Abstract
Background
The impact of iron supplements and iron fortification on diarrhea in children is controversial, with some studies reporting an increase and others reporting no effect.
Objective
The aim of the study was systematically assess the published literature on oral iron supplementation and fortification to evaluate its impact on diarrhea incidence among children aged 4–59 mo.
Methods
Randomized controlled trials of oral iron supplementation or iron fortification that reported diarrheal outcomes in children aged 4–59 mo were identified from a systematic search of 5 databases.
Results
Of the 906 records identified, 19 studies were found to fit the inclusion criteria for this systematic review. However, variable case definitions for diarrhea made meta-analysis impossible. Of the 19 studies, 7 (37%) studies showed a significant increase, either in overall diarrhea incidence or within a specific subgroup of the population, between iron-supplemented and control groups. Subgroups included children who were iron-replete and children undergoing their first month of iron intervention. Two studies reported an increase in bloody diarrhea. The remaining 12 (63%) studies showed no difference between iron-supplemented and control groups.
Conclusions
Studies on iron supplementation and fortification use divergent case definitions for diarrhea. A number of studies (37%) showed an increase in overall diarrhea incidence or within a specific subgroup of the population, between iron-supplemented and control groups, but the majority (63%) did not. In addition, there was no clear relation between diarrhea and type of intervention or amount of iron administered observed. In future studies, we recommend that diarrhea be clearly defined and consistently recorded as a secondary outcome. Antibiotic status of participants receiving iron should also be collected to help assess possible drug interactions resulting in a “red stool effect.” Finally, further microbiome research is required to better understand the effects of oral iron on specific bacterial species in the colon.
Keywords: iron, ferrous sulfate, iron deficiency anemia, diarrhea, anemia, multiple micronutrient powder
Introduction
Iron is required for many essential metabolic processes (1). Pathogens and humans require iron and have developed complex ways to acquire, transport, and store it (2). Bacteria have developed multiple mechanisms for chelating iron and heme directly and for acquiring iron attached to various human iron chaperone molecules (3). In turn, humans tightly regulate free iron at a molar concentration of less than 1024, and bind it with proteins such as ferritin, transferrin, and lactoferrin (4).
Iron deficiency anemia occurs when both intake and total body iron are insufficient to meet the needs of erythropoiesis. A 2011 WHO report estimated a prevalence of 43% of anemia
worldwide (5), with over half of cases attributable to iron deficiency (6). Infants aged 0–5 y, pregnant women, and women of childbearing age are at highest risk (7). Relative iron requirements for children are higher than adults because of the nutritional demands of accelerated growth (8). Among its many uses, iron is essential for brain growth: it is necessary for myelination of oligodendrocytes (9) as well as the production of the key neurotransmitters serotonin (10) and dopamine (11).
In low-income countries, there is concern that untargeted iron supplementation can predispose children to certain infections, including malaria, diarrhea, and respiratory infections. One previous systematic review published in 2002 analyzed 28 randomized controlled trials (no age limits on participants) for the effect of both iron supplementation (oral and parenteral) and fortification on a number of infectious disease outcomes (12). In the analysis, subjects receiving iron had a higher risk of only 1 complication, diarrhea (at an 11% increase).
Iron supplementation and fortification could induce diarrhea by causing intestinal damage through oxidative stress (13–16) or by initiating bacterial dysibiosis and gut inflammation (17–20).
This review focuses specifically on children from the ages of 4 mo to 5 y, a population group that is concurrently at the highest risk of diarrhea and most likely to benefit from iron intervention (8, 21). The primary objective is to systematically assess the published literature on oral iron supplementation and fortification to evaluate its impact on diarrhea incidence among children aged 4–59 mo. Secondary objectives include establishing whether any specific population subgroups are at increased risk of diarrhea and discussing possible potential policy implications based on the results found.
Methods
Search strategy
This systematic review adheres to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) 2009 Checklist (Supplemental Table 1) (22). We published the protocol for this study on 19 May 2017 (CRD42017067297). We conducted a systematic search across 5 different databases; Medline (1946 to July 2017), EMBASE (1974 to 2017 week 31), Global Health (1910 to 2017), Web of Science, and Cochrane Central Register of Controlled Trials. The last search was conducted on 31 July 2017. The search strategy consisted of 4 main concepts: “children,” iron,” “supplementation/fortification,” and “diarrhea” (Supplemental Table 1). Owing to the similarity of Boolean operators, Ovid was used to retrieve searches from 3 databases—Medline, EMBASE, and Global Health—simultaneously. For studies that were indexed by the search but inaccessible, relevant authors were contacted to retrieve full texts. Through this method, 1 further full text was made accessible (23).
Inclusion and exclusion criteria
We restricted the review to double-blind, randomized controlled trials in humans. Searches were limited to the English language. Inclusion criteria were predefined in the published protocol and are reported in the Cochrane endorsed population intervention comparator outcome format (24): population—children between the ages of 4 mo and 5 y at the initiation of iron intervention; intervention—oral iron supplementation or fortification of any kind, any dose and any duration, including multiple micronutrient supplementation if iron was a principal component; comparator—any placebo or control group of the same population receiving no intervention or an intervention containing negligible amounts of additional iron; outcome—diarrhea or dysentery cases reported as either a primary or secondary outcome in any format. We excluded review articles, case studies, and unpublished trials. Studies that obtained participants with existing cases of diarrhea were excluded because they were unrepresentative of the general population. Nonoral iron supplementation, formulated foods, lipid-nutrient supplementation, meat-based iron supplementation, infant formula milk, fortified breast-milk, and bovine lactoferrin were all excluded. Owing to existing evidence that zinc supplementation reduces risk of diarrhea (25, 26), we excluded trials that combined the iron and zinc supplementation arm, unless there was also an iron-only arm. Owing to frequent inconsistencies in the case definitions for diarrhea, we included all case definitions of diarrhea as described in the studies.
Analysis
Owing to substantial heterogeneity of reported outcomes, it was not possible to conduct a meta-analysis, and instead a vote-counting method was used. Studies were classified as either increasing risk of diarrhea with iron formulation/supplementation or having no effect using a significance level of P < 0.05. We described the overall trend of the studies, with a focus on whether any population subgroups or intervention types were particularly affected by iron supplementation or fortification.
Risk of bias
All studies progressing to the extraction phase were assessed using the Cochrane risk of bias tool to ensure adequate quality (27). Categories assessed included: selection bias, detection bias, attrition bias, reporting bias, and “other” biases (such as poor case definitions as well as weak methods of outcome detection).
Every study was assessed for each category of bias individually, and a judgment was made to score the bias as “low risk,” “high risk,” or “unclear risk” if information was insufficient. The bias scores in each category were then used to obtain an overall statement of study quality. Studies were initially considered to be “high quality” and were downgraded to “adequate quality” and “low quality” for each additional category containing a high risk of bias. Studies that scored an “unclear” risk in 4 or more categories were also downgraded in quality.
All studies progressing to the final stage of the review underwent full data extraction regardless of risk of bias. Risk of bias data was recorded and assessed using RevMan v5.2 to display quality outcomes both within and between studies.
