Abstract
Objective:
To explore the effect of goal-setting on physical functioning, quality of life and duration of rehabilitation in geriatric rehabilitation compared to care as usual.
Data sources:
Medline, Embase, CINAHL, PsycINFO and the Cochrane Library were searched from initiation to October 2018.
Methods:
We included randomized controlled trials (RCTs), controlled before–after studies and studies using historic controls of older patients (mean age ⩾55 years) receiving rehabilitation for acquired disabilities. Our primary outcome was physical functioning; secondary outcomes were quality of life and rehabilitation duration. Cochrane guidelines were used to assess the risk of bias of the studies and extract data. Only RCT data were pooled using standardized mean difference (SMD).
Results:
We included 14 studies consisting of a total of 1915 participants with a mean age ranging from 55 to 83 years. Ten out of the 14 studies had a randomized controlled design, 7 of which could be pooled for the primary outcome. The risk of bias was judged high in several domains in all included studies. The meta-analysis showed no statistically significant differences between goal-setting and care as usual for physical functioning (SMD −0.11 (−0.32 to 0.10)), quality of life (SMD 0.09 (−0.56 to 0.75)) and rehabilitation duration (MD 13.46 days (−2.46 to 29.38)).
Conclusion:
We found low-quality evidence that goal-setting does not result in better physical functioning compared to care as usual in geriatric rehabilitation. For quality of life and duration of rehabilitation, we could not exclude a clinically relevant effect.
Keywords: Patient-centred care, geriatric rehabilitation, goal-setting, shared decision-making
Introduction
Goal-setting is regarded as an essential part of rehabilitation.1 It has been defined as the establishment or negotiation of rehabilitation goals and refers to the intended future state of the patient, which will usually involve a change from the current situation.1,2 In 2015, a Cochrane review of randomized controlled trials (RCTs) concluded that goal-setting did not result in higher levels of physical functioning, although there was evidence that goal-setting can result in higher levels of self-efficacy and health-related quality of life in adult rehabilitation patients.1 Because of the limited quality of the 39 included studies, the authors concluded that there is only very low-quality evidence for the beneficial effects of goal-setting for adult rehabilitation patients.
Although this review included a few studies which were conducted in older patients, it did not specifically study the effects of goal-setting in geriatric rehabilitation. Geriatric rehabilitation can be defined and characterized as multidisciplinary treatment to improve independent functioning aimed at older patients who are often frail and have several comorbidities, including cognitive dysfunction and communication problems.3,4 This means that there are both practical and theoretical differences between geriatric and adult rehabilitation which might lead to a different goal-setting process and effect.
This is in accordance with earlier research, which found that this heterogeneous group of older patients with various degrees of frailty find it hard to shape and discuss their personal rehabilitation programme and need guidance in defining their rehabilitation goals.5,6 Furthermore, a systematic review identified several barriers for patient-centred goal-setting, which especially apply to this patient group. It showed that clinicians have difficulty and reservations about involving patients in goal-setting who have problems with communication and cognition.7 In conclusion, there is evidence that the goal-setting process in geriatric rehabilitation is different than that of adult rehabilitation and its effect might therefore be different as well.
The purpose of this review was to systematically identify, critically appraise and synthesize the available evidence on the effects of goal-setting in geriatric rehabilitation. To this end, we conducted a systematic review and meta-analysis to assess the effectiveness of goal-setting versus care as usual on physical functioning, quality of life and duration of rehabilitation of older rehabilitation patients with acquired disabilities.
Methods
A systematic review and meta-analysis was carried out in three stages following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines8: (1) literature search; (2) data extraction and critical appraisal; (3) data synthesis. A review protocol was created before the start of the study. There was one deviation. Originally, we planned to only include studies of inpatient geriatric rehabilitation patients. Because we ended up with a limited number of studies, we decided to also include studies with participants from outpatient settings and combined inpatient and outpatient settings.
Literature search
The primary author conducted a systematic computerized search to identify studies on 15 October 2018. Five electronic databases were searched: Medline, Embase, CINAHL, PsycINFO and the Cochrane Library. The search was not limited by any time restrictions or language (if necessary, a translation service would be used). Search terms were used relating to the following themes: rehabilitation, goal-setting and goal-setting instruments. Rehabilitation was used as a solitary search term and several search term were used to capture the theme goal-setting, like ‘goal-setting’, ‘goal pursuit’ and ‘goal achievement’. In addition, several goal-setting instruments (i.e. ‘Canadian Occupational Performance Measure’ and ‘Talking Mats’) were also used as individual search terms, to make sure studies using these instruments as goal-setting method would be included in our search results. Specific goal-setting instruments which were included in the search were adopted from an earlier review.9 Finally, the reference lists of included articles were scrutinized for other potentially relevant articles. The search terms and strategy for Medline is provided in Supplemental Appendix 1; for the other databases, we adapted the search strings accordingly.
Trials had to report on geriatric rehabilitation to be included in the review, which was defined as a group of rehabilitation patients with an average age of 55 years or older.3 Based on previous reviews, we expected a low number of RCTs that would probably result in too few studies to draw meaningful conclusions; hence, we decided to also include non-randomized studies. Results of the NRSIs will not be included in the meta-analysis but can provide evidence additional to that available from randomized trials.
