Abstract
Survey-based studies show that neighborhood disadvantage is associated with community reported mental health problems. However, fewer studies have examined whether neighborhood characteristics have measurable impact on mental health status of individuals in general and whether neighborhood characteristics impact positive/negative valence processing at both behavioral and brain levels. This study addressed these questions by investigating effects of census-based neighborhood affluence on self-reported symptoms, brain functions, and structures associated with positive/negative valence processing in a sample of individuals with mood and anxiety disorders (n = 262). Employing a Bayesian inference approach, our investigation demonstrates that neighborhood affluence fails to be associated with positive/negative valence processing measured across multiple modalities, with the only effects of neighborhood affluence identified in trait anxiety scores. These findings highlight that while community-based relationships between neighborhood characteristics and mental health problems are strong, it is much less clear that these characteristics have a measurable impact on the individual.
Keywords: Neighborhood, Positive and negative valence systems, Mood and anxiety disorder, Bayes factor, Brain function and structure, Monetary incentive delay task
Highlights
-
•
Neighborhood affluence effects on positive/negative valence processing were studied.
-
•
A sample (n = 262) of patients with anxiety/mood disorders were recruited.
-
•
Symptoms, brain functions and structures of valence processing were recorded.
-
•
Bayesian inference was used to quantify evidence for alternative and null hypotheses.
-
•
Little evidence was found for neighborhood affluence effects across multiple levels.
1. Introduction
A growing literature reports that neighborhood disadvantage has a profoundly negative impact on physical and mental health, above and beyond individual-level effects (Jencks and Mayer, 1990; Rudolph et al., 2014). For instance, neighborhood disadvantage is often associated with coronary heart disease (Chi et al., 2016), cancer (Palumbo et al., 2016), depression (Kim, 2010; Latkin and Curry, 2003; Ross, 2000; Santiago et al., 2011), anxiety (Cerdá et al., 2017; Santiago et al., 2011; Stockdale et al., 2007), and substance use (Boardman et al., 2001; Karriker-Jaffe, 2013). Despite compelling evidence for the relationship between neighborhood disadvantage and poor health, the neural correlates of neighborhood effects remain largely unknown. The goal of this investigation was to determine whether there is a direct relationship between neighborhood defined affluence and individual differences in symptoms and brain processing in a sample of individuals with mood and/or anxiety disorders.
There are at least two potential neuropsychological pathways by which neighborhood disadvantage facilitates mood and anxiety problems, namely altered negative and positive valence processing. On the one hand, neighborhood disadvantage is linked with increased exposure to life stressors (e.g., violence) as well as enhanced vulnerability to deleterious effects of stressful life events (Aneshensel and Sucoff, 1996; Attar et al., 1994; Wilson et al., 2004). Likewise, there is evidence showing that effects of neighborhood disadvantage on mental health problems are mediated by stressful life events at the community level (Boardman et al., 2001; Ross, 2000). Furthermore, recent human brain imaging studies reveal that neighborhood disadvantage exhibits associations with altered brain morphology and functional connectivity in amygdala, hippocampus, and insula (Saxbe et al., 2018; Whittle et al., 2017). Saxbe et al.'s study (2018) indicates that neighborhood disadvantage measured as community violence exposure is related to smaller hippocampus and amygdala volumes as well as stronger resting state connectivity between hippocampus and insula. These regions are important for negative valence processing and often exhibit functional or structural perturbation among mood/anxiety disorders (Brühl et al., 2014; Etkin and Wager, 2007; Soares and Mann, 1997). Therefore, functional and structural alterations in these regions might mediate the effects of neighborhood on mood/anxiety problems. Notably, however, Gianaros et al. (2017) report that neighborhood disadvantage is not correlated to morphology of subcortical regions that are implicated in negative valence processing.
On the other hand, an accumulating body of evidence indicates that residing in disadvantaged neighborhoods accompanies alternations in reward processing, although current evidence is mixed (Gonzalez et al., 2016; Marshall et al., 2018). For instance, several studies demonstrate that family- or neighborhood-level disadvantage mainly impacts reward regulation in prefrontal regions (e.g., medial prefrontal cortex [mPFC]) rather than reward responding in striatal areas such as caudate and nucleus accumbens (NAcc) (Marshall et al., 2018; Muscatell, 2018; Romens et al., 2015). Socioeconomic disadvantage is positively correlated with mPFC activity during reward anticipation, implicating heightened suppression of striatal reward responding that leads to blunted reward sensitivity (Forbes et al., 2009). In accordance with these findings, heightened reward-related mPFC activity could mediate the relationship between socioeconomic disadvantage and depression symptoms (Romens et al., 2015). Likewise, neighborhood disadvantage is associated with reduced resting-state functional connectivity in fronto-striatal circuitry, and the reduced connectivity could mediate the association between neighborhood disadvantage and anxiety (Marshall et al., 2018). Hence, recent evidence suggests that neighborhood disadvantage mainly impacts regulatory responses to reward processing (but see also Gonzalez et al., 2016). Together, recent brain imaging studies suggest the hypothesis that neighborhood disadvantage alters negative and positive valence processing that are closely related to depression and anxiety.
The current work examined this hypothesis by assessing the effects of census-based neighborhood affluence on symptoms and brain functions/structures implicated in negative and positive valence processing among individuals with mood and/or anxiety disorders. We used a latent variable approach to identify factors that comprehensively quantify neighborhood characteristics (for details, see also Forthman et al., 2019). In particular, multivariate quantitative characterization of the neighborhood was derived by performing a factor analysis on the 2011–2015 American Community Survey data. Neighborhood affluence, the focus of the current study, was one of the robust factors showing the greatest loadings from neighborhood-level income and education.
Our work provides several advantages compared to previous studies of neighborhood-health relationships. First, the effects of neighborhood affluence were examined at multiple levels, from self-reported symptoms (e.g., positive and negative affect), to brain functions of reward and loss anticipation during a monetary incentive delay (MID) task (Knutson et al., 2001b), and brain morphology in regions important for positive and negative valence processing (e.g., striatum, amygdala). This multiple-level approach provides more holistic measurements and allows for convergent evidence on neighborhood effects to be revealed, which may provide a better understanding on the complex interplay between neighborhood affluence/disadvantage and symptoms, brain functions and structures. Second, Bayesian inference was employed to quantify the evidence on the presence or the absence of the neighborhood effects. In the Bayesian framework, a Bayes factor (BF10) was computed as a ratio of the amount of evidence the data provides for alternative (H1) and null (H0) hypotheses. Hence, BF10 scores allow for quantifying support both for the H1 hypothesis and for the H0 hypothesis (Jeffreys, 1998; Rouder et al., 2009; Wagenmakers et al., 2010). The application of Bayes factors rather than P-values has been recently advocated in multiple disciplines (Biel and Friedrich, 2018; Dienes, 2016; Han and Park, 2018; Wagenmakers et al., 2018), due to reasons that (i) interpretations of Bayes factors are more straightforward than P-values, since a P quantifies the discrepancy between the data and the null hypothesis without directly involves the alternative hypothesis while a Bayes factor directly evaluates the likelihood of the alternative hypothesis against that of the null hypothesis (Goodman, 2008; Wagenmakers, 2007); and (ii) there is an increasing acknowledgment on the importance of null findings (Atkinson et al., 2018; Moreau et al., 2018; Oldehinkel, 2018), and the Bayesian inference approach allows for accepting H0 hypothesis. Finally, the current study is the first to examine the effects of neighborhood affluence using a clinical sample of patients diagnosed with mood or anxiety disorders, which could offer more direct assessment of the specific clinical impact of neighborhood affluence.
