Abstract
Background: Cancer patients receiving Western medical treatment, frequently seek Traditional Chinese Medicine (TCM) to alleviate adverse effects and prolong survival. Objective: This study evaluated the association between the use of TCM and cancer survival rate. Research into the effect of TCM on patient survival is limited, this analysis focused on 3 patterns of TCM use. Methods: Three retrospective cohorts with different patterns of TCM use were selected from the National Health Insurance Research Database of Taiwan and analyzed. Patients with newly diagnosed cancer between 1997 and 2012 were classified into groups of prediagnosis, postdiagnosis, and continuous TCM use associated with awareness of cancer diagnosis. All demographic and clinical data were analyzed. Results: After propensity score matching, longevity of the postdiagnosis and continuous TCM user was significantly longer than the non-TCM user. The adjusted hazard ratios of death in postdiagnosis and continuous TCM use groups (0.59 and 0.61, respectively) were lower than the non-TCM use group. Conclusion: The analysis suggests that cancer patients using TCM in conjunction with Western medical treatment exhibited a higher survival rate than patients not using TCM treatment.
Keywords: malignancy, Chinese herbal products, traditional Chinese medicine, mortality rate, propensity score, hazard ratio
Introduction
Incidences of all cancer types have been increasing on a global basis. Approximately 14 million newly diagnosed cases were recorded in 2012.1 Globally, the number of new cases of cancer is expected to rise about 70% over the next 2 decades. In Taiwan, there were more than 99 000 patients diagnosed with cancer in 2013; an increase of around 2500 cases from 2012. Cancer is the second leading cause of death in the world, where nearly 1 in 6 deaths is due to cancer. The World Health Organization estimated that 84 million people would die of cancer between 2005 and 2015.2
Successful cancer treatment remains a challenge, not only for improving the survival rates but also for improving the quality of life. Conventional treatments include surgery, chemotherapy, and radiotherapy. Surgical resection is the first-line therapy for most early-stage cancer, while adjuvant therapy such as chemotherapy or radiotherapy are often implemented in the advanced stages.3 Complementary or alternative medicine (CAM) is one of the few choices for cancer patients to improve quality of life, relieve adverse effects of conventional therapy, and to attempt extending survival time.4 The traditional Chinese medicine (TCM) practitioner focuses on changes in the symptoms in conjunction with knowledge of the clinical condition, rather than a single treatment plan or regimen to treat cancer patients. In Taiwan, many patients use TCM to alleviate symptoms of both the cancer as well as side effects of conventional treatments.5 Recent research indicated more than 60% of children with cancer and more than 10% of adult cancer patients in Taiwan used TCM for their complementary treatment.6,7
The current study accessed the National Health Insurance Research Database (NHIRD) to examine the differences of all-cause mortality between the TCM-use patients and non-TCM-use patients. The National Health Insurance (NHI) program provides insurance coverage to more than 99% of Taiwan’s population, and the NHIRD constitutes the usage information of TCM and Western medical treatment of the insured population. TCM therapy has been shown to alleviate the adverse symptoms of conventional treatments such as nausea, vomiting, and fatigue.8,9 Other studies have shown conflicting results with regard to the effect of complementary TCM treatment reducing mortality of cancer patients.10-14
The purpose of this research was to evaluate the effect of adjuvant TCM therapy on survival rate in cancer patients. As research into patient survival and TCM use before or after cancer remains limited, this analysis focused on 3 patterns of TCM use.
Methods
Data Sources
Data were sourced were from the NHIRD between 1997 and 2012. The datasets were provided by the Taiwan National Health Research Institute. The NHI is a compulsory health insurance program effective in Taiwan since 1995. TCM services, including herbal medicines, acupuncture, moxibustion, and chiropractic, have been covered by the NHI since 1996. The NHI has extended its coverage to virtually the entire population of Taiwan, ranging from 96.1% to 99.6% since its implementation.15 The database contains demographic variables, outpatient and inpatient visits, prescription details, and disease diagnoses based on the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM). All cancer patients in the NHI program are registered with catastrophic illness and may then access the NHI approved therapies. This study was approved by the Research Ethics Committee of Hualien Tzu-Chi Hospital in Taiwan (IRB101-98).
Sample Population and Exposure Assessment
Selection criteria for analysis consisted of all patients newly diagnosed with malignancy (ICD-9-CM code: 140-208) from 1997 to 2012. This was refined as patients diagnosed with malignant neoplasm of trachea, bronchus, and lung (ICD-9CM: 162), malignant neoplasm of liver and intrahepatic bile ducts (ICD-9-CM: 155, except patients of liver transplantation), and malignant neoplasm of colon, rectum, rectosigmoid junction, and anus (ICD-9-CM: 153-154).
The study defined TCM users based on patient frequency of TCM access. The TCM users are divided into 3 cohorts, including postdiagnosis (B0A1), prediagnosis (B1A0), and continuous (B1A1) TCM users. Cohort 1 consisted of a control group (B0A0, non-TCM users) and the B0A1 group, which represented patients who initiated TCM use more than or equal to 6 times post cancer diagnosis within the following 6 months. Individuals who used TCM less than 6 times, who used TCM only before the diagnosis, or initiated TCM 6 months post cancer diagnosis were excluded. Cohort 2 was composed of a control (B0A0) and the B1A0 group consisted of patients who used TCM more than or equal to 6 times within the 6 months prior to cancer diagnosis, but not during conventional cancer therapy. Individuals who used TCM less than 6 times or who used TCM only in the 6-month period prior to cancer diagnosis were excluded. Cohort 3 consisted of a control (B0A0) and the B1A1 group, which consisted of patients who used TCM more than or equal to 6 times not only in the 6-month period prior to diagnosis, but also after the cancer diagnosis. Individuals who used TCM less than 6 times, or no TCM in the 6 months prior to or post diagnosis were excluded.
To reduce potential confounding factors, non-TCM users were selected by propensity score matching for gender, age, Charlson Comorbidity Index (CCI), diabetes, hypertension, hyperlipidemia, insurance premium (represents income level), and conventional therapies including surgery, chemotherapy, and radiotherapy. To avoid the effect of the time gap between the initial cancer diagnosis and TCM treatment, the time from initial diagnosis to TCM treatment was matched between B0A1 and B0A0 in cohort 1. For instance, if the enrolled B0A1 patient used TCM treatment initially 30 days after cancer diagnosis, then the follow-up for a patient in the control group would also begin 30 days after cancer diagnosis. Based on the available sample size, cohort 1 (B0A1 vs B0A0) allowed for a 1:4 case-control ratio, while cohort 2 (B1A0 vs B0A0) and cohort 3 (B1A1 vs B0A0) had a ratio of 1:1. Individuals who had died within 90 days post cancer diagnosis were excluded.
