri A., Létourneau M., Djoboulian A., Chatenet D., Déziel E., Castonguay A., Perreault J. Peptide modification results in the formation of a dimer with a 60-fold enhanced antimicrobial activity. PLoS One. 2017;12(3):e0173783. doi: 10.1371/journal.pone.0173783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu B., Huang H., Yang Z., Liu B., Gou S., Zhong C., Han X., Zhang Y., Ni J., Wang R. Design of novel antimicrobial peptide dimer analogues with enhanced antimicrobial activity in vitro and in vivo by intermolecular triazole bridge strategy. Peptides. 2016;88:115–125. doi: 10.1016/j.peptides.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 83.Lorenzon E.N., Sanches P.R.S., Nogueira L.G., Bauab T.M., Cilli E.M. Dimerization of aurein 1.2: Effects in structure, antimicrobial activity and aggregation of Candida albicans cells. Amino Acids. 2013;44(6):1521–1528. doi: 10.1007/s00726-013-1475-3. [DOI] [PubMed] [Google Scholar]