Abstract
Background
In patients with ulcerative colitis (UC), fecal calprotectin (FC) concentrations correlate with endoscopic inflammation evidence. This study investigated the effect of vedolizumab induction on FC concentrations and whether FC concentrations could be a reliable surrogate measure of disease status.
Methods
Data from the placebo-controlled, phase 3 trial GEMINI 1 were used to evaluate week-6 relationships between outcomes (including clinical remission, mucosal healing [MH], and endoscopic remission) and both absolute FC concentration values and relative FC concentration changes from baseline (%FC0-6). Sensitivity and specificity were calculated by cross-tabulation; the value of week-6 FC concentration as surrogate biomarker was measured with Youden J statistic computed for various cut points.
Results
GEMINI 1 induction phase enrolled 895 patients. Fecal calprotectin concentration decreases were deeper in patients with clinical remission, MH, and/or endoscopic remission than in patients without. The best week-6 indicator of clinical or endoscopic remission in this data set was absolute FC concentration ≤150 µg/g. The surrogate biomarker values (based on areas under the curve) for the best-performing cut points (FC0-6 reduction >90%, FC ≤150 µg/g) were fair (range, 0.70–0.77, total population). More patients met the ≤150 µg/g cut point with vedolizumab than with placebo. Baseline FC concentrations were not correlated with clinical outcomes.
Conclusions
Fecal calprotectin concentration reductions were greater with vedolizumab induction than with placebo. Week-6 FC concentrations had only fair surrogate biomarker value for endoscopic status. Our data suggest that, while FC may reflect inflammatory burden, FC concentration after vedolizumab induction may not be a robust biomarker of mucosal inflammation.
Keywords: colitis, ulcerative, calprotectin, vedolizumab
INTRODUCTION
Ulcerative colitis (UC) is an inflammatory bowel disease (IBD) characterized by a relapsing and remitting course. Clinical manifestations of active disease include bloody diarrhea, abdominal cramps, urgency, and fatigue.1, 2 Traditionally, the main goal of therapy in UC has been clinical remission, defined as the absence of symptoms without corticosteroid therapy. Nonetheless, patients in clinical remission may have a significant inflammatory burden, and so endoscopy is an increasingly used measure of disease severity.3 Accordingly, mucosal healing—as assessed endoscopically—has become an additional therapeutic target for treatment of UC because it is associated with a lower risk of treatment escalation, colectomy, and disease relapse.4–7 However, endoscopy is an invasive, time-consuming, and costly procedure, and bowel preparation is uncomfortable for the patient.8 Therefore, there is a role for reliable biomarkers to improve the detection of disease activity, predict relapse, and monitor treatment response.
Fecal calprotectin (FC) is a cytosolic protein released by activated neutrophils and macrophages from the inflamed intestinal mucosa.9 In IBD patients, increases in FC concentrations have been positively correlated with endoscopic and histologic evidence of mucosal inflammation.10, 11 Studies conducted in UC patients who were in clinical remission demonstrated that FC is a strong predictor of clinical relapse and correlates with mucosal disease activity.12–16 Although these studies suggest that FC could be used as a surrogate biomarker for mucosal inflammation in UC patients, most were small studies conducted in single institutions.
Vedolizumab is a humanized monoclonal antibody that specifically binds to the α4β7 integrin heterodimer and selectively blocks gut leukocyte trafficking.17 The safety and efficacy of vedolizumab for the treatment of patients with moderately to severely active UC were demonstrated in the GEMINI 1 trial.18 The initial GEMINI 1 analysis showed a significantly larger decrease in FC concentrations in patients who received vedolizumab than in those who received placebo at week 6 and over the course of therapy to week 52.18 Based upon this finding, we had 3 aims: (1) to assess the value of FC concentrations as a surrogate biomarker of endoscopic outcomes at week 6, (2) to assess the value of baseline FC concentration as predictor of week-6 endoscopic outcomes, and (3) to assess the effect of vedolizumab induction on FC concentration at week 6.
METHODS
Study Design
This post hoc analysis was performed on data from the multicenter, phase 3, randomized, placebo-controlled GEMINI 1 trial of vedolizumab in patients with moderately to severely active UC (ClinicalTrials.gov, NCT00783718). Details of the study design were reported by Feagan et al in 2013.18 Briefly, eligible patients were 18 to 80 years of age, with moderately to severely active UC, defined as a Mayo Clinic score (MCS) of 6 to 12, with endoscopic subscore of ≥2 within 7 days before the first dose of study drug, with disease that extended 15 cm or more from the anal verge. Patients enrolled in the induction portion of the study were assigned to 2 cohorts. In cohort 1, they were randomized in a 3:2 ratio to receive intravenous vedolizumab (300 mg) or placebo in weeks 0 and 2 (double-blind vedolizumab and double-blind placebo, respectively; intent-to-treat [ITT] population) (Supplementary Fig. S1). All patients in the open-label cohort 2 received vedolizumab and used the same induction regimen as in the blinded study (open-label vedolizumab). All patients were permitted use of mesalamine, up to 30 mg of prednisone (or the equivalent) per day, or immunosuppressive agents at stable doses.
