Abstract
Efficacious lifestyle modification programs for children at risk of type 2 diabetes (T2D) have not been well established outside of clinical settings. In this study, the feasibility of a family-focused, YMCA-based prevention program for children at risk of T2D was evaluated between September 2015 and July 2016 in Tucson, Arizona. A 12-week YMCA-led lifestyle intervention was adapted for 9–12-year-old children and their families to encourage healthy eating, physical activity, and supportive home environments. Two YMCA locations were randomized to offer either a face-to-face lifestyle coach-led intervention or an alternating face-to-face and digitally-delivered intervention. Program feasibility and preliminary effects on child anthropometric and behavioral outcomes were assessed at baseline and post-intervention. Changes were assessed using linear regression combining delivery formats, with adjustment for clustering of participants within site/format. Forty-eight children (10.9 ± 1.2 years old; 45% female; 40% Hispanic; 43% White; 87% obese) and their parents enrolled, and 36 (75%) completed 12-week measures. Weekly program attendance averaged 61%. Participants and coaches highly rated program content and engagement strategies. Statistically significant changes in child BMI-z score (−0.05, p = 0.03) and family food and physical activity environment (+5.5% family nutrition and physical activity score, p = 0.01) were observed. A YMCA-led family-focused T2D intervention was feasible for the YMCA and participants and effects on child weight, behavior, and the home environment warranted further investigation.
Keywords: Pediatric obesity, Type 2 diabetes mellitus, Lifestyle intervention, YMCA, Diabetes prevention
Highlights
-
•
Efficacious youth diabetes prevention is not widely available in community settings
-
•
The YMCA offers scalable, sustainable delivery of a family-focused T2D prevention
-
•
E.P.I.C. Kids was feasible and preliminary effects warrant further investigation
1. Introduction
Concomitant with increased obesity (Hales et al., 2018; Ogden et al., 2016), an increased prevalence of type 2 diabetes mellitus (T2D) has been observed in youth. Indeed, T2D has become more prevalent among children and adolescents in the past few decades, increasing by >30% between 2001 and 2012 (Mayer-Davis et al., 2017; Pettitt et al., 2014). Alarmingly, prevalence is projected to quadruple over the next two decades (Pettitt et al., 2008), heralding unprecedented health, social, and financial burden affecting millions. Equally alarming are data suggesting that T2D's manifestation in obese youth is characterized by more rapid disease progression that is less responsive to standard treatments, and higher rates of comorbid conditions and complications (Copeland et al., 2011; TODAY Study Group, 2013a, TODAY Study Group, 2013b, TODAY Study Group, 2013c; TODAY Study Group et al., 2012).
Given the strong association between obesity and risk for T2D, it is generally accepted that weight control and obesity prevention among pediatric populations are critical to T2D prevention. In high-risk adults, clinical and community trials have demonstrated that lifestyle modification is highly effective in preventing T2D (Ackermann et al., 2008; Knowler et al., 2002), however, similar interventions for children with multiple risk factors are rare (Haemer et al., 2014; Ho et al., 2012). Systematic reviews of pediatric obesity prevention studies have identified components of effective interventions, including direct engagement of parents and caregivers (Golan, 2006; Golan and Crow, 2004; Shrewsbury et al., 2011; Waters et al., 2011), targeted focus on improving diet and physical activity behaviors (McGovern et al., 2008; Wilfley et al., 2007), and coaching for parents to increase modeling of desirable behaviors (Cruz et al., 2005). Despite recognition of these efficacious components, systematic integration and testing of these components within the context of youth T2D prevention programs has not occurred. Overall, there remains a paucity of T2D prevention studies outside of school settings, particularly ones which engage parents (Baranowski et al., 2002; Hingle et al., 2010; O'Connor et al., 2009). To our knowledge, few youth-focused T2D prevention studies directly involving family members and led by trained paraprofessionals have been conducted, and those have not been tested at scale (Foster et al., 2012; Hannon et al., 2018; Mantilla et al., 2017) or were tailored to serve very specific racial groups (i.e., African American(Burnet et al., 2011) and Native American (Chambers et al., 2018; Sauder et al., 2018)).
The lack of effective prevention programs adapted for delivery to families in community settings represents a critical barrier to T2D risk reduction among youth. The YMCA, a nationwide organization with a long history of community engagement, offers the opportunity to deliver a sustainable, family-focused T2D prevention at scale. There are 2700 YMCA locations nationwide, collectively serving 30 million people across 10,000 neighborhoods (YMCA of the USA, 2016). Long regarded as a community champion of strong families, youth development and social responsibility, the YMCA has been recognized as an emerging leader in health promotion, most notably, for its national Diabetes Prevention Program(Gakhar, 2015). This backdrop provides an ideal wellness infrastructure from which to implement a YMCA T2D prevention program for youth and families. The objective of this study was to establish the feasibility of a family-focused, YMCA-based intervention on anthropometric and behavioral outcomes in 9–12-year-old children at risk of T2D.
2. Materials and methods
2.1. Study design and Research setting
A pilot trial with pre−/post-intervention measurements at Week 0 (baseline) and Week 12 (post-intervention) evaluated the feasibility of a comprehensive lifestyle intervention on child anthropometric and behavioral outcomes. Two YMCA sites were selected to participate based on existing wellness and prevention infrastructure including an established YMCA Diabetes Prevention Program for adults, membership demographics reflecting a risk profile for T2D (e.g. high ethnic/racial minority and/or low-income membership), and >50% of memberships comprised of families. Additional important features included easy access by public transport, and free childcare and Wi-Fi. Participating families received a YMCA membership for the duration of the study (6 months). Our formative research with YMCA administrators and members suggested that flexibility in program delivery would encourage or support participation(Hingle et al., 2015); thus, two intervention formats, a face-to-face lifestyle coach-led delivery and an alternating face-to-face or digitally-delivered (hybrid program) were evaluated during two 12-week time periods (September to December 2015 and January to April 2016). Both YMCA sites offered both intervention formats; Site A was randomly assigned to offer the face-to-face intervention first (in Fall 2015), and then offered the alternating face-to-face and digitally-delivered content (or hybrid program) in Spring 2016, while Site B offered the hybrid program first (Fall 2015), followed by the face-to-face program (Spring 2016). Sites (versus groups) were chosen as the unit of randomization to minimize the possibility of between-group contacts.
