Highlights
-
•
Neurobehavioral abnormalities afflict up to 90% of patients with MS.
-
•
Neurobehavioral abnormalities cause a greater burden of disability than motor ones.
-
•
Immunological phenomena cause neurobehavioral symptoms through various mechanisms.
-
•
Clinical features of MS like progressive forms predict neurobehavioral abnormalities.
-
•
Currently there aren’t effective therapies to improve neurobehavioral abnormalities.
Keywords: Neurobehavioral abnormality, Cognitive decline, Neuroinflammation, Neurodegeneration, Excitotoxicity, Multiple sclerosis
Abstract
Multiple sclerosis-related neurobehavioral abnormalities are one of the main components of disability in this disease. The same pathological processes that explain demyelination periods and neurodegeneration also allow the comprehension of neurobehavioral abnormalities. Inflammation in the central nervous system caused by cells of the immune system, especially lymphocytes, and by resident cells, such as astrocytes and microglia, directly modulate neurotransmission and synaptic physiology, resulting in behavioral changes (such as sickness behavior) and amplifying the degenerative mechanisms that occur in multiple sclerosis. In addition, neuronal death caused by glutamate-mediated excitotoxicity, alterations in GABAergic, serotonergic, and dopaminergic neurotransmission, and the mechanisms of axon damage are of foremost importance to explain the reduction in brain volume and the associated cognitive decline. Neuroinflammation and neurodegeneration are not isolated phenomena and various instances of interaction between them have been described. This presents attractive targets for the development of therapeutic strategies for this neglected component of multiple sclerosis related disability.
1. Introduction
Multiple sclerosis (MS) is a neuroinflammatory disorder of autoimmune nature, characterized by demyelination and neurodegeneration, which causes neurological impairment. It occurs more frequently in young adults, being the main cause of non-traumatic neurological disability in this population (Giovannoni et al., 2016). Since it usually presents during the most economically productive period of life, the costs incurred and the associated functional decline negatively impact the quality of life of the people affected (Trisolini et al., 2010).
MS can cause disability in any functional system (Giovannoni et al., 2016). However, cognitive impairment and neurobehavioral abnormalities (from now on, both referred as neurobehavioral abnormalities, NBAs) associated with MS has received less attention. In fact, the French neurologist Jean-Martin Charcot mentions it in the original description of the disease as “a marked weakening of the memory, the concepts are formed slowly, and the mental and emotional faculties are completely dull” (Özakbaş et al., 2015). Recent studies have consistently shown that alterations in cognition and behavior provoke and contribute to disability in MS (Glanz et al., 2007, Feuillet et al., 2007, Potagas et al., 2008, Rao et al., 1991) (Table 1).
Table 1.
Main neurobehavioral abnormalities in patients with multiple sclerosis.
| Symptom | Frequency (%) | Reference(s) |
|---|---|---|
| Fatigue | 85–95 | Jongen et al., 2012, Bergendal et al., 2007 |
| Depression | 24 | Marrie et al., 2015 |
| Anxiety | 22–26 | Marrie et al., 2015 |
| Cognitive decline | 45–60 | Bergendal et al., 2007 |
| Decreased processing speed | 20–30 | Nabavi and Sangelaji, 2015 |
| Altered attention | 25 | Nebel et al., 2007 |
It is common to consider that demyelination of inflammatory origin predominates during the first stages of the disease, clinically manifested by episodes of reversible neurological deficit (relapses); while in the late stages neurodegenerative processes lead to irreversible disability (progression) (Correale et al., 2016). Evidence shows that these divisions are artificial and that both processes occur throughout the natural history of the disease (Glanz et al., 2007, Potagas et al., 2008, Rao et al., 1991). For instance, the disease-modifying treatments (DMTs), which target inflammatory demyelination, not only diminish the frequency of relapses but also ameliorate some of the NBAs associated with MS (Mattioli et al., 2015, Utz et al., 2016, Ozakbas et al., 2016). This suggests that both processes operate simultaneously and that their understanding could point to novel therapeutic targets to achieve an integral management of the disease. In agreement, some clinical markers of disease progression are associated with the presence of NBAs (Table 2).
Table 2.
Main clinical factors of MS associated with the onset of neurobehavioral symptoms.
| Progressive form of the disease | Benedict and Zivadinov, 2011, Borghi et al., 2013 |
| Evolution >5 years after diagnosis | Benedict and Zivadinov, 2011, Nebel et al., 2007 |
| Early start (before 18 years of age) | Benedict and Zivadinov, 2011 |
| EDSS >4.0 | Nebel et al., 2007 |
| Altered vocabulary (according to the Weschler Adult Intelligence Scale Vocabulary test) | Borghi et al., 2013 |
| Grey matter degeneration | Borghi et al., 2013 |
| Presence of cortical lesions | Borghi et al., 2013 |
| Increased width of the third ventricle (as an indicator of brain volume loss) | Borghi et al., 2013 |
| Low premorbid intelligence | Borghi et al., 2013 |
Experimental allergic encephalomyelitis (EAE), an experimental model of MS, has been useful to elucidate the pathological mechanisms that underlie NBAs. It is induced by the subcutaneous or intraperitoneal administration of the myelin oligodendrocyte glycoprotein, or its fragments, in mammals (more frequently in rodents). This results in a disease with varied neurological manifestations (weakness, fatigue, paralysis, ataxia) with relapses and recoveries similar to those of MS, which also includes NBAs (Robinson et al., 2014). The resulting demyelination occurs predominantly in the optic nerves and spinal cord, with relative preservation of the brain and hippocampus (Ziehn et al., 2010). However, there is evidence that alterations in synaptic transmission in the non-affected regions cause, at least in part, the NBAs observed in this model (Mandolesi et al., 2010). This fact reinforces the idea that these alterations might explain these symptoms in patients with MS (Centonze et al., 2010).
Although inflammatory demyelination is the pathological hallmark of MS, the resulting defects in action potential speed and spreading can only partially explain the neurobehavioral symptoms of MS. Although several mechanisms have been described to explain NBAs in MS, none of them is unique for MS and are common to other neurodegenerative or neuropsychiatric disorders. In this review, we explore the contribution of mechanisms different to inflammatory demyelination that might cause the NBAs associated with MS, first from an immunopathological perspective, and then from a neuropathological point of view.
2. Contribution of the immunopathological components of MS to NBAs
Broadly, the immunopathological response in multiple sclerosis is considered a process of cellular autoimmunity to antigens characteristic of the central nervous system (CNS) (Dendrou et al., 2015). The origin of the antigens that generate autoreactivity is unknown. One hypothesis is that this antigenic presentation occurs in the periphery; a proposed mechanism is through molecular mimicry of certain Epstein Bar virus antigens with myelin basic protein (Lucas et al, 2011). In support, the induction of EAE requires intraperitoneal (peripheral) administration of CNS autoantigens (Robinson et al., 2014). On the other hand, it has been postulated that autoreactivity could occur within the CNS, since professional antigen-presenting cells, such as monocytes/macrophages and microglia, constantly monitor the CNS (Dendrou et al., 2015). The proteins encoded by reactivation of genomic components of the human endogenous type W viruses (human endogenous retrovirus W), when secreted, act as superantigens for T lymphocytes, which can generate a proinflammatory environment and consequently gliotoxicity (Van Horssen et al., 2016).