Outcome Assessment and Confounding Variables
The Registry for Catastrophic Illness Patients Database identified primary outcome as mortality from the registry files. Patients were followed from the index date to the date of death, date of withdrawal from the NHI system, or December 31, 2012. The index date was defined as the date of cancer diagnosis, except for the B0A1 group. The index date of B0A1 was determined as the date patients incorporated TCM treatment. Confounding variables included gender, age, CCI, baseline comorbidities, and conventional cancer treatments (surgery, chemotherapy, and radiation therapy). Baseline comorbidities included diabetes mellitus (ICD-9-CM code: 250), hypertension (ICD-9-CM code: 401), and hyperlipidemia (ICD-9-CM code: 272). These diseases were determined by at least 2 outpatient visits or one inpatient visit at least 1 year prior to cancer diagnosis. Data regarding insurance premiums as a representative of economic status were also accessed.
Statistical Analysis
Demographic variables in conjunction with the cancer diagnosis between the cohorts were examined for appropriateness of comparison. The variables with a standard difference of >0.1 were deemed clinically meaningful. Hazard ratios (HRs) between treatment groups were estimated using Cox regression. The Kaplan-Meier method was used to assess the fraction of patients surviving 5-years postdiagnosis. The 2-tailed significance level was set at 0.01 in all statistical tests. All analyses were run using SAS statistical software version 9.3.
Results
Patient Data Selection
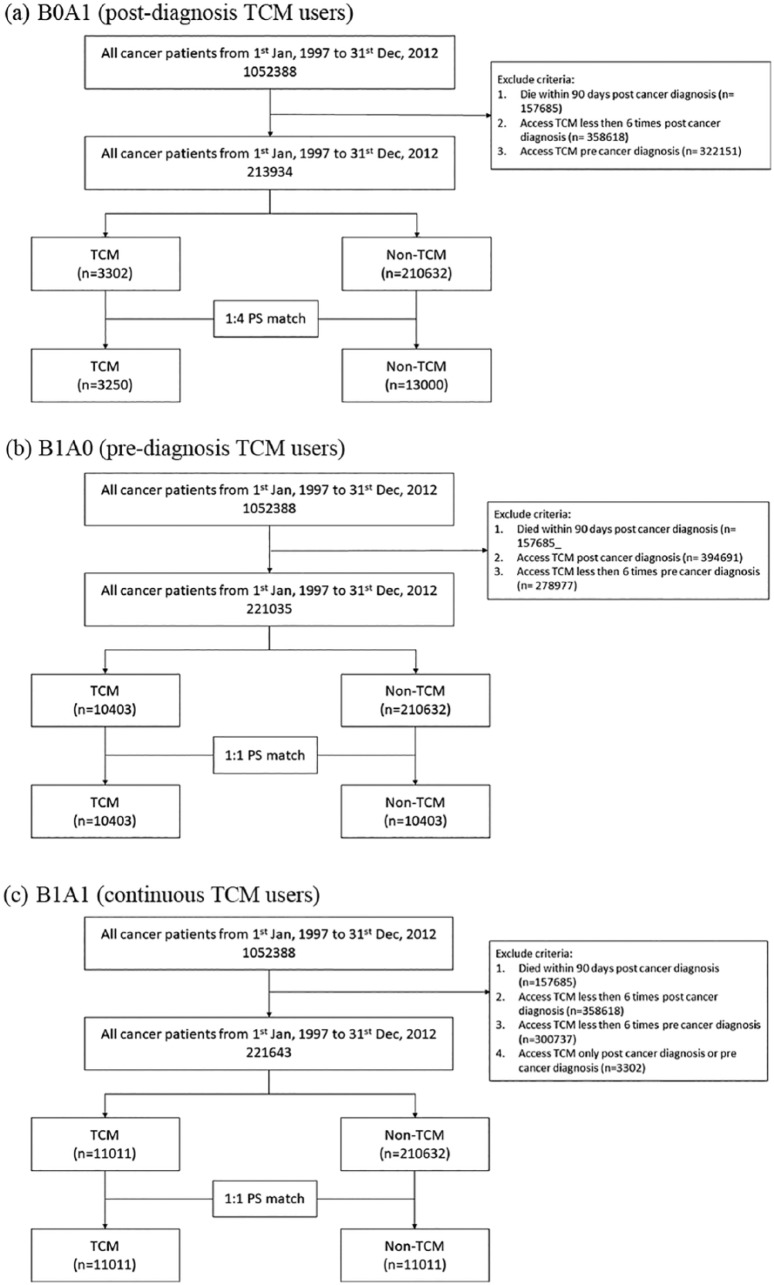
The flowchart in Figure 1 illustrates the differentiation criteria and process for categorizing the 3 cohorts. Cohort 1 (B0A1 vs B0A0), cohort 2 (B1A0 vs B0A0), and cohort 3 (B1A1 vs B0A0). Cohort 1 compared postdiagnosis TCM users (B0A1) with non-TCM (B0A0) users. Cohort 2 represents the comparison of prediagnosis TCM users (B1A0) with non-TCM users. Cohort 3 constitutes continuous TCM users (B1A1) with non-TCM users. One-to-4 matching was performed for cohort 1 due to the small sample size of the B0A1 group, whereas for cohorts 2 and 3, one-to-one matching was applied. After matching, 16 250 (3250:13 000), 20 806 (10 403:10 403), and 22 022 patients (11 011:11 011) constituted cohort 1, cohort 2, and cohort 3, respectively.
Figure 1.
Flowchart of study design: (a) postdiagnosis, (b) prediagnosis, (c) continuous traditional Chinese medicine (TCM) users.
Baseline Characteristics
The basic characteristics of cohort 1 (B0A1 vs B0A0), cohort 2 (B1A0 vs B0A0), and cohort 3 (B1A1 vs B0A0) are delineated as gender, age, CCI score, baseline comorbidities, and use of conventional treatments (Table 1).
Table 1.