Assessments
Stool samples (~20 g) were collected in clinical study sites at screening (21 days to 1 day before first day of study, baseline) and at week 6 using standardized instructions. Quantification of FC concentration in stool samples was conducted using the CALPRO Calprotectin ELISA Test (ALP) (distributed by Calpro, Oslo, Norway).19
Disease outcomes were clinical remission, mucosal healing, and endoscopic remission at week 6 as evaluated by the investigators. Endoscopy was performed at baseline and at week 6 with interpretation by the local investigator. Clinical remission was defined as an MCS of ≤2 with no subscore >1. Mucosal healing was defined as a Mayo Clinic endoscopic subscore of 0 or 1. Endoscopic remission was defined as a Mayo Clinic endoscopic subscore of 0.
Relationship Between FC Concentrations and Disease Outcomes at Week 6
All patients enrolled in the induction portion of GEMINI 1 who completed FC measurements at week 0 were included in the analysis (baseline evaluable population).
To investigate the relationship between baseline FC concentrations and outcomes, baseline FC concentrations from the evaluable population were grouped by quartiles, and clinical outcome rates (eg, clinical remission, mucosal healing, and endoscopic remission) at week 6 were derived for each quartile.
To evaluate the relationship between week 6 relative FC concentration changes from baseline and outcomes, patients in the total population who completed FC measurements at week 6 or at both week 0 and week 6 (week 6 evaluable populations) were stratified by clinical and endoscopic outcomes at week 6, and the FC concentration changes were reported as percentages (%FC0-6).
To determine the effect of vedolizumab treatment on FC and whether baseline FC could predict response to vedolizumab, the analyses that were performed on the overall population were repeated separately for the vedolizumab and placebo groups.
Sensitivity and Specificity Analysis
Logistic regression analyses were performed with clinical remission, mucosal healing, or endoscopic remission at week 6 as dependent variables and week-6 FC concentration or %FC0-6 as independent predictor variables. Specific cut points for week-6 FC concentration and %FC0-6 were then identified based on receiver operating characteristic (ROC) data. Area under the ROC curve (AUROC) was used to estimate predictive value of the different cut points (0.90–1, excellent; 0.80–0.90, good; 0.70–0.80, fair; 0.60–0.70, poor; 0.50–0.60, fail). For each identified cut point, sensitivity and specificity were calculated by cross-tabulation and plotted as a summary of ROC data. Positive predictive values (PPVs) and negative predictive values (NPVs) for each outcome at each cut point were calculated. The Youden J statistic was computed as (sensitivity + specificity) – 100 for each cut point as a measure of predictive value.20 The best cut points were determined by the optimal balance of sensitivity and specificity, as indicated by the maximal Youden J. The larger the J statistic, the better the predictive value of the cut point. All statistical analyses were performed using Statistical Analysis Software Version (SAS) 9.0.
Ethical Considerations
The GEMINI 1 study was designed and implemented by the GEMINI 1 Steering Committee in collaboration with Millennium Pharmaceuticals, which held and analyzed the data. The original protocol was approved by an investigational review board at each center, and all patients gave written, informed consent. Authors made the decision to submit the manuscript for publication and approved the submitted manuscript.
RESULTS
Patient Baseline Characteristics
There were 895 patients (of 1406 screened) included in the study: 746 were treated with vedolizumab (double-blind vedolizumab [cohort 1], n = 225; and open-label vedolizumab [cohort 2], n = 521), and 149 received placebo (double-blind placebo [cohort 1]). Of these patients, 857 represented the baseline evaluable population (with FC measurements at week 0). At baseline, median FC concentrations were similar in both (blinded) cohort 1 placebo (1005.5 μg/g; interquartile range [IQR]: 333–2943) and vedolizumab groups (1111.9 μg/g; IQR: 449–2931) but were numerically lower in the open-label cohort 2 vedolizumab group (782.3 μg/g; IQR: 331–1594 μg/g), resulting in an average of 867.9 μg/g (IQR: 344–1915) for vedolizumab combined. Disease activity at baseline, as determined by MCS, was similar in all treatment groups (Supplementary Table S1).
Relationship Between FC Concentrations and Disease Outcomes at Week 6
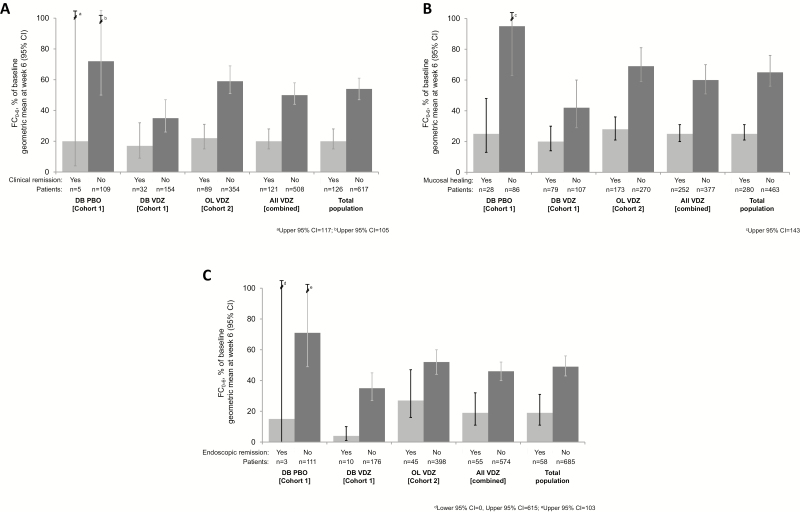
For the analyses performed at week 6, the evaluable populations were 771 patients with FC evaluations at week 6 (for whom absolute FC concentrations could be calculated) and 743 patients with complete FC evaluations at both baseline and week 6, for whom relative reductions from baseline could be calculated. Of these patients, those who achieved clinical remission, mucosal healing, or endoscopic remission at week 6—regardless of treatment group—had larger decreases from baseline in FC concentration than those who had not achieved response (Fig. 1, Supplementary Table S2).
