Abstract
Aims
Patients with acute pulmonary embolism (PE) classified as low risk by the Pulmonary Embolism Severity Index (PESI), its simplified version (sPESI), or the Hestia criteria may be considered for early discharge. We investigated whether the presence of right ventricular (RV) dysfunction may aggravate the early prognosis of these patients.
Methods and results
We did a systematic review and meta-analysis of studies including low-risk patients with acute PE to investigate the prognostic value of RV dysfunction. Diagnosis of RV dysfunction was based on echocardiography or computed tomography pulmonary angiography. In addition, we investigated the prognostic value of elevated troponin or natriuretic peptide levels. The primary outcome was all-cause mortality at 30 days or during hospitalization. We included 22 studies (N = 3295 low-risk patients) in the systematic review: 21 were selected for quantitative analysis. Early all-cause mortality rates in patients with vs. without RV dysfunction on imaging were 1.8% [95% confidence interval (CI) 0.9–3.5%] vs. 0.2% (95% CI 0.03–1.7%), respectively, [odds ratio (OR) 4.19, 95% CI 1.39–12.58]. For troponins, rates were 3.8% (95% CI 2.1–6.8%) vs. 0.5% (95% CI 0.2–1.3%), (OR 6.25, 95% CI 1.95–20.05). For natriuretic peptides, only data on early PE-related mortality were available: rates were 1.7% (95% CI 0.4–6.9%) vs. 0.4% (95% CI 0.1–1.1%), (OR 3.71, 95% CI 0.81–17.02).
Conclusions
In low-risk patients with acute PE, the presence of RV dysfunction on admission was associated with early mortality. Our results may have implications for the management of patients who appear at low risk based on clinical criteria alone, but present with RV dysfunction as indicated by imaging findings or laboratory markers.
Keywords: Pulmonary embolism, Home treatment, Risk stratification, Right ventricular dysfunction, Mortality, Anticoagulation
See page 911 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz018)
Introduction
Acute pulmonary embolism (PE) contributes to the global burden of cardiovascular disease in terms of morbidity, mortality, and financial impact on healthcare systems.1,2 Current management strategies are tailored to the patient’s risk of early death or other serious early complications. In the 2014 European Society of Cardiology (ESC) Guidelines, risk stratification of patients with acute PE is based on three sets of criteria: (i) haemodynamic status and clinical characteristics at baseline, (ii) findings of right ventricular (RV) dysfunction on imaging, notably echocardiography or computed tomography pulmonary angiography (CTPA), and (iii) elevation of cardiac biomarkers, particularly cardiac troponins and natriuretic peptides, indicating myocardial injury and (right) heart failure, respectively.1
A reliable risk classification of PE has immediate clinical implications, notably with regard to the decision on whether to hospitalize the patient or discharge him/her to ambulatory treatment. Home treatment may improve satisfaction with treatment and more generally the quality of life of patients with acute PE; in addition, it may help to reduce costs as well as potential health risks related to unnecessary hospitalization. This is particularly important in view of the fact that, in many countries, patients with acute PE are still hospitalized for several days.3–5 Cohort studies and randomized controlled trials, which investigated the efficacy and safety of ambulatory PE treatment in the vitamin K antagonist (VKA) era, used mainly clinical severity scores for patient selection.6–9 These include the Pulmonary Embolism Severity Index (PESI), its simplified version (sPESI), and the Hestia criteria. In the years to come, non-VKA oral anticoagulants may facilitate early discharge and outpatient treatment for selected patients with acute PE. Consequently, the 2014 ESC Guidelines on the management of acute PE proposed an algorithm, in which a low clinical risk based on the PESI (or sPESI) alone could justify early discharge without the need for further testing.1 However, caution is warranted and maximum safety is imperative when judging a patient to be at sufficiently low risk to be released from medical attention. In this context, accumulating reports appear to suggest that standardized clinical severity scores alone might be insufficient to exclude subclinical RV dysfunction and thus an intermediate risk of early complications.8,10,11 Therefore, we conducted the present systematic review and study-level meta-analysis to determine the prognostic relevance of RV dysfunction resulting from RV pressure overload, detected on echocardiography or CTPA, or of elevated cardiac biomarkers, in patients with acute PE classified into the low-risk category according to current clinical severity scores.
Methods
Literature search and study selection
We conducted a systematic search of the literature for publications in PubMed or Web of Science between October 2008 and May 2018, that is after the derivation and validation of the PESI12 and the publication of the 2008 ESC guidelines on acute PE,13 in which an advanced concept of risk stratification was first introduced. We performed an additional manual search of potentially eligible studies within references of the included studies, international guidelines, relevant (systematic) reviews, and derivation/validation studies of prognostic scores. The literature search strategy is available as Supplementary material online.
Search results were screened independently by two reviewers for the relevance of titles/abstracts and full-texts of the studies fulfilling the inclusion criteria. Potential disagreements were solved by a third reviewer. The authors of publications with missing data were contacted at least twice within a 2-week period. The present work was performed in accordance with the PRISMA and MOOSE guidelines.14,15 We registered the study protocol in PROSPERO (CRD42018103926).
Eligibility criteria
Studies were considered eligible if they fulfilled all the following criteria:
They included normotensive patients (or provided subgroup data for this category of patients) not selected based on the presence/absence of RV dysfunction, with an objectively confirmed diagnosis of acute PE, and categorized as being at low risk according to either the (s)PESI (PESI Classes I or II, PESI < 86 points, or sPESI = 0) or Hestia (all criteria absent);
The study evaluated the prognostic role of RV dysfunction on admission, here primarily defined as the presence of signs of RV pressure overload on imaging tests (echocardiography or CTPA) among low-risk patients, or, alternatively, as myocardial injury based on elevated cardiac troponins or natriuretic peptides.
At least one of the outcomes of interest was assessed during short-term (either at 30 days or, if not available, in-hospital) or 90-day follow-up.
