Abstract
The important role of GH in the control of mammalian longevity was first deduced from extended longevity of mice with genetic GH deficiency (GHD) or GH resistance. Mice with isolated GHD (IGHD) due to GHRH or GHRH receptor mutations, combined deficiency of GH, prolactin, and TSH, or global deletion of GH receptors live longer than do their normal siblings. They also exhibit multiple features of delayed and/or slower aging, accompanied by extension of healthspan. The unexpected, remarkable longevity benefit of severe endocrine defects in these animals presumably represents evolutionarily conserved trade-offs among aging, growth, maturation, fecundity, and the underlying anabolic processes. Importantly, the negative association of GH signaling with longevity extends to other mammalian species, apparently including humans. Data obtained in humans with IGHD type 1B, owing to a mutation of the GHRH receptor gene, in the Itabaianinha County, Brazil, provide a unique opportunity to study the impact of severe reduction in GH signaling on age-related characteristics, health, and functionality. Individuals with IGHD are characterized by proportional short stature, doll facies, high-pitched voices, and central obesity. They have delayed puberty but are fertile and generally healthy. Moreover, these IGHD individuals are partially protected from cancer and some of the common effects of aging and can attain extreme longevity, 103 years of age in one case. We think that low, but detectable, residual GH secretion combined with life-long reduction of circulating IGF-1 and with some tissue levels of IGF-1 and/or IGF-2 preserved may account for the normal longevity and apparent extension of healthspan in these individuals.
Essential Points
GH signaling plays an important role in the control of mammalian aging
Data obtained in laboratory animals indicate that actions of GH on growth, maturation, and metabolism involve significant costs in terms of aging and longevity
Novel information about the role of GH in human aging was derived from studies of a large cohort of individuals with isolated GH deficiency (IGHD) type 1B due to a mutation of the GHRH receptor gene
In this cohort, IGHD represents profound, but not complete, suppression of GH secretion, leading to slow growth and proportional dwarfism
Longevity of these subjects with IGHD is apparently normal whereas various symptoms of aging are attenuated and/or delayed and healthspan seems to be extended in comparison with unaffected siblings
Data obtained in this IGHD cohort resemble findings reported from studies of Laron dwarfism (GH resistance) and some (but not all) congenital GHD cohorts
In our review we consider the question: What is the evidence that GH signaling can influence aging and longevity?
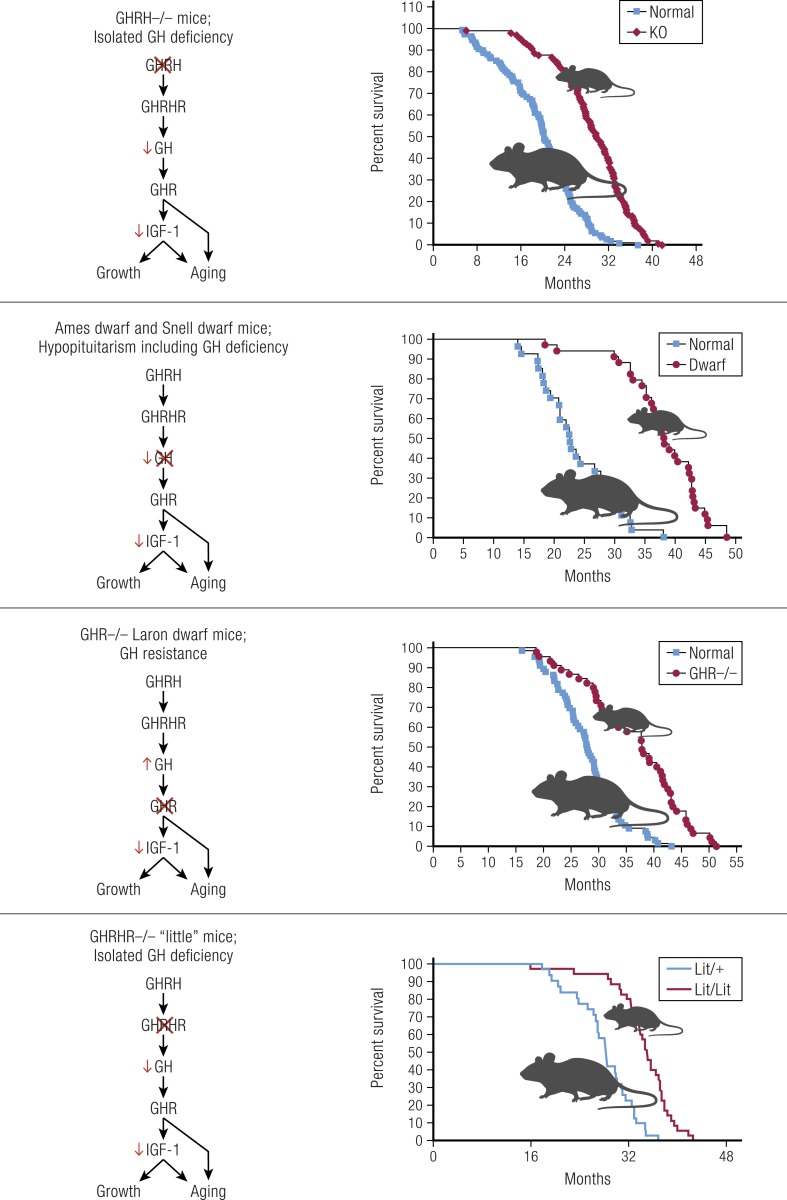
GH-deficient and GH-resistant mice are long-lived
The importance of GH signaling in the control of longevity was established by the demonstration that mice lacking GH or GH receptors (GHRs) live much longer than do their normal siblings (1–4) (Fig. 1). These studies were conducted in GHRH−/−, GHRH receptor (GHRHR)−/−, and GHR−/− mice with isolated GH deficiency (IGHD) or GH resistance, and in hypopituitary Prop1df and Pit1dw mice that lack GH and also prolactin and TSH (1–7). However, it can be debated whether the extension of average lifespan in these animals is due primarily to the altered rate of aging or to reduced incidence, delayed onset, and/or slower progression of fatal neoplasms and other age-related diseases. Using comparative analysis of longevity data, de Magalhães et al. (8) concluded that the rate of aging is reduced by some, but not all, GH-related mutations. More recently, Koopman et al. (9) applied a different approach to analysis of these data and concluded that the rate of aging of GH-deficient and GH-resistant mice is significantly reduced during most of their life and accelerates only at advanced age after most of the normal, usually referred to as wild-type (WT), controls have died. Further support for slower (and/or delayed) aging in GH-related mouse mutants is provided by the evidence that in addition to increases in average and median longevity, the maximal longevity is also significantly increased in these animals (1–4). Moreover, Wang et al. (10) recently reported that the Prop1df mutation (Ames dwarfism) slows molecular changes in the epigenetic clock of aging. This conclusion was based on analysis of age-related increase in the disorder of hepatic methylome. Importantly, effects of Ames dwarfism on this parameter resembled the effects of calorie restriction and treatment with rapamycin, two interventions previously documented to affect the rate of aging (10, 11).
Figure 1.
In mice, isolated GH deficiency, hypopituitarism, and GH resistance lead to reduced growth, diminutive adult body size, and extended longevity (1, 2, 4, 5).
Extension of longevity in GH-related mutants is robust, reproducible, and not sex limited
What deserves particular emphasis is that in GH-related mouse mutants, longevity is extended in both sexes. Moreover, in GHR−/− mice the extension of longevity was shown in four independent studies in two laboratories using animals on three different genetic backgrounds and diets of different macronutrient composition (12, 13). This contrasts with most studies of the impact of gene mutations, deletions, or transgenic expression on longevity, in which effects are often limited to one sex, and testing reproducibility or dependence of the findings on a particular diet or genetic background is rarely attempted. Although this is not surprising in view of the cost and duration of mouse longevity studies, it serves to emphasize the strength of evidence for a role of GH in mammalian aging.
Further support for the role of GH in the control of aging was derived from studies of transgenic mice with massive increase in circulating GH levels. These animals have shorter lifespans than do their normal (genetically unaltered) siblings, and they exhibit numerous symptoms of accelerated aging (14, 15).
GH-deficient and GH-resistant mice have major changes in body composition and extended healthspan as well as lifespan
In addition to reducing the rate of somatic growth and the adult body size, GHD and GH resistance lead to major changes in body composition. This includes increase in adiposity and reduction in the relative weight of the liver, skeletal muscles, and other components of lean body mass. An increase in adiposity is due primarily to a major increase in the amount of subcutaneous white adipose tissue (WAT), and this is particularly prominent in males.
In GHR−/− mice with global, germline deletion of GHR, the amount of subcutaneous (including inguinal) WAT is greatly increased, whereas the amount of retroperitoneal (perinephric) WAT is not altered and the relative weight of epididymal WAT pads is similar to that in control siblings or slightly reduced (likely depending on age and genetic background) (16). Unexpectedly, increased adiposity of GH-deficient and GH-resistant mice coexists with a marked enhancement of insulin sensitivity (13, 17). Moreover, when GHR−/− and Prop1df mice are fed a high-fat diet, they accumulate an additional amount of WAT but remain more insulin sensitive than do their normal siblings (18, 19). This could be due to an anti-inflammatory profile rather than proinflammatory profiles of cytokines secreted by visceral fat of GH-related mutants, including increased expression of adiponectin and reduced expression of IL-6 and TNFα (13, 20). In support of this hypothesis, we have shown that surgical removal of most of the intra-abdominal fat produces the expected improvements in insulin sensitivity in normal animals, but promotes insulin resistance in GH-related mutants (21, 22).
In addition to the overall increase in the mass of WAT, the relative weight of interscapular brown adipose tissue (BAT) is increased in GHR−/− as well as Prop1df mice (23, 24). Increases in the amount of BAT and in the expression of the key mitochondrial uncoupling gene, Ucp1, in BAT and subcutaneous WAT are almost certainly related to the need for increased nonshivering thermogenesis to compensate for increased heat loss in these diminutive mutants (25). Increased adiposity of GHR−/− animals can be ascribed to the absence of lipolytic action of GH locally in WAT, because similar changes in body composition are seen in mice with adipose tissue–specific knockdown of GHR in FaGHRKO mice (16, 26). FaGHRKO mice have a general, rather than depot-specific, increase in WAT accumulation. In contrast to the consistent decrease in lean body mass of GH-deficient and GH-resistant mice, the relative brain weight is increased in these animals. As discussed elsewhere in this review, this has been related to brain growth occurring primarily during the period of GH-independent fetal and early postnatal growth.
Long-lived Prop1df and GHR−/− mice were shown to maintain youthful levels of cognitive function into advanced age (27, 28). Aging-related declines in neuromusculoskeletal function assessed by tests for agility, balance, and strength and age-related increase in insulin resistance are attenuated in GHR−/− and Ames dwarf mice (29, 30). In Snell dwarf mice, extension of longevity is associated with delays of age-dependent changes in collagen cross-linking and in various parameters of immune function (2). Dwarf mice had fewer memory T cells, reduced accumulation of CD4 and CD8 cells expressing P-glycoprotein activity, and preserved responses of T cells to stimulation (2). Moreover, studies of various indices of ovarian function, along with observational data from breeding colonies, indicate that reproductive aging is delayed in both GHR−/− and Ames dwarf mice (31–34). Collectively, these findings indicate that in laboratory mice, GHD and GH resistance extend not only lifespan, but also healthspan (defined as the period of life free of disease and deficits in physical and mental function).
GH signaling can be related to longevity in normal mice and in other mammalian species
It is important to ask whether conclusions derived from studies of mice with genetic alterations and extreme phenotypes also apply to normal (WT) mice and to other species. Studies of longevity of WT mice from different inbred strains, outbred stocks, or selected lines, as well as studies of longevity of individual animals from a genetically heterogeneous population, demonstrated that body size, a strongly GH-dependent trait, is inversely related to lifespan (35–37). These differences have been related to IGF-1 levels (37), further strengthening the evidence that reduced somatotropic signaling predicts longer life in mice.
The longevity advantage of smaller individuals was shown also in other species (13). Particularly striking are the effects in domestic dogs, a species with a huge range of variation in body size (38, 39). Findings concerning growth and aging in different species, including the relationship of height (stature) to life expectancy and extremes of longevity in humans, are discussed later in this article. Interestingly, the inverse relationship of body size to longevity within a species differs from the generally positive impact of body size on longevity in comparisons of different species.
Mechanisms
Conserved mechanisms of aging are related to GH signaling
There is very strong evidence that GH signaling has a major impact on aging and longevity in laboratory mice and that at least some of the effects of GH on aging in mice apply to other mammalian species. Many effects of GH are mediated by IGF-1, and GH is a key regulator of hepatic IGF-1 secretion and circulating IGF-1 levels. Therefore, findings concerning the role of GH in aging expand the present understanding of the evolutionarily conserved role of insulin/IGF-1–like signals in aging of organisms ranging from yeast, worms, and insects to mammals, including our own species (13, 40–42).
Multiple mechanisms link GHD or GH resistance with slow aging and extended longevity
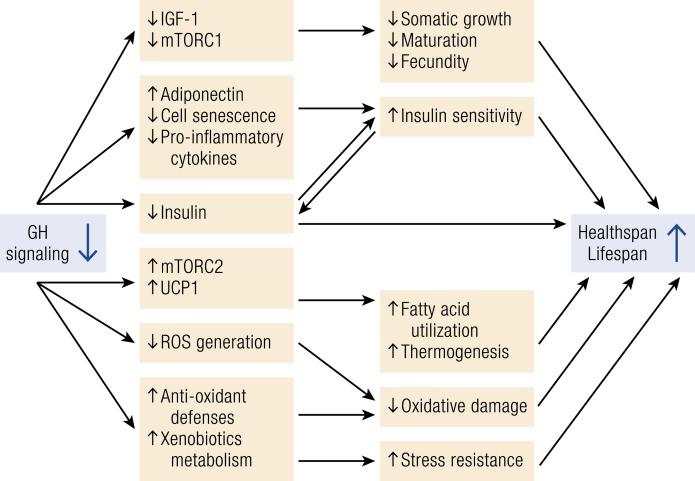
The reports that genetically GH-deficient and GH-resistant mice consistently outlive their normal siblings prompted an intensive search for the underlying mechanisms. These studies involved comparison of multiple phenotypic characteristics of the long-lived GH-related mutants to those of WT animals from the same strain (6, 7, 12, 13), along with studies of the expression of candidate genes (43, 44), analysis of wide gene expression profiles (45–48), comparing responses to various interventions (4, 5, 21, 49, 50), and crosses with other genetically altered mice (51). More recently, this work was expanded to include creation of animals with organ- or age-specific deletion of genes related to GH signaling (26, 52, 53). The picture that emerges from these studies is that existence of a single pathway of molecular and/or cell signaling alterations linking suppression of GH actions to healthy aging and longer life is exceedingly unlikely. Instead, it appears that suppression of GH signals produces a new, longevous phenotype via multiple mechanisms and that these mechanisms interact, forming a complex regulatory network (6, 7, 13). A simplified diagram of some of those interactions is shown in Fig. 2.
Figure 2.
Schematic representation of key mechanistic links between reduced GH signaling and extension of healthspan and lifespan in mice. Arrows identify causal relationships and interactions. Additional mechanisms of extended longevity of GH-related mutants are listed in the text along with references. ROS, reactive oxygen species.
Genetically GH-deficient and GH-resistant mice have normal weight at birth, but their growth rate quickly falls behind that of their normal siblings, puberty is delayed, and adult body size is drastically reduced. These characteristics, along with increased adiposity and other alterations of body composition, are consistent with absence of GH signals, reduced levels of serum IGF-1, and suppression of the activity of the mTORC1 complex. These diminutive animals have less chronic low-grade inflammation (20, 21, 54), are insulin sensitive (5, 17, 55), and are stress resistant (56, 57). They also have apparent improvements in mitochondrial function with reduced production of deleterious reactive oxygen species (7, 58), despite increased daily average metabolic rate per unit of body mass (59). Combined with improved antioxidant defenses, this accounts for reduced oxidative damage to their DNA and other macromolecules (7, 58, 60). Other factors that undoubtedly contribute to the extension of their healthspan and lifespan include enhanced activity of the mTORC2 complex (50, 61), increased hepatic production of hydrogen sulfide (62), improved maintenance of stem cell populations (63), BAT thermogenesis (24, 25), and smaller burden of senescent cells (64).
Interestingly, note that chronic calorie restriction, one of the very few, if not the only, comparably effective antiaging intervention, is also thought to extend longevity via multiple interacting mechanisms (65, 66). Moreover, there is a considerable overlap between mechanisms by which GHD and calorie restriction influence aging (5, 48, 49). A positive impact of calorie restriction, suppression of the somatotropic axis, and inhibition of mTORC1 signaling suggest that some common elements of anabolic and biosynthetic processes, translation, and somatic growth act to accelerate the rate of aging (67). Although multiple molecular mechanisms appear to be underlying this fundamental relationship, several specific mechanisms have been linked to longevity of GH-deficient or GH-resistant organisms. These include alterations in expression of DNA-repair genes (68) and in methylation of multiple genes in the liver (10, 69), reduced inhibitory phosphorylation of insulin substrates and altered activation of PI3K in key insulin target tissues (70), altered expression of cytokines in intra-abdominal WAT and in different brain regions (20, 54, 71), and changes in the expression of AMPK and FOXO (43). Genetic polymorphism of FOXO3 has been associated with extreme longevity in several unrelated human populations (72–74).
Interpretation of the findings concerning mechanisms by which GHD affects aging is complicated by the fact that in most studies the likely mechanisms of aging are difficult to distinguish from the symptoms, signatures, or biomarkers of aging (6, 12, 13). For example, enhanced insulin sensitivity and the coexisting reduction of insulin levels are among the most likely mechanisms of the extension of healthspan and lifespan of GH-deficient and GH-resistant mice, but they also reflect the relatively younger biological age of GH-related mutants. Thus, reduced insulin levels and enhanced insulin sensitivity in GH-deficient and GH-resistant mice can represent both a mechanism of their remarkably extended longevity and an example of retaining youthful phenotypic characteristics into advanced age (51, 55).
The causal (mechanistic) role of reduced insulin levels in the delayed aging of GH-related mutants is emphasized by findings in transgenic mice producing a modified GH molecule that acts as a powerful antagonist of GH actions (3). In these animals, insulin levels are not reduced and lifespan is not extended despite reductions in circulating IGF-1 levels, postnatal growth, and adult body size (3). Observations in animals with organ-specific GHR deletion provide other examples of association of insulin signaling with longevity (26, 53). Some exceptions were also noted in these studies and in mice with extension of longevity induced by mutations not directly related to GH signaling (75, 76). However, female Ins1−/−Ins2+/− mice with a genetic reduction of insulin levels were recently shown to be insulin sensitive and long-lived (77). More work is needed to explain how findings in mice with congenital GHD or global, germline GHR deletion relate to effects of other genetic, as well as nongenetic, interventions on glucose homeostasis vs aging.
The negative impact of somatotropic signaling on longevity discovered in mutant mice applies to mice that have not been genetically modified as well as other species
As indicated in the introduction to this review, the current interest in the role of GH in the control of aging stems primarily from the reports of remarkable extension of longevity in mice with GHD or resistance due to spontaneous mutations or targeted deletions of genes related to GH biosynthesis, secretion, and/or actions (6, 7, 13, 16). Importantly, blocking GH signaling in these animals not only extends lifespan, but also healthspan, and it slows the rate of aging (9, 12, 13). The evidence for slowing the rate of aging is of particular significance, as it indicates that extension of longevity in these mutants is not due simply to reduced IGF levels protecting them from cancer, which is the leading cause of death in most strains of laboratory mice. The findings of reduced longevity in transgenic mice with gross, supraphysiological elevation of circulating GH levels are also robust, reproducible, and based on observations in different independently produced lines of animals and in different laboratories (14, 15, 78).
Alterations in the lifespan of mice with various GH-related genetic alterations are striking and internally consistent. However, it is important to ask whether findings in animals with extreme phenotypes due to loss-of-function mutations, targeted deletion, or ectopic expression of experimental gene constructs apply to mice that have not been genetically altered. It is also important to ask whether observations made in highly domesticated stocks of animals that are naturally short-lived may apply to other species, particularly those that are evolutionarily distant and long-lived.