Results
Included studies
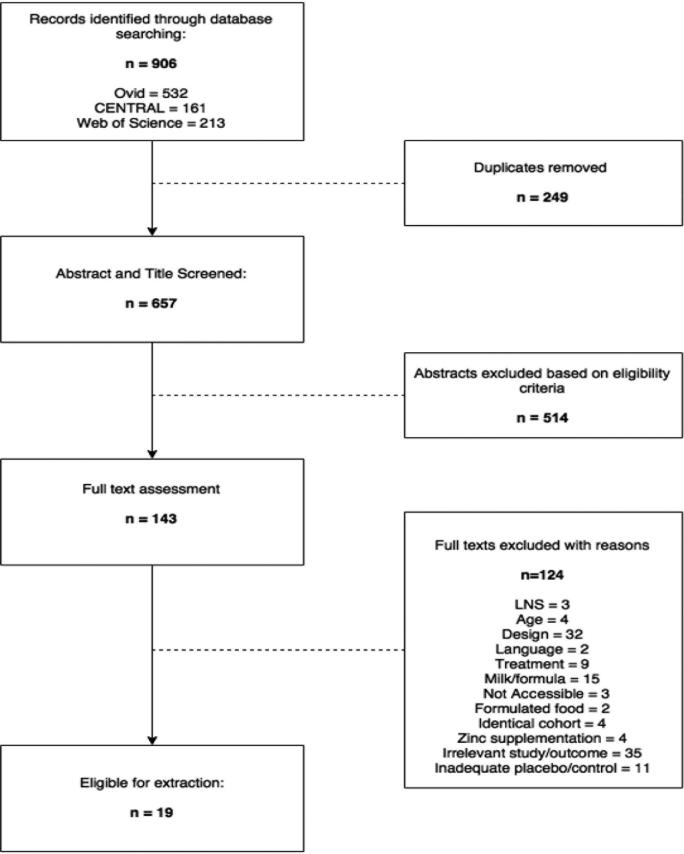
As detailed in Figure 1, a total of 906 records were identified using the predefined search strategy (Supplemental Table 1). A total of 249 duplicates were removed, and of the remaining 657 studies, 143 were eligible for full text appraisal. Four potentially relevant texts were deemed inaccessible. Corresponding authors were contacted, and 1 text was successfully retrieved (23). A full list of excluded studies with reasons for exclusion is available in Supplemental Table 2.
FIGURE 1.
Study selection flow diagram. LNS, lipid-based nutrient supplement.
Nineteen studies progressed to the final stage of review and ranged in publication date from 1991 to 2017. The papers summarized global data; 9/19 studies were from Asia (3 from Bangladesh, 2 from Pakistan, 2 from China, 1 from Cambodia, 1 from India), 4 from Africa (3 from Kenya, 1 from South Africa), 4 from North America (Canada, Honduras, Haiti, Mexico), 1 from South America (Peru), and 1 from Europe (Sweden). One study, undertaken by Dewey et al. (28), included 2 simultaneous cohorts from both Honduras and Sweden. As such, these cohorts have been considered separately in the analyses.
Study design
All 19/19 studies were randomized controlled trials, with 7 being of a simple design, containing a placebo and iron-intervention group only. The remaining studies (12/19) included multiple intervention arms (Tables 1 and 2). Only 2 studies did not randomly assign individual participants: Menon et al. (29) and Soofi et al. (30), who used cluster randomization of villages and food distribution points respectively. The total duration of intervention varied greatly, from 2 to 18 mo. One study, Chen et al. (31), showed a modestly inflated study duration, as the intervention was suspended during weekends and holidays, leading to the reported length of 6 mo being equivalent to 120 supplementation days (4 mo).
TABLE 1.
Summary table of included studies: fortification1
| Study | Age: duration | Iron type | Intervention, n | Control, n | Total, n | Other study arms, n | Effect size | P value | Subgroups of note | Detrimental effect of intervention |
|---|---|---|---|---|---|---|---|---|---|---|
| Barth-Jaeggi et al. (67); 2015; Kenya | 6 mo: 12 mo | 2.5 mg NaFeEDTA | 88 | 82 | 170 | Proportion: intervention 26%, placebo 29% | P > 0.05 | No | ||
| Chen et al. (31); 2011; China | 2–6 y: 6 mo | 12 mg NaFeEDTA | 71 | 61 | 226 | 94 MMN | Risk ratio: intervention 0.95(0.81–1.15), intervention vs. MMN 0.78 (0.61–0.92) | P > 0.05 | No | |
| Christofides et al. (62); 2005; Canada | 4–18 mo: 6 mo | 30 mg ferrous fumarate | 26 | 36 | 62 | Risk ratio: 1.09 (0.61–1.97) | No | |||
| Giovannini et al. (68); 2006; Cambodia | 6 mo: 12 mo | 12.5 mg ferrous fumarate | 68 | 68 | 204 | 68 MMN | Proportion: intervention 10.3%, placebo 5.9%, MMN 10.3% | No | ||
| Jaeggi et al. (17); 2014; Kenya | 6 mo: 4 mo | 12.5 mg ferrous fumarate | 22 | 24 | 46 | 47 ferrous pyrophosphate | Proportion: intervention 27.3%, placebo 8.3% | P = 0.092 | Another formulation of 2.5 mg iron was assessed, but morbidity data not provided | No |
| Javaid et al. (69); 1991; Pakistan | 4.4 mo: 8 mo | 4.1–5.1 mg (mean) ferrous fumarate | 40 | 42 | 129 | Episodes per infant: interventions 2.87, placebo 2.49 | P > 0.05 | A separate control group with no cereal or fortification showed a rate of 4.32 episodes per infant | No | |
| Lemaire et al. (33); 2011; Bangladesh | 12–24 mo: 2 mo | 12.5 mg ferrous fumarate | 132 | 126 | 258 | Number of cases: intervention 126, placebo 135 | P > 0.05 | Two separate cohorts used: winter/summer | No | |
| Menon et al. (29); 2007; Haiti | 9–24 mo: 2 mo | 12.5 mg “iron” | 254 | 161 | 415 | Proportion: intervention 58% control 43% (first month only) | P < 0.05 | Additionally: nonanemic children had a higher prevalence of diarrhea than anemic children in the intervention group during the first month P < 0.05; an increase in diarrhea morbidity from baseline to month 1 was also seen in the fortified nonanemic group P < 0.13 | Yes | |
| Paganini et al. (23); 2017; Kenya | 6.5–9.5 mo: 4 mo | 12.5 mg “iron,” 2.5 mg NaFeEDTA | 52 | 51 | 154 | 52 iron + galacto-oligosacharides | Number of cases in all groups: 74; quote: “no significant group differences in the number of infants treated for diarrhea” | P > 0.05 | No | |
| Soofi et al. (70); 2013; Pakistan | 6 mo: 12 mo | 12.5 mg ferrous fumarate | 746 | 779 | 2271 | 746 MNP + zinc | Incidence rate: intervention 4.16, control 3.73, MNP + zinc 4.32 | P = 0.12 | Increased incidence of bloody diarrhea between 6 and 18 mo in both MNP groups P = 0·003; weaker evidence of an increase in severe diarrhea among children receiving MNPs (≥ 6 stools per day) P = 0·07 | Yes |
MMN, multiple micronutrient; MNP, micronutrient powder; NaFeEDTA, sodium iron ethylene diamine tetraacetate.
