Highlights
-
•
The use of eltrombopag was expanded to cover AA in Japan in August 2017.
-
•
The overall response rate was 55% (6/11).
-
•
Four patients had recovery of all three cell lineages, and two patients had only neutrophil recovery.
-
•
Stage at the initial assessment, the neutrophil-to-lymphocyte ratio and platelet counts were significantly different between the responders and non-responders.
Keywords: Eltrombopag, Aplastic anemia, Neutrophil-to-lymphocyte ratio, Real-world setting
Abstract
Background
Although eltrombopag has recently been approved for treating AA, the effects of its clinical use remain unknown.
Methods
We retrospectively analyzed 11 patients with AA, who had been treated with eltrombopag from August 2017 to May 2018.
Results
Overall response rate was 55%. There was tri-lineage recovery in four patients and platelet recovery in two. The reactive time was within 8 weeks after treatment initiation. Stage at the initial assessment, the neutrophil-to-lymphocyte ratio and platelet counts were significantly different between the responders and non-responders.
Conclusion
Eltrombopag is a promising agent for treating patients with any degree of AA.
1. Introduction
Aplastic anemia is a syndrome of bone marrow failure characterized by marrow hypoplasia and hematopoietic stem cell deficiency. An immunomediated pathophysiology has been inferred based on the response of AA to immunosuppressive therapy, the demonstration of immune activation, and evidence in animal models. [1], [2] The standard treatment for AA is an immunoglobulin (anti-thymocyte globulin, ATG) and cyclosporine, with hematologic responses observed in about two-thirds of patients. [3] Patients with AA refractory to immunosuppression and those who have a relapse after treatment may undergo allogeneic hematopoietic stem cell transplantation. Rapid front-line, bone marrow transplantation with an HLA-identical sibling donor often leads to an excellent outcome. [4], [5], [6] However, 2–40% of patients without a suitable donor for such transplantation continue to have severe cytopenia and are at risk of life-threatening hemorrhage due to thrombocytopenia and severe infection due to neutropenia.
It has recently been reported that eltrombopag, a non-peptide thrombopoietin mimetic oral drug that binds to the transmembrane domain of the MPL receptor, can induce a tri-lineage (platelets, erythrocytes, leukocytes) response in patients with refractory AA. [3] Animal models and insights from congenital marrow failure syndromes indicate involvement of thrombopoietin signaling during hematopoietic stem cell homeostasis as well as thrombopoiesis. The effects of the clinical use of this drug, however, remain largely unknown.
2. Patients and methods
2.1. Patients
This retrospective analysis was conducted in two hematology centers, Kansai Medical University Hospital and Kansai Medical University Medical Center. Patients with AA who had been given eltrombopag from August 2017 to May 2018 were included. Laboratory data were recorded at baseline and at 4, 8, and 12 weeks after eltrombopag treatment was started.
2.2. Treatments
Patients were started eltrombopag at the dose of 25 mg/day for 2 weeks and then were increased the dose every 2 weeks to maximum 100 mg/day.
2.3. Response criteria
Responses to the treatment were assessed according to National Institutes of Health (NIH) criteria. [7] A platelet response was defined by a platelet count increase of 20 × 109/L above baseline or stable platelet counts with no transfusions for at least 8 weeks. An erythroid lineage response was defined as a hemoglobin increase of >1.5 g/dL or a reduction of >4 units of transfused erythrocytes for 8 consecutive weeks. A leukocyte response was defined as an absolute neutrophil increase of 100% or an absolute neutrophil count increase of >0.5 × 109/L. A “robust” response was defined as platelets >50 × 109/L, hemoglobin >10 g/dL, and neutrophils >1 × 109/L for >8 weeks without transfusion support. Not achieving at least one of these criteria during eltrombopag treatment was considered treatment failure.
Paroxysmal nocturnal hemoglobinuria (PNH) clone was assessed by flow cytometry, measuring the frequency of red cell and neutrophils lacking glycosylphosphatidylinositols (GPI)-anchored proteins, and a PNH clone was considered present if red cells deficient were >0.005% or neutrophils deficient were > 0.003%.