We included studies that met all of the following criteria: (1) (quasi- or cluster) randomized controlled trials, non-randomized controlled trials, controlled before–after studies or studies using historic controls; (2) people receiving rehabilitation for disabilities acquired in adulthood; (3) studies involving any type of goal-setting versus care as usual. Studies were excluded based on the following criteria: (1) mean age of the study population under 55 years; (2) studies without data on physical functioning and/or recovery; (3) studies dealing solely with cognitive or psychiatric rehabilitation; (4) mixed or combined intervention studies, that is, when goal-setting was part of a larger intervention.
A full list of articles was composed combining the search results of all five databases and removing duplicates. Two reviewers (E.B.S., H.B.) independently screened titles and abstracts of the full list and agreement had to be reached before the article was subjected to a full-text assessment. In case, an article was only selected by one reviewer a discussion took place between the two reviewers to determine whether the study should be selected for a full-text analysis. A third reviewer (J.vd.W) could be consulted in case that the two reviewers could not reach consensus on inclusion. Next, both reviewers independently assessed the full text of the selected articles. Studies were included in a similar fashion. Our primary outcome was mobility and activities of daily living and the secondary outcomes were quality of life and duration of the rehabilitation.
Data extraction and critical appraisal
The two reviewers independently assessed the study quality and extracted the data from each included study. The results of the quality assessment and data extraction were compared and discrepancies were resolved through discussion. Data were extracted using a standard data extraction form adapted from the Cochrane Consumers and Communication Review Group’s Data Extraction Template and were entered into Covidence (www.covidence.com), a web-based software platform for the production of systematic reviews. The following study characteristics were extracted: study design, patient characteristics, sample size, goal-setting method, functional outcomes and secondary study outcomes. The methodological quality of the individual studies was assessed in accordance with Cochrane guidelines focussing on the following criteria: sequence generation, allocation concealment, blinding of participants and personnel, incomplete data, selective reporting and other sources of bias.10 Thus, we used one tool to assess risk of bias in order to enhance comparability of the risk of bias assessments between the different types of studies. The risk of bias was rated as high, low or unclear.10 The extracted data were entered into Review Manager11 version 5.3 by the primary author; accuracy of the data entry was checked by a second reviewer (H.B.).
Data synthesis
Data synthesis started off by summarizing all available data in order to determine whether statistical pooling of the data was suitable by comparing participants, goal-setting method and outcome measures. For the meta-analysis, we only included studies that randomized individuals, studies using a quasi-randomized design and cluster-randomized studies. We used a mean difference for pooling in cases of similar unit of measurement; otherwise, a standardized mean difference (SMD) was calculated for each study.10 Consequently, we could only include those studies which reported a mean outcome value in the meta-analysis. If a study did not report a standard deviation (SD), we replaced it with the SD of a comparable study which used the same measurement and metric in case that the original authors of the study could not provide it. When a study applied multiple instruments to assess the same outcome, the most appropriate measurement instrument was selected. In addition, when outcomes were assessed at multiple points in time, we preferably used the score at discharge from the intervention; when not available, we used the score obtained at the first follow-up time with a minimum of two weeks. For the cluster-randomized study that did not take the design into account in the analysis,12 we adapted the study size by adjusting for the design effect,10,13 using an intraclass cluster coefficient of 0.08.14 Finally, apart from selecting randomized controlled trials, we did not take additional risks of bias of individual studies into account when excluding studies for pooling.
We used a random effects model to pool the data from all the available studies either with a mean difference or with a SMD.15 Heterogeneity between studies was assessed first by visual inspection of the forest plot. Next, we computed the Q-statistic and I2. Substantial statistical heterogeneity was assumed if the Q-statistic was significant (P < 0.05) and the I2 value was more than 50%.15,16
Results
Study selection
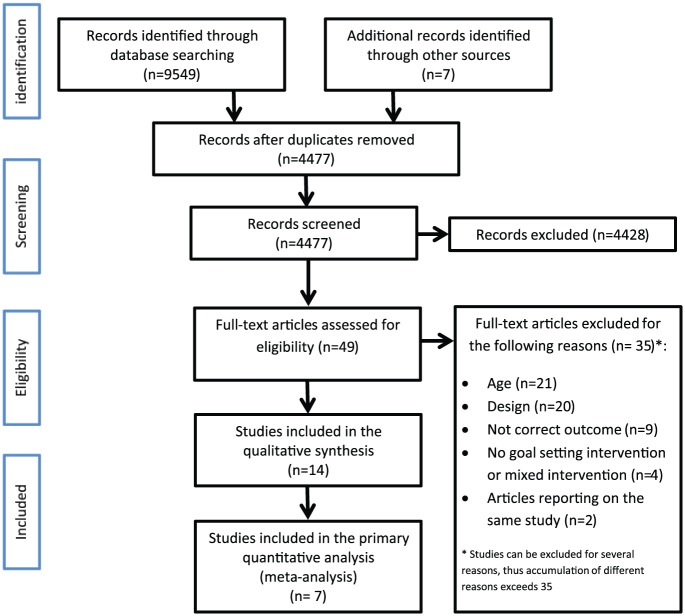
The PRISMA flowchart of the entire search and selection procedure is shown in Figure 1. In summary, 14 out of the 3851 articles met the inclusion criteria and were included in the systematic review; seven of these could be included in the meta-analysis for the primary outcome. Reasons for exclusion in the full-text assessment phase were incorrect age group, no experimental design, not reporting our primary outcome, no goal-setting intervention or a mixed intervention, and finally, we excluded articles containing duplicate outcomes of the same study patients. Three articles reported data from the same study: Guidetti et al.12 and Bertilsson et al.17,18 We only used Guidetti et al.,12 because it reported the most accurate data at three months of follow-up of all the participants.