Based on existing evidence, it was hypothesized that lower neighborhood affluence would be associated with more severe symptoms of depression and anxiety (Cerdá et al., 2017; Santiago et al., 2011). We expected that these potential clinical effects would be accompanied by: (i) enhanced insula activity to loss anticipation (Wu et al., 2014) and attenuated striatal activation to reward anticipation (Arrondo et al., 2015), which are respectively linked to trait negative arousal scores (Wu et al., 2014) and anhedonia/depressive symptoms (Arrondo et al., 2015); and (ii) enhanced mPFC responses to reward anticipation, as consistently observed in previous studies (Marshall et al., 2018; Muscatell, 2018; Romens et al., 2015). Likewise, neighborhood disadvantage was expected to alter brain morphology in regions important for negative/positive processing, such that lower neighborhood affluence might be linked with smaller amygdala, hippocampus, and insula volumes, as identified in several previous studies (Saxbe et al., 2018; Whittle et al., 2017). Finally, we also explored whether neighborhood affluence was associated with altered morphology in mPFC and striatal areas, such as NAcc and caudate, due to critical roles of these regions in reward processing (Haber and Knutson, 2010).
2. Materials and methods
2.1. Participants
Participants were comprised of the first 500 subjects of the Tulsa 1000 (T-1000) study, which assesses and longitudinally tracks 1000 adults. The reason why the first 500 subjects were employed in the current study is that The Tulsa-1000 was still an on-going project when the current manuscript was prepared. We aim to replicate the current results with the second half of the T-1000 participants when the study is completed. The T-1000 study aims to use the National Institute of Mental Health (NIMH) Research Domain Criteria (RDoC) framework to establish a robust and reliable dimensional set of variables quantifying the primary domains proposed by the RDoC, including the positive and negative valence systems (Victor et al., 2018). The T-1000 study was conducted at the Laureate Institute for Brain Research and approved by the Western Institutional Review Board. All participants provided written informed consent and were financially compensated. Participants with mood and/or anxiety issues were categorized using the following dimensional psychopathology cutoffs: Patient Health Questionnaire (PHQ-9) ≥ 10 (Kroenke et al., 2001) and/or Overall Anxiety Severity and Impairment Scale (OASIS) ≥ 8 (Campbell-Sills et al., 2009). In addition, participants completed a diagnostic interview using an abbreviated version of the Mini International Neuropsychiatric Interview (Sheehan et al., 1998). Among the 500 subjects, 262 participants (Fig. S1 and Table S1) met DSM-5 criteria for at least one mood and/or anxiety disorder and were included in the current analyses (Fig. S2 and Table S2). These participants were referred from local treatment facilities or seeking treatment for anxiety and/or depressive symptoms.
2.2. Experimental procedure and task
2.2.1. Demographics
Participants were asked to indicate their age, contact information (e.g. address), ethnicity, gender, average income, education level, and occupational status. Neighborhood affluence was measured based on U.S. Census data, which were geocoded to participants' residential addresses. Specifically, participants' neighborhood affluence was determined in two steps (Forthman et al., 2019): First, the factor neighborhood affluence (and four other factors: singletons in neighborhood, African-Americans in neighborhood, seniors in neighborhood, and noncitizens in neighborhood) was determined by factor analysis using tract-level data from the American Community Survey (ACS). In particular, we used a latent variable approach to identify factors that comprehensively quantify neighborhood characteristics, such that multivariate quantitative characterization of the neighborhood was derived by performing a factor analysis on the 2011–2015 ACS data (for details, see also Forthman et al., 2019). Details on the ACS data extraction, variable selection and factor were provided in the supplementary methods. Second, neighborhood affluence score was then assigned to each participant based on the resident tract.
2.2.2. Self-report questionnaires
Participants completed the following self-report questionnaires to assess positive and negative valence domains: (i) the PHQ-9 rates each of 9 DSM-IV criteria of depression from “0” (not at all) to “3” (nearly every day). Scores of 1–4 indicate minimal depression, 5–9 mild depression, 10–14 moderate depression, 15–19 moderately severe depression, and 20–27 severe depression (Kroenke et al., 2001); (ii) the OASIS is a 5-item questionnaire indexing anxiety-related severity and impairment across anxiety disorders. Each item is scored on a 5-point scale and the ratings are summed to obtain a total score. A cut-score of 8 correctly classifies 87% of individuals with an anxiety diagnosis or not (Campbell-Sills et al., 2009); (iii) the Ruminative Responses Scale (RRS) measures dispositional tendencies to ruminate in response to negative affect, including 22 questions regarding sad mood involving the self, one's symptoms, and possible causes and consequences of sad mood. Responses are rated on a 4-point scale (Nolen-Hoeksema and Morrow, 1991); (iv) the State-Trait Anxiety Inventory (STAI) consists of a “state” subscale to measure temporary anxiety symptoms and a “trait” subscale to measure more long-standing anxiety proneness. Each subscale includes 20 items rating at 4-point scales (Spielberger, 1983); (v) the Positive and Negative Affect Schedule (PANAS) comprises 20-items assessing forms of PA and NA using 5-point scales (Watson and Clark, 1999); (vi) the Temporal Experience of Pleasure Scale (TEPS) is a measure of anticipatory and consummatory pleasure consisting of 18 items rated on a 6-point scale (Gard et al., 2006).
2.2.3. MID task
To measure neural responses to rewards and losses, participants were asked to complete the monetary incentive delay task (MID), a well-established measure of reward processing (Knutson et al., 2001a; Knutson et al., 2001b). This task dissociates anticipatory and consummatory phases of reward processing and has been shown to reliably recruit brain areas associated with regulating approach-related response tendencies and reward sensitivity (e.g. ventral striatum). On each trial, participants were given a cue indicating potential reward (circle), loss (square), or no reward/loss (circle or square). In order to receive a specified reward or avoid a loss, participants are required to press a button within a certain duration of time (adapted for individual participant reaction times [RT]) following presentation of a white square (target cue). Task difficulty was based on RT collected during a practice session, and was calibrated such that each participant should succeed on about 66% of trials. The degree of potential reward or loss was varied on three levels indicated by the number of horizontal lines in a cue. That is, one line denoted the lowest reward/loss value (no reward/loss), two lines an intermediate reward/loss, and three lines the highest reward/loss. Participants could gain or lose points and earned an average of $30 from the task, which they were paid at the end of the session. The primary measures of interest were (i) anticipation of reward vs. no-reward; and (ii) anticipation of loss vs. no-loss. This task included 2 runs each lasting 568 s and consisting of 90 trials.
