Short abstract
Objective
To investigate the impact of medical and obstetric complications associated with mixed connective tissue disease (MCTD) in pregnancy.
Method
We analyzed 68 pregnancies from a systematic literature review and 12 pregnancies affected by MCTD at our centre between 1986 and 2015 for medical and obstetric complications.
Results
During pregnancy 37.1% had active MCTD and 26.7% had relapsed. Maternal complications included caesarean section (31.1%, n = 19), preeclampsia (17.6%, n = 13), thromboembolism events, and death (2.5%, n = 2 for each). Fetal complications included prematurity (48.1%, n = 25), intrauterine growth restriction (38.3%, n = 19), and neonatal lupus (28.6%, n = 18, including chondrodysplasia punctata). More than half (n = 10) of the neonatal lupus cases were explained by anti-U1RNP only. The perinatal mortality rate was 17.7% (n = 14). Pregnant women with active disease had higher rates of prematurity (OR = 7.60; 95%CI [1.93; 29.95]) and perinatal death (OR = 16.83; 95%CI [1.90; 147.70]).
Conclusion
MCTD in pregnancy puts women at risk of medical and obstetric complications, and disease activity probably increases this risk.
Keywords: Mixed connective tissue disease, Sharp syndrome, U1RNP antibody, neonatal systemic lupus erythematosus, chondrodysplasia punctata, pregnancy
Introduction
Mixed connective tissue disease (MCTD) has been recognized as a distinct condition since the discovery of anti-U1RNP antibodies in 1974. Its evolution during pregnancy is less well understood, due to its rarity and the varying clinical presentations associated with U1RNP antibodies (rheumatoid, lupus, polymyositis, and scleroderma pattern). A 2005 review by Kitridou of all MCTD cases in pregnancy since 1979 concludes that overall prognosis is generally favourable, except for rare cases of pulmonary hypertension and scleroderma renal crisis.1 However, this review contains heterogeneous cases: other rheumatologic diagnoses, pregnancies prior to the first symptoms, and a mix of catastrophic cases as well as mild disease cohorts. Moreover, most of these cases date back to the early 1980s, and patient care has evolved significantly.
This study describes the clinical presentation and maternal/fetal outcomes in 12 pregnancies affected by MCTD in our centre from 1986 to 2015 and 68 pregnancies from a systematic literature review. We evaluate if disease activity is associated with worse outcomes.
Methods
Patients
Cases from our centre were selected using files from the Rheumatology and Obstetric Medicine departments, and from a retrospective search of hospital charts from 1986 with the following ICD-9 codes: 443.0 (Raynaud’s syndrome), 710.8 (other specified diffuse diseases of connective tissue), 710.9 (unspecified diffuse connective tissue disease); and with ICD 10th revision codes 173.0 (Raynaud’s syndrome), M35.1 (other overlap syndromes), M35.9 (systemic involvement of connective tissue, unspecified), and pregnancy.
Inclusion criteria included pregnancies of more than 20 weeks’ gestation and a previously-diagnosed MCTD, or a diagnosis made during the current pregnancy or within 6 weeks post-partum. The Alarcon–Segovia criteria or Khan criteria with significant anti-U1RNP levels were used for MCTD diagnosis.2 Exclusion criteria included diagnosis of other rheumatologic diseases, multiple pregnancy, past organ transplant, long-term anticoagulation, antiphospholipid syndrome, other nonendocrine autoimmune and cardiac, liver, or renal disease from other causes.
Systematic literature review
A literature search using PubMed, Medline, and Embase was performed to identify relevant articles published between 1 January 1966 and 30 June 2014. The search was limited to articles in English and French, but articles in other languages were included if a complete English abstract was available.
The search was conducted using the MeSH terms pregnancy, preeclampsia, fetus, premature birth, premature infant, premature labor, maternal mortality, mixed connective tissue disease, MCTD, and Sharp syndrome. We searched in the reference list of the primary articles and reviews for relevant articles not already identified in the literature search.
The same inclusion and exclusion criteria were applied for the systematic review. We contacted six authors for missing data and three replied, but their data were unavailable for our analysis.