Once the autoreactivity and the loss of tolerance ensue, the activated T and B lymphocytes enter the CNS. CD8+ cytotoxic T lymphocytes are the main cellular components found in active circumscribed demyelinating lesions (Correale et al., 2016, Haider et al., 2016). CD8+ T lymphocytes generate these lesions through various mechanisms, such as degranulation and release of lytic proteins (granzymes and perforins), secretion of mediators of cell death (Fas ligand) and synthesis of proinflammatory cytokines (such as tumor necrosis factor alpha, TNFα). These mechanisms become amplified by the subsequent activation of microglia, the formation of reactive oxygen species and mitochondrial dysfunction (Patel and Balabanov, 2012, Mossakowski et al., 2015).
B lymphocytes are more abundant in the perivascular spaces of the CNS than in the brain parenchyma, so their contribution to the demyelination process seems to be indirect. Some studies report that self-reactive immunoglobulins secreted by plasma cells into the CNS facilitate phagocytosis of myelin components and other cellular components by macrophages or microglia, activate complement proteins and produce diffuse neuroinflammation (Dendrou et al., 2015, Siffrin et al., 2010). These immunoglobulins generate the oligoclonal bands in the cerebrospinal fluid (Bankoti et al., 2014) that associate with poor functional prognosis in MS (Rojas et al., 2012).
Tertiary lymphoid structures have been found in the meninges of patients with long-standing MS, as well as in up to 70% of patients who have the secondary progressive form, in whom there are proliferating B cells, plasma cells, helper (or CD4+) T lymphocytes and follicular dendritic cells (Mitsdoerffer and Peters, 2016, Kuerten et al., 2016). These structures express the chemokine CXCL13, which induces the proliferation, antigenic selection, and maturation of autoreactive B lymphocytes (Siffrin et al., 2010).
The effects of inflammatory mediators on peripheral organs (especially those near the vagus nerve), circumventricular organs (vascular organ of the endplate, organ of the postrema area, endocrine hypothalamus) and on the choroidal plexuses result in a series of stereotyped behaviors known as sickness behavior (SB). SB is characterized by fever, secretion of stress hormones (by activation of the hypothalamic-pituitaryadrenal –HPA– axis), anorexia, anhedonia, adynamia and social isolation (Dantzer, 2006). These effects might explain the higher incidence of depression and fatigue in patients with chronic inflammatory disorders (Miller and Raison, 2015). In addition, it has been shown that patients with major depressive disorder have higher levels of inflammation markers in peripheral blood (such as C-reactive protein, IL-1β and TNFα) (Miller et al., 2009). Also, non-steroidal anti-inflammatory drugs have antidepressant effects, as has been shown for acetylsalicylic acid in an animal model of chronic mild stress (Bhatt et al., 2016) and for celecoxib in patients with major depression (Na et al., 2016).
In patients with MS, similar findings have been observed. Recent studies found that 80% of affected people report fatigue as a disabling symptom (Nagaraj et al., 2013), half report other cognitive disorders (Jongen et al., 2012) and another proportion report depression or anxiety (Marrie et al., 2015, Bergendal et al., 2007) (see Table 1). The SB generated in the EAE model is similar to the cognitive impairment associated with MS, which suggests that there are common mechanisms that could represent therapeutic targets. For example, rituximab improves fatigue and mood (Kuerten et al., 2016). Also, vagus nerve stimulation, already used for the clinical management of treatment-resistant depression (Carreno and Frazer, 2017; O’Reardon et al., 2006), reduces the production of proinflammatory cytokines, decreases the permeability of the blood-brain barrier and improves the functional status in an animal model of traumatic brain injury (Neren et al., 2016). These observations suggest that vagus nerve stimulation might have a role in the treatment of some of the neuropsychiatric manifestations of MS.
3. Contribution of neuropathological mechanisms to the NBAs in MS
As previously stated, factors related to brain atrophy, such as grey matter degeneration and increased width of the third ventricle (Borghi et al., 2013) are also linked to NBAs in MS. Brain atrophy in MS is secondary, at least in part, to neuronal death, loss of synaptic density and axonal degeneration (Mandolesi et al., 2010), thus the mechanisms that lead to these phenomena could explain these symptoms and offer therapeutic targets.
One of the most relevant mechanisms of neuronal death is glutamate-mediated excitotoxicity (Kostic et al., 2013). Glutamate is the most abundant excitatory neurotransmitter of the CNS and is involved in multiple cognitive processes, such as learning, memory, attention and the modulation of emotional states. This neurotransmitter is a ligand of two types of receptors: metabotropic (receptors with seven transmembrane domains, coupled to G proteins) and ionotropic. Even though the activity of metabotropic receptors is involved in the cognitive processes previously enumerated, their importance is lower compared to ionotropic receptors (Swanson et al., 2005). The ionotropic glutamate receptors are permeable channels of cations (especially sodium -Na±- and calcium -Ca2+-). Three types of ionotropic receptors have been described according to pharmacological techniques: selective to N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainate. The composition of subunits, relative abundance, synaptic localization and kinetic characteristics of these receptors change according to the studied region of the nervous system, and this serves for the functional purposes of the involved circuits (Barnes and Slevin, 2003).
Although the activation of these ionotropic receptors is necessary for many physiological processes, their excessive activation causes glutamate-mediated excitotoxicity, which may not be limited to neurons, but may also affect glial cells and oligodendrocytes (Domercq et al., 2005). The massive influx of Ca2+ into the intracellular compartment leads to the activation of proteases, lipases, and endonucleases, which degrade cellular components and produce intracellular signaling molecules, which perpetuate these processes. Also, excess Ca2+ is buffered by the mitochondria, in an attempt to regulate its intracellular concentration, which can lead to a loss of transmembrane potential and then to energy failure. The increase of Ca2+ generates stress of the endoplasmic reticulum, activates the response to misfolded proteins, and compromises the ability to maintain postsynaptic structures. Additionally, the entry of cations into the intracellular space causes a loss of transmembrane polarity. This depolarization leads to the uncontrolled release of neurotransmitters, and an inability to maintain the physiological processes of import and export of energy substrates (such as lactate and glucose) and ions (such as potassium, calcium, and sodium). These processes finally, in isolation or together, lead to the activation of pathways of death, such as apoptosis or necrosis (Mehta et al., 2013).