2.3. Data acquisition
Images were acquired with a GE MRI750 3T scanner at the Laureate institute for Brain Research. The MID task scanning consisted of 284 contiguous echo-planar imaging (EPI) volumes using the following parameters: TR/TE = 2000/27 ms, FOV/slice = 240/2.9 mm, 128 × 128 matrix, and 39 axial slices. In addition, high-resolution structural images were acquired through a 3D axial T1-weighted magnetization-prepared rapid acquisition with gradient-echo (MPRAGE) sequence, using the following parameters: TR/TE = 5/2.0 12 ms, FOV/slice = 240 × 192/0 .9mm, and 186 axial slices.
2.4. Statistical analysis
In general, Bayes factor (BF10) was used to quantify evidence in favor of alternative against the null hypothesis (i.e., neighborhood affluence is effective vs. not effective). Here, BF scores above 1 denote evidence for H1 over H0, whereas BF scores below 1 indicate the opposite. By convention (Wagenmakers et al., 2018), the strength of evidence for alternative or null hypothesis is regarded as noteworthy when BF scores are above 3 or below 0.33, respectively. When BF scores are between 0.33 and 3, evidence for alternative or null hypothesis is considered as inconclusive or only anecdotal. Thus, BF scores were thresholded at 3 and 0.33 for supporting the alternative or null hypothesis, respectively; since log (BF) (where natural logarithm was used henceforth) was calculated for all analyses, corresponding thresholds were set at 1.1 and −1.1. In other words, the selected thresholds correspond to the alternative hypothesis being greater than three times more likely than the null hypothesis or in reverse that the null hypothesis is three times more likely than the alternative hypothesis. To obtain the BF scores, we employed the regression BF function from the BayesFactor package (version 0.9.12)1 in R (Morey et al., 2015), with the default setting for all parameters. The default prior distributions for the BayesFactor is the combination of the Cauchy on effect size and the Jeffreys prior on variance, which is also referred as the JZS prior (see also Rouder et al., 2009). The JZS prior was developed to minimize assumptions about the range of effect size (Morey and Rouder, 2011; Rouder et al., 2009).
2.4.1. Symptom analysis
Effects of neighborhood affluence on symptoms (PHQ9, OASIS, RRS, STAI, PANAS, and TEPS) were examined using a regression model with age, gender, ethnicity, individual income, education, and employment status as covariates. The effect of neighborhood affluence on each symptom measure was tested separately.
2.4.2. Functional imaging data
Neuroimaging data analyses were performed with the analysis of functional neuroimaging (AFNI, http://afni. nimh.nih.gov) software package (Cox, 1996). The first 3 volumes of the functional images were discarded for signal equilibrium and participants' adaptation to scanning noise. Subsequent data preprocessing included despiking, slice timing correction, co-registration to anatomical volumes, motion correction, smoothing (5 × 5 × 5 mm3 full width at half maximum), and normalization to the standard Montreal Neurological Institute space (MNI template, resampling voxel size was 2 × 2 × 2 mm3).
A two-level general linear model (GLM) was used to analyze the functional data. For the first level, boxcar regressors were defined for each subject and for each epoch of the time course. The regressors modeled the blood-oxygen-level dependent (BOLD) response to the epoch of anticipation (4 s) in six conditions (15 trials per condition per run): large reward, small reward, no reward, large loss, small loss, and no loss. Furthermore, contrasts associated with anticipation of reward ([1 1 -2 0 0 0]) and loss ([0 0 0 1 1 -2]) were calculated for the second-level analyses. For the second level, effects of neighborhood affluence on neural responses to the anticipation of reward and loss were estimated with age, gender, ethnicity, individual income, education, and employment status as covariates. For the region-of-interest (ROI) analyses, the average BF scores were computed for the voxels within regions important for the anticipation of reward and loss, including the NAcc, caudate, insula, and mPFC (Fouragnan et al., 2018; Knutson et al., 2001a). These functional ROIs were defined as corresponding regions in a widely-used functional brain atlas (Power et al., 2011).
For the whole-brain analyses, BF scores pertaining to the effect of neighborhood affluence were computed for each voxel in the BayesFactor package. The computation was restricted on a group mask generated as the conjunction of all subject masks. To further illustrate brain regions and functions which were modulated by the neighborhood affluence, clusters with no <50 voxels (with cluster-defining threshold of log (BF10) > 1.1) were selected. In addition, functions of brain maps associated with neighborhood effects on reward or loss anticipation were decoded using the Neurosynth Image Decoder (neurosynth.org; Yarkoni et al., 2011). The Neurosynth database (status: 507891 activations reported in 14,371 studies) provides automatically generated meta-analytic maps (activation patterns) for thousands of psychological terms, extracted through text-mining techniques (Yarkoni et al., 2011). Hence, the decoder function permits the calculation of voxel-wise Pearson correlations between a given unthresholded functional map (unthresholded t maps associated with neighborhood effects in our case) and each of the term-based meta-analysis maps in the Neurosynth database. As such, the decoder function allows for identifying the most frequent psychological concepts associated with a given neural pattern (Gorgolewski et al., 2015). The top 10 psychological terms that were associated with neighborhood effects on reward or loss anticipation were illustrated.
2.4.3. Structural MRI data
Data analysis was conducted using the FreeSurfer image analysis suite (version 6.0.0, http://surfer.nmr.mgh.harvard.edu/), with automated algorithms for the volumetric segmentation of subcortical structures and cortical measures. In brief, the MRI data were first normalized to a standard anatomical template and corrected for bias-field inhomogeneities. Resulting volumes were skull stripped with a watershed algorithm (Ségonne et al., 2004) and then segmented into the subcortical white matter and deep GM volumetric structures (Fischl et al., 2002, 2004). The initial tessellation was formed by reconstructing the GM/white matter boundary as well as the outer cortical surface (pial surface) (Dale et al., 1999; Fischl and Dale, 2000). Next, a series of deformation procedures was implemented, including surface inflation (Dale et al., 1999), registration to a spherical atlas (Fischl et al., 1999), and parcellation of the cerebral cortex into units based on gyral and sulcal structures (Fischl et al., 2004). Finally, several morphological features were computed at each vertex on the pial surface, including cortical thickness, cortical volume, and sulcal depth. Specifically, cortical thickness was computed as the closest distance between the white and pial surfaces. Cortical volume was defined as the sum of the volumes of the individual triangles that lie within the neighborhood of the vertex, in which the volume of each triangle was calculated as the product of its area and the thickness at the center of the triangle. Sulcal depth was computed as the displacement from each vertex to the average surface. For subcortical brain structures, automated segmentation yields a volume measurement in units of 1-mm cubic voxels (Fischl et al., 2002). Morphological measures were calculated for brain regions implicated in positive and negative valence systems, including NAcc, caudate, anterior insula, amygdala, hippocampus, and mPFC (Morris and Cuthbert, 2012). Effects of neighborhood affluence on these structural measures were estimated with age, gender, ethnicity, individual income, education, employment status, and intracranial volume as covariates.
