Abstract
Background
People with coronary heart disease (CHD) often require prolonged absences from work to convalesce after acute disease events like myocardial infarctions (MI) or revascularisation procedures such as coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI). Reduced functional capacity and anxiety due to CHD may further delay or prevent return to work.
Objectives
To assess the effects of person‐ and work‐directed interventions aimed at enhancing return to work in patients with coronary heart disease compared to usual care or no intervention.
Search methods
We searched the databases CENTRAL, MEDLINE, Embase, PsycINFO, NIOSHTIC, NIOSHTIC‐2, HSELINE, CISDOC, and LILACS through 11 October 2018. We also searched the US National Library of Medicine registry, clinicaltrials.gov, to identify ongoing studies.
Selection criteria
We included randomised controlled trials (RCTs) examining return to work among people with CHD who were provided either an intervention or usual care. Selected studies included only people treated for MI or who had undergone either a CABG or PCI. At least 80% of the study population should have been working prior to the CHD and not at the time of the trial, or study authors had to have considered a return‐to‐work subgroup. We included studies in all languages. Two review authors independently selected the studies and consulted a third review author to resolve disagreements.
Data collection and analysis
Two review authors extracted data and independently assessed the risk of bias. We conducted meta‐analyses of rates of return to work and time until return to work. We considered the secondary outcomes, health‐related quality of life and adverse events among studies where at least 80% of study participants were eligible to return to work.
Main results
We found 39 RCTs (including one cluster‐ and four three‐armed RCTs). We included the return‐to‐work results of 34 studies in the meta‐analyses.
Person‐directed, psychological counselling versus usual care
We included 11 studies considering return to work following psychological interventions among a subgroup of 615 participants in the meta‐analysis. Most interventions used some form of counselling to address participants' disease‐related anxieties and provided information on the causes and course of CHD to dispel misconceptions. We do not know if these interventions increase return to work up to six months (risk ratio (RR) 1.08, 95% confidence interval (CI) 0.84 to 1.40; six studies; very low‐certainty evidence) or at six to 12 months (RR 1.24, 95% CI 0.95 to 1.63; seven studies; very low‐certainty evidence). We also do not know if psychological interventions shorten the time until return to work. Psychological interventions may have little or no effect on the proportion of participants working between one and five years (RR 1.09, 95% CI 0.88 to 1.34; three studies; low‐certainty evidence).
Person‐directed, work‐directed counselling versus usual care
Four studies examined work‐directed counselling. These counselling interventions included advising patients when to return to work based on treadmill testing or extended counselling to include co‐workers' fears and misconceptions regarding CHD. Work‐directed counselling may result in little to no difference in the mean difference (MD) in days until return to work (MD −7.52 days, 95% CI −20.07 to 5.03 days; four studies; low‐certainty evidence). Work‐directed counselling probably results in little to no difference in cardiac deaths (RR 1.00, 95% CI 0.19 to 5.39; two studies; moderate‐certainty evidence).
Person‐directed, physical conditioning interventions versus usual care
Nine studies examined the impact of exercise programmes. Compared to usual care, we do not know if physical interventions increase return to work up to six months (RR 1.17, 95% CI 0.97 to 1.41; four studies; very low‐certainty evidence). Physical conditioning interventions may result in little to no difference in return‐to‐work rates at six to 12 months (RR 1.09, 95% CI 0.99 to 1.20; five studies; low‐certainty evidence), and may also result in little to no difference on the rates of patients working after one year (RR 1.04, 95% CI 0.82 to 1.30; two studies; low‐certainty evidence). Physical conditioning interventions may result in little to no difference in the time needed to return to work (MD −7.86 days, 95% CI −29.46 to 13.74 days; four studies; low‐certainty evidence). Physical conditioning interventions probably do not increase cardiac death rates (RR 1.00, 95% CI 0.35 to 2.80; two studies; moderate‐certainty evidence).
Person‐directed, combined interventions versus usual care
We included 13 studies considering return to work following combined interventions in the meta‐analysis. Combined cardiac rehabilitation programmes may have increased return to work up to six months (RR 1.56, 95% CI 1.23 to 1.98; number needed to treat for an additional beneficial outcome (NNTB) 5; four studies; low‐certainty evidence), and may have little to no difference on return‐to‐work rates at six to 12 months' follow‐up (RR 1.06, 95% CI 1.00 to 1.13; 10 studies; low‐certainty evidence). We do not know if combined interventions increased the proportions of participants working between one and five years (RR 1.14, 95% CI 0.96 to 1.37; six studies; very low‐certainty evidence) or at five years (RR 1.09, 95% CI 0.86 to 1.38; four studies; very low‐certainty evidence). Combined interventions probably shortened the time needed until return to work (MD −40.77, 95% CI −67.19 to −14.35; two studies; moderate‐certainty evidence). Combining interventions probably results in little to no difference in reinfarctions (RR 0.56, 95% CI 0.23 to 1.40; three studies; moderate‐certainty evidence).
Work‐directed, interventions
We found no studies exclusively examining strictly work‐directed interventions at the workplace.
Authors' conclusions
Combined interventions may increase return to work up to six months and probably reduce the time away from work. Otherwise, we found no evidence of either a beneficial or harmful effect of person‐directed interventions. The certainty of the evidence for the various interventions and outcomes ranged from very low to moderate. Return to work was typically a secondary outcome of the studies, and as such, the results pertaining to return to work were often poorly reported. Adhering to RCT reporting guidelines could greatly improve the evidence of future research. A research gap exists regarding controlled trials of work‐directed interventions, health‐related quality of life within the return‐to‐work process, and adverse effects.
Plain language summary
Interventions to help people return to work after a heart attack, bypass or stent.
What is the aim of this review?
We aimed to find and analyse the results of studies examining programmes to help people with heart disease return to work in order to determine if these programmes really help them return to work, and also if these programmes affect quality of life or have any unwanted effects.
Key messages
Cardiac rehabilitation programmes, including both exercise and counselling components, probably shorten the time needed to return to work (moderate‐certainty evidence) and may increase the number of patients who return to work in the first six months after a heart attack, bypass or stent (low‐certainty evidence), but these programmes may have little or no effect on return to work after six months. Programmes comprising only counselling or exercise may make little to no difference in the number of patients returning to work or in the time needed to return to work (low to very low‐certainty evidence).
What was studied in the review?
People recovering from a heart attack or from a procedure to improve heart disease may have problems returning to work. These procedures could be a bypass (a surgical procedure to bypass narrowed coronary arteries, also called coronary artery bypass graft or CABG) or a nonsurgical intervention, including implanting stents (called percutaneous coronary interventions (PCI)), for example. Physical weakness and emotional problems resulting from heart disease may result in long absences from work or lead to disability retirement. Conditions at work may also make it difficult for patients to return to work. This can have a lasting impact on their quality of life. We looked at programmes that made it easier for people to return to work, for example by modifying their working conditions, or addressing the anxiety that often accompanies heart disease by educating patients on heart health, helping them to exercise or applying a combination of counselling and exercise to help them become healthy enough to return to work.
What are the main results of the review?
We found a total 39 studies that looked at return to work among people with heart disease in programmes designed to support the recovery process or encourage return to work compared to patients receiving usual care.
We found no studies that made changes to the workplace or workplace policies to ease the return to work, for example by reducing patients' working hours or tasks, and gradually increasing the working hours and tasks as health improves.
We found 11 studies evaluating programmes that addressed the fears and depression that often accompany heart disease, by teaching patients about heart disease. We do not know if these counselling and health education programmes increase the number of patients who returned to work or shorten the time patients are away from their jobs (low‐ to very low‐certainty evidence).
We found four studies using programmes that recommended when people with heart disease should return to work or provided counselling to co‐workers to address their concerns regarding the causes of the heart attacks and the patient’s ability to resume working. Work‐directed counselling interventions may make little to no difference to the time patients need to return to work (low‐certainty evidence).
We found nine studies providing exercise programmes alone. Exercise programmes may make little to no difference in the number of patients returning to work between six months and a year (low‐certainty evidence) and may make little to no difference in the number of patients working between one and five years or in the time needed to return to work (low‐certainty evidence).
We found 17 studies that evaluated combined exercise and counselling programmes. These combined programmes may increase the number of patients returning to work up to six months after a heart attack, bypass or stent (low‐certainty evidence): for every five patients enrolled in a combined cardiac rehabilitation programme, one additional patient may return to work. These programs probably shorten the time needed to return to work (moderate‐certainty evidence) by about a month.
How up‐to‐date is this review?
We searched for studies that had been published up to 11 October 2018.
Summary of findings
Summary of findings for the main comparison. Psychological interventions (including health education) compared to usual care for people with coronary heart disease.
| Psychological interventions (including health education) compared to usual care for people with coronary heart disease | ||||||
| Patient or population: people with coronary heart disease Setting: hospital/home Intervention: psychological interventions (including health education) Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with psychological interventions (including health education) | |||||
| Proportion of participants returning to work in the short term (up to 6 months) Follow‐up: range 3 months to 4 months | Study population | RR 1.08 (0.84 to 1.40) | 375 (6 RCTs) | ⊕⊝⊝⊝ Very low1,2,3,4 | We do knot know if psychological interventions (including health education) increase the proportion returning to work in the short term (up to 6 months) | |
| 63 per 100 | 68 per 100 (53 to 88) | |||||
| Proportion of participants returning to work in the medium term (6 months ‐ 1 year) Follow‐up: range 6 months to 1 year | Study population | RR 1.24 (0.95 to 1.63) | 316 (7 RCTs) | ⊕⊝⊝⊝ Very low1,2,3,4 | We do not know if psychological interventions (including health education) increase the proportion returning to work in the medium term (6 months ‐ 1 year). | |
| 63 per 100 | 78 per 100 (59 to 100) | |||||
| Proportion of participants at work in the long term (> 1 to < 5 years) Follow‐up: range 1.5 years to 4 years | Study population | RR 1.09 (0.88 to 1.34) | 239 (3 RCTs) | ⊕⊕⊝⊝ Low2,3 | Psychological interventions (including health education) may make little or no difference in the proportion working in the long term (> 1 to < 5 years) | |
| 74 per 100 | 81 per 100 (65 to 99) | |||||
| Days until return to work Follow‐up: range 6 months to 1.5 years | The mean time to return to work was 9.7 days lower (35.09 lower to 15.69 higher) | ‐ | 125 (2 RCTs) | ⊕⊝⊝⊝ Very low1,2,3 | We do not know if psychological interventions (including health education) lower the days needed until returning to work | |
| *The risk in the Intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level due to substantial heterogeneity that we could not completely explain. 2Downgraded one level due to risk of bias. 3Downgraded one level due to imprecision (pooled confidence interval is wide and includes either a possible appreciable harm or benefit). 4Downgraded one level, because results of a funnel plot indicated possible publication bias.
Summary of findings 2. Work‐directed counselling compared to usual care for people with coronary heart disease.
| Work‐directed counselling compared to usual care for people with coronary heart disease | ||||||
| Patient or population: people with coronary heart disease Setting: hospital/home Intervention: work‐directed counselling Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with work‐directed counselling | |||||
| Days until return to work | The mean time to return to work was 7.52 days lower (20.07 lower to 5.03 higher) | ‐ | 618 (4 RCTs) | ⊕⊕⊝⊝ Low1,2 | Work‐directed counselling may result in little to no difference in days until return to work | |
| Adverse effects: cardiac deaths Follow‐up mean: 6 months | 2 per 100 | 2 per 100 (0 to 8) | RR 1.00 (0.19 to 5.39) | 388 (2 RCTs) | ⊕⊕⊕⊝ Moderate3 | Work‐directed counselling probably results little or no difference in cardiac death rates |
| *The risk in the Intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level due to substantial heterogeneity that we could not completely explain. 2Downgraded one level due to imprecision (two of the four studies did not report the standard deviation). 3Downgraded one level due to imprecision (pooled confidence interval is wide and includes either a possible harm or benefit).
Summary of findings 3. Physical conditioning interventions compared to usual care for people with coronary heart disease.
| Physical conditioning interventions compared to usual care for people with coronary heart disease | ||||||
| Patient or population: people with coronary heart disease Setting: hospital/home Intervention: physical conditioning interventions Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with physical conditioning interventions | |||||
| Proportion of participants returning to work in the short term (up to 6 months) Follow‐up: range 3 months to 5.5 months | Study population | RR 1.17 (0.97 to 1.41) | 460 (4 RCTs) | ⊕⊝⊝⊝ Very low1,2,3 | We do not know if physical conditioning interventions increase the proportion returning to work in the short term (up to 6 months) | |
| 68 per 100 | 80 per 100 (66 to 96) | |||||
| Proportion of participants returning to work in the medium term (6 months‐1 year) Follow‐up: range 0.5 years to 1 years | Study population | RR 1.09 (0.99 to 1.20) | 510 (5 RCTs) | ⊕⊕⊝⊝ Low1 4 | Physical conditioning interventions may result in little to no difference in proportion returning to work in the medium term (6 months‐1 year) | |
| 75 per 100 | 82 per 100 (74 to 90) | |||||
| Proportion of participants at work in the long term (> 1 to < 5 years) Follow‐up: range 3 years to 4 years | Study population | RR 1.04 (0.82 to 1.30) | 156 (2 RCTs) | ⊕⊕⊝⊝ Low1 | Physical conditioning interventions may result in little to no difference in proportion at work in the long term (> 1 to < 5 years) | |
| 64 per 100 | 67 per 100 (53 to 84) | |||||
| Proportion of participants at work in the extended long term (≥ 5 years) Follow‐up: mean 5 years | Study population | RR 1.83 (1.26 to 2.66) | 119 (1 RCT) | ⊕⊕⊝⊝ Low5 | Physical conditioning interventions may increase the proportion at work in the extended long term (≥ 5 years) | |
| 37 per 100 | 68 per 100 (47 to 99) | |||||
| Days until return to work | The mean time to return to work was 7.86 days lower (29.46 lower to 13.74 higher) | ‐ | 430 (4 RCTs) | ⊕⊕⊝⊝ Low1 2 | Physical conditioning interventions appear to result in little to no difference in mean time to return to work (days) | |
|
Adverse effects: cardiac deaths Follow‐up: mean 4.8 years |
8 per 100 | 8 per 100 (3 to 24) | RR 1.00 (0.35 to 2.80) | 285 (2 RCTs) | ⊕⊕⊕⊝ Moderate3 | Physical conditioning interventions probably do not increase adverse effects (cardiac deaths) |
| *The risk in the Intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trials; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level due to risk of bias. 2Downgraded one level due to substantial heterogeneity that we could not completely explain. 3Downgraded one level due to imprecision (pooled confidence interval is wide and includes either a possible appreciable harm or benefit). 4Downgraded one level, because results of funnel plot indicated possible publication bias. 5Downgraded one level because only one study reported the proportion of study participants working five years after the intervention.
Summary of findings 4. Combined interventions compared to usual care for people with coronary heart disease.
| Combined interventions compared to usual care for people with coronary heart disease | ||||||
| Patient or population: people with coronary heart disease Setting: hospital/home Intervention: combined interventions Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with combined interventions | |||||
| Proportion of participants returning to work in the short term (up to 6 months) Follow‐up: range 2.3 months to 4 months | Study population | RR 1.56 (1.23 to 1.98) | 395 (4 RCTs) | ⊕⊕⊝⊝ Low1,2 | Combined rehabilitation interventions may increase the proportion returning to work in the short term (up to 6 months) | |
| 39 per 100 | 61 per 100 (48 to 78) | |||||
| Proportion of participants returning to work in the medium term (6 months ‐ 1 year) Follow‐up: range 6 months to 1 year | Study population | RR 1.06 (1.00 to 1.13) | 992 (10 RCTs) | ⊕⊕⊝⊝ Low3 | Combined interventions may result in little to no difference in the proportion returning to work in the medium term (6 months ‐ 1 year) | |
| 72 per 100 | 76 per 100 (72 to 81) | |||||
| Proportion of participants at work in the long term (> 1 to < 5 years) Follow‐up: range 1.2 years to 3 years | Study population | RR 1.14 (0.96 to 1.37) | 491 (6 RCTs) | ⊕⊝⊝⊝ Very low1,3 | We do not know if combined interventions increase the proportion working long term (> 1 to < 5 years) | |
| 53 per 100 | 60 per 100 (51 to 72) | |||||
| Proportion of participants at work in the extended long term (≥ 5 years) Follow‐up: 5 years | Study population | RR 1.09 (0.86 to 1.38) | 350 (4 RCTs) | ⊕⊝⊝⊝ Very low1,3 | We do not know if combined interventions increase the proportion working after an extended term (≥ 5 years) | |
| 37 per 100 | 41 per 100 (32 to 51) | |||||
| Days until return to work | The mean time to return to work in the intervention group was 40.77 days lower (67.19 lower to 14.35 lower) | ‐ | 181 (2 RCTs) | ⊕⊕⊕⊝ Moderate4 | Combined rehabilitation interventions probably reduce mean time to return to work (days) | |
| Health‐related quality of life assessed with: Angina Pectoris Quality of Life Questionnaire | ‐ | The MD for HrQoL was 0.40 (‐0.03 lower to 0.83 higher) | 87 (1 RCT) | ⊕⊕⊝⊝ Low2,5 | Combined interventions may result in little to no difference in HrQoL | |
|
Adverse effects: reinfarctions Follow‐up: mean 3.8 years |
10 per 100 | 6 per 100 (2 to 15) | RR 0.56 (0.23 to 1.43) | 265 (3 RCTs) | ⊕⊕⊕⊝ Moderate1 | Combined interventions likely result in little to no difference in adverse effects |
| *The risk in the Intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HRQoL: health‐related quality of life; RCT: randomised controlled trial; RR: risk ratio; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level due to imprecision (pooled confidence interval is wide and includes either a possible appreciable harm or benefit). 2Downgraded one level due to risk of bias. 3Downgraded two levels due to risk of bias. 4We detected substantial heterogeneity that we could not completely explain. 5Downgraded one level because only one study reported the effects of the intervention on health‐related quality of life.
Background
Description of the condition
Coronary heart disease (CHD), also called coronary artery disease or ischaemic heart disease, is a narrowing or blockage of the blood vessels supplying the heart muscles (WHO 2012). The most common cause of CHD is atherosclerosis, which is a build‐up of cholesterol and fatty deposits (called plaques) on the inner walls of these arteries. A myocardial infarction (MI) may be the first manifestation of coronary artery disease, but it may also occur in people with established disease. Cardiac ischaemia, that is restriction in blood supply, can often cause chest pain known as angina pectoris when the myocardium, or heart muscle tissue, is starved of oxygen.
CHD is the most important cause of mortality and morbidity in Western industrialised countries. In 2016, with 9.4 million deaths (16.2% of total deaths, all ages) it was the leading cause of deaths in the world (WHO 2018a). In European countries it accounts for 13.6% of total disability adjusted life years (DALYs) and 7.6% of total DALYs internationally (WHO 2018b).
CHD morbidity has economic as well as social implications. Leal 2006 estimated the total costs for the European Union to be EUR 45 billion in 2003, with 51% incurred in health care, 34% in productivity losses and 15% in informal care. Anxiety and depression are often experienced after MI and can have major effects on quality of life and on return to work (Dickens 2006; O'Neil 2010).
People who have experienced cardiac events face many challenges, such as pain and discomfort, fatigue, anxiety, problems with physical activity, cardiac medication, or concerns about diet (Blair 2014). Furthermore, data from qualitative interviews with young patients show that their disease has an impact on establishing a career, meaningful relationships, family, and financial security, thus negatively affecting mental health and health‐related quality of life (Walsh 2018).
Cardiac rehabilitation plays an important role in the overall clinical management of cardiac patients. The National Institute for Health and Care Excellence (NICE) has defined cardiac rehabilitation as a "coordinated and structured programme designed to remove or reduce the underlying causes of cardiovascular disease, as well as to provide the best possible physical, mental and social conditions, so that people can, by their own efforts, continue to play a full part in their community. A healthier lifestyle and slowed or reversed progression of cardiovascular disease can also be achieved" (NICE 2015). Although physical activity is commonly recommended as a core element for people with MI or other acute coronary syndromes, combined (or comprehensive) cardiac rehabilitation consists of interventions with health education, lifestyle advice, stress management and physical exercise components (NICE 2013; Perk 2012; Piepoli 2014). According to the Agency for Healthcare Research and Quality (AHRQ), the programmes are "designed to limit the physiological and psychological effects of cardiac illness, reduce the risk for sudden death or re‐infarction, control cardiac symptoms, stabilise or reverse the atherosclerotic process, and enhance the psychosocial and vocational status of selected patients" (Wenger 1995; Wenger 2008).
The benefits of cardiac rehabilitation have been examined in several systematic reviews. A recently updated Cochrane Review concluded that exercise‐based cardiac rehabilitation for people with CHD is effective in reducing cardiovascular mortality in medium‐ to long‐term studies, and hospital admissions in short‐term studies, but not total MI or need for revascularisation by means of coronary artery bypass surgery (CABG) or percutaneous coronary intervention (PCI) including percutaneous transluminal coronary angioplasty and stents (Anderson 2016). Both PCI and CABG are used to treat blocked coronary arteries. CABG is a surgical procedure to bypass narrowed coronary arteries, whereas PCI is a nonsurgical procedure that opens blocked or narrowed coronary arteries. Another Cochrane Review that focused on psychological interventions for CHD found that psychological interventions may produce small to moderate reductions in depression and anxiety, and may also reduce cardiac mortality. The authors did not find evidence that psychological interventions reduced the rate of MI or the need for cardiac surgery, or total mortality (Richards 2017; Whalley 2011). A third Cochrane Review stated that there is not enough information available to fully understand the impact of educational interventions on mortality, morbidity and health‐related quality of life of people with CHD (Anderson 2017b; Brown 2011).
Although all patients should be offered a cardiac rehabilitation programme with an exercise component (NICE 2013), the majority of CHD patients eligible for cardiac rehabilitation do not enter into these programmes; this is especially true for women, older people, and people with a lower socio‐economic status (Sunamura 2017).
However, it is not sufficient to focus on mortality and morbidity alone. Returning to work is another important outcome of societal and economic significance, especially for younger patients. Although one goal of cardiac rehabilitation is to improve vocational status, it is not known how effective the various properties of cardiac rehabilitation programmes are at enhancing return to work among people with CHD, nor how effective interventions provided by the occupational physicians or other healthcare personnel are when there is no cardiac rehabilitation. According to Hämäläinen 2004 there are also large variations between countries in what proportion of patients (between 40% and 90%) return to work following a MI.
Returning to work is a complex and multi‐factorial process. It has been shown that there are a variety of predictors of returning to work among patient groups, for example, the medical seriousness of the disorder, work‐related factors, personal factors, national compensation policies, and the structure of the healthcare system (Cancelliere 2016; De Vries 2018; Den Bakker 2018). Recent studies examining generic factors that influence return to work found job control, work ability, perceived good health, higher self‐efficacy, the individual's own prediction of their return to work, high socioeconomic status, return‐to‐work co‐ordination, and multidisciplinary interventions facilitate return to work, while job strain, anxiety, depression, comorbidity, long‐term sick leave, older age and low education were identified to be barriers to returning to work (Cancelliere 2016; Gragnano 2018; Vooijs 2015).
Concerning people with CHD, important predictors of returning to work appear to be cardiac factors on admission to the hospital (heart failure, arrhythmia), recurrent cardiac events, and depression scores during hospitalisation (Bhattacharyya 2007), as well as occupational factors, such as the physical intensity of work (Dreyer 2016). Results of a systematic review suggest that depression recorded between admission and up to two months after discharge predicted poorer return to work six to 12 months after a cardiac event (O'Neil 2010). Furthermore peoples' beliefs and perceptions about their illness are considered key determinants of recovery after MI (Petrie 1996). More recently, a study suggested that when patients are satisfied with their job and perceive their work environment positively, they will be more likely to return to work early (Fiabane 2012). An interview survey of a random sample of 2000 people in the UK revealed that being able to work was judged to be the third most important aspect of quality of life for people suffering from an illness, whereas healthy people viewed it as only the sixth most important aspect (Bowling 1995).
While there is a high interest in increasing return to work, the adverse effects of returning to work too early, also called presenteeism, have to be considered (Järvholm 2012). A study by Kivimäki 2005 from the Whitehall II cohort examined the association between sickness absenteeism and the incidence of serious coronary events. The incidence of serious coronary events among unhealthy employees with no sickness absenteeism was twice as high as among unhealthy employees with moderate levels of sickness absenteeism.
Several authors in various countries have proposed additions or alterations to cardiac rehabilitation programmes that are important for work outcomes. In the Netherlands a new guideline on cardiac rehabilitation has been established which includes occupational checklists for determining the need for intervention (NVVC 2011). These checklists and interventions are based on the Dutch guideline for occupational physicians on how to deal with people with CHD (Verbeek 2006). The guidelines strongly advise to start supporting return to work during cardiac rehabilitation, and not after it has finished.
Usually, cardiac rehabilitation programmes focus on the use of aerobic exercise to restore functional capacity after an acute cardiac event. Also resistance training is nowadays standard practice. If the primary goal is return to work, the training programmes should be based on actual job‐related activities (Mital 2004). For example, studies with measurements of functional capacity requirements of firefighters and of police officers have found that a greater functional capacity is required than that typically attained in traditional cardiac rehabilitation programmes (Adams 2009; Adams 2010).
An example of a work‐directed intervention is the stepwise occupational reintegration (SOR) programme. It is an established instrument in Germany intended to support insured workers currently on sick leave to reintegrate back into work step‐by‐step after long‐term illness of more than six weeks duration (Bethge 2016; Bürger 2011). Another programme has been developed for people who were not able to return to work after finishing their regular cardiac rehabilitation called "Interdisciplinary Support Programme (INA)". INA is a combined support programme consisting of exercise training, health education, psychological intervention and expert advice concerning job‐related problems (Karoff 2000a).
Description of the intervention
Based on the International Classification of Functioning, Disability, and Health model (ICF) by the World Health Organization (WHO 1993) there are three opportunities for interventions to enhance return to work (Verbeek 2006):
better treatment of the disease;
work‐directed interventions; and
person‐directed interventions.
This Cochrane Review aims to assess the effects of interventions directed at people with CHD or their environment, specifically their working environment, or combinations of the two, to enhance return to work.
Work‐directed interventions are defined in this review as: workplace adjustments such as modified work hours, modified work tasks, or workplace modifications and improved communication with or between managers, colleagues and health professionals.
Person‐directed interventions consist of:
Physical conditioning interventions that include any type of physical training and physical exercises, and
Psychological interventions that include any type of intervention such as patient counselling and health education; screening and treatment of comorbid psychological disorders; stress management and relaxation training; social support; and gender‐specific interventions.
How the intervention might work
Person‐directed interventions like physical conditioning interventions and intense, occupation‐specific training aim to equip patients with a level of functional capacity that is necessary to perform work tasks safely and successfully (Adams 2010; Adams 2009). Specific psychological interventions, on the other hand, can help by changing people's perception of their illness such that they see themselves again as capable workers and not just as recuperating patients (Petrie 2002).
Work‐directed interventions aim to facilitate return to work by reducing perceived or actual barriers to returning to work by implementing workplace design changes, pauses, etc.
Why it is important to do this review
A range of programmes has been developed to increase the return to work of people with CHD. There are also large variations between countries in the proportion of people that return to work following an MI (ranging from 40% to 90%) (Hämäläinen 2004). While varying cultural and sociopolitical factors may influence people's decisions to return to work (Perk 2004), the variation between countries also seems to suggest that some programmes may be more effective than others.
A number of Cochrane Reviews (Anderson 2016; Anderson 2017a; Anderson 2017b; Brown 2011; Heran 2011; Richards 2017; Whalley 2011) have already assessed the effects of cardiac rehabilitation consisting of: patient education, exercise and psychological interventions in reducing morbidity and mortality of people with CHD. However, none of these reviews have specifically assessed the effects on return to work, which is the aim of our review.
Objectives
To assess the effects of person‐ and work‐directed interventions aimed at enhancing return to work in patients with coronary heart disease compared to usual care or no intervention.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) including cluster‐RCTs and quasi‐RCTs irrespective of publication language or publication status. Quasi‐RCTs are controlled trials that use inappropriate randomisation strategies, accompanied by inadequate allocation concealment, and are therefore at higher risk of bias (Higgins 2017).
Due to the difficulties of performing RCTs at workplaces, we originally intended to include controlled before‐after studies (CBAs). CBAs are non‐randomised studies with one group that receives the intervention and a control group that does not. For a CBA study to have been included, data must have been collected contemporaneously, both at baseline and post‐intervention, so that the timing of the study periods for the control and intervention groups are comparable. Although we found a large number of CBAs examining the effects of person‐directed interventions on return to work, none of the CBA studies that we identified used interventions conducted at workplaces. As CBA studies are more prone to bias than RCTs, and because the CBAs that we found did not contribute information on work‐directed interventions, we deviated from the published protocol and excluded CBAs from the review (see Differences between protocol and review). The CBAs excluded from the review can be found in the Characteristics of excluded studies table.
