Abstract
Background
VYC-15L (Juvéderm Volbella XC) is a nonanimal crosslinked hyaluronic acid (HA) gel with lidocaine.
Objectives
To evaluate safety and effectiveness of repeat treatment with VYC-15L administered 1 year after treatment for lip and perioral enhancement.
Methods
In this prospective multicenter study, 124 subjects with minimal, mild, or moderate lip fullness on the validated 5-point Allergan Lip Fullness Scale (LFS) who received initial/touch-up treatment with VYC-15L received repeat treatment with VYC-15L 1 year after initial treatment. Effectiveness endpoints included LFS responder rates (≥1-point improvement from baseline) and scores on the FACE-Q Satisfaction With Lips and Appraisal of Lip Lines scales at 1 month after repeat treatment. Subjects completed safety diaries for 30 days after repeat treatment.
Results
LFS responder rates were 86.2%, 80.3%, and 65.3% at months 1 and 3 and 1 year, respectively, after initial/touch-up treatment. The responder rate improved to 94.3% 1 month after repeat treatment with VYC-15L and required less median volume vs initial/touch-up treatment (1.5 vs 2.6 mL). FACE-Q scores doubled from baseline at 3 months, remained high through 1 year, and doubled from baseline after repeat treatment. At 1 month after repeat treatment, 96.7% and 89.3% of subjects showed improvement over baseline in FACE-Q Satisfaction With Lips and Appraisal of Lip Lines, respectively. Severe injection site responses were less frequent after repeat treatment than initial/touch-up treatment.
Conclusions
Repeat treatment with VYC-15L at 1 year was safe and effective for lip and perioral enhancement, and required less product volume to achieve similar effectiveness to initial/touch-up treatment.
Level of Evidence: 4

Treatment of the lips and perioral region with hyaluronic acid (HA)-based dermal fillers is a common approach to enhancement or rejuvenation of these areas.1 Volume can be added for lips that are inherently thin, or volume can be restored for lips that have thinned due to the aging process.2,3 Treatment of the perioral region may provide multiple benefits by restoring the structural support of oral commissures and philtral columns and correcting age-related perioral lines.1,4-6
VYC-15L (Juvéderm Volbella XC; Allergan plc, Dublin, Ireland) is a malleable HA-based filler suited for lip and perioral enhancement. In a novel crosslinking process based on the Vycross technology platform (Allergan plc), low- and high-molecular-weight HA are combined to create a tightly crosslinked HA network. The resultant product displays a decreased tendency to absorb water (enabling visualization of results at the time of treatment) and increased duration of effect compared with other facial filler products.1,7 A small amount of lidocaine is added to increase subject comfort during treatment.2,6,8-10 Studies conducted in Europe have demonstrated the safety and effectiveness of VYC-15 with and without lidocaine for the lips and perioral region.1,6,10 The present multicenter US study evaluated the safety and effectiveness of VYC-15L for lip and perioral enhancement. Published results from this study showed that improvement in overall lip fullness (primary endpoint) was similar for VYC-15L compared with a control HA dermal filler with lidocaine at 3 months after initial and touch-up treatment, with treatment effects of VYC-15L persisting through 1 year.11 This report presents results on the safety and effectiveness of repeat treatment administered 1 year after initial treatment with VYC-15L.
METHODS
Study Design and Treatment
Thirteen investigational sites in the United States participated in this prospective, multicenter, controlled study (clinicaltrials.gov identifier NCT01998581) conducted from November 2013 to May 2015. Institutional review board approval was provided by Quorum Review Inc. (Seattle, WA) and Saint Louis University Institutional Review Board (St. Louis, MO) for each site before any subjects were enrolled. The study was conducted in compliance with Good Clinical Practice, and all subjects provided written informed consent. The study design and subject eligibility criteria were previously reported.11 Briefly, the study enrolled subjects aged ≥22 years who had an overall lip fullness score of 0 (minimal), 1 (mild), or 2 (moderate) on the validated photonumeric 5-point Allergan Lip Fullness Scale (LFS),12 and either desired a ≥1-point improvement on the LFS or had Fitzpatrick skin type V or VI with an LFS score of 3 (marked) or 4 (very marked) and desired treatment of the vermilion body of one or both lips. For initial treatment of perioral lines, subjects were required to have an Allergan Perioral Lines Severity Scale (POLSS) score of moderate or severe. Subjects received treatment on a complimentary basis and were compensated for their time based on regional guidelines.
