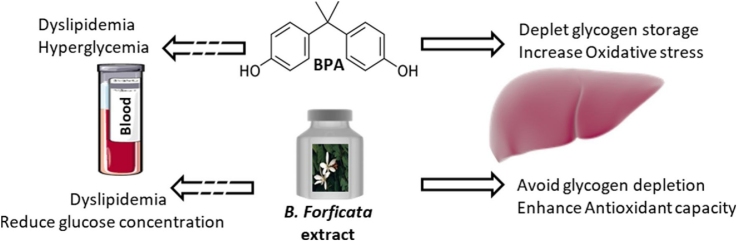
Graphical abstract
Keywords: Bisphenol A, Bauhinia forficata, Hepatoprotection, Oxidative stress, Antioxidant capacity
Highlights
-
•
Elevation in glycemia after Bisphenol A exposure is related to hepatic glycogen storage capacity.
-
•
B. forficata reduces hepatic oxidative stress which may be associated to its inherent free-radical scavenger capacity.
-
•
The association of BPA and B. forficata leads to dyslipidemia thereby suggesting caution with herbal intake.
Abstract
Bisphenol A (BPA) is an abundant raw material applied in the production of daily necessities, such as food cans, baby bottles, electronic and medical equipment. Phytotherapeutic use of plant preparations has long been known for multiple target medicinal uses. The species Bauhinia forficata is widely used as hypoglycemic, anti-inflammatory, antioxidant, diuretic and hypocholesterolemic agent. The aim of this study was to verify the effects of B. forficata extract in association with BPA exposure on serological parameters, hepatic antioxidant status and glycogen store capacity in Wistar rats. B. forficata was able to reduce BPA-induced glucose levels; it also prevented the early glucose elevation in control and BPA-exposed animals after the glucose provocative test. This effect was related to the hepatic glycogen content; while BPA reduced the hepatic glycogen deposits B. forficata treatment contributed to minimize it. BPA and B. forficata singly caused elevation in triacylglycerol and VLDL levels and reduction in cholesterol and LDL concentrations. BPA increased hepatic malondialdehyde levels and reduced catalase activity, thus inducing liver oxidative stress. Conversely, B. forficata treatment reduced malondialdehyde concentration without interfering with catalase activity; this antioxidant capacity is attributed to the flavonoids content (e.g., kaempferol and myricetin). Based on these results, we demonstrated that B. forficata commercial extract has hypoglycemic and antioxidant properties capable of minimizing the effects of BPA. However, it should be considered that the consumption of herbal commercial extract must be judicious to avoid deleterious health effects.
1. Introduction
Bisphenol A (BPA; 2,2-bis(4-hydroxyphenyl)propane; CAS# 80-05-7; 228 Da) is an organic synthetic chemical widely known for its endocrine disrupting effects and is considered a xenoestrogen with similar endogenous estrogen activity interfering with several metabolic processes [1,2]. BPA production is about 2–3 million tons/year, thereby contributing to the exposure of the chemical in the air, water and soil [3]. BPA can still be found in many daily consumable items such as plastic bottles, beverages containers, canned goods, medical devices, dental sealants, eyeglass lenses, PVC gloves, recycled paper, printing inks, toys, consumer electronics, foundry products, and surface of internal water pipes [[4], [5], [6]], thus revealing one of the reasons by which the risks of BPA exposure need a more comprehensive biological assessment. Recently, the European Chemical Agency has classified BPA in the list of substances of very high concern and other agencies have already manifested their concerns on BPA uses [7].
Nanomolar levels of BPA were demonstrated in the blood of adult and fetus, urine, meconium and breast milk, amniotic fluid and hair [4,[8], [9], [10]]. BPA is primarily considered an estrogenic endocrine disrupting chemical (EDC) in such a way that compelling evidences have shown the role of low-dose exposure to BPA in the female reproductive system and during prenatal and/or neonatal periods (for review see Richter et al. [11]). The effects caused by BPA exposure are related to its ability to bind to the classical and non-classical estrogen receptors, thus activating the G-protein-coupled receptor 30 (GPR30) via non-genomic pathways [12,13]. Ropero et al. [14] reported the relationship between estrogen receptors and blood glucose homeostasis, and more recently, researchers have investigated the role of EDC in other tissues and whether BPA is related to the development of metabolic diseases, e.g., type 2 diabetes, obesity and metabolic syndrome, but these associations require a more extensive background [15]. Xenobiotics are able to alter carbohydrate and lipid metabolism [14,16] and, although the precise mechanism whereby BPA can interfere with cell metabolism remains open for debate, BPA could direct or indirectly interfere with cell substrates utilization [1]; this may results in disarrangement of numerous endocrine signaling pathways. Lang et al. [17] demonstrated higher BPA concentrations to be associated with diabetes, and Sabanayagam et al. [8] associated higher urinary BPA levels with prediabetes state. Conversely, Melzer et al. [18] showed no significant association between BPA and diabetes. Howsoever, metabolic diseases are intrinsically related to carbohydrate and lipid metabolism impairment and BPA exposure may be considered a risk factor [13].
The most studies regarding the EDC effects are performed in animal models in which equivalent doses might produce deleterious effects in humans [19]. Experimental data have showed that low-dose exposure to BPA need to be taken into account with regard to the potential adverse effects for humans [9]. Interestingly, BPA and other endocrine-related compounds evoke a nonmonotonic or biphasic dose-response curve [20,21] that could mistakenly lead to the idea that only studies involving low doses of xenoestrogens deserve scientific attention. Because BPA is a ubiquitous agent and causes continuous contamination rather than the expected levels of free BPA exposure, we reinforce the need of studies exploring higher doses in attempt to overcome background contamination.
Beyond the classic role played by the liver in the maintenance of blood glucose concentrations via gluconeogenic pathway and/or via glycogenesis/glycogenolysis, hepatic cells also exert crucial role in regulating BPA metabolism. In brief, liver enzymes are responsible for glucuronidation and sulfation of BPA producing BPA glucuronide and BPA sulfate, respectively, both conjugated metabolites with little or no estrogenic activity. These processes occur in vivo via UDP-glucuronosyltransferases (e.g., UGT2B1) and phenol sulfotransferases (e.g., ST1A3) [21,22]. Reactive oxygen species (ROS) generated in the liver during BPA detoxification can damage the organ [1,23,24], thus evaluation of oxidative stress status in hepatic tissue needs a deeper investigation. Again, studies are divergent regarding oxidative stress-related outcomes and the wide range of tested doses might produce this inconsistence. Therefore, it would be relevant to avoid extremely low doses to better evaluate the consequences of its effects in human health [12].
Treatment of several diseases relies upon medicinal plant preparations and their complex mixtures of biologically active products [25]. For instance, in Brazil, a large part of population has none or limited access to commercial medicines, which makes the use of medicinal plants an easily accessible source of treatment [26] and, certainly, this is not a particular case around the world. In this scenario, Bauhinia forficata Link, also known as “paw of cow” or “cow’s hoof”, has been extensively investigated for its hypoglycemic, antioxidant, and hepatoprotective effects [[27], [28], [29]]. The genus Bauhinia comprises more than 300 species and several environmental factors and/or the plant part used in the phytopreparation may contribute to its therapeutic properties [29]. There are many bioactive compounds in medicinal plants accounting to their biological properties including alkaloids, carbohydrates, glycosides, flavonoids, steroids, terpenoids, peptides and amino acids, lipids, phenolics, glycopeptides and iridoids [25,30,31]. Notwithstanding some studies have achieved different and sometimes opposite outcomes with B. forficata treatment. It is well-accepted that plants’ flavonoids content is responsible for their beneficial effects and kaempferitrin (kampferol-3,7-O-(a)-l-dirhamnoside) is considered one of the main components responsible for B. forficata biological activity [31,32].
In this context, the present study was performed to evaluate whether the exposure to 50 mg/Kg BPA for 15 days is able to impair glucose usage and glycogen storage, lipid metabolism and hepatic oxidative metabolism, and how the treatment with B. forficata extract could improve these alterations.
