Abstract
Background
Anemia in children continues to be a major public health challenge in most developing countries, particularly in Africa.
In the early stages of life, it leads to severe negative consequences on the cognitive functions as well as growth and development of the children, which may persist even after treatment.
Objective
The main aim of this study was to assess the prevalence and associated factors of anemia among hospitalized children attending at university of Gondar comprehensive and specialized referral hospital, Northwest Ethiopia.
Method
A cross sectional study was conducted on 384 hospitalized children, between February and June, 2018. Data of socio demographic characteristics and clinical conditions of the study individuals were collected using questionnaire after taking appropriate written informed consent and assent. Then 3 mL of blood was collected for complete blood count analysis and also stool examination was done for intestinal parasites. Data were coded, cleared and entered into SPSS version 20 for analysis.
Bivariate and multivariate logistic regression models were used to identify associated factors of anemia. P-value ≤ 0.05 was considered as statistically significant.
Result
The overall magnitude of anemia among hospitalized children was 58.6%; of them 56.4% were males. Of anemic children, 28% had mild, 51.1% moderate and 20.9% severe anemia. The magnitude of anemia among children aged 6-59 months, 5-11 years and 12-14 years were 54.1%, 58.9% and 67.5%, respectively.
In this study, anemia was positively associated with parasitic infection (AOR= 2.541; 95% CI: 1.363, 4.737), not eating meat and animal products (AOR = 1.615; 95% CI: 1.014, 2.574).
Conclusion
Anemia among hospitalized children in this study was found to be a severe public health problem. It was strongly associated with intestinal parasitic infection and not eating meat and animal products.
Focused polices and strategies should be designed to reduce anemia among hospitalized children in Ethiopia.
Key words: anemia, hospitalized children, associated factors, Gondar, Northwest Ethiopia
BACKGROUND
Anemia is one of the major public health concerns that cause significant morbidity and mortality in children worldwide (1). It is one of the most prevalent public health problems in the world, affecting both affluent and poor countries with major consequences of human health as well as social and economic developments (1,2). Even its prevalence has shown decrement across the regions; still it remains a serious public health problem around the globe (3).
Although anemia occurs at all stages of human life cycle, its prevalence is higher especially in younger preschool aged children due to increased demand of iron for fast growth (4).
It adversely affects the cognitive and physical development of children which in turn results in a significant impairment of work capacity and school educational performance (3, 5-7).
The magnitude of anemia in children substantially varies across the world’s regions, whereby the global prevalence is estimated to be 42.6%, and its magnitude in Africa, South East Asia, America and European regions is 62.3%, 53.8%, 23.3% and 22.9%, respectively. Globally, on average, around 9.6 million children are severely anemic (1).
Anemia with prevalence of ≥ 40%, 20-39.9%, 5-19.9% and <5% in the community is categorized as severe, moderate, mild and no public health problem, respectively (8).
The 2008 WHO report also has revealed that more than half (56.3%) of the world’s preschool aged children reside in developing countries where anemia is a severe public health problem including sub-Saharan Africa with a prevalence of 40% and above (7, 9, 10).
Similarly, the 2015 WHO report, from the global anemia prevalence in 2011 showed that the highest figure (42.6%) was in children compared to other age groups around the globe and its prevalence in Africa and Ethiopia was 62.3% and 50%, respectively (1).
Anemia is associated with socioeconomic, biological, environmental and nutritional factors. Nutritional deficiencies (iron deficiency, vitamin B12 and folate deficiency), lack of awareness among the mothers about the problem together with their low educational status, unhealthy food habits and parasitic infestations were considered the main factors associated with anemia among children (1, 3, 4, 7, 11-13).
The prevalence of anemia among hospitalized children in different parts of the world has been studied; 33.2% in Lebanon (14), 61.6% in Turkey (15), 56.3% in Uganda (16), 83.2% in Southern Tanzania (17), and 77.2 % in Mwanza, Tanzania (13). However, in our country Ethiopia, there is lack of information about the prevalence and risk factors of anemia among hospitalized children.