Types of participants
We included studies involving adults (18 years or older) who had been diagnosed with CHD, who experienced a MI, or a coronary revascularisation procedure like CABG or PCI, as well as people with angina pectoris or angiographically‐defined CHD. Within each study, at least 80% of participants had to fulfil these criteria.
Participants should also have been employed (either in paid employment or self‐employed) at the time of diagnosis and on sick leave or otherwise not working at the time of the study because of the CHD. This could have been a subgroup of a trial, but at least 80% of the participants should not have been working at the start of the trial.
Types of interventions
We considered all interventions in the following categories that aim to support the return‐to‐work process with individual or group approaches.
Work‐directed interventions: these can include changes in the work environment, work tasks or working methods such as in a stepwise occupational reintegration (SOR) programme
-
Person‐directed interventions:
psychological interventions: all psychological interventions, such as counselling and health education; screening and treatment of comorbid psychological disorders; stress management and relaxation training; social support; gender‐specific interventions undertaken by any qualified professional (e.g. psychologist)
physical conditioning interventions: any supervised or unsupervised inpatient, outpatient, or community‐ or home‐based intervention including some form of physical training or physical exercises that is applied to a cardiac rehabilitation patient population
Any combination of the above
We included studies with a control group receiving no intervention, that is, usual care (as described in study reports). We considered studies involving any pharmacotherapeutic or dietary therapies only if both the intervention and control groups received the same treatment.
Types of outcome measures
Primary outcomes
The primary outcome was return to work, including return to either full‐ or part‐time employment, to the previous job, and to the same role or with changes in work status (change of duties, working location, function).
Return to work could be measured either as event data (e.g. return‐to‐work rates, disability pension rates), or as time‐to‐event data (e.g. time span between reporting sick and resumption of work, number of days on sick leave during the follow‐up period).
Secondary outcomes
Health‐related quality of life within the return‐to‐work process, either measured with generic instruments (SF‐36 and SF‐12, EuroQol EQ‐5D™), or with disease‐specific instruments for participants with angina, MI or heart failure (SAQ, QLMI/MacNew, MLHF, MIDAS, CLASP; Thompson 2003)
Number of participants who returned to work and were still working after an extended period of at least one year
Adverse effects
As we encountered a number of studies reporting the number of participants who were still working after five years during the review process, we added working after five years to the list the secondary outcomes.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases through October 2018 to identify potentially relevant studies:
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 10) in the Cochrane Library;
MEDLINE (PubMed);
EMBASE (OVID);
PsycINFO (ProQuest);
NIOSHTIC (OSH‐UPDATE);
NIOSHTIC‐2 (OSH‐UPDATE);
HSELINE (OSH‐UPDATE);
CISDOC (OSH‐UPDATE); and
LILACS (Virtual Library of Health).
We also searched ClinicalTrials.gov (ClinicalTrials.gov), and the World Health Organization trials portal (www.who.int/ictrp/en/), in May 2018 to identify ongoing trials. We searched all databases from their inception to the present, and we imposed no restriction on language of publication.
The search strategies used for each database and the day of the searches are available in Appendix 1, Appendix 2, Appendix 3, Appendix 4, Appendix 5, and Appendix 6.
Searching other resources
We checked the reference lists of all included studies and key review articles (Anderson 2016; Anderson 2017a; Anderson 2017b; Brown 2011; Heran 2011; O'Brien 2017; Whalley 2011), for additional references. We also contacted experts in the field to identify additional unpublished materials.
Data collection and analysis
Selection of studies
Two review authors (UE, UEW) independently screened titles and abstracts of all the studies we identified as a result of the initial search, and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. Two review authors (PH, AF or PH, JH) also independently screened later search updates. We retrieved the full‐text study reports or publication and two of the review authors (UE, UEW, or JH) independently screened the full‐texts, identified studies for inclusion, and recorded reasons for exclusion of the ineligible studies. We resolved any disagreement through discussion or, if required, we consulted a third person (JA or AS). We identified and excluded duplicates and collated multiple publications of the same study so that each study, rather than each report or publication, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009), and Characteristics of excluded studies table.
We determined the inclusion of articles published in languages other than English or German by having documents professionally translated or with the help of native speakers.
Data extraction and management
We used a data collection form for study characteristics and outcome data, which was piloted on one study in the review. Two of the review authors (UEW, JH, PH) extracted the following study characteristics from included studies.
Methods: study design, total duration of study, study location, study setting, withdrawals, and date of study
Participants: number, mean age or age range, gender, severity of condition, diagnostic criteria if applicable, inclusion criteria, and exclusion criteria
Interventions: description of intervention, comparison, duration, intensity, content of both intervention and control condition, and co‐interventions
Outcomes: description of primary and secondary outcomes specified and collected, and at which time points reported
Notes: references to review for inclusion, funding for trial, and notable conflicts of interest of study authors
Two of the review authors (UEW, PH or PH, JH) independently extracted outcome data from included studies. We noted in Characteristics of included studies if outcome data were not reported in a usable way. We resolved disagreements by consensus or by involving a third person (AF). We extracted multiple publications or reports describing a single study into a single data collection form.
We transferred extracted information into Review Manager 5 (Review Manager 2014), file via Covidence. We originally planned to enter the data directly into Review Manager 5, but during the review we decided to use Covidence to enter and compare extracted data. Two review authors (PH, JH) entered data into Covidence twice and compared entries before importing data into Review Manager 5. A second review author (AF) compared the data presented in the systematic review and study characteristics with study reports for accuracy. Where relevant data were missing or in case of uncertainties, we attempted to contact the authors of the original articles. Articles published in languages other than English, German, or Dutch were translated into English or German for the extraction and 'Risk of bias' assessment.
Assessment of risk of bias in included studies
Two authors (PH, JH) independently assessed the risk of bias in RCTs using the ‘Risk of bias’ tool recommended by Cochrane (Higgins 2017). In case of differences we consulted a third review author (AF). We assessed the risk of bias according to the following domains.
Random sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective outcome reporting
Other bias
We graded each potential source of bias as high‐risk, low‐risk or unclear and provided quotes from the study reports together with a justification for our judgment in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes where necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a patient‐reported health‐related quality‐of‐life scale). If information on risk of bias related to unpublished data or correspondence with a study author, we noted this in the 'Risk of bias' table.
We assessed the risk of bias in cluster‐RCTs with the six domains of the 'Risk of bias' tool as well as recruitment bias, baseline imbalance, loss of clusters, incorrect analysis and compatibility with RCTs randomised by individual.
We originally intended to have two authors (UE, UEW) independently assess the risk of bias in CBAs by using the checklist developed by Downs and Black (Downs 1998). We wanted to only use the items on internal validity and not those on reporting quality or external validity. The instrument has been shown to have good reliability, internal consistency and validity. The thirteen items of the checklist include the domains of the 'Risk of bias' tool recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017), listed above. We planned to modify the answers to the questions of the checklist so that they would fit the 'Risk of bias' tool as implemented in Review Manager 2014 by using 'high risk', 'low risk' or 'unclear' instead of 1 or 0 as proposed by the checklist authors. Due to their increased susceptibility to bias compared to RCTs, we deviated from our protocol and excluded CBA studies (Differences between protocol and review), making the assessment of bias with the Downs and Black checklist unnecessary.
Measures of treatment effect
We entered the outcome data for each study into the data tables in Review Manager 5 to calculate the treatment effects (Review Manager 2014). We expressed dichotomous outcome data as risk ratios with their 95% confidence intervals (CIs). When overall results were statistically significant, we calculated the number needed to treat for an additional beneficial outcome (NNTB).
For continuous variables, such as the number of days until returning to work, we used the mean difference (MD) when outcome measurements in all trials were made on the same scale. We converted results reported in months or weeks into days. If future updates of this review include studies that measure the same concept with different scales, we will calculate the standardised mean difference (SMD) with its 95% CI.
Unit of analysis issues
We originally planned to analyse data from cluster‐RCTs at the level of the individual by accounting for the clustering by using the intracluster correlation coefficient (ICC), as explained in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017). However, because the cluster‐RCTs that we identified did not report the number or size of clusters, it was impossible to include their results. We were unable to contact the authors of the cluster‐RCTs to obtain this information.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study was identified as abstract only). Where this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results with a sensitivity analysis (see Sensitivity analysis).
If numerical outcome data such as standard deviations (SDs) or correlation coefficients were missing, and we could not obtain them from the study authors within six weeks of request, we calculated them from other available statistics such as P values and t‐scores, according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In one case, we calculated the SD from the reported range and sample size using a formula for small studies where n ≤ 15 (Hozo 2005). Where only means and sample sizes were available, we imputed SDs from the pooled SD of the other studies in the same comparison group (Furukawa 2006).
Assessment of heterogeneity
We attempted to assess the clinical homogeneity of the results of included studies based on similarity of intervention, outcome and study designs. We did this by considering study populations with similar distributions of gender, severity of CHD, physically demanding occupational groups or alternatively blue‐collar and white‐collar workers as homogeneous.
During the review process, we found that the heterogeneous reporting of occupational characteristics made it difficult to objectively establish which study populations could be considered as having participant populations with similar physically demanding occupational groups. Therefore, we created a definition for categorising studies into groups with similar physically demanding working conditions that was not a part of the original protocol. We defined physically demanding occupational groups as studies where a majority of study participants (more than 50%) worked in physically demanding employment, manual labour or were described as blue‐collar workers. If 50% or fewer participants worked in physically demanding employment, manual labour or were considered blue‐collar workers, we categorised these study populations as having predominantly non‐physically demanding occupations. We considered all other studies not reporting the characteristics of occupations before the incident CHD to have unknown physical demands.
Likewise, the immense variation in how baseline cardiovascular health was reported made it necessary to create an objective framework for determining which studies could be considered to have study populations with similar CHD severity. We created this decision framework during the review process and it was not included in the original study protocol. We examined study exclusion criteria and the most commonly reported cardiovascular baseline characteristics, in order to create a framework for identifying studies with similar distributions of CHD severity. We categorised study populations as having less severe CHD if the study reported:
-
excluding participants with one or more of the following:
heart failure or systolic dysfunction (i.e. left ventricular ejection fraction (LVEF) < 40%),
unstable or stable angina (often only reported as angina),
positive exercise stress test (i.e. ≥ 2 mm ST segment change, ischaemia) using treadmill or bicycle ergometer,
intracardiac defibrillator (ICD) or atrial fibrillation; or
the study reports that either less than 25% of the participant population had heart failure or the mean LVEF in the study population was more than 40% at baseline.
We included stable angina in the criteria, because studies often used the term angina without explicitly differentiating between unstable and stable anginas. We considered study populations having more severe CHD when patients were not excluded based on cardiovascular criteria and when over 25% of the participant population had heart failure or the average LVEF in the study population was below 40% at baseline. We had a clinical occupational medical doctor specialised in occupational cardiology (JVD) assess and categorise studies that reported excluding participants based on some of the above criteria but including others. We categorised all other studies into a third category of unknown cardiovascular health or CHD severity where we could not determine the severity of CHD from the reported data.
We considered the following interventions as different from each other: work‐directed interventions, physical conditioning interventions, psychological interventions, work‐directed counselling, and combined interventions.
We considered both return‐to‐work outcomes and sick leave‐duration outcomes as similar return‐to‐work outcomes. We planned to combine overall quality‐of‐life outcomes, even if measured with different instruments, with the intention to specifically consider quality of life within the return‐to‐work process. Often studies reported results for subscales or aspects of quality of life (e.g. depression and anxiety) of all study participants, not just study participants in the return‐to‐work subgroups. Similarly, studies also reported adverse events for the entire study populations and not just for participants working prior to returning to work or who were in the return‐to‐work process. Therefore, we presented the results for health‐related quality‐of‐life outcomes and adverse events only for studies where at least 80% of the study participants were eligible to return to work.
For the assessment of statistical heterogeneity, we used the Chi² test with a significance level of P = 0.1 (because of low power of the test in most meta‐analyses), as well as the I² statistic (Higgins 2003). We adopted the values for interpretation proposed in the Cochrane Handbook for Systematic Reviews of Interventions, "0% to 40%: might not be important; 30% to 60%: may represent moderate heterogeneity; 50% to 90%: may represent substantial heterogeneity; 75% to 100%: considerable heterogeneity" (Deeks 2017).
Assessment of reporting biases
Where we were able to pool more than five studies in any single meta‐analysis, we created and visually examined a funnel plot to explore possible small study biases. Asymmetry of the plot may be due to publication bias. Where a sufficient number of studies were available, we additionally tested for funnel plot asymmetry with the test developed by Egger 1997 (Sterne 2017).
Where we detected publication bias, we adjusted for reporting bias using the 'Metatrim' command in Stata. We planned to calculate the failsafe N, which means the estimated number of studies needed to negate the results of the meta‐analysis. However, the results of the analyses where we detected publication bias were not statistically significant.
Data synthesis
Where more than one study provided usable data in any single comparison, we pooled data from studies judged to be clinically homogeneous using Review Manager 5 software (Review Manager 2014), and not version 5.2 as was stated originally in the review protocol. Where studies were statistically heterogenous, we used a random‐effects model; otherwise we used a fixed‐effect model. When using the random‐effects model, we conducted sensitivity checks by using the fixed‐effect model to reveal differences in results. We included a 95% CI for all estimates.
Where there was considerable unexplainable heterogeneity, we refrained from aggregating the studies and instead presented a narrative review.
Where multiple trial arms were reported in a single trial, we included only the relevant arms. Where two comparisons (e.g. intervention A versus usual care and intervention B versus usual care) were combined in the same meta‐analysis, we divided the control group in half to avoid double‐counting.
GRADE and 'Summary of findings' table
We planned to create a 'Summary of findings' table using the following outcomes: return to work, number of participants who were still at work after one year, number of participants still at work after five years, health‐related quality of life, and any adverse effects of interventions, if reported. We expanded the return‐to‐work outcomes to reflect the follow‐up times considered for each of the main comparisons (i.e. up to six months, between six months and one year, number of participants who were still at work after one year, number of participants still at work after five years) as well as the mean time until return to work, and any adverse effects of interventions (i.e. cardiac deaths, total mortality, reinfarctions).
We used the five GRADE considerations (i.e. study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence as it relates to the studies that contributed data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in Section 8.5 (Higgins 2017), and Chapter 12 (Schünemann 2017), of the Cochrane Handbook for Systematic Reviews of Interventions using GRADEpro GDT software (GRADEpro GDT 2015). We justified all decisions to down‐ or upgrade the quality of studies using footnotes and made comments to aid readers' understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We stratified analyses according to the length of follow‐up and conducted subgroup analyses to examine how the gender of the study populations, physically demanding occupational groups or CHD severity in the study population influenced the impact of the interventions. Given sufficient trials in future updates of this review, we will also perform meta‐regression analyses (using Stata® software) to relate the following study characteristics to their sizes of effect:
study population (age, gender, country);
length of follow‐up;
study date; and
physically demanding occupational groups or alternatively blue‐collar versus white‐collar workers.
As we expect that the quality of the usual care applied in the comparison groups is continually improving over time to include forms of cardiovascular rehabilitation in accordance with available guidelines (Price 2016), we performed meta‐regression analysis considering study date with the Stata package metareg (Stata) for outcomes where five or more studies were available. We also ordered the studies in the forest‐plots according to their publication date to visually assess any change in effect over time.
Sensitivity analysis
We performed sensitivity analysis to see what effect study limitations, that is problems in sequence generation, allocation concealment, or blinding, or incomplete outcome data, or selective outcome reporting, might have had on the results by omitting studies we judged to have a high overall risk of bias from meta‐analyses. We considered studies to have a high risk of bias overall if we judged any of the domains: sequence generation, incomplete outcome data, or selective outcome reporting to have a high risk of bias.
Results
Description of studies
Results of the search
Running our systematic search strategies in the chosen electronic reference databases from inception to 11 October 2018 resulted in a total of 10,335 references. After removing duplicates, we screened 9561 titles and abstracts for eligibility. This title and abstract screening identified 199 records where the full text of the articles needed assessment, and we identified an additional 12 records through other sources. Of these 211 articles, we excluded 156 articles (147 studies) with reasons (see Excluded studies; Characteristics of excluded studies) and we were unable to obtain the full text of six arcticles (see Studies awaiting classification; Characteristics of studies awaiting classification). The qualitative synthesis included 39 RCTs described in 49 articles (see Included studies; Characteristics of included studies), and we included 34 of the 39 studies in the quantitative synthesis of data. Figure 1 depicts the study selection process as a PRISMA flow diagram (Moher 2009). We also identified six ongoing studies through searches of clinical trials registries (see Ongoing studies; Characteristics of ongoing studies).
1.
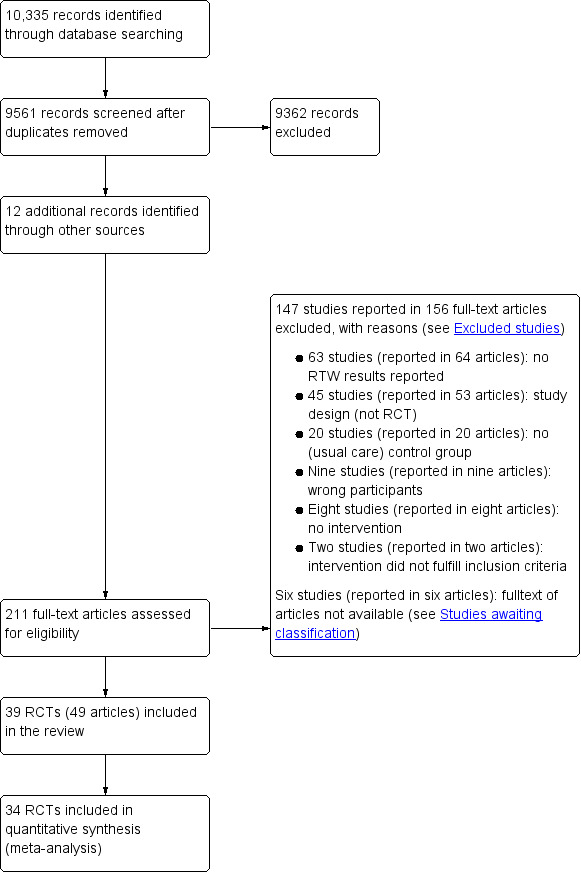
PRISMA flow diagram of study selection process
Included studies
We included 39 RCTs in the review (see Characteristics of included studies).
Design
All of the included studies were RCTs. One study applied cluster randomisation (Geissler 1979), and four studies evaluated more than one form of intervention using a three‐armed design (Froelicher 1994; PRECOR 1991; Rivas 1988; Stern 1983).
Sample sizes
Thirty‐eight of the 39 studies (excluding the multicentre WHO 1983 study) randomised altogether 5944 people with CHD into intervention and control arms. The sample sizes of the studies ranged from 10 to 456 participants. Excluding two studies lacking any information on the number of study participants who had been working prior to CHD (Carson 1982; Hämäläinen 1991), the return‐to‐work subgroups of the studies comprised 3660 participants. The studies included in the quantitative analysis randomised altogether 4661 participants and the return‐to‐work subgroups followed up comprised 3290 participants.
Setting
Studies had been conducted mostly in North America and Europe (31 of 38 studies, excluding the international multicentre WHO 1983 study). The countries contributing the most studies were the USA (eight studies), Sweden (five studies), UK (five studies), and Australia (three studies). Finland, the Netherlands, New Zealand, and Norway each contributed two studies. We also found single studies originating from Canada, Cuba, Denmark, France, the former German Democratic Republic, Germany, Italy, Portugal, and Switzerland. Of the 39 included studies, 32 recruited patients admitted to hospitals or cardiac care units, where they were being treated for CHD. Of the remaining studies, three recruited PCI patients (Higgins 2001; Hofman‐Bang 1999; Pfund 2001), one recruited patients before elective CABG (Engblom 1997), one recruited participants from what seemed to be a post‐MI outpatient clinic (Holmbäck 1994), one recruited patients referred to the study by their attending cardiologist (Erdman 1986), and one study recruited study participants among patients surviving the first (possibly in‐hospital) rehabilitation phase (Geissler 1979). Six studies conducted inpatient interventions before the participants were discharged from hospital (sometimes beginning shortly before a planned cardiac procedure). Twenty‐four studies conducted interventions as outpatient programmes, and nine studies began their interventions in the hospital before discharge and continued the intervention with either outpatient rehabilitation sessions or some sort of post‐discharge contact with the participants.
The oldest study was published in 1974 and the most recent study was published in 2017. Six of the 39 included studies first published results in the 1970s. We also observed a peak in study publication in the 1980s (13 studies) and 1990s (12 studies) that subsided in the decades beginning in 2000 (six studies) and 2010 (two studies).
Participants
Most trials (24 of the 39 included studies) included both men and women, where women typically made up a smaller proportion of the recruited participant population. Andersson 2010 was the only study to include only women, whereas 12 studies included only men (Andersen 1981; Bethell 1990; Carson 1982; Engblom 1997; Erdman 1986; Fielding 1980; Geissler 1979; Picard 1989; PRECOR 1991; Vermeulen 1988; WHO 1983; Worcester 1993), and two studies did not report the sex of participants (Bertie 1992; Marra 1985).
Only 15 of the 39 studies provided any information regarding the types of employment prior to the intervention or how many of the study participants worked in physically strenuous jobs. Based on the information provided, we classified six studies as having examined interventions among a study population of predominantly manual (blue‐collar) workers (Dugmore 1999; Haerem 2000; Lidell 1996; Maeder 1977; Vermeulen 1988; Worcester 1993) and nine studies as having considered a more sedentary (white‐collar) working population (Burgess 1987; Engblom 1997; Higgins 2001; Holmbäck 1994; Horlick 1984; Marra 1985; Picard 1989; Pilote 1992; Rivas 1988). The remaining studies did not provide enough information to judge the physical demands of work among the study population.
Most studies had been conducted among people who had suffered an acute MI (34 of 39 studies). Three studies included only PCI patients, one study included CABG patients, and one study included patients who had either suffered a MI or had undergone CABG or PCI (Andersson 2010). The severity of CHD in the participant populations was difficult to assess with the information reported, however we judged 14 studies to have included only participants with less severe CHD (Andersson 2010; Bertie 1992; Burgess 1987; Erdman 1986; Hall 2002; Holmbäck 1994; Maeder 1977; Marra 1985; Oldridge 1991; Pfund 2001; Pilote 1992; PRECOR 1991; Stern 1983; Vermeulen 1988), and 12 studies to have included participants with more severe CHD (Bengtsson 1983; Carson 1982; Dugmore 1999; Engblom 1997; Froelicher 1994; Hofman‐Bang 1999; Petrie 2002; Picard 1989; Pozen 1977; Rahe 1979; WHO 1983; Worcester 1993). Although Pozen 1977 considered participants with less severe CHD separately, we categorised this study in the more severe category but examined the results of both categories separately in subgroup analyses of CHD severity. We could not determine the severity of CHD among participant populations of the remaining 13 studies.
Interventions
We compared interventions to usual care. Usual care for CHD may have sometimes also included some lesser forms of cardiovascular rehabilitation, and participants receiving usual care might have sought other sources of cardiac rehabilitation. Some studies described usual care as having included the provision of brochures on risk factors, individual risk factor counselling or recommendations for physical training, while other studies only described usual care as comprising the clinical care of patients or provided no further description of usual care. Descriptions of the care received by participants included in the control group are included in the Characteristics of included studies tables.
Comparisons
We compared studies according to the type of intervention(s) implemented compared to usual care. We defined categories of intervention comparisons as follows.
Work‐directed interventions versus usual care
Psychological interventions (including health education) versus usual care
Work‐directed counselling versus usual care
Physical conditioning interventions versus usual care
Combined interventions applying both psychological counselling and physical conditioning versus usual care
We included four three‐armed RCTs. One study randomised participants into one of two combined intervention groups with varying intensities of exercise and a control group receiving usual care (Rivas 1988), and three randomised participants into an exercise intervention, a counselling intervention, and usual care groups (Froelicher 1994; PRECOR 1991; Stern 1983). We considered the study arms of the latter studies in the appropriate comparison groups and divided the control groups in half to avoid double counting.
The control group of one included study also received a light exercise programme instead of usual care (Worcester 1993), but the results of this study were comparable to the results of the other exercise intervention studies.
Work‐directed interventions
None of the studies implemented work‐directed interventions at the organisational level, meaning changes in the work environment, work tasks or working methods, or a stepwise occupational reintegration programme.
Person‐directed psychological interventions
Eleven studies examined the impact of psychological counselling, risk factor educational interventions or a combination of both on return to work compared to usual care (Broadbent 2009; Fielding 1980; Figueiras 2017; Haerem 2000; Hanssen 2009; Horlick 1984; Petrie 2002; Pozen 1977; PRECOR 1991; Rahe 1979; Stern 1983). We included in our meta‐analyses the return‐to‐work results for a total of 615 participants receiving psychological counselling interventions or usual care.
Person‐directed work‐directed counselling interventions
Four studies (641 participants) applied work‐directed counselling, either by recommending a time frame for return to work based on the results of a symptom‐limited treadmill test (Picard 1989; Pilote 1992), by recommending a specific workday for return to work (within a week of the counselling session) to participants and their family physicians (Pfund 2001), or by extending the counselling offered to address concerns regarding the causes of the CHD and return to work after CHD to include participants' co‐workers (Burgess 1987).
Person‐directed physical conditioning interventions
Ten studies evaluated the impact of some form of physical conditioning or physical exercises on return to work compared to usual care (Andersen 1981; Bethell 1990; Carson 1982; Dugmore 1999; Froelicher 1994; Holmbäck 1994; Maeder 1977; Marra 1985; Stern 1983; Worcester 1993). We included the return‐to‐work results of 920 participants altogether (nine studies) in our meta‐analyses. We excluded one study from the meta‐analysis because the authors did not report information regarding the number of participants returning to work in each arm of the study (Carson 1982).
Person‐directed combined interventions
Seventeen studies reported return to work following combined cardiac rehabilitation programmes including both counselling and exercise interventions compared to usual care studies (Andersson 2010; Bengtsson 1983; Bertie 1992; Engblom 1997; Erdman 1986; Froelicher 1994; Geissler 1979; Hall 2002; Hämäläinen 1991; Higgins 2001; Hofman‐Bang 1999; Lidell 1996; Oldridge 1991; PRECOR 1991; Rivas 1988; Vermeulen 1988; WHO 1983). We included the return‐to‐work results of 1230 study participants (13 studies) in our meta‐analyses.
We excluded four studies of combined interventions from our meta‐analysis (Geissler 1979; Hall 2002; Hämäläinen 1991; WHO 1983). We excluded Hall 2002 because they did not provide, and we could not obtain, the numbers of participants rejoining the workforce at various time points. We also excluded Hämäläinen 1991 from our meta‐analysis because it was unclear how many study participants had been in employment prior to the MI. We could not include the cluster‐randomised study by Geissler 1979 in our meta‐analysis, because we could not determine the number of clusters and the size of the clusters. We also excluded the WHO 1983 multicentre study from our meta‐analysis because the interventions and study methods varied greatly between centres, details about the study procedures, interventions, and characteristics of study participants of each individual centre were lacking, and results were ‐ at least in part ‐ published elsewhere by the individual studies.
Outcomes
Primary Outcomes
Most of the included studies reported the number or proportion of study participants working at follow‐ups using a subgroup of study participants who were working before their CHD. We did not include studies that did not consider return to work at least as a secondary outcome. In 10 studies, all of the participants were working or on sick leave prior to their CHD (Dugmore 1999; Fielding 1980; Froelicher 1994; Hofman‐Bang 1999; Marra 1985; Pfund 2001; Picard 1989; Pilote 1992; Rivas 1988; Vermeulen 1988). When authors reported the proportion of participants working only as percentages, we calculated the number of participants using the total number of participants in the return‐to‐work subgroups (working before CHD) where this was possible. We could not determine the number of participants working prior to CHD and at the follow‐ups in two studies (Hall 2002; Hämäläinen 1991), and the follow‐up time and number of participants who returned to work was unclear in one study that reported the mean time until return to work (Carson 1982). Although Hall 2002 applied a survival analysis to evaluate differences in return‐to‐work rates, the reported results included only the P values of Wilcoxon and log‐rank tests. Thirteen studies also reported mean time on sick leave or until return to work (Bengtsson 1983; Bethell 1990; Burgess 1987; Carson 1982; Fielding 1980; Hanssen 2009; Higgins 2001; Holmbäck 1994; Maeder 1977; Marra 1985; Pfund 2001; Picard 1989; Pilote 1992).
Secondary Outcomes
The studies reporting adverse effects and aspects of health‐related quality of life often reported results for the entire study population and not just among those eligible to return to work (health‐related quality of life within the return‐to‐work process). Therefore, we considered the adverse effects and health‐related quality of life results only among studies where the population eligible to return to work exceeded 80%.