TABLE 2.
Summary table of included studies: supplementation1
| Study | Age: duration | Iron type | Intervention, n | Control, n | Total, n | Other study arms, n | Effect size | P value | Subgroups of note | Detrimental effect of intervention |
|---|---|---|---|---|---|---|---|---|---|---|
| Abdelrazik et al. (38); 2007; India | 6 mo: 12 mo | 43 mg ferrous gluconate | 198 | 50 | 348 | Proportion: intervention 75.8%, placebo 50% | P = 0.03 | Of the group that received iron, those with normal ferritin at baseline had higher rates of diarrhea; P = 0.04 | Yes | |
| Baqui et al. (36); 2003; Bangladesh | 6 mo: 6 mo | 20 mg ferrous sulfate | 165 | 157 | 476 | 154 MMN | Adjusted odds: iron 1.01(0.91–1.13), MMN 1.15 (1.02–1.29), control 1.0 | P < 0.05 | A zinc and zinc + iron arm was excluded from extraction; MMN was not well tolerated with a 41% drop out rate; iron alone had little effect on diarrhea incidence, but MMN containing iron had a significant impact | Yes |
| Chang et al. (34); 2010; Bangladesh | 6–18 mo: 6 mo | 6.25 mg “Iron” | 201 | 201 | 799 | 199 iron and zinc, 198 zinc only | Incidence rate: iron 2.7, placebo 2.3, combined iron and zinc 2.0, iron and zinc alternative days 2.1, zinc alone 2.3. | P < 0.05 | Iron alone significantly increases risk of diarrhea in children; this effect is mitigated by the addition of zinc; giving iron to underweight children had less detrimental effects on diarrhea than those who were of normal weight. | Yes |
| Chen et al. (71); 2013; China | 3–6 y: 6 mo | 1–2 mg/kg ferrous sulfate | 98 | 104 | 292 | 90 vitamin A + iron | Incidence rate: iron 0.4, placebo 0.43, vitamin A + iron 0.28 | P > 0.05 | Significant decrease when iron is combined with vitamin A P < 0.05 | No |
| Dewey et al. (28); 2002; Honduras Cohort | 4 mo: 3 or 5 mo | 1 mg/kg ferrous sulfate | 36/40 (3/5 mo) | 42 | 118 | Proportion (over whole study duration): intervention 64%/58% placebo 50% | Iron supplementation reduced the risk of diarrhea among infants with Hb < 110 g/L at 4 mo, but led to an increase in diarrhea among infants with Hb > 110 g/L at 4 mo P = 0.03; NB: combined cohorts used: Sweden/Honduras; morbidity data specific to 4–6 mo and 6–9 mo reported, but overall morbidity incidence extracted only | Yes | ||
| Dewey et al. (28); 2002; Swedish Cohort | 4 mo: 3 or 5 mo | 1 mg/kg ferrous sulfate | 30/30 (3/5 mo) | 36 | 96 | Proportion (over whole study duration): intervention 27%/30% placebo 14% | Combined data, as above | Yes | ||
| Luabeya et al. (32); 2007; South Africa | 6 mo: 18 mo | 10 mg ferrous fumarate | 109 | 113 | 335 | 113 zinc + vitamin A | Number of cases: intervention 89 placebo 98 vitamin A + zinc 92 | P = 0.484 | No | |
| Mitra et al. (35); 1997; Bangladesh | 29 mo: 15 mo | 125 mg ferrous gluconate | 118 | 131 | 249 | Diarrhea episodes per child per year: intervention 2.8 (1.6–4.8), control 2.5 (1.6–5.0) dysentery episodes per child per year: intervention 2.5 (0.9–4.8), control 2.5 (0.9–4.8) | Diarrhea P = 0.32, dysentery P = 0.84 | Dysentery episodes per child per year, under 12 months of age: iron 5.2 (2.4–7.8), control 3.5 (2.1–4.8); P = 0.03 | Yes | |
| Richard et al. (72); 2006; Peru | 0–4 y (multiple strata): 7 mo | 15 mg ferrous sulfate | 60 | 61 | 187 | 66 zinc | Risk ratio: intervention 0.97 (0.78–1.21), iron + zinc 0.89 (0.70–1.12), control 1.0. | P = 0.32 | No | |
| Rosado and Allen (73); 1997: Mexico | 1.5–3 y: 12 mo | 20 mg ferrous sulfate | 54 | 56 | 165 | 55 iron + zinc | Episodes per year: intervention 76, iron + zinc 46, placebo 62 | P > 0.05 intervention P < 0.05 iron + zinc | No |
Hb, hemoglobin; MMN, multiple micronutrient.
Population
Four out of 19 studies recruited their participants in a hospital setting either through routine infant clinics or at birth, 13/19 studies recruited from predefined geographical areas, and 2/19 enrolled nursery attendees. Eleven out of 19 studies selected children under the age of 12 mo, with 9 of these selecting children 6 mo old or older, as they began complementary feeding. Two studies, Luabeya et al. (32) and Lemaire et al. (33), recruited participants with existing comorbidities: HIV infection and moderate acute malnutrition respectively. All other studies detailed specific inclusion and exclusion criteria, excluding participants with congenital malformations or chronic diseases (Supplemental Table 3).
Intervention style
Nine out of 19 studies involved direct supplementation of iron through the use of syrups, tablets, capsules, and solutions. Five studies involved traditional fortification: 2 studies using maize, 1 study using wheat, and 2 studies using a nondescript cereal for fortification. “Point of use fortification,” often interchangeably termed “sprinkles,” “micronutrient powders,” or “at-home fortification,” was used by 5 studies (Table 3).
TABLE 3.
Reported effect of intervention on diarrhea incidence by iron type1
| Iron type | Increased incidence | No effect |
|---|---|---|
| Ferrous sulfate | Dewey et al. (28) | Richard et al. (72) |
| Ferrous sulfate | Baqui et al. (36) | Chen et al. (2013) (71) |
| Ferrous sulfate | Rosado and Allen (73) | |
| Ferrous gluconate | Abdelrazik et al. (38) | |
| Ferrous gluconate | Mitra et al. (35)2 | |
| Ferrous fumarate | Soofi et al. (30)2 | Jaeggi et al. (17) |
| Ferrous fumarate | Luabeya et al. (32) | |
| Ferrous fumarate | Lemaire et al. (33) | |
| Ferrous fumarate | Christofides et al. (62) | |
| Ferrous fumarate | Javaid et al. (69) | |
| Ferrous fumarate | Giovannini et al. (68) | |
| NaFeEDTA + ferrous fumarate | Paganini et al. (23) | |
| NaFeEDTA | Barth-Jaeggi et al. (67) | |
| NaFeEDTA | Chen et al. (2011) (31) | |
| Nondescript iron | Chang et al. (34) | |
| Nondescript iron | Menon et al. (29)2 |
NaFeEDTA, sodium iron ethylene diamine tetraacetate.
Significant increase within population subgroup only.