FIGURE 1.
Change from baseline in FC concentrations at week 6 (%FC0-6) by clinical outcome status at week 6 and by treatment group: (A) clinical remission, (B) mucosal healing, and (C) endoscopic remission. Error bars represent 95% CIs. aUpper 95% CI, 117; bUpper 95% CI, 105; cUpper 95% CI, 143; dLower 95% CI, 0, Upper 95% CI, 615; eUpper 95% CI, 103. Abbreviations: CI, confidence interval; DB, double-blind; PBO, placebo; VDZ, vedolizumab.
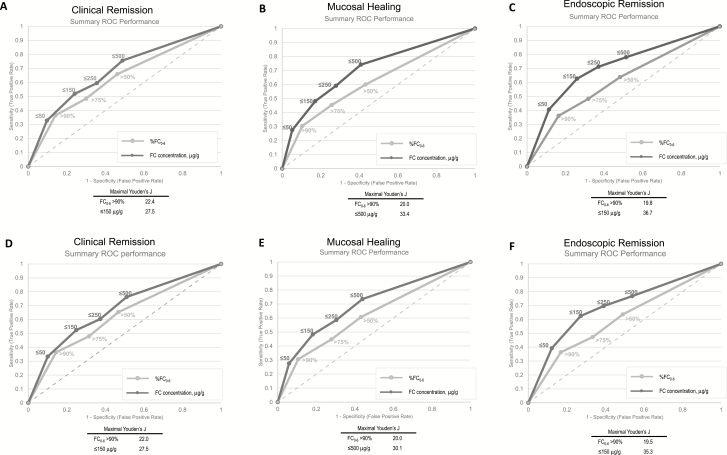
Specific cut points for FC reduction from baseline to week 6, FC0-6 (>50%, >75%, >90%), or for absolute FC concentration at week 6 (≤50, ≤150, ≤250, and ≤500 μg/g) were determined based on ROC data. Summaries of ROC curve data for the total population are shown in Fig. 2, Supplementary Table S3A, and Supplementary Table S3B. For each cut point and disease outcome sensitivity, the specificity, area under the curve (AUC), and Youden J statistic were examined. The AUC was fair (range: 0.70–0.77) across all cut points and disease outcomes. In general, the highest sensitivity was associated with the smallest FC0-6 (≥60% at FC0-6 >50%) and the highest FC concentration cutoff at week 6 (>74% for ≤500 μg/g) across all measures of disease status. Sensitivity declined with increasing change from baseline and with decreasing FC concentration. The opposite trends were observed for specificity: the largest change from baseline (FC0-6 >90%) and the lowest FC concentrations (≤50 μg/g) at week 6 were associated with the highest specificity for each measure of disease status, with specificity >83% for FC0-6 >90% and >88% for absolute value concentrations ≤50 μg/g. Limiting the analysis to patients who received vedolizumab yielded similar results. The AUC was fair (AUC range: 0.67–0.75) across cut points and measures of outcomes in patients receiving vedolizumab (Supplementary Table 3B).
FIGURE 2.
Summary of ROC performance at week 6 for clinical remission, mucosal healing, and endoscopic remission: total population (A–C) and vedolizumab-treated population (D–F).
Youden J values were generally higher for absolute cut points of FC than for percent reductions. The largest Youden’s J for reduction from baseline FC was at FC0-6 >90% (Fig. 2). Overall in this study, the most promising outcome indicators based on Youden’s J were absolute FC concentration ≤150 μg/g for clinical remission and endoscopic remission, and FC concentration ≤500 μg/g for mucosal healing.
These analyses identified multiple values that were informative; however, no robust cut point was identifiable. An FC concentration ≤50 µg/g had a PPV of 0.76 for mucosal healing, and an FC concentration >500 µg/g had an NPV of 0.79. The prevalence of these values in the overall population was 12% and 40%, respectively. Similar results were observed when analyses were restricted to vedolizumab-treated patients (Fig. 2 and Supplementary Table 3B).
In the analyses by outcome status and treatment group, there were larger reductions in FC concentrations from baseline among patients who achieved clinical remission, mucosal healing, or endoscopic remission at week 6 than in nonresponders in both treatment groups (Fig. 1). There were similar decreases in FC concentrations from baseline across all 3 outcomes.
Relationship Between Vedolizumab Treatment and FC Concentration
Because a cut point FC concentration of 150 μg/g was identified as the most promising cut point as surrogate biomarker for clinical and endoscopic remission, we compared the proportion of patients achieving this concentration of FC between the treatment groups at week 6. The proportion of patients with ≤150 µg/g FC at week 6 was higher in the vedolizumab group than in the placebo group (29.3% [95% CI, 23.4–35.3] vs 16.8% [95% CI, 10.8–22.8], respectively).