The description of the items that are part of the clinical scores are summarized in the Supplementary material online. Two authors independently assessed the quality of studies focusing on clinical outcomes in accordance with the Newcastle–Ottawa Scale for cohort studies.16
Right ventricular dysfunction and elevated cardiac biomarkers
Visualization of RV dysfunction (due to pressure overload) at presentation was based on echocardiography or CTPA. Since the definition of the parameters of RV dysfunction, and the cut-off values that were used, varied over time, we included studies requiring at least one of the following criteria: dilatation of the right ventricle (e.g. diastolic diameter ≥30 mm in the parasternal short-axis view) on echocardiography or an elevated RV/left ventricular end-diastolic diameter ratio (cut-off of 0.9 or 1.0) on echocardiography, or CTPA; hypokinesis of the RV free wall or abnormal motion of the interventricular septum on echocardiography; tricuspid valve regurgitation velocity (cut-off 2.7 or 2.8 m/s) on echocardiography.1,17
We further defined myocardial injury and (right) heart failure based on elevated circulating levels of cardiac troponins [troponin I (TnI) or T (TnT), by standard or high-sensitivity (hs) assays] and natriuretic peptides [B-type natriuretic peptide (BNP), N-terminal pro B-type natriuretic peptide (NT-proBNP)], respectively. Since current ESC guidelines do not recommend specific assays or cut-offs, we based our classification on the cut-offs reported in the selected studies.1
Study outcomes
We defined early all-cause mortality, i.e. death during the hospital stay or within the first 30 days following diagnosis of acute PE, as the primary outcome of our meta-analysis. The secondary outcomes were (i) early PE-related adverse outcome, defined as (PE-related) mortality, haemodynamic collapse (with need for cardiopulmonary resuscitation, mechanical ventilation, or catecholamine administration), or recurrent venous thromboembolism (VTE); (ii) early PE-related mortality; and (iii) long-term all-cause mortality.
Statistical analysis
We calculated pooled odds ratios (ORs), weighed rates, and absolute risk differences between groups of patients with vs. without RV dysfunction applying a random-effect model [95% confidence interval (CI)]. An exact binomial approach was preferred since the proportions were expected to be close to the low boundary. We assessed (statistical) heterogeneity of exposure effects by calculating the I2 statistic, which summarizes the amount of variance among studies beyond chance. Heterogeneity was defined as low (I2 < 25%), moderate (I2 = 25–75%), or high (I2 > 75%). The presence of publication bias was evaluated by visually inspecting funnel plots if >10 studies were analysed. We performed predefined subgroup analyses investigating the prognostic value of different cut-offs and tests, the rate of complications in inpatient vs. outpatient, and differences between studies grouped by study design (prospective or management studies vs. post hoc analyses). RevMan 5.3 (Cochrane Collaboration) and R 3.4.3 (mada, metafor) served for data analysis.
Results
Systematic review of the literature
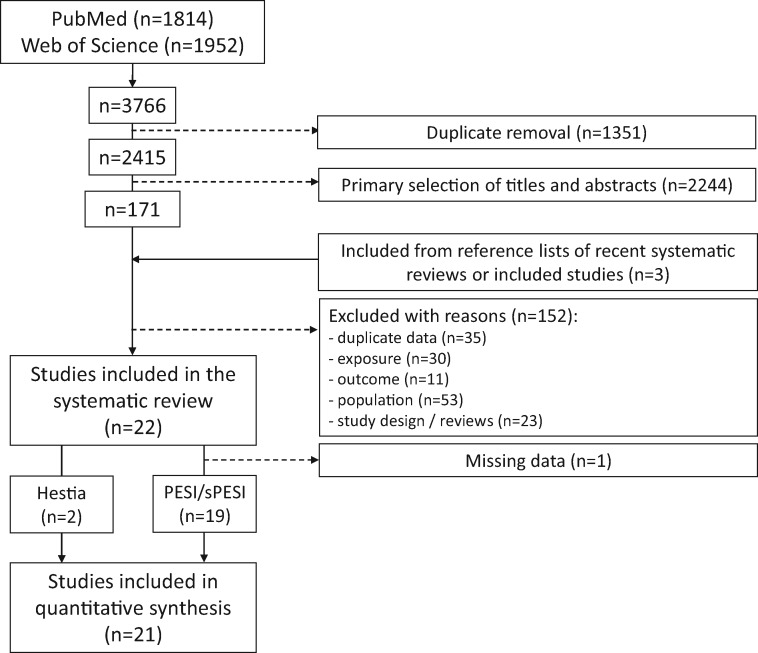
The literature search identified 1814 records in PubMed and 1952 in Web of Science, leaving 2415 records after duplicate removing. The process of study selection is summarized in Figure 1. We identified three additional studies not retrieved by our initial search.18–20 Eventually, we included 22 studies in the systematic review for a total of 7536 patients diagnosed with acute PE (Table 1). Of these patients, 3295 were classified as being at low risk according to the PESI [1516/3265 (46%)], the sPESI [932/2639 (35%)], or the Hestia criteria [847/1632 (52%)]. Additional information on the individual studies included in the systematic review of the literature is shown in the Supplementary material online, Tables S1 and S2. The study outcomes and quality assessment are summarized in the Supplementary material online, Tables S3 and S4.
Figure 1.
Study selection.
Table 1.