During neuroinflammation episodes, the concentration of glutamate in the cerebral parenchyma increases due to overexpression in macrophages, dendritic cells and the microglia of the xc- system transporter, which works by importing cysteine into the cells in exchange for glutamate (Scannevin and Huganir, 2000). However, in a recent study, blocking the expression of the xc- system transporter protected from demyelination and attenuated the infiltration of inflammatory cells in the CNS, contrary to what was expected, suggesting that this antiporter is involved in the migration of T lymphocytes to CNS (Pampliega et al., 2011). On the other hand, Domercq et al. (2005) found that the increase in glutamate concentration due to inhibition of GLT-1 and GLAST transporters is toxic to oligodendrocytes, which could contribute to immune-mediated demyelination.
Besides, inflammatory mediators potentiate glutamate-mediated excitotoxicity. For example, TNFα decreases the expression of the GluR2 subunit of the AMPA type receptor in neurons, which causes these receptors to be permeable to Ca2+ (Mehta et al., 2013). The activation of the TNFα receptor in astrocytes decreases the expression of the excitatory amino acids transporter type 1 (EAAT1) in patients with progressive MS (Pitt et al., 2003). Also, the activation of microglia by TNF induces the secretion of glutamate, brain-derived neurotrophic factor (Takeuchi et al., 2006) and of TNF (Kuno et al., 2005), which could stimulate the secretion of more glutamate through positive feedback loop.
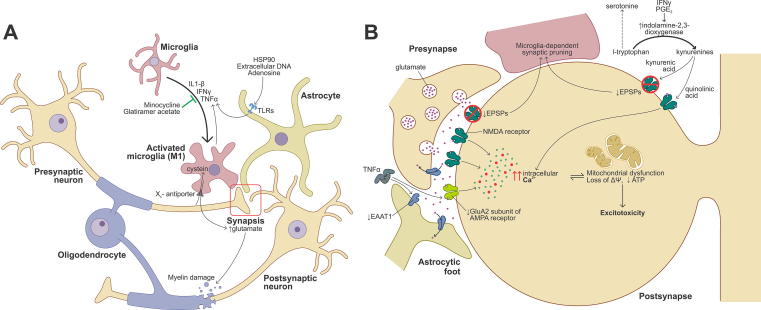
Alterations in neurotransmission and the synaptic structure, even when there is no evidence of neuronal death, are involved in cognitive deterioration in animal models of MS. For example, the induction of EAE in rodents decreases the density of excitatory postsynaptic structures in the CA1 area of the hippocampus, a region of the brain involved with memory, learning, and spatial orientation. This finding is dependent on the phagocytic activity of the microglia. These alterations were avoided with the in vivo administration of NMDA receptor agonists. It is worth noting that this protection became evident only when the blockade of these receptors was carried out in the initial stages of the EAE induction (Bellizzi et al., 2016). In another study, the administration of glatiramer acetate (GA), one of the first DMTs developed for MS, prevented changes in some electrophysiological parameters of spontaneous excitatory postsynaptic potentials (EPSPs) of striatum median spiny neurons in EAE mice, even since presymptomatic stages. These changes were partially dependent on the activation of microglia by a Th1 cytokine profile. Also, GA decreased the density of microglia and its surface, measures indicative of activation, as well as the amount of TNFα in these cells (Gentile et al., 2013). (Fig. 1).
Fig. 1.
Summary of the interplay of immune and neural mechanisms associated with NBAs in multiple sclerosis. A) Soluble factors released by microglia, astrocytes or immune cells (such as lymphocytes, not depicted) modulate synaptic neurotransmission, in this case mediated by glutamate, and induce myelin damage. These soluble factors can directly cause oligodendrocyte death, as well. Some therapeutic strategies operate by blocking the release of these factors. B) Alterations in synaptic transmission in MS explain NBAs. Glutamate-mediated excitotoxicity leads to neuronal death, which could be caused by TNFα through enhanced Ca2+ permeability in GluA2-deficient AMPA receptors or decreased glutamate uptake by EAAT1. Also, IFNγ and PGE2 induce indolamine-2,3-dioxygenase expression which results in serotonin deficiency and quinolinic synthesis, the latter causing NMDA receptor activation and excitotoxicity. On the contrary, kynurenic acid blocks NMDA receptors and might contribute to microglia-dependent synaptic pruning. Excessive intracellular Ca2+ disrupts mitochondrial buffering capacity and causes energy failure.
Excitotoxicity results not only from excessive glutamatergic neurotransmission, but also from insufficient inhibitory inputs to counterbalance excitatory signals. The main inhibitory neurotransmitter in the mammalian CNS is γ-amino-butyric acid (GABA). GABA mediates its effects through a ligand-gated chloride-permeable channel (the GABA-A receptor -GABAAR-) or through a metabotropic G-protein-coupled receptor (the GABA-B receptor). GABAAR opening results in chloride influx and membrane hyperpolarization, which inhibits neuronal activity (Tatti et al., 2017). Whatever causes insufficient GABAAR activation might also cause hyperexcitability and, ultimately, excitotoxicity. GABA failure as a cause of excitotoxicity has been previously implicated in other neurodegenerative disorders, such as motor neuron disease (Ramírez-Jarquín et al., 2014) and its role in MS is accumulating.
Reduced levels of extracellular GABA are found in the white matter of patients with MS, regardless of the clinical phenotype (Paul et al., 2014, Cawley et al., 2015, Cao et al., 2018). Lower levels of GABA, as assessed by MRI spectroscopy, are associated with motor disability and cognitive impairment (Cawley et al., 2015, Nantes et al., 2017). Indeed, a very recent study found that reduced GABA concentrations in the posterior cingulate cortex were correlated with altered executive function while low levels in the hippocampus were linked to deficits in verbal memory (Cao et al., 2018).
These findings might be explained by the effects of secreted interleukin (IL) 1β from infiltrating autoreactive T lymphocytes. In the hippocampus of EAE mice IL1β reduces GABAergic neurotransmission due to a loss of inhibitory synaptic inputs (Mori et al., 2014). Similar observations have been made in the cerebellum (Mandolesi et al., 2012) and striatum (Rossi et al., 2011). These results suggest that the modulation of GABAergic neurotransmission is a potential target for the development of therapeutic strategies for NBAs in MS. For example, siponimod, a sphingosine-1-phosphate receptor antagonist in approval for secondary progressive MS (Kappos et al., 2018), prevents the degeneration of parvalbumine-positive GABAergic interneurons in the EAE mice (Gentile et al., 2016). Also, ganaxolone, a synthetic neurosteroid agonist of GABAAR, ameliorated the NBAs and the neuroinflammation associated with the induction of EAE in mice (Paul et al., 2014). However, other classical agonists of GABAAR, such as benzodiazepines and barbiturates, have failed to improve the clinical scores in animal models of MS (Gilani et al., 2014).