In general, even among patients who had not achieved clinical remission, mucosal healing, or endoscopic remission at week 6, there was a more pronounced decline in FC concentrations in those receiving vedolizumab than in patients receiving placebo in cohort 1 (Fig. 1A-C).
Relationship Between Baseline Fecal Calprotectin and Disease Status at Week 6
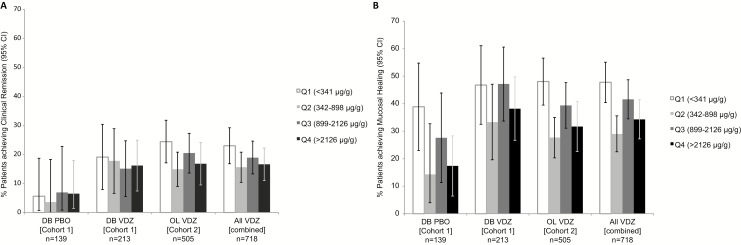
The baseline FC quartiles were calculated to be in the 25th percentile (341 µg/g), 50th percentile (898 µg/g), and 75th percentile (2126 µg/g) in the GEMINI 1 population. As shown in Fig. 3, there were no apparent trends of association between baseline FC quartiles and clinical remission or mucosal healing either in general or among vedolizumab-treated patients. In general, mucosal healing rates were greater in the subgroup of patients with lower baseline FC concentrations (within the 25th percentile, <341 µg/g) than in patients with higher baseline FC concentrations. The potential association with endoscopic remission was not assessed.
FIGURE 3.
Week 6 clinical outcomes by baseline FC concentration quartiles: (A) rates of clinical remission, (B) rates of mucosal healing. Abbreviations: OL, open-label; PBO, placebo; Q, quartile; VDZ, vedolizumab.
DISCUSSION
In this study, we evaluated FC as a biomarker of disease activity and treatment response during induction in a large population of patients with active UC enrolled in a phase 3 study. Fecal calprotectin has generally been considered to be a sensitive, noninvasive method of measuring inflammation in the gastrointestinal (GI) tract.13, 14, 21, 22 Although not specific for IBD, FC concentrations can be used as a diagnostic screening assay to rule out UC and Crohn’s disease (CD) among patients presenting with GI symptoms (at a threshold <50 μg/g).21 In addition, for patients with quiescent UC, it has been reported that FC concentrations <150 µg/g suggest endoscopic remission, and concentrations >150 µg/g are associated with an elevated risk of relapse within 2 months.12, 23 Whether FC can also be used to monitor treatment response and predict clinical and endoscopic outcomes in induction has not been extensively studied.
In our study, we observed that vedolizumab treatment was associated with a reduction in FC concentrations from baseline, regardless of clinical status at week 6. This finding is consistent with other induction studies that have reported reductions in FC concentrations with vedolizumab or golimumab.24, 25 Notably, even patients who did not achieve clinical response in our study were more likely to have FC <150 µg/g at week 6 if they received vedolizumab than if they received placebo. Indeed in GEMINI 1, the vedolizumab treatment group had both a higher proportion of patients below the threshold FC concentration of 150 μg/g and a larger mean change in FC concentration during the induction period compared with the placebo group. The decrease in FC in vedolizumab treatment groups likely reflects an active anti-inflammatory effect regardless of clinical response, and thus vedolizumab may advance the transition from relapsing to remitting states. The apparent lack of coincidence of FC reductions and endoscopic changes may reflect the different time course that these events follow, resulting in FC reductions in apparent nonresponders. According to this interpretation, FC reductions could be observed first at a given time point in nonresponders, only to be precursors to a potential future response.26
Fecal calprotectin concentration at baseline or week 6 was not strongly associated with clinical and endoscopic outcomes, and the sensitivity and specificity of FC for endoscopically defined mucosal healing was suboptimal. Based on our results, we conclude that, during induction therapy, week-6 FC concentration measurements are not clinically useful indicators of week-6 outcomes such as clinical remission, endoscopic remission, and mucosal healing.
Other studies have also evaluated the relationship between FC and UC disease status during induction therapy, but the studies have varied designs, and results are somewhat conflicting. A recent meta-analysis of 16 trials in UC patients calculated a combined sensitivity and specificity for FC of 88% and 79%, respectively, for the diagnosis of endoscopically active UC in symptomatic patients.27 The authors of the meta-analysis noted that cut points and initial disease status of patients varied from study to study. They concluded that FC could be a useful biomarker but may be specific to clinical context, which is in line with our failure to find a single cut point for FC concentration that could indicate disease state.
We attempted to determine cut points to use FC as a biomarker for clinical remission, mucosal healing status, or endoscopic remission but were unable to locate any that were of great clinical utility. For instance, in our analysis, a 90% reduction in FC concentration had 89% specificity for mucosal healing, but only a few patients (15%) achieved such a substantial percent reduction in FC concentration in GEMINI 1. Cut points for absolute FC concentrations were similarly inappropriate for real-world practice. For example, FC concentrations ≤50 µg/g reliably correlated with the presence of mucosal healing, but this value was observed in only 14% of the patients evaluated. Even for the most promising cut point identified by this study (150 μg/g), the PPV and NPV for mucosal healing in vedolizumab-treated patients were 63% and 72%, respectively, which would leave approximately one third of patients misclassified. Therefore, the results of our analysis did not show that FC concentration would have a high clinical utility potential during induction therapy, which is in line with the interpretation of the meta-analysis previously described.