2.2. Recruitment, screening, eligibility, and enrollment
Participants were recruited through the YMCA membership listserv, pediatric and family medicine practices, and the broader community using electronic announcements, flyers, posters, and word-of-mouth. Respondents completed a brief phone- or web-based screening (depending on respondent preference) to assess eligibility. Families meeting the study criteria were invited to attend study information sessions held at YMCA intervention sites to confirm interest and eligibility. Eligible participants were 9–12-years-old, body mass index (BMI) at or above the 85th percentile for age and sex and reported one or more additional T2D risk factors including: family history of T2D in first- or second-degree relative, race/ethnicity Native American, African American, Latino, Asian American, Pacific Islander, or medical diagnosis of insulin resistance or impaired glucose tolerance (American Diabetes Association, 2018; Jolliffe and Janssen, 2007). Participants agreed to use a study-provided mobile device (Kindle Fire HDX7, Amazon) throughout the intervention, were able to speak and read English and have a primary caregiver (defined as the adult who most frequently prepares/obtains food, regulates media use, and provides physical activity opportunities) willing to take part in all intervention sessions and activities. Children were excluded if they had previously diagnosed type 1 or type 2 diabetes mellitus, psychiatric illness, limitations preventing physical activity, or were taking medications known to affect weight or appetite. Concomitant participation in a weight management, lifestyle behavior modification, or similar nutrition program were also reason for exclusion.
Eligibility was confirmed at study information sessions, where child height and weight were measured by trained research technicians and sex- and age-specific BMI was determined. Interested and eligible respondents completed the informed consent process following University of Arizona Institutional Review Board-approved materials and methods. Written consent was obtained from parents and verbal assent was obtained from children and documented in writing by study staff prior to enrollment. Families who were not yet YMCA members were assigned a study location based on proximity to their home and all participants (regardless of current YMCA membership status) were provided a 6-month family membership free of charge.
2.3. Intervention
The goal of the intervention was to promote adoption of behaviors associated with an improved weight trajectory in youth while supporting normal growth and development. Congruent with a 2012 Institute of Medicine report ((IOM), 2012) and guidelines set forth by an expert committee in child and adolescent obesity(Barlow and Expert, 2007), intervention content supported participants in making physical activity an integral and routine part of life, eating a healthier diet (both quantity and quality), and creating a home food and physical activity environment in which healthy options and behaviors were the routine, easy choice. Formative research activities engaged six YMCA administrators and staff, five youth YMCA members between the ages of 9 and 12, and an advisory board comprised of experts in diet, physical activity, youth development, endocrinology, digital education, and medicine whose input collectively informed and refined program content and delivery methods prior to the start of the intervention (Hingle et al., 2015).
Families participated in weekly group-based intervention sessions led by two trained YMCA lifestyle coaches and attended by up to 9 other families. Program activities were conducted at times convenient to parents (early weekday evenings and Saturday morning). All activities promoted active learning through hands-on activities requiring movement and interaction with others and provided opportunities for the family to acquire and practice skills related to healthy eating and physical activity. Sessions were 1.5 h long and conducted over 12 consecutive weeks at participating YMCAs. (Table 1) A similar format was followed each week featuring a structured physical activity that encouraged families to get moving upon arrival; small group discussions focused on setting and achieving behavioral goals and fostering family interactions; hands-on practice with food preparation and tasting opportunities focused on vegetables, whole grains, and legumes; opportunities to engage in family physical activities requiring little or no equipment (or, on alternating weeks, child-only physical activities plus separate coach-led parenting discussions focused on food, physical activity or media use); an energy balance topic to increase foundational knowledge and skills related to healthy food selection and the benefits of physical activity and structuring the home environment to support healthy habits. Each week concluded with opportunities to set new weekly goals or revise previous goals.
Table 1.
E.P.I.C. kids intervention sessions and topics.
| Week 1 | What to expect from this program; meet your coaches; setting and achieving realistic goals |
| Week 2 | Get kids the energy they need to grow, learn, and play; energy density of foods |
| Week 3 | Swap screen time for active time, and make sedentary time more active; why moderate-to-vigorous physical activity matters |
| Week 4 | Making healthy food more available and accessible in the home; How to prepare, eat, and enjoy vegetables, whole grains, and legumes |
| Week 5 | How to choose tasty, low-calorie beverages and drink less sugary drinks; label reading |
| Week 6 | Making healthy food more available and accessible in the home; Have more fun staying active as a family; benefits of physical activity |
| Week 7 | Serve just the right amount of food to keep weights healthy; healthy eating/MyPlate; noticing/taking advantage of food and activity cues |
| Week 8 | Eating out made easy; enjoy calm, healthy, relaxed meals; use positive practices to teach kids to enjoy new foods |
| Week 9 | Make mealtime family time: secrets to successful family meals; focus on positive change and be a good nutrition role model |
| Week 10 | Finding the best stuff at your grocery store; talking back to negative thoughts |
| Week 11 | Learn and practice healthy sleep habits; managing stress |
| Week 12 | Making family physical activity happen; problem solving |
Biweekly digital delivery of the intervention was offered to participants enrolled in the hybrid program through the study's mobile-optimized website (Moodle Pty Ltd., Perth, Australia) and a study-provided Wi-Fi-enabled tablet (one per family, Kindle Fire HDX7, Amazon). Intervention content and activities were equivalent to the face-to-face-only sessions, while interactions with coaches and other families were fostered via online synchronous meetings, hosted discussions, and program−/coach−/participant-posted content. Digital delivery represented a previously untested enhancement compared to the more traditional face-to-face delivery, thus, a series of focus groups and in-depth interviews with YMCA administrators and members (including five youth) were conducted to understand user preferences related to hardware, degree of interactivity with coaches and other families, and meeting “locations” (asynchronous vs synchronous, and days/times). Input from the research team's instructional media design specialists was also obtained throughout this development process (Hingle et al., 2015).