Intervention type
Two studies, Menon et al. (29) and Chang et al. (34), did not specify the type of iron used. The remaining studies used iron sulfate, fumarate, gluconate, sulfate, or sodium iron ethylene diamine tetraacetate (NaFeEDTA). One study, Paganini et al. (23), used a novel combination of 2 forms of iron: ferrous fumarate and NaFeEDTA (Table 4).
TABLE 4.
Reported effect of intervention on diarrhea incidence by intervention style1
| Intervention category | Intervention form | Increased incidence | No effect |
|---|---|---|---|
| Fortification | Cereal | Chen et al. (2011) (31) | |
| Cereal | Javaid et al. (69) | ||
| Wheat soy blend | Menon et al. (29)1 | ||
| Maize | Jaeggi et al. (17) | ||
| Maize | Barth-Jaeggi et al. (67) | ||
| Point-of-use fortification | Sprinkles | Soofi et al. (30)1 | Christofides et al. (62) |
| Sprinkles | Lemaire et al. (33) | ||
| Sprinkles | Paganini et al. (23) | ||
| Sprinkles | Giovannini et al. (68) | ||
| Supplementation | Syrup | Abdelrazik et al. (38) | Richard et al. (72) |
| Syrup | Mitra et al. (35)1 | ||
| Syrup | Dewey et al. (28) | ||
| Tablet/capsule | Baqui et al. (36) | Luabeya et al. (32) | |
| Tablet/capsule | Chen et al. (2013) (71) | ||
| Dissolvable tablet/solution | Chang et al. (34) | Rosado and Allen (73) |
Significant increase within population subgroup only.
Intervention frequency
All studies provided participants with a regime of daily iron supplementation, besides Chang et al. (34) who supplemented on alternate days (Supplemental Table 4). Four out of 19 studies adjusted their iron dose according to either weight or age, with the remainder using a fixed dose of daily iron. Significant heterogeneity in amount of iron delivered was seen between studies. A full list of detailed ingredients and intervention types for each intervention is provided in Supplemental Table 5.
Outcome
Five out of 19 studies did not provide a specific case definition for the diagnosis of diarrhea, with only 10 of 19 studies using the standard definition of “three or more watery stools within a 24 hour period.” In all studies, cases were reported by infants’ mothers to a fieldworker or study personnel via informal interviews or written questionnaires. One study, Mitra et al. (35), deviated from this method by additionally including physician's records when measuring diarrhea incidence (Supplemental Table 3).
Effect of intervention
Twelve out of 19 (63%) studies showed no significant difference in diarrheal outcomes between intervention and placebo groups. Four out of 19 (21%) studies showed a significant difference in diarrhea incidence between groups, with all reporting an increase in morbidity. Reported outcome formats varied greatly with a variety of rates, ratios, proportions, and raw numbers all being presented. A further 3 out of 19 (16%) studies showed a higher rate of diarrhea in specific subgroups only. Mitra et al. (35) demonstrated strong evidence of an increased rate of dysentery (defined as mucus or blood-containing stool) in children under 1 year of age. Menon et al. (29) reported a significant increase in diarrheal incidence in iron-replete infants during the initial month of supplementation. Soofi et al. (30) also presented a strongly significant increase in bloody diarrhea within those taking iron-containing micronutrient powders.
Although no formal statistical analyses were conducted, no clear relation between style of supplementation and diarrheal incidence was evident. Baqui et al. (36) showed no effect of 20 mg ferrous sulfate supplementation alone but an increased incidence in diarrheal morbidity when iron was delivered as a multiple micronutrient formation. None of the 3 studies that used NaFeEDTA led to increases in diarrheal incidence. There was also no clear relation between volume of iron administered and diarrheal outcomes.
Risk of bias
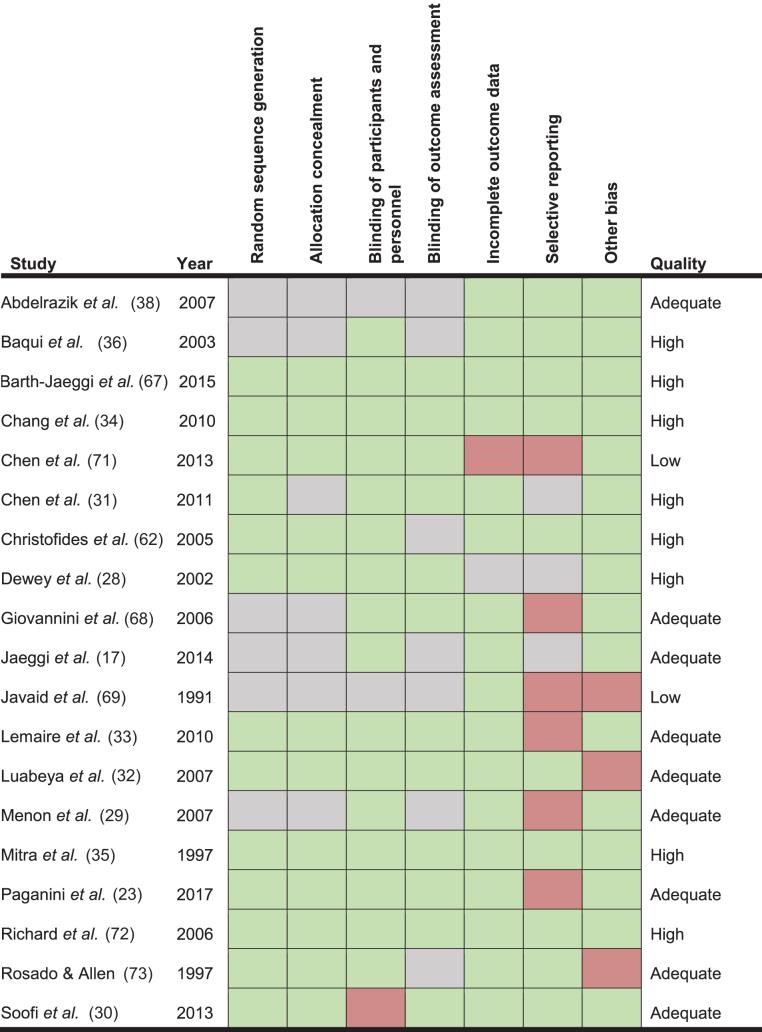
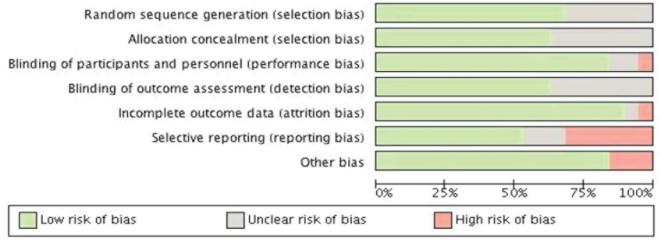
The risk of bias assessment was determined on all 19 studies (Table 5 and Figure 2). The overall risk of bias was low, with 9/19 (47%) studies considered “high” quality, a further 8/19 (42%) being of “adequate,” and just 2/19 (11%) being considered “low” quality. Between studies, the most common risk of bias was that of reporting bias with 6/19 (31%) studies scoring inadequately in this area. For the majority of papers, diarrhea was not a primary outcome and often only added as an aside to the original results if deemed noteworthy.
