Abstract
Purpose
To evaluate the effect of the overexpression of miR-21 on the properties of pterygium and examine whether miR-21 promotes the proliferation of pterygium cells through targeting the PTEN/AKT signaling pathway.
Methods
Information regarding patient gender, age, and pterygium severity was gathered. Expression of miR-21 was obtained through examination of excised pterygium tissues and superior conjunctiva tissues with real-time PCR. Human pterygium fibroblasts (HPFs) were obtained from pterygium surgery and subjected to primary culture. The HPF cell lines were divided into a negative control group, an miR-21 inhibitor group, and an miR-21 inhibitor + VO-Ohpic trihydrate group, and then the cell viability and apoptosis and the expression of PTEN and AKT were examined.
Results
Fifty-eight subjects with unilateral primary pterygium were included. An increase in the miR-21 levels in pterygium tissue was evident compared with that in the paired normal conjunctival tissues (independent-samples t test, p<0.01). As the severity of the pterygium increased, the miR-21 levels increased (p=0.004, rs=0.373, Spearman’s rank correlation coefficient). The miR-21 inhibitor suppressed the proliferation and induced apoptosis of HPF cells through increasing the PTEN expression, and further decreasing the expression of p-AKT, which could be reversed by the PTEN inhibitor VO-Ohpic trihydrate.
Conclusions
Aberrant miR-21 overexpression in the pterygium could target PTEN, which contributes to abnormal proliferation of the HPF cells through depressing the PTEN/AKT pathway. The results also suggested the potential of miR-21 and the PTEN/AKT pathway as a novel therapeutic strategy for pterygium.
Introduction
Pterygium is a wing-shaped ocular tissue that consists of conjunctival epithelia and fibrovascular tissue with a prevalence of 2% in temperate regions and up to 20% in tropical parts of the world [1]. Pterygium is thought to be a type of benign proliferative lesion and is characterized by activation of myofibroblast cells and excess production of the extracellular matrix (ECM) [2].
Various theories have been postulated for the genesis and development of pterygium, including ultraviolet exposition, immunological mechanisms, viral infections, neoplasm, and limbal stem cell deficiency [3]. Thus far, the precise pathogenesis of this disorder has not been fully understood yet. Furthermore, despite the various operative procedures that have been used for treating primary pterygium, postoperative recurrence remains a major challenge [4]. New and more effective treatment strategies should be explored.
MicroRNAs are a group of endogenous non-coding RNAs that can modulate various biologic processes. The function is accomplished through complementation with the 3′ untranslated region (UTR) of a target mRNA totally or partially, or through post-translational repression of encoded proteins [5,6]. The expression of miR-21 has been found to be high in cervical cancer, colorectal cancer, breast cancer, and other tumors [7-9].
PTEN (Gene ID 5728, OMIM 601728) was found to be one of the target genes of miR-21 [10-13]. Accumulating evidence suggests that miR-21 promotes cell proliferation via the activation of PTEN/AKT signaling in cancer cells [14,15]. Previous studies showed that transducing the Tat-PTEN fusion protein into subconjunctival fibroblasts results in the suppression of Akt phosphorylation and further suppresses activation of myofibroblasts [16].
The progression of pterygium is significantly associated with the activation of myofibroblast cells [17,18]. Therefore, this study aimed to investigate the expression of miR-21 in primary pterygium tissues compared to normal conjunctival tissues and to clarify whether miR-21 plays a role in the pathogenesis of pterygium through the PTEN/AKT signaling pathway.
Methods
Study design
Subjects with unilateral pterygium who underwent excision with conjunctival autografting from January 2016 to August 2016 were enrolled in the study. A detailed ophthalmic examination and slit-lamp photography (Topcon SL-D Digital Slit-Lamp; Topcon, Tokyo, Japan) were performed for each patient. A 16X magnification image was taken for further analysis. Those who had ocular diseases other than pterygium and those who had undergone ocular surgeries were excluded in the study. A small rectangular piece of normal conjunctival tissue was taken from the autograft from the superior conjunctiva as control. Pterygium tissues and conjunctival tissues were collected at the time of surgery and frozen in liquid nitrogen for use. This study followed the tenets of the Declaration of Helsinki and was approved by the ethics committee of Shanghai Heping Ophthalmological Hospital. Written informed consent was obtained from all patients and Declaration of Helsinki and Declaration of ARVO statement on human subjects and was approved.
Primary pterygium severity was graded based on the slit-lamp examination and slit-lamp photography [19]. The extension, vascularity, and thickness of the pterygium were evaluated in this grading system. Then the grades were converted into a severity score. Based on this severity score, all subjects were stratified into mild (3–5), moderate (6–8), and severe (9–11) pterygium subgroups.
Reagents
Reagents were purchased as follows: PBS (1X; 137 mM NaCl, 2.7 mM KCl, 10 mM NaPO4, 2 mM KPO4, pH 7.4; Hyclone, Logan UT), miR-21 inhibitor and miR-21 negative control (NC; Sangon Biotech Co., Ltd., Shanghai, China), primary antibodies against PTEN and p-AKT (Epitomics, Carlsbad, CA), fluorescent-dye conjugated secondary antibodies (ThermoFisher, Carlsbad, CA), PTEN inhibitor VO-Ohpic trihydrate (MedchemExpress, Monmouth Junction, NJ), fetal bovine serum (FBS, ThermoFisher), Dulbecco’s modified Eagle’s medium/F12 (Gibco, Grand Island, NY), Lipofectamine 3000 (Invitrogen, Carlsbad, CA), and Annexin V-FITC Apoptosis Detection Kit (Becton Dickinson, Franklin Lake, NJ).