Most studies in Ethiopia focus on the prevalence of anemia under age five and for school aged children (18-20). Therefore, this study was aimed to determine the prevalence and associated factors of anemia among hospitalized children attending university of Gondar referral and comprehensive hospital, Gondar, Northwest Ethiopia. Identifying risk factors and determining the magnitude of anemia have paramount importance to reform the regional and national public policy for ensuring sustainable improvement.
METHODS
Study setting and population
A cross sectional study was done among 384 hospitalized children aged 6 months to 14 years at university of Gondar comprehensive and specialized referral hospital, between February and June, 2018. Gondar is located at a distance of 737 km from the country’s capital, Addis Ababa–in the North Gondar zone of the Amhara regional state, in North-Western Ethiopia. The city‘s latitude and longitude coordinates are 12°03′61″ N and 37°02′81″ E, respectively; and it is located at elevation of 2,133 m above sea level. Children with chronic kidney disease, active bleeding, HIV/AIDS, those who have undergone surgery in the previous one month and who had chronic illness were excluded from the study.
Data collection procedures
Upon obtaining a signed consent form and additional assent from children aged 7 years and above, the children who fulfilled the inclusion criteria were enrolled into the study. A pre-tested and standardized questionnaire was used to collect social demographics and social economic history. The caretaker/ family’s level of income was assessed by asking the caretaker how much they earned per month including donation or handout from other persons or organizations. Other clinical data of hospitalized children were also collected by nurses working in pediatric ward.
Laboratory analysis
Hematology analysis
About 3mL of venous blood was collected from each subject for the analysis of hematological parameters; hemoglobin (Hgb), hematocrit (%), mean cell volume (MCV), mean cell hemoglobin (MCH), mean cell hemoglobin concentration (MCHC), red blood cell count (RBC) and red cell distribution width (RDW) were determined using Cell-Dyn1800 automated blood analyzer.
Stool examinations
For intestinal parasite examination, stool samples were collected and both wet mount and concentration technique were employed by an experienced laboratory technologist.
Anemia definition and severity classification
Since the study area altitude is 2133 meter above sea level, results of Hgb values were adjusted by subtracting 0.8g/dl to its respective sea level as it is recommended by WHO. Then Hgb cutoff value 11g/dl for children 6-59 months, 11.5g/dl for children 5-11 years and 12g/dl for children 12-15 years were considered to define anemia. Regarding to its severity of anemia, Hgb value of 10.0-10.9 g/dl, 7.0-9.9 g/dl and less than 7 g/dl were considered as mild, moderate and severe anemia, respectively for children aged 6-59 months and Hgb value of 11.0-11.4 g/dl for children 5-11 years and 11.0-11.9 g/dl for children aged 12-14 years was considered as mild type of anemia while Hgb value of 8.0-10.9 g/dl and below 8.0 g/dl were considered as moderate and mild type of anemia for both age groups; children aged 5-11 years and 12-14 years (8).
Statistical analysis methods
Data were cleaned, edited, checked for completeness and entered into SPSS version 20 statistical software for analysis. Descriptive statistics were used to summarize the characteristics of the study population. To determine factors associated with anemia binary logistic regression analysis was done; and odds ratio with its 95% confidence level was used to determine the strength of association between the predictors and dependent variables. A p-value of less than 0.05 were considered as statistically significant.
RESULTS
Socio-demographic and clinical characteristics of study participants
In this study, a total of 384 hospitalized children aged between 6 months and 15 years were included, of which 212 (55.2%) are males in gender and 55.7% were from rural setting. The median age of the children was 5 years with interquartile range (IQR) of 2-10 years. Nearly half (45%) of the hospitalized children were in the age group between 6 months to 5 years. From the total hospitalized children, 18% were infected with intestinal parasites, 20% were hospitalized for five or more days and 21.6% (83/384) were malnourished. From malnourished hospitalized children, 45.8% (38/83) were severely malnourished. Regarding educational status, 58.1% of mothers and 51.6% fathers of hospitalized children were unable to read and write. For nearly two thirds (64.3%) of the hospitalized children, their family/care giver monthly household income was below 2,000 Ethiopian Birr (70 US dollars) (Table 1).
Table 1.
