CaWRKY41 plays dual roles in increasing resistance to Ralstonia solanacearum infection and susceptibility to Cd stress in pepper by indirectly and directly increasing H2O2 accumulation and Zn transporter expression.
Keywords: Capsicum annuum, CaWRKY41, cadmium, H2O2, Ralstonia solanacearum, reactive oxygen species
Abstract
WRKY transcription factors have been implicated in both plant immunity and plant responses to cadmium (Cd); however, the mechanism underlying the crosstalk between these processes is unclear. Here, we characterized the roles of CaWRKY41, a group III WRKY transcription factor, in immunity against the pathogenic bacterium Ralstonia solanacearum and Cd stress responses in pepper (Capsicum annuum). CaWRKY41 was transcriptionally up-regulated in response to Cd exposure, R. solanacearum inoculation, and H2O2 treatment. Virus-induced silencing of CaWRKY41 increased Cd tolerance and R. solanacearum susceptibility, while heterologous overexpression of CaWRKY41 in Arabidopsis impaired Cd tolerance, and enhanced Cd and zinc (Zn) uptake and H2O2 accumulation. Genes encoding reactive oxygen species-scavenging enzymes were down-regulated in CaWRKY41-overexpressing Arabidopsis plants, whereas genes encoding Zn transporters and enzymes involved in H2O2 production were up-regulated. Consistent with these findings, the ocp3 (overexpressor of cationic peroxidase 3) mutant, which has elevated H2O2 levels, displayed enhanced sensitivity to Cd stress. These results suggest that a positive feedback loop between H2O2 accumulation and CaWRKY41 up-regulation coordinates the responses of pepper to R. solanacearum inoculation and Cd exposure. This mechanism might reduce Cd tolerance by increasing Cd uptake via Zn transporters, while enhancing resistance to R. solanacearum.
Introduction
Plants are frequently exposed to various biotic and abiotic stresses in their natural habitats. A variety of defense response mechanisms have evolved that protect the plant against particular stresses. These mechanisms are mediated by complex signaling pathways, which must be coordinately and tightly regulated. Common signaling pathways such as MAPK cascades (Rodriguez et al., 2010; Meng and Zhang, 2013) and pathways involving calcium (Knight, 2000; Bose et al., 2011) and reactive oxygen species (ROS; (Qi et al., 2017) are ubiquitously involved in plant responses to various biotic or abiotic stresses, suggesting that they coordinate these responses. However, the exact roles of most of these signaling components and how they are functionally linked are poorly understood.
ROS, including the superoxide radical (O2−), hydrogen peroxide (H2O2), hydroxyl radical (OH•), and singlet oxygen (1O2), are partially reduced forms of molecular oxygen (O2) that typically result from the transfer of one, two, or three electrons to O2. H2O2 is the most stable ROS, with a relatively long half-life (~1 ms in the cell), and often acts as an intercellular and intracellular signal that triggers downstream responses (Baxter et al., 2014; Camejo et al., 2016). ROS homeostasis is modulated by various enzymes; ROS production in multiple subcellular locations is associated with the activities of NADPH oxidases [or respiratory burst oxidase homologs (RBOHs)], glycolate oxidases, and peroxidases (Mittler, 2002; Suzuki et al., 2011; Marino et al., 2012; Gupta et al., 2017). ROS are scavenged by the antioxidant system, including non-enzymatic antioxidants such as ascorbic acid and glutathione, and several antioxidant enzymes, such as catalase (CAT), ascorbate peroxidase (APX), monodehydroascorbate reductase, dehydroascorbate reductase, glutathione reductase, glutathione peroxidase, and glutathione-S-transferase (Romero-Puertas et al., 2007; Dinakar et al., 2010). The production and decomposition of ROS are balanced under non-stress conditions. However, under various environmental stress conditions, this balance frequently breaks down, resulting in a burst of ROS (Lv et al., 2017). Although excess ROS cause oxidative injury, these molecules also act as second messengers that regulate physiological and developmental processes in plants under both stress and non-stress conditions (Apel and Hirt, 2004; Baxter et al., 2014; Qi et al., 2017).
Accumulating evidence indicates that ROS bursts are crucial regulators of plant immunity (Torres et al., 2006; Mersmann et al., 2010; Vellosillo et al., 2010). The perception of pathogen-associated molecular patterns by pattern recognition receptors, and of specific pathogen effectors (either directly or indirectly) by specific nucleotide-binding leucine-rich repeat receptors, triggers ROS bursts in the plant through the activation of RBOHs and peroxidases (Schwizer et al., 2017). ROS bursts are thought to reinforce the cell wall around points of infection and activate downstream responses including defense gene expression, the production of antimicrobial compounds, and the hypersensitive response (Alvarez et al., 1998; Torres et al., 2006). Virulent pathogens possess effectors that are capable of suppressing ROS bursts in various ways and thereby suppressing downstream immune responses during infection (Shidore et al., 2017). Thus, ROS may act as overlapping components in pathogen-associated molecular pattern-triggered immunity and effector-triggered immunity, and serve as crucial nodes connecting these processes (Tsuda and Katagiri, 2010; Adachi et al., 2015).
ROS bursts are also a primary effect of exposure to excess cadmium (Cd). This element, which is released into the agricultural ecological system as a result of urbanization and industrialization, is considered to be one of the most toxic heavy metals in the environment (Gupta et al., 2017). Cd is thought to induce the formation of ROS indirectly by inhibiting the activity of antioxidant enzymes, impairing the respiratory chain, or displacing copper and iron ions from metalloenzymes and interfering with the redox status of the cell (Valko et al., 2005). ROS production in response to Cd exposure may cause oxidative injury to plants, but the exact roles of ROS in the plant response to Cd exposure are poorly understood. As ROS are associated with the plant response to pathogen infection and Cd toxicity, these processes are thought to be linked via ROS. Indeed, treatment with salicylic acid (SA), a defense-signaling molecule, alleviates Cd toxicity in barley (Hordeum vulgare) seedlings (Metwally et al., 2003). Moreover, Cd concentrations close to the toxicity threshold induce defense-signaling pathways mediated by SA and jasmonic acid (Cabot et al., 2013). However, the exact roles of ROS in plant responses to Cd tolerance, and whether and how plant immunity and responses to Cd stress are coordinated by ROS, remain to be elucidated.
A key step in plant responses to diverse stresses is the transcriptional reprogramming of a multitude of defense-associated genes by various transcription factors (TFs). WRKY proteins, which are characterized by the presence of one or two highly conserved WRKY domains, constitute one of the largest TF families. WRKY TFs are important positive and negative regulators of plant growth and development, and of defense responses to environmental stimuli (Eulgem et al., 2000; Rushton et al., 2010). While this large family of TFs is mainly involved in regulating plant immune responses (Sarris et al., 2015), a few WRKY members, including Tamarix hispida WRKY7 (Yang et al., 2016) and Zea mays WRKY4 (Hong et al., 2017), have been implicated in plant responses to Cd toxicity. In addition, some WRKY TFs are involved in more than one biological process, suggesting that WRKYs are crucial nodes in the crosstalk between plant immunity and other biological processes (Rushton et al., 2010). Moreover, the expression of most group III WRKY genes is modified in response to pathogen attack and treatment with SA (Kalde et al., 2003). As recent studies have shown that group III WRKY genes play important roles in plant responses to abiotic stress (Li et al., 2013; Ding et al., 2014; Chen et al., 2017), we reasoned that these genes might be involved in the crosstalk between plant responses to pathogen attack and abiotic stress, possibly coordinating plant responses to these stresses.
Pepper (Capsicum annuum) is a solanaceous vegetable crop widely grown around the world. Blight and bacterial wilt caused by the soil-borne pathogens Phytophthora capsici and Ralstonia solanacearum, respectively, frequently reduce pepper production. Heavy metal contamination is another factor that inhibits pepper growth. Heavy metal residues are present in soils as a result of sewage irrigation and the use of heavy-metal-containing products such as pesticides and fertilizers. A better understanding of how pepper responds to heavy metal contamination would lay the foundations for developing effective countermeasures.
In the present study, we investigated the transcriptional responses of group III WRKYs to Cd toxicity and R. solanacearum inoculation. We also investigated the responses of these genes to iron (Fe) deficiency, because Cd toxicity-induced chlorosis resembles Fe deficiency-induced chlorosis (Sun et al., 2015; Chen et al., 2016; Li et al., 2016), and plant responses to Fe deficiency are related to responses to excess Cd (Nakanishi et al., 2006; Han et al., 2014; Mendoza-Cozatl et al., 2014). Among the eight group III WRKY genes we examined, only CaWRKY41 was synergistically up-regulated in pepper plants challenged by Cd toxicity, Fe deficiency, or R. solanacearum inoculation. We identified a positive feedback loop between CaWRKY41 and H2O2 accumulation during the response to R. solanacearum inoculation and excess Cd exposure in pepper.
Materials and methods
Plant materials and growth conditions
Seeds of pepper (Capsicum annuum) 8# (an inbred line provided by the pepper breeding group at Fujian Agriculture and Forestry University) and CM334 (Mexican landrace of C. annuum cv. CM334), and tobacco (Nicotiana benthamiana) were imbibed in sterile water at 25±2 °C overnight and sown in a steam-sterilized soil mix (peat moss, vermiculite, and perlite, 2:1:1 by volume) in plastic pots.
Pepper plants were grown in a growth room maintained at 25±2 °C with a light intensity of ~100 µmol photons m−2 s−1 and a relative humidity of 70% under a 16 h light/8 h dark cycle. For liquid cultivation, 21-day-old pepper seedlings were transferred to 1.2 l black plastic beakers containing modified one-fifth Hoagland solution. The initial nutrient solution contained the macronutrients KNO3 (1 mM), Ca (NO3)2·4H2O (1 mM), MgSO4·7H2O (1.4 mM), and KH2PO4 (0.2 mM), and the micronutrients Fe-EDTA (20 µM), H3BO3 (3 µM), (NH4)6Mo7O24 (1 µM), MnCl2 (0.5 µM), ZnSO4 (0.4 µM), and CuSO4 (0.2 µM). The pH of the solution was adjusted to 5.8, and the nutrient solution was renewed every 3 days.
For Arabidopsis thaliana cultivation, wild-type (WT; Col-0), ocp3 (Coego et al., 2005), CaWRKY41-OE1, and CaWRKY41-OE4 transgenic Arabidopsis seeds were treated by exposure to 4 °C in darkness for 3 days and then sown on vertically placed Petri dishes containing ½ Murashige and Skoog (MS) medium (PhytoTechnology, product ID M524) supplemented with 1% (w/v) sucrose and 0.8% agar (Sigma, cat. no. A1296) in the absence or presence of heavy metals or other supplements in a growth chamber maintained at 22±2 °C with a light intensity of ~100 µmol photons m−2 s−1 and a relative humidity of 70%, under a 16 h light/8 h dark cycle.