Health‐related quality of life
For psychological intervention studies where more than 80% of the population were eligible to return to work, one study measured anxiety with a Catell Self‐Analysis Form and nine‐point rating scale (reporting only results of the paired t‐test; Fielding 1980), and a second study measured perceived health with a self‐developed personal adjustment questionnaire (Horlick 1984). We did not find enough studies reporting total health‐related quality of life to perform a meta‐analysis of health‐related quality of life for psychological interventions.
One study of work‐directed counselling assessed aspects of health‐related quality of life within the return‐to‐work process using the Impact of Events Scale, the Taylor Manifest Anxiety Survey, and the Zung Depression Scale at baseline and at the three‐ and 13‐month follow‐ups (Burgess 1987). A second study assessed health‐related quality of life with the EuroQoL Questionnaire at baseline and the four‐month follow‐up, but reported only the baseline values (Pfund 2001). All work‐directed counselling studies included only participants eligible for return to work. We did not find enough studies reporting total health‐related quality of life to perform a meta‐analysis of health‐related quality of life of work‐directed counselling interventions.
Two physical conditioning intervention studies assessed aspects of health‐related quality of life where at least 80% of the study population was considered eligible to return to work; one used the Toronto attitude scale (TAS) and the profile of mood states (POMS) checklists to assess depression, anxiety and vigour or activity, as well as a 10‐item quality‐of‐life questionnaire at the 12‐month follow‐up, stratifying the results according to prognosis (Dugmore 1999), and the other used a self‐report questionnaire on perceived physical performance and psychological well‐being but did not report the individual results (Holmbäck 1994). We were not able perform a meta‐analysis of health‐related quality of life of physical conditioning interventions.
Three studies of combined interventions where at least 80% of the study population was considered eligible to return to work assessed aspects of health‐related quality of life (Bengtsson 1983; Erdman 1986; Hofman‐Bang 1999). One study used the Minnesota Multiphasic Personality Inventory (MMPI) and questions on anxiety (Bengtsson 1983), a second study used a self‐developed well‐being questionnaire at the six‐month and five‐year follow‐ups to measure mean well‐being, feelings of disability, despondency, and social inhibition at the six‐month and five‐year follow‐ups (Erdman 1986), and the third study used the Angina Pectoris Quality of Life Questionnaire (APQLQ), Beck Depression Inventory, and Trait anxiety questionnaires, and reported the mean health‐related quality of life scores at the one‐ and two‐year follow‐ups (Hofman‐Bang 1999). We did not find enough studies reporting total health‐related quality of life scores to perform a meta‐analysis of combined interventions.
Adverse effects
We considered severe adverse effects, such as deaths, reinfarctions, cardiac surgeries, and hospital readmissions reported by studies where at least 80% of the study population was considered eligible to return to work.
Two studies of psychological and educational interventions considered adverse outcomes in study populations where at least 80% of all study participants were eligible to return to work. One reported total mortality up to six months (Broadbent 2009), and the other reported reinfarctions (Fielding 1980). We did not find enough studies to perform a meta‐analysis of adverse effects for psychological interventions.
Two studies assessed cardiac mortality or reinfarction rates up to six months following work‐directed counselling versus usual care (Picard 1989; Pilote 1992).
Three physical conditioning studies reported adverse effects as total mortality (Holmbäck 1994), cardiac deaths or fatal reinfarctions (Dugmore 1999; Marra 1985), and reinfarctions (Holmbäck 1994; Marra 1985) in study populations where at least 80% of all study participants were eligible to return to work (Dugmore 1999; Holmbäck 1994; Marra 1985). We considered fatal MI together with cardiac deaths in one meta‐analysis, and reinfarction rates in a second meta‐analysis.
Studies of combined interventions reported adverse effects as all deaths (Bengtsson 1983; Erdman 1986; Hofman‐Bang 1999; Rivas 1988), cardiac deaths (Vermeulen 1988), hospital readmissions (Hofman‐Bang 1999), or reinfarctions (Bengtsson 1983; Erdman 1986; Vermeulen 1988) in study populations where at least 80% of all study participants were eligible to return to work. We evaluated results for all deaths (total mortality) in one meta‐analysis and reinfarction rates in a second meta‐analysis.
Working after an extended period of at least one year
We found a total of 15 studies reporting on the rates of participants still working after an extended period of at least one year that could be included in a meta‐analysis (Andersen 1981; Andersson 2010; Bengtsson 1983; Bertie 1992; Burgess 1987; Dugmore 1999; Engblom 1997; Erdman 1986; Hanssen 2009; Hofman‐Bang 1999; Lidell 1996; Maeder 1977; PRECOR 1991; Rahe 1979; WHO 1983). Three studies reported extended working rates after psychological counselling and education programmes (Hanssen 2009; PRECOR 1991; Rahe 1979), one study reported extended working rates after work‐directed counselling (Burgess 1987), three studies reported working rates after physical conditioning interventions (Andersen 1981; Dugmore 1999; Maeder 1977), and eight studies reported extended working rates after combined interventions (Andersson 2010; Bengtsson 1983; Bertie 1992; Engblom 1997; Erdman 1986; Hofman‐Bang 1999; Lidell 1996; PRECOR 1991).
Follow‐up
The included studies reported return‐to‐work rates for various follow‐up times, so we categorised results into similar periods of time to examine the short‐term (up to six months), medium‐term (six to 12 months), long‐term (between one and five years), and extended long‐term (five years or longer) effects of the interventions on return to work. Where studies reported results for more than one time point we considered the data for the longest follow‐up in the range. For example, if a study reported the number of participants returning to work at both eight and 12 months, we only included the 12‐month results in the analysis of medium‐term results. Single studies sometimes provided data for more than one follow‐up range.
Five studies considered only shorter follow‐up times up to six months (Broadbent 2009; Froelicher 1994; Marra 1985; Petrie 2002; Pfund 2001), while nine studies reported both short‐term results and at least one additional follow‐up time (Andersen 1981; Bertie 1992; Dugmore 1999; Figueiras 2017; Higgins 2001; Horlick 1984; Rahe 1979; Rivas 1988; Worcester 1993).
A total of 22 studies reported return‐to‐work results for follow‐ups between six and 12 months: eight studies reported the six‐month follow‐up (Dugmore 1999; Erdman 1986; Fielding 1980; Horlick 1984; Picard 1989; Pilote 1992; Pozen 1977; Rivas 1988), eight reported the 12‐month follow‐up (Andersson 2010; Figueiras 2017; Higgins 2001; Hofman‐Bang 1999; Holmbäck 1994; Oldridge 1991; Stern 1983; Worcester 1993), and five studies reported both (Engblom 1997; Geissler 1979; Haerem 2000; Lidell 1996; Rahe 1979).
We also differentiated the secondary outcome of working after an extended period of time of at least one year to consider follow‐ups conducted between one and five years and at five years or later. Thirteen studies reported working rates between one and five years (Andersen 1981; Andersson 2010; Bengtsson 1983; Bertie 1992; Burgess 1987; Engblom 1997; Geissler 1979; Hanssen 2009; Hofman‐Bang 1999; Maeder 1977; PRECOR 1991; Rahe 1979; WHO 1983), and five studies reported results for five‐year follow‐up (Andersen 1981; Dugmore 1999; Engblom 1997; Erdman 1986; Lidell 1996).
Excluded studies
We excluded 147 studies (published in 156 articles), and listed the most critical reasons for exclusion in the Characteristics of excluded studies table. We excluded studies for the following reasons:
63 studies (64 articles) did not consider the outcome return to work or did not report the return to work results;
45 studies (53 articles) were not RCTs;
20 studies lacked a usual care control group;
eight studies lacked an intervention;
seven studies did not meet the requirement that at least 80% of study participants had to be employed at the time of diagnosis and on sick leave because of the CHD, or that study authors considered a subgroup of previously employed study participants (Bar 1992; Cay 1981; Gutschker 1977; Kittel 2008; Nelson 1994; Schiller 1976; Yonezawa 2009);
two studies applied interventions that did not satisfy our inclusion criteria (Heller 1993; Kagan‐Ponomarev 1994);
two study populations did not meet our requirements regarding CHD indications (Christensen 2017; Huber 2014).
Six futher studies reported in six articles are awaiting classification because our library staff could not locate or obtain the full‐text articles (see Characteristics of studies awaiting classification).
Risk of bias in included studies
Due to poor reporting, we often judged studies to have an unclear risk of bias for one or more domains. We considered studies to have an overall high risk of bias if we judged them to have a high risk of bias in any of the following domains: sequence generation (selection bias), incomplete outcome data (attrition bias), or selective outcome reporting (reporting bias). According to these criteria, 15 studies had an overall high risk of bias (Andersen 1981; Andersson 2010; Bertie 1992; Broadbent 2009; Carson 1982; Erdman 1986; Geissler 1979; Hanssen 2009; Hofman‐Bang 1999; Holmbäck 1994; Horlick 1984; Lidell 1996; Pozen 1977; WHO 1983; Worcester 1993). Interventions requiring the active participation of the participants, such as cardiac rehabilitation interventions are difficult, if not impossible, to conduct completely without the knowledge of the study participants (i.e. blinding of participants). Therefore, we did not consider the domains for blinding (performance bias and detection bias) in our determination of the overall risk of bias.
Of the 24 studies we considered not to have an overall high risk of bias, we assigned six studies a low risk of bias for random sequence generation, and low or unclear risk of bias for allocation concealment, blinding of outcome assessors (detection bias), incomplete outcome data (attrition bias) and selective outcome reporting (reporting bias) (Figueiras 2017; Maeder 1977; Petrie 2002; Picard 1989; Pilote 1992; Rivas 1988). We assigned the remaining 18 studies an unclear risk of bias for random sequence generation and low or unclear risk of bias for incomplete outcome data (attrition bias), incomplete outcome data (attrition bias), or selective outcome reporting (reporting bias) (Figure 2).
2.
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
Allocation
We judged three studies to have a low risk of selection bias, that is, they used a suitable random sequence generation method and concealed allocation (Broadbent 2009; Picard 1989; Pilote 1992), and seven further studies used a suitable sequence generation method (Bethell 1990; Erdman 1986; Figueiras 2017; Maeder 1977; Petrie 2002; Rivas 1988; Worcester 1993). Usually studies did not describe the method of random sequence generation and did not mention allocation concealment, and we judged these studies to have an unclear risk of bias. One study was cluster‐randomised, by region according to hospital districts (Geissler 1979). We considered this study to have a high risk of bias, as they did not report the number and size of the clusters or further details regarding the method of randomisation. Also we judged the multicentre WHO 1983 study to have a high risk of bias, because the authors reported that only half of the centres appeared to have achieved suitable randomisation.
Blinding
We gave all but three of the included studies a rating of high risk of performance bias, because the study participants and personnel were aware of the rehabilitation intervention. One exception is the study by Figueiras 2017, where the authors reported that the caregivers did not know the group allocation and we judged the risk of bias to be low. Where the form of the intervention made it less likely that participants in either group would have realised their allocated group, we judged the risk of performance bias to be unclear. This was the case for the studies where follow‐up counselling was provided with telephone calls (Hanssen 2009), and the counselling intervention was integrated within the inpatient care (Petrie 2002).
We gave six studies a low risk of bias rating for blinding of outcome assessors (Engblom 1997; Froelicher 1994; Haerem 2000; Hofman‐Bang 1999; Lidell 1996; Picard 1989). Although only Picard 1989 reported that the data co‐ordinator assessing the outcome (employment status) was not involved with performing the intervention (and presumably blinded to group allocation), we also judged detection bias to be low if work status was obtained from official documents, registries or validated questionnaires (Engblom 1997; Froelicher 1994; Haerem 2000; Hofman‐Bang 1999; Lidell 1996). We judged studies to have a high risk of bias for blinding of outcome assessors if the study descriptions stated that the outcome assessors were aware of the group allocation. This applied to four studies (Bethell 1990; Marra 1985; Oldridge 1991; Worcester 1993).
Incomplete outcome data
We assigned studies a high risk of attrition bias rating if there were unbalanced losses to follow‐up (i.e. over 5%‐point difference between groups), overall attrition exceeded 10% (without information regarding group allocation), information pertaining to the number of participants in the return‐to‐work analyses or follow‐up times for the return‐to‐work analyses were incomplete, study participants who suffered adverse outcomes were excluded from the return‐to‐work analyses, or participants' reported reasons for dropping out of the study could have biased the results, and no intention‐to‐treat analysis was conducted. Altogether this pertained to 11 studies (Andersen 1981; Andersson 2010; Bertie 1992; Broadbent 2009; Erdman 1986; Hanssen 2009; Holmbäck 1994; Horlick 1984; Lidell 1996; Pozen 1977; WHO 1983). We judged 12 studies with low losses to follow‐up and balanced attrition or studies that conducted intention‐to‐treat analyses to have a low risk of attrition bias (Bengtsson 1983; Bethell 1990; Dugmore 1999; Engblom 1997; Figueiras 2017; Geissler 1979; Hall 2002; Marra 1985; Oldridge 1991; Picard 1989; PRECOR 1991; Vermeulen 1988). We judged the remaining 13 studies as having an unclear risk of attrition bias because not enough information was provided to determine if attrition was balanced or there where discrepancies in the reported number of persons followed.
Selective reporting
Reporting bias was difficult to assess, because none of the studies cited any prior study protocol or registration with a clinical trials database. Therefore, we considered studies reporting non‐significant results and without indications of unplanned subgroup analyses as having a low risk of selective reporting bias (Andersson 2010; Vermeulen 1988). We judged the remaining studies to have an unclear risk of reporting bias.
Other potential sources of bias
We evaluated the following additional sources of bias for the Geissler 1979 cluster‐RCT: recruitment bias, baseline imbalance, loss of clusters, incorrect analysis, comparability with individually randomised trials. We included our judgements and the reasons for these judgements in the risk of bias table under other bias. Otherwise, we did not find any other potential sources of bias among the studies.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
1. Work‐directed interventions
We could not consider the effect of work‐directed interventions alone, as we found no studies examining only work‐directed interventions conducted at the organisational level. Only one study integrated a work‐directed intervention into their combined cardiac rehabilitation programme by providing employers with recommendations for work modifications when it was deemed necessary (Bengtsson 1983). We examined the results of this study in the combined interventions category.
2. Person‐directed psychological interventions
2.1 Psychological counselling and risk factor education versus usual care: primary outcomes
Eleven studies examined the impact of psychological counselling, risk factor educational interventions or a combination of both on return to work (Broadbent 2009; Fielding 1980; Figueiras 2017; Haerem 2000; Hanssen 2009; Horlick 1984; Petrie 2002; Pozen 1977; PRECOR 1991; Rahe 1979; Stern 1983).
2.1.1 Short term (less than six months)
Psychological interventions had little or no effect on the proportions of study participants returning to work up to six months (RR 1.08, 95% CI 0.84 to 1.40; I² = 69%; very low‐certainty evidence), and both the I² values and the Chi²‐test (P = 0.007) indicated substantial heterogeneity (Analysis 1.1). The severity of CHD in the study populations seemed to explain some of this heterogeneity (Analysis 1.2). To determine the possible impact of small‐study effects, we also conducted a fixed‐effect meta‐analysis, where smaller studies received less weight. The fixed‐effect model resulted in a summarised RR of 1.04 (95% CI 0.92 to 1.17). When we excluded the two studies with an overall high risk of bias (Broadbent 2009; Horlick 1984), the pooled effect of counselling interventions on the proportion of participants returning to work up to six months was RR 1.13 (95% CI 0.91 to 1.41; I² = 1%). Exclusion of these two studies also seemed to explain much of the observed heterogeneity. Considering changes to study results over time, we found that newer studies appeared less likely to find an effect. However, a meta‐regression considering the linear relationship between publication year and the log RR did not indicate any change in the impact of interventions over time (slope β = 0.006, P = 0.623). The asymmetry of the funnel plot for the short‐term results (Figure 3), indicated a presence of publication bias. However, the results of the Egger's test did not indicate any small‐study effects (P = 0.502), so we did not apply the 'trim and fill' method.
1.1. Analysis.
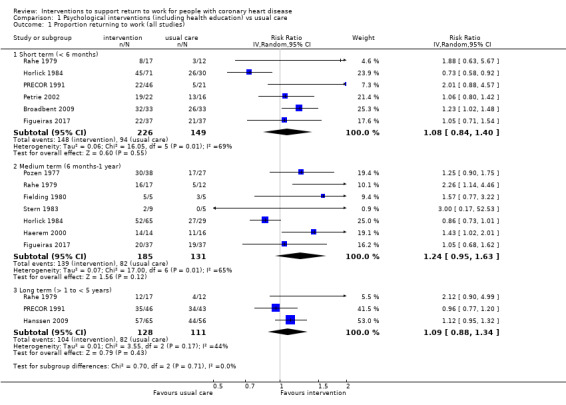
Comparison 1 Psychological interventions (including health education) vs usual care, Outcome 1 Proportion returning to work (all studies).
1.2. Analysis.
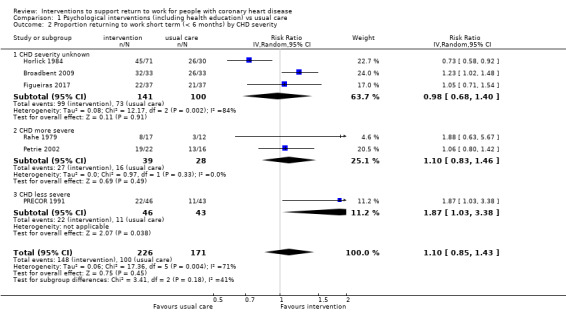
Comparison 1 Psychological interventions (including health education) vs usual care, Outcome 2 Proportion returning to work short term (< 6 months) by CHD severity.
3.
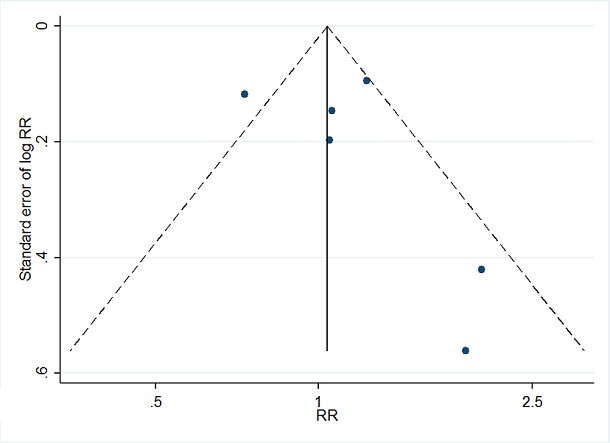
Funnel plot of comparison 2. Psychological interventions (including health education) vs usual care, outcome: 2.3 proportion returning to work (short term)
2.1.2 Medium term (six months to one year)
Seven studies reported the number of participants in work at follow‐ups from six to 12 months following psychological counselling and risk factor education (Analysis 1.1). These interventions resulted in a pooled RR for medium‐term return to work of 1.24 (95% CI 0.95 to 1.63; I² = 65%; very low‐certainty evidence). The I² value indicated substantial heterogeneity and a P value < 0.1 for the Chi²‐test was detected for the results of the medium‐term follow‐up period. A sensitivity analysis with fixed‐effect analysis to detect small‐study effects lowered the observed RR to 1.03 (95% CI 0.91 to 1.16). As a sensitivity analysis we also excluded the two studies we judged to have an overall high risk of bias (Horlick 1984; Pozen 1977), which increased the RR to 1.40 (95% CI 1.11 to 1.77; I² = 0%). Excluding the studies with an overall high risk of bias also appeared to explain some of the heterogeneity. Since interventions focused primarily on risk factor education may produce smaller effects, we also excluded the one study applying a predominantly informative intervention as a further sensitivity analysis. However, excluding Haerem 2000 lowered the pooled effect (RR 1.20, 95% CI 0.89 to 1.63; I² = 61%).
Visually, the intervention effects on return to work between six and 12 months appear to be decreasing with time (Analysis 1.1). However, the results of the meta‐regression considering the linear relationship between study year and the log RR did not indicate any time‐dependency (slope β = −0.004, P = 0.668).
A funnel plot of the seven studies included also indicated the presence of reporting biases, which was supported by the Egger test (P = 0.034). We applied a 'trim and fill' method to correct for the asymmetry, and the corrected random‐effects estimate was RR 0.97 (95% CI 0.74 to 1.27), after filling with four 'missing' studies. The pooled results of the seven studies were not statistically significant, so we did not calculate a failsafe N. We did not conduct a subgroup analysis for the sex of the study participants, because with the exception of two studies including only male participants (Fielding 1980; PRECOR 1991), all of the studies included both women and men. Similarly, we did not perform any subgroup analyses based on physically demanding occupations, because only two studies reported having study populations with either predominantly physically demanding occupations (Haerem 2000) or less physically active occupations (Horlick 1984). The remaining five studies did not describe the physical demands of the study populations' occupations (Fielding 1980; Figueiras 2017; Pozen 1977; Rahe 1979; Stern 1983).
Subgroup analysis
We also considered return to work at six to 12 months for subgroups of studies with similar severity of CHD, where we considered the low‐ and high‐risk subpopulations of Pozen 1977 separately (Analysis 1.3). Among the two study populations with higher severity of CHD (Pozen 1977; Rahe 1979), we found a summarised effect of RR 1.61 (95% CI 0.97 to 2.67; I² = 43%). The summarised effect was RR 1.17 (95% CI 0.67 to 2.03; I² = 0%) for the subgroup with less severe CHD (Pozen 1977; Stern 1983). Among the studies where we could not determine the severity of CHD among the study participants, we found a summarised effect was RR 1.12 (95% CI 0.82 to 1.53; I² = 67%; Fielding 1980; Figueiras 2017; Haerem 2000; Horlick 1984). The severity of CHD in the study populations also explained some of the heterogeneity, where both the Chi² tests and I² values indicated lower heterogeneity in the subgroups where we were able to classify the general severity of CHD.
1.3. Analysis.
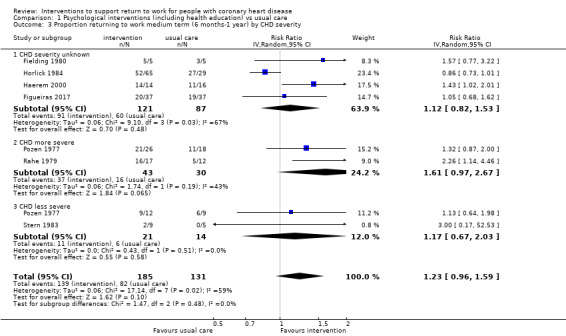
Comparison 1 Psychological interventions (including health education) vs usual care, Outcome 3 Proportion returning to work medium term (6 months‐1 year) by CHD severity.
2.1.3 Mean days until return to work
Two studies considering psychological interventions also reported the mean or median days until returning to work (Fielding 1980; Hanssen 2009). We pooled these two studies using SDs derived from the reported range (Fielding 1980), or imputed (Hanssen 2009). We observed a pooled MD for time until return to work of −9.70 days (95% CI −35.09 to 15.69, very low‐certainty evidence; Analysis 1.4).
1.4. Analysis.
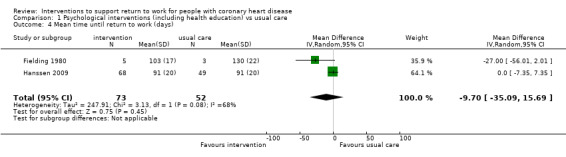
Comparison 1 Psychological interventions (including health education) vs usual care, Outcome 4 Mean time until return to work (days).
2.2 Psychological counselling interventions versus usual care: secondary outcomes
Working after an extended period of at least one year
We found three studies reporting the rates of people working more than one year and up to four years (long‐term) after hospitalisation (Hanssen 2009; PRECOR 1991; Rahe 1979). Psychological interventions may have little or no effect on the proportion of participants working at follow‐ups between one and four years (RR 1.09, 95% CI 0.88 to 1.34; I² = 44%; low‐certainty evidence). Excluding the one study with overall high risk of bias (Hanssen 2009) from the analysis resulted in a RR of 1.28 (95% CI 0.61 to 2.67; I² = 67%). Pooling with the fixed‐effect model did little to change the summarised effect estimate (RR 1.08, 95% CI 0.94 to 1.23). There were not enough studies to perform a meta‐regression.
2.3 Work‐directed counselling versus usual care: primary outcomes
Four studies applied work‐directed counselling, either by recommending a time‐frame for returning to work based on the results of a symptom‐limited treadmill test (Picard 1989; Pilote 1992), recommending a specific workday for return to work to participants and their family physicians (Pfund 2001), or by extending the offered counselling to participants' social networks (including co‐workers) to address their concerns regarding the causes of CHD and the ability of participants to return to work (Burgess 1987). Due to the variation in follow‐up times, we could not summarise the effects of these interventions on the relative proportions of study participants returning to work (Analysis 2.1).
2.1. Analysis.
Comparison 2 Work‐directed counselling vs usual care, Outcome 1 Proportion returning to work (all studies).
We pooled the MD in days of the four studies by using imputed SDs for two studies (Burgess 1987; Picard 1989). We observed a pooled MD of −7.52 days (95% CI −20.07 to 5.03; low‐certainty evidence; Analysis 2.2) for mean time until return to work following work‐directed counselling interventions compared to usual care. The results of the four studies showed considerable heterogeneity (Chi² = 20.36, df = 3 (P = 0.0001); I² = 85%). Excluding the one study population categorised as having a more severe CHD (and the only study population consisting of only men) from the analysis (Picard 1989), reduced the observed heterogeneity (Chi² = 2.48, df = 2 (P = 0.29); I² = 19%) and the observed effect estimate (MD −2.02 days, 95% CI −8.53 to 4.49). We considered none of the four work‐directed counselling studies to have a high overall risk of bias, and we found no visual indication of any time‐dependency (Analysis 2.2).
2.2. Analysis.
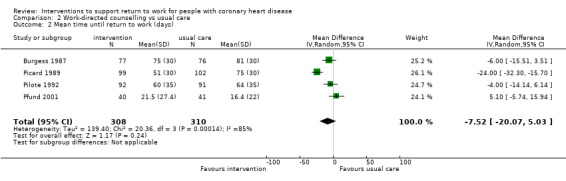
Comparison 2 Work‐directed counselling vs usual care, Outcome 2 Mean time until return to work (days).
2.4 Work‐directed counselling versus usual care: secondary outcomes
Adverse effects
Two studies reported the rates of cardiac deaths (i.e. sudden death, death following MI) and reinfarctions up to six months after work‐directed counselling (Picard 1989; Pilote 1992). Work‐directed counselling probably makes little or no difference to cardiac death rate (RR 1.00, 95% CI 0.19 to 5.39, I² = 0%; moderate‐certainty evidence; Analysis 2.3). Work‐directed counselling may make little or no difference to reinfarction rate (RR 0.67, 95% CI 0.21 to 2.11; I² = 3%; Analysis 2.4).
2.3. Analysis.
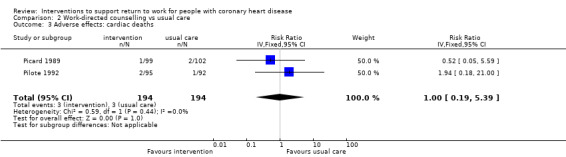
Comparison 2 Work‐directed counselling vs usual care, Outcome 3 Adverse effects: cardiac deaths.
2.4. Analysis.
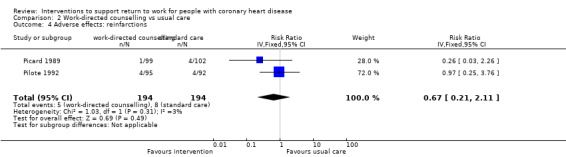
Comparison 2 Work‐directed counselling vs usual care, Outcome 4 Adverse effects: reinfarctions.
3. Person‐directed physical conditioning interventions versus usual care
3.1 Person‐directed physical conditioning interventions versus usual care: primary outcomes
We included nine studies comparing the impact of some form of physical training or exercises versus usual care on return to work in the meta‐analysis shown in Analysis 3.1 (Andersen 1981; Bethell 1990; Dugmore 1999; Froelicher 1994; Holmbäck 1994; Maeder 1977; Marra 1985; Stern 1983; Worcester 1993).
3.1. Analysis.
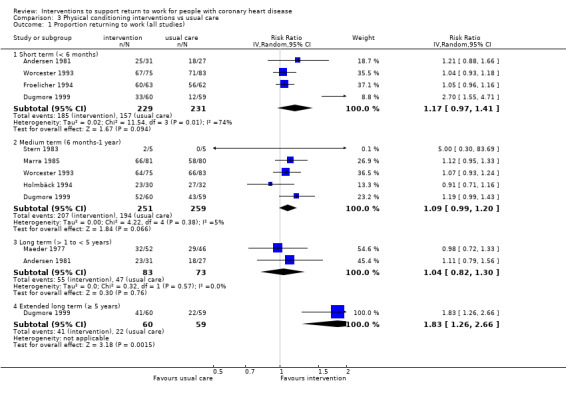
Comparison 3 Physical conditioning interventions vs usual care, Outcome 1 Proportion returning to work (all studies).