Characteristics of the studies included after systematic review of the literature
| Study | N | Low risk, n (%) | Men, n (%) | Age (standard deviation or interquartile range) | Early discharge, n (%) | Exposure | Study population |
|---|---|---|---|---|---|---|---|
| Erol et al.18 | 110 | 34 (31), sPESI | 14 (42) | 47 (range 21–79) | 0 | RV dysfunction (echo), TnI, NT-proBNP | Pneumology Department |
| Singanayagam et al.20 | 585 | 288 (49), PESI | 251 (43)a | 55% >65 yearsa | — | RV dysfunction (CTPA) | Without known thrombophilia, haematological malignancy, or ongoing anticoagulation (Emergency Room) |
| Palmieri et al.19 | 89 | 27 (30), PESI | 6 (22) | 24 (18) PESI I, 57 (21) PESI II | — | RV dysfunction (echo), Troponin | Acute central without renal failure, recent acute coronary syndrome, or haemodynamic instability on admission (Emergency Room) |
| PROTECT27,28 | 848 | 313 (37), sPESI | 416 (49)a | 72 (59–80)a | — | RV dysfunction (echo and CTPA), BNP | Without haemodynamic instability, life expectancy <3 months, pregnancy, geographic inaccessibility |
| PREP21 | 529 | 329 (62), PESI | 247 (47)a | 67 (52–77)a | — | RV dysfunction (echo), TnI, BNP | Patients not anticoagulated for >24 h or with cardiogenic shock at admission |
| Côté et al.11 | 779 | 779 (100), sPESI | 347 (45)a | 61 (44–72)a | — | RV dysfunction (CTPA) | Patients from PREP study, PROTECT study, and consecutive patients from a single-centre prospective registry |
| Hakemi et al.22 | 298 | 173 (58), PESI | 151 (51)a | 56 (13)a | — | hsTnI | Patients admitted for acute PE |
| Lankeit et al.29 | 526 | 198 (37), sPESI | 266 (51)a | 71 (55–79)a | — | TnI | Normotensive patients at presentation; PERGO, PROTECT, and Polish cohorts |
| Lankeit et al.30 | 688 | 258 (38), sPESI | 326 (47)a | 70 (54–78)a | — | NT-proBNP | |
| Vanni et al.23 | 540 | 145 (31), PESI | 65 (45) | 73% >65 years | — | RV dysfunction (echo) | Emergency Room |
| Kartal et al.31 | 68 | 30 (44), sPESI | 30 (44)a | 60 (18)a | 23 (34) | TnI | Without prior pulmonary hypertension, suboptimal imaging tests, and pregnancy (Emergency Room) |
| Moores et al.24 | 567 | 191 (34), PESI | 245 (43)a | 74% >65 yearsa | 191 (100) | TnI | Outpatients (Emergency Room) |
| Ozsu et al.32 | 121 | 45 (38), sPESI | 52 (43)a | 70 (55–76)a | — | hsTnT, TnT | Emergency Room |
| Ozsu et al.33 | 206 | 59 (29), sPESI | 82 (40)a | 71 (58–80) | 31 (15) | TnT | Emergency Room |
| SWIVTER34 | 369 | 106 (29), sPESI | 66 (62) | 72 (13) | 49 (46) | TnI, TnT, hsTnT | With available troponin measurement |
| Polo Friz et al.35 | 106 | 34 (32), sPESI | 35 (33)a | 74 (14)a | — | hsTNT | Emergency Department |
| Weekes et al.36 | 123 | 53 (43), sPESI | 63 (51)a | 59 (43–69)a | — | RV dysfunction (CTPA), TnI, BNP | Emergency Room |
| Choi et al.25 | 657 | 363 (64), PESI | 295 (45) | 68 | — | RV dysfunction (echo), TnI, NT-proBNP | Hospitalized because of acute PE |
| HESTIA38 | 530 | 297 (56), Hestia | 159 (58) | 55 (16) | 275 (100) | RV dysfunction (echo) | Selected for anticoagulant treatment at home |
| VESTA37 | 1102 | 550 (50), Hestia | 297 (54) | 54 (15) | 275 (100) | NT-proBNP | Selected for anticoagulant treatment at home, life expectancy >3 months |
| Systematic review only | |||||||
| Singanayagam et al.26 | 411 | 214 (52), PESI | 177 (43)a | 55% >65 yearsa | — | TnI | First PE; without thrombophilia, haematological malignancy, or ongoing anticoagulation (Emergency Room) |
BNP, B-type natriuretic peptide; CTPA, computed tomography pulmonary angiography; hsTnI, (high sensitivity) troponin I; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PE, pulmonary embolism; RV, right ventricular; (s)PESI, (simplified) pulmonary embolism severity index; TnT, troponinT.
Baseline characteristics referring to the overall study population.
Twenty cohort studies adopted PESI19–26 or sPESI11,18,27–36 for risk stratification of the patients, while two adopted the Hestia criteria.37,38 One study did not provide complete information on outcomes, and it was therefore excluded from the meta-analysis.26 Eventually, a total of 21 studies were selected for the quantitative analysis.11,18–25,27–36
Early all-cause mortality
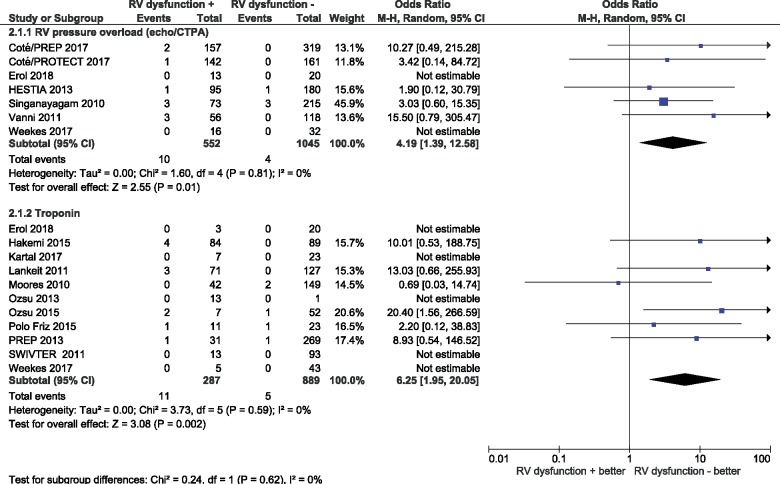
Among patients classified as being at low risk by the PESI, sPESI, or the Hestia criteria, 34% (95% CI 30–39%) were reported to have signs of RV dysfunction on echocardiography or CTPA (n = 9 studies, n = 1987 patients). Of these, seven studies (n = 1597 patients) provided data on early all-cause mortality. As shown in Table 2 and Figure 2, early all-cause mortality rates of this ‘low-risk’ population were 1.8% (95% CI 0.9–3.5%) and 0.2% (0.03–1.7%) in the presence and absence of RV dysfunction, respectively, corresponding to an OR of 4.19 (95% CI 1.39–12.58; I2 0%; Figure 3).
Table 2.