Figure 1.
PRISMA flowchart.
Study characteristics
A total of 14 studies met the selection criteria for the current review12,19–31; the summary of the study characteristics can be found in Table 1. The mean age ranged from 55 to 93 years and the patients were admitted for various reasons. One study reported cognitive dysfunction in 26.6% of the participants19; four studies reported an average score on the Mini-Mental State Examination with average scores ranging from 23 to 27.6.12,20,23,27 Finally,three of the included studies in the systematic review reported data on the proportion of patients having at least one comorbid condition ranging from 4.5% to 68.5%.12,19,24
Table 1.
Study characteristics.
| First author (year of publication) | Rehabilitation setting | Population (mean age; n randomized intervention/control) | Design | Intervention | Relevant outcome measures | Analysis | Findings | Considerations |
|---|---|---|---|---|---|---|---|---|
| Beckerman et al.19 (2004) | Rehabilitation centre (inpatient) | 651 rehabilitation patients admitted for the following diagnosis: amputation of a lower limb, spinal cord injury, Guillain–Barré syndrome, postpoliomyelitis syndrome (mean age 56.0; 214/437) | Non-randomized trial in three prospective cohorts: RAP (n = 214), partial RAP (=282) and care as usual group (=437) | Rehabilitation Activities Profile (RAP). An interdisciplinary goal-setting and evaluation tool, which offers the team a structured method to goal-setting | Barthel Index Duration of rehabilitation |
Descriptive statistics at discharge Multilevel logistic regression analysis for duration of rehabilitation, linear multilevel regression analysis for Barthel scores at discharge |
Intervention group (RAP) had lower Barthel scores compared
to control (−3.7%, 95% confidence interval (CI): −7.0 to
−0.3). No significant effect on duration of rehabilitation (odds ratio RAP versus control: 1.98, 95% CI 0.59 to 6.67) |
Not included in the meta-analysis because of the
design Data of partial RAP group not included in review |
| Colquhoun et al.20 (2010) | Rehabilitation unit (inpatient) | 103 geriatric rehabilitation patients admitted for longer than two weeks (mean age 81.6; 45/58) | Historical controlled study | Canadian Occupational Performance Measure (COPM) | Functional Independence Measure Duration of rehabilitation |
Descriptive statistics at discharge. Independent sample t-test and generalized linear modelling | No significant difference for any outcome | Not included in the meta-analysis because of the design |
| Duncan and Pozehl21 (2003) | Cardiac rehabilitation facility (outpatient) | 15 heart failure patients who had permission from their attending cardiologist and were able to attend week exercise programme three times a week (mean age 66.4; 8/7) | Randomized controlled trial | Goal-setting with visual feedback and problem-solving support | 6-minute Walk Test Minnesota Living With Heart Failure (quality of life) |
Descriptive statistics at 12 and 24 weeks after the start of the study. Two-tailed, independent t-tests. | No statistically significant differences | The meta-analysis included data from 12 weeks follow-up |
| Gagné and Hoppes22 (2003) | Rehabilitation hospital (inpatient) | 31 rehabilitation patients admitted longer than two weeks and a FIM score <26 (no mean age reported, range: 56–93; 15/16) | Randomized controlled trial | Structured goal-setting by daily discussion of goal notebook by means of a standard format in describing rehabilitation goals | Functional Independence Measure | Descriptive statistics two weeks after the start of the study. Mann–Whitney U test | The intervention group had a higher FIM sub score for upper-body dressing (P = 0.019). No other significant differences | Not included in meta-analysis because total FIM score lacking |
| Guidetti et al.23 (2010) | 3 Rehabilitation clinics (inpatient) | 40 stroke rehabilitation patients (mean age 67.6; 19/21) | Randomized controlled trial | Client-Centred Self Care Intervention (CCSCI); a nine-step programme | Barthel Index Functional Independence Measure Duration of rehabilitation |
Descriptive statistics at three months of follow-up. T-test for independent continuous samples data. Wilcoxon matched pairs test for within groups analysis | There were no significant differences for any outcome | Not included in the primary meta-analysis because functional outcomes were reported in medians instead of means |
| Guidetti et al.12 (2015) | 16 Rehabilitation units (inpatient) | 280 stroke rehabilitation patients (mean age 72.4; 129/151) | Cluster-randomized controlled trial | Client-centred activities of daily living intervention (CADL). A nine-step structured goal-setting method | Barthel Index | Descriptive statistics at 3, 6 and 12 months. Linear mixed-effects models were used for continuous outcomes | There were no significant differences between CADL and care as usual | The meta-analysis included data from three months