Phylogenetic analysis of group III WRKY TFs across three plant species
The WRKY TFs were described previously (Eulgem et al., 2000). The amino acid sequences of proteins and domains of group III CaWRKYs, SlWRKYs, and AtWRKYs from the C. annuum, Solanum lycopersicum, and A. thaliana genomes were downloaded from Plant TFDB V4.0 (http://planttfdb.cbi.pku.edu.cn/index.php).
Pathogens and inoculation procedures
Ralstonia solanacearum strain FJ150501 was isolated from pepper plants showing symptoms of bacterial wilt infection in Guangdong Province, China. For soil-drenching inoculation, PYL-279 and PYL-279-wrky41 pepper plants grown in pots, with the roots partially and mechanically damaged, were inoculated with a 108 cfu/ml (OD600=0.8) suspension of R. solanacearum. A disease index (from 0 to 5) was scored daily in the R. solanacearum-inoculated pepper plants, as follows: 0 (no wilting), 1 (1 to 20% wilted), 2 (21 to 40% wilted), 3 (41 to 60% wilted), 4 (61 to 80% wilted), and 5 (81 to 100% wilted or dead). The average values reported are based on three independent replicates, each comprising six plants. Electrolyte leakage was measured in pepper leaves at 0, 24, and 48 h post infection. For suspension inoculation, pepper plants were grown in 1.2 l black plastic beakers containing one-fifth Hoagland solution. Eight of the lateral roots were removed from each plant with a pair of scissors, and the plants were then transferred to one-fifth Hoagland solution with 108 cfu/ml R. solanacearum suspension.
Plasmid construction and plant transformation
To construct the vector 35S::CaWRKY41, the full-length open reading frame was cloned into pDONR207 and transferred into the pGWB2 expression vector (Invitrogen, USA). To construct the reporter vector (pCaWRKY41::GUS) for histochemical β-glucuronidase (GUS) analysis, the promoter of CaWRKY41 of ~2 kb in length (pCaWRKY41) was amplified via PCR from pepper genomic DNA and cloned into the pMDC163 vector (Invitrogen). The constructs 35S::CaWRKY41 and pCaWRKY41::GUS were then transformed into Agrobacterium tumefaciens strain GV3101 using the freeze–thaw method. A. tumefaciens-mediated transformation of Arabidopsis was performed using the floral dip method (Clough and Bent, 1998), and transgenic plants were identified by sowing seeds on ½ MS agar plates containing 50 mg l−1 hygromycin and selecting hygromycin-resistant seedlings.
Subcellular localization and transcriptional activity analysis
The coding region of CaWRKY41 without the stop codon was cloned into the pCambia1300-GFP/C vector by In-Fusion Cloning (Clontech, USA). The pCambia1300-CaWRKY41-GFP construct was transformed into A. tumefaciens GV3101 and infiltrated into the fully expanded leaves of 5-week-old N. benthamiana plants. At 2 days post inoculation, green fluorescent protein (GFP) fluorescence was observed by confocal laser-scanning microscopy (Zeiss LSM710, Germany). For the transactivation assay, the open reading frames of CaWRKY41 (1–329) and the mutant genes CaWRKY41 (61–329), CaWRKY41 (131–329), and CaWRKY41 (192–329) were generated by PCR with specific primer pairs and cloned into pGBKT7 (Clontech) to generate various CaWRKY41 constructs (BD-CaWRKY41,-1,-2 and -3). Transcriptional activation activity was determined in yeast cells transformed with these constructs grown on SD medium lacking Trp for 3 days, and a colony-lift filter assay (X-gal assay) was performed.
Virus-induced gene silencing
CaWRKY41-silenced pepper plants were generated using tobacco rattle virus-based virus-induced gene silencing (VIGS) as described previously (Dang et al., 2013). Briefly, a specific 328 bp fragment of CaWRKY41 was identified by homologous searching via BLAST analysis against the genome sequences of pepper cultivars CM334 (http://peppergenome.snu.ac.kr/) and Zunla-1 (http://peppersequence.genomics.cn/page/species/blast.jsp). The fragment was cloned into the entry vector pDONR207 and then into the PYL279 vector. The vectors (PYL-279 and PYL-279-wrky41) were separately transformed into A. tumefaciens GV3101 cells, which were subsequently mixed with A. tumefaciens cells harboring PYL-192 and injected into fully expanded pepper seedling cotyledons. PYL-279-wrky41 pepper plants were subjected to experimental analysis, with PYL-279 plants (transformed with empty vector) serving as a control. Levels of H2O2 and of the expression of various genes were measured in CaWRKY41-silenced PYL-279 and PYL-279-wrky41 pepper plants grown in liquid culture.
Treatment of plants with Cd and exogenous application of H2O2
To test the effect of Cd on seed germination and growth in Arabidopsis, seeds were treated by exposure to 4 °C in darkness for 3 days and then grown on ½ MS medium containing 25 µM, 50 µM, or 100 µM CdSO4 for 8 days. To measure the expression of various genes in plants in the presence of excess Cd supply, 7-day-old Arabidopsis seedlings were transferred to ½ MS medium without or with 25 µM CdSO4, cultured for 6 or 72 h, and harvested for use. To investigate the expression of the eight CaWRKY group III genes, pepper plants at the six-leaf stage grown in liquid culture were treated with Cd stress (2.5, 5, 25, and 60 µM CdSO4) and Fe deficiency (0 µM Fe-EDTA). Pepper plants at the six-leaf stage were sprayed with H2O2 (1 mM) and incubated for 0, 1, 3, 6, 12, 24, 36, and 48 h, and leaf tissue was harvested for CaWRKY41 expression analysis.
Histochemical staining
Leaves were stained with Trypan blue and 3, 3′-diaminobenzidine (DAB) as described previously (Dang et al., 2013, 2014; Cai et al., 2015). For GUS staining, seedlings or tissues were incubated overnight in GUS staining solution (1 mg·ml−1 X-Gluc, 1 mM K3Fe(CN)6, 1 mM K4Fe(CN)6, 50 mM sodium phosphate buffer pH 7.0, 10 mM Na2EDTA, and 0.1% Triton X-100) at 37 °C, destained several times in 75% (v/v) ethanol, and observed under a stereomicroscope (Leica, Germany).
Measurement of H2O2 and Cd contents and enzyme activity
Seedlings were grown on ½ MS medium for 7 days, treated with 25 µM CdSO4 for 3 and 5 days, and sampled for H2O2 and Cd analysis and enzymatic assays. For H2O2 measurements, seedlings were harvested, ground in liquid nitrogen, and examined using an Amplex Red H2O2-peroxidase Assay Kit (Molecular Probes). This one-step assay uses Amplex Red reagent (10-acetyl-3,7-dihydroxyphenoxazine) to detect H2O2. Briefly, approximately 80 mg of sample was processed and measured using an H2O2 standard curve. The fluorescence emission spectrum (590 nm) was detected at an excitation wavelength of 530 nm using a Tecan Infinite 200 Pro (Tecan, Switzerland).
To measure the Cd contents in roots and shoots, the roots were rinsed three times (for 4 min each time) with Milli-Q water to remove Cd attached to the root surface. The root and shoot samples were weighed and digested with 0.5 ml (for roots) and 1 ml (for shoots) concentrated HNO3. Each sample was adjusted to 10 ml with Milli-Q water and then filtered through filter paper. Cd in the samples was detected by inductively coupled plasma-atomic emission spectrometry (IRIS/AP Optical Emission Spectrometer, Thermo Scientific, USA). The experiment was performed in three biological replicates.
For enzymatic activity analysis, approximately 80 mg of sample was ground in liquid nitrogen using a TissueLyserII, and milled samples were homogenized in phosphate buffer (600 μl, 50 mM, pH 7.0) and centrifuged at 3000 × g at 4 °C for 10 min. Then, peroxidase (POD), CAT, and APX activity were analyzed using an ELISA kit (Shanghai Bangyi Biotechnology Co. Ltd, China) according to the manufacturer’s instructions. Microtiter plate wells were coated with purified POD, CAT, and APX antibody, to make a solid-phase antibody, and then samples were added to the wells together with an antibody labeled with horseradish peroxidase, and an antibody–antigen–enzyme complex formed. Substrate solution was added after thorough washing, and then, using a blank well as the zero control, the absorbance was measured at 450 nm in a Tecan Infinite 200 Pro Plate Reader (Tecan).
RNA extraction and reverse transcription–quantitative PCR (RT–qPCR)
Total RNA was isolated from Arabidopsis and pepper tissues using a TaKaRa Mini BEST Universal RNA Extraction Kit (TaKaRa, Dalian, China). RNA (1 µg) was used as a template to synthesize cDNA with a TaKaRa PrimeScript RT-PCR Kit (TaKaRa) according to the manufacturer’s instructions. Gene expression levels were measured on a CFX96 Real-Time PCR System (Bio-Rad, USA) using SYBR® Premix Ex Taq™ II (TaKaRa); specific primers are listed in Supplementary Table S1 at JXB online. Arabidopsis UBIQUITIN10 (AtUBQ10) and pepper Actin1 (CaActin1) were used for normalization.
Results
Phylogenetic analysis of CaWRKY group III genes
To identify the phylogenetic relationships among the eight CaWRKY group III genes, we compared their nucleotide sequences to those of WRKY genes from tomato and Arabidopsis. We constructed an unrooted phylogenetic tree based on an alignment of the amino acid sequences of the group III WRKY proteins and domains from the three plant species using the neighbor-joining method. Based on this analysis, WRKYs from pepper share higher sequence similarity with WRKYs from tomato than with those from Arabidopsis (see Supplementary Fig. S1). Detailed information about the CaWRKY group III genes is provided in Supplementary Tables S2 and S3.
Expression analysis of eight CaWRKY group III genes during exposure to excess Cd or Fe deficiency
Cd is a highly toxic heavy metal that is readily absorbed by plant roots, loaded into the xylem, and transported to leaves, leading to the generation of ROS (Valko et al., 2005; Perez-Chaca et al., 2014; Keunen et al., 2015). ROS production has been detected in sunflower (Helianthus annuus L.) and maize (Z. mays) under conditions of Fe deficiency (Ranieri et al., 2001; Sun et al., 2007).