Rates of short-term adverse events in low-risk patients with or without imaging and laboratory indicators of right ventricular dysfunction or myocardial injury
| RV dysfunction (exposure) | Study population (n studies) | With RV dysfunction, % (95% CI) | Without RV dysfunction, % (95% CI) | |
|---|---|---|---|---|
| Early all-cause mortality | RV pressure overload (echo/CTPA) | 1597 (7) | 1.8 (0.9–3.5) | 0.2 (0.03–1.7) |
| Troponin | 1176 (11) | 3.8 (2.1–6.8) | 0.5 (0.2–1.3) | |
| BNP/NT-proBNP | — | — | — | |
| Early PE-related adverse outcome | RV pressure overload (echo/CTPA) | 1488 (6) | 3.7 (0.9–14.4) | 0.7 (0.06–6.4) |
| Troponin | 1137 (8) | 10.2 (7.2–14.3) | 0.6 (0.1–5.6) | |
| BNP/NT-proBNP | 1405 (6) | 5.4 (1.8–14.6) | 1.3 (0.6–2.6) |
The PE-related adverse outcome includes PE-related mortality, haemodynamic collapse, and/or recurrent venous thromboembolism.
BNP, B-type natriuretic peptide; CTPA, computed tomography pulmonary angiography; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PE, pulmonary embolism; RV, right ventricular.
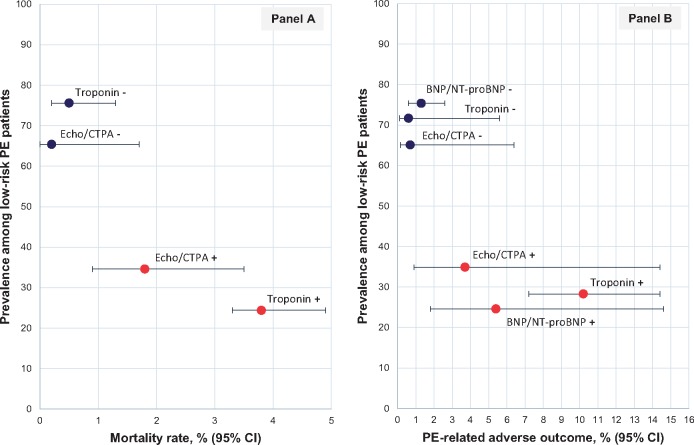
Figure 2.
(A) Early mortality and early pulmonary embolism-related adverse outcome rates in patients with vs. without right ventricular dysfunction. An early pulmonary embolism-related adverse outcome (B) was defined as early death, haemodynamic collapse, and/or recurrent venous thromboembolism. Red dots (with corresponding confidence intervals) represent estimates in the presence of right ventricular dysfunction; blue dots, in its absence. BNP, B-type natriuretic peptide; CTPA, computed tomography pulmonary angiography; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PE, pulmonary embolism; 95% CI, 95% confidence interval.
Figure 3.
Prognostic value of imaging and laboratory indicators of right ventricular dysfunction or myocardial injury for early all-cause mortality in low-risk patients. CTPA, computed tomography pulmonary angiography; RV, right ventricular; 95% CI, 95% confidence interval.
Among low-risk PE patients based on the PESI or sPESI, 26% (95% CI 17–39%) tested positive for cardiac troponin I or T (n = 13 studies, n = 1458 patients). Of these, eleven studies (n = 1176 patients) provided data on early all-cause mortality. Early all-cause mortality rates were 3.8% (95% CI 2.1–6.8%) and 0.5% (95% CI 0.2–1.3%) in patients with and without elevated troponin levels, respectively, corresponding to an OR of 6.25 (95% CI 1.95–20.05; I2 0%). The corresponding proportions of low-risk patients testing positive for BNP and NT-proBNP were 30% (95% CI 26–33%) and 23% (95% CI 12–40%) of tested (n = 7 studies, n = 1663 patients). No data on all-cause mortality were reported in these latter studies.
Early pulmonary embolism-related outcome
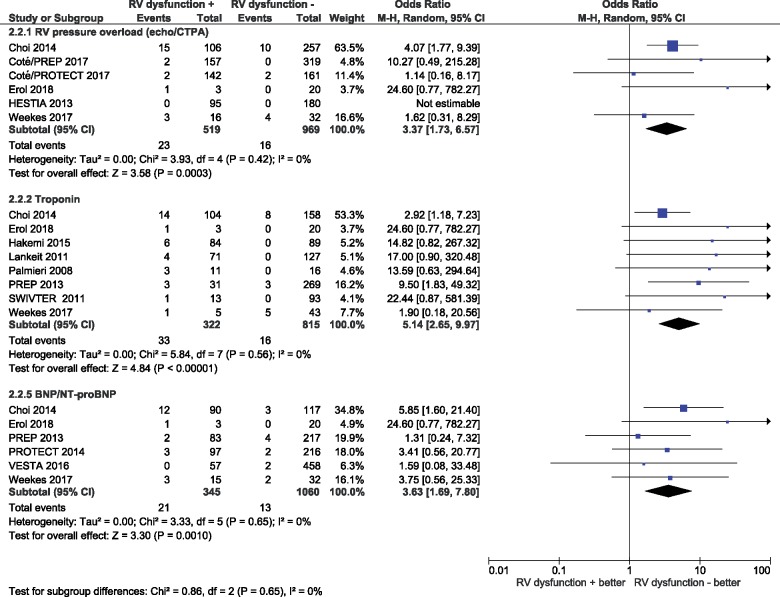
As shown in Table 2 and Figure 2, the rates of an early adverse outcome (PE-related mortality, haemodynamic collapse, or recurrent VTE) were 3.7% (95% CI 0.9–14.4%) and 0.7% (95% CI 0.06–6.4%) in patients with vs. without RV dysfunction on echocardiography or CTPA (n = 6 studies, n = 1488 patients), respectively, corresponding to an OR of 3.37 (95% CI 1.73–6.57; I2 0%; Figure 4).
Figure 4.
Prognostic value of imaging and laboratory indicators of right ventricular dysfunction or myocardial injury for early pulmonary embolism-related adverse outcome in low-risk patients. The outcome includes mortality, haemodynamic collapse, and/or recurrent venous thromboembolism. BNP, B-type natriuretic peptide; CTPA, computed tomography pulmonary angiography; NT-proBNP, N-terminal pro-B-type natriuretic peptide; RV, right ventricular; 95% CI, 95% confidence interval.