Available evidence allows answering both of these questions in the affirmative. The evidence comes mostly from studies relating longevity to body size, a strongly GH-dependent trait. Analysis of longevity of different strains, stocks, or selected lines of laboratory mice demonstrated that larger animals do not live as long as smaller ones (35, 37, 79). The most persuasive evidence for negative relationship of body size and longevity in this species came from a study comparing adult body weight and longevity in individual mice from a genetically heterogeneous population (37). Differences in longevity of mice from different inbred strains have also been related to differences in circulating IGF-1 levels (80), another strongly GH-dependent trait. Evidence for a negative relationship of body size to longevity was also obtained in other mammalian species, including rats (81), dogs (38, 82), horses (83), and, more recently, humans (36, 84), although evidence for survival benefits of shorter human stature is considered controversial by some authors (85). The role of GH, GHD, and GH resistance in human aging is discussed elsewhere in this review.
The evidence for longer survival of smaller individuals is particularly strong in domestic dogs, a species in which selective breeding produced a truly astonishing range of variation in body size. In addition to being common knowledge among veterinarians and dog owners, the reciprocal relationship of body size and longevity in this species was documented in a number of large studies in different populations of various purebred or mixed-breed animals (38, 39, 82). Differences in body size between different dog breeds have been shown to reflect differences in circulating IGF-1 levels (39, 86).
Importantly, however, note that in comparisons between different species rather than within an individual mammalian species, the relationship of longevity to body size is generally direct rather than inverse. Thus, the largest species such as whales or elephants are remarkably long-lived, whereas the smallest mammals (with the exception of some bats) usually live just a few years. This would imply a positive association of GH signaling and its downstream targets, including levels of endocrine IGF-1, with longevity. Quantitative aspects of GH, as well as systemic and local IGF-1 signaling in different species, have not been systematically explored and compared, but available data include some intriguing exceptions from the expected relationships. Thus, one recent study reported a counterintuitive finding that IGF-1 levels in larger mammals tend to be lower than in smaller mammals (87). Rodents are a group of mammals with a particularly large range of differences in body size and longevity. In these animals, longevity does not seem to be related to circulating levels of IGF-1 (88), but it was shown to be inversely related to expression of IGF-1 receptors in the brain (89).
Although the somatotropic axis is involved in the control of aging in mammals from various taxonomic orders, important species differences also exist. Severe suppression or absence of GH signals in the syndromes of hereditary dwarfism leads to extension of longevity in mice that can exceed 50%, but has no or very little effect on human longevity (see details later in this review). Major quantitative differences between the impact of GH on longevity in men vs mice are likely related to life-course differences in energy partitioning between growth, reproduction, maintenance, and repair. With some notable exceptions, large species of endothermic (warm-blooded) animals live longer than do small species, with the differences roughly corresponding to different metabolic rates. Laboratory mice, similarly to most other small rodents, are among the shortest lived mammals. They are characterized by rapid postnatal growth and maturation, early life reproduction, and high fecundity, with less resources devoted to repair and maintenance. This cluster of characteristics, sometimes referred to as pace-of-life syndrome (90, 91), is likely associated with less efficient mitochondrial function and greater production of harmful amounts of reactive oxygen species, leading to greater damage to DNA and other macromolecules. This, in turn, promotes faster aging and shorter lifespan. In contrast, humans are an extreme example of a long-living species with longevity exceeding what could be predicted from the resting (basal) metabolic rate and body size. Compared with species with shorter lifespans, humans grow and mature slowly, start to reproduce relatively late in life, and produce few offspring. This is associated with a very large time and energy investment in the care of each offspring, as well as improved mechanisms of maintenance and repair. These characteristics would reduce accumulation of molecular damage to DNA and other cell components consistent with the potential of human organs to function for periods approaching and occasionally exceeding 100 years. In strong support of species differences in the accumulation of DNA damage, the expression of genome maintenance genes was shown to be higher and the frequency of somatic mutations lower in humans than in mice (92, 93). Against this background, it is not surprising that reduced activity of GH, a predominantly anabolic hormone, has greater impact on longevity of mice than humans. The role of GH-dependent growth in early life in shaping the trajectory of aging is supported by the demonstration that treatment of GH-deficient Ames dwarf mice with GH injections during this period significantly shortens their longevity (94, 95).
Aging in Humans With IGHD
The concept that normal growth involves some costs in terms of aging and longevity is based primarily on results obtained in experimental animals. Determining to what extent this concept applies to human aging is of paramount importance but presents considerable challenges. GH-dependent developmental processes are involved not only in attainment of normal body size, but also in regulation of key body functions, including nutrient storage and utilization, strength, morbidity, sensory perception, cognition, and reproduction. Table 1 lists key effects of the GH and IGF system on body size and function.
Table 1.
Simplified Scheme of Physiological Roles of Components of the GH System in Body Size and Body Function
| Role | Component of the GH System |
|---|---|
| Body size | |
| Stature | Pituitary GH and circulating IGF-1 |
| Amount of fat | |
| Amount of muscle | |
| Amount of bone | |
| Body function | |
| Brain function | |
| Reproduction | |
| Immune function | Pituitary GH and circulating IGF-1 |
| Voice | Extrapituitary GH |
| Teeth | Tissue growth factors |
| Feeding | IGF-1/IGF-2 |
| Sense organs | |
| Rapid eye movement sleep | |
| Somatotroph expansion | GHRH |
| Non–rapid eye movement sleep | |
| Enteroendocrine connections | Ghrelin and somatostatin |
Although increased GH secretion in childhood or early adulthood may have provided an evolutionary advantage by assuring adequate body size and composition, it might be deleterious in terms of longevity. Thus, the gradual decline in GH secretion after early adulthood may have evolutionary advantages by slowing aging and extending longevity. Pituitary GH and circulating IGF-1 are critical for body size, whereas body functions are also influenced by extrapituitary GH (e.g., brain, eye, immune cells) and by locally produced growth factors. Although the actions of pituitary GH and circulating IGF-1 are well studied, the role of local (tissue) levels of growth factors in determining body function is largely unknown. It is also possible that individuals with reduced body size, due to a reduction of GH secretion or lack of GH action, may display an array of compensatory mechanisms, which could positively impact lifespan as well as healthspan. Pathways evolutionarily conserved from yeast to mice seem to protect human cells from age-related pathologies, insulin resistance, and cancer as suggested by findings in GH-resistant individuals (96). In the following sections, we discuss aging and longevity in humans with IGHD and with the primary hereditary form of GH insensitivity (GHI), the Laron syndrome.
Etiology of IGHD
IGHD prevalence varies from 1:3500 to 1:8700 (97). In some individuals, childhood IGHD is an idiopathic, relative, and transitory state, which leads to short stature when not diagnosed and treated. In others, IGHD results from a variety of hypothalamic or pituitary insults or functional defects. Nevertheless, anatomic abnormalities are found in only 12% of such patients examined by MRI (98). This suggests that genetic rather than structural defects may account for GHD in a significant proportion of cases, and the estimated incidence of genetic IGHD is between 5% and 30% of cases. There are four types of inherited IGHD: IA, IB, II, and III (Table 2) (99). Type IA causes profound growth failure, usually earlier than in type IB, the most frequent inherited type of IGHD. Although most cases are due to yet unknown genetic defects, mutations in the GHRHR gene (GHRHR) are present in at least 10% of these cases, making polymorphisms of this gene the most frequent genetic IGHD (100). In the development of IGHD type II, deficiency of TSH and ACTH may also occur, and the pituitary function of all such patients should continue to be monitored closely over the years (101). IGHD type III may be associated with agammaglobulinemia (102). These last two types are not appropriate for studying the interaction between longevity and IGHD, because of their associated conditions and rarity. Therefore, we will use IGHD type 1A (103) and type 1B (104) to analyze these relationships. We think that it is crucial to study the key phenotypic characteristics of humans with proven genetic IGHD to understand the true consequences of IGHD in the whole organism. In this manner, we avoid the uncertainties of the idiopathic IGHD. Alternatively, organic GHD can occur in pan-hypopituitarism due to genetic and acquired causes. These include pituitary surgery and or radiation, which may affect several body functions. Additionally, it is well known that suboptimal (or excessive) substitution of other hormones (glucocorticoids, thyroid hormones, and sex hormones) in either genetic or acquired cases may influence various metabolic and vascular risk factors and ultimately influence longevity.
Table 2.
Types of Inherited IGHD
| Type | Inheritance | Serum GH | Development of GH Antibodies | Genes Involved |
|---|---|---|---|---|
| IA | AR | Absent | Frequent | GH1 (large deletions) |
| IB | AR | Low | No | GH1 (rare), GHRHR |
| II | AD | Low | No | GH1 (exon 3 deletion), GHRHR signal peptide mutation |
| III | X-linked | Low | No | Unknown |
Abbreviations: AD, autosomal dominant; AR, autosomal recessive.
The main difficulty in identifying the links between IGHD and aging is to find a sufficient number of subjects with genetic IGHD living without GH treatment. We were fortunate to describe a cohort of 105 individuals with IGHD type 1B in the Itabaianinha County in northeastern Brazil and to follow them for 24 years (105). In this cohort, IGHD is due to the inactivating mutation of the GHRHR gene, c.57+1G>A GHRHR. In the following section, we summarize the phenotypic characteristics of these individuals using the Itabaianinha IGHD cohort. When necessary, we comment on differences from IGHD type 1A and GHI. We first describe the hormonal and MRI data, and then the clinical data.
Hormonal and MRI data
The Itabaianinha subjects with IGHD exhibit marked reductions of serum concentrations of IGF-1, IGF-2, and IGF-binding protein type 3 (IGFBP-3) throughout their life. The reduction of IGF-1 is more pronounced than the IGF-2, with IGF-1 levels being mostly undetectable at all ages (106).
In an attempt to measure the bioavailability of IGF-1 and IGF-2, we calculated molar ratios to IGFBP-3. We found a profound reduction in the IGF-1/IGFBP-3 ratio, accompanied by increase in the IGF-2/IGFBP-3 ratio, so that the combined IGF-1 plus IGF-2/IGFBP-3 ratio was twice as high in adults with IGHD compared with controls. This indicates an increase of IGF-2 relative to IGFBP-3 in IGHD, likely representing a compensatory mechanism for the diminished IGF-1 levels (106). We hypothesized that this IGF-2 upregulation may have physiological implications, contributing to the IGF bioavailability to some vital tissues such as the brain and the eye, in which growth seems to be less dependent on the pituitary GH and circulating IGF-1 (107). Although it is unknown whether this upregulation of IGF-2 occurs with GHD of other etiologies, it was shown that height SD score (SDS) in Laron dwarfism has a strong positive correlation with IGF-1/IGF-2 levels (108). It is interesting that IGF-2 secretion persists through life in humans, whereas it virtually disappears in rodents after birth (109).
Peak serum GH values were <1 ng/mL in both clonidine and insulin tolerance tests, indicating a severe GHD (105, 110). We also demonstrated a small but significant GH increase during insulin tolerance tests, showing that some GH response to hypoglycemia can occur despite complete lack of GHRHR function (110). Likewise, we found a small but significant GH response to a GH secretagogue (GHRP-2) that acts on the ghrelin receptor (111). In a study of four Sindh dwarfs with the E72X mutation in the GHRHR gene, similar findings were reported in two of the subjects with another GH secretagogue, hexarelin (112). Additionally, these four patients exhibited nocturnal plasma GH levels significantly higher than during daytime, with maintained pulsatility (113). These data suggest that in subjects with severe IGHD, there is a residual GH secretion, which can be modified by some stimuli. This residual low GH secretion could influence the balance between body size and composition, consistent with the primordial elongation action of GH, as well as the functional impact of the local actions of GH or growth factors, most likely affecting immune and brain function, sensory organs efficiency, and so on.
The low but detectable serum GH response identifies this type of IGHD as 1B (autosomal recessive with low but measurable serum GH). This type of IGHD differs from IGHD type 1A (also autosomal recessive) caused by deletion or nonsense mutations in the gene encoding GH (GH1), with no detectable GH secretion and severe IGF-1 deficiency and from the GHI syndrome (Laron dwarfism), with similar profound IGF-1 deficiency, but without any GH action (108, 114). Although these syndromes are very similar, subtle differences may occur in terms of body composition, glucose metabolism, cancer incidence, and ultimately longevity. These differences are discussed later in this review.
Individuals from our cohort with IGHD have higher basal cortisol levels, probably due to the increased activity of the enzyme 11β-hydroxysteroid dehydrogenase, which converts cortisone to cortisol (115). They also have moderately elevated serum TSH levels (111), likely an indication of reduced effect of IGF-1 on hypothalamic somatostatin. Furthermore, they also exhibit a reduced serum total T3 and increased serum free T4, owing to a reduction in the function of the GH-dependent deiodinase system (111). Serum prolactin and gonadotropin levels are normal, but in males with IGHD, total testosterone and SHBG levels are higher than in controls, without a difference in free testosterone (116).
Subjects with IGHD from the Itabaianinha cohort exhibit marked anterior pituitary hypoplasia (117), without abnormalities of the stalk or neurohypophysis (98), which was also described in mice with GHRHR gene mutation (little mouse) (118). This is undoubtedly related to the lack of the GHRH effect on the expansion of somatotroph cells. In our experience, normal size of the anterior pituitary as determined by MRI rules out homozygosity for a GHRHR mutation at least in subjects who are 8 years of age or older.
Physical findings
The main findings in IGHD individuals from this cohort are proportional short stature, doll facies, high-pitched prepubertal voice (119–121), reduced muscle mass, and central adiposity. The first four of these features stem from the lack of the synergistic effect of pituitary GH and IGF-1 in bones and muscles. Proportional short stature is a landmark feature of this IGHD type 1B. In contrast, the patients with Laron syndrome and individuals with IGHD due to hGH-1 gene deletion have a disproportional body, with the lower limbs being short relative to the spine and head (122). This suggests that GH and IGF-1 act differentially on the spine and limbs. Alternatively, having very little, but active GH seems to ensure a proportional ratio of the upper and lower body segments.
Newborns with IGHD from this cohort are of normal size, whereas newborns with Laron syndrome have mildly reduced birth length and birth weight (108), suggesting that the GH-resistance has a greater impact on human birth size than does IGHD type 1B. In the latter condition, the postnatal growth failure becomes evident during the first year of age and is progressive, consistent with cumulative GHD. As age increases, there is a marked reduction of adult height. Measured adult height in untreated individuals is 128.7 ± 5.9 cm in males (range, 117 to 137 cm) and 117.6 ± 5.7 cm in females (range, 107 to 126 cm). Adult height SDSs range from −9.6 to −5.1 (123). The reduction of the head perimeter is less pronounced than the reduction of stature (−2.7 SDS) (121), similar to the findings in patients with Laron syndrome (124). Greater impact of IGHD on adult height than on head size may reflect the fact that much of the growth of the brain and the skull occurs early when growth is less dependent on GH, whereas somatic growth of the rest of the body is mainly postnatal and strongly dependent on GH and endocrine IGF-1. Relative brain size is consistently increased in genetically dwarf mice and reduced in giant GH-transgenic mice. Anterior facial height (−4.3 SDS) is less reduced than maxillary length (−6.5 SDS) (119), causing a disproportion between the calvarium and the face, resulting in a doll-like or cherubim angel facies. These cephalometric features together with the underdevelopment of the larynx lead to a typically high-pitched voice with high fundamental frequency in both sexes (120, 121, 125). This is a very characteristic trait of this syndrome. Voice-related quality-of-life (QOL) scores were more severely reduced in individuals with IGHD than in those with short stature not related to GHD. The impact of severe IGHD on voice is important and tends to abolish the effects of puberty and aging on the structure of the vocal folds, thereby preventing the fundamental frequency variation normally associated with aging (125). These findings suggest that although bone is an important target for GH action, the consequences of IGHD on bone development are not uniform and seem to be influenced by local factors.
Adult subjects with IGHD also have reduced values of quantitative ultrasound of the heel and reduced areal bone mineral density scores, reflecting the small size of the bones. However, calculated volumetric bone mineral density values are similar to controls and there is no increase in the prevalence of fractures (126–128). These results agree with data of bone status and fracture prevalence in Russian adults with childhood-onset GHD, in which subjects with IGHD had no fractures, whereas their age-matched counterparts with deficiency of multiple pituitary hormones and poorly substituted gonadal steroids had markedly increased prevalence of fractures (129). In our cohort with IGHD, the prevalence of vertebral fractures was actually reduced in older individuals with IGHD compared with age-matched controls (109).
Interestingly, this congenital IGHD causes hip joint problems and genu valgum, without apparent clinical consequences (126). In spite of the pivotal role of the GH–IGF axis in bone metabolism and the correlation between IGF-1 levels and bone mineral density in population studies (130), we did not find an increased prevalence of either osteoporosis or fractures in these subjects (126, 128). Instead, the bones of these individuals, although smaller, are apparently appropriate for their muscles. This could explain why these individuals do not seem to be prone to fractures, even when exerting extreme physical effort in sport (e.g., soccer, horse racing) or professional tasks (e.g., farm workers, mechanics, etc.). Unpublished data of our group indicate that subjects with IGHD from this cohort, with a mean age of 46.5 ± 12.4 (SD) years and an age range from 18 to 78 years, have better muscular function than do their normal counterparts, matched by sex, age, and physical activity.
The growth of nonosseous structures is also unevenly affected, exhibiting tissue-specific consequences of IGHD. When corrected by body surface area, thyroid volume (111) and left ventricular mass (131) are significantly smaller than those from normal controls. This is probably due to the reduced trophic effect of IGF-1 on the thyrocytes (132) and cardiomyocytes (133). Corrected sizes of the spleen and uterus are also reduced, indicating a dependency on the pituitary GH or circulating IGF-1. However, the lower number of pregnancies also may contribute to the reduced uterine size (134). Alternatively, prostate and ovaries are approximately normal sized, whereas pancreas, liver, and kidneys appear larger compared with controls (135), suggesting an overgrowth in these three organs.
Compared with normal controls, subjects with IGHD from this population have similar visual acuity, intraocular pressure, and lens thickness, higher values of spherical equivalent and corneal curvature, and lower measures of axial length, anterior chamber depth, and central corneal thickness (136). They also have an increase of optic disc size, but with similar thickness of the macula, and moderate reduction of vascular retinal branching points. This vascular retinal hypoplasia may be protective from diabetic retinopathy (137). This is important, as these individuals can develop diabetes, with a prevalence in adults (assessed by the oral glucose tolerance test) of 15% (138). Although the stature of IGHD was 75% of the normal controls, the ocular axial length corresponded to 96%, similar to the head circumference (93%), suggesting a parallelism between eye and head growth (136). Adequate eye and head (brain) development likely represent important elements of the environmental adaptation and survival capacity of these individuals. Eye and brain growth may involve different patterns of temporal regulation than does the whole-body growth, suggesting an important role of IGF-2 or other local growth factors in these systems, and reflecting a physiologic hierarchy controlling body size and function (107). Accordingly, the brain, different from height, has 83.6% of its growth completed within the first year of life, with essentially full growth achieved during the first 3 years of life (139), showing a lesser dependency on pituitary GH or endocrine IGF-1 for its growth.
The external appearance of these subjects with IGHD includes a contrast between the wrinkled skin and youthful hair with delayed pigmentation in children and teenagers and virtual absence of graying, even in old age. Moreover, these individuals generally do not complain of tiredness, even at advanced age, and look younger than their chronological age (123). Table 3 summarizes the principal physical findings in IGHD and Laron dwarfism.
Table 3.
Physical Findings in Human GHRH Resistance (IGHD) and GH Resistance (Laron Syndrome)
| Physical Findings | IGHD | Laron Syndrome |
|---|---|---|
| Neonatal size | Normal | Mildly reduced |
| Microphallus | Absent | Sometimes present |
| Short stature | Proportionate | Disproportional (lower limbs) |
| Final adult height | 116 to 142 cm in males | 117 to 137 cm in males |
| 108 to 136 cm in females | 107 to 126 cm in females | |
| Reduction of head | Less pronounced than height | Less pronounced than height |
| Facies | Doll: disproportion between the calvarium and the face | Doll: disproportion between the calvarium and the face |
| Hair | Sparse | Sparse |
| Skin | Wrinkled | Wrinkled |
| Voice | High-pitched | High-pitched |
| Obesity | More severe | Less pronounced |
| Bones | Smaller but strong | Smaller but strong |
| Muscular function | Appropriate | Reduced |
| Eyes | Shorter eyes, increased optic disc and vascular retinal hypoplasia | Shorter eyes, increased optic disc and vascular retinal hypoplasia |
Reproductive function
Subjects with IGHD have no obvious abnormalities in sexual development or function. Unlike the findings in Laron dwarfism, they do not exhibit microphallus, suggesting that IGF-1 has only a modulatory role on the postnatal development of genitalia. Similarly to patients with Laron syndrome, they reach full sexual development despite delayed puberty (140). These findings are congruent with the report that a 15-year-old boy with severe congenital IGF-1 deficiency due to IGF-1 gene deletion had genitalia of normal size (141).