TABLE 5.
Risk of bias assessment for included studies1
FIGURE 2.
Cochrane risk of bias graph for included studies.
All studies (19/19) reported random sequence generation, but 6/19 did not disclose their exact methods of randomization. Performance bias was adequately addressed in 16/19 papers, with only Soofi et al. (30) being downgraded to “high risk” because of a lack of use of an adequate placebo. Papers with the lowest risk of bias in this category, such as Jaeggi et al. (17), used triangle taste testing to ensure participants could not discriminate between iron compounds and placebo. Blinding of outcome assessors was described in 12/19 studies and unreported in the remainder. Studies not reporting this bias category tended to be older, predating the 2010 Consolidated Standards of Reporting Trials (CONSORT) criteria (37). Seventeen out of 19 studies provided attrition data, usually via an annotated flow diagram accounting for loss to follow-up for each individual.
Six out of 19 studies were categorized as being at high risk of reporting bias; this was mainly due to the incomplete presentation of both absolute and relative values for diarrhea incidence. In many cases, this was because diarrhea was not an intended primary outcome. One study by Lemaire et al. (33) was suspected of reporting bias due to the presentation of a composite outcome consisting of dysentery, diarrhea, and lower respiratory tract infections as a single figure. In order to account for this, supplementary data for this study were located to retrieve outcome-specific results. When considering “other” sources of bias, 3/19 studies were penalized for having weak or nondescript case definitions. Supporting statements for each risk of bias judgment are provided in Supplemental Table 6.
Discussion
In summary, of the 19 studies extracted, 12 showed no effect of iron on diarrheal incidence, 4 showed a significant increase, and a further 3 showed an increase within a specific subpopulation.
Iron and pathogen-induced diarrhea
Iron supplement/fortificant-induced diarrhea could be due to 2 candidate mechanisms: first, through the production of reactive oxygen species and second through bacterial dysibiosis.
Iron itself has the potential to produce copious reactive oxygen species within the intestinal tract through both the Haber–Weiss and Fenton reactions (14). This has the unintended side-effect of causing intestinal damage through oxidative stress, thus precipitating lipid peroxidation and inflammatory diarrhea (13). This mechanism has been demonstrated in in vitro studies with enterocyte-like cells exhibiting a degradation in epithelial integrity after iron exposure (15, 16).
Two recent randomized controlled trials have shown that iron intervention can alter the gut microbiome (17, 18). Specifically, both studies observed a trend toward increase in E.coli as well as a concurrent decrease in Lactobacillaceae (19). Both studies also showed a significant increase calprotectin within the intervention group, a biomarker for gut inflammation.
Because there are multiple biologically plausible mechanisms by which oral iron supplementation could cause diarrhea and conflicting clinical data, we attempted to perform an analysis of the current literature to assess the possibility that a causal relation exists.
Diarrhea in the iron-replete
Four cohorts, Abdelrazik et al. (38), Menon et al. (29), Dewey et al. Sweden/Honduras (28), extracted in this review displayed a higher incidence of diarrhea specifically in children who were iron-replete as defined by the study. This review's findings, that iron-replete individuals may be more susceptible to iron-induced diarrhea, support current WHO guidelines that recommend the use of iron fortificant or supplements only in areas that have an anemia prevalence of 40% and 20% respectively (39, 40).
Bloody diarrhea
Two studies, by Soofi et al. (30) and Mitra et al. (35), showed a significantly increased incidence of acute bloody diarrhea within those who received iron interventions. Acute bloody diarrhea, commonly referred to as dysentery, is a symptom commonly associated with toxin-producing bacteria such as Shigella, E.coli, Salmonella, or Campylobacter (41). The results presented by the Pakistan study were alarming enough to prompt a correspondence in The Lancet in 2013 with Tobe-Gai et al., who called for an “urgent need… (for) robust evidence on age-specific doses” of micronutrient powder (42). Although it is tempting to attribute the results to iron administration, 1 further possibility is that of antibiotic interactions. Unlike other cohorts, such as those in Jaeggi et al. (17) and Paganini et al. (23), the Pakistan study included participants receiving antibiotic treatments at baseline. A candidate antibiotic that may be accountable for the significant difference in dysenteric outcomes is Cefdinir (43). Cefdinir is a third-generation cephalosporin often used in pediatric populations for the treatment of penicillin-resistant infections such as otitis media, sinusitis, and pharyngitis (44–46) These infections are extremely common in infants and also have an increasingly high resistance to first-line antibiotics with recent reports estimating between 30% and 70% resistance (47). One side-effect of Cefdinir that is becoming increasingly well documented is its ability to cause the formation of red stools, especially when coadministered with iron (48). Based on case reports, the volume of iron needed to form these red-iron complexes is relatively low (49). One small randomized controlled trial described the frequency of stool discoloration from Cefdinir to be as high as 10%, with a concurrent significant increase in diarrhea at higher doses (50). This relatively common Cefdinir side-effect may falsely promote an apparent association between iron administration and bloody diarrhea.
It is plausible that, especially in a sample of almost 3000 infants, a plethora of antibiotics were prescribed, dependent on availability and prescribing patterns of the region. It could be argued that both the Pakistan cohort and the Mitra et al. (35) Bangladesh cohort did not show an increase in bloody diarrhea for all children, but only those younger than 18 mo. If cephalosporin administration was accountable for this relation, differential prescribing between age groups would have to be demonstrated. Alternatively the association could be explained by the epidemiology of otitis media itself, which has a peak incidence during the first year of life, specifically 6–18 mo (51). During this period, we would expect the prescription of cephalosporins to be most frequent and thus the incidence of reported bloody diarrhea to be higher, as is the case with both studies. Although unlikely, if antibiotic prescription were liable for some of the results observed, the ramifications of these findings would be significant.
Risk of diarrhea by type of intervention
Five out of 9 iron supplementation studies showed a significant increase in the diarrhea. There is little consensus on which iron type should be used. However, ferrous fumarate provides the most iron per gram, ferrous sulfate is the cheapest, and ferrous gluconate is known for its minimal side-effect profile (52). Fourteen studies utilized conventional iron salts as a form of iron intervention. These include ferrous fumarate, ferrous sulfate, and ferrous gluconate, in order of decreasing bioavailability (53).
Three studies utilized NaFeEDTA, with all of these studies showing no effect on diarrheal morbidity. The benefits of NaFeEDTA are 3-fold. First, within the lumen of the intestine, the unconventional manner in which the EDTA complex binds iron may sequester iron from iron-dependent pathogens, thus withholding iron desperately needed for survival (17). Second, it is well established through in vitro experimentation that EDTA itself exhibits antimicrobial properties and is commonly used to prevent the formation of biofilm. Recent studies have pertinently shown that EDTA can induce the deterioration of both E. coli and Salmonellaenterica cell membranes (54, 55). Finally, when used as a fortificant, the EDTA component also protects iron from the inhibitory effect of phytates and polyphenols (56). Moreover, NaFeEDTA has been reported to be absorbed 2–4 times more efficiently than ferrous sulfate, the compound once considered the benchmark of iron bioavailability (57, 58). Its use has been recently endorsed by the WHO/FAO Expert Committee on Food Additives and is recommended for use specifically with corn and condiments (56). Despite its inherent benefits, NaFeEDTA is expensive, its effective cost per milligram reported to be 16 times that of ferrous sulfate alone, making it less viable for resource-poor programs (59).