Data collection
Charts from Centre Hospitalier de l'Université de Montréal (CHUM) were reviewed to collect information on maternal characteristics (age, parity, medical, and obstetric history), clinical presentation (signs and symptoms, disease length, disease control from six months prior to pregnancy, use of steroids or immunosuppressive drugs, and disease activity during pregnancy), disease outcomes, maternal outcomes (delivery mode and obstetric complications), and fetal outcomes (gestational age at birth, birthweight, and neonatal complications). A well-controlled disease was defined as presenting no myositis, synovitis, serositis, or active digit ulcer, no deterioration of baseline organ dysfunction using 5 mg or less prednisone daily, and with normal complete blood count and C-reactive protein, if available. Conversely, MCTD was considered active if not defined by the above criteria, and included relapses (deterioration of a pre-existing condition, new symptoms or starting/increasing immunosuppression drugs), de novo diagnosis and symptoms during pregnancy leading to a diagnosis within six weeks of delivery. Chronic, nonprogressing disease features or organ dysfunction were not considered as active disease.
Disease outcomes were documented including relapses (as above), pulmonary arterial hypertension (PAH: ≥25 mmHg by cardiac catheterisation or echocardiography), interstitial lung disease (ILD: classic imaging, or a restrictive pattern or reduced diffusion on pulmonary function tests), non-preeclamptic proteinuria (caused by glomerulonephritis or unknown origin provided that there was no preeclampsia), and renal scleroderma crisis (RSC: blood pressure >140/90 mmHg or >30/20 mmHg above patient’s baseline or acute renal failure without hypertension, with one of the following: >50% increase in creatinine level or >120% of upper limit of normal, non-nephrotic range proteinuria, microscopic haematuria, haemolysis, hypertensive encephalopathy, pulmonary edema, or renal biopsy showing thrombotic microangiopathy).
Obstetric complications were documented as caesarean section, preeclampsia, thromboembolic event, and maternal death, and fetal complications as prematurity, intrauterine growth restriction (IUGR), neonatal lupus (NL), and perinatal death. Preeclampsia was defined by gestational hypertension (systolic >140 mmHg or diastolic >90 mmHg beginning at >20 weeks’ gestation) with de novo proteinuria or chronic hypertension (pre-pregnancy or beginning at < 20 weeks’ gestation) aggravating or with de novo proteinuria, or gestational or chronic hypertension with at least one adverse condition or severe complication, as defined by the Society of Obstetricians and Gynecologists of Canada (SOGC).3 A thromboembolic event was defined as new venous clot diagnosed by ultrasound, computerized tomography pulmonary angiogram, or ventilation/perfusion scintigraphy. We used the WHO definition of maternal mortality (ICD-10, WHO, 1994). An IUGR diagnosis was given for estimated fetal weight <10th percentile for gestational age on a third trimester ultrasound, or if the newborn was small for gestational age (birthweight <10th percentile).4 If both data were available and conflicting, the latter was prioritised. Prematurity was defined as birth before 37 weeks’ gestation, calculated from date of last menstrual period or adjusted with first trimester ultrasound. NL describes a fetus or a neonate born from an anti-Ro, anti-La, or anti-U1RNP-positive mother that develops heart block and/or typical rash and/or liver or haematologic manifestations. Chondrodysplasia punctata (CP) is a heterogeneous disorder, diagnosed in utero or after delivery, characterized by epiphyseal or vertebral stippling, with or without other severe features like facial dysmorphism, dwarfism, joint contractures, cataracts, ichthyosis, and mental retardation. Autoimmune disease is one of several a etiologies that is associated with the development of this syndrome.5 Perinatal death includes stillbirth (intrauterine fetal death at 20 weeks’ gestation or later, which is not a therapeutic abortion), therapeutic abortions (TA: termination of pregnancy for serious maternal or fetal condition) and neonatal mortality (newborn death up to 28 days after its birth).
Statistics
Appropriate summary statistics were calculated to describe patient characteristics and their medical and obstetric complications (medians and ranges for normally-distributed continuous variables and proportions for binary or categorical variables). Pregnancies from the same woman were analysed as two different events. A sensitivity analysis did not alter the results. Sample sizes and denominators vary due to missing data. Differences between active and non-active disease with odds ratios and 95% confidence intervals were generated with a pooled logistic regression estimated with a generalized estimating equation model to take account of dependence in observations. Analyses were performed using SPSS software version 22.
This study was approved by the institutional ethics and review board of the Centre de Recherche du Centre Hospitalier de l’Université de Montréal.