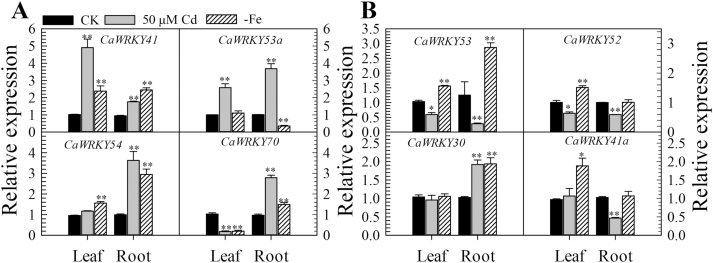
In the present study, H2O2 accumulation was detected in DAB-stained pepper leaves after 24, 36, and 48 h of Cd stress and Fe deficiency treatments (see Supplementary Fig. S2A, B). Similar to the response to Cd stress, the newly emerged leaves of pepper plants at the eight-leaf stage displayed yellowing after Fe deficiency treatment (Supplementary Fig. S2C, D). To identify the group III WRKY TFs involved in Cd stress, we measured the expression of the eight group III WRKY genes by RT–qPCR analysis in pepper plants exposed to Cd stress or Fe deficiency. CaWRKY41 and CaWRKY53a expression significantly increased under Cd stress in both the roots and leaves of pepper plants (Fig. 1A). Furthermore, CaWRKY41, CaWRKY53, and CaWRKY54 expression markedly increased under Fe deficiency treatment in both roots and leaves (Fig. 1). Therefore, among the eight group III WRKY genes in pepper, only CaWRKY41 expression was up-regulated by both Cd toxicity and Fe deficiency in roots and leaves, pointing to the involvement of CaWRKY41 in the response of pepper to excess Cd and Fe deficiency, which might be associated with the production of H2O2.
Fig. 1.
(A, B) Expression of eight group III WRKY genes in the leaves and roots of pepper plants after 24 h of exposure to Cd stress and Fe deficiency, as determined by RT–qPCR analysis. The relative expression of the genes in stressed plants was compared with that of control untreated (CK) plants, which was set to a value of 1. Data represent the mean ±SE of three biological replicates. Asterisks indicate significant differences compared with CK plants (Student’s t-test; *P<0.05, **P<0.01).
CaWRKY41 is up-regulated in response to Cd and H2O2
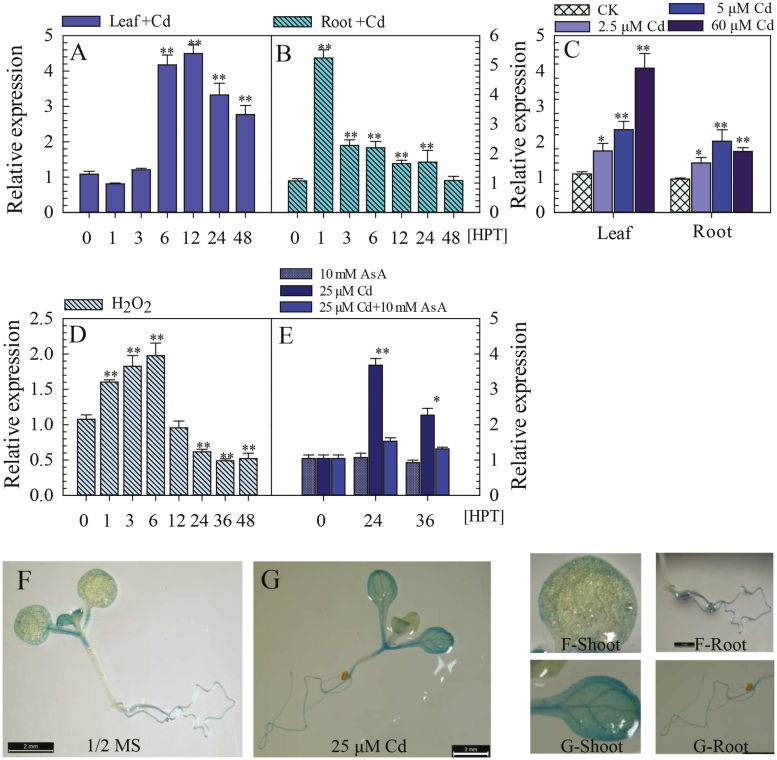
To further investigate the involvement of CaWRKY41 in the response of pepper to Cd toxicity, we measured the time course and dose-responsive patterns of CaWRKY41 expression in response to Cd stress by RT–qPCR analysis. After exposure to excess Cd, CaWRKY41 expression was strongly enhanced, peaking at 12 h post treatment (HPT) in the leaves and 1 HPT in the roots (Fig. 2A, B). CaWRKY41 expression was also increased in response to treatment with 2.5, 5, and 60 µM Cd compared with the control (Fig. 2C). Additionally, CaWRKY41 expression was significantly up-regulated in response to exogenous application of H2O2 (Fig. 2D). However, the CaWRKY41 expression in pepper leaves triggered by excess Cd was reduced when samples were treated with the H2O2 scavenger ascorbic acid (Fig. 2E, Supplementary Fig. S2E).
Fig. 2.
CaWRKY41 was transcriptionally induced by Cd and H2O2 treatment in pepper. (A, B) CaWRKY41 expression in pepper leaves and roots determined by RT–qPCR at the indicated time points after treatment with 25 µM CdSO4. HPT, hours post treatment. (C) CaWRKY41 expression in pepper leaves and roots determined by RT–qPCR at 12 HPT with 2.5, 5, or 60 µM CdSO4. CK, control untreated. (D) CaWRKY41 expression in pepper leaves analyzed at 0, 1, 3, 6, 12, 24, 36, and 48 HPT with 1 mM H2O2. Relative expression levels of CaWRKY41 in stressed plants were compared with those of control plants, which were set to a value of 1. Data represent the mean ±SE of three biological replicates. Asterisks indicate significant differences compared with control plants (Student’s t-test; *P<0.05, **P<0.01). (E) Excess Cd-induced expression of CaWRKY41 in pepper leaves was inhibited by treatment with 10 mM ascorbic acid (AsA). (F, G) GUS expression in transgenic Arabidopsis plants carrying the pCaWRKY41::GUS construct. Seven-day-old pCaWRKY41::GUS seedlings were transferred to ½ MS medium without (F) or with (G) 25 µM CdSO4 for 12 h, followed by staining. Panels labeled F-shoot, F-root, G-shoot, and G-root show magnifications of the corresponding plant parts shown in panel F or G, respectively, to show details of the GUS staining patterns of shoots and roots of pCaWRKY41::GUS seedlings. Plants were grown on ½ MS medium under 16 h light/8 h dark conditions.
To confirm the expression pattern of CaWRKY41, we generated pCaWRKY41::GUS transgenic Arabidopsis plants. Seven-day-old pCaWRKY41::GUS seedlings were transferred to ½ MS medium without or with excess Cd for 12 h and then stained to analyze GUS activity. When pCaWRKY41::GUS seedlings were transferred to conditions of excess Cd, increased GUS activity was observed in the shoot and root (Fig. 2F, G). When pCaWRKY41::GUS seedlings were grown under normal conditions, GUS staining was consistently detected in the roots, shoots, mature leaves, and petioles (Supplementary Fig. S3A–G). Intensive GUS staining was also observed in the flowers (Supplementary Fig. S3H, I) but not in the siliques (Supplementary Fig. S3J). These results imply that CaWRKY41 might be involved in the response of pepper to excess Cd and H2O2 accumulation.
Analysis of the subcellular localization and transcriptional activity of CaWRKY41
As the function of a given protein is closely related to its subcellular localization, we investigated the subcellular localization of CaWRKY41 in transiently transformed N. benthamiana leaves harboring the open reading frame of this gene, without the translation terminator, driven by the 35S promoter and fused to the GFP gene. The CaWRKY41-GFP fusion protein was exclusively localized to the nuclei of epidermal cells when heterologously expressed in N. benthamiana (Supplementary Figs S3K and S4A).
In addition, we assayed the transcriptional activity of CaWRKY41 in yeast via a transcriptional activation assay. The expression of the LacZ reporter gene driven by the GAL4 upstream activating sequence was significantly increased by the presence of the BD-CaWRKY41 fusion protein in yeast, but LacZ expression was not induced in the negative control (Supplementary Fig. S4B). These results indicate that CaWRKY41 is a nuclear protein with transcriptional activity.
CaWRKY41 silencing increases Cd tolerance and reduces H2O2 accumulation in pepper
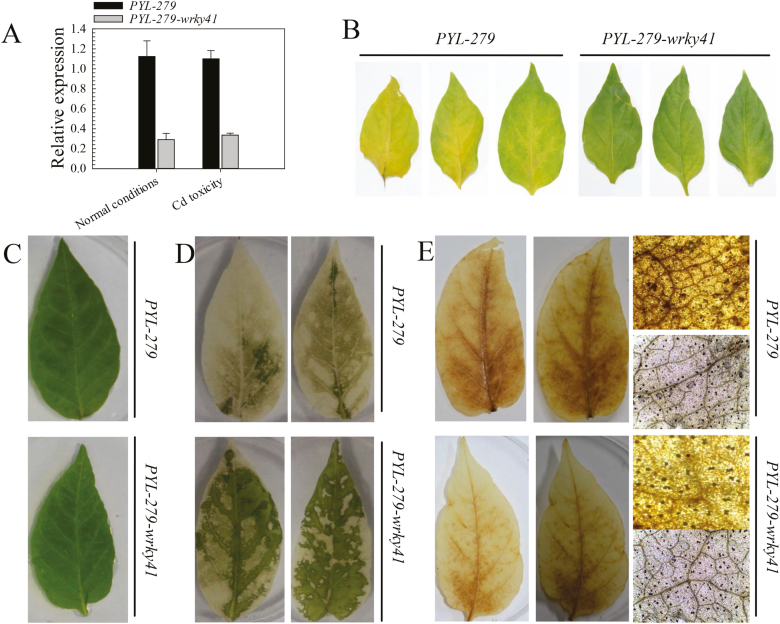
The induction of CaWRKY41 expression by excess Cd points to its involvement in the Cd stress response. To test this possibility, we examined the effect of VIGS of CaWRKY41 on the response of pepper to Cd stress. To avoid possible off-target silencing, we inserted a specific 328 bp fragment of CaWRKY41 into the PYL-279-wrky41 vector [tobacco rattle virus (PYL-279): wrky41] to silence CaWRKY41 in pepper. CaWRKY41 was expressed at a level approximately 3.8- and 3.2-fold lower in CaWRKY41-silenced plants than in control plants (PYL-279), in the presence and absence of Cd stress, respectively (Fig. 3A), respectively, indicating that we had successfully silenced CaWRKY41 via VIGS.
Fig. 3.
CaWRKY41 silencing enhances tolerance to Cd stress in pepper. (A) CaWRKY41 expression in PYL-279 (control) and PYL-279-wrky41 pepper leaves. (B) Less yellowing was observed in PYL-279-wrky41 compared with PYL-279 pepper leaves. Pepper plants were grown in one-fifth Hoagland solution. When photobleaching was observed in PYL-279-pds leaves, PYL-279-wrky41 and PYL-279 plants were transferred to fresh nutrient solution containing 50 µM CdSO4 for 4 days. (C, D) Leaves from PYL-279 and PYL-279-wrky41 cultured on 1/5 MS medium without (C) or with (D) 25 µM CdSO4 for 4 days. (E) H2O2 production observed after 3, 3′-diaminobenzidine staining in leaves of PYL-279 and PYL-279-wrky41 plants at 3 days post treatment with 25 µM CdSO4.