Fortification is often considered as a safer alternative to supplementation because of its smaller dose and a more physiological uptake when combined with foods (58). This safety is somewhat represented in our results, with only 1/5 “traditional” fortification studies and 2/5 sprinkle studies leading to an increased incidence of diarrhea [Table 4]. Although it appears that, when compared to fortificants, supplements have a higher risk of diarrheal morbidity, it is important to note that the data on the effectiveness of each intervention were not extracted. For example, a study providing low-dose iron fortification may have no effect on morbidity but also no effect on the intended outcome of interest; usually serum ferritin. This limitation of this review makes it difficult to recommend a specific form of intervention.
Limitations
The search term “iron” was an essential keyword in the search strategy used. It is possible that a select few multiple micronutrient studies would not have included the keyword “iron,” as it may have been an assumed “micronutrient” in the collective whole. This constraint was unavoidable if all iron interventions were to be captured, and a number of reviews already exist that assess the safety of multiple micronutrient interventions (60).
“Conventional vote-counting” (61) was the method used to describe the results of this review. This involves counting the number of trials that showed an adverse effect of the intervention on diarrhea (7/19), those that had a protective effect (0/19) and those that had no effect (12/19). A great deal of literature has been published on vote counting and its inherent flaws, which this study is also fallible to (62, 63). In order to mitigate these effects, this review only “counted” positive associations that were statistically significant at a significance level of P < 0.05, whether that be in a specific subgroup or overall. This adaptation provides a more robust overview of relations than older forms of conventional vote-counting that often use a baseline cutoff of P < 0.5 (64).
There was significant heterogeneity in how diarrheal outcomes were reported. Proportions, risk ratios, incidence rates, and raw numbers were all variously reported. However, “incidence” definitions varied, with some studies reporting total frequency of diarrheal episodes and others reporting the number of children who ever suffered from diarrhea within a given period. The latter value always gives a number below that of the total study population (n), whereas the former could be much higher, as it accounts for children who suffer from multiple discrete episodes of diarrhea throughout the study duration.
Conclusions
Undoubtedly there is a strong need for effective treatments for iron deficiency. However, a delicate balance between providing iron to host and increasing pathogen growth needs to be maintained, particularly in the gut. Factors such as genetics, gut integrity, diet, hygiene, and inflammation status all contribute to the complex interplay between iron and the gut (65, 66).
We recommend that future iron-intervention studies consider 3 key recommendations. First, diarrhea as defined by the WHO should be recorded as a clearly reported secondary outcome, preferably as a crude number. Second, antibiotic status of individuals enrolled in an iron study should be collected, with those taking antibiotics at baseline excluded. This would help account for possible drug interactions and the possibility of the “red stool effect.” Finally, fecal bacteria should be analyzed where possible to help contribute to the burgeoning field of microbiome research and to further understand the selective effects of iron on specific bacterial species. We hope that these recommendations are modest yet sufficiently achievable to ensure that diarrhea is adequately assessed in iron-intervention studies.
Supplementary Material
Acknowledgments
We thank Frances Bird and Tina Sorensen for their help with design of data-extraction templates.
The authors’ responsibilities were as follows—AG: conducted the review design, search, and analyses; AG, PJ, and CC: wrote the paper; and all authors: read and approved the final manuscript.
Notes
Funded by Bill and Melinda Gates Foundation and core funding MC-A760-5QX00 to the International Nutrition Group by the UK Medical Research Council and the UK Department for International Development under the Medical Research Council/Department for International Development Concordat agreement.
Author disclosures: None of the authors report a conflict of interest related to research presented in this article.
Supplemental Tables 1–6 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
References
- 1. Messenger AJ, Barclay R.. Bacteria, iron and pathogenicity. Biochem Educ 1983;11:54–63. [Google Scholar]
- 2. Drakesmith H, Prentice AM. Hepcidin and the iron–infection axis. Science (80-) 2012;338:768–72. [DOI] [PubMed] [Google Scholar]
- 3. Clarke TE, Tari LW, Vogel HJ. Structural biology of bacterial iron uptake systems. Curr Top Med Chem 2001;1:7–30. [DOI] [PubMed] [Google Scholar]
- 4. Krewulak KD, Vogel HJ. Structural biology of bacterial iron uptake. Biochim Biophys Acta Biomembr 2008;1778:1781–804. [DOI] [PubMed] [Google Scholar]
- 5. WHO. The global prevalence of anaemia in 2011. WHO Report. Geneva: WHO; 2015. [Google Scholar]
- 6. Soofi S, Habib MA, Bhutta Z, I. H Evaluation of zinc status and community perceptions in Pakistan: The National Nutrition Survey 2011. Ann Nutr Metab 2013;63:207. [Google Scholar]
- 7. Mahoney DH. Anemia in at-risk populations—what should be our focus? Am J Clin Nutr 2008;88:1457–8. [DOI] [PubMed] [Google Scholar]
- 8. Domellöf M. Iron requirements, absorption and metabolism in infancy and childhood. Curr Opin Clin Nutr Metab Care 2007;10:329–35. [DOI] [PubMed] [Google Scholar]
- 9. Todorich B, Pasquini JM, Garcia CI, Paez PM, Connor JR. Oligodendrocytes and myelination: The role of iron. GLIA 2009;57:467–78. [DOI] [PubMed] [Google Scholar]
- 10. Kim J, Wessling-Resnick M. Iron and mechanisms of emotional behavior. J Nutr Biochem 2014;25:1101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Youdim MB, Ben-Shachar D, Ashkenazi R, Yehuda S. Brain iron and dopamine receptor function. Adv Biochem Psychopharmacol 1983;37:309–21. [PubMed] [Google Scholar]
