Abstract
Regulation of cellular volume is a critical homeostatic process that is intimately linked to ionic and osmotic balance in the brain tissue. Because the brain is encased in the rigid skull and has a very complex cellular architecture, even minute changes in the volume of extracellular and intracellular compartments have a very strong impact on tissue excitability and function. The failure of cell volume control is a major feature of several neuropathologies, such as hyponatremia, stroke, epilepsy, hyperammonemia, and others. There is strong evidence that such dysregulation, especially uncontrolled cell swelling, plays a major role in adverse pathological outcomes. To protect themselves, brain cells utilize a variety of mechanisms to maintain their optimal volume, primarily by releasing or taking in ions and small organic molecules through diverse volume-sensitive ion channels and transporters. In principle, the mechanisms of cell volume regulation are not unique to the brain and share many commonalities with other tissues. However, because ions and some organic osmolytes (e.g., major amino acid neurotransmitters) have a strong impact on neuronal excitability, cell volume regulation in the brain is a surprisingly treacherous process, which may cause more harm than good. This topical review covers the established and emerging information in this rapidly developing area of physiology.
12.1. COMMON AND UNIQUE ASPECTS OF OSMOTIC BALANCE WITHIN THE CNS
Much like any other cell type in the human and animal body, brain cells are subjected to the actions of osmotic forces. The underlying mechanisms and principles are discussed in detail in the first chapter of this book (Delpire & Gagnon, 2018) and, therefore, are only briefly recapitulated here.
Prototypical lipid membranes of animal cells have high passive permeability for water, but in practical terms are essentially impermeant to ions and polar extra- and intracellular solutes. Owing to the semi-permeability of the plasmalemma, water moves freely in and out of the cell as dictated by osmotic gradients set by membrane-impermeant molecules (Finkelstein, 1987). Any net accumulation or loss of solutes and metabolites is immediately followed by water flow and ensuing changes in hydrostatic pressure. Due to the absence of a rigid cell wall, animal cells are poorly equipped to withstand hydrostatic forces. Therefore, they swell or shrink, and cope with changes in cell volume and ionic homeostasis using several distinct strategies.
Moderate degrees of cell shrinkage produce relatively limited strain on cell architecture and, in the short-term, are accommodated by folding of the cell membrane. Moderate degrees of cell swelling are more problematic because the plasma membrane has a very limited stretch capacity and is tethered to extensive cytoskeletal networks. Nevertheless, upon encountering an acute osmotic challenge, many cell types can rapidly increase their volume up to several-fold using extensive membrane reserves and, in extreme cases, via exocytotic recruitment of intracellular membranes (Morris & Homann, 2001; Groulx, Boudreault, Orlov, & Grygorczyk, 2006). Movement of water and changes in cell volume dissipate osmotic gradients on the scale of seconds to several minutes, which is then followed by cell volume regulation.
Osmotic changes in cell volume are typically compensated via the act of active volume regulation. Cell volume control is a vital homeostatic property of living organisms, which emerged at early stages of evolution and is characteristic for organisms belonging to all branches of the phylogenetic tree (Chamberlin & Strange, 1989). The process of volume restoration is much slower than the initial osmotic swelling or shrinkage. Depending on conditions and cell type, it takes minutes to hours to complete. In principle, osmotic imbalances are counteracted via the accumulation or loss of small solute molecules. Osmolyte fluxes are mediated by diverse “volume-sensitive” membrane transporters and ion channels, the majority of which have been studied at length. Unfortunately, we know very little about how cells actually sense changes in their volume [the nature of cell volume sensor(s)] and have insufficient knowledge of how cell volume sensing is transduced to changes in solute transport [the nature of downstream intracellular signals]. For detailed discussion of these topics, the reader can be addressed to several comprehensive reviews (Lang et al., 1998; Wehner, Olsen, Tinel, Kinne-Saffran, & Kinne, 2003; Mongin & Orlov, 2001; Hoffmann, Lambert, & Pedersen, 2009).
12.1.1. Common mechanisms of regulatory volume decrease (RVD)
As schematically depicted in Fig. 12.1A, osmotic cell swelling is counteracted via the process of RVD. In general, volume recovery in swollen cells is mediated by the coupled efflux of cytosolic K+ and the inorganic anions, Cl− and bicarbonate (HCO3−). K+ is the main intracellular osmolyte, but its movement must be accompanied by anions to preserve electroneutrality and sustain the electrochemical driving force for K+. In most cell types, RVD is dominated by K+ and Cl− channels, which are activated independently but are coupled at the functional level via changes in the membrane potential. Among the diverse K+ channels, many subtypes show sensitivity to cell swelling, including the two-pore domain TREKs (products of the KCNK1 and KCNK2 genes), TASKs (KCNK3, 5 and 9), and TRAAK (KCNK4), the voltage-sensitive Kv1.1 and Kv1.3 (KCNA1 and KCNA3), Kv4.1 and Kv4.3 (KCND1 and KCND3), Kv7.1, 4 and 5 (KCNQ1, KCNQ4, and KCNQ5), and the Ca2+- and voltage-sensitive BK (KCNMA1) and IK1 (KCNN4) [reviewed in (Lang et al., 1998; Wehner et al., 2003; Hoffmann et al., 2009)]. It is important to note that due to the dominant nature of K+ permeability in most mammalian cells, K+ channels need not be cell-volume sensitive to contribute to cell volume control.
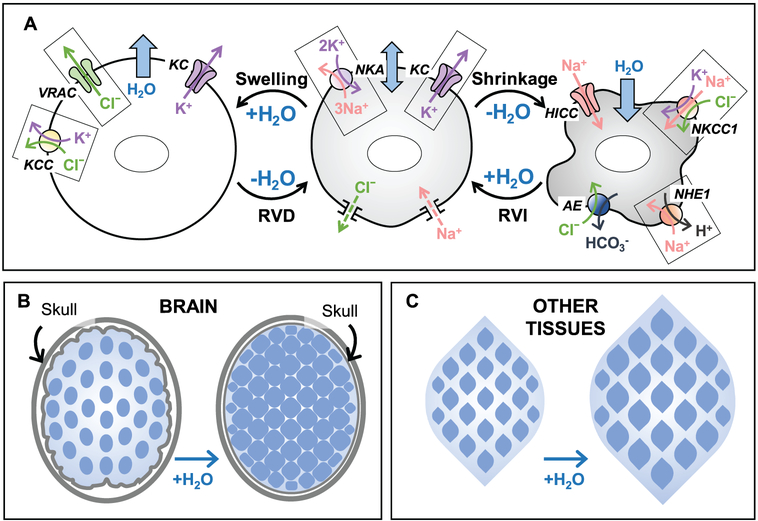
Figure 12.1.
Basic principles of cell volume regulation in mammalian cells and major differences in the osmotic homeostasis within the brain and in other tissues. (A) Ion transport mechanisms responsible for cell volume regulation. Center panel: Under isosmotic conditions, the work of the Na+,K+-ATPase (NKA) and the dominant activity of K+ channels (KC), set the transmembrane ionic gradients and negative membrane potential, as well as compensate for the persistent Na+ uptake via a variety of mechanisms (Na+ leak). Importantly, the negative membrane potential drives out intracellular Cl−, offsetting the presence of the negatively charged impermeant macromolecules and metabolites in the cytosol. Left panel: Hypoosmotic cell swelling triggers activation of the volume-regulated Cl−/anion channel (VRAC) and several K+,Cl− cotransporters (KCC). Cooperative activity of VRAC, KC, and KCC mediates the loss of cytosolic KCl and powers regulatory volume decrease (RVD). Right panel: Cell shrinkage upon exposure to hyperosmotic media stimulates the ubiquitous Na+,K+,Cl− cotransporter 1 (NKCC1) and/or the Na+/H+ exchanger 1 (NHE1). NHE works in cooperation with the volume-insensitive Cl−/HCO3−anion exchangers (AE). In some cell types, cell shrinkage also opens hypertonicity induced non-selective cation channels (HICC). The combined activity of these transporters and channels leads to cytosolic accumulation of NaCl and KCl and mediates regulatory volume increase (RVI). (B and C) The major differences in cell volume control between the brain and other tissues are due to the fixed volume of (extracellular + intracellular) space in the CNS. (B) In the brain, due to spatial limitations imposed by the rigid skull, cell swelling under hypoosmotic conditions or in pathologies occurs at the expense of the interstitial volume and may also compress blood vessels and cause ischemia. Also, due to the restricted ion transport across the blood-brain barrier, there is a “fixed” total pool of extracellular and intracellular ions. Therefore, during cell volume regulation, the electrochemical driving forces for ionic fluxes dissipate very quickly. (C) In contrast to the brain, the majority of peripheral tissues are not restricted in terms of their osmotic expansion or shrinkage, and allow for the relatively rapid exchange of electrolytes between the interstitial space and the blood.
In contrast to K+ conductance, the typical basal Cl− permeability of the plasma membrane is low and must be augmented during RVD. This is accomplished via activation of a volume-sensitive chloride current (ICl,vol or ICl,swell), which was initially characterized in the pioneer work done by the groups of Y. Okada, K. Strange, and B. Nilius. Because the biophysical properties of ICl,vol are similar in different cell types, this led to the hypothesis that they are mediated by the same ion channel or a group of similar channels. The putative ICl,vol channel was christened volume-regulated anion channel (VRAC), volume-sensitive outwardly rectifying Cl− channel (VSOR), or volume-sensitive organic osmolyte-anion channel (VSOAC) (Strange, Emma, & Jackson, 1996; Okada, 1997; Nilius et al., 1997). In this review we will use the acronym VRAC. Despite detailed studies of ICl,vol currents in many cell types, the molecular identity of VRAC remained an enigma until a few years ago. In 2014, the laboratories of A. Patapoutian and T.J. Jentsch independently identified VRAC as a heteromeric complex of proteins from the family of leucine-rich repeat-containing 8 (LRRC8) (Qiu et al., 2014; Voss et al., 2014). The functional properties and physiological roles for the LRRC8-containing VRAC are discussed in several specialized reviews (Stauber, 2015; Pedersen, Okada, & Nilius, 2016; Mongin, 2016; Jentsch, 2016) and for the CNS tissue are additionally covered in sections 12.2 and 12.8.
One of the properties of VRAC, which is reflected in their alternative name VSOAC, is the ability to conduct not only inorganic anions (Cl−, HCO3−, and others), but also small, negatively charged and neutral organic molecules. The first evidence that Cl− channels, which were later identified as VRAC, can constitute a route for transmembrane amino acid fluxes was collected in MDCK cells by U. Banderali and G. Roy, who measured anion currents mediated by aspartic acid, glutamic acid, and the amino sulfonic acid taurine in excised membrane patches (Banderali & Roy, 1992). Subsequent whole-cell electrophysiology studies measured amino acid currents in a variety of cell types subjected to hypoosmotic swelling (Jackson & Strange, 1993; Jackson, Morrison, & Strange, 1994; Strange et al., 1996; Manolopoulos, Voets, Declercq, Droogmans, & Nilius, 1997). Although the relative permeability of VRAC to organic osmolytes is a fraction of that for Cl− (in the order of 0.05–0.3), the loss of organic molecules is strongly favored by their electrochemical gradients and may play a significant role in RVD. In some cell types, the loss of organic osmolytes accounts for about 50% of the total osmolyte loss during RVD, but such contribution is likely cell type specific [reviewed in (Wehner et al., 2003)]. What is essential in the context of the present discussion is that some of the VRAC-permeable osmolytes, such as glutamate, aspartate, taurine, and GABA, are major neurotransmitters in the CNS. Therefore, release of these molecules exerts a dramatic impact on neural functions, irrespectively of their relative contribution to RVD [see (Mongin, 2016) and sections 12.2 and 12.8].
Besides channel-mediated ion fluxes, in many cell types, RVD is supplemented by KCl loss via the electroneutral KCl cotransporters (KCC1–4, encoded by SLC12A4–6). Discussion of the physiological roles of KCC and their contributions to cell volume control can be found in specialized reviews (Lauf & Adragna, 2000; Gamba, 2005; Kahle et al., 2015; Kahle et al., 2008), and in a brain-specific context in section 12.2.
12.1.2. Generic mechanisms of regulatory volume increase (RVI)
Following osmotic or metabolic shrinkage, cells recover their volume through the process of RVI (see Fig. 12.1A). The two main ion transporters which drive RVI are the electroneutral Na+,K+,2Cl− co-transporter NKCC1 (gene product of SLC12A2) and the ubiquitous isoform of the Na+/H+-exchanger NHE1 (SLC9A1). Through their combined work, NKCC1 and NHE1 mediate the net accumulation of NaCl and KCl and drive the osmotically obligated movement of water inside the cell (Lang et al., 1998; Wehner et al., 2003; Mongin & Orlov, 2001; Hoffmann et al., 2009). Activity of the shrinkage-activated NHE1 (and other cell type-specific NHEs) causes alkalization of the cytosol and stimulates the pH-sensitive anion exchangers (AE), which are not directly regulated by cell volume changes. Ubiquitous AE2 (SLC4A2) and the brain- and heart-enriched AE3 (SLC4A3) take Cl− inside the cell in exchange for HCO3− out, and in such a way contribute to the accumulation of intracellular Cl− and removal of alkaline equivalents (Alper, 1991; O’Neill, 1999). In contrast to NHEs, shrinkage-activated NKCC1 works “autonomously”, but its efficacy in RVI depends on the preservation of the combined inwardly-directed gradient for [Na+ plus K+ plus Cl−]. In certain cell types, hyperosmolar media strongly activate NKCC1, but this is not accompanied by RVI due to an insufficient electrochemical driving force for ionic movement. In contrast, the same cells can effectively regulate their volume in response to isosmotic cell shrinkage (e.g., caused by the receptor-mediated loss of intracellular electrolytes or the metabolic decrease of osmolyte content) or as a result of the so-called “RVI after RVD” protocol (when cells are returned to isosmotic medium after prolonged adaptation to hypoosmotic conditions) (O’Neill, 1999).
A relatively rare, but thermodynamically-effective, mechanism of RVI, which has been identified in only a few cell types, involves the opening of hypertonicity-induced non-selective cation channels (HICCs). HICCs mediate the rapid accumulation of monovalent cations, primarily Na+, and very fast RVI (Bohmer et al., 2000; Wehner, Shimizu, Sabirov, & Okada, 2003; Wehner et al., 2006; Koos et al., 2018). The molecular composition of HICCs remains rather obscure, but depending on the cell type they may incorporate heteromeric assemblies of the transient receptor potential (TRP) channels from the melastatin family (e.g., TRPM2 and TRPM5), and/or one or several types of the “epithelial” Na+ channels from the degenerin family (ENaCα through δ, SCNN1A-D) (Bondarava, Li, Endl, & Wehner, 2009; Koos et al., 2018).
The long-term adaptation to hyperosmotic conditions involves the intracellular accumulation of small, nonionic organic molecules, which have the collective name ‘idiogenic’ or ‘compatible’ osmolytes. The compatible osmolytes are beneficial for cell volume control cells because, unlike ions, they do not modify ionic strength and composition in the cytosol, and in such a way preserve the optimal conditions for intracellular enzymatic reactions. The relevant compounds include amino acids (e.g., glutamine and alanine), amino acid derivatives (e.g., the aminosulfonic acid taurine), methylamines (betaine and glycerophosphorylcholine), and polyalcohols (sorbitol and myo-inositol). Changes in the cytosolic levels of compatible osmolytes are in part due to the increase in expression of Na+-dependent membrane transporters (such as SMIT for myo-inositol, BGT1 for betaine, and TauT for taurine), which lead to net osmolyte accumulation. Alternatively, there are increases in the expression of certain synthetic enzymes (aldose reductase for sorbitol, phospholipase for glycerophosphorylcholine, etc.) that lead to the net increase in osmolyte content inside of the cell. In all cases, the main driving mechanism for the long-term adaptation to hyperosmolarity are changes in gene expression, which are initiated by the osmosensitive transcription factor TonEBP, also known as OREBR and NFAT5 (Burg, Ferraris, & Dmitrieva, 2007; Hoffmann et al., 2009). As in the case of RVD, changes in transmembrane fluxes of certain organic osmolytes can have a dramatic impact on brain functions.
12.1.3. Donnan cell swelling
Besides the above-mentioned mechanisms responsible for “emergency” cell volume control (RVD and RVI), it is very important to recognize that under normotonic steady-state conditions all animal cells, including cells in the brain, are also constantly subjected to a persistent osmotic challenge. Due to the high content of impermeant organic macromolecules in the cytosol and intracellular organelles which create significant osmotic (or colloid) load, cells continuously accumulate water. The resulting osmotic behavior, which is often referred to as Donnan swelling or the Gibbs-Donnan effect, was theoretically predicted at the turn of the 20th century by J.W. Gibbs and experimentally tested by F.G. Donnan [reviewed in (Macknight & Leaf, 1977)]. Animal cells are capable of resisting Donnan swelling via mechanisms which were first recognized in the studies by E.B. Wilson (Wilson, 1954) and A. Leaf (Leaf, 1956). These investigators found that metabolically compromised cells and tissue slices steadily accumulate water, Na+, and Cl−, suggesting that cell volume maintenance involves an energy-dependent process. The subsequent work led to the development of the “double Donnan” or “pump-and-leak” model (Macknight & Leaf, 1977; Lang et al., 1998; Hoffmann et al., 2009). This model proposes that Donnan cell swelling is counteracted by the osmotic and electrical work of the Na+,K+-pump, which sets the negative membrane potential and drives the passive extrusion of intracellular Cl− (Fig. 12.1A). The low intracellular [Cl−] compensates for the presence of the net-negatively charged macromolecules (e.g., proteins and polynucleotides) and numerous organic metabolites [see discussion in (Lang et al., 1998; Hoffmann et al., 2009) and the “neurocentric” view in (Wilson & Mongin, 2019)].
12.1.4. The peculiarities of cell volume regulation in the brain
Several unique features of brain physiology strongly influence the execution and efficacy of cell volume control within the CNS. For the purposes of our discussion, it is helpful to briefly mention some of the relevant factors upfront:
The brain is contained within a rigid skull, which dramatically limits to what extent the CNS tissue and its functional components can expand or shrink (see Fig. 12.1B). In the context of fluid-electrolyte homeostasis, the brain can be divided into several distinct compartments [reviewed in (Strange, 1992; Kimelberg, 2004; Thrane, Rangroo Thrane, & Nedergaard, 2014)]. (i) An intracellular partition is filled with the intracellular fluid (ICF). Within CNS, there are four major functionally distinct classes of cells: neurons, astrocytes, oligodendrocytes, and microglia; additionally, ciliated ependymal cells line the brain ventricles and spinal cord canal. (ii) The interstitial extracellular fluid (ECF) bathes all brain cells and creates a milieu for their physiological activities. (iii) The cerebrospinal fluid (CSF) is produced by the specialized epithelial cells of the choroid plexus in the brain ventricles; it serves as a precursor for ECF. CSF is moved through the ventricles into the subarachnoid space by positive hydrostatic pressure generated by the choroid plexus and with the assistance of ependymal cells. From there it is pushed via the periarterial spaces deep into the brain, where it is slowly equilibrated with ECF. The movement and equilibration of CSF and ECF is mediated by the glymphatic system, the idea of which has been largely shaped by M. Nedergaard and co-workers (Iliff et al., 2012; Iliff et al., 2013; Thrane et al., 2014). The combination of CSF plus ESF represents the extracellular space of the brain. (iv) Finally, the segregated blood compartment contains vessels of various diameters and circulating blood cells, which assist in delivering oxygen and nutrients to the brain tissue. All hydrophilic components (ions and nutrients) are transported inside the brain in a highly regulated manner via either the choroid plexus or the endothelial blood-brain barrier (BBB). The main message here is that in the brain, any change in the volume of an individual fluid compartment can happen only at the expense of its counterparts, unlike other tissues which can expand in a relatively uniform fashion (see Fig. 12.1C).
The total volume of the ECF is very small, which puts significant constraints on cell volume regulation. Due to the restricted transport of electrolytes across the BBB, brain tissue contains a rather small pool of extracellular osmolytes. For the sake of argument, let us simplistically assume that the extracellular space occupies 20%, and the intracellular space 80%, of the total brain volume. A moderate, 10% increase in volume of the cellular compartment (swelling) will occur at the expense of the extracellular space and reduce the latter from the initial 20 to 12% of the total brain volume (or nearly two-fold). This on its own would concurrently increase the concentration of extracellular ions and signaling molecules and modify their diffusion parameters. Now, consider that swollen brain cells engage in the process of RVD (see section 12.1.1) and release 10% of their cytosolic K+ content. Based on the intracellular [K+] in the range of 130–140 mM, and taking into account the above-mentioned volume ratios, we should expect a rise in the extracellular [K+] to well above 50 mM. Needless to say, this would dramatically diminish the driving force for RVD and be incompatible with brain function. This overly-simplistic example can explain why cell volume regulation in the brain is likely significantly less effective than in other tissues, but also disproportionally impacts tissue functions.
The morphological complexity of brain cells may allow for “local” fluctuations in the volume of their subcellular compartments. As an extreme example, we can consider projection neurons, which have exceptionally long and voluminous processes (both axonal and dendritic). The volume of the neuronal cell body represents <1% and in many cases as little as 0.1% of the total cell volume (Morfini, Stenoien, & Brady, 2006). Therefore, local ionic fluxes in the distal processes (such as during action potentials or pathological depolarization) are likely to change local volume independently of the cell body.
Release of typical cellular osmolytes (both inorganic and organic) has a strong physiological impact on brain cells. Since the brain is an excitable tissue, changes in the intracellular and the extracellular levels of K+, Na+, and Cl− not only contribute to cell volume control, but also dramatically modify neuronal excitability (see example above). Furthermore, many of the “classical” compatible organic osmolytes are important signaling molecules in the brain. For instance, the most abundant cytosolic amino acid and organic osmolyte, glutamate, is the main excitatory neurotransmitter in the brain playing a signaling role in 90% of the excitatory synapses (Meldrum, 2000). Another highly enriched cytosolic molecule, taurine, is an endogenous ligand for the two most important inhibitory receptors, GABA and glycine receptor-channels (Albrecht & Schousboe, 2005).
12.2. RVD IN THE CNS CELLS
Even in the face of the abovementioned limitations and caveats, brain cells are capable of regulating their volume much like the majority of cells in other tissues. The idea of active cell volume control within the brain was initially proposed based on early in vivo observations of the compensatory loss of inorganic ions and organic osmolytes from brain tissue in response to systemic hyponatremia (Yannet, 1940; Melton & Nattie, 1983; Thurston & Hauhart, 1987; Melton, Patlak, Pettigrew, & Cserr, 1987; Verbalis & Gullans, 1991). These latter studies were matched with numerous clinical and basic science findings that reductions in local or systemic osmolarity (hyponatremia) lead to the modification of neuronal excitability and the development of life-threatening neurological deficits, which tend to resolve over time as the brain adapts [see (Andrew, 1991; Adrogue & Madias, 2000; Sterns, 2015) and section 12.6.1 for references and further discussion].
12.2.1. Neuronal RVD and its ionic mechanisms
To the best of our knowledge, the first direct observation of neuronal RVD was made in cultures of dorsal root ganglion (DRG) cells by H. Horie and colleagues (Horie, Ikuta, Takenaka, & Ito, 1989). Most of the subsequent early mechanistic studies were done in cultures of cerebellar granular cells by the group of H. Pasantes-Morales. They used a combination of Coulter counter and radiotracer assays to quantify RVD and osmolyte fluxes. Once subjected to hypoosmotic media, DRG and cerebellar neurons rapidly swell and then completely or partially regulate their volume to the same or a new steady-state level within 15 min (Horie et al., 1989; Pasantes-Morales, Maar, & Moran, 1993; Pasantes-Morales, Chacon, Murray, & Moran, 1994). The RVD process is driven by the loss of cytosolic K+, because disruption of the transmembrane K+ gradient with either high [K+]o or high [Rb+]o media completely blocks compensatory volume decrease (Pasantes-Morales et al., 1993). As in many other cells, K+ permeability is the rate-limiting step in this process because the K+ ionophore gramicidin dramatically increases the rate of RVD (Pasantes-Morales et al., 1994). Nevertheless, K+ fluxes have to be supplemented by the efflux of Cl− and other organic and inorganic osmolytes, the functional importance of which is underscored by the fact that RVD is strongly inhibited by Cl− channel blockers or by adding high millimolar levels of extracellular amino acids (Pasantes-Morales et al., 1993; Pasantes-Morales et al., 1994).