2. Material and methods
2.1. Experimental groups
Twenty-eight male Wistar rats (90-days-old) were housed in an environmentally controlled clean-air room with standard temperature (22 ± 3 °C), 12 h light-and-dark cycles and relative humidity of 60 ± 5%. Filtered water in glass bottles and standard chow (Nuvilab-CR1®, Nuvital Ltda, Colombo, Paraná, Brazil) were supplied ad libitum. Animals were randomly assigned to one of the four groups (n = 7): C + PL group - received every other day water with 0.02% ethanol (vehicle) at the same dose of BPA intercalated with the same dose of saline (0,9%); C + BF group - received every other day 10% alcoholic extract of B. forficata (0,1 mL/10 g of body weight), intercalated with saline; BPA + PL group - received 50 mg/Kg BPA every other day intercalated with saline; BPA + BF group - received the same treatment of BPA + PL, but instead of saline solution, 10% alcoholic extract of BF was used. The period of treatment was 30 days. BPA (Sigma-Aldrich, USA) was freshly prepared and administered orally via gavage with 0.02% ethanol (vehicle) dissolved in water [1]. The commercial alcoholic B. forficata extract (made with leaves) was obtained from natural’ products pharmacy located in Londrina, Paraná, Brazil. The quality control of the alcoholic extract was carried out by identifying the compounds through mass spectrometry. Humidity was measured during the preparation, which was maintained at 10%. This formulation was chosen because of the indiscriminative use of this compound by population so that one of our aims was to verify whether this extract has any significant biological effect, regardless of its exact chemical composition. Animal body weight was evaluated every week along the experiment. All animals were handled in line with the ethical principles for animal research established by the Brazilian Council for Control of Animal Experimentation and all experimental procedures were approved by the Animal Ethics Committee of North of Parana State University, UENP, Brazil (protocol number: CEUA 3364). We also have conducted mass spectrometry analysis to determine the main components of B. forficata commercial extract as described elsewhere [33]. The results showed seven major components among them myricetin-O-(O-galloyl)-hexoside and kaempferol-3-O-(2-rhamnosyl) rutinoside, which has been related to its pharmacological activities [34].
2.2. Glycemic index and intraperitoneal glucose tolerance test
Blood glucose concentration was determined on the first day to certify there is no difference among the experimental groups. Two days before euthanasia, rats were fasted overnight (10–12 h) and then submitted to intraperitoneal glucose tolerance test (IpGTT). Glucose was administered (2 g/kg) at a single dose as 20% aqueous solution and then blood samples were obtained from tail vein before and at 30, 60 and 120 min after glucose administration. Blood glucose was measured by automatic glucose analyzer (Accu-Check Active, Roche®, SP, Brazil). The trapezoidal rule was used to determine the area under the curve (AUC).
2.3. Morphometric and serum biochemical determinations
After 30 days of experimental procedures and in fasted state, all rats were anesthetized with i.p. injections of 0.1 mL of 1% sodium pentobarbital for the measurement of body length (nose-to-anus or nose-anal length). The body weight and body length were used to calculate body mass index ((BMI, g/cm2) = body weight/length2) and Lee-index ((g/cm) = cube root of body weight/length) [35]. Then, rats were euthanized using barbiturate overdose (150 mg/kg of sodium pentobarbital). Blood samples were collected via cardiac puncture, placed into centrifuge tubes, allowed to clot to obtain the serum and centrifuged at 6.000 rpm for 15 min. Triacylglycerol (TG), total cholesterol (TC) and high-density lipoprotein cholesterol (HDL) were determined in serum by enzymatic methods (test Kit CELM diagnosis, Modern Laboratory Equipment Company, São Paulo, SP, Brazil). The very-low-density lipoprotein cholesterol (VLDL) was calculated by Friedewald equation and total protein was also quantified [36]. Low-density lipoprotein cholesterol (LDL) concentration was measured by the sodium phosphotungstate/Mg2+ method [37], using an enzymatic colorimetric method. Tissue samples ( ± 200 mg) of liver were dissected, washed with ice-cold saline solution and immediately frozen at −80 °C and other samples of hepatic tissue were excised for histological preparations.
2.4. Hepatic tissue determinations
Liver samples were homogenized with 0.01 M sodium phosphate buffer (pH 7.4), using l-beader cell disrupter with 3 mm zirconium beads (3.720 rpm, 1 min). Thereafter, the homogenate was centrifuged at 10.000 rpm for 15 min at 4 °C, and the supernatant was collected and processed for triacylglycerol determination and oxidative stress analyses. Catalase activity was determined with phosphate buffer (pH 7.0) in an assay mixture containing 0.019 M hydrogen peroxide and buffer solution in a final volume of 0.3 mL [38]. Malondialdehyde (MDA) was measured by the thiobarbituric acid reactive substances (TBARS) assay. Briefly, liver homogenate was prepared with H2SO4, 0.05 M, centrifuged in 10% trichloroacetic acid, and then reacted with 0.67% thiobarbituric acid. The samples were read in a spectrophotometer at 535 nm. MDA is considered an important marker for lipid peroxidation as a result of oxidative stress injury [39].
2.5. Histological procedures
Small pieces of the left lobes of liver were fixed in methacarn for 3 h, stocked in 70% alcohol and then routinely processed for paraffin-embedded. The blocks were cut into 4-μm-thick, obtaining semi-serial sections that were disposed on light microscopy slides. Detection of glycogen was obtained by the immersion of the slides in 1% periodic acid solution for 10 min and, after subsequent washes in water, sections were submitted to Schiff-reactive for 30 min. The sections were counter-stained with Harris’ hematoxylin. Slides were analyzed by common light microscopy connected to digital camera (Zeiss) and photomicrographs were obtained under 20x objective.
For glycogen analysis in hepatic samples, two different zones were considered as follows: zone I, located around the portal tracts and zone III, encircle the central veins. According to the intensity of zones staining, the amount of glycogen was classified as light, moderate or intense.
2.6. Statistical analysis
Statistical comparisons were performed by two-way analysis of variance (ANOVA - two factors: BPA and B. forficata treatment) complemented by Tukey’s test. The results are presented as the means ± standard deviations (SD). Statistical significance was set at p < 0.05. Sigma Plot version 11.0 was used for all graphic design and statistics.
3. Results
3.1. B. forficata ameliorated the hyperglycemic effects of BPA
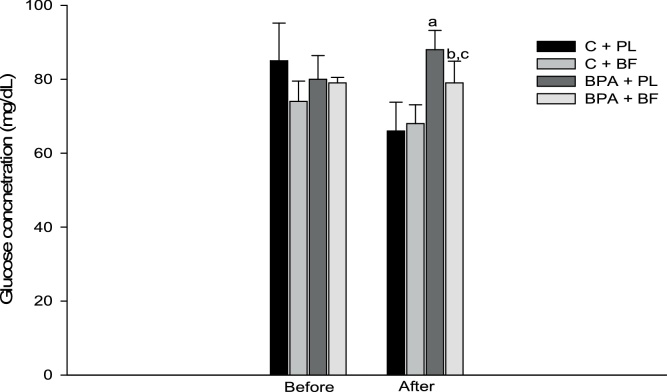
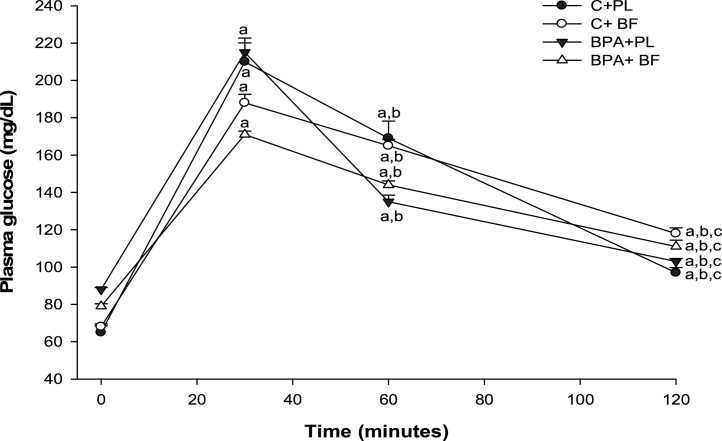
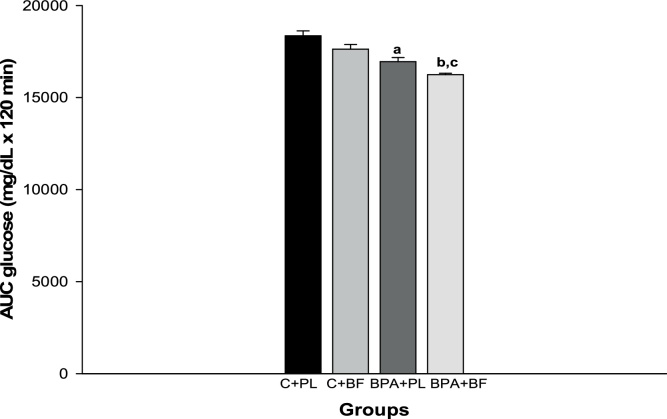
There was no difference in the initial body weight, but all animals had higher final body weight and neither BPA nor B. forficata treatment influenced the body weight gain (Table 1). BMI, Lee index and body length were similar among the groups and liver weight and liver weight/body weight ratio was higher in control animals. The initial blood glucose concentrations did not differ among groups. After 30 days of treatment and in fasting state, BPA + PL group had higher blood glucose levels compared with C + PL (Fig. 1). After 30 min of glucose administration, IpGTT results showed that all animals presented elevated glycaemia and B. forficata-treated animals had lower glucose levels than their respective control groups (Fig. 2). As shown by the area under the curve, the animals receiving BPA alone or associated with B. forficata had lower glucose levels as a result of impaired glucose utilization caused by BPA (Fig. 3). Briefly, we have demonstrated different responses for glucose metabolism during fasting or feeding states and B. forficata extract seems to avoid or minimize the hyperglycemia in both conditions. Importantly, BPA effects appear to be more correlated with a depletion of carbohydrate storages.