The individuals with IGHD from our cohort have delayed puberty. The beginning of climacteric is anticipated, as assessed by the increased number of middle-aged women with elevated FSH. Age at menopause (expressed as the age of the complete disappearance of menstruation) was not statistically different between the IGHD and control groups, and within the age range of normality. These women with IGHD did not report late-life fertility, but they had a fewer children (107, 134). Reduced parity might be related to becoming sexually active at a later age and to the reliance on caesarian deliveries due to cephalic/pelvic disproportion. Lower parity may be advantageous to women with IGHD, because the number of children seems to reduce maternal longevity in normal subjects (142). These human data exhibit strong similarity with data in dwarf rodents, that is, delayed puberty, smaller litters, and longer times between litters (143, 144), suggesting that GH or IGF-1 deficiency reduces the number of primordial follicles. Curiously, ovarian aging in GHR−/− and Prop1df mice seems to be delayed (31–34).
The women with IGHD experience no difficulties in breastfeeding. Although GH and prolactin share strong similarities in structure and function, adequate mammary development and lactation are apparently possible in the absence of GH signals (134).
QOL and sleep
Our data indicate that these subjects with IGHD, living throughout their lifespan with very low GH levels, have normal QOL, as measured by a standardized, self-weighted questionnaire (145). These data seem conflicting with guidelines consensus which are based on the concept that GHD, especially in adults, impairs QOL, suggesting that GH replacement therapy may improve it (146, 147). However, most of these studies examined heterogeneous groups of patients with GHD of varying etiologies, severities, and associated conditions. These include pituitary surgery, radiotherapy, and deficiency of other pituitary hormones, with thyroid, gonadal, and adrenal insufficiencies, whose deficits and replacements can also influence QOL. Although social and genetic differences may occur between this unique cohort with IGHD and other patients with GHD, we hypothesize that with respect to the QOL and several physiological functions, having very little GH throughout life may be more advantageous than having normal GH secretion with subsequent decline (147). Moreover, studies showing beneficial effects of GH on QOL were done in a retrospective, open-label fashion. Thus, the placebo effect was not excluded. In contrast, all studies done in a prospective, randomized, placebo-controlled fashion failed to demonstrate any difference between GH and placebo (148–151).
Individuals with IGHD from our cohort exhibit impairment in both non–rapid eye movement and rapid eye movement sleep, more conspicuous in the former, but this apparently does not affect their QOL (152). They also exhibit higher prevalence of dizziness (without a direct impact on balance and with low frequency of falls) and mild high-tone sensorineural hearing loss (153). They have no increase in the frequency of infections (154), but they have higher prevalence of periodontal disease (155). The apparently normal immune function suggests that many immune cells have local growth factors that are apparently independent from an intact somatotropic axis (pituitary GH and endocrine IGF-1).
Metabolic and cardiovascular function and age-related disease
GH is an anabolic, lipolytic, and hyperglycemic hormone, which generates IGF-1, an anabolic, lipotropic, and hypoglycemic peptide. Therefore, the body composition and the metabolic and cardiovascular phenotypes of subjects with IGHD reflect synergistic as well as opposing actions of GH and IGF-1. The consequences of reduced GH and IGF-1 signaling are found already in early childhood and persist throughout life. They include decreased fat free mass and increased percent body fat (131, 156). Obesity of subjects with IGHD in our cohort is predominantly truncal, owing to the reduced lipolysis by the GH-sensitive lipase in this body region (115). Truncal obesity may be a direct effect of the lack of GH, as it is not reversed by IGF-1 treatment in subjects with GHI syndrome (157). Although obesity is a finding common to both GHI syndrome and IGHD, it seems to be more pronounced in the former (35, 115), possibly reflecting some residual lipolysis in IGHD due to the low, but detectable, GH secretion. Another difference between the effects of these two syndromes on adiposity is the regional distribution of fat, with an increase in adiposity especially in the arms in the GHI syndrome (157), and mainly in the visceral adipose tissue in the Itabaianinha subjects with IGHD (115). Despite visceral adiposity, subjects with IGHD from our cohort have increased insulin sensitivity. This was thoroughly assessed (158) and is associated with an increase in serum adiponectin (159). The increased insulin sensitivity, in association with increased visceral adiposity, suggests that there is a threshold of GH secretion necessary for visceral adiposity to impair insulin sensitivity (115). Alternatively, the biological impact of the visceral adiposity may be different in states of GHD or GH resistance. GH-resistant GHR−/− mice exhibit a profoundly altered secretory activity of visceral fat (reduced IL-6 levels in the epididymal fat) and expression of genes related to fat metabolism, associated with a marked increase in adiponectin levels and insulin sensitivity (21, 43). Interestingly, a transplant of visceral fat from GH-insensitive GHR−/− mice improves insulin sensitivity of normal mice (160). Thus, the available evidence suggests a beneficial metabolic role of visceral adipose tissue in states of abolished GH action and severe IGF-1 deficiency. Remarkably, the subjects with IGHD we studied had reduced β-cell function, which could contribute to the occurrence of diabetes in this cohort (158). Reduced development and secretory function of β-cells also characterizes Ames dwarf (161) and GHR−/− mice (162).
Growth, energy balance, and alimentary function depend on a complex regulatory network. This network includes GH, a hyperglycemic, anabolic and lipolytic peptide; its stimulatory factors, GHRH and ghrelin; inhibitory factor, somatostatin; and principal mediator of GH actions, IGF-1, a hypoglycemic, anabolic, and lipotropic agent. Insulin and gastrointestinal hormones, including GLP-1, are also involved in this network. Insulin and GH are essential in homoeothermic animals to adapt the energy utilization to the amount of food, promoting anabolism when calorie supply exceeds demands and catabolism in the opposite situation. Insulin is the main metabolic hormone in the fed state, but GH assumes a key role as a stimulator of lipolysis during prolonged fasting, providing an evolutionary advantage in times of food scarcity (163). Somatostatin, in addition to its role in the hypothalamic control of GH release, also suppresses the secretion of various gastrointestinal hormones such as glucagon, GLP-1, and insulin. Ghrelin, an orexigenic peptide and potent stimulatory GH factor, produced mostly by the stomach, enhances feeding and weight gain. These pathways provide a simplified view of the interactions between the GH–IGF-1 axis, the alimentary system, and the energy balance.
Because the GH–IGF-1 axis has an important role in the balance between energy intake (EI) and energy requirement, we decided to study this balance in the subjects with IGHD. In contrast to the findings in adult-onset GHD, as well as Laron dwarfism (158), in which reduced EI has been described, our subjects with IGHD had higher EI (mainly derived from proteins; corrected by body weight) than did the controls (164). Greater caloric intake could have adaptive advantages for small-sized individuals in an environment with limited access to food. The higher EI per body weight suggests a possible increase of the orexigenic hormone ghrelin in these subjects with IGHD.
In our cohort, the subjects with IGHD had high serum total and low-density lipoprotein (LDL) cholesterol levels throughout life (131, 165). This was secondary to a decrease in hepatic LDL receptor activity due to GHD (not IGF-1 deficiency) (166), exemplifying a direct GH effect. Subjects with IGHD also presented with a mild increase in systolic blood pressure in adults, as well as arterial hypertension in the elderly, without evidence of cardiac hypertrophy. This suggests that the very low GH/IGF-1 levels might counterbalance the effects of the increased blood pressure on left ventricular mass (131). Similarly, these individuals do not exhibit increases in carotid intima media thickness or evidence of coronary atherosclerosis when assessed by stress echocardiograms (167), and they have coronary (168) and abdominal aortic calcification scores similar to controls (128). These findings could be attributed to a dual action of IGF-1, which may promote atherogenesis by increasing proliferation of vascular smooth muscle cells or prevent it by increasing nitric oxide formation, vascular compliance, and insulin sensitivity. Accordingly, a persistent very low IGF-1 level might have a protective role, whereas a milder decrease (as seen in adult-onset GHD) might be noxious (167). Prevalence of nonalcoholic fatty liver disease (NAFLD) is increased in the IGHD adults. However, they do not progress to advanced forms of hepatitis. This finding contrasts with the findings of severe forms of NAFLD in acquired hypopituitarism and challenges the concept of a direct role of GH/IGF-1 deficiency in the pathogenesis of advanced NAFLD (169).
Enhanced insulin sensitivity constitutes a significant physiological characteristic of long-lived GH-deficient and GH-resistant mice (5, 16, 17). It is very plausible that the high insulin sensitivity of subjects with IGHD leads to increased or at least normal longevity, as detected in this cohort (104). Additionally, there were no differences in the rate of cardiovascular deaths, and we found only one cancer death in our IGHD cohort. However, there were four skin tumors (three of them were malignant), suggesting a vulnerability of their skin to tumor development (170). This contrasts with the apparent absence of breast, colon, and prostate cancers in the whole Itabaianinha cohort with IGHD during 24 years of medical care by our team (107). The absence of these common neoplasms in this cohort agrees with the hypothesis that IGF-1 deficiency protects against DNA damage and favors apoptosis of damaged cells, thereby reducing the risk of cancer, as shown in patients with Laron syndrome (96, 171).
Aging and longevity
Longevity was not significantly affected by IGHD in our cohort. However, the subjects with IGHD with very low, but active, GH secretion and virtually no IGF-1 production have long lives and some became centenarians (104) (Fig. 3). This contrasts with findings in several types of GH-deficient and GH-resistant mice (including animals with GHRH gene deletion or GHRHR mutation and IGHD) in which longevity is markedly extended (see details and references earlier in this review). Perhaps it is easier to extend life of animals that are inherently short-lived, or in animals living under controlled and highly protected laboratory conditions, than in the inherently long-lived humans exposed to a variety of risks unrelated to biological aging. Another reason for this apparent discrepancy may arise from distinct pathophysiological impacts of GH signaling in humans and mice. For instance, we demonstrated an IGF-1/IGF-2 upregulation in human subjects with IGHD (107), which may have physiological implications, contributing to increased IGF bioavailability, brain function, and extended maximal lifespan. Because IGF-2 secretion virtually disappears in rodents after birth, no upregulation of IGF is possible in this species (172). Other differences between the two species can cause a difference in longevity outcomes and they deserved to be studied. The number of individuals close to and >100 years of age in the relatively small population of subjects with IGHD is remarkable considering that centenarians are rare in normal contemporaneous human populations. In Brazil, the recent statistic is 1.27 per 10,000 (173, 174). This exceptional finding is possibly indicative of increased maximum lifespan in these subjects with IGHD, despite similar mean lifespan compared with controls.
Figure 3.
Three elderly IGHD individuals, homozygous for the c.57+1G>A GHRHR mutation. The man (photograph on the left), 123 cm tall (48.4 inches), died at age 94. At the age of 89 years, he suffered traumatic brain injury riding a horse without a harness to aid a cow in labor. Beside him, his sister (photograph in the middle), 116 cm (45.6 inches) tall, died at age 103. Both did not exhibit kyphosis or gray hair, and they died due to natural causes. The other woman (photograph on the right), 107 cm tall (42.1 inches), died at age 65, probably due to stroke. The author M.H.A.-O., who is 174 cm (68.5 inches) tall, is the normal control.
Although the subjects with IGHD in our cohort have deleterious age-related conditions, such as atherosclerosis, diabetes, NAFLD, and skin cancer, these conditions very rarely progress to advanced stages that would compromise their longevity. Although the increased insulin sensitivity is one plausible explanation for this beneficial profile, studies of other putative mechanisms, such as mTOR pathway or mitochondrial function in these subjects with IGHD, are lacking. However, our data indicate that the healthspan of these subjects is remarkably increased in comparison with their normal siblings, living in the same environmental conditions. In this cohort, subjects with IGHD maintain normal memory, and apparently normal brain function, until they reach their 90s. Unfortunately, we have not yet done a systematic comparison of brain function between the subjects with IGHD and their siblings. However, the normal bone and muscle function and the lack of tiredness exposes these individuals to the risk of accidents, even in advanced ages. Accordingly, from the 105 dwarfs initially registered in our cohort in 1997 (104), four individuals (3.8%) died of causes that were not health related, whereas from the 49,821 inhabitants of the Itabaianinha County, 354 (0.7%) individuals with normal stature died due to this group of causes (P = 0.02). Thus, the older subjects with IGHD apparently exhibit a different profile of senescence, maintaining a more youthful level of morbidity and mortality. It is interesting that accidents were also a relatively frequent cause of death in the Ecuadorian cohort of Laron dwarfs (96), and an accident was responsible for at least one death in the Israeli cohort (175).
“The healthspan of these subjects is remarkably increased in comparison with their normal siblings.”
Longevity data (often described in hypopituitarism and discussed later in this review) are scarce in genetic IGHD. The finding of normal longevity in our cohort of IGHD type 1B subjects with the c.57+1G>A GHRHR mutation (104) contrasts with the reduced longevity described in a smaller kindred of subjects with IGHD type 1A due to a large homozygous deletion in the GH-1 gene who lived in the 19th and 20th centuries in the Swiss Alps (103). The causes of deaths, which occurred in the middle of the 20th century, were analyzed by interviewing still living children of families with affected members and going through the church and community records. The authors, although admitting that these data are not in strictly medical terms, concluded that there was no difference in cause of death between the affected and unaffected brothers and sisters (103). As the recollections of offspring are not strictly a scientifically valid argument, the conclusions of this interesting study warrant caution in the interpretation.
It is difficult to compare differences in longevity between 1A and 1B IGHD owing to the scarcity and validity of data in 1A. We can speculate that different genetic and environmental backgrounds, historical period of observation, and pattern of residual GH secretion may influence longevity in both conditions. Further studies may clarify whether the difference between our subjects with very low, but measurable, serum GH levels and the IGHD type 1A, with no detectable GH secretion, might contribute to different impacts on longevity. It is also unknown whether GH profiles in the Itabaianinha cohort exhibit an age-related decline, making their GH levels virtually absent in late stages of life.
In the data from the Itabaianinha cohort analyzed until 2006, there was a higher frequency of deaths before 20 years of age. Once IGHD individuals reach adulthood, there is no difference in their lifespan compared with siblings or the general population. At that time, there were seven people with IGHD in Itabaianinha aged >70 years (104). Currently the oldest person with IGHD is an 88-year-old woman. At the time of this writing, 30 deaths have been recorded: 14 were from undetermined causes, 4 were due to stroke, 4 were due to external causes, 3 were due to infection, 2 represented natural deaths at the ages of 104 and 94 years, 1 was due to heart attack, 1 was due to hepatic cirrhosis, and 1 was due to skin cancer, with an apparent predominance of non-age-related causes.
These data also suggest that IGHD is not a risk factor for cardiovascular mortality in middle or advanced age. Alternatively, we do not have the same certainty for cerebrovascular mortality, which is apparently more frequent than cardiovascular mortality in this IGHD cohort. Similarly, a nationwide autopsy database of Japanese patients with hypopituitarism, and without GH replacement therapy, has shown a decreased relative frequency of death from all heart diseases, without difference in the frequency of death from ischemic heart disease in comparison with controls (176). However, there was a twofold increase in cerebrovascular mortality and a fourfold to fivefold increase in intracerebral hemorrhage (176). Although cerebral hemorrhage is often associated with cranial radiation therapy, a discrepancy between coronary and cerebral arteries status in lifetime untreated congenital IGHD is possible (176).
Another genetic example of long-lived GHD subjects has been reported on Krk Island in the Adriatic Sea, studied since 1864 (177). This population is GH deficient, but this is associated with deficits of prolactin, TSH, and gonadotropins due to a PROP-1 gene mutation. The “little people of Krk” could survive to a very advanced age, even 91 years old without GH and sex hormones replacement and with irregular thyroid hormone treatment. From the 25 patients, 6 died of cardiovascular disease, 2 by unknown causes, and 1 from an accident (177).
In summary, deficiency in GH signaling in mice delays aging and remarkably extends lifespan and healthspan, with delays in cognitive decline and in the onset of age-related disease. The role of IGFs in the control of aging and lifespan is evolutionarily conserved from worms to mammals. The combination of reduced GH, IGF-1, and insulin signaling likely contributes to extended longevity in GH- or GHR-deficient organisms. Diminutive body size and reduced fecundity of GH-deficient and GH-resistant mice can be viewed as trade-offs for extended longevity. Mechanisms responsible for delayed aging of GH-related mutants include enhanced stress resistance and xenobiotic metabolism, reduced inflammation, improved insulin signaling, and various metabolic adjustments (13). In the next section we discuss several of these aging aspects in humans with a severe, unequivocal, and untreated GHD.
Aging in Other Human Cohorts With Altered GH Signaling
Longevity in Laron syndrome
Longevity in Laron syndrome could be assessed in the Israeli (175) and the Ecuadorian cohorts (96, 178) owing to the relatively large number of subjects followed in each cohort (64 and 99 individuals, respectively). In both cohorts, longevity was apparently normal. In the first cohort, the oldest living individual was near his 60s and four deaths were registered: one girl near the age of 4, two patients with diabetes with cardiovascular complications (one at age 50 and another at age 75 after a car accident), and one woman at age 54 presumably of coronary heart disease. In the second cohort, the oldest living individual was in his 90s. In addition to the early deaths that occurred before the age of 10 due to common childhood diseases, there were 30 deaths among patients with Laron syndrome: 9 due to age-related diseases (8 due to cardiac disease and 1 to stroke) and 21 were due to non–age-related causes. Patients with Laron syndrome from the Ecuadorian cohort died much more frequently from accidents, alcohol-related causes, and convulsive disorders (96), featuring a youthful pattern of mortality, associated with increased insulin sensitivity, as shown in our subjects with IGHD with an inactivating GHRHR gene mutation. Remarkably, cancer was not a cause of death in either the Israeli or Ecuadorian cohorts (1, 171, 179). In conclusion, both GHRH resistance (IGHD) and GH resistance confer protection against age-related pathologies, but not extension of lifespan.
Longevity in GHD in the syndromes of hypopituitarism
Rosén and Bengtsson (180) reported increased cardiovascular mortality in 333 consecutive deaths of Swedish patients with hypopituitarism (diagnosed between 1956 and 1987 and with deaths between 1987 and 1994), which solidified the concept of the detrimental effects of GHD on life expectancy. Nevertheless, a subsequent study of 1014 UK patients with hypopituitarism did not find evidence of association between GHD and increased mortality (181). In the latter study, independent risk factors for excess mortality were female sex, craniopharyngioma, and untreated gonadotropin deficiency (181). Importantly, a more recent study with 1286 Swedish patients with hypopituitarism (deaths from 1995 to 2009) revealed a mildly increased overall mortality (182). Two important causes of excess mortality were identified: adrenal crisis and an increased risk of a late appearance of de novo malignant brain tumors in patients who previously received radiotherapy. Cardiovascular or cerebrovascular mortality did not significantly exceed those of the reference population (182). Similarly, cardiovascular mortality was not above that expected in a Danish nationwide study (183) and in another multinational study (184). Cerebrovascular disease–related deaths were modestly elevated in one study (184), and in women only in another study (183).
As a whole, the previously published literature does not support the concept that GHD associated with hypopituitarism reduces longevity. Several confounders, for example, sex, etiology, radiotherapy, and suboptimal (or excessive) replacement of glucocorticoid, thyroid, and sex hormones, can influence this relationship. The only way to answer the question of whether untreated IGHD influences longevity in humans is provided by experiments of nature, such as the Itabaianinha dwarfs. Our data do not seem to support the concept that genetic, severe, and not treated IGHD, per se, compromises longevity (104). Finally, the Endocrine Society’s guidelines on hypopituitarism consolidated the concept that untreated congenital IGHD does not reduce life expectancy (147).