The initial observations of neuronal swelling and RVD have been reproduced in many neuronal cell types, including acutely isolated peripheral sympathetic neurons (Leaney, Marsh, & Brown, 1997), cerebellar granular cells (Patel, Lauritzen, Lazdunski, & Honore, 1998), cultured cortical neurons (Inoue, Mori, Morishima, & Okada, 2005), and the neuroblastoma N1E-115 cell line (Falke & Misler, 1989; Altamirano, Brodwick, & Alvarez-Leefmans, 1998). In these latter studies, cell volume dynamics in the substrate-attached cells was assessed using phase microscopy, or indirectly, based on the changes in intracellular calcein fluorescence. Several publications have explored the biophysical properties and possible mechanisms of activation of volume-sensitive Cl− channels and 125I− efflux in neuroblastoma cell lines (Basavappa et al., 1995; Bond, Basavappa, Christensen, & Strange, 1999; Cheema, Pettigrew, & Fisher, 2007) and primary neuronal cells (Leaney et al., 1997; Inoue et al., 2005). The conclusions of these early reports were that (i) neurons respond to hypoosmotic gradients with predictable osmotic swelling, (ii) in the majority of cases, neurons are capable of active cell volume control, (iii) neuronal RVD is driven by volume-sensitive K+ and Cl− fluxes. However, the molecular identities of swelling-activated K+ and Cl− channels in neuronal cells remain poorly unexplored. They are inferred based on gene expression profiles and functional data obtained in other cell types. Due to the high background K+ conductance and diverse expression, many types of K+ channels may assist in neuronal RVD, and these need not be necessarily cell-volume sensitive. In contrast, since the resting Cl− conductance is low, RVD is only possible with significant increases in Cl− permeability, which are mediated by VRAC. Brain VRAC channels are assembled from the proteins belonging to the LRRC8 family, but, with the exception of one study in primary sensory neurons (Wang et al., 2017), this yet to be directly tested in diverse neuronal cells [see section 12.8 and review (Mongin, 2016)].
Besides the functionally coupled K+ and Cl− channels, neuronal RVD can be additionally powered by one or more electroneutral K+,Cl− cotransporters. The “classical” KCC1, KCC3, and KCC4 are silent under basal conditions but are activated by increases in cell volume, while the neuron-specific KCC2 is basally active and additionally stimulated by cell swelling (Lauf & Adragna, 2000; Gamba, 2005). The contribution of KCCs to neuronal cell volume control has been suggested in the past but never definitively tested (Kahle et al., 2015; Wilson & Mongin, 2019). In one study, cultured hippocampal pyramidal neurons from KCC3-null mice showed a lack of RVD in hypoosmotic media (Boettger et al., 2003). Also, one of the phenotypic features of the KCC3-knockout animals is the enlarged diameter of peripheral axons, possibly due to defective cell volume regulation (Byun & Delpire, 2007).
The views on the osmotic behavior of neuronal cells have been strongly influenced by a number of studies coming from the groups of R.D. Andrew, G.G. Somjen, and S.A. Kirov, who found that pyramidal neurons in the hippocampus and cortex (acutely isolated or in brain slices), do not swell in response to moderate hypoosmotic challenge or swell with a significant delay (Somjen, Faas, Vreugdenhil, & Wadman, 1993; Aitken et al., 1998; Andrew, Labron, Boehnke, Carnduff, & Kirov, 2007; Caspi, Benninger, & Yaari, 2009). Interestingly, these publications also reported that the same neuronal cells readily increase their volume in response to spreading depolarizations or ischemia-like conditions. Another study performed side-by-side comparison of acutely isolated hippocampal pyramidal cells and the hypothalamic magnocellular neurons and found that only magnocellular neurons swell to the predicted degree, while pyramidal cells do not behave as typical osmometers (Zhang & Bourque, 2003). Furthermore, intravital work in transgenic animals expressing EGFP in neuronal cells, registered no swelling of dendritic processes in response to hypoosmotic gradients as large as −100 mOsm (Steffensen, Sword, Croom, Kirov, & MacAulay, 2015). Overall, these findings have been explained by the very low water permeability of neuronal membranes due to the lack of aquaporins (Andrew et al., 2007; Caspi et al., 2009), and/or by the paradoxical osmotic shutdown of the major ion permeability pathways in swollen pyramidal cells (Somjen et al., 1993). Although this narrative has become very dominant, is hard to say if low water permeability is a general property of pyramidal neurons, or the result of select experimental conditions. The idea of osmotic resistance of cortical and hippocampal pyramidal cells has been recently challenged by the work of T. Fiacco and co-workers, who found in brain slices that pyramidal neurons swell and shrink to the same degree as nearby “water-permeable” astrocytes (Murphy et al., 2017). Additionally, the already mentioned KCC3 study in hippocampal pyramidal cells exposed to hypoosmotic conditions showed complete RVD within 20 minutes (Boettger et al., 2003). In brain slices, the ability to regulate cell volume under hypoosmotic conditions has been attributed to a dependence on intracellular taurine content, as it was regained in slices preincubated with taurine-containing media (Kreisman & Olson, 2003). Another already mentioned factor (see section 12.1.4) are the limitations imposed by small extracellular space in vivo, which may reduce the driving force of RVD.
As briefly outlined in the introduction (section 12.1.4), brain cells, specifically neurons, have such an elaborate morphology that the distant parts of the cell are likely to experience “local” hydration gradients and require “decentralized” mechanisms of cell volume control. Neuronal hyperexcitation is associated with significant fluxes of ions and water. Dendrites, which are endowed with a high density of postsynaptic ionotropic receptors, have especially high propensity to swell. This phenomenon is known as dendritic beading or the formation of synaptic varicosities; which is particularly pronounced under pathological conditions [reviewed in (Fiala, Spacek, & Harris, 2002; Greenwood & Connolly, 2007)]. Consequently, an important question is; are neuronal processes capable of “local” volume control? The few studies that have explored this topic suggest an affirmative answer. For instance, swollen synaptosomes (isolated presynaptic nerve endings) completely recover their volume within 2 minutes after exposure to hypoosmotic media (Babila et al., 1990). Within the same time frame, swollen synaptosomes strongly upregulate their Ca2+ uptake via activation of unidentified cation channels (Mongin, Aksentsev, Orlov, & Konev, 1997). Hypoosmolarity-induced Ca2+ influx may explain the swelling-induced exocytosis of neurotransmitters, however, other mechanisms of release have also been suggested (Tuz, Pena-Segura, Franco, & Pasantes-Morales, 2004; Waseem, Rakovich, Lavrukevich, Konev, & Fedorovich, 2005; Tuz & Pasantes-Morales, 2005). In cortical neurons, acute exposure to glutamate or NMDA causes rapid (within 10 min), swelling of their somata and formation of dendritic varicosities (Inoue & Okada, 2007). For both compartments, this process is completely [Na+]o-dependent, but also inhibited by non-selective VRAC blockers. Once NMDA-receptor agonists are removed, swelling of the cell body and dendritic varicosities is resolved within 20–60 min, and the latter volume recovery is also sensitive to VRAC inhibitors (Inoue & Okada, 2007). These data suggest that in neuronal processes, VRAC contributes to both cellular swelling and compensatory RVD during and after treatment with excitotoxins. Collectively, studies in synaptosomes and neuronal cultures suggest that in neuronal cells, cell volume-sensitive ion channels are distributed throughout their distal cell processes, in both the dendritic and the axonal compartments, and mediate cell volume control. The Cl− movement in local volume control appears to be largely mediated by VRAC.
12.2.2. Astrocytic RVD and swelling-activated ion channels
Although neurons represent the main cell type in the CNS, they are significantly outnumbered by several classes of non-neuronal cells – neuroglia. Among neuroglia, astrocytes are the most numerous and functionally complex, and the most studied in the context of cell volume regulation (Haydon & Carmignoto, 2006; Mongin & Kimelberg, 2009; Sofroniew & Vinters, 2010; Verkhratsky & Nedergaard, 2018). The keen interest in astrocytic cell volume homeostasis and its dysregulation was prompted by the very early knowledge that many diverse factors that lead to brain edema – such as toxins, hypoxia and metabolic inhibition, the Na+,K+-pump blocker ouabain, and brain traumas – all trigger profound swelling of astrocytes and corresponding reductions in the extracellular space [e.g., (Cornog, Gonatas, & Feierman, 1967; Bakay & Lee, 1968; Bourke & Nelson, 1972; Baethmann & Van Harreveld, 1973; Bakay, Lee, Lee, & Peng, 1977)]. The pathological significance of these and other relevant findings is discussed in section 12.6, while the potential reasons for preferential swelling of astrocytes are considered in section 12.5. The first observations of RVD in glial cells have been made in the C6 glioma cell line by the laboratory of A. Baethmann (Kempski, Chaussy, Gross, Zimmer, & Baethmann, 1983), and primary astrocyte cultures by H.K. Kimelberg and co-workers (Kimelberg & Frangakis, 1985; O’Connor, Kimelberg, Keese, & Giaever, 1993) and J.E. Olson et al. (Olson, Sankar, Holtzman, James, & Fleischhacker, 1986). The bulk of the subsequent mechanistic studies were carried out by the laboratories of H. Pasantes-Morales (Sanchez-Olea, Pena, Moran, & Pasantes-Morales, 1993; Pasantes-Morales, Murray, Lilja, & Moran, 1994; Pasantes-Morales, Murray, Sanchez-Olea, & Moran, 1994) and M.D. Norenberg (Bender, Neary, Blicharska, Norenberg, & Norenberg, 1992; Bender, Neary, & Norenberg, 1993).
Much like in neuronal cells, astrocytic RVD is mediated by the loss of K+ and Cl− via functionally coupled K+ and Cl− channels. These were initially explored using radiotracer assays and pharmacological inhibitors (Sanchez-Olea et al., 1993; Sanchez-Olea, Moran, Martinez, & Pasantes-Morales, 1993; Vitarella, DiRisio, Kimelberg, & Aschner, 1994; Pasantes-Morales et al., 1994). The biophysical properties and regulation of swelling-activated ion channels have been further explored using an electrophysiological approach. Surprisingly, the molecular nature and properties of swelling-activated glial K+ channels received little attention, perhaps because the membrane conductance in swollen cells is dominated by Cl− channels. Yet, it is known that two types of channels dominate K+ permeability in astroglia: the volume-insensitive, inward-rectifier channels, such as Kir4.1 (KCNJ10), and the swelling-activated two-pore domain TWIK-1 and TREK-1 channels [reviewed in (Kofuji & Newman, 2004; Olsen et al., 2015); but see the alternative opinion on Kir4.1 volume sensitivity in (Soe, MacAulay, & Klaerke, 2009)]. In contrast, numerous laboratories studied the biophysical properties of astroglial VRAC as a pathway for Cl− release (Jackson & Strange, 1993; Jackson et al., 1994; Olson & Li, 1997; Parkerson & Sontheimer, 2004; Crepel, Panenka, Kelly, & MacVicar, 1998; Abdullaev, Rudkouskaya, Schools, Kimelberg, & Mongin, 2006; Liu, Tashmukhamedov, Inoue, Okada, & Sabirov, 2006; Minieri, Pivonkova, Harantova, Anderova, & Ferroni, 2015). VRAC properties and functions in astrocytes and other CNS cells are summarized in two recent comprehensive reviews (Akita & Okada, 2014; Mongin, 2016). Here, we present for illustrative purposes our own electrophysiological recordings of typical VRAC currents in primary astrocytes (Fig. 12.2A–B). Besides VRAC, other types of swelling activated Cl− channels, such as the voltage gated ClC-2 channels and maxi-Cl− channels, also exist and are expressed in astrocytes. These may provide additional minor contributions to RVD (Parkerson & Sontheimer, 2004; Liu et al., 2006). The molecular nature of astrocytic VRAC has been uncovered only recently (Hyzinski-Garcia, Rudkouskaya, & Mongin, 2014; Schober, Wilson, & Mongin, 2017) and is further discussed in section 12.8.
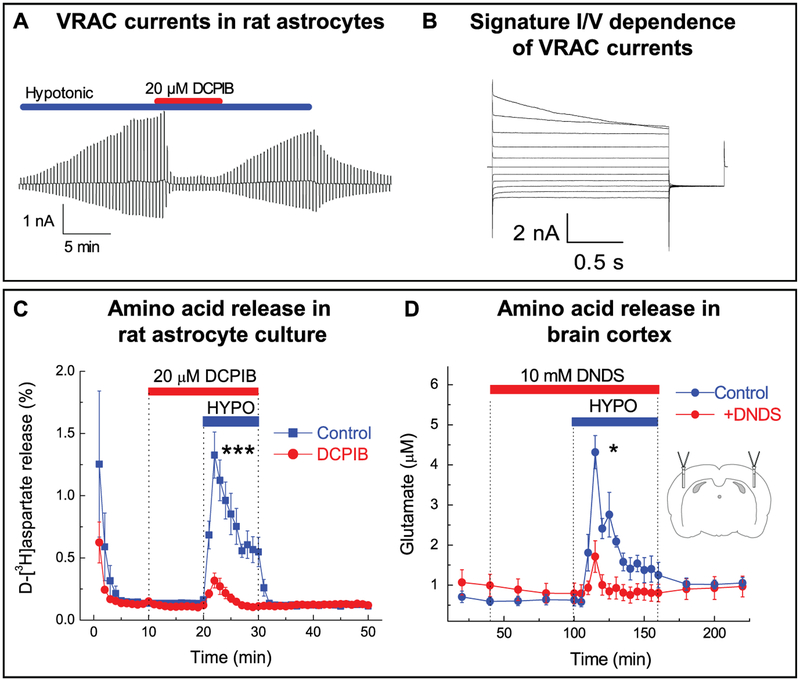
Figure 12.2.
Swelling-activated Cl− currents and amino acid release through VRAC in vitro and in vivo. (A) Representative whole-cell recordings of Cl− currents in primary rat astrocytes exposed to hypoosmotic medium (−60 mOsm). Activity of Cl− channels was measured by holding cells at 0 mV and alternately administering ± 40 mV voltage pulses. Currents were inhibited by treatment with the VRAC blocker DCPIB (20 μm). (B) Swelling-activated Cl− currents in astrocytes in response to 20 mV step pulses from −100 to +100 mV, displaying the characteristic outward rectification and time-dependent inactivation at positive potentials. (C) Effect of DCPIB on swelling-activated glutamate release in primary astrocytes, traced with the non-metabolizable glutamate analog D-[3H]aspartate. ***p < 0.001, effect of DCPIB. (D) Effect of the non-specific VRAC blocker DNDS on swelling-activated glutamate release in the rat cortex after stimulation with hypoosmotic artificial cerebrospinal fluid, measured by microdialysis approach and analyzed with HPLC. *p <0.05, effect of DNDS. (A-C) adapted from I.F. Abdullaev et al. (2006), with permission. (D) Reproduced from R.E. Haskew-Layton et al., 2008, under the Creative Commons Attribution (CC BY) license.
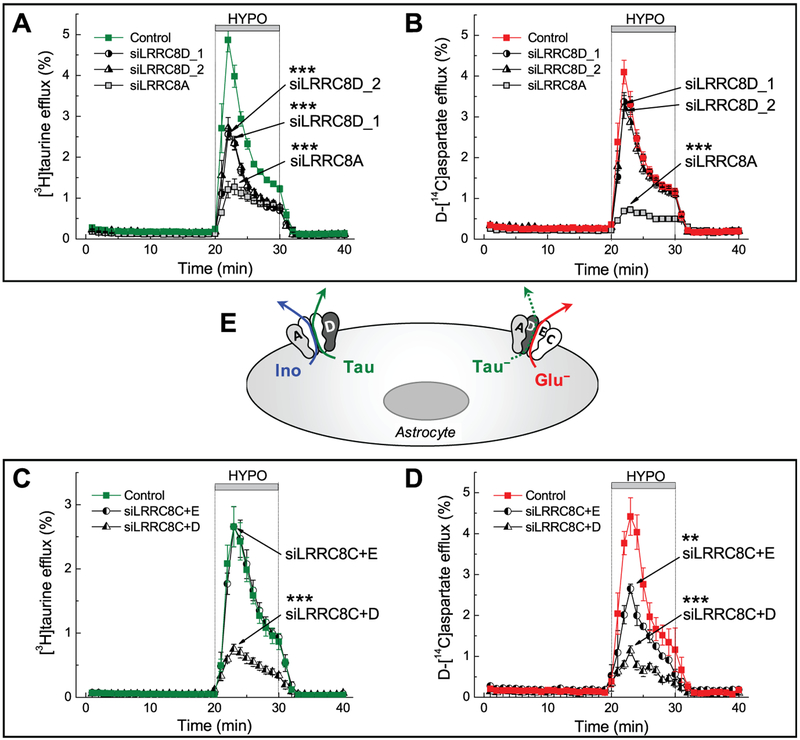
As already discussed, besides conducting inorganic anions, VRAC is permeable to a variety of organic osmolytes. The first publications on glial swelling-activated release of organic osmolytes putatively linked this process to VRAC based on sensitivity to diverse chloride channel blockers (Pasantes Morales & Schousboe, 1988; Pasantes-Morales, Moran, & Schousboe, 1990; Martin, Madelian, Seligmann, & Shain, 1990; Kimelberg, Goderie, Higman, Pang, & Waniewski, 1990; Strange & Morrison, 1992; Strange, Morrison, Shrode, & Putnam, 1993). The release of compatible osmolytes was initially considered a solely beneficial process, assisting in cell volume control. However, H.K. Kimelberg introduced a very counterintuitive at the time idea on the potentially harmful aspects of such release (Kimelberg et al., 1990). Because two cytosolic amino acids, glutamate and aspartate, are the excitatory neurotransmitters, their release can be toxic and drive tissue damage in a variety of brain pathologies. This aspect of VRAC activity has been the subject of several comprehensive reviews (Kimelberg & Mongin, 1998; Kimelberg, 2005; Mongin, 2007; Mongin, 2016) and is further discussed in sections 12.6 and 12.7. The link between organic osmolyte release and VRAC has been extensively tested in glial cells in several electrophysiological studies, which directly measured amino acid currents (Jackson & Strange, 1993; Roy, 1995; Olson & Li, 1997; Abdullaev et al., 2006; Liu et al., 2006). Here, we present our own data on the swelling-activated glutamate release in cultured astrocytes, and the microdialysis study of the VRAC-mediated glutamate release in the brain cortex in vivo (Fig. 12.2C, D). Interestingly, our recent molecular biology work strongly suggests that there are at least two different heteromeric VRAC complexes, which have preferential selectivity for uncharged and charged organic osmolytes [see (Schober et al., 2017) and additional discussion in section 12.8].
Another important idea related to the functional consequences of cell volume control in the CNS is VRAC regulation by G-protein-coupled receptors (GPCRs). In the brain, the degree of cell swelling under physiological conditions is estimated to be in the order of 2–5% of “normal” cell volume. This is unlikely to be sufficient to open VRAC to any significant degree. However, we and others found that astroglial VRAC can be potently modulated by GPCR signaling. E.g., in astrocytes, activation of purinergic P2Y receptors causes partial, Ca2+-dependent stimulation of VRAC, even when cells are not swollen (Mongin & Kimelberg, 2002; Darby, Kuzmiski, Panenka, Feighan, & MacVicar, 2003; Takano et al., 2005; Mongin & Kimelberg, 2005; Akita, Fedorovich, & Okada, 2011). ATP and ADP act at P2Y1, P2Y2 and/or P2Y4 receptors, prompt the release of intracellular Ca2+, activation of classical PKC α and βI, and additionally Ca2+/calmodulin-dependent PK II (Mongin & Kimelberg, 2005; Rudkouskaya, Chernoguz, Haskew-Layton, & Mongin, 2008; Akita et al., 2011). Interestingly, pharmacological inhibitors and molecular biology tools that block the GPCR-driven VRAC opening have little to no effect on VRAC activation by hypoosmotic media. Based on our work, we proposed a model in which purinergic signaling activates or modulates channel activity via phosphorylation of a small fraction of VRAC channels that are already open or “primed” for opening. Although ATP effects are measurable in non-swollen cells, the main, larger pool of VRAC molecules remains inaccessible for purinergic stimulation. The concurrent cell swelling, even as small as 5%, recruits from the “silent”, volume-sensitive pool of VRAC, and acts synergistically with the purinergic receptors (Mongin & Kimelberg, 2005).
Following our findings on the purinergic modulation of VRAC activity in astrocytes, several other groups uncovered very similar synergistic effects of cell swelling and diverse GPCR receptors, linked to either Ca2+ or cAMP signaling, on VRAC-like Cl− currents and organic osmolyte release. For astrocytes and astrocytic cell lines, the reader can be referred to the following significant studies: (Rosso, Peteri-Brunback, Poujeol, Hussy, & Mienville, 2004; Cheema, Ward, & Fisher, 2005; Ramos-Mandujano, Vazquez-Juarez, Hernandez-Benitez, & Pasantes-Morales, 2007; Liu, Akita, Shimizu, Sabirov, & Okada, 2009; Akita & Okada, 2011). Findings in non-CNS cells are covered by three comprehensive reviews (Fisher, Cheema, Foster, & Heacock, 2008; Franco, Panayiotidis, & de La Paz, 2008; Fisher, Heacock, Keep, & Foster, 2010). Recently, to definitively discriminate between VRAC and other potential mechanisms of ATP-stimulated amino acid release (such as vesicular release and others), we demonstrated that ATP-stimulated glutamate and taurine release is abolished by knocking down the essential VRAC subunit LRRC8A (Hyzinski-Garcia et al., 2014).
12.3. RVI IN THE CNS CELLS
Severe systemic hyperosmolarity, such as increases from ~290 to 410–420 mOsm, places mammals (studied mostly in rodent models) in danger of death (Arieff, Guisado, & Lazarowitz, 1977; Gullans & Verbalis, 1993). Milder elevations in systemic osmolarity are typically associated with behavior abnormalities, such as restlessness and increased startle response (Arieff et al., 1977; Gullans & Verbalis, 1993). Numerous early studies, most notably by H.F. Cserr and colleagues, found that in response to systemic hypertonic perturbations the brain as an organ shrinks but then effectively adapts, and regulates its volume via the accumulation of osmolytes and osmotically obligated water on the scale of a few hours to days [e.g., (Cserr, DePasquale, & Patlak, 1987; DePasquale, Patlak, & Cserr, 1989; Cserr et al., 1991)}(Gullans & Verbalis, 1993)]. The measurable tissue volume regulation occurs as rapidly as 90 min after hypertonic insult and is associated with the uptake of Na+, Cl−, and K+, with the bulk of these ions accumulating in the intracellular rather than extracellular compartment (Cserr, DePasquale, & Patlak, 1987; Cserr et al., 1991). These results strongly suggest that brain cells undergo successful RVI in vivo. The secondary phase of adaptation to chronic hyperosmotic conditions involves the accumulation of idiogenic (compatible) organic osmolytes, which slowly replace the excess of inorganic ions within one or two weeks [see for example (Chan & Fishman, 1979; Lohr, McReynolds, Grimaldi, & Acara, 1988) and review (Gullans & Verbalis, 1993)].
In contrast to the whole brain, studies in cultured glial and neuronal cells found no evidence of RVI when cells have been exposed to hyperosmotic media for 10–90 min (Kimelberg & Frangakis, 1985; Horie et al., 1989; O’Connor et al., 1993; Zhang & Bourque, 2003). Similarly, pyramidal neurons in brain slices prepared from either neocortex or hippocampus readily shrunk under hyperosmotic conditions but did not demonstrate RVI, at least during incubation times under 1 h (Andrew et al., 2007). To the best of our knowledge, the only two known reports of RVI in brain cells come from the studies of Kemplski et al. in a C6 glioma cell line (Kempski et al., 1983) and D. Sun and colleagues in immature rat neurons (Schomberg et al., 2003). The former work found complete cell volume recovery (RVI) in C6 cells incubated in hyperosmotic media for a full three hours, but only if medium osmolarity was increased by adding non-ionic compounds (mannitol). When osmolarity was increased with NaCl, RVI was not observed (Kempski et al., 1983). The latter publication (Schomberg et al., 2003) reported relatively fast (within 20 min) and effective (60%) volume recovery in immature rat neurons exposed to osmolarity increased by adding sucrose. Additionally, murine astrocytes also demonstrated a trend for RVI upon exposure to sucrose-containing hyperosmotic media, but this process was efficacious only when cells were co-stimulated with adrenergic agonists (Song, Xu, Hertz, & Peng, 2015). Overall, it appears that cultured neuronal and glial cells do not have transmembrane ionic gradients and ion transporter levels which would favor RVI (see section 12.1.2).
Due to the lack of RVI in model experiments, and because hypertonicity represents a relatively rare and not life-threatening phenomenon in the CNS, there have been surprisingly few studies exploring volume-sensitive ion transporters in brain cells. We could recall only one paper on hypertonic activation of the Na+/H+ exchanger in rodent astrocytes (Shrode, Klein, O’Neill, & Putnam, 1995). NKCC1 stimulation under hyperosmotic conditions has been reported in the already mentioned study in immature rat neurons (Schomberg et al., 2003). In contrast, there are numerous studies linking NHE transporters and NKCC1 to adverse outcomes in various neuropathologies, particularly stroke [reviewed in (Mongin, 2007; Kintner, Wang, & Sun, 2007)].