Socio-demographic and clinical characteristics of hospitalized children attending University of Gondar comprehensive and specialized referral hospital, Northwest Ethiopia
| Characteristics | Frequency | Percentage | |
|---|---|---|---|
| Sex | Male | 212 | 55.2 |
| Female | 172 | 48.8 | |
| Age in years | 0.5-5 | 172 | 45.0 |
| 5-11 | 129 | 33.1 | |
| 12-14 | 83 | 21.9 | |
| Residence | Rural | 214 | 55.7 |
| Urban | 170 | 44.3 | |
| Nutritional status | Severe malnutrition | 38 | 9.9 |
| Moderate malnutrition | 45 | 11.7 | |
| Normal | 301 | 78.4 | |
| Intestinal parasite infection | Yes | 69 | 18.0 |
| No | 315 | 82.0 | |
| Length of hospitalization | Up to 2 days | 112 | 29.2 |
| 3-5 days | 195 | 50.8 | |
| Above 5 days | 77 | 20.0 | |
| Maternal educational status | Unable read and write | 223 | 58.1 |
| Primary education | 44 | 11.5 | |
| Secondary education | 47 | 12.2 | |
| College/University | 70 | 18.2 | |
| Paternal educational status | Unable read and write | 198 | 51.6 |
| Primary education | 42 | 10.9 | |
| Secondary education | 57 | 14.8 | |
| College/University | 87 | 22.7 | |
| Monthly household income in ETB | <1000 | 66 | 17.2 |
| 1001-2000 | 181 | 47.1 | |
| Above 2000 | 137 | 35.1 | |
ETB: Ethiopian Birr; 28ETB = 1 $USD.
Prevalence of anemia
The mean (± SD) value of altitude adjusted Hgb level among hospitalized children was 10.5 ± 2.6 g/dL, with values ranging from 2.3 g/dL to 19.3 g/dL. The overall prevalence of anemia in this study was 58.6% (225/384), with 65.9% (141/214) and 49.4% (84/170) magnitude among rural and urban hospitalized children, respectively.
Of the 225 anemic cases, 127 (56.4%) were males. Of the total anemic hospitalized children, 28% (63/225), 51.1% (115/225) and 20.9% (47/225) had mild, moderate, and severe anaemia, respectively. Ninety-three (41.3 %) anemic cases were in the age group between 6 months to 5 years, and the rest 76 (33.8%) and 56 (24.9%) are in the age group 5 to 12 years and 12 to 14 years, respectively.
The most common type of anaemia was the microcytic hypochromic anemia observed in 62.2% (140/225) of children, followed by 36.9% (83/225) with normocytic normochromic anaemia. Two children (0.9%) had macrocytic normochromic anaemia. There was no significant difference in the prevalence of anemia between females and males which was 57.0% and 59.9%, respectively. The prevalence of anemia was higher among older children (65.7%) in 12-15 years, and it had shown increased as the child’s age increased (Figure 1).
Figure 1.
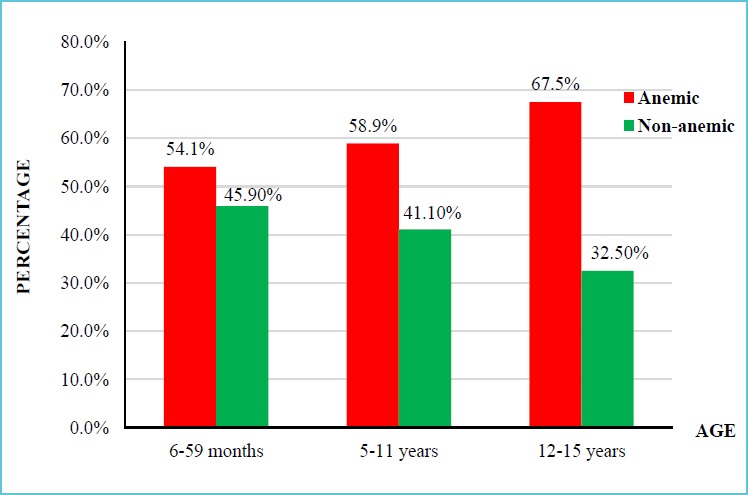
Distribution of anemia among different age groups of hospitalized children
Factors associated with anemia
In order to determine child related factors that were associated with anemia, bivariate logistic regression analysis followed by multivariate logistic analysis was done.