In patients with a positive vs. negative cardiac troponin test (n = 8 studies, n = 1137 patients), the corresponding rates were 10.2% (95% CI 7.2–14.3%) vs. 0.6% (95% CI 0.1–5.6%), for an OR of 5.14 (95% CI 2.65–9.97; I2 0%). Finally, for those with BNP/NT-proBNP values above and below the cut-off used (n = 6 studies, n = 1405 patients), the rates were 5.4% (95% CI 1.8–14.6%) and 1.3% (95% CI 0.6–2.6%), respectively, resulting in an OR of 3.63 (95% CI 1.69–7.80; I2 0%).
We calculated separately the pooled rates of early PE-related mortality. These rates were 1.3% (95% CI 0.5–3.1%) vs. 0.02% (95% CI 0.01–21.4%) in patients with and without RV dysfunction on imaging (n = 8 studies, n = 1699 patients), respectively; 1.3% (95% CI 0.3–5.4%) vs. 0.4% (95% CI 0.1–2.0%) in those with and without elevated troponin levels (n = 10 studies, n = 1199 patients); and 1.7% (95% CI 0.4–6.9%) vs. 0.4% (95% CI 0.1–1.1%) in patients with vs. without elevated BNP or NT-proBNP levels (n = 5 studies, n = 1092 patients). Of note, pooled ORs for RV dysfunction on imaging (OR 2.95, 95% CI 1.00–8.65; I2 0%), elevated troponin (OR 1.71, 95% CI 0.50–5.86; I2 0%), and elevated BNP/NT-proBNP (OR 3.71, 95% CI 0.81–17.02; I2 0%) were based on only four, three, and three studies, respectively, since the other studies recorded no events.
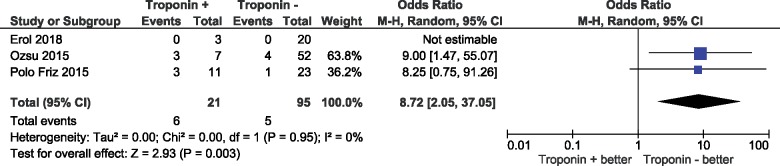
Three-month mortality in patients with low-risk pulmonary embolism
A total of three studies provided data on all-cause mortality at 90 days. Mortality rates in low-risk patients by PESI or sPESI with a positive vs. negative cardiac troponin test were 28.6% (95% CI 13.4–50.8%) and 5.3% (95% CI 2.2–12.0%), respectively, (OR 8.72, 95% CI 2.05–37.05; I2 0%); Figure 5.
Figure 5.
Prognostic value of troponin for 3-month all-cause mortality in patients classified to the low risk category according to the pulmonary embolism severity index (PESI) or (simplified) pulmonary embolism severity index (sPESI).
Publication bias and subgroup analyses
We did not identify publication bias for all-cause mortality, whereas this cannot be excluded for the PE-related adverse outcome (Supplementary material online, Figure S1).
We found no differences in the additive prognostic value of RV dysfunction on imaging vs. that of laboratory biomarkers (P-values for subgroup differences reported in Figures 2 and 3). This was also the case if only studies comparing multiple markers within the same study population were analysed (data not shown). The results after exclusion of the two Hestia studies are provided in the Supplementary material online, Table S5 and Figures S2–S3.
Low-risk patients classified using standardized troponin cut-off levels or high sensitivity assays had a mortality rate similar to that of patients classified by different cut-off levels and assays [absolute risk difference −1.0% (95% CI −3.3% to +0.3%)]. The absolute risk difference of mortality rates for NT-proBNP (cut-off 600 pg/mL) vs. BNP (cut-off 100 pg/mL) was −0.3% (95% CI −1.6% to +1.2%).
Discussion
The results of the present systematic review of the literature and meta-analysis indicate that RV dysfunction, primarily defined by RV pressure overload assessed on imaging tests, may have a significant impact on the early prognosis of patients classified as being at low risk based on the PESI, sPESI, or the Hestia criteria. Similar findings were obtained when the elevation of laboratory cardiac biomarkers, notably cardiac troponin or natriuretic peptides, served as a parameter of myocardial injury and (subclinical) RV dysfunction, respectively. Our results may have implications for the acute-phase management of patients who appear to be at a low risk of early death or severe complications based on clinical criteria alone. These findings also support the concept that optimal risk stratification of acute PE should include the assessment of RV status on admission.
The spectrum of PE severity largely varies and early mortality rates can range from <1% among stable patients to over 50% in the presence of haemodynamic instability.39 Consequently, the management of acute PE is tailored to the patient’s early risk and may include early discharge and home treatment for low-risk patients.17 Risk assessment models are practical and helpful for clinicians, permitting a standardized categorization of patients with acute PE into risk classes.1 In particular, the PESI and sPESI have been extensively validated for the identification of low-risk PE. In a randomized trial, 344 patients with acute PE and a PESI low-risk risk Class of I–II were assigned to outpatient or inpatient treatment; all patients received subcutaneous enoxaparin followed by oral VKA anticoagulation.40 In this study, outpatient care appeared to be non-inferior when compared with standard inpatient treatment. Based on these and other findings, the 2014 guidelines of the ESC proposed that patients with a low PESI or a negative sPESI be considered for home treatment.1 However, the use of clinical scores does not directly take into account the functional status of the right ventricle, which has repeatedly been shown to be a key determinant of PE prognosis.11,41,42 Consequently, it cannot be excluded that patients initially classified into the low-risk category, based on the parameters and cut-off values of a formal clinical severity score calculated on admission, may deteriorate few hours later due to the decompensation of previously unnoticed RV dysfunction.
The results of the present meta-analysis indicate that low-risk patients are at higher risk of early mortality if RV dysfunction is present. Due to the low absolute rate of events in this population, this question could not be adequately addressed by single studies.11,21,24,26,27 Of note, the early death risk of ‘low-risk’ patients with RV dysfunction appears similar to that previously reported for intermediate-risk PE39 The mortality rates yielded by our meta-analysis are clearly higher than those reported for low-risk PE patients in studies that validated the PESI or sPESI,43 and raise doubts on whether such patients could be sent home safely. The paucity of data in the literature regarding event rates after early discharge and home treatment (as opposed to standard in-hospital management), or of data reported in prospective management trials (vs. post hoc analyses) did not allow us to perform additional subgroup analyses. Finally, it must be noted that the association between RV dysfunction and the outcomes was consistent across the studies, at least in terms of statistical heterogeneity (I2 = 0%). However, I2 may have been underestimated due to the imprecision of single studies.