Table 1.
Morphometric characteristics of rats receiving saline (C + PL); saline solution and B. forficata (C + BF); Bisphenol A and saline solution (BPA + PL); Bisphenol A and B. forficata (BPA + BF).
| PARAMETERS | GROUPS |
|||
|---|---|---|---|---|
| C + PL | C + BF | BPA + PL | BPA + BF | |
| IBW (g) | 291.12 ± 30.09 | 286.11 ± 25.06 | 287.83 ± 32.42 | 284.23 ± 22.68 |
| FBW (g) | 346.56 ± 46.95* | 345.44 ± 15.32* | 335.10 ± 44.11* | 328.79 ± 35.23* |
| WG (g) | 54.64 ± 20.34 | 59.33 ± 24.26 | 47.27 ± 17.02 | 44.56 ± 13.91 |
| BMI (g/cm2) | 1.73 ± 0.11 | 1.71 ± 0.18 | 1.74 ± 0.19 | 1.69 ± 0.19 |
| Lee index (g/cm3) | 0.49 ± 0.02 | 0.49 ± 0.03 | 0.50 ± 0.02 | 0.49 ± 0.03 |
| LW(g) | 10.32 ± 2.09 | 10.01 ± 1.46 | 8.26 ± 0.89a | 8.44 ± 1.12 |
| FBW/LW | 0.030 ± 0.004 | 0.029 ± 0.004 | 0.025 ± 0.003a | 0.026 ± 0.002 |
| BC (cm) | 24.14 ± 0.69 | 24.14 ± 0.38 | 23.57 ± 1.13 | 24.00 ± 0.82 |
| BL (cm) | 14.14 ± 0.90 | 14.28 ± 0.95 | 13.86 ± 0.69 | 14.00 ± 1.15 |
Data expressed as mean ± standard deviation; p < 0.05. * - differs significantly from IBW; Letter a - differs significantly from the C + PL group. Initial body weight (IBW), final body weight (FBW), weight gain (WG), body mass index (BMI), liver weight (LW), FBW/LW ratio, body circumference (BC), body length (BL).
Fig. 1.
Fasting glucose concentrations before and after BPA and B. forficata treatment of animals receiving saline solution (C + PL); B. forficata and saline solution (C + BF); Bisphenol A and saline (BPA + PL); Bisphenol A + B. forficata (BPA + BF). Data expressed as mean ± standard deviation; p < 0.05. Letters: a - differs significantly from the C + PL group; b - differs significantly from the BPA + PL group; c - differs significantly from the C + BF group.
Fig. 2.
Intraperitoneal glucose tolerance test of animals receiving saline solution (C + PL); B. forficata and saline solution (C + BF); Bisphenol A and saline (BPA + PL); Bisphenol A + B. forficata (BPA + BF). Data expressed as mean ± standard deviation; p < 0.05. Letters: a - differs significantly from the C + PL group; b - differs significantly from the BPA + PL group; c - differs significantly from the C + BF group.
Fig. 3.
Area under the curve for intraperitoneal glucose tolerance test of animals receiving saline solution (C + PL); B. forficata and saline solution (C + BF); Bisphenol A and saline (BPA + PL); Bisphenol A + B. forficata (BPA + BF). Data expressed as mean ± standard deviation; p < 0.05. Letters: a - differs significantly from the C + PL group; b - differs significantly from the BPA + PL group; c - differs significantly from the C + BF group.
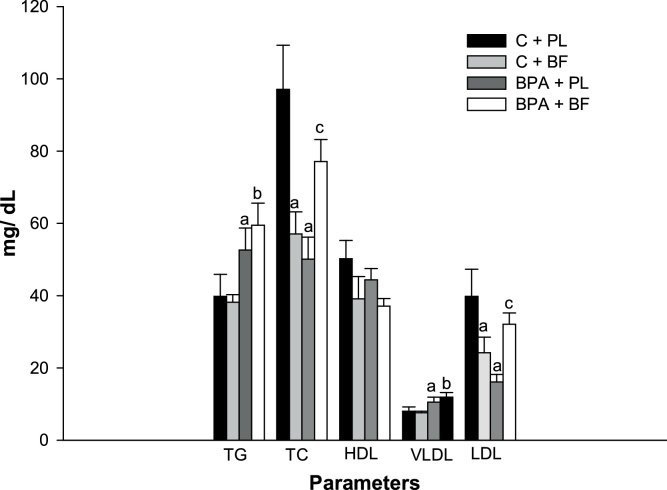
3.2. B. forficata and BPA acted synergistically to elevate total cholesterol and LDL concentrations
Lipid profile is presented in Fig. 4. BPA exposure increased triacylglycerol and VLDL-cholesterol concentrations and B. forficata extract did not improve these parameters. Total cholesterol and LDL fraction were lower in the BPA + PL and C + BF groups whereas this effect was not seen in BPA + BF. In fact, BPA and B. forficata together actually increased LDL levels indicating this association to have an undesirable impact on lipid profile, enhancing the risk factors for metabolic diseases. Overall, HDL cholesterol was unchanged following the treatments (Fig. 4).
Fig. 4.
Lipid profile of animals receiving saline solution (C + PL); B. forficata and saline solution (C + BF); Bisphenol A and saline (BPA + PL); Bisphenol A + B. forficata (BPA + BF). Data expressed as mean ± standard deviation; p ≤ 0.05. Letters: a - differs statistically from the C + PL group; b - differs statistically from the C + BF group; c - differs statistically from the BPA + PL group. TG: Triacylglycerol; TC: Total cholesterol.
3.3. BPA increased hepatic oxidative injury and B. forficata acted as antioxidant
Table 2 presents the hepatic parameters such as triacylglycerol content, MDA and catalase activity. After treatments, B. forficata and BPA promoted higher levels of triacylglycerol. The levels of MDA were similar between the control groups but were higher after BPA exposure; notably, treatment with B. forficata was efficient to reduce the levels around normal values. The antioxidant activity of catalase was significantly increased by the treatment of B. forficata; otherwise, catalase activity was reduced in the presence of BPA.
Table 2.
Hepatic concentrations of protein, triacylglycerol, malondialdehyde and catalase activity of animals receiving saline (C + PL); saline solution and B. forficata (C + BF); Bisphenol A and saline solution (BPA + PL); Bisphenol A and B. forficata (BPA + BF).
| PARAMETERS | GROUPS |
|||
|---|---|---|---|---|
| C + PL | C + BF | BPA + PL | BPA + BF | |
|
Protein (mg/mg tissue) |
123.29 ± 19.33 | 105.80 ± 20.79 | 134.88 ± 11.08 | 128.47 ± 16.90 |
|
Triacylglycerol (mg /mg tissue) |
7.36 ± 2.79 | 11.89 ± 3.50a | 13.47 ± 2.02a | 11.53 ± 2.05 |
| Malondialdehyde (nmol/mg tecido) | 79.13 ± 22.35 | 73.61 ± 15.50 | 217.06 ± 35.45a | 65.63 ± 14.13c |
|
Catalase (μmol/mg protein) |
1280.36 ± 259.71 | 994.28 ± 162.29a | 713.33 ± 57.46a | 740.89 ± 133.45b |
Data expressed as mean ± standard deviation; p ≤ 0.05. Letters: a - differs statistically from the C + PL group; b - differs statistically from group C + BF; c - differs statistically from group BPA + PL.