Human pterygium fibroblast cell culture and human conjunctival fibroblast cell culture
Human pterygium fibroblast (HPF) cell lines were cultivated from subconjunctival connective tissue collected from patients with pterygium during the surgical removal. In less than 4 h after the surgical excision, under aseptic conditions and in a laminar flow, the subconjunctival connective tissues were placed in 35 mm Petri dishes and washed with DMEM/F12 for removal of blood and cut into minor pieces. Then the small pieces were placed in polystyrene cell culture flasks with DMEM/F12 (Gibco) supplemented with 10% FBS; Gibco Life Technologies), 100 U/ml penicillin, and 100 g/ml streptomycin (Gibco Life Technologies). The cells were maintained at 37 °C with 5% CO2 in a humidified atmosphere, with exchanges of nutrient medium every 3 days until they reached semiconfluence, when they were subcultured. Cells between the third and sixth passages were used for all experiments. Human conjunctival fibroblast cell lines were cultivated from subconjunctival connective tissue collected from patients with pterygium during the surgical removal. Then the procedure was the same as that for the culture of the HPF cells.
Immunohistochemistry
The pterygium tissue and conjunctival tissue were fixed with 4% buffered paraformaldehyde for 24 h and then washed in PBS for 2 min. The washing procedure was repeated three times. The tissues were dehydrated with a series of graded sucrose and embedded in optimum cutting temperature (OCT) compound. Sections were cut to a thickness of 8 µm. Then the sections were incubated in blocking buffer for 1 h at room temperature to block non-specific binding. The antibody against PTEN (1:200 dilution, Epitomics) was used to show the expression of PTEN in the pterygium and conjunctival tissues. The sections were incubated in primary antibody solution for 24 h at −4 °C. Then the samples were washed in PBS for 5 min, and the washing procedure was repeated three times. All samples were incubated for 2 h with fluorescent-dye conjugated secondary antibodies at room temperature. Samples were incubated for 5 min using Hoechst to stain the cell nuclei. Then the samples were washed in PBS for 5 min, and the washing procedure was repeated three times. The confocal laser-scanning microscope (CLSM) was performed to evaluate the expression of PTEN. The cultured cells was fixed using 4% buffered paraformaldehyde for 24 h and then washed in PBS for 2 min. The washing procedure was repeated three times. From the blocking non-specific binding step on, the procedure was the same as that for the pterygium and conjunctival tissues.
miRNA transfection
When the density of the cultured HPF cells reached 50–70%, the cells were transfected with the miR-21 inhibitor or NC sequence using Lipofectamine 3000 according to the manufacturer’s instructions. Fresh medium was added after transfection for 6 h.
Cell grouping and treatment methods
The HPF cells were divided into the NC group, the miR-21 inhibitor group, and the miR-21 inhibitor + VO-Ohpic trihydrate group. The NC group was transfected only with negative control miRNA sequences, the miR-21 inhibitor group was transfected with miR-21 inhibitor sequences, and the miR-21 inhibitor+ VO-Ohpic trihydrate group was transfected with miR-21 inhibitor sequences followed by the addition of the PTEN inhibitor VO-Ohpic trihydrate.
Quantitative real-time reverse transcription PCR
Quantitative real-time reverse transcription PCR (qRT-PCR; conditions for amplification were as follows: one cycle at 95 °C for 15 min, 40 cycles at 94 °C for 20 s, 60 °C for 34 s) reactions were performed using the ViiA 7 Real-Time PCR System (Applied Biosystems, Carlsbad, CA) to validate the expression level of PTEN and miR-21. Total RNA was isolated using TRIzol reagents (Tiangen Biotech Co., Beijing, China). A miRcute miRNA isolation kit (Tiangen Biotech Co.) was used to isolate small RNAs from the total RNA according to the manufacturer’s instructions. cDNAs of the mRNAs were obtained using M-MLV reverse transcriptase (Invitrogen) and oligo(dT)18. For miRNA, cDNA was then synthesized using miRcute plus miRNA First-strand cDNA Synthesis Kit (Tiangen Biotech Co.) according to the manufacturer’s instructions. For mRNA quantification, qRT-PCR was performed using the Tiangen Biotech Talent qPCR PreMix Detection Kit (FP209). For microRNA quantification, qRT-PCR was performed using the miRcute qPCR Detection Kit (SYBR Green; Tiangen Biotech Co.) with specific primer sets. PCR amplification was performed with the following primers: PTEN, 5′-TTT GAG AGT TGA GCC GCT GT-3′, and 5′-ATG CTT TGA ATC CAA AAA CCT TAC T-3′. The internal control for mRNA was GAPDH with the forward primer 5′-AGC CAC ATC GCT CAG ACA-3′ and the reverse primer 5′-TGG ACT CCA CGA CGT ACT-3′. The mature miR-21 sequence was 5′-UAG CUU AUC AGA CUG AUG UUG A-3′. The miR-21 primer and the internal control U6 (Catalog Number: CD201–0145) were obtained from Tiangen Biotech Co. Conditions for amplification were adjusted in accordance with the manufacturer’s instructions. The relative expression of the gene of interest was analyzed using the 2-ΔΔCT method.
Cell growth tested using the Cell Counting Kit-8 (CCK-8)
The medium was renewed with 100 µl/well (96-well), and then 10 µl CCK-8 was added into each well (Research Institute of Tongren Chemistry, Kyushu, Japan), and the blank control group was set (with medium only). Both groups were developed for 1 h at 37 °C. At 24, 48, and 72 h, the cell cultural supernatants were transferred to new tubes for use. The absorbance of each well at 450 nm was recorded on a microplate reader. In each group, the average value of three wells was obtained.