In summary, data derived from patients with acquired syndromes of hypopituitarism are not sufficient for arriving at firm conclusions about GHD and longevity. However, data from congenital IGHD (Itabaianinha cohort), combined deficiency of GH and other adenohypophyseal hormones (Krk cohort), and GHI syndromes (Israeli and Ecuadorian cohorts) suggest a role of suppressed GH signaling and the resulting IGF-1 deficiency in the control of human aging. These endocrine changes appear to have greater impact on the chances of achieving exceptional longevity than on the average lifespan. Subjects with congenital defects of GH signaling have some protection from age-related pathologies and appear to maintain a youthful profile of morbidity and causes of death into advanced age.
Collectively, available evidence suggests that GH exerts various direct and IGF-mediated effects on healthspan, lifespan, and, by implication, the rate of the underlying process of biological aging in humans. These effects appear to be particularly relevant to mortality rate in late life and the chances of attaining extremely old age, as well as familial longevity, or perhaps are likely to be detected in these types of studies. However, quantitative impact of extremes of GH signaling (GHD, GH resistance, pathologic excess) on human aging is much smaller than could be expected from data obtained in laboratory stocks of house mice. This is likely related to major differences in reproductive strategies, energy partitioning, and trade-offs between short-lived and long-lived species of mammals. This is discussed in more detail earlier in this review.
Impact of GH excess on longevity
The reduced life expectancy associated with chronic GH excess in transgenic mice (14, 15) seems to mirror historical data of increased mortality in untreated patients with acromegaly. Indeed, without appropriate therapy, the lifespan of patients with acromegaly may be reduced by 10 years (185). Mortality in this disease is principally due to cardiovascular (biventricular hypertrophy, hypertension, metabolic abnormalities, diastolic and systolic dysfunction, arrhythmias, atherosclerosis, and endothelial dysfunction) and respiratory (sleep apnea and ventilatory dysfunction) diseases (186, 187). Although neoplastic complications have been associated with the increased risk of death in this disease (188, 189), some studies challenge this concept (190). Before current therapies became available, the standard mortality rate for these patients was more than double (186). However, more recent meta-analyses report that mortality of patients with normal GH and IGF-1 after successful therapy is similar to that in the general population (187). Reduction of mortality in acromegaly to expected normal levels was obtained with improvements of pituitary surgery, radiotherapy, and medical therapy (somatostatin receptor analogs, dopamine agonists, and GHR antagonists). Case studies of individuals afflicted with the rare syndrome of gigantism suggest extreme reduction of life expectancy (191). Thus, chronic GH exposure can reduce longevity in humans as it does mice. A report of accelerated telomere shortening in acromegaly (192) raises an intriguing possibility that increased risk of age-associated disease and life expectancy in these subjects may represent symptoms of accelerated aging.
Does GH have a physiological role in the control of human aging and longevity?
In view of the data concerning age-related diseases and mortality in syndromes of GH excess, deficiency, or resistance, it is interesting to ask whether GH might have a physiological role in the control of human aging. Available evidence suggests that, similarly to what was discovered in mice, GH does have an impact on human aging, with its physiological effects on growth, maturation, and metabolism being associated with some costs in terms of healthspan and longevity. However, some of the findings relevant to this issue are surprisingly inconsistent and controversial. This is perhaps best illustrated by a contrast between increased disease risk and mortality of individuals with untreated acromegaly or gigantism (see details in the preceding section) and the ability of GH therapy to reverse some age-related changes in body composition of elderly individuals (193).
The interpretation of longevity data in relationship to GH or GH-dependent traits is further complicated by the evidence that IGF-1, a key mediator of many GH actions, protects from some age-related diseases and pathological changes (cognitive decline and cardiovascular disease) while increasing the risk of others (primarily cancer) (194, 195). Discussion of the role of circulating (systemic) vs tissue (local) actions of IGF-1 and its binding proteins in the control of aging and in the risk of chronic diseases is outside the scope of this review.
Consistent with the suspected role of GH in human aging, Samaras (36) provided many examples of negative association of height and longevity in different human populations. More recently, He et al. (84) reported such association in a large cohort of American men of Japanese ancestry, along with the evidence that height was negatively associated with a FOXO3 longevity allele and with fasting insulin levels, both of which are thought to be causally related to aging and lifespan. Numerous studies linked genetic polymorphisms related to the secretion and actions of GH and IGF-1 to human longevity (196–200). Moreover, low serum IGF-1 levels have been associated with extreme survival (201).
Some of the strongest evidence for a physiological role of GH in human aging was obtained in recent studies of the offspring of long-lived families in the Netherlands (202). These individuals were previously shown to have reduced incidence of chronic disease and lower mortality than did their spouses or partners who share the same environment and are closely matched for age, education, and socioeconomical history but differ with respect to genes affecting longevity (203). Careful analysis of GH pulses in their serum demonstrated that the offspring of long-lived families, that is, individuals genetically predisposed to familial longevity, secrete less GH than do their spouses or partners (202). Offspring of centenarians were previously shown to have reduced circulating IGF-1 bioactivity (204).
Controversies and GH Therapy
GH treatment
Since biosynthetic GH became readily available in the mid-1980s, there was a rapid expansion of its uses. In the United States, the Food and Drug Administration, in addition to the GH replacement therapy indication in pediatric, transition, and adult patients with GHD, approved its use for several non-GHD states. In children, they include Turner syndrome, Noonan syndrome, chronic renal insufficiency, Prader-Willi syndrome, idiopathic short stature, short stature homeobox-containing gene, and failure to show catch-up growth after being born small for gestational age (SGA). In adults, these states included wasting and cachexia due to AIDS related to HIV infection. Although still controversial (205–207), long-term use of GH for its growth-promoting action seems to have a good safety profile (208–211). When increasing height is the purpose of treatment, the benefits of GH replacement surpass the safety concerns. Although idiopathic short stature is an approved indication for GH therapy, this issue continues to be debated and psychological counseling is viewed as a worthwhile option (212). Children with idiopathic short stature generally require higher doses of GH than do those with GHD, apparently due to GH and IGF-1 resistance (213), and they seem to benefit from GH treatment in terms of height gain without any severe negative metabolic outcomes. Metabolic effects are similar to those observed in children with GHD and include a transient decrease in insulin sensitivity and a dose-dependent increase in IGF-1, without any increase in the risk of diabetes (214, 215).
Childhood, as well as adult, GHD can develop following traumatic brain injury and, in fact, is the most common type of pituitary dysfunction in these patients (216). GH therapy was shown to improve QOL in patients with traumatic brain injury (217) and, curiously, beneficial effects were also detected in those that had no evidence of GHD (218).
However, GH replacement therapy in adults must be restricted to individuals with proven organic GHD (147, 219, 220). Positive effects are predicted in body composition, exercise capacity, and QOL. Although GH replacement therapy in adults improves some surrogate markers of cardiovascular disease, there is no evidence of reduction in cardiovascular mortality. On the contrary, it tends to increase insulin resistance, a well-recognized cardiovascular risk factor and the major cause of morbidity and mortality (221). In our patients with lifetime IGHD due to the GHRHR gene mutation, 6 months of treatment with a long-acting GH (Nutropin Depot) given every 15 days had reversible beneficial effects on body composition and metabolic profile, but this caused a progressive increase in intima media thickness and in the number of atherosclerotic carotid plaques (222). Subsequently, we showed arrest of progression of atherosclerosis 5 years after therapy discontinuation, proving a deleterious effect of GH replacement therapy on the ordinarily slow evolution of atherosclerosis in these subjects with IGHD (223).
Children and adults with Prader-Willi syndrome may receive GH replacement therapy to improve body composition, namely increasing lean body mass and decreasing fat mass (224, 225). Athletes often desire this therapy, especially for its anabolic actions. GH has been promoted to achieve faster recovery from injury and muscle exertion, although there is no evidence that GH or IGF-1 actually improves physical performance in healthy young adults. Additionally, it may worsen exercise capacity and increase adverse events. Its use in athletic competition is banned by the World Anti-Doping Agency, and athletes are required to submit to testing for GH exposure (226, 227). Similarly, GH use for sports is strongly discouraged by the Endocrine Society and American Association of Clinical Endocrinologists (219, 220). As previously noted, subjects with IGHD from the Itabaianinha cohort have better muscular function than do their normal controls, indicating that their very low GH and IGF-1 levels throughout life can be sufficient for good physical performance. In conclusion, without the objective of increasing height, the concerns about risks and detrimental effects of GH replacement override the possible benefits.
“Without appropriate therapy, the lifespan of patients with acromegaly may be reduced by 10 years.”
Use of GH as an antiaging agent?
Numerous studies in humans and in experimental animals suggest that GH actions could be characterized as “pro-aging.” Importantly, this applies to both normal and pathologically elevated levels of GH. Key findings concerning the role of GH in aging are discussed earlier in this review. As viewed against the background of these observations, it is rather curious that the potential use of GH as an antiaging agent attracted enormous interest and publicity. GH itself, as well as various GH-related products (primarily nutritional supplements designed to increase endogenous GH secretion), is widely and aggressively advertised with lists of rejuvenation and health benefits that appear to be, to put it very mildly, grossly exaggerated. The rationale for the use of GH to slow down or even reverse aging is based on the well-documented decline in GH levels during aging together with the beneficial changes in body composition and bone mineral density in GH-treated elderly men studied by Rudman et al. (193) and the overlap of some symptoms of adult GHD with the effects of aging. These symptoms include expansion of WAT, reduced mass and function of skeletal muscles, and changes in mood, energy, and well-being, leading to worsening of the QOL. More recent studies confirmed the ability of GH treatment to reduce adiposity and increase muscle mass in middle-aged or older adults, but this treatment produced no benefits in muscle strength and had a number of undesirable side effects (150, 151, 228). The current consensus is that treatment of endocrinologically normal individuals with GH to reduce or reverse the effects of aging is not justified and is ineffective with the risks outweighing the benefits (219). In the United States, GH treatment based solely on chronological age and a desire to control aging is illegal.
The potential utility of GH in the treatment of sarcopenia, a key component of frailty in the elderly, remains to be fully explored. This would seem to merit large, well-designed studies, but would also require careful assessment of the risks related to GH-induced insulin resistance, diabetes, and cancer, as well as potential shifts in the profile of proinflammatory and anti-inflammatory cytokines.
Growth Hormone and Development
Impact of prenatal development on the somatotropic axis
Children with low birth weight resulting from maternal starvation or extreme malnutrition during pregnancy are at increased risk for developing type 2 diabetes, hypertension, and other types of chronic disease in adult life. This relationship and the broader concept of the developmental origins of health and disease was discovered in adults born during or after a period of extreme World War II food shortages in the Netherlands (220, 230) and subsequently verified in numerous studies in other populations (231–233). To facilitate identification of the underlying mechanisms, pregnant mice, rats, sheep, and baboons have been exposed to calorie restriction or low-protein diets to produce SGA and lower birthweight offspring. Reduced rate of fetal growth in these studies led to adult abnormalities of body composition, glucose homeostasis, serum lipids, and cardiac function closely resembling the epidemiological findings in humans exposed to prenatal malnutrition. Reduced fetal and postnatal growth reflect altered function of the somatotropic axis. This includes reduced hepatic IGF-1 expression and circulating IGF-1 levels (234–237) as well as reduction of IGF-1 bioavailability due to increased production of IGF-binding proteins (237). Also involved is an increase of adult hepatic expression of miR-29, an miRNA capable of suppressing IGF-1 expression in hepatoma cells (234), and reduced IGF-1 response to GH stimulation (235), which in rats has been related to altered DNA methylation around the GH response element of the IGF-1 gene (236). The important role of GH in mediating the effects of fetal undernutrition was shown in a series of studies from Vickers and colleagues (238) in which pregnant rats had their food intake reduced by 50%, and their offspring were given daily bovine GH injections starting at 3 days of age. GH treatment rescued (partially normalized) numerous characteristics of the SGA offspring, including body composition, insulin resistance, inflammation, adipogenesis, and endothelial and cardiovascular function (238). The beneficial effects of GH treatment on the metabolic dysregulation in these animals offer interesting contrast to the reports that nutritionally induced catch-up growth tends to amplify rather than ameliorate the negative impacts of intrauterine growth retardation on adult health (238). Actually, similar negative effects on adult health (abdominal obesity, hyperinsulinemia, and probably increased risk of diabetes) seem to occur in infants with a rapid weight gain, especially in the first 3 months of life (240). A timely, healthy catch-up growth may be beneficial in preventing metabolic consequences and also guaranteeing neurodevelopmental and cognitive functions. GH replacement therapy can be an option to increase height and improve cognitive function (without insulin resistance), as well as improve body fat distribution (241).
The consensus statement of the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society (242) addresses the issue of GH therapy of children born SGA. According to this statement, although the classic GHD is rare in this population, children with severe growth retardation from 2 to 4 years of age should be considered for daily GH treatment. In children born SGA, growth responses to GH treatment, as well as spontaneous postnatal growth, are variably related to insulin secretion, sensitivity to insulin, and expression of genetic markers of these characteristics (243–245). Analysis of somatotropic and insulin signaling suggests that SGA children may exhibit some resistance to the actions of GH and IGF-1 (246).
Prolonged GH treatment of individuals born SGA increased bone mineral density, but these gains were gradually lost after treatment was stopped (247). Treatment of SGA children with GH did not increase their mortality (248), similar to findings in children with idiopathic GHD or idiopathic short stature. In contrast to individuals with low birth weight due to maternal undernutrition, very preterm children have high postnatal GH levels, which have been linked to neurodevelopmental abnormalities and cognitive deficits (249).
Additional studies are needed to clarify the impact of maternal nutrition during pregnancy on the postnatal function of the GHRH–GH–IGF-1 axis and somatic growth, and to elucidate the role of GH in mediating the impact of developmental events on adult health. Separating the impact of nutritional and hormonal factors is particularly challenging because their complex interactions include a major role of nutrients in determining sensitivity of hepatic IGF-1 expression to GH stimulation. Moreover, nutritional and GH/IGF-1 signals combine to regulate the activation of the mTORC1 complex and its downstream targets likely involved in mediating the effects of maternal undernutrition (250). The complexity of these relationships is further emphasized by the evidence that maternal overnutrition similarly to undernutrition can lead to metabolic dysregulation in adult offspring (251). In recent analyses of data from the Demographic Health Survey in Brazil, childhood mortality was increased at both extremes of maternal and childhood nutrition (252, 253). The impact of GH signaling during early life on longevity is discussed in the next section of this review.
Actions of GH during early life can impact adult phenotype and longevity
In hypopituitary Ames dwarf mice, congenital absence of GH is associated with a remarkable extension of longevity. Six weeks of GH replacement therapy started at 1 or 2 weeks of age shortens lifespan of these animals (94, 95), implying that absence of GH signals during this period of postnatal development is causally linked to extended longevity. In addition to the shortening of lifespan, early life GH replacement partially or fully normalized (rescued) many adult metabolic characteristics in dwarfs who had been treated with GH as juveniles. These metabolic characteristics included levels of blood glucose, plasma insulin, adiponectin, ketone bodies, LDL, as well as activation of stress-responsive pathways and expression of various genes related to inflammation and detoxification of xenobiotics (95). The crucial time window for modifying longevity of these animals by a relatively brief period of GH replacement therapy apparently has to include the preweaning period. Replacement of GH started a few weeks after weaning did not affect longevity in Snell dwarf mice studied by Vergara et al. (254) or in pilot studies in Ames dwarfs in our laboratory. Moreover, identical GH treatment twice a day for 6 weeks administered to middle-aged Ames dwarf mice did not alter their longevity. In support of the impact of early life somatotropic signaling on aging, Ashpole et al. (255) recently reported that knockdown of IGF-1 in 10-day-old mice increased longevity of females.
However, there is also evidence for the impact of GH and IGF-1 actions during adult life on aging and longevity. Inducing global GHR deletion in 6-week-old-mice significantly extended longevity of females (256). Moreover, a robust increase in female longevity was reported in mice in which IGF-1 was knocked down at 5 months of age (255).
Studies of aging in genetically GH-deficient Lewis dwarf rats and responses of these animals to early life GH treatment revealed interesting differences from dwarf mice. Dwarf rats, similar to dwarf mice, have reduced IGF-1 levels and improved capacity for DNA repair and are significantly protected from cancer (68, 144, 257), but they do not live longer, apparently because of increased incidence of intracranial hemorrhage (144). Several weeks of GH treatment starting at 4 or 5 weeks of age reversed their enhanced DNA repair capacity but, surprisingly, increased their lifespan. The authors related lifespan extension to promotion of normal developmental events by GH replacement therapy and temporal delay in cerebral hemorrhage (144).
Many questions concerning the impact of the somatotropic axis during various stages of life history on health and longevity remain to be answered. As mentioned earlier in this review, this includes an important question of potential benefits of GH therapy or increasing endogenous GH release in the elderly (193, 258). However, data available to date indicate that the actions of GH during postnatal development and adult life are generally pro-aging and that the levels of GH during the rapid period of peripubertal growth may be particularly important in this regard.
“Actions of GH during postnatal development and adult life are general pro-aging.”
Conclusions and Future Directions
What do we know?
Research conducted during the past 25 years provided much evidence for a role of GH in the control of mammalian aging. In populations of laboratory mice, GHD and GH resistance led to slower and/or delayed aging, multiple indications of extended healthspan, and robust increase in longevity. Surprisingly, the longevity benefits of these endocrine defects match or exceed the effects of all known dietary, genetic, or pharmacological antiaging interventions.
GH signaling appears to affect aging and longevity by a variety of interacting mechanisms, some of which overlap the actions of calorie restriction, interference with IGF-1 signaling, or suppression of mTORC1. Negative correlation of longevity with body size, a GH-dependent trait, is striking in mice with GH-related mutations or targeted gene deletions and is also seen in mice that have not been genetically altered. Importantly, the negative correlation of body size and longevity extends to other species of rodents, carnivores, and equids, as well as various (although not all) of the examined human cohorts.
In humans, reduced GH and IGF-1 signaling was associated with increased old age survival and probability of attaining exceptional longevity. Chronic massive increase of GH secretion reduces life expectancy in both humans and mice. However, no increase of longevity was seen in the large cohort of subjects with IGHD-I (described in detail in this review) or in other cohorts of humans with IGHD, GHI, or hypopituitarism. Instead, reduced GH signaling was associated with protection from some age-related diseases, improvements in various measures of QOL, and changes in the profile of common causes of death. It would appear that the potentially beneficial effects of reduced GH signaling on human longevity are relatively subtle and may be obscured or even reversed by features of the underlying endocrine syndromes unrelated to GH or by iatrogenic factors.
Why some of the findings in mice differ from findings in humans
The difference between the normal life expectancy of humans with various GH-related dwarfing syndromes and the extended lifespan of mice with genetic GHD or GH resistance, prompted the question of whether the role of GH in aging is species specific? Information reviewed in this article suggests that similarly to its action on growth, body composition, and metabolism, the actions of GH on aging are not limited to mice, and that variations between species in their responses to GH, GHD, or GHI are quantitative rather than qualitative. However, some major differences exist and it is interesting to ask why. We suspect that a greater impact of GHD on aging in mice than in people reflects different relative roles of growth and metabolism in the life history of these species. Mice are a primary example of living fast with rapid postnatal growth and maturation, high fecundity, and short life, whereas humans have opposite characteristics (90, 91). This may explain why reduced GH signaling has a much greater impact on aging of mice than humans. Another likely reason for greater impact of GHD and GHI on longevity of mice is that most mice die of cancer, whereas cancer accounts for less than half of deaths in most human populations. Removing GH signals reduces circulating IGF-1 levels, and the combination of these endocrine changes provides protection from cancer. Calorie restriction and rapamycin treatment also reduce cancer incidence and progression and produce a major extension of longevity in mice. We would like to suggest that the trade-offs among GH-dependent growth and anabolic processes, reproduction, cancer, and aging are fundamentally similar in different species. The differential impact of reduced GH signaling on longevity reflects primarily the quantitative differences between regulation of aging in fast-living and slow-living animals (90, 91). The fact that excessive GH levels reduce longevity in both mice and humans would seem to support this interpretation.