follow-up A design effect correction was calculated for inclusion in the meta-analyse |
| Harwood et al.24 (2011) | Community stroke rehabilitation (outpatient) | 172 stroke patients recruited 6 to 12 weeks after stroke and living in the community (mean age 61.4; 46/39) | Randomized controlled trial | Take Charge Session (TCS) a structured individual assessment to facilitate self-directed rehabilitation (n = 46), ‘Inspirational’ DVD (iDVD) (n = 48), combination of TCS and iDVD (n = 39) | Barthel Index Physical Component Summary Score |
Descriptive statistics at 12 months of
follow-up Analysis of variance (ANOVA) for continuous outcome and logistic regression with odds ratio for categorical outcome. Kruskal–Wallis test for Barthel Index |
TCS had higher PCS scores compared to control (6.0; 95% CI: 2.0–10.0, P = 0.004). No other significant differences | The meta-analysis only included the TCS (goal-setting intervention) compared to the control group |
| O’Brien et al.25 (2013) | Physiotherapy clinic (outpatient) | 27 patients with hip and/or knee osteoarthritis (mean age 63.4; 17/10) | Randomized controlled trial | Goal-setting by means of completing action and coping planning under guidance of a research assistant | 6-minute Walk Test | Descriptive statistics at discharge using ANOVA | There were no significant differences for any outcome | |
| Oestergaard et al.26 (2012) | Rehabilitation at the hospital (inpatient) | 87 patients with degenerative disc disease who had undergone lumbar fusion surgery (mean age 55; 40/47) | Randomized controlled trial | Canadian Occupational Performance measure (COPM) | Activities of daily living (ADL) performance
questionnaire Duration of rehabilitation |
Descriptive statistics at one week, 1 and three months and three years after the start of the study. The chi-square or Fisher’s exact test were used for comparison of proportions | No significant difference for any outcome | Not included in the meta-analysis because functional outcomes were reported in median instead of means |
| Ogawa et al.27 (2016) | Rehabilitation at the hospital (inpatient) | 44 patients with disabling diseases (mean age 78.6; 22/22) | Quasi-randomized controlled trial | Goal Attainment Scaling (GAS) | Functional Independence Measure | Descriptive statistics at four weeks. Analysis of variance was used for continuous data | No significant difference in change score of the Functional Independence Measure between the two groups | In the systematic review and meta-analysis we only included control group I (real control) and control group II (goal-setting). Intervention group was excluded |
| Taylor et al.28 (2011) | 4 inpatient rehabilitation services (inpatient) | 41 stroke patients (mean age 61.3; 18/23) | Cluster-randomized controlled trial | Canadian Occupational Performance (COPM) | Functional Independence Measure SF-36 (quality of life) Duration of rehabilitation |
Descriptive statistics at 12 weeks after start of the study
and at discharge No statistical test used for comparing outcome data |
A statistically significant longer duration of rehabilitation in the intervention group | |
| Tomori et al.29 (2015) | 10 sub-acute rehabilitation units (inpatient) | 54 patients with disabling diseases (mean age 66.2; 27/27) | Randomized controlled trial | Aid for Decision-making in Occupation Choice (ADOC) | Functional Independence Measure SF-36 (quality of life) Duration of rehabilitation |
Descriptive statistics at two months. Two-tailed independent t-test for between-group comparison | There were no significant differences in any post-intervention outcomes between the groups | |
| Verhoef et al.30 (2007) | Rheumatology rehabilitation clinic (inpatient and outpatient) | 165 rheumatology patients (median age 61a, 85/80) | Controlled before-and-after study in a prospective cohort study with a pre-post-test non-equivalent design | Rehabilitation Activities Profile (RAP). An interdisciplinary goal-setting tool, which offers the team a structured method to goal-setting | Health Assessment Questionnaire (functional
ability) Rheumatoid Arthritis Quality of Life questionnaire Duration of rehabilitation |
Descriptive statistics at discharge and six weeks after
discharge. Chi-square test, Fischer Exact Test, Mann–Whitney U test |
There were no significant differences for any outcome | Not included in the meta-analysis because of the design |
| Wressle et al.31 (2002) | 2 rehabilitation hospitals (inpatient and outpatient) | 206 rehabilitation patients (median age 79a, 151/55) | Non-randomized trial in a prospective cohort study | Canadian Occupational Performance (COPM) | Klein–Bell ADL Scale | Descriptive statistics at discharge Mann–Whitney U test, Wilcoxon signed-rank test |
Significant change in score on Klein–Bell scale in favour of control (P = 0.002) | Not included in the meta-analysis because functional outcomes were reported in median instead of means |
In case of absence of a total median age, the lowest age median was given of either the control or intervention group.