Results
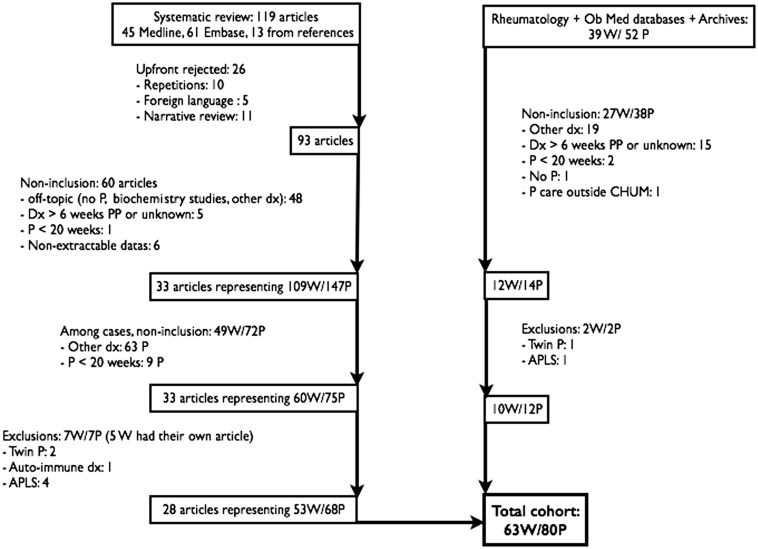
We retrieved 93 articles for assessment: 65 were rejected due to repetition, foreign language, narrative review, incomplete or inaccessible data, multiple pregnancies, other autoimmune disease, or antiphospholipid syndrome. The analysis included 28 articles, including a total of 53 women and 68 pregnancies.6–33 With the cases from our centre and those found in the literature, a total of 63 patients and 80 pregnancies were analysed (17 women had two pregnancies; Figure 1). No randomized controlled trials, cohort, or case-control studies were identified.
Figure 1.
Case selection.
W: women; P: pregnancy; Dx: diagnosis; PP: post-partum; APLS: antiphospholipid syndrome.
Baseline characteristics
Patient characteristics are described in Table 1. Median age was 29 years. A diagnosis was made 5 years before pregnancy (median) and 37.7% were primiparous. Disease was well-controlled in 21 women (80.8%) 6 months prior to pregnancy, but data were only available for 26 women. Steroids or other immunosuppressive drugs were used by 42.9% at the start of pregnancy. For those with available data, 70 (37.1%) had active disease in pregnancy, including 16 relapses from 60 previously-identified women with MCTD, 7 de novo diagnoses in pregnancy, and 3 in the six-week period following delivery. The breakdown of positive antibodies (apart from anti-U1RNP) is detailed in Table 1.
Table 1.
Baseline features.
|
Literature review and CHUM cohort(63 W, 80 P) |
CHUM cohort only(10 W, 12 P) |
|||
|---|---|---|---|---|
| N | Median (range) | N | Median (range) | |
| Age (y) | 63 | 29 (17–41) | 12 | 31.5 (18–41) |
| Disease duration (y) | 61 | 5 (0–13) | 11 | 7 (0–13) |
|
P/N |
% |
P/N |
% |
|
| Primiparous | 20/53 | 37.7 | 7/12 | 58.3 |
| Good disease controla | 21/26 | 80.8 | 9/10 | 90.0 |
| Steroids/Immunosupp.b | 15/35 | 42.9 | 3/10 | 30.0 |
| Active diseasec | 26/70 | 37.1 | 1/12 | 8.8 |
| ANA | 46/47 | 97.9 | 11/12 | 91.7 |
| Anti-Ro | 14/44 | 31.8 | 5/10 | 50.0 |
| Anti-La | 6/36 | 16.7 | 3/10 | 30.0 |
| Anti-Smith | 9/22 | 40.9 | 5/10 | 50.0 |
| Anti-dsDNA | 5/19 | 26.3 | 4/9 | 44.4 |
| Low complement | 4/16 | 25.0 | 1/9 | 11.1 |
N: available sample size per category; P: pregnancy; W: women.
aGood control of mixed connective tissue disease six months prior to pregnancy.
bSteroids or immunosuppressive drugs.
cIncluding relapses in pregnancy and de novo diagnosis up to six weeks PP.
Medical complications
Among women suffering from MCTD before pregnancy (n = 60), 26.7% relapsed during pregnancy (n = 16). Prevalence of PAH was described in seven cases (8.8% from the overall cohort), including a mild case (35 mmHg) in the CHUM cohort and six severe cases from the literature, one of which was diagnosed at the time of autopsy for a woman who collapsed suddenly immediately post-partum,23 and one which was confirmed by right heart catheterisation during caesarean section.22 Among other maternal major organ dysfunction during pregnancy, ILD affected 11 patients (13.8%), non-preeclamptic proteinuria was found in 11 (13.8%), and RSC affected 1.3% (one pregnancy from the literature;22 Table 2).
Table 2.
Medical and obstetric complications.
|
Literature review and CHUM cohort (63 W, 80 P) |
CHUM cohort only(10 W, 12 P) |
|||
|---|---|---|---|---|
| P/N | % | P/N | % | |
| Medical complications | ||||
| Relapse | 16/60 | 26.7 | 0/11 | 0 |
| PAHa | 7/80 | 8.8 | 1/12 | 8.3 |
| ILDa | 11/80 | 13.8 | 2/12 | 16.6 |
| Non-preeclamptic proteinuriaa | 11/80 | 13.8 | 0/12 | 0 |
| SRCa | 1/80 | 1.3 | 0/12 | 0 |
| Obstetric complications | ||||
| Preeclampsia | 13/74 | 17.6 | 3/11 | 27.2 |
| Caesarean section | 19/61 | 31.1 | 3/11 | 27.2 |
| VTE | 2/80 | 2.5 | 0/12 | 0 |
| Maternal mortality | 2/80 | 2.5 | 0/12 | 0 |
| Prematurity | 25/52 | 48.1 | 2/11 | 18.2 |
| IUGR | 19/49 | 38.8 | 2/11 | 18.2 |
| NL | 6/63 | 9.5 | 0/9 | 0 |
| CP | 13/63 | 20.6 | 0/9 | 0 |
| Perinatal mortalityb | 14/79 | 17.7 | 0/12 | 0 |
CP: chondrodysplasia punctata; ILD: interstitial lung disease; IUGR: intrauterine growth restriction; N: available sample size per category; NL: neonatal lupus; PAH: pulmonary arterial hypertension; P: pregnancy; SRC: scleroderma renal crisis; VTE: venous thromboembolism; W: women.
aPrevalence based on total cohort as no systematic screening was made.
bFetus and neonates combined.
Obstetric complications
The rate of caesarean section (31.1%), preeclampsia (17.6%), and thromboembolic events (2.5%) was noted. Two maternal deaths occurred. One was from previously undiagnosed pulmonary hypertension as described above,23 and one was due to severe right cardiac insufficiency eight months after a post-partum pulmonary embolism.21 This death was included in our study as it was secondary to an obstetric complication, bringing the maternal mortality rate to 2.5%. Fetal complications included prematurity (48.1%), IUGR (38.8%), and NL (9.5%). Concerning NL, 3 of 6 were from mothers having anti-U1RNP alone, without Anti-Ro or La antibodies. The mother of the only child with congenital heart block was positive for anti-U1RNP and anti-Smith only; however, Anti-Ro and La were not routinely screened in 1970.18 The last two NL cases were exposed to anti-U1RNP in conjunction with Anti-Ro and La and anti-La respectively. Thirteen infants (20.6%) had CP. There were 14 perinatal deaths (17.7%): four had a CP diagnosis with concurrent maternal condition such as premature rupture of membranes, three resulted from severe relapses leading to stillbirth, two were TA, and two others followed severe preeclampsia (one stillbirth and one TA). Seven other cases are from the same article from Kitridou in 1988: three stillbirths and four TA for whom circumstances are not reported.15
Effect of disease activity
To study the impact of MCTD activity on obstetric course, we compared 26 pregnancies with active disease against 44 pregnancies with quiescent disease. Women with active MCTD had a disease of shorter duration (an average of 3.04 vs. 5.80 years; OR = 0.80; 95%CI [0.65; 0.97]). Mean age was similar and there was no significant difference between groups with the use of immunosuppression at the beginning of pregnancy. There was insufficient data concerning immunosuppressive agent use to measure if this had an effect on activity. Women in the active group were less frequently well-controlled prior to pregnancy than in the quiescent group, although the difference was not significant (71.4% vs. 83.3%; OR = 0.79; 95%CI [0.48; 1.32]). Active disease led to significantly more prematurity (80.0% vs. 29.0%; OR = 7.60; 95CI% [1.93; 29.95]) and perinatal mortality (42.3% vs. 2.3%; OR = 16.83; 95%CI [1.90; 148.70]). There was a non-significant trend towards more caesareans (OR = 1.95; 95%CI [0.54; 7.13]), IUGR (OR = 1.48; 95%CI [0.04; 5.49]) and more NL with CP (grouped together – see discussion) (OR = 1.27; 95%CI [0.35; 4.66]). Preeclampsia risk was not significantly increased (OR = 0.88; 95%CI [0.18; 4.23]).
Discussion
To our knowledge this is the most extensive MCTD series examined in pregnancy. We demonstrate that this condition may increase the risk of medical and obstetric complications for the mother and her fetus. This risk is similar to what is seen with other autoimmune diseases, especially systemic lupus erythematosus and systemic sclerosis.
The relapse rate of 26.7% approximates that of lupus (25% to 67%) and rheumatoid arthritis (10% to 25%).34–36 The PAH prevalence rate is 8.8%, but prevalence could be higher, since systematic screening in non-pregnant populations with MCTD and scleroderma show a prevalence of 13.3%.37 Similarly, ILD affects 13.8% of pregnancies, which is less than the value obtained from systematic screening (52% to 66%) for MCTD in a non-pregnant population.38–40 Furthermore, non-preeclamptic proteinuria was noted, but RSC was infrequent. For optimal care, these data suggest systematic screening for PAH and ILD before pregnancy and careful follow-up of blood pressure and renal parameters during pregnancy. Screening for these severe complications was rarely done or reported in our review, so the prevalence is an estimation and may be inaccurate.
The maternal complication rates (Table 3, including for preeclampsia, thromboembolic events, and mortality) from our data exceed what is expected from the general population, but are comparable to that for other rheumatologic diseases in pregnancy.34–36,41–43 Aspirin may help to prevent preeclampsia in this population.
Table 3.
Impact of disease activity.
| N (P = 70) | Active MCTD(P = 26; 37.1%) |
Quiescent MCTD(P = 44; 62.9%) |
OR | 95%CI | |||
|---|---|---|---|---|---|---|---|
| P | Mean (range) | P | Mean (range) | ||||
| Maternal age (y) | 56 | 16 | 28.8 (17–36) | 40 | 28.5 (18–41) | 0.99 | 0.87–1.13 |
| Disease duration (y) | 56 | 20 | 3.0 (0–9.3) | 36 | 5.8 (0.1–1.13) | 0.80a | 0.65–0.97 |
|
Ratio |
% |
Ratio |
% |
||||
| Good disease controlb | 25 | 5/7 | 71.4 | 15/18 | 83.3 | 0.79 | 0.48–1.32 |
| Steroids/Immunosupp.c | 32 | 4/9 | 44.4 | 10/23 | 43.5 | 1.01 | 0.29–3.52 |
| Preeclampsia | 68 | 5/25 | 20.0 | 7/43 | 16.3 | 0.88 | 0.18–1.23 |
| Caesarean section | 56 | 8/17 | 47.1 | 11/39 | 28.2 | 1.95 | 0.54–7.13 |
| VTE | 70 | 1/26 | 3.8 | 0/44 | 0 | – | – |
| Maternal mortality | 70 | 1/26 | 3.8 | 0/44 | 0 | – | – |
| Prematurity | 46 | 12/15 | 80.0 | 9/31 | 29.0 | 7.60a | 1.93–29.95 |
| IUGR | 43 | 7/16 | 43.8 | 10/29 | 34.5 | 1.48 | 0.04–5.49 |
| NL and CP | 54 | 4/16 | 25.0 | 8/38 | 21.1 | 1.27 | 0.35–4.66 |
| Perinatal mortality | 69 | 11/26 | 42.3 | 1/43 | 2.3 | 16.83a | 1.90–148.7 |
CI: confidence intervals, CP: chondrodysplasia punctata, IUGR: intrauterine growth restriction, N: sample size available, NL: neonatal lupus, OR: odds ratio, P: pregnancy, VTE: venous thromboembolic event.
aStatistically significant on alpha 0.05 threshold.
bSix months prior to pregnancy.
cSteroids or immunosuppressive drugs.
Fetal complication rates (Table 3), including prematurity, IUGR, and perinatal mortality, are also higher than in the general population, but are similar to other rheumatologic diseases in pregnancy.35,36,41–44 Neonatal lupus was frequently seen in pregnant women with MCTD (28.6%). This rate is similar to that for cutaneous (10–20%) or haematologic and hepatic (27%) manifestations of offspring from mothers positive for anti-Ro or La antibodies.45 We highlight three NL cases due to anti-U1RNP antibodies only. Congenital heart block rate seen was 1.6% and reflects the 1–2% rate from the literature.45 We also demonstrate that CP is a potential risk for these fetuses.7,17,19,27,30 CP has numerous etiologies, but when this condition is linked to autoimmune diseases, it may result from maternal antibodies affecting specific proteins in the fetal cartilage, among other potential mechanisms.7 Moreover, others consider it as a bone manifestation of NL.5 In fact, some CP cases from mothers with Lupus and one from a mother with MCTD were also associated with cutaneous and hematologic NL manifestations.7 Four deaths were linked to CP in the literature cohort. We did not find any CP cases in our data: the higher rate of CP in the literature may be due do a publication bias.
MCTD activity seems to influence the risk of obstetric complications, as we have shown for prematurity and fetal mortality. Moreover, women with active disease during pregnancy showed a trend, although not significant, for a more active disease pre-pregnancy. Pre-pregnancy counselling to improve disease control may improve outcomes.
Our study has several limitations. First, many outcomes were missing from the cases found in the literature. Data were available on disease activity for only 70 pregnancies and was rarely detailed (Table 3). Second, the retrospective chart review is limited by the ICD system since it is mainly used for billing, and MCTD is not specifically coded for. We feel that we identified these women adequately by using broad and sensitive coding, then by reviewing charts to select only appropriate diagnoses. Third, late complications among offspring (e.g. NL and mortality) could have been missed in the CHUM population in the absence of any paediatric care in our centre, but none were noted when reviewing maternal charts. Fourth, only case series and reports were retrieved. Case series are prone to publication bias that overestimates complications: successful pregnancies may not be published. We used historical controls to compare the complication rates with our data. Without a group control, selection and publication bias may have occurred. Trends highlighted by our study should be corroborated through a multicentre MCTD prospective cohort, as was done for lupus in pregnancy.42 Fifth, medical and obstetric practices have improved over time, which could influence outcomes. Since our aim was to inventory all the previous existing cases, we kept articles considered older (eight articles were from 1979 to 1995, the others from 2001 to 2014). However, we cannot conclude that older cases resulted in worse outcomes: two of these articles are case series with 12 and 11 patients for whom we have very few details. Of the remaining six case reports, one responded well to steroids, one responded well without treatment, one had a post-partum flare, and three provide no disease details. In our cases, three delivered before 1995: two without complications and one that had a de novo post-partum diagnosis. Finally, we were unable to analyse outcomes according to different antibody profiles or clinical subtypes due to lack of data.
Conclusion
Despite limitations inherent to case series and systematic reviews, our study demonstrates that MCTD in pregnancy is not a benign condition. The relapse rate is similar to that of other autoimmune diseases, and PAH, ILD and renal dysfunction are important comorbidities to consider. These women have a higher risk of preeclampsia, thromboembolism, and death. Their offspring risk NL, prematurity, IUGR, and death. Proper disease control could contribute to reducing some of these risks.
Acknowledgements
The authors thank Ms Daniela Ziegler for the MEDLINE search, Ms Line Sergerie for the chart review, Dr Alexandra Albert and Dr Martial Koenig for submitting cases, Mr Miguel Chagnon for the statistical analysis, and Mr John Davison for editing the manuscript. This study was presented as an oral presentation at the combined ISOM/NASOM meeting held in New Orleans, LO, USA, on 25–27 October 2014.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
This study was approved by the institutional Research Ethics Board (Comité d'éthique de la recherché) of the Centre de Recherche du Centre Hospitalier de l’Université de Montréal (CE 14.104-AS). The ethical board of the author’s institution did not require patient consent since as this was a retrospective case series.
Guarantor
MM.
Contributorship
MM guarantees the manuscript’s accuracy and the contributorship of all the authors. Both authors created the protocol, analysed the data, and contributed to writing the manuscript.
References
- 1.Kitridou RC. Pregnancy in mixed connective tissue disease. Rheum Dis Clin North Am 2005; 31: 497–508, vii. [DOI] [PubMed] [Google Scholar]
- 2.Alarcón-Segovia D, Cardiel MH. Comparison between 3 diagnostic criteria for mixed connective tissue disease. Study of 593 patients. J Rheumatol 1989; 16: 328–334. [PubMed] [Google Scholar]
- 3.Magee LA, Pels A, Helewa M, et al. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: Executive summary. J Obstet Gynaecol Can 2014; 36: e1–e27. [DOI] [PubMed] [Google Scholar]
- 4.Kramer MS, Platt RW, Wen SW, et al. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics 2001; 108: E35. [DOI] [PubMed] [Google Scholar]
- 5.Silverman E, Jaeggi E. Non-cardiac manifestations of neonatal lupus erythematosus. Scand J Immunol 2010; 72: 223–225. [DOI] [PubMed] [Google Scholar]
- 6.Behluli I, Riehl J, Rath W. Mixed connective tissue disease and HELLP syndrome in pregnancy. Dtsch Med Wochenschr 2007; 132: 878–880. [DOI] [PubMed] [Google Scholar]
- 7.Chitayat D, Keating S, Zand DJ, et al. Chondrodysplasia punctata associated with maternal autoimmune diseases: Expanding the spectrum from systemic lupus erythematosus (SLE) to mixed connective tissue disease (MCTD) and scleroderma report of eight cases. Am J Med Genet 2008; 146A: 3038–3053. [DOI] [PubMed] [Google Scholar]
- 8.Chung L, Flyckt R, Colon I, et al. Outcome of pregnancies complicated by systemic sclerosis and mixed connective tissue disease. Lupus 2006; 15: 595–599. [DOI] [PubMed] [Google Scholar]
- 9.Cimaz R, Biggioggero M, Catelli L, et al. Ultraviolet light exposure is not a requirement for the development of cutaneous neonatal lupus. Lupus 2002; 11: 257–260. [DOI] [PubMed] [Google Scholar]
- 10.Dezeros G, Duchatel F, Kerneis Y, et al. Association of Sharp’s syndrome and pregnancy. Anesthesie Analgesie Reanimation 1981; 38: 559–561. [PubMed] [Google Scholar]
- 11.Fujiwaki T, Urashima R, Urushidani Y, et al. Neonatal lupus erythematosus associated with maternal mixed connective tissue disease. Pediatr Int 2003; 45: 210–213. [DOI] [PubMed] [Google Scholar]
- 12.Hasmuller S, Ehrhardt H, Kahlert S, et al. Peripartum bilateral uterine rupture in a patient with mixed connective tissue disease with favorable outcome for the severely asphyctic newborn after hypothermia. Arch Gynecol Obstet 2010; 281: 617–621. [DOI] [PubMed] [Google Scholar]
- 13.Horita Y, Tsunoda S, Inenaga T, et al. Pregnancy outcome in nephrotic syndrome with mixed connective tissue disease. Nephron 2001; 89: 354–356. [DOI] [PubMed] [Google Scholar]
- 14.Hoshino T, Kita M, Takahashi T, et al. Management of two pregnancies in a woman with mixed connective tissue disease, pulmonary fibrosis, frequent pneumothorax and oxygen inhalation therapy along with a published work review. J Obstet Gynaecol Res 2008; 34: 613–618. [DOI] [PubMed] [Google Scholar]
- 15.Kitridou RC. Pregnancy in mixed connective tissue disease, poly/dermatomyositis and scleroderma. Clin Exp Rheumatol 1988; 6: 173–178. [PubMed] [Google Scholar]
- 16.Lundberg I, Hedfors E. Pregnancy outcome in patients with high titer anti-RNP antibodies. A retrospective study of 40 pregnancies. J Rheumatol 1991; 18: 359–362. [PubMed] [Google Scholar]
- 17.Nayak SS, Adiga PK, Rai L, et al. Severe rhizomelic chondrodysplasia punctata in a fetus due to maternal mixed connective tissue disorder. Genet Couns 2012; 23: 487–491. [PubMed] [Google Scholar]
- 18.Nolan RJ, Shulman ST, Victorica BE. Congenital complete heart block associated with maternal mixed connective tissue disease. J Pediatr 1979; 95: 420–422. [DOI] [PubMed] [Google Scholar]
- 19.Schulz SW, Bober M, Johnson C, et al. Maternal mixed connective tissue disease and offspring with chondrodysplasia punctata. Semin Arthritis Rheum 2010; 39: 410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strandberg L, Salomonsson S, Bremme K, et al. Ro52, Ro60 and La IgG autoantibody levels and Ro52 IgG subclass profiles longitudinally throughout pregnancy in congenital heart block risk pregnancies. Lupus 2006; 15: 346–353. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe R, Tatsumi K, Uchiyama T, et al. Puerperal secondary pulmonary hypertension in a patient with mixed connective tissue disease. Nihon Kyobu Shikkan Gakkai Zasshi 1995; 33: 883–887. [PubMed] [Google Scholar]
- 22.Yamaguchi T, Ohshima S, Tanaka T, et al. Renal crisis due to intimal hyperplasia in a patient with mixed connective tissue disease (MCTD) accompanied by pulmonary hypertension. Intern Med 2001; 40: 1250–1253. [DOI] [PubMed] [Google Scholar]
- 23.Morton A. Pulmonary hypertension imitating HELLP syndrome. Obstet Med 2013; 6: 169–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu G, Wang S, Liang M. The early diagnosis and treatment of immune disease in pregnancy. Int J Gynecol Obstet 2009; 107: S245. [Google Scholar]
- 25.Heelan K, Watson R, Collins S. RNP antibody-positive neonatal lupus erythematosus: A case report and review of the literature. Br J Dermatol 2012; 167: 128. [Google Scholar]
- 26.Goya M, Meseguer ML, Merced C, et al. Successful pregnancy in a patient with pulmonary hypertension associated with mixed collagen vascular disease. J Obstet Gynaecol 2014; 34: 191–192. [DOI] [PubMed] [Google Scholar]
- 27.Colin E, Touraine R, Levaillant JM, et al. Binder phenotype in mothers affected with autoimmune disorders. J Matern Fetal Neonatal Med 2012; 25: 1413–1418. [DOI] [PubMed] [Google Scholar]
- 28.Goudzwaard C, Tandon R, Chen E. 17-Year-old pregnant female with pulmonary arterial hypertension: Successful maternal-fetal outcome with inhaled treprostinil therapy. Chest 2011; 140: 51A. [Google Scholar]
- 29.Boggess KA, Easterling TA, Raghu G. Management and outcome of pregnant women with interstitial and restrictive lung disease. Am J Obstet Gynecol 1995; 173: 1007–1014. [DOI] [PubMed] [Google Scholar]
- 30.Lim K, Pugash D, Friedman JM, et al. Just images: Four consecutive pregnancies affected by chondrodysplasia punctata in a woman with mixed connective tissue disease. Oral communication abstracts. Ultrasound Obstet Gynecol 2005; 26: 309–375.16152007 [Google Scholar]
- 31.Sheth AP, Esterly NB, Ratoosh SL, et al. U1RNP positive neonatal lupus erythematosus: Association with anti-La antibodies? Br J Dermatol 1995; 132: 520–526. [DOI] [PubMed] [Google Scholar]
- 32.Selby C, Richfield M, Croft S, et al. Postpartum flare in mixed connective tissue disease. J Rheumatol 1982; 9: 332–334. [PubMed] [Google Scholar]
- 33.Bonnin M, Mercier FJ, Sitbon O, et al. Severe pulmonary hypertension during pregnancy: Mode of delivery and anesthetic management of 15 consecutive cases. Anesthesiology 2005; 102: 1133–1137. [DOI] [PubMed] [Google Scholar]
- 34.Østensen M, Cetin I. Autoimmune connective tissue diseases. Best Pract Res Clin Obstet Gynaecol 2015; 29: 658–670. [DOI] [PubMed] [Google Scholar]
- 35.Smyth A, Oliveira GH, Lahr BD, et al. A systematic review and meta-analysis of pregnancy outcomes in patients with systemic lupus erythematosus and lupus nephritis. Clin J Am Soc Nephrol 2010; 5: 2060–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar S, Suri V, Wanchu A. Pregnancy and rheumatic disorders. Indian J Rheumatol 2010; 5: 35–41. [Google Scholar]
- 37.Wigley FM, Lima JA, Mayes M, et al. The prevalence of undiagnosed pulmonary arterial hypertension in subjects with connective tissue disease at the secondary health care level of community-based rheumatologists (the UNCOVER study). Arthritis Rheum 2005; 52: 2125–2132. [DOI] [PubMed] [Google Scholar]
- 38.Végh J, Szilasi M, Soós G, et al. Interstitial lung disease in mixed connective tissue disease. Orv Hetil 2005; 146: 2435–2443. [PubMed] [Google Scholar]
- 39.Bodolay E, Szekanecz Z, Dévényi K, et al. Evaluation of interstitial lung disease in mixed connective tissue disease (MCTD). Rheumatology (Oxford) 2005; 44: 656–661. [DOI] [PubMed] [Google Scholar]
- 40.Gunnarsson R, Aaløkken TM, Molberg Ø, et al. Prevalence and severity of interstitial lung disease in mixed connective tissue disease: A nationwide, cross-sectional study. Ann Rheum Dis 2012; 71: 1966–1972. [DOI] [PubMed] [Google Scholar]
- 41.Barnabe C, Faris P, Quan H. Canadian pregnancy outcomes in rheumatoid arthritis and systemic lupus erythematosus. Int J Rheumatol 2011; 345727: 6. DOI: 10.1155/2011/345727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clowse ME, Jamison M, Myers E, et al. A national study of the complications of lupus in pregnancy. Am J Obstet Gynecol 2008; 199: 127.e121–127.e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cervera R, Serrano R, Pons-Estel GJ, et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: A multicentre prospective study of 1000 patients. Ann Rheum Dis 2015; 74: 1011–1018. [DOI] [PubMed] [Google Scholar]
- 44.Taraborelli M, Ramoni V, Brucato A, et al. Brief report: Successful pregnancies but a higher risk of preterm births in patients with systemic sclerosis: An Italian multicenter study. Arthritis Rheum 2012; 64: 1970–1977. [DOI] [PubMed] [Google Scholar]
- 45.Brucato A, Cimaz R, Caporali R, et al. Pregnancy outcomes in patients with autoimmune diseases and anti-Ro/SSA antibodies. Clinic Rev Allerg Immunol 2011; 40: 27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]