3.1.1 Short term (less than six months)
Physical conditioning interventions resulted in a pooled RR estimate for short‐term return to work of 1.17 (95% CI 0.97 to 1.41; very low‐certainty evidence), with indications of substantial statistical heterogeneity (Chi² = 11.54, df = 3 (P = 0.009); I² = 74%). Excluding the Dugmore 1999 results, which we extracted from a graph, eliminated much of the observed heterogeneity (Chi² = 0.73, df = 2 (P = 0.69); I² = 0%) and reduced the pooled effect (RR 1.06, 95% CI 0.98 to 1.14). A sensitivity analysis excluding studies with overall high risk of bias (Andersen 1981; Worcester 1993), resulted in a pooled RR of 1.62 (95% CI 0.65 to 4.06; I² = 91%), due to the increased influence of the Dugmore 1999 study results.
3.1.2 Medium term (six months to one year)
Physical conditioning interventions made little or no difference in the medium‐term return‐to‐work rates (RR 1.09, 95% CI 0.99 to 1.20; I² = 5%; low‐certainty evidence). When we excluded studies with an overall high risk of bias (Holmbäck 1994; Worcester 1993) in a sensitivity analysis, physical conditioning interventions increased return‐to‐work rates at six to 12 months (RR 1.16, 95% CI 1.02 to 1.31; I² = 0%). The funnel plot of the five study results suggested some potential publication bias (Figure 4). The results of the Egger's test (P = 0.60), gave no indication of potential publication bias for this outcome, so we did not apply the 'trim and fill' method. However tests of publication bias may be underpowered when few studies are available. The meta‐regression considering changes of log RR over study time gave no indication of a time‐dependency (slope β = 0.00, P = 0.951).
4.
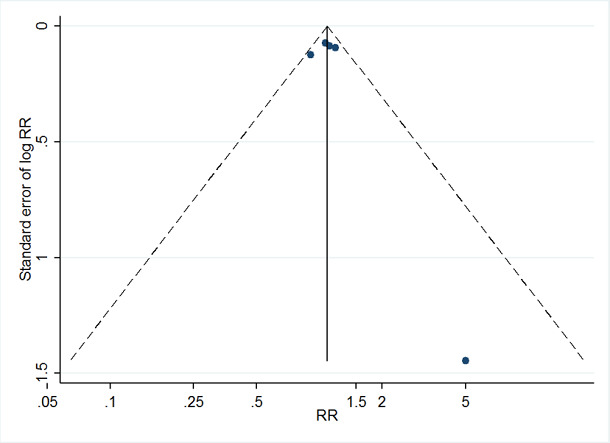
Funnel plot of comparison 4. Physical conditioning interventions vs usual care, outcome: 4.3 proportion returning to work (medium term)
Subgroup analysis
Pooling only study populations with similar CHD severity (Analysis 3.2) resulted in physical interventions having a bigger effect on the return‐to‐work rate in the two studies where the CHD was generally more severe (RR 1.12, 95% CI 1.00 to 1.25; I² = 0%; Dugmore 1999; Worcester 1993), and made little to no difference to return to work among the three study populations where the CHD was less severe (RR 1.04, 95% CI 0.84 to 1.29, I² = 36%; Holmbäck 1994; Marra 1985; Stern 1983).
3.2. Analysis.
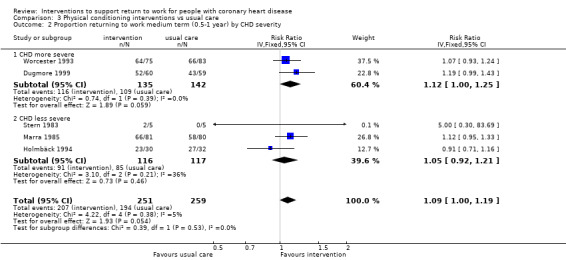
Comparison 3 Physical conditioning interventions vs usual care, Outcome 2 Proportion returning to work medium term (0.5‐1 year) by CHD severity.
3.1.5 Mean days until return to work
Four studies reported the mean time until return to work after MI either in weeks (Bethell 1990; Holmbäck 1994), or months (Maeder 1977; Marra 1985). We converted the reported results into mean days until return to work and pooled the mean differences. Using the SDs reported by Bethell 1990 and the interquartile ranges reported by Holmbäck 1994 we imputed the SD for the remaining two studies. Marra 1985 reported the results separately for study participants previously working in blue‐ or white‐collar professions, and we combined these results for Analysis 3.3. This analysis found that physical interventions made little or no difference in the time needed until return to work compared to usual care (MD −7.86 days, 95% CI −29.46 to 13.74; low‐certainty evidence). Due to the considerable statistical heterogeneity observed for this analysis (Chi² = 12.38, df = 3 (P = 0.006); I² = 76%), we also conducted a sensitivity analysis with the fixed‐effect model. The fixed‐effect model resulted in a smaller MD of −2.84 days (95% CI −10.43 to 4.75), giving some indication of the presence of a small‐study effect.
3.3. Analysis.
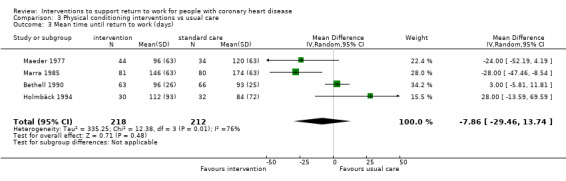
Comparison 3 Physical conditioning interventions vs usual care, Outcome 3 Mean time until return to work (days).
Subgroup analysis
We conducted subgroup analyses of study populations with more physically demanding occupations (blue‐collar workers) or less physically demanding occupations (white‐collar workers), using the stratified results reported by Marra 1985 (Analysis 3.4). We observed no mean difference in return‐to‐work times and considerably heterogeneity (Chi² = 4.50, df = 1; P = 0.03; I² = 78%) for white‐collar workers, while physical conditioning interventions reduced the mean time until return to work for blue‐collar workers (MD 28.29 days, 95% CI −48.68 to −7.91; I² = 0%).
3.4. Analysis.
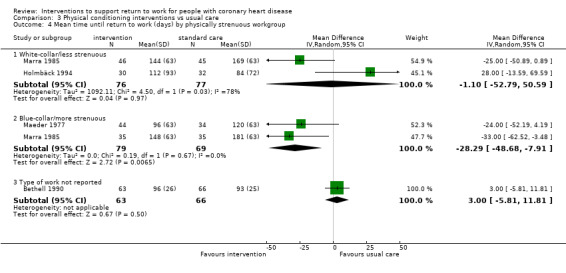
Comparison 3 Physical conditioning interventions vs usual care, Outcome 4 Mean time until return to work (days) by physically strenuous workgroup.
3.2 Person‐directed physical conditioning interventions versus usual care: secondary outcomes
Working after an extended period of at least one year
Two studies reported the number or proportions of participants working between one and five years (Andersen 1981; Maeder 1977). Physical conditioning interventions had little to no effect on the proportion of participants at work in the long term (RR 1.04, 95% CI 0.82 to 2.66; I² = 0%; low‐certainty evidence). Excluding Andersen 1981, which we judged to have an overall high risk of bias, left only Maeder 1977, where the authors detected no effect of the intervention. However, Maeder 1977 applied an early, in‐hospital mobilisation intervention, and it is reasonable to expect more moderate effects on return to work by such a mild intervention. Only Dugmore 1999 reported the proportions of participants working five years after study completion. Dugmore 1999 alone reported the effects of a physical conditioning intervention on return to work compared to usual care at five years' follow‐up (RR 1.83, 95% 1.26 to 2.66; low‐certainty evidence).
Adverse effects
One study reported the rates of cardiac deaths (Marra 1985), and a second reported the rate of fatal MI (Dugmore 1999). We found that physical conditioning interventions may make little or no difference to the rate of cardiac deaths (RR 1.00, 95% CI 0.35 to 2.80; I² = 0%; moderate‐certainty evidence; Analysis 3.5). Two studies reported reinfarction rate (RR 0.70, 95% CI 0.26 to 1.88; I² = 47%; Analysis 3.6).
3.5. Analysis.
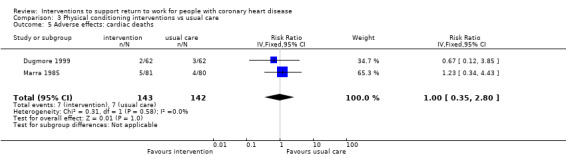
Comparison 3 Physical conditioning interventions vs usual care, Outcome 5 Adverse effects: cardiac deaths.
3.6. Analysis.
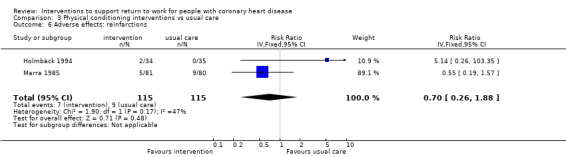
Comparison 3 Physical conditioning interventions vs usual care, Outcome 6 Adverse effects: reinfarctions.
4. Combined interventions versus usual care
4.1 Combined interventions versus usual care: primary outcomes
We included 13 studies evaluating combined (comprehensive) cardiac rehabilitation programmes combining both counselling and physical exercise components in the meta‐analysis (Andersson 2010; Bengtsson 1983; Bertie 1992; Engblom 1997; Erdman 1986; Froelicher 1994; Higgins 2001; Hofman‐Bang 1999; Lidell 1996; Oldridge 1991; PRECOR 1991; Rivas 1988; Vermeulen 1988).
4.1.1 Short term (less than six months)
Four studies reported rate of return to work up to six months following a combined cardiac rehabilitation programme (Bertie 1992; Higgins 2001; PRECOR 1991; Rivas 1988). Combined cardiac rehabilitation programmes may increase the short‐term return‐to‐work rate (RR 1.56, 95% CI 1.23 to 1.98; I² = 20%; low‐certainty evidence; Analysis 4.1). This corresponds with a NNTB of 5, meaning one additional person will return to work up to six months after CHD hospitalisation for every five people receiving combined cardiac rehabilitation. Rivas 1988 considered two combined cardiac rehabilitation arms with varying intensities of the exercise component versus a single control group receiving usual care, and we combined the results of both intervention arms for the data synthesis. A sensitivity analysis excluding the one study with an overall high risk of bias (Bertie 1992) did not substantially alter the pooled estimate (RR 1.51, 95% CI 1.09 to 2.09, I² = 42%). The forest plot gave no visual indications of any time‐dependency for short‐term effects of combined interventions, and there were not enough studies considering short‐term return to work to conduct a meta‐regression.
4.1. Analysis.
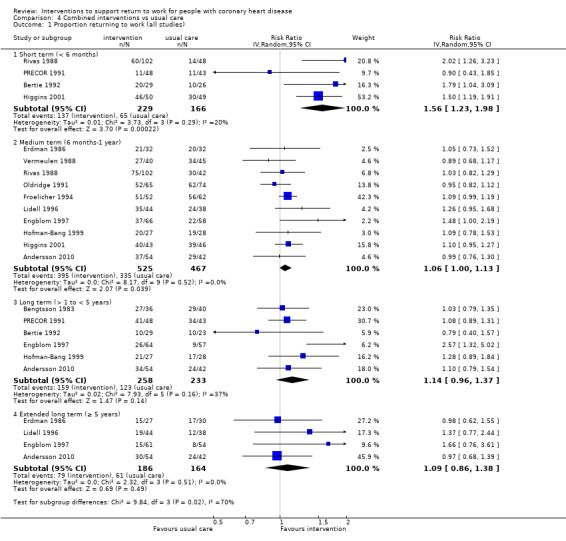
Comparison 4 Combined interventions vs usual care, Outcome 1 Proportion returning to work (all studies).
4.1.2 Medium term (six months to one year)
Ten studies reported medium‐term return to work following combined interventions (Andersson 2010; Engblom 1997; Erdman 1986; Froelicher 1994; Higgins 2001; Hofman‐Bang 1999; Lidell 1996; Oldridge 1991; Rivas 1988; Vermeulen 1988). Combined interventions may make little to no difference in the medium‐term return‐to‐work rate (RR 1.06, 95% CI 1.00 to 1.13; I² = 0%; low‐certainty evidence; Analysis 4.1). As a sensitivity analysis, we omitted the four studies with an overall high risk of bias (Andersson 2010; Erdman 1986; Hofman‐Bang 1999; Lidell 1996) from the analysis, and this caused little change to the pooled effect estimate (RR 1.05, 95% CI 0.97 to 1.14; I² = 23%). Both a funnel plot (Figure 5), and the results of the Egger's Test (P = 0.843), showed no indications of publication bias for this outcome. We discerned no clear pattern of changing effect over time from the forest plot, and the meta‐regression of the log RR and study year also did not indicate any time‐dependency (slope β = 0.005, P = 0.409).
5.
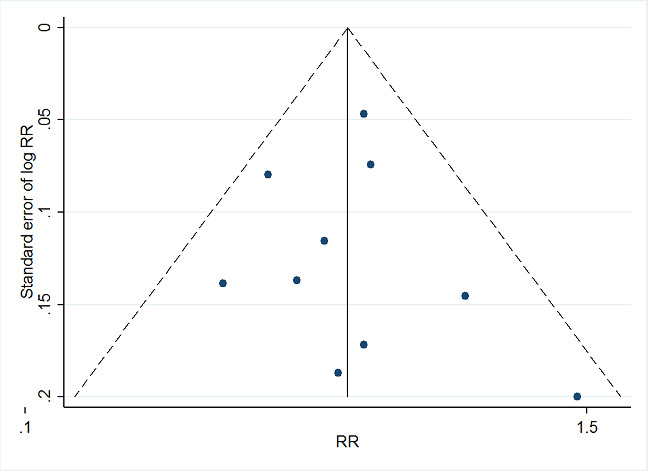
Funnel plot of comparison 5. Combined conditioning interventions vs usual care, outcome: 5.3 proportion returning to work (medium term)
Subgroup analysis
The subgroup analysis of participant populations with similar severities of CHD (Analysis 4.2), resulted in a larger pooled effect estimate for participant populations with more severe CHD (RR 1.12, 95% CI 0.99 to 1.25; I² = 11%). We also considered study populations with similar physical work demands in a subgroup analysis (Analysis 4.3). The three study populations with more sedentary (white‐collar) workers (Engblom 1997; Higgins 2001; Rivas 1988), resulted in a pooled RR of 1.11 (95% CI 0.97 to 1.28; I² = 20%), while the two studies with study populations predominantly comprised of physical labourers (Lidell 1996; Vermeulen 1988), resulted in a pooled RR of 1.06 (95% CI 0.76 to 1.48; I² = 66%). Results did not differ notably according to the sex of the participants included in the studies (Analysis 4.4).
4.2. Analysis.
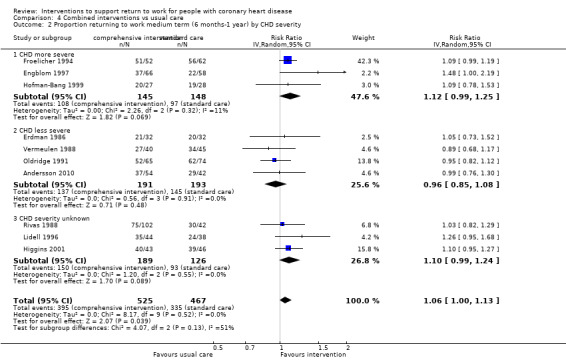
Comparison 4 Combined interventions vs usual care, Outcome 2 Proportion returning to work medium term (6 months‐1 year) by CHD severity.
4.3. Analysis.
Comparison 4 Combined interventions vs usual care, Outcome 3 Proportion returning to work medium term (6 months‐1 year) by physically strenuous work.
4.4. Analysis.
Comparison 4 Combined interventions vs usual care, Outcome 4 Proportion returning to work medium term (6 months‐1 year) by sex.
4.1.3 Mean days until return to work
Two studies reported the time until return to work following a combined intervention (Bengtsson 1983; Higgins 2001). We obtained SDs from the t‐test results of Higgins 2001 and applied this to both studies. Combined interventions shortened the mean length of time until return to work to a MD of −40.77 days (95% CI −67.19 to −14.35; I² = 66%; moderate‐certainty evidence; Analysis 4.5). We considered neither of these studies to have an overall high risk of bias. A sensitivity analysis with the fixed‐effect model resulted in a pooled MD of −39.32 days (95% CI −54.49 to −24.16).
4.5. Analysis.
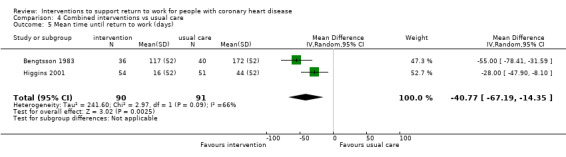
Comparison 4 Combined interventions vs usual care, Outcome 5 Mean time until return to work (days).
4.2 Combined interventions versus usual care: secondary outcomes
Working after an extended period of at least one year
Aggregation of the six studies reporting results from long‐term follow‐ups of one to five years (Andersson 2010; Bengtsson 1983; Bertie 1992; Engblom 1997; Hofman‐Bang 1999; PRECOR 1991), resulted in a RR of 1.14 (95% CI 0.96 to 1.37; I² = 37%; very low‐certainty evidence; Analysis 4.1). Excluding the three studies with an overall high risk of bias (Andersson 2010; Bertie 1992; Hofman‐Bang 1999) increased the RR and the heterogeneity (RR 1.23, 95% CI 0.88 to 1.70; I² = 69%). Both a funnel plot (Figure 6), and the Egger's test (P = 0.406), showed no indications of publication bias or small‐study effects for long‐term return‐to‐work rates following combined interventions. We observed low heterogeneity for return‐to‐work rates following combined interventions, and sensitivity analyses with fixed‐effect models resulted in similar estimates. Regarding changes of effect over time, the meta‐regression of the log RRs and study year indicated no time‐dependency (slope β = 0.006, P = 0.614), and we discerned no clear pattern of changes over time from the forest plot.
6.
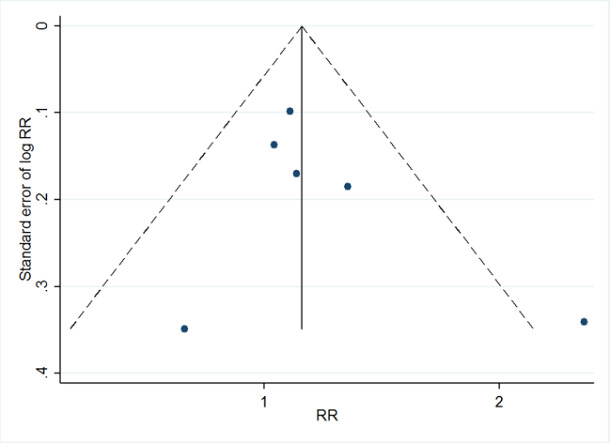
Funnel plot of comparison: 5 combined conditioning interventions vs usual care, outcome: 5.6 proportion returning to work (long term)
Four studies reported the effects of combined interventions on the working status at five‐year follow‐up (Andersson 2010; Engblom 1997; Erdman 1986; Lidell 1996). Combined interventions resulted in a summarised RR for working after five years of 1.09 (95% CI 0.86 to 1.38, I² = 0%, very low‐certainty evidence; Analysis 4.1). When we excluded the three studies with an overall high risk of bias from the meta‐analysis, only Engblom 1997 remained. This study found the highest beneficial effect of the interventions on working status at five years (RR 1.66, 95% CI 0.76 to 3.61). There were also too few studies to be considered in a meta‐regression, and we discerned no pattern of changes over time from the forest plot.
Health‐related quality of life
One study reported a total score for health‐related quality of life using the Angina Pectoris Quality of Life Questionnaire among study participants primarily eligible to return to work at a follow‐up time of two years (Hofman‐Bang 1999). The studied combined intervention appeared to have little to no effect on health‐related quality of life score when compared to usual care group at two years (MD 0.40, 95% CI −0.03 to 0.83; low‐certainty evidence; Analysis 4.6).
4.6. Analysis.
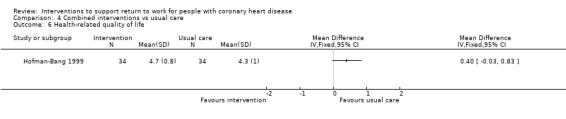
Comparison 4 Combined interventions vs usual care, Outcome 6 Health‐related quality of life.
Adverse effects
Four studies reported total mortality rates after combined interventions for follow‐up times of one to five years (Bengtsson 1983; Erdman 1986; Hofman‐Bang 1999; Rivas 1988). Combined interventions resulted in a summarised RR for total mortality of 1.43 (95% CI 0.59 to 3.51; I² = 5%; Analysis 4.7). Three studies also reported reinfarction rates after combined interventions for follow‐up times of one to five years (Bengtsson 1983; Erdman 1986; Hofman‐Bang 1999; Vermeulen 1988). Combined interventions resulted in a summarised RR for reinfarctions of 0.56 (95% CI 0.23 to 1.40; I² = 0%; moderate‐certainty evidence; Analysis 4.8).
4.7. Analysis.
Comparison 4 Combined interventions vs usual care, Outcome 7 Adverse effects: total mortality.
4.8. Analysis.
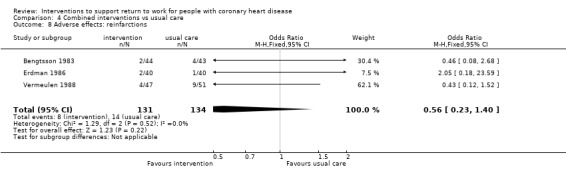
Comparison 4 Combined interventions vs usual care, Outcome 8 Adverse effects: reinfarctions.
Discussion
Summary of main results
This review included 39 studies applying a randomised controlled study design to investigate the impact of various interventions on the rate and timing of return to work following a MI, CABG or PCI. We do not know if counselling interventions (including health education) that addressed fears or concerns related to CHD increase the proportion of people with CHD returning to work at follow‐ups of up to one year (very low‐certainty evidence), and these interventions may make little or no difference in the proportion working in the long term (low‐certainty evidence). We also do not know if psychological counselling interventions reduce the time needed to return to work due to the very low certainty of the evidence. We did not find studies reporting total health‐related quality of life during the return‐to‐work process or adverse effects following person‐directed psychological interventions, so we could not assess these secondary outcomes.
Counselling directed specifically at encouraging returning to work, for example by providing a physician‐sanctioned goal for returning to work based on the results of symptom‐limited treadmill testing or by attempting to assuage concerns of co‐workers regarding their colleague’s ability to return to work, may result in little to no difference in days until return to work. Work‐directed counselling probably results in little or no difference in cardiac death rates. Why counselling to encourage return to work did not have more of an impact on return to work is unclear. Perhaps interventions providing a concrete time frame for when it should be physically safe to return to work do not adequately address other personal and work‐related obstacles that may affect an individual's decision to return to work.
Cardiac rehabilitation comprising only some form of physical conditioning following CHD may result in little to no difference in the medium‐term (six to 12 months) return‐to‐work rate (low‐certainty evidence) and may result in little to no difference in the proportion at work after one year and up to five years (low‐certainty evidence). We do not know if physical conditioning interventions alone increase short‐term (up to six months) return to work (very low‐certainty evidence). Considering the timing of return to work among participants returning to work, physical conditioning interventions may result in little to no difference in mean time needed to return to work (low‐certainty evidence). Physical conditioning interventions appeared to reduce time away from work particularly among blue‐collar workers. Physical conditioning interventions probably do not increase adverse effects (cardiac deaths).
Combined (comprehensive) cardiac rehabilitation programmes combining physical conditioning with counselling and risk factor education appeared to have some effect on return to work. Combined cardiac rehabilitation programmes may increase the proportion of participants resuming work up to six months following hospitalisation for CHD (low‐certainty evidence). For about every five participants receiving combined rehabilitation, one additional participant returned to work within six months of hospitalisation (NNTB 5). Combined rehabilitation programmes may result in little to no difference in the proportion returning to work after six months and up to one year (low‐certainty evidence). We do not know if combined cardiac rehabilitation programmes increase the long‐term (one to five years) or the extended long‐term (five or more years) proportion of participants working after hospitalisation (very low‐certainty evidence). Combined interventions probably reduce the average time needed to return to work (moderate‐certainty evidence) by about 40 days when compared to receiving usual care. Combined interventions may result in little to no difference in health‐related quality of life and probably result in little to no difference in adverse effects (assessed as reinfarctions).
Overall completeness and applicability of evidence
We found 39 RCTs examining the effect of person‐directed interventions on return to work among people with CHD conducted since the 1970s. However, none of the studies focused on the evaluation of work‐directed programmes. Overall the evidence of the studies was directly applicable to the aim of this review and its study questions. Studies were conducted for the most part in North America, Western Europe, and Australia. Although women were more often included in the study populations (25 studies) than not, women generally comprised a very small portion of the included participants. Only one study explicitly examined the effect of an intervention on return to work among women (Andersson 2010). Studies predominantly considered people who had been hospitalised for a MI, and less frequently considered people undergoing CABG or PCI. This may mean that the results are less applicable to people undergoing revascularisation procedures.
The studies considering health‐related quality of life reported results for the entire study populations and not just those eligible to return to work. Therefore, to assess our secondary outcome of health‐related quality of life within the return‐to‐work process, we considered health‐related quality of life results of studies where at least 80% of study participants were eligible to return to work. We did not find enough studies conducted predominantly among participants eligible to return to work that had assessed health‐related quality of life to conduct a meta‐analysis of this secondary outcome. Likewise, we also considered cardiac death rates, reinfarction rates and total mortality as severe adverse effects of interventions among studies where at least 80% of study participants were eligible to return to work in order to increase the applicability of the results to the return‐to‐work process.
Quality of the evidence
We included 39 studies, but once we aggregated studies according to intervention form and follow‐up times, the highest number of studies that we could aggregate was 10. We judged the overall risk of bias to be high for 16 of the studies. However, excluding studies judged to have an overall high risk of bias did not seem to systematically alter the results. The way that studies described many aspects of the study characteristics of interest, including return‐to‐work results and details regarding the severity of CHD at baseline were heterogeneous and often lacking important details, such as the actual number of participants considered in the return‐to‐work analysis. Also studies rarely provided information regarding study participants' employment characteristics prior to CHD (i.e. how many participants worked in physically strenuous occupations). Studies considering return to work among a subgroup of previously employed participants for the return‐to‐work analyses, reported loss to follow‐up for the entire study populations, which made it difficult for us to determine the outcome‐specific losses to follow‐up in these studies. Some studies also reported that the desire to return to work reduced compliance with the rehabilitation programmes, however withdrawals during the interventions should not have affected the results among studies conducting intention‐to‐treat analyses, and we considered intention‐to‐treat in our 'Risk of bias' assessments.
Our assessment of the quality of evidence was also hindered by poorly reported methods. Even studies published after the publication of the first proposal of standards for reporting of RCTs (Standards of Reporting Trials Group 1994) lacked adequate descriptions of allocation methods to permit clear 'Risk of bias' judgements, and none of the included studies cited a study protocol that would have permitted more objective assessments of selective reporting bias. Although we initially intended to contact all study authors to obtain additional information to aid in the 'Risk of bias' assessments, this often proved to be impossible, as many studies were conducted more than 20 years ago.
We downgraded nearly all outcomes by at least one level due to risk of bias. We also downgraded many outcomes because of imprecision due to wide confidence intervals that could include possible appreciable harm or benefit, and because of inconsistency due to substantial heterogeneity that could not completely be explained. We did not downgrade any outcomes for indirectness.
Return to work was often a secondary outcome of the studies, and as such, the results pertaining to return to work were not always clearly reported. There may have been additional cardiac rehabilitation studies that considered return to work, but did not report these results in any published document. It is possible that such omissions may be more likely to involve results for secondary outcomes when these were not statistically significant, and this selective reporting could result in a form of publication bias. We found some indications of publication bias among the studies of psychological interventions considering short‐term (Figure 3), and medium‐term return to work. We also observed visual indications of publication bias among physical conditioning intervention studies reporting medium‐term return to work (Figure 4). We did not detect publication bias for the pooled analyses of combined interventions reporting medium‐term (Figure 5) and long‐term (Figure 6) return to work.
Potential biases in the review process
Although we conducted an extensive search, our review process may have some limitations. We excluded studies mentioning return to work somewhere in the abstract or introduction without reporting any return‐to‐work results. We included studies reporting return‐to‐work results as percentages without providing the absolute number of study participants working prior to their CHD. We tried to obtain unpublished data from study authors regarding numbers of people working prior to CHD, but we were not always able to contact them. While we still included these studies in the review, we could not include the results of these studies in the meta‐analysis.
We also found registered clinical trials mentioning return to work as an outcome, that according to their registered start dates, should have produced results by now. However, we did not find any results that we could link to these studies. Publication bias may be leading to an underreporting of return‐to‐work results.