3.4. BPA and B. forficata altered the capacity of hepatic glycogen storage
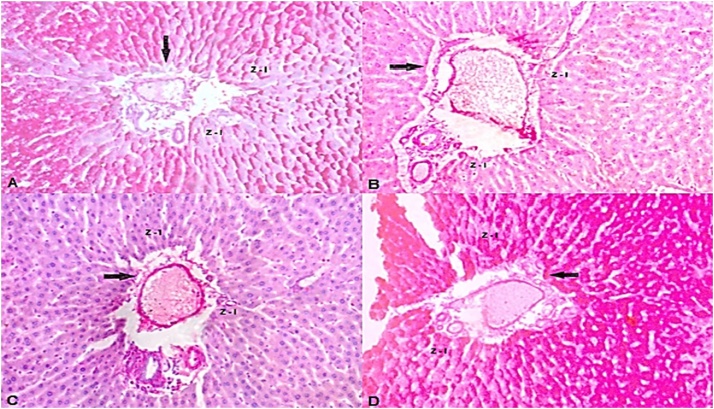
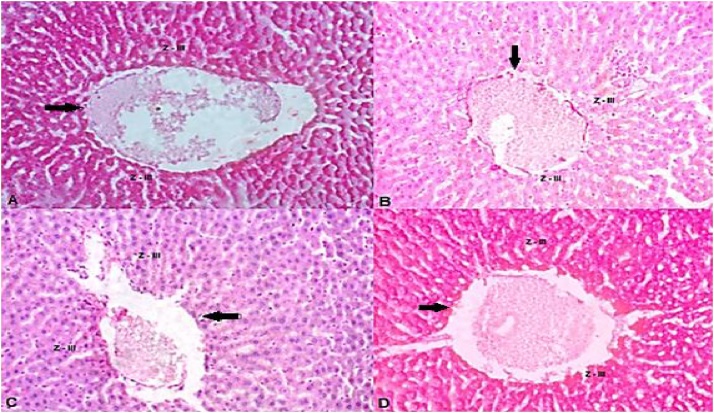
Histopathological data have showed that hepatocytes located in zone III, in control group, contained great amount of glycogen granules that were PAS-positive, while at the zone I they were classified as light (Table 3). The two zones of hepatic lobules of animals receiving B. forficata alone were classified as intense and moderate, respectively. Both groups that received BPA had negative reactions to PAS, in both zones, being classified as light, which reveals the absence of glycogen in the liver of these animals. (Figs. 5 e 6).
Table 3.
Amount of glycogen in hepatic tissue of animals receiving saline (C + PL); saline solution and B. forficata (C + BF); Bisphenol A and saline solution (BPA + PL); Bisphenol A and B. forficata (BPA + BF).
| GROUPS | HEPATIC LOBES |
|
|---|---|---|
| ZONE I | ZONE III | |
| C + PL | + | + + |
| C + BF | + + + | + + |
| BPA + PL | + | + |
| BPA + BF | + | + |
Glycogen content measured by the intensity of the histochemical reaction. According to the intensity of the staining the amount of glycogen was considered light (+), moderate (++) and intense (+++).
Fig. 5.
Photomicrography of the hepatic tissue corresponding to Zone I, around the portal system, pointed by the arrows. A - Animals that received saline solution; (C + PL); B - Animals that received Bisphenol A and saline; (BPA + PL); C - Animals that received Bisphenol A and B. forficata (BPA + BF); D - Animals that received B. forficata and saline solution (C + BF).
4. Discussion
The aim of this study was first to determine whether exposure to 50 mg/kg BPA impair glucose and lipid metabolism and what would be the effects of BPA over hepatic histology and oxidative stress profile; second, we verified whether the treatment with commercial extract of B. forficata, a potential natural agent, is able to ameliorate these eventual deleterious effects. There are several studies evaluating the effects of lower doses of BPA on the reproductive system in rats [21,[40], [41], [42], [43], [44], [45]]; however, few studies have reported the high-dose effects of 50 mg/Kg BPA [1,46,47]. This fact brings to the light an interesting paradigm in which if low doses of a drug induce deleterious effects, increasing it would be even more harmful (“the dose makes the poison”). Just like for other EDCs, BPA evokes a nonmonotonic dose response [21,40] and we have found that some effects caused by low doses of BPA were not seen with 50 mg/kg and vice-versa, as described below. Moreover, BPA exposure may come from a diversity of sources [48]; therefore, the effects of higher dose of BPA seem to be quite plausible. It is worth mentioning that 50 mg/kg is considered the lowest observed adverse effect level (LOAEL) for BPA [40]. Bauhinia forficata or “cow’s foot” was first described by Link in 1821 and belongs to the fabacea family; it is abundantly used because of its phytotherapeutic properties [26]. Several patents were deposited with B. forficata being the active principle [25] but is still very common the use of B. forficata extract from ordinary pharmaceutical manipulations. The extract used in this study was elaborated with plant’ leaves which are rich in flavonoids and the results from mass spectrometry analysis [33] showed that the commercial alcoholic extract of B. forficata contains, among other compounds, the kaempferitrin which is considered the chemical marker for B. forficata [25,28,34].
Following BPA exposure, the changes in body weight are still a controversial issue. Rubin et al. [40] showed that perinatal male rats exposed to different low doses of BPA (0.25–250 μg) had elevation in body weight, but when BPA exposure was extended to peripubertal period, this difference was attenuated. Interestingly, mice exposed to BPA alone during peripubertal period had elevation in body weight [20]. In our study, BPA or B. forficata did not alter the final body weight similarly with the results obtained by Hassan et al. [1]; otherwise, these same authors demonstrated that lower doses of BPA significantly affect body weight gain. Taken together, these results reinforce the biphasic dose-response effect of BPA [49] and indicate that exposure window should be considered. Other morphometric parameters, such as BMI and Lee Index, were similar among the experimental groups (Table 1), but the use of these markers is still relevant [20,50]. For instance, Harley et al. [51] showed the association of BPA levels with BMI and waist circumference in children. In contrast to the study of Hassan et al. [1], rats that received 50 mg/kg BPA presented reduced liver sizes than control animals. Although no significant differences in protein content was found we believe that BPA can influence the structural components of the organ, but a deeper investigation is needed.
Fasting glucose levels are presented in Fig. 1. Both groups receiving BPA showed elevated glucose concentrations and B. forficata treatment reduced this parameter. IpGTT was performed to evaluate the glycemic response in animal receiving BPA, B. forficata or both, after a glucose overload (Fig. 2). Again, rats that received BPA showed increased glucose basal levels pointing out to BPA-induced hyperglycemia. Treatment with B. forficata in both C + BF and BPA + BF groups was able to avoid glucose elevation after 30 min compared with their respective controls; glucose levels were decreased after 60 min in a similar way in all animals. Our results demonstrate B. forficata to influence the initial stages of glucose response, since after 120 min the four groups had similar glucose levels. Following glucose overload, BPA also reduced glycemia as it can be seen in the area under the curve (Fig. 3). in vitro assays have demonstrated that BPA can increase insulin-stimulated glucose uptake, which was attributed to increased amount of GLUT4 protein; this effect was not directly mediated by the estrogen receptor but seems to be involved with other nuclear receptors [52]. Interestingly, B. forficata in association with BPA reduced glucose circulating levels and this apparent synergic effect requires more investigation. Therefore, our results demonstrate that B. forficata extract reduces the fasting hyperglycemia caused by BPA and avoids glycemic elevation after glucose administration, suggesting its capacity to minimize hyperglycemia followed by glucose overload rather than a glucose-reducing effect. Exposure to higher dose of BPA reduced glucose concentration after glucose offering and this effect was more accentuated with BPA combined with BF. Similar results for BPA were corroborated by Alonso-Magdalena et al. (2006) following a glucose provocative test.