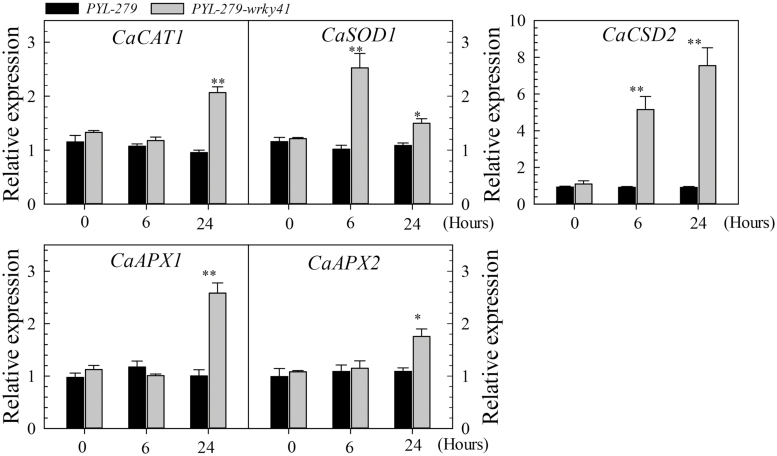
Upon exposure to Cd stress, PYL-279-wrky41 plants and detached leaves consistently exhibited attenuated Cd stress-induced chlorosis compared with controls (Fig. 3B–D). CaWRKY41-silenced leaves also accumulated less H2O2 than control leaves under Cd stress (Fig. 3E). Consistently, genes encoding antioxidant enzymes, including CAT (CaCAT1), superoxide dismutase (CaSOD1), copper zinc superoxide dismutase (CaCSD2), and APX (CaAPX1 and CaAPX2), were up-regulated at 24 HPT with Cd stress in the youngest leaves of PYL-279-wrky41 plants compared with the control. However, no difference in the expression of these genes was detected between the youngest leaves of PYL-279 and PYL-279-wrky41 under normal growth conditions (Fig. 4). These results suggest that CaWRKY41 negatively regulates Cd tolerance, likely by mediating the accumulation of H2O2 through the transcriptional regulation of antioxidant genes.
Fig. 4.
Expression of genes encoding ROS-scavenging enzymes determined by RT–qPCR analysis in CaWRKY41-silenced plants 0, 6, and 24 h after treatment with 25 µM CdSO4. Data represent the mean ±SE of three biological replicates. Asterisks indicate significant differences compared with control plants (Student’s t-test; *P<0.05, **P<0.01).
Overexpression of CaWRKY41 increases sensitivity to Cd in Arabidopsis in an H2O2-dependent manner
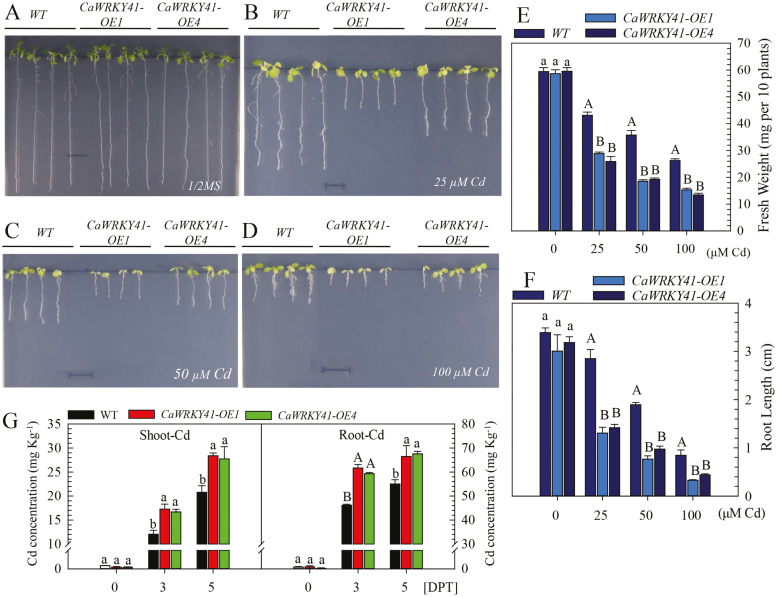
To confirm the results of the CaWRKY41-silencing experiments, we performed a gain-of-function analysis by ectopically overexpressing CaWRKY41 in Arabidopsis. None of the seven CaWRKY41-overexpressing T4 homozygous transgenic Arabidopsis lines exhibited significant differences in seed germination, seedling growth, or development compared with WT plants under normal conditions (Supplementary Fig. S4C, D), although, as expected, CaWRKY41-overexpressing plants exhibited high expression of CaWRKY41, as revealed by semi-quantitative PCR (Supplementary Fig. S4E). We randomly selected two independent overexpressing lines (CaWRKY41-OE1 and CaWRKY41-OE4) for further analysis. These CaWRKY41-OE lines were more sensitive than the WT to Cd stress (Fig. 5A–D), and had lower fresh weights and shorter roots (Fig. 5E, F).
Fig. 5.
Overexpression of CaWRKY41 reduces tolerance to Cd stress in transgenic Arabidopsis plants. (A–D) Seedling growth in WT, CaWRKY41-OE1, and CaWRKY41-OE4 lines on ½ MS medium containing (A) 0, (B) 25, (C) 50, and (D) 100 µM CdSO4. Representative photographs were taken 8 days after germination. (E) Fresh weight and (F) root length in WT, CaWRKY41-OE1, and CaWRKY41-OE4 plants exposed to Cd stress. (G) Cd concentration in the shoots and roots of WT, CaWRKY41-OE1, and CaWRKY41-OE4 plants after 3 and 5 days of treatment. Data represent the mean ±SE of three biological replicates. Different letters indicate significant differences compared with the control (Tukey’s test; lowercase letters indicate P<0.05 and uppercase letters indicate P<0.01).
Next, we compared the growth status of CaWRKY41-OE1 and OE4 plants with that of WT plants exposed to excess Cd, or to no Cd, via rapid noninvasive chlorophyll fluorescence imaging. Under normal conditions, there was no marked difference in the fluorescence characteristics of WT and CaWRKY41-OE plants (Supplementary Fig.S5A, C, E); however, under Cd stress, CaWRKY41-OE1 and OE4 plants exhibited lower chlorophyll fluorescence parameters than WT plants (Supplementary Fig. S5 B, D, F). Furthermore, higher Cd (Fig. 5G) and zinc (Zn) (Supplementary Fig. S6A, B) contents were detected in both the roots and shoots of CaWRKY41-overexpressing plants (OE1 and OE4) than in those of the WT after 3 or 5 days of Cd treatment. By contrast, the Fe contents in roots and shoots were similar in CaWRKY41-OE and WT plants (Supplementary Fig. S6C, D). Additionally, the CaWRKY41-OE lines were more sensitive than the WT plants to excess Zn (Supplementary Fig. S6 E, F).
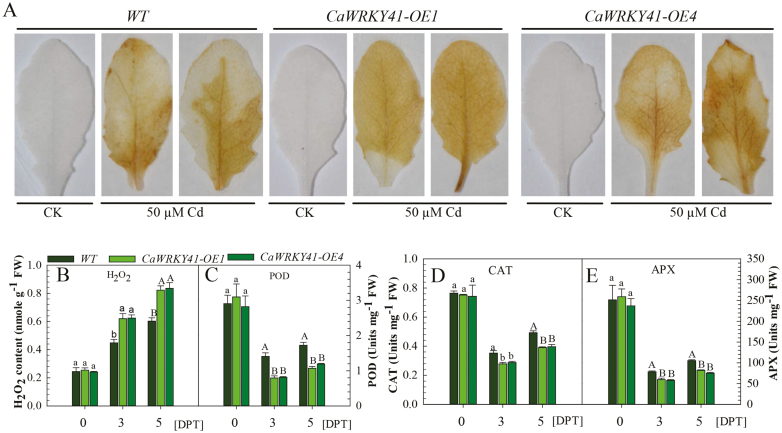
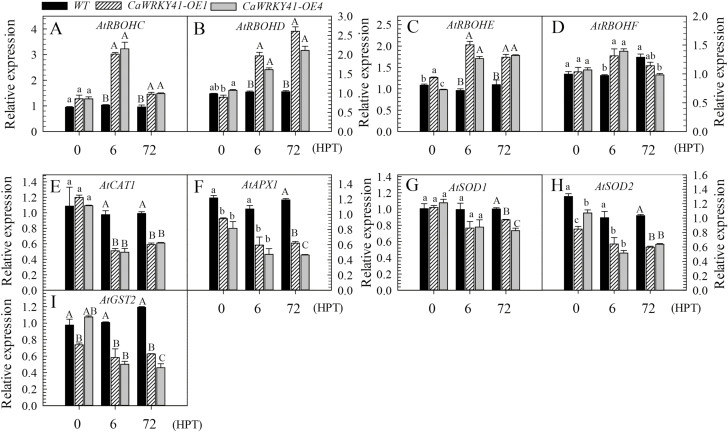
The reduced accumulation of H2O2 in CaWRKY41-silenced leaves compared with control plants under Cd stress suggests that H2O2 might be involved in CaWRKY41-mediated responses to Cd in pepper. To investigate this possibility, we analyzed the effect of CaWRKY41 overexpression on H2O2 accumulation in Arabidopsis plants subjected to Cd stress. H2O2 levels were higher in the leaves of CaWRKY41-overexpressing lines (OE1 and OE4) than in those of the WT, as revealed by DAB staining and direct H2O2 measurements (Fig. 6A, B). Accordingly, the activities of the ROS-scavenging enzymes POD, CAT, and APX were reduced in OE1 and OE4 plants compared with WT plants (Fig. 6C–E). By contrast, higher expression of genes associated with ROS production, such as AtRBOHC (Macho et al., 2012), AtRBOHD (Li et al., 2014; Kadota et al., 2015), AtRBOHE, and AtRBOHF (Chaouch et al., 2012) (Fig. 7A–D), and lower expression of the ROS-scavenging enzyme genes AtCAT1, AtAPX1, AtSOD1, AtSOD2, and AtGST2 (Fig. 7E–I), were detected in OE1 and OE4 plants compared with WT plants at 6 and 72 HPT with Cd. These results suggest that the enhanced accumulation of ROS including H2O2 in response to CaWRKY41 overexpression might be due to enhanced ROS production and reduced ROS scavenging, and that elevated H2O2 levels might contribute to Cd sensitivity in pepper plants.
Fig. 6.
H2O2 accumulation and ROS-scavenging enzymatic activity in response to Cd stress. (A) H2O2 production observed via 3, 3′-diaminobenzidine staining in leaves of WT, CaWRKY41-OE1, and CaWRKY41-OE4 plants at 24 h post treatment with 50 µM CdSO4. CK, control untreated. (B) Seedling H2O2 content. DPT, days post treatment. (C) Peroxidase (POD) activity. (D) Catalase (CAT) activity. (E) Ascorbate peroxidase (APX) activity. For B–E, 7-day-old WT, CaWRKY41-OE1, and CaWRKY41-OE4 seedlings were transferred to ½ MS medium without or with 25 µM CdSO4 for 3 or 5 days before analysis. Data represent the mean ±SE of three biological replicates. Different letters indicate significant differences compared with the control (Tukey’s test; lowercase letters indicate P<0.05 and uppercase letters indicate P<0.01).