2.4. Statistics
Association baseline characteristics between responders and non-responders were analyzed using the Fisher's exact test. All statistical tests were two-sided, statistical significance was defined as p < 0.05, and 95% confidence intervals (CI) were calculated. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R version 2.13.0 (The R Foundation). Specifically, EZR is a modified version of R Commander (version 1.6–3), which adds statistical functions frequently used in biostatistics [8].
3. Results
3.1. Patients’ characteristics
The clinical characteristics of the 11 patients (median age 64 years, range 17–90 years; 55% male) included in this study are shown in Table 1. Patients’ degrees of AA were staged according to Japanese criteria of AA [9]. One patient was at stage 1, three patients were at stage 2; one patient was at stage 3; and six patients at stage 5. Eight patients underwent karyotype analysis, which showed that one patient had a chromosomal abnormality(t(8;14)(q11.2; p11.2)(1/20 cells), and the other seven had a normal karyotype. Five patients were analyzed for a PNH clone. Three patients were positive for it (0.05%, 0.024%, 0.02%, respectively), and two were negative. Seven patients were previously treated with cyclosporine and ATG and four with cyclosporine alone. The median maximum dose of eltrombopag was 50 (25–75) mg. The median follow-up period was 12.0 (1.2–73.6) months.
Table 1.
Patients’ characteristics.
| n | 11 |
|---|---|
| Median age(y/o) | 64 (17–90) |
| Male/female(n) | 6/5 |
| Stage at treatment (n) | |
| 1 | 1 |
| 2 | 3 |
| 3 | 1 |
| 4 | 0 |
| 5 | 6 |
| Chromosomal abnormality | |
| Positive or negative | 1/7 |
| Unknown | 3 |
| PNH | |
| Positive or negative | 3/2 |
| Unknown | 6 |
| Pretreatment | |
| ATG | 7 |
| CyA | 11 |
PNH: paroxysmal nocturnal hemoglobinuria.
ATG: antithymocyte globulin.
CyA: cyclosporine.
3.2. Response to treatment
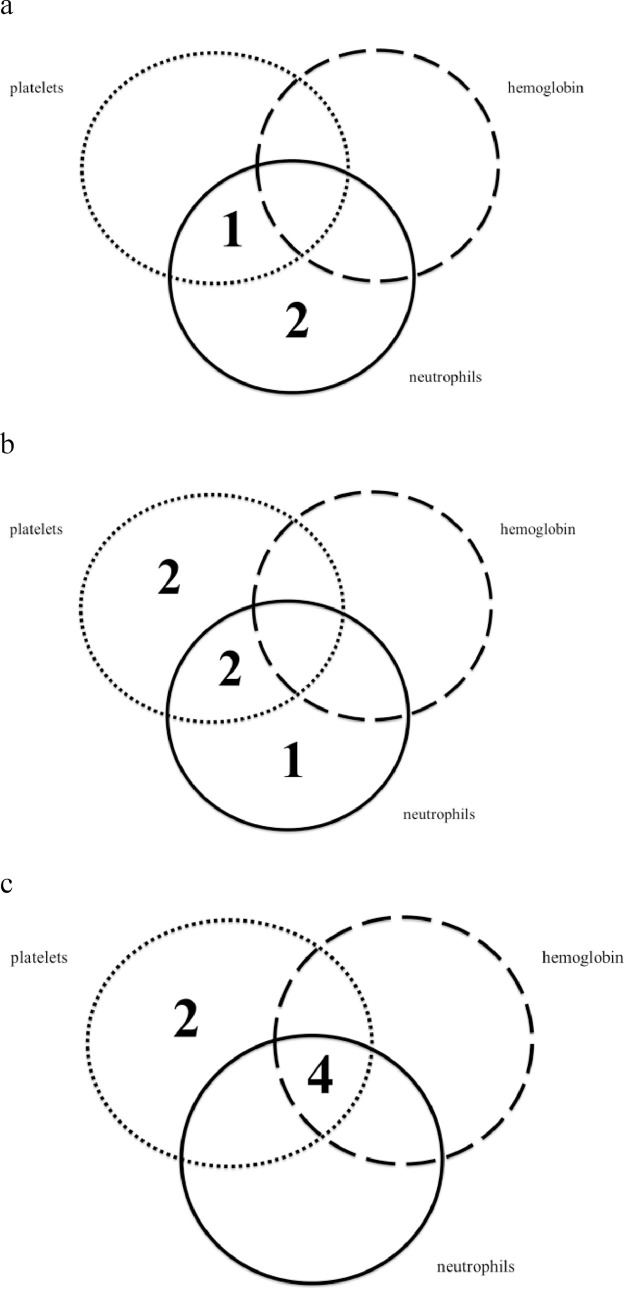
Four patients had recovery of all three cell lineages, and two patients had only neutrophil recovery. The overall response rate was 55% (6/11). Temporal changes are shown in Fig. 1. The neutrophil response was seen relatively early, and the response to erythroid response occurred relatively late. Most of the six patients showed at least some reaction at 8 weeks.