Agreements and disagreements with other studies or reviews
Like the Anderson 2017a review, we found no evidence that psychological interventions for CHD had any impact, positive or otherwise, on adverse reactions such as mortality or non‐fatal MI in the studies examining return to work. The Anderson 2017a review also reported that four of 10 studies examining health‐related quality of life observed improvements in at least one dimension of health‐related quality of life in the intervention group receiving a psychological intervention that differed significantly from that observed in the comparison groups. We were unable to examine health‐related quality of life following psychological interventions among studies examining return to work.
In contrast to the Anderson 2016 review, which found evidence that exercise‐based rehabilitations reduced cardiac mortality, we did not observe any meaningful differences across study groups with regard to cardiac mortality in the studies examining return to work in populations predominantly eligible to return to work.
Authors' conclusions
Implications for practice.
We found low‐certainty evidence that cardiac rehabilitation, including both physical conditioning and psychological aspects, may promote return to work up to six months following coronary heart disease (CHD), but we also found low‐certainty evidence that these programmes may have little or no effect on the proportion of participants returning to work between six months and one year. Due to the very low certainty of evidence found, we do not know if these programmes increase the proportion of participants at work after a year.
Regarding single‐component, person‐directed interventions, we do not know if programmes including only a counselling component make any difference in return to work up to six months or between six months and one year (very low‐certainty evidence). We found low‐certainty evidence that work‐directed counselling alone may result in little to no difference in the time needed to return to work. We found very low‐certainty evidence regarding the effect of physical conditioning programmes up to six months, so we do not know if physical conditioning alone has any effect on return to work. Physical conditioning programmes may result in little to no difference in return to work between six months and one year (low‐certainty evidence).
Implications for research.
Our review identified several aspects that future research could address.
Population
In our analysis, pooling the effect estimates of psychological interventions (including health education) and physical conditioning interventions resulted in risk ratios 1.24 and 1.17, respectively, for short‐term return to work, but the pooled confidence intervals were imprecise. According to our power analysis, the pooled confidence intervals for these two results should not have included a null effect 83% to 84% of the time. To find precise estimates of smaller effects 80% of the time with 95% confidence, such as the RR 1.06 we observed for medium‐term return to work following combined interventions, new studies need to recruit altogether 3774 study participants (compared to the 992 study participants included in our analysis). Since sick leave is costly for employers and paid sick leave may be limited or even unavailable for some workers, we consider even small increases in return to work to be relevant. However, detecting small effects requires conducting very large trials.
In addition, we still need high quality studies that directly address the return‐to‐work process and adequately report the vocational status and job characteristics of study participants prior to the onset of CHD. In a subgroup analysis of physical conditioning interventions, we found that physical conditioning lowered the time needed to return to work only among the two study populations where physically strenuous working conditions or blue‐collar occupations were predominant (Analysis 3.4). More information is needed to corroborate this finding and to determine if interventions may be more effective at promoting return to work for certain employee populations.
When working situations are beneficial and supportive of health, return to work can be considered an important component of regaining full health and improving health‐related quality of life. Delayed return to work or early retirement following CHD can have long‐lasting detrimental financial consequences on individuals and their families, especially where social systems are lacking to provide adequate financial support following a prolonged illness. For some people, such financial factors may be the main impulse to decide if and when they will return to work. Additional research is needed to determine if health outcomes are comparable between people who feel compelled to return to work and those who want to return to work of their own accord.
Interventions and comparisons
Additional evidence is also needed to determine if cardiac rehabilitation including both physical conditioning and psychological components truly promotes return to work up to six months following CHD. In addition to containing exercise, as well as anxiety and risk factor education, future combined interventions may also need to develop better ways to assist transitions back into the workforce without inadvertently promoting presenteeism. Returning to work is a complex and multi‐factorial process, and combined interventions that better address work‐related factors, possibly by providing return‐to‐work coordination, could eliminate further barriers to returning to work. Cardiac rehabilitation interventions also need to make accommodations for people who have to or want to return to work. There is a need to concurrently support the recovery process while alleviating any difficulties that can occur during the return‐to‐work process. This may require the development of strategies that improve access to cardiac rehabilitation centres.
None of the studies exclusively considered work‐directed interventions such as stepwise occupational reintegration (SOR). We also found no controlled studies on the effectiveness of coaching by an occupational physician or on the effects of structured communication between occupational physicians, employers, and the cardiac rehabilitation team. Few combined rehabilitation programmes (three studies) mentioned providing individual work‐directed recommendations to patients or employers as part of the rehabilitation programme. Similarly, only a few studies directly addressed the return‐to‐work process by offering a recommendation for when to return to work (three studies) or by counselling patients and their co‐workers to assuage their concerns about working with heart disease (one study). Although studies sometimes reported changes in working status (full versus part time), reductions in working hours seemed to have been initiated by the patients themselves and were not part of the intervention.
In view of the variation of the single interventions implemented to address either physical or psychological condition following CHD, more research is also needed. Effective single interventions are advantageous, because they are cheaper and simpler to organise than the combined interventions and can also take place outside cardiac rehabilitation centres. Studies considering single components of combined interventions also help explain how much return to work is impacted by either focusing on psychological or physical recovery following CHD among study participants with specific risks.
Outcomes
Return to work was often a secondary outcome of the studies, and as such, the results pertaining to return to work were often poorly reported. Providing the complete results of secondary analyses, at least as on‐line supplements (even when the results were not statistically significant), would help future assessments of return to work among people with CHD. Adhering to recommended reporting guidelines for RCTs could also greatly improve the evidence obtained from future research of return to work following cardiac rehabilitation programmes.
A priori registration of protocols in online RCT registries, which would assist in the objective assessment of selective reporting, may already be improving, as we found seven ongoing registered studies. We also encountered difficulties in identifying participant populations with comparable CHD severity due to the greatly varying selection of cardiac health measures and comorbidities reported. Using core outcome sets when assessing cardiac health of study populations will help alleviate this problem.
Acknowledgements
We thank Jani Ruotsalainen, Managing Editor, Cochrane Work for providing administrative and logistical support for the development of the review protocol, and Kaisa Neuvonen, Information Specialist, Cochrane Work for developing the search strategies. We also thank Jochen Schmitt for his contributions to the planning of the study and his contributions to the protocol.
We also thank Cochrane Work Co‐ordinating Editor, Jos Verbeek; Managing Editor, Jani Ruotsalainen; Information Specialist, Kaisa Neuvonen; Editors, Anneli Ojajärvi and Wim van Veelen; and external peer referees Andrew Beswick, Eira Viikari‐Juntura, Lars Hermann Tang, and Tomi‐Pekka Tuomainen for their comments on the protocol and the review. Last but not least, we thank Megan Prictor and Denise Mitchell for copy‐editing the text.
Thank you to the many wonderful people who helped us to screen and translate documents in various languages, including Giorgio Maria Agazzi, Julie Cwikel, Nikolaos Stilianakis, and Shangqing Wang. And a special thanks to Soja Nazarov for her help obtaining and translating Russian papers. And our most heartfelt thanks to Evgeniya Regner, Alejandra Rodriguez Sanchez, Hermann Burr, and Ronny Staudte for their tremendous support with the literature collection. We wish you all the best in all of your future endeavours!
And most of all, our thanks go to the courageous people that help science by agreeing to participate in research studies.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Heart Diseases] explode all trees
#2 MeSH descriptor: [Heart Failure] explode all trees
#3 MeSH descriptor: [Coronary Disease] explode all trees
#4 MeSH descriptor: [Myocardial Infarction] explode all trees
#5 MeSH descriptor: [Myocardial Ischemia] explode all trees
#6 MeSH descriptor: [Angina Pectoris] explode all trees
#7 MeSH descriptor: [Angina, Unstable] explode all trees
#8 MeSH descriptor: [Acute Coronary Syndrome] explode all trees
#9 MeSH descriptor: [Coronary Artery Bypass] explode all trees 4
#10 "percutaneous intervention"
#11 "pci"
#12 "percutaneous coronary angioplasty"
#13 "ptca"
#14 thrombolysis
#15 "cabg"
#16 {or #1‐#15}
#17 "return to work"
#18 MeSH descriptor: [Employment] explode all trees
#19 MeSH descriptor: [Unemployment] explode all trees
#20 MeSH descriptor: [Sick Leave] explode all trees
#21 MeSH descriptor: [Absenteeism] explode all trees
#22 retirement
#23 MeSH descriptor: [Work] explode all trees
#24 MeSH descriptor: [Occupations] explode all trees
#25 MeSH descriptor: [Occupational Medicine] explode all trees
#26 MeSH descriptor: [Occupational Health] explode all trees
#27 MeSH descriptor: [Occupational Health Services] explode all trees
#28 "disability management"
#29 "disability prevention"
#30 occupation*
#31 vocational*
#32 "work ability"
#33 "work capacity"
#34 "work activity"
#35 "work disability"
#36 "work rehabilitation"
#37 "work status"
#38 "work retention"
#39 workability
#40 employability
#41 employable
#42 employee*
#43 {or #17‐#42}
#44 "modified duty" or "modified duties"
#45 "modified duties"
#46 MeSH descriptor: [Work Capacity Evaluation] explode all trees
#47 MeSH descriptor: [Vocational Guidance] explode all trees
#48 "vocational training" or "vocational placement" or "vocational counseling" (Word variations have been searched)
#49 "solution focused intervention" or "work adjustment" (Word variations have been searched)
#50 "work visit" or "work site visit" (Word variations have been searched)
#51 "light duty" or "work reintegration plan" or "supported employment" or "modified work" or "workplace accommodation" or "job accommodation" or "on the job programs" (Word variations have been searched)
#52 "ergonomic counseling" or "ergonomic education" or "ergonomic training" or "ergonomic approach" (Word variations have been searched)
#53 MeSH descriptor: [Human Engineering] explode all trees
#54 "case manager" or "case management" or "vocational guidance" or "workplace intervention" or "occupational intervention" (Word variations have been searched)
#55 {or #44‐#54}
#56 MeSH descriptor: [Rehabilitation] explode all trees
#57 MeSH descriptor: [Exercise] explode all trees
#58 exercise or "exercise therapy" (Word variations have been searched)
#59 MeSH descriptor: [Sports] explode all trees
#60 MeSH descriptor: [Physical Education and Training] explode all trees
#61 exertion* (Word variations have been searched)
#62 rehabilitation and physical* (Word variations have been searched)
#63 rehabilitation and train* (Word variations have been searched)
#64 rehabilitation and exercise* (Word variations have been searched)
#65 rehabilitation and aerobic* (Word variations have been searched)
#66 MeSH descriptor: [Physical Therapy Modalities] explode all trees
#67 {or #56‐#66}
#68 MeSH descriptor: [Gender Identity] explode all trees
#69 MeSH descriptor: [Social Support] explode all trees
#70 "autogenic training" (Word variations have been searched)
#71 "stress management" (Word variations have been searched)
#72 "relaxation techniques" (Word variations have been searched)
#73 "patient counseling" (Word variations have been searched)
#74 MeSH descriptor: [Psychotherapy] explode all trees
#75 MeSH descriptor: [Psychology, Applied] explode all trees
#76 MeSH descriptor: [Health Education] explode all trees
#77 {or #68‐#76}
#78 {or #55, #67, #77}
#79 {and #16, #43, #78}
#80 #79 limit to trials
Searched on 11 October 2018
Appendix 2. MEDLINE search strategy
#1)
(heart diseases[MeSH Terms]) OR (heart diseases) OR (heart failure[MeSH Terms]) OR (heart failure) OR (coronary disease[MeSH Terms]) OR (coronary disease) OR (myocardial infarction[MeSH Terms]) OR (myocardial infarction) OR (myocardial ischaemia[MeSH Terms]) OR (myocardial ischaemia) OR (angina pectoris[MeSH Terms]) OR (angina pectoris) OR (angina pectoris, unstable[MeSH Terms]) OR (acute coronary syndrome[MeSH Terms]) OR (acute coronary syndrome) OR (percutaneous intervention) OR ("pci") OR (percutaneous coronary angioplasty) OR ("ptca") OR (thrombolysis) OR (coronary artery bypass grafting[MeSH Terms]) OR (coronary artery bypass grafting) OR ("cabg")
#2)
(return‐to‐work) OR (employment[MeSH Terms]) OR (employment) OR (unemployment[MeSH Terms]) OR (unemployment) OR (unemployed) OR (retirement) OR (sick leave[MeSH Terms]) OR (sick leave) OR (sickness absence) OR (absenteeism[MeSH Terms]) OR (absenteeism) OR (work[MeSH Terms]) OR (occupations[MeSH Terms]) OR (occupational medicine[MeSH Terms]) OR (occupational health[MeSH Terms]) OR (occupational health services[MeSH Terms]) OR ("disability management") OR (“disability prevention”) OR (occupation*) OR (vocational*) OR (work ability) OR ("work ability") OR ("work capacity") OR ("work activity") OR ("work disability") OR ("work rehabilitation") OR ("work status") OR ("work retention") OR (workability) OR (employability) OR (employable) OR (employee*)
#3)
(modified duty) OR (modified duties) OR (work capacity evaluations[MeSH Terms]) OR (vocational guidance[MeSH Terms]) OR (vocational training) OR (vocational placement) OR (vocational counseling) OR (solution focused intervention) OR (work adjustment) OR (work visit) OR (work site visit) OR (light duty) OR (work reintegration plan) OR (supported employment) OR (modified work) OR (workplace accommodation) OR ("on the job programs") OR (job accommodation) OR (ergonomic counseling) OR (ergonomic education) OR (ergonomic training) OR (ergonomic approach) OR (ergonomics[MeSH Terms]) OR (case manager) OR (case management) OR (vocational guidance) OR (workplace intervention) OR (occupational intervention)
#4)
(rehabilitation[MeSH Terms]) OR (exercise[MeSH Terms]) OR (exercise) OR (exercise therapy) OR (sports[MeSH Terms]) OR ((physical education and training[MeSH Terms])) OR (exertion*) OR ((rehabilitation) AND physical*)) OR ((rehabilitation) AND train*)) OR ((rehabilitation) AND exercise*)) OR ((rehabilitation) AND aerobic*)) OR (physical therapy modalities[MeSH Terms])
#5)
(gender[MeSH Terms]) OR (social support[MeSH Terms]) OR (autogenic training) OR (stress management) OR (relaxation techniques) OR (patient counseling) OR (psychotherapies[MeSH Terms]) OR (applied psychology[MeSH Terms]) OR (health education[MeSH Terms])
#6) #3 OR #4 OR #5
#7)
(randomized controlled trial OR controlled clinical trial OR clinical trial OR comparative study OR evaluation studies[Publication Type]))) OR (randomized controlled trial[MeSH Terms]) OR (random allocation[MeSH Terms]) OR (double blind method[MeSH Terms]) OR (single blind method[MeSH Terms]) OR (clinical trial[MeSH Terms]) OR (((singl* OR doubl* OR trebl* OR tripl*)) AND (mask* OR blind*))) OR (placebos[MeSH Terms]) OR (placebo) OR random*) OR (research design[MeSH Terms]) OR (studies, follow up[MeSH Terms]) OR (prospective studies[MeSH Terms]) OR (cross over studies[MeSH Terms]) OR (prospectiv*) OR (volunteer*) OR (evaluate*) OR (compare*) OR (programs) OR (effects) OR ((control OR controls* OR controla* OR controle* OR controli* OR controll*))
#8) #1 AND #2 AND #6 AND #7
#9) #8 NOT (animals NOT humans)
Searched on 11 October 2018
Appendix 3. Embase search strategy
#1) 'return to work'/exp OR 'employment'/de OR 'unemployment'/de OR unemployed OR 'retirement'/exp OR 'medical leave'/de OR 'sick leave'/exp OR 'sickness absence'/exp OR 'absenteeism'/de OR 'work'/de OR 'occupation'/de OR 'occupational medicine'/de OR 'occupational health'/de OR 'occupational health service'/de OR 'disability management' OR 'disability prevention' OR occupation* OR vocational* OR 'work ability'/exp OR 'work capacity'/exp OR 'work activity' OR 'work disability'/exp OR 'work rehabilitation' OR 'work status' OR 'work retention' OR 'workability' OR 'employability'/exp OR employable OR employee*
#2) 'modified duty' OR 'modified duties' OR ('work capacity'/exp AND 'evaluation'/exp) OR 'vocational guidance'/de OR 'vocational training'/exp OR 'vocational placement' OR 'vocational counseling'/exp OR 'solution focused intervention' OR 'work adjustment'/exp OR 'work visit' OR 'work site visit' OR 'light duty' OR 'work reintegration plan' OR 'supported employment' OR 'modified work' OR 'workplace accommodation' OR 'on the job programs' OR 'job accommodation'/exp OR 'ergonomic counseling' OR 'ergonomic education' OR 'ergonomic training' OR 'ergonomic approach' OR 'ergonomics'/de OR 'case manager'/exp OR 'case management'/exp OR 'vocational guidance'/exp OR 'workplace intervention' OR 'occupational intervention'
#3) 'rehabilitation'/de OR 'exercise'/de OR 'exercise therapy'/exp OR 'sport'/de OR 'physical education'/de OR exertion* OR (rehabilitation NEAR/5 physical*) OR (rehabilitation NEAR/5 train*) OR (rehabilitation NEAR/5 exercise*) OR ('rehabilitation'/exp AND aerobic*) OR 'physiotherapy'/de OR 'physical therapy modalities'/exp
#4) 'gender'/de OR 'social support'/de OR 'autogenic training'/exp OR 'stress management'/exp OR 'relaxation techniques'/exp OR 'patient counseling'/exp OR 'psychotherapy'/de OR 'psychology'/de OR 'health education'/de
#5) #2 OR #3 OR #4
#6) 'randomized controlled trial'/de OR 'randomization'/de OR 'double blind procedure'/de OR 'single blind procedure'/de OR 'clinical trial'/de OR (singl* OR doubl* OR trebl* OR tripl* AND (mask* OR blind*)) OR 'placebo'/exp OR random* OR 'methodology'/de OR 'follow up'/de OR 'prospective study'/de OR 'crossover procedure'/de OR (prospectiv* OR volunteer* OR evaluate* OR compare* OR programs OR effects OR 'control'/exp OR controls* OR controla* OR controle* OR controli* OR controll*)
#7) ('heart'/exp AND 'diseases'/exp) OR ('heart'/exp AND failure) OR 'coronary artery disease'/exp OR 'heart infarction'/exp OR (myocardial AND 'infarction'/exp) OR 'heart muscle ischaemia'/exp OR (myocardial AND 'ischaemia'/exp) OR 'angina pectoris'/exp OR ('angina'/exp AND pectoris) OR 'unstable angina pectoris'/exp OR 'acute coronary syndrome'/exp OR (acute AND coronary AND 'syndrome'/exp) OR 'percutaneous intervention' OR 'pci' OR 'percutaneous coronary angioplasty' OR 'ptca'/exp OR 'thrombolysis'/exp OR 'coronary artery bypass graft'/exp OR 'cabg'
#8) #1 AND #5 AND #6 AND #7
#9) #8 AND [humans]/lim AND [embase]/lim
Searched on 11 October 2018
Appendix 4. PsycINFO search strategy
#1) "Cardiovascular Disorders" OR (heart disease) OR (heart failure) OR (coronary artery disease) OR (heart infarction) OR (myocardial AND infarction) OR (heart muscle ischaemia) OR (myocardial AND ischaemia) OR (angina AND pectoris) OR "unstable angina pectoris" OR "acute coronary syndrome" OR (acute AND coronary AND syndrome) OR ("Heart Disorders") OR ("Myocardial Infarctions") OR ("Angina Pectoris") OR (percutaneous intervention) OR "percutaneous intervention" OR "pci" OR "percutaneous coronary angioplasty" OR "ptca" OR thrombolysis OR "coronary artery bypass graft" OR "cabg"
#2) ("return to work") OR ("Employment Status") OR ("Unemployment") OR unemployed OR ("Retirement") OR "medical leave" OR "sick leave" OR "sickness absence" OR absenteeism OR work OR occupation* OR (occupational medicine) OR (occupational health) OR (occupational health service) OR (disability management) OR (disability prevention) OR vocational* OR (work ability) OR (work capacity) OR (work activity) OR (work disability) OR (work rehabilitation) OR (work status) OR (work retention) OR workability OR employability OR employable OR employee*
#3) (modified duty) OR (modified duties) OR ((work capacity) AND (evaluation)) OR (vocational guidance) OR (vocational training) OR (vocational placement) OR (vocational counsel*) OR (solution focused intervention) OR (work adjustment) OR (work visit) OR (work site visit) OR (light duty) OR (work reintegration plan) OR (supported employment) OR (modified work) OR (workplace accommodation) OR (on the job programs) OR "on the job program" OR (job accommodation) OR (ergonomic counsel*) OR (ergonomic education) OR (ergonomic training) OR (ergonomic approach) OR ergonomics OR (case manager) OR (case management) OR ("Occupational Guidance") OR (workplace intervention) OR (occupational intervention)
#4) "Exercise" OR (exercise therapy) OR (AB sport) OR (TI sport) OR "Physical Education" OR exertion* OR ("rehabilitation" N5 physical*) OR ("rehabilitation" N5 train*) OR ("rehabilitation" N5 exercise*) OR ("Rehabilitation" N5 aerobic*) OR "Physical Therapy" OR physiotherapy OR "Rehabilitation"
#5) gender OR (social support) OR "Autogenic Training" OR "Stress Management" OR ("Relaxation" AND techniques) OR "Client Education" OR "psychotherapy" OR "psychology" OR (health education)
#6) (randomized controlled trial) OR randomization OR (double blind procedure) OR (single blind procedure) OR (clinical trial) OR ((singl* OR doubl* OR trebl* OR tripl*) AND (mask* OR blind*)) OR ("Placebo") OR random* OR methodology OR (follow up) OR (prospective study) OR (crossover procedure) OR prospectiv* OR volunteer* OR evaluat* OR compare* OR programs OR effects OR ("Experiment Controls") OR control*
#7) 3 OR 4 OR 5
#8) 1 AND 2 AND 6 AND 7
Searched on 11 October 2018
Appendix 5. OSH Update + Fire search strategy
#1) GW{(heart disease*) OR (heart failure) OR (coronary disease) OR (myocardial infarction) OR (myocardial ischaemia) OR (angina pectoris) OR (acute coronary syndrome) OR (percutaneous intervention) OR (“pci”) OR (percutaneous coronary angioplasty) OR (“ptca”) OR (thrombolysis) OR (coronary artery bypass grafting) OR (“cabg”)}
#2) GW{(return‐to‐work) OR (employment) OR (unemployment) OR (unemployed) OR (retirement) OR (sick leave) OR (sickness absence) OR (absenteeism) OR (“disability management”) OR (“disability prevention”) OR (occupation*) OR (vocational*) OR (work ability) OR (“work ability”) OR (“work capacity”) OR (“work activity”) OR (“work disability”) OR (“work rehabilitation”) OR (“work status”) OR (“work retention”) OR (workability) OR (employability) OR (employable) OR (employee*)}
#3) GW{((modified duty) OR (modified duties) OR (work capacity evaluation*) OR (vocational guidance) OR (vocational training) OR (vocational placement) OR (vocational counseling) OR (solution focused intervention) OR (work adjustment) OR (work visit) OR (work site visit) OR (light duty) OR (work reintegration plan) OR (supported employment) OR (modified work) OR (workplace accommodation) OR (“on the job programs”) OR (job accommodation) OR (ergonomic counseling) OR (ergonomic education) OR (ergonomic training) OR (ergonomic approach) OR (ergonomics) OR (case manager) OR (case management) OR (workplace intervention) OR (occupational intervention)) OR ((exercise) OR (exercise therapy) OR (sports) OR (physical education and training) OR (exertion*) OR (rehabilitation)) OR ((social support) OR (autogenic training) OR (stress management) OR (relaxation techniques) OR (patient counseling) OR (psychotherap*) OR (health education))}
#4) GW{(randomized controlled trial) OR (controlled clinical trial) OR (clinical trial) OR (comparative study) OR (evaluation studies) OR (random allocation) OR (double blind method) OR (single blind method) OR (clinical trial) OR ((singl* OR doubl* OR trebl* OR tripl*) AND (mask* OR blind*)) OR (placebo) OR (random*) OR (prospectiv*) OR (volunteer*) OR (evaluate*) OR (compare*) OR (programs) OR (effects) OR (control OR controls* OR controla* OR controle* OR controli* OR controll*)}
#5) GW{#1 AND #2 AND #3 AND #4}
Searched on 17 October 2018
Appendix 6. LILACS search strategy
tw:((return TO work OR employment OR occupation) (coronary heart disease OR coronary disease OR myocardial infarction OR myocardial ischaemia OR heart disease*)) AND (instance:"regional") AND ( db:("LILACS"))
Searched on 11 October 2018
Data and analyses
Comparison 1. Psychological interventions (including health education) vs usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion returning to work (all studies) | 11 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Short term (< 6 months) | 6 | 375 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.84, 1.40] |
| 1.2 Medium term (6 months‐1 year) | 7 | 316 | Risk Ratio (IV, Random, 95% CI) | 1.24 [0.95, 1.63] |
| 1.3 Long term (> 1 to < 5 years) | 3 | 239 | Risk Ratio (IV, Random, 95% CI) | 1.09 [0.88, 1.34] |
| 2 Proportion returning to work short term (< 6 months) by CHD severity | 6 | 397 | Risk Ratio (IV, Random, 95% CI) | 1.10 [0.85, 1.43] |
| 2.1 CHD severity unknown | 3 | 241 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.68, 1.40] |
| 2.2 CHD more severe | 2 | 67 | Risk Ratio (IV, Random, 95% CI) | 1.10 [0.83, 1.46] |
| 2.3 CHD less severe | 1 | 89 | Risk Ratio (IV, Random, 95% CI) | 1.87 [1.03, 3.38] |
| 3 Proportion returning to work medium term (6 months‐1 year) by CHD severity | 7 | 316 | Risk Ratio (IV, Random, 95% CI) | 1.23 [0.96, 1.59] |
| 3.1 CHD severity unknown | 4 | 208 | Risk Ratio (IV, Random, 95% CI) | 1.12 [0.82, 1.53] |
| 3.2 CHD more severe | 2 | 73 | Risk Ratio (IV, Random, 95% CI) | 1.61 [0.97, 2.67] |
| 3.3 CHD less severe | 2 | 35 | Risk Ratio (IV, Random, 95% CI) | 1.17 [0.67, 2.03] |
| 4 Mean time until return to work (days) | 2 | 125 | Mean Difference (IV, Random, 95% CI) | ‐9.70 [‐35.09, 15.69] |
Comparison 2. Work‐directed counselling vs usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion returning to work (all studies) | 4 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Short term (< 6 months) | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Medium term (6 months‐1 year) | 2 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Long term (> 1 to < 5 years) | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Mean time until return to work (days) | 4 | 618 | Mean Difference (IV, Random, 95% CI) | ‐7.52 [‐20.07, 5.03] |
| 3 Adverse effects: cardiac deaths | 2 | 388 | Risk Ratio (IV, Fixed, 95% CI) | 1.00 [0.19, 5.39] |
| 4 Adverse effects: reinfarctions | 2 | 388 | Risk Ratio (IV, Fixed, 95% CI) | 0.67 [0.21, 2.11] |
Comparison 3. Physical conditioning interventions vs usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion returning to work (all studies) | 8 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Short term (< 6 months) | 4 | 460 | Risk Ratio (IV, Random, 95% CI) | 1.17 [0.97, 1.41] |
| 1.2 Medium term (6 months‐1 year) | 5 | 510 | Risk Ratio (IV, Random, 95% CI) | 1.09 [0.99, 1.20] |
| 1.3 Long term (> 1 to < 5 years) | 2 | 156 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.82, 1.30] |
| 1.4 Extended long term (≥ 5 years) | 1 | 119 | Risk Ratio (IV, Random, 95% CI) | 1.83 [1.26, 2.66] |
| 2 Proportion returning to work medium term (0.5‐1 year) by CHD severity | 5 | 510 | Risk Ratio (IV, Fixed, 95% CI) | 1.09 [1.00, 1.19] |
| 2.1 CHD more severe | 2 | 277 | Risk Ratio (IV, Fixed, 95% CI) | 1.12 [1.00, 1.25] |
| 2.2 CHD less severe | 3 | 233 | Risk Ratio (IV, Fixed, 95% CI) | 1.05 [0.92, 1.21] |
| 3 Mean time until return to work (days) | 4 | 430 | Mean Difference (IV, Random, 95% CI) | ‐7.86 [‐29.46, 13.74] |
| 4 Mean time until return to work (days) by physically strenuous workgroup | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 White‐collar/less strenuous | 2 | 153 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐52.79, 50.59] |
| 4.2 Blue‐collar/more strenuous | 2 | 148 | Mean Difference (IV, Random, 95% CI) | ‐28.29 [‐48.68, ‐7.91] |
| 4.3 Type of work not reported | 1 | 129 | Mean Difference (IV, Random, 95% CI) | 3.0 [‐5.81, 11.81] |
| 5 Adverse effects: cardiac deaths | 2 | 285 | Risk Ratio (IV, Fixed, 95% CI) | 1.00 [0.35, 2.80] |
| 6 Adverse effects: reinfarctions | 2 | 230 | Risk Ratio (IV, Fixed, 95% CI) | 0.70 [0.26, 1.88] |
Comparison 4. Combined interventions vs usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion returning to work (all studies) | 13 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Short term (< 6 months) | 4 | 395 | Risk Ratio (IV, Random, 95% CI) | 1.56 [1.23, 1.98] |
| 1.2 Medium term (6 months‐1 year) | 10 | 992 | Risk Ratio (IV, Random, 95% CI) | 1.06 [1.00, 1.13] |
| 1.3 Long term (> 1 to < 5 years) | 6 | 491 | Risk Ratio (IV, Random, 95% CI) | 1.14 [0.96, 1.37] |
| 1.4 Extended long term (≥ 5 years) | 4 | 350 | Risk Ratio (IV, Random, 95% CI) | 1.09 [0.86, 1.38] |
| 2 Proportion returning to work medium term (6 months‐1 year) by CHD severity | 10 | 992 | Risk Ratio (IV, Random, 95% CI) | 1.06 [1.00, 1.13] |
| 2.1 CHD more severe | 3 | 293 | Risk Ratio (IV, Random, 95% CI) | 1.12 [0.99, 1.25] |
| 2.2 CHD less severe | 4 | 384 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.85, 1.08] |
| 2.3 CHD severity unknown | 3 | 315 | Risk Ratio (IV, Random, 95% CI) | 1.10 [0.99, 1.24] |
| 3 Proportion returning to work medium term (6 months‐1 year) by physically strenuous work | 10 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.1 White‐collar/less strenuous | 3 | 357 | Risk Ratio (IV, Random, 95% CI) | 1.11 [0.97, 1.28] |
| 3.2 Blue‐collar/more strenuous | 2 | 167 | Risk Ratio (IV, Random, 95% CI) | 1.06 [0.76, 1.48] |
| 3.3 Type of work not reported | 5 | 468 | Risk Ratio (IV, Random, 95% CI) | 1.05 [0.98, 1.13] |
| 4 Proportion returning to work medium term (6 months‐1 year) by sex | 10 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Men only | 3 | 273 | Risk Ratio (IV, Random, 95% CI) | 1.09 [0.81, 1.45] |
| 4.2 Women and men | 6 | 623 | Risk Ratio (IV, Random, 95% CI) | 1.07 [1.00, 1.14] |
| 4.3 Women only | 1 | 96 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.76, 1.30] |
| 5 Mean time until return to work (days) | 2 | 181 | Mean Difference (IV, Random, 95% CI) | ‐40.77 [‐67.19, ‐14.35] |
| 6 Health‐related quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Adverse effects: total mortality | 4 | 438 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.59, 3.51] |
| 8 Adverse effects: reinfarctions | 3 | 265 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.23, 1.40] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andersen 1981.