Hepatocyte is the most common liver cell and among their many functions stands out the accumulation of glycogen. Hepatocytes are polyhedral cells with acidophilic cytoplasm and small basophilic points that represent clusters of granular endoplasmic reticulum and free ribosomes. Asahi et al. [53] analyzed the effects of BPA in non-parenchymal hepatocytes and related endoplasmic reticulum stress-associated apoptosis thereby reinforcing the role of BPA on oxidative stress. Besides, hepatocytes are arranged in cords that branch to form a continuous three-dimensional meshwork. These structures are in the inner of hepatic lobules, which are bordered by connective tissue, formed by branches of portal vein, lymphatic vessel and biliary duct. The region around the portal triads, named zone I, is the first to receive nutrients and oxygen; therefore, these hepatocytes firstly capture glucose, converting into glycogen and are the first to release glucose during fasting periods. The zone III is located around the central vein of the hepatic lobules. These hepatocytes are the last to respond to toxin exposure. In the present work, the light presence of glycogen in both zones of liver of BPA + PL and BPA + BF animals (Fig. 5, Fig. 6) indicates that these animals had more pronounced glycogen depletion in periods of fasting. This finding can partly explain why those animals not challenged with glucose overload showed circulating glucose elevation and B. forficata was able to minimize it. Although the underlying mechanism by which B. forficata regulates glycogen deposit was not the focus of this study, the effect over hepatic tissue caused by natural compounds can ameliorate liver processes, thus contributing to the regulation of glycogen metabolism. Jayashree et al. [54] showed that decreased glycogen concentration caused by BPA is likely due to the reduction in Akt phosphorylation which leads to decreased glycogen synthesis. In agreement with our results, Zaulet et al. [55] demonstrated silymarin, a natural compound, was able to restore the decreased glycogen deposits following BPA exposure. These same authors stated that, in response to harmful toxicant effect, hepatic glycogenolysis is activated thus protecting cells from oxidative injury. Although B. forficata was able to augment glycogen deposition in control animals, these results were not found in BPA-treated animals; this explains the lower glucose level in animals from control compared to BPA groups. Our results demonstrated B. forficata extract to induce hepatic glycogenesis or inhibit glycogenolysis in animals unexposed to BPA, which can contribute to B. forficata hypoglycemic effect. Also, BPA has diminished the hepatic capacity to store the polysaccharide and the treatment did not reverse this effect.
Fig. 6.
Photomicrography of the hepatic tissue corresponding to Zone III, around the vein, pointed by the arrows. A - Animals that received saline solution; (C + PL); B - Animals that received Bisphenol A and saline; (BPA + PL); C - Animals that received Bisphenol A and B. forficata (BPA + BF); D - Animals that received B. forficata and saline solution (C + BF).
BPA elevated serum triacylglycerol concentration and B. forficata did not reduce this parameter. Similarly, triacylglycerol levels in control groups were not altered by B. forficata and this pattern was observed also in VLDL results (Fig. 4). In accordance with Rubin et al. [40], lower doses of BPA did not alter triacylglycerol levels in females exposed during perinatal and peribubertal periods, reinforcing the dose- and time-dependent BPA’ effects. On the other hand, Meng et al. [56] showed that BPA exposure promoted the expression of hepatic lipid synthesis and fatty acid accumulation-related genes. Our tests were carried out with animals in fasting state so the only source for circulating triacylglycerol is the liver endogenous biosynthesis, which is build up and transported in the VLDL form. It can be assumed, therefore, that BPA influences lipoprotein formation and B. forficata has no influence over this parameter. Total cholesterol was lower in C + BF and BPA + PL, but when these two compounds were offered together, cholesterol levels were increased. Neither BPA nor B. forficata affected HDL concentrations but the same profile found for cholesterol occurred for the LDL fraction. These results suggest the potential health risks of BPA should be deeply evaluated. Studies relating lipidemic profile with BPA and mainly with B. forficata are not so numerous and the results are still controversial. For instance, Olsen et al. [57] evaluating an elderly population found no association between BPA and LDL, but Metwally et al. [50] showed higher levels of triacylglycerol and LDL-cholesterol in patients with higher concentrations of BPA. These same authors attributed this effect to the adipokines pattern found in these patients and the participation of this adipocyte-specific hormone has already been proposed by Hugo et al. [16]. The alterations found after BPA exposure can also be related to the capacity of this EDC to accelerate adipocyte differentiation, at least in vitro [58]. Another possible biochemical explanation for BPA effects over lipid profile involves its estrogenic properties and the ability to regulate key proteins involved with cholesterol homeostasis, such as liver receptor homologue-1 (LRH-1) or is involved with adipocyte maintenance since estrogens are potent regulators of fat metabolism [15]. BPA exposure have altered cholesterolemia of mice, which was related to the overexpression of key genes involved with cholesterol biosynthesis [59] or with hepatic lipid synthesis and fatty acid accumulation [56]. Our results have demonstrated that even for a short period of time, 50 mg/kg of BPA can alter lipid profile in male rats, similarly to what was found in female animals [46]. These last authors demonstrated that long- term exposure to BPA influences lipid profile as well as liver and antioxidant enzymes. Lino Cde et al. [60], studying alloxan-induced diabetes rats, found no alterations in LDL levels after B. forficata treatment and Damasceno et al. [61] showed elevation in total cholesterol and triacylglycerol concentrations in diabetic pregnant rats caused by B. forficata treatment. However, there are still no studies evaluating the toxicological effects of plants from genus Bauhinia [34]. In this context, our results shed light on these possible metabolic adverse effects and stimulate future investigations. Interestingly, Ecker et al. [62] already described that B. forficata consumption should be better evaluated for its capacity to induce mitochondrial damages.
Hepatic parameters are presented in Table 2. Triacylglycerol content were higher in animals receiving BPA and B. forficata, which is in accordance with Rubin et al. [40] who demonstrated lower doses of BPA to increase triacylglycerol accumulation in hepatic tissue of female rats during earlier stages of life. Our results indicate BPA as a potential EDC to be implicated in the elevation of risk for metabolic liver disease. Since no signal of hepatic steatosis was observed, we assumed that the triacylglycerol elevation caused by the extract is not a relevant side effect. Oxidative stress is directly related to tissue injury in different organs [63], and the capacity of BPA to increase ROS has already been described in addition to the antioxidant properties of B. forficata [12,64]. Although B. forficata elevated triacylglycerol content in control animals, this was not accompanied by elevation in MDA levels, even with the reduced catalase activity observed in this group. BPA exposure has caused a considerable reduction in catalase activity and increased liver MDA concentration, thus indicating oxidative damage. As expected, B. forficata reduced MDA levels induced by BPA exposure, but this protective effect was not associated with elevation in catalase activity (Table 2). BPA metabolism can generate sufficient ROS to exceed the capacity of intracellular defenses. In accordance with other studies, our results evidenced the BPA capacity to induce damage to biomolecules mainly in membrane lipids [12,65]. Treatment with B. forficata can ameliorate oxidative stress damage, which can be explained by the inherent antioxidant capacities of flavonoids and phenolic acids present in B. forficata leaf extract, which can act as non-enzymatic antioxidants [27,28,34,[66], [67], [68]]. This seems quite plausible since C + BF animals also showed elevation in triacylglycerol content suggesting increased substrate for peroxidation, but MDA concentration was not altered even with catalase activity reduction. Corroborating these findings, BPA itself appears to cause reduction in both expression and activity of catalase [1,69] and B. forficata also demonstrated to reduce these parameters [70]. A limitation of our study is that we have not determined other antioxidant enzymes activities. Bindhumol et al. [71] showed reduction of superoxide dismutase (SOD) as well as glutathione reductase and peroxidase activities in the liver of rats treated with lower doses of BPA. On the other hand, an increase in glutathione levels were found in animals exposed to 25 μg of BPA [41]. Curiously, mice receiving 50 mg/kg BPA had reduction of hepatic SOD and increase in catalase activity, but no alteration in the glutathione system [72]. A review of the effects of BPA on antioxidant enzymes can be found in Gassman [12]. Diabetic rats treated with B. forficata showed no alteration in GSH content but had a drastic reduction in SOD activity (Damasceno et al. [61]. Conversely, diabetic mice treated with B. forficata tea showed no differences in SOD and catalase activities [27]. Taken together, these outcomes clearly demonstrate that more studies are necessary to achieve a consensus about pro and antioxidant effects of BPA and B. forficata.