A few small (n range: 20–53), open-label studies have investigated the biomarker value of FC concentrations for clinical outcomes.28–31 In one study with 53 patients treated with 5 mg/kg of infliximab, both the Mayo Clinic score (= 0) and FC (<50 mg/kg) correlated well with endoscopic remission at week 10. An AUC of ROC analyses gave 0.94 for Mayo score and 0.91 for FC.28 However, although patients who achieved endoscopic remission at week 10 showed a significant decrease in FC between baseline and week 2 (P < 0.001) compared with patients who did not show a remission, FC concentrations at week 2 had little predictive value (specificity: 67%; sensitivity: 54%, AUC: 0.59) for the protocol-defined remission (endoscopic remission and FC normalization to <50 mg/kg or >80% decrease from baseline) at week 10.28 These results may support our contention that FC values and endoscopic values may be fluctuating at different times within the relapsing and remitting cycle of UC.
One pilot study evaluated FC as an early biomarker of clinical remission at 6 weeks in UC patients receiving infliximab therapy. The study yielded sensitivity and specificity of 90% and 64%, respectively, using a cut point of 10,000 µg/mL for the FC concentration between days 1 and 3 of therapy (AUC during that period).31 Establishing a composite score with calprotectin levels, partial Mayo score, and serum infliximab <120 mg/mL increased the specificity to 79%.31 This small study suggested that biomarkers, individual or composite, were potentially useful in predicting outcomes 6 weeks after treatment but would need larger scale confirmation.
Thus, although some studies have yielded what may be promising results for using FC as a biomarker, differences in study design, the selection of cut points, and the time point studied make it difficult to gain clarity or generalize conclusions. Based on our results, we conclude that FC concentration measured at week 6 of vedolizumab induction therapy is not a clinically useful indicator of clinical remission, endoscopic remission, or mucosal healing at this visit, since we could not find a cut point that could be used in general clinical practice. Fecal calprotectin concentration at baseline was not a strong predictor of clinical and endoscopic outcomes at week 6.
The lack of a clinically robust cut point to date is just one barrier to the use of absolute values of FC or change scores as biomarkers of endoscopic healing in induction therapy. Although FC concentrations generally correlate with inflammation, there is substantial intrapatient variability.32 Moreover, specific cut points are dependent on consistency in detection methods. At present, multiple manufacturers produce testing kits based on enzyme-linked immunosorbent assay (ELISA) with distinct compositions of detection antibodies, ancillary reagents, and testing protocols.14
It is also possible that FC concentrations reflect subclinical inflammation or other processes (eg, barrier function, cytokine expression) that may not correlate well with endoscopic measures of disease activity. Knowing that UC is a disease that cycles between relapse and remission, rising FC concentrations may reflect the trafficking of inflammatory cells into the colon, setting the stage for relapse before endoscopically active or symptomatic disease.33–35 Our finding that mean FC concentrations decreased in patients receiving vedolizumab treatment, regardless of response, suggests that the α4β7 integrin inhibitor may block the trafficking of memory T cells to the gut, reducing the subsequent inflammation.26
Our study has several strengths including a large sample size, prospective collection of outcome, and the randomized design of GEMINI 1. However, a limitation was the use of the site investigators to assess endoscopic disease status. Although endoscopic assessment of mucosal healing is subjective, use of centralized, blinded readers can minimize bias.36 Baseline assessment of disease severity is often overestimated by local readers in comparison to assessment by central readers.36 Conversely, site readers systematically down code endoscopic scoring in comparison with central readers following induction therapy.37 Such biases may have contributed to measurement variances and obscured relationships between FC-defined and endoscopic outcomes.
CONCLUSIONS
Although vedolizumab induction was associated with larger reductions in FC concentrations compared with placebo, week-6 FC concentrations had only fair value as indicators of endoscopic status. Our data suggest that, although FC may reflect inflammatory burden, FC concentration measured shortly after vedolizumab induction may not be a clinically useful biomarker of mucosal inflammation or endoscopic outcomes. Additional research could help evaluate whether the surrogate biomarker value of serial FC measurements during the first 12 to 24 weeks of therapy with vedolizumab may help reduce the need for invasive procedures to monitor mucosal healing. Until such data are available, endoscopy will remain the gold standard for assessing mucosal healing in patients treated with vedolizumab.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank the patients, their families, and the investigators who participated in this study. The authors would like to thank Dirk Lindner of Takeda Pharmaceuticals International AG for his assistance in the statistical analyses during the development of the manuscript. Medical writing assistance was provided by Katy Favorite, PhD, and Julia Saiz, PhD, for inVentiv Medical Communications, LLC, a Syneos Health Group company and funded by Takeda Development Center Americas, Inc.
Supported by Takeda Development Center Americas, Inc. in Deerfield, Illinois, USA.