The intervention was delivered by ten YMCA lifestyle coaches (2 men, 8 women; 5 bilingual English/Spanish; ages ranging from 25 to 65 years old) selected from existing YMCA staff trained to deliver the adult-focused Diabetes Prevention Program. Coaches were recommended by their supervisor), or self-nominated based on an interest in working with youth and families. All had prior experience leading group education sessions focused on nutrition, fitness and/or health. Coaches completed 16 h of training across four sessions in July and November 2015. Training sessions were a combination of didactic presentations and mock group sessions led by the research team. This type and amount of training has been used in previous studies successfully translating clinical T2D prevention programs to community settings (Ackermann et al., 2008; Katula et al., 2011) and allowed coaches to practice competencies related to T2D prevention when working with youth and families (Finch et al., 2009).
2.4. Program feasibility
Given the YMCA's interest in replication, dissemination, and scaling of effective family-focused T2D prevention programs across other sites, feasibility metrics were framed within the context of RE-AIM, a program evaluation tool designed to assess the public health impact of an intervention across five dimensions – reach, efficacy, adoption, implementation, and maintenance (Glasgow et al., 1999). Data aligned with these dimensions were collected from multiple sources by the researchers and lifestyle coaches. Recruitment, enrollment and retention rates were tracked and calculated by the research team based on number of recruitment events and activities, reach versus number of respondents from these events, and numbers of respondents screened, eligible, and enrolled. Program adherence and engagement were assessed using weekly attendance logs (a proxy for adherence) and through session observations by researchers following a standard rubric. Moodle software analytics allowed for characterization of time spent with mobile content for hybrid program participants. Program satisfaction was rated weekly by all participants using brief surveys, which asked families to rate the relevance of intervention content with regard to daily life, report promoters and barriers to attendance and engagement, and, estimate the degree to which program strategies were applied to make recommended lifestyle changes. Fidelity to the intervention was assessed by research team members observing intervention sessions (three per intervention group) and through audits of session logs kept by coaches in which departures from the intervention were documented. Program-related costs were tracked by the research team and YMCA administrators and cost in order to calculate costs per participant and per family. Frequency of YMCA facility use by participants was captured through final surveys and the YMCA's electronic membership logs.
2.5. Anthropometric and behavioral measurements
Anthropometric and behavioral outcomes were assessed at baseline and 12 weeks (post-intervention). Body weight and height was measured in children wearing light clothing and no shoes using an electronic calibrated scale (SECA 876, Chino CA) rounded to the nearest 0.1 kg and a portable stadiometer (ShorrBoard, Olney MD) rounded to the nearest 0.1 cm. Waist circumference was measured at the umbilicus using a non-stretching tape to the nearest 0.1 cm. Anthropometric measures were taken in duplicate and averaged. BMI percentile was determined using age- and sex-specific growth charts developed by the Centers for Disease Control and Prevention (Kuczmarski et al., 2000).
Child dietary intake was assessed using two non-consecutive 24-h dietary recalls (one weekday, one weekend day) administered by trained nutritionists and analyzed using Nutrient Data System for Research (Minneapolis, MN, v. 2012) (Feskanich et al., 1989). Recalls were conducted with the child only. Overall diet quality (and its components: total vegetables, whole fruit, whole grains, plant, animal and seafood proteins, fatty acids, sodium, sugar, and refined grains) was calculated using the Healthy Eating Index-2010, a valid and reliable measure of diet quality designed to assess the degree to which intake conforms to dietary recommendations (Guenther et al., 2014). The HEI is appropriate for use with children's dietary data (Center for Nutrition Policy and Promotion, 2013). Changes in physical activity were measured over a 7-day period using waist-worn Actigraph GT3X accelerometers (Actigraph, Pensacola FL); valid wear time was set at 10 h/day for at least 4 of the 7 days of assessment (Tudor-Locke et al., 2012), and data were processed using 60-s epochs and intensity cut points developed by Evenson et al. (Evenson et al., 2008). Caregivers completed the Family Nutrition and Physical Activity Tool (FNPA), a 20-item survey of the family home environment and practices associated with children's obesity risk (Ihmels et al., 2009a). The predictive validity of the FNPA on child body weight status was established in studies of parents of school-age children in which BMI was measured (Ihmels et al., 2009b). Maturity, an important covariate when evaluating adiposity changes in peripubertal youth, was assessed using Tanner's validated self-report which presents illustrations that agree with pubertal staging by a physician (Morris and Udry, 1980; Tanner, 1962).
2.6. Statistical analyses
The primary outcome was change in BMI z-score. The target sample size of 48 children was informed by results of Foster et al. (Foster et al., 2012), who observed a mean decrease of 4.3% in percentage overweight (SE = 1.1) in a YMCA-based pediatric obesity intervention over 6 months and was based on the number of participants needed to detect an effect size of this magnitude with 80% power assuming a one-sided test at alpha = 0.05. A recruitment goal of sixty participants was set to allow for up to 20% attrition. Changes in anthropometric and behavioral outcomes at 12 weeks across intervention formats were assessed using a linear regression model with adjustment for the potential correlation of responses within a site/format. Exploratory descriptive analyses stratified by delivery format were performed to provide an assessment of differences between the intervention formats. All p-values reported are two-sided.
3. Results
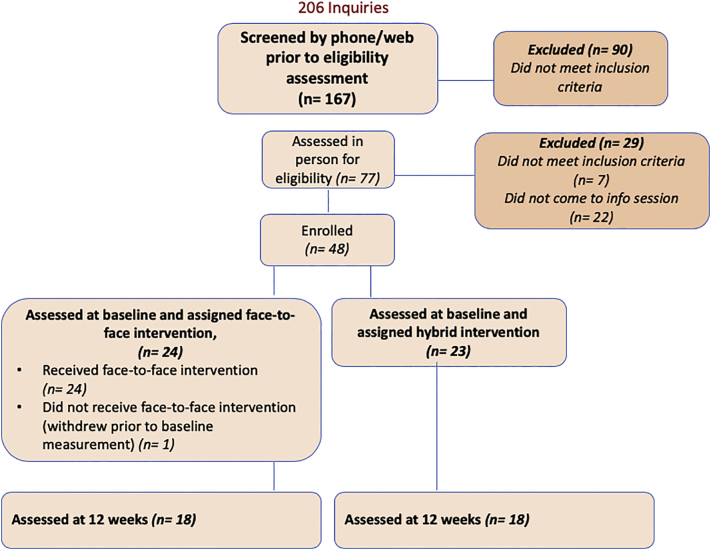
Recruitment activities yielded 206 inquiries, 167 of which were screened by the research team. Of these, 48 families enrolled, 47 were measured at baseline (1 family withdrew prior to baseline measurements), and 36 completed post-intervention (12-week) measures (Fig. 1).