There were two distinct approaches to goal-setting in the included studies. Eight studies used a goal-setting instrument to set goals.19,20,26–31 These instruments were the Canadian Occupational Performance Measure (COPM), the Rehabilitation Activities Profile (RAP), the Goal Attainment Scaling (GAS) and the Aid for Decision-making in Occupation Choice (ADOC). The other six studies used a standardized approach to goal-setting with predefined intervention.12,21–25 These were the Client-Centred Self Care Intervention (CCSCI), the Client-centred Activities of Daily Living (CADL) and the Take Charge Session (TCS).
The study designs of the included studies were individually randomized controlled trials (RCTs) (n = 7), cluster RCTs (n = 2), non-randomized controlled trials (n = 2), quasi-RCT; controlled before-after study and historic control study (each n = 1).
Critical appraisal
A summary of the risk of bias assessment of the included studies is presented in Supplemental Table 1. The most frequent source of methodological bias was lack of blinding for the intervention, which was classified as high in all studies. In addition, ‘other sources of bias’ were classified as high in 12 of the 14 included studies. The main reason was the presence of baseline imbalances in patient characteristics between control group and intervention group, which was found in six studies.
Primary outcome
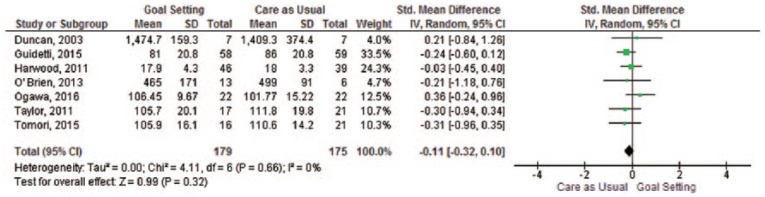
All of the 14 included studies (1915 participants) reported data on physical functioning at follow-up. The 14 studies in the systematic review showed mixed results, 11 found no differences between the intervention group and the control group on our primary outcome (Table 1). Two studies reported a statistically significant difference in favour of the control group19,31 and one study found a statistically significant higher level for the upper-body dressing subscale of the Functional Independent Measure in the goal-setting group.22 The meta-analysis included seven (n = 354 participants analysed) studies (Figure 2) showed no significant difference in physical functioning between goal-setting and care as usual (SMD −0.11, 95% confidence interval (CI) –0.32 to 0.10).
Figure 2.
Meta-analysis – physical functioning.
Secondary outcomes
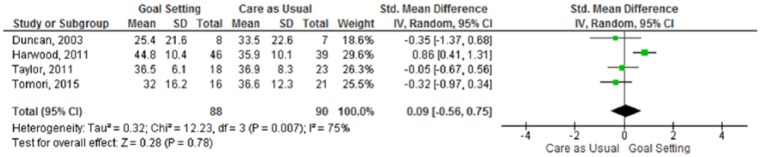
Four of the included studies reported data on quality of life and these studies (n = 178 participants analysed) could all be used for data pooling. Only one individual study reported a significant difference in quality of life between the two groups, in favour of the goal-setting intervention.24 The meta-analysis (Figure 3) showed no statistically significant difference in quality of life between goal-setting and care as usual (SMD 0.09, 95% CI −0.56 to 0.75). There was evidence of substantial heterogeneity between the studies.
Figure 3.
Meta-analysis – quality of life.
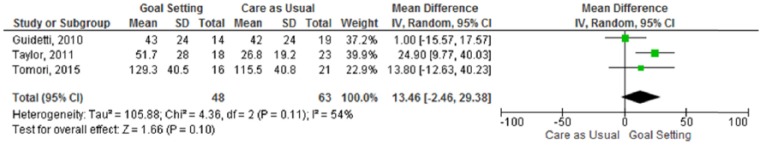
Data on duration of rehabilitation (days) were reported in four studies, one of which found a statistically significant difference: duration of the rehabilitation was significantly longer in the intervention group.28 We used the mean difference to pool all the data, because the unit of measurement was the same for all included studies. The meta-analysis (Figure 4) included three studies (n = 111 participants analysed) and showed a non-significant difference between goal-setting and care as usual for the duration of rehabilitation (MD 13.46 days, 95% CI −2.46 to 29.38).
Figure 4.
Meta-analysis – duration of rehabilitation.
Discussion
This systematic review and meta-analysis studied the effect of goal-setting on rehabilitation outcomes in older rehabilitation patients. The current meta-analysis did not show a statistically significant effect of goal-setting in geriatric rehabilitation for any of the primary and secondary outcomes. The power of our meta-analysis was sufficient to exclude a clinically relevant effect on our primary outcome, as the 95% confidence interval excluded a clinically relevant effect, that is, a SMD >0.5.32 In conclusion, our study found low-quality evidence that goal-setting does not have a relevant effect on physical functioning. For quality of life and duration of rehabilitation, the available studies could not exclude clinically relevant effects of goal-setting. The overall risk of bias of the included studies was judged to be considerable.
