Abstract
Leprosy is a neglected endemic infectious disease in the Pacific region. In French Polynesia (FP), leprosy is no longer a public health problem at the national level, defined by the World Health Organization as a prevalence rate below 1 case per 10,000 population. However, even if its incidence has dramatically declined in FP in the last decades, leprosy is still endemic at a low level. Here we present a case of leprosy in a 34-year-old man from FP diagnosed in 2018. Clinical and microbiologic examinations, including fluorescence in-situ hybridization, led to the diagnosis of a multibacillary leprosy, and multidrug therapy was initiated. There is a need to maintain leprosy surveillance and trained medical staff for the detection and treatment of new cases.
Keywords: French Polynesia, leprosy, Pacific
Introduction
Leprosy is a chronic bacterial disease caused by Mycobacterium leprae that predominantly affects skin and peripheral nerves. Leprosy is responsible for disabilities and deformities, and thus is associated with stigma. More than 200,000 new cases of leprosy are reported annually in the world, with a slow decrease in the detection of new cases globally during the past decade [1], [2].
The goal of eliminating leprosy as a public health problem at the national level was defined by the World Health Organization (WHO) in 1991 as a prevalence rate below 1 case per 10,000 [1], [2], [3]. The goal of WHO for 2020 is to have no new children diagnosed with grade 2 leprosy (visible damage/deformity or disability), fewer than 1 newly diagnosed leprosy patient with grade 2 leprosy per million population and no countries with legislation that allows discrimination against people with leprosy [4].
French Polynesia (FP) is a remote French overseas territory located in the South Pacific. The French Polynesian population comprises about 280,000 inhabitants distributed in 72 inhabited islands grouped in five archipelagoes. FP belongs to the 22 Pacific island countries and territories.
Leprosy is endemic in the Pacific region, including FP, and is considered to be a neglected disease in the region, with a stable incidence in the past decade [1], [3], [5], [6].
We report a case of leprosy in FP diagnosed in 2018 and report data about the epidemiology of leprosy in this country since the early 20th century.
Patient and Methods
A 34-year-old man living in Raiatea Island, FP, was admitted in September 2018. The patient had no underlying disease and no known contact with people with leprosy.
Clinical signs appeared in mid-2016. At examination he had erythaematous plaques (improperly classified as urticarial plaques) on trunk and arms, hand and foot oedema with dyschromic plaques, and asthenia. At this time he was treated for toxocariasis because serology for Toxocara canis was positive.
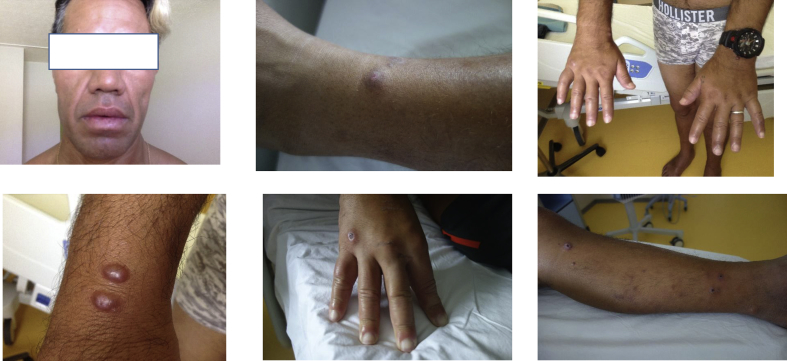
Clinical examination at admission in September 2018 (Fig. 1) yielded leonine facies, bilateral conjunctivitis, loss of ends of the eyebrows, oedema of the feet and hands, nodules in different parts of the body (hypochromic and hyperchromic), small nodules on extremities and earlobes, loss of feeling in the feet, enlarged nerves on the ankles, small inguinal and axillary adenopathies (< 1 cm), hypertrophy of mammary glands, nasal congestion with nose bleeding after sample collection and asthenia. Cardiac and pulmonary examination detected no abnormalities. Hepatic enzymes, urea, creatinine and thyroid laboratory markers results were within the normal range.
Fig. 1.
Leprosy in 34-year-old French Polynesian man.
This patient had two of the three WHO diagnosis criteria for leprosy: thickened or enlarged peripheral nerve with loss of sensation and/or weakness of the muscles supplied by the nerve and presence of acid-fast bacilli in a slit-skin smear [1]. This case was classified as multibacillary leprosy according to the clinical WHO classification (more than five skin lesions or more than one nerve trunk involvement or with bacilli in a skin smear) and grade 1 disability (loss of sensation but not yet visible damage, deformation or disability [1].