12.4. CELL VOLUME CHANGES DURING NORMAL BRAIN FUNCTIONING
Brain tissue exists in an environment of near constant osmolarity, which is accomplished via the tight control of systemic water-electrolyte homeostasis and is due to restrictions of electrolyte transport across the blood-brain barrier [reviewed in (Strange, 1992; Bourque & Oliet, 1997; Kimelberg, 2004)]. Nevertheless, brain cells experience constant fluctuations of their volume due to ionic and osmolyte fluxes resulting from electrical activities, reuptake and recycling of neurotransmitter molecules, and dynamic changes in tissue metabolism. The first study directly demonstrating nerve swelling upon electrical stimulation was performed in isolated nerve fibers of invertebrates (Iwasa, Tasaki, & Gibbons, 1980). This pioneer work was followed by numerous observations in higher organisms. One approach, which has been widely utilized in brain slices, is based on detecting variations in light scattering or the intrinsic optical signal (IOS) of the tissue, which indirectly reflect changes in cell volume (Lipton, 1973; Andrew & MacVicar, 1994; Aitken, Fayuk, Somjen, & Turner, 1999). Using IOS signals, several groups demonstrated that electrical stimulation of cortical and hippocampal brain slices causes cellular swelling (MacVicar & Hochman, 1991; Andrew & MacVicar, 1994; Holthoff & Witte, 1996).
An alternative technique allowing for the measurement of interstitial volume is dynamic monitoring of the extracellular concentrations of poorly membrane permeant cations, such as tetramethylammonium (TMA+) or tetraethylammonium (TEA+) [reviewed in (Nicholson & Sykova, 1998; Sykova, 2004; Sykova & Nicholson, 2008)]. The relevant chemical probes are introduced into the CNS electrophoretically, and then detected with ion-selective electrodes. The rate of diffusion of TMA+/TEA+ from the point of injection to the registering electrode gives information on the tortuosity of the extracellular space. Fluctuations of TMA+/TEA+ concentrations under steady-state conditions reflects changes in the volume of the interstitial liquid. Using this type of assay, several groups found that electrical stimulation of neurons causes significant reductions in the extracellular space (cell swelling) in brain preparations from lower vertebrates and mammals [see for example (Svoboda & Sykova, 1991; Jing, Aitken, & Somjen, 1994; Larsen et al., 2014; Larsen & MacAulay, 2017) and comprehensive review by (Sykova & Nicholson, 2008)].
The oldest method for monitoring changes in the volume of interstitial liquid is the electrical impedance assay, which was developed by A. Van Harreveld and co-workers (Van Harreveld, Dafny, & Khattab, 1971; Van Harreveld, 1972). This approach employs measuring changes in the extracellular resistance (impedance) upon the delivery of electric currents with their parameters selected to not allow charge transfer across cellular membranes. Using this technique or its modifications, several groups demonstrated dynamic activity-dependent changes in ionic concentrations and volume of the extracellular space (Dietzel, Heinemann, Hofmeier, & Lux, 1982; Dietzel, Heinemann, & Lux, 1989; Amzica & Neckelmann, 1999). F. Amzica and D. Neckelmann were able to deduct which cell type is swollen, by adding an additional approach and measuring the activity-dependent changes in capacitance (proportional to cell volume) in both neurons and astrocytes in the cortex of anesthetized cats. The capacitance changes of patched astrocytes closely followed neuronal activity and were most pronounced during seizure-like events, suggesting that swelling of astroglia modifies ECF volume. In contrast, apparent changes in neuronal cell volume were smaller and did not correlate with degree of excitation (Amzica & Neckelmann, 1999).
Overall, each of the abovementioned methodologies reveal measurable changes in the volume of the intracellular and extracellular compartments in response to neuronal activation. These are mediated by the activity-dependent fluxes of ions and movement of osmotically obligated water. Changes in the volume and geometry of the extracellular compartment are sufficient to impact neuronal excitability in a normal brain, but are particularly profound in neuropathologies [see review by (Sykova & Nicholson, 2008) and section 12.6]. Depending on the study, cellular swelling has been ascribed to either neurons or astrocytes, but most likely multiple cell types are affected. Thus, N.N. Haj-Yasein et al. analyzed ECF shrinkage (inversely related to cell swelling) in response to high-frequency stimulation in hippocampal slices prepared from either wild type animals or animals carrying deletion of the astrocytic water channel aquaporin-4 (AQP4). In the regions enriched with astrocytic processes, nerve stimulation triggered shrinkage of the extracellular space that was strongly influenced by the deletion of AQP4, suggesting that it occurred in astrocytes. In contrast, in the areas devoid of astrocytic processes, changes in the extracellular space were more profound but independent of AQP4 expression, pointing to their neuronal origin (Haj-Yasein et al., 2012).
Another important example of physiologically relevant cell volume changes can be found in hypothalamic neuroendocrine structures, which sense minor osmolarity changes in systemic circulation. The supraoptic (SON) and the paraventricular (PVN) nuclei of the hypothalamus contain magnocellular neurons, whose main function is regulation of the body’s water-electrolyte homeostasis (Bourque & Oliet, 1997; Hussy, Deleuze, Desarmenien, & Moos, 2000; Bourque, 2008). These neurons project to the pituitary gland and secrete the antidiuretic hormones, vasopressin and oxytocin. Once released into systemic circulation, vasopressin in humans and vasopressin and oxytocin in rodents regulate water reabsorption in the kidney. Neuronal activity in the SON and PVN and release of antidiuretics are activated by hyperosmolarity and inhibited by hypoosmolarity via several distinct mechanisms, which are comprehensively discussed in several specialized reviews (Hussy et al., 2000; Bourque, 2008; Noda & Hiyama, 2015). Briefly: (i) Magnocellular neurons in the SON and PVN are regulated via the excitatory inputs from the upstream osmosensitive neurons located in the organum vasculosum lamina terminalis (OVLT) and the subfornical organ (SFO). (ii) Both the OVLT and SFO cells and the SON and PVN neurons are intrinsically osmosensitive, via the mechanism deciphered in the laboratory of C.W. Bourque. This group discovered that hyperosmolarity directly stimulates osmosensitive neurons via opening the volume-sensitive splice variant of the TRPV1 channel, which lacks a portion of its amino terminus (ΔN-TRPV1) (Bourque, Oliet, & Richard, 1994; Voisin, Chakfe, & Bourque, 1999; Ciura & Bourque, 2006; Ciura, Liedtke, & Bourque, 2011). (iii) An alternative, non-cell-autonomous mechanism responsible for sensing hypoosmolarity was identified by the group of N. Hussy, who found that even moderate reductions in medium osmolarity stimulate specialized populations of SON and PVN astrocytes, which contain extremely high cytosolic levels of taurine. Once swollen, astrocytes release taurine via a VRAC-like channel (Deleuze, Duvoid, & Hussy, 1998; Bres et al., 2000). Taurine inhibits the activity of SON and PVN magnocellular neurons by acting as the endogenous ligand for their inhibitory glycine receptors (Hussy, Deleuze, Pantaloni, Desarmenien, & Moos, 1997). A similar, astrocyte-specific mechanism was found in OVLT, where swelling triggers Ca2+ influx via TRPV4 and amplifies astrocytic taurine release via VRAC (Ciura et al., 2018). Overall, the direct activation of neurons by shrinkage and their indirect inhibition by taurine release from swollen astrocytes mediate the CNS osmolarity sensing.
12.5. A SPECIAL CASE FOR ASTROCYTIC SWELLING AND CELL VOLUME CONTROL
A large variety of brain pathologies – including hyponatremia, stroke, traumatic brain and spinal cord injuries, epilepsy, hepatic failure, and hypoglycemia – are associated with the prominent pathological swelling of astrocytes [see reviews (Norenberg, 1994; Kimelberg, 1995; Mongin & Kimelberg, 2005; Stokum, Gerzanich, & Simard, 2016) and section 12.6 for further discussion and references]. The phenomenon of pathological swelling in the CNS has been historically termed cytotoxic brain edema (Klatzo, 1967; Kimelberg, 1995; Stokum et al., 2016). This unusual name originates from the early studies on the effects of certain toxins, such as dinitrophenol, triethyltin, and hexachlorophene, on brain tissue. The latter toxic substances trigger the shift of electrolytes and water from the extracellular to the intracellular space, without the net accumulation of water in the brain or swelling of brain tissue. To avoid confusion, H.K. Kimelberg introduced the more accurate term of cellular edema (Kimelberg, 1995). Cellular/cytotoxic edema is usually explained by oncotic (Donnan) swelling of metabolically compromised cells. This is in contrast to vasogenic edema, which is caused by disruption of the integrity of the BBB at the blood-brain interface. Vasogenic edema causes the accumulation of plasma proteins and net water influx into the brain tissue; and is associated with massive and potentially life-threatening brain swelling. Cellular edema typically precedes vasogenic edema, but these two processes are connected. Despite much work in the field, the molecular mechanisms underlying cellular edema are incompletely understood. The major unanswered question is why cell swelling is preferentially seen in one cell type – astrocytes. In this section text and Fig. 12.3, we present several prevalent hypotheses.
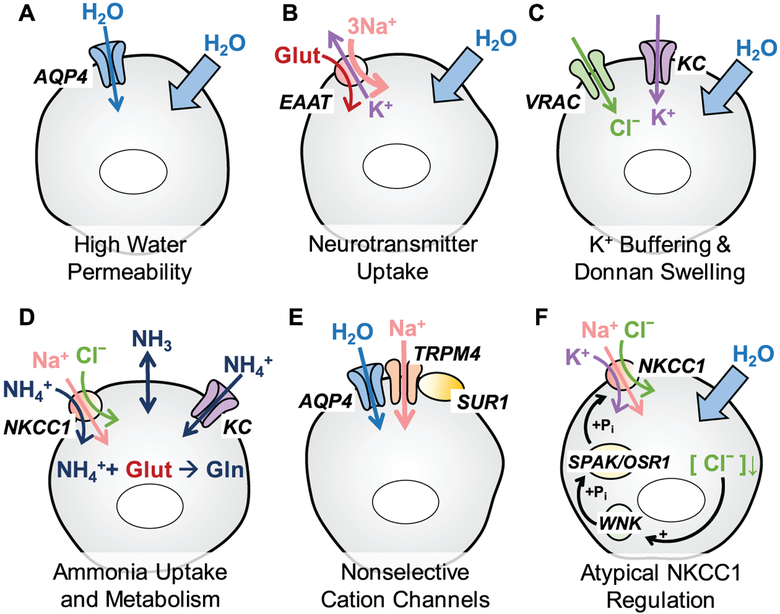
Figure 12.3.
Astrocytic properties that may be responsible for preferential propensity of this cell type to cell swelling in neuropathologies. (A) Astrocyte membranes are highly permeable to water due to expression of the water channel aquaporin-4 (AQP4); (B) Astrocytes take up neurotransmitters from the extracellular space in order to maintain normal neuronal activity. Excitatory amino acid transporters (EAAT) take one glutamate in together with 3 Na+ and in exchange of 1 K+, and promote the accumulation of osmotically obligated water; (C) Buffering of [K+]o through potassium channels (KC) can be associated with the concomitant influx of Cl− through VRAC and promote Donnan cell swelling; (D) In normal brain and neuropathologies, astrocytes accumulate extracellular ammonia (NH3) via passive transmembrane diffusion and ammonium ions (NH4+) through potassium channels (KCs) and NKCC1. Inside the cell, NH3/NH4+ is then assimilated to produce glutamine from glutamate. (E) Upregulation and activation of the nonselective SUR1-TRPM4 channels and their assembly with AQP4 promotes Na+ uptake and water accumulation; (F) Astrocytic NKCC1 cotransporter contributes to electrolyte and water accumulation in response to high [K+]o. Cell swelling may paradoxically activate NKCC1 via the low [Cl−]i-sensing WNK/SPAK/OSR1 cascade and in such a way further amplify the persistent astrocytic swelling.
12.5.1. Water permeability and aquaporin channels
As shown in Fig. 12.3A, one popular explanation for the preferential swelling of astrocytes is the high water permeability of their membranes, which is facilitated by the presence of water channels, aquaporins (AQP). Astrocytes abundantly express the AQP4 protein, particularly in their processes contacting brain capillaries and larger vessels (Amiry-Moghaddam & Ottersen, 2003). Deletion of AQP4 in astrocytes reduces their water permeability by ~sevenfold at 12oC but only twofold at 37oC (Solenov, Watanabe, Manley, & Verkman, 2004). It is well known that water can move in and out of the cell through many alternative routes, passively or via assorted channels and transporters conducting major osmolytes [see (MacAulay, Hamann, & Zeuthen, 2004) and section 12.5.2]. Nonetheless, despite the relatively modest decrease in water permeability at physiological temperatures, AQP4-null animals show high resistance to systemic water intoxication, (hyponatremia) as compared to their wild type counterparts (Manley et al., 2000). Specific enrichment of AQP4 in astrocytic endfeet is dependent on the adaptor proteins, α-syntrophin and dystrophin (Amiry-Moghaddam et al., 2003). Deletion of α-syntrophin impedes water movement from the blood to the brain and reduces tissue edema in an animal model of stroke (Amiry-Moghaddam et al., 2003). Because brain and cell swelling in neuropathologies develops on a slow scale, it is not clear if the twofold changes in water permeability in AQP-null astrocytes or disruption of AQP4 localization in the astrocytic endfeet seen in α-syntrophin knockouts can explain the observed resistance to cellular and/or tissue edema. Perhaps, AQP4 plays more complex roles in regulating water movement and osmotic gradients, via modulating activities of ion channels and transporters. E.g., AQP4 can form functional complexes with the non-selective cation channel TRPV4 (Benfenati et al., 2011), the SUR1-TRPM4 channel complex (Stokum et al., 2018), and also regulates the activity of VRAC (Benfenati et al., 2007).
12.5.2. Neurotransmitter uptake
A well-supported mechanism for astrocytic swelling is related to the critical role for these cells in the re-uptake of the major amino acid neurotransmitters: glutamate, aspartate, and GABA (Danbolt, 2001; Zhou & Danbolt, 2013). Excitatory glutamate and aspartate molecules are taken inside the cell by excitatory amino transporters (EAATs) with 3 Na+ ions in exchange for 1 K+ out per each transport cycle, along with the uncoupled conduction of Cl− (Machtens et al., 2015; Fahlke, Kortzak, & Machtens, 2016) (Fig. 12.3B). Uptake of the inhibitory amino acid GABA occurs through GAT transporters along with ~2–3 Na+ and 1 Cl− (Willford, Anderson, Spencer, & Eskandari, 2015). There is an extensive literature on glutamate-induced astrocyte swelling in cultures and in situ, indicating that such swelling involves the accumulation of Na+ and Cl−, and is Ca2+-dependent (Koyama, Sugimoto, Shigenaga, Baba, & Iwata, 1991; Schneider, Baethmann, & Kempski, 1992; Bender, Schousboe, Reichelt, & Norenberg, 1998; Izumi, Kirby, Benz, Olney, & Zorumski, 1999; Koyama et al., 2000)). Under pathologic conditions such as epilepsy or ischemic stroke, high extracellular glutamate levels can significantly contribute to astrocytic swelling via transporter or metabotropic receptor-mediated mechanisms (Hansson, Johansson, Westergren, & Ronnback, 1994; Bender et al., 1998). Importantly, in addition to increasing the osmotic load of the cell, the neurotransmitter transporters also readily move water (Zeuthen & MacAulay, 2002; MacAulay et al., 2004). E.g., the astrocytic glutamate transporter EAAT1/GLAST has the water permeability of roughly one tenth of that of the water channel AQP1 (MacAulay, Gether, Klaerke, & Zeuthen, 2001).
12.5.3. K+ buffering and Donnan swelling
Another well-recognized function of astroglial cells is the buffering of extracellular K+, which is accomplished via the activity of K+ channels, the Na+,K+-pump and the NKCC1 cotransporter, and further facilitated by redistributing the intracellular K+ throughout the astrocytic syncytium (Kofuji & Newman, 2004; MacAulay & Zeuthen, 2012). Their high K+ uptake capacity and negative membrane potential make astrocytes prone to K+-induced swelling (Fig. 12.3C). Moderate elevations in [K+]o during physiological excitation are thought to be the key reason for the dynamic changes in astrocyte volume in healthy brain tissue [section 12.4, but see alternative view in (Larsen & MacAulay, 2017)]. Work in astrocyte cultures suggests that supraphysiologic increases in [K+]o (up to 10–15 mM) trigger cell swelling via stimulation of the bumetanide-sensitive NKCC1 (Larsen et al., 2014). The same mechanism has been identified for the activity-dependent astrocyte swelling in optic nerve (MacVicar, Feighan, Brown, & Ransom, 2002). However, in hippocampal slices, bumetanide does not prevent the activity-dependent shrinkage of the interstitial space (and by extension, astrocytic swelling), suggesting that other mechanisms may dominate this process in situ and in vivo (Larsen et al., 2014).
In pathologies such as cerebral hypoxia and ischemia, extracellular K+ levels can rise as high as 80 mM (Hansen, 1985). This leads to robust astrocytic swelling via the Donnan mechanism: membrane depolarization creates an inward driving force for Cl−, which in turn sustains the electromotive force for the additional influx of Na+ and K+ and continued cell swelling (Rutledge & Kimelberg, 1996; Mongin, Cai, & Kimelberg, 1999). In astrocyte cultures, high K+-induced cell swelling depends on the extracellular [Cl−], and is completely blocked by Cl− channel blockers and strongly reduced by [Ca2+]i buffering, perhaps because [Ca2+]i facilitates K+ accumulation via Ca2+-dependent K+ channels or other Ca2+-dependent pathways (Rutledge, Aschner, & Kimelberg, 1998; Mongin et al., 1999). There is experimental evidence that the NKCC1-dependent mechanism appears as equally important as the role of Donnan swelling in promoting astrocyte swelling in situ and in vivo (see section 12.5.6).
12.5.4. Ammonia uptake and metabolism
Acute and chronic liver diseases frequently lead to increases in the systemic levels of ammonia, which at physiological pH exists in a balance between the charged and uncharged forms (NH4+ ↔ NH3). NH3 freely crosses the blood-brain barrier, and NH4+/NH3 buildup in the brain tissue results in the development of hepatic encephalopathy (see section 12.6.3). Astrocytic swelling is a major feature of this disease and its degree highly correlates with blood ammonia levels (Norenberg, 1977; Swain, Butterworth, & Blei, 1992; Norenberg, 1998). Uncharged NH3 enters the cell via passive diffusion through the cell membrane, in a manner dependent on the intracellular pH (Antonenko, Pohl, & Denisov, 1997). In contrast, the charged NH4+ enters the cell through NKCC1 (taken up at the K+-binding site) and via K+ channels (Kinne, Kinne-Saffran, Schutz, & Scholermann, 1986; Nagaraja & Brookes, 1998; Allert, Koller, & Siebler, 1998; Marcaggi & Coles, 2001) (see Fig. 12.3D). Ammonia influx through K+ channels causes astrocytic membrane depolarization and leads to Donnan swelling (Allert et al., 1998). Extensive in vitro and in vivo studies, the majority of which have been done in the laboratory of M.D. Norenberg, have shown that elevated levels of ammonia cause persistent astrocytic swelling (Norenberg et al., 1991; Norenberg, 1998). The mechanisms of such swelling are multifaceted and complex. However, it is known that they largely depend on ammonia assimilation by the astrocytic enzyme glutamine synthetase. For further information on ammonia transport, metabolism, and their relationship to cell swelling, see (Marcaggi & Coles, 2001; Norenberg, 1998) and section 12.6.3.
12.5.5. SUR1/TRPM4 non-selective cation channels
A series of studies performed by J.M. Simard and colleagues identified an additional mechanism for astrocytic swelling, which may be selectively relevant to pathological states. Biophysical studies in reactive astrocytes revealed a nonselective cation permeability (NCCa-ATP), which was activated in metabolically inhibited cells and caused their swelling and blebbing (Chen & Simard, 2001). The NCCa-ATP channel contains the sulfonylurea receptor SUR1 that is typically coupled with Kir6.x proteins to form KATP channels (Chen, Dong, & Simard, 2003). However, in the case of NCCa-ATP the non-selective cation permeability is mediated by the transient receptor potential melastatin 4 (TRPM4) channel (Woo, Kwon, Ivanov, Gerzanich, & Simard, 2013). In an animal stroke model, SUR1 expression steadily increased up to 6 hours after the initiation of ischemia, and treatment with the sulfonylurea inhibitor glibenclamide reduced brain edema and tissue damage (Simard et al., 2006). Their most recent work demonstrated that SUR1-TRPM4 can form a tripartite complex with AQP4, and such association mediates the pathological, high-capacity influx of water that is ~7.6-fold higher than that stimulated by TRPM4 alone, and ~3.2-fold higher than that induced by the SUR1-TRPM4 assembly (Stokum et al., 2018) (Fig. 12.3E). TRPM4 knockout mice displayed reduced astrocytic cell swelling following cold-injury (Stokum et al., 2018), but additional studies are needed to determine if this heteromeric complex is critical for cell swelling in other pathologies.
12.5.6. NKCC1 activity
As mentioned in section 12.1.2, NKCC1 is one of the primary shrinkage-activated transporters, which contribute to RVI in a variety of cell types (Russell, 2000). In astrocyte cultures, pharmacological inhibition or genetic deletion of NKCC1 prevents the high [K+]o-induced astrocytic swelling and swelling-activated glutamate release (Su, Kintner, & Sun, 2002; Su, Kintner, Flagella, Shull, & Sun, 2002). This is paradoxical because NKCC1 is inhibited by cell swelling. This transporter is regulated by a protein kinase cascade, which incorporates the With No Lysine (K) (WNK) protein kinases WNK1–4 and the two homologous downstream protein kinases, Sterile20-related Proline-Alanine-rich Kinase (SPAK) and Oxidative Stress Responsive kinase 1 (OSR1) (Kahle, Ring, & Lifton, 2008; Alessi et al., 2014). Typically, the activity of the WNK/SPAK/OSR is augmented by cell shrinkage and inhibited by cell swelling with matching effects on NKCC1 (Russell, 2000; Hoffmann et al., 2009). However, astrocytes represent an interesting exception: in these cells, swelling activates NKCC1 nearly as potently as cell shrinkage (Mongin et al., 1994; Mongin et al., 1996). One potential mechanism is the activation of WNK kinases by low intracellular Cl−. Astrocytes are high-Cl− cells, in which swelling and subsequent RVD likely reduce [Cl−]i and relieve the known inhibition of WNK kinases by this anion (Kahle et al., 2008; Pacheco-Alvarez & Gamba, 2011; Piala et al., 2014; Terker et al., 2016). The end result of this unusual regulation would be an amplification of cell swelling due to additional osmolyte intake via NKCC1 (Fig. 12.3F).
12.5.7. Na+-bicarbonate cotransporters and other emerging mechanisms
Recent studies from the N. MacAulay laboratory (Larsen et al., 2014; Larsen & MacAulay, 2017), proposed alternative mechanisms of astrocytic swelling in hippocampal slices. They found that electrical stimulation of neuronal networks causes increases in intracellular [K+] and shrinkage of the interstitial space, reflecting cellular (astrocyte?) swelling. In brain slices, cell swelling was largely insensitive to the inhibitors of glutamate transporters (TBOA), K+ channels (Ba2+), and NKKC1 (bumetanide). Instead, it was lessened by the non-selective inhibitor of the Na+-bicarbonate cotransporter NBCe1, DIDS, and poorly selective monocarboxylate transporter blocker, α-cyano-4-hydroxycinnamate (Larsen & MacAulay, 2017), suggesting the contribution of these two latter transport mechanisms. Although this work remains to be validated using more selective molecular biology and genetics tools, the underlying ideas are supported by the literature. Thus, S.J. Mulligan and co-workers found that the K+-induced astrocytic swelling in hippocampal slices is largely dependent on the presence of bicarbonate in the media, and is strongly reduced by blockers of NBCe1 and GABAA receptors (Florence, Baillie, & Mulligan, 2012). To what extent these additional mechanisms can contribute to pathological swelling of astroglia remains to be tested.
12.6. FAILURE OF CELL VOLUME CONTROL IN BRAIN PATHOLOGIES
In this section, we discuss several examples of neuropathologies which are associated with defective cell volume control (persistent cell swelling), as well as the potential relevance of cell volume changes to disease outcomes.