Gaps in knowledge
Despite extensive research during the last 25 years, many questions concerning aging of subjects with IGHD in our cohort remain to be answered:
What factors are responsible for extended healthspan of the subjects with IGHD? We are planning to study the involvement of ghrelin, fibroblast growth factor 21, and klotho.
What are the main causes of death in older subjects with IGHD (in addition to the identified increase of deaths due to nonmedical causes, likely due to the youthful profile of morbidity and mortality)? We will first focus on cerebrovascular disease.
Why are individuals with IGHD vulnerable to skin cancer even though they are generally protected from neoplastic disease? Detailed studies of their skin are ongoing.
Other unanswered questions concerning GHD and aging include the following:
Is the trajectory of human aging and mortality significantly altered in individuals with IGHD, GHI, or hypopituitarism that includes GHD?
Does GH replacement therapy alter longevity and the risk of age-related disease in subjects with IGHD or hypopituitarism?
Can dietary or pharmacological suppression of GH signaling promote healthy aging and longevity in endocrinologically normal individuals?
Acknowledgments
The authors thank the IGHD individuals for their participation in all these studies, the Associação do Crescimento Físico e Humano de Itabaianinha for assistance, and all the collaborators who participated in the several studies, in particular Prof. Roberto Salvatori of Johns Hopkins University (Baltimore, MD) and Prof. Ayrton C. Moreira of the Faculty of Medicine of Ribeirão Preto of the University of São Paulo (Ribeirão Preto, Brazil). We are grateful for editorial assistance provided by Dr. Tracy Evans and Lisa Hensley. We apologize to those whose work pertinent to the issues discussed was not cited due to limitations of the format or to inadvertent omissions.
Financial Support: This work was supported by National Institute on Aging/National Institutes of Health Grants P01AG031736, R01AG019899, and R21AG051869 (to A.B.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations
- BAT
brown adipose tissue
- EI
energy intake
- GHD
GH deficiency
- GHI
GH insensitivity
- GHR
GH receptor
- GHRHR
GHRH receptor
- IGFBP-3
IGF-binding protein type 3
- IGHD
isolated GHD
- LDL
low-density lipoprotein
- NAFLD
nonalcoholic fatty liver disease
- QOL
quality of life
- SDS
SD score
- SGA
small for gestational age
- WAT
white adipose tissue
- WT
wild-type
References
- 1. Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384(6604):33. [DOI] [PubMed] [Google Scholar]
- 2. Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci USA. 2001;98(12):6736–6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144(9):3799–3810. [DOI] [PubMed] [Google Scholar]
- 4. Sun LY, Spong A, Swindell WR, Fang Y, Hill C, Huber JA, Boehm JD, Westbrook R, Salvatori R, Bartke A. Growth hormone-releasing hormone disruption extends lifespan and regulates response to caloric restriction in mice. eLife. 2013;2:e01098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci USA. 2006;103(20):7901–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bartke A. Growth hormone and aging: updated review. World J Mens Health. 2018;36:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown-Borg HM. The somatotropic axis and longevity in mice. Am J Physiol Endocrinol Metab. 2015;309(6):E503–E510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Magalhães JP, Cabral JA, Magalhães D. The influence of genes on the aging process of mice: a statistical assessment of the genetics of aging. Genetics. 2005;169(1):265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koopman JJ, van Heemst D, van Bodegom D, Bonkowski MS, Sun LY, Bartke A. Measuring aging rates of mice subjected to caloric restriction and genetic disruption of growth hormone signaling. Aging (Albany NY). 2016;8(3):539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang T, Tsui B, Kreisberg JF, Robertson NA, Gross AM, Yu MK, Carter H, Brown-Borg HM, Adams PD, Ideker T. Epigenetic aging signatures in mice livers are slowed by dwarfism, calorie restriction and rapamycin treatment. Genome Biol. 2017;18(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, Javors MA, Li X, Nadon NL, Nelson JF, Pletcher S, Salmon AB, Sharp ZD, Van Roekel S, Winkleman L, Strong R. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13(3):468–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bartke A. Single-gene mutations and healthy ageing in mammals. Philos Trans R Soc Lond B Biol Sci. 2011;366(1561):28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bartke A, Sun LY, Longo V. Somatotropic signaling: trade-offs between growth, reproductive development, and longevity. Physiol Rev. 2013;93(2):571–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pendergrass WR, Li Y, Jiang D, Wolf NS. Decrease in cellular replicative potential in “giant” mice transfected with the bovine growth hormone gene correlates to shortened life span. J Cell Physiol. 1993;156(1):96–103. [DOI] [PubMed] [Google Scholar]
- 15. Wolf E, Kahnt E, Ehrlein J, Hermanns W, Brem G, Wanke R. Effects of long-term elevated serum levels of growth hormone on life expectancy of mice: lessons from transgenic animal models. Mech Ageing Dev. 1993;68(1–3):71–87. [DOI] [PubMed] [Google Scholar]
- 16. Basu R, Qian Y, Kopchick JJ. Mechanisms in endocrinology: lessons from growth hormone receptor gene-disrupted mice: are there benefits of endocrine defects? Eur J Endocrinol. 2018;178(5):R155–R181. [DOI] [PubMed] [Google Scholar]
- 17. Masternak MM, Panici JA, Bonkowski MS, Hughes LF, Bartke A. Insulin sensitivity as a key mediator of growth hormone actions on longevity. J Gerontol A Biol Sci Med Sci. 2009;64(5):516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Berryman DE, List EO, Kohn DT, Coschigano KT, Seeley RJ, Kopchick JJ. Effect of growth hormone on susceptibility to diet-induced obesity. Endocrinology. 2006;147(6):2801–2808. [DOI] [PubMed] [Google Scholar]
- 19. Hill CM, Fang Y, Miquet JG, Sun LY, Masternak MM, Bartke A. Long-lived hypopituitary Ames dwarf mice are resistant to the detrimental effects of high-fat diet on metabolic function and energy expenditure. Aging Cell. 2016;15(3):509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang Z, Al-Regaiey KA, Masternak MM, Bartke A. Adipocytokines and lipid levels in Ames dwarf and calorie-restricted mice. J Gerontol A Biol Sci Med Sci. 2006;61(4):323–331. [DOI] [PubMed] [Google Scholar]
- 21. Masternak MM, Bartke A, Wang F, Spong A, Gesing A, Fang Y, Salmon AB, Hughes LF, Liberati T, Boparai R, Kopchick JJ, Westbrook R. Metabolic effects of intra-abdominal fat in GHRKO mice. Aging Cell. 2012;11(1):73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Menon V, Zhi X, Hossain T, Bartke A, Spong A, Gesing A, Masternak MM. The contribution of visceral fat to improved insulin signaling in Ames dwarf mice. Aging Cell. 2014;13(3):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li Y, Knapp JR, Kopchick JJ. Enlargement of interscapular brown adipose tissue in growth hormone antagonist transgenic and in growth hormone receptor gene-disrupted dwarf mice. Exp Biol Med (Maywood). 2003;228(2):207–215. [DOI] [PubMed] [Google Scholar]
- 24. Darcy J, McFadden S, Fang Y, Huber JA, Zhang C, Sun LY, Bartke A. Brown adipose tissue function is enhanced in long-lived, male Ames dwarf mice. Endocrinology. 2016;157(12):4744–4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Darcy J, McFadden S, Fang Y, Berryman DE, List EO, Milcik N, Bartke A. Increased environmental temperature normalizes energy metabolism outputs between normal and Ames dwarf mice. Aging (Albany NY). 2018;10(10):2709–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. List EO, Berryman DE, Funk K, Gosney ES, Jara A, Kelder B, Wang X, Kutz L, Troike K, Lozier N, Mikula V, Lubbers ER, Zhang H, Vesel C, Junnila RK, Frank SJ, Masternak MM, Bartke A, Kopchick JJ. The role of GH in adipose tissue: lessons from adipose-specific GH receptor gene-disrupted mice. Mol Endocrinol. 2013;27(3):524–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kinney BA, Meliska CJ, Steger RW, Bartke A. Evidence that Ames dwarf mice age differently from their normal siblings in behavioral and learning and memory parameters. Horm Behav. 2001;39(4):277–284. [DOI] [PubMed] [Google Scholar]
- 28. Kinney BA, Coschigano KT, Kopchick JJ, Steger RW, Bartke A. Evidence that age-induced decline in memory retention is delayed in growth hormone resistant GH-R-KO (Laron) mice. Physiol Behav. 2001;72(5):653–660. [DOI] [PubMed] [Google Scholar]
- 29. Arum O, Rasche ZA, Rickman DJ, Bartke A. Prevention of neuromusculoskeletal frailty in slow-aging Ames dwarf mice: longitudinal investigation of interaction of longevity genes and caloric restriction. PLoS One. 2013;8(10):e72255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arum O, Rickman DJ, Kopchick JJ, Bartke A. The slow-aging growth hormone receptor/binding protein gene-disrupted (GHR-KO) mouse is protected from aging-resultant neuromusculoskeletal frailty. Age (Dordr). 2014;36(1):117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schneider A, Zhi X, Bartke A, Kopchick JJ, Masternak MM. Effect of growth hormone receptor gene disruption and PMA treatment on the expression of genes involved in primordial follicle activation in mice ovaries. Age (Dordr). 2014;36(4):9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bachelot A, Monget P, Imbert-Bolloré P, Coshigano K, Kopchick JJ, Kelly PA, Binart N. Growth hormone is required for ovarian follicular growth. Endocrinology. 2002;143(10):4104–4112. [DOI] [PubMed] [Google Scholar]
- 33. Schneider A, Matkovich SJ, Saccon T, Victoria B, Spinel L, Lavasani M, Bartke A, Golusinski P, Masternak MM. Ovarian transcriptome associated with reproductive senescence in the long-living Ames dwarf mice. Mol Cell Endocrinol. 2017;439:328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saccon TD, Moreira F, Cruz LA, Mondadori RG, Fang Y, Barros CC, Spinel L, Bartke A, Masternak MM, Schneider A. Ovarian aging and the activation of the primordial follicle reserve in the long-lived Ames dwarf and the short-lived bGH transgenic mice. Mol Cell Endocrinol. 2017;455:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miller RA, Chrisp C, Atchley W. Differential longevity in mouse stocks selected for early life growth trajectory. J Gerontol A Biol Sci Med Sci. 2000;55(9):B455–B461. [DOI] [PubMed] [Google Scholar]
- 36. Samaras TT, ed. Human Body Size and the Laws of Scaling: Physiological, Performance, Growth, Longevity and Ecological Ramifications. New York, NY: Nova Science Publishers; 2007. [Google Scholar]
- 37. Miller RA, Harper JM, Galecki A, Burke DT. Big mice die young: early life body weight predicts longevity in genetically heterogeneous mice. Aging Cell. 2002;1(1):22–29. [DOI] [PubMed] [Google Scholar]
- 38. Greer KA, Canterberry SC, Murphy KE. Statistical analysis regarding the effects of height and weight on life span of the domestic dog. Res Vet Sci. 2007;82(2):208–214. [DOI] [PubMed] [Google Scholar]
- 39. Greer KA, Hughes LM, Masternak MM. Connecting serum IGF-1, body size, and age in the domestic dog. Age (Dordr). 2011;33(3):475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299(5611):1346–1351. [DOI] [PubMed] [Google Scholar]
- 41. Longo VD, Finch CE. Genetics of aging and diseases: from rare mutations and model systems to disease prevention. Arch Neurol. 2002;59(11):1706–1708. [DOI] [PubMed] [Google Scholar]
- 42. McElwee JJ, Schuster E, Blanc E, Piper MD, Thomas JH, Patel DS, Selman C, Withers DJ, Thornton JM, Partridge L, Gems D. Evolutionary conservation of regulated longevity assurance mechanisms. Genome Biol. 2007;8(7):R132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Al-Regaiey KA, Masternak MM, Bonkowski M, Sun L, Bartke A. Long-lived growth hormone receptor knockout mice: interaction of reduced insulin-like growth factor I/insulin signaling and caloric restriction. Endocrinology. 2005;146(2):851–860. [DOI] [PubMed] [Google Scholar]
- 44. Masternak MM, Bartke A. PPARs in calorie restricted and genetically long-lived mice. PPAR Res. 2007;2007:28436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dozmorov I, Bartke A, Miller RA. Array-based expression analysis of mouse liver genes: effect of age and of the longevity mutant Prop1df. J Gerontol A Biol Sci Med Sci. 2001;56(2):B72–B80. [DOI] [PubMed] [Google Scholar]
- 46. Swindell WR. Gene expression profiling of long-lived dwarf mice: longevity-associated genes and relationships with diet, gender and aging. BMC Genomics. 2007;8(1):353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stout MB, Swindell WR, Zhi X, Rohde K, List EO, Berryman DE, Kopchick JJ, Gesing A, Fang Y, Masternak MM. Transcriptome profiling reveals divergent expression shifts in brown and white adipose tissue from long-lived GHRKO mice. Oncotarget. 2015;6(29):26702–26715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tsuchiya T, Dhahbi JM, Cui X, Mote PL, Bartke A, Spindler SR. Additive regulation of hepatic gene expression by dwarfism and caloric restriction. Physiol Genomics. 2004;17(3):307–315. [DOI] [PubMed] [Google Scholar]
- 49. Bartke A, Wright JC, Mattison JA, Ingram DK, Miller RA, Roth GS. Extending the lifespan of long-lived mice. Nature. 2001;414(6862):412. [DOI] [PubMed] [Google Scholar]
- 50. Fang Y, Hill CM, Darcy J, Reyes-Ordoñez A, Arauz E, McFadden S, Zhang C, Osland J, Gao J, Zhang T, Frank SJ, Javors MA, Yuan R, Kopchick JJ, Sun LY, Chen J, Bartke A. Effects of rapamycin on growth hormone receptor knockout mice. Proc Natl Acad Sci USA. 2018;115(7):E1495–E1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Arum O, Boparai RK, Saleh JK, Wang F, Dirks AL, Turner JG, Kopchick JJ, Liu JL, Khardori RK, Bartke A. Specific suppression of insulin sensitivity in growth hormone receptor gene-disrupted (GHR-KO) mice attenuates phenotypic features of slow aging. Aging Cell. 2014;13(6):981–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. List EO, Berryman DE, Funk K, Jara A, Kelder B, Wang F, Stout MB, Zhi X, Sun L, White TA, LeBrasseur NK, Pirtskhalava T, Tchkonia T, Jensen EA, Zhang W, Masternak MM, Kirkland JL, Miller RA, Bartke A, Kopchick JJ. Liver-specific GH receptor gene-disrupted (LiGHRKO) mice have decreased endocrine IGF-I, increased local IGF-I, and altered body size, body composition, and adipokine profiles. Endocrinology. 2014;155(5):1793–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. List EO, Berryman DE, Ikeno Y, Hubbard GB, Funk K, Comisford R, Young JA, Stout MB, Tchkonia T, Masternak MM, Bartke A, Kirkland JL, Miller RA, Kopchick JJ. Removal of growth hormone receptor (GHR) in muscle of male mice replicates some of the health benefits seen in global GHR−/− mice. Aging (Albany NY). 2015;7(7):500–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hascup ER, Wang F, Kopchick JJ, Bartke A. Inflammatory and glutamatergic homeostasis are involved in successful aging. J Gerontol A Biol Sci Med Sci. 2016;71(3):281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Arum O, Saleh JK, Boparai RK, Kopchick JJ, Khardori RK, Bartke A. Preservation of blood glucose homeostasis in slow-senescing somatotrophism-deficient mice subjected to intermittent fasting begun at middle or old age [published correction appears in Age (Dordr). 2014;36(4):9695]. Age (Dordr). 2014;36(3):9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Salmon AB, Murakami S, Bartke A, Kopchick J, Yasumura K, Miller RA. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am J Physiol Endocrinol Metab. 2005;289(1):E23–E29. [DOI] [PubMed] [Google Scholar]
- 57. Bokov AF, Lindsey ML, Khodr C, Sabia MR, Richardson A. Long-lived ames dwarf mice are resistant to chemical stressors. J Gerontol A Biol Sci Med Sci. 2009;64(8):819–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Brown-Borg HM, Johnson WT, Rakoczy SG. Expression of oxidative phosphorylation components in mitochondria of long-living Ames dwarf mice. Age (Dordr). 2012;34(1):43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Westbrook R, Bonkowski MS, Strader AD, Bartke A. Alterations in oxygen consumption, respiratory quotient, and heat production in long-lived GHRKO and Ames dwarf mice, and short-lived bGH transgenic mice. J Gerontol A Biol Sci Med Sci. 2009;64(4):443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sanz A, Bartke A, Barja G. Long-lived Ames dwarf mice: oxidative damage to mitochondrial DNA in heart and brain. J Am Aging Assoc. 2002;25(3):119–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dominick G, Bowman J, Li X, Miller RA, Garcia GG. mTOR regulates the expression of DNA damage response enzymes in long-lived Snell dwarf, GHRKO, and PAPPA-KO mice. Aging Cell. 2017;16(1):52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hine C, Kim HJ, Zhu Y, Harputlugil E, Longchamp A, Matos MS, Ramadoss P, Bauerle K, Brace L, Asara JM, Ozaki CK, Cheng SY, Singha S, Ahn KH, Kimmelman A, Fisher FM, Pissios P, Withers DJ, Selman C, Wang R, Yen K, Longo VD, Cohen P, Bartke A, Kopchick JJ, Miller R, Hollenberg AN, Mitchell JR. Hypothalamic-pituitary axis regulates hydrogen sulfide production. Cell Metab. 2017;25(6):1320–1333.e5. [DOI] [PMC free article] [PubMed]
- 63. Ratajczak MZ, Shin DM, Ratajczak J, Kucia M, Bartke A. A novel insight into aging: are there pluripotent very small embryonic-like stem cells (VSELs) in adult tissues overtime depleted in an Igf-1-dependent manner? Aging (Albany NY). 2010;2(11):875–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Stout MB, Tchkonia T, Pirtskhalava T, Palmer AK, List EO, Berryman DE, Lubbers ER, Escande C, Spong A, Masternak MM, Oberg AL, LeBrasseur NK, Miller RA, Kopchick JJ, Bartke A, Kirkland JL. Growth hormone action predicts age-related white adipose tissue dysfunction and senescent cell burden in mice. Aging (Albany NY). 2014;6(7):575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Anderson RM, Weindruch R. Metabolic reprogramming, caloric restriction and aging. Trends Endocrinol Metab. 2010;21(3):134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Collino S, Martin FP, Montoliu I, Barger JL, Da Silva L, Prolla TA, Weindruch R, Kochhar S. Transcriptomics and metabonomics identify essential metabolic signatures in calorie restriction (CR) regulation across multiple mouse strains. Metabolites. 2013;3(4):881–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Blagosklonny MV. Calorie restriction: decelerating mTOR-driven aging from cells to organisms (including humans). Cell Cycle. 2010;9(4):683–688. [DOI] [PubMed] [Google Scholar]