Fig. 7.
Expression of genes encoding ROS-producing and ROS-scavenging enzymes detected by RT–qPCR analysis in WT, CaWRKY41-OE1, and CaWRKY41-OE4 plants at 0, 6, and 72 h post treatment with Cd. (A–D) Expression of ROS-producing enzyme genes (A) AtRBOHC, (B) AtRBOHD, (C) AtRBOHE, and (D) AtRBOHF. (E–I) Expression of ROS-scavenging enzyme genes (E) AtCAT1, (F) AtAPX1, (F) AtSOD1, AtSOD2, and (G) AtGST2. Data represent the mean ±SE of three biological replicates. Different letters indicate significant differences compared with the control (Tukey’s test; lowercase letters indicate P<0.05 and uppercase letters indicate P<0.01).
To test this possibility, we examined whether there was a relationship between H2O2 accumulation and Cd sensitivity in the A. thaliana ocp3 (overexpressor of cationic peroxidase 3) mutant, which harbors a T-DNA insertion in a homeodomain TF gene involved in increased H2O2 production in healthy plants (Coego et al., 2005). Mutant ocp3 plants exhibited shorter primary roots than WT plants under Cd stress (Supplementary Fig. S7), supporting the notion that Cd sensitivity is associated with H2O2 accumulation. Collectively, these results suggest that the CaWRKY41-mediated Cd sensitivity observed in transgenic Arabidopsis is caused by H2O2 accumulation due to increased H2O2 production and reduced H2O2 scavenging.
Overexpression of CaWRKY41 increases Cd levels in Arabidopsis by activating Zn transporters
Since we detected higher levels of Cd but not Fe in both the roots and shoots of CaWRKY41-OE plants compared with WT upon excess Cd supply, we reasoned that the enhanced Cd sensitivity in response to CaWRKY41 overexpression might be due to enhanced uptake of Cd. A Cd-specific transporter has not yet been identified, and Cd is thought to be transported by Fe and Zn transporters in plants (Saraswat and Rai, 2011; Barabasz et al., 2016). Therefore, we reasoned that, since Fe levels were not elevated in CaWRKY41-OE Arabidopsis plants compared with control plants, Cd might enter CaWRKY41-OE Arabidopsis plants via Zn transporters.
To test this hypothesis, we measured the expression of genes encoding Zn transporters, including AtZIP1 (Kawachi et al., 2009), AtZIP3 (Gustin et al., 2009), AtZIP4 (Gustin et al., 2009), AtZIP5 (Gustin et al., 2009), and AtZIP9 (Gustin et al., 2009) in CaWRKY41-OE Arabidopsis plants. Only AtZIP3, AtZIP4, and AtZIP9 (Supplementary Fig. S8 B, C, E), were up-regulated in these plants compared with controls; the expression of the other genes did not differ from those of controls upon exposure to excess Cd. These results suggest that increased Cd uptake might be due at least in part to the enhanced expression of genes encoding Zn transporters.
Silencing of CaWRKY41 confers reduced resistance to R. solanacearum inoculation
Our results indicate that H2O2, which has been implicated in plant immunity (Levine et al., 1994; Alvarez et al., 1998; Torres et al., 2006), is involved in CaWRKY41-mediated Cd sensitivity. NADPH oxidases, which contribute to ROS production, have frequently been shown to be involved in plant immunity (Kadota et al., 2015). Thus, we reasoned that CaWRKY41 might also participate in plant immunity.
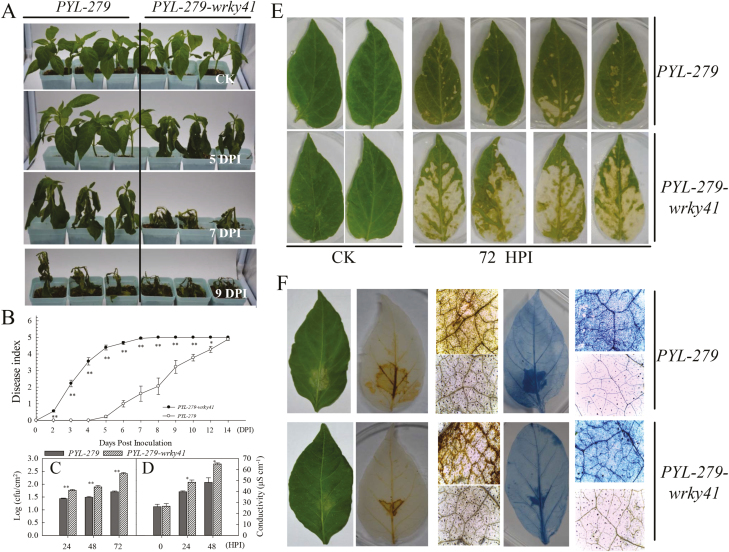
To test this possibility, we monitored changes in CaWRKY41 expression in response to R. solanacearum inoculation. CaWRKY41 was strongly induced by R. solanacearum inoculation, with peak expression detected at 6 h post inoculation (Supplementary Fig. S9A). In addition, analysis of CaWRKY41-silenced pepper plants, in which CaWRKY41 expression was approximately 3.2- and 3.3-fold lower than the control (PYL-279) under pathogen inoculation and non-inoculation conditions, respectively (Supplementary Fig. S9B), showed that CaWRKY41 silencing increased susceptibility to R. solanacearum compared with PYL-279 plants at 5, 7, and 9 days post inoculation (Fig. 8A). Consistently, PYL-279-wrky41 plants had a higher disease index, higher rate of R. solanacearum growth, and higher level of electrolyte leakage compared with PYL-279 plants (Fig. 8B–D). In addition, more serious symptoms of bacterial wilt were observed in the detached youngest leaves of PYL-279-wrky41 compared with PYL-279 plants after infiltration of an R. solanacearum suspension for at least 30 min, while no difference was observed in untreated leaves (Fig. 8E).
Fig. 8.
CaWRKY41 silencing enhances susceptibility to Ralstonia solanacearum FJ150501. (A) Appearance of PYL-279 and PYL-279-wrky41 pepper plants at 0, 5, 7, and 9 days post inoculation (DPI) with R. solanacearum. (B) Disease index scored daily for R. solanacearum-inoculated PYL-279 and PYL-279-wrky41 pepper plants. (C) Bacterial growth and (D) conductivity (as a measure of electrolyte leakage) in PYL-279 and PYL-279-wrky41 pepper leaves following R. solanacearum inoculation. HPI, hours post inoculation. Data represent the mean ±SE of three biological replicates. Asterisks indicate significant differences compared with control plants (Student’s t-test; *P<0.05, **P<0.01). (E) Effect of R. solanacearum on leaves isolated from PYL-279 and PYL-279-wrky41 plants. R. solanacearum was collected from stem exudates or the vascular portions of infected pepper leaves, and the appearance of symptoms was observed 72 HPI. CK, control untreated. (F) Decreased H2O2 levels and cell death in the leaves of PYL-279-wrky41 pepper plants compared with PYL-279 24 h after inoculation with R. solanacearum.
DAB staining revealed R. solanacearum-triggered H2O2 production in PYL-279 plants, but much less H2O2 accumulation was detected in R. solanacearum-inoculated CaWRKY41-silenced pepper leaves than in PYL-279 leaves. Similarly, much higher levels of cell death (as revealed by Trypan blue staining) were triggered by R. solanacearum inoculation in the youngest leaves of PYL-279 plants at 24 h post inoculation (Fig. 8F) compared with PYL-279-wrky41. These results indicate that the role of CaWRKY41 as a positive regulator of plant immunity is also associated with H2O2 signaling.
The response of pepper to Cd stress is closely associated with the response to R. solanacearum inoculation
Our data show that overexpression of CaWRKY41 increases sensitivity to Cd in Arabidopsis in an H2O2-dependent manner, and that silencing of CaWRKY41 enhances susceptibility to R. solanacearum infection and reduces H2O2 accumulation. Specifically, we found that AtOCP3, an important modulator of plant immunity that encodes a protein that catalyzes H2O2 production (Coego et al., 2005; Ramirez et al., 2010; Garcia-Andrade et al., 2011), also confers Cd sensitivity. We reasoned that H2O2 might act as a crucial signaling component that coordinates the response to Cd stress and R. solanacearum inoculation in pepper and, if so, that these responses are closely related.
To test this possibility, we monitored the growth of R. solanacearum in the leaves of pepper plants under Cd stress, and found that the growth of the pathogen was significantly repressed by Cd stress (Supplementary Fig. S9C). Furthermore, expression of CaPR1, CaPR4, and CaNPR1 was induced under Cd toxicity in pepper plants (Supplementary Fig. S9 D–F). On the other hand, when pepper plants were challenged with R. solanacearum, the Cd contents in the roots and leaves of R. solanacearum-inoculated pepper plants were significantly higher than those of mock-treated control plants (Supplementary Fig. S9G, H). Together, these data indicate that the responses of pepper to Cd stress and R. solanacearum inoculation are closely related.
Discussion
Although plant immunity and Cd tolerance have been intensively studied in the past few decades, and several proteins have been implicated in both of these processes (Mirouze et al., 2006; Kim et al., 2007; Kuhnlenz et al., 2015; Campe et al., 2016; Peris-Peris et al., 2017), little is known about the connections between the two processes. In the present study, we provide evidence that both immunity and Cd uptake in pepper are coordinately regulated by CaWRKY41 and are dependent on the ROS signaling pathway.
Responses of pepper to R. solanacearum inoculation and Cd are coordinately regulated by CaWRKY41
We analyzed the expression of eight group III WRKY genes in the roots and leaves of pepper plants grown in the presence of excess Cd or under Fe deficiency, since the response of plants to Fe deficiency was previously shown to be related to the response to excess Cd (Nakanishi et al., 2006; Han et al., 2014; Mendoza-Cozatl et al., 2014). Among these eight genes, only CaWRKY41 was up-regulated in roots and leaves by both excess Cd exposure and Fe deficiency (Fig. 1). In addition, CaWRKY41 was induced by R. solanacearum inoculation (Supplementary Fig. S9A), pointing to a role for CaWRKY41 in the crosstalk between the response to excess Cd exposure and R. solanacearum inoculation in pepper. Gain- and loss-of-function analyses confirmed this speculation: CaWRKY41-silenced pepper plants showed substantially enhanced sensitivity to R. solanacearum inoculation (Fig. 8A), as also revealed by lighter Trypan blue staining compared with PYL-279 plants when challenged with R. solanacearum (Fig. 8F). In addition, the growth rate of R. solanacearum and the disease index (indicative of the severity of symptoms of infection) was higher in CaWRKY41-silenced pepper plants than in PYL-279 plants (Fig. 8B, C). Moreover, the leaves of CaWRKY41-silenced pepper plants showed enhanced tolerance to Cd (Fig. 3B–D), while CaWRKY41-overexpressing Arabidopsis plants exhibited enhanced sensitivity to Cd (Fig. 5A–D), with these plants having a lower fresh weight and shorter primary root than WT plants (Fig. 5E, F).