Fig. 1.
Participant screening, enrollment, and assessment.
Paired diet quality data were available for 44 participants, 8 of whom did not complete the other post-intervention measures. Additionally, 5 participants were excluded from the physical activity comparison due to invalid accelerometer data. Children were 10.9-years-old ±1.2, 45% female, 40% Hispanic, 43% White, 87% obese, and 83% had a parent or sibling with T2D. More than half of parents reported that their combined household income was less than $40,000 per year. A majority of parents had completed at least 2 years of college (60%) and were employed at least part time (74%). Just over one-third were current YMCA members. (Table 2) Statistically significant differences in ethnicity and race were observed between the two sites, which was expected given their geographical locations and respective demographics of those regions.
Table 2.
Baseline participant characteristics, n = 47 families.
| Overall |
Site A |
Site B |
|||||
|---|---|---|---|---|---|---|---|
| Characteristic | Mean or Freq. | SD or % | Mean or Freq. | SD or % |
Mean or Freq. | SD or % |
p |
| Child | |||||||
| Age (years) | 10.9 | 1.2 | 10.8 | 1.2 | 11.1 | 1.3 | 0.5 |
| Sex | |||||||
| Male | 26 | 55% | 18 | 56% | 8 | 53% | |
| Female | 21 | 45% | 14 | 44% | 7 | 47% | 0.85 |
| Ethnicity | |||||||
| Hispanic | 19 | 40% | 14 | 44% | 5 | 33% | |
| Non-Hispanic | 15 | 32% | 6 | 19% | 9 | 60% | |
| Prefer not to reply | 13 | 28% | 12 | 37% | 1 | 7% | 0.01 |
| Race | |||||||
| White | 20 | 43% | 8 | 25% | 12 | 80% | |
| Black | 10 | 22% | 8 | 25% | 2 | 13% | |
| Asian | 0 | 0% | 0 | 0% | 0 | 0% | |
| American Indian | 2 | 4% | 2 | 6% | 0 | 0% | |
| Prefer not to reply | 14 | 30% | 14 | 44% | 1 | 7% | 0.004 |
| Weight status | |||||||
| Overweight (BMI 85th–95th percentiles) | 6 | 13% | 5 | 16% | 1 | 7% | |
| Obese (BMI at or above 95th percentile) | 41 | 87% | 27 | 84% | 14 | 93% | 0.39 |
| Waist circumference (cm) | 91.1 | 13.4 | 92.3 | 13.6 | 92.3 | 14.4 | 0.99 |
| Tanner Stage (score of 1–5) | |||||||
| Female | 2.9 | 1.2 | 2.9 | 1.1 | 2.9 | 1.3 | 0.9 |
| Male | 1.8 | 1.2 | 1.9 | 1.1 | 1.6 | 1.4 | 0.62 |
| First or second degree relative with T2D | 39 | 83% | |||||
| Parent/household | |||||||
| Ethnicity | |||||||
| Hispanic | 29 | 62% | 24 | 75% | 5 | 33% | |
| Non-Hispanic | 18 | 38% | 8 | 25% | 10 | 67% | 0.006 |
| Race | |||||||
| White | 35 | 74% | 20 | 63% | 15 | 100% | |
| Black | 3 | 6% | 3 | 9% | 0 | 0% | |
| Asian | 0 | 0% | 0 | 0% | 0 | 0% | |
| American Indian | 4 | 9% | 4 | 12% | 0 | 0% | |
| Prefer not to reply | 5 | 11% | 5 | 16% | 0 | 0% | 0.06 |
| Combined household income | |||||||
| <$40,000 | 24 | 51% | 20 | 62% | 4 | 27% | |
| $40,000–$79,999 | 11 | 23% | 7 | 22% | 4 | 27% | |
| >$80,000 | 12 | 26% | 5 | 16% | 7 | 46% | 0.04 |
| Primary caregiver education | |||||||
| High school or less | 7 | 15% | 6 | 19% | 1 | 7% | |
| Some college or 2-year degree | 28 | 60% | 20 | 62% | 8 | 53% | |
| 4-year degree or more | 12 | 25% | 6 | 19% | 6 | 40% | 0.23 |
| Primary caregiver employment | |||||||
| Not employed | 10 | 21% | 7 | 22% | 3 | 20% | |
| Employed | 37 | 79% | 25 | 78% | 12 | 80% | 0.88 |
| YMCA member | |||||||
| Yes | 16 | 34% | 6 | 19% | 10 | 67% | |
| No | 26 | 55% | 22 | 69% | 4 | 27% | |
| Past member | 5 | 11% | 4 | 12% | 1 | 6% | 0.005 |
Program attendance averaged 61% across both intervention formats. Session observations confirmed high fidelity to the intervention, although 17% of sessions ran over the 1.5-h allotted time frame. Coaches provided participants with evidence-based information, encouraged participant questions, and kept a majority of families engaged in program activities “all or almost all the time (>90%).” Both participants and lifestyle coaches highly rated the program content and engagement strategies. A majority of participants strongly agreed that weekly activities were enjoyable (85%), applicable (72%) and useful (78%) to their daily lives and motivated them to make lifestyle changes (70%). In particular, participants enjoyed the hands-on food demonstrations, family physical activity, and parenting skills discussions. Participants rated the effectiveness of their lifestyle coaches as moderately high to high (average 3.1–3.8/4.0), and their likelihood to continue the lifestyle changes they initiated during the program as moderate. Forty-four percent of families reported using the YMCA facilities outside of weekly intervention sessions. YMCA member analytics data confirmed that 29% of families used their membership credentials a minimum of once per week to access the facility. Half of participants said that they saved money compared to pre-intervention food costs as a result of participating in the intervention and reported that their participation took the place of time previously spent in leisure time activities, chores, and homework/work. Program delivery costs were estimated at $326 per child.
Coaches rated ease of program implementation as moderately high to high (average 4.0/5.0). A majority (83%) thought the program was effective in promoting positive health behavior change in children and families, and eight out of ten coaches expressed interest in leading future programs.