- 12. Gera T, Sachdev HPS. Effect of iron supplementation on incidence of infectious illness in children: Systematic review. BMJ 2002;325:1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang C-J, Lin J-F, Chang H-H, Lee G-A, Hung C-F. Lutein protects against methotrexate-induced and reactive oxygen species-mediated apoptotic cell injury of IEC-6 cells. PLoS One 2013;8:e72553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harel A, Bromberg Y, Falkowski PG, Bhattacharya D. Evolutionary history of redox metal-binding domains across the tree of life. Proc Natl Acad Sci 2014;111:7042–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Natoli M, Felsani A, Ferruzza S, Sambuy Y, Canali R, Scarino ML. Mechanisms of defence from Fe(II) toxicity in human intestinal Caco-2 cells. Toxicol Vitr 2009;23:1510–5. [DOI] [PubMed] [Google Scholar]
- 16. Ferruzza S, Scarino ML, Gambling L, Natella F, Sambuy Y. Biphasic effect of iron on human intestinal Caco-2 cells: Early effect on tight junction permeability with delayed onset of oxidative cytotoxic damage. Cell Mol Biol 2003;49:89–99. [PubMed] [Google Scholar]
- 17. Jaeggi T, Kortman GAM, Moretti D, Chassard C, Holding P, Dostal A, Boekhorst J, Timmerman HM, Swinkels DW, Tjalsma H et al.. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 2014;64:731–42. [DOI] [PubMed] [Google Scholar]
- 18. Zimmermann MB, Chassard C, Rohner F, N'Goran EK, Nindjin C, Dostal A, Utzinger J, Ghattas H, Lacroix C, Hurrell RF. The effects of iron fortification on the gut microbiota in African children: A randomized controlled trial in Côte d'Ivoire. Am J Clin Nutr 2010;92:1406–15. [DOI] [PubMed] [Google Scholar]
- 19. Felis GE, Pot B. The family Lactobacillaceae. Lactic acid bacteria. Chichester, UK: John Wiley & Sons; 2014. p. 245–7. [Google Scholar]
- 20. Paganini D, Zimmermann MB. The effects of iron fortification and supplementation on the gut microbiome and diarrhea in infants and children: A review. Am J Clin Nutr 2017;106:1688S–93S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr 2009;12:444. [DOI] [PubMed] [Google Scholar]
- 22. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paganini D, Uyoga MA, Kortman GAM, Cercamondi CI, Moretti D, Barth-Jaeggi T, Schwab C, Boekhorst J, Timmerman HM, Lacroix C et al.. Prebiotic galacto-oligosaccharides mitigate the adverse effects of iron fortification on the gut microbiome: A randomised controlled study in Kenyan infants. Gut 2017;66:1956–67. [DOI] [PubMed] [Google Scholar]
- 24. Huang X, Lin J, Demner-Fushman D. Evaluation of PICO as a knowledge representation for clinical questions. AMIA Annu Symp Proc 2006;2006:359–63. [PMC free article] [PubMed] [Google Scholar]
- 25. Fischer Walker C, Black RE. Zinc and the risk for infectious disease. Annu Rev Nutr 2004;24:255–75. [DOI] [PubMed] [Google Scholar]
- 26. Mayo-Wilson E, Junior JA, Imdad A, Dean S, Chan XHS, Chan ES, Jaswal A, Bhutta ZA. Zinc supplementation for preventing mortality, morbidity, and growth failure in children aged 6 months to 12 years of age. Cochrane Database Syst Rev 2014;5:CD009384. [DOI] [PubMed] [Google Scholar]
- 27. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JAC. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dewey KG, Domellof M, Cohen RJ, Rivera LL, Hernell O, Lonnerdal B. Iron supplementation affects growth and morbidity of breast-fed infants: Results of a randomized trial in Sweden and Honduras. J Nutr 2002;132:3249–55. [DOI] [PubMed] [Google Scholar]
- 29. Menon P, Ruel MT, Loechl CU, Arimond M, Habicht J-P, Pelto G, Michaud L. Micronutrient sprinkles reduce anemia among 9- to 24-mo-old children when delivered through an integrated health and nutrition program in rural Haiti. J Nutr 2007;137:1023–30. [DOI] [PubMed] [Google Scholar]
- 30. Soofi S, Cousens S, Iqbal SP, Akhund T, Khan J, Ahmed I, Zaidi AKM, Bhutta ZA. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: A cluster-randomised trial. Lancet 2013;382:29–40. [DOI] [PubMed] [Google Scholar]
- 31. Chen K, Zhang X, Li TY, Chen L, Wei XP, Qu P, Liu YX. Effect of vitamin A, vitamin A plus iron and multiple micronutrient-fortified seasoning powder on infectious morbidity of preschool children. Nutrition 2011;27:428–34. [DOI] [PubMed] [Google Scholar]
- 32. Luabeya KKA, Mpontshane N, Mackay M, Ward H, Elson I, Chhagan M, Tomkins A, van den Broeck J, Bennish ML. Zinc or multiple micronutrient supplementation to reduce diarrhea and respiratory disease in South African children: A randomized controlled trial. PLoS One 2007;2:e541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lemaire M, Islam QS, Shen H, Khan MA, Parveen M, Abedin F, Haseen F, Hyder Z, Cook RJ, Zlotkin SH. Iron-containing micronutrient powder provided to children with moderate-to-severe malnutrition increases hemoglobin concentrations but not the risk of infectious morbidity: A randomized, double-blind, placebo-controlled, noninferiority safety trial. Am J Clin Nutr 2011;94:585–93. [DOI] [PubMed] [Google Scholar]
- 34. Chang S, El Arifeen S, Bari S, Wahed MA, Rahman KM, Rahman MT, Mahmud ABA, Begum N, Zaman K, Baqui AH et al.. Supplementing iron and zinc: Double blind, randomized evaluation of separate or combined delivery. Eur J Clin Nutr 2010;64:153–60. [DOI] [PubMed] [Google Scholar]
- 35. Mitra AK, Akramuzzaman SM, Fuchs GJ, Rahman MM, Mahalanabis D. Long-term oral supplementation with iron is not harmful for young children in a poor community of Bangladesh. J Nutr 1997;127:1451–5. [DOI] [PubMed] [Google Scholar]
- 36. Baqui AH, Zaman K, Persson LA, El-Arifeen S, Yunus M, Begum N, Black RE. Simultaneous weekly supplementation of iron and zinc is associated with lower morbidity due to diarrhea and acute lower respiratory infection in Bangladeshi infants. J Nutr 2003;133:4150–7. [DOI] [PubMed] [Google Scholar]
- 37. Moher D, Schulz K, Altman D; CONSORT Group. The CONSORT statement: Revised recommendations for improving the quality of reports of parallel-group randomised trials. Clin Oral Investig 2003;7:2–7. [DOI] [PubMed] [Google Scholar]
- 38. Abdelrazik N, Al-Haggar M, Al-Marsafawy H, Abdel-Hadi H, Al-Baz R, Mostafa A-H. Impact of long-term oral iron supplementation in breast-fed infants. Indian J Pediatr 2007;74:739–45 [DOI] [PubMed] [Google Scholar]
- 39. WHO. Guideline: Daily iron supplementation in infants and children. Geneva: WHO; 2016. [PubMed] [Google Scholar]
- 40. WHO. Use of multiple micronutrient powders for point-of-use fortification of foods consumed by infants and young children aged 6–23 months and children aged 2–12 years. Geneva: WHO; 2016. [PubMed] [Google Scholar]
- 41. Murphy MS. Management of bloody diarrhoea in children in primary care. BMJ 2008;336:1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tobe-Gai R, Mori R. Micronutrient powders for young children. Lancet 2013;382:1171–2. [DOI] [PubMed] [Google Scholar]
- 43. Graves R, Weaver SP. Cefdinir-associated “bloody stools” in an infant. J Am Board Fam Med 2008;21:246–8. [DOI] [PubMed] [Google Scholar]
- 44. Lieberthal AS, Carroll AE, Chonmaitree T, Ganiats TG, Hoberman A, Jackson MA, Joffe MD, Miller DT, Rosenfeld RM, Sevilla XD et al.. The diagnosis and management of acute otitis media. Pediatrics 2013;131:e964–99. [DOI] [PubMed] [Google Scholar]
- 45. Bisno AL. Primary care: Acute pharyngitis. N Engl J Med 2001;344:205–11. [DOI] [PubMed] [Google Scholar]
- 46. Slavin RG, Spector SL, Bernstein IL, Kaliner MA, Kennedy DW, Virant FS, Wald ER, Khan DA, Blessing-Moore J, Lang DM et al.. The diagnosis and management of sinusitis: A practice parameter update. J Allergy Clin Immunol 2005;116:S13–47 [DOI] [PubMed] [Google Scholar]
- 47. Leibovitz E, Broides A, Greenberg D, Newman N. Current management of pediatric acute otitis media. Expert Rev Anti Infect Ther 2010;8:151–61. [DOI] [PubMed] [Google Scholar]
- 48. Lancaster J, Sylvia LM, Schainker E. Nonbloody, red stools from coadministration of cefdinir and iron-supplemented infant formulas. Pharmacotherapy 2008;28:678–81. [DOI] [PubMed] [Google Scholar]
- 49. Nelson JS. Red stools and omnicef. J Pediatr 2000;136:853–4. [DOI] [PubMed] [Google Scholar]
- 50. Bowlware KL, McCracken GH, Lozano-Hernandez J, Ghaffar F. Cefdinir pharmacokinetics and tolerability in children receiving 25 mg/kg once daily. Pediatr Infect Dis J 2006;25:208–10. [DOI] [PubMed] [Google Scholar]
- 51. McWilliams CJ, Goldman RD. Update on acute otitis media in children younger than 2 years of age. Can Fam Physician 2011;57:1283–5. [PMC free article] [PubMed] [Google Scholar]
- 52. Cancelo-Hidalgo MJ, Castelo-Branco C, Palacios S, Haya-Palazuelos J, Ciria-Recasens M, Manasanch J, Pérez-Edo L. Tolerability of different oral iron supplements: A systematic review. Curr Med Res Opin 2013;29:291–303. [DOI] [PubMed] [Google Scholar]
- 53. Nilson Alberto Piza J. Food fortification: A tool for fighting hidden hunger. Food Nutr Bull 1998;19:49–60. [Google Scholar]
- 54. Finnegan S, Percival SL.. EDTA: An antimicrobial and antibiofilm agent for use in wound care. Adv Wound Care 2015;4:415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Leive L. Release of lipopolysaccharide by EDTA treatment of E.coli. Biochem Biophys Res Commun 1965;21:290–6. [DOI] [PubMed] [Google Scholar]
- 56. Bothwell TH, MacPhail AP. The potential role of NaFeEDTA as an iron fortificant. Int J Vitam Nutr Res 2004;74:421–34. [DOI] [PubMed] [Google Scholar]
- 57. Hurrell RF, Reddy MB, Burri J, Cook JD. An evaluation of EDTA compounds for iron fortification of cereal-based foods. Br J Nutr 2000;84:903–10. [PubMed] [Google Scholar]
- 58. Hurrell R, Bothwell T, Cook JD, Dary O, Davidsson L, Fairweather-Tait S, Hallberg L, Lynch S, Rosado J, Walter T et al.. The usefulness of elemental iron for cereal flour fortification: A SUSTAIN Task Force report. Nutr Rev 2002;60:391–406. [DOI] [PubMed] [Google Scholar]
- 59. Galetti V, Kujinga P, Mitchikpe CES, Zeder C, Tay F, Tossou F, Hounhouigan JD, Zimmermann MB, Moretti D. Efficacy of highly bioavailable zinc from fortified water: A randomized controlled trial in rural Beninese children. Am J Clin Nutr 2015;102:1238–48. [DOI] [PubMed] [Google Scholar]
- 60. De-Regil LM, Suchdev PS, Vist GE, Walleser S, Peña-Rosas JP. Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age (Review). Evid Based Child Health 2013;8:112–201 [DOI] [PubMed] [Google Scholar]
- 61. Light R, Smith P.. Accumulating evidence: Procedures for resolving contradictions among different research studies. Harv Educ Rev 1971;41:429–71. [Google Scholar]
- 62. Christofides A, Schauer C, Sharieff W, Zlotkin SH. Acceptability of micronutrient sprinkles: A new food-based approach for delivering iron to First Nations and Inuit children in Northern Canada. Chronic Dis Can 2005;26:114–20. [PubMed] [Google Scholar]
- 63. Bushman BJ, Wang MC. Vote-counting procedures in meta-analysis, in Handbook of research synthesis and meta-analysis. Cooper H. (ed.) New York: Russell Sage Foundation; 2009, pp. 207–20. [Google Scholar]
- 64. Conover WJ. Practical nonparametric statistics. New York: Wiley; 1980. 120 p. [Google Scholar]
- 65. Dainty JR, Berry R, Lynch SR, Harvey LJ, Fairweather-Tait SJ. Estimation of dietary iron bioavailability from food iron intake and iron status. PLoS One 2014;9:e111824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bannerman RM. Genetic defects of iron transport. Fed Proc 1976;35:2281–5. [PubMed] [Google Scholar]
- 67. Barth-Jaeggi T, Moretti D, Kvalsvig J, Holding PA, Njenga J, Mwangi A, Chhagan MK, Lacroix C, Zimmermann MB. In-home fortification with 2.5 mg iron as NaFeEDTA does not reduce anaemia but increases weight gain: A randomised controlled trial in Kenyan infants. Matern Child Nutr 2015;11 Suppl 4:151–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Giovannini M, Sala D, Usuelli M, Livio L, Francescato G, Braga M, Radaelli G, Riva E. Double-blind, placebo-controlled trial comparing effects of supplementation with two different combinations of micronutrients delivered as sprinkles on growth, anemia, and iron deficiency in Cambodian infants. J Pediatr Gastroenterol Nutr 2006;42:306–12. [DOI] [PubMed] [Google Scholar]
- 69. Javaid N, Haschke F, Pietschnig B, Schuster E, Huemer C, Shebaz A, Ganesh P, Steffan I, Hurrel R, Secretin M. Interactions between infections, malnutrition and iron nutritional status in Pakistani infants. Acta Paediatr Scand Suppl 1991;374:141–50. [DOI] [PubMed] [Google Scholar]
- 70. Soofi S, Cousens S, Iqbal SP, Akhund T, Khan J, Ahmed I, Zaidi AK, Bhutta ZA. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: A cluster-randomised trial. Lancet 2013;382:29–40. [DOI] [PubMed] [Google Scholar]
- 71. Chen K, Chen XR, Zhang L, Luo HY, Gao N, Wang J, Fu GY, Mao M. Effect of simultaneous supplementation of vitamin A and iron on diarrheal and respiratory tract infection in preschool children in Chengdu City, China. Nutrition 2013;29:1197–203. [DOI] [PubMed] [Google Scholar]
- 72. Richard SA, Zavaleta N, Caulfield LE, Black RE, Witzig RS, Shankar AH. Zinc and iron supplementation and malaria, diarrhea, and respiratory infections in children in the Peruvian Amazon. Am J Trop Med Hyg 2006;75:126–32. [DOI] [PubMed] [Google Scholar]
- 73. Rosado L, Allen H. Zinc supplementation reduced morbidity, but neither zinc nor iron supplementation affected growth or body composition of Mexican preschoolers. Am J Clin Nutr 1997;65:13–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.