Demographic Characteristics of All Cancer Patients by Pattern of TCM Treatment Use.
| Variables | TCM (B0A1) | Control (B0A0) | Standardized Difference | TCM (B1A0) | Control (B0A0) | Standardized Difference | TCM (B1A1) | Control (B0A0) | Standardized Difference |
|---|---|---|---|---|---|---|---|---|---|
| N | 3250 | 13 000 | 10 403 | 10 403 | 11 011 | 11 011 | |||
| Gender, n (%) | |||||||||
| Female | 1425 (43.8) | 5716 (44) | 0.00 | 4997 (48) | 5030 (48.4) | 0.01 | 6178 (56.1) | 6130 (55.7) | −0.01 |
| Male | 1825 (56.2) | 7284 (56) | 5406 (52) | 5373 (51.6) | 4833 (43.9) | 4881 (44.3) | |||
| Age (years), mean ± SD | 56.0 ± 15.0 | 55.7 ± 14.6 | 0.02 | 61.8 ± 15.0 | 61.9 ± 16.4 | 0.00 | 59.9 ± 14.1 | 59.8 ± 17.1 | 0.00 |
| ≤40, n (%) | 418 (12.9) | 1649 (12.7) | 0.00 | 827 (7.9) | 903 (8.7) | 0.05 | 904 (8.2) | 1268 (11.5) | 0.16 |
| Age group (years), n (%) | |||||||||
| 41-50 | 730 (22.5) | 3053 (23.5) | 1501 (14.4) | 1413 (13.6) | 1966 (17.9) | 1687 (15.3) | |||
| 51-60 | 768 (23.6) | 3247 (25) | 2080 (20) | 1988 (19.1) | 2505 (22.7) | 2258 (20.5) | |||
| 61-70 | 697 (21.4) | 2675 (20.6) | 2394 (23) | 2340 (22.5) | 2593 (23.5) | 2330 (21.2) | |||
| >70 | 637 (19.6) | 2376 (18.3) | 3601 (34.6) | 3759 (36.1) | 3043 (27.6) | 3468 (31.5) | |||
| CCI, mean ± SD | 5.9 ± 3.4 | 5.8 ± 3.4 | 0.03 | 7.6 ± 3.7 | 7.6 ± 3.7 | 0.00 | 6.0 ± 3.1 | 5.9 ± 3.3 | 0.01 |
| Diabetes mellitus, n (%) | 749 (23) | 2930 (22.5) | 0.01 | 2601 (25) | 2553 (24.5) | 0.01 | 3423 (31.1) | 3381 (30.7) | 0.01 |
| Hypertension, n (%) | 1340 (41.2) | 5295 (40.7) | 0.01 | 5049 (48.5) | 5043 (48.5) | 0.00 | 6043 (54.9) | 6111 (55.5) | −0.01 |
| Hyperlipidemia, n (%) | 772 (23.8) | 2988 (23) | 0.02 | 2632 (25.3) | 2622 (25.2) | 0.00 | 4168 (37.9) | 4125 (37.5) | 0.01 |
| Chemotherapy, n (%) | 1452 (44.7) | 5811 (44.7) | 0.00 | 4494 (43.2) | 4483 (43.1) | 0.00 | 3057 (27.8) | 3029 (27.5) | 0.01 |
| Radiation therapy, n (%) | 886 (27.3) | 3567 (27.4) | 0.00 | 2766 (26.6) | 2674 (25.7) | 0.02 | 1983 (18) | 1951 (17.7) | 0.01 |
| Insurance premium, n (%) | |||||||||
| Dependent | 855 (26.3) | 3054 (23.5) | 0.13 | 3261 (31.3) | 3087 (29.7) | 0.08 | 3210 (29.2) | 3058 (27.8) | 0.04 |
| 0-19 100 | 759 (23.4) | 3448 (26.5) | 2314 (22.2) | 2656 (25.5) | 2466 (22.4) | 2586 (23.5) | |||
| 19 100-42 000 | 1350 (41.5) | 5690 (43.8) | 4407 (42.4) | 4247 (40.8) | 4697 (42.7) | 4775 (43.4) | |||
| >42 000 | 286 (8.8) | 808 (6.2) | 421 (4) | 413 (4) | 638 (5.8) | 592 (5.4) | |||
| Days from initial diagnosis to treatment, mean ± SD | 51.4 ± 38.1 | 51.6 ± 38.1 | −0.01 | ||||||
| Median survival (years) | NAa | NAa | 1.9 | 3.4 | NAa | NAa | |||
Abbreviations: TCM, traditional Chinese medicine; CCI, Charlson Comorbidity Index; NA, not available.
Not available because the corresponding survival probability was greater than 50% during the whole 5-year observation period.
The mean days from initial diagnosis of cancer to TCM treatment was 51 days in cohort 1. The age distribution, gender, comorbidities, CCI, and the percentage of conventional treatments received were not clinically meaningful between the postdiagnosis TCM user (B0A1) and non-TCM user (B0A0) groups. However, the insurance premium of cohort 1 showed a slight difference (standard difference = 0.13), and a higher male/female ratio with a greater age distribution in the 40+ years range.
In cohort 2, no clinically meaningful differences were observed between the prediagnosis TCM users (B1A0) and the control, regardless of age, gender, comorbidities, insurance premium, or the percentage use of conventional therapies. Similar to cohort 1, there was a greater male to female ratio, and an age distribution where slightly more than 90% of patients were in the 40+ years range. The median survival time was 1.9 years for prediagnosis TCM users (B1A0), and 3.4 years for non-TCM users.
In cohort 3, TCM users (B1A1) and controls (B0A0) showed similar characteristics as cohorts 1 and 2. Slightly more than 90% of patients were in the 40+ years age range. However, a larger portion of female to male patients was observed.
Cancer Type
Survival rates are highly dependent on the type of cancer. The 10 most common types of cancer in Taiwan account for 3 quarters of the mortality rate (Table 2.) In B0A1 group, the frequency by type was 10.9% lung cancer, 11% liver cancer, 13.4% colorectal cancer, 15.4% breast cancer, and 11.9% oral cancer in B0A1 group. In B1A0 group, lung cancer was 18.5%, liver cancer 13.8%, colorectal cancer 13.3%, breast cancer 7.9%, and oral cancer 5.1% in frequency. As well as in B1A1 group, the frequencies were 8.2% lung cancer, 12.9% liver cancer, 12.7% colorectal cancer, 16.9% breast cancer, and 6.4% oral cancer.
Table 2.