The study comprised initial treatment, an optional touch-up treatment at 30 days, and repeat treatment with VYC-15L administered during the 1-year follow-up visit after completion of the 1-year safety and effectiveness assessments. Eligible subjects were randomly assigned using a central block randomization schedule and an automated interactive voice/web response system (Perceptive Informatics; Deerfield, IL) in a 3:1 ratio to receive VYC-15L or control, with randomization stratified by Fitzpatrick skin phototype.11 Data from subjects initially randomized to the VYC-15L treatment arm of the study and who received repeat treatment are reported here. Each site had an unblinded treating investigator who performed all treatment injections and a blinded evaluating investigator who performed safety and effectiveness assessments. For repeat treatment, the treating investigator injected VYC-15L into the lips and mid to deep dermis of the perioral area using a 30-gauge half-inch needle. The treating investigator selected treatment sites (vermilion body, perioral lines, oral commissures, vermilion border, Cupid’s bow, and philtral columns) and treatment volume as needed for lip and perioral enhancement based on clinical experience. Injection volume was not to exceed 1.5 mL for each lip (upper and lower) at each of the initial, optional touch-up, and repeat treatments. Total treatment volume allowed was a maximum of 6 mL for initial and touch-up treatments combined and 6 mL for repeat treatment. Subjects returned for follow-up visits at 3 and 14 days and 1 month after repeat treatment.
Effectiveness Assessments
The blinded evaluating investigator assessed: overall lip fullness using the 5-point LFS (scored as 0 = minimal, 1 = mild, 2 = moderate, 3 = marked, and 4 = very marked); severity of perioral lines at rest and at maximal contraction using the validated Allergan POLSS and Perioral Lines at Maximal Contraction (POLM) scales, respectively, and severity of oral commissures using the validated Allergan Oral Commissures Severity Scale (OCSS) (each scored as 0 = none, 1 = mild, 2 = moderate, and 3 = severe)13; and global aesthetic improvement using the 5-point Global Aesthetic Improvement Scale (GAIS; 2 = much improved to −2 = much worse). Subjects completed the Satisfaction With Lips and Appraisal of Lip Lines scales of the validated FACE-Q questionnaire14,15 (items on the scales were combined to create a score range of 0-100) at the initial treatment study visit. The treating investigator used 11-point scales to rate ease of injection (0 = difficult to 10 = easy) and product moldability (0 = stiff to 10 = moldable); the subject used 11-point scales to rate natural look and natural feel of the lips (0 = unnatural looking/feeling to 10 = natural looking/feeling).
Safety Assessments
Subjects recorded the occurrence and severity of injection site responses (ISRs) in a daily diary for 30 days after repeat treatment and attended follow-up visits at days 3 and 14 after repeat treatment. Adverse events (AEs) were monitored throughout the month after repeat treatment. The evaluating investigators assessed Tyndall effect (bluish hue in the treated area) and lip sensation (by 2-point discrimination and light touch tests). Subjects rated procedural pain of the treatment on an 11-point scale (0 = no pain to 10 = worst pain imaginable) and completed the Recovery Early Life Impact scale of FACE-Q16 on day 3 after repeat treatment.
Statistics
Responder rates for the LFS, POLSS, POLM, and OCSS were defined as the proportion of subjects with a ≥1-point improvement from baseline in evaluating investigator-assessed LFS, POLSS, POLM, and OCSS scores, respectively. Only subjects with baseline POLSS scores of moderate or severe who received perioral line treatment were included in the analysis of perioral line severity. All FACE-Q scale scores were transformed by the conversion tables created by the FACE-Q developers, in which the lowest and highest scores were 0 and 100, respectively. Paired t tests were used to test the statistical significance of changes from baseline in mean scores on the LFS, POLSS, and FACE-Q.
RESULTS
Subject Disposition
Of the 168 subjects who received initial treatment with VYC-15L, 124 (73.8%) subjects received repeat treatment with VYC-15L at the 1-year visit. Twenty subjects declined repeat treatment at the 1-year visit; 65.0% (13/20) of these subjects declined repeat treatment because they were satisfied with their current lip fullness. Additional reasons for discontinuation were loss to follow-up (7.1%; 12/168), withdrawal of consent (4.8%; 8/168), other (1.8%; relocated to the East Coast, n = 2; subject decision, n = 1), AE (0.6%; 1/168), and protocol violation (0.6%; 1/168). Nearly all (98.4%; 122/124) of the subjects who received repeat treatment completed the 1-month follow-up visit within the prescribed time.
Demographic and Baseline Characteristics
Demographic and baseline characteristics of the study population are shown in Table 1. For subjects who received initial treatment with VYC-15L, median age was 53 years (range, 22-78 years), and mean age was 52.7 years; most subjects were female (97.6%) and white (85.7%), and the majority had LFS scores of mild or moderate at baseline (78.6%). Most subjects had Fitzpatrick skin type II or III (61.3%), but all Fitzpatrick skin types were represented. Most subjects were nonsmokers (88.1%), and mean exposure to sunlight was 2 hours per day.
Table 1.