Fig. 1.
Temporal response to eltrombopag, by cell lineage. These venn diagrams show the numbers of patients with uni-lineage and multi-lineage responses at 4 weeks (a), 8 weeks (b), and 12 weeks (c) from the start of eltrombopag treatment.
3.3. Comparison of responders and non-responders
We classified the patients into two groups: responders and non-responders (Table 2). The AA stage at the initial assessment was severe in the non-responder group. The median treatment period from diagnosis to eltrombopag start was clearly shorter in the responders, whose reticulocyte count were higher than in the non-responders at the start of treatment and their ferritin levels were lower.
Table 2.
Comparison between responder and non-responder.
| Factors | R(n) | NR(n) | p |
|---|---|---|---|
| Age>65 | 2 | 2 | 1 |
| Male | 2 | 4 | 0.567 |
| Stage 5 at treatment | 1 | 5 | 0.015 |
| Ret>30,000 | 3 | 1 | 0.242 |
| PLT>2.0 | 5 | 1 | 0.015 |
| Ferritin>700 | 4 | 2 | 0.242 |
| Time to Tx>65mon | 3 | 3 | 1 |
R: responder.
NR: non-responder.
Ret: reticulocyte.
PLT: platelet.
Tx: treatment.
4. Discussion
AA is a bone marrow failure syndrome with a wide range of severity. Patients with mild disease do not require transfusion and need only to be observed. Those with severe AA, however, require weekly transfusion and often transplantation. The results of younger-age transplantation between matched siblings generally lead to a good prognosis. The few patients who cannot undergo transplantation because of the lack of a matched donor or ineligibility must undergo continuous immunosuppression and transfusion. The effectiveness of immunosuppression is about 60%, so patients with failed immunosuppression have no other promising treatment other than transfusion.
Eltrombopag was approved to use for AA in Japan in August 2017. To date, however, there have been no reports about eltrombopag use in the clinical setting in Japan and thus no consensus about how to use it. Questions could include how severe the AA should be before it is used and how to use it most effectively, the best time to start it in an AA patient, and what is the most effective dose, among other unknowns.
In our cohort of 11 patients, 6 responded to eltrombopag, with 4 of them recovering all three cell lineages. The most reactive time from the start of treatment was 8 weeks. Neutrophils recovered the fastest, and erythrocytes were slowest.
We tried to identify the difference in the responders’ and non-responders’ characteristics. Age, sex, the period from diagnosis to treatment, and the ferritin level showed no differences. Factors that were significant regarding response were the AA severity, and platelet count.
Although these data must be confirmed by a multivariate analysis with a large cohort, we believed that our results provide some hints about characteristics of the patients who will respond to eltrombopag. For example, higher platelet counts were consistent with milder disease.