As indicated in Table 2, in the bivariate logistic regression analysis, children related factors such being 12-15 years old, infection with intestinal parasites, not eating meat and animal products, edema and fever were significantly associated with anemia. Then those variables having p-value less than 0.25 were subjected to multivariate logistic regression.
Table 2.
Association of child related factors with anemia among hospitalized children
| Characteristics | Anemic | Non-anemic | COR (95% Cl) | AOR (95% Cl) | |
|---|---|---|---|---|---|
| Sex | Male | 127 (59.9) | 85 (40.1) | 1 | - |
| Female | 98(57) | 74(43) | 0.886 (0.589,1.333) |
- | |
| Age in years | 0.5-5 | 93 (54.1) | 79 (45.9) | 1 | 1 |
| 5-11 | 76 (58.9) | 53 (41.1) | 1.218 (0.768,1.932) |
1.02 (0.603,1.726) |
|
| 12-15 | 56 (67.5) | 27 (32.5) | 1.762 (1.018,3.049) |
1.53 (0.829,2.822) |
|
| Nutritional status | Malnourished | 49(59) | 34(41) | 1.024 (0.625,1.677) |
- |
| Normal | 176 (58.5) | 125 (41.5) | 1 | - | |
| Intestinal parasites | Yes | 50 (72.5) | 19 (27.5) | 2.105 (1.187,3.734) |
2.541 (1.363,4.737)* |
| No | 175 (55.6) | 140 (44.4) | 1 | 1 | |
| Hospitalization length | Up to 2 days | 69 (61.6) | 43 (38.4) | 1 | 1 |
| 3-5 days | 105 (53.8) | 90 (46.2) | 0.727 (0.453,1.167) |
0.685 (0.414,1.132) |
|
| Above 5 days | 51 (66.2) | 26 (33.8) | 1.222 (0.666,2.242) |
1.29 (0.681,2.442) |
|
| Do you eat meat | Yes | 101 (51.8) | 94 (48.2) | 1 | 1 |
| No | 124 (65.6) | 65 (34.4) | 1.775 (1.177,2.678) |
1.615 (1.014,2.574)* |
|
| Edema | Yes | 71 (70.3%) | 30 (29.7) | 1.982 (1.219,3.225) |
1.56 (0.898,2.71) |
| No | 154 (54.4) | 129 (45.6) | 1 | 1 | |
| Fever | Yes | 27 (45.8) | 32 (54.2) | 0.541 (0.31,0.946) |
0.606 (0.324,1.136) |
| No | 198 (60.9) | 127 (39.2) | 1 | 1 | |
| Vomiting | Yes | 18 (46.2) | 21 (53.8) | 0.571 (0.294,1.112) |
0.446 (0.208,0.96)* |
| No | 207(60) | 138(40) | 1 | 1 | |
| Diarrhea >1 week | Yes | 14 (43.8) | 18 (56.3) | 0.52 (0.25,1.079) |
0.402 (0.178,0.907)* |
| No | 214 (58.8) | 150 (41.2) | 1 | 1 | |
* Indicates statistically significant association;
COR: Crude Odds Ratio; AOR: Adjusted Odds Ratio; CI: Confidence interval
In multivariate logistic regression analysis, being infected with parasitic infection (AOR = 2.541; 95% CI: 1.363, 4.737) and not eating meat and animal products (AOR = 1.615; 95% CI: 1.014, 2.574) were statistically associated with anemia. In this study, vomiting (AOR = 0.446; 95% CI: 0.208, 0.96) and diarrhea for more than one week (AOR = 0.402; 95% CI: 0.178, 0.907) were also showed a statistically significant association with anemia. This is due to the fact that during vomiting and diarrhea, there will be a decrease in body fluid and mask the low level of Hgb value.
Family/care giver-related factor association with anemia was determined as indicated in Table 3. In bivariate logistic analysis, being rural in residence, having no formal maternal education and a monthly income below 2,000.00 Ethiopian Birr (28 Ethiopian Birr = 1 $USD) were statistically associated with anemia among hospitalized children. However, there is no family/care giver related factors associated with anemia in multivariate logistic regression analysis.
Table 3.