Determination of cell apoptosis with flow cytometry with Annexin V-FITC/PI reagent
HPF cells in the logarithmic growth phase were diluted to 1 × 104 cells/ml, seeded in a six-well plate, and cultured with 5% CO2 at 37 °C. After the treatment with the miR-21 NC, miR-21 inhibitor, or miR-21 inhibitor together with VO-Ohpic for 48 h, the cells were treated with the Annexin V-FITC/PI reagent according to the manufacturer’s protocol. Within 1 h, cell apoptosis was analyzed via flow cytometry (FACSCanto II; BD Biosciences, Franklin Lake, NJ).
Western blot
Cells were collected and total proteins were extracted using the radioimmunoprecipitation assay (RIPA) lysis buffer. The cell lysis was centrifuged at 10947 ×g 4 °C for 15 min. Equal amounts of protein were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to a polyvinylidene difluoride (PVDF) membrane. After being blocked with 5% non-fat milk in Tris-buffered saline containing 0.05% Tween-20 (TBST), the membranes were incubated overnight using corresponding antibodies at 4 °C. Next, the membranes were washed with TBST three times and incubated with the corresponding horseradish peroxidase–conjugated secondary antibody at 1:1,000 dilution in TBST. The membranes were developed with electrochemiluminescence (ECL) solution (Pierce, Rockford, IL) and photographed as images. Target protein bands were quantitatively analyzed using Quantity One software (Appendix 1).
Statistical methods
The difference in the expression of miR-21 between pterygia tissue and conjunctival tissue was analyzed with an independent-samples t test. Correlation between pterygium clinical characteristics and the level of miR-21 was determined with the Spearman rank correlation test or Pearson correlation analysis. Western blot results were normalized using β-actin. One-way ANOVA followed by post hoc Bonferroni’s test was used to compare the differences among the three groups. Independent experiments were performed three times. A p value of less than 0.05 was considered statistically significant. All analyses were performed using SPSS 22.0 (IBM Corp, Amun, NY).
Results
The demographic data of the patients with pterygium and the severity grades
At the end of the investigation, 58 patients were enrolled in this study. The age range of was from 26 to 82 years old, with a median of 66±9.0 years on average. All patients were graded according to the criterion mentioned in a previous section (Table 1).
Table 1. The grade of the subjects with primary pterygium.
| Number of subjects | Female subjects(Number) | Patients age | |
|---|---|---|---|
| Extension(E) |
|
|
|
| Grade 1 |
17 |
12 |
26-79(62.88±13.22) |
| Grade 2 |
15 |
12 |
60-78(67.27±6.01) |
| Grade 3 |
17 |
12 |
49-80(66.24±7.73) |
| Grade 4 |
9 |
3 |
52-82(69.11±9.96) |
| Vascularity(V) |
|
|
|
| Grade 1 |
19 |
16 |
47-82(64.58±9.00) |
| Grade 2 |
26 |
16 |
26-80(67.545±11.22) |
| Grade 3 |
13 |
7 |
52-81(64.85±7.10) |
| Thickness(T) |
|
|
|
| Grade 1 |
17 |
11 |
26-80(62.06±13.32) |
| Grade 2 |
15 |
10 |
59-80(69.27±6.20) |
| Grade 3 |
15 |
11 |
49-82(68.73±8.31) |
| Grade 4 | 11 | 7 | 52-75(63.73±6.25) |
Nineteen pterygium cases (32.8%) were classified as mild, 27 cases (46.6%) were classified as moderate, and 12 cases (20.7%) were classified as severe. Patient age and gender had no effect on the classifications of severity. Patient age and gender showed no correlation with the miR-21 levels (Pearson correlation analysis, Pearson r1=0.112, P1=0.404, Pearson r2=–0.04, P2=0.764).
miR-21 was highly expressed in pterygium tissues and correlates with the severity grades
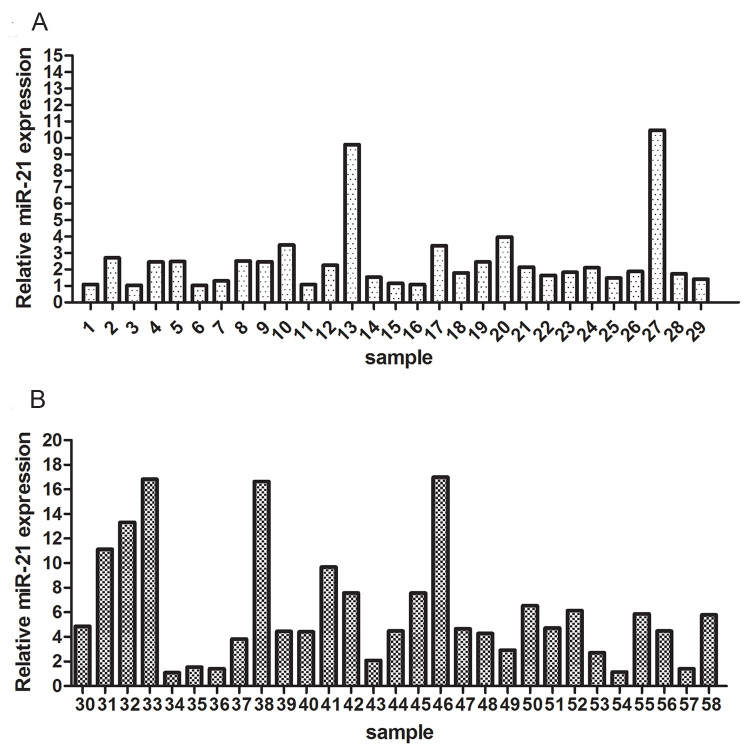
To explore the expression of miR-21 in pterygium, the relative levels of miR-21 in pterygium tissues and adjacent normal conjunctival tissues were analyzed with qRT-PCR. The expression of miR-21 in the pterygium tissues was more than fourfold that in the normal conjunctival tissues (independent-samples t test, p<0.01; Figure 1). A positive correlation was found between severity and the level of expression of miR-21 (p=0.004, rs=0.373, Spearman’s rank correlation analysis).