Conflicts of interest: AP, MR, HY, TW, and JX are all employees of Takeda Pharmaceuticals; JX also holds Takeda stock. RC is a former employee of Takeda Development Centre Europe Ltd. BB served as an advisor/speaker for Shire, Ferring, Janssen, Abbvie, Takeda, and Pfizer and as an advisor for Amgen, Pendopharm, Genentech, Merck, and Allergan and has received research support from Janssen, Abbvie, GSK, BMS, Amgen, Genentech, Merck, RedHill Biopharm, BI, Qu Biologic, Celgene, and Alvine and holds stock options from Qu Biologic. SD receives lecture fee(s) from and is a consultant for AbbVie, Ferring, Hospira, Johnson and Johnson, Merck, MSD, Takeda, Mundipharma, Pfizer Inc, Tigenix, UCB Pharma, Vifor, Biogen, Celgene, Allergan, Celltrion, Sandoz, and Boehringer Ingelheim. BF receives grant/research support from Abbvie Inc., Amgen Inc., AstraZeneca/MedImmune Ltd., Atlantic Pharmaceuticals Ltd., Boehringer Ingelheim, Celgene Corporation, Celltech, Genentech Inc/Hoffmann-La Roche Ltd., Gilead Sciences Inc., GlaxoSmithKline, Janssen Research & Development LLC., Pfizer Inc., Receptos Inc./Celgene International, Sanofi, Santarus Inc., Takeda Development Center Americas Inc., and Tillotts Pharma AG, UCB; is a consultant for Abbott/AbbVie, Ablynx, Akebia Therapeutics, Allergan, Amgen, Applied Molecular Transport Inc., Aptevo Therapeutics, Astra Zeneca, Atlantic Pharma, Avir Pharma, Baxter Healthcare Corp., Biogen Idec, Boehringer Ingelheim, Bristol-Myers Squibb, Calypso Biotech, Celgene, Elan/Biogen, EnGene, Ferring Pharma, Roche/Genentech, Galapagos, GiCare Pharma, Gilead, Given Imaging Inc., GSK, Inception IBD Inc, Ironwood Pharma, Janssen Biotech (Centocor), JnJ/Janssen, Kyowa Kakko Kirin Co Ltd., Lexicon, Lilly, Lycera BioTech, Merck, Mesoblast Pharma, Millennium, Nektar, Nestles, Nextbiotix, Novonordisk, Pfizer, Prometheus Therapeutics and Diagnostics, Progenity, Protagonist, Receptos, Roche/Genentech, Salix Pharma, Serano, Shire, Sienna Biologics, Sigmoid Pharma, Sterna Biologicals, Synergy Pharma Inc., Takeda, Teva Pharma, TiGenix, Tillotts, UCB Pharma, Vertex Pharma, Vivelix Pharma, VHsquared Ltd., Warner-Chilcott, Wyeth, Zealand, and Zyngenia and is on the speakers’ bureau of Abbott/AbbVie, JnJ/Janssen, Lilly, Takeda, Tillotts, and UCB Pharma; is a member of the Scientific Advisory Board for Abbott/AbbVie, Allergan, Amgen, Astra Zeneca, Atlantic Pharma, Avaxia Biologics Inc., Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Centocor Inc., Elan/Biogen, Ferring, Galapagos, Genentech/Roche, JnJ/Janssen, Merck, Nestles, Novartis, Novonordisk, Pfizer, Prometheus Laboratories, Protagonist, Salix Pharma, Sterna Biologicals, Takeda, Teva, TiGenix, Tillotts Pharma AG, and UCB Pharma; and is a member of the board of directors and senior science officer for Robarts Clinical Trials Inc, Western University, London. JL received personal fees from Shire, Janssen Pharmaceuticals, AbbVie, Immune Pharmaceuticals, AstraZenecca, Amgen, MedImmune, Merck, Nestle Health Science, Takeda Pharmaceuticals North America, Pfizer, Lilly, Gilead, Celgene, Samsung Bioepis, and Johnson and Johnson; he has research funding from Takeda Pharmaceuticals North America and Nestle Health Science and nonfinancial research support from AbbVie. WR is a speaker for Abbott Laboratories, Abbvie, Aesca, Aptalis, Astellas, Centocor, Celltrion, Danone Austria, Elan, Falk Pharma GmbH, Ferring, Immundiagnostik, Mitsubishi Tanabe Pharma Corporation, MSD, Otsuka, PDL, Pharmacosmos, PLS Education, Schering-Plough, Shire, Takeda, Therakos, Vifor, and Yakult; WR is a consultant for Abbott Laboratories, Abbvie, Aesca, Amgen, AM Pharma, AOP Orphan, Arena Pharmaceuticals, Astellas, Astra Zeneca, Avaxia, Roland Berger GmBH, Bioclinica, Biogen IDEC, Boehringer Ingelheim, Bristol-Myers Squibb, Cellerix, Chemocentryx, Celgene, Centocor, Celltrion, Covance, Danone Austria, Elan, Eli Lilly, Ernest & Young, Falk Pharma GmbH, Ferring, Galapagos, Genentech, Gilead, Grünenthal, ICON, Index Pharma, Inova, Janssen, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, LivaNova, Mallinckrodt, Medahead, MedImmune, Millennium, Mitsubishi Tanabe Pharma Corporation, MSD, Nestle, Novartis, Ocera, Otsuka, Parexel, PDL, Periconsulting, Pharmacosmos, Philip Morris Institute, Pfizer, Procter & Gamble, Prometheus, Protagonist, Provention, Robarts Clinical Trial, Sandoz, Schering-Plough, Second Genome, Seres Therapeutics, Setpointmedical, Sigmoid, Takeda, Therakos, Tigenix, UCB, Vifor, Zealand, Zyngenia, and 4SC; WR is an advisory board member for Abbott Laboratories, Abbvie, Aesca, Amgen, AM