Demographic and Baseline Characteristics of Subjects Treated With VYC-15L
| Parameter | VYC-15L (N = 168) |
|---|---|
| Age, median (range), years | 53 (22-78) |
| Female, n (%) | 164 (97.6) |
| Race, n (%) | |
| White | 144 (85.7) |
| Black or African American | 15 (8.9) |
| Other | 9 (5.4) |
| Ethnicity, n (%) | |
| Not Hispanic or Latino | 156 (92.9) |
| Hispanic or Latino | 12 (7.1) |
| Fitzpatrick skin type, n (%) | |
| I | 18 (10.7) |
| II | 50 (29.8) |
| III | 53 (31.5) |
| IV | 26 (15.5) |
| V | 11 (6.5) |
| VI | 10 (6.0) |
| Overall lip fullness on LFS, n (%) | |
| Minimal (0) | 33 (19.6) |
| Mild (1) | 73 (43.5) |
| Moderate (2) | 59 (35.1) |
| Marked (3) | 3 (1.8) |
| Very Marked (4) | 0 (0) |
| Perioral lines severity on POLSS, n (%) | (n = 94) |
| Severe (3) | 40 (42.6) |
| Moderate (2) | 46 (48.9) |
| Mild (1) | 7 (7.4) |
| None (0) | 1 (1.1) |
Treatment Administration
Characteristics for the initial and touch-up treatment have been published.11 For repeat treatment at 1 year, the median total volume of VYC-15L injected into the perioral region (1.5 mL) (Table 2) was less than the median total volume administered for initial plus touch-up treatment (2.6 mL). The median volume injected to the lips was 0.95 mL (upper lip, 0.50 mL; lower lip, 0.45 mL) for repeat treatment compared with 1.8 mL (upper lip, 1.0 mL; lower lip, 0.8 mL) for initial plus touch-up treatment. Repeat treatment injection sites included the upper lip in 96.0% of subjects (vermilion body: 85.5%; vermilion border: 60.5%; Cupid’s bow: 49.2%), philtral columns in 42.7% of subjects, the lower lip in 95.2% of subjects (vermilion body: 91.9%; vermilion border: 50.8%), the oral commissures in 86.3% of subjects, and the perioral lines in 53.2% of subjects. Anesthesia was administered to 63.7% of subjects before repeat treatment, with topical anesthesia administered to 40.3% of subjects and ice applied to 32.3% of subjects. The median duration of anesthesia (time from application of anesthesia to treatment start) was 5.0 minutes for ice, 23.5 minutes for topical anesthesia, and 5.0 minutes for local anesthesia. The mean score for procedural pain during repeat treatment was 3.6 out of 10.
Table 2.
Treatment Sites and Volume Injected for Repeat Treatment With VYC-15L
| Treatment site | Subjects treatedn (%) | Volume injected,median (range), mL |
|---|---|---|
| Total | 124 (100) | 1.50 (0.40-4.0) |
| Upper lip | 119 (96.0) | 0.50 (0.05-1.3) |
| Lower lip | 118 (95.2) | 0.45 (0.15-1.4) |
| Oral commissures | 107 (86.3) | 0.35 (0.05-2.0) |
| Perioral lines | 66 (53.2) | 0.30 (0.05-1.6) |
| Philtral columns | 53 (42.7) | 0.10 (0.05-0.3) |
The most frequent injection techniques used across all injection sites for repeat treatment were tunneling (83.1%) and serial puncture (75.8%); fanning was used infrequently for the oral commissures (14.0%) and perioral lines (7.6%); crosshatching was not used. Nearly all injections to the vermilion body of the upper lip (100%) and lower lip (98.2%) were subdermal, and nearly all injections to the vermilion border of the upper lip (98.7%) and lower lip (100%) were intradermal. All injections to perioral lines, Cupid’s bow, philtral columns, and oral commissures were done intradermally.
Treating investigators rated ease of injection (11-point scale: 0 = difficult, 10 = easy) with scores of 8 to 10 in 100% of subjects who received repeat treatment with VYC-15L, with a score of 10 in 91.9% (114/124) of subjects. For treating investigator-rated moldability (11-point scale: 0 = stiff, 10 = moldable) during repeat treatment, the proportion receiving scores of 8 to 10 was 98.4% (122/124), with a score of 10 in 81.5% (101/124) of subjects.
Effectiveness
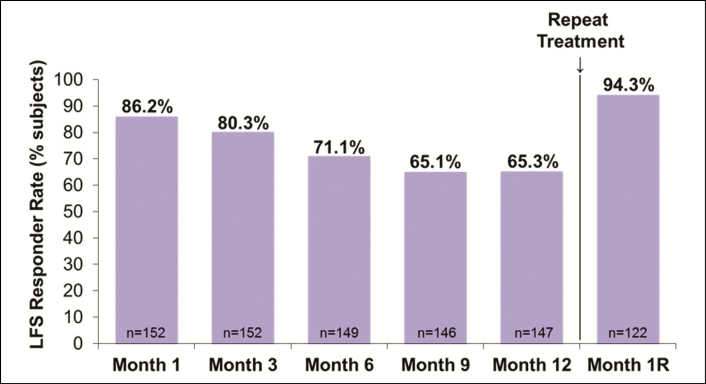
The LFS responder rate (≥1-point improvement from baseline) was 80.3% at 3 months after initial treatment or touch-up treatment if performed, decreased to 65.3% at 1 year, and increased to 94.3% at 1 month after repeat treatment (Figure 1). Mean LFS scores significantly improved from baseline (1.2) at 3 months after initial plus touch-up treatment (2.2), remained significantly higher than baseline at all time points through 1 year, and increased again 1 month after repeat treatment (2.6), surpassing the mean score at 3 months after initial treatment (P < 0.001 vs baseline for all time points). Representative photographs of 2 subjects' lips and perioral areas at baseline, 3 months, and 1 year after initial/touch-up treatment, and 1 month after repeat treatment with VYC-15L are shown in Figures 2 and 3.
Figure 1.
LFS responder rate by study visit. LFS, Allergan Lip Fullness Scale; Month 1R, Month 1 after repeat treatment; VYC-15L, Juvéderm Volbella XC.
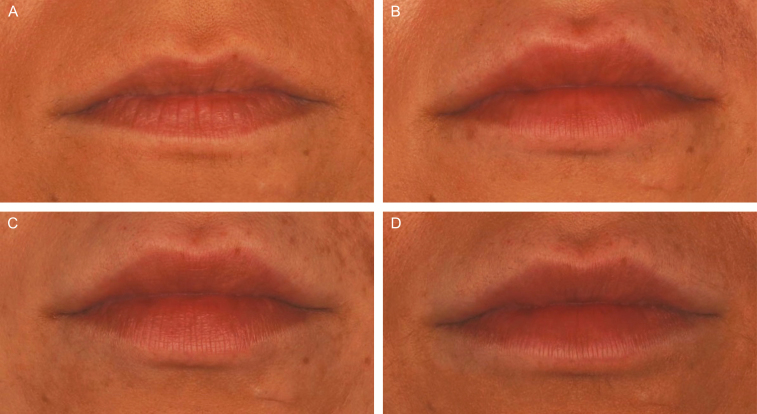
Figure 2.
Representative photographs of a 29-year-old female subject's lips and perioral area at (A) baseline, (B) 3 months, and (C) 1 year after treatment, and at 1 month after repeat treatment (D) with VYC-15L. She received initial treatment with 0.9 and 0.65 mL in the upper and lower lips, respectively, and touch-up treatment with 0.6 and 0.4 mL in the upper and lower lips, respectively. At repeat treatment, she received 0.85 and 0.75 mL in the upper and lower lips, respectively. (A) LFS at Baseline = Moderate; (B) LFS at Month 3 = Marked; (C) LFS at Month 12 = Moderate; (D) LFS at Month 1R = Marked. LFS, Allergan Lip Fullness Scale; Month 1R, Month 1 after repeat treatment; VYC-15L, Juvéderm Volbella XC.
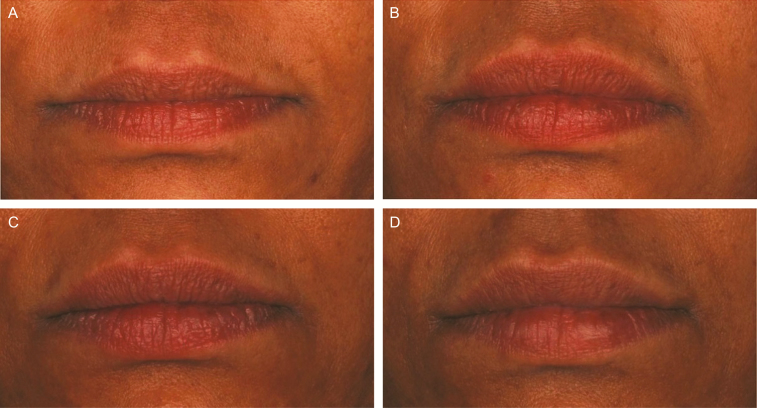
Figure 3.
Representative photographs of a 47-year-old female subject's lips and perioral area at (A) baseline, (B) 3 months, and (C) 1 year after treatment, and at 1 month after repeat treatment (D) with VYC-15L. She received initial treatment with 0.6 and 0.4 mL in the upper and lower lips, respectively, and touch-up treatment with 0.4 and 0.2 mL in the upper and lower lips, respectively. At repeat treatment, she received 0.95 and 0.65 mL in the upper and lower lips, respectively, 1 mL in the oral commissures, and 0.3 mL in the philtral columns. (A) LFS at Baseline = Moderate; (B) LFS at Month 3 = Marked; (C) LFS at Month 12 = Moderate; (D) LFS at Month 1R = Marked. LFS, Allergan Lip Fullness Scale; Month 1R, Month 1 after repeat treatment; VYC-15L, Juvéderm Volbella XC.
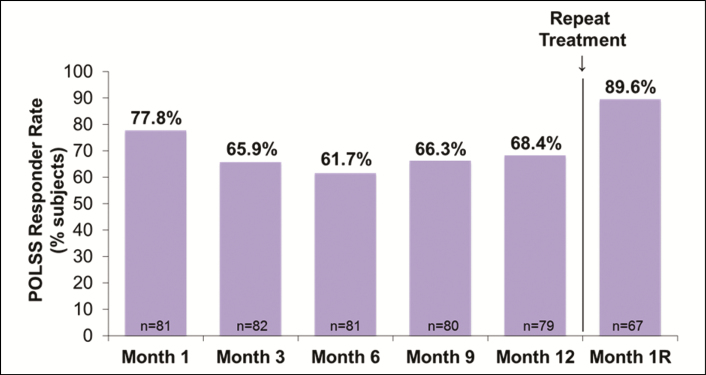
For subjects receiving treatment in the perioral lines, the POLSS responder rate at 1 month after repeat treatment (89.6%) was greater than at any other time point after initial treatment with VYC-15L (65.9% at 3 months and 68.4% at 1 year) (Figure 4). The mean change from baseline in POLSS scores was −0.9 at 3 months (P < 0.001), −0.8 at 1 year (P < 0.001), and −1.2 at 1 month after repeat treatment (P < 0.001). The POLM responder rate was 51.2% at 3 months, 31.6% at 1 year, and 67.2% at 1 month after repeat treatment. Among subjects receiving treatment in oral commissures, the OCSS responder rate was 59.2% at 3 months, 54.4% at 1 year, and 78.1% at 1 month after repeat treatment.