There is evidence that other neurotransmitters are altered in animal models and patients with MS. Serotonin, a neurotransmitter derived from the essential amino acid tryptophan, participates in cognitive processes, such as attention (Wingen et al., 2008). During inflammatory events, stimulation by interferons and prostaglandin E2 increases the expression of indoleamine-2,3-dioxygenase, which synthesizes kynurenic acid from tryptophan, in the cells of the innate immune system. Overexpression of this enzyme may limit the availability of substrate for the synthesis of serotonin and, at the same time, the accumulation of kynurenines, which have significant effects on the functioning of the CNS. For example, kynurenic acid inhibits the release of dopamine (which is involved in the reward system) and is an antagonist of glutamate receptors, whereas quinolinic acid is an agonist of glutamate receptors and can potentiate glutamate-mediated excitotoxicity (Vécsei et al., 2012). Importantly, in patients with MS, high concentrations of kynurenine are observed in early stages of the disease (Török et al., 2016). In addition, the kynurenine profile in CSF correlates with the progression of disability and has potential as a biomarker, since it can differentiate between progressive and recurrent forms of the disease with an accuracy close to 90% (Lim et al., 2017).
Dopamine is one of the essential neurotransmitters for attention and motivation (Felger et al., 2016). The proinflammatory conditions promote the oxidation of tetrahydrobiopterin, which can block the synthesis of dopamine (Dobryakova et al., 2015). In patients with MS, alterations in dopaminergic neurotransmission between the striatum and the prefrontal cortex are related to some cognitive symptoms, such as fatigue, depression and decreased attention (Haider et al., 2011).
Several mechanisms underlying axonal degeneration in MS have been described, including oxidative stress. In fact, in demyelinating lesions of patients with MS, there is a high concentration of nitric oxide (NO) (Smith et al., 2001), whereas it has been observed that NO causes axonal degeneration in an EAE animal model, which decreases with antioxidants (Campbell et al., 2012). Evidence shows that free radicals produce mitochondrial dysfunction since they increase the permeability of the internal membrane, damage mitochondrial DNA and generate pro-apoptotic complexes in axons of patients with MS (Campbell et al., 2011). Similarly, in the cerebral cortex of patients with MS deletions in the mitochondrial genome and alterations in the electron transport chain support mitochondrial dysfunction as causal of axonal degeneration (Trapp and Stys, 2009). Demyelination and mitochondrial dysfunction generate energy failure that compromises the viability of the affected axons and, finally, of the neurons (Nicholls, 2004).
Another key point to mention is the compensatory mechanisms to maintain the driving speed in the axons, which consist of modifications in the expression and distribution of ion channels activated by voltage, and which can be deleterious. In particular, the sodium channels, which under normal conditions locate in the Ranvier nodes, increase their expression and are located diffusely after demyelination. These changes promote the accumulation of intracellular sodium, with the consequent loss of transmembrane potential, reverse functioning of the Na±/Ca2+ antiporter and excessive calcium intake to the intracellular compartment (Dutta and Trapp, 2007). Persistent axonal damage leads to the retrograde Wallerian degeneration observed in white matter with acute demyelination (Singh et al., 2017) as well as in chronic active lesions (Trapp and Stys, 2009). However, this process of degeneration has also been documented in gray matter and even in neurons without myelin, suggesting that this process develops independently of damage to myelin (Singh et al., 2017).
Microglial cells can have a protective or harmful effect on the CNS, which depends in large part on the microenvironment, that is, on the relative local abundance of cytokines and signaling molecules. The activation by IFN-γ produces differentiation, or polarization, towards a pro-inflammatory phenotype associated with neurodegeneration (M1). On the other hand, IL-4 allows polarization towards a state that favors neurogenesis, oligodendrogenesis and is anti-inflammatory (M2) (Cherry et al., 2014). A recent study found that most of the activated microglia have the M1 phenotype in the cerebrospinal fluid of patients with MS; however, multiple clones with M2 phenotype were also identified. The authors concluded that the CNS of patients with MS presents an environment that promotes the activation of microglia towards both phenotypes, probably as a compensatory mechanism against tissue damage (Vogel et al., 2013).
Another mechanism that might add to the development of NBAs in MS mediated by microglia is synaptic pruning. Complement proteins C5a and C1q, among others, mark dendritic spines without synaptic activity in order to be phagocytosed by microglial cells (Paolicelli et al., 2011). Although this process was first described in the developing nervous system, a direct association exists between the activation of complement proteins and the loss of brain volume in pathologies characterized by cognitive impairment, such as Alzheimer-type dementia (Hong et al., 2016). A high concentration of C1q, C3b and C4d complement proteins are present in demyelinating lesions, and to a lesser extent in gray and white matter, in necropsies performed in patients with MS compared to healthy controls. In addition, a relationship between protein levels and the time of diagnosis suggest their involvement in neurodegeneration associated with MS (Michailidou et al., 2017).
Given these findings, it has been proposed that microglia could be a therapeutic strategy for neuroinflammation and cognitive impairment associated with MS. For example, the administration of minocycline, which inhibits the activation of microglia, has neuroprotective effects in animal models of brain damage (Scholz et al., 2015, Madeira et al., 2015). Since this drug has an acceptable and clinically proven safety profile, there are ongoing clinical trials in humans, which show a delay in the progression of CIS to MS (Metz et al., 2017).
Although astrocytes are the most abundant glial cells of the CNS, their role in the pathophysiology of MS was not clear until recently. Astrocytes react early to molecules associated with cell damage (e.g., heat shock protein 90, extracellular DNA and adenosine) of the CNS through Toll-like receptors, these being the first to respond during activation of the innate immune system, as well as to cytokines in the tissue. In response, astrocytes secrete cytokines (such as IL6, IL1β, TNFα, and tumor growth factor β) and chemokines (such as CCL2, CCL5, CXCL10, CXCL12, and IL8). These molecules modulate the permeability of the blood-brain barrier, promote the activation of leukocytes and microglia, and stimulate the migration of the latter towards the injured tissue (Correale and Farez, 2015). Moreover, activation of astrocytes could have a neurotoxic effect, because astrocytes increase secretion of glutamate and adenosine triphosphate in sites where there is damage to myelin (Brosnan and Raine, 2013).
The mechanisms of neuronal and glial death discussed so far impact the efficiency of neuronal networks to integrate information, which leads to network collapse. A wealth of data obtained from humans by several methods based on MRI supports the idea that alterations in connectivity are the substrate of NBAs in neurodegenerative disorders (Ahmed et al., 2016), and in MS (Nave, 2010, Schoonheim et al., 2015). However, the diversity of methods and inconsistencies in subject selection have hindered reaching conclusions on the characteristic network alterations underlying NBAs in MS. Brains of people with MS show increased activation and connectivity, which reflect compensatory changes to demyelination, axonal loss and neuron death (Schoonheim et al., 2010). NBAs are clinically apparent when these changes are not enough to cope with the pathologic process underlying MS, which is a consequence of decreased network efficiency (Schoonheim et al., 2015). No specific networks are preferentially targeted in MS, rather graph analysis approaches have highlighted that whole-brain network inefficiency is causal to NBAs in MS (Helekar et al., 2010).