3. Results
3.1. Symptoms
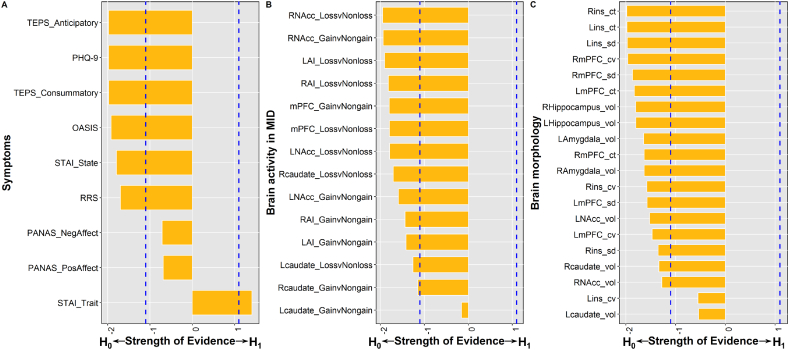
Among questionnaires (Fig. 1A and Table S3), log (BF) scores were below −1.1 for anticipatory and consummatory subscales of the TEPS, STAI state anxiety, RRS, PHQ-9, and OASIS scores, providing the evidence that there was no effect of neighborhood affluence on these measures. For PA and NA, log (BF) scores were between −1.1 and 0, providing anecdotal evidence that neighborhood affluence did not impact these measures. Finally, log (BF) scores were above 1.1 for STAI trait anxiety, revealing evidence that neighborhood affluence was negatively correlated with trait anxiety. The scatter plots of corresponding results are illustrated in Fig. S3.
Fig. 1.
Strength of evidence (i.e. log (BF) scores) regarding the effect of neighborhood affluence on symptoms (A), brain activity in the monetary incentive delay (MID) task (B), and brain morphology (C). Blue dashed lines indicate thresholds (i.e., −1.1 and 1.1). BF, Bayes factor; TEPS, temporal experience of pleasure scale; PHQ, Patient Health Questionnaire; OASIS, overall anxiety severity and impairment scale; STAI, State-Trait Anxiety Inventory; RRS, ruminative responses scale; PANAS, Positive and Negative Affect Schedule; L, left; R, right; NAcc, nucleus accumbens; AI, anterior insula; mPFC, medial prefrontal cortex; ins, insula; ct, cortical thickness, cv, cortical volumes; sd, sulcal depth; vol, volume. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Brain activations
3.2.1. ROI analysis
Log (BF) scores of brain activity in all ROIs were below zero (Fig. 1B and Table S3), providing anecdotal to moderate evidence that neighborhood affluence had no effect on brain activity of these ROIs in response to reward or loss anticipation. The scatter plots of corresponding results are illustrated in Fig. S4 and S5.
3.2.2. Whole brain
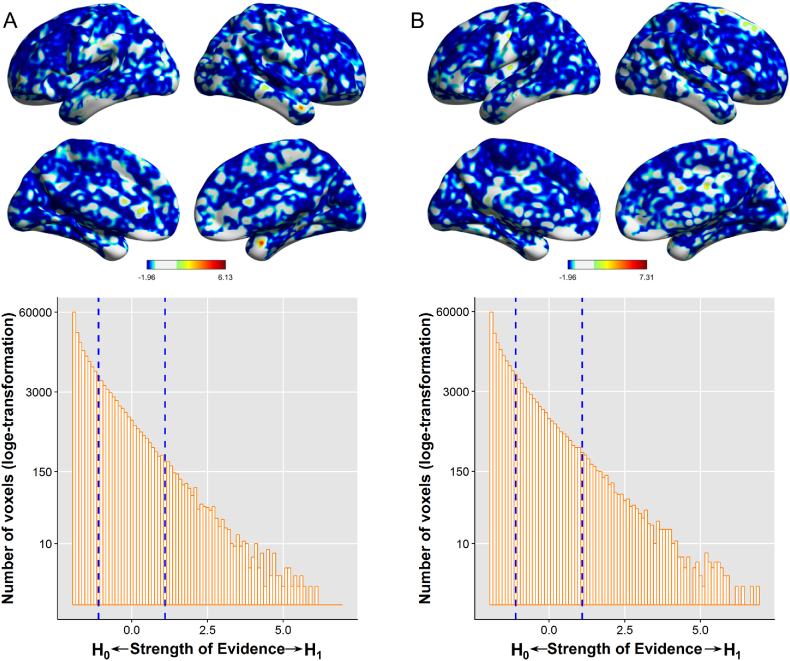
For both reward and loss anticipation (Fig. 2), log (BF) scores associated with neighborhood effects were below zero in most brain regions.
Fig. 2.
Whole-brain analysis for the effects of neighborhood affluence on neural responses to reward (A) or loss (B) anticipation in the monetary incentive delay (MID) task. Brain maps illustrate log (BF) scores at each voxel (thresholded at −1.1 and 1.1). Histograms illustrate the distribution of log (BF) scores across the whole brain, the pattern of which shows that there are more voxels showing strong evidence in favor of no effects of neighborhood affluence than those showing strong evidence in favor of neighborhood affluence effects. Blue dashed lines indicate thresholds. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
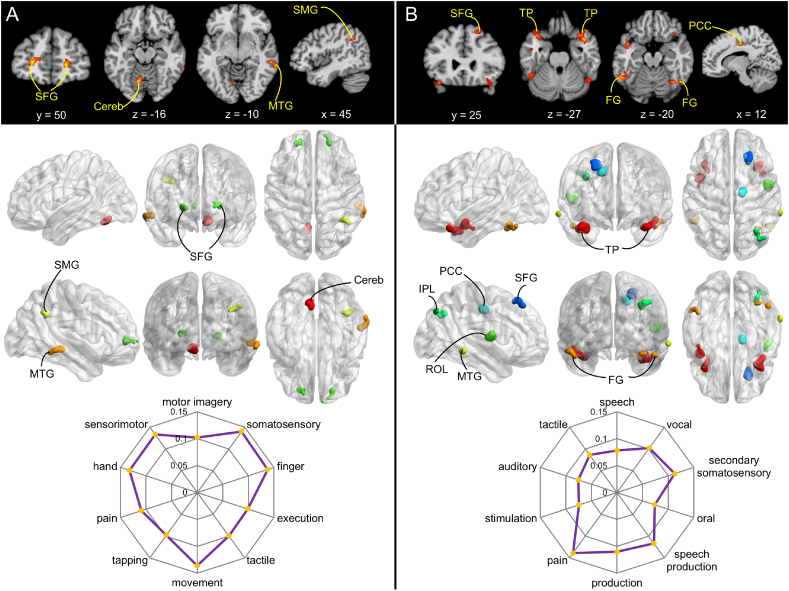
For reward anticipation, clusters (voxel number > 50) that exhibited effects of neighborhood affluence were mainly located in superior frontal gyrus (SFG), supramarginal gyrus (SMG), middle temporal gyrus (MTG), and cerebellum (Fig. 3A and Table 1). Neighborhood affluence exhibited positive correlations with neural responses in SFG and cerebellum (Fig. S6), and negative correlations with neural responses in SMG and MTG (Fig. S6). Furthermore, a one-sample t-test revealed that activations of all of these regions were enhanced in response to reward anticipation compared to the no reward condition (Fig. S7A and C). Finally, functional decoding revealed that effects of neighborhood affluence on reward anticipation were mainly associated with terms related to "somatosensory", "finger", "movement", "sensorimotor", "hand", "pain", "motor imagery", "tactile", "execution", and "tapping" (Fig. 3A).
Fig. 3.