Figure 1.
The expression of miR-21 in human pterygium tissues from 58 patients. A and B: These pterygium tissues exhibited significantly higher expression of miR-21 compared to the control.
The expression of PTEN was lower and p-AKT was higher in pterygium tissues compared to conjunctival tissues
The expression of PTEN was lower in the pterygium tissues (one-way ANOVA) followed by the Bonferroni test, P1<0.001, P2<0.001, P3<0.001) compared to the normal conjunctival tissues. The p-AKT exhibited higher expression in pterygium tissues compared to the conjunctival tissues (one-way ANOVA followed by the Bonferroni test, P1=0.647, P2=0.012, P3<0.001; Figure 2, Figure 3).
Figure 2.
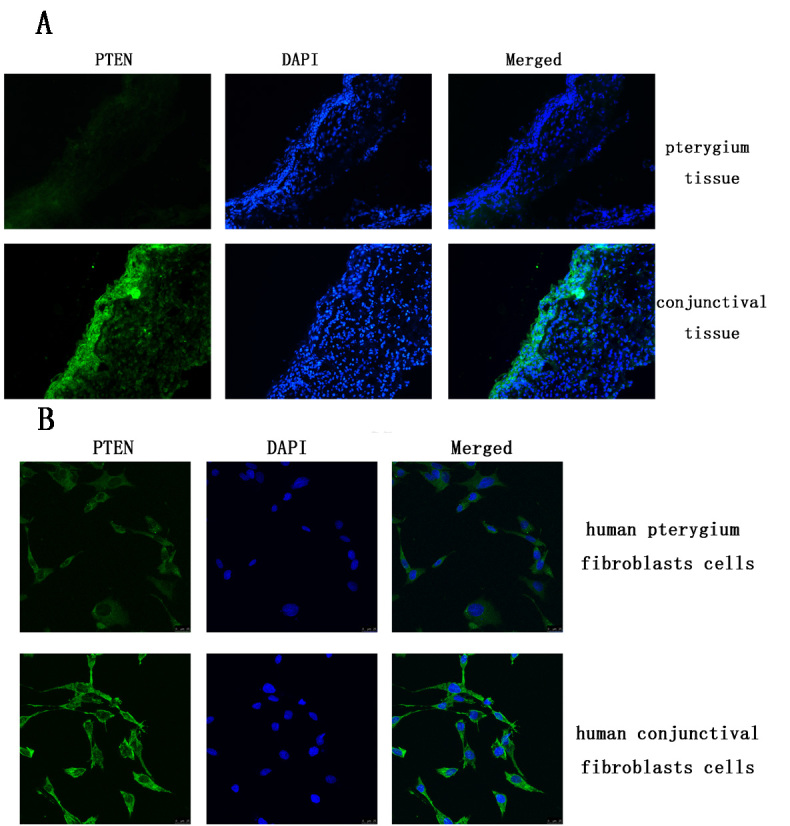
The expression of PTEN in the pterygium tissue and cultured pterygium fibroblast cells. A: The expression of PTEN was lower in pterygium tissue than that in conjunctival tissues. B: The expression of PTEN was lower in cultured pterygium fibroblast cells than that in cultured conjunctival fibroblast cells.
Figure 3.
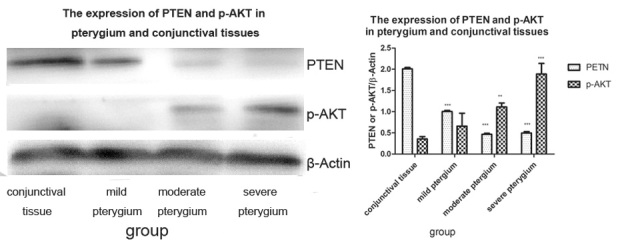
The expression of PTEN was lower and the expression of p-AKT was higher in pterygium tissues compared to conjunctival tissues. **p<0.05, ***p<0.001.
miR-21 inhibitor inhibited cell proliferation and promoted cell apoptosis
Compared with the miR-21 NC sequence transfection group, the miR-21 inhibitor significantly suppressed the expression of miR-21 (Figure 4). These data showed that the miR-21 inhibitor could effectively suppress the expression of miR-21 in HPF cells.
Figure 4.