- 68. Podlutsky A, Valcarcel-Ares MN, Yancey K, Podlutskaya V, Nagykaldi E, Gautam T, Miller RA, Sonntag WE, Csiszar A, Ungvari Z. The GH/IGF-1 axis in a critical period early in life determines cellular DNA repair capacity by altering transcriptional regulation of DNA repair-related genes: implications for the developmental origins of cancer. Geroscience. 2017;39(2):147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cole JJ, Robertson NA, Rather MI, Thomson JP, McBryan T, Sproul D, Wang T, Brock C, Clark W, Ideker T, Meehan RR, Miller RA, Brown-Borg HM, Adams PD. Diverse interventions that extend mouse lifespan suppress shared age-associated epigenetic changes at critical gene regulatory regions. Genome Biol. 2017;18(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bonkowski MS, Dominici FP, Arum O, Rocha JS, Al Regaiey KA, Westbrook R, Spong A, Panici J, Masternak MM, Kopchick JJ, Bartke A. Disruption of growth hormone receptor prevents calorie restriction from improving insulin action and longevity. PLoS One. 2009;4(2):e4567. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71. Sadagurski M, Landeryou T, Cady G, Kopchick JJ, List EO, Berryman DE, Bartke A, Miller RA. Growth hormone modulates hypothalamic inflammation in long-lived pituitary dwarf mice. Aging Cell. 2015;14(6):1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, Masaki KH, Willcox DC, Rodriguez B, Curb JD. FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci USA. 2008;105(37):13987–13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Anselmi CV, Malovini A, Roncarati R, Novelli V, Villa F, Condorelli G, Bellazzi R, Puca AA. Association of the FOXO3A locus with extreme longevity in a southern Italian centenarian study. Rejuvenation Res. 2009;12(2):95–104. [DOI] [PubMed] [Google Scholar]
- 74. Flachsbart F, Caliebe A, Kleindorp R, Blanché H, von Eller-Eberstein H, Nikolaus S, Schreiber S, Nebel A. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc Natl Acad Sci USA. 2009;106(8):2700–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Blüher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299(5606):572–574. [DOI] [PubMed] [Google Scholar]
- 76. Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D, Withers DJ. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326(5949):140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Templeman NM, Flibotte S, Chik JHL, Sinha S, Lim GE, Foster LJ, Nislow C, Johnson JD. Reduced circulating insulin enhances insulin sensitivity in old mice and extends lifespan. Cell Reports. 2017;20(2):451–463. [DOI] [PubMed] [Google Scholar]
- 78. Bartke A. Can growth hormone (GH) accelerate aging? Evidence from GH-transgenic mice. Neuroendocrinology. 2003;78(4):210–216. [DOI] [PubMed] [Google Scholar]
- 79. Wirth-Dzieçiołowska E, Czumińska K. Longevity and aging of mice from lines divergently selected for body weight for over 90 generations. Biogerontology. 2000;1(2):171–178. [DOI] [PubMed] [Google Scholar]
- 80. Yuan R, Meng Q, Nautiyal J, Flurkey K, Tsaih SW, Krier R, Parker MG, Harrison DE, Paigen B. Genetic coregulation of age of female sexual maturation and lifespan through circulating IGF1 among inbred mouse strains. Proc Natl Acad Sci USA. 2012;109(21):8224–8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rollo CD. Growth negatively impacts the life span of mammals. Evol Dev. 2002;4(1):55–61. [DOI] [PubMed] [Google Scholar]
- 82. Patronek GJ, Waters DJ, Glickman LT. Comparative longevity of pet dogs and humans: implications for gerontology research. J Gerontol A Biol Sci Med Sci. 1997;52(3):B171–B178. [DOI] [PubMed] [Google Scholar]
- 83. Brosnahan MM, Paradis MR. Demographic and clinical characteristics of geriatric horses: 467 cases (1989–1999). J Am Vet Med Assoc. 2003;223(1):93–98. [DOI] [PubMed] [Google Scholar]
- 84. He Q, Morris BJ, Grove JS, Petrovitch H, Ross W, Masaki KH, Rodriguez B, Chen R, Donlon TA, Willcox DC, Willcox BJ. Shorter men live longer: association of height with longevity and FOXO3 genotype in American men of Japanese ancestry [published correction appears in PLoS One. 2014;9(8):e105839]. PLoS One. 2014;9(5):e94385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Vangipurapu J, Stancáková A, Jauhiainen R, Kuusisto J, Laakso M. Short adult stature predicts impaired β-cell function, insulin resistance, glycemia, and type 2 diabetes in Finnish men. J Clin Endocrinol Metab. 2017;102(2):443–450. [DOI] [PubMed] [Google Scholar]
- 86. Jimenez AG. Physiological underpinnings in life-history trade-offs in man’s most popular selection experiment: the dog. J Comp Physiol B. 2016;186(7):813–827. [DOI] [PubMed] [Google Scholar]
- 87. Swanson EM, Dantzer B. Insulin-like growth factor-1 is associated with life-history variation across Mammalia. Proc Biol Sci. 2014;281(1782):20132458. [DOI] [PMC free article] [PubMed]
- 88. Tian X, Seluanov A, Gorbunova V. Molecular mechanisms determining lifespan in short- and long-lived species. Trends Endocrinol Metab. 2017;28(10):722–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Azpurua J, Yang JN, Van Meter M, Liu Z, Kim J, Lobo Ladd AA, Coppi AA, Gorbunova V, Seluanov A. IGF1R levels in the brain negatively correlate with longevity in 16 rodent species. Aging (Albany NY). 2013;5(4):304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ricklefs RE, Wikelski M. The physiology/life-history nexus. Trends Ecol Evol. 2002;17(10):462–468. [Google Scholar]
- 91. Salzman TC, McLaughlin AL, Westneat DF, Crowley PH. Energetic trade-offs and feedbacks between behavior and metabolism influence correlations between pace-of-life attributes [published correction appears in Behav Ecol Sociobiol. 2018;72:76]. Behav Ecol Sociobiol. 2018;72(3):54. [Google Scholar]
- 92. MacRae SL, Zhang Q, Lemetre C, Seim I, Calder RB, Hoeijmakers J, Suh Y, Gladyshev VN, Seluanov A, Gorbunova V, Vijg J, Zhang ZD. Comparative analysis of genome maintenance genes in naked mole rat, mouse, and human. Aging Cell. 2015;14(2):288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Milholland B, Dong X, Zhang L, Hao X, Suh Y, Vijg J. Differences between germline and somatic mutation rates in humans and mice. Nat Commun. 2017;8:15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Panici JA, Harper JM, Miller RA, Bartke A, Spong A, Masternak MM. Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long-lived mutant mice. FASEB J. 2010;24(12):5073–5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sun LY, Fang Y, Patki A, Koopman JJ, Allison DB, Hill CM, Masternak MM, Darcy J, Wang J, McFadden S, Bartke A. Longevity is impacted by growth hormone action during early postnatal period. eLife. 2017;6:e24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3(70):70ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ranke MB, Reiter E, Price D. Idiopathic growth hormone deficiency in KIGS: selected aspects In: Ranke MB, Price DA, Reiter EO, eds. Growth Hormone Therapy in Pediatrics–20 Years of KIGS. 1st ed.Basel, Switzerland: Karger; 2007:116–135. [Google Scholar]
- 98. Cacciari E, Zucchini S, Carlà G, Pirazzoli P, Cicognani A, Mandini M, Busacca M, Trevisan C. Endocrine function and morphological findings in patients with disorders of the hypothalamo-pituitary area: a study with magnetic resonance. Arch Dis Child. 1990;65(11):1199–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Pérez Jurado LA, Argente J. Molecular basis of familial growth hormone deficiency. Horm Res. 1994;42(4–5):189–197. [DOI] [PubMed] [Google Scholar]
- 100. Martari M, Salvatori R. Diseases associated with growth hormone-releasing hormone receptor (GHRHR) mutations. Prog Mol Biol Transl Sci. 2009;88:57–84. [DOI] [PubMed] [Google Scholar]
- 101. Mullis PE, Robinson IC, Salemi S, Eblé A, Besson A, Vuissoz JM, Deladoey J, Simon D, Czernichow P, Binder G. Isolated autosomal dominant growth hormone deficiency: an evolving pituitary deficit? A multicenter follow-up study. J Clin Endocrinol Metab. 2005;90(4):2089–2096. [DOI] [PubMed] [Google Scholar]
- 102. Dattani MT. Growth hormone deficiency and combined pituitary hormone deficiency: does the genotype matter? Clin Endocrinol (Oxf). 2005;63(2):121–130. [DOI] [PubMed] [Google Scholar]
- 103. Besson A, Salemi S, Gallati S, Jenal A, Horn R, Mullis PS, Mullis PE. Reduced longevity in untreated patients with isolated growth hormone deficiency. J Clin Endocrinol Metab. 2003;88(8):3664–3667. [DOI] [PubMed] [Google Scholar]
- 104. Aguiar-Oliveira MH, Oliveira FT, Pereira RM, Oliveira CR, Blackford A, Valenca EH, Santos EG, Gois-Junior MB, Meneguz-Moreno RA, Araujo VP, Oliveira-Neto LA, Almeida RP, Santos MA, Farias NT, Silveira DC, Cabral GW, Calazans FR, Seabra JD, Lopes TF, Rodrigues EO, Porto LA, Oliveira IP, Melo EV, Martari M, Salvatori R. Longevity in untreated congenital growth hormone deficiency due to a homozygous mutation in the GHRH receptor gene. J Clin Endocrinol Metab. 2010;95(2):714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Salvatori R, Hayashida CY, Aguiar-Oliveira MH, Phillips JA III, Souza AH, Gondo RG, Toledo SP, Conceicão MM, Prince M, Maheshwari HG, Baumann G, Levine MA. Familial dwarfism due to a novel mutation of the growth hormone-releasing hormone receptor gene. J Clin Endocrinol Metab. 1999;84(3):917–923. [DOI] [PubMed] [Google Scholar]
- 106. Aguiar-Oliveira MH, Gill MS, de A Barretto ES, Alcântara MR, Miraki-Moud F, Menezes CA, Souza AH, Martinelli CE, Pereira FA, Salvatori R, Levine MA, Shalet SM, Camacho-Hubner C, Clayton PE. Effect of severe growth hormone (GH) deficiency due to a mutation in the GH-releasing hormone receptor on insulin-like growth factors (IGFs), IGF-binding proteins, and ternary complex formation throughout life. J Clin Endocrinol Metab. 1999;84(11):4118–4126. [DOI] [PubMed] [Google Scholar]
- 107. Aguiar-Oliveira MH, Souza AHO, Oliveira CRP, Campos VC, Oliveira-Neto LA, Salvatori R. Mechanisms in endocrinology: the multiple facets of GHRH/GH/IGF-I axis: lessons from lifetime, untreated, isolated GH deficiency due to a GHRH receptor gene mutation. Eur J Endocrinol. 2017;177(2):R85–R97. [DOI] [PubMed] [Google Scholar]
- 108. Savage MO, Blum WF, Ranke MB, Postel-Vinay MC, Cotterill AM, Hall K, Chatelain PG, Preece MA, Rosenfeld RG. Clinical features and endocrine status in patients with growth hormone insensitivity (Laron syndrome). J Clin Endocrinol Metab. 1993;77(6):1465–1471. [DOI] [PubMed] [Google Scholar]
- 109. van Buul-Offers SC, Bloemen RJ, Reijnen-Gresnigt MG, van Leiden HA, Hoogerbrugge CM, Van den Brande JL. Insulin-like growth factors-I and -II and their binding proteins during postnatal development of dwarf Snell mice before and during growth hormone and thyroxine therapy. J Endocrinol. 1994;143(1):191–198. [DOI] [PubMed] [Google Scholar]
- 110. Salvatori R, Serpa MG, Parmigiani G, Britto AV, Oliveira JL, Oliveira CR, Prado CM, Farias CT, Almeida JC, Vicente TA, Aguiar-Oliveira MH. GH response to hypoglycemia and clonidine in the GH-releasing hormone resistance syndrome. J Endocrinol Invest. 2006;29(9):805–808. [DOI] [PubMed] [Google Scholar]
- 111. Gondo RG, Aguiar-Oliveira MH, Hayashida CY, Toledo SP, Abelin N, Levine MA, Bowers CY, Souza AH, Pereira RM, Santos NL, Salvatori R. Growth hormone-releasing peptide-2 stimulates GH secretion in GH-deficient patients with mutated GH-releasing hormone receptor. J Clin Endocrinol Metab. 2001;86(7):3279–3283. [DOI] [PubMed] [Google Scholar]
- 112. Maheshwari HG, Rahim A, Shalet SM, Baumann G. Selective lack of growth hormone (GH) response to the GH-releasing peptide hexarelin in patients with GH-releasing hormone receptor deficiency. J Clin Endocrinol Metab. 1999;84(3):956–959. [DOI] [PubMed] [Google Scholar]
- 113. Maheshwari HG, Pezzoli SS, Rahim A, Shalet SM, Thorner MO, Baumann G. Pulsatile growth hormone secretion persists in genetic growth hormone-releasing hormone resistance. Am J Physiol Endocrinol Metab. 2002;282(4):E943–E951. [DOI] [PubMed] [Google Scholar]
- 114. Laron Z. Laron syndrome (primary growth hormone resistance or insensitivity): the personal experience 1958–2003. J Clin Endocrinol Metab. 2004;89(3):1031–1044. [DOI] [PubMed] [Google Scholar]
- 115. Gomes-Santos E, Salvatori R, Ferrão TO, Oliveira CR, Diniz RD, Santana JA, Pereira FA, Barbosa RA, Souza AH, Melo EV, Epitácio-Pereira CC, Oliveira-Santos AA, Oliveira IA, Machado JA, Santana-Júnior FJ, Barreto-Filho JA, Aguiar-Oliveira MH. Increased visceral adiposity and cortisol to cortisone ratio in adults with congenital lifetime isolated GH deficiency. J Clin Endocrinol Metab. 2014;99(9):3285–3289. [DOI] [PubMed] [Google Scholar]
- 116. Menezes M, Salvatori R, Melo LD, Rocha IE, Oliveira CR, Pereira RM, Souza AH, Valença EH, Melo EV, Campos VC, Costa FO, Aguiar-Oliveira MH. Prolactin and sex steroids levels in congenital lifetime isolated GH deficiency. Endocrine. 2013;44(1):207–211. [DOI] [PubMed] [Google Scholar]
- 117. Oliveira HA, Salvatori R, Krauss MP, Oliveira CR, Silva PR, Aguiar-Oliveira MH. Magnetic resonance imaging study of pituitary morphology in subjects homozygous and heterozygous for a null mutation of the GHRH receptor gene. Eur J Endocrinol. 2003;148(4):427–432. [DOI] [PubMed] [Google Scholar]
- 118. Wilson DB, Wyatt DP. Histopathology of the pituitary gland in neonatal little (lit) mutant mice. Histol Histopathol. 1992;7(3):451–455. [PubMed] [Google Scholar]
- 119. Oliveira-Neto LA, Melo MF, Franco AA, Oliveira AH, Souza AH, Valença EH, Britto IM, Salvatori R, Aguiar-Oliveira MH. Cephalometric features in isolated growth hormone deficiency. Angle Orthod. 2011;81(4):578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Barreto VM, D’Avila JS, Sales NJ, Gonçalves MI, Seabra JD, Salvatori R, Aguiar-Oliveira MH. Laryngeal and vocal evaluation in untreated growth hormone deficient adults. Otolaryngol Head Neck Surg. 2009;140(1):37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Valença EH, Salvatori R, Souza AH, Oliveira-Neto LA, Oliveira AH, Gonçalves MI, Oliveira CR, D’Ávila JS, Melo VA, de Carvalho S, de Andrade BM, Nascimento LS, Rocha SB, Ribeiro TR, Prado-Barreto VM, Melo EV, Aguiar-Oliveira MH. Voice formants in individuals with congenital, isolated, lifetime growth hormone deficiency. J Voice. 2016;30(3):281–286. [DOI] [PubMed] [Google Scholar]
- 122. Laron Z, Silbergeld A, Kauli R. Differential effects of hGH and IGF-I on body proportions. Anthropol Anz. 2012;69(3):255–259. [DOI] [PubMed] [Google Scholar]
- 123. Aguiar-Oliveira MH, Salvatori R. Lifetime growth hormone deficiency: impact on growth, metabolism, body composition, and survival capacity In: Preedy VR, ed. Handbook of Growth and Growth Monitoring in Health and Disease. New York, NY: Springer; 2012:2699–2710. [Google Scholar]
- 124. Laron Z, Iluz M, Kauli R. Head circumference in untreated and IGF-I treated patients with Laron syndrome: comparison with untreated and hGH-treated children with isolated growth hormone deficiency. Growth Horm IGF Res. 2012;22(2):49–52. [DOI] [PubMed] [Google Scholar]
- 125. Valença EH, Souza AH, Oliveira AH, Valenca SL, Salvatori R, Goncalves MI, Oliveira-Neto LA, Barros AD, Nascimento UN, Oliveira CR, Cardoso DF, Melo VA, Aguiar-Oliveira MH. Voice quality in short stature with and without GH deficiency. J Voice. 2012;26(5):673.e13-9. [DOI] [PubMed] [Google Scholar]
- 126. Epitácio-Pereira CC, Silva GM, Salvatori R, Santana JA, Pereira FA, Gois-Junior MB, Britto AV, Oliveira CR, Souza AH, Santos EG, Campos VC, Pereira RM, Valença EH, Barbosa RA, Farias MI, de Paula FJ, Ribeiro TV, Oliveira MC, Aguiar-Oliveira MH. Isolated GH deficiency due to a GHRH receptor mutation causes hip joint problems and genu valgum, and reduces size but not density of trabecular and mixed bone. J Clin Endocrinol Metab. 2013;98(11):E1710–E1715. [DOI] [PubMed] [Google Scholar]
- 127. de Paula FJ, Góis-Júnior MB, Aguiar-Oliveira MH, Pereira FA, Oliveira CR, Pereira RM, Farias CT, Vicente TA, Salvatori R. Consequences of lifetime isolated growth hormone (GH) deficiency and effects of short-term GH treatment on bone in adults with a mutation in the GHRH-receptor gene. Clin Endocrinol (Oxf). 2009;70(1):35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Souza AH, Farias MI, Salvatori R, Silva GM, Santana JA, Pereira FA, de Paula FJ, Valença EH, Melo EV, Barbosa RA, Pereira RM, Gois-Junior MB, Aguiar-Oliveira MH. Lifetime, untreated isolated GH deficiency due to a GH-releasing hormone receptor mutation has beneficial consequences on bone status in older individuals, and does not influence their abdominal aorta calcification. Endocrine. 2014;47(1):191–197. [DOI] [PubMed] [Google Scholar]
- 129. Bouillon R, Koledova E, Bezlepkina O, Nijs J, Shavrikhova E, Nagaeva E, Chikulaeva O, Peterkova V, Dedov I, Bakulin A, Oganov V, Attanasio AF. Bone status and fracture prevalence in Russian adults with childhood-onset growth hormone deficiency. J Clin Endocrinol Metab. 2004;89(10):4993–4998. [DOI] [PubMed] [Google Scholar]
- 130. Zofková I. Pathophysiological and clinical importance of insulin-like growth factor-I with respect to bone metabolism. Physiol Res. 2003;52(6):657–679. [PubMed] [Google Scholar]
- 131. Barreto-Filho JA, Alcântara MR, Salvatori R, Barreto MA, Sousa AC, Bastos V, Souza AH, Pereira RM, Clayton PE, Gill MS, Aguiar-Oliveira MH. Familial isolated growth hormone deficiency is associated with increased systolic blood pressure, central obesity, and dyslipidemia. J Clin Endocrinol Metab. 2002;87(5):2018–2023. [DOI] [PubMed] [Google Scholar]
- 132. Bian D, Li Z, Ma H, Mu S, Ma C, Cui B, Gao L, Zhao J. Effects of diosgenin on cell proliferation induced by IGF-1 in primary human thyrocytes. Arch Pharm Res. 2011;34(6):997–1005. [DOI] [PubMed] [Google Scholar]