The 10 Leading Causes of Cancer Death in Taiwan.a
| Variables | TCM (B0A1) | Control (B0A0) | TCM (B1A0) | Control (B0A0) | TCM (B1A1) | Control (B0A0) |
|---|---|---|---|---|---|---|
| Cancers of trachea, bronchus, and lung | 355 (10.9) | 1523 (11.7) | 1928 (18.5) | 1466 (14.1) | 903 (8.2) | 1028 (9.3) |
| Cancers of liver and intrahepatic bile ducts | 357 (11) | 1815 (14) | 1433 (13.8) | 1163 (11.2) | 1422 (12.9) | 1247 (11.3) |
| Cancers of colon, rectum, and anus | 435 (13.4) | 1366 (10.5) | 1383 (13.3) | 1665 (16) | 1403 (12.7) | 1630 (14.8) |
| Cancer of breast (female) | 502 (15.4) | 1587 (12.2) | 822 (7.9) | 1014 (9.7) | 1861 (16.9) | 1316 (12) |
| Cancer of oral cavity | 386 (11.9) | 1409 (10.8) | 528 (5.1) | 842 (8.1) | 700 (6.4) | 921 (8.4) |
| Cancer of prostate | 104 (3.2) | 243 (1.9) | 325 (3.1) | 328 (3.2) | 695 (6.3) | 403 (3.7) |
| Cancer of stomach | 173 (5.3) | 634 (4.9) | 566 (5.4) | 661 (6.4) | 308 (2.8) | 542 (4.9) |
| Cancer of pancreas | 52 (1.6) | 222 (1.7) | 273 (2.6) | 185 (1.8) | 56 (0.5) | 149 (1.4) |
| Cancer of esophagus | 57 (1.8) | 439 (3.4) | 230 (2.2) | 260 (2.5) | 71 (0.6) | 181 (1.6) |
| Cancer of ovary | 64 (2) | 225 (1.7) | 146 (1.4) | 177 (1.7) | 203 (1.8) | 188 (1.7) |
| Others | 765 (23.5) | 3537 (27.2) | 2769 (26.6) | 2642 (25.4) | 3389 (30.8) | 3406 (30.9) |
Abbreviation: TCM, traditional Chinese medicine.
Data are presented as number (percentage).
All-Cause Death Incidence
In cohort 1, there were 1105 deaths per 10 725 person-years in the postdiagnosis TCM user (B0A1) group; meaning the incidence rate was 103 cases per 1000 person-years. The control had a total 4297 deaths per 43 515 person-years, resulting in an incidence rate of 98.7 cases per 1000 person-years. The incidence ratio was 1.0 and exhibited no statistically significant different incidence of mortality in post–cancer diagnosis TCM users (B0A1) to non-TCM users (Table 3).
Table 3.
Incidence (1000 Person-Years) and Incidence Ratio of Mortality for Cancer Patients With and Without TCM Treatment.
| Comparison | TCM |
Control (B0A0) |
Ratioc (99% CI) | ||||
|---|---|---|---|---|---|---|---|
| PY | Eventa | Rateb (99% CI) | PY | Eventa | Rateb (99% CI) | ||
| B0A1 vs B0A0 | 10 725.0 | 1105 | 103.0 (95.0–111.0) | 43 515.0 | 4297 | 98.7 (94.9–102.6) | 1.0 (1.0–1.1) |
| B1A0 vs B0A0 | 24 145.0 | 5574 | 230.9 (222.9–238.8) | 28 645.0 | 4674 | 163.2 (157.0–169.3) | 1.4 (1.3–1.5)d |
| B1A1 vs B0A0 | 40 895.0 | 2832 | 69.3 (65.9–72.6) | 3602.0 | 3807 | 105.7 (202.3–1110.1) | 0.7 (0.6–0.7)d |
Abbreviations: TCM, traditional Chinese medicine; PY, total person-years.
Event indicates number of events.
Rate indicates incidence.
Ratio indicates incidence ratio.
Statistically significant at P < .01.
In cohort 2, a total 5574 deaths occurred in the prediagnosis TCM users (B1A0), and the incidence rate was 230.9 cases per 1000 person-years. The incidence ratio of 1.4 demonstrated a statistically significant higher incidence of mortality in prediagnosis TCM users (B1A0) compared with the controls.
In the continuous TCM users (B1A1) of the third cohort, the incidence rate was 69.3 per 1000 person-years, and the incidence rate of the control group was 163.2 per 1000 person-years. The incidence ratio of 0.7 exhibits a lower incidence of mortality in continuous TCM users (B1A1), and was statistically significant compared with the controls.
Hazard Ratio and 5-Year Survival
A Cox proportional hazard model was used to adjust the patient covariate variable, and the HRs for mortality are shown in Table 4. Compared with non-TCM users, postdiagnosis patients (B0A1) and continuous (B1A1) TCM users showed a lower risk of death, 0.59 (HR 99% confidence interval (CI) = 0.54-0.65, P < .001) and 0.61 (HR 99% CI = 0.58-0.66, P < .001), respectively. Pre–cancer diagnosis TCM users (B1A0) showed a high risk of death at 1.68 (HR 99% CI = 1.60-1.72, P < .001). Moreover, advanced age, male gender, and high CCI score were significantly related to an increased risk of death from cancer.
Table 4.
HR for All Causes Mortality in Patients With Cancer Receiving TCM Treatment in the Stratification of After (B0A1), Before (B1A0), and Continuous (B1A1) Use Pattern.
| Variables | B0A1 |
B1A0 |
B1A1 |
|||
|---|---|---|---|---|---|---|
| HR (99% CI) | P | HR (99% CI) | P | HR (99% CI) | P | |
| Group | 0.59 (0.54–0.65) | <.001 | 1.68 (1.60–1.72) | <.001 | 0.61 (0.58–0.66) | <.001 |
| Age, years | 1.01 (1.01–1.02) | <.001 | 1.01 (1.01–1.02) | <.001 | 1.02 (1.01–1.02) | <.001 |
| Gender | 1.87 (1.73–2.01) | <.001 | 1.27 (1.21–1.34) | <.001 | 1.52 (1.43–1.63) | <.001 |
| CCI | 1.14 (1.13–1.15) | <.001 | 1.11 (1.10–1.12) | <.001 | 1.17 (1.15–1.18) | <.001 |
Abbreviations: HR, hazard ratio; TCM, traditional Chinese medicine; CCI, Charlson Comorbidity Index; CI, confidence interval.
The Kaplan-Meier survival curves for both the postdiagnosis TCM users (B0A1, Figure 2a), cohort 1, and the continuous TCM use group (B1A1, Figure 2c), cohort 3 exhibits better survival curves than controls without the use of TCM (log-rank test: P < .05). The 5-year survival probabilities for cohort 1 were 60% for postdiagnosis TCM users (B0A1) and 52% for the control. For cohort 3, the 5-year survival probabilities were 67% for continuous TCM users (B1A1) and 57% for non-TCM users. However, compared with the non-TCM users, the prediagnosis TCM users (B1A0, Figure 2), cohort 2 exhibited a lower survival estimate, for those who ceased TCM use after the cancer diagnosis. The 5-year survival probability of the prediagnosis TCM users (B1A0) was 26%, and 44% for the control.
Figure 2.
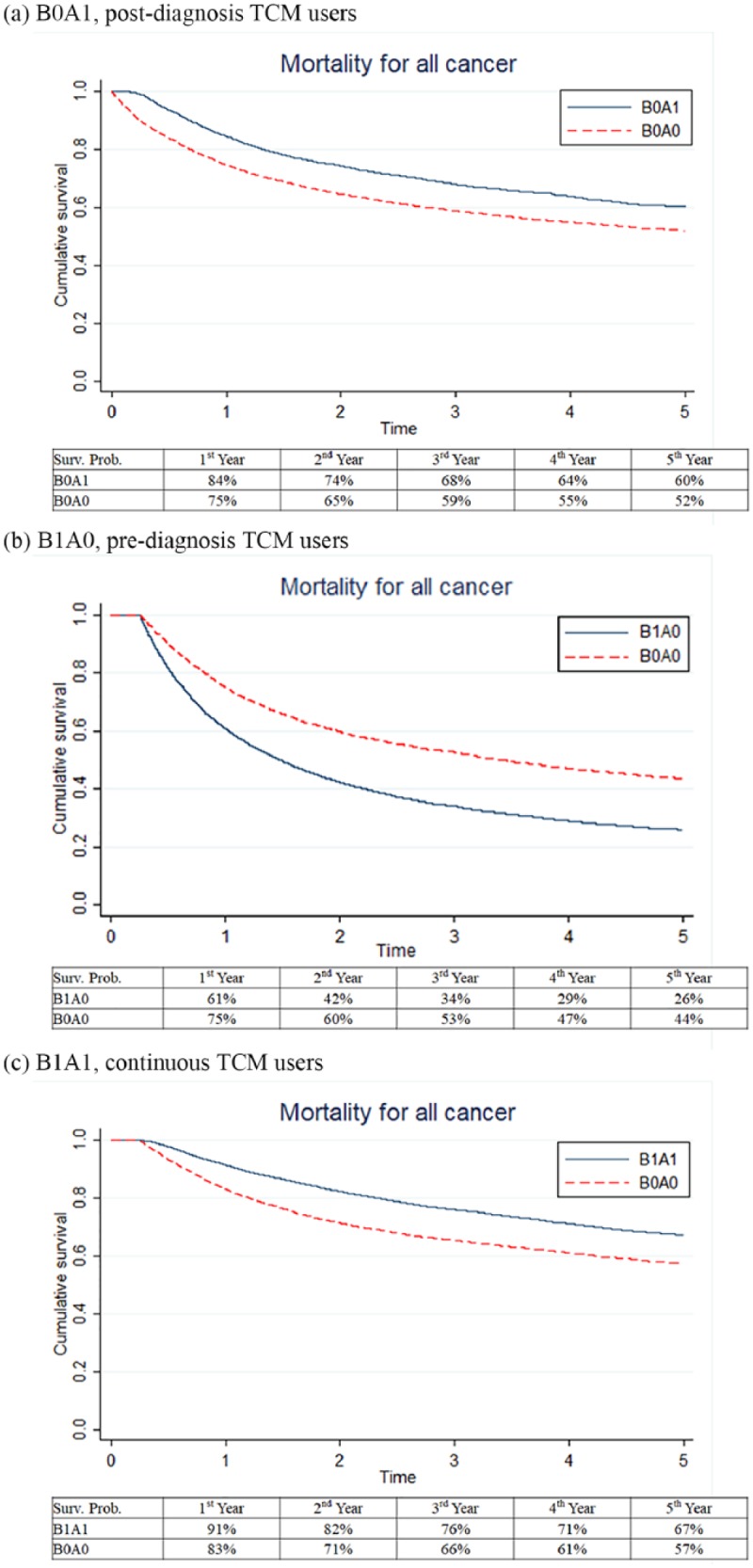
Kaplan-Meier survival curves for cancer patients receiving traditional Chinese medicine (TCM) treatment use stratified by use pattern: (a) postdiagnosis, (b) prediagnosis, (c) continuous TCM users.
Subgroup Analysis
To further investigate the effect of TCM on patients, 3 cancer types were selected to conduct subgroup analysis. Cancer of the trachea, bronchus, and lung (lung cancer); cancer of the liver and intrahepatic bile ducts (liver cancer); and cancer of the colon, rectum, and anus (colorectal cancer) were chosen as these were the 3 leading causes of cancer death in Taiwan in 2016. Propensity score matching was performed with 1:4 case-control ratio selected for the B0A1 group, and 1:1 ratio for both the B1A0 and the B1A1 groups.
Among lung cancer patients, there were 284, 1842, and 784 cases using TCM enrolled in the B0A1, the B1A0, and the B1A1 groups, respectively (Table 5). The HR of B0A1 was 0.70 (99% CI = 0.56-0.87, P < .001), HR of B1A1 was 0.78 (99% CI = 0.66-0.94, P < .001), and an increased risk of death from B1A0 (HR = 1.31, 99% CI = 1.18-1.45, P < .001) group after adjusting for covariates. For liver cancer patients, risk of death was lower in both B0A1 (HR = 0.73, 99% CI = 0.59-0.92, P < .001) and B1A1 (HR = 0.56, 99% CI = 0.48-0.65, P < .001) groups, and higher in B1A0 (HR = 1.49, 99% CI = 1.32-1.70, P < .001). The results of the colorectal cancer subgroup analysis showed reduced risk of death in cohorts 1 and 3 (B0A1 [HR = 0.83, 99% CI = 0.65-1.07, P = .057], B1A1 [HR = 0.77, 99% CI = 0.64-0.94, P < .001]), but a higher risk in cohort 2, B1A0 (HR = 1.44, 99% CI = 1.23-1.69, P < .001).
Table 5.
Subgroup Analysis for HR of Death in Patients With Different Types of Cancer Receiving TCM Treatment.
| Type of Cancer | B0A1 |
B1A0 |
B1A1 |
|||
|---|---|---|---|---|---|---|
| TCM:Control | HR (99% CI) | TCM:Control | HR (99% CI) | TCM:Control | HR (99% CI) | |
| Lung cancer | 284:1136 | 0.70 (0.56–0.87)a | 1842:1842 | 1.31 (1.18–1.45)a | 784:784 | 0.78 (0.66–0.94)a |
| Liver cancer | 309:1236 | 0.73 (0.59–0.92)a | 1378:1378 | 1.49 (1.32–1.70)a | 1296:1296 | 0.56 (0.48–0.65)a |
| Colorectal cancer | 391:1564 | 0.83 (0.65–1.07) | 1315:1315 | 1.44 (1.23–1.69)a | 1299:1299 | 0.77 (0.64–0.94)a |
Abbreviations: HR, hazard ratio; TCM, traditional Chinese medicine; CI, confidence interval.
Statistically significant at P < .01.
Discussion
The use of CAM for cancer is widespread and the interest in pursuing this form of adjunct therapy has increased globally from 25% to almost 50%.16 In the United States, 10% to more than 60% of cancer patients have used CAM.17 In Canada, more than 80% of all women with breast cancer use CAM.18 In Germany, 50.1% of breast cancer patients, and 44% of gynecological cancer patients used CAM.19
TCM modalities in conjunction with standard western medical practice include acupuncture and herbalism. TCM has long been accepted in Taiwan and covered by the NHI program since 1996. Previous research demonstrated that cancer patients use TCM as their preferred CAM therapy in Taiwan.7,20-23 Therefore, the TCM therapy has been the most accepted and dominant option among cancer patients who received CAM treatment.
The effect of TCM treatment on patient survival of various cancer types has been reported for gastric cancer,10 liver cancer,22 head and neck cancer,23 breast cancer,24 lung cancer,12,25,26 prostate cancer,11 myeloid leukemia,21 and pancreatic cancer.27 The literature to date supports the effectiveness of TCM for alleviating chemotherapy, radiotherapy, and surgery-induced side effects. However, there is limited evidence to suggest TCM efficacy as a treatment for cancer.13,14,28 The present research suggests that TCM treatment is positively associated with increased cancer survival rates. Multiple mechanisms of action are associated with TCM in cancer treatment, including (1) immunomodulation,29,30 (2) altered inflammation response,31,32 (3) angiogenesis suppression,33-35 and (4) microenvironment modification.36 A significant proportion of TCM research focuses on botanical preparations; however, TCM is often a combination of techniques that have been shown to alter immune response and anti-inflammatory effect.37-40 Due to the multiplicity of interventions available within TCM, this study focused on botanical/herbal medication and acupuncture.
The usage pattern of TCM and its association with survival rates has not previously been investigated. This is the first nationwide cohort study to assess the association between survival rate of cancer patients and TCM usage pattern. This retrospective cancer study is a population-based TCM pharmacoepidemiological analysis: the primary finding is that postdiagnosis and continuous TCM treatment use are associated with a significant decrease in HR of all cancer types. This finding remained true even with the exclusion of individuals expiring within 90 days post cancer diagnosis. The crude HRs were statistically significant, and remained consistent after adjusting for age, gender, comorbidities, monthly income, and use of conventional therapy. Patients from TCM-use groups of the B0A1 (postdiagnosis use) and the B1A1 (continuous use) exhibited a lower HR when compared with non-TCM users within a 5-year follow-up period.
Of particular interest is the B1A0 (prediagnosis use) group, which exhibited a higher risk of mortality than the control group. This may be due to delayed diagnosis in the B1A0 group, or an artefact of poor compliance to follow-up. Consistent outpatient TCM treatments are crucial for TCM treatment to be effective, as such, loss of follow-up is a plausible reason for the increased mortality. The potential for positive effect via TCM intervention is dependent on the duration of the therapy and skill of the practitioner. While TCM is not a proven curative therapy for any cancers, the extended therapy may potentiate a positive outcome due to placebo effect. Late-stage colon cancer patients reported protective effects of TCM41; however, no sustained effect on survival was noted. Cancer prevention using TCM is supported by multiple fields of research including: cell studies, animal studies, and clinical trials.42,43 Botanical extracts, herbal preparations, multiple antioxidant sources, including regular ginseng ingestion prior to cancer diagnosis was associated with significantly improved overall and disease-free survival.44 The literature is vast with studies demonstrating the positive effect of TCM treatment. TCM appears to have a significant impact in the prevention of relapse, metastasis, radiation injury prevention, chemotherapy-induced adverse effects, and other negative pharmacologically induced side effects.45 The chemical complexity of TCM preparations must not be underestimated, as the compounds encompassing TCM prescriptions, in conjunction with acupuncture remains largely unknown. The current research suggests increased longevity for patients that accessed TCM prior to cancer diagnosis and continued in conjunction with western medical interventions. A similar positive effect exists for postdiagnosis TCM treatment and is comparable to findings in other studies.7,21,25 This analysis of the national cancer data suggests TCM treatment is a viable therapy for reducing mortality of cancer patients. Based on the current analysis, and subgroup investigation, TCM appears to be more effective for treating specific cancers. The data implies that TCM shows the greatest efficacy for treating liver cancer, followed by lung cancer and colorectal malignancy. TCM enhanced survival in liver, lung, and colorectal malignancy patients for both postdiagnosis and continuous TCM use groups. A similar effect was found in all-cancer analysis. This study showed that patients undergoing chemotherapy or radiotherapy were at higher risk of mortality, which is in agreement with other research. In general, patients receiving either of these treatment modalities will exhibit advanced disease stages. Patients with stage III-IV cancer undergoing chemotherapy are at greater risk of suffering adverse effects due to immunosuppression. Opportunistic infections as a result of cancer treatment, and subsequent side effects may contribute to cancer progression and mortality.46-48 Notwithstanding the adverse effects of conventional treatment, the higher mortality rate may be associated with advanced stages of disease at the time of diagnosis. This study found that a higher CCI corresponded with higher risk of mortality. By contrast, hypertension and hyperlipidemia showed a lower risk of death. Based on the NHI database, patients with poor health status, regular exercise, or chronic disease were more likely to access TCM clinics.49 The TCM treatment of comorbidities may explain the more frequent access to TCM clinical services and its beneficial effects.
This research is based on the NHIRD data from the NHI program, which covers over 99% of the Taiwanese population. This ensures that the study is representative of the general population of Taiwan. Unfortunately, several limitations exist, which may account for some of the conflicting results. Some datasets were not available, or were nonexistent, so that potential confounding variables remain unknown. Because of access restrictions to the NHI database or limited data, variables including performance status of patients, biochemical data, and cancer clinical stage, were not available. Patient medication compliance data were not accessible.
Conclusions
TCM adjuvant treatment appears to benefit cancer patients of all ages. Longevity and survival rates increased for all cancer types, regardless of the stage of cancer or Western medical intervention. The current study provides a strong basis for further research into the biochemical mechanisms of TCM therapy for cancer patients
Footnotes
Author Contributions: Shao-Yi Lu and Tsung-Cheng Hsieh had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design: Shao-Yi Lu, Tsung-Cheng Hsieh
Acquisition of data: Jiann-I Pan, Zi-Xuan Fu, Jung-Lun Wu, Tsung-Cheng Hsieh
Analysis and interpretation of data: Shao-Yi Lu, Jain-Jung Chen, Tsung-Cheng Hsieh
Statistical analysis of data: Jung-Lun Wu, Tsung-Cheng Hsieh
Drafting the article: Shao-Yi Lu
Revising the article critically for important intellectual content: Shao-Yi Lu and Tsung-Cheng Hsieh.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012: Cancer Incidence and Mortality Worldwide in 2012. IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer; 2012. [Google Scholar]
- 2. Danhier F, Feron O, Preat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148:135-146. [DOI] [PubMed] [Google Scholar]
- 3. Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654-2664. [DOI] [PubMed] [Google Scholar]
- 4. McCall MD, Graham PJ, Bathe OF. Quality of life: a critical outcome for all surgical treatments of gastric cancer. World J Gastroenterol. 2016;22:1101-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pu CY, Lan VM, Lan CF, Lang HC. The determinants of traditional Chinese medicine and acupuncture utilization for cancer patients with simultaneous conventional treatment. Eur J Cancer Care (Engl). 2008;17:340-349. [DOI] [PubMed] [Google Scholar]
- 6. Yen HR, Lai WY, Muo CH, Sun MF. Characteristics of Traditional Chinese medicine use in pediatric cancer patients: a nationwide, retrospective, Taiwanese-Registry, population-based study. Integr Cancer Ther. 2017;16:147-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kuo YT, Chang TT, Muo CH, et al. Use of complementary traditional Chinese medicines by adult cancer patients in Taiwan: a nationwide population-based study. Integr Cancer Ther. 2018;17:531-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garcia MK, McQuade J, Haddad R, et al. Systematic review of acupuncture in cancer care: a synthesis of the evidence. J Clin Oncol. 2013;31:952-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu SH, Cheng YC. Old formula, new Rx: the journey of PHY906 as cancer adjuvant therapy. J Ethnopharmacol. 2012;140:614-623. [DOI] [PubMed] [Google Scholar]
- 10. Hung KF, Hsu CP, Chiang JH, et al. Complementary Chinese herbal medicine therapy improves survival of patients with gastric cancer in Taiwan: a nationwide retrospective matched-cohort study. J Ethnopharmacol. 2017;199:168-174. [DOI] [PubMed] [Google Scholar]
- 11. Liu JM, Lin PH, Hsu RJ, et al. Complementary traditional Chinese medicine therapy improves survival in patients with metastatic prostate cancer. Medicine (Baltimore). 2016;95:e4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shen HS, Wen SH. Effect of early use of Chinese herbal products on mortality rate in patients with lung cancer. J Ethnopharmacol. 2018;211:1-8. [DOI] [PubMed] [Google Scholar]
- 13. Chen X, Deng L, Jiang X, Wu T. Chinese herbal medicine for oesophageal cancer. Cochrane Database Syst Rev. 2016;(1):CD004520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taixiang W, Munro AJ, Guanjian L. Chinese medical herbs for chemotherapy side effects in colorectal cancer patients. Cochrane Database Syst Rev. 2005;(1):CD004540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin GM, Chen YJ, Kuo DJ, et al. Cancer incidence in patients with schizophrenia or bipolar disorder: a nationwide population-based study in Taiwan, 1997-2009. Schizophr Bull. 2013;39:407-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Horneber M, Bueschel G, Dennert G, Less D, Ritter E, Zwahlen M. How many cancer patients use complementary and alternative medicine: a systematic review and metaanalysis. Integr Cancer Ther. 2012;11:187-203. [DOI] [PubMed] [Google Scholar]
- 17. Cassileth BR, Deng G. Complementary and alternative therapies for cancer. Oncologist. 2004;9:80-89. [DOI] [PubMed] [Google Scholar]
- 18. Boon HS, Olatunde F, Zick SM. Trends in complementary/alternative medicine use by breast cancer survivors: comparing survey data from 1998 and 2005. BMC Womens Health. 2007;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fasching PA, Thiel F, Nicolaisen-Murmann K, et al. Association of complementary methods with quality of life and life satisfaction in patients with gynecologic and breast malignancies. Support Care Cancer. 2007;15:1277-1284. [DOI] [PubMed] [Google Scholar]
- 20. Fleischer T, Chang TT, Chiang JH, Hsieh CY, Sun MF, Yen HR. Integration of Chinese herbal medicine therapy improves survival of patients with chronic lymphocytic leukemia: a nationwide population-based cohort study. Medicine (Baltimore). 2016;95:e3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fleischer T, Chang TT, Chiang JH, Sun MF, Yen HR. Improved survival with integration of Chinese herbal medicine therapy in patients with acute myeloid leukemia: a nationwide population-based cohort study. Integr Cancer Ther. 2017;16:156-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liao YH, Lin CC, Lai HC, Chiang JH, Lin JG, Li TC. Adjunctive traditional Chinese medicine therapy improves survival of liver cancer patients. Liver Int. 2015;35:2595-2602. [DOI] [PubMed] [Google Scholar]
- 23. Lin HC, Lin CL, Huang WY, et al. The use of adjunctive traditional Chinese medicine therapy and survival outcome in patients with head and neck cancer: a nationwide population-based cohort study. QJM. 2015;108:959-965. [DOI] [PubMed] [Google Scholar]
- 24. Lee YW, Chen TL, Shih YR, et al. Adjunctive traditional Chinese medicine therapy improves survival in patients with advanced breast cancer: a population-based study. Cancer. 2014;120:1338-1344. [DOI] [PubMed] [Google Scholar]
- 25. Li TM, Yu YH, Tsai FJ, et al. Characteristics of Chinese herbal medicine usage and its effect on survival of lung cancer patients in Taiwan. J Ethnopharmacol. 2018;213:92-100. [DOI] [PubMed] [Google Scholar]
- 26. Liao YH, Li CI, Lin CC, Lin JG, Chiang JH, Li TC. Traditional Chinese medicine as adjunctive therapy improves the long-term survival of lung cancer patients. J Cancer Res Clin Oncol. 2017;143:2425-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang X, Hao J, Zhu CH, et al. Survival benefits of Western and traditional Chinese medicine treatment for patients with pancreatic cancer. Medicine (Baltimore). 2015;94:e1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang M, Liu X, Li J, He L, Tripathy D. Chinese medicinal herbs to treat the side-effects of chemotherapy in breast cancer patients. Cochrane Database Syst Rev. 2007;(2):CD004921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yoshida Y, Wang MQ, Liu JN, Shan BE, Yamashita U. Immunomodulating activity of Chinese medicinal herbs and Oldenlandia diffusa in particular. Int J Immunopharmacol. 1997;19:359-370. [DOI] [PubMed] [Google Scholar]
- 30. Ayeka PA, Bian Y, Mwitari PG, et al. Immunomodulatory and anticancer potential of Gan cao (Glycyrrhiza uralensis Fisch.) polysaccharides by CT-26 colon carcinoma cell growth inhibition and cytokine IL-7 upregulation in vitro. BMC Complement Altern Med. 2016;16:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ye L, Jia Y, Ji KE, et al. Traditional Chinese medicine in the prevention and treatment of cancer and cancer metastasis. Oncol Lett. 2015;10:1240-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hofseth LJ, Wargovich MJ. Inflammation, cancer, and targets of ginseng. J Nutr. 2007;137(1 suppl):183S-185S. [DOI] [PubMed] [Google Scholar]
- 33. Jiang WG, Ye L, Ji K, et al. Antitumour effects of Yangzheng Xiaoji in human osteosarcoma: the pivotal role of focal adhesion kinase signalling. Oncol Rep. 2013;30:1405-1413. [DOI] [PubMed] [Google Scholar]
- 34. Ye L, Ji K, Frewer N, Ji J, Jiang WG. Impact of Yangzheng Xiaoji on the adhesion and migration of human cancer cells: the role of the AKT signalling pathway. Anticancer Res. 2012;32:2537-2543. [PubMed] [Google Scholar]
- 35. Jiang WG, Ye L, Ji K, Frewer N, Ji J, Mason MD. Inhibitory effects of Yangzheng Xiaoji on angiogenesis and the role of the focal adhesion kinase pathway. Int J Oncol. 2012;41:1635-1642. [DOI] [PubMed] [Google Scholar]
- 36. Nie J, Zhao C, Deng LI, et al. Efficacy of traditional Chinese medicine in treating cancer. Biomed Rep. 2016;4:3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brustin R, Toledano M, Geffen T, et al. Immune modulation and treatment of human papilloma virus-related warts with energetics of living systems acupuncture. Med Acupunct. 2017;29:145-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kavoussi B, Ross BE. The neuroimmune basis of anti-inflammatory acupuncture. Integr Cancer Ther. 2007;6:251-257. [DOI] [PubMed] [Google Scholar]
- 39. Mori H, Nishijo K, Kawamura H, Abo T. Unique immunomodulation by electro-acupuncture in humans possibly via stimulation of the autonomic nervous system. Neurosci Lett. 2002;320:21-24. [DOI] [PubMed] [Google Scholar]
- 40. Wang Z, Yi T, Long M, Ding F, Ouyang L, Chen Z. Involvement of the negative feedback of IL-33 signaling in the anti-inflammatory effect of electro-acupuncture on allergic contact dermatitis via targeting microRNA-155 in mast cells. Inflammation. 2018;41:859-869. [DOI] [PubMed] [Google Scholar]
- 41. Lin TH, Yen HR, Chiang JH, Sun MF, Chang HH, Huang ST. The use of Chinese herbal medicine as an adjuvant therapy to reduce incidence of chronic hepatitis in colon cancer patients: a Taiwanese population-based cohort study. J Ethnopharmacol. 2017;202:225-233. [DOI] [PubMed] [Google Scholar]
- 42. Hu B, Wang SS, Du Q. Traditional Chinese medicine for prevention and treatment of hepatocarcinoma: from bench to bedside. World J Hepatol. 2015;7:1209-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu J, Wang S, Zhang Y, Fan HT, Lin HS. Traditional Chinese medicine and cancer: history, present situation, and development. Thorac Cancer. 2015;6:561-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cui Y, Shu XO, Gao YT, Cai H, Tao MH, Zheng W. Association of ginseng use with survival and quality of life among breast cancer patients. Am J Epidemiol. 2006;163:645-653. [DOI] [PubMed] [Google Scholar]
- 45. Li X, Yang G, Li X, et al. Traditional Chinese medicine in cancer care: a review of controlled clinical studies published in Chinese. PLoS One. 2013;8:e60338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kishida Y, Kawahara M, Teramukai S, et al. Chemotherapy-induced neutropenia as a prognostic factor in advanced non-small-cell lung cancer: results from Japan Multinational Trial Organization LC00-03. Br J Cancer. 2009;101:1537-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Whiteside TL. Immune suppression in cancer: effects on immune cells, mechanisms and future therapeutic intervention. Semin Cancer Biol. 2006;16:3-15. [DOI] [PubMed] [Google Scholar]
- 48. Lyman GH, Michels SL, Reynolds MW, Barron R, Tomic KS, Yu J. Risk of mortality in patients with cancer who experience febrile neutropenia. Cancer. 2010;116:5555-5563. [DOI] [PubMed] [Google Scholar]
- 49. Shih CC, Lin JG, Liao CC, Su YC. The utilization of Traditional Chinese medicine and associated factors in Taiwan in 2002. Chin Med J (Engl). 2009;122:1544-1548. [PubMed] [Google Scholar]