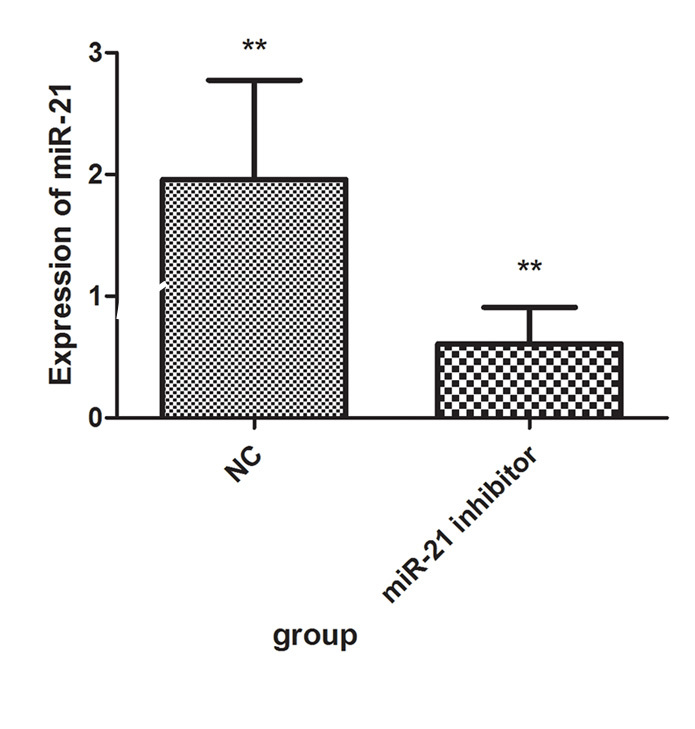
After HPF cells were transfected with miR-21 inhibitor for 24 h, the expression of miR-21 was statistically significantly suppressed compared to the negative control (NC). **p<0.01.
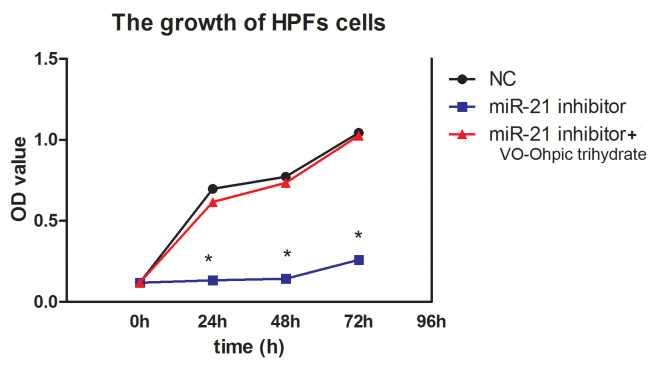
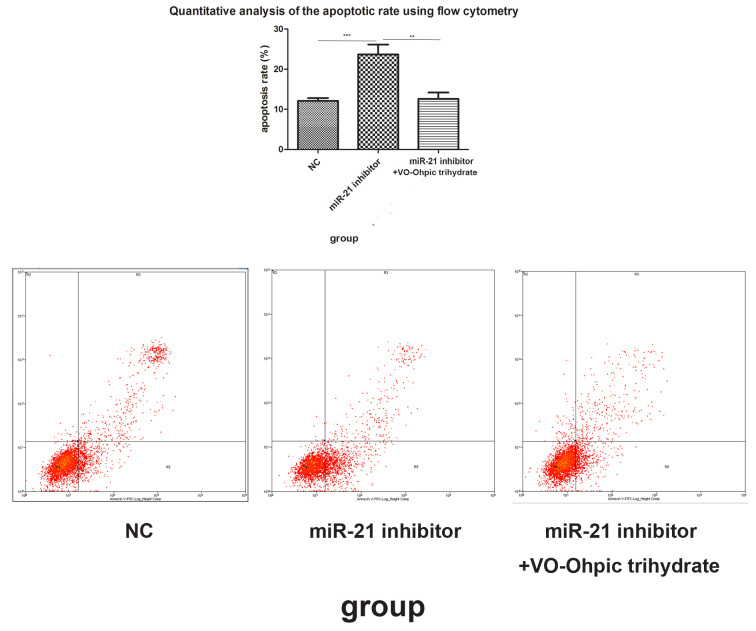
Compared with the cells transfected with the NC group, the miR-21 inhibitor statistically significantly suppressed cell proliferation at different time points (p<0.001; Figure 5). Flow cytometry with the Annexin V-FITC/PI reagent showed that the cell apoptosis rate in the miR-21 inhibitor transfected group was higher than that in the NC group (p=0.003; Figure 6).
Figure 5.
The miR-21 inhibitor inhibited cell proliferation. The miR-21 inhibitor suppressed the cell relative viability. NC, negative control. *p<0.05.
Figure 6.
Cell apoptosis was detected with flow cytometry with the Annexin V-FITC/PI reagent. The fourth quadrant and the second quadrant indicated the early apoptotic cells and the late apoptotic cells, respectively. Compared to the miR-21 negative control (NC) group, the miR-21 inhibitor promoted cell apoptosis. **p<0.01, ***p<0.001.
miR-21 modulated PTEN/AKT signaling in HPF cells
VO-Ohpic trihydrate is a highly selective small-molecule inhibitor of PTEN. To elucidate the effect of miR-21 toward HPF cells through PTEN, 1.0 uM VO-Ohpic trihydrate was administered to the miR-21 inhibitor–transfected cells. The CCK-8 and flow cytometry with Annexin V-FITC/PI reagent were performed to determine cell viability and apoptosis. Compared with the cells transfected with the miR-21 inhibitor, the addition of VO-Ohpic trihydrate statistically significantly recovered the cell proliferation (p<0.001; Figure 5) and reduced the cell apoptosis rate (p=0.001; Figure 6).