12.6.1. Hyponatremia
Hyponatremia is the most prevalent electrolyte disorder, which manifests as a drop in serum Na+ levels below the threshold of ~135 mM (Adrogue & Madias, 2000). On average, this condition affects 1.72% of the U.S. population, but hospitalized patients, elderly people, and individuals with neurological disorders are disproportionally affected (Upadhyay, Jaber, & Madias, 2006; Mohan, Gu, Parikh, & Radhakrishnan, 2013). Most often, hyponatremia develops as a result of impaired capacity of the kidney to excrete water. It could be caused by underlying kidney pathologies, severe hormonal imbalance, or due to the syndrome of inappropriate antidiuretic hormone secretion (SIADH). SIADH is very common and in hospital settings; it can be triggered by anesthesia and several commonly prescribed medications or may develop in an idiogenic fashion. In neurological patients and endurance athletes, hyponatremia can also be caused by excessive water intake (Fraser & Arieff, 1997; Adrogue & Madias, 2000).
Although all tissues are affected, the brain represents the target organ of this disorder and is responsible for all major symptoms. Acute hyponatremia is typically associated with headaches, nausea, fatigue, confusion and hallucinations. In its most severe form, acute hyponatremia progresses to seizures and coma and puts patients at significant risk for brain damage and death. The latter outcomes are driven by expansion of the brain tissue and compression of blood vessels, particularly in the brainstem, leading to the life-threatening dysregulation of blood pressure, heart rate, thermal regulation and respiratory control (Fraser & Arieff, 1997; Adrogue & Madias, 2000; Podesta et al., 2015). Cell swelling in hyponatremia is the closest analogue of the model osmotic cell swelling utilized in cell cultures and other model studies. As already mentioned, in the hyponatremic brain swelling is mainly seen in astrocytes, particularly in the astrocytic processes surrounding blood vessels [e.g., (Wasterlain & Torack, 1968; Manley et al., 2000; Risher, Andrew, & Kirov, 2009)]. There is conflicting information on neuronal swelling, with some studies showing no swelling of neuronal somata or processes (Andrew et al., 2007; Steffensen et al., 2015), while others report typical hypoosmotic increases in neuronal volume in brain slice models (Murphy et al., 2017).
Irrespectively of which cell types are swollen (or not), rapid adaptation of the brain to hyponatremia is mediated by the loss of inorganic ions, Na+, K+, and Cl−, which move from the cytosol to the interstitial space to the circulatory system (Melton et al., 1987; Gullans & Verbalis, 1993). The loss of K+ is the main driving mechanism of RVD but, when it occurs in the brain within the constraints of a small extracellular space, it leads to rapid dissipation of K+ gradients, membrane depolarization, and neuronal excitation that can precipitate seizure development (Andrew, Fagan, Ballyk, & Rosen, 1989; Andrew, 1991; Somjen, 2004). Swollen cells also release a variety of small organic molecules – including glutamate, aspartate, myo-inositol, and the amino sulfonic acid taurine – and these can be measured in the extracellular space using a microdialysis approach (Wade, Olson, Samson, Nelson, & Pazdernik, 1988; Lehmann, 1989; Verbalis & Gullans, 1991; Haskew-Layton et al., 2008). On a short time-scale, organic osmolytes are released predominantly via VRAC, but the long-term adaptation to hyponatremia likely involves additional processes (Estevez, O’Regan, Song, & Phillis, 1999; Haskew-Layton et al., 2008; Hyzinski-Garcia et al., 2011). It has been estimated that the loss of organic osmolytes is responsible for >50% of the regulatory loss of osmotically obligated water during compensatory changes in hyponatremia (Sterns et al., 1993). Yet, because aspartate and glutamate are the excitatory neurotransmitters, their release is not benign as it causes activation of neuronal glutamate receptors, with the NMDA subtype likely being the main target (Lauderdale et al., 2015; Mongin, 2016). Furthermore, activation of VRAC in astrocytes severely impairs extracellular glutamate scavenging because it interrupts its removal and metabolic conversion into the non-toxic glutamine (Haskew-Layton et al., 2008; Hyzinski-Garcia et al., 2011).
One interesting aspect of brain adaptation to hyponatremia is the preferential loss of one organic osmolyte, taurine. This phenomenon has been reported in vitro and in vivo and interpreted in the context of the privileged role of taurine in brain cell volume control and with the implied existence of taurine-selective volume regulatory pathways (Wade et al., 1988; Pasantes Morales & Schousboe, 1988; Lehmann, 1989; Verbalis & Gullans, 1991; Moran, Maar, & Pasantes-Morales, 1994; Pasantes-Morales, Lezama, Ramos-Mandujano, & Tuz, 2006). We have recently tested this hypothesis and found that the differences between the osmotic behavior of taurine and excitatory amino acids are determined by the rates of their reuptake and synthesis. Once glutamate uptake and de novo synthesis via transamination are pharmacologically blocked, astrocytes release cytosolic taurine and aspartate at very similar rates (Schober & Mongin, 2015). This suggests that the preservation of cytosolic glutamate and aspartate during RVD are homeostatic mechanisms related to their numerous roles in metabolism and brain signaling.
In contrast to the acute disease, chronic hyponatremia, which develops and/or persists for more than 48 hours, is typically “asymptomatic”. This happens because the brain tissue slowly adapts to new osmotic conditions via the already mentioned loss of inorganic and particularly organic osmoles. Despite an apparent lack of symptoms, chronic hyponatremia still requires correction because it is strongly associated with a host of mild neurological deficits and increases morbidity and mortality of co-existing clinical conditions (Adrogue & Madias, 2000; Podesta et al., 2015). However, its treatment often comes with a separate set of dangers. Rapid correction of systemic and Na+ levels leads to osmotic demyelination syndrome, a demyelinating pathology that most commonly affects the central basis of pontis (pontine myelinosis) but also the nearby regions of thalamus and subcortical areas (Adrogue & Madias, 2000; Sterns & Silver, 2006; Podesta et al., 2015). To avoid demyelination, correction of chronic hyponatremia is performed by introducing additional electrolytes at a very slow rate. The etiology of demyelination is likely related to lowered levels of organic osmolytes, particularly myo-inositol, which play incompletely understood protective roles in the preservation of myelin integrity. In one experimental study, the intravenous supplementation of myo-inositol dramatically decreased mortality rates and preserved myelin structures in rats subjected to rapid correction of chronic hyponatremia (Silver, Schroeder, Sterns, & Rojiani, 2006; Sterns & Silver, 2006).
12.6.2. Epilepsy
Epilepsy is the fourth most common chronic neurologic disorder, which affects more than 2 million individuals in the U.S. and 65 million people worldwide (Hirtz et al., 2007; Thurman et al., 2011). The term epilepsy encompasses a constellation of diseases with somewhat different etiologies, all of which manifest as recurrent, unprovoked seizures which are usually initiated in the cortical areas or the hippocampus and then spread throughout the brain (McNamara, 1994; McNamara, Huang, & Leonard, 2006). While the main focus of epilepsy research is on changes in neuronal cells, emerging studies also implicate the complex interactions between neurons and various classes of glia, with the potentially strong involvement of changes in glial gene expression, glutamate metabolism, cellular morphology and cell volume (McNamara et al., 2006; David et al., 2009; Eid, Williamson, Lee, Petroff, & de Lanerolle, 2008; Murphy, Binder, & Fiacco, 2017). As mentioned in the prior section, a hypoosmotic challenge on its own can cause brain hyperexcitability and seizures, strongly suggesting that cell volume is a highly relevant factor.
The experimental evidence for cellular swelling in epilepsy is derived from three types of findings: (i) electron microscopy studies in fixed brain tissue after chemically-induced status epilepticus, (ii) intravital magnetic resonance measurements of water mobility, and (iii) intravital or ex vivo monitoring of volume dynamics in the extracellular space. In the study by (Fabene et al., 2006), the first two parameters were compared side-by-side. As reproduced in Fig. 12.4, rats with status epilepticus develop profound swelling of astrocytic cell bodies and processes, and additionally show swelling of dendritic boutons. Interestingly, neuronal cell bodies appear to maintain their volume, or even moderately shrink with an apparent condensation of cytoplasm (Fabene et al., 2006). The same study correlated the phenomenon of cellular swelling with MRI-registered changes in water mobility. Increased signal of diffusion-weighted imaging (DWI) and the inversely related apparent diffusion coefficient (ADC) of water show a consistent and long lasting (for at least 24 hours) decrease in water mobility in the epileptogenic brain areas (Fabene et al., 2006). Similar in nature and amplitude DWI and ADC changes are reliably associated with the movement of water from the extracellular to the intracellular space (Sotak, 2004). Other publications demonstrated that the development of seizure activity is preceded by shrinkage of the extracellular space, signifying increases in cellular volume [e.g., (Binder, Papadopoulos, Haggie, & Verkman, 2004; Slais et al., 2008)]. In brain slice studies, application of hyperosmotic media ameliorates the K+-induced electrographic seizures; and, conversely, introduction of mild hypoosmolar media dramatically increases epileptogenic activity, again suggesting that cell volume and/or volume of extracellular space are highly relevant (Traynelis & Dingledine, 1989). Likely causes for hyperexcitability and cellular swelling are discussed below.
Figure 12.4.
Cell swelling in status epilepticus revealed by electron microscopy (EM). (A) Structural analysis of the control rat neocortex reveals neuronal cell bodies [N], capillaries [Cap], and pericytes [P]. (B and C) Two hours following injection of 4-aminopyridine, EM shows swollen perivascular astrocytic endfeet [asterisks in (B)], which surround an endothelial cell [E]. Swollen astrocytic processes [asterisks in (C)] also border an apparently shrunken pyramidal neuronal body [N]. (D) A higher magnification image in the CA3 layer of the hippocampus displays a swollen dendrite [D] with apparently normal mitochondrion [M], and adjacent electron-transparent swollen astrocytic processes [asterisks]. Additionally labeled are: an axon terminal [A] and dendritic spines [1,2]. The scale bars in the fields (A-C) = 5 μm, and in (D) = 1 μm. Reproduced from P.F. Fabene et al., 2006, with permission.
The core reason for neuronal dysfunction in epilepsy is the modification of cellular excitability, which is complex in its nature. Still, the initial triggers of disease are ionic in nature. Excessive neuronal activity leads to the accumulation of K+ and neurotransmitter molecules in the extracellular space. High [K+]o causes partial membrane depolarization, increases neuronal excitation, and removes the Mg2+ block of the glutamate NMDA receptors, which are all strongly associated with epileptogenic activities (McNamara, 1994). Additionally, K+-induced depolarization alters the electrochemical driving force for Cl− in neurons and dampens the activity of the inhibitory GABAA and glycine receptors (McNamara, 1994). The importance of changes in Cl− gradients is strongly supported by studies of the functional roles for electroneutral KCC transporters. In neuronal cells, these transporters set low intracellular Cl− levels, and in such a way allow for the inhibitory actions of GABA and glycine (Kahle et al., 2008; Kaila, Price, Payne, Puskarjov, & Voipio, 2014). Deletion or downregulation of the activities of KCC2 and KCC3 result in neuronal hyperexcitability in humans and model organisms (Woo et al., 2002; Huberfeld et al., 2007; Kahle et al., 2014; Kaila et al., 2014). KCC2-null mice develop spontaneous seizures and have high postnatal mortality (Woo et al., 2002), whereas KCC3-null phenotypes are more diverse. In humans, KCC3 mutations cause mental retardation, severe peripheral neuropathy, and loss of the corpus callosum (Howard et al., 2002). In mice, the loss of KCC3 has been alternatively associated with defects in peripheral myelination and neurological deficits, or alternatively, hyperexcitability, reduced seizure threshold, and deafness (Howard et al., 2002; Boettger et al., 2003). The KCC3 phenotypes have been interpreted in the context of defective ability of neurons to regulate their volume (Kahle et al., 2015), but this remains to be proven. Many of the mechanisms listed in section 12.5 can contribute to astrocytic swelling in epilepsy. These may include neurotransmitter uptake, the [K+]o-induced Donnan swelling, and anomalous NKCC1 activity [e.g., (Bender et al., 1998; Palma et al., 2006; Hubel & Ullah, 2016)].
An additional interesting topic is the link between the astrocytic water channel AQP4 and seizure susceptibility. Several studies have identified changes in the volume of the extracellular compartment and its dynamics during hyperexcitability, and found modified seizure thresholds in AQP4 knockout animals (Binder, Oshio, Ma, Verkman, & Manley, 2004; Binder et al., 2006; Lee et al., 2012). These data have been interpreted in the context of reduced water fluxes and/or defective cell volume homeostasis in AQP4-null mice (Verkman, Binder, Bloch, Auguste, & Papadopoulos, 2006; Binder, Nagelhus, & Ottersen, 2012; Murphy et al., 2017). However, even without AQP4, astrocytes retain fairly high water permeability (Solenov et al., 2004). Besides direct effect on water fluxes, the observed changes in AQP4-null animals can be explained by other factors, such as adaptive changes in the expression of other genes or the indirect effects of AQP4 on activities of ion transporters and channels. This protein has been shown to physically associate with or functionally regulate activities of the K+ channel Kir4.1, volume-sensitive VRAC, and nonselective cation channels TRPV4 and TRPM4/SUR1 [(Nagelhus, Mathiisen, & Ottersen, 2004; Benfenati et al., 2007; Benfenati et al., 2011; Stokum et al., 2018), but see an alternative opinion for Kir4.1 (Zhang & Verkman, 2008)]. Therefore, effects of AQP4 deletion may develop independent of or be upstream of cell volume changes [see (Assentoft, Larsen, & MacAulay, 2015) for detailed discussion].
12.6.3. Hepatic encephalopathy
Another syndrome that disproportionally affects the CNS and is strongly linked to astrocytic swelling is hyperammonemia – systemic increases in ammonia levels above the approximate threshold of ~100 μM, which lead to neurologic deficits described as hyperammonemia or hepatic encephalopathy (Blei & Larsen, 1999; Norenberg, Rao, & Jayakumar, 2005; Haussinger & Schliess, 2008; Brusilow, Koehler, Traystman, & Cooper, 2010). In hyperammonemias, arterial ammonia levels frequently reach 1 mM, and can be as high as 5 mM, leading to severe brain edema and high mortality rates due to herniation of the brainstem (Blei & Larsen, 1999; Norenberg et al., 2005; Brusilow et al., 2010). Most frequently, hyponatremia develops due to acute liver failure or chronic liver disease. Depending on the clinical population, 20 to 80% of patients with liver cirrhosis suffer from moderate to severe hepatic encephalopathy. However, other conditions – such as inherited deficiencies of the urea cycle, certain bacterial infections of the gut, and Reye’s syndrome – may also lead to the same type of neuropathology (Felipo & Butterworth, 2002; Brusilow et al., 2010).
The main mechanism of the hepatic encephalopathy is thought to be osmolyte accumulation in the brain due to fixation of ammonia by the astrocytic enzyme glutamine synthetase (Norenberg et al., 2005; Brusilow et al., 2010). Glutamine synthetase produces glutamine from ammonia and glutamate in an ATP-dependent fashion, as a part of its normal physiological activity in the brain, the recycling of excitatory neurotransmitters (McKenna, 2007; Sofroniew & Vinters, 2010; Verkhratsky & Nedergaard, 2018). Because ammonia circulates systemically and freely diffuses into the brain, in hyperammonemia this process serves as an unlimited osmotic sink as long as the brain has energy reserves. The mechanisms of ammonia transport across cell membranes are summarized in section 12.5 and Fig. 12.3D. The osmotic burden due to the activity of glutamine synthetase is further accentuated by the accumulation of brain alanine produced via the work of the alternative detoxification mechanism incorporating glutamate dehydrogenase and alanine aminotransferase (Tofteng et al., 2006; Dadsetan et al., 2013). In experimental hyperammonemias, treatments with the glutamine synthetase inhibitor methionine sulfoximine reduced the amount of brain edema and cell swelling, supporting the causal link of glutamine synthetase to disease development (Blei, Olafsson, Therrien, & Butterworth, 1994; Tanigami et al., 2005).
Because glutamine and alanine synthesis occur in astrocytes, it is not surprising that the main cellular pathology in hepatic encephalopathy is astrocytic. Human pathological studies show the appearance of swollen astrocytes in acute disease or so-called Alzheimer type II astrocytes in chronic pathology, with the latter characterized by enlarged cell somata and nuclei with significant nucleolus. The frequencey of observed pathological cells correlates with the severity of encephalopathy (Norenberg, 1998). The mechanisms of astrocytic swelling have been thoroughly explored in primary astrocyte cultures with main contributions coming from the laboratory of M.D. Norenberg. Surprisingly, these cell culture studies indicate that the osmotic effect of glutamine accumulation is not the leading reason for cell swelling. Instead, they propose a variety of alternative mechanisms, including membrane depolarization via ammonia uptake through K+ channels followed by Donnan swelling (Allert et al., 1998), osmotic load due to NKCC1 activity (Jayakumar, Valdes, & Norenberg, 2011), and secondary swelling due to opening of mitochondrial permeability transition pore (Bai et al., 2001) followed by production of reactive oxygen and nitrogen species (Murthy, Rama Rao, Bai, & Norenberg, 2001). To reconcile the conflicting results on the role of astrocytic glutamine in cellular swelling, J. Albrecht and M.D. Norenberg proposed the “Trojan horse” hypothesis, in which the glutamine synthetase-derived glutamine causes astrocytic swelling via its secondary metabolic effects within the cells, particularly in mitochondria (Albrecht & Norenberg, 2006; Norenberg, Jayakumar, Rama Rao, & Panickar, 2007)).
12.6.4. Stroke
Stroke is the fifth most prevalent source of mortality in the U.S. and the second most common cause of death worldwide, with 6.5 million deaths reported globally in 2013 (Feigin, Norrving, & Mensah, 2017). A stroke occurs due to the interruption of blood flow to an area of the brain. Because brain metabolism is almost exclusively dependent on oxidation of blood-derived glucose, reductions in cerebral blood flow below a threshold of ~50% of their normal levels cause a rapid decline in tissue metabolism, and anything below 20% of normal blood flow levels is followed by severe tissue damage (Dirnagl, Iadecola, & Moskowitz, 1999; Brott & Bogousslavsky, 2000; Lo, Dalkara, & Moskowitz, 2003). The majority of strokes (>85%) are ischemic and involve embolic or thrombotic occlusion of a larger blood vessel. A smaller (<15%), but deadlier fraction of strokes are hemorrhagic and result from bleeding through a rupture in the parenchyma or subarachnoid space. The mechanisms of tissue damage are not identical between the two types of stroke because of the nature of the initial insult, but they all share characteristics of ionic dysfunction and cell swelling. In this section we focus on ischemic stroke.
There is a significant body of work demonstrating astrocytic cell swelling in ischemic stroke, through (i) electron microscopy in animal stroke models, (ii) measurements of brain interstitial volume in ischemia, (iii) two-photon microscopy imaging of cell swelling through a cranial window, and (iv) diffusion and perfusion weighted MRI imaging, which are widely utilized in clinical settings. The most commonly used animal model of stroke is focal ischemia induced by transient or permanent occlusion of the middle cerebral artery occlusion (MCAo) (Longa, Weinstein, Carlson, & Cummins, 1989). EM analysis reveals astrocytic swelling in fixed MCAo tissue after only 30 min of ischemia and such swelling persists up to 24 hours after reperfusion (Garcia et al., 1993). Under physiological conditions, the extracellular space makes up ~20% of the brain volume (Thrane et al., 2014). Early methods of measuring interstitial space in the brain used radiotracer diffusion studies or the ion-selective microelectrodes detecting the extracellular marker tetramethylammonium (TMA+), and found that during severe ischemia the volume fraction drops to as low as 5% [reviewed in (Sykova & Nicholson, 2008)]. More recently, studies using 2-photon microscopy allowed for the direct observation of cell swelling in both animal slices and in vivo. The laboratory of S.A. Kirov found that the cross-sectional area of astrocytes in hippocampal slices increased by ~40% upon 3-min depolarization with high extracellular [K+], and by ~30% when slices were exposed to oxygen/glucose deprivation (Risher et al., 2009). The same study used a cranial window and 2-photon microscopy to demonstrate that global ischemia, which was induced by cardiac arrest, prompted very rapid swelling of astrocytic soma by ~30% (Risher et al., 2009). Another publication, which used similar methodology for measuring astrocytic swelling in the ischemic penumbra of the photothrombotic stroke model, found the degree of astrocytic swelling to be in the range of 20–35% (Risher, Croom, & Kirov, 2012).
In clinical settings, tissue edema is visualized using the MRI-derived DWI or ADC signals. The early hyperintensity in MRI signal or matching hypointensity of the ADC signal serve as a reliable representation of the ischemic area and are fairly predictive of the final infarction volume (Neumann-Haefelin et al., 1999; Thijs, Adami, Neumann-Haefelin, Moseley, & Albers, 2002; Kucinski et al., 2002). Acute ischemia-induced changes in DWI and ADC are shown in Fig. 12.5. The human MRI data have been extensively validated by crosschecking DWI imaging with histological outcomes in animal stroke models [e.g., (Neumann-Haefelin et al., 2000)]. The time-dependent development of DWI signal in human stroke correlates well with the evolution of infarction, suggesting that cellular edema contributes to progression of tissue damage (Battey et al., 2014).
Figure 12.5.
MRI imaging modes which are used in clinical settings to visualize changes in water mobility (cell swelling) following ischemic stroke. Left panel: At early times after the onset of focal ischemic stroke, fast spin-echo T2-weighted MRI imaging has yet to show brain damage. Center panel: In contrast to the T2 imaging, diffusion weighted imaging (DWI) shows a large hyperintense area in the territories of the prefrontal artery (arrowhead) and a smaller signal in the territory of the anterior cerebral artery (arrow). Hyperintensity reflects a decrease in water mobility upon its shift from the extracellular to the intracellular space. Right panel: Apparent diffusion coefficient (ADC) of water identifies the regions of low water mobility as hypointense signals. In 2–3 days, the DWI/ADC positive areas will likely develop full infarction that will be apparent in T2 imaging (not shown). Reproduced from M.G. Lansberg et al., 1999, with permission.
The reasons for ischemic swelling of astroglial cells are likely plural and may vary significantly depending on the proximity to the ischemic core. In the ischemic core, cessation of the blood flow and resulting lack of oxygen and glucose lead to the rapid (within 5 min) and complete depletion of energy reserves, inhibition of the Na+,K+-pump, and collapse of ionic gradients (Dirnagl et al., 1999). A dramatic rise in the intracellular [Na+] and [Ca2+] and extracellular [K+] lead to Donnan cell swelling, which for poorly understood reasons is largely restricted to astrocytes. Astrocytes may retain their membrane potentials, and consequently the electrochemical driving force for ion accumulation, for a longer time than neurons. Furthermore, they are linked into syncytium by gap junctions, and at least in the beginning of the ischemic episode, work as one unified ion sink. Neurons also swell, but their swelling is largely limited to beading in the dendritic arbors, where a high density of ionotropic receptor-channels and water-permeable electroneutral transporters (NKKC1, KCC2, MCT, etc.) facilitate rapid osmotic loading (Steffensen et al., 2015). In contrast, neuronal cell bodies in the ischemic core typically retain their initial volume or even appear to shrink, perhaps due to the rapid redistribution of osmolytes into astrocytic compartments (Garcia et al., 1993).
In the ischemic penumbra, tissue ATP levels are partially preserved, or even elevated in the areas distal to occlusion. Nevertheless, the penumbral tissue is electrically silent as a result of presynaptic failure (Bolay & Dalkara, 1998; Neumann-Haefelin & Witte, 2000; Bolay et al., 2002). This is explained by the difficulties in sustaining metabolism in remote axonal processes and oxidative/nitrosative modification in presynaptic processes [reviewed in (Mongin, Dohare, & Jourd’heuil, 2012)]. Astrocytic swelling in the penumbra likely involves numerous mechanisms, including but not limited to osmolyte fluxes via the Na+-dependent transporters (GLT-1, GLAST, and others). Moreover, astrocytic swelling is promoted by NKCC1 activity. Treatment with the NKCC1 inhibitor bumetanide given prior to the induction of animal ischemia reduces the infarction volume and tissue edema in a MCAo model of stroke (Yan, Dempsey, & Sun, 2001). In astrocyte cultures, bumetanide and genetic deletion of NKCC1 make astrocytes resilient to K+-induced cell swelling and obliterates the K+-swelling-induced release of excitatory amino acids (Su et al., 2002; Su et al., 2002). The major physiological signal for NKCC1 activation is phosphorylation by the WNK signaling cascade [see section 12.5.6 and reviews (Kahle et al., 2008; McCormick & Ellison, 2011)]. This cascade is typically inhibited by cell swelling, rendering NKCC1 silent. Yet, paradoxically, astrocytes and glioma C6 cells respond to hypoosmotic cell swelling with an increase in NKCC1 activity (Mongin et al., 1994; Mongin et al., 1996). As already mentioned, one possible explanation is that cell swelling and RVD reduce the intracellular Cl− levels and relieve Cl− inhibition of protein kinases belonging to the WNK family (Kahle et al., 2008; Pacheco-Alvarez & Gamba, 2011; Piala et al., 2014; Terker et al., 2016). Genetic deletion of the brain-enriched WNK3 reduces cerebral edema and tissue injury following MCAo stroke (Begum et al., 2015).
