Abstract
Cisplatin induces anorexia, weight loss, loss of adipose tissue, skeletal muscle atrophy, and serious adverse effects that can cause premature termination of chemotherapy. The aim of this study was to use an animal model to assess cisplatin therapy (3 cycles) with and without d-methionine to investigate its protective effects on cisplatin-induced anorexia and skeletal muscle wasting. Wistar rats were divided into 3 groups and treated as follows: saline as control (group 1), intraperitoneal cisplatin once a week for 3 weeks (group 2), and intraperitoneal cisplatin once a week for 3 weeks plus oral administration of d-methionine (group 3). Tissue somatic index (TSI), gastric emptying index (GEI), and feeding efficiency were measured. Both hepatic lipid metabolism and muscle atrophy-related gene expressions and C2C12 myotubes were determined by polymerase chain reaction. Micro–computed tomography (micro-CT) was used to conduct assessment of bone microarchitecture indices. Pathological changes of the gastric mucosa were assessed by hematoxylin and eosin staining after euthanizing the animals. d-Methionine increased food intake, weight gain, gastric emptying, and feeding efficiency, as well as decrease stomach contents, after cisplatin injections. Cisplatin caused shortening of myofibers. Cisplatin-induced muscle mass wasting was mediated by the elevation of mRNA expressions of MAFbx and MuRF-1 in ubiquitin ligases in muscle tissue homogenate. The mRNA expressions of MyoD and myogenin, markers of muscle differentiation, declined following cisplatin administration. The administration of d-methionine not only led to significant improvements in myofiber diameter and cross-sectional fiber areas but also reversed muscle atrophy-related gene expression. However, there were no significant changes in stomach histology or microarchitecture of trabecular bone among the study groups. The results indicate that d-methionine has an appetite-enhancing effect and ameliorates cisplatin-induced adipose and muscle tissue loss during cisplatin-based chemotherapy.
Keywords: cisplatin, d-methionine, anorexia, cachexia, muscle atrophy, MuRF-1
Introduction
Cancer-associated cachexia is characterized by anorexia, body weight decrease, and loss of adipose tissue and skeletal muscle.1 Cancer cachexia prevalence ranges from 50% to 80% in patients with advanced cancer.2 In addition to tumor-induced anorexia and cachexia, there is evidence of chemotherapy-induced anorexia and cachexia.3-5 Among chemotherapeutic agents, cisplatin is a first-line, standard care agent for lung cancer with strong emetic activity. There is evidence that cisplatin induces anorexia, loss of adipose tissue and skeletal muscle atrophy,3,5-7 indicating that cisplatin produces serious adverse effects that can lead patients to discontinuation of treatment. The incidence of anorexia is approximately 15% among patients with cancer receiving high-dose cisplatin treatment.8 These adverse effects can markedly decrease the quality of patients’ lives, aggravate suffering and even lead to discontinue their treatments. The development of an adjuvant or chemopreventive agent without decreased antitumor action that ameliorates these adverse responses is vital for building patient confidence and continuing treatment.
d-Methionine is commonly found in fermented dairy products like cheese and yogurt. d-methionine is the dextro isomer of the essential amino acid l-methionine and a sulfur-containing nucleophile that is able to inactivate the toxic cisplatin-derived Pt-species by the formation of Pt–d-methionine adducts in plasma.9 Campbell et al10 were the first to demonstrate that d-methionine provides protection against cisplatin ototoxicity in rats. The mechanisms of d-methionine’s otoprotective action were through its specific antioxidant enzyme activities and its ability to counteract oxidative stress.11-13 Previous studies conducted both in vitro and in vivo have suggested that d-methionine is a potential protector against radiation-induced oral mucositis, but does not alter response to cisplatin-based chemotherapy.14 Oral administration of MRX-1024, a high concentration (200 mg/mL) bioavailable suspension formulation of d-methionine (Molecular Therapeutics, Ann Arbor, MI), has been shown to be appropriate for head and neck cancer patients receiving concurrent radiation and cisplatin (the molar ratio of d-methionine:cisplatin is 52).15 Based on these findings, d-methionine is an excellent chemopreventive agent candidate.
A short-term animal study has shown that d-methionine administered 30 minutes before cisplatin injection reduces weight loss and improves survival (molar ratio 38:1, d-methionine:cisplatin).10 The aim of the present study was to determine if d-methionine, a sulfur-containing antioxidant, protects against cisplatin-induced anorexia, adipose tissue, and skeletal muscle wasting. First, we investigated the effects of oral d-methionine administration on food intake, body weight, gastric emptying, feeding efficiency, and stomach histopathology in cisplatin-induced anorexic rats. Second, we investigated the effects of d-methionine on cisplatin-induced myotube formation and myotube atrophy in vivo and in vitro. Additionally, we also tested the effect of cisplatin on trabecular microarchitecture as this may help gain insight into bone fractures which are a chemotherapy-associated clinical problem.
Materials and Methods
Drugs and Chemicals
Cisplatin and d-methionine were purchased from Sigma-Aldrich Chemical (St Louis, MO). All other chemicals and reagents used in this study were of analytical grade.
Animals
Twenty-four male Wistar rats (initial average body weight between 176 and 200 g) were purchased from BioLASCO Taiwan Co, Ltd. Rats were housed in cages with a maximum of 4 rats per cage with a 12-hour light/12-hour dark cycle and fed an autoclaved diet with ad libitum access to standard laboratory rodent diet 5001 during the study period.
Experimental Design
After 2 weeks of acclimatization, animals were randomly divided into 3 equal groups of 8 animals each. Based on our previous study,16 anorexia was induced by intraperitoneal administration of cisplatin at dose of 5 mg/kg body weight once a week for 3 weeks. The experimental design was as follows:
Saline group: Phosphate buffered saline (PBS) was orally administered daily with a single intraperitoneal injection of 0.9% (w/v) NaCl solution on the 1st, 8th, and 15th days.
Cisplatin group: A single intraperitoneal injection of cisplatin (5 mg/kg) dissolved in normal saline was administered on the 1st, 8th, and 15th days.
d-Methionine (300 mg/kg/day) + cisplatin (5 mg/kg): d-methionine was started 3 days before the first cisplatin injection. d-Methionine was dissolved in PBS and administered daily by gavage at a dose of 300 mg/kg/day. Cisplatin (5 mg/kg) was injected on days 1, 8, and 15. In the present study, the molar ratio of d-methionine to cisplatin is 121:1. Each animal’s body weight, food intake, and water consumption were monitored daily during the experimental period. All animals were sacrificed one day after the last cisplatin injection. Blood samples were collected and centrifuged at 4°C. The plasma was frozen at −80°C until analysis. The stomach was immediately removed and weighed. The stomach was opened and the contents removed and weighed. Meanwhile, kidney, liver, heart, spleen, brain, and visceral adipose tissue (epididymal, mesenteric, and perirenal) were immediately removed and weighed. The fur was stripped from the hind limbs of all rats for gross comparisons of muscle mass and the hind limbs were photographed. The various muscle tissues (ie, diaphragm, gastrocnemius, soleus, extensor digitorum longus, psoas, and quadriceps) were removed and weighted. Tissue somatic index (TSI), gastric emptying index (GEI), and feeding efficiency were calculated according to the following formulas:
C2C12 Cell Cultures
The C2C12 murine skeletal muscle cell line (BCRC number 60083) was purchased from the Bioresource Collection and Research Center (Hsinchu, Taiwan) and cultured in Dulbecco’s modified Eagle medium (DMEM) (GIBCO, Rockville, MD) with 10% fetal bovine serum (HyClone, Logan, UT), glutamine (2 mM), penicillin (100 units/mL), and streptomycin (100 μg/mL) (Invitrogen, Carlsbad, CA). To induce differentiation, at 80% to 90% confluence, the medium was changed to the differentiating medium, consisting of DMEM supplemented with 2% horse serum (HyClone, Logan, UT), to induce myotube formation. After 5 days of culture, differentiated C2C12 myotubes were incubated in DMEM or cisplatin (50 μM) or pretreated with 5 mM d-methionine for 1 hour followed by exposure to 5 mM cisplatin for 24 hours.