Our result revealed that eltrombopag in a clinical setting produced a 55% response rate. This overall response rate is almost equivalent to the results in the first Phase 2 study (their overall response rate was 44%) [3]. In western countries, however, the maximum dose was increased to 150 mg, whereas in Japan it is 100 mg. Reactivity was observed between 8 and 12 weeks, similar to that in the Phase 2 study. Recently, a French group reported that patients who were treated with ATG prior to being given eltrombopag had a better response to it [10]. The patients in our study, however, showed no such tendency with prior ATG. Another Phase 1/2 study reported that early use of eltrombopag with ATG and cyclosporine produced a better response [11]. Our study failed to reveal differences between patients regarding the time from diagnosis to treatment, although it would be likely to be a longer period in non-responders. Thus, this period might influence the response to eltrombopag.
Our study revealed that eltrombopag is a promising agent to treat patients with AA at any grade of severity. Nevertheless, we should continue to search for particular characteristics of patients that might influence who could receive the most benefit from this treatment.
Authors' contributions
-
(1)
Conception and design of the study, acquisition of data, and/or analysis and interpretation of data: A.K., A.N., S.F., A.S., T.N., Y.T., Y.A., M.H., H.Y., K.I., T.I.
-
(2)
Drafting the article and/or revising it critically for important intellectual content: A.N., S.N.
-
(3)
Final approval of the version to be submitted: A.N., S.N.
Conflict of interest
The authors declare no competing financial interests in relation to this study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lrr.2019.03.002.
Appendix. Supplementary materials
References
- 1.Young N.S., Calado R.T., Scheinberg P. Review in translational hematology current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108(8):2509–2519. doi: 10.1182/blood-2006-03-010777.Supported. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solomou E.E., Rezvani K., Mielke S., Malide D., Keyvanfar K., Kajigaya S. Anemia brief report deficient CD4 ϩ CD25 ϩ FOXP3 ϩ T regulatory cells in acquired aplastic anemia. Hematology. 2007;110(5):1603–1606. doi: 10.1182/blood-2007-01-066258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olnes M.J., Scheinberg P., Calvo K.R., Desmond R., Tang Y., Dumitriu B. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N. Engl. J. Med. 2012;367(1):11–19. doi: 10.1056/NEJMoa1200931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.M. J.C., K. A.G. Management of the refractory aplastic anemia patient: what are the options? Am. Soc. Hematol. 2013;2013(22):87–94. doi: 10.1182/asheducation-2013.1.87. Education Program. [DOI] [PubMed] [Google Scholar]
- 5.A Bacigalupo, J. Hows, Gluckman E., Nissen C., Marsh J., Van Lint M.T., Congiu M., De Planque M.M., Ernst P., McCann S. Bone marrow transplantation (BMT) versus immunosuppression for the treatment of severe aplastic anaemia (SAA): a report of the EBMT SAA working party. Br. J. Haematol. 1988;70(Oct (2)):177–182. doi: 10.1111/j.1365-2141.1988.tb02460.x. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida N., Kobayashi R., Yabe H., Kosaka Y., Yagasaki H., Watanabe K.I. First-line treatment for severe aplastic anemia in children: bone marrow transplantation from a matched family donor versus immunosuppressive therapy. Haematologica. 2014;99(12):1784–1791. doi: 10.3324/haematol.2014.109355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desmond R., Townsley D.M., Dumitriu B., Olnes M.J., Sscheinberg P., Bevans M. Eltrombopag restores trilineage hematopoiesis in refractory severe aplastic anemia that can be sustained on discontinuation of drug. Blood. 2014;123(12):1818–1825. doi: 10.1182/blood-2013-10-534743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transpl. 2013;48(March (3)):452–458. doi: 10.1038/bmt.2012.244. Epub 2012 Dec 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.http://zoketsushogaihan.com/file/guideline_H30/02.pdf (Accessed 5 January 2019).
- 10.Lengline E., Drenou B., Peterlin P., Tournilhac O., Abraham J., Berceanu A. Nationwide survey on the use of eltrombopag in patients with severe aplastic anemia: a report on behalf of the french reference center for aplastic anemia. Haematologica. 2018;103(2):212–220. doi: 10.3324/haematol.2017.176339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desmond R., Dumitriu B., Rios O., Weinstein B., Valdez J., Lotter J. Eltrombopag added to standard immunosuppression for aplastic anemia. 2017. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.