Association of family/caregiver related factors with anemia in among hospitalized children
| Characteristics | Anemic | Non-anemic | CIR (95% Cl) | AOR (95% Cl) | |
|---|---|---|---|---|---|
| Residence | Urban | 84 (49.4) | 86 (50.6) | 1 | 1 |
| Rural | 141 (65.9) | 73 (34.1) | 1.977 (1.309,2.988) |
1.638 (0.844,3.177) |
|
| Maternal educational status | Unable read & write | 144 (64.6) | 79 (35.4) | 1.823 (1.059,3.137) |
1.657 (0.299,9.182) |
| Primary education | 21 (47.7) | 23 (52.3) | 0.913 (0.429,1.942) |
0.908 (0.191,4.305) |
|
| Secondary education | 25 (53.2) | 22 (46.8) | 1.136 (0.542,2.382) |
1.113 (0.278,4.455) |
|
| College / University | 35(50) | 35(50) | 1 | 1 | |
| Paternal educational status | Unable read & write | 125 (63.1) | 73 (36.9) | 1.673 (1.005,2.786) |
0.318 (0.064– 1.588) |
| Primary education | 25 (59.5) | 17 (40.5) | 1.437 (0.682,3.03) |
0.455 (0.103,2.003) |
|
| Secondary education | 31 (54.4) | 26 (45.6) | 1.165 (0.597,2.276) |
0.558 (0.156,1.996) |
|
| College / University | 44 (50.6) | 43 (49.4) | 1 | 1 | |
| Mother occupation | Government employee | 32 (51.6) | 30 (48.4) | 0.66 (0.38-0 1.145) |
1.112 (0.241,5.128) |
| Private | 12 (41.4) | 17 (58.6) | 0.437 (0.201,0.949) |
0.467 (0.166,1.315) |
|
| House wife | 181 (61.8) | 112 (38.2) | 1 | 1 | |
| Father occupation | Government employee | 40 (48.2) | 43 (51.8) | 1 | 1 |
| Farmer | 134 (63.5) | 77 (36.5) | 1.871 (1.119,3.127) |
1.553 (0.305,7.892) |
|
| Private | 51 (56.7) | 39 (43.3) | 1.406 (0.772,2.56) |
1.955 (0.463,8.255) |
|
| Monthly income in ETB | <1000 | 43 (65.2) | 23 (534.8) | 1.842 (1.004,3.381) |
1.528 (0.569,4.102) |
| 1001-2000 | 113 (62.4) | 68 (37.6) | 1.638 (1.044,2.569) |
1.283 (0.563,2.926) |
|
| Above 2000 | 69 (50.4) | 68 (49.6) | 1 | 1 | |
ETB = Ethiopian Birr, 28.40 ETB = 1 $USD;
COR: Crude Odds Ratio; AOR: Adjusted Odds Ratio; CI: Confidence interval.
DISCUSSION
The findings from this study indicate that the prevalence of anemia among hospitalized children aged 6 months to 15 is high. The prevalence was found to be 58.6 %. The overall prevalence is higher than reported from other studies in India (21), Lebanon (14), Nepal (22) and Nigeria (23), where the prevalence were between 32 and 50%. The variations in percentage in different regions might be due to heterogeneity of the studied population, dietary habits, different nutritional status and incidence of worm infestation in a defined geographical spots (21).
In our study, prevalence of anemia was elevated as age increase and it was quite high among older children aged 12-14 years (65.7%). This finding is contrary to that describe by others where the prevalence of anemia was highest among children under age of five (14). The highest prevalence in age group between 12 and 15 may be attributed to low intake of iron rich food or inappropriate dietary choices in children, poor iron absorption due to iron absorption inhibiting factors such as tannine in tea which reduces hemoglobin synthesis or inadequate iron absorption enhancers (vitamin C and hydrochloric acid) (24). Based on our finding, anemia prevalence in children under five which is 54.1% is lower than the result found in Ghana, Brazil, Bangalore, and Tanzania (13, 17, 25-27).
Different studies had different result on the association between anemia and gender. This study found insignificant difference in anemia prevalence between male and female, 57.0% and 59.9% respectively. While others have demonstrated that the prevalence of anemia can vary between male and female children (28). They argued that the high prevalence of anemia in boys may be due to the faster growth of preschool boys than girls that has high iron demand which cannot be met by diet alone.