- 133. Ock S, Lee WS, Ahn J, Kim HM, Kang H, Kim HS, Jo D, Abel ED, Lee TJ, Kim J. Deletion of IGF-1 receptors in cardiomyocytes attenuates cardiac aging in male mice. Endocrinology. 2016;157(1):336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Menezes M, Salvatori R, Oliveira CR, Pereira RM, Souza AH, Nobrega LM, Cruz EA, Menezes M, Alves EO, Aguiar-Oliveira MH. Climacteric in untreated isolated growth hormone deficiency. Menopause. 2008;15(4 Pt 1):743–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Oliveira CR, Salvatori R, Nóbrega LM, Carvalho EO, Menezes M, Farias CT, Britto AV, Pereira RM, Aguiar-Oliveira MH. Sizes of abdominal organs in adults with severe short stature due to severe, untreated, congenital GH deficiency caused by a homozygous mutation in the GHRH receptor gene. Clin Endocrinol (Oxf). 2008;69(1):153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Faro ACN, Pereira-Gurgel VM, Salvatori R, Campos VC, Melo GB, Oliveira FT, Oliveira-Santos AA, Oliveira CRP, Pereira FA, Hellström A, Oliveira-Neto LA, Valença EHO, Aguiar-Oliveira MH. Ocular findings in adult subjects with an inactivating mutation in GH releasing hormone receptor gene. Growth Horm IGF Res. 2017;34:8–12. [DOI] [PubMed] [Google Scholar]
- 137. Pereira-Gurgel VM, Faro AC, Salvatori R, Chagas TA, Carvalho-Junior JF, Oliveira CR, Costa UM, Melo GB, Hellström A, Aguiar-Oliveira MH. Abnormal vascular and neural retinal morphology in congenital lifetime isolated growth hormone deficiency. Growth Horm IGF Res. 2016;30–31:11–15. [DOI] [PubMed] [Google Scholar]
- 138. Vicente TA, Rocha IE, Salvatori R, Oliveira CR, Pereira RM, Souza AH, Campos VC, Santos EG, Diniz RD, Valença EH, Epitácio-Pereira CC, Oliveira MC, Mari A, Aguiar-Oliveira MH. Lifetime congenital isolated GH deficiency does not protect from the development of diabetes. Endocr Connect. 2013;2(2):112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Nellhaus G. Head circumference from birth to eighteen years. Practical composite international and interracial graphs. Pediatrics. 1968;41(1):106–114. [PubMed] [Google Scholar]
- 140. Laron Z, Kauli R. Sexual development in patients with Laron syndrome In: Laron Z, Kopchick JJ, eds. Laron Syndrome—From Man to Mouse: Lessons in Clinical Experience. Berlin, Germany: Springer-Verlag; 2011:101–118. [Google Scholar]
- 141. Woods KA, Camacho-Hübner C, Savage MO, Clark AJ. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N Engl J Med. 1996;335(18):1363–1367. [DOI] [PubMed] [Google Scholar]
- 142. Jasienska G, Nenko I, Jasienski M. Daughters increase longevity of fathers, but daughters and sons equally reduce longevity of mothers. Am J Hum Biol. 2006;18(3):422–425. [DOI] [PubMed] [Google Scholar]
- 143. Danilovich N, Wernsing D, Coschigano KT, Kopchick JJ, Bartke A. Deficits in female reproductive function in GH-R-KO mice; role of IGF-I. Endocrinology. 1999;140(6):2637–2640. [DOI] [PubMed] [Google Scholar]
- 144. Sonntag WE, Carter CS, Ikeno Y, Ekenstedt K, Carlson CS, Loeser RF, Chakrabarty S, Lee S, Bennett C, Ingram R, Moore T, Ramsey M. Adult-onset growth hormone and insulin-like growth factor I deficiency reduces neoplastic disease, modifies age-related pathology, and increases life span. Endocrinology. 2005;146(7):2920–2932. [DOI] [PubMed] [Google Scholar]
- 145. Barbosa JA, Salvatori R, Oliveira CR, Pereira RM, Farias CT, Britto AV, Farias NT, Blackford A, Aguiar-Oliveira MH. Quality of life in congenital, untreated, lifetime isolated growth hormone deficiency. Psychoneuroendocrinology. 2009;34(6):894–900. [DOI] [PubMed] [Google Scholar]
- 146. Cook DM, Yuen KC, Biller BM, Kemp SF, Vance ML; American Association of Clinical Endocrinologists . American Association of Clinical Endocrinologists medical guidelines for clinical practice for growth hormone use in growth hormone-deficient adults and transition patients—2009 update. Endocr Pract. 2009;15(Suppl 2):1–29. [DOI] [PubMed] [Google Scholar]
- 147. Fleseriu M, Hashim IA, Karavitaki N, Melmed S, Murad MH, Salvatori R, Samuels MH. Hormonal replacement in hypopituitarism in adults: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(11):3888–3921. [DOI] [PubMed] [Google Scholar]
- 148. Chihara K, Kato Y, Kohno H, Takano K, Tanaka T, Teramoto A, Shimatsu A. Safety and efficacy of growth hormone (GH) during extended treatment of adult Japanese patients with GH deficiency (GHD). Growth Horm IGF Res. 2008;18(4):307–317. [DOI] [PubMed] [Google Scholar]
- 149. Hoffman AR, Kuntze JE, Baptista J, Baum HB, Baumann GP, Biller BM, Clark RV, Cook D, Inzucchi SE, Kleinberg D, Klibanski A, Phillips LS, Ridgway EC, Robbins RJ, Schlechte J, Sharma M, Thorner MO, Vance ML. Growth hormone (GH) replacement therapy in adult-onset GH deficiency: effects on body composition in men and women in a double-blind, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89(5):2048–2056. [DOI] [PubMed] [Google Scholar]
- 150. Blackman MR, Sorkin JD, Münzer T, Bellantoni MF, Busby-Whitehead J, Stevens TE, Jayme J, O’Connor KG, Christmas C, Tobin JD, Stewart KJ, Cottrell E, St Clair C, Pabst KM, Harman SM. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA. 2002;288(18):2282–2292. [DOI] [PubMed] [Google Scholar]
- 151. Papadakis MA, Grady D, Black D, Tierney MJ, Gooding GA, Schambelan M, Grunfeld C. Growth hormone replacement in healthy older men improves body composition but not functional ability. Ann Intern Med. 1996;124(8):708–716. [DOI] [PubMed] [Google Scholar]
- 152. Oliveira FT, Salvatori R, Marcondes J, Macena LB, Oliveira-Santos AA, Faro ACN, Campos VC, Oliveira CRP, Costa UMM, Aguiar-Oliveira MH. Altered sleep patterns in patients with non-functional GHRH receptor. Eur J Endocrinol. 2017;177(1):51–57. [DOI] [PubMed] [Google Scholar]
- 153. Prado-Barreto VM, Salvatori R, Santos Júnior RC, Brandão-Martins MB, Correa EA, Garcez FB, Valença EH, Souza AH, Pereira RM, Nunes MA, D’Avila JS, Aguiar-Oliveira MH. Hearing status in adult individuals with lifetime, untreated isolated growth hormone deficiency. Otolaryngol Head Neck Surg. 2014;150(3):464–471. [DOI] [PubMed] [Google Scholar]
- 154. Campos VC, Barrios MR, Salvatori R, de Almeida RP, de Melo EV, Nascimento AC, de Jesus AR, Aguiar-Oliveira MH. Infectious diseases and immunological responses in adult subjects with lifetime untreated, congenital GH deficiency. Endocrine. 2016;54(1):182–190. [DOI] [PubMed] [Google Scholar]
- 155. Britto IM, Aguiar-Oliveira MH, Oliveira-Neto LA, Salvatori R, Souza AH, Araujo VP, Corraini P, Pannuti CM, Romito GA, Pustiglioni FE. Periodontal disease in adults with untreated congenital growth hormone deficiency: a case-control study. J Clin Periodontol. 2011;38(6):525–531. [DOI] [PubMed] [Google Scholar]
- 156. de A Barretto ES, Gill MS, De Freitas ME, Magalhães MM, Souza AH, Aguiar-Oliveira MH, Clayton PE. Serum leptin and body composition in children with familial GH deficiency (GHD) due to a mutation in the growth hormone-releasing hormone (GHRH) receptor. Clin Endocrinol (Oxf). 1999;51(5):559–564. [DOI] [PubMed] [Google Scholar]
- 157. Laron Z, Ginsberg S, Lilos P, Arbiv M, Vaisman N. Body composition in untreated adult patients with Laron syndrome (primary GH insensitivity). Clin Endocrinol (Oxf). 2006;65(1):114–117. [DOI] [PubMed] [Google Scholar]
- 158. Oliveira CR, Salvatori R, Barreto-Filho JA, Rocha IE, Mari A, Pereira RM, Campos VC, Menezes M, Gomes E, Meneguz-Moreno RA, Araújo VP, Leite NT, Nascimento-Junior AC, Farias MI, Viscente TA, Araújo RD, Melo EV, Aguiar-Oliveira MH. Insulin sensitivity and β-cell function in adults with lifetime, untreated isolated growth hormone deficiency. J Clin Endocrinol Metab. 2012;97(3):1013–1019. [DOI] [PubMed] [Google Scholar]
- 159. Oliveira CR, Salvatori R, Meneguz-Moreno RA, Aguiar-Oliveira MH, Pereira RM, Valença EH, Araujo VP, Farias NT, Silveira DC, Vieira JG, Barreto-Filho JA. Adipokine profile and urinary albumin excretion in isolated growth hormone deficiency. J Clin Endocrinol Metab. 2010;95(2):693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Bennis MT, Schneider A, Victoria B, Do A, Wiesenborn DS, Spinel L, Gesing A, Kopchick JJ, Siddiqi SA, Masternak MM. The role of transplanted visceral fat from the long-lived growth hormone receptor knockout mice on insulin signaling. Geroscience. 2017;39(1):51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Bartke A, Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol. 2004;63:189–225. [DOI] [PubMed] [Google Scholar]
- 162. Liu JL, Coschigano KT, Robertson K, Lipsett M, Guo Y, Kopchick JJ, Kumar U, Liu YL. Disruption of growth hormone receptor gene causes diminished pancreatic islet size and increased insulin sensitivity in mice. Am J Physiol Endocrinol Metab. 2004;287(3):E405–E413. [DOI] [PubMed] [Google Scholar]
- 163. Sakharova AA, Horowitz JF, Surya S, Goldenberg N, Harber MP, Symons K, Barkan A. Role of growth hormone in regulating lipolysis, proteolysis, and hepatic glucose production during fasting. J Clin Endocrinol Metab. 2008;93(7):2755–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Oliveira-Santos AA, Salvatori R, Gomes-Santos E, Santana JA, Leal AC, Barbosa RA, Oliveira CR, Souza AH, Valença EH, Aguiar-Oliveira MH. Subjects with isolated GH deficiency due to a null GHRHR mutation eat proportionally more, but healthier than controls. Endocrine. 2016;51(2):317–322. [DOI] [PubMed] [Google Scholar]
- 165. Gleeson HK, Souza AH, Gill MS, Wieringa GE, de A Barretto ES, Barretto-Filho JA, Shalet SM, Aguiar-Oliveira MH, Clayton PE. Lipid profiles in untreated severe congenital isolated growth hormone deficiency through the lifespan. Clin Endocrinol (Oxf). 2002;57(1):89–95. [DOI] [PubMed] [Google Scholar]
- 166. Rudling M, Olivecrona H, Eggertsen G, Angelin B. Regulation of rat hepatic low density lipoprotein receptors. In vivo stimulation by growth hormone is not mediated by insulin-like growth factor I. J Clin Invest. 1996;97(2):292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Menezes Oliveira JL, Marques-Santos C, Barreto-Filho JA, Ximenes Filho R, de Oliveira Britto AV, Oliveira Souza AH, Prado CM, Pereira Oliveira CR, Pereira RM, Ribeiro Vicente TA, Farias CT, Aguiar-Oliveira MH, Salvatori R. Lack of evidence of premature atherosclerosis in untreated severe isolated growth hormone (GH) deficiency due to a GH-releasing hormone receptor mutation. J Clin Endocrinol Metab. 2006;91(6):2093–2099. [DOI] [PubMed] [Google Scholar]
- 168. Costa UM, Oliveira CR, Salvatori R, Barreto-Filho JA, Campos VC, Oliveira FT, Rocha IE, Oliveira JL, Silva WA, Aguiar-Oliveira MH. Brazilian adult individuals with untreated isolated GH deficiency do not have accelerated subclinical atherosclerosis. Endocr Connect. 2016;5(1):41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Diniz RD, Souza RM, Salvatori R, Franca A, Gomes-Santos E, Ferrão TO, Oliveira CR, Santana JA, Pereira FA, Barbosa RA, Souza AH, Pereira RM, Oliveira-Santos AA, Silva AM, Santana-Júnior FJ, Valença EH, Campos VC, Aguiar-Oliveira MH. Liver status in congenital, untreated, isolated GH deficiency. Endocr Connect. 2014;3(3):132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Marinho CG, Mermejo LM, Salvatori R, Assirati JAJ, Oliveira CRP, Santos EG, Leal ÂCGB, Barros-Oliveira CS, Damascena NP, Lima CA, Farias CT, Moreira AC, Aguiar-Oliveira MH. Occurrence of neoplasms in individuals with congenital, severe GH deficiency from the Itabaianinha kindred. Growth Horm IGF Res. 2018;41:71–74. [DOI] [PubMed] [Google Scholar]
- 171. Steuerman R, Shevah O, Laron Z. Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies. Eur J Endocrinol. 2011;164(4):485–489. [DOI] [PubMed] [Google Scholar]
- 172. DeChiara TM, Efstratiadis A, Robertson EJ. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature. 1990;345(6270):78–80. [DOI] [PubMed] [Google Scholar]
- 173. Instituto Brasileiro de Geografia e Estatística. Censo demográfico: 2000. Available at: https://www.ibge.gov.br/estatisticas-novoportal/sociais/saude/9663-censo-demografico-2000.html?edicao=9771&t=downloads. Accessed 24 October 2018. [Google Scholar]
- 174. Instituto Brasileiro de Geografia e Estatística. Censo demográfico: 2010. Available at: https://www.ibge.gov.br/estatisticas-novoportal/sociais/saude/9663-censo-demografico-2000.html?edicao=9771&t=downloads. Accessed 24 October 2018. [Google Scholar]
- 175. Laron Z. Life span and mortality of patients with Laron syndrome. In: Laron Z, Kopchick JJ, eds. Laron Syndrome—From Man to Mouse: Lessons from Clinical and Experimental Experience. Berlin: Springer; 2011. [Google Scholar]
- 176. Kaji H, Chihara K. Direct causes of death in Japanese patients with hypopituitarism as analyzed from a nation-wide autopsy database. Eur J Endocrinol. 2004;150(2):149–152. [DOI] [PubMed] [Google Scholar]
- 177. Krzisnik C, Grgurić S, Cvijović K, Laron Z. Longevity of the hypopituitary patients from the island Krk: a follow-up study. Pediatr Endocrinol Rev. 2010;7(4):357–362. [PubMed] [Google Scholar]
- 178. Rosenfeld RG, Rosenbloom AL, Guevara-Aguirre J. Growth hormone (GH) insensitivity due to primary GH receptor deficiency. Endocr Rev. 1994;15(3):369–390. [DOI] [PubMed] [Google Scholar]
- 179. Shevah O, Laron Z. Patients with congenital deficiency of IGF-I seem protected from the development of malignancies: a preliminary report. Growth Horm IGF Res. 2007;17(1):54–57. [DOI] [PubMed] [Google Scholar]
- 180. Rosén T, Bengtsson BA. Premature mortality due to cardiovascular disease in hypopituitarism. Lancet. 1990;336(8710):285–288. [DOI] [PubMed] [Google Scholar]
- 181. Tomlinson JW, Holden N, Hills RK, Wheatley K, Clayton RN, Bates AS, Sheppard MC, Stewart PM; West Midlands Prospective Hypopituitary Study Group . Association between premature mortality and hypopituitarism. Lancet. 2001;357(9254):425–431. [DOI] [PubMed] [Google Scholar]
- 182. Burman P, Mattsson AF, Johannsson G, Höybye C, Holmer H, Dahlqvist P, Berinder K, Engström BE, Ekman B, Erfurth EM, Svensson J, Wahlberg J, Karlsson FA. Deaths among adult patients with hypopituitarism: hypocortisolism during acute stress, and de novo malignant brain tumors contribute to an increased mortality. J Clin Endocrinol Metab. 2013;98(4):1466–1475. [DOI] [PubMed] [Google Scholar]
- 183. van Bunderen CC, van Nieuwpoort IC, Arwert LI, Heymans MW, Franken AA, Koppeschaar HP, van der Lely AJ, Drent ML. Does growth hormone replacement therapy reduce mortality in adults with growth hormone deficiency? Data from the Dutch National Registry of Growth Hormone Treatment in adults. J Clin Endocrinol Metab. 2011;96(10):3151–3159. [DOI] [PubMed] [Google Scholar]
- 184. Gaillard RC, Mattsson AF, Akerblad AC, Bengtsson BA, Cara J, Feldt-Rasmussen U, Koltowska-Häggström M, Monson JP, Saller B, Wilton P, Abs R. Overall and cause-specific mortality in GH-deficient adults on GH replacement. Eur J Endocrinol. 2012;166(6):1069–1077. [DOI] [PubMed] [Google Scholar]
- 185. Colao A, Ferone D, Marzullo P, Lombardi G. Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev. 2004;25(1):102–152. [DOI] [PubMed] [Google Scholar]
- 186. Holdaway IM, Rajasoorya RC, Gamble GD. Factors influencing mortality in acromegaly. J Clin Endocrinol Metab. 2004;89(2):667–674. [DOI] [PubMed] [Google Scholar]
- 187. Holdaway IM, Bolland MJ, Gamble GD. A meta-analysis of the effect of lowering serum levels of GH and IGF-I on mortality in acromegaly. Eur J Endocrinol. 2008;159(2):89–95. [DOI] [PubMed] [Google Scholar]
- 188. Wolinski K, Stangierski A, Dyrda K, Nowicka K, Pelka M, Iqbal A, Car A, Lazizi M, Bednarek N, Czarnywojtek A, Gurgul E, Ruchala M. Risk of malignant neoplasms in acromegaly: a case-control study. J Endocrinol Invest. 2017;40(3):319–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Dal J, Leisner MZ, Hermansen K, Farkas DK, Bengtsen M, Kistorp C, Nielsen EH, Andersen M, Feldt-Rasmussen U, Dekkers OM, Sørensen HT, Jørgensen JOL. Cancer incidence in patients with acromegaly: a cohort study and meta-analysis of the literature. J Clin Endocrinol Metab. 2018;103(6):2182–2188. [DOI] [PubMed] [Google Scholar]
- 190. Petroff D, Tönjes A, Grussendorf M, Droste M, Dimopoulou C, Stalla G, Jaursch-Hancke C, Mai M, Schopohl J, Schöfl C. The incidence of cancer among acromegaly patients: results from the German Acromegaly Registry. J Clin Endocrinol Metab. 2015;100(10):3894–3902. [DOI] [PubMed] [Google Scholar]
- 191. Radian S, Diekmann Y, Gabrovska P, Holland B, Bradley L, Wallace H, Stals K, Bussell AM, McGurren K, Cuesta M, Ryan AW, Herincs M, Hernández-Ramírez LC, Holland A, Samuels J, Aflorei ED, Barry S, Dénes J, Pernicova I, Stiles CE, Trivellin G, McCloskey R, Ajzensztejn M, Abid N, Akker SA, Mercado M, Cohen M, Thakker RV, Baldeweg S, Barkan A, Musat M, Levy M, Orme SM, Unterländer M, Burger J, Kumar AV, Ellard S, McPartlin J, McManus R, Linden GJ, Atkinson B, Balding DJ, Agha A, Thompson CJ, Hunter SJ, Thomas MG, Morrison PJ, Korbonits M. Increased population risk of AIP-related acromegaly and gigantism in Ireland. Hum Mutat. 2017;38(1):78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Matsumoto R, Fukuoka H, Iguchi G, Odake Y, Yoshida K, Bando H, Suda K, Nishizawa H, Takahashi M, Yamada S, Ogawa W, Takahashi Y. Accelerated telomere shortening in acromegaly; IGF-I induces telomere shortening and cellular senescence. PLoS One. 2015;10(10):e0140189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Rudman D, Feller AG, Nagraj HS, Gergans GA, Lalitha PY, Goldberg AF, Schlenker RA, Cohn L, Rudman IW, Mattson DE. Effects of human growth hormone in men over 60 years old. N Engl J Med. 1990;323(1):1–6. [DOI] [PubMed] [Google Scholar]