Multidrug therapy for multibacillary leprosy was initiated with rifampicin 600 mg per day, clofazimine 100 mg per day and dapsone 100 mg per day, a regimen slightly different from the WHO recommendations [1]. This therapeutic regimen has been used by French Polynesian leprologists for many years.
Written consent was obtained from the patient to report this case.
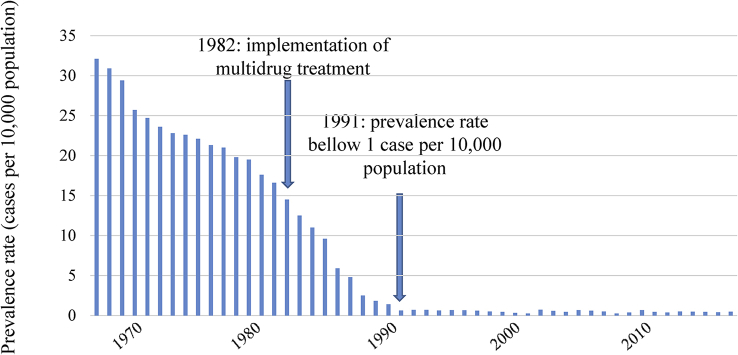
The incidence rate of leprosy in FP from 1967 to 2017 is reported in Fig. 2.
Fig. 2.
Incidence rate of leprosy in French Polynesia (cases per 10,000 population) from 1967 to 2017.
Results
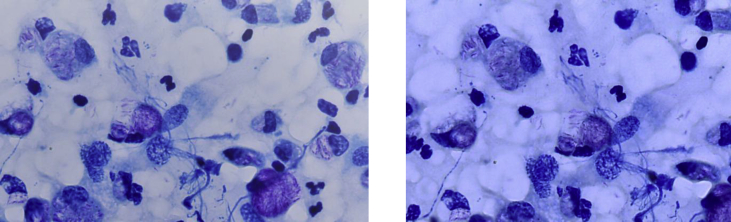
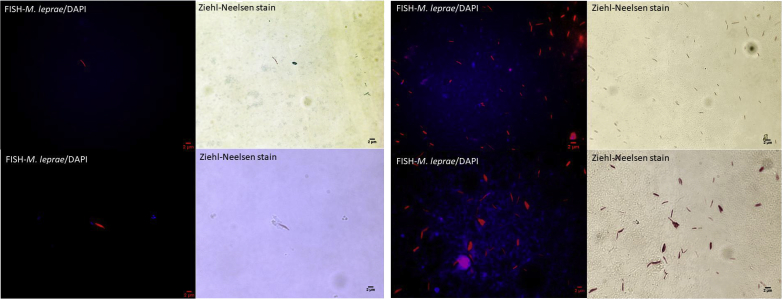
Ziehl-Neelsen–stained skin smears from the earlobes and nasal mucosa yielded numerous acid-fast bacilli with a bacterial index of 5 (100–1000 bacilli in every field at 100× magnification) and intra- and extracellular globi (clumps of bacilli in capsular material). Histopathologic examination of skin lesions revealed a nonspecific inflammatory infiltrate with predominance of histiocytes, with numerous acid-fast bacilli evident upon Ziehl-Neelsen staining (Fig. 3). Diagnosis was confirmed by the specific detection of M. leprae in nasal mucosa and skin smears by combining fluorescence in-situ hybridization (FISH) and Ziehl-Neelsen staining using a modified protocol already tested on mycobacteria [7], [8]. Briefly, smear slides were fixed in 4% paraformaldehyde and covered with 10 mg/mL lysozyme, then 10 μg/mL proteinase K (respectively at 37°C for 30 minutes and 37°C for 5 minutes). Slides were incubated overnight with a 10 μL suspension containing the specific probe targeting the M. leprae rpoB gene (Alexa 555-GCCAGAGCAAGACAGACGTT-3′). After washing steps, smears were stained with Ziehl-Neelsen staining (Kit Quick-TB; RAL Diagnostics, Martillac, France) and mounted with ProLong Diamond Antifade (Fisher Scientific, Illkirch, France) containing 4,6-diamidino-2-phenylindole. Microscopic observation was performed using the 100× objective lens of a Leica DMI6000 fluorescence microscope (Leica Microsystems, Nanterre, France). FISH and Ziehl-Neelsen staining combination yielded specific detection of M. leprae as red fluorescent and Ziehl-Neelsen–positive bacilli (Fig. 4).
Fig. 3.
Smears showing numerous acid-fast bacilli with intracellular and extracellular globi.
Fig. 4.
Microscopic images of smears from nasal mucosa (A) and skin biopsy (B) combining FISH with DAPI and Ziehl-Neelsen staining. Image acquisition was performed for same microscopic field using Hamamatsu Orca AG camera (Hamamatsu Photonics, Herrsching-am-Ammersee, Germany) to visualize FISH-positive mycobacteria (left panels) and using DFC425C Digital Microscope Camera (Leica Microsystems, Nanterre, France) for Ziehl-Neelsen–positive mycobacteria (right panels). DAPI, 4′,6-diamidino-2-phenylindole; FISH, fluorescence in-situ hybridization.
Discussion
Leprosy was probably introduced in FP during past migrations from Asia, long before European migration. The disease is known as oovi in the Tahitian language and koovi in the Marquesian language [9]. The disease was subsequently described in the 18th century by explorers. The last introductions probably occurred by Chinese immigrants in the Marquesas archipelago in the 19th century. From the 19th century, patients with leprosy were isolated in leper colonies. The first leper colonies were in Tahiti (the main FP island) and Marquesas in 1914. The last one, located in Tahiti, was closed in 1976 [6], [9]. In FP, notification of leprosy cases has been systematic since 1902, and leprosy has been a mandatory reporting communicable disease since 1911. Up to 1946, diagnosis was based on clinical examination only. Lepromin skin test, search for acid-fast bacilli and biopsy for pathologic examination were available from 1946 [10]. Dapsone monotherapy was implemented in 1952 and multidrug therapy including rifampicin in 1982. Case detection rate of leprosy in FP decreased from 50 per 100,000 population in 1902 to 25 per 100,000 in 1946 to 8 per 100,000 in 1991, to 1.8 per 100,000 population in 2017, with an average annual rate of decrease of 2% between 1902 and 1991 [10]. Introduction of multidrug therapy contributed to the decline of leprosy in FP, but it is difficult to evaluate the respective contribution of multidrug therapy, economic improvement of the country and natural decline of leprosy [11].
The prevalence of leprosy in FP is under the rate of 1 case per 10,000 population since 1991 and has been stable since then, with a mean prevalence of 0.44 per 10,000 population and a mean incidence of 1.6 cases per 100,000 population from 2000 to 2017 [6].
Even if data are lacking from some remote Pacific islands, leprosy is endemic in the region [12], [13], [14], [15]. The overall prevalence of leprosy in the Pacific island countries and territories in 2010 was 1.64 per 10,000 population, so the WHO threshold of 1 per 10,000 population has not yet been achieved [3]. However, disparities exist within the Pacific areas. The Federated States of Micronesia, Marshall Islands and Kiribati failed to reach leprosy elimination, with a prevalence over 10 cases per 100,000 population; in Nauru and Palau the annual prevalence ranges from 1 to 10 cases per 10,000 population [3]. These Pacific island countries and territories are located in the Northwest Pacific. If we consider the East Pacific island countries and territories (which includes FP), the overall prevalence is below 1 per 10,000 population [6]. The higher prevalence of leprosy in the Northwest Pacific is possibly due to its close location to Southeast Asia, the area in the world were three quarters of leprosy cases are reported [2].
Data about drug resistance and genetic diversity of M. leprae strains circulating in the Pacific and FP are scarce. Four French Polynesian strain genotyped using single nucleotide polymorphism (SNP) belonged to SNP genotype 3; however, the four SNP types have been isolated in New Caledonia [16]. FISH has been reported to successfully detect M. leprae in paraffin-embedded tissue sections from skin biopsy samples [17]. In this case, FISH allowed detection and visualization of M. leprae bacilli directly on skin smears and nasal mucosa smears, offering an additional method for leprosy diagnosis.
With fewer than ten cases per year in the past decade, leprosy has become a rare disease in FP, and new physicians are not trained to detect its early clinical signs. Consequently, leprosy can remain undetected or misdiagnosed, as illustrated by this case. It highlights the need to maintain leprosy surveillance as well as to have specialists and general healthcare staff trained to detect and report cases, as timely diagnosis and proper implementation of treatment will prevent development of nerve damages and disabilities, and reduce the disease burden of leprosy [1], [3].
Conflict of Interest
None declared.
References
- 1.World Health Organization Guidelines for the diagnosis, treatment and prevention of leprosy: executive summary. 2018. https://zeroleprosy.org/wp-content/uploads/2018/08/WHO-Guidelines-Web-Version.pdf Available at:
- 2.World Health Organization Global leprosy update, 2017: reducing the disease burden due to leprosy. World Heal Organ Wkly Epidemiol Rec. 2018;35:445–456. [Google Scholar]
- 3.World Health Organization Epidemiological review of leprosy in the Western Pacific region, 2008–2010. 2010. http://www.wpro.who.int/leprosy/documents/leprosy_report_2008_2010.pdf Available at:
- 4.World Health Organization Global leprosy strategy, 2016–2020. 2016. http://www.wpro.who.int/leprosy/documents/globalleprosystrategy2016-2020.pdf Available at:
- 5.Kline K., McCarthy J.S., Pearson M., Loukas A., Hotez P.J. Neglected tropical diseases of Oceania: review of their prevalence, distribution, and opportunities for control. PLoS Negl Trop Dis. 2013;7:e1755. doi: 10.1371/journal.pntd.0001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen N., Mallet H., Segalin J., Lasarde C., Buluc A., Daudens E. La lèpre dans le Pacifique et en Polynésie française. Bulletin d’informations sanitaires, épidemiologiques et statistique. 2011;4:5–6. [Google Scholar]
- 7.Loukil A., Kirtania P., Bedotto M., Drancourt M. FISHing Mycobacterium tuberculosis complex by use of a rpoB DNA Probe bait. J Clin Microbiol. 2018;56 doi: 10.1128/JCM.00568-18. e00568-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darriet F., Bernioles P., Loukil A., Saidani N., Eldin C., Drancourt M. Fluorescence in situ hybridization microscopic detection of bacilli Calmette Guérin mycobacteria in aortic lesions: a case report. Medicine (Baltimore) 2018;97:e11321. doi: 10.1097/MD.0000000000011321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaban A. La lèpre en Polynésie française (dépistage, disgnostic, traitement et évolution de 1996 à 2006) Mémoire pour la Capacité en médecine tropicale. 2006:1–46. [Google Scholar]
- 10.Glaziou P., Cartel J.L., Moulia-Pelat J.P., Ngoc L.N., Chanteau S., Plichart R. Tuberculosis in leprosy patients detected between 1902 and 1991 in French Polynesia. Int J Lepr Other Mycobact Dis. 1993;61:199–204. [PubMed] [Google Scholar]
- 11.Cartel J.L., Spiegel A., Nguyen Ngoc L., Moulia-Pelat J.P., Martin P.M., Grosset J.H. Leprosy in French Polynesia. The possible impact of multidrug therapy on epidemiological trends. Lepr Rev. 1992;63:223–230. doi: 10.5935/0305-7518.19920027. [DOI] [PubMed] [Google Scholar]
- 12.Woodall P., Scollard D., Rajan L. Hansen disease among Micronesian and Marshallese persons living in the United States. Emerg Infect Dis. 2011;17:1202–1208. doi: 10.3201/eid1707.102036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Worth R.M. Leprosy in Hawaii; the end of an epidemic. Int J Lepr Other Mycobact Dis. 1996;64:441–447. [PubMed] [Google Scholar]
- 14.Louis F.J., Louis J.P., Schill H., Parc F. The last offensive of leprosy in the South Pacific Ocean: the epidemic on Rapa (1922–1950) Bull Soc Pathol Exot Filiales. 1987;80:306–319. [PubMed] [Google Scholar]
- 15.Monchy D., Huerre M., Crouzat M., Dubourdieu D., Duval P., Sottil J.P. Leprosy in New Caledonia from 1983 to 1992. Histopathological and epidemiological data. Bull Soc Pathol Exot. 1994;87:28–32. [PubMed] [Google Scholar]
- 16.Reibel F., Chauffour A., Brossier F., Jarlier V., Cambau E., Aubry A. New insights into the geographic distribution of Mycobacterium leprae SNP genotypes determined for isolates from leprosy cases diagnosed in metropolitan France and French territories. PLoS Negl Trop Dis. 2015;9:e0004141. doi: 10.1371/journal.pntd.0004141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lefmann M., Schweickert B., Buchholz P., Gobel U.B., Ulrichs T., Seiler P. Evaluation of peptide nucleic acid–fluorescence in situ hybridization for identification of clinically relevant mycobacteria in clinical specimens and tissue sections. J Clin Microbiol. 2006;44:3760–3767. doi: 10.1128/JCM.01435-06. [DOI] [PMC free article] [PubMed] [Google Scholar]