| Methods |
Study design: parallel RCT Recruitment: recruited at hospital discharge Allocation: not reported Blinding: none reported Randomisation: random numbers Follow‐up(s): 3 years Description: supervised training programme |
|
| Participants |
Baseline characteristics Intervention group
Control group
Inclusion criteria
Exclusion criteria
The study authors report that participants had no signs of heart failure, severe ventricular ectopies or atrioventricular blockages at discharge. It is unclear if this was an inclusion criteria. Baseline imbalances: ‐ Physically demanding work (i.e. white‐ vs blue‐collar): unknown Severity of CHD: unknown |
|
| Interventions |
Intervention characteristics Training group
Control group
|
|
| Outcomes | Proportion at work at < 6 months (short term): 4 months Proportion at work at > 12 months to < 5 years (long term): 3 years Number returning to previous work Adverse events (mortality, non‐fatal reinfarctions) |
|
| Identification |
Sponsorship source: none reported Country: Denmark Setting: single‐centre, ambulant Possible conflicts of interest: no information provided Ethics committee approval: no information provided regarding participant consent or ethics committee approval |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised using "random numbers". No further information about generation of random numbers |
| Allocation concealment (selection bias) | Unclear risk | No allocation method was reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the study, blinding of participants was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | None reported. It is unclear how return to employment was assessed. If assessed with official records, it may not be subject to detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of participants with fatal or non‐fatal reinfarctions was reported in the text, but these participants were excluded from the analysis. (no loss‐to‐follow‐up analysis) |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol was available. |
| Other bias | Unclear risk | None identified |
Andersson 2010.
| Methods |
Study design: parallel RCT Recruitment: AMI, CABG, PCI patients recruited from April 1997‐October 2000 Allocation: envelope Blinding: not blinded Randomisation: no Information provided Follow‐up(s): yearly up to 5 years Description: combined inpatient rehabilitation programme for women |
|
| Participants |
Baseline characteristics Intervention group
Control group
Inclusion criteria
Exclusion criteria
Baseline imbalances: ‐ Physically demanding work (i.e. white‐ vs blue‐collar): unknown Severity of CHD: less |
|
| Interventions |
Intervention characteristics Group programme (6‐10 women) aimed at promoting and maintain lifestyle changes
Control group
|
|
| Outcomes | Proportion at work at 6–12 months (medium term): 12 months Proportion at work at > 12 months to < 5 years (long term): 3 years Proportion at work at 5 years (extended long term): 5 years Number of participants at work calculated from proportions provided and number of participants working at baseline Becks Depression Inventory, Gothenburg QoL Inventory (only baseline results reported) Adverse events (mortality, emergency room visits) |
|
| Identification |
Sponsorship source: supported by Swedish Research Council, Swedish Heart & Lung Foundation, regional agreement on medical training & clinical research (ALF), Stockholm County Council, Saltsjöbaden Hospital and the Dept. of Cardiology at the Karolinska Univ. Hospital Country: Sweden Setting: single‐centre: Saltsjöbaden Hospital near Stockholm; inpatient Possible conflicts of interest: none reported Ethics committee approval: approved by the Karolinska Hospital Ethics Committee and all participants gave informed written consent. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The randomisation was not stratified as the number of eligible patients was presumed to be too small for a stratified randomisation.” No further information provided. |
| Allocation concealment (selection bias) | Low risk | Quote: “All baseline examinations were performed before randomisation… Patients were logged into the study and then called to baseline examination. After that, a biomedical scientist, not involved in the study, opened the envelope that revealed the group allocation.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the study, blinding of participants was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding mentioned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Results only given as the proportion (%) employed, on sick leave or with disability pension (not mutually exclusive) for year 1, 3 and 5 after study onset. No information regarding the actual number of study participants employed at the 1‐, 3‐ or 5‐year follow‐ups were reported or how many study participants were followed at each of the follow‐up time points (loss‐to‐follow‐up). Study authors contacted, no further information provided. No information about how the drop‐outs (n = 19) were distributed across the groups ⇢ imbalanced group sizes (I: n = 69; C: n = 61) |
| Selective reporting (reporting bias) | Low risk | No study protocol available, however no difference in proportion of employed study participants was detected and still was reported, suggesting there was no reporting bias (towards only reporting statistically significant results). |
| Other bias | Unclear risk | None identified |
Bengtsson 1983.
| Methods |
Study design: parallel RCT Recruitment: October 1973‐January 1975 Allocation: no information provided Blinding: not reported Randomisation: “…allocated at random"; no further information provided Follow‐up(s): 8‐19 months (average 14 months) Description: combined rehabilitation programme with recommendations for work modifications |
|
| Participants |
Baseline characteristics Intervention group
Control group
Inclusion criteria
Exclusion criteria
Baseline imbalances: ‐ Physically demanding work (i.e. white‐ vs blue‐collar): unknown Severity of CHD: severe (severe cardiac failure excluded, angina not excluded) |
|
| Interventions |
Intervention characteristics Rehabilitation programme
Control group
|
|
| Outcomes | Proportion at work at > 12 months to < 5 years (long term): about 13.5 months Working status ascertained at follow‐up examination between 8 and 19 months Mean sick leave (days) Minnesotal Multiphasic Personality Inventory Adverse events (mortality, reinfarction) |
|
| Identification |
Sponsorship source: none reported Country: Sweden Setting: single‐centre; outpatient Possible conflicts of interest: no information provided Ethics committee approval: no information provided regarding patient consent or ethics committee approval |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “…allocated at random to either the rehabilitation (81) or the control (90) group…” No further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information regarding allocation concealment was reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the study, blinding of participants was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Neither blinding of outcome assessors, nor how work status was assessed was reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study authors attempted an intention‐to‐treat analysis. Quote: “Seven who were invited to take part [in the treatment programme] declined; 6 of these were seen at follow‐up examination, and were included in the rehabilitation group because the control group probably also comprised a comparable number of patients who would no doubt also have declined further treatment.” However the impact of adverse effects was not assessed. Quote: “Those patients who developed a new infarction during the investigation period were excluded, because the follow‐up interview was focused on experiences of MI at time of entry to the study.” |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol available |
| Other bias | Unclear risk | None identified |
Bertie 1992.
| Methods |
Study design: parallel RCT Recruitment: patients who were admitted to a single centre after AMI Allocation: not reported Blinding: not reported Randomisation: no information provided Follow‐up(s): after rehabilitation, 4 months, 1‐2 years Description: combined rehabilitation programme |
|
| Participants |
Baseline characteristics Intervention group
Control group
Inclusion criteria: ‐ Exclusion criteria
Baseline imbalances: ‐ Physically demanding work (i.e. white‐ vs blue‐collar): unknown Severity of CHD: less severe (patients with uncontrolled heart failure excluded) |
|
| Interventions |
Intervention characteristics
Control group
|
|
| Outcomes | Proportion at work at < 6 months (short term): 4 months Proportion at work at > 12 months to < 5 years (long term): 1‐2 years Well‐being and anxiety about health Adverse events (mortality, MI) |
|
| Identification |
Sponsorship source: The British Heart Foundation and the Chest, Heart and Stroke Association Country: UK Setting: single centre: the Plymouth cardiac care unit Possible conflicts of interest: no information provided Ethics committee approval: no information provided |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “On their final hospital day, 110 patients who had suffered acute myocardial infarction and had been admitted to the Plymouth coronary care unit were randomised into two groups…”. No further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the study, blinding of participants was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | None reported. Employment status was assessed with a questionnaire filled out by the study participants (with help from a physiotherapist if necessary). Study participants were aware of their group allocation, which could have affected reporting. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “Patients were withdrawn from the study because of death, increasing angina, coronary artery surgery, reinfarction at their own request, and failure to complete assessments 2 or 4.” No ITT analysis was conducted; however, attrition: 28% of controls and 25% of exercise group was similar. |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol was available |
| Other bias | Unclear risk | None identified |
Bethell 1990.
| Methods |
Study design: parallel RCT Recruitment: patients admitted to CCU were recruited from December 1979‐March 1984 Allocation: not reported Blinding: not reported Randomisation: random letter sequence Follow‐up(s): 3 months post‐interview (about 4 months after admission) Description: supervised exercise programme (3 months) at a community sports centre |
|
| Participants |
Baseline characteristics Invervention group
Control group
Inclusion criteria
Exclusion criteria
Baseline imbalances: ‐ Physically demanding work (i.e. white‐ vs blue‐collar): unknown Severity of CHD: unknown |
|
| Interventions |
Intervention characteristics Circuit training at Alton Sports Centre 3 x/week
Control group
|
|
| Outcomes | Mean time to RTW (weeks): 4 months Adverse events (mortality) |
|
| Identification |
Sponsorship source: Grand from British Heart Foundation, Wessex Regional Health Authority Country: UK Setting: single‐centre; outpatient Possible conflicts of interest: not reported Ethics committee approval: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The qualifying patients were randomised by order of admission into treatment and control groups by means of a random letter sequence.” |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the study, blinding of participants was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: “Three months from the initial interview the patient was seen again by the research assistant who repeated the initial interview and examination.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the end only 73 of the 99 participants in the treatment group completed the exercise course (8 participants left the exercise group to return to work), but all participants attending the 3‐month assessment appear to have been included in the analyses. |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol available |
| Other bias | Unclear risk | None identified |
Broadbent 2009.
| Methods |
Study design: parallel RCT Recruitment: June 2002‐June 2003 Allocation: sealed, consecutively numbered envelopes Blinding: not reported Randomisation: randomisation sequence was generated using a computerised random number generator Follow‐up(s): 3 and 6 months Description: inpatient illness perception intervention |
|
| Participants |
Baseline characteristics Intervention group
Control group
Inclusion criteria
Exclusion criteria: a serious comorbid psychiatric or medical condition Baseline imbalances: ‐ Physically demanding work (i.e. white‐ vs blue‐collar): unknown Severity of CHD: unknown |
|
| Interventions |
Intervention characteristics "Standard care plus/intervention group"
Control group Standard hospital care
|
|
| Outcomes | Proportion at work at < 6 months (short term): 3 months Days to RTW: Cox proportional hazards model was used to determine if RTW rate differed between groups. Intervention group returned to work faster (log rank statistic Chi2(1)=19.31, P=.001)). Adverse events (mortality) |
|
| Identification |
Sponsorship source: a grant from the Heart Foundation of New Zealand Country: New Zealand Setting: single‐centre at Auckland City Hospital; inpatient and self‐administered (tape listening) Possible conflicts of interest: no information provided Ethics committee approval: approval was gained from the Auckland Ethics Committees (AKY/02/00/092) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The randomisation sequence was generated using a computerized random number generator…” |
| Allocation concealment (selection bias) | Low risk | Quote: “…allocation was kept in sealed consecutively numbered envelopes.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “There was no blinding of group assignment.” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessed with questionnaires |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Although the loss to follow‐up at 6 months was 19% in the intervention group and 27% in the control group, at 3 months it was 3% in the intervention group and 10% in the control group. RTW results were only reported for the 3‐month follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol available |
| Other bias | Unclear risk | None identified |
Burgess 1987.
| Methods |
Study design: parallel RCT Recruitment: patients recruited from CCU admissions log Allocation: sealed envelope Blinding: not reported Randomisation: stratified by sex for each hospital site Follow‐up(s): 3‐4 months, 13 months Description: multi‐centred RCT for people < 62 years compared to conventional care |
|
| Participants |
Baseline characteristics Intervention group
Control group
Inclusion criteria
Exclusion criteria
Baseline imbalances: ‐ Physically demanding work (i.e. white‐ vs blue‐collar): white‐collar (54% white‐collar vs 46% blue‐collar) Severity of CHD: less severe (participants with unstable postinfarction angina excluded) |
|
| Interventions |
Intervention Conventional care plus the experimental cardiac RTW intervention beginning during the last week of the hospitalisation with the following goals. Quote:
Control group
|
|
| Outcomes | Proportion at work at > 12 months to < 5 years (long term): 13 months Impact of Events, Taylor Manifest anxiety, Zung Depression |
|
| Identification |
Sponsorship source: a grant from The Robert Wood Johnson Foundation, Princeton, New Jersey, USA Country: USA Setting: multi‐centred; 11 hospitals in eastern Massachusetts as well as outpatient (visits at home) and workplace Possible conflicts of interest: not reported Ethics committee approval: informed consent was obtained prior to the patient interview |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The exact randomisation method is not clearly stated, however, the use of stratification and a central allocation centre do suggest that much consideration went into the planning of the randomisation. Quote: "Randomization was conducted by telephone from the study's central office, .... The randomisation stratified by sex for each hospital site to assure a proportionate mix of males and females in the usual care and rehab groups." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was conducted by telephone from the study's central office, where a research assistant opened a sealed envelope containing the subject's group assignment." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the study, blinding of participants was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "A full patient medical and work history was obtained from hospital medical records and from patient interviews at baseline. Measures taken at baseline and at each of the two follow‐up interviews provided information on variables looking at each patient’s demographic, medical, psychological, social, and occupational status." ‐ data gained from medical records and via interviews; no information about blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The attrition of study participants was similar in both treatment groups, and the number of study participants with complete data were the same for the groups at the final follow‐up. However, there appeared to be discrepancies in the reported numbers of study subject followed. |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol was available. |
| Other bias | Unclear risk | None identified |
Carson 1982.
| Methods |
Study design: parallel RCT Recruitment: men with MI who were admitted to CCU Allocation: not reported Blinding: not reported Randomisation: not reported Follow‐up(s): 3.5 years Description: supervised exercise programme (12 weeks, 2 x/week) |
|
| Participants |
Baseline characteristics Intervention group
Control group
Inclusion criteria MI diagnosis based on ECG changes and/or elevation of SGOT or LD) taken on 3 consecutive days, and admitted to the CCU Exclusion criteria
Baseline imbalances: ‐ Physically demanding work (i.e. white‐ vs blue‐collar): unknown Severity of CHD: severe (prevalence of angina reported) |
|
| Interventions |
Intervention characteristics Exercise
Control group
|
|
| Outcomes | Not enough information provided to be included in the meta‐analysis (number of study participants working before MI): Quote: "Eighty‐one per cent of both exercise and control groups who were working before MI returned to work after MI. There was no significant difference between the two groups in the mean time of return to work following MI (exercise 13 weeks, control 12 weeks)." Adverse events (mortality) |
|
| Identification |
Sponsorship source: DHHS (Department of Health and Social Security) Country: UK Setting: single‐centre, outpatient (hospital gym) Possible conflicts of interest: no information provided Ethics committee approval: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The 303 patients who accepted were then randomly allocated to an exercise group (151) and a control group (152).” No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the study, blinding of participants was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not clear how work status was assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The study authors write, "eighty‐one per cent of both exercise and control groups who were working before MI returned to work after MI", but do not state at what within what time‐frame or at which rate they returned to work. Study follow‐ups were done at 5 months, 1, 2 and 3 years after the MI, but the loss‐to follow‐up is unclear. It is also unclear how many study participants were working prior to the MI. |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol available |
| Other bias | Unclear risk | None identified |
Dugmore 1999.
| Methods |
Study design: parallel RCT Recruitment: patients of consultant physicians with clinically documented MI; 1984‐1988 Allocation: not reported Blinding: not reported Randomisation: not reported Follow‐up(s): 3 weeks, 4 months, 8 months, 12 months, 5 years Description: aerobic and local muscular endurance training |
|
| Participants |
Baseline characteristics Intervention group Treatment (good/poor prognosis)
Control (good / poor prognosis
Inclusion criteria clinically documented MI Exclusion criteria ‐ Baseline imbalances: ‐ Physically demanding work (i.e. white vs. blue collar): blue‐collar Severity of CHD: severe (> 2 mm ST segment depression included and classified into poor prognosis group; RTW results combined the groups) |
|
| Interventions |
Intervention characteristics Good prognosis group:
Poor prognosis group:
Control group
|
|
| Outcomes | Proportion at work at < 6 months (short term): 3 months Proportion at work at 6 months–12 months (medium term): 6 months Proportion at work at 5 years (extended long term): 5 years Results included in the meta‐analyses were derived from percentages provided in the figures and text. Toronto attitude scale (TAS); Profile of Mood States (POMS); Quality of life (10‐item) Adverse events (mortality, non‐fatal reinfarctions) |
|
| Identification |
Sponsorship source: grant from British Heart Foundation, Wessex Regional Health Authority Country: UK Setting: single‐centre; outpatient Possible conflicts of interest: not reported Ethics committee approval: not reported |
|
| Notes | Groups were matched based on prognosis, severity of their infarcts (cardiac enzymes/ECG changes), age, sex, Peel index score | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided. Quote: “Following an uncomplicated response to early exercise testing and subsequent random allocation to a treatment group, the 36 patients who formed the good prognosis group immediately begin anaerobic training three times a week for 12 months.” |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the study, blinding of participants was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐assessment of RTW at 5‐year follow‐up could have also introduced recall bias to the outcome assessment. Quote: “Vocational status/lifestyle change (five year follow‐up)— Selected aspects reflecting changes in vocational status and lifestyle were measured five years after completing the initial 12 month study. The instrument used for this assessment was a self‐administered questionnaire designed in accordance with the principles listed in the symposium on methodology for this investigative procedure.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “In all, 119 completed questionnaires were received from this research population (n = 124), representing a 95.6% compliance rate for this investigative procedure.” The only attrition reported at the five‐year follow‐up was due to deaths in the study population and these were similar in both study arms (2 treatment, 3 controls). |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol was available |
| Other bias | Unclear risk | None identified |
Engblom 1997.
| Methods |
Study design: parallel RCT Recruitment: men scheduled for elective CABS February 1986‐December 1987 were recruited consecutively Allocation: not reported Blinding: none reported Randomisation: none reported Follow‐up(s): 6 months, 1 year Description: combined rehabilitation programme for CABS patients |
|
| Participants |
Baseline characteristics Intervention group
Control group
Inclusion criteria
Exclusion criteria
Baseline imbalances: ‐ Physically demanding work (i.e. white vs. blue collar): white‐collar (42% manual workers) Severity of CHD: severe (LVEF: intervention group: 70%; control group: 71%) |
|
| Interventions |
Intervention characteristics
Control group
|
|
| Outcomes | Proportion at work at 6 months–12 months (medium term): 12 months Proportion at work at > 12 months to < 5 years (long term): 3 years Proportion at work at 5 years (extended long term): 5 years Nottingham Health Profile (NHP) Adverse events (death due to cardiac arrest, reinfarction) |
|
| Identification |
Sponsorship source: none reported. Country: Finland Setting: single centre; outpatient Possible conflicts of interest: none reported Ethics committee approval: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided to determine risk of bias. The method for generating the random sequence was not described. |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment procedure was described. Risk of bias cannot be determined |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the study, blinding of participants was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | None described, but the outcome time until returning to work is not likely to be falsely assessed and cross‐checked with the social registries. Quote: “The employment status of each patient was asked by the physician and later checked from the registries of the Social Insurance Institution of Finland.” (Engblom 1997) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One person in the control "usual care" (H) group died 7 months post‐CABS. In the 5‐year follow‐up, deaths and loss to follow‐up were reported for the entire study population (not just men working at baseline). There appeared to be no notable differences between treatment groups. Quote: “Twelve patients in group R and 13 patients in group H (no significant difference between groups [NS]) died either peri‐ or postoperatively. Two patients in group R and three patients in group H were lost during the follow‐up.” |
| Selective reporting (reporting bias) | Unclear risk | A study protocol was not available to permit assessment of reporting bias. However, a number of non‐statistically significant results were reported, indicating a reporting bias might not have been a serious problem. |
| Other bias | Unclear risk | None identified |
Erdman 1986.
| Methods |
Study design: parallel RCT Recruitment: cardiac patients in greater Rotterdam were referred by their treating cardiologist; September 1976‐March 1978 Allocation: not reported Blinding: not reported Randomisation: random number tables Follow‐up(s): 6 months, 5 years Description: a combined and interactive rehabilitation programme with sport games for men < 65 years of age |
|
| Participants |
Baseline characteristics Intervention group
Control group
Total
Inclusion criteria
Exclusion criteria
Baseline imbalances: ‐ Physically demanding work (i.e. white vs. blue collar): unknown (73% skilled labourers and low‐level employees) Severity of CHD: less severe (patients with unstable angina excluded) |
|
| Interventions |
Intervention characteristics Outpatient interactional rehabilitation programme
Control group Standard cardiologic care, and referring physician suggested a home rehabilitation programme, i.e. brochure with guidelines and advice for physical fitness training and jogging |
|
| Outcomes | Proportion at work at 6 months–12 months (medium term): 6 months Proportion at work at 5 years (extended long term): 5 years Well‐being questionnaire Adverse events (reinfarction deaths, non‐fatal reinfarctions) |
|
| Identification |
Sponsorship source: The Dutch Heart Foundation grant #75.066 and the Rotterdam Foundation for Cardiac Rehabilitation Country: Netherlands Setting: single centre: a conventional gymnasium; outpatient Possible conflicts of interest: no information provided Ethics committee approval: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Immediately after fulfilment of the selection criteria for this study, the 80 patients were randomly allocated (by means of a table for random numbers) either to participation in the Rehab programme or to the home rehabilitation (Home) with the encouragement of their referring physicians.” |
| Allocation concealment (selection bias) | Unclear risk | No information reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the study, blinding of participants was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding was conducted and work resumption was self‐reported. The study authors do not report any verification of working status using employment registry data. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Although the study authors report similar attrition in both study arms (about 20%), the reasons for the loss to follow‐up seem to differ. Reasons given for loss to follow‐up in the treatment group were primarily cardiovascular in nature, while the reasons in the control group were not (lack of motivation to participate in the follow‐up evaluations). |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol was available |
| Other bias | Unclear risk | None identified |
Fielding 1980.
| Methods |
Study design: parallel RCT Recruitment: participants were recruited at discharge from the CCU Allocation: not reported Blinding: not blinded Randomisation: not reported Follow‐up(s): 6 months Description: weekly group meetings with a psychologist with relaxation training |
|
| Participants |
Baseline characteristics Intervention group "Heart Club"
Control group
Inclusion criteria
Exclusion criteria
Baseline imbalances: ‐ Physically demanding work (i.e. white vs. blue collar): unknown Severity of CHD: unknown |
|
| Interventions |
Intervention "Heart Club"
Control group
|
|
| Outcomes | Proportion at work at 6 months–12 months (medium term): 6 months Mean length of illness (sick leave) in days Anxiety with the Catell Self‐Analysis Form; 9‐point rating scale Adverse events (reinfarctions) |
|
| Identification |
Sponsorship source: no information Country: UK Setting: single setting, outpatient Possible conflicts of interest: none reported Ethics committee approval: |
|
| Notes | Personal communication: information regarding occupational status of the study participants provided by the study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A randomisation method was not described. Quote: "Ten patients were assessed and randomly allocated to experimental or control groups." |
| Allocation concealment (selection bias) | Unclear risk | None described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the study, blinding of participants was not possible. Control participants were placed on a ‘waiting list’, which might have influenced their decisions of when to return to work |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There is no mention of blinding and information regarding the assessment of working status and illness duration is insufficient to determine if these were prone to detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts reported; no percentage of 'number working' reported, unclear how many participants remained in each group |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol was available |
| Other bias | Unclear risk | None identified |
Figueiras 2017.
| Methods |
Study design: parallel RCT Recruitment: recruitment at three CCUs Allocation: not reported Blinding: care‐givers were blinded Randomisation: computer block randomisation Follow‐up(s): 4, 8, and 12 months Description: an inpatient individual psychological counselling with telephone follow‐ups |
|
| Participants |
Baseline characteristics Intervention group
Control group
Inclusion criteria
Exclusion criteria
Baseline imbalances: ‐ Physically demanding work (i.e. white vs. blue collar): unknown Severity of CHD: unknown |
|
| Interventions |
Intervention In‐hospital individual participant session (about 45 min) with health psychologist including:
Participants were mailed a manual with illness and recovery information Weekly phone calls were made in the first 4 weeks after discharge to discuss strategies to change behavior and recovery goals
Control group
|
|
| Outcomes | Proportion at work at < 6 months (short term): 4 months* Proportion at work at 6‐12 months (medium term): 8 months* *Provided by personal communication Hospital Anxiety & Depression Scale |
|
| Identification |
Sponsorship source: FEDER through COMPETE and FCT – Fundação para a Ciência e a Tecnologia – reference PTDC/PSI‐PCL/112503/2009 Country: Portugal Setting: single setting, inpatient/outpatient (phone calls) Possible conflicts of interest: none reported Ethics committee approval: “The study was approved by the Ethics Commissions of all hospitals involved and by the Portuguese Data Protection Authority (CNPD) and registered with the number n º17,523/2011 – ‘Programa Coração Saudável’” |
|
| Notes | RTW results were obtained through personal communication. It is unclear how many people actually responded to the follow‐ups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The randomisation sequence was generated using a computer block randomisation to allocate the patients either to the control or the Intervention group after the baseline assessment.” |
| Allocation concealment (selection bias) | Unclear risk | Allocation and randomisation were conducted after the baseline assessment. No information regarding allocation concealment was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Caregivers were blinded to the group assignment.” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | None reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants not replying at all time‐points (loss‐to‐follow‐up) were similar in both groups. However, it is unclear how many actually responded regarding the RTW results. |
| Selective reporting (reporting bias) | Unclear risk | No mention of an a priori published study protocol. Non‐significant results for RTW not provided in the published articles |
| Other bias | Unclear risk | None identified |
Froelicher 1994.
| Methods |
Study design: 3‐armed RCT Recruitment: patients admitted to CCUs of 7 hospitals, September 1977‐December1979 Allocation: not reported Blinding: not reported Randomisation: not reported Follow‐up(s): 6 months Description: a 3‐arm RCT with exercise or exercise with education counselling, relaxation therapy, and family support provided |
|
| Participants |
Baseline characteristics Intervention group 1 – exercise
Intervention group 2 – exercise and teaching counselling
Control group
Inclusion criteria
Exclusion criteria
Baseline imbalances: ‐ Description and recruitment methods: all consecutively admitted patients ≤ 70 years of age diagnosed AMI admitted to CCUs of 7 participating Seattle hospitals during 1977 through 1979 were screened for inclusion in the study. Physically demanding work (i.e. white vs. blue collar): unknown Severity of CHD: severe (patients with angina included) |
|
| Interventions |
Intervention characteristics Intervention 1 – exercise
Intervention 2 – exercise and counselling
Control group
|
|
| Outcomes | Proportion at work at < 6 months (short term): 5.5 months (24 weeks) Sickness impact profile Adverse events (mortality, cardiac surgery) |
|
| Identification |
Sponsorship source: study was supported by Research Grant 5 ROI NU 00589‐04 from the Bureau of Health Professions, Division of Nursing, Department of Health and Human sciences Country: USA Setting: multicentre: seven North‐Western hospitals; in‐ and outpatient Possible conflicts of interest: not reported Ethics committee approval: the participants gave an informed consent to participate in the study. "Human subjects review committee requirements for human informed consent were observed." |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The randomisation method is not clearly stated. Quote: "Randomization was designed to provide patients in each hospital with an equal chance to be assigned to one of three groups..." |
| Allocation concealment (selection bias) | Unclear risk | No allocation method is reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the study, blinding of participants and personnel was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Although no blinding of outcome assessors is reported, a validated standardised questionnaire (Activity Summary Questionnaire) was used at regular intervals to determine if participants had returned to work. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition due to withdrawal and the medical reasons for withdrawal reported (i.e. surgery, death) were similar across all three groups. However, "of the remaining 207 patients eligible for follow ‐up, 177 (86%) had completed questions pertaining to return to work, defined as return to the same job as before AMI." 14% of the participants eligible for follow‐up had not completed questions pertaining to RTW |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol was available. |
| Other bias | Unclear risk | None identified |
Geissler 1979.
| Methods |
Study design: parallel RCT Recruitment: MI patients surviving the first phase of rehabilitation (in‐hospital treatment) in one East German district; June 1973‐June 1975 Allocation: not reported Blinding: not reported Randomisation: cluster‐randomisation according to hospital region Follow‐up(s): 6 months, 12 months, 2 years Description: combined rehabilitation with an inpatient and outpatient phase |
|
| Participants |
Baseline characteristics Intervention group
Control group
Inclusion criteria < 70 years of age at the time of the MI Exclusion criteria ‐ Baseline imbalances: ‐ Physically demanding work (i.e. white vs. blue collar): unknown Severity of CHD: unknown |
|
| Interventions |
Intervention characteristics Inpatient (Phase II) and outpatient (Phase III) rehabilitation:
Control group
|
|
| Outcomes | Proportion at work at 6 months–12 months (medium term): 12 months Proportion at work at > 12 months to < 5 years (long term): 2 years Adverse events (cardiac deaths, reinfarctions) |
|
| Identification |
Sponsorship source: no information provided Country: Former East Germany (GDR) Setting: inpatient and outpatient Possible conflicts of interest: not reported Ethics committee approval: not reported |
|
| Notes | The number of people working before MI is not explicitly reported, but RTW is reported for all men aged < 65 years. Due to the sociopolitical policies in place at the time of this study, presumed that all of the participants presented in the RTW table were working prior to their heart attack. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The study authors write that "regional cluster randomisation" was used. No further description of the randomisation method was reported. |
| Allocation concealment (selection bias) | Unclear risk | No method of allocation concealment was reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the study, blinding of participants was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No outcome assessor blinding was reported, nor is it reported how RTW was assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2 participants from the control group refused the 2‐year follow‐up and the participants' 2‐year survival was similar in both groups. |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol available |
| Other bias | Unclear risk | Additional sources of bias from cluster‐RCT:
|
Haerem 2000.
| Methods |
Study design: parallel RCT Recruitment: ‐ Allocation: not reported Blinding: not reported Randomisation: not reported ("carried out in blocks of 4 patients") Follow‐up(s): 1, 8, and 52 weeks Description: tape‐recorded discharge counselling provided for 4 weeks of listening |
|
| Participants |
Baseline characteristics Intervention group "tape"
Control group
Inclusion criteria
Exclusion criteria ‐ Baseline imbalances: ‐ Physically demanding work (i.e. white vs. blue collar): blue‐collar (13 light work vs 17 physical work) Severity of CHD: unknown |
|
| Interventions |
Intervention "Audiotape" Intervention participants received a structured recorded conversation regarding MI, risk factors, medication, treatment options, etc. with a doctor on audiotape and a tape player (returned at the 1‐week follow‐up)
Control group
|
|
| Outcomes | Proportion at work at 6–12 months (medium term): 6 and 12 months Adverse events (hospital readmissions) |
|
| Identification |
Sponsorship source: a grant from the Norwegian Medical Association Country: Norway Setting: single centre: Hedmark Central Hospital; inpatient and self‐administered (tape‐listening). Possible conflicts of interest: not reported Ethics committee approval: an informed consent was obtained from each participant |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Other than that the randomisation was done in blocks of 4 participants, no method of randomisation is described. |
| Allocation concealment (selection bias) | Unclear risk | No method of allocation concealment is described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the study, blinding of participants was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding of outcome assessors is mentioned. However, information regarding lifestyle was collected using a questionnaire comprising 8 questions. Additionally, information regarding sick leave was obtained from, "the patients, their private doctors, the local health insurance offices, and hospital records". This suggests that information on employment was validated with data from unbiased sources. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The group allocation of people not assessed at the follow‐up is not described. |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol was available |
| Other bias | Unclear risk | None identified |
Hall 2002.
| Methods |
Study design: parallel RCT Recruitment: low‐risk AMI patients admitted to Westmead and Blacktown Hospitals; April 1994‐December 1996 Allocation: not reported Blinding: not reported Randomisation: not reported Follow‐up(s): 1 year Description: combined outpatient rehabilitation programme |
|
| Participants |
Baseline characteristics Intervention group
Control group
Inclusion criteria
Exclusion criteria: high‐risk patients Baseline imbalances: ‐ Physically demanding work (i.e. white vs. blue collar): unknown Severity of CHD: less severe (excl. unstable angina post infarction, cardiac failure, LVEF > 40%, negative exercise stress test (> 2mm ST depression)) |
|
| Interventions |
Intervention characteristics Rehabilitation group (REHAB)
Control group
|
|
| Outcomes | Graphics of cumulative proportions of people returning to any paid work presented. Participants in the control group returned to work sooner (survival analysis: Wilcoxon test P = 0.007; log‐rank test P = 0.038), but the cumulative percentages were approximately the same by 12 months. Cardiovascular extension of the Health Measurement Questionnaire |
|
| Identification |
Sponsorship source: Australian National Health and Medical Research Council Country: Australia Setting: outpatient Possible conflicts of interest: none reported Ethics committee approval: approved by the Western Sydney Area Ethics Committee. Consent obtained from participants and physicians |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No method of randomisation was reported |
| Allocation concealment (selection bias) | Unclear risk | No method of allocation concealment was reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the study, blinding of participants was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No outcome assessor blinding was reported, RTW was assessed with questionnaires asking how many hours of paid work the participants worked in the previous week. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | By the 12‐month follow‐up, loss to follow‐up was similar in both groups. |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol was available. |
| Other bias | Unclear risk | None identified |
Hanssen 2009.
| Methods |
Study design: parallel RCT Recruitment: starting 2001, patients hospitalised (≥ 2 days) for AMI Allocation: not reported Blinding: not reported Randomisation: "Simple randomisation procedure" Follow‐up(s): 12 and 18 months Description: telephone follow‐up |
|
| Participants |
Baseline characteristics Intervention group
Control group
Inclusion criteria
Exclusion criteria
Baseline imbalances: ‐ Physically demanding work (i.e. white vs. blue collar): unknown Severity of CHD: unknown |
|
| Interventions |
Intervention
Control group
|
|
| Outcomes | Proportion at work at > 12 months to < 5 years (long term): 18 months SF‐36 Adverse events (mortality) |
|
| Identification |
Sponsorship source: the study was supported by grants from the Haukeland University Hospital, the Norwegian Nurse Association, the Meltzer Foundation for grants and the Norwegian Lung and Heart Foundation Country: Norway Setting: single‐centre: Haukeland University Hospital; outpatient Possible conflicts of interest: no information provided Ethics committee approval: this study was approved by The Regional Committee for Medical Research Ethics and the Privacy Issues Unit at Norwegian Social Science Data Services |
|
| Notes | RTW results for the subgroup study participants working prior to the intervention were provided through personal communications. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "simple randomisation procedure." The sequence generation method used was described only as a "simple randomisation procedure". This unfortunately, does not provide an insight regarding the susceptibility of the method to bias. |
| Allocation concealment (selection bias) | Unclear risk | No method of allocation concealment is described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Once group allocation was disclosed each subject was informed orally and in writing what his or her participation in the study involved." Blinding not possible due to study design. No blinding of participants and personnel is described, however the fact that the participants were first made aware of the what their "participation in the study involved" for their allocated group, and since the intervention comprised mainly of a weekly telephone calls from a nurse, participants might not have been aware of being in a treatment or control group. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Endpoints were assessed by self‐report using mailed questionnaires and from the medical records 12 and 18 months after discharge." Assessment via questionnaires |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "From randomisation to the fifth measurement point after 18 months, the loss to follow‐up was 26% in the control group and 35% in the Intervention group." The proportion of study participants not followed until 18 months is moderately high and unevenly distributed between the intervention and control groups. The researchers also report that the participants lost to follow‐up had "significantly longer hospital stays, poorer HRQoL scores at baseline, a larger proportion of non‐smokers and smokers and a smaller proportion of ex‐smokers". |
| Selective reporting (reporting bias) | Unclear risk | No study protocol was available. However, none of the results/analyses described a statistically significant difference, suggesting a lack of selective outcome reporting. |
| Other bias | Unclear risk | None identified |
Higgins 2001.
| Methods |
Study design: parallel RCT Recruitment: consecutive PCI patients; June 1995‐January 1997 Allocation: not reported Blinding: not reported Randomisation: no method described Follow‐up(s): 10 weeks (range: 8‐26 weeks); 51 weeks (range: 36‐56 weeks) Description: combined rehabilitation programme |
|
| Participants |
Baseline characteristics Intervention group
Control group
Inclusion criteria
Exclusion criteria
Baseline imbalances:
Physically demanding work: white‐collar Severity of CHD: unknown |
|
| Interventions |
Intervention characteristics
Control group
|
|
| Outcomes | Proportion at work at < 6 months (short term): 2 months Proportion at work at 6 months–12 months (medium term): 12 months Psychological adjustment to illness scale: self‐report (PAIS‐SR) |
|
| Identification |
Sponsorship source: Prince Charles Hospital Private Practice Fund Country: Australia Setting: home‐based intervention Possible conflicts of interest: no information provided Ethics committee approval: informed written consent was obtained |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants “were randomly assigned to either control (standard care and telephone follow‐up) or intervention (individualized, comprehensive, home‐based, cardiac rehabilitation) groups.” No further information was provided. |
| Allocation concealment (selection bias) | Unclear risk | No method of allocation concealment was reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the study, blinding of participants was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding reported in data collection Quote: "Occupational information was obtained from the hospital medical records and from interviews with participants at T1, T2 , and T3. Information obtained at T2 and T3 was collected using telephone interviews and mailed questionnaires." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Although 15 participants were excluded after recruitment due to complications or due to the need for additional surgical procedures, it is unclear how these cases were distributed among the treatment groups. Presumably, the randomisation resulted in evenly distributed groups of 60 participants each, so the attrition of participants would have been comparable in both treatment arms (9 control and 6 intervention participants). |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol available |
| Other bias | Unclear risk | None identified |
Hofman‐Bang 1999.
| Methods |
Study design: parallel RCT Recruitment: participants recruited among those referred to the outpatient clinic of the Department of Cardiology, Karolinska Hospital for PCI Allocation: not reported Blinding: not reported Randomisation: no method described Follow‐up(s): 12 months, 2 years Description: combined inpatient rehabilitation with 11‐month maintenance programme |
|
| Participants |
Baseline characteristics Intervention group
Control group
Inclusion criteria
Exclusion criteria
Baseline imbalances: beta‐blockers (P < 0.05): intervention group: 70; control group: 90 Recruitment Methods: Physically demanding work: unknown Severity of CHD: severe (included patients with angina, congestive heart failure) |
|
| Interventions |
Intervention characteristics
Control group Usual care after a PTCA procedure (one outpatient visit at the clinic), followed by family physician care for further secondary preventive efforts |
|
| Outcomes | Proportion at work at 6 months–12 months (medium term): 12 months Proportion at work at > 12 months to < 5 years (long term): 2 years APQLQ; Beck Depression Inventory; Trait anxiety Adverse events (mortality, hospital readmissions) |
|
| Identification |
Sponsorship source: supported by AMF insurance company, SPP insurance company and the Swedish Heart and Lung Foundation Country: Sweden Setting: single‐centre: rehabilitation centre HälsoInvest Föllinge; in‐ and outpatient Possible conflicts of interest: no information provided Ethics committee approval: the study protocol was approved by the ethical review board of the Karolinska Hospital, Stockholm |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation is not described. |
| Allocation concealment (selection bias) | Unclear risk | No method of allocation concealment is described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of this study (intervention was a 4‐week residential rehabilitation), blinding of participants was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessors is not mentioned, but RTW and sick leave information from the self‐administered questionnaires was confirmed with official registry data. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of study participants followed was relatively evenly distributed between groups. However, roughly half of the participants were not included in the reported proportions of participants returning to work by 12 and 18 months (all of the included study participants were employed at baseline). |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol was available |
| Other bias | Unclear risk | None identified |
Holmbäck 1994.
| Methods |
Study design: parallel RCT Recruitment: MI patients attending a post‐MI clinic during 2‐year period Allocation: sealed envelopes Blinding: not reported Randomisation: no method described Follow‐up(s): 4 within 12 months Description: supervised exercise programme |
|
| Participants |
Baseline characteristics Exercise/training group
Non‐exercise/control group
Inclusion criteria
Exclusion criteria
Baseline imbalances:
Physically demanding work: white collar Severity of CHD: less severe |
|
| Interventions |
Intervention characteristics Exercise/training group
Control group
|
|
| Outcomes | Proportion at work at 6–12 months (medium term): 12 months Median (and IQR) RTW time (weeks) Adverse events (mortality, reinfarction) |
|
| Identification |
Sponsorship source: Malmöhus County Council Country: Sweden Setting: single‐centre: Hospital Post‐MI Clinic/Lund University Hospital; outpatient Possible conflicts of interest: none reported Ethics committee approval: approved by the Ethical Research Committee of the Medical Faculty |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was performed according to random numbers" no information about randomisation method |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed according to random numbers in sealed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the intervention (supervised training) blinding of participants would not have been possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not mentioned, and it is unclear how employment status was determined. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6 dropouts in Intervention group, 4 dropouts in control group until 1‐year follow‐up. Total study attrition was 14.5%. Half of the participants lost to follow‐up in the intervention group did not finish the exercise training programme due to lack of motivation, time or severe lumbago. Also, 2 participants in the intervention group suffered a reinfarction, compared to no reinfarctions in the control group. |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol was available |
| Other bias | Unclear risk | None identified |
Horlick 1984.
| Methods |
Study design: parallel RCT Recruitment: consecutive post‐MI patients Allocation: not reported Blinding: not reported Randomisation: no method described Follow‐up(s): 6 months Description: educational‐group discussion programme |
|
| Participants |
Baseline characteristics Intervention group
Control group
Inclusion criteria
Exclusion criteria: none Baseline imbalances: ‐ Physically demanding work: white‐collar Severity of CHD: unknown |
|
| Interventions |
Intervention
Control group
|
|
| Outcomes | Proportion at work at < 6 months (short term): 3 months Proportion at work at 6 months–12 months (medium term): 6 months Self‐developed personal adjustment questionnaires |
|
| Identification |
Sponsorship source: grant from the Saskatchewan Heart Foundation Country: Canada Setting: multi‐centred: three Saskatoon hospitals; in‐ and outpatient Possible conflicts of interest: no information provided Ethics committee approval: not reported |
|
| Notes | Number of participants returning to work calculated from percents given in text and number of study participants followed at 3 and 6 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only the ratios of distribution to treatment versus control group over time are described, i.e. 3:1 and later 2.5:1. The method of sequence generation is not described. |
| Allocation concealment (selection bias) | Unclear risk | No method of allocation concealment is described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the intervention (outpatient educational group discussions), blinding of participants would not have been possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No concealment of outcome assessors is described, and assessment of "intention to return to work or retire" was described as self‐reported. Information regarding RTW was also obtained from a physician's report, but it is unclear if the physicians were aware of their participant's group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The proportion of study participants lost to follow‐up was 12% in the control group and 22% in the Intervention group. |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol was available |
| Other bias | Unclear risk | None identified |
Hämäläinen 1991.
| Methods |
Study design: parallel RCT Recruitment: patients < 65 years of age, treated for their first AMI at 1 of 5 hospitals; April 1978‐March 1980 Allocation: randomly Blinding: not reported Randomisation: not reported Follow‐up(s): 1, 2, 3 months, 1 and 6 years Description: 2‐week inpatient combined rehabilitation programme |
|
| Participants |
Baseline characteristics Intervention group (residential rehabilitation)
Intrvention group (hospital outpatient)
Inclusion criteria
Exclusion criteria ‐ Baseline imbalances: ‐ Physically demanding work (i.e. white vs. blue collar): unknown Severity of CHD: unknown |
|
| Interventions |
Intervention characteristics
Control group
|
|
| Outcomes | Proportion at work at 6 months–12 months: 12 months Adverse events (mortality, reinfarctions) |
|
| Identification |
Sponsorship source: not reported Country: Finland Setting: inpatient rehabilitation Possible conflicts of interest: no information provided Ethics committee approval: not reported |
|
| Notes | Study authors write that only people who were working (also part‐time while receiving half‐pension), on sick leave, or unemployed at the time of their MI were included in the RTW analysis. The number of participants included in the analysis was provided, and the study authors could not be reached. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No method of sequence generation is described. |
| Allocation concealment (selection bias) | Unclear risk | No method of allocation concealment is described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of this programme the blinding of participants was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding of outcome assessors is described and it is unclear how employment status/RTW was determined. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "The participation rates at the check‐ups were 97%, 92%, and 89% at 1, 3, and 6 years"; no overall allocation of dropouts between intervention and control group indicated. |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol was available |
| Other bias | Unclear risk | None identified |
Lidell 1996.
| Methods |
Study design: parallel RCT Recruitment: consecutive MI patients from 700‐bed hospital in south west Sweden Allocation: not reported Blinding: cardiologist performing the exercise test was not aware of which group the participants belonged to; further blinding not reported Randomisation: no method described Follow‐up(s): 1 year, 5 years Description: combined rehabilitation programme |
|
| Participants |
Baseline characteristics Intervention group
Control group
Inclusion criteria
Exclusion criteria
Baseline imbalances:
Physically demanding work: blue‐collar Severity of CHD: unknown |
|
| Interventions |
Intervention characteristics
Control group
|
|
| Outcomes | Proportion at work at 6 months–12 months (medium term): 12 months Proportion at work at 5 years (extended long term): 5 years WHO QoL questionnaire scale A: life situation, scale B: life habits, and scale C: physical and psychological complaints Adverse events (mortality) |
|
| Identification |
Sponsorship source: Swedish National association for Heart and Lung Patients and the County Council Halland, Sweden Country: Sweden Setting: single‐centre, outpatient Possible conflicts of interest: no information provided Ethics committee approval: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No method of randomisation was reported. |
| Allocation concealment (selection bias) | Unclear risk | No method of allocation concealment was reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the study, blinding of participants was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No outcome assessor blinding was reported, however the outcome RTW was assessed with a standardised assessment tool (WHO Questionnaire). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 11 participants in the control group and 2 participants in the intervention group declined to take part in the 5‐year follow‐up. This imbalance could have caused attrition bias. |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol available |
| Other bias | Unclear risk | None identified |
Maeder 1977.
| Methods |
Study design: parallel RCT Recruitment: participants hospitalised for AMI October 1971‐ December 1972 Allocation: not reported Blinding: not reported Randomisation: random numbers table Follow‐up(s): 12 months, 4 years Description: participants in the intervention group encouraged to move and walk more in the weeks post‐MI |
|
| Participants |
Baseline characteristics Intervention group
Control group
Inclusion criteria
Exclusion criteria
Baseline imbalances: higher frequency of anamnestic angina in the early mobilisation group Recruitment methods: all participants < 70 years hospitalised for AMI were included in the study. Physically demanding work: blue‐collar Severity of CHD: less severe |
|
| Interventions |
Intervention characteristics Early mobilisation group
Control group
|
|
| Outcomes | Proportion at work (part‐/full‐time) at 6 months–12 months (medium term): 12 months Proportion at work at > 12 months to < 5 years (long term): 4 years Mean sick leave duration in months Depression‐ transient and prolonged (clinical information from attending physician) Adverse events (mortality, non‐fatal reinfarctions) |
|
| Identification |
Sponsorship source: not reported Country: Switzerland Setting: single centre: the Cantonal Hospital of Geneva; inpatient Possible conflicts of interest: no information provided Ethics committee approval: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using “random number tables in serial subgroups of six” |
| Allocation concealment (selection bias) | Unclear risk | No allocation method was reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the study, blinding of participants was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | None reported, and it is unclear how RTW was assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | An ITT analysis was not conducted, and the study authors do not report if the attrition of study participants was evenly distributed across groups. Quantities of dropout cases (also in combination of the 2 studies) did not match with the combined numbers provided in the text. |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol was available |
| Other bias | Unclear risk | None identified |
Marra 1985.
| Methods |
Study design: parallel RCT Recruitment: patients referred to the hospital rehabilitation centre; July 1977‐1980 Allocation: not reported Blinding: no blinding of assessors Randomisation: no method described Follow‐up(s): 2 months, 4.5 years Description: supervised training programme |
|
| Participants |
Baseline characteristics Intervention group
Control group
Inclusion criteria
Exclusion criteria
Baseline imbalances: hypercholesterolaemia (P < 0.02): intervention group: 42; control group: 29 Physically demanding work: white‐collar Severity of CHD: less severe |
|
| Interventions |
Intervention characteristics
Control group
|
|
| Outcomes | Proportion at work (blue/white collar) at 6‐12 months (medium term):about 6 months Mean months till RTW Adverse events (cardiac deaths, MIs) |
|
| Identification |
Sponsorship source: not reported Country: Italy Setting: single‐centred: rehabilitation centre, the San Giovanni Battista main Hospital; inpatient/outpatient Possible conflicts of interest: no information provided Ethics committee approval: not reported |
|
| Notes | Timepoint of RTW assessment unclear, average time until RTW between 4 and 6 months for each group reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No method of sequence generation was described. |
| Allocation concealment (selection bias) | Unclear risk | No method of allocation concealment was described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "All the patients were evaluated, followed up and cared for by four physicians (the authors) who could not keep the study blinded because of practical and ethical reasons."; participants couldn't be blinded due to study design |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "All the patients were evaluated, followed up and cared for by four physicians (the authors) who could not keep the study blinded because of practical and ethical reasons." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Three patients in both groups (see Table 1) dropped out during the rehabilitation programme or the equivalent self managed physical activity. No drop out was observed during long term follow‐up." |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol was available |
| Other bias | Unclear risk | None identified |
Oldridge 1991.
| Methods |
Study design: parallel RCT Recruitment: all patients admitted with a diagnosis of AMI to any 1 of 6 local hospitals Allocation: not reported Blinding: investigators were not blinded to allocation; blinding of participants it is not described Randomisation: not described Follow‐up(s): 2, 4, 8 and 12 months Description: combined outpatient rehabilitation programme |
|
| Participants |
Baseline characteristics Intervention group
Control group
Inclusion criteria AMI patient Exclusion criteria
Baseline imbalances: ‐ Physically demanding work: unknown Severity of CHD: less severe |
|
| Interventions |
Intervention characteristics
Control group
|
|
| Outcomes | Proportion at work at 6–12 months (medium term): 12 months QoL after AMI questionnaire (self‐developed), quality of well‐being questionnaire Adverse events (mortality) |
|
| Identification |
Sponsorship source: Grant 6606‐2724‐44 from the National Health Research and Development Programme, Health and Welfare, Canada Country: USA Setting: single‐centre: the Health Sciences Centre; the intervention sessions were held in a hospital gymnasium; outpatient Possible conflicts of interest: no information provided Ethics committee approval: ethics committees of the University and each hospital |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It is not clear how the allocation sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | The study authors report that participants received the next available study number with the associated group allocation. It is unclear if this method is sufficient to prevent bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the intervention (supervised exercise, cognitive behavioral intervention), blinding of participants would not have been possible. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Another possible limitation to the present study is that the investigators were not blinded to allocation, although such bias would be expected to favour the rehabilitation group." Quote: "Mortality and work status were monitored throughout the study." ‐ unclear how this was done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A low number of study participants were lost to follow‐up. The number of study participants who died during the follow‐up period was also similar in both study arms (intervention n = 3, control group n = 4). |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol was available |
| Other bias | Unclear risk | None identified |
Petrie 2002.
| Methods |
Study design: parallel RCT Recruitment: consecutive first‐time MI patients admitted to Auckland Hospital over 12‐month period (time period not given) Allocation: not reported Blinding: not reported Randomisation: computer‐generated Follow‐up(s): 3 months Description: individualised education to change illness perception |
|
| Participants |
Baseline characteristics Intervention
Usual care
Inclusion criteria ≤ 65 years of age at the time of the MI Exclusion criteria: none Baseline imbalances: time in hospital (days): intervention group: 7.7 (4.0); control group: 9.3 (6.2); number working at baseline: intervention group: 80.7%; control group; 58.9% Physically demanding work: unknown Severity of CHD: severe |
|
| Interventions |
Intervention In‐hospital individualised illness perception counselling
Control group
|
|
| Outcomes | Proportion at work at < 6 months (short term): 3 months Illness perception questionnaire |
|
| Identification |
Sponsorship source: Heart Foundation of New Zealand Country: New Zealand Setting: inpatient Possible conflicts of interest: no information provided Ethics committee approval: study authors report obtaining consent and ethics committee approval |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “...patients were randomly assigned into either an intervention or control group using a computer‐generated allocation code.” |
| Allocation concealment (selection bias) | Unclear risk | No method of allocation concealment was reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Due to the nature of the study, blinding of participants was not possible. However, it is unclear if the participants in the intervention group would have realised they were in the intervention group, since the intervention was integrated into the inpatient hospital care. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No outcome assessor blinding was reported, time until returning to work was assessed with a questionnaire at 3 months. It is unclear if a validated questionnaire item was used to determine the time point of the participants’ RTW |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “[The 12‐week follow‐up] questionnaire was returned by 56 patients (86%), and non‐respondents did not differ significantly from respondents on any baseline variables.” It is unclear if group assignment (intervention vs control) is considered to be one of the baseline variables. |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol available |
| Other bias | Unclear risk | None identified |
Pfund 2001.
| Methods |
Study design: parallel RCT Recruitment: continuously employed patients (≤ 60 years) after successful coronary catheter revascularisation; March1998‐December 1999 Allocation: not reported Blinding: not reported Randomisation: no method described Follow‐up(s): 4 months Description: intervention group received a RTW consultation regarding RTW including a proposed date for RTW in the 1st week after the intervention |
|
| Participants |
Baseline characteristics Intervention group
Control group
Inclusion criteria
Exclusion criteria
Baseline imbalances: ‐ Recruitment Methods: Physically demanding work: unknown Severity of CHD: less severe |
|
| Interventions |
Intervention characteristics
Control group
|
|
| Outcomes | Proportion at work at < 6 months (short term): 4 months Duration of sick leave EuroQOL (only baseline reported) |
|
| Identification |
Sponsorship source: Ernst und Berta‐Grimmke‐Stiftung, Düsseldorf Country: Germany Setting: multi‐centred: medical clinic III of the University of Cologne and the joint practice Haubrichhof, Cologne; inpatient Possible conflicts of interest: no information provided Ethics committee approval: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation is not described. |
| Allocation concealment (selection bias) | Unclear risk | No method of allocation concealment is described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of the study participants was not mentioned. The hospital personnel and study researchers would have been aware of the group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding of outcome assessors is mentioned, and RTW was assessed with an interview. No additional checks of work status with external sources is mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Overall there were 104 patients included in the study (intention‐to‐treat) of which 100 (96%) were interviewed after 4 months." Unclear what happened to the 4 dropouts and how they were allocated |
| Selective reporting (reporting bias) | Unclear risk | No study protocol was available. Although the intervention did not result in a statistically significant difference in short‐term (4‐month) RTW rates, the results were reported. However, the statistically significant differences between RTW among private vs pubically insured participants (which did not directly address the study aims) are overemphasised |
| Other bias | Unclear risk | None identified |
Picard 1989.