In conclusion, we have demonstrated that the dose of 50 mg/Kg BPA is able to cause hepatic deleterious effects mainly associated with oxidative stress and may alter hepatic glycogen storage thus reflecting directly in glucose circulating levels. Bauhinia forficata minimize the hyperglycemic effect caused by BPA and seems to act synergistically in reducing glycaemia after glucose overload. B. forficata extract and BPA alone reduced total cholesterol and LDL levels; however, cholesterol and LDL levels were elevated after BPA and B. forficata treatment, suggesting wariness for the indiscriminate consumption of natural compounds. The most beneficial effect promoted by B. forficata extract was related to its free-radical scavenger capacity that may be attributed to their extract components rather than any other enzymatic induction.
Authors’ contributions
MSF and JPFS carried out the histological analysis. PRB and LBG conducted all experiment procedures and biochemical analysis. FGC conducted statistical analysis and help with the discussion. GSFA and LGAC participated in the elaboration and revision of the manuscript. FRFS conceived and designed the study, wrote, and revised the manuscript.
Conflict of interest
Authors warrant that the article is original, does not infringe upon any copyright or other proprietary right of any third party and has not been previously published. Moreover, the final version of the manuscript is in accordance with the authors. All authors declare that there are no competing interests.
Transparency document
Acknowledgements
This study received financial support from CNPq and Fundação Araucária, Brazil.
References
- 1.Hassan Z.K., Elobeid M.A., Virk P., Omer S.A., ElAmin M., Daghestani M.H., Alolayan E.M. Bisphenol A induces hepatotoxicity through oxidative stress in rat model. Oxid. Med. Cell. Longev. 2012:1–6. doi: 10.1155/2012/194829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goloubkova T., Spritzer P.M. Xenoestrogênios: o exemplo do Bisfenol-A. Arq. Bras. Endocrinol. Metabol. 2000;44(4):323–330. [Google Scholar]
- 3.Menale C., Mita D.G., Diano N., Diano S. Adverse effects of bisphenol a exposure on glucose metabolism regulation. Open Biotechnol. J. 2016;10(Suppl-1, M10):122. 130. [Google Scholar]
- 4.Karzi V., Tzatzarakis M.N., Vakonaki E., Alegakis T., Katsikantami I., Sifakis S., Rizos A., Tsatsakis A.M. Biomonitoring of bisphenol A, triclosan and perfluorooctanoic acid in hair samples of children and adults. J. Appl. Toxicol. 2018;38(8):1144–1152. doi: 10.1002/jat.3627. [DOI] [PubMed] [Google Scholar]
- 5.Tzatzarakis M.N., Karzi V., Vakonaki E., Goumenou M., Kavvalakis M., Stivaktakis P., Tsitsimpikou C., Tsakiris I., Rizos A.K., Tsatsakis A.M. Bisphenol A in soft drinks and canned foods and data evaluation. Food Addit. Contam. Part B Surveill. 2017;10(2):85–90. doi: 10.1080/19393210.2016.1266522. [DOI] [PubMed] [Google Scholar]
- 6.Tzatzarakis M.N., Vakonaki E., Kavvalakis M.P., Barmpas M., Kokkinakis E.N., Xenos K., Tsatsakis A.M. Biomonitoring of bisphenol A in hair of Greek population. Chemosphere. 2015;118:336–341. doi: 10.1016/j.chemosphere.2014.10.044. [DOI] [PubMed] [Google Scholar]
- 7.Nakanishi J., Miyamoto K.-I., Kawasaki H., Bisphenol A. Risk assessment document. AIST) NIoAISaT. 2007 [Google Scholar]
- 8.Sabanayagam C., Teppala S., Shankar A. Relationship between urinary bisphenol A levels and prediabetes among subjects free of diabetes. Acta Diabetol. 2013;50(4):625–631. doi: 10.1007/s00592-013-0472-z. [DOI] [PubMed] [Google Scholar]
- 9.vom Saal F.S., Akingbemi B.T., Belcher S.M., Birnbaum L.S., Crain D.A., Eriksen M., Farabollini F., Guillette L.J., Jr., Hauser R., Heindel J.J., Ho S.M., Hunt P.A., Iguchi T., Jobling S., Kanno J., Keri R.A., Knudsen K.E., Laufer H., LeBlanc G.A., Marcus M., McLachlan J.A., Myers J.P., Nadal A., Newbold R.R., Olea N., Prins G.S., Richter C.A., Rubin B.S., Sonnenschein C., Soto A.M., Talsness C.E., Vandenbergh J.G., Vandenberg L.N., Walser-Kuntz D.R., Watson C.S., Welshons W.V., Wetherill Y., Zoeller R.T. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod. Toxicol. 2007;24(2):131–138. doi: 10.1016/j.reprotox.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welshons W.V., Nagel S.C., vom Saal F.S. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147(6 Suppl):S56–S69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- 11.Richter C.A., Birnbaum L.S., Farabollini F., Newbold R.R., Rubin B.S., Talsness C.E., Vandenbergh J.G., Walser-Kuntz D.R. F.S. vom Saal, In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 2007;24(2):199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gassman N.R. Induction of oxidative stress by bisphenol A and its pleiotropic effects. Environ. Mol. Mutagen. 2017;58(2):60–71. doi: 10.1002/em.22072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rochester J.R. Bisphenol A and human health: a review of the literature. Reprod. Toxicol. 2013;42:132–155. doi: 10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Ropero A.B., Alonso-Magdalena P., Garcia-Garcia E., Ripoll C., Fuentes E., Nadal A. Bisphenol-A disruption of the endocrine pancreas and blood glucose homeostasis. Int. J. Androl. 2008;31(2):194–200. doi: 10.1111/j.1365-2605.2007.00832.x. [DOI] [PubMed] [Google Scholar]
- 15.Chevalier N., Fenichel P. Endocrine disruptors: new players in the pathophysiology of type 2 diabetes? Diabetes Metab. 2015;41(2):107–115. doi: 10.1016/j.diabet.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Hugo E.R., Brandebourg T.D., Woo J.G., Loftus J., Alexander J.W., Ben-Jonathan N. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ. Health Perspect. 2008;116(12):1642–1647. doi: 10.1289/ehp.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lang I.A., Galloway T.S., Scarlett A., Henley W.E., Depledge M., Wallace R.B., Melzer D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300(11):1303–1310. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- 18.Melzer D., Rice N.E., Lewis C., Henley W.E., Galloway T.S. Association of urinary bisphenol a concentration with heart disease: evidence from NHANES 2003/06. PLoS One. 2010;5(1) doi: 10.1371/journal.pone.0008673. e8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groff T. Bisphenol A: invisible pollution. Curr. Opin. Pediatr. 2010;22(4):524–529. doi: 10.1097/MOP.0b013e32833b03f8. [DOI] [PubMed] [Google Scholar]
- 20.Yang M., Chen M., Wang J., Xu M., Sun J., Ding L., Lv X., Ma Q., Bi Y., Liu R., Hong J., Ning G. Bisphenol a promotes adiposity and inflammation in a nonmonotonic dose-response way in 5-week-old male and female C57BL/6J mice fed a low-calorie. Diet Endocrinol. 2016;157(6):2333–2345. doi: 10.1210/en.2015-1926. [DOI] [PubMed] [Google Scholar]
- 21.Vandenberg L.N., Maffini M.V., Sonnenschein C., Rubin B.S., Soto A.M. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr. Rev. 2009;30(1):75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tyl R.W. Commentary to the CERHR expert panel report on bisphenol A birth defects. Dev. Reprod. Toxicol. 2008;83(3):152. doi: 10.1002/bdrb.20155. [DOI] [PubMed] [Google Scholar]