Morphological classification of anemia showed microcytic hypochromic anemia was the most predominant type occurring in 62.2% of children. This finding is similar to the study done in Tanzania, and it is assumed that iron depletion is the main factor responsible for the high percentage of microcytic-hypochromic anemia (13). On the other hand, normocytic normochromic anemia was the second type of anemia in this study. This may be due to acute blood loss, drug therapy and chronic diseases (anemia of inflammation) (29, 30).
Child related factors like being infected with parasitic infection and not eating meat and animal products were statistically associated with anemia. The lower immune response in children compared to adults, poor hygiene and environmental conditions favor the susceptibility of children to parasitic infections (31). In our finding, children with parasitic infestation are two and half times more likely to have anemia than children who are free from parasitic infection, (AOR= 2.541; 95% CI: 1.363, 4.737). This finding is similar to study done in Bangladesh (32). The mechanism behind this relation can be due to the fact that the parasite directly induces iron deficiency through blood loss by mechanical rupture of host capillaries and arterioles followed by the release of a battery of pharmacologically active polypeptides including anticoagulants, antiplatelet agents, and antioxidants (31).
Not eating meat and animal products were also found to be related to a risk of acquiring anemia in our study and this is similar to the study done in Uganda (12). Iron can be found in two forms in foods heme and non-heme. Heme iron is only found in animal products, whereas non-heme iron is only found in plants (33). Iron deficiency and/or low bioavailability account for half of the anemia in developing countries (34) accessed 28 November, 2018.
In this study univariate analysis showed anemia that was significantly associated with maternal related variables like being rural in residence (COR= 1.977 (1.309, 2.988)), have no formal education (COR= 1.823 (1.059, 3.137)) and low monthly income (COR= 1.842 (1.004, 3.381)), but in multivariate analysis this factor was not significant statistically. Since mothers are mostly care givers for the child, maternal education has always been linked to many child health outcomes. It may also affect health decision making and thus influence the probability of a child meeting certain nutrition-related requirements.
In addition, anemia among children was also associated with household income. Children living in household with lower monthly income (<2000 Ethiopian Birr) were more likely to have anemia compared to those with higher income. Similar finding was from study conducted in Brazil and northern Ethiopia (9). The reason might be due to children from poor households are less likely to get iron-rich foods.
This study has some limitations in that we lack data on the prevalence of blood parasite like plasmodium falciparum, one of the risk factors considered to be the principal cause of severe anemia in malaria-endemic areas in Africa. The clinical diagnoses of the children were also not included in this study therefore clinical causes of anemia could not be identified.
CONCLUSIONS
Prevalence of anemia in hospital admitted children aged between 6 months and 15 years is high and it was found to be a severe public health problems. Being more common in children may predispose this vulnerable population to future infections, hematological and developmental disorder. Microcytic, hypochromic and normocytic normochromic pattern were the common morphological types of anemia. Parasitic infection and not eating meat and animal products significantly associate with anemia. Deworming and interventions like iron supplementation and nutritional education activities are important to decrease the prevalence of anemia.
Acknowledgments
The authors would like to extend their gratitude to the University of Gondar comprehensive and specialized referral hospital pediatrics ward staffs for their support during this study. We also thank the data collectors, children and their parents/caregivers who participated in this study.
Footnotes
Abbreviations
AOR: Adjusted odds ratio
COR: Crude odds ratio
EDTA: Ethylene Die-amine Tetra-acetic Acid
FA: Fanconi Anemia
HCT: Hematocrit
Hgb: Hemoglobin
IDA: Iron Deficiency Anemia
MCH: Mean Cell Hemoglobin
MCHC: Mean Cell Hemoglobin Concentration
MCV: Mean Cell Volume
RBC: Red Blood Cell
RDW: Red Cell Distribution width
WHO: World Health Organization
Ethical approval and consent to participate
Ethical clearance and approval was granted from University of Gondar, Collage of Medicine and Health Sciences, School of Biomedical and Laboratory Sciences Ethical Review Committee. Permission letter was also obtained from the hospital administrators. The purpose of the research was explained to the study subjects and written informed consent and assent was obtained from each hospitalized children and their care givers and then those who were willing to participate were included in the study. Participation was fully voluntarily, refusal at any time during data collection was permitted. Confidentiality was kept. When haemoglobin level of the children was below 11 g/dl, the result was communicated with the physicians.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Competing interests
The authors have declared that they have no existing competing interests.
Funding
No specific funding was received for this work.
- BE & AA: conceived and designed the experiments
- YW, ST, EM, MM: performed the experiments
- BE & MG: analyzed and interpreted results
- All authors contributed to the writing and editing of the manuscript and approved the final version submitted.
REFERENCES
- 1.WHO. The global prevalence of anaemia in 2011. Geneva: World Health Organization; 2015; Available from: http://www.who.int/iris/handle/10665/177094. [Google Scholar]
- 2.Chatterjee A, Bosch RJ, Kupka R, Hunter DJ, Msamanga GI, Fawzi WW. Predictors and consequences of anaemia among antiretroviral-naïve HIV-infected and HIV-uninfected children in Tanzania. Public Health Nutr. 2010;13(2):289-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brabin BJ, Premji Z, Verhoeff F. An analysis of anemia and child mortality. J Nutr. 2001;131(2S-2):636S-45S. [DOI] [PubMed] [Google Scholar]
- 4.WHO/UNICEF/UNU. Iron deficiency anaemia assessment, prevention, and control:aguide for programme managers. Geneva, World Health Organization; 2001; Available from: http://www.who.int/nutrition/publications/en/idaassessmentpreventioncontrol.pdf. [Google Scholar]
- 5.Das P. Can we eliminate nutritional anemia in the near future? South East Asia J Public Health. 2015;5(1):1-3. [Google Scholar]
- 6.Ekwochi U, Osuorah DIC, Odetunde OI, Egbonu I, Ezechukwu CC. Prevalence of iron deficiency anaemia in anaemic under-5 children in Enugu South East Nigeria. Niger J Paed. 2014;41(2):129-132. [Google Scholar]
- 7.Sanou D, Ngnie-Teta I. Risk factors for anemia in preschool children in sub-Saharan Africa. Silverberg D, editor. Anemia: InTech; 2012. Available from: http://www.intechopen.com/books/anemia/risk-factors-for-anemia-in-preschool-children-in-sub-saharan-africa. [Google Scholar]
- 8.WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization; 2011; Available from: http://www.who.int/vmnis/indicators/haemoglobin.pdf. [Google Scholar]
- 9.Adish A, Esrey SA, Gyorkos TW, Johns T. Risk factors for iron deficiency anemia in preschool children in northern Ethiopia. Public Health Nutr. 1999;2(3):243-252. [DOI] [PubMed] [Google Scholar]
- 10.Zein Z. Hematocrit levels and anemia in Ethiopian children. East Afr Med J. 1991;68(6):412-419. [PubMed] [Google Scholar]
- 11.Alaofè H, Zee J, Dossa R, O’Brien HT. Education and improved iron intakes for treatment of mild iron-deficiency anemia in adolescent girls in southern Benin. Food Nutr Bull. 2009;30(1):24–36. [DOI] [PubMed] [Google Scholar]
- 12.Kikafunda JK, Lukwago FB, Turyashemererwa F. Anaemia and associated factors among under-fives and their mothers in Bushenyi district, Western Uganda. Public Health Nutr. 2009;12(12):2302-2308. [DOI] [PubMed] [Google Scholar]
- 13.Simbauranga RH, Kamugisha E, Hokororo A, Kidenya BR, Makani J. Prevalence and factors associated with severe anaemia amongst under-five children hospitalized at Bugando Medical Centre, Mwanza, Tanzania. BMC Hematol. 2015;15:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salami A, Bahmad HF, Ghssein G, Salloum L, Fakih H. Prevalence of anemia among Lebanese hospitalized children: Risk and protective factors. PLoS One. 2018;13(8):e0201806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cetinkaya F, Yildirmak Y, Kutluk G. Severe iron-deficiency anemia among hospitalized young children in an urban hospital. Pediatr Hematol Oncol. 2005;22(1):77–81. [DOI] [PubMed] [Google Scholar]