Clusters exhibiting effects of neighborhood affluence during reward (A) or loss (B) anticipation. These maps present the same results as those in the Fig. 2, except that only clusters showing the presence of the effects of neighborhood affluence (log (BF) >1.1) and consisting of at least 50 voxels are illustrated. Radar charts illustrate the top 10 topics associated with effects of neighborhood affluence on reward or loss anticipation. SFG, superior frontal gyrus; Cereb, Cerebellum; MTG, middle temporal gyus; SMG, supramarginal gyrus; TP, temporal pole; FG, fusiform gyrus; PCC, posterior cingulate cortex; IPL, inferior parietal lobule; ROL, rolandic operculum.
Table 1.
Clusters exhibiting effects of neighborhood affluence during reward or loss anticipation. Only clusters consisting of at least 50 voxels are illustrated.
| Laterality | Brain regions | MNI coordinates (mm) |
Peak log (BF) score | Cluster size (voxels) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Reward anticipation | ||||||
| R | Superior frontal gyrus | 21 | 53 | 3 | 5.18 | 75 |
| L | Superior frontal gyrus | −23 | 51 | 3 | 5.56 | 62 |
| R | Supramarginal gyrus | 41 | −51 | 35 | 3.24 | 60 |
| R | Middle temporal gyrus | 59 | −31 | −9 | 3.56 | 53 |
| L | cerebellum | −9 | −65 | −13 | 5.69 | 65 |
| Loss anticipation | ||||||
| R | Superior frontal gyrus | 27 | 31 | 55 | 3.33 | 76 |
| R | Rolandic operculum | 49 | −5 | 9 | 7.31 | 52 |
| R | Posterior cingulate cortex | 15 | −13 | 43 | 6.93 | 52 |
| R | Inferior parietal lobule | 33 | −65 | 39 | 3.42 | 60 |
| R | Temporal pole | 43 | 11 | −25 | 6.67 | 147 |
| L | Temporal pole | −35 | 15 | −25 | 4.37 | 55 |
| L | Temporal pole | −43 | 7 | −21 | 4.56 | 76 |
| R | Middle temporal gyrus | 67 | −41 | −9 | 6.19 | 52 |
| R | Fusiform gyrus | 51 | −59 | −23 | 5.17 | 101 |
| L | Fusiform gyrus | −55 | −53 | −17 | 6.42 | 139 |
L, left; R, right; MNI, Montreal Neurological Institute; BF, Bayes factor.
For loss anticipation, clusters (voxel number >50) that exhibited effects of neighborhood affluence were mainly located in SFG, rolandic operculum (ROL), posterior cingulate cortex (PCC), temporal pole (TP), MTG, and fusiform gyrus (FG) (Fig. 3B and Table 1). Neighborhood affluence was positively correlated with neural responses in ROL and PCC, and negatively correlated with neural responses in other regions (Fig. S8). Furthermore, one-sample t-tests revealed that none of these regions except ROL showed different activity in response to loss anticipation versus the no loss condition (Fig. S7B and D). The activity of ROL showed lower activations in response to loss anticipation than the no loss condition. Finally, functional decoding revealed that neighborhood effects on loss anticipation were primarily associated with "pain", "speech production", "secondary somatosensory", "production", "vocal", "tactile", "speech", "auditory", "stimulation", and "oral" terms (Fig. 3B).
Together, these findings complement ROI analyses suggesting that neighborhood affluence had no effect on neural responses in brain regions that are previously implicated in reward or loss anticipation.
3.3. Brain morphology
Log (BF) scores of structural measures in all ROIs were below zero (Fig. 1C and Table 1), providing anecdotal to moderate evidence that there was no effect of neighborhood affluence on the brain morphology in these ROIs (The correlation coefficients of corresponding results are illustrated in Figs. S9–S11).
4. Discussion
This investigation aimed to examine the effects of neighborhood affluence on symptoms, brain functions and structures associated with positive and negative valence processing in a sample of adults with mood and/or anxiety disorders. The Bayesian inference approach enabled us to quantify evidence for both the presence and absence of the effects of neighborhood. Our findings provide evidence that – on an individual subject level - neighborhood affluence is not associated with most behavioral and brain measures of positive and negative processing, from self-reported symptoms to brain functions and structures. This finding contrasts with those of others who have reported strong association on a community level between neighborhood characteristics and mental health. There are several potential explanations for the discrepancy between current null findings and prior significant results of neighborhood disadvantage effects.
First, while our study measured the neighborhood disadvantage/affluence using census-based data from the ACS, several other studies employ subjective appraisal of the neighborhood based on self-reports (Gonzalez et al., 2016; Saxbe et al., 2018). Specifically, participants in Gonzalez et al. (2016) are asked to report neighborhood quality using a questionnaire, and participants in Saxbe et al. (2018) report on community violence exposure. Hence, it is possible that objective and subjective measures of neighborhood disadvantage are associated with differential neuropsychological consequences within residents. In line with this possibility, another study employing census-based measures of neighborhood disadvantage also fails to identify associations between neighborhood disadvantage and brain morphology in subcortical regions, similar to the current findings (Gianaros et al., 2017). Furthermore, there is evidence showing differential effects of objective and subjective neighborhood disadvantage on mental health, such that perceived neighborhood quality is most strongly linked with self-reported symptoms and mediates the association between objective neighborhood disadvantage and health (O'neil et al., 2001; Weden et al., 2008; Wen et al., 2006). Further studies are needed to examine the interplay between objective and subjective measures of neighborhood disadvantage at both behavioral and brain levels.
Second, it is possible that effects of neighborhood disadvantage are more profound during early developmental periods (e.g. childhood and adolescence) compared to adulthood (examined in the current study). Indeed, several neuroimaging studies demonstrate the impact of neighborhood disadvantage among childhood or adolescence on brain functions and structures associated with positive and negative valence processing (Gonzalez et al., 2016; Marshall et al., 2018; Saxbe et al., 2018; Whittle et al., 2017). In contrast, other studies focusing on adult neighborhood disadvantage mainly identify neighborhood effects on brain morphology in cortical regions, such as language-related areas (Gianaros et al., 2017; Krishnadas et al., 2013). Therefore, recent neuroimaging evidence seems to support a differential role of neighborhood disadvantage at childhood/adolescence and adulthood stages. Notably, however, many survey-based studies demonstrate the relationship between neighborhood disadvantage and positive/negative valence processing across a wide age range (e.g. Ellen and Turner, 1997; Latkin and Curry, 2003; Menec et al., 2010). Further efforts are thus required to symmetrically and directly compare the effects of neighborhood disadvantage across the lifespan. For instance, it would be important to record historical neighborhood disadvantage at different developmental stages to examine their distinct or common effects on the current psychiatric symptoms.
Third, the current study focused on neighborhood affluence showing the greatest loadings from neighborhood-level income and education, the effects of which are most extensively examined in the literature (Arcaya et al., 2016; Leventhal and Brooks-Gunn, 2000). However, many other neighborhood characteristics not examined may have profound effects on mental health and associated alterations in positive/negative valence processing. For instance, higher neighborhood green space is related to lower depression and anxiety symptoms, since green space may facilitate recovery from mental fatigue and reductions in stress (Beyer et al., 2014; Brown et al., 2018). Likewise, pollution in the neighborhood is another important determinant of mental health, such that both air and noise pollution are positively correlated with individual psychological distress (Dzhambov et al., 2017; Sass et al., 2017). Moreover, social cohesion is associated with lower levels of depression (Echeverría et al., 2008), whereas residential stability is positively correlated with depressive symptoms (Aneshensel et al., 2007). Therefore, future brain imaging studies may examine whether these physical and social characteristics of neighborhood on mental health are mediated by alterations in positive/negative valence processing at multiple levels.