- 23.Seachrist D.D., Bonk K.W., Ho S.M., Prins G.S., Soto A.M., Keri R.A. A review of the carcinogenic potential of bisphenol A. Reprod. Toxicol. 2016;59:167–182. doi: 10.1016/j.reprotox.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korkmaz A., Ahbab M.A., Kolankaya D., Barlas N. Influence of vitamin C on bisphenol A, nonylphenol and octylphenol induced oxidative damages in liver of male rats. Food Chem. Toxicol. 2010;48(10):2865–2871. doi: 10.1016/j.fct.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 25.de Souza B.V.C., Moreira Araujo R., Silva O.A., Faustino L.C., Goncalves M.F.B., Dos Santos M.L., Souza G.R., Rocha L.M., Cardoso M.L.S., Nunes L.C.C. Bauhinia forficata in the treatment of diabetes mellitus: a patent review. Expert Opin. Ther. Pat. 2018;28(2):129–138. doi: 10.1080/13543776.2018.1409208. DOI: /13543776.2018.1409208. [DOI] [PubMed] [Google Scholar]
- 26.Trojan-Rodrigues M., Alves T.L., Soares G.L., Ritter M.R. Plants used as antidiabetics in popular medicine in Rio Grande do Sul, southern Brazil. J. Ethnopharmacol. 2012;139(1):155–163. doi: 10.1016/j.jep.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 27.Salgueiro A.C., Folmer V., da Silva M.P., Mendez A.S., Zemolin A.P., Posser T., Franco J.L., Puntel R.L., Puntel G.O. Effects of Bauhinia forficata tea on oxidative stress and liver damage in diabetic mice. Oxid. Med. Cell. Longev. 2016 doi: 10.1155/2016/8902954. 8902954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miceli N., Buongiorno L.P., Celi M.G., Cacciola F., Dugo P., Donato P., Mondello L., Bonaccorsi I., Taviano M.F. Role of the flavonoid-rich fraction in the antioxidant and cytotoxic activities of Bauhinia forficata Link. (Fabaceae) leaves extract. Nat. Prod. Res. 2016;30(11):1229–1239. doi: 10.1080/14786419.2015.1050671. [DOI] [PubMed] [Google Scholar]
- 29.Cechinel Filho V. Chemical composition and biological potential of plants from the genus Bauhinia. Phytother. Res. 2009;23(10):1347–1354. doi: 10.1002/ptr.2756. [DOI] [PubMed] [Google Scholar]
- 30.Vinayagam R., Jayachandran M., Xu B. Antidiabetic effects of simple phenolic acids: a comprehensive review. Phytother. Res. 2016;30(2):184–199. doi: 10.1002/ptr.5528. [DOI] [PubMed] [Google Scholar]
- 31.Vinayagam R., Xu B. Antidiabetic properties of dietary flavonoids: a cellular mechanism review. Nutr. Metab. (Lond). 2015;12(60) doi: 10.1186/s12986-015-0057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franco R.R., da Silva Carvalho D., de Moura F.B.R., Justino A.B., Silva H.C.G., Peixoto L.G., Espindola F.S. Antioxidant and anti-glycation capacities of some medicinal plants and their potential inhibitory against digestive enzymes related to type 2 diabetes mellitus. J. Ethnopharmacol. 2018;215:140–146. doi: 10.1016/j.jep.2017.12.032. [DOI] [PubMed] [Google Scholar]
- 33.Sampaio C.F., Lucchetta N.R., Punhagui A.P.F., Banedetti P.R., Arakawa N.S., Seiva F.R.F., Fernandes G.S.A. Alcohol extract of Bauhinia forficata link reduces lipid peroxidation in the testis and epididymis of adult Wistar rats. Microsc. Res. Technol. 2018:1–7. doi: 10.1002/jemt.23175. [DOI] [PubMed] [Google Scholar]
- 34.Cechinel-Zanchett C.C.D.A., Cechinel-Filho S.F. Ethnopharmacological, phytochemical, pharmacological and toxicological aspects os Bauhinia forficata: a mini-review covering the last five years. Nat. Prod. Commun. 2018;13(7):6. [Google Scholar]
- 35.Souza G.A., Ebaid G.X., Seiva F.R., Rocha K.H., Galhardi C.M., Mani F., Novelli E.L. N-acetylcysteine an allium plant compound improves high-sucrose diet-induced obesity and related effects. Evid. Complement. Alternat. Med. 2011;(2011) doi: 10.1093/ecam/nen070. 643269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 37.Seiva F.R., Chuffa L.G., Braga C.P., Amorim J.P., Fernandes A.A. Quercetin ameliorates glucose and lipid metabolism and improves antioxidant status in postnatally monosodium glutamate-induced metabolic alterations. Food Chem. Toxicol. 2012;50(10):3556–3561. doi: 10.1016/j.fct.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 38.Aebi H. Catalase. In: Bergmeyer H.-U., editor. Methods of Enzymatic Analysis Methods of Enzymatic Analysis Academic. 105. 2nd ed. Elsevier Inc; 1974. [Google Scholar]
- 39.Asghari A., Akbari G., Meghdadi A., Mortazavi P. Protective effect of metformin on testicular ischemia/reperfusion injury in rats. Acta Cir. Bras. 2016;31(6):411–416. doi: 10.1590/S0102-865020160060000008. [DOI] [PubMed] [Google Scholar]
- 40.Rubin B.S., Paranjpe M., DaFonte T., Schaeberle C., Soto A.M., Obin M., Greenberg A.S. Perinatal BPA exposure alters body weight and composition in a dose specific and sex specific manner: the addition of peripubertal exposure exacerbates adverse effects in female mice. Reprod. Toxicol. 2017;68:130–144. doi: 10.1016/j.reprotox.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cabaton N.J., Canlet C., Wadia P.R., Tremblay-Franco M., Gautier R., Molina J., Sonnenschein C., Cravedi J.P., Rubin B.S., Soto A.M., Zalko D. Effects of low doses of bisphenol A on the metabolome of perinatally exposed CD-1 mice. Environ. Health Perspect. 2013;121(5):586–593. doi: 10.1289/ehp.1205588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vandenberg L.N., Maffini M.V., Wadia P.R., Sonnenschein C., Rubin B.S., Soto A.M. Exposure to environmentally relevant doses of the xenoestrogen bisphenol-A alters development of the fetal mouse mammary gland. Endocrinology. 2007;148(1):116–127. doi: 10.1210/en.2006-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Markey C.M., Michaelson C.L., Veson E.C., Sonnenschein C., Soto A.M. The mouse uterotrophic assay: a reevaluation of its validity in assessing the estrogenicity of bisphenol A. Environ. Health Perspect. 2001;109(1):55–60. doi: 10.1289/ehp.0110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakagawa Y., Tayama S. Metabolism and cytotoxicity of bisphenol A and other bisphenols in isolated rat hepatocytes. Arch. Toxicol. 2000;74(2):99–105. doi: 10.1007/s002040050659. [DOI] [PubMed] [Google Scholar]
- 45.Steinmetz R., Mitchner N.A., Grant A., Allen D.L., Bigsby R.M., Ben-Jonathan N. The xenoestrogen bisphenol A induces growth, differentiation, and c-fos gene expression in the female reproductive tract. Endocrinology. 1998;139(6):2741–2747. doi: 10.1210/endo.139.6.6027. [DOI] [PubMed] [Google Scholar]
- 46.Moustafa G.G., Ahmed A.A.M. Impact of prenatal and postnatal exposure to bisphenol A on female rats in a two generational study: genotoxic and immunohistochemical implications. Toxicol. Rep. 2016;3:685–695. doi: 10.1016/j.toxrep.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stump D.G., Beck M.J., Radovsky A., Garman R.H., Freshwater L.L., Sheets L.P., Marty M.S., Waechter J.M., Jr., Dimond S.S., Van Miller J.P., Shiotsuka R.N., Beyer D., Chappelle A.H., Hentges S.G. Developmental neurotoxicity study of dietary bisphenol A in Sprague-Dawley rats. Toxicol. Sci. 2010;115(1):167–182. doi: 10.1093/toxsci/kfq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caporossi L., Papaleo B. Bisphenol a and metabolic diseases: challenges for occupational medicine. Int. J. Environ. Res. Public Health. 2017;14(9) doi: 10.3390/ijerph14090959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rubin B.S., Soto A.M. Bisphenol A: perinatal exposure and body weight. Mol. Cell. Endocrinol. 2009;304(1-2):55–62. doi: 10.1016/j.mce.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Metwally F.M., Mohamed M.M., Sharaf N.E., Ghazy M.A., El Mishad A.M., Elfiky A. The impact of bisphenol a (BPA) As environmental obesogen on lipids and lipids metabolism. Int. J. Pharm. Clin. Res. 2016;8(9):1323–1330. [Google Scholar]
- 51.Harley K.G., Aguilar Schall R., Chevrier J., Tyler K., Aguirre H., Bradman A., Holland N.T., Lustig R.H., Calafat A.M., Eskenazi B. Prenatal and postnatal bisphenol A exposure and body mass index in childhood in the CHAMACOS cohort. Environ. Health Perspect. 2013;121(4):514–520. doi: 10.1289/ehp.1205548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sakurai K., Kawazuma M., Adachi T., Harigaya T., Saito Y., Hashimoto N., Mori C. Bisphenol A affects glucose transport in mouse 3T3-F442A adipocytes. Br. J. Pharmacol. 2004;141(2):209–214. doi: 10.1038/sj.bjp.0705520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Asahi J., Kamo H., Baba R., Doi Y., Yamashita A., Murakami D., Hanada A., Hirano T. Bisphenol A induces endoplasmic reticulum stress-associated apoptosis in mouse non-parenchymal hepatocytes. Life Sci. 2010;87(13-14):431–438. doi: 10.1016/j.lfs.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 54.Jayashree S., Indumathi D., Akilavalli N., Sathish S., Selvaraj J., Balasubramanian K. Effect of Bisphenol-A on insulin signal transduction and glucose oxidation in liver of adult male albino rat. Environ. Toxicol. Pharmacol. 2013;35(2):300–310. doi: 10.1016/j.etap.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 55.Zaulet M., Kevorkian S.E.M., Dinescu S., Cotoraci C., Suciu M., Herman H., Buburuzan L., Badulescu L., Ardelean A., Hermenean A. Protective effects of silymarin against bisphenol A-induced hepatotoxicity in mouse liver. Exp. Ther. Med. 2017;13(3):821–828. doi: 10.3892/etm.2017.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meng Z., Wang D., Yan S., Li R., Yan J., Teng M., Zhou Z., Zhu W. Effects of perinatal exposure to BPA and its alternatives (BPS, BPF and BPAF) on hepatic lipid and glucose homeostasis in female mice adolescent offspring. Chemosphere. 2018;212:297–306. doi: 10.1016/j.chemosphere.2018.08.076. DOI: 6/j.chemosphere.2018.08.076. [DOI] [PubMed] [Google Scholar]
- 57.Olsen L., Lind L., Lind P.M. Associations between circulating levels of bisphenol A and phthalate metabolites and coronary risk in the elderly. Ecotoxicol. Environ. Saf. 2012;80:179–183. doi: 10.1016/j.ecoenv.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 58.Masuno H., Kidani T., Sekiya K., Sakayama K., Shiosaka T., Yamamoto H., Honda K. Bisphenol A in combination with insulin can accelerate the conversion of 3T3-L1 fibroblasts to adipocytes. J. Lipid Res. 2002;43(5):676–684. [PubMed] [Google Scholar]
- 59.Marmugi A., Lasserre F., Beuzelin D., Ducheix S., Huc L., Polizzi A., Chetivaux M., Pineau T., Martin P., Guillou H., Mselli-Lakhal L. Adverse effects of long-term exposure to bisphenol A during adulthood leading to hyperglycaemia and hypercholesterolemia in mice. Toxicology. 2014;325:133–143. doi: 10.1016/j.tox.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 60.Lino Cde S., Diogenes J.P., Pereira B.A., Faria R.A., Andrade Neto M., Alves R.S., de Queiroz M.G., de Sousa F.C., Viana G.S. Antidiabetic activity of Bauhinia forficata extracts in alloxan-diabetic rats. Biol. Pharm. Bull. 2004;27(1):125–127. doi: 10.1248/bpb.27.125. [DOI] [PubMed] [Google Scholar]
- 61.Damasceno D.C., Volpato G.T., Calderon Ide M., Aguilar R., Rudge M.V. Effect of Bauhinia forficata extract in diabetic pregnant rats: maternal repercussions. Phytomedicine. 2004;11(2-3):196–201. doi: 10.1078/0944-7113-00348. [DOI] [PubMed] [Google Scholar]
- 62.Ecker A., Araujo Vieira F., de Souza Prestes A., Mulling Dos Santos M., Ramos A., Dias Ferreira R., Teixeira de Macedo G., Vargas Klimaczewski C., Lopes Seeger R., Teixeira da Rocha J.B., de Vargas N.B., Barbosa Effect of Syzygium cumini and Bauhinia forficata aqueous-leaf extracts on oxidative and mitochondrial parameters in vitro. EXCLI J. 2015;14:1219–1231. doi: 10.17179/excli2015-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oo P.S., Yamaguchi Y., Sawaguchi A., Tin Htwe Kyaw M., Choijookhuu N., Noor Ali M., Srisowanna N., Hino S.I., Hishikawa Y. Estrogen regulates mitochondrial morphology through phosphorylation of dynamin-related protein 1 in MCF7 human breast Cancer cells. Acta Histochem. Cytochem. 2018;51(1):21–31. doi: 10.1267/ahc.17034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gassman N.R., Coskun E., Jaruga P., Dizdaroglu M., Wilson S.H. Combined Effects of high-dose bisphenol a and oxidizing agent (KBrO3) on cellular microenvironment, gene expression, and chromatin structure of Ku70-deficient mouse embryonic fibroblasts. Environ. Health Perspect. 2016;124(8):1241–1252. doi: 10.1289/EHP237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tiwari D., Kamble J., Chilgunde S., Patil P., Maru G., Kawle D., Bhartiya U., Joseph L., Vanage G. Clastogenic and mutagenic effects of bisphenol A: an endocrine disruptor. Mutat. Res. 2012;743(1-2):83–90. doi: 10.1016/j.mrgentox.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 66.da Cunha A.M., Menon S., Menon R., Couto A.G., Burger C., Biavatti M.W. Hypoglycemic activity of dried extracts of Bauhinia forficata Link. Phytomedicine. 2010;17(1):37–41. doi: 10.1016/j.phymed.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 67.Khalil N.M., Pepato M.T., Brunetti I.L. Free radical scavenging profile and myeloperoxidase inhibition of extracts from antidiabetic plants: bauhinia forficata and Cissus sicyoides. Biol. Res. 2008;41(2):165–171. DOI: /S0716-97602008000200006. [PubMed] [Google Scholar]
- 68.de Sousa E., Zanatta L., Seifriz I., Creczynski-Pasa T.B., Pizzolatti M.G., Szpoganicz B., Silva F.R. Hypoglycemic effect and antioxidant potential of kaempferol-3,7-O-(alpha)-dirhamnoside from Bauhinia forficata leaves. J. Nat. Prod. 2004;67(5):829–832. doi: 10.1021/np030513u. [DOI] [PubMed] [Google Scholar]
- 69.Tajbakhsh A., Pasdar A., Rezaee M., Fazeli M., Soleimanpour S., Hassanian S.M., FarshchiyanYazdi Z., Younesi Rad T., Ferns G.A., Avan A. The current status and perspectives regarding the clinical implication of intracellular calcium in breast cancer. J. Cell. Physiol. 2017 doi: 10.1002/jcp.26277. [DOI] [PubMed] [Google Scholar]
- 70.Ecker A., Gonzaga T., Seeger R.L., Santos M.M.D., Loreto J.S., Boligon A.A., Meinerz D.F., Lugokenski T.H., Rocha J., Barbosa N.V. High-sucrose diet induces diabetic-like phenotypes and oxidative stress in Drosophila melanogaster: protective role of Syzygium cumini and Bauhinia forficata. Biomed. Pharmacother. 2017;89:605–616. doi: 10.1016/j.biopha.2017.02.076. [DOI] [PubMed] [Google Scholar]
- 71.Bindhumol V., Chitra K.C., Mathur P.P. Bisphenol A induces reactive oxygen species generation in the liver of male rats. Toxicology. 2003;188(2-3):117–124. doi: 10.1016/s0300-483x(03)00056-8. [DOI] [PubMed] [Google Scholar]
- 72.Kabuto H., Hasuike S., Minagawa N., Shishibori T. Effects of bisphenol A on the metabolisms of active oxygen species in mouse tissues. Environ. Res. 2003;93(1):31–35. doi: 10.1016/s0013-9351(03)00062-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.