Together, our findings indicate that CaWRKY41 is a positive regulator of immunity and a negative regulator of Cd tolerance in pepper. Crosstalk between biotic and abiotic stress responses is thought to be involved in coordinately regulating plant responses to multiple environmental stresses (Fujita et al., 2006; Wu et al., 2009). Although the synergistic effect of Cd and Botrytis infection on PDF1.2 expression (Cabot et al., 2013) and the differential regulation of Cd uptake in response to SA application in plants (Kovacik et al., 2009) have been previously reported, little is known about the crosstalk between Cd toxicity and pathogen responses. Furthermore, members of the WRKY TF family have been implicated in plant immunity, but only a few WRKY TFs, such as T. hispida WRKY7 (Yang et al., 2016) and Z. mays WRKY4 (Hong et al., 2017), have been shown to positively regulate plant tolerance to Cd toxicity. The results of the current study strongly suggest that CaWRKY41 plays a role in the crosstalk between the response of pepper to R. solanacearum infection and excess Cd exposure.
R. solanacearum inoculation and excess Cd activate a positive feedback loop between CaWRKY41 expression and H2O2 accumulation
Although bursts of ROS including H2O2 have been shown to be involved in plant responses to pathogen attack (Torres et al., 2006; Vellosillo et al., 2010) and exposure to Cd toxicity (Garnier et al., 2006; Heyno et al., 2008), and the role of H2O2 as a signaling molecule in plant immunity is well established (Alvarez et al., 1998; Qi et al., 2017), the role of H2O2 in plant responses to Cd toxicity has remained elusive.
The results of the current study indicate that both exposure to excess Cd and R. solanacearum inoculation trigger H2O2 accumulation in pepper plants. The enhanced H2O2 accumulation might induce the expression of CaWRKY41, as exogenous application of H2O2 significantly increases CaWRKY41 expression (Fig. 2D), which in turn triggers H2O2 accumulation in Arabidopsis under Cd stress, as revealed by DAB staining and direct H2O2 measurements (Fig. 6A, B). These results suggest that there is a positive feedback loop between CaWRKY41 expression and H2O2 accumulation during the response to R. solanacearum inoculation and excess Cd exposure in pepper. Similar positive feedback loops are common in plant responses to pathogens or other abiotic stresses and are believed to be crucial for amplifying defense signaling (Wang et al., 2014; Cai et al., 2015; Shen et al., 2016; Guo et al., 2017; Ren et al., 2018). In plants, H2O2 is a general signaling molecule in the response to pathogen or abiotic stresses and is coupled with large-scale transcriptional reprogramming (Yang et al., 2013). However, it is unclear how H2O2 signaling is linked to specific TFs. It was recently reported that oxidation of the BRASSINAZOLE-RESISTANT1 (BZR1) transcription factor can be induced by H2O2, and that this plays a major role in regulating gene expression (Tian et al., 2018).
Further research is required to elucidate the mechanism underlying H2O2-mediated transcriptional modulation of CaWRKY41 expression during the response to Cd stress and R. solanacearum infection in pepper. H2O2 accumulation was attributed to its enhanced production and reduced degradation due to the enhanced expression of CaWRKY41, since the genes encoding NADPH oxidases (associated with ROS production), including AtRBOHC (Macho et al., 2012), AtRBOHD (Li et al., 2014; Kadota et al., 2015), AtRBOHE, and AtRBOHF (Chaouch et al., 2012) were up-regulated in Arabidopsis plants overexpressing CaWRKY41 (Fig. 7A–D). These results are consistent with the finding that NADPH oxidases differentially regulate ROS production and are significantly up-regulated by Cd exposure (Gupta et al., 2017). Furthermore, H2O2 accumulation has been found to be dependent on or closely correlated to NADPH oxidase (Foreman et al., 2003). By contrast, genes encoding antioxidant enzymes, including POD, CAT, and APX (Smeets et al., 2013), were significantly down-regulated in response to CaWRKY41 overexpression in Arabidopsis (Fig. 6C–E, Fig. 7E–I). Similarly, it was reported that repression of CATALASE2 (CAT2) resulted in H2O2 accumulation, and that inhibition of H2O2 degradation conferred enhanced disease resistance (Yuan et al., 2017).
We speculate that exposure to excess Cd triggers H2O2 accumulation, and that H2O2, and therefore the expression of CaWRKY41, might confer Cd sensitivity and resistance to R. solanacearum. In support of this notion, the Arabidopsis ocp3 mutant, which produces high levels of H2O2 and exhibits increased resistance to the necrotrophic pathogens Botrytis cinerea and Plectosphaerella cucumerina (Coego et al., 2005), exhibited enhanced sensitivity to excess Cd compared with control plants in the present study (Supplementary Fig. S7). In addition, Cd exposure repressed the growth of R. solanacearum in inoculated pepper plants (Supplementary Fig. S9C). By contrast, R. solanacearum inoculation increased Cd uptake by the roots and leaves of pepper plants exposed to excess Cd (Supplementary Fig. S9G, H). Together, these results strongly suggest that H2O2 accumulation increases plant immunity and plant sensitivity to excess Cd.
CaWRKY41 likely mediates Cd sensitivity by enhancing Cd uptake via enhanced Zn transporter activity
Increased Cd uptake or reduced levels of Cd detoxification result in cellular damage in plants (Schutzendubel et al., 2001). We found that Cd levels in both the roots and shoots of CaWRKY41-overexpressing Arabidopsis plants were significantly higher than those of WT plants (Fig. 5G), indicating that the susceptibility of CaWRKY41-overexpressing Arabidopsis plants to Cd stress is due to their high Cd contents.
Our findings suggest that the enhanced Cd contents might be due to the up-regulation of various Zn transporter genes, such as AtZIP3, AtZIP4, and AtZIP9, by CaWRKY41 (Supplementary Fig. S8B, C, E). Indeed, uptake of Cd by Zn and Fe transporters has previously been suggested (Saraswat and Rai, 2011; Barabasz et al., 2016), and Fe content was found to increase in Arabidopsis roots and to vary in accordance with the period and concentration of Cd treatment (Gupta et al., 2017). However, although CaWRKY41 was activated by Fe deficiency, the Fe content of CaWRKY41-overexpressing Arabidopsis plants did not significantly differ from that of control plants (Supplementary Fig. S6C, D). It is puzzling from an evolutionary point of view why CaWRKY41 would positively regulate disease resistance in pepper plants but promote the absorption of Cd and enhance sensitivity to Cd, which might reduce the adaptability of the plant to a heavy-metal-contaminated environment. We speculate that CaWRKY41 might have evolved to coordinate plant immunity and the absorption of essential ions, including Zn, by modulating the activity of specific ion transporters. Indeed, Zn is required for the functioning of Zn binding motif-containing proteins associated with disease resistance, including WRKY TFs (Duan et al., 2007), Rar1 (Shirasu et al., 1999; Muskett et al., 2002; Wang et al., 2017), and R proteins (Yang et al., 2010; Bi et al., 2011), which play important roles in plant immunity. However, some of these ion transporters can be hijacked by Cd, which has only recently been released into the environment as a result of modern industrial practices, suggesting that plants have not yet evolved a counterstrategy to distinguish between Zn and Cd.
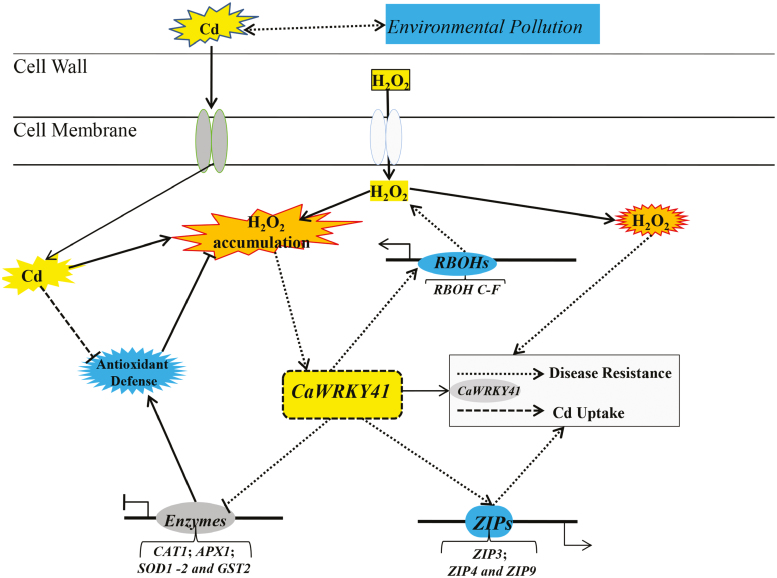
Based on these findings, we propose a working model (Fig. 9) in which H2O2 accumulation and the expression of CaWRKY41, as well as a positive feedback loop between these processes, are induced by R. solanacearum infection or excess Cd exposure. The increase in H2O2 accumulation and CaWRKY41 expression enhance plant immunity and sensitivity to excess Cd exposure by increasing Cd uptake via Zn transporters.
Fig. 9.
Working model for the role of CaWRKY41 in regulating Cd sensitivity and R. solanacearum resistance in pepper. Cd toxicity induces H2O2 production and inhibits the activity of ROS-scavenging enzymes, leading to accumulation of H2O2 and up-regulation of CaWRKY41. Subsequently, CaWRKY41 directly or indirectly activates the expression of ROS-producing genes (RBOH C-F) and Zn transporters (ZIP3, ZIP5, and ZIP9), and inhibits the expression of ROS-scavenging enzymes (CAT1, APX1, SOD1, SOD2, and GST2). Finally, a positive feedback loop between H2O2 accumulation and CaWRKY41 up-regulation coordinates the responses of pepper to R. solanacearum infection and Cd toxicity.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Phylogenetic analysis of eight pepper group III WRKY proteins and Arabidopsis and tomato group III WRKY proteins.
Fig. S2. Cd stress and Fe deficiency promotes H2O2 accumulation.
Fig. S3. GUS expression in transgenic pCaWRKY41::GUS Arabidopsis plants under normal growth conditions.
Fig. S4. CaWRKY41 is a transcriptional activator localized to the nucleus.
Fig. S5. Analysis of the effects of Cd stress on plant growth using chlorophyll fluorescence imaging before the appearance of visible effects on plant growth.
Fig. S6. Effect of Cd treatment on Zn concentrations in Arabidopsis.
Fig. S7. The Arabidopsis ocp3 mutant shows reduced tolerance to Cd stress.