Determinations of Hepatic Lipid Metabolism and Muscle Atrophy–Related Gene Expressions by RT-PCR
Muscles and liver were weighed to the nearest 0.1 g. Muscle and liver samples were flash-frozen in liquid nitrogen and ground to a powder in a stainless steel mortar and pestle homogenizer. One milliliter RareRNA (GenePure Technology Co, Taichung, Taiwan) was used to homogenize parts of the frozen crushed muscle and liver samples to extract RNA. Analysis of RT-PCR (reverse transcription polymerase chain reaction) was carried out as described previously with some modifications.17 The concentration of each RNA sample and the 260/280-nm absorbance (A260/A280) ratio was measured using the Amersham-Ultrosrec 2100 Pro Spectrophotometer. Only samples with A260/A280 ratios between 1.8 and 2.0 were used for further analyses. cDNA was reverse-transcribed from 2 µg total cellular RNA and amplified in a reaction volume of 50 µL. High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) was used for reverse transcription. PCR was performed with 1 µL RT products, 10 × PCR buffer 2.5 µL, 10 mM dNTPs (1 µL), 1 µL of each primer of β-actin, 1 µL of each primer of gene to be tested and ProTaq DNA polymerase, in a final volume of 25 µL. The primer sequences for PCR amplification are presented in Table 1. Samples were equally loaded onto 2% or 1.5% agarose gel, stained with ethidium bromide, and visualized with UV transilluminator. The PCR reactions were performed on programmable thermal controller instrument-thermal cycler model 2400. Sequences of primers are presented in Table1. The relative mRNA levels of stearoyl-CoA desaturase-1 (SCD-1), fatty acid synthase (FAS), carnitine palmitoyltransferase-1α (CPT-1α), insulin-like growth factor gene 1 (IGF-1), myostatin, forkhead box O1 transcription factor (FOXO-1), muscle atrophy F-box protein (MAFbx), muscle RING finger-containing protein 1 (MURF-1), Myogenin and MyoD in each sample were normalized to β-actin content using the ImageJ software.
Table 1.
Sequences of Primers Used in This Study.
| Source | Gene | Sequence 5’-3’ (Forward) | Sequence 5’-3’ (Reverse) |
|---|---|---|---|
| Rat | FAS | GTGTCTGGGTGGGTGTGAGT | GCTAGAGGAGCAGGCTGTGT |
| SCD1 | CCAGGGCACTGATAAGGT | GATATCCACGACCCCAGCT | |
| CPT-1a | ATGACGGCTATGGTGTCTCC | GTGAGGCCAAACAAGGTGAT | |
| FOXO-1 | AATTTGCTAAGAGCCGAGGA | AAGTCATCATTGCTGTGGGA | |
| Myostatin | GCTCAAACAGCCTGAATCCAAC | TCACAGTCAAGCCCAAAGTCTC | |
| MAFbx | GGAGCTGATAGCAAAGTCAC | GGAGAAGTTCCCGTATGAGTC | |
| MuRF-1 | CTCGCAGCTGGAGGACTCC | CTCGTCCAGGATGGCGTAG | |
| IGF-1 | TCGTCTTCACATCTCTTCTACC | GTGTACTTCCTTTCCTTCTCCT | |
| Myogenin | CTACCTTCCTGTCCACCTTC | CTCCAGTGCATTGCCCCACT | |
| MyoD | GGAGACAATCCTCAAGCGATGC | AGCACCTGGT AAATCGGATTGG | |
| β-actin | CAACCTTCTTGCAGCTCCTC | TTCTGACCCATACCCACCAT |
Isolation of RNA and Real-Time RT-PCR
RNA from cultured C2C12 myotubes was isolated using RareRNA (GenePure Technology, Taiwan). Total RNA was reverse transcribed by High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, 4368813) to cDNA. The reverse transcription reaction conditions were 25°C for 10 minutes, 37°C for 120 minutes, and 85°C for 5 minutes. Real-time PCR was performed using ABI StepOnePlus real-time PCR system with gene-specific primers and Smart Quant Green Master Mix with dUTP and ROX (Protech, PT-GL-SQGLR-V3) using StepOne real-time PCR machine (Applied Biosystems, Foster City, USA). Ct values for both the target and internal control genes were calculated, and the relative changes in gene expression were analyzed by 2−ΔΔCt method. The mRNA of β-actin was used to normalize the total amount of cDNA on real-time PCR.
Primers were designed as follows:
Myogenin forward: 5′-ACAGCATCACGGTGGAGGATATGT-3′, reverse: 5′-CCCTGCTACAGAAGTGATGGCTTT-3′;
MyoD forward: 5′-ACGACTGCTTTCTTCACCACTCCT-3′, reverse: 5′-TCGTCTTAACTTTCTGCCACTCCG-3′;
MAFbx forward: 5′-CGACCTGCCTGTGTGCTTAC-3′, reverse: 5′-CTTGCGAATCTGCCTCTCTG-3′;
MuRF-1 forward: 5′-GGTGCCTACTTGCTCCTTGT-3′, reverse: 5′-CTGGTGGCTATTCTCCTTGG-3′.
Determination of Bone Microarchitecture Indices
For the assessment of bone microarchitecture indices in bone marrow, femur, and tibia were collected and fixed in 70% alcohol solution. Micro–computed tomography (micro-CT; Skyscan 1076) was used to conduct trabecular bone volume/total tissue volume (BV/TV; %), trabecular number (Tb.N; mm−1), trabecular thickness (Tb.Th; mm), and trabecular separation (Tb.Sp; mm) assays.
Histological Analysis
For the assessment of pathological changes of the stomach, stomach tissues were obtained and fixed in 10% formalin for 48 hours. Then, stomach samples embedded in paraffin were stained with hematoxylin and eosin (H&E) dye. After staining, slides were observed under Olympus BX60 microscope. In addition, gastrocnemius muscles were formalin fixed, embedded in paraffin, and cross-sectioned at the midpoint of the muscle. Sections were mounted on slides and stained with H&E. Digital images were captured using Zeiss Axio Imager A2 microscope and analyzed with ImageJ software for myofiber diameter (original magnification, 200) and cross-sectional area (CSA). Myofiber diameter was determined as described previously5 by measuring the longest minor axis (the perpendicular bisector of the major axis) of at least 100 neighboring myofibers per animal. CSA of at least 50 muscle fibers/animal were measured.
Statistical Analysis
IBM SPSS Statistics 19 was used for all statistical analysis. All data are presented as mean ± standard deviation (SD). Statistical comparisons of the different treatment groups were carried out by 1-way analysis of variance followed by Tukey’s test adjustments for multiple comparisons. P < .05 was considered statistically significant.