Pharma, Astellas, Astra Zeneca, Avaxia, Biogen IDEC, Boehringer-Ingelheim, Bristol-Myers Squibb, Cellerix, Chemocentryx, Celgene, Centocor, Celltrion, Danone Austria, Elan, Ferring, Galapagos, Genentech, Grünenthal, Inova, Janssen, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, MedImmune, Millenium, Mitsubishi Tanabe Pharma Corporation, MSD, Nestle, Novartis, Ocera, Otsuka, PDL, Pharmacosmos, Pfizer, Procter & Gamble, Prometheus, Sandoz, Schering-Plough, Second Genome, Setpointmedical, Takeda, Therakos, Tigenix, UCB, Zealand, Zyngenia, and 4SC and has received research funding from Abbott Laboratories, Abbvie, Aesca, Centocor, Falk Pharma GmbH, Immundiagnsotik, and MSD. BES receives financial support for research from Pfizer, Janssen, MedImmune, and Takeda and is a consultant for Takeda, Janssen, AbbVie, Pfizer, Shire, Lilly, and Amgen. AR has received consultant fees from Bühlmann Laboratories AG, Switzerland. PG has served as a consultant for Abbvie Immunology, Janssen Pharmaceuticals, Takeda Pharmaceuticals, and Pfizer, Inc., and on speaker bureaus for Abbvie Immunology, Takeda Pharmaceuticals, and Pfizer, Inc.; he currently receives research funding from Abbvie, Lilly, Takeda, Pfizer, Seres, Prometheus, UCB, BMS, Boehringer Ingelheim, and Corrona. TD has no conflicts of interest to disclose.
REFERENCES
- 1. Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365:1713–1725. [DOI] [PubMed] [Google Scholar]
- 2. Ungaro R, Mehandru S, Allen PB, et al. . Ulcerative colitis. Lancet. 2017;389:1756–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. . Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110:1324–1338. [DOI] [PubMed] [Google Scholar]
- 4. Ardizzone S, Cassinotti A, Duca P, et al. . Mucosal healing predicts late outcomes after the first course of corticosteroids for newly diagnosed ulcerative colitis. Clin Gastroenterol Hepatol. 2011;9:483–489.e3. [DOI] [PubMed] [Google Scholar]
- 5. Frøslie KF, Jahnsen J, Moum BA, et al. ; IBSEN Group Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133:412–422. [DOI] [PubMed] [Google Scholar]
- 6. Lichtenstein GR, Rutgeerts P. Importance of mucosal healing in ulcerative colitis. Inflamm Bowel Dis. 2010;16:338–346. [DOI] [PubMed] [Google Scholar]
- 7. Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012;61:1619–1635. [DOI] [PubMed] [Google Scholar]
- 8. Ristvedt SL, McFarland EG, Weinstock LB, et al. . Patient preferences for CT colonography, conventional colonoscopy, and bowel preparation. Am J Gastroenterol. 2003;98:578–585. [DOI] [PubMed] [Google Scholar]
- 9. Johne B, Fagerhol MK, Lyberg T, et al. . Functional and clinical aspects of the myelomonocyte protein calprotectin. Mol Pathol. 1997;50:113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Costa F, Mumolo MG, Bellini M, et al. . Role of faecal calprotectin as non-invasive marker of intestinal inflammation. Dig Liver Dis. 2003;35:642–647. [DOI] [PubMed] [Google Scholar]
- 11. Røseth AG, Aadland E, Jahnsen J, et al. . Assessment of disease activity in ulcerative colitis by faecal calprotectin, a novel granulocyte marker protein. Digestion. 1997;58:176–180. [DOI] [PubMed] [Google Scholar]
- 12. Costa F, Mumolo MG, Ceccarelli L, et al. . Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut. 2005;54:364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Røseth AG, Aadland E, Grzyb K. Normalization of faecal calprotectin: a predictor of mucosal healing in patients with inflammatory bowel disease. Scand J Gastroenterol. 2004;39:1017–1020. [DOI] [PubMed] [Google Scholar]
- 14. D’Haens G, Ferrante M, Vermeire S, et al. . Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2218–2224. [DOI] [PubMed] [Google Scholar]
- 15. Mosli MH, MacDonald JK, Bickston SJ, et al. . Vedolizumab for induction and maintenance of remission in ulcerative colitis: a Cochrane systematic review and meta-analysis. Inflamm Bowel Dis. 2015;21:1151–1159. [DOI] [PubMed] [Google Scholar]
- 16. Yamaguchi S, Takeuchi Y, Arai K, et al. . Fecal calprotectin is a clinically relevant biomarker of mucosal healing in patients with quiescent ulcerative colitis. J Gastroenterol Hepatol. 2016;31:93–98. [DOI] [PubMed] [Google Scholar]
- 17. Soler D, Chapman T, Yang LL, et al. . The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009;330:864–875. [DOI] [PubMed] [Google Scholar]
- 18. Feagan BG, Rutgeerts P, Sands BE, et al. ; GEMINI 1 Study Group Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 19. CALPRO Calprotectin ELISA Test (ALP) [package insert]. Dietzenbach, Germany: NovaTec Immunodiagnostica GmbH, 2011. [Google Scholar]