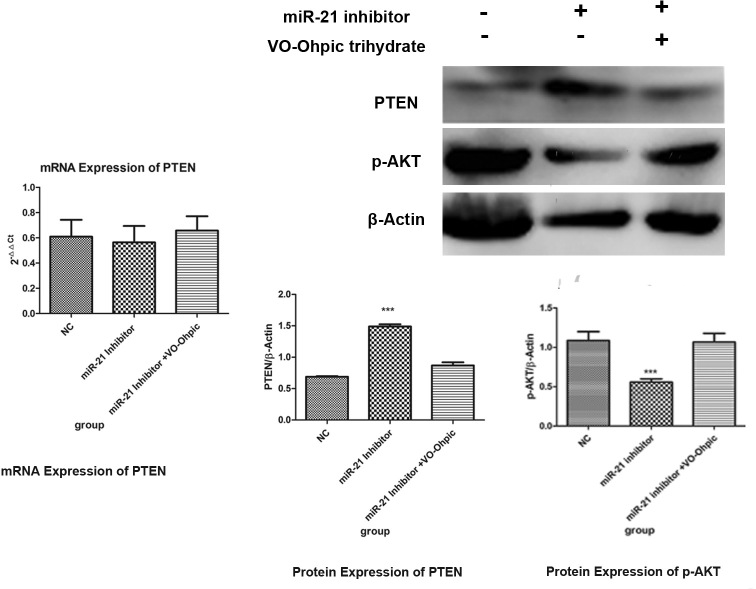
Then the expression of PTEN and p-AKT in the three different cell groups was examined. Compared with the NC group, the expression of the PTEN protein but not the PTEN mRNA levels in the miR-21 inhibitor group increased (p<0.001). While for the miR-21 inhibitor + VO-Ohpic trihydrate group, the protein expression of PTEN did not change compared with the NC group (p=0.098). Compared with the NC group, the miR-21 inhibitor suppressed the protein level of phosphorylation AKT (p-AKT; p<0.001), which was reversed by the PTEN inhibitor VO-Ohpic trihydrate (p<0.001), and made the expression of p-AKT return to the level of the NC group (p=0.177). These results revealed that miR-21 enhances cell proliferation and inhibits cell apoptosis through regulating the PTEN/AKT signaling pathway in the HPF cell line (Figure 7).
Figure 7.
MiR-21 induces cell proliferation through the PTEN/AKT dependent pathway. The miR-21 inhibitor promoted the expression of the PTEN protein and inhibited the expression of p-AKT, and VO-Ohpic trihydrate reversed the effect. NC, negative control. **p<0.01, ***p<0.001.
Discussion
The critical role for miR-21 in the development and progression of cancer has been well established [7], and it has been found that miR-21 mediates cellular proliferation through inhibiting the expression of PTEN [14]. Results of the present study showed that miR-21 was upregulated in human pterygium tissues compared with normal conjunctiva tissues. A positive correlation was found between severity and the level of expression of miR-21. The protein expression of PTEN in pterygium was lower than that in the conjunctival tissues, while the protein expression of p-AKT was higher. The Inhibition of miR-21 inhibited the proliferation of HPF cells and led to apoptosis, which can be reversed by the PTEN inhibitor VO-Ohpic trihydrate. The inhibition of miR-21 upregulated PTEN and then resulted in the decreased expression of p-AKT.
In this study, the higher level of miR-21 corresponded to the severity of pterygium. For pterygium, the proliferative growth of HPF cells was the main histopathologic change [20-23], and the proliferation of HPF cells contributes to the development of pterygium [18]. miR-21 has been shown to be related to multiple cell processes, including cell proliferation, apoptosis, and invasion [24,25], and acts as a cancer-promoting molecule [26-31] in several different types of human cancers, including neuroblastoma, lung, breast, colorectal, pancreas, and lymphoma.
When the miR-21 inhibitor was transferred into cultured HPF cells, decreased proliferation of HPF cells and increased apoptosis followed. miR-21 played an essential part in the proliferative growth of HPFs, which was consistent with the in vivo results.
Previous studies demonstrated that miR-21 represses PTEN through translational inhibition, and the miR-21 binding site in the PTEN 3′ UTR is crucial [32]. In accordance with these findings, we found that miR-21 interfered with the translation of PTEN without reducing its mRNA level. Suppression of miR-21 decreased proliferation and induced apoptosis in HPF cells, and the PTEN inhibitor VO-Ohpic trihydrate partly reversed the effect. Moreover, the fact that inhibition of miR-21 resulted in the increase in PTEN protein expression further confirmed the conclusion that regulation of PTEN was by miR-21 in HPF cells. These findings suggest that miR-21 promotes the growth of HPF cells by targeting PTEN.
PTEN modulates cell biologic processes via its target molecule Akt [33,34]. The present study indicated that knocking down miR-21 expression led to the inactivation of AKT in HPF cells while the PTEN inhibitor neutralized the suppressive effects of miR-21 inhibitor.
Taken together, the results indicate that the miRNA-21/PTEN/AKT signaling pathway plays an important role in the development of pterygium. Similar to the western blot results, a higher grade of pterygium, lower expression of the PTEN protein, and higher expression of p-AKT protein were observed. miR-21 acted as a growth promoter, and the relative level of miR-21 in pterygium compared to conjunctival tissues determined the severity of the pterygium through regulation of the PTEN/p-AKT pathway.
The involvement of miR-21 in pterygium pathogenesis may suggest an alternative avenue for developing treatments [35] [36]. Recent research found that miR-21/vascular endothelial growth factor (VEGF) plays an important role in the development of ocular angiogenesis [37]. Previous studies showed that anti-VEGF therapy was effective in some patients with pterygium [38] taking into account that miR-21 is the relative upstream factor and anti-miR-21 may be a possible treatment strategy for pterygium. Of course, a delivery system is required to ensure stability in vivo and to efficiently and safely introduce nucleic acid–based drugs into cells [39].
Acknowledgments
The authors thank Xumin Shang for excellent operation assistance. This study was funded by Hongkou health-bureau project funding (Hongwei140339) and had no commercial relationship with any organization.
Appendix 1. Quantity One software.
To access the data, click or select the words “Appendix 1.”