The Hestia criteria have been proposed as an alternative tool for selecting low-risk patients.7 Although some of these criteria are less standardized than the items included in the (s)PESI, this set of questions integrates familial, social, and broad medical factors. In light of this fact, the Hestia criteria and PESI are not directly comparable. In our meta-analysis, we could not provide pooled estimates only for the Hestia criteria due to the paucity of data in the literature. The ongoing Hospitalization or Out-treatment ManagEment of Pulmonary Embolism (HOME-PE) trial (NCT02811237) aspires to directly compare these two strategies.
Limitations
The present meta-analysis has a number of limitations that need to be discussed. First, several of the included studies were post hoc analyses of existing cohorts, having adopted heterogeneous criteria of RV dysfunction as well as non-prespecified cut-off values. On the other hand, it can also be argued that previous cohort studies which validated the value of the PESI, sPESI, or other criteria for defining low risk, may have included pre-selected ‘lower-risk’ populations with PE and were thus able to show such favourable results for clinical assessment alone. Second, we acknowledge that the CIs of our estimates remain wide, despite statistical significance, due to the low absolute rates of early adverse events encountered in this patient population. It is also important to mention that the present study was not designed to test if there is a single optimal biomarker or imaging parameter to quantify the early risk in patients with a low clinical score. Therefore, our results suggesting that RV pressure overload on imaging possesses prognostic value similar to the elevation of cardiac biomarkers must be considered as hypothesis-generating. An ongoing prospective management trial is investigating whether early discharge and home treatment is feasible, effective, and safe in patients who are classified into the low-risk category using the combination of clinical (modified Hestia) criteria and the absence of RV dysfunction or free-floating thrombi on an imaging test.44
Conclusion
In conclusion, in patients with acute PE classified into the low-risk category according to clinical criteria alone, the presence of RV dysfunction was associated with an increased risk of early complications including all-cause death and PE-related serious adverse events. The estimated risk was in the lower range of that previously reported for patients with intermediate-risk PE. Right ventricular pressure overload and dysfunction on imaging appeared to possess a prognostic value comparable to the elevation of cardiac troponins or natriuretic peptides. Our results reinforce the concept of integrating the functional status of the right ventricle into the risk stratification of acute PE.
Supplementary Material
Acknowledgements
We would like to acknowledge the help received from Dr Cha (Kyungpook National University Hospital, Jung-Gu, Daegu, South Korea), Dr Polo Friz (Vimercate Hospital, Italy), Dr Côté (Hôpital de l'Enfant-Jésus du CHU de Québec, Université Laval, Canada), Dr Weekes (Carolinas Medical Center, Charlotte, North Carolina, USA), and Dr Singanayagam (Royal Infirmary of Edinburgh, UK), who retrieved data from the studies they co-authored.
Funding
This work was supported by the German Federal Ministry of Education and Research (BMBF-01EO1003 and 01EO1503 to S.B., S.H.M., and S.V.K.).
Conflict of interest: S.B.: congress and travel payments (Daiichi Sankyo, Bayer HealthCare), lecture honoraria (EKOS Corporation/BTG). S.V.K.: consultancy and lecture honoraria from Bayer HealthCare, Boehringer Ingelheim, Daiichi-Sankyo, and Pfizer-Bristol-Myers-Squibb; payment for travel accommodation/meeting expenses from Bayer HealthCare; institutional grants from Boehringer Ingelheim, Bayer HealthCare, and Daiichi Sankyo. G.M.: travel and accommodation funding from Leo Pharma, Pfizer-Bristol-Myers-Squibb, Daiichi Sankyo and Bayer HealthCare; institutional grants from Leo Pharma and Boehringer Ingelheim. All the other authors declared no conflict of interest.
References
- 1. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk NA, Zamorano JL, Zompatori M.. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism: the task force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC)Endorsed by the European Respiratory Society (ERS). Eur Heart J 2014;35:3033–3073.25173341 [Google Scholar]
- 2. Barco S, Woersching AL, Spyropoulos AC, Piovella F, Mahan CE.. European Union-28: an annualised cost-of-illness model for venous thromboembolism. Thromb Haemost 2016;115:800–808. [DOI] [PubMed] [Google Scholar]
- 3. Dentali F, Di Micco G, Giorgi Pierfranceschi M, Gussoni G, Barillari G, Amitrano M, Fontanella A, Lodigiani C, Guida A, Visona A, Monreal M, Di Micco P.. Rate and duration of hospitalization for deep vein thrombosis and pulmonary embolism in real-world clinical practice. Ann Med 2015;47:546–554. [DOI] [PubMed] [Google Scholar]
- 4. Konstantinides SV. Trends in pulmonary embolism outcomes: are we really making progress? J Am Coll Cardiol 2016;67:171–173. [DOI] [PubMed] [Google Scholar]
- 5. Jimenez D, de Miguel-Diez J, Guijarro R, Trujillo-Santos J, Otero R, Barba R, Muriel A, Meyer G, Yusen RD, Monreal M.. Trends in the management and outcomes of acute pulmonary embolism: analysis from the RIETE registry. J Am Coll Cardiol 2016;67:162–170. [DOI] [PubMed] [Google Scholar]
- 6. Aujesky D, Obrosky DS, Stone RA, Auble TE, Perrier A, Cornuz J, Roy PM, Fine MJ.. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005;172:1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zondag W, Mos IC, Creemers-Schild D, Hoogerbrugge AD, Dekkers OM, Dolsma J, Eijsvogel M, Faber LM, Hofstee HM, Hovens MM, Jonkers GJ, van Kralingen KW, Kruip MJ, Vlasveld T, DE Vreede MJ, Huisman MV.. Outpatient treatment in patients with acute pulmonary embolism: the Hestia study. J Thromb Haemost 2011;9:1500–1507. [DOI] [PubMed] [Google Scholar]
- 8. Otero R, Uresandi F, Jimenez D, Cabezudo MA, Oribe M, Nauffal D, Conget F, Rodriguez C, Cayuela A.. Home treatment in pulmonary embolism. Thromb Res 2010;126:e1–e5. [DOI] [PubMed] [Google Scholar]
- 9. Agterof MJ, Schutgens RE, Snijder RJ, Epping G, Peltenburg HG, Posthuma EF, Hardeman JA, van der Griend R, Koster T, Prins MH, Biesma DH.. Out of hospital treatment of acute pulmonary embolism in patients with a low NT-proBNP level. J Thromb Haemost 2010;8:1235–1241. [DOI] [PubMed] [Google Scholar]
- 10. Hellenkamp K, Kaeberich A, Schwung J, Konstantinides S, Lankeit M.. Risk stratification of normotensive pulmonary embolism based on the sPESI—does it work for all patients? Int J Cardiol 2015;197:162–163. [DOI] [PubMed] [Google Scholar]
- 11. Côté B, Jimenez D, Planquette B, Roche A, Marey J, Pastre J, Meyer G, Sanchez O.. Prognostic value of right ventricular dilatation in patients with low-risk pulmonary embolism. Eur Respir J 2017;50. pii: 1701611. [DOI] [PubMed] [Google Scholar]
- 12. Donze J, Le Gal G, Fine MJ, Roy PM, Sanchez O, Verschuren F, Cornuz J, Meyer G, Perrier A, Righini M, Aujesky D.. Prospective validation of the Pulmonary Embolism Severity Index. A clinical prognostic model for pulmonary embolism. Thromb Haemost 2008;100:943–948. [DOI] [PubMed] [Google Scholar]
- 13. Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galie N, Pruszczyk P, Bengel F, Brady AJ, Ferreira D, Janssens U, Klepetko W, Mayer E, Remy-Jardin M, Bassand JP; ESC Committee for Practice Guidelines (CPG). Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J 2008;29:2276–2315. [DOI] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P.. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB.. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 16. Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (12 December 2018).
- 17. Konstantinides SV, Barco S, Lankeit M, Meyer G.. Management of pulmonary embolism: an update. J Am Coll Cardiol 2016;67:976–990. [DOI] [PubMed] [Google Scholar]
- 18. Erol S, Batum O, Yılmaz, U.. Is advanced risk stratification unnecessary in patients with simplified pulmonary embolism severity index (sPESI) of 0? Araștırma Makalesi 2018;71:55–59. [Google Scholar]
- 19. Palmieri V, Gallotta G, Rendina D, De Bonis S, Russo V, Postiglione A, Martino S, Di Minno MN, Celentano A.. Troponin I and right ventricular dysfunction for risk assessment in patients with nonmassive pulmonary embolism in the Emergency Department in combination with clinically based risk score. Intern Emerg Med 2008;3:131–138. [DOI] [PubMed] [Google Scholar]
- 20. Singanayagam A, Chalmers JD, Scally C, Akram AR, Al-Khairalla MZ, Leitch L, Hill LE, Hill AT.. Right ventricular dilation on CT pulmonary angiogram independently predicts mortality in pulmonary embolism. Respir Med 2010;104:1057–1062. [DOI] [PubMed] [Google Scholar]
- 21. Sanchez O, Trinquart L, Planquette B, Couturaud F, Verschuren F, Caille V, Meneveau N, Pacouret G, Roy PM, Righini M, Perrier A, Bertoletti L, Parent F, Lorut C, Meyer G.. Echocardiography and pulmonary embolism severity index have independent prognostic roles in pulmonary embolism. Eur Respir J 2013;42:681–688. [DOI] [PubMed] [Google Scholar]
- 22. Hakemi EU, Alyousef T, Dang G, Hakmei J, Doukky R.. The prognostic value of undetectable highly sensitive cardiac troponin I in patients with acute pulmonary embolism. Chest 2015;147:685–694. [DOI] [PubMed] [Google Scholar]
- 23. Vanni S, Nazerian P, Pepe G, Baioni M, Risso M, Grifoni G, Viviani G, Grifoni S.. Comparison of two prognostic models for acute pulmonary embolism: clinical vs. right ventricular dysfunction-guided approach. J Thromb Haemost 2011;9:1916–1923. [DOI] [PubMed] [Google Scholar]
- 24. Moores L, Aujesky D, Jimenez D, Diaz G, Gomez V, Marti D, Briongos S, Yusen R.. Pulmonary embolism severity index and troponin testing for the selection of low-risk patients with acute symptomatic pulmonary embolism. J Thromb Haemost 2010;8:517–522. [DOI] [PubMed] [Google Scholar]
- 25. Choi KJ, Cha SI, Shin KM, Lim J, Yoo SS, Lee J, Lee SY, Kim CH, Park JY, Lee WK.. Prognostic implications of computed tomographic right ventricular dilation in patients with acute pulmonary embolism. Thromb Res 2014;133:182–186. [DOI] [PubMed] [Google Scholar]
- 26. Singanayagam A, Scally C, Al-Khairalla MZ, Leitch L, Hill LE, Chalmers JD, Hill AT.. Are biomarkers additive to pulmonary embolism severity index for severity assessment in normotensive patients with acute pulmonary embolism? QJM 2011;104:125–131. [DOI] [PubMed] [Google Scholar]
- 27. Barrios D, Morillo R, Lobo JL, Nieto R, Jaureguizar A, Portillo AK, Barbero E, Fernandez-Golfin C, Yusen RD, Jiménez D.. Assessment of right ventricular function in acute pulmonary embolism. Am Heart J 2017;185:123–129. [DOI] [PubMed] [Google Scholar]
- 28. Jimenez D, Kopecna D, Tapson V, Briese B, Schreiber D, Lobo JL, Monreal M, Aujesky D, Sanchez O, Meyer G, Konstantinides S, Yusen RD, On Behalf of the Protect Investigators. Derivation and validation of multimarker prognostication for normotensive patients with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med 2014;189:718–726. [DOI] [PubMed] [Google Scholar]
- 29. Lankeit M, Jimenez D, Kostrubiec M, Dellas C, Hasenfuss G, Pruszczyk P, Konstantinides S.. Predictive value of the high-sensitivity troponin T assay and the simplified pulmonary embolism severity index in hemodynamically stable patients with acute pulmonary embolism: a prospective validation study. Circulation 2011;124:2716–2724. [DOI] [PubMed] [Google Scholar]
- 30. Lankeit M, Jimenez D, Kostrubiec M, Dellas C, Kuhnert K, Hasenfuss G, Pruszczyk P, Konstantinides S.. Validation of N-terminal pro-brain natriuretic peptide cut-off values for risk stratification of pulmonary embolism. Eur Respir J 2014;43:1669–1677. [DOI] [PubMed] [Google Scholar]
- 31. Kartal M, Unal A, Goksu E, Yilmaz D, Gungor F.. Outpatient treatment of pulmonary embolism: sPESI score and highly sensitive troponin may prove helpful. Hong Kong J Emerg Med 2017;24:132–137. [Google Scholar]
- 32. Ozsu S, Abul Y, Orem A, Oztuna F, Bulbul Y, Yaman H, Ozlu T.. Predictive value of troponins and simplified pulmonary embolism severity index in patients with normotensive pulmonary embolism. Multidiscip Respir Med 2013;8:34.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ozsu S, Bektas H, Abul Y, Ozlu T, Orem A.. Value of cardiac troponin and sPESI in treatment of pulmonary thromboembolism at outpatient setting. Lung 2015;193:559–565. [DOI] [PubMed] [Google Scholar]
- 34. Spirk D, Aujesky D, Husmann M, Hayoz D, Baldi T, Frauchiger B, Banyai M, Baumgartner I, Kucher N.. Cardiac troponin testing and the simplified pulmonary embolism severity index. The SWIss Venous ThromboEmbolism Registry (SWIVTER). Thromb Haemost 2011;106:978–984. [DOI] [PubMed] [Google Scholar]
- 35. Polo Friz H, Molteni M, Del Sorbo D, Pasciuti L, Crippa M, Villa G, Meloni DF, Primitz L, Galli A, Rognoni M, Cavalieri d’Oro L, Arpaia G, Cimminiello C.. Mortality at 30 and 90 days in elderly patients with pulmonary embolism: a retrospective cohort study. Intern Emerg Med 2015;10:431–436. [DOI] [PubMed] [Google Scholar]
- 36. Weekes AJ, Johnson AK, Troha D, Thacker G, Chanler-Berat J, Runyon M.. Prognostic value of right ventricular dysfunction markers for serious adverse events in acute normotensive pulmonary embolism. J Emerg Med 2017;52:137–150. [DOI] [PubMed] [Google Scholar]
- 37. den Exter PL, Zondag W, Klok FA, Brouwer RE, Dolsma J, Eijsvogel M, Faber LM, van Gerwen M, Grootenboers MJ, Heller-Baan R, Hovens MM, Jonkers GJ, van Kralingen KW, Melissant CF, Peltenburg H, Post JP, van de Ree MA, Vlasveld LT, de Vreede MJ, Huisman MV.. Efficacy and safety of outpatient treatment based on the Hestia clinical decision rule with or without N-terminal pro-brain natriuretic peptide testing in patients with acute pulmonary embolism. A randomized clinical trial. Am J Respir Crit Care Med 2016;194:998–1006. [DOI] [PubMed] [Google Scholar]
- 38. Zondag W, Vingerhoets LMA, Durian MF, Dolsma A, Faber LM, Hiddinga BI, Hofstee HMA, Hoogerbrugge ADM, Hovens MMC, Labots G, Vlasveld T, de Vreede MJM, Kroft LJM, Huisman MV; Hestia Study Investigators. Hestia criteria can safely select patients with pulmonary embolism for outpatient treatment irrespective of right ventricular function. J Thromb Haemost 2013;11:686–692. [DOI] [PubMed] [Google Scholar]
- 39. Becattini C, Agnelli G, Lankeit M, Masotti L, Pruszczyk P, Casazza F, Vanni S, Nitti C, Kamphuisen P, Vedovati MC, De Natale MG, Konstantinides S.. Acute pulmonary embolism: mortality prediction by the 2014 European Society of Cardiology risk stratification model. Eur Respir J 2016;48:780–786. [DOI] [PubMed] [Google Scholar]
- 40. Aujesky D, Roy PM, Verschuren F, Righini M, Osterwalder J, Egloff M, Renaud B, Verhamme P, Stone RA, Legall C, Sanchez O, Pugh NA, N'gako A, Cornuz J, Hugli O, Beer HJ, Perrier A, Fine MJ, Yealy DM.. Outpatient versus inpatient treatment for patients with acute pulmonary embolism: an international, open-label, randomised, non-inferiority trial. Lancet 2011;378:41–48. [DOI] [PubMed] [Google Scholar]
- 41. Becattini C, Agnelli G, Vedovati MC, Pruszczyk P, Casazza F, Grifoni S, Salvi A, Bianchi M, Douma R, Konstantinides S, Lankeit M, Duranti M.. Multidetector computed tomography for acute pulmonary embolism: diagnosis and risk stratification in a single test. Eur Heart J 2011;32:1657–1663. [DOI] [PubMed] [Google Scholar]
- 42. Kucher N, Rossi E, De Rosa M, Goldhaber SZ.. Prognostic role of echocardiography among patients with acute pulmonary embolism and a systolic arterial pressure of 90 mm Hg or higher. Arch Intern Med 2005;165:1777–1781. [DOI] [PubMed] [Google Scholar]
- 43. Zhou XY, Ben SQ, Chen HL, Ni SS.. The prognostic value of pulmonary embolism severity index in acute pulmonary embolism: a meta-analysis. Respir Res 2012;13:111.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barco S, Lankeit M, Binder H, Schellong S, Christ M, Beyer-Westendorf J, Duerschmied D, Bauersachs R, Empen K, Held M, Schwaiblmair M, Fonseca C, Jimenez D, Becattini C, Quitzau K, Konstantinides S.. Home treatment of patients with low-risk pulmonary embolism with the oral factor Xa inhibitor rivaroxaban. Rationale and design of the HoT-PE Trial. Thromb Haemost 2016;116:191–197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.