Participants preferred the flexibility of the hybrid intervention sessions, attended these sessions at similar rates to the face-to-face format, and commented that sessions were fun. At the same time, they were less satisfied overall with the quality of the online program compared to the face-to-face format. Criticisms were largely focused on technical problems with the study-provided tablet, the mastery of which took valuable time away from the intervention activities.
Significant decreases in child BMI z-score were observed for the overall sample at 12 weeks (−0.05, p = 0.03). (Table 3) There were no significant differences in BMI z-score change between the two formats, data not shown.
Table 3.
Changes in BMI z-score and waist circumference in child participants at 12 weeks, overall and by program format (face-to-face and hybrid).
| Variable | Program format | n | Mean | 95% CI | 2-sided p-value |
|
|---|---|---|---|---|---|---|
| BMI z-score | ||||||
| Week 12-Baseline | Overall | 36 | −0.05 | −0.08 | −0.1 | 0.03 |
| Face-to-face | 18 | −0.05 | −0.36 | 0.27 | 0.3 | |
| Hybrid | 18 | −0.04 | −0.15 | 0.06 | 0.13 | |
| Waist circumference | ||||||
| Week 12-Baseline | Overall | 36 | 0.45 | −2.44 | 1.5 | 0.52 |
| Face-to-face | 18 | 0.25 | −8.61 | 9.11 | 0.778 | |
| Hybrid | 18 | −1.15 | −15.68 | 13.37 | 0.49 | |
Modest improvements in diet quality and moderate-to-vigorous physical activity were observed, although these changes were not statistically significant. Significant positive changes in healthy home environment occurred at 12 weeks (+5.5% in healthy home score, p = 0.01) with improvements reported in the domains of home nutrition (p = 0.03), and physical activity (p = 0.01). (Table 4).
Table 4.
Changes in child diet quality, physical activity, and the home environment at 12 weeks, overall and by program format (face-to-face and hybrid).
| Baseline |
Week 12 |
Delta |
2-sided p-value |
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Overall | ||||||
| Healthy Eating Index (score out of 100), n = 44 | 48.1 | 9.1 | 49.6 | 9.8 | 1.5 | 0.41 |
| Moderate-to-vigorous physical activity (minutes per day), n = 31 | 28.9 | 15.3 | 30.6 | 18.7 | 1.6 | 0.64 |
| Family nutrition and physical activity (score out of 80), n = 36 | 56.3 | 7.3 | 60.6 | 6.6 | 4.4 | 0.01 |
| Nutrition subscale | 26.8 | 4 | 28.8 | 3.3 | 2.1 | 0.03 |
| - Breakfast and family meals | ||||||
| - Parent modeling of nutrition | ||||||
| - Nutrient-dense foods | ||||||
| - High-kcal beverages | ||||||
| Physical activity subscale | 13 | 3.2 | 14.1 | 3.2 | 1.1 | 0.01 |
| - Child's physical activity | ||||||
| - Parent modeling of physical activity | ||||||
| Sedentary behavior subscale | 7.5 | 2.2 | 8.2 | 2.3 | 0.7 | 0.18 |
| - Screen time | ||||||
| - TV in child's bedroom | ||||||
| - Sleep schedule | ||||||
| Face-to-Face | ||||||
| Healthy Eating Index (score out of 100), n = 44 | 48.6 | 9 | 47.8 | 9.4 | 0.8 | 0.46 |
| Moderate-to-vigorous physical activity (minutes per day), n = 31 | 22.6 | 11.1 | 27.8 | 20 | 5.2 | 0.28 |
| Family nutrition and physical activity (score out of 80), n = 36 | 57.6 | 8.2 | 62.8 | 5.8 | 5.2 | 0.01 |
| Nutrition subscale | 27.5 | 4.4 | 29.4 | 3 | 1.9 | 0.1 |
| - Breakfast and family meals | ||||||
| - Parent modeling of nutrition | ||||||
| - Nutrient-dense foods | ||||||
| - High-kcal beverages | ||||||
| Physical activity subscale | 13.2 | 3.5 | 14.5 | 2.8 | 1.3 | 0.05 |
| - Child's physical activity | ||||||
| - Parent modeling of physical activity | ||||||
| Sedentary behavior subscale | 7.7 | 2.1 | 9.1 | 1.8 | 1.4 | 0.07 |
| - Screen time | ||||||
| - TV in child's bedroom | ||||||
| - Sleep schedule | ||||||
| Hybrid | ||||||
| Healthy Eating Index (score out of 100), n = 44 | 47.7 | 9.5 | 51.4 | 10.2 | −3.7 | 0.27 |
| Moderate-to-vigorous physical activity (minutes per day), n = 31 | 35.8 | 16.6 | 33.5 | 17.4 | −2.2 | 0.73 |
| Family nutrition and physical activity (score out of 80), n = 36 | 54.9 | 6.3 | 58.5 | 6.7 | 3.6 | 0.2 |
| Nutrition subscale | 26 | 3.5 | 28.2 | 3.5 | 2.2 | 0.33 |
| - Breakfast and family meals | ||||||
| - Parent modeling of nutrition | ||||||
| - Nutrient-dense foods | ||||||
| - High-kcal beverages | ||||||
| Physical activity subscale | 12.8 | 3.1 | 13.6 | 3.6 | 0.8 | 0.15 |
| - Child's physical activity | ||||||
| - Parent modeling of physical activity | ||||||
| Sedentary behavior subscale | 7.4 | 2.4 | 7.4 | 2.4 | 0 | 0.79 |
| - Screen time | ||||||
| - TV in child's bedroom | ||||||
| - Sleep schedule | ||||||
4. Discussion
This YMCA-based intervention demonstrated promising, albeit modest, effects on child weight and weight-related lifestyle behaviors, with reductions in child BMI z-scores comparable to more intensive pediatric obesity prevention interventions (Foster et al., 2012; Sacher et al., 2010). Improvements in diet quality and moderate-to-vigorous physical activity were also observed, although these changes were not statistically significant. Multiple behavioral changes were targeted during the course of the intervention, so it is possible that participants had initiated recommended behavioral changes and their modest size and incremental nature were difficult to detect within the short duration of this pilot. Parent-reported changes to the home environment were significant and positive, which suggested increased parental involvement and support, as well as potential collateral benefits for other household members. This was encouraging, as small changes across multiple factors, supported by parents, are likely to be more sustainable over time compared to programs that only engage the child.