4. Conclusions
Neurobehavioral abnormalities and cognitive impairment are common throughout the natural history of MS, although they are not well acknowledged in the clinical setting. Together, they negatively impact the quality of life of people who suffer from MS. However, some advances have been made, as in recent clinical guidelines, there is greater emphasis on the recognition of NBAs as a marker of suboptimal response to treatment.
NBAs originate from the complex interaction between cells of the immune system, glia, and neurons in the context of autoimmunity characteristic of MS. Most current DMTs control to a greater or lesser extent the inflammatory component of the disease, but show have not shown to halt neurodegeneration. Also, their impact of NBAs have not been thoroughly studied and the few available results are unsatisfactory. There is an unmet need in developing therapeutic strategies to ameliorate disability associated with NBAs. Understanding the relationship between the immune and nervous system in MS offer new therapeutic avenues, such as the modulation of glutamate, GABA or monoamine neurotransmission. Given the intricate and complex interplay of the pathophysiologic mechanisms involved treatments aimed to NBAs will have to address both components, immune and neural.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Competing interests
Rafael Lazo-Gomez and Diego Mireles-Jacobo work for Novartis Pharma Mexico.
Funding
The preparation of this review did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
Gloria Llamosa and Marco Antonio Sotomayor-Sobrino wrote and critically reviewed the manuscript. Rafael Lazo-Gómez wrote, critically reviewed the manuscript and designed the figure. Diego Mireles-Jacobo critically reviewed and corrected the english version of the manuscript.
References
- Ahmed R.M., Devenney E.M., Irish M., Ittner A., Naismith S., Ittner L.M., Rohrer J.D., Halliday G.M., Eisen A., Hodges J.R., Kiernan M.C. Neuronal network disintegration: common pathways linking neurodegenerative diseases. J. Neurol. Neurosurg. Psychiatry. 2016;87:1234–1241. doi: 10.1136/jnnp-2014-308350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankoti J., Apeltsin L., Hauser S.L., Allen S., Albertolle M.E., Witkowska H.E. In multiple sclerosis, oligoclonal bands connect to peripheral B-cell responses. Ann. Neurol. 2014;75:266–276. doi: 10.1002/ana.24088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes G.N., Slevin J.T. Ionotropic glutamate receptor biology: effect on synaptic connectivity and function in neurological disease. Curr. Med. Chem. 2003;10:2059–2072. doi: 10.2174/0929867033456800. [DOI] [PubMed] [Google Scholar]
- Bellizzi M.J., Geathers J.S., Allan K.C., Gelbard H.A. Platelet-activating factor receptors mediate excitatory postsynaptic hippocampal injury in experimental autoimmune encephalomyelitis. J. Neurosci. 2016;36:1336–1346. doi: 10.1523/JNEUROSCI.1171-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict R.H.B., Zivadinov R. Risk factors for and management of cognitive dysfunction in multiple sclerosis. Nat Rev Neurol. 2011;7:332–342. doi: 10.1038/nrneurol.2011.61. [DOI] [PubMed] [Google Scholar]
- Bergendal G., Fredrikson S., Almkvist O. Selective decline in information processing in subgroups of multiple sclerosis: an 8-year longitudinal study. Eur. Neurol. 2007;57:193–202. doi: 10.1159/000099158. [DOI] [PubMed] [Google Scholar]
- Borghi M., Cavallo M., Carletto S., Ostacoli L., Zuffranieri M., Picci R.L. Presence and significant determinants of cognitive impairment in a large sample of patients with multiple sclerosis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0069820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan C.F., Raine C.S. The astrocyte in multiple sclerosis revisited. Glia. 2013;61:453–465. doi: 10.1002/glia.22443. [DOI] [PubMed] [Google Scholar]
- Campbell G.R., Ohno N., Turnbull D.M. Mahad DJ. Mitochondrial changes within axons in multiple sclerosis: an update. Curr. Opin. Neurol. 2012:221–230. doi: 10.1097/WCO.0b013e3283533a25. [DOI] [PubMed] [Google Scholar]
- Campbell G.R., Ziabreva I., Reeve A.K., Krishnan K.J., Reynolds R., Howell O. Mitochondrial DNA deletions and neurodegeneration in multiple sclerosis. Ann. Neurol. 2011;69:481–492. doi: 10.1002/ana.22109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G., Edden R.A.E., Gao F., Li H., Gong T., Chen W., Liu X., Wang G., Zhao B. Reduced GABA levels correlate with cognitive impairment in patients with relapsing-remitting multiple sclerosis. Eur. Radiol. 2018;28:1140–1148. doi: 10.1007/s00330-017-5064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawley N., Solanky B.S., Muhlert N., Tur C., Edden R.A., Wheeler-Kingshott C.A., Miller D.H., Thompson A.J., Ciccarelli O. Reduced gamma-aminobutyric acid concentration is associated with physical disability in progressive multiple sclerosis. Brain. 2015;138:2584–2595. doi: 10.1093/brain/awv209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D., Muzio L., Rossi S., Furlan R., Bernardi G., Martino G. The link between inflammation, synaptic transmission and neurodegeneration in multiple sclerosis. Cell Death Differ. 2010;17:1083–1091. doi: 10.1038/cdd.2009.179. [DOI] [PubMed] [Google Scholar]
- Cherry J.D., Olschowka J.A., O’Banion M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflammation. 2014;11:98. doi: 10.1186/1742-2094-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correale J., Farez M.F. The role of astrocytes in multiple sclerosis progression. Front. Neurol. 2015;6:180. doi: 10.3389/fneur.2015.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correale J., Gaitán M.I., Ysrraelit M.C., Fiol M.P. Progressive multiple sclerosis: from pathogenic mechanisms to treatment. Brain. 2016;140:527–546. doi: 10.1093/brain/aww258. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine, sickness behavior, and depression. Neurol. Clin. 2006;24:441–460. doi: 10.1016/j.ncl.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dendrou C.A., Fugger L., Friese M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015;15(9):545–558. doi: 10.1038/nri3871. [DOI] [PubMed] [Google Scholar]
- Dobryakova E., Genova H.M., DeLuca J., Wylie G.R. The dopamine imbalance hypothesis of fatigue in multiple sclerosis and other neurological disorders. Front. Neurol. 2015;6:52. doi: 10.3389/fneur.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domercq M., Etxebarria E., Pérez-Samartín A., Matute C. Excitotoxic oligodendrocyte death and axonal damage induced by glutamate transporter inhibition. Glia. 2005;52:36–46. doi: 10.1002/glia.20221. [DOI] [PubMed] [Google Scholar]
- Dutta R., Trapp B.D. Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology. 2007;68(Suppl 3):S22–S31. doi: 10.1212/01.wnl.0000275229.13012.32. [DOI] [PubMed] [Google Scholar]
- Felger J.C., Li Z., Haroon E., Woolwine B.J., Jung M.Y., Hu X. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatry. 2016;21:1358–1365. doi: 10.1038/mp.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuillet L., Reuter F., Audoin B., Malikova I., Barrau K., Ali Cherif A. Early cognitive impairment in patients with clinically isolated syndrome suggestive of multiple sclerosis. Mult Scler. 2007;13:124–127. doi: 10.1177/1352458506071196. [DOI] [PubMed] [Google Scholar]
- Gentile A., Rossi S., Studer V., Motta C., De Chiara V., Musella A. Glatiramer acetate protects against inflammatory synaptopathy in experimental autoimmune encephalomyelitis. J. Neuroimmune Pharmacol. 2013;8:651–663. doi: 10.1007/s11481-013-9436-x. [DOI] [PubMed] [Google Scholar]
- Gentile A., Musella A., Bullitta S., Fresegna D., De Vito F., Fantozzi R., Piras E., Gargano F., Borsellino G., Battistini L., Schubart A., Mandolesi G., Centonze D. Siponimod (BAF312) prevents synaptic neurodegeneration in experimental multiple sclerosis. J Neuroinflammation. 2016;13:207. doi: 10.1186/s12974-016-0686-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilani A.A., Dash R.P., Jivrajani M.N., Thakur S.K., Nivsarkar M. Evaluation of GABAergic transmission modulation as a novel functional target for management of multiple sclerosis: exploring inhibitory effect of GABA on glutamate-mediated excitotoxicity. Adv. Pharmacol. Sci. 2014;2014 doi: 10.1155/2014/632376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni G., Butzkueven H., Dhib-Jalbut S., Hobart J., Kobelt G., Pepper G. Brain health: time matters in multiple sclerosis. Mult. Scler. Relat. Disord. 2016;9:S5–S48. doi: 10.1016/j.msard.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Glanz B.I., Holland C.M., Gauthier S.A., Amunwa E.L., Liptak Z., Houtchens M.K. Cognitive dysfunction in patients with clinically isolated syndromes or newly diagnosed multiple sclerosis. Mult Scler. 2007;13:1004–1010. doi: 10.1177/1352458507077943. [DOI] [PubMed] [Google Scholar]
- Haider L., Fischer M.T., Frischer J.M., Bauer J., Höftberger R., Botond G. Oxidative damage in multiple sclerosis lesions. Brain. 2011;134:1914–1924. doi: 10.1093/brain/awr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider L., Zrzavy T., Hametner S., Höftberger R., Bagnato F., Grabner G. The topograpy of demyelination and neurodegeneration in the multiple sclerosis brain. Brain. 2016;139:807–815. doi: 10.1093/brain/awv398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helekar S.A., Shin J.C., Mattson B.J., Bartley K., Stosic M., Saldana-King T., Montague P.R., Hutton G.J. Functional brain network changes associated with maintenance of cognitive function in multiple sclerosis. Front. Hum. Neurosci. 2010;4:219. doi: 10.3389/fnhum.2010.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S., Dissing-Olesen L., Stevens B. New insights on the role of microglia in synaptic pruning in health and disease. Curr. Opin. Neurobiol. 2016;36:128–134. doi: 10.1016/j.conb.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongen P.J., Ter Horst A.T., Brands A.M. Cognitive impairment in multiple sclerosis. Minerva Med. 2012;103:73–96. [PubMed] [Google Scholar]
- Kappos L., Bar-Or A., Cree B.A.C., Fox R.J., Giovannoni G., Gold R., Vermersch P., Arnold D.L., Arnould S., Scherz T., Wolf C., Wallström E., Dahlke F. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet. 2018;391:1263–1273. doi: 10.1016/S0140-6736(18)30475-6. [DOI] [PubMed] [Google Scholar]
- Kostic M., Zivkovic N., Stojanovic I. Multiple sclerosis and glutamate excitotoxicity. Rev. Neurosci. 2013;24:71–88. doi: 10.1515/revneuro-2012-0062. [DOI] [PubMed] [Google Scholar]
- Kuerten S., Schickel A., Kerkloh C., Recks M., Addicks K., Ruddle N. The development of tertiary lymphoid organs in the central nervous system facilitates determinant spreading of the MP4-specific T cell response (P4164) J. Immunol. 2016;190(172):9. [Google Scholar]
- Kuno R., Wang J., Kawanokuchi J., Takeuchi H., Mizuno T., Suzumura A. Autocrine activation of microglia by tumor necrosis factor-alpha. J. Neuroimmunol. 2005;162:89–96. doi: 10.1016/j.jneuroim.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Lim C.K., Bilgin A., Lovejoy D.B., Tan V., Bustamante S., Taylor B.V. Kynurenine pathway metabolomics predicts and provides mechanistic insight into multiple sclerosis progression. Sci. Rep. 2017;7:41473. doi: 10.1038/srep41473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas R.M., Hughes A.M., Lay M.-L.J., Ponsonby A.-L., Dwyer D.E., Taylor B.V. Epstein-Barr virus and multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 2011;82:1142–1148. doi: 10.1136/jnnp-2011-300174. [DOI] [PubMed] [Google Scholar]
- Madeira M.H., Boia R., Santos P.F., Ambrósio A.F., Santiago A.R. Contribution of microglia-mediated neuroinflammation to retinal degenerative diseases. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/673090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandolesi G., Grasselli G., Musumeci G., Centonze D. Cognitive deficits in experimental autoimmune encephalomyelitis: Neuroinflammation and synaptic degeneration. Neurol Sci. 2010;31(Suppl 2):S255–S259. doi: 10.1007/s10072-010-0369-3. [DOI] [PubMed] [Google Scholar]
- Mandolesi G., Grasselli G., Musella A., Gentile A., Musumeci G., Sepman H., Haji N., Fresegna D., Bernardi G., Centonze D. GABAergic signaling and connectivity on Purkinje cells are impaired in experimental autoimmune encephalomyelitis. Neurobiol. Dis. 2012;46:414–424. doi: 10.1016/j.nbd.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Marrie R.A., Reingold S., Cohen J., Stuve O., Trojano M., Sorensen P.S. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler J. 2015;21:305–317. doi: 10.1177/1352458514564487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli F., Stampatori C., Bellomi F., Scarpazza C., Capra R. Natalizumab significantly improves cognitive impairment over three years in MS: Pattern of disability progression and preliminary MRI Findings. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0131803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta A., Prabhakar M., Kumar P., Deshmukh R., Sharma P.L. Excitotoxicity: Bridge to various triggers in neurodegenerative disorders. Eur. J. Pharmacol. 2013;698:6–18. doi: 10.1016/j.ejphar.2012.10.032. [DOI] [PubMed] [Google Scholar]
- Metz L.M., Li D.K.B., Traboulsee A.L., Duquette P., Eliasziw M., Cerchiaro G. Trial of Minocycline in a Clinically Isolated Syndrome of Multiple Sclerosis. N. Engl. J. Med. 2017;376:2122–2133. doi: 10.1056/NEJMoa1608889. [DOI] [PubMed] [Google Scholar]
- Michailidou I., Naessens D.M.P., Hametner S., Guldenaar W., Kooi E.J., Geurts J.J.G. Complement C3 on microglial clusters in multiple sclerosis occur in chronic but not acute disease: Implication for disease pathogenesis. Glia. 2017;65:264–277. doi: 10.1002/glia.23090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.H., Raison C.L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2015;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsdoerffer M., Peters A. Tertiary lymphoid organs in central nervous system autoimmunity. Front. Immunol. 2016;7:1–12. doi: 10.3389/fimmu.2016.00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori F., Nisticò R., Mandolesi G., Piccinin S., Mango D., Kusayanagi H., Berretta N., Bergami A., Gentile A., Musella A., Nicoletti C.G., Nicoletti F., Buttari F., Mercuri N.B., Martino G., Furlan R., Centonze D. Interleukin-1β promotes long-term potentiation in patients with multiple sclerosis. Neuromolecular Med. 2014;16:38–51. doi: 10.1007/s12017-013-8249-7. [DOI] [PubMed] [Google Scholar]
- Mossakowski A.A., Pohlan J., Bremer D., Lindquist R., Millward J.M., Bock M. Tracking CNS and systemic sources of oxidative stress during the course of chronic neuroinflammation. Acta Neuropathol. 2015;130:799–814. doi: 10.1007/s00401-015-1497-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi S.M., Sangelaji B. Cognitive dysfunction in multiple sclerosis: Usually forgotten in the clinical assessment of MS patients. J. Res. Med. Sci. 2015;20:533–534. doi: 10.4103/1735-1995.163984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj K., Taly A.B., Gupta A., Prasad C., Christopher R. Prevalence of fatigue in patients with multiple sclerosis and its effect on the quality of life. J. Neurosci. Rural. Pract. 2013;4:278–282. doi: 10.4103/0976-3147.118774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nantes J.C., Proulx S., Zhong J., Holmes S.A., Narayanan S., Brown R.A., Hoge R.D., Koski L. GABA and glutamate levels correlate with MTR and clinical disability: Insights from multiple sclerosis. Neuroimage. 2017;157:705–715. doi: 10.1016/j.neuroimage.2017.01.033. [DOI] [PubMed] [Google Scholar]
- Nave K.A. Myelination and support of axonal integrity by glia. Nature. 2010;468:244–252. doi: 10.1038/nature09614. [DOI] [PubMed] [Google Scholar]
- Nebel K., Wiese H., Seyfarth J., Gizewski E.R., Stude P., Diener H.C. Activity of attention related structures in multiple sclerosis patients. Brain Res. 2007;1151:150–160. doi: 10.1016/j.brainres.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Neren D., Johnson M.D., Legon W., Bachour S.P., Ling G., Divani A.A. Vagus nerve stimulation and other neuromodulation methods for treatment of traumatic brain injury. Neurocrit. Care. 2016;24:308–319. doi: 10.1007/s12028-015-0203-0. [DOI] [PubMed] [Google Scholar]
- Nicholls D.G. Mitochondrial dysfunction and glutamate excitotoxicity studied in primary neuronal cultures. Curr. Mol. Med. 2004;4:149–177. doi: 10.2174/1566524043479239. [DOI] [PubMed] [Google Scholar]
- O’Reardon J.P., Cristancho P., Peshek A.D. Vagus Nerve Stimulation (VNS) and Treatment of Depression: To the Brainstem and Beyond. Psychiatry (Edgmont). 2006;3:54–63. [PMC free article] [PubMed] [Google Scholar]
- Ozakbas, S., Cinar, B.P., Kosehasanogullari, G., Yigit, P. Abstract CG35. Effects of Fingolimod on Cognitive Status in Patients with Multiple Sclerosis: Prospective, Controlled Trial. Presented at: CMSC Annual Meeting 2016. June 1-4, 2016; National Harbor, MD.
- Özakbaş S., Üniversitesi D.E., Fakültesi T., Tıp D., Bölümü B., Dalı N.A. Cognitive impairment in multiple sclerosis: historical aspects, current status, and beyond. Arch Neuropsychiatr. 2015;52:12–15. doi: 10.5152/npa.2015.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampliega O., Domercq M., Soria F.N., Villoslada P., Rodríguez-Antigüedad A., Matute C. Increased expression of cystine/glutamate antiporter in multiple sclerosis. J Neuroinflammation. 2011;8:63–66. doi: 10.1186/1742-2094-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli R.C., Bolasco G., Pagani F., Maggi L., Scianni M., Panzanelli P. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Patel J., Balabanov R. Molecular mechanisms of oligodendrocyte injury in multiple sclerosis and experimental autoimmune encephalomyelitis. Int. J. Mol. Sci. 2012;13:10647–10659. doi: 10.3390/ijms130810647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A.M., Branton W.G., Walsh J.G., Polyak M.J., Lu J.Q., Baker G.B., Power C. GABA transport and neuroinflammation are coupled in multiple sclerosis: regulation of the GABA transporter-2 by ganaxolone. Neuroscience. 2014;273:24–38. doi: 10.1016/j.neuroscience.2014.04.037. [DOI] [PubMed] [Google Scholar]
- Pitt D., Nagelmeier I.E., Wilson H.C., Raine C.S. Glutamate uptake by oligodendrocytes: Implications for excitotoxicity in multiple sclerosis. Neurology. 2003;61:1113–1120. doi: 10.1212/01.wnl.0000090564.88719.37. [DOI] [PubMed] [Google Scholar]
- Potagas C., Giogkaraki E., Koutsis G., Mandellos D., Tsirempolou E., Sfagos C. Cognitive impairment in different MS subtypes and clinically isolated syndromes. J. Neurol. Sci. 2008;267:100–106. doi: 10.1016/j.jns.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Ramírez-Jarquín U.N., Lazo-Gómez R., Tovar-Y-Romo L.B., Tapia R. Spinal inhibitory circuits and their role in motor neuron degeneration. Neuropharmacology. 2014;82:101–107. doi: 10.1016/j.neuropharm.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Rao S.M., Leo G.J., Bernardin L., Unverzagt F. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology. 1991;41:685–691. doi: 10.1212/wnl.41.5.685. [DOI] [PubMed] [Google Scholar]
- Robinson A.P., Harp C.T., Noronha A., Miller S.D. The experimental autoimmune encephalomyelitis (EAE) model of MS. utility for understanding disease pathophysiology and treatment. Handb. Clin. Neurol. 2014;122:179–189. doi: 10.1016/B978-0-444-52001-2.00008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas J.I., Tizio S., Patrucco L., Cristiano E. Oligoclonal bands in multiple sclerosis patients: worse prognosis? Neurol. Res. 2012;34:889–892. doi: 10.1179/1743132812Y.0000000088. [DOI] [PubMed] [Google Scholar]
- Rossi S., Muzio L., De Chiara V., Grasselli G., Musella A., Musumeci G., Mandolesi G., De Ceglia R., Maida S., Biffi E., Pedrocchi A., Menegon A., Bernardi G., Furlan R., Martino G., Centonze D. Impaired striatal GABA transmission in experimental autoimmune encephalomyelitis. Brain Behav. Immun. 2011;25:947–956. doi: 10.1016/j.bbi.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Scannevin R.H., Huganir R.L. Postsynaptic organization and regulation of excitatory synapses. Nat. Rev. Neurosci. 2000;1:133–141. doi: 10.1038/35039075. [DOI] [PubMed] [Google Scholar]
- Scholz R., Sobotka M., Caramoy A., Stempfl T., Moehle C., Langmann T. Minocycline counter-regulates pro-inflammatory microglia responses in the retina and protects from degeneration. J. Neuroinflammation. 2015;12:209. doi: 10.1186/s12974-015-0431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonheim M.M., Geurts J.J., Barkhof F. The limits of functional reorganization in multiple sclerosis. Neurology. 2010;74:1246–1247. doi: 10.1212/WNL.0b013e3181db9957. [DOI] [PubMed] [Google Scholar]
- Schoonheim M.M., Meijer K.A., Geurts J.J. Network collapse and cognitive impairment in multiple sclerosis. Front. Neurol. 2015;6:82. doi: 10.3389/fneur.2015.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siffrin V., Vogt J., Radbruch H., Nitsch R., Zipp F. Multiple sclerosis – candidate mechanisms underlying CNS atrophy. Trends Neurosci. 2010;33:202–210. doi: 10.1016/j.tins.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Singh S., Dallenga T., Winkler A., Roemer S., Maruschak B., Siebert H. Relationship of acute axonal damage, Wallerian degeneration, and clinical disability in multiple sclerosis. J. Neuroinflammation. 2017;14:57. doi: 10.1186/s12974-017-0831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.J., Kapoor R., Hall S.M., Davies M. Electrically active axons degenerate when exposed to nitric oxide. Ann. Neurol. 2001;49:470–476. [PubMed] [Google Scholar]
- Swanson C.J., Bures M., Johnson M.P., Linden A.-M., Monn J.A., Schoepp D.D. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat. Rev. Drug. Discov. 2005;4:131–144. doi: 10.1038/nrd1630. [DOI] [PubMed] [Google Scholar]
- Takeuchi H., Jin S., Wang J., Zhang G., Kawanokuchi J., Kuno R. Tumor necrosis factor-α induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J. Biol. Chem. 2006;281:21362–21368. doi: 10.1074/jbc.M600504200. [DOI] [PubMed] [Google Scholar]
- Tatti R., Haley M.S., Swanson O.K., Tselha T., Maffei A. Neurophysiology and regulation of the balance between excitation and inhibition in neocortical circuits. Biol. Psychiatry. 2017;81:821–831. doi: 10.1016/j.biopsych.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Török N., Majláth Z., Fülöp F., Toldi J., Vécsei L. Brain aging and disorders of the central nervous system: Kynurenines and drug metabolism. Curr. Drug Metab. 2016;17:412–429. doi: 10.2174/1389200217666151222155043. [DOI] [PubMed] [Google Scholar]
- Trapp B.D., Stys P.K. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol. 2009;8:280–291. doi: 10.1016/S1474-4422(09)70043-2. [DOI] [PubMed] [Google Scholar]
- Trisolini M., Honeycutt A., Wiener J., Lesesne S. Global economic impact of multiple sclerosis global economic impact of multiple sclerosis. Mult. Scler. Int. Fed. 2010;May:1–104. [Google Scholar]
- Utz K.S., Lee D.-H., Lämmer A., Waschbisch A., Linker R.A., Schenk T. Cognitive functions over the course of 1 year in multiple sclerosis patients treated with disease modifying therapies. Ther. Adv. Neurol. Disord. 2016;9:269–280. doi: 10.1177/1756285616643892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Horssen J., Van Der Pol S., Nijland P., Amor S., Perron H. Human endogenous retrovirus W in brain lesions: Rationale for targeted therapy in multiple sclerosis. Mult. Scler. Relat. Disord. 2016;8:11–18. doi: 10.1016/j.msard.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Vécsei L., Szalárdy L., Fülöp F., Toldi J. Kynurenines in the CNS: recent advances and new questions. Nat. Rev. Drug. Discov. 2012;12:64–82. doi: 10.1038/nrd3793. [DOI] [PubMed] [Google Scholar]
- Vogel D.Y.S., Vereyken E.J.F., Glim J.E., Heijnen P.D.A.M., Moeton M., van der Valk P. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J. Neuroinflammation. 2013;10:35. doi: 10.1186/1742-2094-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingen M., Kuypers K.P.C., van de Ven V., Formisano E., Ramaekers J.G. Sustained attention and serotonin: a pharmaco-fMRI study. Hum. Psychopharmacol. 2008;23:221–230. doi: 10.1002/hup.923. [DOI] [PubMed] [Google Scholar]
- Ziehn M.O., Avedisian A.A., Tiwari-Woodruff S., Voskuhl R.R. Hippocampal CA1 atrophy and synaptic loss during experimental autoimmune encephalomyelitis, EAE. Lab Investig. 2010;90:774–786. doi: 10.1038/labinvest.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further reading
- Achiron A., Chapman J., Magalashvili D., Dolev M., Lavie M., Bercovich E. Modeling of cognitive impairment by disease duration in multiple sclerosis: a cross-sectional study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0071058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A., Smith M.C., Yin X., Fox R.J., Staugaitis S.M., Trapp B.D. Neurogenesis in the chronic lesions of multiple sclerosis. Brain. 2008;131:2366–2375. doi: 10.1093/brain/awn157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evonuk K.S., Baker B.J., Doyle R.E., Moseley C.E., Sestero C.M., Johnston B.P. Inhibition of system Xc− transporter attenuates autoimmune inflammatory demyelination. J. Immunol. 2015;195:450–463. doi: 10.4049/jimmunol.1401108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman M.S., Selchen D., Arnold D.L., Prat A., Banwell B., Yeung M. Treatment optimization in MS: Canadian MS Working Group updated recommendations. Can. J. Neurol. Sci. 2013;40:307–323. doi: 10.1017/s0317167100014244. [DOI] [PubMed] [Google Scholar]
- Giannakopoulou A., Grigoriadis N., Bekiari C., Lourbopoulos A., Dori I., Tsingotjidou A.S. Acute inflammation alters adult hippocampal neurogenesis in a multiple sclerosis mouse model. J. Neurosci. Res. 2013;91:890–900. doi: 10.1002/jnr.23226. [DOI] [PubMed] [Google Scholar]
- Werner P., Pitt D., Raine C.S. Multiple sclerosis: Altered glutamate homeostasis in lesions correlates with oligodendrocyre and axonal damage. Ann. Neurol. 2001;50:169–180. doi: 10.1002/ana.1077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.