This review identified three studies with a positive outcome in favour of the control group and two studies in favour of the intervention group. There are some differences between these studies, which appear to be minor, like research design and goal-setting method. For example, all the studies favouring the control group used a specific goal-setting measurement instrument as a means to implement the intervention, namely the RAP or COPM, instead of only prescribing actions how to perform the intervention. Still, it is likely that this difference is due to chance since there are also two studies in the review which used the COPM and found no significant differences. The same goes for the custom approaches to goal-setting, two of these studies found statistical differences in favour of the intervention groups and the other four found no differences.
Similar to the Cochrane review, our study found that goal-setting does not lead to higher levels of physical functioning.1 Three studies from the Cochrane review were also included in the current review.21,24,25 In addition, we included four other and newer studies and found similar evidence that goal-setting does not yield better results than care as usual in terms of physical functioning.
Regarding quality of life, our results differ from those of the Cochrane review.1 Our study suggests that goal-setting does not result in higher levels of quality of life, although we could not to exclude a clinically relevant effect in either direction, as shown by the boundaries of the confidence interval (−0.56 to 0.75). Nonetheless, the Cochrane review found some evidence that goal-setting can lead to improved psychosocial outcomes like health-related quality of life in adult rehabilitation. Three studies from the Cochrane review were also included in our meta-analysis21,24,28 and one individually randomized RCT we included was not included in the Cochrane review.29 Pooling these four studies resulted in a non-significant effect; there was, however, considerable statistical heterogeneity between the studies.
Furthermore, our review differs in several ways from the Cochrane review which necessitates the use of an independent search and review.1 Most importantly, our review specifically studied the effect of goal-setting on older rehabilitation patients, whereas the Cochrane review included patients from the age of 18 years. Second, the Cochrane review included several psychosocial outcomes, whereas our review focused exclusively on quality of life as psychosocial outcome. In addition, our review also studied the effect of goal-setting on duration of rehabilitation, while the Cochrane review did not. Third, the Cochrane review included the study of Sewell et al.,33 while we excluded this study, because goal-setting was not compared to care as usual. Finally, as mentioned, our search was updated in 15 October 2018; the latest update search for the Cochrane review was in January 2014.
There are several potential explanations for not finding a significant result in this review. First, all 14 included studies lacked a process evaluation, including an assessment of adherence to protocol. Process evaluation is considered an essential part of designing and testing complex interventions.34,35 The absence of a proper process evaluation prohibits drawing conclusions on the extent and quality of the implementation and the level of protocol adherence of the goal-setting interventions in the included studies. And so it is not surprising that a significant effect cannot be demonstrated in a study in which the goal-setting intervention was implemented incorrectly or incompletely.
Second, goal-setting could already have been integrated in care as usual to some degree. A recent study which explored goal-setting during inpatient rehabilitation actually found that all participating rehabilitation units in their study conducted at least therapist-led goal-setting.36 In therapist-led goal-setting, it is the therapist who identifies the problems, defines rehabilitation goals and evaluates the process.36 At the same time, there is evidence that patients are not always involved in goal-setting, and that the goal-setting process itself is often incomplete.36,37 Goal-setting is not merely about establishing rehabilitation goals but also includes negotiation of goals, that is, involving the patient in defining and evaluating them. In short, there is some evidence that care as usual might not be an entirely true control group because to some extent goal-setting is already integrated in usual care. In other words, perhaps we were only able to study the additive effect of standardized goal-setting, that is, goal-setting by means of an instrument or a predefined approach, compared to non-standardized goal-setting in care as usual.
In conclusion, this study found low-quality evidence that goal-setting does not result in better physical functioning compared to care as usual in geriatric rehabilitation. In addition, we found low-quality evidence that goal-setting does not result in higher levels of quality of life and/or shortened duration of rehabilitation. However, because of the wide 95% confidence interval, we could not exclude a clinically relevant effect for these secondary outcomes.
The current review has several limitations. First, we used a basic operationalization to define geriatric rehabilitation patients, namely a group of rehabilitation patients with an average age of 55 years or older (cf. Bachmann et al.3). It should be noted that only a minority of the included studies reported data on the prevalence of comorbidity and cognitive functioning. Thus, the included studies contain a heterogeneous group of older patients of varying complexity. We still believe that this mix of the patients with varying comorbidity is an accurate reflection of the current practice of geriatric rehabilitation.3,4,38
Second, we included studies with a variety of approaches to goal-setting. Despite this heterogeneity, these studies, in our opinion, cover the broad spectrum of goal-setting.
Third, most of the studies lacked a clear description of what was considered usual care. A recent study showed that goal-setting in clinical practice is often therapist-led and does not include monitoring progress and revising goals with the patient.36 This makes it difficult to get an idea about the level of goal-setting in the control group.
Based on our results, we cannot recommend the implementation of standardized approaches to goal-setting in rehabilitation of older adults in order to improve physical recovery and quality of life. However, within the framework of shared decision-making, goal-setting may be considered desirable or even imperative from an ethical point of view, since goal-setting involves patients in decision-making and is therefore a means to respect the preferences, values and autonomy of patients.39,40 Future studies should aim at improving quality of evidence by reducing the risk of bias using clear study outcomes and publishing trial protocols and using sufficient sample sizes in the trials to reduce baseline imbalance. Furthermore, these studies should conduct a process evaluation to check the implementation and the level of protocol adherence of the goal-setting intervention.