Results
d-Methionine Alleviates Weight Loss, Promotes Food and Water Intake During Cisplatin Treatment
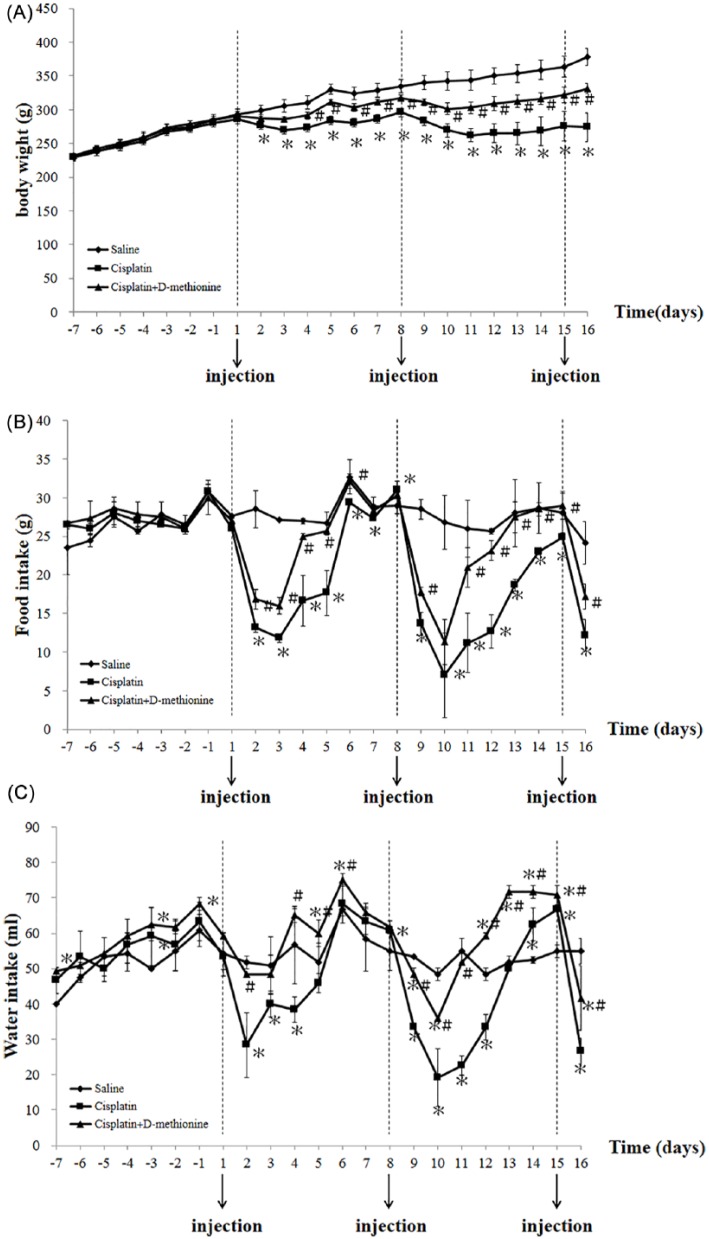
A previous study has shown that guinea pigs treated with d-methionine combined with cisplatin lose less body weight than those treated with cisplatin alone in a model of short-term exposure to cisplatin (at the molar ratio of 121:1, d-methionine:cisplatin).13 To evaluate the effects of d-methionine on cisplatin-induced body weight loss under long-term conditions, cisplatin was administered intraperitoneally once a week for 3 successive weeks. In our study, anorexia, loss of adipose tissue and skeletal muscle wasting were generated in a model of repeated exposure to 5 mg/kg of cisplatin once a week for 3 weeks. We observed an obvious decline in body weight after cisplatin injections (Figure 1A). It is worth noting that in rats receiving cisplatin treatment alone, weight loss progressed with increasing numbers of injections. In the cisplatin combined with d-methionine group, body weight also decreased, but this reduction was less prominent compared with cisplatin-treated group. The final body weights of cisplatin-treated rats and cisplatin combined with d-methionine rats showed decreases of 27.5% and 12.4%, respectively, when compared with the saline group. Similar results were observed for food and water intakes. The food intakes of cisplatin-treated rats and cisplatin combined with d-methionine rats decreased by 49.5% and 29%, respectively. Water consumption by cisplatin-treated rats and cisplatin combined with d-methionine rats was reduced by 51.5% and 24.2%, respectively. Thus, oral administration of d-methionine ameliorates cisplatin-induced weight loss and promotes greater intakes of food and water.
Figure 1.
Changes in body weight, food intake, and water intake during the experimental period among the 3 groups. (A) Body weights, (B) food intake, and (C) water intake were recorded daily. Data are presented as mean ± SD, n = 8. Differences were analyzed by 1-way analysis of variance. *Indicates statistical significance when compared with the saline-treated group (P < .05). #Indicates statistical significance when compared with the cisplatin-treated group (P < .05).
d-Methionine Alleviates the Decreases in Spleen and Liver Weights and Increases Some Relative Organ Weights
Table 2 shows that cisplatin treatment resulted not only in significant reductions in body weight and spleen and liver weights but also an increase in kidney relative weight compared with the saline-treated group. In the group receiving cisplatin combined with d-methionine, decreases in spleen and liver weights were significantly alleviated with increases in the ratios of heart/body weight, kidney/body weight, and stomach/body weight compared with the cisplatin group.
Table 2.
Changes in Organ Weights and Tissue Somatic Indices (TSI) After Cisplatin and d-Methionine Administration.a
| Saline (n = 8) | Cisplatin (n = 8) | Cisplatin + d-Methionine (n = 8) | |
|---|---|---|---|
| (A) Organ weight: | |||
| Heart (g) | 1.1 ± 0.1 | 1.0 ± 0.2 | 1.1 ± 0.1 |
| Spleen (g) | 0.9 ± 0.1 | 0.6 ± 0.1* | 0.7 ± 0.1*# |
| Liver (g) | 14.6 ± 1.0 | 10.9 ± 1.0* | 13.3 ± 0.7# |
| Kidney (g) | 3.0 ± 0.4 | 2.8 ± 0.6 | 2.8 ± 0.4 |
| Stomach (g) | 2.0 ± 0.2 | 1.9 ± 0.2 | 2.0 ± 0.2 |
| (B) TSIb | |||
| Heart/body weight ratio (%) | 0.30 ± 0.03 | 0.38 ± 0.02* | 0.35 ± 0.03*# |
| Spleen/body weight ratio (%) | 0.23 ± 0.03 | 0.20 ± 0.04 | 0.22 ± 0.03 |
| Liver/body weight ratio (%) | 3.65 ± 0.42 | 3.81 ± 0.27 | 3.75 ± 0.37 |
| Kidney/body weight ratio (%) | 0.77 ± 0.04 | 1.10 ± 0.23* | 0.83 ± 0.08# |
| Stomach/body weight ratio (%) | 0.51 ± 0.03 | 0.72 ± 0.05* | 0.60 ± 0.06*# |
Values are presented as mean ± SD.
TSI means relative tissue weight (tissue weight/body weight, g/g).
Indicates statistical significance when compared with the saline-treated group (P < .05).
Indicates statistical significance when compared with the cisplatin-treated group (P < .05).
d-Methionine Administration Improves Gastrointestinal Dysfunction
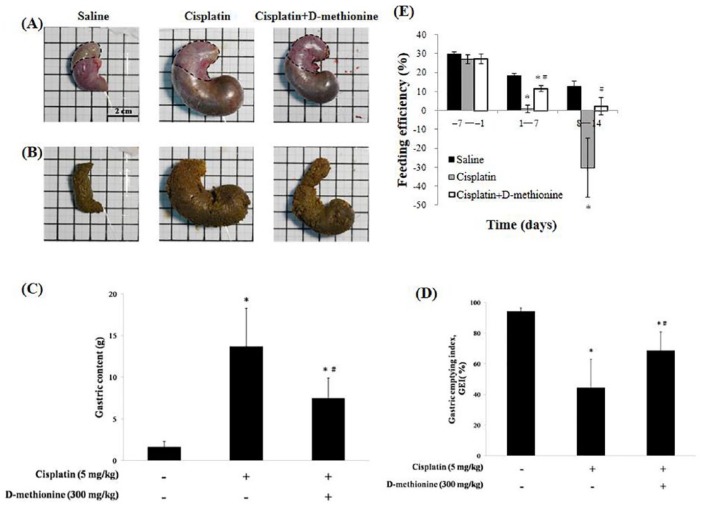
Anorexia and early satiety are 2 main factors for decreased food intake after cisplatin treatment. It is widely accepted that cisplatin frequently induces gastrointestinal dysfunction.18 To determine whether cisplatin treatment affect feeding efficiency and gastric emptying, we recorded the gastric content load and calculated the GEI on the day of euthanasia. There were visible signs of stomach distension and gastric stasis in cisplatin-treated rats when compared with saline and cisplatin combined with d-methionine rats (Figure 2). As shown in Figure 2, the feeding efficiency markedly declined from the 8th to the 15th day in cisplatin-treated rats due to increased gastric content, which also led to decreased GEI. In contrast to the cisplatin alone group, oral administration of d-methionine increased feeding efficiency. The rats treated with cisplatin showed gastric emptying delay (Figure 2D). Delayed gastric emptying was alleviated by d-methionine administration. Based on these results, d-methionine administration improves gastrointestinal tract function in rats treated with cisplatin.
Figure 2.
Effects of d-methionine on gastrointestinal toxicity after cisplatin treatment. (A) Views of stomachs of the 3 groups. The dotted line borders the gastric fundus. (B) Gastric contents. (C) Quantity of gastric contents. (D) Gastric emptying index (GEI). (E) Feeding efficiency. Data are presented as mean ± SD, n = 8. Differences were analyzed by 1-way analysis of variance. The asterisk (*) indicates significant differences when compared with the saline group, and the hashtag (#) with cisplatin group (P < .05).
Effects of Cisplatin and d-Methionine on the Weights of Visceral Fat and Skeletal Muscle
We examined whether reduced body weight results in marked depletion of visceral fat and skeletal muscle, by measuring the weights of visceral adipose tissue (epididymal, mesenteric, and perirenal) and skeletal muscle (gastrocnemius muscle, soleus muscle, tibialis anterior muscle, and extensor digitorum longus). We found that the weights of visceral adipose tissue and skeletal muscle mass were significantly reduced in cisplatin-treated rats when compared with saline group (Table 3). In the cisplatin combined with d-methionine group, there were significantly increased visceral adipose tissues and skeletal muscle mass when compared with cisplatin group.
Table 3.
Weights of Visceral Adipose Tissue (Epididymal, Mesenteric, and Perirenal Fat) and Hind Leg Muscles (Gastrocnemius Muscle, Soleus Muscle, Tibialis Anterior Muscle, and Extensor Digitorum Longus) of the 3 Groups.a
| Saline (n = 8) | Cisplatin (n = 8) | Cisplatin + d-Methionine (n = 8) | |
|---|---|---|---|
| Epididymal fat (g) | 3.8 ± 1.2 | 1.7 ± 0.4* | 3.1 ± 0.3# |
| Mesenteric fat (g) | 4.3 ± 0.5 | 2.2 ± 0.7* | 3.2 ± 0.4*# |
| Perirenal fat (g) | 3.9 ± 1.3 | 0.30 ± 0.49* | 1.7 ± 0.2*# |
| Gastrocnemius muscle (g) | 4.2 ± 0.2 | 3.1 ± 0.5* | 3.7 ± 0.2*# |
| Soleus muscle (g) | 0.34 ± 0.04 | 0.21 ± 0.75* | 0.32 ± 0.03# |
| Tibialis anterior muscle (g) | 2.0 ± 0.3 | 1.0 ± 0.2* | 1.7 ± 0.3# |
| Extensor digitorum longus (g) | 0.32 ± 0.03 | 0.22 ± 0.01* | 0.29 ± 0.02# |
Values are presented as mean ± SD.
Indicates statistical significance when compared with the saline-treated group (P < .05).
Indicates statistical significance when compared with the cisplatin-treated group (P < .05).
Effects of d-Methionine on Cisplatin-Induced Muscle Atrophy
The C2C12 cell line differentiates rapidly, forming contractile myotubes and producing characteristic muscle proteins. Hence, C2C12 myoblasts derived from satellite cells are commonly used as an in vitro system to study muscle development and differentiation.3 The differentiation of C2C12 myoblasts into multinucleated myotubes is through cell fusion process. Terminally differentiated myotubes will form multinucleated myofibers which fill with myofibrils. Thus, skeletal muscles generally consist of bundles of multinucleated myofibers (fibroblast-like; fusiform).19
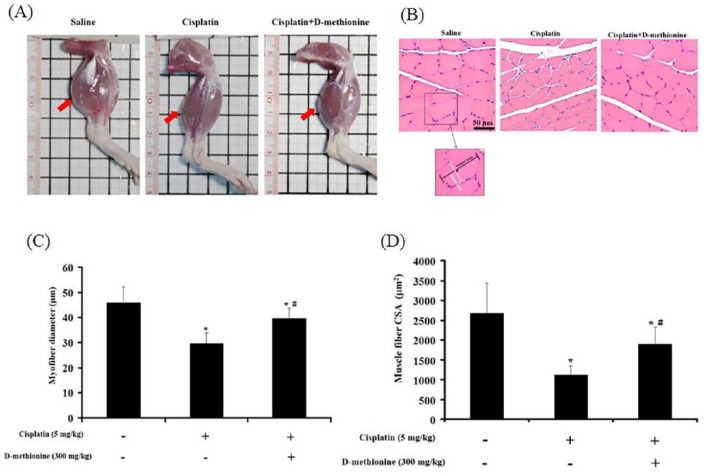
Hind limbs in cisplatin-induced group were smaller than in saline-treated group and cisplatin combined with d-methionine-treated group (Figure 3A). Next, morphometric analysis of the gastrocnemius muscle was performed in the three groups. The diameter of myofibers and CSAs of the gastrocnemius muscle were significantly reduced in cisplatin-treated rats when compared with saline-treated rats. The administration of d-methionine resulted in significant improvements in myofiber diameter and cross-sectional fiber areas (Figure 3B-D). Histopathological analysis of skeletal muscle tissues showed no significant evidence of inflammatory infiltrates, necrotic degeneration, or fibrosis in any of the tested animals.
Figure 3.
d-Methionine alleviated cisplatin-induced muscle mass loss and muscle atrophy. (A) Images of hind limbs. Arrowheads indicate gastrocnemius muscle. (B) Hematoxylin and eosin–stained cross sections of gastrocnemius muscle are shown at 200× magnification (bar scale = 150 µm) for calculation of fiber diameters. (C) Typical length of myofiber diameter (minor axis), the areas of 100 myotubes were measured in at least 10 fields. (D) Cross-sectional areas of at least 50 muscle fibers/animal were measured, with 6 animals analyzed for each determination. Values are presented as mean ± SD. *Indicates statistical significance when compared with the saline-treated group (P < .05). #Indicates statistical significance when compared with the cisplatin-treated group (P < .05).
Effects of d-Methionine on Hepatic Lipid Metabolism–Related gene Expressions Following Cisplatin Administration
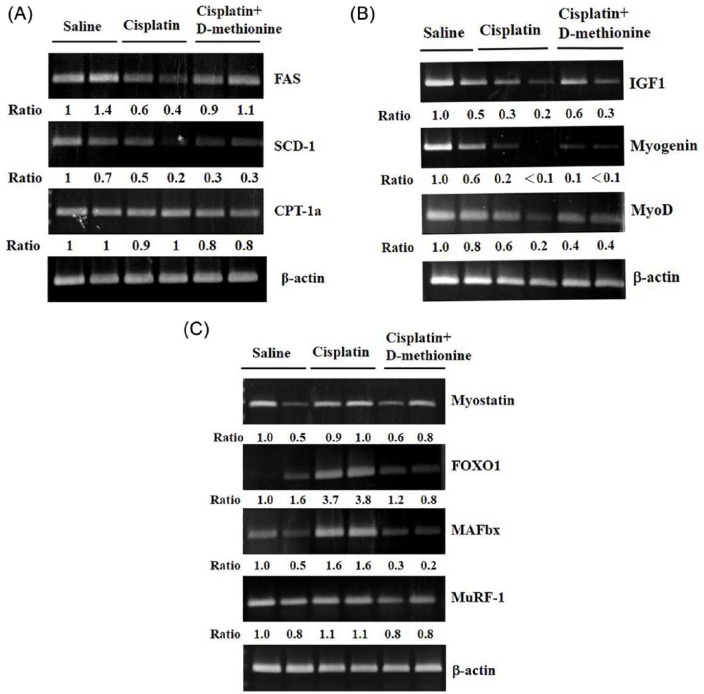
Garcia et al6 reported that cisplatin suppresses lipogenesis in liver. To understand the effects of d-methionine on hepatic lipid metabolism in cisplatin-induced cachexia condition, we used RT-PCR to investigate hepatic regulation of lipid metabolism genes, including those involved in de novo lipogenesis, polyunsaturated fatty acid synthesis, and fatty acid β-oxidation. SCD-1 is a component of the polyunsaturated fatty acid synthesis system. RT-PCR analysis showed that mRNA expression of SCD-1 decreases following cisplatin (Figure 4A). FAS, a key enzyme required for de novo lipogenenesis, is involved in long-chain fatty acid synthesis. FAS mRNA expression was lower in the liver tissues of cisplatin-treated rats than in the liver tissues of saline rats. Interestingly, d-methionine administration increased FAS mRNA expression, but not SCD-1 mRNA expression. There was no significant change in mRNA level of CPT-1α, the key regulatory enzyme in fatty acid β-oxidation, among the 3 groups (Figure 4A).
Figure 4.
Effects of d-methionine on hepatic lipid gene expression, muscle protein degradation, and protein synthesis–related mRNA expressions after cisplatin treatment. (A) Lipid metabolism gene expressions in liver. (B) Protein degradation– and (C) protein synthesis–related gene expressions in gastrocnemius muscle.
Effects of d-Methionine on Muscle Atrophy–Related Gene Expressions Following Cisplatin Administration
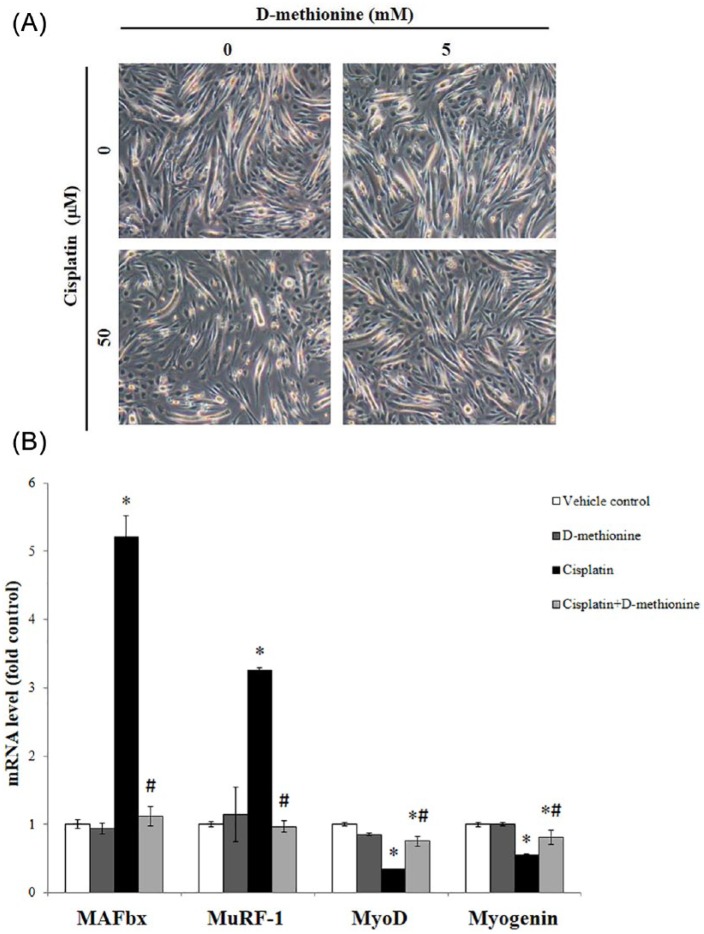
To further investigate the possible anti-muscle atrophy mechanisms of d-methionine, muscle atrophy-related gene mRNA expressions in gastrocnemius muscle were measured using RT-PCR. Cisplatin decreased IGF-1 mRNA expression level relative to saline rats (Figure 4B). Administration of d-methionine slightly reversed IGF-1 mRNA expression. Additionally, mRNA levels of myogenin and MyoD, muscle protein synthesis–related genes, decreased following the administration of cisplatin. However, d-methionine administration did not result in increases in these 2 gene mRNA expressions. The level of myostatin mRNA expression slightly increased in cisplatin group and cisplatin combined with d-methionine groups when compared with saline group. However, there were no significant differences between cisplatin and cisplatin combined with d-methionine group. FOXO1 and MAFbx mRNA expressions were elevated in gastrocnemius muscle in cisplatin-treated rats, compared with the saline group. There were no significant increases in mRNA levels of FOXO1 or MAFbx with d-methioine administration (Figure 4C). There were also no significant changes in the expression of MuRF-1 mRNA among the 3 groups. These results indicated that cisplatin-induced muscle mass wasting is mediated by elevation of mRNA expression of MAFbx/atrogin-1 in ubiquitin ligases and by decreases in mRNA expressions of MyoD and Myogenin. Administration with d-methionine inhibited muscle atrophy-related gene expressions and had a tendency toward slight recovery of MyoD and myogenin expressions.
The Effect of Cisplatin and d-Methionine on Gene Expression of Myogenesis (Myogenin and MyoD) and Specific E3-Ubiquitin Ligases (MAFbx and MuRF-1) in C2C12 Myocytes
To further investigate whether there is a direct preventive effect of d-methionine against myogenesis and specific E3-ubiquitin ligases in skeletal muscle, real-time PCR was employed to test cultured C2C12 myocytes. According to the work of Fanzani et al,20 50 μM cisplatin was the ideal dose in our experiments. After 50 µM cisplatin exposure for 24 hours, myotubes dramatically changed in size, becoming thinner, longer, and narrower. Interestingly, pretreatment with d-methionine for 1 hour led to the nearly full restoration of the morphology of myotubes to resemble that of controls in cisplatin-treated cells. Remarkably, d-methionine treatment did not affect myotube morphology (Figure 5A). In addition, cisplatin increased the mRNA levels of MAFbx and MuRF-1. Meanwhile, the mRNA expressions of myogenin and MyoD were significantly downregulated in cisplatin-induced cells when compared with control cells. Pretreatment with d-methionine for 1 hour led to the downregulation of the expressions of MAFbx and MuRF-1 and the upregulation of the expressions of myogenin and MyoD when compared with cells treated with cisplatin alone (Figure 5B).
Figure 5.
Effects of d-methionine on myogenesis (myogenin and MyoD) and specific E3-ubiquitin ligase (MAFbx and MuRF-1) mRNA levels in cisplatin-induced C2C12 myotube atrophy. C2C12 myotubes were treated with Dulbecco’s modified Eagle medium (DMEM), 50 µM cisplatin or 50 µM cisplatin + 5 mM d-methionine for up to 24 hours. (A) Cellular morphological changes. (B) Real-time polymerase chain reaction. The mRNA expression levels of MAFbx, MuRF-1, myogenin, and MyoD were normalized according to the amount of β-actin. Results are representative of 3 independent experiments. Values are presented as Mean ± SD. *Indicates statistical significance when compared with the saline-treated group (P < .05). #Indicates statistical significance when compared with the cisplatin-treated group (P < .05).
Trabecular Bone Microarchitecture Analysis
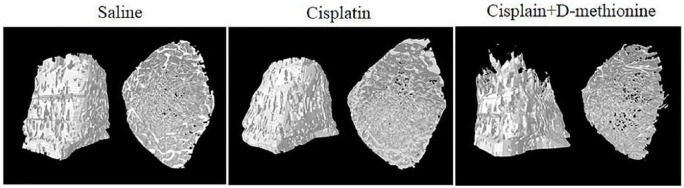
Micro-CT images demonstrated no differences in the microarchitecture of trabecular bone of tibia among the 3 groups of rats (Figure 6). Bone structural integrity and compactness were evident in the saline group. There were no differences in the degree of bone density in cisplatin and cisplatin combined with d-methionine groups when compared with saline group. We also performed quantification of bone microarchitecture. There were no significant changes in Tb.N, BS/TV, or BV/TV, but there was an increase in Tb.Th. in the cisplatin-treated rats when compared with the saline group. With d-methionine administration, Tb.Th. and Tb.Sp. increased. However, there were no significant differences in BV/TV, Tb.Th, Tb.N. or SMI between cisplatin and cisplatin combined with d-methionine-treated rats (Table 4). Hence, cisplatin did not cause bone damage in our animal model.
Figure 6.
Micro–computed tomography 3-dimensional image analysis of the tibia.
Table 4.
Morphological Parameters of Trabecular Bone Microarchitecture.a
| Saline (n = 8) | Cisplatin (n = 8) | Cisplatin + d-Methionine (n = 8) | |
|---|---|---|---|
| Percent bone volume (BV/TV; %) | 26.5 ± 4.1 | 26.6 ± 9.6 | 24.6 ± 2.3 |
| Trabecular thickness (Tb.Th; mm) | 0.08 ± 0.006 | 0.10 ± 0.004* | 0.09 ± 0.003* |
| Trabecular separation (Tb.Sp; mm) | 0.27 ± 0.07 | 0.47 ± 0.09 | 0.56 ± 0.14* |
| Trabecular number (Tb. N; mm−1) | 3.24 ± 0.29 | 2.65 ± 0.85 | 2.63 ± 0.32 |
| Structure model index (SMI) | 1.44 ± 0.17 | 1.18 ± 0.61 | 1.29 ± 0.13 |
Values are presented as mean ± SD.
Indicates statistical significance when compared with the saline-treated group (P < .05).
Indicates statistical significance when compared with the cisplatin-treated group (P < .05).
Histopathological Examination
As depicted in Figure 2, cisplatin inhibits food ingestion. Analytical results of H&E staining of mouse gastric fundus mucosa have revealed decreases in mucosal thickness and diffuse edema of submucosa after cisplatin administration.21 We sought to see if cisplatin can lead to stomach injury, H&E staining was performed to evaluate histopathological changes to stomach tissues. No marked histopathological morphology was detected in the stomach tissues of the 3 groups (data not shown). Functional dyspepsia is a common functional gastrointestinal disorder without identifiable cause on conventional diagnostic evaluation. The symptoms are usually associated with food ingestion and include early satiety, feeling of fullness, bloating, belching, nausea, vomiting, and loss of appetite.22 Thus, we speculated that cisplatin would cause functional dyspepsia rather than stomach injury.
Discussion
In the present study, we evaluated the efficacy of d-methionine in the prevention of cisplatin-induced anorexia-cachexia in a rat model of chronic cisplatin administration. We observed that d-methionine improves cisplatin-induced anorexia, mainly by promoting feeding efficiency and gastric emptying (Figure 2). d-Methionine was also found to lessen the loss of visceral adipose tissue and reduce atrophy of skeletal muscle (Table 3). The administration of d-methionine resulted in significant improvements in myofiber diameter and cross-sectional fiber areas (Figure 3B-D). d-Methionine protects against muscle atrophy/muscle mass loss, at least partially, by alleviating the increases in FOXO1 and MAFbx mRNA expressions (Figure 4).
Anorexia is usually defined as loss of desire to eat. Numerous studies have shown that cisplatin adversely affects food intake,3,5,7,23 which is significantly associated with declining body weight. In the present study, the toxic effects of cisplatin on appetite, body weight and feeding efficiency increased with accumulated treatment times and doses (Figure 2 E). This finding confirms that cisplatin-induced toxicity was dose dependent.7 Changes in body weight and food intake after each cisplatin injection were reported by Malik et al,24 who showed that administration of cisplatin significantly reduces food intake on the first day following cisplatin, reaching a nadir at 2 days and progressively recovering thereafter. We also observed the same phenomenon, as shown in Figure 1A and B. Our results revealed that the animals treated with d-methionine combined with cisplatin lose less weight than cisplatin only; these results are in agreement with previous studies.10-13,25 Based on these results, d-methionine has antianorexic effect under the cisplatin treatment condition.
In addition, cisplatin has been shown to decrease feeding efficiency3,6 and to induce gastric stasis and stomach distension defined as an abnormal accumulation of gases and food in the stomach.26 The relation of stomach distension to gastric emptying is shown in Figure 2A and D and reveals that gastric emptying is delayed by cisplatin, which is consistent with a previous report by Abalo et al.27 Their radiographic study demonstrated that cisplatin induces notable gastric distension and this response is strongly visible for the first 2 hours after cisplatin injection.27 Cisplatin may induce early satiety (the perceived feeling that the stomach is full soon after starting to eat) which is associated with reduced gastric emptying, and leads to appetite loss afterward. Our results revealed that d-methionine administration enhances gastric emptying and feeding efficiency decreases gastric contents. In an aquaculture study, the supplementation of optimal dietary methionine improved the growth performance and feed utilization in juvenile yellow catfish.28
The complex multifactorial etiology of cisplatin-induced anorexia has yet to be fully elucidated. The results of in situ hybridization histochemistry and RT-PCR have shown that many feeding-regulating peptides in the hypothalamus, such as neuropeptide (NPY) and proopiomelanocortin (POMC), are involved in cisplatin-induced anorexia.24,29 In addition, cisplatin-induced anorexia is mediated by decreases in plasma ghrelin level and serotonin (5-HT).30 The mechanisms of the protective effect of d-methionine against cisplatin-induced anorexia are not fully understood. In vitro studies using a metallomics approach9 have revealed that the products of Pt–d-methionine complex may form in human plasma when cisplatin and d-methionine are added. These Pt–d-methionine complexes may play an important role in ameliorating the toxic side effects of cisplatin in animal models. In addition, we are currently studying the relationship between d-methionine and appetite-regulating factors to determine how d-methionine exerts its antianorexic effect.
l-Methionine, one of the sulfur-containing indispensable amino acids, participates in protein synthesis, methyl transfer and the synthesis of cysteine and cystine. l-Methionine is converted to cysteine, taurine, and glutathione via transsulfuration.31 These molecules, acting as antioxidants, may regulate intestinal epithelial antioxidant function, and may improve intestinal mucosal integrity and gut function.32,33 Meanwhile, the aquaculture study of juvenile yellow catfish indicated that the appropriate methionine level may be beneficial to enhance antioxidant status, innate immune response and protect against the infection of pathogenic bacteria.28 These results could also partially explain the present results. d-Methionine, through the enhancing of enterocyte antioxidant capacity may establish a prophylactic barrier against cisplatin-induced gastrointestinal dysfunction.
Increase in kidney/body weight ratio has been suggested as a parameter of nephrotoxicity induced by cisplatin.34 As shown in Table 2, the weights of spleen and liver decreased, and the ratios of heart/body weight, kidney/body weight and stomach/body weight increased, in cisplatin-treated rats. Orally administered d-methionine alleviated these changes. It is worth mentioning that the weight of heart did not gain, but the ratio of heart/body weight did increase in the cisplatin only group in comparison with saline group. Cisplatin generates reactive oxygen species, which damages various organs, including the heart.35 Further studies are needed to verify the correlation between increased heart relative body weight and cardiotoxicity. The results of the current study are consistent with those of previous reports3,5,36 in that cisplatin produced substantial weight loss associated with pronounced reduction in skeletal muscle mass (Figure 1A and B) and caused shortening of myofibers.5,36 Our study showed that preadministration with d-methionine prevents muscle atrophy/muscle mass loss.
Loss of skeletal muscle mass is an important characteristic of cachexia.3 FOXO-1, a member of the Foxo forkhead type transcription factors, plays a critical role in skeletal muscle atrophy signaling pathways and is markedly upregulated in skeletal muscle in energy-deprived conditions such as cisplatin-induced anorexia cachexia.5 MAFbx and MuRF-1, ubiquitin-ligating enzymes, are involved in the proteolysis of myofibrillar proteins.1 FOXO-1 can bind to the promoter regions of either the MuRF-1 or MAFbx gene, leading to increases in their expression levels within muscle. Increased expressions of MuRF-1 and MAFbx following atrophy stimulant are thought to be responsible for the shift in protein balance from net synthesis to net degradation, inducing a loss of muscle mass.37 The cisplatin-induced wasting of muscle mass is mediated by the elevation of mRNA levels of MAFbx and MuRF-1, and by the decline in expressions of MyoD and myogenin.3,5 Consistent with these studies, we observed that the gene expressions of FOXO-1 and MAFbx in skeletal muscles are upregulated by cisplatin, resulting in muscle protein degradation. Long-term oral administration of d-methionine effectively decreased FOXO-1 and MAFbx mRNA expressions, indicating that d-methionine attenuates protein degradation. However, MuRF-1 mRNA expression in gastrocnemius muscle did not significantly differ among the three groups. It has been demonstrated that MyoD and myogenin (myogenic regulatory factors) are involved in the regulation of muscle regeneration.38 MyoD and myogenin were downregulated by cisplatin, with no markedly reverse effect by d-methionine, suggesting that d-methionine does not affect protein synthesis. Activation of IGF-1 and activation of myostatin signaling are involved in muscle hypertrophy and atrophy, respectively.5 Moreover, cisplatin increases myostatin mRNA expression level and abolishes IGF-1 expression in the quadriceps muscle.5 Our findings did not provide evidence in favor of a regulation of the expression of myostatin or IGF-1 after cisplatin treatment, suggesting that, at least in the present experimental model, other mediators drive cisplatin-induced muscle atrophy. In vivo, cisplatin induced the activation of FOXO-1 and MAFbx and decreases in myogenin and MyoD led to an increased proteolysis and decreased muscle mass. To date, the research on d-methionine in the modulation of muscle atrophy is lacking. d-Methionine protects against muscle atrophy/muscle mass loss, at least partially, by alleviating the increases in FOXO-1 and MAFbx mRNA expressions (Figure 4).
Cisplatin was found to induce C2C12 myotube atrophy either through the downregulation of Akt and myogenic differentiation (MyoD and myogenin) or the activation of autophagy system, myostatin pathways and ubiquitin ligases.3,20 A previous study demonstrated that the induction of C2C12 myotube atrophy is due to the activation of the NF-κB signaling pathway, not the ubiquitin-proteasome system.39 The mechanisms of muscle wasting by cisplatin are complex and multifactorial. In agreement with the findings of previous reports,3 cisplatin caused direct atrophy of C2C12 myotubes, characterized by increases in the levels of ubiquitin ligase (MAFbx and MuRF-1) mRNA expressions36 and decreases in the mRNA levels of myogenic regulatory factors (MyoD and myogenin). The effects of cisplatin treatment on C2C12 cells are considered predictive of impairment of the process of muscle differentiation. d-Methionine blunts cisplatin-induced suppression of protein synthesis and activation of protein degradation. These in vitro studies suggest that pretreatment with d-methionine effectively prevents the formation of muscle atrophy by mediating the proteolysis pathways independent of its orexigenic (appetite-stimulating) actions. These findings suggested that d-methionine alleviates cisplatin toxic side effects because of its antioxidant abilities12,13 or the formation of Pt–d-methionine complex,9 which neutralizes toxicity.
From Table 3, the rats treated with cisplatin combined with d-methionine had greater visceral adipose tissue content than the rats treated with cisplatin alone. Garcia et al6 reported that cisplatin induces fat mass loss in correlation with inhibition of de novo lipogenesis in white adipose tissue, liver, and muscle.6 In our rat model, we also observed that cisplatin decreases the expressions of hepatic lipogenic enzymes FAS and SCD-1. The decrease in the expressions of FAS was attenuated by oral d-methionine administration. Recent in vitro studies using human plasma have found that d-methionine may react with a highly reactive cisplatin hydrolysis product in the bloodstream and/or in tissues. Accordingly, it is possible that highly toxic cisplatin hydrolysis products are neutralized by d-methionine,9 which would explain d-methionine-induced increase in FAS mRNA expression in the liver. In cancer cachexia, adipose tissue wasting results from increase in lipolytic activity and decreases in activities of lipoprotein lipase and de novo lipogenesis in adipose tissue.1 In the current study, we did not explore lipid metabolism in adipose tissue. Because of the lack of research on lipid metabolism in adipose tissue, there is no evidence to explain the relationship between loss of adipose tissue and lipogenesis/lipolysis in cisplatin-induced anorexia and cachexia in rats.
It is also well known that chemotherapy can disrupt the bone microenvironment, leading to changes in marrow cellular composition.40 Our results indicated that cisplatin treatment with/without d-methionine may not have increased the risks of chemotherapy-induced microarchitectural deterioration of bone in our low-dose, long-term experimental model (Table 4 and Figure 6). Analyses for hematopoietic cells within the bone marrow are required in cancer patients over the course of high-dose chemotherapy regimens to routinely monitor chemotherapy-induced hematopoietic defects.
In conclusion, the chemopreventive efficacy of d-methionine, as evidenced by the improvement in appetite and recovery of lost body weight, helps prevent muscle mass loss. Furthermore, d-methionine protects against muscle atrophy/muscle mass loss, at least partially, by alleviating the increases in FOXO-1 and MAFbx mRNA expressions.
Acknowledgments
The authors would like to thank Dr Kai-Li Liu (Department of Nutrition, Chung Shan Medical University) for her kind instructions and advice in maintaining the muscle cell culture and muscle fiber analysis. We also thank the Taiwan Mouse Clinic for technical support in micro-CT experiment.
Footnotes
Ethical Approval and Consent to Participate: The experimental protocol for this study was approved by the Instituted Animal Care and Use Committee, Chung Shan Medical University Experimental Animal Center, Taichung, Taiwan (Approval No: 1439). The treatment of the rats was according to the guidelines of the Instituted Animal Care and Use Committee of Chung Shan Medical University for the care and use of laboratory animals.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Chung Shan Medical University Grant (CSMU-SHOW-105-02 and CSMU-JAH-106-03).
References
- 1. Argiles JM, Busquets S, Stemmler B, Lopez-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer. 2014;14:754-762. [DOI] [PubMed] [Google Scholar]
- 2. von Haehling S, Anker SD. Prevalence, incidence and clinical impact of cachexia: facts and numbers—update 2014. J Cachexia Sarcopenia Muscle. 2014;5:261-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen JA, Splenser A, Guillory B, et al. Ghrelin prevents tumour- and cisplatin-induced muscle wasting: characterization of multiple mechanisms involved. J Cachexia Sarcopenia Muscle. 2015;6:132-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang H, Li TL, Hsia S, Su IL, Chan YL, Wu CJ. Skeletal muscle atrophy is attenuated in tumor-bearing mice under chemotherapy by treatment with fish oil and selenium. Oncotarget. 2015;6:7758-7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sakai H, Sagara A, Arakawa K, et al. Mechanisms of cisplatin-induced muscle atrophy. Toxicol Appl Pharmacol. 2014;278:190-199. [DOI] [PubMed] [Google Scholar]
- 6. Garcia JM, Scherer T, Chen JA, et al. Inhibition of cisplatin-induced lipid catabolism and weight loss by ghrelin in male mice. Endocrinology. 2013;154:3118-3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shiomi Y, Ohira Y, Yoshimura M, Ozaki T, Takei M, Tanaka T. Z-505 hydrochloride ameliorates chemotherapy-induced anorexia in rodents via activation of the ghrelin receptor, GHSR1a. Eur J Pharmacol. 2018;818:148-157. [DOI] [PubMed] [Google Scholar]
- 8. Schmoll HJ, Aapro MS, Poli-Bigelli S, et al. Comparison of an aprepitant regimen with a multiple-day ondansetron regimen, both with dexamethasone, for antiemetic efficacy in high-dose cisplatin treatment. Ann Oncol. 2006;17:1000-1006. [DOI] [PubMed] [Google Scholar]
- 9. Sooriyaarachchi M, White WM, Narendran A, Gailer J. Chemoprotection by d-methionine against cisplatin-induced side-effects: insight from in vitro studies using human plasma. Metallomics. 2014;6:532-541. [DOI] [PubMed] [Google Scholar]
- 10. Campbell KC, Rybak LP, Meech RP, Hughes L. d-Methionine provides excellent protection from cisplatin ototoxicity in the rat. Hear Res. 1996;102:90-98. [DOI] [PubMed] [Google Scholar]
- 11. Campbell KC, Meech RP, Rybak LP, Hughes LF. The effect of d-methionine on cochlear oxidative state with and without cisplatin administration: mechanisms of otoprotection. J Am Acad Audiol. 2003;14:144-156. [PubMed] [Google Scholar]
- 12. Cheng PW, Liu SH, Young YH, Lin-Shiau SY. d-Methionine attenuated cisplatin-induced vestibulotoxicity through altering ATPase activities and oxidative stress in guinea pigs. Toxicol Appl Pharmacol. 2006;215:228-236. [DOI] [PubMed] [Google Scholar]
- 13. Lo WC, Chang CM, Liao LJ, et al. Assessment of d-methionine protecting cisplatin-induced otolith toxicity by vestibular-evoked myogenic potential tests, ATPase activities and oxidative state in guinea pigs. Neurotoxicol Teratol. 2015;51:12-20. [DOI] [PubMed] [Google Scholar]
- 14. Vuyyuri SB, Hamstra DA, Khanna D, et al. Evaluation of d-methionine as a novel oral radiation protector for prevention of mucositis. Clin Cancer Res. 2008;14:2161-2170. [DOI] [PubMed] [Google Scholar]
- 15. Hamstra DA, Eisbruch A, Naidu MU, et al. Pharmacokinetic analysis and phase 1 study of MRX-1024 in patients treated with radiation therapy with or without cisplatinum for head and neck cancer. Clin Cancer Res. 2010;16:2666-2676. [DOI] [PubMed] [Google Scholar]
- 16. Lin MT, Ko JL, Liu TC, Chao PT, Ou CC. Protective effect of d-methionine on body weight loss, anorexia, and nephrotoxicity in cisplatin-induced chronic toxicity in rats. Integr Cancer Ther. 2018;17:813-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ou CC, Hsiao YM, Wu WJ, Tasy GJ, Ko JL, Lin MY. FIP-fve stimulates interferon-gamma production via modulation of calcium release and PKC-alpha activation. J Agric Food Chem. 2009;57:11008-11013. [DOI] [PubMed] [Google Scholar]
- 18. Badary OA, Awad AS, Sherief MA, Hamada FM. In vitro and in vivo effects of ferulic acid on gastrointestinal motility: inhibition of cisplatin-induced delay in gastric emptying in rats. World J Gastroenterol. 2006;12:5363-5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Endo T. Molecular mechanisms of skeletal muscle development, regeneration, and osteogenic conversion. Bone. 2015;80:2-13. [DOI] [PubMed] [Google Scholar]
- 20. Fanzani A, Zanola A, Rovetta F, Rossi S, Aleo MF. Cisplatin triggers atrophy of skeletal C2C12 myotubes via impairment of Akt signalling pathway and subsequent increment activity of proteasome and autophagy systems. Toxicol Appl Pharmacol. 2011;250:312-321. [DOI] [PubMed] [Google Scholar]
- 21. Pini A, Garella R, Idrizaj E, Calosi L, Baccari MC, Vannucchi MG. Glucagon-like peptide 2 counteracts the mucosal damage and the neuropathy induced by chronic treatment with cisplatin in the mouse gastric fundus. Neurogastroenterol Motil. 2016;28:206-216. [DOI] [PubMed] [Google Scholar]
- 22. Feinle-Bisset C, Vozzo R, Horowitz M, Talley NJ. Diet, food intake, and disturbed physiology in the pathogenesis of symptoms in functional dyspepsia. Am J Gastroenterol. 2004;99:170-181. [DOI] [PubMed] [Google Scholar]
- 23. Song MY, Ku SK, Kim HJ, Han JS. Low molecular weight fucoidan ameliorating the chronic cisplatin-induced delayed gastrointestinal motility in rats. Food Chem Toxicol. 2012;50:4468-4478. [DOI] [PubMed] [Google Scholar]
- 24. Malik NM, Moore GB, Smith G, Liu YL, Sanger GJ, Andrews PL. Behavioural and hypothalamic molecular effects of the anti-cancer agent cisplatin in the rat: a model of chemotherapy-related malaise? Pharmacol Biochem Behav. 2006;83:9-20. [DOI] [PubMed] [Google Scholar]
- 25. Cheng PW, Liu SH, Hsu CJ, Lin-Shiau SY. Correlation of increased activities of Na+, K+-ATPase and Ca2+-ATPase with the reversal of cisplatin ototoxicity induced by d-methionine in guinea pigs. Hear Res. 2005;205:102-109. [DOI] [PubMed] [Google Scholar]
- 26. Cabezos PA, Vera G, Castillo M, Fernandez-Pujol R, Martin MI, Abalo R. Radiological study of gastrointestinal motor activity after acute cisplatin in the rat. Temporal relationship with pica. Auton Neurosci. 2008;141:54-65. [DOI] [PubMed] [Google Scholar]
- 27. Abalo R, Cabezos PA, Vera G, Lopez-Perez AE, Martin MI. Cannabinoids may worsen gastric dysmotility induced by chronic cisplatin in the rat. Neurogastroenterol Motil. 2013;25:373-382.e292. [DOI] [PubMed] [Google Scholar]
- 28. Elmada CZ, Huang W, Jin M, Liang X, Maik K, Zhou Q. The effect of dietary methionine on growth, antioxidant capacity, innate immune response and disease resistance of juvenile yellow catfish (Pelteobagrus fulvidraco). Aquaculture Nutr. 2016;22:1163-1173. [Google Scholar]
- 29. Yoshimura M, Matsuura T, Ohkubo J, et al. The gene expression of the hypothalamic feeding-regulating peptides in cisplatin-induced anorexic rats. Peptides. 2013;46:13-19. [DOI] [PubMed] [Google Scholar]
- 30. Takeda H, Sadakane C, Hattori T, et al. Rikkunshito, an herbal medicine, suppresses cisplatin-induced anorexia in rats via 5-HT2 receptor antagonism. Gastroenterology. 2008;134:2004-2013. [DOI] [PubMed] [Google Scholar]
- 31. Metayer S, Seiliez I, Collin A, et al. Mechanisms through which sulfur amino acids control protein metabolism and oxidative status. J Nutr Biochem. 2008;19:207-215. [DOI] [PubMed] [Google Scholar]
- 32. Shoveller AK, Stoll B, Ball RO, Burrin DG. Nutritional and functional importance of intestinal sulfur amino acid metabolism. J Nutr. 2005;135:1609-1612. [DOI] [PubMed] [Google Scholar]
- 33. Chen Y, Li D, Dai Z, et al. l-Methionine supplementation maintains the integrity and barrier function of the small-intestinal mucosa in post-weaning piglets. Amino Acids. 2014;46:1131-1142. [DOI] [PubMed] [Google Scholar]
- 34. Guindon J, Hohmann AG. Use of sodium bicarbonate to promote weight gain, maintain body temperature, normalize renal functions and minimize mortality in rodents receiving the chemotherapeutic agent cisplatin. Neurosci Lett. 2013;544:41-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. El-Awady el-SE, Moustafa YM, Abo-Elmatty DM, Radwan A. Cisplatin-induced cardiotoxicity: mechanisms and cardioprotective strategies. Eur J Pharmacol. 2011;650:335-341. [DOI] [PubMed] [Google Scholar]
- 36. Sakai H, Kimura M, Tsukimura Y, et al. Dexamethasone exacerbates cisplatin-induced muscle atrophy. Clin Exp Pharmacol Physiol. 2019;46:19-28. [DOI] [PubMed] [Google Scholar]
- 37. Bodine SC, Baehr LM. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am J Physiol Endocrinol Metab. 2014;307:E469-E484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sabourin LA, Rudnicki MA. The molecular regulation of myogenesis. Clin Genet. 2000;57:16-25. [DOI] [PubMed] [Google Scholar]
- 39. Damrauer JS, Stadler ME, Acharyya S, Baldwin AS, Couch ME, Guttridge DC. Chemotherapy-induced muscle wasting: association with NF-κB and cancer cachexia. Basic Appl Myol. 2008;18:139-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Georgiou KR, King TJ, Scherer MA, Zhou H, Foster BK, Xian CJ. Attenuated Wnt/β-catenin signalling mediates methotrexate chemotherapy-induced bone loss and marrow adiposity in rats. Bone. 2012;50:1223-1233. [DOI] [PubMed] [Google Scholar]