The intervention successfully attracted participants at high risk of T2D; this was both encouraging and important, particularly when considering national recommendations for T2D screening and intervention (American Diabetes Association, 2018). Program attendance and attrition was similar to other programs intervening with youth and families for the purpose of T2D and obesity prevention (Baranowski et al., 2003; Foster et al., 2012; Gentile et al., 2009; Sanigorski et al., 2008).
Our study had several strengths. To our knowledge, this was among the first T2D prevention programs for youth and families offered at the YMCA, led by the YMCA. The YMCA is a recognized leader in health promotion with a long history of community engagement, most recently for the successful YMCA Diabetes Prevention Program (Gakhar, 2015). Partnering with the YMCA expands program reach to >2700 YMCA locations nationwide, providing an ideal wellness infrastructure from which to implement T2D prevention to millions at risk. In contrast to previous community-based pediatric obesity or T2D prevention interventions in which researcher-led activities were typical, engagement of YMCA lifestyle coaches to lead the intervention introduced sustainable and scalable elements to the intervention.
An a priori focus on scalability, replication, dissemination, and sustainability allowed capture of numerous factors believed to influence intervention delivery and uptake including weekly attendance, participant engagement and satisfaction, implementation costs and administrative support. Across these metrics, the program was rated moderate to high, signifying potential for expansion within the YMCA network once efficacy is demonstrated.
This study also had several limitations. Its duration was relatively brief (12-week), presenting a narrow window of opportunity within which to intervene and evaluate the intervention's impact. There was no true control group. Several of our outcome measures were self-reported, raising the potential for social desirability of response. Participant retention was a challenge, reaching 25% attrition at 12 weeks; while disappointing, this was consistent with similar studies in the literature (Baranowski et al., 2003; Foster et al., 2012; Gentile et al., 2009; Hannon et al., 2018; Sanigorski et al., 2008).
5. Conclusions
A YMCA-led family-focused T2D intervention was feasible for the YMCA and participants and effects on child weight, behavior, and the home environment warranted further investigation. Future research will test the efficacy of the intervention at 6 months (post-intervention) and maintenance effects at 12 months using a cluster-randomized controlled trial design in partnership with four YMCA, with a long-term goal of providing accessible, affordable prevention to children and families at risk of T2D across the Southwest.
Funding
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health [award number R21DK100805] and by the National Cancer Institute of the National Institutes of Health [award number P30 CA023074].
Author contributions
MDH and SBG conceived of the study. MDH, RK, SBG, CS, and DR initiated the study design and TT and CU helped with implementation. MDH, SBG, RK, CS, and DR are grant holders. DR and KS provided statistical expertise in clinical trial design and KS conducted the primary statistical analysis. All authors contributed to refinement of the study protocol and approved the final manuscript.
Competing interests
The authors have no competing interests to declare.
ClinicalTrials.gov, NCT02421198 on April 15, 2015.
Acknowledgments
YMCA of Southern Arizona. Annemarie Medina, Vivian Cullen, Dane Woll, Ronnye Bertoglio, Yvonne Corral, Gina Gant, Alejandra Gonzalez, Pam Hector, Kerry Hewitt, Judy Johnson, Rebecca Martin, Manny Smith, Gloria White E.P.I.C. Kids Advisory Board. Barbara Chamberlin, New Mexico State University; Valerie Lawson, Y-USA; Matt Longjohn, Y-USA; Teresia O'Connor, Baylor College of Medicine and USDA-ARS Children's Nutrition Research Center; Heather Patrick, Live Healthier; Donna Spruijt-Metz, University of Southern California; Jessica Schultz, Grow2Bfit.
References
- Ackermann R.T., Finch E.A., Brizendine E., Zhou H., Marrero D.G. Translating the Diabetes Prevention Program into the community. The DEPLOY pilot study. Am. J. Prev. Med. 2008;35:357–363. doi: 10.1016/j.amepre.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association Prevention or delay of type 2 diabetes: standards of medical Care in Diabetes - 2018. Diabetes Care. 2018;41:S51–S54. doi: 10.2337/dc18-S005. [DOI] [PubMed] [Google Scholar]
- Baranowski T., Cullen K.W., Nicklas T., Thompson D., Baranowski J. School-based obesity prevention: a blueprint for taming the epidemic. Am. J. Health Behav. 2002;26:486–493. doi: 10.5993/ajhb.26.6.9. [DOI] [PubMed] [Google Scholar]
- Baranowski T., Baranowski J.C., Cullen K.W., Thompson D.I., Nicklas T., Zakeri I.E., Rochon J. The fun, food, and fitness project (FFFP): the Baylor GEMS pilot study. Ethn Dis. 2003;13:S30–S39. [PubMed] [Google Scholar]
- Barlow S.E., Expert C. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl. 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- Burnet D.L., Plaut A.J., Wolf S.A., Huo D., Solomon M.C., Dekayie G., Quinn M.T., Lipton R., Chin M.H. Reach-out: a family-based Diabetes Prevention Program for African American youth. J. Natl. Med. Assoc. 2011;103:269–277. doi: 10.1016/s0027-9684(15)30290-x. [DOI] [PubMed] [Google Scholar]
- Center for Nutrition Policy and Promotion . Diet quality of children age 2–17 years as measured by the healthy eating index. In: USDA, editor. Nutrition Insight. Vol. 52. 2013. https://www.cnpp.usda.gov/sites/default/files/nutrition_insights_uploads/Insight52.pdf Available at: [Google Scholar]