Clinical messages.
The evidence reviewed found that standardized goal-setting did not result in better physical functioning or quality of life in geriatric rehabilitation.
The included studies showed a high risk of bias and process evaluation and adherence to protocol was lacking in all studies.
Supplemental Material
Supplemental material, Supplemental_Material for Goal-setting in geriatric rehabilitation: a systematic review and meta-analysis by Ewout B Smit, Hylco Bouwstra, Cees MPM Hertogh, Elizabeth M Wattel and Johannes C van der Wouden in Clinical Rehabilitation
Acknowledgments
Study concept and design: Ewout Smit, Hylco Bouwstra, Johannes van der Wouden, Cees Hertogh. Acquisition of data: Ewout Smit. Analysis and interpretation of data: Ewout Smit, Hylco Bouwstra, Johannes van der Wouden, Lizette Wattel, Cees Hertogh. Drafting of the manuscript: Ewout Smit. Critical revision of the manuscript for important intellectual content: Ewout Smit, Hylco Bouwstra, Johannes van der Wouden, Lizette Wattel, Cees Hertogh. There are no other people who participated in or made contribution to the study.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iDs: Ewout B Smit  https://orcid.org/0000-0002-3904-1251
https://orcid.org/0000-0002-3904-1251
Johannes C van der Wouden  https://orcid.org/0000-0001-6639-6050
https://orcid.org/0000-0001-6639-6050
Supplemental material: Supplemental Material for this article is available online.
References
- 1. Levack WM, Weatherall M, Hay-Smith JC, et al. Goal setting and strategies to enhance goal pursuit for adults with acquired disability participating in rehabilitation. Cochrane Database Syst Rev 2015; 20(7): CD009727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wade D. Goal setting in rehabilitation: an overview of what, why and how. Clin Rehabil 2009; 23(4): 291–295. [DOI] [PubMed] [Google Scholar]
- 3. Bachmann S, Finger C, Huss A, et al. Inpatient rehabilitation specifically designed for geriatric patients: systematic review and meta-analysis of randomised controlled trials. BMJ 2010; 340: c1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bouwstra H, Wattel LM, de Groot AJ, et al. The influence of activity-based funding on treatment intensity and length of stay of geriatric rehabilitation patients. J Am Med Dir Assoc 2017; 18(6): 549.e15–549.e22. [DOI] [PubMed] [Google Scholar]
- 5. Huby G, Stewart J, Tierney A, et al. Planning older people’s discharge from acute hospital care: linking risk management and patient participation in decision-making. Health Risk Soc 2004; 6(2): 115–132. [Google Scholar]
- 6. Leach E, Cornwell P, Fleming J, et al. Patient centered goal-setting in a subacute rehabilitation setting. Disabil Rehabil 2010; 32(2): 159–172. [DOI] [PubMed] [Google Scholar]
- 7. Rosewilliam S, Roskell CA, Pandyan AD. A systematic review and synthesis of the quantitative and qualitative evidence behind patient-centred goal setting in stroke rehabilitation. Clin Rehabil 2011; 25(6): 501–514. [DOI] [PubMed] [Google Scholar]
- 8. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stevens A, Beurskens A, Koke A, et al. The use of patient-specific measurement instruments in the process of goal-setting: a systematic review of available instruments and their feasibility. Clin Rehabil 2013; 27(11): 1005–1019. [DOI] [PubMed] [Google Scholar]
- 10. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions, version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011, www.handbook.cochrane.org [Google Scholar]
- 11. Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014. [Google Scholar]
- 12. Guidetti S, Ranner M, Tham K, et al. A ‘Client-centred activities of daily living’ intervention for persons with stroke: one-year follow-up of a randomized controlled trial. J Rehabil Med 2015; 47(7): 605–611. [DOI] [PubMed] [Google Scholar]
- 13. Donner A, Klar N. Issues in the meta-analysis of cluster randomized trials. Stat Med 2002; 21(19): 2971–2980. [DOI] [PubMed] [Google Scholar]
- 14. Campbell MK, Fayers PM, Grimshaw JM. Determinants of the intracluster correlation coefficient in cluster randomized trials: the case of implementation research. Clin Trials 2005; 2(2): 99–107. [DOI] [PubMed] [Google Scholar]
- 15. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7(3): 177–188. [DOI] [PubMed] [Google Scholar]
- 16. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327(7414): 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bertilsson AS, Ranner M, von Koch L, et al. A client-centred ADL intervention: three-month follow-up of a randomized controlled trial. Scand J Occup Ther 2014; 21(5): 377–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bertilsson AS, Eriksson G, Ekstam L, et al. A cluster randomized controlled trial of a client-centred, activities of daily living intervention for people with stroke: one year follow-up of caregivers. Clin Rehabil 2016; 30(8): 765–775. [DOI] [PubMed] [Google Scholar]