Fig. S8. RT–qPCR analysis of the ZIP members involved in Zn uptake.
Fig. S9. Cd inhibits R. solanacearum growth and R. solanacearum infection increases Cd uptake.
Table S1. Sequences of primers used in this study.
Table S2. CaWRKY group III genes.
Table S3. Analysis of the C/S-elements in the 2 kb promoter fragment of CaWRKY group III genes.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (31572136, 31372061) and the R&D project from the Ministry of Science and Technology of China (2016YFD0100704).
Glossary
Abbreviations:
- ROS
reactive oxygen species
- TF
Transcription factor
- VIGS
virus-induced gene silencing
References
- Adachi H, Nakano T, Miyagawa N, Ishihama N, Yoshioka M, Katou Y, Yaeno T, Shirasu K, Yoshioka H. 2015. WRKY transcription factors phosphorylated by MAPK regulate a plant immune NADPH oxidase in Nicotiana benthamiana. The Plant Cell 27, 2645–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez ME, Pennell RI, Meijer PJ, Ishikawa A, Dixon RA, Lamb C. 1998. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92, 773–784. [DOI] [PubMed] [Google Scholar]
- Apel K, Hirt H. 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology 55, 373–399. [DOI] [PubMed] [Google Scholar]
- Barabasz A, Klimecka M, Kendziorek M, Weremczuk A, Ruszczyńska A, Bulska E, Antosiewicz DM. 2016. The ratio of Zn to Cd supply as a determinant of metal-homeostasis gene expression in tobacco and its modulation by overexpressing the metal exporter AtHMA4. Journal of Experimental Botany 67, 6201–6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter A, Mittler R, Suzuki N. 2014. ROS as key players in plant stress signalling. Journal of Experimental Botany 65, 1229–1240. [DOI] [PubMed] [Google Scholar]
- Bi D, Johnson KC, Zhu Z, Huang Y, Chen F, Zhang Y, Li X. 2011. Mutations in an atypical TIR-NB-LRR-LIM resistance protein confer autoimmunity. Frontiers in Plant Science 2, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose J, Pottosin II, Shabala SS, Palmgren MG, Shabala S. 2011. Calcium efflux systems in stress signaling and adaptation in plants. Frontiers in Plant Science 2, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabot C, Gallego B, Martos S, Barceló J, Poschenrieder C. 2013. Signal cross talk in Arabidopsis exposed to cadmium, silicon, and Botrytis cinerea. Planta 237, 337–349. [DOI] [PubMed] [Google Scholar]
- Cai H, Yang S, Yan Y, et al. 2015. CaWRKY6 transcriptionally activates CaWRKY40, regulates Ralstonia solanacearum resistance, and confers high-temperature and high-humidity tolerance in pepper. Journal of Experimental Botany 66, 3163–3174. [DOI] [PubMed] [Google Scholar]
- Camejo D, Guzmán-Cedeño Á, Moreno A. 2016. Reactive oxygen species, essential molecules, during plant–pathogen interactions. Plant Physiology and Biochemistry 103, 10–23. [DOI] [PubMed] [Google Scholar]
- Campe R, Langenbach C, Leissing F, Popescu GV, Popescu SC, Goellner K, Beckers GJ, Conrath U. 2016. ABC transporter PEN3/PDR8/ABCG36 interacts with calmodulin that, like PEN3, is required for Arabidopsis nonhost resistance. New Phytologist 209, 294–306. [DOI] [PubMed] [Google Scholar]
- Chaouch S, Queval G, Noctor G. 2012. AtRbohF is a crucial modulator of defence-associated metabolism and a key actor in the interplay between intracellular oxidative stress and pathogenesis responses in Arabidopsis. The Plant Journal 69, 613–627. [DOI] [PubMed] [Google Scholar]
- Chen J, Nolan TM, Ye H, Zhang M, Tong H, Xin P, Chu J, Chu C, Li Z, Yin Y. 2017. Arabidopsis WRKY46, WRKY54, and WRKY70 transcription factors are involved in brassinosteroid-regulated plant growth and drought responses. The Plant Cell 29, 1425–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Yang L, Yan X, et al. 2016. Zinc-finger transcription factor ZAT6 positively regulates cadmium tolerance through the glutathione-dependent pathway in Arabidopsis. Plant Physiology 171, 707–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Coego A, Ramirez V, Gil MJ, Flors V, Mauch-Mani B, Vera P. 2005. An Arabidopsis homeodomain transcription factor, OVEREXPRESSOR OF CATIONIC PEROXIDASE 3, mediates resistance to infection by necrotrophic pathogens. The Plant Cell 17, 2123–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang F, Wang Y, She J, et al. 2014. Overexpression of CaWRKY27, a subgroup IIe WRKY transcription factor of Capsicum annuum, positively regulates tobacco resistance to Ralstonia solanacearum infection. Physiologia Plantarum 150, 397–411. [DOI] [PubMed] [Google Scholar]
- Dang FF, Wang YN, Yu L, et al. 2013. CaWRKY40, a WRKY protein of pepper, plays an important role in the regulation of tolerance to heat stress and resistance to Ralstonia solanacearum infection. Plant, Cell & Environment 36, 757–774. [DOI] [PubMed] [Google Scholar]
- Dinakar C, Abhaypratap V, Yearla SR, Raghavendra AS, Padmasree K. 2010. Importance of ROS and antioxidant system during the beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Planta 231, 461–474. [DOI] [PubMed] [Google Scholar]
- Ding ZJ, Yan JY, Li GX, Wu ZC, Zhang SQ, Zheng SJ. 2014. WRKY41 controls Arabidopsis seed dormancy via direct regulation of ABI3 transcript levels not downstream of ABA. The Plant Journal 79, 810–823. [DOI] [PubMed] [Google Scholar]
- Duan MR, Nan J, Liang YH, Mao P, Lu L, Li L, Wei C, Lai L, Li Y, Su XD. 2007. DNA binding mechanism revealed by high resolution crystal structure of Arabidopsis thaliana WRKY1 protein. Nucleic Acids Research 35, 1145–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulgem T, Rushton PJ, Robatzek S, Somssich IE. 2000. The WRKY superfamily of plant transcription factors. Trends in Plant Science 5, 199–206. [DOI] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JH, et al. 2003. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422, 442–446. [DOI] [PubMed] [Google Scholar]
- Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K. 2006. Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Current Opinion in Plant Biology 9, 436–442. [DOI] [PubMed] [Google Scholar]
- García-Andrade J, Ramírez V, Flors V, Vera P. 2011. Arabidopsis ocp3 mutant reveals a mechanism linking ABA and JA to pathogen-induced callose deposition. The Plant Journal 67, 783–794. [DOI] [PubMed] [Google Scholar]
- Garnier L, Simon-Plas F, Thuleau P, Agnel JP, Blein JP, Ranjeva R, Montillet JL. 2006. Cadmium affects tobacco cells by a series of three waves of reactive oxygen species that contribute to cytotoxicity. Plant, Cell & Environment 29, 1956–1969. [DOI] [PubMed] [Google Scholar]
- Guo P, Li Z, Huang P, Li B, Fang S, Chu J, Guo H. 2017. A tripartite amplification loop involving the transcription factor WRKY75, salicylic acid, and reactive oxygen species accelerates leaf senescence. The Plant Cell 29, 2854–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta DK, Pena LB, Romero-Puertas MC, Hernández A, Inouhe M, Sandalio LM. 2017. NADPH oxidases differentially regulate ROS metabolism and nutrient uptake under cadmium toxicity. Plant, Cell & Environment 40, 509–526. [DOI] [PubMed] [Google Scholar]
- Gustin JL, Loureiro ME, Kim D, Na G, Tikhonova M, Salt DE. 2009. MTP1-dependent Zn sequestration into shoot vacuoles suggests dual roles in Zn tolerance and accumulation in Zn-hyperaccumulating plants. The Plant Journal 57, 1116–1127. [DOI] [PubMed] [Google Scholar]
- Han B, Yang Z, Xie Y, Nie L, Cui J, Shen W. 2014. Arabidopsis HY1 confers cadmium tolerance by decreasing nitric oxide production and improving iron homeostasis. Molecular Plant 7, 388–403. [DOI] [PubMed] [Google Scholar]
- Heyno E, Klose C, Krieger-Liszkay A. 2008. Origin of cadmium-induced reactive oxygen species production: mitochondrial electron transfer versus plasma membrane NADPH oxidase. New Phytologist 179, 687–699. [DOI] [PubMed] [Google Scholar]
- Hong C, Cheng D, Zhang G, Zhu D, Chen Y, Tan M. 2017. The role of ZmWRKY4 in regulating maize antioxidant defense under cadmium stress. Biochemical and Biophysical Research Communications 482, 1504–1510. [DOI] [PubMed] [Google Scholar]
- Kadota Y, Shirasu K, Zipfel C. 2015. Regulation of the NADPH oxidase RBOHD during plant immunity. Plant & Cell Physiology 56, 1472–1480. [DOI] [PubMed] [Google Scholar]
- Kalde M, Barth M, Somssich IE, Lippok B. 2003. Members of the Arabidopsis WRKY group III transcription factors are part of different plant defense signaling pathways. Molecular Plant-Microbe Interactions 16, 295–305. [DOI] [PubMed] [Google Scholar]
- Kawachi M, Kobae Y, Mori H, Tomioka R, Lee Y, Maeshima M. 2009. A mutant strain Arabidopsis thaliana that lacks vacuolar membrane zinc transporter MTP1 revealed the latent tolerance to excessive zinc. Plant & Cell Physiology 50, 1156–1170. [DOI] [PubMed] [Google Scholar]
- Keunen E, Schellingen K, Van Der Straeten D, Remans T, Colpaert J, Vangronsveld J, Cuypers A. 2015. ALTERNATIVE OXIDASE1a modulates the oxidative challenge during moderate Cd exposure in Arabidopsis thaliana leaves. Journal of Experimental Botany 66, 2967–2977. [DOI] [PubMed] [Google Scholar]
- Kim DY, Bovet L, Maeshima M, Martinoia E, Lee Y. 2007. The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. The Plant Journal 50, 207–218. [DOI] [PubMed] [Google Scholar]
- Knight H. 2000. Calcium signaling during abiotic stress in plants. International Review of Cytology 195, 269–324. [DOI] [PubMed] [Google Scholar]
- Kovacik J, Grúz J, Hedbavny J, Klejdus B, Strnad M. 2009. Cadmium and nickel uptake are differentially modulated by salicylic acid in Matricaria chamomilla plants. Journal of Agricultural and Food Chemistry 57, 9848–9855. [DOI] [PubMed] [Google Scholar]
- Kuhnlenz T, Westphal L, Schmidt H, Scheel D, Clemens S. 2015. Expression of Caenorhabditis elegans PCS in the AtPCS1-deficient Arabidopsis thaliana cad1-3 mutant separates the metal tolerance and non-host resistance functions of phytochelatin synthases. Plant, Cell & Environment 38, 2239–2247. [DOI] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. 1994. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79, 583–593. [DOI] [PubMed] [Google Scholar]