Finally, it may be important to point out that the current findings are based on a Tulsa Oklahoma sample. Although the effects of neighborhood disadvantage have been frequently examined as a universal phenomenon, it is possible that cultural and geographic characteristics across different areas may interact with the neighborhood effects. For instance, Oklahoma has experienced a rise in seismicity since 2010 due to wastewater injection, which is associated with increased proportion of Google search episodes for anxiety (Casey et al., 2018). It remains unclear how this might interact with effects of community-level affluence, but such environmental factors would be important to consider in future studies. Therefore, current and previous findings derived from a single area need to be interpreted with caution. This issue could be addressed by future meta-analytic studies synthesizing results from multiple areas and increased number of multi-site neuroimaging studies (e.g. the ABCD study). Likewise, another characteristic of the current sample is that participants were drawn from a clinical population, which differed from previous studies employing participants sampled from general populations (e.g., Gianaros et al., 2017). Therefore, future studies are needed to examine whether effects of neighborhood affluence are more profound among general or subclinical populations compared to clinical samples.
Despite evidence showing that neighborhood affluence had no effect on most measures associated with positive and negative processing, we did identify evidence regarding neighborhood effects on trait anxiety and brain activations within frontal, temporal, and parietal regions. First, the negative correlation between neighborhood affluence and trait anxiety is broadly in line with previous observations showing that residing in advantageous neighborhood accompanies lower levels of mental health problems (Cerdá et al., 2017; Kim, 2010; Latkin and Curry, 2003; Ross, 2000; Santiago et al., 2011; Stockdale et al., 2007). However, one should interpret this finding with caution, since the anxiety-neighborhood affluence relationship did not converge with results for other symptom measures or functional/structural brain data. A possible interpretation of the trait anxiety result is that the current study conducted a series of tests, and this result may have emerged by chance. The Bayesian inference does not implement multiple comparison correction (Han and Park, 2018), and we will address this issue by examining whether this particular finding can be replicated in the second half T1000 participants. Second, we identified neighborhood effects within several frontal, parietal, and temporal regions during reward and loss anticipation. However, functional decoding analyses indicate that these regions may be more recruited by action preparation or anticipation of motor responding than by reward/loss anticipation per se.
Taken together, our results identify little evidence supporting that neighborhood affluence modulates positive/negative valence processing measured across multiple modalities. These findings suggest that varying definitions of neighborhood affluence may contribute to mixed findings with respect to brain and symptom relationships; as a result, the reliability of neighborhood effects on individual mental health requires further investigation.
The following are the supplementary data related to this article.
A map that shows where the T500 subjects are located. Each participant is represented only once. The color of the tract shows how many participants are in the tract. Clicking on a tract will open a popup that says the geoCode of the tract and the exact number of participants in the tract. A tract will be grey if there are no T500 participants living in that tract.
Supplementary material
Conflict of interest
None of the authors has conflicts of interests to declare.
Funding
This work was supported by the William K Warren Foundation. The sponsor has no role in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
References
- Aneshensel C.S., Sucoff C.A. The neighborhood context of adolescent mental health. J. Health Soc. Behav. 1996:293–310. [PubMed] [Google Scholar]
- Aneshensel C.S., Wight R.G., Miller-Martinez D., Botticello A.L., Karlamangla A.S., Seeman T.E. Urban neighborhoods and depressive symptoms among older adults. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2007;62:S52–S59. doi: 10.1093/geronb/62.1.s52. [DOI] [PubMed] [Google Scholar]
- Arcaya M.C., Tucker-Seeley R.D., Kim R., Schnake-Mahl A., So M., Subramanian S. Research on neighborhood effects on health in the United States: a systematic review of study characteristics. Soc. Sci. Med. 2016;168:16–29. doi: 10.1016/j.socscimed.2016.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrondo G., Segarra N., Metastasio A., Ziauddeen H., Spencer J., Reinders N.R., Dudas R.B., Robbins T.W., Fletcher P.C., Murray G.K. Reduction in ventral striatal activity when anticipating a reward in depression and schizophrenia: a replicated cross-diagnostic finding. Front. Psychol. 2015;6:1280. doi: 10.3389/fpsyg.2015.01280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson E.G., Audesse A.J., Palacios J.A., Bobo D.M., Webb A.E., Ramachandran S., Henn B.M. No evidence for recent selection at FOXP2 among diverse human populations. Cell. 2018;174:1424–1435. doi: 10.1016/j.cell.2018.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attar B.K., Guerra N.G., Tolan P.H. Neighborhood disadvantage, stressful life events and adjustments in urban elementary-school children. J. Clin. Child Psychol. 1994;23:391–400. [Google Scholar]
- Beyer K.M., Kaltenbach A., Szabo A., Bogar S., Nieto F.J., Malecki K.M. Exposure to neighborhood green space and mental health: evidence from the survey of the health of Wisconsin. Int. J. Environ. Res. Public Health. 2014;11:3453–3472. doi: 10.3390/ijerph110303453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biel A.L., Friedrich E. Why you should report Bayes factors in your transcranial brain stimulation studies. Front. Psychol. 2018;9:1125. doi: 10.3389/fpsyg.2018.01125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman J.D., Finch B.K., Ellison C.G., Williams D.R., Jackson J.S. Neighborhood disadvantage, stress, and drug use among adults. J. Health Soc. Behav. 2001:151–165. [PubMed] [Google Scholar]
- Brown S.C., Perrino T., Lombard J., Wang K., Toro M., Rundek T., Gutierrez C.M., Dong C., Plater-Zyberk E., Nardi M.I. Health disparities in the relationship of neighborhood greenness to mental health outcomes in 249,405 US Medicare beneficiaries. Int. J. Environ. Res. Public Health. 2018;15:430. doi: 10.3390/ijerph15030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brühl A.B., Delsignore A., Komossa K., Weidt S. Neuroimaging in social anxiety disorder—a meta-analytic review resulting in a new neurofunctional model. Neurosci. Biobehav. Rev. 2014;47:260–280. doi: 10.1016/j.neubiorev.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L., Norman S.B., Craske M.G., Sullivan G., Lang A.J., Chavira D.A., Bystritsky A., Sherbourne C., Roy-Byrne P., Stein M.B. Validation of a brief measure of anxiety-related severity and impairment: the Overall Anxiety Severity and Impairment Scale (OASIS) J. Affect. Disord. 2009;112:92–101. doi: 10.1016/j.jad.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey J.A., Goldman-Mellor S., Catalano R. Association between Oklahoma earthquakes and anxiety-related Google search episodes. Environ. Epidemiol. 2018;2:e016. doi: 10.1097/EE9.0000000000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdá M., Nandi V., Frye V., Egan J.E., Rundle A., Quinn J.W., Sheehan D., Hoover D.R., Ompad D.C., Van Tieu H. Neighborhood determinants of mood and anxiety disorders among men who have sex with men in New York City. Soc. Psychiatry Psychiatr. Epidemiol. 2017;52:749–760. doi: 10.1007/s00127-017-1379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi G.C., Hajat A., Bird C.E., Cullen M.R., Griffin B.A., Miller K.A., Shih R.A., Stefanick M.L., Vedal S., Whitsel E.A. Individual and neighborhood socioeconomic status and the association between air pollution and cardiovascular disease. Environ. Health Perspect. 2016;124:1840. doi: 10.1289/EHP199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dienes Z. How Bayes factors change scientific practice. J. Math. Psychol. 2016;72:78–89. [Google Scholar]
- Dzhambov A., Tilov B., Markevych I., Dimitrova D. Residential road traffic noise and general mental health in youth: the role of noise annoyance, neighborhood restorative quality, physical activity, and social cohesion as potential mediators. Environ. Int. 2017;109:1–9. doi: 10.1016/j.envint.2017.09.009. [DOI] [PubMed] [Google Scholar]
- Echeverría S., Diez-Roux A.V., Shea S., Borrell L.N., Jackson S. Associations of neighborhood problems and neighborhood social cohesion with mental health and health behaviors: the Multi-Ethnic Study of Atherosclerosis. Health & Place. 2008;14:853–865. doi: 10.1016/j.healthplace.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Ellen I.G., Turner M.A. Does neighborhood matter? Assessing recent evidence. Hous. Policy Debate. 1997;8:833–866. [Google Scholar]
- Etkin A., Wager T.D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatr. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis: II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., Van Der Kouwe A., Killiany R., Kennedy D., Klaveness S. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B., Van Der Kouwe A., Destrieux C., Halgren E., Ségonne F., Salat D.H., Busa E., Seidman L.J., Goldstein J., Kennedy D. Automatically parcellating the human cerebral cortex. Cereb. Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Forbes E.E., Hariri A.R., Martin S.L., Silk J.S., Moyles D.L., Fisher P.M., Brown S.M., Ryan N.D., Birmaher B., Axelson D.A. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am. J. Psychiatr. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forthman K.L., Yeh H.-w., Kuplicki R., Paulus M.P. 2019. Dimensions of neighborhood tracts and their associations with mental health problems. bioRxiv, 518258. [Google Scholar]
- Fouragnan E., Retzler C., Philiastides M.G. Separate neural representations of prediction error valence and surprise: evidence from an fMRI meta-analysis. Hum. Brain Mapp. 2018;39:2887–2906. doi: 10.1002/hbm.24047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard D.E., Gard M.G., Kring A.M., John O.P. Anticipatory and consummatory components of the experience of pleasure: a scale development study. J. Res. Pers. 2006;40:1086–1102. [Google Scholar]
- Gianaros P.J., Kuan D.C.-H., Marsland A.L., Sheu L.K., Hackman D.A., Miller K.G., Manuck S.B. Community socioeconomic disadvantage in midlife relates to cortical morphology via neuroendocrine and cardiometabolic pathways. Cereb. Cortex. 2017;27:460–473. doi: 10.1093/cercor/bhv233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M.Z., Allen J.P., Coan J.A. Lower neighborhood quality in adolescence predicts higher mesolimbic sensitivity to reward anticipation in adulthood. Dev. Cogn. Neurosci. 2016;22:48–57. doi: 10.1016/j.dcn.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman S. Elsevier; 2008. A Dirty Dozen: Twelve P-Value Misconceptions. Seminars in Hematology; pp. 135–140. [DOI] [PubMed] [Google Scholar]
- Gorgolewski K.J., Varoquaux G., Rivera G., Schwarz Y., Ghosh S.S., Maumet C., Sochat V.V., Nichols T.E., Poldrack R.A., Poline J.-B. NeuroVault. Org: a web-based repository for collecting and sharing unthresholded statistical maps of the human brain. Front. Neuroinformatics. 2015;9:8. doi: 10.3389/fninf.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N., Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H., Park J. Using SPM 12's second-level Bayesian inference procedure for fMRI analysis: practical guidelines for end users. Front. Neuroinformatics. 2018;12:1. doi: 10.3389/fninf.2018.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys H. OUP Oxford; 1998. The Theory of Probability. [Google Scholar]
- Jencks C., Mayer S.E. 1990. The Social Consequences of Growing up in a Poor Neighborhood. Inner-City Poverty in the United States 111; p. 186. [Google Scholar]
- Karriker-Jaffe K.J. Neighborhood socioeconomic status and substance use by US adults. Drug Alcohol Depend. 2013;133:212–221. doi: 10.1016/j.drugalcdep.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. Neighborhood disadvantage and mental health: the role of neighborhood disorder and social relationships. Soc. Sci. Res. 2010;39:260–271. [Google Scholar]
- Knutson B., Adams C.M., Fong G.W., Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J. Neurosci. 2001;21 doi: 10.1523/JNEUROSCI.21-16-j0002.2001. RC159-RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Fong G.W., Adams C.M., Varner J.L., Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Krishnadas R., McLean J., Batty G.D., Burns H., Deans K.A., Ford I., McConnachie A., McLean J.S., Millar K., Sattar N. Socioeconomic deprivation and cortical morphology: psychological, social, and biological determinants of ill health study. Psychosom. Med. 2013;75:616–623. doi: 10.1097/PSY.0b013e3182a151a7. [DOI] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latkin C.A., Curry A.D. Stressful neighborhoods and depression: a prospective study of the impact of neighborhood disorder. J. Health Soc. Behav. 2003:34–44. [PubMed] [Google Scholar]
- Leventhal T., Brooks-Gunn J. The neighborhoods they live in: the effects of neighborhood residence on child and adolescent outcomes. Psychol. Bull. 2000;126:309. doi: 10.1037/0033-2909.126.2.309. [DOI] [PubMed] [Google Scholar]
- Marshall N.A., Marusak H.A., Sala-Hamrick K.J., Crespo L.M., Rabinak C.A., Thomason M.E. Socioeconomic disadvantage and altered corticostriatal circuitry in urban youth. Hum. Brain Mapp. 2018;39:1982–1994. doi: 10.1002/hbm.23978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menec V.H., Shooshtari S., Nowicki S., Fournier S. Does the relationship between neighborhood socioeconomic status and health outcomes persist into very old age? A population-based study. J. Aging Health. 2010;22:27–47. doi: 10.1177/0898264309349029. [DOI] [PubMed] [Google Scholar]
- Moreau D., Wilson A.J., McKay N.S., Nihill K., Waldie K.E. No evidence for systematic white matter correlates of dyslexia and dyscalculia. NeuroImage: Clin. 2018;18:356–366. doi: 10.1016/j.nicl.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey R.D., Rouder J.N. Bayes factor approaches for testing interval null hypotheses. Psychol. Methods. 2011;16:406. doi: 10.1037/a0024377. [DOI] [PubMed] [Google Scholar]
