Abstract
Introduction
The impact of immune checkpoint blockade on immunity in cancer patients is not completely elucidated due to the complexity of the immune network. Recent studies have revealed a significant role of programed cell death‐ligand 2 (PD‐L2) in negatively controlling the production of CD4+ T helper type 2 (Th2) cytokines and airway hypersensitiveness, suggesting hypo‐responsive Th2 cells via the PD‐1/PD‐L2 inhibitory pathway in lung could be reawaken by PD‐1 blockade therapy.
Methods
We describe the first report of acute eosinophilic pneumonia (AEP), which is known as Th2‐associated pulmonary disease, triggered by nivolumab, an anti‐PD‐1 antibody, in an advanced non‐small cell lung cancer patient. Based on the current case report and literature, the present study proposes a potential mechanism of the onset of AEP as an immune‐related adverse event (irAE).
Results
A 62‐year‐old man was diagnosed with lung adenocarcinoma and nivolumab was selected as the third‐line regimen. After three cycles of nivolumab treatment, chest computed tomography revealed pulmonary infiltrates in both lungs. The patient was diagnosed with AEP based on the diagnostic criteria for AEP. Nivolumab was suspended and the patient was started on oral prednisolone. His symptoms and radiological findings had rapidly improved.
Conclusions
Given the increasing frequency of the use of anti‐PD‐1 antibodies, clinicians should be aware of the risk of AEP as a potential irAE. This study may improve our understanding of the pathophysiology underlying Th2‐associated irAEs and AEP.
Keywords: Acute eosinophilic pneumonia, immune checkpoint blockade, immune‐related adverse event, lung cancer, programed cell death‐ligand 2 (PD‐L2)
1. INTRODUCTION
The impact of immune checkpoint blockade on immunity in cancer patients is not completely elucidated due to the complexity of the immune network. Recent studies have revealed a significant role of programed cell death‐ligand 2 (PD‐L2) in negatively controlling the production of CD4+ T helper type 2 (Th2) cytokines and airway hypersensitiveness,1, 2, 3 suggesting hypo‐responsive T helper 2 cells (Th2 cells) via the programed cell death‐1 (PD‐1)/programed cell death‐ligand 2 (PD‐L2) inhibitory pathway in lung could be reawaken by PD‐1 blockade therapy.
Acute eosinophilic pneumonia (AEP) is a Th2 inflammation associated lung disease with a remarkable increase in bronchoalveolar lavage (BAL) eosinophils, first described in 1989.4, 5 Although AEP has been associated with tobacco smoke, environmental or occupational dust exposures, toxin inhalations, and medications including NSAIDs, minocycline, cephalosporins, and phenytoin,5, 6, 7 nivolumab‐induced AEP has not been reported. In addition, the precise mechanism of significant eosinophil accumulation in AEP remains to be elucidated.
Here we describe the first report of AEP triggered by nivolumab, an anti‐PD‐1 antibody, in an advanced non‐small cell lung cancer patient. Based on the current case, present study proposes a potential mechanism of the onset of AEP as an immune‐related adverse event (irAE).
2. CASE PRESENTATION
A 62‐year‐old man was diagnosed with lung adenocarcinoma and had right lower lobectomy (pT2bN2M0 stage III A, PD‐L1 tumor proportion score <1%; Figure 1A). Thereafter he received postoperative adjuvant therapy of cisplatin and vinorelbine. After 5 months, he was diagnosed with postoperative recurrence of lung adenocarcinoma with multiple metastasis in both lungs. He received platinum‐based chemotherapy as the first‐line chemotherapy regimen and nivolumab was selected as the third‐line regimen. The patient had no history of asthma, atopy, and drug allergy. The patient had not begun taking any new medications and had no history of cigarette smoking.
Figure 1.
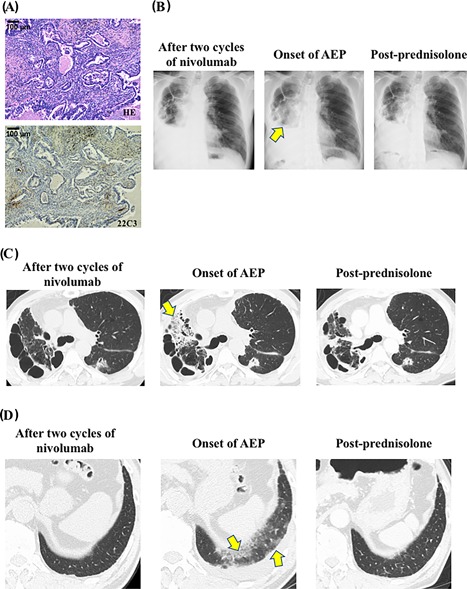
Key pathology and imaging (A) A hematoxylin and eosin staining and an immunohistochemical staining of primary lung tumor negative staining for PD‐L1 (clone 22C3 pharmDx kit, tumor proportion score <1%). B, Chest radiograph after two cycles of nivolumab (left panel), at the onset of acute eosinophilic pneumonia (AEP) (middle panel), and 7‐days after treatment with prednisolone (post‐prednisolone; right panel). Chest radiograph at the onset of AEP shows consolidation in the right upper lobe (Arrow). C and D, Chest computed tomography (CT) after two cycles of nivolumab (left panels), at the onset of AEP (middle panels), and 1‐month after treatment with prednisolone (post‐prednisolone; right panels). Consolidation in right upper lobe (C) and ground‐glass opacity in left lower lobe at the onset of AEP (D) are shown. Arrows indicate consolidation (C) and ground‐glass opacity (D)
After three cycles of nivolumab treatment (3 mg/kg every 2 weeks), he presented with cough and chest computed tomography revealed pulmonary infiltrates in both lungs (Figure 1B–D). Levofloxacin was administered for 12 days; however, antibiotics did not improve his symptom or radiological findings. Thus, bronchoalveolar lavage was performed from the right upper lobe. Bronchoalveolar lavage cellular analysis showed a significant increase of total cell count of 12.1 × 105 mL, of which 27.1% were eosinophils (normal upper limit, 1.3%), 8.3% were lymphocytes (normal upper limit, 11%). No pathogenic bacterial organism was cultured. The patient was diagnosed with AEP based on the diagnostic criteria for AEP.7 Nivolumab was suspended and the patient was started on oral prednisolone (0.5 mg/kg/day, 30 mg/day), which is tapered 5 mg weekly. His symptoms and radiological findings had rapidly improved (Figure 1B–D), which is consistent with AEP.5, 6, 7 No sign of lung cancer worsening has been observed since the initiation of treatment with nivolumab or prednisolone.
3. DISCUSSION
The pathophysiology underlying irAEs has not been fully elucidated.8, 9, 10 Elucidating mechanisms of irAEs is needed to develop precise treatments for irAEs.8 PD‐1 and the ligands, PD‐L1 and PD‐L2 have been implicated in playing an important role in maintaining immune homeostasis not only in cancer but also in lung allergic diseases.2, 3 Th2 cells predominantly produce interleukin‐4 (IL), IL‐5, and IL‐13, which have essential roles in the development of Th2 associated lung diseases, enhancing the growth, differentiation, and eosinophil recruitment.1 Recent studies have shown that PD‐L2 is highly expressed on pulmonary dendritic cells (DCs) and inhibits responses of Th2 cells, which express PD‐1, suggesting that blocking the PD‐1–PD‐L2 interaction by anti‐PD‐1 antibodies could facilitate a Th2 inflammation.1, 2, 3, 11, 12
Antibodies against PD‐1 inhibit binding of both PD‐L1 and PD‐L2 to PD‐1 expressed on activated T cells.3, 8, 12 This raises the possibility that blockade of PD‐1–PD‐L2 interaction by anti‐PD‐1 antibodies mediates Th2‐associated pulmonary diseases such as AEP: anti‐PD‐1 antibody activates Th2 cells and promotes the production of Th2‐type cytokines in the lung. One of the Th2 cytokines, IL‐5, supports the development of eosinophils in the bone marrow. Eosinophilia in lung tissue is driven by the recruitment of eosinophils to the lung mucosa and interstitium via production of eotaxin induced by Th2 cytokines, which could result in the onset of AEP as an irAE (Figure 2).
Figure 2.
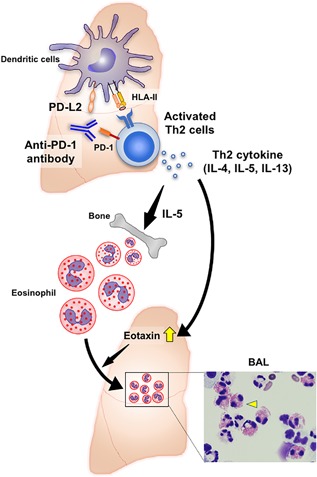
A hypothetical mechanism of the onset of AEP as an irAE in an advanced non‐small cell lung cancer patient based on the current case report and references. Antibodies against PD‐1 inhibit binding of not only PD‐L1 but also PD‐L2 to PD‐1. PD‐L2 is highly expressed on pulmonary DCs and inhibits responses of Th2 cells, which express PD‐1. The blocking the PD‐1–PD‐L2 interaction facilitates a pulmonary Th2‐type inflammation. Th2 cells predominantly produce IL‐4, IL‐5, and IL‐13, which have essential roles in the development of Th2‐associated lung diseases, enhancing the growth, differentiation, and recruitment of eosinophils. One of the Th2 cytokines, IL‐5, supports the development of eosinophils in the bone marrow. Eosinophilia in lung tissue is driven by the recruitment of eosinophils to the lung mucosa and interstitium via production of eotaxin induced by Th2 cytokines in the lung, which results in the onset of AEP as an irAE in lung cancer patients treated with an anti‐PD‐1 antibody therapy. Right lower imaging indicates a photomicrograph of representative bronchoalveolar lavage cytospin preparation, which shows eosinophilia observed in the current case. Yellow arrowhead indicates an eosinophil
4. CONCLUSION
In conclusion, the present study describes the first case of AEP as an irAE in a lung cancer patient who had received PD‐1 blockade therapy. The present study also proposes a potential mechanism of the onset of AEP as an irAE. Given the increasing frequency of the use of anti‐PD‐1 antibodies, clinicians should be aware of the risk of AEP as a potential irAE. This study may improve our understanding of the pathophysiology underlying Th2‐associated irAEs and AEP.
CONFLICT OF INTEREST
No potential conflicts of interest were disclosed.
ETHICAL STATEMENT
This study was approved by the patient and we obtained written consent form the patient. This report was prepared in accordance with the Helsinki Declaration.
ACKNOWLEDGMENT
This work was supported by JSPS KAKENHI Grant Number JP18K15928.
Jodai T, Yoshida C, Sato R, et al. A potential mechanism of the onset of acute eosinophilic pneumonia triggered by an anti‐PD‐1 immune checkpoint antibody in a lung cancer patient. null. 2019;7:3–6. 10.1002/iid3.238
Takayuki Jodai and Chieko Yoshida contributed equally to this work.
REFERENCES
- 1. Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015; 16:45–56. [DOI] [PubMed] [Google Scholar]
- 2. Bratke K, Fritz L, Nokodian F, et al. Differential regulation of PD‐1 and its ligands in allergic asthma. Clin Exp Allergy. 2017; 47:1417–1425. [DOI] [PubMed] [Google Scholar]
- 3. Chen DS, Irving BA, Hodi FS. Molecular pathways: next‐generation immunotherapy‐inhibiting programmed death‐ligand 1 and programmed death‐1. Clin Cancer Res. 2012; 18:6580–6587. [DOI] [PubMed] [Google Scholar]
- 4. Mato N, Bando M, Kusano A, et al. Clinical significance of interleukin 33 (IL‐33) in patients with eosinophilic pneumonia. Allergol Int. 2013; 62:45–52. [DOI] [PubMed] [Google Scholar]
- 5. Allen JN, Pacht ER, Gadek JE, Davis WB. Acute eosinophilic pneumonia as a reversible cause of noninfectious respiratory failure. N Engl J Med. 1989; 321:569–574. [DOI] [PubMed] [Google Scholar]
- 6. Rhee CK, Min KH, Yim NY, et al. Clinical characteristics and corticosteroid treatment of acute eosinophilic pneumonia. Eur Respir J. 2013; 41:402–409. [DOI] [PubMed] [Google Scholar]
- 7. De Giacomi F, Decker PA, Vassallo R, Ryu JH. Acute eosinophilic pneumonia: correlation of clinical characteristics with underlying cause. Chest 2017; 152:379–385. [DOI] [PubMed] [Google Scholar]
- 8. Postow MA, Sidlow R, Hellmann MD. Immune‐related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018; 378:158–168. [DOI] [PubMed] [Google Scholar]
- 9. Tomita Y, Sueta D, Kakiuchi Y, et al. Acute coronary syndrome as a possible immune‐related adverse event in a lung cancer patient achieving a complete response to anti‐PD‐1 immune checkpoint antibody. Ann Oncol. 2017; 28:2893–2895. [DOI] [PubMed] [Google Scholar]
- 10. Horio Y, Takamatsu K, Tamanoi D, et al. Trousseau's syndrome triggered by an immune checkpoint blockade in a non‐small cell lung cancer patient. Eur J Immunol. 2018; 48:1764–1767. [DOI] [PubMed] [Google Scholar]
- 11. van der Werf N, Redpath S A, Azuma M, Yagita H, Taylor MD. Th2 cell‐intrinsic hypo‐responsiveness determines susceptibility to helminth infection. PLoS Pathog. 2013; 9:e1003215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Y, Chung Y, Bishop C, et al. Regulation of T cell activation and tolerance by PDL2. Proc Natl Acad Sci USA. 2006; 103:11695–11700. [DOI] [PMC free article] [PubMed] [Google Scholar]