Figure 4.
POLSS responder rate by study visit among subjects who received treatment in perioral lines. POLSS, Perioral Lines Severity Scale; Month 1R, Month 1 after repeat treatment.
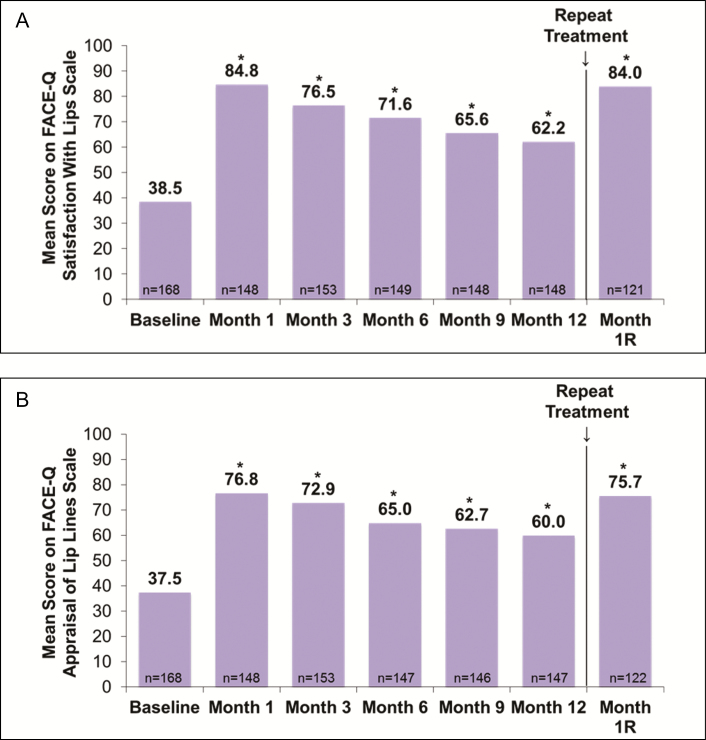
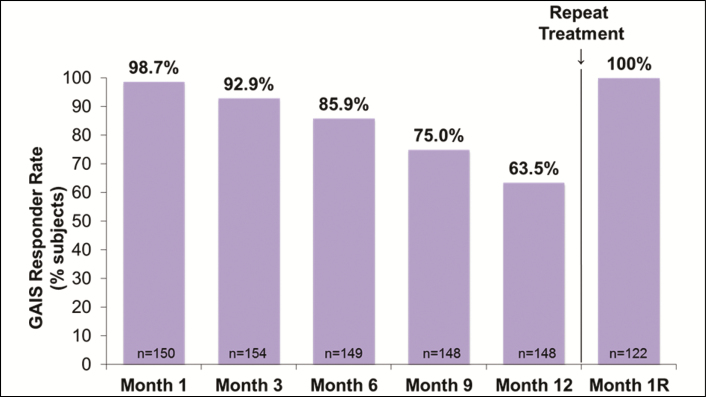
The mean scores on the FACE-Q Satisfaction With Lips scale improved from baseline to 1 month after initial/touch-up treatment (P < 0.001 vs baseline), remained significantly higher than baseline through 1 year (P < 0.001 for all time points), and increased again at 1 month after repeat treatment (P < 0.001) to values similar to those observed at 1 month after initial and touch-up treatment (Figure 5A). Results were comparable on the FACE-Q Appraisal of Lip Lines scale (Figure 5B). At 1 month after repeat treatment, 96.7% and 89.3% of subjects showed improvement over baseline in FACE-Q Satisfaction With Lips and Appraisal of Lip Lines, respectively. The proportion of subjects rated by evaluating investigators on the GAIS as “improved” or “much improved” in appearance was 92.9% at 3 months, 63.5% at 1 year, and 100% at 1 month after repeat treatment with VYC-15L (Figure 6).
Figure 5.
Mean scores of (A) FACE-Q Satisfaction With Lips scale and (B) Appraisal of Lip Lines scale by study visit. Month 1R, Month 1 after repeat treatment; *P < 0.001 vs baseline.
Figure 6.
Proportion of responders (subjects who were “improved” or “much improved”) on GAIS by study visit. GAIS, Global Aesthetic Improvement Scale; Month 1R, Month 1 after repeat treatment.