Another important cause for ischemic cell swelling is the pathological opening of ATP-sensitive nonselective cation channels. One type, which is assembled from the TRP channels belonging to the melastatin family (TRPM4) and the sulfonylurea receptor-1 (SUR1) appears to dominate brain edema and stroke outcomes (Chen & Simard, 2001; Simard et al., 2006; Simard et al., 2010). The SUR1 subunit expression is upregulated during ischemic hypoxia by HIF1-α activation, and in an animal model of stroke can be detected in neurons, astrocytes, and endothelial cells (Simard et al., 2006). Treatment with the sulfonylurea glibenclamide inhibits these channels, and has been shown to reduce cerebral edema and stroke volume by as much as 50% and increase viability in the malignant MCAo model of stroke (Simard et al., 2006; Simard et al., 2010). Recently, it has been shown that astrocytic SUR1-TRPM4 forms a tripartite complex with AQP4, and in such a way significantly increases the rate of water influx into cells exposed to hypoosmotic media (Stokum et al., 2018) (see section 12.5.5). Importantly, sulfonylureas, the SUR1 antagonists, protect not only animals but also humans and are now in clinical trials for treatment of ischemic stroke (Kunte et al., 2007; Sheth et al., 2016).
12.7. VRAC AS A VILLAIN IN NEUROPATHOLOGIES
Persistent cell swelling in neuropathologies disrupts normal neural functions via numerous mechanisms, including volume-dependent changes in ionic gradients and cell excitability. There are strong reasons to believe that among many relevant processes, opening of VRAC is the key to the pathological tissue damage. The uncontrolled release of glutamate and aspartate activates highly abundant receptors on neurons and all classes of glial cells, including the Na+/K+ permeable ionotropic AMPA and kainate receptors, the Na+/K+/Ca2+-permeable NMDA receptors, and a variety of metabotropic mGluRs (Meldrum, 2000). Their combined actions cause sustained membrane depolarization and increases in the intracellular [Ca2+], which set in motion multiple neurodegenerative cascades causing immediate or delayed neuronal damage and demise. This process is termed excitotoxicity (Choi, 1988; Choi, 1992; Arundine & Tymianski, 2004). In principle, activation of VRAC can impact extracellular excitotoxin levels via at least three interconnected mechanisms: (i) VRAC serves as a conduit for excitatory amino acid release from swollen astrocytes and other cell types; (ii) opening of VRAC shunts glutamate to the extracellular space and renders astrocytic glutamate uptake ineffective; (iii) shunting of glutamate to the extracellular space interrupts glutamate processing via the glutamate-glutamine cycle, also a major mechanism regulating extracellular glutamate levels (Haskew-Layton et al., 2008; Hyzinski-Garcia et al., 2011). Pathological contributions of VRAC have been most extensively studied in stroke.
In vivo studies in animal stroke models consistently demonstrated dramatic increases in extracellular glutamate levels both in the ischemic core and the penumbra (Benveniste, Drejer, Schousboe, & Diemer, 1984; Phillis & O’Regan, 1996; Phillis, Song, & O’Regan, 1997; Seki, Feustel, Keller, Jr., Tranmer, & Kimelberg, 1999; Feustel, Jin, & Kimelberg, 2004; Dohare et al., 2014). This pathological glutamate can be contributed to by several mechanisms, which are not limited to VRAC. Besides VRAC, the best candidates for release routes include reversal of glutamate transporters, opening of connexin hemichannels, and stimulation of P2X7-receptor associated pannexin hemichannels; (Szatkowski, Barbour, & Attwell, 1990; Rossi, Oshima, & Attwell, 2000; Ye, Wyeth, Baltan-Tekkok, & Ransom, 2003; Duan et al., 2003; Orellana, Avendano, & Montero, 2014). It should be noted that not all of these mechanisms have been extensively tested in vivo. The relative contribution of VRAC was probed with diverse anion channel blockers, including the most selective among the available VRAC inhibitors, DCPIB. These compounds strongly reduced the intraischemic glutamate levels clearly pointing to VRAC activity in the pathological brain (Phillis et al., 1997; Phillis, Song, & O’Regan, 1998; Seki et al., 1999; Feustel et al., 2004; Zhang, Zhang, Feustel, & Kimelberg, 2008). However, because the specificity of VRAC blockers, including DCPIB, has been questioned (Ye, Oberheim, Kettenmann, & Ransom, 2009; Benfenati et al., 2009; Bowens, Dohare, Kuo, & Mongin, 2013), further work in transgenic animals and/or with more specific molecular biology tools, is needed to unequivocally link VRAC to glutamate release in stroke.
Its is important to note the fact that VRAC contributions to pathological glutamate levels are likely context-specific. The inhibitory effects of VRAC blockers and the inferred mechanisms of the intraischemic glutamate release vary depending on the study. The major determinant appears to be the site of detection, e.g., position of the microdialysis probe within the ischemic brain. In the ischemic core, the main source of glutamate release is reversal of the dihydrokainate-sensitive glutamate transporter GLT1 (Seki et al., 1999). In contrast, in the penumbra, glutamate release is dominated by the tamoxifen- and DCPIB-sensitive VRAC (Feustel et al., 2004; Zhang et al., 2008). There are several potential explanations for paradoxical “silence” of VRAC the ischemic core, where cell swelling is the most pronounced. VRAC opening requires the non-hydrolytic binding of ATP, and therefore its activity and associated glutamate release are abolished in metabolically-inhibited cells (Jackson et al., 1994; Oike, Droogmans, & Nilius, 1994; Rutledge, Mongin, & Kimelberg, 1999). VRAC is also potently inhibited by arachidonic acid and other polyunsaturated fatty acids, which are abundantly produced in the ischemic tissue (Kubo & Okada, 1992; Nilius, Sehrer, & Droogmans, 1994). In contrast, on the periphery of the ischemic tissue, collateral blood flow partially sustains brain cells metabolism, allowing for sustained VRAC activity. It is in the ischemic penumbra, tamoxifen and DCPIB suppress glutamate release by up to 70% (Feustel et al., 2004; Zhang et al., 2008).
Even if we accept the link between VRAC and pathological glutamate release, the potential impact of this process on tissue viability is not certain due to the very complex nature of ischemic brain injury. Due to the role of VRAC in cell volume control, its activity in stroke tissue may beneficial, harmful, or net-neutral. The question about the therapeutic value of this channel has been addressed in animal stroke models by H.K. Kimelberg and co-workers. The systemic administration of the blood-brain barrier-permeable tamoxifen potently protected against brain injury in both the transient and the permanent cerebral ischemia, with maximal protection reaching >80% and an extended therapeutic window up to 3 h after occlusion of the MCA (Kimelberg et al., 2000; Kimelberg, Jin, Charniga, & Feustel, 2003). Due to limited tamoxifen selectivity, its protective effects have been further verified with the more specific (but not blood-brain barrier-permeable) VRAC blocker DCPIB (Zhang et al., 2008). In Fig. 12.6A, we present representative data from the latter study illustrating DCPIB-mediated protection in the ischemic brain. Because DCPIB is not BBB permeable, it required intracerebral injection to achieve neuroprotection. As shown in Fig. 12.6B, systemic administration of the same compound was ineffective, further suggesting that it acts in the brain parenchyma but not on blood vessels. The protective effects of VRAC blockers (tamoxifen) have been next validated in larger animal species, in a canine model of stroke (Boulos et al., 2011).
Figure 12.6.
Intracerebral treatment with the VRAC blocker DCPIB potently protects against ischemic brain damage in a rat model of stroke induced by 2-hour occlusion of the middle cerebral artery (MCAo). (A) Representative images of brain sections prepared from MCAo animals after intracerebral injection of vehicle (DMSO) or 20 μg/kg DCPIB. (B) Brain sections from MCAo animal which were treated systemically with intravenous injection of vehicle or 10 mg/kg DCPIB. Animals were euthanized 3 days after ischemia and the extent of brain infarction was revealed by staining brain sections with 2,3,5-triphenyltetrazolium chloride (viable tissue is red). Modified from Y. Zhang et al., 2008, with permission.
Altogether, animal data support the major pathological significance for VRAC in stroke and warrant giving it the title of the “villain” in the ischemic brain. The idea of the therapeutic utility of VRAC in stroke and other neuropathologies has been extensively covered in several relatively recent reviews (Kimelberg, 2005; Mongin, 2007; Mongin, 2016).
12.8. THE EVOLVING UNDERSTANDING OF VRAC IDENTITY: PROGRESS AND PERSPECTIVES
Despite much progress in the field, many aspects of cell volume regulation in the brain require further mechanistic exploration. One avenue which carries the most promise is the recent and ongoing work on establishing the molecular nature of VRAC and the mechanisms contributing to its activation in swollen cells.
For nearly thirty years, the molecular identity of VRAC remained unknown. In 1988, two electrophysiological studies resulted in the first recordings of swelling-activated Cl− currents in human lymphocytes and intestinal epithelial 407 cells (Cahalan & Lewis, 1988; Hazama & Okada, 1988). Since these two pioneer publications, the biophysical properties of VRAC have been a subject of intense interest and were characterized in numerous cell and tissue types [reviewed in (Strange et al., 1996; Okada, 1997; Nilius et al., 1997; Pedersen et al., 2016; Mongin, 2016)]. Nevertheless, despite numerous attempts to clone VRAC, its molecular identity was established only in 2014. The laboratories of A. Patapoutian and T.J. Jentsch performed independent genome-wide siRNA screens and identified VRAC as heteromeric protein complexes homologous to mammalian pannexins and assembled from the gene products belonging to the leucine-rich repeat containing family 8 (LRRC8) (Qiu et al., 2014; Voss et al., 2014). Both labs discovered that VRAC activity is critically dependent on the expression of the LRRC8A isoform, while the four other members of the same family, LRRC8B through E, play dispensable roles (Qiu et al., 2014; Voss et al., 2014). However, T.J. Jentsch and colleagues made an additional conceptual step and established that LRRC8A has to heteromerize with at least one alternative type of the LRRC8 subunits in order to form a fully active VRAC (Voss et al., 2014). Moreover, they found that LRRB8C-E partners modify the biophysical properties of VRAC (Voss et al., 2014). These latter finding addressed the long-standing question of why VRACs have similar but not identical profiles in different cell types (Okada, 1997; Nilius & Droogmans, 2003; Okada, Sato, & Numata, 2009). Also importantly, deletion of LRRC8A and disruption of VRAC function blocked the RVD process in model cell lines (Qiu et al., 2014; Voss et al., 2014). The amazing progress in our knowledge of VRAC is covered in the two invited reviews in this book (Okada, Okada, Islam & Sabirov, 2018; Osei-Owusu, Yang, Vitery & Qiu, 2018). The most recent development in this area is the report on the 3D structure of VRAC resolved with X-ray crystallography and cryo-electron microscopy (Deneka, Sawicka, Lam, Paulino, & Dutzler, 2018).
In this chapter, we narrowly focus on the recent discovery of the heterogeneous VRAC complexes with distinct permeability profiles for organic molecules. As seen in the previous sections, our interest in this topic is motivated by the high relevance of organic osmolyte release to normal brain signaling and pathological states. The two original papers which linked VRAC to LRRC8 proteins, based their work on measurements of currents and fluxes of Cl− and other halides. However, they also additionally found that the LRRC8A-containing channels are responsible for swelling-activated release of the zwitterionic taurine (Qiu et al., 2014; Voss et al., 2014). Our laboratory was the first to affirm the critical role of LRRC8A for VRAC activity in brain cells (astrocytes), but also made the side-by-side comparisons of the LRRC8A-dependent release of taurine and the negatively charged amino acids, glutamate and aspartate (Hyzinski-Garcia et al., 2014). The principal finding in our study was that presence of LRRC8A is equally critical for release of both taurine and glutamate/aspartate (Hyzinski-Garcia et al., 2014). On one hand, this was highly encouraging because the VRAC involvement in the release of organic osmolytes has been questioned in the past (Kirk, 1997; Franco, 2003; Shennan, 2008). On the other hand, the finding of a uniform dependence on LRRC8A expression contradicted the enduring idea about the existence of plural swelling-activated release pathways for inorganic anions and organic osmolytes. Over the years, many groups accrued strong, albeit indirect evidence that Cl− and certain organic molecules, such as taurine, may be transported via distinct volume-sensitive pathways [e.g., (Lambert & Hoffmann, 1994; Shennan, McNeillie, & Curran, 1994; Stutzin et al., 1999; Mongin, Reddi, Charniga, & Kimelberg, 1999)]. The hypothesis that taurine has its own independent release route was particularly influential, but not rooted in any molecular knowledge. This has changed with the discovery of the LRRC8 proteins.
The first molecular evidence for multiple VRAC channels responsible for the release of inorganic and organic osmolytes came from the laboratory of T.J. Jentsch. They discovered that LRRC8A/LRRC8D-containing VRAC plays a unique role in transport of the chemotherapy drugs, cisplatin and carboplatin (Planells-Cases et al., 2015). The same study established that expression of the LRRC8D subunit is essential for the release of taurine, but dispensable for swelling-activated Cl− currents (Planells-Cases et al., 2015). These findings led to the conclusion that LRRC8A/LRRC8D-containing heteromers are the long-sought after swelling-activated taurine channel. Several subsequent publications explored the requirements of LRRC8 composition for the release of taurine and other structurally diverse organic osmolytes, including glutamate, GABA glycine, lactate, and myo-inositol (Gaitan-Penas et al., 2016; Lutter, Ullrich, Lueck, Kempa, & Jentsch, 2017; Schober et al., 2017). The emerging picture is interesting but rather complex. In the most definitive study, Lutter et al. used an erase-and-replace approach in model cell lines to establish that (i) LRRC8A+C-containing VRAC can fully sustain swelling-activated Cl− currents but are poorly permeable to organic osmolytes, (ii) LRRC8A+D heteromers have very limited Cl− conductance but transport all charged and uncharged organic osmolytes, and (iii) LRRC8A+E heteromers exert slightly reduced Cl− conductance and are permeable to anionic amino acids, but not uncharged organic osmolytes (Lutter et al., 2017). As in the LRRC8 discovery studies, the combination of LRRC8A+B did not support Cl− currents or organic osmolyte release. More complex permutations of the LRRC8A, C, D, and E subunits containing three or more LRRC8 isoforms demonstrated some quantitative differences, suggesting that introduction of additional subunits (with the exception of B) modifies the permeability properties of VRAC. Yet, the take home message from this model work was that LRRC8D is indispensable for for release of uncharged and net-neural molecules, while LRRC8D and LRRC8E or their combination can sustan the release of excitatory amino acids.
Our work explored the requirements of endogenous LRRC8 subunits for organic osmolyte release in brain astrocytes (Schober et al., 2017). The principal observations of this study are presented in Fig. 12.7. Although there were strong similarities between our study and report of Lutter et al. (Lutter et al., 2017), we also found interesting qualitative differences, which may reflect the cell type-specific variations in VRAC composition and properties. Much like Lutter et al., we found that swelling activated release of non-polar and uncharged zwitterionic osmolytes, myo-inositol and taurine, was either strongly (taurine) or fully (myo-inositol) suppressed by siRNA knockdown of LRRC8D. The same transport processes were insensitive to the deletion of LRRC8C, LRRC8E, or their combination. In contrast, the release of negatively charged D-aspartate demonstrated very different sensitivity profile. It was strongly (but not completely) reduced by the combined deletion of LRRC8C+LRRC8E. Unlike myo-inositol and taurine, D-aspartate fluxes were insensitive to the downregulation of LRRC8D. Finally and surprisingly, D-aspartate release was fully sensitive to the combined deletion of LRRC8C+ LRRC8D. The last bit of information is at odds with the findings of Lutter et al., and strongly suggests that LRRC8C is present in VRAC heteromers that are responsible for release of negatively charged amino acids. The simplest explanation for these rather complex findings is presented in Fig. 12.7E. We hypothesize that astrocytes express two types of VRAC channels: (a) the LRRC8A+D-containing heteromers, which dominate the release of uncharged molecules, and (b) the LRRC8A+C+D+E-containing heteromers, which mediate the movement of charged amino acids. It appears that in the latter “LRRC8A+C+D+E” channel the C, D and E subunits can partially substitute for each other, because their individual deletions had little effect on D-aspartate fluxes. The functional role for the LRRC8E subunit is particularly interesting and unexpected. In our hands, astrocytes have at least ten-fold lower expression of the LRRC8E mRNA as compared to other LRRC8 transcripts (Hyzinski-Garcia et al., 2014; Schober et al., 2017). Similarly, transcriptomics assays reveal extremely low LRRC8E expression levels in astrocyte and other brain cells (Zhang et al., 2014). Further work is needed to establish the LRRC8 stoichiometry for astrocytic VRAC, and whether the post-transcriptional stability of LRRC8E is different from other LRRC8 family members.
Figure 12.7.
RNAi analysis reveals that astrocytes express two types of heteromeric LRRC8-containing VRAC channels with differential permeability to charged and uncharged organic osmolytes. (A–D) Primary rat astrocytes were transfected with negative control siRNA (Control), or the gene-specific siRNA targeting the expression of one or more members of the proteins belonging to the family of leucine-rich repeat-containing 8 (LRRC8) proteins. After a 4-day incubation with the gene specific siRNA constructs, astrocytes were simultaneously loaded with [3H]taurine and the glutamate analog D-[14C]aspartate, and superfused with isosmotic or hypoosmotic (HYPO) media to activate VRAC and quantify the amino acid release. Taurine and glutamate fluxes have been measured simultaneously but, for clarity, are presented in separate panels (A-B) and (C-D). Knockdown of LRRC8A reduced release of all amino acids. Knockdown of LRRC8D reduced [3H]taurine release by ~50% (A), but had no effect on the release of D-[14C]aspartate (B). Conversely, the combined knockdown of LRRC8C + LRRC8E inhibited the efflux of D-[14C]aspartate (D), but not [3H]taurine (C). Finally, and surprisingly, the combined knockdown of LRRC8C + LRRC8D inhibited the release of all amino acids to the same extent as the knockdown of LRRC8A. (E) The proposed composition of the two populations of LRRC8-containing VRAC heteromers which explains the data in (A-D). The LRRC8A+D combination is responsible for the movement of uncharged molecules such as taurine and myo-inositol (Ino) (data not shown). Another channel composed of LRRC8A+C+D+E is permeable to negatively charged molecules, including glutamate. Because zwitterionic taurine is partially negatively charged, it shares both pathways. **p<0.01, ***p<0.001, amino acid release rates vs. cells treated with the negative control siRNA. Adapted from A.L. Schober et al. (2017), with permission.
It is fitting that this admittedly non-exhaustive review is concluded with the discussion of structure, functions, and (patho)physiological roles of VRAC. In our opinion, the discovery of the LRRC8 proteins has been and continues to be a powerful catalyst for further progress in this field. Although VRAC studies can be dated back to the late 1980s, only now do we have the molecular biology and genetics tools to tackle some of the perennial questions about this channel and cell volume control in the brain. More work is needed to establish if VRAC and other volume-sensitive channels and transporters, such as KCC, NKCC, and SUR1/TRPM4, are friends or foes in the pathological brain. The emerging clinical studies certainly provide a lot of hope that the relevant information will soon be translated to new treatment modalities.
AKNOWLEDGEMENTS
The work in Authors’ laboratory was funded in part by NIH/NINDS grant R01 NS061953.
ABBREVIATIONS:
- ADC
apparent diffusion coefficient [of water]
- BBB
blood-brain barrier
- CNS
central nervous system
- CSF
cerebrospinal fluid
- DRG
dorsal root ganglion
- DWI
diffusion-weighted imaging
- EGFP
enhanced green fluorescent protein
- EM
electron microscopy
- ENaC
epithelial Na+ channels
- GPCR
G protein-coupled receptor
- HICCs
hypertonicity-induced cation channels
- ICF
intracellular fluid
- ISF
interstitial fluid
- MRI
magnetic resonance imaging
- PK
protein kinase
- PKC
protein kinase C
- RVD
regulatory volume decrease
- RVI
regulatory volume increase
- TRP
transient receptor potential [channels]
- VRAC
volume-regulated anion channels
REFERENCES
- Abdullaev IF, Rudkouskaya A, Schools GP, Kimelberg HK, & Mongin AA (2006). Pharmacological comparison of swelling-activated excitatory amino acid release and Cl- currents in rat cultured astrocytes. J.Physiol, 572, 677–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrogue HJ & Madias NE (2000). Hyponatremia. N.Engl.J.Med, 342, 1581–1589. [DOI] [PubMed] [Google Scholar]
- Aitken PG, Borgdorff AJ, Juta AJ, Kiehart DP, Somjen GG, & Wadman WJ (1998). Volume changes induced by osmotic stress in freshly isolated rat hippocampal neurons. Pflugers Arch., 436, 991–998. [DOI] [PubMed] [Google Scholar]
- Aitken PG, Fayuk D, Somjen GG, & Turner DA (1999). Use of intrinsic optical signals to monitor physiological changes in brain tissue slices. Methods, 18, 91–103. [DOI] [PubMed] [Google Scholar]
- Akita T, Fedorovich SV, & Okada Y (2011). Ca2+ nanodomain-mediated component of swelling-induced volume-sensitive outwardly rectifying anion current triggered by autocrine action of ATP in mouse astrocytes. Cell Physiol Biochem., 28, 1181–1190. [DOI] [PubMed] [Google Scholar]
- Akita T & Okada Y (2011). Regulation of bradykinin-induced activation of volume-sensitive outwardly rectifying anion channels by Ca2+ nanodomains in mouse astrocytes. J.Physiol, 589, 3909–3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akita T & Okada Y (2014). Characteristics and roles of the volume-sensitive outwardly rectifying (VSOR) anion channel in the central nervous system. Neuroscience, 275, 211–231. [DOI] [PubMed] [Google Scholar]
- Albrecht J & Norenberg MD (2006). Glutamine: a Trojan horse in ammonia neurotoxicity. Hepatology, 44, 788–794. [DOI] [PubMed] [Google Scholar]
- Albrecht J & Schousboe A (2005). Taurine interaction with neurotransmitter receptors in the CNS: an update. Neurochem.Res, 30, 1615–1621. [DOI] [PubMed] [Google Scholar]
- Alessi DR, Zhang J, Khanna A, Hochdorfer T, Shang Y, & Kahle KT (2014). The WNK-SPAK/OSR1 pathway: master regulator of cation-chloride cotransporters. Sci.Signal, 7, re3. [DOI] [PubMed] [Google Scholar]
- Allert N, Koller H, & Siebler M (1998). Ammonia-induced depolarization of cultured rat cortical astrocytes. Brain Res., 782, 261–270. [DOI] [PubMed] [Google Scholar]
- Alper SL (1991). The band 3-related anion exchanger (AE) gene family. Annu.Rev.Physiol, 53, 549–564. [DOI] [PubMed] [Google Scholar]
- Altamirano J, Brodwick MS, & Alvarez-Leefmans FJ (1998). Regulatory volume decrease and intracellular Ca2+ in murine neuroblastoma cells studied with fluorescent probes. J.Gen.Physiol, 112, 145–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiry-Moghaddam M, Otsuka T, Hurn PD, Traystman RJ, Haug FM, Froehner SC et al. (2003). An alpha-syntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proc.Natl.Acad.Sci.U.S.A, 100, 2106–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiry-Moghaddam M & Ottersen OP (2003). The molecular basis of water transport in the brain. Nat.Rev.Neurosci, 4, 991–1001. [DOI] [PubMed] [Google Scholar]
- Amzica F & Neckelmann D (1999). Membrane capacitance of cortical neurons and glia during sleep oscillations and spike-wave seizures. J.Neurophysiol, 82, 2731–2746. [DOI] [PubMed] [Google Scholar]
- Andrew RD (1991). Seizure and acute osmotic change: clinical and neurophysiological aspects. J.Neurol.Sci, 101, 7–18. [DOI] [PubMed] [Google Scholar]
- Andrew RD, Fagan M, Ballyk BA, & Rosen AS (1989). Seizure susceptibility and the osmotic state. Brain Res., 498, 175–180. [DOI] [PubMed] [Google Scholar]
- Andrew RD, Labron MW, Boehnke SE, Carnduff L, & Kirov SA (2007). Physiological evidence that pyramidal neurons lack functional water channels. Cereb.Cortex, 17, 787–802. [DOI] [PubMed] [Google Scholar]
- Andrew RD & MacVicar BA (1994). Imaging cell volume changes and neuronal excitation in the hippocampal slice. Neuroscience, 62, 371–383. [DOI] [PubMed] [Google Scholar]
- Antonenko YN, Pohl P, & Denisov GA (1997). Permeation of ammonia across bilayer lipid membranes studied by ammonium ion selective microelectrodes. Biophys.J, 72, 2187–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arieff AI, Guisado R, & Lazarowitz VC (1977). Pathophysiology of hyperosmolar states In Andereoli TE, Grantham JJ, & Rector FC (Eds.), Disturbances in Body Fluid Osmolality (pp. 227–250). Bethesda, MD: American Physiological Society. [Google Scholar]
- Arundine M & Tymianski M (2004). Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell Mol.Life Sci, 61, 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assentoft M, Larsen BR, & MacAulay N (2015). Regulation and Function of AQP4 in the Central Nervous System. Neurochem.Res, 40, 2615–2627. [DOI] [PubMed] [Google Scholar]
- Babila T, Atlan H, Fromer I, Schwalb H, Uretzky G, & Lichtstein D (1990). Volume regulation of nerve terminals. J.Neurochem, 55, 2058–2062. [DOI] [PubMed] [Google Scholar]
- Baethmann A & Van Harreveld A (1973). Water and electrolyte distribution in gray matter rendered edematous with a metabolic inhibitor. J.Neuropathol.Exp.Neurol, 32, 408–423. [DOI] [PubMed] [Google Scholar]
- Bai G, Rama Rao KV, Murthy CR, Panickar KS, Jayakumar AR, & Norenberg MD (2001). Ammonia induces the mitochondrial permeability transition in primary cultures of rat astrocytes. J.Neurosci.Res, 66, 981–991. [DOI] [PubMed] [Google Scholar]
- Bakay L & Lee JC (1968). The effect of acute hypoxia and hypercapnia on the ultrastructure of the central nervous system. Brain, 91, 697–706. [DOI] [PubMed] [Google Scholar]
- Bakay L, Lee JC, Lee GC, & Peng JR (1977). Experimental cerebral concussion. Part 1: An electron microscopic study. J.Neurosurg, 47, 525–531. [DOI] [PubMed] [Google Scholar]
- Banderali U & Roy G (1992). Anion channels for amino-acids in Mdck cells. Am.J.Physiol, 263, C1200–C1207. [DOI] [PubMed] [Google Scholar]
- Basavappa S, Chartouni V, Kirk K, Prpic V, Ellory JC, & Mangel AW (1995). Swelling-induced chloride currents in neuroblastoma cells are calcium dependent. J.Neurosci, 15, 3662–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battey TW, Karki M, Singhal AB, Wu O, Sadaghiani S, Campbell BC et al. (2014). Brain edema predicts outcome after nonlacunar ischemic stroke. Stroke, 45, 3643–3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum G, Yuan H, Kahle KT, Li L, Wang S, Shi Y et al. (2015). Inhibition of WNK3 Kinase Signaling Reduces Brain Damage and Accelerates Neurological Recovery After Stroke. Stroke, 46, 1956–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender AS, Neary JT, Blicharska J, Norenberg LO, & Norenberg MD (1992). Role of calmodulin and protein kinase C in astrocytic cell volume regulation. J.Neurochem, 58, 1874–1882. [DOI] [PubMed] [Google Scholar]
- Bender AS, Neary JT, & Norenberg MD (1993). Role of phosphoinositide hydrolysis in astrocyte volume regulation. J.Neurochem, 61, 1506–1514. [DOI] [PubMed] [Google Scholar]
- Bender AS, Schousboe A, Reichelt W, & Norenberg MD (1998). Ionic mechanisms in glutamate-induced astrocyte swelling: role of K+ influx. J.Neurosci.Res, 52, 307–321. [DOI] [PubMed] [Google Scholar]
- Benfenati V, Caprini M, Dovizio M, Mylonakou MN, Ferroni S, Ottersen OP et al. (2011). An aquaporin-4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell-volume control in astrocytes. Proc.Natl.Acad.Sci.U.S.A, 108, 2563–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfenati V, Caprini M, Nicchia GP, Rossi A, Dovizio M, Cervetto C et al. (2009). Carbenoxolone inhibits volume-regulated anion conductance in cultured rat cortical astroglia. Channels (Austin.), 3, 323–336. [DOI] [PubMed] [Google Scholar]
- Benfenati V, Nicchia GP, Svelto M, Rapisarda C, Frigeri A, & Ferroni S (2007). Functional down-regulation of volume-regulated anion channels in AQP4 knockdown cultured rat cortical astrocytes. J.Neurochem, 100, 87–104. [DOI] [PubMed] [Google Scholar]
- Benveniste H, Drejer J, Schousboe A, & Diemer NH (1984). Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J.Neurochem, 43, 1369–1374. [DOI] [PubMed] [Google Scholar]
- Binder DK, Nagelhus EA, & Ottersen OP (2012). Aquaporin-4 and epilepsy. Glia, 60, 1203–1214. [DOI] [PubMed] [Google Scholar]
- Binder DK, Oshio K, Ma T, Verkman AS, & Manley GT (2004). Increased seizure threshold in mice lacking aquaporin-4 water channels. Neuroreport, 15, 259–262. [DOI] [PubMed] [Google Scholar]
- Binder DK, Papadopoulos MC, Haggie PM, & Verkman AS (2004). In vivo measurement of brain extracellular space diffusion by cortical surface photobleaching. J.Neurosci, 24, 8049–8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DK, Yao X, Zador Z, Sick TJ, Verkman AS, & Manley GT (2006). Increased seizure duration and slowed potassium kinetics in mice lacking aquaporin-4 water channels. Glia, 53, 631–636. [DOI] [PubMed] [Google Scholar]
- Blei AT & Larsen FS (1999). Pathophysiology of cerebral edema in fulminant hepatic failure. J.Hepatol, 31, 771–776. [DOI] [PubMed] [Google Scholar]
- Blei AT, Olafsson S, Therrien G, & Butterworth RF (1994). Ammonia-induced brain edema and intracranial hypertension in rats after portacaval anastomosis. Hepatology, 19, 1437–1444. [PubMed] [Google Scholar]
- Boettger T, Rust MB, Maier H, Seidenbecher T, Schweizer M, Keating DJ et al. (2003). Loss of K-Cl co-transporter KCC3 causes deafness, neurodegeneration and reduced seizure threshold. EMBO J., 22, 5422–5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohmer C, Wagner CA, Beck S, Moschen I, Melzig J, Werner A et al. (2000). The shrinkage-activated Na(+) conductance of rat hepatocytes and its possible correlation to rENaC. Cell Physiol.Biochem, 10, 187–194. [DOI] [PubMed] [Google Scholar]
- Bolay H & Dalkara T (1998). Mechanisms of motor dysfunction after transient MCA occlusion: persistent transmission failure in cortical synapses is a major determinant. Stroke, 29, 1988–1993. [DOI] [PubMed] [Google Scholar]
- Bolay H, Gursoy-Ozdemir Y, Sara Y, Onur R, Can A, & Dalkara T (2002). Persistent defect in transmitter release and synapsin phosphorylation in cerebral cortex after transient moderate ischemic injury. Stroke, 33, 1369–1375. [DOI] [PubMed] [Google Scholar]
- Bond T, Basavappa S, Christensen M, & Strange K (1999). ATP dependence of the ICl,swell channel varies with rate of cell swelling. Evidence for two modes of channel activation. J.Gen.Physiol, 113, 441–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondarava M, Li T, Endl E, & Wehner F (2009). alpha-ENaC is a functional element of the hypertonicity-induced cation channel in HepG2 cells and it mediates proliferation. Pflugers Arch., 458, 675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulos AS, Deshaies EM, Dalfino JC, Feustel PJ, Popp AJ, & Drazin D (2011). Tamoxifen as an effective neuroprotectant in an endovascular canine model of stroke. J.Neurosurg, 114, 1117–1126. [DOI] [PubMed] [Google Scholar]
- Bourke RS & Nelson KM (1972). Further studies on the K + -dependent swelling of primate cerebral cortex in vivo: the enzymatic basis of the K + -dependent transport of chloride. J.Neurochem, 19, 663–685. [DOI] [PubMed] [Google Scholar]
- Bourque CW (2008). Central mechanisms of osmosensation and systemic osmoregulation. Nat.Rev.Neurosci, 9, 519–531. [DOI] [PubMed] [Google Scholar]
- Bourque CW & Oliet SH (1997). Osmoreceptors in the central nervous system. Annu.Rev.Physiol, 59, 601–619. [DOI] [PubMed] [Google Scholar]
- Bourque CW, Oliet SH, & Richard D (1994). Osmoreceptors, osmoreception, and osmoregulation. Front Neuroendocrinol., 15, 231–274. [DOI] [PubMed] [Google Scholar]
- Bowens NH, Dohare P, Kuo YH, & Mongin AA (2013). DCPIB, the proposed selective blocker of volume-regulated anion channels, inhibits several glutamate transport pathways in glial cells. Mol.Pharmacol, 83, 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bres V, Hurbin A, Duvoid A, Orcel H, Moos FC, Rabie A et al. (2000). Pharmacological characterization of volume-sensitive, taurine permeable anion channels in rat supraoptic glial cells. Br.J.Pharmacol, 130, 1976–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brott T & Bogousslavsky J (2000). Treatment of acute ischemic stroke. N.Engl.J.Med, 343, 710–722. [DOI] [PubMed] [Google Scholar]
- Brusilow SW, Koehler RC, Traystman RJ, & Cooper AJ (2010). Astrocyte glutamine synthetase: importance in hyperammonemic syndromes and potential target for therapy. Neurotherapeutics, 7, 452–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg MB, Ferraris JD, & Dmitrieva NI (2007). Cellular response to hyperosmotic stresses. Physiol Rev., 87, 1441–1474. [DOI] [PubMed] [Google Scholar]
- Byun N & Delpire E (2007). Axonal and periaxonal swelling precede peripheral neurodegeneration in KCC3 knockout mice. Neurobiol.Dis, 28, 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan MD & Lewis RS (1988). Role of potassium and chloride channels in volume regulation by T lymphocytes. Soc.Gen.Physiol Ser, 43, 281–301. [PubMed] [Google Scholar]
- Caspi A, Benninger F, & Yaari Y (2009). KV7/M channels mediate osmotic modulation of intrinsic neuronal excitability. J.Neurosci, 29, 11098–11111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin ME & Strange K (1989). Anisosmotic cell volume regulation: a comparative view. Am.J.Physiol, 257, C159–C173. [DOI] [PubMed] [Google Scholar]
- Chan PH & Fishman RA (1979). Elevation of rat brain amino acids, ammonia and idiogenic osmoles induced by hyperosmolality. Brain Res., 161, 293–301. [DOI] [PubMed] [Google Scholar]
- Cheema TA, Pettigrew VA, & Fisher SK (2007). Receptor regulation of the volume-sensitive efflux of taurine and iodide from human SH-SY5Y neuroblastoma cells: Differential requirements for Ca2+ and protein kinase C. J.Pharmacol.Exp.Ther, 320, 1068–1077. [DOI] [PubMed] [Google Scholar]
- Cheema TA, Ward CE, & Fisher SK (2005). Subnanomolar concentrations of thrombin enhance the volume-sensitive efflux of taurine from human 1321N1 astrocytoma cells. J.Pharmacol.Exp.Ther, 315, 755–763. [DOI] [PubMed] [Google Scholar]
- Chen M, Dong Y, & Simard JM (2003). Functional coupling between sulfonylurea receptor type 1 and a nonselective cation channel in reactive astrocytes from adult rat brain. J.Neurosci, 23, 8568–8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M & Simard JM (2001). Cell swelling and a nonselective cation channel regulated by internal Ca2+ and ATP in native reactive astrocytes from adult rat brain. J.Neurosci, 21, 6512–6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW (1988). Glutamate neurotoxicity and diseases of the nervous system. Neuron, 1, 623–634. [DOI] [PubMed] [Google Scholar]
- Choi DW (1992). Excitotoxic cell death. J.Neurobiol, 23, 1261–1276. [DOI] [PubMed] [Google Scholar]
- Ciura S & Bourque CW (2006). Transient receptor potential vanilloid 1 is required for intrinsic osmoreception in organum vasculosum lamina terminalis neurons and for normal thirst responses to systemic hyperosmolality. J.Neurosci, 26, 9069–9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciura S, Liedtke W, & Bourque CW (2011). Hypertonicity sensing in organum vasculosum lamina terminalis neurons: a mechanical process involving TRPV1 but not TRPV4. J.Neurosci, 31, 14669–14676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciura S, Prager-Khoutorsky M, Thirouin ZS, Wyrosdic JC, Olson JE, Liedtke W et al. (2018). Trpv4 mediates hypotonic inhibition of central osmosensory neurons via taurine gliotransmission. Cell Rep., 23, 2245–2253. [DOI] [PubMed] [Google Scholar]
- Cornog JL, Gonatas NK, & Feierman JR (1967). Effects of intracerebral injection of ouabain on the fine structure of rat cerebral cortex. Am.J.Pathol, 51, 573–590. [PMC free article] [PubMed] [Google Scholar]
- Crepel V, Panenka W, Kelly ME, & MacVicar BA (1998). Mitogen-activated protein and tyrosine kinases in the activation of astrocyte volume-activated chloride current. J.Neurosci, 18, 1196–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserr HF, DePasquale M, Nicholson C, Patlak CS, Pettigrew KD, & Rice ME (1991). Extracellular volume decreases while cell volume is maintained by ion uptake in rat brain during acute hypernatremia. J.Physiol, 442, 277–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserr HF, DePasquale M, & Patlak CS (1987). Regulation of brain water and electrolytes during acute hyperosmolality in rats. Am.J.Physiol, 253, F522–F529. [DOI] [PubMed] [Google Scholar]
- Cserr HF, DePasquale M, & Patlak CS (1987). Volume regulatory influx of electrolytes from plasma to brain during acute hyperosmolality. Am.J.Physiol, 253, F530–F537. [DOI] [PubMed] [Google Scholar]
- Dadsetan S, Kukolj E, Bak LK, Sorensen M, Ott P, Vilstrup H et al. (2013). Brain alanine formation as an ammonia-scavenging pathway during hyperammonemia: effects of glutamine synthetase inhibition in rats and astrocyte-neuron co-cultures. J.Cereb.Blood Flow Metab, 33, 1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC (2001). Glutamate uptake. Prog.Neurobiol, 65, 1–105. [DOI] [PubMed] [Google Scholar]
- Darby M, Kuzmiski JB, Panenka W, Feighan D, & MacVicar BA (2003). ATP released from astrocytes during swelling activates chloride channels. J.Neurophysiol, 89, 1870–1877. [DOI] [PubMed] [Google Scholar]
- David Y, Cacheaux LP, Ivens S, Lapilover E, Heinemann U, Kaufer D et al. (2009). Astrocytic dysfunction in epileptogenesis: consequence of altered potassium and glutamate homeostasis? J.Neurosci, 29, 10588–10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleuze C, Duvoid A, & Hussy N (1998). Properties and glial origin of osmotic-dependent release of taurine from the rat supraoptic nucleus. J.Physiol, 507, 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpire E & Gagnon KB (2018). Water homeostasis and cell volume maintenance and regulation. Current Topics in Membranes, 81, 3–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneka D, Sawicka M, Lam AKM, Paulino C, & Dutzler R (2018). Structure of a volume-regulated anion channel of the LRRC8 family. Nature, 558, 254–259. [DOI] [PubMed] [Google Scholar]
- DePasquale M, Patlak CS, & Cserr HF (1989). Brain ion and volume regulation during acute hypernatremia in Brattleboro rats. Am.J.Physiol, 256, F1059–F1066. [DOI] [PubMed] [Google Scholar]
- Dietzel I, Heinemann U, Hofmeier G, & Lux HD (1982). Stimulus-induced changes in extracellular Na+ and Cl- concentration in relation to changes in the size of the extracellular space. Exp.Brain Res, 46, 73–84. [DOI] [PubMed] [Google Scholar]
- Dietzel I, Heinemann U, & Lux HD (1989). Relations between slow extracellular potential changes, glial potassium buffering, and electrolyte and cellular volume changes during neuronal hyperactivity in cat brain. Glia, 2, 25–44. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, & Moskowitz MA (1999). Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci., 22, 391–397. [DOI] [PubMed] [Google Scholar]
- Dohare P, Hyzinski-Garcia MC, Vipani A, Bowens NH, Nalwalk JW, Feustel PJ et al. (2014). The neuroprotective properties of the superoxide dismutase mimetic tempol correlate with its ability to reduce pathological glutamate release in a rodent model of stroke. Free Radic.Biol.Med, 77, 168–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S, Anderson CM, Keung EC, Chen Y, Chen Y, & Swanson RA (2003). P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J.Neurosci, 23, 1320–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid T, Williamson A, Lee TS, Petroff OA, & de Lanerolle NC (2008). Glutamate and astrocytes--key players in human mesial temporal lobe epilepsy? Epilepsia, 49 (Suppl 2), 42–52. [DOI] [PubMed] [Google Scholar]
- Estevez AY, O’Regan MH, Song D, & Phillis JW (1999). Effects of anion channel blockers on hyposmotically induced amino acid release from the in vivo rat cerebral cortex. Neurochem.Res, 24, 447–452. [DOI] [PubMed] [Google Scholar]
- Fabene PF, Weiczner R, Marzola P, Nicolato E, Calderan L, Andrioli A et al. (2006). Structural and functional MRI following 4-aminopyridine-induced seizures: a comparative imaging and anatomical study. Neurobiol.Dis, 21, 80–89. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Kortzak D, & Machtens JP (2016). Molecular physiology of EAAT anion channels. Pflugers Arch., 468, 491–502. [DOI] [PubMed] [Google Scholar]
- Falke LC & Misler S (1989). Activity of ion channels during volume regulation by clonal N1E115 neuroblastoma cells. Proc.Natl.Acad.Sci.U.S.A, 86, 3919–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigin VL, Norrving B, & Mensah GA (2017). Global burden of stroke. Circ.Res, 120, 439–448. [DOI] [PubMed] [Google Scholar]
- Felipo V & Butterworth RF (2002). Neurobiology of ammonia. Prog.Neurobiol, 67, 259–279. [DOI] [PubMed] [Google Scholar]
- Feustel PJ, Jin Y, & Kimelberg HK (2004). Volume-regulated anion channels are the predominant contributors to release of excitatory amino acids in the ischemic cortical penumbra. Stroke, 35, 1164–1168. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Spacek J, & Harris KM (2002). Dendritic spine pathology: cause or consequence of neurological disorders? Brain Res.Rev, 39, 29–54. [DOI] [PubMed] [Google Scholar]
- Finkelstein A (1987). Water Movement Through Lipid Bilayers, Pores, and Plasma Membranes: Theory and Reality. (1 ed.) New York: Willey Interscience. [Google Scholar]
- Fisher SK, Cheema TA, Foster DJ, & Heacock AM (2008). Volume-dependent osmolyte efflux from neural tissues: regulation by G-protein-coupled receptors. J.Neurochem, 106, 1998–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SK, Heacock AM, Keep RF, & Foster DJ (2010). Receptor regulation of osmolyte homeostasis in neural cells. J.Physiol, 588, 3355–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florence CM, Baillie LD, & Mulligan SJ (2012). Dynamic volume changes in astrocytes are an intrinsic phenomenon mediated by bicarbonate ion flux. PLoS ONE, 7, e51124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco R (2003). Osmosensitive taurine release: does taurine share the same efflux pathway with chloride and other amino acid osmolytes? Adv.Exp.Med.Biol, 526, 189–196. [PubMed] [Google Scholar]
- Franco R, Panayiotidis MI, & de La Paz LD (2008). Autocrine signaling involved in cell volume regulation: the role of released transmitters and plasma membrane receptors. J.Cell Physiol, 216, 14–28. [DOI] [PubMed] [Google Scholar]
- Fraser CL & Arieff AI (1997). Epidemiology, pathophysiology, and management of hyponatremic encephalopathy. Am.J.Med, 102, 67–77. [DOI] [PubMed] [Google Scholar]
- Gaitan-Penas H, Gradogna A, Laparra-Cuervo L, Solsona C, Fernandez-Duenas V, Barrallo-Gimeno A et al. (2016). Investigation of LRRC8-mediated volume-regulated anion currents in Xenopus oocytes. Biophys.J, 111, 1429–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamba G (2005). Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol.Rev, 85, 423–493. [DOI] [PubMed] [Google Scholar]
- Garcia JH, Yoshida Y, Chen H, Li Y, Zhang ZG, Lian J et al. (1993). Progression from ischemic injury to infarct following middle cerebral artery occlusion in the rat. Am.J.Pathol, 142, 623–635. [PMC free article] [PubMed] [Google Scholar]
- Greenwood SM & Connolly CN (2007). Dendritic and mitochondrial changes during glutamate excitotoxicity. Neuropharmacology, 53, 891–898. [DOI] [PubMed] [Google Scholar]
- Groulx N, Boudreault F, Orlov SN, & Grygorczyk R (2006). Membrane reserves and hypotonic cell swelling. J.Membr.Biol, 214, 43–56. [DOI] [PubMed] [Google Scholar]
- Gullans SR & Verbalis JG (1993). Control of brain volume during hyperosmolar and hypoosmolar conditions. Annu.Rev.Med, 44, 289–301. [DOI] [PubMed] [Google Scholar]
- Haj-Yasein NN, Jensen V, Ostby I, Omholt SW, Voipio J, Kaila K et al. (2012). Aquaporin-4 regulates extracellular space volume dynamics during high-frequency synaptic stimulation: a gene deletion study in mouse hippocampus. Glia, 60, 867–874. [DOI] [PubMed] [Google Scholar]
- Hansen AJ (1985). Effect of anoxia on ion distribution in the brain. Physiol Rev., 65, 101–148. [DOI] [PubMed] [Google Scholar]
- Hansson E, Johansson BB, Westergren I, & Ronnback L (1994). Glutamate-induced swelling of single astroglial cells in primary culture. Neuroscience, 63, 1057–1066. [DOI] [PubMed] [Google Scholar]
- Haskew-Layton RE, Rudkouskaya A, Jin Y, Feustel PJ, Kimelberg HK, & Mongin AA (2008). Two distinct modes of hypoosmotic medium-induced release of excitatory amino acids and taurine in the rat brain in vivo. PLoS ONE, 3, e3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussinger D & Schliess F (2008). Pathogenetic mechanisms of hepatic encephalopathy. Gut, 57, 1156–1165. [DOI] [PubMed] [Google Scholar]
- Haydon PG & Carmignoto G (2006). Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev., 86, 1009–1031. [DOI] [PubMed] [Google Scholar]
- Hazama A & Okada Y (1988). Ca2+ sensitivity of volume-regulatory K+ and Cl- channels in cultured human epithelial cells. J.Physiol, 402, 687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, & Zalutsky R (2007). How common are the “common” neurologic disorders? Neurology, 68, 326–337. [DOI] [PubMed] [Google Scholar]
- Hoffmann EK, Lambert IH, & Pedersen SF (2009). Physiology of cell volume regulation in vertebrates. Physiol.Rev, 89, 193–277. [DOI] [PubMed] [Google Scholar]
- Holthoff K & Witte OW (1996). Intrinsic optical signals in rat neocortical slices measured with near-infrared dark-field microscopy reveal changes in extracellular space. J.Neurosci, 16, 2740–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie H, Ikuta S, Takenaka T, & Ito S (1989). Adaptation of cultured mammalian neurons to a hypotonic environment with age-related response. Brain Res., 477, 233–240. [DOI] [PubMed] [Google Scholar]
- Howard HC, Mount DB, Rochefort D, Byun N, Dupre N, Lu J et al. (2002). The K-Cl cotransporter KCC3 is mutant in a severe peripheral neuropathy associated with agenesis of the corpus callosum. Nat.Genet, 32, 384–392. [DOI] [PubMed] [Google Scholar]
- Hubel N & Ullah G (2016). Anions govern cell volume: a case study of relative astrocytic and neuronal swelling in spreading depolarization. PLoS ONE, 11, e0147060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberfeld G, Wittner L, Clemenceau S, Baulac M, Kaila K, Miles R et al. (2007). Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J.Neurosci, 27, 9866–9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussy N, Deleuze C, Desarmenien MG, & Moos FC (2000). Osmotic regulation of neuronal activity: a new role for taurine and glial cells in a hypothalamic neuroendocrine structure. Prog.Neurobiol, 62, 113–134. [DOI] [PubMed] [Google Scholar]
- Hussy N, Deleuze C, Pantaloni A, Desarmenien MG, & Moos F (1997). Agonist action of taurine on glycine receptors in rat supraoptic magnocellular neurones: possible role in osmoregulation. J.Physiol, 502, 609–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyzinski-Garcia MC, Rudkouskaya A, & Mongin AA (2014). LRRC8A protein is indispensable for swelling-activated and ATP-induced release of excitatory amino acids in rat astrocytes. J.Physiol, 592, 4855–4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyzinski-Garcia MC, Vincent MY, Haskew-Layton RE, Dohare P, Keller RW Jr., & Mongin AA (2011). Hypoosmotic swelling modifies glutamate-glutamine cycle in the cerebral cortex and in astrocyte cultures. J.Neurochem, 118, 140–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M et al. (2013). Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J.Clin.Invest, 123, 1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA et al. (2012). A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci.Transl.Med, 4, 147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Mori S, Morishima S, & Okada Y (2005). Volume-sensitive chloride channels in mouse cortical neurons: characterization and role in volume regulation. Eur.J.Neurosci, 21, 1648–1658. [DOI] [PubMed] [Google Scholar]
- Inoue H & Okada Y (2007). Roles of volume-sensitive chloride channel in excitotoxic neuronal injury. J.Neurosci, 27, 1445–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa K, Tasaki I, & Gibbons RC (1980). Swelling of nerve fibers associated with action potentials. Science, 210, 338–339. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Kirby CO, Benz AM, Olney JW, & Zorumski CF (1999). Muller cell swelling, glutamate uptake, and excitotoxic neurodegeneration in the isolated rat retina. Glia, 25, 379–389. [PubMed] [Google Scholar]
- Jackson PS, Morrison R, & Strange K (1994). The volume-sensitive organic osmolyte-anion channel VSOAC is regulated by nonhydrolytic ATP binding. Am.J.Physiol, 267, C1203–C1209. [DOI] [PubMed] [Google Scholar]
- Jackson PS & Strange K (1993). Volume-sensitive anion channels mediate swelling-activated inositol and taurine efflux. Am.J.Physiol, 265, C1489–C1500. [DOI] [PubMed] [Google Scholar]
- Jayakumar AR, Valdes V, & Norenberg MD (2011). The Na-K-Cl cotransporter in the brain edema of acute liver failure. J.Hepatol, 54, 272–278. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ (2016). VRACs and other ion channels and transporters in the regulation of cell volume and beyond. Nat.Rev.Mol.Cell Biol, 17, 293–307. [DOI] [PubMed] [Google Scholar]
- Jing J, Aitken PG, & Somjen GG (1994). Interstitial volume changes during spreading depression (SD) and SD-like hypoxic depolarization in hippocampal tissue slices. J.Neurophysiol, 71, 2548–2551. [DOI] [PubMed] [Google Scholar]
- Kahle KT, Khanna AR, Alper SL, Adragna NC, Lauf PK, Sun D et al. (2015). K-Cl cotransporters, cell volume homeostasis, and neurological disease. Trends Mol.Med, 21, 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle KT, Merner ND, Friedel P, Silayeva L, Liang B, Khanna A et al. (2014). Genetically encoded impairment of neuronal KCC2 cotransporter function in human idiopathic generalized epilepsy. EMBO Rep., 15, 766–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle KT, Ring AM, & Lifton RP (2008). Molecular physiology of the WNK kinases. Annu.Rev.Physiol, 70, 329–355. [DOI] [PubMed] [Google Scholar]
- Kahle KT, Staley KJ, Nahed BV, Gamba G, Hebert SC, Lifton RP et al. (2008). Roles of the cation-chloride cotransporters in neurological disease. Nat.Clin.Pract.Neurol, 4, 490–503. [DOI] [PubMed] [Google Scholar]
- Kaila K, Price TJ, Payne JA, Puskarjov M, & Voipio J (2014). Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat.Rev.Neurosci, 15, 637–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempski O, Chaussy L, Gross U, Zimmer M, & Baethmann A (1983). Volume regulation and metabolism of suspended C6 glioma cells: an in vitro model to study cytotoxic brain edema. Brain Res., 279, 217–228. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK (1995). Current concepts of brain edema. Review of laboratory investigations. J.Neurosurg, 83, 1051–1059. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK (2004). Water homeostasis in the brain: basic concepts. Neuroscience, 129, 851–860. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK (2005). Astrocytic swelling in cerebral ischemia as a possible cause of injury and target for therapy. Glia, 50, 389–397. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Feustel PJ, Jin Y, Paquette J, Boulos A, Keller RW Jr. et al. (2000). Acute treatment with tamoxifen reduces ischemic damage following middle cerebral artery occlusion. Neuroreport, 11, 2675–2679. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK & Frangakis MV (1985). Furosemide- and bumetanide-sensitive ion transport and volume control in primary astrocyte cultures from rat brain. Brain Res., 361, 125–134. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Goderie SK, Higman S, Pang S, & Waniewski RA (1990). Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J.Neurosci, 10, 1583–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK, Jin Y, Charniga C, & Feustel PJ (2003). Neuroprotective activity of tamoxifen in permanent focal ischemia. J.Neurosurg, 99, 138–142. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK & Mongin AA (1998). Swelling-activated release of excitatory amino acids in the brain: Relevance for pathophysiology. Contrib.Nephrol, 123, 240–257. [DOI] [PubMed] [Google Scholar]
- Kinne R, Kinne-Saffran E, Schutz H, & Scholermann B (1986). Ammonium transport in medullary thick ascending limb of rabbit kidney: involvement of the Na+,K+,Cl(−)-cotransporter. J.Membr.Biol, 94, 279–284. [DOI] [PubMed] [Google Scholar]
- Kintner DB, Wang Y, & Sun D (2007). Role of membrane ion transport proteins in cerebral ischemic damage. Front Biosci., 12, 762–770. [DOI] [PubMed] [Google Scholar]
- Kirk K (1997). Swelling-activated organic osmolyte channels. J.Membr.Biol, 158, 1–16. [DOI] [PubMed] [Google Scholar]
- Klatzo I (1967). Presidential Address: Neuropathological aspects of brain edema. J.Neuropathol.Exp.Neurol, 26, 1–13. [DOI] [PubMed] [Google Scholar]
- Kofuji P & Newman EA (2004). Potassium buffering in the central nervous system. Neuroscience, 129, 1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koos B, Christmann J, Plettenberg S, Kading D, Becker J, Keteku M et al. (2018). Hypertonicity-induced cation channels in HepG2 cells: architecture and role in proliferation vs. apoptosis. J.Physiol, 596, 1227–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama Y, Ishibashi T, Okamoto T, Matsuda T, Hashimoto H, & Baba A (2000). Transient treatments with L-glutamate and threo-beta-hydroxyaspartate induce swelling of rat cultured astrocytes. Neurochem.Int, 36, 167–173. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Sugimoto T, Shigenaga Y, Baba A, & Iwata H (1991). A morphological study on glutamate-induced swelling of cultured astrocytes: involvement of calcium and chloride ion mechanisms. Neurosci.Lett, 124, 235–238. [DOI] [PubMed] [Google Scholar]
- Kreisman NR & Olson JE (2003). Taurine enhances volume regulation in hippocampal slices swollen osmotically. Neuroscience, 120, 635–642. [DOI] [PubMed] [Google Scholar]
- Kubo M & Okada Y (1992). Volume-regulatory Cl- channel currents in cultured human epithelial cells. J.Physiol, 456, 351–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucinski T, Vaterlein O, Glauche V, Fiehler J, Klotz E, Eckert B et al. (2002). Correlation of apparent diffusion coefficient and computed tomography density in acute ischemic stroke. Stroke, 33, 1786–1791. [DOI] [PubMed] [Google Scholar]
- Kunte H, Schmidt S, Eliasziw M, del Zoppo GJ, Simard JM, Masuhr F et al. (2007). Sulfonylureas improve outcome in patients with type 2 diabetes and acute ischemic stroke. Stroke, 38, 2526–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert IH & Hoffmann EK (1994). Cell swelling activates separate taurine and chloride channels in Ehrlich mouse ascites tumor cells. J.Membr.Biol, 142, 289–298. [DOI] [PubMed] [Google Scholar]
- Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E et al. (1998). Functional significance of cell volume regulatory mechanisms. Physiol Rev., 78, 247–306. [DOI] [PubMed] [Google Scholar]
- Lansberg MG, Tong DC, Norbash AM, Yenari MA, & Moseley ME (1999). Intra-arterial rtPA treatment of stroke assessed by diffusion-and perfusion-weighted MRI. Stroke, 30, 678–680 [DOI] [PubMed] [Google Scholar]
- Larsen BR, Assentoft M, Cotrina ML, Hua SZ, Nedergaard M, Kaila K et al. (2014). Contributions of the Na(+)/K(+)-ATPase, NKCC1, and Kir4.1 to hippocampal K(+) clearance and volume responses. Glia, 62, 608–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen BR & MacAulay N (2017). Activity-dependent astrocyte swelling is mediated by pH-regulating mechanisms. Glia, 65, 1668–1681. [DOI] [PubMed] [Google Scholar]
- Lauderdale K, Murphy T, Tung T, Davila D, Binder DK, & Fiacco TA (2015). Osmotic edema rapidly increases neuronal excitability through activation of NMDA receptor-dependent slow inward currents in juvenile and adult hippocampus. ASN Neuro., 7 (5), 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauf PK & Adragna NC (2000). K-Cl cotransport: properties and molecular mechanism. Cell Physiol Biochem., 10, 341–354. [DOI] [PubMed] [Google Scholar]
- Leaf A (1956). On the mechanism of fluis exchange of tissues in vitro. Biochem.J, 62, 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaney JL, Marsh SJ, & Brown DA (1997). A swelling-activated chloride current in rat sympathetic neurones. J.Physiol, 501, 555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DJ, Amini M, Hamamura MJ, Hsu MS, Seldin MM, Nalcioglu O et al. (2012). Aquaporin-4-dependent edema clearance following status epilepticus. Epilepsy Res., 98, 264–268. [DOI] [PubMed] [Google Scholar]
- Lehmann A (1989). Effects of microdialysis-perfusion with anisoosmotic media on extracellular amino acids in the rat hippocampus and skeletal muscle. J.Neurochem, 53, 525–535. [DOI] [PubMed] [Google Scholar]
- Lipton P (1973). Effects of membrane depolarization on light scattering by cerebral cortical slices. J.Physiol, 231, 365–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HT, Akita T, Shimizu T, Sabirov RZ, & Okada Y (2009). Bradykinin-induced astrocyte-neuron signalling: glutamate release is mediated by ROS-activated volume-sensitive outwardly rectifying anion channels. J.Physiol, 587, 2197–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HT, Tashmukhamedov BA, Inoue H, Okada Y, & Sabirov RZ (2006). Roles of two types of anion channels in glutamate release from mouse astrocytes under ischemic or osmotic stress. Glia, 54, 343–357. [DOI] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, & Moskowitz MA (2003). Mechanisms, challenges and opportunities in stroke. Nat.Rev.Neurosci, 4, 399–415. [DOI] [PubMed] [Google Scholar]
- Lohr JW, McReynolds J, Grimaldi T, & Acara M (1988). Effect of acute and chronic hypernatremia on myoinositol and sorbitol concentration in rat brain and kidney. Life Sci., 43, 271–276. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, & Cummins R (1989). Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke, 20, 84–91. [DOI] [PubMed] [Google Scholar]
- Lutter D, Ullrich F, Lueck JC, Kempa S, & Jentsch TJ (2017). Selective transport of neurotransmitters and modulators by distinct volume-regulated LRRC8 anion channels. J.Cell Sci, 130, 1122–1133. [DOI] [PubMed] [Google Scholar]
- MacAulay N, Gether U, Klaerke DA, & Zeuthen T (2001). Water transport by the human Na+-coupled glutamate cotransporter expressed in Xenopus oocytes. J.Physiol, 530, 367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAulay N, Hamann S, & Zeuthen T (2004). Water transport in the brain: role of cotransporters. Neuroscience, 129, 1031–1044. [DOI] [PubMed] [Google Scholar]
- MacAulay N & Zeuthen T (2012). Glial K(+) clearance and cell swelling: key roles for cotransporters and pumps. Neurochem.Res, 37, 2299–2309. [DOI] [PubMed] [Google Scholar]
- Machtens JP, Kortzak D, Lansche C, Leinenweber A, Kilian P, Begemann B et al. (2015). Mechanisms of anion conduction by coupled glutamate transporters. Cell, 160, 542–553. [DOI] [PubMed] [Google Scholar]
- Macknight ADC & Leaf A (1977). Regulation of cellular volume. Physiol Rev., 57, 510–573. [DOI] [PubMed] [Google Scholar]
- MacVicar BA, Feighan D, Brown A, & Ransom B (2002). Intrinsic optical signals in the rat optic nerve: role for K(+) uptake via NKCC1 and swelling of astrocytes. Glia, 37, 114–123. [DOI] [PubMed] [Google Scholar]
- MacVicar BA & Hochman D (1991). Imaging of synaptically evoked intrinsic optical signals in hippocampal slices. J.Neurosci, 11, 1458–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW et al. (2000). Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat.Med, 6, 159–163. [DOI] [PubMed] [Google Scholar]
- Manolopoulos VG, Voets T, Declercq PE, Droogmans G, & Nilius B (1997). Swelling-activated efflux of taurine and other organic osmolytes in endothelial cells. Am.J.Physiol, 273, C214–C222. [DOI] [PubMed] [Google Scholar]
- Marcaggi P & Coles JA (2001). Ammonium in nervous tissue: transport across cell membranes, fluxes from neurons to glial cells, and role in signalling. Prog.Neurobiol, 64, 157–183. [DOI] [PubMed] [Google Scholar]
- Martin DL, Madelian V, Seligmann B, & Shain W (1990). The role of osmotic pressure and membrane potential in K(+)-stimulated taurine release from cultured astrocytes and LRM55 cells. J.Neurosci, 10, 571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick JA & Ellison DH (2011). The WNKs: atypical protein kinases with pleiotropic actions. Physiol Rev., 91, 177–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna MC (2007). The glutamate-glutamine cycle is not stoichiometric: fates of glutamate in brain. J.Neurosci.Res, 85, 3347–3358. [DOI] [PubMed] [Google Scholar]
- McNamara JO (1994). Cellular and molecular basis of epilepsy. J.Neurosci, 14, 3413–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara JO, Huang YZ, & Leonard AS (2006). Molecular signaling mechanisms underlying epileptogenesis. Sci.STKE, 2006, re12. [DOI] [PubMed] [Google Scholar]
- Meldrum BS (2000). Glutamate as a neurotransmitter in the brain: review of physiology and pathology 2. J.Nutr, 130, 1007S–1015S. [DOI] [PubMed] [Google Scholar]
- Melton JE & Nattie EE (1983). Brain and CSF water and ions during dilutional and isosmotic hyponatremia in the rat. Am.J.Physiol, 244, R724–R732. [DOI] [PubMed] [Google Scholar]
- Melton JE, Patlak CS, Pettigrew KD, & Cserr HF (1987). Volume regulatory loss of Na, Cl, and K from rat brain during acute hyponatremia. Am.J.Physiol, 252, F661–F669. [DOI] [PubMed] [Google Scholar]
- Minieri L, Pivonkova H, Harantova L, Anderova M, & Ferroni S (2015). Intracellular Na(+) inhibits volume-regulated anion channel in rat cortical astrocytes. J.Neurochem, 132, 286–300. [DOI] [PubMed] [Google Scholar]
- Mohan S, Gu S, Parikh A, & Radhakrishnan J (2013). Prevalence of hyponatremia and association with mortality: results from NHANES. Am.J.Med, 126, 1127–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongin AA (2007). Disruption of ionic and cell volume homeostasis in cerebral ischemia: The perfect storm. Pathophysiology, 14, 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongin AA (2016). Volume-regulated anion channel--a frenemy within the brain. Pflugers Arch., 468, 421–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongin AA, Aksentsev SL, Orlov SN, & Konev SV (1997). Hypoosmotic shock activates Ca2+ channels in isolated nerve terminals. Neurochem.Int, 31, 835–843. [DOI] [PubMed] [Google Scholar]
- Mongin AA, Aksentsev SL, Orlov SN, Kvacheva ZB, Mezen NI, Fedulov AS et al. (1996). Swelling-induced activation of Na+,K+,2Cl− cotransport in C6 glioma cells: Kinetic properties and intracellular signalling mechanisms. Biochim.Biophys.Acta, 1285, 229–236. [DOI] [PubMed] [Google Scholar]
- Mongin AA, Aksentsev SL, Orlov SN, Slepko NG, Kozlova MV, Maximov GV et al. (1994). Swelling-induced K+ influx in cultured primary astrocytes. Brain Res., 655, 110–114. [DOI] [PubMed] [Google Scholar]
- Mongin AA, Cai Z, & Kimelberg HK (1999). Volume-dependent taurine release from cultured astrocytes requires permissive [Ca2+]i and calmodulin. Am.J.Physiol.Cell Physiol, 277, C823–C832. [DOI] [PubMed] [Google Scholar]
- Mongin AA, Dohare P, & Jourd’heuil D (2012). Selective vulnerability of synaptic signaling and metabolism to nitrosative stress. Antioxid.Redox.Signal, 17, 992–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongin AA & Kimelberg HK (2002). ATP potently modulates anion channel-mediated excitatory amino acid release from cultured astrocytes. Am.J.Physiol.Cell Physiol, 283, C569–C578. [DOI] [PubMed] [Google Scholar]
- Mongin AA & Kimelberg HK (2005). Astrocytic swelling in neuropathology In Kettenmann H & Ransom BR (Eds.), Neuroglia (2nd. ed., pp. 550–562). Oxford/New York: Oxford University Press. [Google Scholar]
- Mongin AA & Kimelberg HK (2005). ATP regulates anion channel-mediated organic osmolyte release from cultured rat astrocytes via multiple Ca2+-sensitive mechanisms. Am.J.Physiol.Cell Physiol, 288, C204–C213. [DOI] [PubMed] [Google Scholar]
- Mongin AA & Kimelberg HK (2009). Regulation of cell volume in neural cells In Squire LR (Ed.), Encyclopedia of Neuroscience (pp. 81–87). Amsterdam/Heidelberg/London/New York/Paris/Sidney/Tokyo: Elsevier. [Google Scholar]
- Mongin AA & Orlov SN (2001). Mechanisms of cell volume regulation and possible nature of the cell volume sensor. Pathophysiology, 8, 77–88. [DOI] [PubMed] [Google Scholar]
- Mongin AA, Reddi JM, Charniga C, & Kimelberg HK (1999). [3H]Taurine and D-[3H]aspartate release from astrocyte cultures are differently regulated by tyrosine kinases. Am.J.Physiol, 276, C1226–C1230. [DOI] [PubMed] [Google Scholar]
- Moran J, Maar T, & Pasantes-Morales H (1994). Cell volume regulation in taurine deficient cultured astrocytes. Adv.Exp.Med.Biol, 359, 361–367. [DOI] [PubMed] [Google Scholar]
- Morfini GA, Stenoien DL, & Brady ST (2006). Axonal transport In Siegel GJ, Albers RW, Brady ST, & Price DL (Eds.), Basic Neurochemistry: Molecular, Cellular and Medical Aspects (7th ed., pp. 485–501). London: Elsevier Academic Press. [Google Scholar]
- Morris CE & Homann U (2001). Cell surface area regulation and membrane tension. J.Membr.Biol, 179, 79–102. [DOI] [PubMed] [Google Scholar]
- Murphy TR, Binder DK, & Fiacco TA (2017). Turning down the volume: Astrocyte volume change in the generation and termination of epileptic seizures. Neurobiol.Dis, 104, 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TR, Davila D, Cuvelier N, Young LR, Lauderdale K, Binder DK et al. (2017). Hippocampal and cortical pyramidal neurons swell in parallel with astrocytes during acute hypoosmolar stress. Front Cell Neurosci., 11, 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy CR, Rama Rao KV, Bai G, & Norenberg MD (2001). Ammonia-induced production of free radicals in primary cultures of rat astrocytes. J.Neurosci.Res, 66, 282–288. [DOI] [PubMed] [Google Scholar]
- Nagaraja TN & Brookes N (1998). Intracellular acidification induced by passive and active transport of ammonium ions in astrocytes. Am.J.Physiol, 274, C883–C891. [DOI] [PubMed] [Google Scholar]
- Nagelhus EA, Mathiisen TM, & Ottersen OP (2004). Aquaporin-4 in the central nervous system: cellular and subcellular distribution and coexpression with KIR4.1. Neuroscience, 129, 905–913. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin T, Kastrup A, de CA, Yenari MA, Ringer T, Sun GH et al. (2000). Serial MRI after transient focal cerebral ischemia in rats: dynamics of tissue injury, blood-brain barrier damage, and edema formation. Stroke, 31, 1965–1972. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin T & Witte OW (2000). Periinfarct and remote excitability changes after transient middle cerebral artery occlusion. J.Cereb.Blood Flow Metab, 20, 45–52. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin T, Wittsack HJ, Wenserski F, Siebler M, Seitz RJ, Modder U et al. (1999). Diffusion- and perfusion-weighted MRI. The DWI/PWI mismatch region in acute stroke. Stroke, 30, 1591–1597. [DOI] [PubMed] [Google Scholar]
- Nicholson C & Sykova E (1998). Extracellular space structure revealed by diffusion analysis. Trends Neurosci., 21, 207–215. [DOI] [PubMed] [Google Scholar]
- Nilius B & Droogmans G (2003). Amazing chloride channels: an overview. Acta Physiol Scand., 177, 119–147. [DOI] [PubMed] [Google Scholar]
- Nilius B, Eggermont J, Voets T, Buyse G, Manolopoulos V, & Droogmans G (1997). Properties of volume-regulated anion channels in mammalian cells. Prog.Biophys.Mol.Biol, 68, 69–119. [DOI] [PubMed] [Google Scholar]
- Nilius B, Sehrer J, & Droogmans G (1994). Permeation properties and modulation of volume-activated Cl(−)-currents in human endothelial cells. Br.J.Pharmacol, 112, 1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M & Hiyama TY (2015). Sodium sensing in the brain. Pflugers Arch., 467, 465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norenberg MD (1977). A light and electron microscopic study of experimental portal-systemic (ammonia) encephalopathy. Progression and reversal of the disorder. Lab Invest, 36, 618–627. [PubMed] [Google Scholar]
- Norenberg MD (1994). Astrocyte responses to CNS injury. J.Neuropathol.Exp.Neurol, 53, 213–220. [DOI] [PubMed] [Google Scholar]
- Norenberg MD (1998). Astroglial dysfunction in hepatic encephalopathy. Metab.Brain Dis, 13, 319–335. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Baker L, Norenberg LO, Blicharska J, Bruce-Gregorios JH, & Neary JT (1991). Ammonia-induced astrocyte swelling in primary culture. Neurochem.Res, 16, 833–836. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Jayakumar AR, Rama Rao KV, & Panickar KS (2007). New concepts in the mechanism of ammonia-induced astrocyte swelling. Metab Brain Dis., 22, 219–234. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Rao KV, & Jayakumar AR (2005). Mechanisms of ammonia-induced astrocyte swelling. Metab Brain Dis., 20, 303–318. [DOI] [PubMed] [Google Scholar]
- O’Connor ER, Kimelberg HK, Keese CR, & Giaever I (1993). Electrical resistance method for measuring volume changes in monolayer cultures applied to primary astrocyte cultures. Am.J.Physiol, 264, C471–C478. [DOI] [PubMed] [Google Scholar]
- O’Neill WC (1999). Physiological significance of volume-regulatory transporters. Am.J.Physiol, 276, C995–C1011. [DOI] [PubMed] [Google Scholar]
- Oike M, Droogmans G, & Nilius B (1994). The volume-activated chloride current in human endothelial cells depends on intracellular ATP. Pflugers Arch., 427, 184–186. [DOI] [PubMed] [Google Scholar]
- Okada Y (1997). Volume expansion-sensing outward-rectifier Cl- channel: fresh start to the molecular identity and volume sensor. Am.J.Physiol, 273, C755–C789. [DOI] [PubMed] [Google Scholar]
- Okada Y (2018). Molecular identities of volume-regulatory anion channels. Curr.Top.Membr, 81, [DOI] [PubMed] [Google Scholar]
- Okada Y, Sato K, & Numata T (2009). Pathophysiology and puzzles of the volume-sensitive outwardly rectifying anion channel. J.Physiol, 587, 2141–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen ML, Khakh BS, Skatchkov SN, Zhou M, Lee CJ, & Rouach N (2015). New Insights on Astrocyte Ion Channels: Critical for Homeostasis and Neuron-Glia Signaling. J.Neurosci, 35, 13827–13835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JE & Li GZ (1997). Increased potassium, chloride, and taurine conductances in astrocytes during hypoosmotic swelling. Glia, 20, 254–261. [DOI] [PubMed] [Google Scholar]
- Olson JE, Sankar R, Holtzman D, James A, & Fleischhacker D (1986). Energy-dependent volume regulation in primary cultured cerebral astrocytes. J.Cell Physiol, 128, 209–215. [DOI] [PubMed] [Google Scholar]
- Orellana JA, Avendano BC, & Montero TD (2014). Role of connexins and pannexins in ischemic stroke. Curr.Med.Chem, 21, 2165–2182. [DOI] [PubMed] [Google Scholar]
- Pacheco-Alvarez D & Gamba G (2011). WNK3 is a putative chloride-sensing kinase. Cell Physiol.Biochem, 28, 1123–1134. [DOI] [PubMed] [Google Scholar]
- Palma E, Amici M, Sobrero F, Spinelli G, Di AS, Ragozzino D et al. (2006). Anomalous levels of Cl- transporters in the hippocampal subiculum from temporal lobe epilepsy patients make GABA excitatory. Proc.Natl.Acad.Sci.U.S.A, 103, 8465–8468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkerson KA & Sontheimer H (2004). Biophysical and pharmacological characterization of hypotonically activated chloride currents in cortical astrocytes. Glia, 46, 419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasantes Morales H & Schousboe A (1988). Volume regulation in astrocytes: a role for taurine as an osmoeffector. J.Neurosci.Res, 20, 503–509. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Chacon E, Murray RA, & Moran J (1994). Properties of osmolyte fluxes activated during regulatory volume decrease in cultured cerebellar granule neurons. J.Neurosci.Res, 37, 720–727. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Lezama RA, Ramos-Mandujano G, & Tuz KL (2006). Mechanisms of cell volume regulation in hypo-osmolality. Am.J.Med, 119, S4–S11. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Maar TE, & Moran J (1993). Cell volume regulation in cultured cerebellar granule neurons. J.Neurosci.Res, 34, 219–224. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Moran J, & Schousboe A (1990). Volume-sensitive release of taurine from cultured astrocytes: properties and mechanism. Glia, 3, 427–432. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Murray RA, Lilja L, & Moran J (1994). Regulatory volume decrease in cultured astrocytes. I. Potassium- and chloride-activated permeability. Am.J.Physiol, 266, C165–C171. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Murray RA, Sanchez-Olea R, & Moran J (1994). Regulatory volume decrease in cultured astrocytes. II. Permeability pathway to amino acids and polyols. Am.J.Physiol, 266, C172–C178. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Lauritzen I, Lazdunski M, & Honore E (1998). Disruption of mitochondrial respiration inhibits volume-regulated anion channels and provokes neuronal cell swelling. J.Neurosci, 18, 3117–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen SF, Okada Y, & Nilius B (2016). Biophysics and physiology of the volume-regulated anion channel (VRAC)/volume-sensitive outwardly rectifying anion channel (VSOR). Pflugers Arch., 468, 371–383. [DOI] [PubMed] [Google Scholar]
- Phillis JW & O’Regan MH (1996). Mechanisms of glutamate and aspartate release in the ischemic rat cerebral cortex. Brain Res., 730, 150–164. [DOI] [PubMed] [Google Scholar]
- Phillis JW, Song D, & O’Regan MH (1997). Inhibition by anion channel blockers of ischemia-evoked release of excitotoxic and other amino acids from rat cerebral cortex. Brain Res., 758, 9–16. [DOI] [PubMed] [Google Scholar]
- Phillis JW, Song D, & O’Regan MH (1998). Tamoxifen, a chloride channel blocker, reduces glutamate and aspartate release from the ischemic cerebral cortex. Brain Res., 780, 352–355. [DOI] [PubMed] [Google Scholar]
- Piala AT, Moon TM, Akella R, He H, Cobb MH, & Goldsmith EJ (2014). Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci.Signal, 7, ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planells-Cases R, Lutter D, Guyader C, Gerhards NM, Ullrich F, Elger DA et al. (2015). Subunit composition of VRAC channels determines substrate specificity and cellular resistance to Pt-based anti-cancer drugs. EMBO J., 34, 2993–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podesta MA, Faravelli I, Cucchiari D, Reggiani F, Oldani S, Fedeli C et al. (2015). Neurological counterparts of hyponatremia: pathological mechanisms and clinical manifestations. Curr.Neurol.Neurosci.Rep, 15, 18. [DOI] [PubMed] [Google Scholar]
- Osei-Owusu J, Yang J, Vitery MDC, & Qiu Z (2018). Molecular biology and physiology of volume-regulated anion channel (VRAC). Current Topics in Membranes, 81, 177–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Z, Dubin AE, Mathur J, Tu B, Reddy K, Miraglia LJ et al. (2014). SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell, 157, 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Mandujano G, Vazquez-Juarez E, Hernandez-Benitez R, & Pasantes-Morales H (2007). Thrombin potently enhances swelling-sensitive glutamate efflux from cultured astrocytes. Glia, 55, 917–925. [DOI] [PubMed] [Google Scholar]
- Risher WC, Andrew RD, & Kirov SA (2009). Real-time passive volume responses of astrocytes to acute osmotic and ischemic stress in cortical slices and in vivo revealed by two-photon microscopy. Glia, 57, 207–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risher WC, Croom D, & Kirov SA (2012). Persistent astroglial swelling accompanies rapid reversible dendritic injury during stroke-induced spreading depolarizations. Glia, 60, 1709–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Oshima T, & Attwell D (2000). Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature, 403, 316–321. [DOI] [PubMed] [Google Scholar]
- Rosso L, Peteri-Brunback B, Poujeol P, Hussy N, & Mienville JM (2004). Vasopressin-induced taurine efflux from rat pituicytes: a potential negative feedback for hormone secretion. J.Physiol, 554, 731–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy G (1995). Amino-acid current through anion channels in cultured human glial-cells. J.Membr.Biol, 147, 35–44. [DOI] [PubMed] [Google Scholar]
- Rudkouskaya A, Chernoguz A, Haskew-Layton RE, & Mongin AA (2008). Two conventional protein kinase C isoforms, alpha and betaI, are involved in the ATP-induced activation of volume-regulated anion channel and glutamate release in cultured astrocytes. J.Neurochem, 105, 2260–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JM (2000). Sodium-potassium-chloride cotransport. Physiol Rev., 80, 211–276. [DOI] [PubMed] [Google Scholar]
- Rutledge EM, Aschner M, & Kimelberg HK (1998). Pharmacological characterization of swelling-induced D-[3H]aspartate release from primary astrocyte cultures. Am.J.Physiol, 274, C1511–C1520. [DOI] [PubMed] [Google Scholar]
- Rutledge EM & Kimelberg HK (1996). Release of [3H]-D-aspartate from primary astrocyte cultures in response to raised external potassium. J.Neurosci, 16, 7803–7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge EM, Mongin AA, & Kimelberg HK (1999). Intracellular ATP depletion inhibits swelling-induced D-[3H]aspartate release from primary astrocyte cultures. Brain Res., 842, 39–45. [DOI] [PubMed] [Google Scholar]
- Sanchez-Olea R, Moran J, Martinez A, & Pasantes-Morales H (1993). Volume-activated Rb+ transport in astrocytes in culture. Am.J.Physiol, 264, C836–C842. [DOI] [PubMed] [Google Scholar]
- Sanchez-Olea R, Pena C, Moran J, & Pasantes-Morales H (1993). Inhibition of volume regulation and efflux of osmoregulatory amino acids by blockers of Cl- transport in cultured astrocytes. Neurosci.Lett, 156, 141–144. [DOI] [PubMed] [Google Scholar]
- Schneider GH, Baethmann A, & Kempski O (1992). Mechanisms of glial swelling induced by glutamate. Can.J.Physiol Pharmacol, 70 Suppl, S334–S343. [DOI] [PubMed] [Google Scholar]
- Schober AL & Mongin AA (2015). Intracellular levels of glutamate in swollen astrocytes are preserved via neurotransmitter reuptake and de novo synthesis: implications for hyponatremia. J.Neurochem, 135, 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober AL, Wilson CS, & Mongin AA (2017). Molecular composition and heterogeneity of the LRRC8-containing swelling-activated osmolyte channels in primary rat astrocytes. J.Physiol, 595, 6939–6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomberg SL, Bauer J, Kintner DB, Su G, Flemmer A, Forbush B et al. (2003). Cross talk between the GABA(A) receptor and the Na-K-Cl cotransporter is mediated by intracellular Cl-. J.Neurophysiol, 89, 159–167. [DOI] [PubMed] [Google Scholar]
- Seki Y, Feustel PJ, Keller RW Jr., Tranmer BI, & Kimelberg HK (1999). Inhibition of ischemia-induced glutamate release in rat striatum by dihydrokinate and an anion channel blocker. Stroke, 30, 433–440. [DOI] [PubMed] [Google Scholar]
- Shennan DB (2008). Swelling-induced taurine transport: relationship with chloride channels, anion-exchangers and other swelling-activated transport pathways. Cell Physiol Biochem., 21, 15–28. [DOI] [PubMed] [Google Scholar]
- Shennan DB, McNeillie SA, & Curran DE (1994). The effect of a hyposmotic shock on amino acid efflux from lactating rat mammary tissue: stimulation of taurine and glycine efflux via a pathway distinct from anion exchange and volume-activated anion channels. Exp.Physiol, 79, 797–808. [DOI] [PubMed] [Google Scholar]
- Sheth KN, Elm JJ, Molyneaux BJ, Hinson H, Beslow LA, Sze GK et al. (2016). Safety and efficacy of intravenous glyburide on brain swelling after large hemispheric infarction (GAMES-RP): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol., 15, 1160–1169. [DOI] [PubMed] [Google Scholar]
- Shrode LD, Klein JD, O’Neill WC, & Putnam RW (1995). Shrinkage-induced activation of Na+/H+ exchange in primary rat astrocytes: role of myosin light-chain kinase. Am.J.Physiol, 269, C257–C266. [DOI] [PubMed] [Google Scholar]
- Silver SM, Schroeder BM, Sterns RH, & Rojiani AM (2006). Myoinositol administration improves survival and reduces myelinolysis after rapid correction of chronic hyponatremia in rats. J.Neuropathol.Exp.Neurol, 65, 37–44. [DOI] [PubMed] [Google Scholar]
- Simard JM, Chen M, Tarasov KV, Bhatta S, Ivanova S, Melnitchenko L et al. (2006). Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat.Med, 12, 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Tsymbalyuk N, Tsymbalyuk O, Ivanova S, Yurovsky V, & Gerzanich V (2010). Glibenclamide is superior to decompressive craniectomy in a rat model of malignant stroke. Stroke, 41, 531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slais K, Vorisek I, Zoremba N, Homola A, Dmytrenko L, & Sykova E (2008). Brain metabolism and diffusion in the rat cerebral cortex during pilocarpine-induced status epilepticus. Exp.Neurol, 209, 145–154. [DOI] [PubMed] [Google Scholar]
- Soe R, MacAulay N, & Klaerke DA (2009). Modulation of Kir4.1 and Kir4.1-Kir5.1 channels by small changes in cell volume. Neurosci.Lett, 457, 80–84. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV & Vinters HV (2010). Astrocytes: biology and pathology. Acta Neuropathol, 119, 7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solenov E, Watanabe H, Manley GT, & Verkman AS (2004). Sevenfold-reduced osmotic water permeability in primary astrocyte cultures from AQP-4-deficient mice, measured by a fluorescence quenching method. Am.J.Physiol.Cell Physiol, 286, C426–C432. [DOI] [PubMed] [Google Scholar]
- Somjen GG (2004). Ions in the Brain: Normal Functions, Seizures, and Stroke. New York: Oxford University Press. [Google Scholar]
- Somjen GG, Faas GC, Vreugdenhil M, & Wadman WJ (1993). Channel shutdown: a response of hippocampal neurons to adverse environments. Brain Res., 632, 180–194. [DOI] [PubMed] [Google Scholar]
- Song D, Xu J, Hertz L, & Peng L (2015). Regulatory volume increase in astrocytes exposed to hypertonic medium requires beta1 -adrenergic Na(+) /K(+) -ATPase stimulation and glycogenolysis. J.Neurosci.Res, 93, 130–139. [DOI] [PubMed] [Google Scholar]
- Sotak CH (2004). Nuclear magnetic resonance (NMR) measurement of the apparent diffusion coefficient (ADC) of tissue water and its relationship to cell volume changes in pathological states. Neurochem.Int, 45, 569–582. [DOI] [PubMed] [Google Scholar]
- Stauber T (2015). The volume-regulated anion channel is formed by LRRC8 heteromers - molecular identification and roles in membrane transport and physiology. Biol.Chem, 396, 975–990. [DOI] [PubMed] [Google Scholar]
- Steffensen AB, Sword J, Croom D, Kirov SA, & MacAulay N (2015). Chloride cotransporters as a molecular mechanism underlying spreading depolarization-induced dendritic beading. J.Neurosci, 35, 12172–12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterns RH (2015). Disorders of plasma sodium--causes, consequences, and correction. N.Engl.J.Med, 372, 55–65. [DOI] [PubMed] [Google Scholar]
- Sterns RH, Baer J, Ebersol S, Thomas D, Lohr JW, & Kamm DE (1993). Organic osmolytes in acute hyponatremia. Am.J.Physiol, 264, F833–F836. [DOI] [PubMed] [Google Scholar]
- Sterns RH & Silver SM (2006). Brain volume regulation in response to hypo-osmolality and its correction. Am.J.Med, 119, S12–S16. [DOI] [PubMed] [Google Scholar]
- Stokum JA, Gerzanich V, & Simard JM (2016). Molecular pathophysiology of cerebral edema. J.Cereb.Blood Flow Metab, 36, 513–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokum JA, Kwon MS, Woo SK, Tsymbalyuk O, Vennekens R, Gerzanich V et al. (2018). SUR1-TRPM4 and AQP4 form a heteromultimeric complex that amplifies ion/water osmotic coupling and drives astrocyte swelling. Glia, 66, 108–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange K (1992). Regulation of solute and water balance and cell volume in the central nervous system. J.Am.Soc.Nephrol, 3, 12–27. [DOI] [PubMed] [Google Scholar]
- Strange K, Emma F, & Jackson PS (1996). Cellular and molecular physiology of volume-sensitive anion channels. Am.J.Physiol, 270, C711–C730. [DOI] [PubMed] [Google Scholar]
- Strange K & Morrison R (1992). Volume regulation during recovery from chronic hypertonicity in brain glial cells. Am.J.Physiol, 263, C412–C419. [DOI] [PubMed] [Google Scholar]
- Strange K, Morrison R, Shrode L, & Putnam R (1993). Mechanism and regulation of swelling-activated inositol efflux in brain glial cells. Am.J.Physiol, 265, C244–C256. [DOI] [PubMed] [Google Scholar]
- Stutzin A, Torres R, Oporto M, Pacheco P, Eguiguren AL, Cid LP et al. (1999). Separate taurine and chloride efflux pathways activated during regulatory volume decrease. Am.J.Physiol, 277, C392–C402. [DOI] [PubMed] [Google Scholar]
- Su G, Kintner DB, Flagella M, Shull GE, & Sun D (2002). Astrocytes from Na(+)-K(+)-Cl(−) cotransporter-null mice exhibit absence of swelling and decrease in EAA release. Am.J.Physiol.Cell Physiol, 282, C1147–C1160. [DOI] [PubMed] [Google Scholar]
- Su G, Kintner DB, & Sun D (2002). Contribution of Na(+)-K(+)-Cl(−) cotransporter to high-[K(+)](o)- induced swelling and EAA release in astrocytes. Am.J.Physiol Cell Physiol, 282, C1136–C1146. [DOI] [PubMed] [Google Scholar]
- Svoboda J & Sykova E (1991). Extracellular space volume changes in the rat spinal cord produced by nerve stimulation and peripheral injury. Brain Res., 560, 216–224. [DOI] [PubMed] [Google Scholar]
- Swain M, Butterworth RF, & Blei AT (1992). Ammonia and related amino acids in the pathogenesis of brain edema in acute ischemic liver failure in rats. Hepatology, 15, 449–453. [DOI] [PubMed] [Google Scholar]
- Sykova E (2004). Extrasynaptic volume transmission and diffusion parameters of the extracellular space. Neuroscience, 129, 861–876. [DOI] [PubMed] [Google Scholar]
- Sykova E & Nicholson C (2008). Diffusion in brain extracellular space. Physiol Rev., 88, 1277–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatkowski M, Barbour B, & Attwell D (1990). Non-vesicular release of glutamate from glial cells by reversed electrogenic glutamate uptake. Nature, 348, 443–446. [DOI] [PubMed] [Google Scholar]
- Takano T, Kang J, Jaiswal JK, Simon SM, Lin JH, Yu Y et al. (2005). Receptor-mediated glutamate release from volume sensitive channels in astrocytes. Proc.Natl.Acad.Sci.U.S.A, 102, 16466–16471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanigami H, Rebel A, Martin LJ, Chen TY, Brusilow SW, Traystman RJ et al. (2005). Effect of glutamine synthetase inhibition on astrocyte swelling and altered astroglial protein expression during hyperammonemia in rats. Neuroscience, 131, 437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terker AS, Zhang C, Erspamer KJ, Gamba G, Yang CL, & Ellison DH (2016). Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int., 89, 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijs VN, Adami A, Neumann-Haefelin T, Moseley ME, & Albers GW (2002). Clinical and radiological correlates of reduced cerebral blood flow measured using magnetic resonance imaging. Arch.Neurol, 59, 233–238. [DOI] [PubMed] [Google Scholar]
- Thrane AS, Rangroo Thrane V, & Nedergaard M (2014). Drowning stars: reassessing the role of astrocytes in brain edema. Trends Neurosci., 37, 620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman DJ, Beghi E, Begley CE, Berg AT, Buchhalter JR, Ding D et al. (2011). Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia, 52 Suppl 7, 2–26. [DOI] [PubMed] [Google Scholar]
- Thurston JH & Hauhart RE (1987). Brain amino acids decrease in chronic hyponatremia and rapid correction causes brain dehydration: possible clinical significance. Life Sci., 40, 2539–2542. [DOI] [PubMed] [Google Scholar]
- Tofteng F, Hauerberg J, Hansen BA, Pedersen CB, Jorgensen L, & Larsen FS (2006). Persistent arterial hyperammonemia increases the concentration of glutamine and alanine in the brain and correlates with intracranial pressure in patients with fulminant hepatic failure. J.Cereb.Blood Flow Metab, 26, 21–27. [DOI] [PubMed] [Google Scholar]
- Traynelis SF & Dingledine R (1989). Role of extracellular space in hyperosmotic suppression of potassium-induced electrographic seizures. J.Neurophysiol, 61, 927–938. [DOI] [PubMed] [Google Scholar]
- Tuz K & Pasantes-Morales H (2005). Hyposmolarity evokes norepinephrine efflux from synaptosomes by a depolarization- and Ca2+ -dependent exocytotic mechanism. Eur.J.Neurosci, 22, 1636–1642. [DOI] [PubMed] [Google Scholar]
- Tuz K, Pena-Segura C, Franco R, & Pasantes-Morales H (2004). Depolarization, exocytosis and amino acid release evoked by hyposmolarity from cortical synaptosomes. Eur.J.Neurosci, 19, 916–924. [DOI] [PubMed] [Google Scholar]
- Upadhyay A, Jaber BL, & Madias NE (2006). Incidence and prevalence of hyponatremia. Am.J.Med, 119, S30–S35. [DOI] [PubMed] [Google Scholar]
- Van Harreveld A (1972). The extracellular space in the vertebrate central nervous system In Bourne GH (Ed.), The Structure and Function of Nervous System (pp. 449–512). Academic Press. [Google Scholar]
- Van Harreveld A, Dafny N, & Khattab FI (1971). Effects of calcium on the electrical resistance and the extracellular space of cerebral cortex. Exp.Neurol, 31, 358–367. [DOI] [PubMed] [Google Scholar]
- Verbalis JG & Gullans SR (1991). Hyponatremia causes large sustained reductions in brain content of multiple organic osmolytes in rats. Brain Res., 567, 274–282. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A & Nedergaard M (2018). Physiology of astroglia. Physiol Rev., 98, 239–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkman AS, Binder DK, Bloch O, Auguste K, & Papadopoulos MC (2006). Three distinct roles of aquaporin-4 in brain function revealed by knockout mice. Biochim.Biophys.Acta, 1758, 1085–1093. [DOI] [PubMed] [Google Scholar]
- Vitarella D, DiRisio DJ, Kimelberg HK, & Aschner M (1994). Potassium and taurine release are highly correlated with regulatory volume decrease in neonatal primary rat astrocyte cultures. J.Neurochem, 63, 1143–1149. [DOI] [PubMed] [Google Scholar]
- Voisin DL, Chakfe Y, & Bourque CW (1999). Coincident detection of CSF Na+ and osmotic pressure in osmoregulatory neurons of the supraoptic nucleus. Neuron, 24, 453–460. [DOI] [PubMed] [Google Scholar]
- Voss FK, Ullrich F, Munch J, Lazarow K, Lutter D, Mah N et al. (2014). Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science, 344, 634–638. [DOI] [PubMed] [Google Scholar]
- Wade JV, Olson JP, Samson FE, Nelson SR, & Pazdernik TL (1988). A possible role for taurine in osmoregulation within the brain. J.Neurochem, 51, 740–745. [DOI] [PubMed] [Google Scholar]
- Wang R, Lu Y, Gunasekar S, Zhang Y, Benson CJ, Chapleau MW et al. (2017). The volume-regulated anion channel (LRRC8) in nodose neurons is sensitive to acidic pH. JCI Insight, 2, e90632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waseem TV, Rakovich AA, Lavrukevich TV, Konev SV, & Fedorovich SV (2005). Calcium regulates the mode of exocytosis induced by hypotonic shock in isolated neuronal presynaptic endings. Neurochem.Int, 46, 235–242. [DOI] [PubMed] [Google Scholar]
- Wasterlain CG & Torack RM (1968). Cerebral edema in water intoxication. II. An ultrastructural study. Arch.Neurol, 19, 79–87. [DOI] [PubMed] [Google Scholar]
- Wehner F, Bondarava M, ter VF, Endl E, Nurnberger HR, & Li T (2006). Hypertonicity-induced cation channels. Acta Physiol (Oxf), 187, 21–25. [DOI] [PubMed] [Google Scholar]
- Wehner F, Olsen H, Tinel H, Kinne-Saffran E, & Kinne RKH (2003). Cell volume regulation: osmolytes, osmolyte transport, and signal transduction. Rev.Physiol.Biochem.Pharmacol, 148, 1–80. [DOI] [PubMed] [Google Scholar]
- Wehner F, Shimizu T, Sabirov R, & Okada Y (2003). Hypertonic activation of a non-selective cation conductance in HeLa cells and its contribution to cell volume regulation. FEBS Lett., 551, 20–24. [DOI] [PubMed] [Google Scholar]
- Willford SL, Anderson CM, Spencer SR, & Eskandari S (2015). Evidence for a revised ion/substrate coupling stoichiometry of GABA transporters. J.Membr.Biol, 248, 795–810. [DOI] [PubMed] [Google Scholar]
- Wilson CS & Mongin AA (2019). The signaling role for chloride in the bidirectional communication between neurons and astrocytes. Neurosci.Lett, 689, 33–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TH (1954). Ionic permeability and osmotic swelling of cells. Science, 120, 104–105. [DOI] [PubMed] [Google Scholar]
- Woo NS, Lu J, England R, McClellan R, Dufour S, Mount DB et al. (2002). Hyperexcitability and epilepsy associated with disruption of the mouse neuronal-specific K-Cl cotransporter gene. Hippocampus, 12, 258–268. [DOI] [PubMed] [Google Scholar]
- Woo SK, Kwon MS, Ivanov A, Gerzanich V, & Simard JM (2013). The sulfonylurea receptor 1 (Sur1)-transient receptor potential melastatin 4 (Trpm4) channel. J.Biol.Chem, 288, 3655–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Dempsey RJ, & Sun D (2001). Na+-K+-Cl- cotransporter in rat focal cerebral ischemia. J.Cereb.Blood Flow Metab, 21, 711–721. [DOI] [PubMed] [Google Scholar]
- Yannet H (1940). Changes in the brain resulting from depletion of extracellular electrolytes. Am.J.Physiol, 128, 683–689. [Google Scholar]
- Ye ZC, Oberheim N, Kettenmann H, & Ransom BR (2009). Pharmacological “cross-inhibition” of connexin hemichannels and swelling activated anion channels. Glia, 57, 258–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZC, Wyeth MS, Baltan-Tekkok S, & Ransom BR (2003). Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J.Neurosci, 23, 3588–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuthen T & MacAulay N (2002). Cotransporters as molecular water pumps. Int.Rev.Cytol, 215, 259–284. [DOI] [PubMed] [Google Scholar]
- Zhang H & Verkman AS (2008). Aquaporin-4 independent Kir4.1 K+ channel function in brain glial cells. Mol.Cell Neurosci, 37, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S et al. (2014). An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J.Neurosci, 34, 11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang H, Feustel PJ, & Kimelberg HK (2008). DCPIB, a specific inhibitor of volume regulated anion channels (VRACs), reduces infarct size in MCAo and the release of glutamate in the ischemic cortical penumbra. Exp.Neurol, 210, 514–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z & Bourque CW (2003). Osmometry in osmosensory neurons. Nat.Neurosci, 6, 1021–1022. [DOI] [PubMed] [Google Scholar]
- Zhou Y & Danbolt NC (2013). GABA and Glutamate Transporters in Brain. Front Endocrinol. (Lausanne), 4, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]