| Methods |
Study design: parallel RCT Recruitment: recruited at Kaiser Foundation Hospitals in Redwood City, Santa Clara, San Jose, Hayward and South San Francisco, USA. July 1983‐ September 1985 Allocation: sealed envelopes Blinding: not reported Randomisation: sealed envelopes with an equal number of group assignments were shuffled into random order and each new patient was assigned with the top envelope on the stack Follow‐up(s): 6 months Description: intervention provided AMI patients eligible for treadmill testing with a RTW consultation including a recommendation for RTW based on results of treadmill testing |
|
| Participants |
Baseline characteristics Intervention group
Usual Care Group
Inclusion criteria
Exclusion criteria
Baseline imbalances: ‐ Physically demanding work: white‐collar Severity of CHD: severe (patients with ventricular fibrillation included; intervention group n = 8; control group n = 3) |
|
| Interventions |
Intervention characteristics
Control group
|
|
| Outcomes | Proportion at work (part‐/full‐time) at 6 months–12 months (medium term): 6 months Median and range of days until RTW: 6 months Working hours per week: 6 months Adverse events (mortality, cardiac events, non‐fatal reinfarctions) |
|
| Identification |
Sponsorship source: National Heart, Lung and Blood Institute, Bethesda, Maryland; Robert Wood Johnson Foundation, Princeton, New Jersey; Dr. Picard‐ National Research Service Award Fellowship Country: USA Setting: single‐centre, outpatient Possible conflicts of interest: no information provided Ethics committee approval: institutional review boards at Stanford University and Kaiser Foundation Hospitals approved the study |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using randomly sorted envelopes. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used to allocate participants. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the study, blinding of participants was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | RTW was assessed with questionnaires at 6 months or with an exit interview conducted by a data co‐ordinator. The researchers report that the data co‐ordinator had not been involved with performing the intervention, and this suggests an attempt to blind the outcome assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “All analyses were done by intention to treat.” |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol was available |
| Other bias | Unclear risk | None identified |
Pilote 1992.
| Methods |
Study design: parallel RCT Recruitment: patients hospitalised for AMI in the CCUs of 4 San Francisco Bay Area Kaiser‐Foundation Medical Centres from August 1987‐December 1989 Allocation: sealed envelopes Blinding: all cardiac events were confirmed by a cardiologist blinded to the randomisation. Randomisation: computer programme Follow‐up(s): 6 months Description: "Occupational Work Evaluation" including a recommendation of when to return to work based on treadmill testing |
|
| Participants |
Baseline characteristics Intervention
Usual care
Inclusion criteria
Exclusion criteria
Baseline imbalances: white race (P < 0.05): intervention group: 82%; control group: 65% Physically demanding work: white‐collar Severity of CHD: less severe |
|
| Interventions |
Intervention characteristics
Usual care
|
|
| Outcomes | Proportion at work (part‐/full‐time) at 6–12 months (medium term): 6 months Days until RTW: 6 months Adverse events (mortality, non‐fatal reinfarctions) |
|
| Identification |
Sponsorship source: Grant HL36734 from the National Heart, Lung and Blood Institute of Health, Bethesda, Maryland Country: USA Setting: multicentre: 4 Kaiser‐Foundation Medical Centres, San Francisco Bay Area; evaluation (the Occupational Work Evaluation) at a university research clinic Possible conflicts of interest: no information provided Ethics committee approval: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer programme was used to randomly assign participants. |
| Allocation concealment (selection bias) | Low risk | Quote: "The assignments were placed in sealed envelopes and drawn in sequence as patients were randomised." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the study intervention (treadmill tests with counselling session), blinding was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Information regarding return to work for both groups was obtained by a Stanford‐based data coordinator... The occupational status of all patients was ascertained by telephone by the data coordinator at 6 months after the myocardial infarction." Quote: "All cardiac events were confirmed by a cardiologist blinded to the randomisation." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of participants lost to follow‐up was altogether low. However, 3 (2 deaths) participants were lost to follow‐up in the intervention group vs 1 participant in the control group by 6 months, and 15 people in the intervention group developed contraindications and withdrew from the study vs 10 (1 death due to cancer) in the control group. |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol was available |
| Other bias | Unclear risk | None identified |
Pozen 1977.
| Methods |
Study design: parallel RCT Recruitment: consecutive patients over 16 months (dates of recruitment not reported) Allocation: not reported Blinding: not reported Randomisation: no method described Follow‐up(s): 6 months Description: counselling with a nurse rehabilitator |
|
| Participants |
Baseline characteristics Intervention group (high‐risk)
Intervention group (low‐risk)
Control group (high‐risk)
Control group (low‐risk)
Inclusion criteria
Exclusion criteria
Baseline imbalances: ‐ Physically demanding work: unknown Severity of CHD: severe (we considered high‐ and low‐risk groups together and allocated all participants to the high‐risk group, except in subgroup analysis considering CHD severity.) |
|
| Interventions |
Intervention (low‐ and high‐risk groups)
Control group (low‐ and high‐risk)
|
|
| Outcomes | Proportion at work at 6–12 months (medium term): 6 months IPAT anxiety score; Hopkins Symptom Checklist‐90 |
|
| Identification |
Sponsorship source: by funds from the Health Services Research and Development Grant #HS 000429 of the Johns Hopkins Health Services Research and Development Center, the Robert Wood Johnson Clinical Scholars Program, a Baltimore City Hospitals administered grant from the Department of Health, Education and Welfare, Grant # 5 501 RRO 5556 Country: USA Setting: CCU of the Baltimore City Hospitals; in‐ and outpatient Possible conflicts of interest: no information provided Ethics committee approval: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "One hundred and seventy‐nine patients categorized as high risk were randomly assigned in equal proportions to the study and control groups. One hundred and thirty‐four patients categorized as low risk were randomly assigned in a 2: 1 ratio to the study and control groups, respectively." No further information |
| Allocation concealment (selection bias) | Unclear risk | None described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of this study, blinding of participants and personnel would not have been possible. The study authors do write that study and control participants were assigned to different rooms when possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors is not described, and it is not entirely clear how work status was assessed ‐ presumably with a questionnaire. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "follow‐up consisted of phone calls, letters, and personal visits before these 26 patients were considered 'lost to follow‐up' (representing 15 per cent of the 174 patients with true MIs)." Quote: "The 15 per cent loss to follow‐up may slightly bias the results in the positive direction by selecting a somewhat more compliant patient population." |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol was available |
| Other bias | Unclear risk | None identified |
PRECOR 1991.
| Methods |
Study design: 3‐armed RCT Recruitment: patients admitted to the CCUs from February 1981‐ May 1984 Allocation: not reported Blinding: no blinding (participants were informed about the principle of the study) Randomisation: no method described Follow‐up(s): 2 months, 2 years Description: combined rehabilitation programme with exercise and education, or counselling (only) programme (CP) |
|
| Participants |
Baseline characteristics Intervention group (rehabilitation)
Intervention group (counselling)
Control group (usual care)
Inclusion criteria
Exclusion criteria
Baseline imbalances: ‐ Physically demanding work: unknown Severity of CHD: less severe |
|
| Interventions |
Intervention characteristics Rehabilitation programme
Counselling programme
Control group
|
|
| Outcomes | Proportion at work at < 6 months (short term): 2 months Proportion at work at > 12 months to < 5 years (long term): 2 years Adverse events (reinfarction, cardiac surgery) |
|
| Identification |
Sponsorship source: by a grant from the Institut National de la Santé et de la Recherche Médicale, by the Hospices Civils de Lyon and by the Association pour la Promotion et la Réalisation d'Essais Thérapeutiques Country: France Setting: multi‐centred (4 clinical CCUs): Hospital Cardiovasculaire Louis Pradel; Centre Hospialier de Vienne; Hospital Lyon Sud; Clinique Mutualiste Eugene Andre Possible conflicts of interest: no information provided Ethics committee approval: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients who could perform an exercise test adequately were randomised between a rehabilitation (RP) and a counselling programme (CP) or usual care (UC)." ‐ no further information |
| Allocation concealment (selection bias) | Unclear risk | None described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the intervention (group sessions), the blinding of participants and personnel would not have been feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding of outcome assessors is described, and no details are given regarding how RTW was assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants appear to have been followed. Quote: "No patient was lost to follow‐up" |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol was available |
| Other bias | Unclear risk | None identified |
Rahe 1979.
| Methods |
Study design: parallel RCT Recruitment: October 1971‐June 1972 (January‐July1973 17 additional post‐MI patients were referred for treatment) Allocation: not reported Blinding: not reported Randomisation: no method described Follow‐up(s): 18 months, 3‐4 years Description: a randomised treatment group and a non‐randomised group of volunteers (not included in the meta‐analysis) received group therapy |
|
| Participants |
Baseline characteristics Intervention group 1 (randomised)
Intervention group 2 (non‐randomised volunteers, not included in the analysis)
Control group (CG)
Inclusion criteria
Exclusion criteria: not reported Baseline imbalances: average age: intervention group 1 50.9 years; control group 55.2 years Physically demanding work: unknown Severity of CHD: severe |
|
| Interventions |
Intervention characteristics
Control group
|
|
| Outcomes | Proportion at work at < 6 months (short term): 3 months Proportion at work at 6–12 months (medium term): 6, 12 months Participants working full‐time after > 12 months to < 5 years: 4 years Clinical anxiety (general and cardiac‐specific) Adverse events (mortality, reinfarction, bypass) |
|
| Identification |
Sponsorship source: by the Naval Medical Research and Development Command, Department of the Navy, under Research Work Unit ZF 51.524.002.‐5020 Country: USA Setting: Naval Regional Medical Centre/US Naval Hospital Possible conflicts of interest: no information provided Ethics committee approval: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Twenty‐two patients were randomly assigned to the treatment group and 22 to the control group." No further information provided |
| Allocation concealment (selection bias) | High risk | No allocation concealment was described, and the study authors report that, "17 additional post‐MI patients who met the research criteria were referred for treatment." Although these participants should have been randomly allocated to the study arms, the study authors explain that these participants joined the study expecting the intervention (and examined as a separate treatment group). The results of these non‐randomised people are nevertheless excluded from the quantitative synthesis of results. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the intervention (group therapy), blinding of participants and personnel would not have been possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding of outcome assessors is described, and it is unclear how exactly RTW was assessed. The study authors state that standardised interviews and research questionnaires were used at the follow‐ups. However, no validated questionnaire or validation with independent (unbiased) occupational records is described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up was low across all groups. Three people died before the 3‐4‐year follow‐up, but the study authors do not describe to which of the three study groups these participants were allocated. |
| Selective reporting (reporting bias) | Unclear risk | Although the study authors do mention a study protocol, it does not appear to have been published. |
| Other bias | Unclear risk | None identified |
Rivas 1988.
| Methods |
Study design: 3‐arm RCT Recruitment: first AMI patients admitted to CCU Allocation: not reported Blinding: not reported Randomisation: no method described Follow‐up(s): 3 months, 6 months, 9 months, 12 months Description: combined outpatient rehabilitation including supervised training with psychological and vocational counselling with more (group A) and less (group B) intense physical training |
|
| Participants |
Baseline characteristics Intervention group (rehabilitation group A)
Interventions group (rehabilitation group B)
Control group
Inclusion criteria
Exclusion criteria: none Baseline imbalances: ‐ Physically demanding work: white‐collar Severity of CHD: unclear |
|
| Interventions |
Intervention characteristics
Control group
|
|
| Outcomes | Proportion at work at < 6 months (short term): 3 months Proportion at work at 6–12 months (medium term): 12 months Adverse events (mortality) |
|
| Identification |
Sponsorship source: Institute of Cardiology and Cardiovascular Surgery, Rehabilitation Center Country: Cuba Setting: single centre (Institute of Cardiology and Cardiovascular Surgery, Rehabilitation Center), ambulant Possible conflicts of interest: no information provided Ethics committee approval: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...patients were randomly distributed according to a table of random numbers in three groups" |
| Allocation concealment (selection bias) | Unclear risk | No method of allocation concealment is described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the intervention (outpatient exercise programmes), blinding of participants and personnel would not have been possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding of outcome assessors, nor is the method of assessing RTW is mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | For intervention group A and the control group, the number of study participants reported to be lost to follow‐up due to death was the same (n = 1). Together in groups A and B a total of 3 study participants were lost due to retirement compared to 5 study participants in the control group. |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol was available |
| Other bias | Unclear risk | None identified |
Stern 1983.
| Methods |
Study design: 3‐armed RCT Recruitment: 3‐year recruitment period. Recruited from the CCU, internists, and the larger community. MI documented within 6 weeks‐1 year prior to study admission Allocation: not reported Blinding: not reported Randomisation: block randomisation; no method described Follow‐up(s): 3, 6, and 12 months Description: intervention included either supervised exercise or group counselling |
|
| Participants |
Baseline characteristics Study participants were predominantly white, married, middle to upper‐middle class men admitted to the study 7 months (mean) after MI Intervention group (counselling)
intervention group (exercise therapy)
Control group (CG)
Inclusion criteria
Exclusion criteria
Baseline imbalances:
Physically demanding work: unknown Severity of CHD: less severe |
|
| Interventions |
Intervention characteristics
Control group
|
|
| Outcomes | Proportion at work at 6–12 months (medium term): 12 months Taylor Manifest anxiety, Zung Depression Adverse events (mortality, MI, bypass) |
|
| Identification |
Sponsorship source: grant G008003044 from the National Institute of Handicapped Research, Department of Education, Washington DC Country: USA Setting: George Washington University Hospital; single‐centre; outpatient Possible conflicts of interest: no information provided Ethics committee approval: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised into 3 study groups in blocks of 6, but the method of sequence generation is not described. |
| Allocation concealment (selection bias) | Unclear risk | No method of allocation concealment is described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the interventions examined (supervised exercise training or group counselling), the blinding of study participants and personnel would not have been possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding of outcome assessors is mentioned and it is unclear if a validated questionnaire was used to assess RTW at the follow‐up. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It seems as if no participants were lost to follow‐up for the evaluation of RTW. However, it is unclear if non‐compliant study participants in the intervention groups were excluded from some or all of the outcome assessments. |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol was available |
| Other bias | Unclear risk | None identified |
Vermeulen 1988.
| Methods |
Study design: parallel RCT Recruitment: hospitalised for AMI during the period January 1971‐May 1975 Allocation: not reported Blinding: not reported Randomisation: no method described Follow‐up(s): 12 months Description: combined outpatient rehabilitation programme |
|
| Participants |
Baseline characteristics Intervention group
Control group
Inclusion criteria
Exclusion criteria
Baseline imbalances: ‐ Physically demanding work: blue‐collar Severity of CHD: less severe |
|
| Interventions |
Intervention characteristics Rehabilitation interventions consisted of physical training, social counselling, group meetings and, when necessary, individual psychological advice
Control group
|
|
| Outcomes | Proportion at work at 6– 12 months (medium term): 12 months Adverse events (mortality, reinfarction) |
|
| Identification |
Sponsorship source: no information provided Country: The Netherlands Setting: single‐centred, outpatient: Revalidatie Instituut Muiderpoort Possible conflicts of interest: no information provided Ethics committee approval: not reported |
|
| Notes | The proportion of blue‐collar workers returning to work was lower but the difference was not statistically significant. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...the patients were randomly assigned to either a control group (N = 47) or a rehabilitation group (N = 51)." No further information given |
| Allocation concealment (selection bias) | Unclear risk | None described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the study, blinding of participants was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported, and unclear how RTW was assessed (questionnaire or register) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss‐to‐follow‐up reported. RTW results at 1‐year follow‐up describe 85 participants. Examination of exercise tolerance at 5‐year follow‐up according to working status describes 89 participants |
| Selective reporting (reporting bias) | Low risk | No study protocol was available. However, the results were not statistically significant, and little differences were observed regarding RTW between the intervention and control group |
| Other bias | Unclear risk | None identified |
WHO 1983.
| Methods |
Study design: multi‐centre, RCT Recruitment: 24 centres; hospitalised AMI patients from June 1973‐October 1975 Allocation: not reported Blinding: not reported Randomisation: random numbers table (some centres applied cluster‐randomisation or a non‐randomised control group) Follow‐up(s): 3, 6, 12 months, 2 and 3 years Description: combined rehabilitation programme |
|
| Participants |
Baseline characteristics Intervention group (rehabilitation)
Control group
Inclusion criteria
Exclusion criteria ‐ Baseline imbalances: ‐ Physically demanding work: unknown Severity of CHD: severe |
|
| Interventions |
Intervention characteristics
Control group
|
|
| Outcomes | Proportion at work at < 6 months (short term): 3 months Proportion at work at 6–12 months (medium term): 12 months Proportion at work at > 12 months to < 5 years (long term): 3 years Adverse events (mortality, reinfarctions, non‐fatal reinfarctions) |
|
| Identification |
Sponsorship source: WHO Country: international Setting: ‐ Possible conflicts of interest: no information provided Ethics committee approval: not reported |
|
| Notes | The results of the individual centres were often published separately and the number of people included in the RTW results were not reported, therefore this study is not included in the meta‐analyses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Although the study protocol called for the randomisation of participants according to random number tables, some study centres applied cluster‐randomisation or a selected a non‐randomised control group (i.e. control hospital). Also the study authors write, "Only 12 centres out of the 24 seemed to have achieved proper randomisation in their groups of R and C patients". |
| Allocation concealment (selection bias) | Unclear risk | None described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the study, blinding of participants was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | None described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "By 1 April 1970 data on follow‐up over a three‐year period were available for about 78% of all patients initially enrolled in the study." Overall 22% loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All of the results' proposed analyses seem to have been reported. |
| Other bias | Unclear risk | None identified |
Worcester 1993.
| Methods |
Study design: parallel RCT Recruitment: patients admitted to CCU with AMI over 3 years Allocation: not reported Blinding: not reported/open‐management trial Randomisation: not reported Follow‐up(s): 4 and 12 months Description: intense versus light exercise in men < 70 years of age |
|
| Participants |
Baseline characteristics Intervention group (exercise training)
Intervention group (light exercise)
Included criteria
Excluded criteria
Baseline imbalances: ‐ Description and recruitment methods: during the 3 years of enrolment 339 men satisfied the criteria for entry to the study. Men < 70 years who had been admitted consecutively to a single CCU with AMI were eligible for the study. Physically demanding work (i.e. white‐ vs blue‐collar): blue‐collar Severity of CHD: severe (included clinical heart failure) |
|
| Interventions |
Intervention characteristics
Control group
|
|
| Outcomes | Proportion at work at < 6 months (short term): 4 months Proportion at work at 6 –12 months (medium term): 12 months Spielberger state anxiety trait inventory; IPAT depression scale; Hackett‐Cassern denial scale; Eysenck personality inventory Adverse events (mortality) |
|
| Identification |
Sponsorship source: National Heart Foundation of Australia Country: Australia Setting: Australian teaching hospital: single centre, at the Austin Hospital, Melbourne; outpatient Possible conflicts of interest: not reported Ethics committee approval: all participants gave their informed consent. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation method is not explicitly described. However, the study authors cite a paper by Peto et al (1976) that describes randomisation techniques and includes a random numbers table |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment is described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the study, blinding of participants (and personnel) is not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessors is described, and the assessment of occupational status seems to have been accomplished with semi‐structured interviews and not with a validated questionnaire or an independent external source, such as employment records. However, several validated instruments measuring depression and anxiety were used to assess quality of life. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The loss to follow‐up was greater in the Intervention group at both the 4‐ and 12‐month reviews. No ITT analysis was conducted. |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine, no study protocol was available |
| Other bias | Unclear risk | None identified |
AMI: acute myocardial infarction; APQLQ: Angina Pectoris Quality of Life Questionnaire; BMI: body mass index; BP: blood pressure; CABG: coronary artery bypass grafting CABS: coronary artery bypass surgery; CAD: coronary artery disease; CCU: coronary care unit; CHD: coronary heart disease; CPK: creatine phosphokinase; CPK‐MB: creatine kinase‐muscle/brain; ECG: electrocardiogram; ERNA: Early Return to Normal Activities; GP: general practitioner; HRQoL: health‐related quality of life; IPAT: Institute for Personality and Ability Testing; ITT: intention‐to‐treat; IQR: interquartile range; LDH: lactate dehydrogenase; LVEF: left ventricular ejection fraction; MI: myocardial infarction; NYHA: New York Heart Association; PCI: percutaneous coronary intervention; PTCA: percutaneous transluminal coronary angioplasty; QoL: quality of life; RCT: randomised controlled trial; RTW: return to work; SF‐36: 36‐item short form survey; S‐ASAT: serum aspartate aminotransferase; SGOT: serum glutamic oxaloacetic transaminase; W: Watts; WHO: World Health Organization; YMCA: Young Men's Christian Association
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahlmark 1979 | No intervention |
| Al'khimovich 1990 | No RTW |
| Ali 2018 | Not a RCT (cohort) |
| Aronov 1991 | No RTW |
| Aronov 2006 | No RTW |
| Bar 1992 | Participants (not stated how many working prior to MI). By 20 May 2016 no answer to mail. Only 1 centre randomised patients |
| Ben‐Ari 1986 | Not a RCT |
| Bjarnason‐Wehrens 1999 | No control group |
| Boszormenyi 1984 | Control group did not receive standard care |
| Boulay 1983 | Not a RCT |
| Bounhoure 2014 | Not a RCT |
| Buchwalsky 2002 | Not a RCT |
| Burns 2007 | No control group |
| Carlsson 1998 | No RTW |
| Cay 1981 | Participants ‐ only approximately 50% were working prior to MI, no subgroup analysis |
| Christensen 2017 | Participants included received implantable cardioverter defibrillator and unclear if > 80% had the indications of MI, CABG or PCI |
| Danchin 1988 | Not a RCT |
| David 2011 | No RTW |
| Davies 1991 | Not a RCT |
| Dimopoulos 1999 | Not a RCT |
| Dominiak 2011 | No RTW |
| Dorn 1999 | No RTW |
| Dorn 2001 | No RTW |
| Dumont 1999 | Not a RCT |
| Espinosa 2004 | Not a RCT |
| Fattirolli 1998 | Not RCT (protocol only) |
| Ferrario 2010 | Not a RCT |
| Follick 1988 | No intervention |
| Foster 1984 | No RTW |
| Fujita 1983 | No control group |
| Gallagher 2003 | No RTW |
| Garrity 1973 | Not a RCT |
| Giannuzzi 1992 | No RTW |
| Giannuzzi 1993 | No RTW |
| Giannuzzi 1997 | No RTW |
| Goeminne 1989 | Not a RCT |
| Grief 1995 | No RTW |
| Griffo 1983 | Not a RCT |
| Groden 1967 | No control group |
| Gutschker 1977 | Only an abstract available; not enough information to include: participants only % RTW reported no number of workers; possibly same study as Geissler 1979 |
| Gysan 1999 | No control group |
| Gysan 2004 | No RTW |
| Hakkila 1965 | Not a RCT |
| Hare 1983 | Only abstract available; not enough information provided to include ‐ no RTW results |
| Haussler 1997 | Not a RCT |
| Havelkova 2010 | No RTW |
| Hedback 1993 | Not a RCT; same study as Hedback 1987 (excluded; see Hedback 1993, secondary reference) |
| Heller 1990 | No RTW |
| Heller 1993 | Main intervention focused on providing information on healthy nutrition (dietary intervention), and primary physicians were provided information on the benefits of prescribing beta‐blockers (pharmaceutical co‐intervention) |
| Henritze 1989 | Not a RCT |
| Hertzeanu 1993 | No RTW |
| Huber 2014 | Participants: < 70% of patients included were diagnosed with an ischaemic heart disease (ICD‐10 I20‐I25) |
| Hui 2006 | No RTW |
| Iacovino 1997 | No usual care control group |
| Isaaz 2010 | No intervention |
| Jette 1991 | No RTW |
| Johnson 2014 | Not a RCT |
| Kadda 2015 | No RTW, self‐efficacy scores for total population (including participants not employed at baseline) |
| Kagan‐Ponomarev 1994 | Intervention does not fulfil inclusion criteria: early discharge vs normal rehabilitation |
| Kallio 1979 | No RTW |
| Kamath 2012 | No RTW |
| Karoff 2000b | Not a RCT, same study as Karoff 1997 Karoff 1999, Karoff 2000 (see Karoff 2000b, secondary references) |
| Kelbaek 1981 | No RTW |
| Kellermann 1968 | No control group |
| Kellermann 1975 | Not a RCT |
| Kittel 2008 | Study participants were on sick‐leave for > 3 months prior to rehabilitation and were selected because the patient or attending physician anticipated work reintegration would be difficult; 21.5% control group unemployed |
| Kokutsov 1990 | Not a RCT |
| Korzeniowska‐Kubacka 2004 | Not a RCT |
| Korzeniowska‐Kubacka 2015 | Not a RCT |
| Kovoor 2006 | No RTW |
| Krasemann 1979 | Not a RCT |
| Kushnir 1976 | Not a RCT |
| Laaksovirta 1985 | No intervention |
| Lamberti 2016 | Not a RCT |
| Lamm 1982 | Not a RCT |
| Langosch 1982 | No control group (in the follow‐up) |
| Lautamaki 2017 | CABG group compared with PCI, not a control group receiving usual care |
| Lear 2002 | No RTW |
| Li 2004 | No RTW |
| Liang 1988 | No control group |
| Lie 2009 | No RTW |
| Lisspers 1999 | No intervention |
| Liu 1997 | No RTW |
| Maeland 1987 | No RTW |
| Maeland 1989 | No control group |
| Marchionni 1994 | No RTW |
| Maroto 1996 | No RTW |
| Mayou 1981 | Not a RCT |
| Miller 1988 | No RTW comparison |
| Mirmohammadi 2014 | Not a RCT |
| Mital 1999 | No RTW (see Mital 1999 secondary reference) |
| Mital 2000 | Not a RCT |
| Mulcahy 1971 | No control group |
| Nelson 1994 | Participants (< 80% working prior to MI and no RTW subgroup analysis) |
| Ng 2000 | No RTW |
| Nikolaeva 1986 | Not a RCT |
| Nikrahan 2016 | No RTW |
| Ohm 1987 | Not RCT (CBA) |
| Palatsi 1976 | Not a RCT |
| Pegus 2002 | No RTW |
| Petrie 1996 | No control group; all participants were offered rehabilitation programme |
| Pierson 2001 | No RTW |
| Pitscheider 1995 | No control group |
| Price 2005 | Not a RCT |
| Rakowska 2015 | No RTW |
| Rauscha 1988 | No intervention |
| Redfern 2007 | No RTW |
| Reid 2012 | No RTW |
| Roviaro 1984 | No RTW |
| Rudnicki 1977 | No RTW |
| Rugulies 2003 | No RTW |
| Salonen 1980 | No control group |
| Salvetti 2008 | No RTW |
| Saner 1999 | No control group |
| Sanne 1973 | Difference in RTW between treatment groups not reported (only total RTW in the entire population) |
| Schaller 1977 | No RTW comparison |
| Schiller 1976 | Unclear how many participants were working; intervention unclear; not RCT |
| Schlierf 1995 | No RTW |
| Schuster 1995 | Not a RCT |
| Schwartze 1991 | Not an RCT, no control group |
| Shapiro 1972 | No control group |
| Shrey 2000 | Review |
| Sieber 1986 | Not a RCT |
| Siggeirsdottir 2016 | Not a RCT |
| Simchen 2001 | Not a RCT |
| Sledzevskaia 1994 | No RTW |
| Smirnov 1989 | No RTW |
| Speiser 1982 | No intervention |
| Steinacker 2011 | No RTW |
| Stepanova 1975 | No RTW |
| Sturchio 2012 | No RTW |
| Sundin 1994 | No RTW |
| Szalewska 2015a | Not a RCT |
| Szalewska 2015b | Not a RCT |
| Tarasov 1998 | No RTW |
| Toms 2003 | Self‐selection into intervention group |
| Tooth 1998 | No RTW |
| Van der Peijl 2004 | No RTW |
| Van Dixhoorn 1989 | No RTW, no control group |
| Varvaro 2000 | No RTW |
| Velasco 1982 | No true control group without intervention |
| Vibulchai 2016 | No RTW |
| Wallach 1969 | No intervention |
| Wieslander 2005 | No control group |
| Yonezawa 2009 | Participants already returned to work at start of trial |
| Yoshida 1999 | No RTW |
| Yu 2003 | No RTW |
CABG: coronary artery bypass grafting; CBA: controlled before‐after study; MI: myocardial infarction; PCI: percutaneous coronary intervention; RTC: randomised controlled trial; RTW: return to work
Characteristics of studies awaiting assessment [ordered by study ID]
Franklin 2012.
| Methods | RCT |
| