Abstract
Introduction:
Cefoxitin has a good in vitro activity and stability in resistance to hydrolysis by extended-spectrum beta-lactamases and is a good candidate for the treatment of urinary tract infection. However, data are scarce regarding its use in clinical practice.
Methods:
We conducted a retrospective study from September 2014 to November 2017, in a tertiary care hospital in Garches (France). We gathered all prescriptions of cefoxitin for urinary tract infection due to extended-spectrum beta-lactamase isolates. We compared the clinical outcomes between Escherichia coli and Klebsiella pneumoniae extended-spectrum-beta-lactamase-producing isolates after a 90-day follow-up. When available, we assessed whether cefoxitin-based regimen was associated with an emergence of resistance.
Results:
The treatment of 31 patients with a mean age of 60 ± 18 years was analyzed. We observed a clinical cure of 96.7% (n = 30/31) at day 30 and of 81.2% (n = 13/16) and 85.7% (12/14) at day 90 for extended-spectrum beta-lactamase Escherichia coli and Klebsiella pneumoniae isolates, respectively (p = 0.72). No adverse events were reported. One patient who relapsed carried a Klebsiella pneumoniae isolate that became intermediate to cefoxitin in the follow-up.
Conclusion:
In a period of major threat with a continuous increase of extended-spectrum beta-lactamase obliging to a policy of carbapenem-sparing regimens, it seems detrimental to deprive physicians of using cefoxitin for extended-spectrum beta-lactamase Enterobacteriaceae for the treatment of urinary tract infection while our data show its efficacy.
Keywords: Cefoxitin, Escherichia coli, extended-spectrum beta-lactamase, Klebsiella pneumoniae, urinary tract infection
Introduction
Nowadays, we are experiencing a worldwide proliferation of extended-spectrum beta-lactamase (ESBL) strains which is a major public health threat, according to the Centers for Disease Control and Prevention (CDC).1 Carbapenems are deemed to be the standard regimen, but alternatives are currently evaluated considering the emergence of resistance to carbapenems with the risk of deadlock situation.
Cefoxitin (FOX), a cephamycin, was rapidly replaced by third-generation cephalosporins in the 1980s because of a better efficacy against gram-negative bacteria.2 Nevertheless, FOX has a good in vitro activity and stability because of a grouping 7-α-methoxy that inhibits the action of ESBLs by its shape.3
Therefore, FOX has been repurposed to treat ESBL infections, especially after the study of Lepeule and colleagues4 in 2012 that highlighted its comparable activity to carbapenems against CTX-M-15-producing Escherichia coli strains, with similar bactericidal activities and selective pressure.
On the other hand, the use of FOX in Klebsiella pneumoniae infections has been controversial since 1989 due to a case report describing a porin-deficient mutant of a TEM-3 beta-lactamase (Omp K35) after a cephamycin exposure.5 Recently, Ananthan and colleagues6 showed that such resistance (mediated by Omp K35) could be observed also for ESBL E. coli.
The aim of this study was to evaluate FOX in ESBL urinary tract infections (UTIs) and whether the type of microorganism impacts the outcome.
Methods
Setting and design
We conducted a retrospective study from September 2014 to November 2017 in Raymond-Poincaré Teaching Hospital with 255 beds of acute care, located in Garches (France). The hospital is a center of expertise in neurological impairment, including spinal cord–injured patients. They are subject to bladder dysfunction with UTI and are frequently colonized by multidrug-resistant (MDR) organisms with an incidence that can reach up to twice the expected value. In 2013, ESBL-producing Enterobacteriaceae colonization revealed an incidence of 1.1 per 1000 patient-days, compared with 0.55 in other healthcare facilities.7
FOX was chosen because of its low price (€35 for a 7-day treatment with 1 g/6 h) and considering there was no other possible alternative outside of carbapenems, especially fluoroquinolone or trimethoprim-sulfamethoxazole.
Patients were adults treated by intermittent intravenous infusion of FOX for a UTI caused by an ESBL isolate with an administration of FOX ⩾ 50% of the total duration of antibiotics. Those data were extracted by a hospital pharmacist and thereafter data were analyzed by two independent infectious disease specialists according to cytobacteriological examination of the urine (CBEU) and medical charts. Finally, to carry out analyses, the cohort was divided into two arms depending on the microorganism (E. coli or K. pneumoniae).
Microbiological definitions
Isolates for which the minimal inhibitory concentration (MIC) of cefotaxime decreased by three serial twofold dilutions when tested in the presence of clavulanate or for which zone diameters increased by 5 mm in the presence of clavulanate were considered positive for ESBL, as described previously.8
Definition and endpoints
The clinical information gathered included age, sex, underlying diseases to calculate a Charlson comorbidity score,9 urinary system pathology, bacteremia, posology, and the duration of antibiotic regimen (including FOX and eventually oral relay).
Definitions of orchitis, pyelonephritis, and prostatitis were based on the French guidelines for the treatment of UTI.10
UTI was defined by a positive urinalysis (CFU ⩾ 105/mL) and at least one clinical sign: dysuria, frequency, urgency, suprapubic or costovertebral tenderness, and clinical autonomic dysreflexia (which is associated with throbbing headaches, profuse sweating, nasal stuffiness, flushing of the skin above the level of the lesion, and slow heart rate). In the absence of fever, with persistent symptoms of more than 72 h, patient was considered as suffering from cystitis.
Clinical cure was defined as resolution of symptoms without recurrence during the follow-up.
Clinical failure was defined by the persistence of symptoms during treatment or by the recurrence of symptoms within 90 days after the end of treatment. In such condition, failure was assessed by a positive urine culture during the follow-up to rule out a possible emergence of a FOX-resistant strain.
Clinical outcomes were assessed at the end of treatment and for 90 days after discontinuation of antibiotics. A follow-up CBEU was not systematically performed.
Statistical analysis
Results are expressed as n (%) or median (minimum–maximum) and outcomes were assessed using Fisher’s exact test. Statistical analyses were carried out using Prism v.7.0d (GraphPad Software Inc., La Jolla, CA).
Results
Overall 31 patients had an ESBL UTI and were analyzed. Mean age was 60 ± 18 years. The flowchart is detailed in Figure 1. Patients’ characteristics are summarized in Table 1. No patient presented with sepsis according to the latest guidelines.
Figure 1.
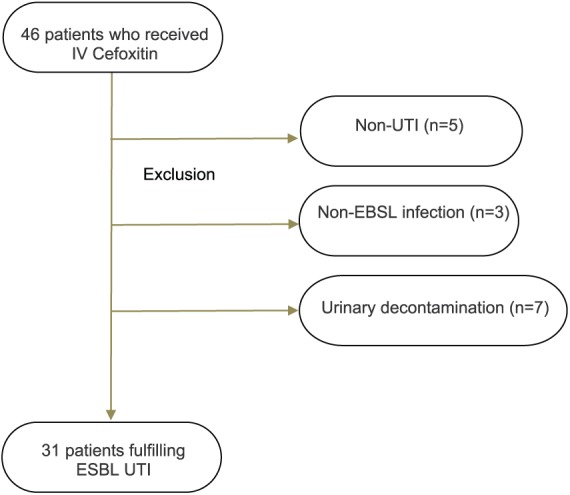
Flowchart of the studied population before inclusion in the study. All patients presented with UTI due to an ESBL isolate.
ESBL, extended-spectrum beta-lactamase; UTI, urinary tract infection.
Table 1.
Patients’ characteristics – comparison between E. coli- and K. pneumoniae-infected patients.
| ESBL E. coli (n = 17) | ESBL K. pneumoniae (n = 14) | p value | |
|---|---|---|---|
| Patients | |||
| Sex (male), n (%) | 12 (70.6) | 11 (78.6) | 0.69 |
| Age, mean (±SD) (in years) | 62 ± 17.9 | 57 ± 18.1 | 0.41 |
| Underlying condition, n (%) | 0.99 | ||
| Neurological disorder* | 7 | 8 | – |
| Immunocompromised$ | 2 | 2 | – |
| Urological disease‡ | 5 | 6 | – |
| Diabetes | 2 | 1 | – |
| Chronic kidney failure | 2 | 1 | – |
| Median creatinine plasma level | 112 (78–143) | 107 (68–121) | 0.99 |
| Home resident/LTCF, n (%) | 13 (76.5)/4 | 10 (71.4)/4 | 0.99 |
| Median Charlson comorbidity index (min–max) | 4 (0–10) | 4.5 (1–9) | 0.29 |
| Median length of stay (min–max) | 8 (4–31) | 10 (5–60) | 0.27 |
| Infection characteristics | |||
| Site of infection, n (%) | |||
| Pyelonephritis | 11 (64.7) | 11 (78.7) | 0.45 |
| Prostatitis | 4 (23.5) | 1 (7.1) | 0.34 |
| Orchitis | 2 (11.8) | 1 (7.1) | 0.99 |
| Cystitis | – | 1 (7.1) | 0.45 |
| Abscesses§, n (%) | 1 (5.9) | 1 (7.1) | 0.99 |
| Concomitant bacteremia, n (%) | 3 (17.7) | 2 (14.2) | 0.99 |
| Antibiotic regimen | |||
| Median duration of Cefoxitin therapy (min–max) | 10 (5–21) | 10 (5–21) | 0.41 |
| Pyelonephritis | 10 (5–21) | 10 (7–21) | |
| Prostatitis | 19.5 (16–21) | 21 | |
| Orchitis | 15.5 (10–21) | 14 | |
| Cystitis | – | 6 | |
| Median daily dose (min–max) | 4 (2–8) | 6 (3–6) | 0.53 |
ESBL, extended-spectrum beta-lactamase; SD, standard deviation; LTCF, long-term care facility.
Including severe cranial trauma, spine cord injury, multiple sclerosis, paraplegia/tetraplegia, and stroke under intermittent bladder catheterization (n = 4 in each arm).
HIV, multiple myeloma, hematological malignancy including lymphoma and cancer.
Urinary tract abnormality including urological cancer and recurrent urinary tract infection.
Abscesses were perinephric abscess or prostatic abscess of medical treatment.
Prior to the administration of FOX, 12.9% (n = 4) received another effective antimicrobial therapy (imipenem/cilastine) within the 48 h (for three E. coli isolates). Of note, three cases received an inactive therapy with a third-generation cephalosporin.
Median daily dose of FOX was 4 g (2–8). Only one patient infected by ESBL E. coli received an additional oral relay with 4 days of levofloxacin.
During the follow-up no patient reported any adverse event.
One patient who had a favorable outcome at day 30 died at day 40 of a cancer in palliative care and was lost to follow-up at day 90.
Overall, we noted an efficacy of FOX of 96.7% (n = 30/31) at day 30 and 83.3% (n = 25/30) at day 90. Statistical analysis revealed no particular risk factor for failure (n = 5), including in the case of ESBL K. pneumoniae-related infection (Table 2).
Table 2.
Characteristics of patients who failed to cefoxitin regimen at day 90.
| Success (n = 25) | Failure (n = 5) | p value | |
|---|---|---|---|
| Patients | |||
| Age, mean (±SD) (in years) | 58 ± 18.8 | 62 ± 13.9 | 0.41 |
| Charlson comorbidity index median (min–max) | 4 (0–10) | 4 (0–9) | 0.45 |
| Infection characteristics | |||
| Site of infection, n | |||
| Pyelonephritis | 18 | 3 | 0.59 |
| Prostatitis | 4 | 1 | 0.99 |
| Orchitis | 2 | 1 | 0.43 |
| Cystitis | 1 | – | 0.99 |
| Abscess, n | 1 | 1 | 0.31 |
| Concomitant bacteremia, n | 5 | 0 | 0.56 |
| Due to a K. pneumoniae isolate | 12 | 1 | 0.35 |
| Antibiotic regimen | |||
| Median duration of Cefoxitin therapy (min–max) | 10 (5–21) | 10 (5–21) | 0.41 |
| Median daily dose (min–max) | 4 (2–8) | 6 (3–6) | 0.16 |
SD: standard deviation.
Outcomes at day 90 were similar between the E. coli and K. pneumoniae groups with a favorable outcome in 13/16 (81.2%) and 12/14 (85.7%), respectively (p = 0.72; Figure 2).
Figure 2.
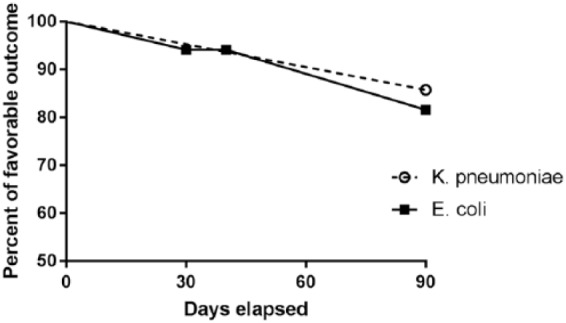
Outcomes after cefoxitin therapy, considering one lost to follow-up at day 40 for the treatment of a K. pneumoniae UTI.