- 19. Beckerman H, Roelofsen E, Knol D, et al. The value of the Rehabilitation Activities Profile (RAP) as a quality sub-system in rehabilitation medicine. Disabil Rehabil 2004; 26(7): 387–400. [DOI] [PubMed] [Google Scholar]
- 20. Colquhoun H, Letts L, Law M, et al. Routine administration of the Canadian Occupational Performance Measure: effect on functional outcome. Aust Occup Ther J 2010; 57(2): 111–117. [DOI] [PubMed] [Google Scholar]
- 21. Duncan K, Pozehl B. Effects of an exercise adherence intervention on outcomes in patients with heart failure. Rehabil Nurs 2003; 28(4): 117–122. [DOI] [PubMed] [Google Scholar]
- 22. Gagne DE, Hoppes S. The effects of collaborative goal-focused occupational therapy on self-care skills: a pilot study. Am J Occup Ther 2003; 57(2): 215–219. [DOI] [PubMed] [Google Scholar]
- 23. Guidetti S, Andersson K, Andersson M, et al. Client-centred self-care intervention after stroke: a feasibility study. Scand J Occup Ther 2010; 17(4): 276–285. [DOI] [PubMed] [Google Scholar]
- 24. Harwood M, Weatherall M, Talemaitoga A, et al. Taking charge after stroke: promoting self-directed rehabilitation to improve quality of life – a randomized controlled trial. Clin Rehabil 2011; 26(6): 493–501. [DOI] [PubMed] [Google Scholar]
- 25. O’Brien D, Bassett S, McNair P. The effect of action and coping plans on exercise adherence in people with lower limb osteoarthritis: a feasibility study. NZ J Physiother 2013; 41(2): 49–57. [Google Scholar]
- 26. Oestergaard LG, Maribo T, Bunger CE, et al. The Canadian Occupational Performance Measure’s semi-structured interview: its applicability to lumbar spinal fusion patients. A prospective randomized clinical study. Eur Spine J 2012; 21(1): 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ogawa T, Omon K, Yuda T, et al. Short-term effects of goal-setting focusing on the life goal concept on subjective well-being and treatment engagement in subacute inpatients: a quasi-randomized controlled trial. Clin Rehabil 2016; 30(9): 909–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Taylor WJ, Brown M, William L, et al. A pilot cluster randomized controlled trial of structured goal-setting following stroke. Clin Rehabil 2011; 26(4): 327–338. [DOI] [PubMed] [Google Scholar]
- 29. Tomori K, Nagayama H, Ohno K, et al. Comparison of occupation-based and impairment-based occupational therapy for subacute stroke: a randomized controlled feasibility study. Clin Rehabil 2015; 29(8): 752–762. [DOI] [PubMed] [Google Scholar]
- 30. Verhoef J, Toussaint PJ, Zwetsloot-Schonk JH, et al. Effectiveness of the introduction of an International Classification of Functioning, Disability and Health-based rehabilitation tool in multidisciplinary team care in patients with rheumatoid arthritis. Arthritis Rheum 2007; 57(2): 240–248. [DOI] [PubMed] [Google Scholar]
- 31. Wressle E, Eeg-Olofsson AM, Marcusson J, et al. Improved client participation in the rehabilitation process using a client-centred goal formulation structure. J Rehabil Med 2002; 34(1): 5–11. [DOI] [PubMed] [Google Scholar]
- 32. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Erlbaum, 1988. [Google Scholar]
- 33. Sewell L, Singh SJ, Williams JE, et al. Can individualized rehabilitation improve functional independence in elderly patients with COPD. Chest 2005; 128(3): 1194–1200. [DOI] [PubMed] [Google Scholar]
- 34. Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008; 337: a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moore GF, Audrey S, Barker M, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015; 350: h1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Plant S, Tyson SF. A multicentre study of how goal-setting is practised during inpatient stroke rehabilitation. Clin Rehabil 2018; 32(2): 263–272. [DOI] [PubMed] [Google Scholar]
- 37. Meyer T, Pohontsch N, Raspe H. Goal setting in inpatient medical rehabilitation – the challenge persists. Rehabilitation 2009; 48(3): 128–134. [DOI] [PubMed] [Google Scholar]
- 38. Hoenig H, Nusbaum N, Brummel-Smith K. Geriatric rehabilitation: state of the art. J Am Geriatr Soc 1997; 45(11): 1371–1381. [DOI] [PubMed] [Google Scholar]
- 39. Levack WM. Ethics in goal planning for rehabilitation: a utilitarian perspective. Clin Rehabil 2009; 23(4): 345–351. [DOI] [PubMed] [Google Scholar]
- 40. Munthe C, Sandman L, Cutas D. Person centred care and shared decision making: implications for ethics, public health and research. Health Care Anal 2012; 20(3): 231–249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Material for Goal-setting in geriatric rehabilitation: a systematic review and meta-analysis by Ewout B Smit, Hylco Bouwstra, Cees MPM Hertogh, Elizabeth M Wattel and Johannes C van der Wouden in Clinical Rehabilitation