- Chambers R., Rosenstock S., Walls M., Kenney A., Begay M., Jackson K., Nelson L., Neault N., Goklish N. Engaging native American caregivers in youth-focused Diabetes Prevention and management. Prev. Chronic Dis. 2018;15:170521. doi: 10.5888/pcd15.170521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland K.C., Zeitler P., Geffner M., Guandalini C., Higgins J., Hirst K., Kaufman F.R., Linder B., Marcovina S. Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J. Clin. Endocrinol. Metab. 2011;96:159–167. doi: 10.1210/jc.2010-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz M.L., Shaibi G.Q., Weigensberg M.J., Spruijt-Metz D., Ball G.D., Goran M.I. Pediatric obesity and insulin resistance: chronic disease risk and implications for treatment and prevention beyond body weight modification. Annu. Rev. Nutr. 2005;25:435–468. doi: 10.1146/annurev.nutr.25.050304.092625. [DOI] [PubMed] [Google Scholar]
- Evenson K.R., Catellier D.J., Gill K., Ondrak K.S., McMurray R.G. Calibration of two objective measures of physical activity for children. J. Sports Sci. 2008;26:1557–1565. doi: 10.1080/02640410802334196. [DOI] [PubMed] [Google Scholar]
- Feskanich D., Sielaff B.H., Chong K., Buzzard I.M. Computerized collection and analysis of dietary intake information. Comput. Methods Prog. Biomed. 1989;30:47–57. doi: 10.1016/0169-2607(89)90122-3. [DOI] [PubMed] [Google Scholar]
- Finch E.A., Kelly M.S., Marrero D.G., Ackermann R.T. Training YMCA wellness instructors to deliver an adapted version of the Diabetes Prevention Program lifestyle intervention. Diabetes Educ. 2009;35(224–8):32. doi: 10.1177/0145721709331420. [DOI] [PubMed] [Google Scholar]
- Foster G.D., Sundal D., McDermott C., Jelalian E., Lent M.R., Vojta D. Feasibility and preliminary outcomes of a scalable, community-based treatment of childhood obesity. Pediatrics. 2012;130:652–659. doi: 10.1542/peds.2012-0344. [DOI] [PubMed] [Google Scholar]
- Gakhar M. Measureable Progress, unlimited support: YMCA's Diabetes Prevention Program overview in: YMCA of the USA (Ed.). March 18, 2015. 2015. https://www.midiabetesprevention.org/documents/YUSA-Presentation-3-18-15_FINAL.pdf Available at:
- Gentile D.A., Welk G., Eisenmann J.C., Reimer R.A., Walsh D.A., Russell D.W., Callahan R., Walsh M., Strickland S. Evaluation of a multiple ecological level child obesity prevention program: switch what you do, view, and chew. BMC. 2009;7:49. doi: 10.1186/1741-7015-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow R.E., Vogt T.M., Boles S.M. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am. J. Public Health. 1999;89:1322–1327. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan M. Parents as agents of change in childhood obesity--from research to practice. Int. J. Pediatr. Obes. 2006;1:66–76. doi: 10.1080/17477160600644272. [DOI] [PubMed] [Google Scholar]
- Golan M., Crow S. Parents are key players in the prevention and treatment of weight-related problems. Nutr. Rev. 2004;62:39–50. doi: 10.1111/j.1753-4887.2004.tb00005.x. [DOI] [PubMed] [Google Scholar]
- Guenther P.M., Kirkpatrick S.I., Reedy J., Krebs-Smith S.M., Buckman D.W., Dodd K.W., Casavale K.O., Carroll R.J. The healthy eating Index-2010 is a valid and reliable measure of diet quality according to the 2010 dietary guidelines for Americans. J. Nutr. 2014;144:399–407. doi: 10.3945/jn.113.183079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haemer M.A., Grow H.M., Fernandez C., Lukasiewicz G.J., Rhodes E.T., Shaffer L.A., Sweeney B., Woolford S.J., Estrada E. Addressing prediabetes in childhood obesity treatment programs: support from research and current practice. Child Obes. 2014;10:292–303. doi: 10.1089/chi.2013.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales C.M., Fryar C.D., Carroll M.D., Freedman D.S., Ogden C.L. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. JAMA. 2018;319:1723–1725. doi: 10.1001/jama.2018.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon T.S., Saha C., Carroll A.E., Palmer K.N., Phillips E.O., Marrero D.G. The ENCOURAGE healthy families study: a comparative effectiveness trial to reduce risk for type 2 diabetes in mothers and children. Pediatr. Diabetes. 2018;19:1041–1049. doi: 10.1111/pedi.12692. [DOI] [PubMed] [Google Scholar]
- Hingle M.D., O'Connor T.M., Dave J.M., Baranowski T. Parental involvement in interventions to improve child dietary intake: a systematic review. Prev. Med. 2010;51:103–111. doi: 10.1016/j.ypmed.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingle M.D., Turner T., Kutob R., Merchant N., Roe D.J., Stump C., Going S.B. The EPIC kids study: a randomized family-focused YMCA-based intervention to prevent type 2 diabetes in at-risk youth. BMC Public Health. 2015;15:1253. doi: 10.1186/s12889-015-2595-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M., Garnett S.P., Baur L., Burrows T., Stewart L., Neve M., Collins C. Effectiveness of lifestyle interventions in child obesity: systematic review with meta-analysis. Pediatrics. 2012;130:e1647–e1671. doi: 10.1542/peds.2012-1176. [DOI] [PubMed] [Google Scholar]
- Ihmels M.A., Welk G.J., Eisenmann J.C., Nusser S.M. Development and preliminary validation of a family nutrition and physical activity (FNPA) screening tool. Int. J. Behav. Nutr. Phys. Act. 2009;6:14. doi: 10.1186/1479-5868-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihmels M.A., Welk G.J., Eisenmann J.C., Nusser S.M., Myers E.F. Prediction of BMI change in young children with the family nutrition and physical activity (FNPA) screening tool. Ann. Behav. Med. 2009;38:60–68. doi: 10.1007/s12160-009-9126-3. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine . National Academies Press; 2012. Accelerating Progress in Obesity Prevention: Solving the Weight of the Nation.http://www.nationalacademies.org/hmd/Reports/2012/Accelerating-Progress-in-Obesity-Prevention.aspx Available at: (May 8, 2012. Accessed December 20, 2018) [PubMed] [Google Scholar]
- Jolliffe C.J., Janssen I. Development of age-specific adolescent metabolic syndrome criteria that are linked to the adult treatment panel III and international Diabetes federation criteria. J. Am. Coll. Cardiol. 2007;49:891–898. doi: 10.1016/j.jacc.2006.08.065. [DOI] [PubMed] [Google Scholar]