UTI: urinary tract infection.
Finally, 11 cases (4 E. coli and 7 K. pneumoniae isolates) benefited from microbiological follow-up. We observed only one failure in a patient suffering from prostatitis due to a K. pneumoniae isolate which has become intermediate to FOX later on and was retreated.
Discussion
Our study showed a remarkable clinical cure (83.3% at day 90) for ESBL UTI treated by FOX. It brings new relevant data considering that our cohort is exclusively composed of UTI unlike Kerneis and colleagues11 (n = 23), concerns both sexes unlike Demonchy and colleagues12 (n = 23), and is mainly composed of pyelonephritis unlike Mambie and colleagues13 (n = 15). Our results are consistent with their findings with a clinical cure in 83% at day 90,12 whereas Kerneis and colleagues11 reported a favorable outcome also in 83% of cases but with a different endpoint (after a median follow-up of 14 days).
Moreover, Kim and colleagues14 reported a favorable outcome of 93.2% (n = 83/89) at day 90 using carbapenems for ESBL UTIs, thereby being not different from our series.
To our knowledge, this is the biggest cohort of UTI reported. Moreover, some of these cited studies share heterogeneous clinical and microbiological populations11,13,15 that may constitute a selective bias.
Interestingly, in our study, failure was not associated with lower dosing of FOX (Table 2). Yet, the median dose was relatively low (4 g). Although our study does not provide any arguments for lower efficacy on K. pneumoniae infections (p = 0.35), we observed the appearance of one isolate that became intermediate to FOX after a 6 g regimen of FOX for prostatitis in a patient weighting 95 kg. Therefore, physician should be worried that an Omp K35 mutation remains possible, maybe in a higher proportion for K. pneumoniae than E. coli isolates6 despite the fact two different case series12,13 reported no emergence of resistance with K. pneumoniae isolates in UTI. To answer this question, a plasma and tissue pharmacokinetics of FOX would be helpful. Indeed, Guet-Revillet and colleagues16 suggested that only high dosage (8 g per day) and continuous perfusion will able to reach pharmacological targets. Nevertheless, there is no MIC breakpoint issued by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) for the treatment of ESBL isolates with FOX, and only zone diameters are acknowledged.17 Another limit is the absence of a systematic control urinalysis to evaluate a potential acquisition of resistance, but it is not recommended by guidelines.
In a period of major threat obliging to a policy of carbapenem-sparing regimens, it seems detrimental to deprive physicians of using FOX while our data show its efficacy. Furthermore, limiting the prescription of FOX only to E. coli isolates could wrongly encourage the use of carbapenems.
Further studies with larger sample size are necessary in order to confirm our findings, particularly in patients infected by ESBL K. pneumoniae isolates.
Acknowledgments
All the listed authors have contributed to this work and approved the paper. This manuscript also fulfills the ethics committee approval. B.D., O.S., and F.B. designed the study. A.D., L.F., and M.M. supervised data collection and management. B.D., O.S., C.P., M.R., and A.D. analyzed the data. O.S. prepared the first draft of the manuscript. All the authors participated in manuscript preparation and approved the final manuscript for publications. This work was carried out as part of our routine work.
Footnotes
Compliance with ethical standards: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Contributor Information
Olivia Senard, Maladies Infectieuses, Hôpital Universitaire Raymond-Poincaré, AP-HP, Garches, France.
Frédérique Bouchand, Pharmacie Hospitalière, Hôpital Universitaire Raymond-Poincaré, AP-HP, Garches, France.
Laurene Deconinck, Maladies Infectieuses, Hôpital Universitaire Raymond-Poincaré, AP-HP, Garches, France.
Morgan Matt, Maladies Infectieuses, Hôpital Universitaire Raymond-Poincaré, AP-HP, Garches, France.
Lesly Fellous, Pharmacie Hospitalière, Hôpital Universitaire Raymond-Poincaré, AP-HP, Garches, France.
Martin Rottman, Laboratoire de Microbiologie, Hôpital Universitaire Raymond-Poincaré, AP-HP, Garches, France.
Christian Perronne, Maladies Infectieuses, Hôpital Universitaire Raymond-Poincaré, AP-HP, Garches, France.
Aurélien Dinh, Maladies Infectieuses, Hôpital Universitaire Raymond-Poincaré, AP-HP, Garches, France.
Benjamin Davido, Maladies Infectieuses, Hôpital Universitaire Raymond-Poincaré, AP-HP, Garches, France.
References
- 1. European Centre for Disease Prevention and Control. Annual epidemiological report 2014: antimicrobial resistance and healthcare-associated infections. Stockholm: European Centre for Disease Prevention and Control, 2015. [Google Scholar]
- 2. Forsgren A. Comparative in vitro activity of first, second and third generation cephalosporins. Acta Pathol Microbiol Scand B 1981; 89: 221–225. [DOI] [PubMed] [Google Scholar]
- 3. Jacoby GA, Carreras I. Activities of beta-lactam antibiotics against Escherichia coli strains producing extended-spectrum beta-lactamases. Antimicrob Agents Chemother 1990; 34: 858–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lepeule R, Ruppé E, Le P, et al. Cefoxitin as an alternative to carbapenems in a murine model of urinary tract infection due to Escherichia coli harboring CTX-M-15-type extended-spectrum β-lactamase. Antimicrob Agents Chemother 2012; 56: 1376–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pangon B, Bizet C, Buré A, et al. In vivo selection of a cephamycin-resistant, porin-deficient mutant of Klebsiella pneumoniae producing a TEM-3 beta-lactamase. J Infect Dis 1989; 159: 1005–1006. [DOI] [PubMed] [Google Scholar]
- 6. Ananthan S, Subha A. Cefoxitin resistance mediated by loss of a porin in clinical strains of Klebsiella pneumoniae and Escherichia coli. Indian J Med Microbiol 2005; 23: 20–23. [DOI] [PubMed] [Google Scholar]
- 7. Institut de veille Sanitaire. Surveillance des bactéries multirésistantes dans les établissements de santé en France. Réseau BMR – Raisin – Résultats 2013, http://invs.santepubliquefrance.fr/Publications-et-outils/Rapports-et-syntheses/Maladies-infectieuses/2015/Surveillance-des-bacteries-multiresistantes-dans-les-etablissements-de-sante-en-France (2015, accessed October 2016).
- 8. Tenover FC, Raney PM, Williams PP, et al. Evaluation of the NCCLS extended-spectrum beta-lactamase confirmation methods for Escherichia coli with isolates collected during Project ICARE. J Clin Microbiol 2003; 41: 3142–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 10. Société de Pathologie Infectieuse de Langue Francaise (SPILF). Diagnostic et antibiothérapie des infections urinaires bactériennes communautaires de l’adulte, 2015, http://www.infectiologie.com/UserFiles/File/spilf/recos/infections-urinaires-spilf.pdf
- 11. Kernéis S, Valade S, Geri G, et al. Cefoxitin as a carbapenem-sparing antibiotic for infections caused by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Infect Dis 2015; 47: 789–795. [DOI] [PubMed] [Google Scholar]
- 12. Demonchy E, Courjon J, Ughetto E, et al. Cefoxitin-based antibiotic therapy for ESBL-producing Enterobacteriaceae prostatitis: a prospective pilot-study. Int J Antimicrob Agents 2018; 52: 836–841. [DOI] [PubMed] [Google Scholar]
- 13. Mambie A, Vuotto F, Poitrenaud D, et al. Cefoxitin: an alternative to carbapenems in urinary tract infections due to extended-spectrum beta-lactamase-producing Enterobacteriaceae. Médecine Mal Infect 2016; 46: 215–219. [DOI] [PubMed] [Google Scholar]
- 14. Kim SA, Altshuler J, Paris D, et al. Cefepime versus carbapenems for the treatment of urinary tract infections caused by extended-spectrum β-lactamase-producing Enterobacteriaceae. Int J Antimicrob Agents 2018; 51: 155–158. [DOI] [PubMed] [Google Scholar]
- 15. Matsumura Y, Yamamoto M, Nagao M, et al. Multicenter retrospective study of cefmetazole and flomoxef for treatment of extended-spectrum-β-lactamase-producing Escherichia coli bacteremia. Antimicrob Agents Chemother 2015; 59: 5107–5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guet-Revillet H, Emirian A, Groh M, et al. Pharmacological study of cefoxitin as an alternative antibiotic therapy to carbapenems in treatment of urinary tract infections due to extended-spectrum-β-lactamase-producing Escherichia coli. Antimicrob Agents Chemother 2014; 58: 4899–4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Committee on Antimicrobial Susceptibility Testing: breakpoint tables for interpretation of MICs and zone diameters, http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_7.1_Breakpoint_Tables.pdf