- 16.Kiggundu VL, O’Meara WP, Musoke R, Nalugoda FK, Kigozi G, Baghendaghe E, et al. High prevalence of malaria parasitemia and anemia among hospitalized children in Rakai, Uganda. PLoS One. 2013;8(12):e82455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mghanga FP, Genge CM, Yeyeye L, Twalib Z, Kibopile W, Rutalemba FJ, et al. Magnitude, severity, and morphological types of anemia in hospitalized children under the age of five in Southern Tanzania. Cureus. 2017;9(7):e1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Assefa S, Mossie A, Hamza L. Prevalence and severity of anemia among school children in Jimma Town, Southwest Ethiopia. BMC Hematol. 2014;14(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Getaneh Z, Enawgaw B, Engidaye G, Seyoum M, Berhane M, Abebe Z, et al. Prevalence of anemia and associated factors among school children in Gondar town public primary schools, northwest Ethiopia: A school based cross-sectional study. PLoS One. 2017;12(12):e0190151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melku M, Alene KA, Terefe B, Enawgaw B, Biadgo B, Abebe M, et al. Anemia severity among children aged 6-59 months in Gondar town, Ethiopia: a community-based cross-sectional study. Ital J Pediatr. 2018;44(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ritu S, Ashok D, Vithal TP, Shivani R, Rajaram N. A hospital based study on anemia prevalence in children of an Indian Island. Int J Pediatr. 2017;5(12):6245-6252. [Google Scholar]
- 22.Sharma A, Giri A, Pudasaini S. Prevalence of anemia in children aged 6 months to 15 years: a hospital based study. J Pathol Nep. 2017;7(2):1168-1171. [Google Scholar]
- 23.Mainasara A, Ibrahim K, Uko E, Jiya N, Erhabor O, Umar A, et al. Prevalence of anaemia among children attending paediatrics department of UDUTH, Sokoto, North-Western Nigeria. IBRR. 2017;7(1):1-10. [Google Scholar]
- 24.Bagchi K. Iron deficiency anaemia-an old enemy. East Mediterr Health J. 2004;10(6):754-760. [PubMed] [Google Scholar]
- 25.Adu-Amankwaah J, Allotey EA, Kwasie DA, Afeke I, Owiafe PK, Adiukwu PC, et al. Prevalence and morphological types of anaemia among children under-five years in the Volta regional hospital of Ghana. Open Access Lib J. 2018;5(2):1-10. [Google Scholar]
- 26.Dos Santos RF, Gonzalez ES, de Albuquerque EC, de Arruda IK, Diniz Ada S, Figueroa JN, et al. Prevalence of anemia in under five-year-old children in a children’s hospital in Recife, Brazil. R Rev Bras Hematol Hemoter. 2011;33(2):100-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahuja S, Nagaraj N. Prevalence and risk factors of anemia in under five-year-old children in children’s hospital. Int J Contemp Pediatr. 2018;5(2):499-502. [Google Scholar]
- 28.Lehmann F, Gray-Donald K, Mongeon M, Di Tommaso S. Iron deficiency anemia in 1-year-old children of disadvantaged families in Montreal. CMAJ. 1992;146(9):1571-1577. [PMC free article] [PubMed] [Google Scholar]
- 29.Bonnet JD. Normocytic normochromic anemia. Postgrad Med. 1977;61(6):139-142. [DOI] [PubMed] [Google Scholar]
- 30.Nemeth E, Ganz T. Anemia of Inflammation. Hematol Oncol Clin North Am. 2014;28(4):671-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Odebunmi JF, Adefioye OA, Adeyeba OA. Hookworm infection among school children in Vom, Plateau state Nigeria. Am Eurasian J Sci Res. 2007;2(1):39-42. [Google Scholar]
- 32.Banu H, Khanum H, Hossain MA. Relationships between anaemia and parasitic infections in adolescent girls of Bangladesh. Bangladesh J Zool. 2014;42(1):91-103. [Google Scholar]
- 33.Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health. J Res Med Sci. 2014;19(2):164-174. [PMC free article] [PubMed] [Google Scholar]
- 34.Allen LH, De Benoist B, Dary O, Hurrell R. Guidelines on food fortification with micronutrients. World Health Organization and Food and Agriculture Organization of the United Nations; 2006; Available from: http://apps.who.int/iris/bitstream/handle/10665/43412/9241594012eng.pdf?sequence=1 [Google Scholar]