References
- 1.Papadia M, Barabino S, Valente C, Rolando M. Anatomical and immunological changes of the cornea in patients with pterygium. Curr Eye Res. 2008;33:429–34. doi: 10.1080/02713680802130354. [DOI] [PubMed] [Google Scholar]
- 2.Mauro J, Foster CS. Pterygia: pathogenesis and the role of subconjunctival bevacizumab in treatment. Semin Ophthalmol. 2009;24:130–4. doi: 10.1080/08820530902801106. [DOI] [PubMed] [Google Scholar]
- 3.Chui J, Di Girolamo N, Wakefield D, Coroneo MT. The pathogenesis of pterygium: current concepts and their therapeutic implications. Ocul Surf. 2008;6:24–43. doi: 10.1016/s1542-0124(12)70103-9. [DOI] [PubMed] [Google Scholar]
- 4.Ang LP, Chua JL, Tan DT. Current concepts and techniques in pterygium treatment. Curr Opin Ophthalmol. 2007;18:308–13. doi: 10.1097/ICU.0b013e3281a7ecbb. [DOI] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song N, Liang B, Wang D. The function of miR-21 expression differences and pathogenesis on familial and triple negative breast cancer serum. Pak J Pharm Sci. 2016;29:679–84. [PubMed] [Google Scholar]
- 8.Y. Gómez-Gómez Y Organista-Nava J, Ocadiz-Delgado R, García-Villa E, Leyva-Vazquez MA, Illades-Aguiar B, Lambert PF, García-Carrancá A, Gariglio P. The expression of miR-21 and miR-143 is deregulated by the HPV16 E7 oncoprotein and 17β-estradiol. Int J Oncol. 2016;49:549–58. doi: 10.3892/ijo.2016.3575. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z, Liu H, Jin W, Ding Z, Zheng S, Tissue YYu. microRNA-21 expression predicted recurrence and poor survival in patients with colorectal cancer-A meta-analysis. Onco Targets Ther. 2016;4:2615–24. doi: 10.2147/OTT.S103893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–7. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 11.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 12.Qi W, Li H, Cai XH, Gu JQ, Meng J, Xie HQ, Zhang JL, Chen J, Jin XG, Tang Q, Hao Y, Gao Y, Wen AQ, Xue XY, Gao Smith F, Jin SW. Lipoxin A4 activates alveolar epithelial sodium channel gamma via the microRNA-21/PTEN/AKT pathway in lipopolysaccharide-induced inflammatory lung injury. Lab Invest. 2015;95:1258–68. doi: 10.1038/labinvest.2015.109. [DOI] [PubMed] [Google Scholar]
- 13.Wu T, Liu Y, Fan Z, Xu J, Jin L, Gao Z, Wu Z, Hu L, Wang J, Zhang C, Chen W, Wang S. miR-21 modulates the immunoregulatory function of bone marrow mesenchymal stem cells through the PTEN/Akt/TGF-β 1 pathway. Stem Cells. 2015;33:3281–90. doi: 10.1002/stem.2081. [DOI] [PubMed] [Google Scholar]
- 14.Bai H, Xu R, Cao Z, Wei D, Wang C. Involvement of miR-21 in resistance to daunorubicin by regulating PTEN expression in the leukaemia K562 cell line. FEBS Lett. 2011;585:402–8. doi: 10.1016/j.febslet.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 15.Ou H, Li Y, Kang M. Activation of miR-21 by STAT3 induces proliferation and suppresses apoptosis in nasopharyngeal carcinoma by targeting PTEN gene. PLoS One. 2014;9:e109929. doi: 10.1371/journal.pone.0109929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung EJ, Lee HK, Jung SA, Lee SJ, Chee HY, Sohn YH, Lee JH. Transduction of PTEN proteins using the tat domain modulates TGF-β1-mediated signaling pathways and transdifferentiation in subconjunctival fibroblasts. Invest Ophthalmol Vis Sci. 2012;53:379–86. doi: 10.1167/iovs.11-8491. [DOI] [PubMed] [Google Scholar]
- 17.Karahan N, Baspinar S, Ciris M, Baydar CL, Kapucuoglu N. Cyclooxygenase-2 expression in primary and recurrent pterygium. Indian J Ophthalmol. 2008;56:279–83. doi: 10.4103/0301-4738.39663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim KW, Park SH, Lee SH, Kim JC. Upregulated stromal cell-derived factor 1 (SDF-1) expression and its interaction with CXCR4 contribute to the pathogenesis of severe pterygia. Invest Ophthalmol Vis Sci. 2013;54:7198–206. doi: 10.1167/iovs.13-13044. [DOI] [PubMed] [Google Scholar]
- 19.Chien KH, Chen SJ, Liu JH, Woung LC, Chen JT, Liang CM, Chiou SH, Tsai CY, Cheng CK, Hu CC, Peng CH. Correlation of microRNA-145 levels and clinical severity of pterygia. Ocul Surf. 2013;11:133–8. doi: 10.1016/j.jtos.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Dushku N, Reid TW. Immunohistochemical evidence that human pterygia originate from an invasion of vimentin expressing altered limbal epithelial basal cells. Curr Eye Res. 1994;13:473–81. doi: 10.3109/02713689408999878. [DOI] [PubMed] [Google Scholar]