- 20. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. [DOI] [PubMed] [Google Scholar]
- 21. Kennedy NA, Clark A, Walkden A, et al. . Clinical utility and diagnostic accuracy of faecal calprotectin for IBD at first presentation to gastroenterology services in adults aged 16-50 years. J Crohns Colitis. 2015;9:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kawashima K, Ishihara S, Yuki T, et al. . Fecal calprotectin level correlated with both endoscopic severity and disease extent in ulcerative colitis. BMC Gastroenterol. 2016;16:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sandborn WJ, Panés J, Zhang H, et al. . Correlation between concentrations of fecal calprotectin and outcomes of patients with ulcerative colitis in a phase 2 trial. Gastroenterology. 2016;150:96–102. [DOI] [PubMed] [Google Scholar]
- 24. Tursi A, Allegretta L, Buccianti N, et al. . Effectiveness and safety of golimumab in treating outpatient ulcerative colitis: a real-life prospective, multicentre, observational study in primary inflammatory bowel diseases centers. J Gastrointestin Liver Dis. 2017;26:239–244. [DOI] [PubMed] [Google Scholar]
- 25. Baumgart DC, Bokemeyer B, Drabik A, et al. ; Vedolizumab Germany Consortium Vedolizumab induction therapy for inflammatory bowel disease in clinical practice–a nationwide consecutive German cohort study. Aliment Pharmacol Ther. 2016;43:1090–1102. [DOI] [PubMed] [Google Scholar]
- 26. Rosario M, Dirks NL, Milch C, et al. . A review of the clinical pharmacokinetics, pharmacodynamics, and immunogenicity of vedolizumab. Clin Pharmacokinet. 2017;56:1287–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mosli MH, Zou G, Garg SK, et al. . C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110:802–819; quiz 820. [DOI] [PubMed] [Google Scholar]
- 28. De Vos M, Dewit O, D’Haens G, et al. ; behalf of BIRD Fast and sharp decrease in calprotectin predicts remission by infliximab in anti-TNF naïve patients with ulcerative colitis. J Crohns Colitis. 2012;6:557–562. [DOI] [PubMed] [Google Scholar]
- 29. Magro F, Lopes SI, Lopes J, et al. ; Portuguese IBD group [GEDII] Histological outcomes and predictive value of faecal markers in moderately to severely active ulcerative colitis patients receiving infliximab. J Crohns Colitis. 2016;10:1407–1416. [DOI] [PubMed] [Google Scholar]
- 30. Frin AC, Filippi J, Boschetti G, et al. . Accuracies of fecal calprotectin, lactoferrin, M2-pyruvate kinase, neopterin and zonulin to predict the response to infliximab in ulcerative colitis. Dig Liver Dis. 2017;49:11–16. [DOI] [PubMed] [Google Scholar]
- 31. Beswick L, Rosella O, Rosella G, et al. . Exploration of predictive biomarkers of early infliximab response in acute severe colitis: a prospective pilot study. J Crohns Colitis. 2018;12:289–297. [DOI] [PubMed] [Google Scholar]
- 32. Toyonaga T, Kobayashi T, Nakano M, et al. . Usefulness of fecal calprotectin for the early prediction of short-term outcomes of remission-induction treatments in ulcerative colitis in comparison with two-item patient-reported outcome. Plos One. 2017;12:e0185131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guardiola J, Lobatón T, Rodríguez-Alonso L, et al. . Fecal level of calprotectin identifies histologic inflammation in patients with ulcerative colitis in clinical and endoscopic remission. Clin Gastroenterol Hepatol. 2014;12:1865–1870. [DOI] [PubMed] [Google Scholar]
- 34. Magro F, Lopes J, Borralho P, et al. . Comparison of different histological indexes in the assessment of UC activity and their accuracy regarding endoscopic outcomes and faecal calprotectin levels. Gut. 2018; doi:10.1136/gutjnl-2017-315545. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 35. Molander P, Färkkilä M, Ristimäki A, et al. . Does fecal calprotectin predict short-term relapse after stopping TNFα-blocking agents in inflammatory bowel disease patients in deep remission?J Crohns Colitis. 2015;9:33–40. [DOI] [PubMed] [Google Scholar]
- 36. Feagan BG, Sandborn WJ, D’Haens G, et al. . The role of centralized reading of endoscopy in a randomized controlled trial of mesalamine for ulcerative colitis. Gastroenterology. 2013;145:149–157.e2. [DOI] [PubMed] [Google Scholar]
- 37. Feagan B, Vermeire S, Sandborn W, et al. . Tofacitinib for maintenance therapy in patients with active ulcerative colitis in the phase 3 OCTAVE Sustain trial: results by local and central endoscopic assessments. United European Gastroenterol J. 2017;5(5_suppl):A25–A26. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.