The majority of subjects rated the natural look and natural feel of the lips with scores of 7 to 10 on an 11-point scale (0 = unnatural looking/feeling and 10 = natural looking/feeling). For natural look of the lips, the proportions of subjects with ratings of 7 to 10 were 84.4% at 3 months, 93.2% at 1 year, and 90.9% at 1 month after repeat treatment. For natural feel of the lips, the proportions of subjects with ratings of 7 to 10 were 87.7% at 3 months, 91.9% at 1 year, and 89.3% at 1 month after repeat treatment.
Safety
ISRs occurring after initial and touch-up treatment, as recorded in the daily diaries, were reported previously.11 Briefly, the most frequent ISRs reported by subjects in the VYC-15L group after the initial and touch-up treatments combined were swelling (94.9%), lumps/bumps (91.8%), firmness (90.5%), and bruising (90.5%). After repeat treatment with VYC-15L, the most common ISRs were swelling (93.3%), tenderness (90.0%), firmness (85.0%), and lumps/bumps (85.0%) (Table 3). The majority of ISRs were mild or moderate in severity. The incidence of severe ISRs was lower after repeat treatment (36.2%) compared with initial and touch-up treatment (45.8%). Most (71.6%) ISRs reported after repeat treatment resolved within 2 weeks. The most common ISRs lasting for 15 to 30 days after repeat treatment were lumps and bumps (24.5%; 25/102) and firmness (13.7%; 14/102).
Table 3.
Injection Site Responses Reported by Subjects in Diaries After Repeat Treatment
| ISRs | Incidence of ISRs, n (%)(N = 120)a | Distribution of ISRs by severityn/N (%)b | |
|---|---|---|---|
| Mild/moderate | Severe | ||
| Subjects with any ISR | 116 (96.7) | 74/116 (63.8) | 42/116 (36.2) |
| Swelling | 112 (93.3) | 87/112 (77.7) | 25/112 (22.3) |
| Tenderness to touch | 108 (90.0) | 91/108 (84.2) | 17/108 (15.7) |
| Firmness | 102 (85.0) | 80/102 (78.4) | 22/102 (21.6) |
| Lumps/bumps | 102 (85.0) | 83/102 (81.4) | 19/102 (18.6) |
| Bruising | 98 (81.7) | 78/98 (79.6) | 20/98 (20.4) |
| Pain after injection | 92 (76.7) | 85/92 (92.4) | 7/92 (7.6) |
| Redness | 90 (75.0) | 77/90 (85.5) | 13/90 (14.4) |
| Discoloration | 41 (34.2) | 37/41 (90.2) | 4/41 (9.8) |
| Itching | 33 (27.5) | 32/33 (97.0) | 1/33 (3.0) |
ISRs, injection site responses.
aNumber of subjects who received repeat treatment with VYC-15L and recorded in their diaries after repeat treatment.
bDenominator is the number of subjects who experienced the indicated ISR.
ISRs that were ongoing at the end of the diary completion period (day 30 after repeat treatment) were classified as AEs. Treatment-related AEs occurred in 50.0% of subjects after initial and touch-up treatment, most commonly lumps/bumps (32.1%), injection site bruising (17.9%), and injection site pain (12.5%). After repeat treatment, the incidence of treatment-related AEs was 13.7% (17/124); the most common AEs were injection site mass (diary term lumps/bumps; 7.3%) and injection site induration (diary term firmness; 4.0%). Most treatment-related AEs after repeat treatment occurred within 1 day, were mild or moderate in severity, required no treatment, and resolved within 60 days without sequelae. One case each of injection site edema (2 occurrences in 1 subject) and injection site pain required treatment with ibuprofen and paracetamol, respectively. Treatment-related AEs lasted longer than 60 days after repeat treatment in 2.4% (3/124) of subjects (injection site mass, 1.6%; injection site induration and chapped lips, 0.8%). One of 124 subjects (0.8%) reported a treatment-related AE after repeat treatment that was ongoing at study exit: moderate upper lip injection site mass. No treatment was required for this ongoing event. There were no treatment-related serious AEs or deaths.
Mean scores on the FACE-Q Recovery Early Life Impact scale after VYC-15L treatment were 81.1 three days after initial treatment, 89.2 three days after touch-up treatment, and 86.5 three days after repeat treatment, demonstrating that treatment was not disruptive to normal daily activities.
No Tyndall effect was noted, and there was no reduction in lip sensation after repeat treatment with VYC-15L.
DISCUSSION
The results of this study contribute to the growing literature on the long-term use of HA-based fillers, demonstrating that repeat treatment with VYC-15L is safe and effective for lip and perioral enhancement, with subjects achieving high levels of clinical response and satisfaction with treatment. Repeat treatment with VYC-15L administered 1 year after the initial treatment required less product volume to restore lip fullness to levels higher than those seen after the initial and touch-up treatments.11 Similarly, in a European study conducted at 12 sites in 280 subjects, improvements in lip fullness were maintained in 56.1% of subjects at 1 year and increased to over 95% of subjects after repeat treatment with VYC-15L.10 In subjects treated with HYC-24L (Juvéderm Ultra XC; Allergan plc), improvements in lip fullness were maintained in over 50% of subjects at 1 year after initial and touch-up treatment, and repeat treatment resulted in a return to levels seen at 1 month after initial treatment.4
Other effectiveness measures showed improvements comparable to those observed for the LFS after repeat treatment, including mean scores on the FACE-Q Satisfaction With Lips and Appraisal of Lip Lines scales, and the GAIS rating of “improved” or “much improved.” At 1 month after repeat treatment with VYC-15L, responder rates and scores on these measures returned to levels seen at 1 month after the initial and touch-up treatments. The POLSS and OCSS responder rates mirror those observed in the European study of VYC-15L described earlier.10 In the same study, approximately 85% of subjects treated with VYC-15L reported achievement of their treatment goals at 1 month, with the proportion declining gradually to 52% at 1 year and increasing to 96% at 1 month after repeat treatment with VYC-15L.10 More than 80% of investigators indicated that they were “very satisfied” with the aesthetic features of the lips and mouth in repose and in animation through month 6; investigator-rated satisfaction gradually declined from 6 to 12 months, but increased to 100% at 1 month after repeat treatment. Responder rates for oral commissures severity were generally lower than those for lip fullness in our study. This may be because this study enrolled subjects based on lip fullness criteria, not oral commissure severity, and, therefore, the LFS was the scale most likely to show response to treatment.
Safety outcomes, including ISRs and treatment-related AEs, were consistent with those in previous studies with VYC-15L and other Juvéderm products in the lips and perioral areas.1,4,6,17 Notably, the incidence of severe ISRs, as well as the incidence of treatment-related AEs, was substantially lower after repeat treatment with VYC-15L than after the initial and touch-up treatment. In part, this may reflect the lower median volume of filler used for repeat treatment with VYC-15L compared with the volume used for initial and touch-up treatment (1.5 and 2.6 mL, respectively). The shorter follow-up duration after repeat treatment may contribute to the lower AE rate compared to initial and touch-up treatment. However, most AEs occurring for each treatment period were reported within the first few days after treatment. Nonetheless, a limitation of this study is the short duration of follow-up (1 month) after repeat treatment.
Repeat treatment of midface volume deficit with another Vycross product (VYC-20L; Juvéderm Voluma; Allergan plc) was shown to be effective and associated with high levels of subject satisfaction.18 Similar to our observations for VYC-15L treatment of the lips and perioral area, the mean injection volume for repeat treatment with VYC-20L in the midface was approximately half the mean volume injected for initial/touch-up treatment. In addition, the incidence, severity, and duration of common treatment-site responses were lower after repeat treatment compared with initial treatment. Taken together, these findings suggest that repeat treatment with Vycross HA-based fillers is safe and effective for the long-term maintenance of desired aesthetic results.
CONCLUSION
This study shows that repeat treatment with VYC-15L administered 1 year after initial treatment is safe and effective for lip and perioral enhancement and requires a lower volume of product to achieve effectiveness similar to that obtained after the initial and touch-up treatment.
Disclosures
Dr Rivkin is a consultant and investigator for Allergan plc and Merz Aesthetics USA. Dr Weinkle is an investigator for Allergan plc, Alphaeon, DermAvance, and Revance, and a speaker for Sinclair and Teoxane. Dr Hardas and Ms Murphy were employees and stockholders of Allergan plc at the time of manuscript preparation. Dr Weiss is a consultant and investigator for Allergan plc. Dr Glaser has served as a remunerated advisor, consultant, and speaker for Allergan plc; has received research grants from Allergan plc, Brickell Biotech, Dermira, Galderma, Suneva, and Ulthera; and has served as a paid consultant for Brickell Biotech and Miramar. Dr Biesman is an investigator for Allergan plc. Dr Schumacher is an employee of Allergan plc.
Funding
This study was funded by Allergan plc (Dublin, Ireland). Editorial support for this article was provided by Peloton Advantage (Parsippany, NJ) and was funded by Allergan plc.
Acknowledgments
Treating and evaluating investigators, respectively, at each study site were: David Bank, MD and William Nolan, MD, Mt Kisco, NY; Ashish Bhatia, MD and Te Shao Hsu, MD, Naperville, IL; Brian Biesman, MD and Jennifer Martin, MD, Nashville, TN; Lisa Donofrio, MD and Ronald Savin, MD, New Haven, CT; Roy Geronemus, MD and Jeremy Brauer, MD, New York, NY; Dee Anna Glaser, MD, Natalie Semchyshyn, MD, and Ian Maher, MD, St. Louis, MO; Richard Glogau, MD, Patricia Engasser, MD, and Kristin Hudacek, MD, San Francisco, CA; Mary Lupo, MD and Katherine Holcomb, MD, New Orleans, LA; Alexander Rivkin, MD and Robert Cohen, MD, Los Angeles, CA; Ava Shamban, MD and Soheil Simzar, MD, Santa Monica, CA; Susan Taylor, MD, Philadelphia, PA and Allison Britt-Kimmins, MD, Chadds Ford, PA; Susan Weinkle, MD and John Demetree, MD, Bradenton, FL; and Robert Weiss, MD and Margaret Weiss, MD, Hunt Valley, MD.
REFERENCES
- 1. Eccleston D, Murphy DK. Juvéderm(®) Volbella™ in the perioral area: a 12-month prospective, multicenter, open-label study. Clin Cosmet Investig Dermatol. 2012;5:167-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gassia V, Raspaldo H, Niforos FR, Michaud T. Global 3-dimensional approach to natural rejuvenation: recommendations for perioral, nose, and ear rejuvenation. J Cosmet Dermatol. 2013;12(2):123-136. [DOI] [PubMed] [Google Scholar]
- 3. Sarnoff DS, Gotkin RH. Six steps to the “perfect” lip. J Drugs Dermatol. 2012;11(9):1081-1088. [PubMed] [Google Scholar]
- 4. Dayan S, Bruce S, Kilmer S, et al. . Safety and Effectiveness of the Hyaluronic Acid Filler, HYC-24L, for Lip and Perioral Augmentation. Dermatol Surg. 2015;41Suppl 1:S293-S301. [DOI] [PubMed] [Google Scholar]
- 5. Philipp-Dormston WG, Hilton S, Nathan M. A prospective, open-label, multicenter, observational, postmarket study of the use of a 15 mg/mL hyaluronic acid dermal filler in the lips. J Cosmet Dermatol. 2014;13(2):125-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Raspaldo H, Chantrey J, Belhaouari L, Saleh R, Murphy DK. Juvéderm volbella with lidocaine for lip and perioral enhancement: a prospective, randomized, controlled trial. Plast Reconstr Surg Glob Open. 2015;3(3):e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hee CK, Shumate GT, Narurkar V, Bernardin A, Messina DJ. Rheological Properties and In Vivo Performance Characteristics of Soft Tissue Fillers. Dermatol Surg. 2015;41(Suppl 1):S373-S381. [DOI] [PubMed] [Google Scholar]
- 8. Raspaldo H, De Boulle K, Levy PM. Longevity of effects of hyaluronic acid plus lidocaine facial filler. J Cosmet Dermatol. 2010;9(1):11-15. [DOI] [PubMed] [Google Scholar]
- 9. Smith L, Cockerham K. Hyaluronic acid dermal fillers: can adjunctive lidocaine improve patient satisfaction without decreasing efficacy or duration?Patient Prefer Adherence. 2011;5:133-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Raspaldo H, Chantrey J, Belhaouari L, et al. . Lip and Perioral Enhancement: A 12-Month Prospective, Randomized, Controlled Study. J Drugs Dermatol. 2015;14(12):1444-1452. [PubMed] [Google Scholar]
- 11. Geronemus RG, Bank DE, Hardas B, Shamban A, Weichman BM, Murphy DK. Safety and Effectiveness of VYC-15L, a Hyaluronic Acid Filler for Lip and Perioral Enhancement: One-Year Results From a Randomized, Controlled Study. Dermatol Surg. 2017;43(3):396-404. [DOI] [PubMed] [Google Scholar]
- 12. Werschler WP, Fagien S, Thomas J, Paradkar-Mitragotri D, Rotunda A, Beddingfield FC 3rd. Development and validation of a photographic scale for assessment of lip fullness. Aesthet Surg J. 2015;35(3):294-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cohen JL, Thomas J, Paradkar D, et al. . An interrater and intrarater reliability study of 3 photographic scales for the classification of perioral aesthetic features. Dermatol Surg. 2014;40(6):663-670. [DOI] [PubMed] [Google Scholar]
- 14. Pusic AL, Klassen AF, Scott AM, Cano SJ. Development and psychometric evaluation of the FACE-Q satisfaction with appearance scale: a new patient-reported outcome instrument for facial aesthetics patients. Clin Plast Surg. 2013;40(2):249-260. [DOI] [PubMed] [Google Scholar]
- 15. Klassen AF, Cano SJ, Schwitzer JA, et al. . Development and Psychometric Validation of the FACE-Q Skin, Lips, and Facial Rhytids Appearance Scales and Adverse Effects Checklists for Cosmetic Procedures. JAMA Dermatol. 2016;152(4):443-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Klassen AF, Cano SJ, Schwitzer JA, Scott AM, Pusic AL. FACE-Q scales for health-related quality of life, early life impact, satisfaction with outcomes, and decision to have treatment: development and validation. Plast Reconstr Surg. 2015;135(2):375-386. [DOI] [PubMed] [Google Scholar]
- 17. Fagien S, Maas C, Murphy DK, Thomas JA, Beddingfield FC III. Juvéderm Ultra for lip enhancement: an open-label, multicenter study. Aesthet Surg J. 2013;33(3):414-420. [DOI] [PubMed] [Google Scholar]
- 18. Baumann L, Narins RS, Beer K, et al. . Volumizing Hyaluronic Acid Filler for Midface Volume Deficit: Results After Repeat Treatment. Dermatol Surg. 2015;41Suppl 1:S284-S292. [DOI] [PubMed] [Google Scholar]