- 21.Kase S, Kitaichi N, Furudate N, Yoshida K, Ohno S. Increased expression of mucinous glycoprotein KL-6 in human pterygium. Br J Ophthalmol. 2006;90:1208–9. doi: 10.1136/bjo.2006.094300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kase S, Takahashi S, Sato I, Nakanishi K, Yoshida K, Ohno S. Expression of p27(KIP1) and cyclin D1, and cell proliferation in human pterygium. Br J Ophthalmol. 2006;91:958–61. doi: 10.1136/bjo.2006.110387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garfias Y, Bautista-de Lucio VM, Garcia C, Nava A, Villalvazo L, Jimenez-Martinez MC. Study of the expression of CD30 in pterygium compared to healthy conjunctivas. Mol Vis. 2009;15:2068–73. [PMC free article] [PubMed] [Google Scholar]
- 24.Bradley JC, Yang W, Bradley RH, Reid TW, Schwab IR. The science of pterygia. Br J Ophthalmol. 2010;94:815–20. doi: 10.1136/bjo.2008.151852. [DOI] [PubMed] [Google Scholar]
- 25.Bhagat TD, Zhou L, Sokol L, Kessel R, Caceres G, Gundabolu K, Tamari R, Gordon S, Mantzaris I, Jodlowski T, Yu Y, Jing X, Polineni R, Bhatia K, Pellagatti A, Boultwood J, Kambhampati S, Steidl U, Stein C, Ju W, Liu G, Kenny P, List A, Bitzer M, Verma A. miR-21 mediates hematopoietic suppression in MDS by activating TGF-beta signaling. Blood. 2013;121:2875–81. doi: 10.1182/blood-2011-12-397067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schee K, Boye K, Abrahamsen TW, Fodstad O, Flatmark K. Clinical relevance of microRNA miR-21, miR-31, miR-92a, miR-101, miR-106a and miR-145 in colorectal cancer. BMC Cancer. 2002;12:505. doi: 10.1186/1471-2407-12-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Capodanno A, Boldrini L, Proietti A, Alì G, Pelliccioni S, Niccoli C, D’Incecco A, Cappuzzo F, Chella A, Lucchi M, Mussi A, Fontanini G. Let-7g and miR-21 expression in non-small cell lung cancer: Correlation with clinicopathological and molecular features. Int J Oncol. 2013;43:765–74. doi: 10.3892/ijo.2013.2003. [DOI] [PubMed] [Google Scholar]
- 28.Seca H, Lima RT, Lopes-Rodrigues V, Guimaraes JE, Almeida GM, Vasconcelos MH. Targeting miR-21 induces autophagy and chemosensitivity of leukemia cells. Curr Drug Targets. 2013;14:1135–43. doi: 10.2174/13894501113149990185. [DOI] [PubMed] [Google Scholar]
- 29.Sicard F, Gayral M, Lulka H, Buscail L, Cordelier P. Targeting miR-21 for the therapy of pancreatic cancer. Mol Ther. 2013;21:986–94. doi: 10.1038/mt.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tao T, Wang Y, Luo H, Yao L, Wang L, Wang J, Yan W, Zhang J, Wang H, Shi Y, Yin Y, Jiang T, Kang C, Liu N, You Y. Involvement of FOS-mediated miR-181b/miR-21 signalling in the progression of malignant gliomas. Eur J Cancer. 2013;49:3055–63. doi: 10.1016/j.ejca.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Soria-Valles C, Gutiérrez-Fernández A, Guiu M, Mari B, Fueyo A, Gomis RR, López-Otín C. The anti-metastatic activity of collagenase-2 in breast cancer cells is mediated by a signaling pathway involving decorin and miR-21. Oncogene. 2014;33:3054–63. doi: 10.1038/onc.2013.267. [DOI] [PubMed] [Google Scholar]
- 32.Yang SM, Huang C, Li XF, Yu MZ, He Y, Li J. miR-21 confers cisplatin resistance in gastric cancer cells by regulating PTEN. Toxicology. 2013;306:162–8. doi: 10.1016/j.tox.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 33.Chappell WH, Steelman LS, Long JM, Kempf RC, Abrams SL, Franklin RA, Bäsecke J, Stivala F, Donia M, Fagone P, Malaponte G, Mazzarino MC, Nicoletti F, Libra M, Maksimovic-Ivanic D, Mijatovic S, Montalto G, Cervello M, Laidler P, Milella M, Tafuri A, Bonati A, Evangelisti C, Cocco L, Martelli AM, McCubrey JA. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health. Oncotarget. 2011;2:135–64. doi: 10.18632/oncotarget.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hyun T, Yam A, Pece S, Xie X, Zhang J, Miki T, Gutkind JS, Li W. Loss of PTEN expression leading to high Akt activation in human multiple myelomas. Blood. 2000;96:3560–8. [PubMed] [Google Scholar]
- 35.Krützfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–9. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 36.Haraguchi T, Ozaki Y, Iba H. Vectors expressing efficient RNA decoys achieve the long-term suppression of specific microRNA activity in mammalian cells. Nucleic Acids Res. 2009;37:e43. doi: 10.1093/nar/gkp040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merrigan SL, Kennedy BN, Vitamin D. Receptor Agonists Regulate Ocular Developmental Angiogenesis and Modulate Expression of dre-miR-21 and VEGF. Br J Pharmacol. 2017;174:2636–51. doi: 10.1111/bph.13875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poenaru Sava MG, Raica ML, Cimpean AM. VEGF mRNA assessment in human pterygium: a new ‘scope’ for a future hop. Ophthalmic Res. 2014;52:130–5. doi: 10.1159/000363142. [DOI] [PubMed] [Google Scholar]
- 39.Ochiya T, Takahama Y, Nagahara S, Sumita Y, Hisada A, Itoh H, Nagai Y, Terada M. New delivery system for plasmid DNA in vivo using atelocollagen as a carrier material: the Minipellet. Nat Med. 1999;5:707–10. doi: 10.1038/9560. [DOI] [PubMed] [Google Scholar]