- Li J, Besseau S, Törönen P, Sipari N, Kollist H, Holm L, Palva ET. 2013. Defense-related transcription factors WRKY70 and WRKY54 modulate osmotic stress tolerance by regulating stomatal aperture in Arabidopsis. New Phytologist 200, 457–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Li M, Yu L, et al. 2014. The FLS2-associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host & Microbe 15, 329–338. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang H, Ai Q, Liang G, Yu D. 2016. Two bHLH transcription factors, bHLH34 and bHLH104, regulate iron homeostasis in Arabidopsis thaliana. Plant Physiology 170, 2478–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv W, Yang L, Xu C, Shi Z, Shao J, Xian M, Chen J. 2017. Cadmium disrupts the balance between hydrogen peroxide and superoxide radical by regulating endogenous hydrogen sulfide in the root tip of Brassica rapa. Frontiers in Plant Science 8, 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macho AP, Boutrot F, Rathjen JP, Zipfel C. 2012. Aspartate oxidase plays an important role in Arabidopsis stomatal immunity. Plant Physiology 159, 1845–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino D, Dunand C, Puppo A, Pauly N. 2012. A burst of plant NADPH oxidases. Trends in Plant Science 17, 9–15. [DOI] [PubMed] [Google Scholar]
- Mendoza-Cózatl DG, Xie Q, Akmakjian GZ, et al. 2014. OPT3 is a component of the iron-signaling network between leaves and roots and misregulation of OPT3 leads to an over-accumulation of cadmium in seeds. Molecular Plant 7, 1455–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Zhang S. 2013. MAPK cascades in plant disease resistance signaling. Annual Review of Phytopathology 51, 245–266. [DOI] [PubMed] [Google Scholar]
- Mersmann S, Bourdais G, Rietz S, Robatzek S. 2010. Ethylene signaling regulates accumulation of the FLS2 receptor and is required for the oxidative burst contributing to plant immunity. Plant Physiology 154, 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metwally A, Finkemeier I, Georgi M, Dietz KJ. 2003. Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiology 132, 272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirouze M, Sels J, Richard O, et al. 2006. A putative novel role for plant defensins: a defensin from the zinc hyper-accumulating plant, Arabidopsis halleri, confers zinc tolerance. The Plant Journal 47, 329–342. [DOI] [PubMed] [Google Scholar]
- Mittler R. 2002. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science 7, 405–410. [DOI] [PubMed] [Google Scholar]
- Muskett PR, Kahn K, Austin MJ, Moisan LJ, Sadanandom A, Shirasu K, Jones JD, Parker JE. 2002. Arabidopsis RAR1 exerts rate-limiting control of R gene-mediated defenses against multiple pathogens. The Plant Cell 14, 979–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi H, Ogawa I, Ishimaru Y, Mori S, Nishizawa NK. 2006. Iron deficiency enhances cadmium uptake and translocation mediated by the Fe2+ transporters OsIRT1 and OsIRT2 in rice. Soil Science and Plant Nutrition 52, 464–469. [Google Scholar]
- Perez-Chaca MV, Rodríguez-Serrano M, Molina AS, Pedranzani HE, Zirulnik F, Sandalio LM, Romero-Puertas MC. 2014. Cadmium induces two waves of reactive oxygen species in Glycine max (L.) roots. Plant, Cell & Environment 37, 1672–1687. [DOI] [PubMed] [Google Scholar]
- Peris-Peris C, Serra-Cardona A, Sánchez-Sanuy F, Campo S, Ariño J, San Segundo B. 2017. Two NRAMP6 isoforms function as iron and manganese transporters and contribute to disease resistance in rice. Molecular Plant-Microbe Interactions 30, 385–398. [DOI] [PubMed] [Google Scholar]
- Qi J, Wang J, Gong Z, Zhou JM. 2017. Apoplastic ROS signaling in plant immunity. Current Opinion in Plant Biology 38, 92–100. [DOI] [PubMed] [Google Scholar]
- Ramírez V, Van der Ent S, García-Andrade J, Coego A, Pieterse CM, Vera P. 2010. OCP3 is an important modulator of NPR1-mediated jasmonic acid-dependent induced defenses in Arabidopsis. BMC Plant Biology 10, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranieri A, Castagna A, Baldan B, Soldatini GF. 2001. Iron deficiency differently affects peroxidase isoforms in sunflower. Journal of Experimental Botany 52, 25–35. [PubMed] [Google Scholar]
- Ren T, Wang J, Zhao M, Gong X, Wang S, Wang G, Zhou C. 2018. Involvement of NAC transcription factor SiNAC1 in a positive feedback loop via ABA biosynthesis and leaf senescence in foxtail millet. Planta 247, 53–68. [DOI] [PubMed] [Google Scholar]
- Rodriguez MC, Petersen M, Mundy J. 2010. Mitogen-activated protein kinase signaling in plants. Annual Review of Plant Biology 61, 621–649. [DOI] [PubMed] [Google Scholar]
- Romero-Puertas MC, Corpas FJ, Rodríguez-Serrano M, Gómez M, Del Río LA, Sandalio LM. 2007. Differential expression and regulation of antioxidative enzymes by cadmium in pea plants. Journal of Plant Physiology 164, 1346–1357. [DOI] [PubMed] [Google Scholar]
- Rushton PJ, Somssich IE, Ringler P, Shen QJ. 2010. WRKY transcription factors. Trends in Plant Science 15, 247–258. [DOI] [PubMed] [Google Scholar]
- Saraswat S, Rai JPN. 2011. Complexation and detoxification of Zn and Cd in metal accumulating plants. Reviews in Environmental Science and Bio/Technology 10, 327–339. [Google Scholar]
- Sarris PF, Duxbury Z, Huh SU, et al. 2015. A plant immune receptor detects pathogen effectors that target WRKY transcription factors. Cell 161, 1089–1100. [DOI] [PubMed] [Google Scholar]
- Schützendübel A, Schwanz P, Teichmann T, Gross K, Langenfeld-Heyser R, Godbold DL, Polle A. 2001. Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and differentiation in Scots pine roots. Plant Physiology 127, 887–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwizer S, Kraus CM, Dunham DM, Zheng Y, Fernandez-Pozo N, Pombo MA, Fei Z, Chakravarthy S, Martin GB. 2017. The tomato kinase Pti1 contributes to production of reactive oxygen species in response to two flagellin-derived peptides and promotes resistance to Pseudomonas syringae infection. Molecular Plant-Microbe Interactions 30, 725–738. [DOI] [PubMed] [Google Scholar]
- Shen L, Liu Z, Yang S, et al. 2016. Pepper CabZIP63 acts as a positive regulator during Ralstonia solanacearum or high temperature–high humidity challenge in a positive feedback loop with CaWRKY40. Journal of Experimental Botany 67, 2439–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shidore T, Broeckling CD, Kirkwood JS, Long JJ, Miao J, Zhao B, Leach JE, Triplett LR. 2017. The effector AvrRxo1 phosphorylates NAD in planta. PLoS Pathogens 13, e1006442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasu K, Lahaye T, Tan MW, Zhou F, Azevedo C, Schulze-Lefert P. 1999. A novel class of eukaryotic zinc-binding proteins is required for disease resistance signaling in barley and development in C. elegans. Cell 99, 355–366. [DOI] [PubMed] [Google Scholar]
- Smeets K, Opdenakker K, Remans T, Forzani C, Hirt H, Vangronsveld J, Cuypers A. 2013. The role of the kinase OXI1 in cadmium- and copper-induced molecular responses in Arabidopsis thaliana. Plant, Cell & Environment 36, 1228–1238. [DOI] [PubMed] [Google Scholar]
- Sun B, Jing Y, Chen K, Song L, Chen F, Zhang L. 2007. Protective effect of nitric oxide on iron deficiency-induced oxidative stress in maize (Zea mays). Journal of Plant Physiology 164, 536–543. [DOI] [PubMed] [Google Scholar]
- Sun N, Liu M, Zhang W, Yang W, Bei X, Ma H, Qiao F, Qi X. 2015. Bean metal-responsive element-binding transcription factor confers cadmium resistance in tobacco. Plant Physiology 167, 1136–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R. 2011. Respiratory burst oxidases: the engines of ROS signaling. Current Opinion in Plant Biology 14, 691–699. [DOI] [PubMed] [Google Scholar]
- Tian Y, Fan M, Qin Z, et al. 2018. Hydrogen peroxide positively regulates brassinosteroid signaling through oxidation of the BRASSINAZOLE-RESISTANT1 transcription factor. Nature Communications 9, 1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Jones JD, Dangl JL. 2006. Reactive oxygen species signaling in response to pathogens. Plant Physiology 141, 373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K, Katagiri F. 2010. Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Current Opinion in Plant Biology 13, 459–465. [DOI] [PubMed] [Google Scholar]
- Valko M, Morris H, Cronin MT. 2005. Metals, toxicity and oxidative stress. Current Medicinal Chemistry 12, 1161–1208. [DOI] [PubMed] [Google Scholar]
- Vellosillo T, Vicente J, Kulasekaran S, Hamberg M, Castresana C. 2010. Emerging complexity in reactive oxygen species production and signaling during the response of plants to pathogens. Plant Physiology 154, 444–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, El-Shetehy M, Shine MB, Yu K, Navarre D, Wendehenne D, Kachroo A, Kachroo P. 2014. Free radicals mediate systemic acquired resistance. Cell Reports 7, 348–355. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang Y, Liu P, et al. 2017. TaRar1 is involved in wheat defense against stripe rust pathogen mediated by YrSu. Frontiers in Plant Science 8, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Deng Z, Lai J, et al. 2009. Dual function of Arabidopsis ATAF1 in abiotic and biotic stress responses. Cell Research 19, 1279–1290. [DOI] [PubMed] [Google Scholar]
- Yang F, Li W, Jørgensen HJ. 2013. Transcriptional reprogramming of wheat and the hemibiotrophic pathogen Septoria tritici during two phases of the compatible interaction. PLoS One 8, e81606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Shi Y, Liu J, Guo L, Zhang X, Yang S. 2010. A mutant CHS3 protein with TIR-NB-LRR-LIM domains modulates growth, cell death and freezing tolerance in a temperature-dependent manner in Arabidopsis. The Plant Journal 63, 283–296. [DOI] [PubMed] [Google Scholar]
- Yang GY, Wang C, Wang YC, Guo YC, Zhao YL, Yang CP, Gao CQ. 2016. Overexpression of ThVHAc1 and its potential upstream regulator, ThWRKY7, improved plant tolerance of Cadmium stress. Scientific Reports 6, 18752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan HM, Liu WC, Lu YT. 2017. CATALASE2 coordinates SA-mediated repression of both auxin accumulation and JA biosynthesis in plant defenses. Cell Host & Microbe 21, 143–155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.