- 194. Teumer A, Qi Q, Nethander M, Aschard H, Bandinelli S, Beekman M, Berndt SI, Bidlingmaier M, Broer L, Cappola A, Ceda GP, Chanock S, Chen MH, Chen TC, Chen YD, Chung J, Del Greco Miglianico F, Eriksson J, Ferrucci L, Friedrich N, Gnewuch C, Goodarzi MO, Grarup N, Guo T, Hammer E, Hayes RB, Hicks AA, Hofman A, Houwing-Duistermaat JJ, Hu F, Hunter DJ, Husemoen LL, Isaacs A, Jacobs KB, Janssen JA, Jansson JO, Jehmlich N, Johnson S, Juul A, Karlsson M, Kilpelainen TO, Kovacs P, Kraft P, Li C, Linneberg A, Liu Y, Loos RJ, Lorentzon M, Lu Y, Maggio M, Magi R, Meigs J, Mellström D, Nauck M, Newman AB, Pollak MN, Pramstaller PP, Prokopenko I, Psaty BM, Reincke M, Rimm EB, Rotter JI, Saint Pierre A, Schurmann C, Seshadri S, Sjögren K, Slagboom PE, Strickler HD, Stumvoll M, Suh Y, Sun Q, Zhang C, Svensson J, Tanaka T, Tare A, Tönjes A, Uh HW, van Duijn CM, van Heemst D, Vandenput L, Vasan RS, Völker U, Willems SM, Ohlsson C, Wallaschofski H, Kaplan RC, Wallaschofski H, Kaplan RC; CHARGE Longevity Working Group; Body Composition Genetics Consortium . Genomewide meta-analysis identifies loci associated with IGF-I and IGFBP-3 levels with impact on age-related traits. Aging Cell. 2016;15(5):811–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Guevara-Aguirre J, Guevara A, Palacios I, Pérez M, Prócel P, Terán E. GH and GHR signaling in human disease. Growth Horm IGF Res. 2018;38:34–38. [DOI] [PubMed] [Google Scholar]
- 196. Bonafè M, Olivieri F. Genetic polymorphism in long-lived people: cues for the presence of an insulin/IGF-pathway-dependent network affecting human longevity. Mol Cell Endocrinol. 2009;299(1):118–123. [DOI] [PubMed] [Google Scholar]
- 197. van Heemst D, Beekman M, Mooijaart SP, Heijmans BT, Brandt BW, Zwaan BJ, Slagboom PE, Westendorp RG. Reduced insulin/IGF-1 signalling and human longevity. Aging Cell. 2005;4(2):79–85. [DOI] [PubMed] [Google Scholar]
- 198. Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci USA. 2008;105(9):3438–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Dato S, Soerensen M, De Rango F, Rose G, Christensen K, Christiansen L, Passarino G. The genetic component of human longevity: new insights from the analysis of pathway-based SNP-SNP interactions. Aging Cell. 2018;17(3):e12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Revelas M, Thalamuthu A, Oldmeadow C, Evans TJ, Armstrong NJ, Kwok JB, Brodaty H, Schofield PR, Scott RJ, Sachdev PS, Attia JR, Mather KA. Review and meta-analysis of genetic polymorphisms associated with exceptional human longevity. Mech Ageing Dev. 2018;175:24–34. [DOI] [PubMed] [Google Scholar]
- 201. Milman S, Atzmon G, Huffman DM, Wan J, Crandall JP, Cohen P, Barzilai N. Low insulin-like growth factor-1 level predicts survival in humans with exceptional longevity. Aging Cell. 2014;13(4):769–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. van der Spoel E, Jansen SW, Akintola AA, Ballieux BE, Cobbaert CM, Slagboom PE, Blauw GJ, Westendorp RGJ, Pijl H, Roelfsema F, van Heemst D. Growth hormone secretion is diminished and tightly controlled in humans enriched for familial longevity. Aging Cell. 2016;15(6):1126–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Westendorp RG, van Heemst D, Rozing MP, Frölich M, Mooijaart SP, Blauw GJ, Beekman M, Heijmans BT, de Craen AJ, Slagboom PE; Leiden Longevity Study Group . Nonagenarian siblings and their offspring display lower risk of mortality and morbidity than sporadic nonagenarians: the Leiden Longevity Study. J Am Geriatr Soc. 2009;57(9):1634–1637. [DOI] [PubMed] [Google Scholar]
- 204. Vitale G, Brugts MP, Ogliari G, Castaldi D, Fatti LM, Varewijck AJ, Lamberts SW, Monti D, Bucci L, Cevenini E, Cavagnini F, Franceschi C, Hofland LJ, Mari D, Janssen J. Low circulating IGF-I bioactivity is associated with human longevity: findings in centenarians’ offspring. Aging (Albany NY). 2012;4(9):580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Carel JC, Ecosse E, Landier F, Meguellati-Hakkas D, Kaguelidou F, Rey G, Coste J. Long-term mortality after recombinant growth hormone treatment for isolated growth hormone deficiency or childhood short stature: preliminary report of the French SAGhE study. J Clin Endocrinol Metab. 2012;97(2):416–425. [DOI] [PubMed] [Google Scholar]
- 206. Sävendahl L, Maes M, Albertsson-Wikland K, Borgström B, Carel JC, Henrard S, Speybroeck N, Thomas M, Zandwijken G, Hokken-Koelega A. Long-term mortality and causes of death in isolated GHD, ISS, and SGA patients treated with recombinant growth hormone during childhood in Belgium, the Netherlands, and Sweden: preliminary report of 3 countries participating in the EU SAGhE study. J Clin Endocrinol Metab. 2012;97(2):E213–E217. [DOI] [PubMed] [Google Scholar]
- 207. Krzyzanowska-Mittermayer K, Mattsson AF, Maiter D, Feldt-Rasmussen U, Camacho-Hübner C, Luger A, Abs R. New neoplasm during GH replacement in adults with pituitary deficiency following malignancy: a KIMS analysis. J Clin Endocrinol Metab. 2018;103(2):523–531. [DOI] [PubMed] [Google Scholar]
- 208. Cohen P, Bright GM, Rogol AD, Kappelgaard AM, Rosenfeld RG; American Norditropin Clinical Trials Group . Effects of dose and gender on the growth and growth factor response to GH in GH-deficient children: implications for efficacy and safety. J Clin Endocrinol Metab. 2002;87(1):90–98. [DOI] [PubMed] [Google Scholar]
- 209. Bell J, Parker KL, Swinford RD, Hoffman AR, Maneatis T, Lippe B. Long-term safety of recombinant human growth hormone in children. J Clin Endocrinol Metab. 2010;95(1):167–177. [DOI] [PubMed] [Google Scholar]
- 210. Kemp SF, Kuntze J, Attie KM, Maneatis T, Butler S, Frane J, Lippe B. Efficacy and safety results of long-term growth hormone treatment of idiopathic short stature. J Clin Endocrinol Metab. 2005;90(9):5247–5253. [DOI] [PubMed] [Google Scholar]
- 211. Cook DM, Rose SR. A review of guidelines for use of growth hormone in pediatric and transition patients. Pituitary. 2012;15(3):301–310. [DOI] [PubMed] [Google Scholar]
- 212. Cohen P, Rogol AD, Deal CL, Saenger P, Reiter EO, Ross JL, Chernausek SD, Savage MO, Wit JM; 2007 ISS Consensus Workshop participants . Consensus statement on the diagnosis and treatment of children with idiopathic short stature: a summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop. J Clin Endocrinol Metab. 2008;93(11):4210–4217. [DOI] [PubMed] [Google Scholar]
- 213. Cohen P, Germak J, Rogol AD, Weng W, Kappelgaard AM, Rosenfeld RG; American Norditropin Study Group . Variable degree of growth hormone (GH) and insulin-like growth factor (IGF) sensitivity in children with idiopathic short stature compared with GH-deficient patients: evidence from an IGF-based dosing study of short children. J Clin Endocrinol Metab. 2010;95(5):2089–2098. [DOI] [PubMed] [Google Scholar]
- 214. Saenger P, Attie KM, DiMartino-Nardi J, Hintz R, Frahm L, Frane JW; Genentech Collaborative Study Group . Metabolic consequences of 5-year growth hormone (GH) therapy in children treated with GH for idiopathic short stature. J Clin Endocrinol Metab. 1998;83(9):3115–3120. [DOI] [PubMed] [Google Scholar]
- 215. Dahlgren J. Metabolic benefits of growth hormone therapy in idiopathic short stature. Horm Res Paediatr. 2011;76(Suppl 3):56–58. [DOI] [PubMed] [Google Scholar]
- 216. Munoz A, Urban R. Neuroendocrine consequences of traumatic brain injury. Curr Opin Endocrinol Diabetes Obes. 2013;20(4):354–358. [DOI] [PubMed] [Google Scholar]
- 217. Gardner CJ, Mattsson AF, Daousi C, Korbonits M, Koltowska-Haggstrom M, Cuthbertson DJ. GH deficiency after traumatic brain injury: improvement in quality of life with GH therapy: analysis of the KIMS database. Eur J Endocrinol. 2015;172(4):371–381. [DOI] [PubMed] [Google Scholar]
- 218. Devesa J, Díaz-Getino G, Rey P, García-Cancela J, Loures I, Nogueiras S, Hurtado de Mendoza A, Salgado L, González M, Pablos T, Devesa P. Brain Recovery after a plane crash: treatment with growth hormone (GH) and neurorehabilitation: a case report. Int J Mol Sci. 2015;16(12):30470–30482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Gharib H, Cook DM, Saenger PH, Bengtsson BA, Feld S, Nippoldt TB, Rodbard HW, Seibel JA, Vance ML, Zimmerman D; American Association of Clinical Endocrinologists Growth Hormone Task Force . American Association of Clinical Endocrinologists medical guidelines for clinical practice for growth hormone use in adults and children—2003 update [published correction appears in Endocr Pract. 2008;14(6):802–803]. Endocr Pract. 2003;9(1):64–76. [DOI] [PubMed] [Google Scholar]
- 220. Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML; Endocrine Society . Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(6):1587–1609. [DOI] [PubMed] [Google Scholar]
- 221. Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106(4):453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Oliveira JL, Aguiar-Oliveira MH, D’Oliveira A Jr, Pereira RM, Oliveira CR, Farias CT, Barreto-Filho JA, Anjos-Andrade FD, Marques-Santos C, Nascimento-Junior AC, Alves EO, Oliveira FT, Campos VC, Ximenes R, Blackford A, Parmigiani G, Salvatori R. Congenital growth hormone (GH) deficiency and atherosclerosis: effects of GH replacement in GH-naive adults. J Clin Endocrinol Metab. 2007;92(12):4664–4670. [DOI] [PubMed] [Google Scholar]
- 223. Araujo VP, Aguiar-Oliveira MH, Oliveira JL, Rocha HM, Oliveira CR, Rodrigues TM, Nunes MA, Britto IM, Ximenes R, Barreto-Filho JA, Meneguz-Moreno RA, Pereira RM, Valença EH, Oliveira-Neto LA, Vicente TA, Blackford A, Salvatori R. Arrest of atherosclerosis progression after interruption of GH replacement in adults with congenital isolated GH deficiency. Eur J Endocrinol. 2012;166(6):977–982. [DOI] [PubMed] [Google Scholar]
- 224. Wolfgram PM, Carrel AL, Allen DB. Long-term effects of recombinant human growth hormone therapy in children with Prader-Willi syndrome. Curr Opin Pediatr. 2013;25(4):509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Vogt KS, Emerick JE. Growth hormone therapy in adults with Prader-Willi syndrome. Diseases. 2015;3(2):56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Liu H, Bravata DM, Olkin I, Friedlander A, Liu V, Roberts B, Bendavid E, Saynina O, Salpeter SR, Garber AM, Hoffman AR. Systematic review: the effects of growth hormone on athletic performance. Ann Intern Med. 2008;148(10):747–758. [DOI] [PubMed] [Google Scholar]
- 227. Baumann GP. Growth hormone doping in sports: a critical review of use and detection strategies. Endocr Rev. 2012;33(2):155–186. [DOI] [PubMed] [Google Scholar]
- 228. Liu H, Bravata DM, Olkin I, Nayak S, Roberts B, Garber AM, Hoffman AR. Systematic review: the safety and efficacy of growth hormone in the healthy elderly. Ann Intern Med. 2007;146(2):104–115. [DOI] [PubMed] [Google Scholar]
- 229. Bleker LS, de Rooij SR, Painter RC, van der Velde N, Roseboom TJ. Prenatal undernutrition and physical function and frailty at the age of 68 years: the Dutch Famine Birth Cohort Study. J Gerontol A Biol Sci Med Sci. 2016;71(10):1306–1314. [DOI] [PubMed] [Google Scholar]
- 230. Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301(6761):1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Lumey LH, Khalangot MD, Vaiserman AM. Association between type 2 diabetes and prenatal exposure to the Ukraine famine of 1932–33: a retrospective cohort study. Lancet Diabetes Endocrinol. 2015;3(10):787–794. [DOI] [PubMed] [Google Scholar]
- 232. Thurner S, Klimek P, Szell M, Duftschmid G, Endel G, Kautzky-Willer A, Kasper DC. Quantification of excess risk for diabetes for those born in times of hunger, in an entire population of a nation, across a century. Proc Natl Acad Sci USA. 2013;110(12):4703–4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Wang PX, Wang JJ, Lei YX, Xiao L, Luo ZC. Impact of fetal and infant exposure to the Chinese Great Famine on the risk of hypertension in adulthood. PLoS One. 2012;7(11):e49720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Sohi G, Revesz A, Ramkumar J, Hardy DB. Higher hepatic miR-29 expression in undernourished male rats during the postnatal period targets the long-term repression of IGF-1. Endocrinology. 2015;156(9):3069–3076. [DOI] [PubMed] [Google Scholar]
- 235. Donzeau A, Bouhours-Nouet N, Fauchard M, Decrequy A, Mathieu E, Boux de Casson F, Gascoin G, Coutant R. Birth weight is associated with the IGF-1 response to GH in children: programming of the anabolic action of GH? J Clin Endocrinol Metab. 2015;100(8):2972–2978. [DOI] [PubMed] [Google Scholar]
- 236. Fu Q, McKnight RA, Callaway CW, Yu X, Lane RH, Majnik AV. Intrauterine growth restriction disrupts developmental epigenetics around distal growth hormone response elements on the rat hepatic IGF-1 gene. FASEB J. 2015;29(4):1176–1184. [DOI] [PubMed] [Google Scholar]
- 237. Li C, Schlabritz-Loutsevitch NE, Hubbard GB, Han V, Nygard K, Cox LA, McDonald TJ, Nathanielsz PW. Effects of maternal global nutrient restriction on fetal baboon hepatic insulin-like growth factor system genes and gene products. Endocrinology. 2009;150(10):4634–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Reynolds CM, Li M, Gray C, Vickers MH. Early-life growth hormone treatment to offspring of undernourished mothers alters metabolic parameters in primary adipocytes in adulthood. Growth Factors. 2014;32(1):34–40. [DOI] [PubMed] [Google Scholar]
- 239. Tarry-Adkins JL, Ozanne SE. Nutrition in early life and age-associated diseases. Ageing Res Rev. 2017;39:96–105. [DOI] [PubMed] [Google Scholar]
- 240. Leunissen RW, Kerkhof GF, Stijnen T, Hokken-Koelega A. Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA. 2009;301(21):2234–2242. [DOI] [PubMed] [Google Scholar]
- 241. Zanelli SA, Rogol AD. Short children born small for gestational age outcomes in the era of growth hormone therapy. Growth Horm IGF Res. 2018;38:8–13. [DOI] [PubMed] [Google Scholar]
- 242. Clayton PE, Cianfarani S, Czernichow P, Johannsson G, Rapaport R, Rogol A. Management of the child born small for gestational age through to adulthood: a consensus statement of the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society. J Clin Endocrinol Metab. 2007;92(3):804–810. [DOI] [PubMed] [Google Scholar]
- 243. Gies I, Thomas M, Tenoutasse S, De Waele K, Lebrethon MC, Beckers D, Francois I, Maes M, Rooman R, de Beaufort C, Massa G, De Schepper J. Insulin sensitivity modulates the growth response during the first year of high-dose growth hormone treatment in short prepubertal children born small for gestational age. Horm Res Paediatr. 2012;78(1):24–30. [DOI] [PubMed] [Google Scholar]
- 244. Jensen RB, Thankamony A, Day F, Scott RA, Langenberg C, Kirk J, Donaldson M, Ivarsson SA, Söder O, Roche E, Hoey H, Juul A, Ong KK, Dunger DB. Genetic markers of insulin sensitivity and insulin secretion are associated with spontaneous postnatal growth and response to growth hormone treatment in short SGA children: the North European SGA Study (NESGAS). J Clin Endocrinol Metab. 2015;100(3):E503–E507. [DOI] [PubMed] [Google Scholar]
- 245. Jensen RB, Thankamony A, O’Connell SM, Salgin B, Kirk J, Donaldson M, Ivarsson SA, Söder O, Roche E, Hoey H, Dunger DB, Juul A; NESGAS Group . Baseline IGF-I levels determine insulin secretion and insulin sensitivity during the first year on growth hormone therapy in children born small for gestational age. Results from a North European Multicentre Study (NESGAS). Horm Res Paediatr. 2013;80(1):38–46. [DOI] [PubMed] [Google Scholar]
- 246. Woods KA, van Helvoirt M, Ong KK, Mohn A, Levy J, de Zegher F, Dunger DB. The somatotropic axis in short children born small for gestational age: relation to insulin resistance. Pediatr Res. 2002;51(1):76–80. [DOI] [PubMed] [Google Scholar]
- 247. Smeets CCJ, van der Steen M, Renes JS, Hokken-Koelega ACS. Bone mineral density after cessation of GH treatment in young adults born SGA: a 5-year longitudinal study. J Clin Endocrinol Metab. 2017;102(9):3508–3516. [DOI] [PubMed] [Google Scholar]
- 248. Quigley CA, Child CJ, Zimmermann AG, Rosenfeld RG, Robison LL, Blum WF. Mortality in children receiving growth hormone treatment of growth disorders: data from the Genetics and Neuroendocrinology of Short Stature International Study. J Clin Endocrinol Metab. 2017;102(9):3195–3205. [DOI] [PubMed] [Google Scholar]
- 249. Scratch SE, Anderson PJ, Doyle LW, Thompson DK, Ahmadzai ZM, Greaves RF, Inder TE, Hunt RW. High postnatal growth hormone levels are related to cognitive deficits in a group of children born very preterm. J Clin Endocrinol Metab. 2015;100(7):2709–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Abu Shehab M, Damerill I, Shen T, Rosario FJ, Nijland M, Nathanielsz PW, Kamat A, Jansson T, Gupta MB. Liver mTOR controls IGF-I bioavailability by regulation of protein kinase CK2 and IGFBP-1 phosphorylation in fetal growth restriction. Endocrinology. 2014;155(4):1327–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Ozanne SE, Fernandez-Twinn D, Hales CN. Fetal growth and adult diseases. Semin Perinatol. 2004;28(1):81–87. [DOI] [PubMed] [Google Scholar]
- 252. Felisbino-Mendes MS, Moreira AD, Velasquez-Melendez G. Association between maternal nutritional extremes and offspring mortality: a population-based cross-sectional study, Brazil, Demographic Health Survey 2006. Midwifery. 2015;31(9):897–903. [DOI] [PubMed] [Google Scholar]
- 253. Pereira IF, Andrade LM, Spyrides MH, Lyra CO. Nutritional status of children under 5 years of age in Brazil: evidence of nutritional epidemiological polarisation. Cien Saude Colet. 2017;22(10):3341–3352. [DOI] [PubMed] [Google Scholar]
- 254. Vergara M, Smith-Wheelock M, Harper JM, Sigler R, Miller RA. Hormone-treated Snell dwarf mice regain fertility but remain long lived and disease resistant. J Gerontol A Biol Sci Med Sci. 2004;59(12):1244–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Ashpole NM, Logan S, Yabluchanskiy A, Mitschelen MC, Yan H, Farley JA, Hodges EL, Ungvari Z, Csiszar A, Chen S, Georgescu C, Hubbard GB, Ikeno Y, Sonntag WE. IGF-1 has sexually dimorphic, pleiotropic, and time-dependent effects on healthspan, pathology, and lifespan. Geroscience. 2017;39(2):129–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Junnila RK, Duran-Ortiz S, Suer O, Sustarsic EG, Berryman DE, List EO, Kopchick JJ. Disruption of the GH receptor gene in adult mice increases maximal lifespan in females. Endocrinology. 2016;157(12):4502–4513. [DOI] [PubMed] [Google Scholar]
- 257. Ramsey MM, Ingram RL, Cashion AB, Ng AH, Cline JM, Parlow AF, Sonntag WE. Growth hormone-deficient dwarf animals are resistant to dimethylbenzanthracine (DMBA)-induced mammary carcinogenesis. Endocrinology. 2002;143(10):4139–4142. [DOI] [PubMed] [Google Scholar]
- 258. Thorner MO, Chapman IM, Gaylinn BD, Pezzoli SS, Hartman ML. Growth hormone-releasing hormone and growth hormone-releasing peptide as therapeutic agents to enhance growth hormone secretion in disease and aging. Recent Prog Horm Res. 1997;52:215–244. [PubMed] [Google Scholar]