- Morey R.D., Rouder J.N., Jamil T. R Package Version 0.9 9. 2015. BayesFactor: Computation of Bayes factors for common designs; p. 2014. [Google Scholar]
- Morris S.E., Cuthbert B.N. Research domain criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin. Neurosci. 2012;14:29. doi: 10.31887/DCNS.2012.14.1/smorris. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell K.A. Annals of the New York Academy of Sciences; 2018. Socioeconomic Influences on Brain Function: Implications for Health. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S., Morrow J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: the 1989 Loma Prieta earthquake. J. Pers. Soc. Psychol. 1991;61:115. doi: 10.1037//0022-3514.61.1.115. [DOI] [PubMed] [Google Scholar]
- Oldehinkel A.J. The importance of taking no for an answer. Nat. Hum. Behav. 2018;1 doi: 10.1038/s41562-018-0393-5. [DOI] [PubMed] [Google Scholar]
- O'neil R., Parke R.D., McDowell D.J. Objective and subjective features of children's neighborhoods: relations to parental regulatory strategies and children's social competence. J. Appl. Dev. Psychol. 2001;22:135–155. [Google Scholar]
- Palumbo A., Michael Y., Hyslop T. Latent class model characterization of neighborhood socioeconomic status. Cancer Causes Control. 2016;27:445–452. doi: 10.1007/s10552-015-0711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Cohen A.L., Nelson S.M., Wig G.S., Barnes K.A., Church J.A., Vogel A.C., Laumann T.O., Miezin F.M., Schlaggar B.L. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romens S.E., Casement M.D., McAloon R., Keenan K., Hipwell A.E., Guyer A.E., Forbes E.E. Adolescent girls' neural response to reward mediates the relation between childhood financial disadvantage and depression. J. Child Psychol. Psychiatry. 2015;56:1177–1184. doi: 10.1111/jcpp.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross C.E. Neighborhood disadvantage and adult depression. J. Health Soc. Behav. 2000:177–187. [Google Scholar]
- Rouder J.N., Speckman P.L., Sun D., Morey R.D., Iverson G. Bayesian t tests for accepting and rejecting the null hypothesis. Psychon. Bull. Rev. 2009;16:225–237. doi: 10.3758/PBR.16.2.225. [DOI] [PubMed] [Google Scholar]
- Rudolph K.E., Stuart E.A., Glass T.A., Merikangas K.R. Neighborhood disadvantage in context: the influence of urbanicity on the association between neighborhood disadvantage and adolescent emotional disorders. Soc. Psychiatry Psychiatr. Epidemiol. 2014;49:467–475. doi: 10.1007/s00127-013-0725-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago C.D., Wadsworth M.E., Stump J. Socioeconomic status, neighborhood disadvantage, and poverty-related stress: prospective effects on psychological syndromes among diverse low-income families. J. Econ. Psychol. 2011;32:218–230. [Google Scholar]
- Sass V., Kravitz-Wirtz N., Karceski S.M., Hajat A., Crowder K., Takeuchi D. The effects of air pollution on individual psychological distress. Health & Place. 2017;48:72–79. doi: 10.1016/j.healthplace.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxbe D., Khoddam H., Piero L.D., Stoycos S.A., Gimbel S.I., Margolin G., Kaplan J.T. Community violence exposure in early adolescence: longitudinal associations with hippocampal and amygdala volume and resting state connectivity. Dev. Sci. 2018;21:1–11. doi: 10.1111/desc.12686. (e12686) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ségonne F., Dale A.M., Busa E., Glessner M., Salat D., Hahn H.K., Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G.C. The MINI-international neuropsychiatric interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Soares J.C., Mann J.J. The anatomy of mood disorders—review of structural neuroimaging studies. Biol. Psychiatry. 1997;41:86–106. doi: 10.1016/s0006-3223(96)00006-6. [DOI] [PubMed] [Google Scholar]
- Spielberger C.D. Consulting Psychologists Press; Palo Alto, CA: 1983. Manual for the State-Trait Anxiety Inventory STAI (form Y)("Self-Evaluation Questionnaire") [Google Scholar]
- Stockdale S.E., Wells K.B., Tang L., Belin T.R., Zhang L., Sherbourne C.D. The importance of social context: Neighborhood stressors, stress-buffering mechanisms, and alcohol, drug, and mental health disorders. Soc. Sci. Med. 2007;65:1867–1881. doi: 10.1016/j.socscimed.2007.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor T.A., Khalsa S.S., Simmons W.K., Feinstein J.S., Savitz J., Aupperle R.L., Yeh H.-W., Bodurka J., Paulus M.P. Tulsa 1000: a naturalistic study protocol for multilevel assessment and outcome prediction in a large psychiatric sample. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-016620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenmakers E.-J. A practical solution to the pervasive problems ofp values. Psychon. Bull. Rev. 2007;14:779–804. doi: 10.3758/bf03194105. [DOI] [PubMed] [Google Scholar]
- Wagenmakers E.-J., Lodewyckx T., Kuriyal H., Grasman R. Bayesian hypothesis testing for psychologists: a tutorial on the Savage–Dickey method. Cogn. Psychol. 2010;60:158–189. doi: 10.1016/j.cogpsych.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Wagenmakers E.-J., Marsman M., Jamil T., Ly A., Verhagen J., Love J., Selker R., Gronau Q.F., Šmíra M., Epskamp S. Bayesian inference for psychology. Part I: theoretical advantages and practical ramifications. Psychon. Bull. Rev. 2018;25:35–57. doi: 10.3758/s13423-017-1343-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D., Clark L.A. 1999. The PANAS-X: Manual for the Positive and Negative Affect Schedule-Expanded Form. [Google Scholar]
- Weden M.M., Carpiano R.M., Robert S.A. Subjective and objective neighborhood characteristics and adult health. Soc. Sci. Med. 2008;66:1256–1270. doi: 10.1016/j.socscimed.2007.11.041. [DOI] [PubMed] [Google Scholar]
- Wen M., Hawkley L.C., Cacioppo J.T. Objective and perceived neighborhood environment, individual SES and psychosocial factors, and self-rated health: an analysis of older adults in Cook County, Illinois. Soc. Sci. Med. 2006;63:2575–2590. doi: 10.1016/j.socscimed.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Whittle S., Vijayakumar N., Simmons J.G., Dennison M., Schwartz O., Pantelis C., Sheeber L., Byrne M.L., Allen N.B. Role of positive parenting in the association between neighborhood social disadvantage and brain development across adolescence. JAMA Psychiatry. 2017;74:824–832. doi: 10.1001/jamapsychiatry.2017.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D.K., Kirtland K.A., Ainsworth B.E., Addy C.L. Socioeconomic status and perceptions of access and safety for physical activity. Ann. Behav. Med. 2004;28:20–28. doi: 10.1207/s15324796abm2801_4. [DOI] [PubMed] [Google Scholar]
- Wu C.C., Samanez-Larkin G.R., Katovich K., Knutson B. Affective traits link to reliable neural markers of incentive anticipation. Neuroimage. 2014;84:279–289. doi: 10.1016/j.neuroimage.2013.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T., Poldrack R.A., Nichols T.E., Van Essen D.C., Wager T.D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods. 2011;8:665. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A map that shows where the T500 subjects are located. Each participant is represented only once. The color of the tract shows how many participants are in the tract. Clicking on a tract will open a popup that says the geoCode of the tract and the exact number of participants in the tract. A tract will be grey if there are no T500 participants living in that tract.
Supplementary material