- Katula J.A., Vitolins M.Z., Rosenberger E.L., Blackwell C.S., Morgan T.M., Lawlor M.S., Goff D.C., Jr. One-year results of a community-based translation of the Diabetes Prevention Program: healthy-living partnerships to prevent Diabetes (HELP PD) project. Diabetes Care. 2011;34:1451–1457. doi: 10.2337/dc10-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowler W.C., Barrett-Connor E., Fowler S.E., Hamman R.F., Lachin J.M., Walker E.A., Nathan D.M., Diabetes Prevention Program Research G. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski R.J., Ogden C.L., Grummer-Strawn L.M., Flegal K.M., Guo S.S., Wei R., Mei Z., Curtin L.R., Roche A.F. CDC growth charts: United States. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- Mantilla C., Jones T., Decker K.M., Jacobo A.M., Sontheimer S.Y., Mirro M.R., Hare M.E., Han J.C. DIabetes Prevention Program in youth (insulin superheroes Club) pilot: improvement in metabolic parameters and physical fitness after 16 weeks of Lifetyle intervention. Diabetes Care. 2017;40:e63–e64. doi: 10.2337/dc16-2678. [DOI] [PubMed] [Google Scholar]
- Mayer-Davis E.J., Lawrence J.M., Dabelea D., Divers J., Isom S., Dolan L., Imperatore G., Linder B., Marcovina S. Incidence trends of type 1 and type 2 Diabetes among youths, 2002-2012. N. Engl. J. Med. 2017;376:1419–1429. doi: 10.1056/NEJMoa1610187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern L., Johnson J.N., Paulo R., Hettinger A., Singhal V., Kamath C., Erwin P.J., Montori V.M. Clinical review: treatment of pediatric obesity: a systematic review and meta-analysis of randomized trials. J. Clin. Endocrinol. Metab. 2008;93:4600–4605. doi: 10.1210/jc.2006-2409. [DOI] [PubMed] [Google Scholar]
- Morris N.M., Udry J.R. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9:271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- O'Connor T.M., Jago R., Baranowski T. Engaging parents to increase youth physical activity a systematic review. Am. J. Prev. Med. 2009;37:141–149. doi: 10.1016/j.amepre.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Ogden C.L., Carroll M.D., Lawman H.G., Fryar C.D., Kruszon-Moran D., Kit B.K., Flegal K.M. Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA. 2016;315:2292–2299. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettitt D.J., Lawrence J.M., Beyer J., Hillier T.A., Liese A.D., Mayer-Davis B., Loots B., Imperatore G., Liu L. Association between maternal diabetes in utero and age at offspring's diagnosis of type 2 diabetes. Diabetes Care. 2008;31:2126–2130. doi: 10.2337/dc08-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettitt D.J., Talton J., Dabelea D., Divers J., Imperatore G., Lawrence J.M., Liese A.D., Linder B., Mayer-Davis E.J. Prevalence of diabetes in U.S. youth in 2009: the SEARCH for diabetes in youth study. Diabetes Care. 2014;37:402–408. doi: 10.2337/dc13-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher P.M., Kolotourou M., Chadwick P.M., Cole T.J., Lawson M.S., Lucas A., Singhal A. Randomized controlled trial of the MEND program: a family-based community intervention for childhood obesity. Obesity (Silver Spring) 2010;18(Suppl. 1):S62–S68. doi: 10.1038/oby.2009.433. [DOI] [PubMed] [Google Scholar]
- Sanigorski A.M., Bell A.C., Kremer P.J., Cuttler R., Swinburn B.A. Reducing unhealthy weight gain in children through community capacity-building: results of a quasi-experimental intervention program, be active eat well. Int. J. Obes. 2008;32:1060–1067. doi: 10.1038/ijo.2008.79. [DOI] [PubMed] [Google Scholar]
- Sauder K.A., Dabelea D., Bailey-Callahan R., Kanott Lambert S., Powell J., James R., Percy C., Jenks B.F., Testaverde L. Targeting risk factors for type 2 diabetes in American Indian youth: the tribal turning point pilot study. Pediatr Obes. 2018;13:321–329. doi: 10.1111/ijpo.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrewsbury V.A., Steinbeck K.S., Torvaldsen S., Baur L.A. The role of parents in pre-adolescent and adolescent overweight and obesity treatment: a systematic review of clinical recommendations. Obes. Rev. 2011;12:759–769. doi: 10.1111/j.1467-789X.2011.00882.x. [DOI] [PubMed] [Google Scholar]
- Tanner J. 2nd ed. Oxford Blackwell Scientific London; United Kingdom: 1962. Growth at Adolescence. [Google Scholar]
- TODAY Study Group Lipid and inflammatory cardiovascular risk worsens over 3 years in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care. 2013;36:1758–1764. doi: 10.2337/dc12-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODAY Study Group Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care. 2013;36:1735–1741. doi: 10.2337/dc12-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODAY Study Group Retinopathy in youth with type 2 diabetes participating in the TODAY clinical trial. Diabetes Care. 2013;36:1772–1774. doi: 10.2337/dc12-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODAY Study Group, Zeitler P., Hirst K., Pyle L., Linder B., Copeland K., Arslanian S., Cuttler L., Nathan D.M. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N. Engl. J. Med. 2012;366:2247–2256. doi: 10.1056/NEJMoa1109333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor-Locke C., Camhi S.M., Troiano R.P. A catalog of rules, variables, and definitions applied to accelerometer data in the National Health and nutrition examination survey, 2003-2006. Prev. Chronic Dis. 2012;9 doi: 10.5888/pcd9.110332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters E., de Silva-Sanigorski A., Hall B.J., Brown T., Campbell K.J., Gao Y., Armstrong R., Prosser L., Summerbell C.D. Interventions for preventing obesity in children. Cochrane Database Syst. Rev. 2011;12 doi: 10.1002/14651858.CD001871.pub3. [DOI] [PubMed] [Google Scholar]
- Wilfley D.E., Tibbs T.L., Van Buren D.J., Reach K.P., Walker M.S., Epstein L.H. Lifestyle interventions in the treatment of childhood overweight: a meta-analytic review of randomized controlled trials. Health Psychol. 2007;26:521–532. doi: 10.1037/0278-6133.26.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YMCA of the USA Organizational profile: facts and figures. 2016. http://www.ymca.net/organizational-profile/ Available at: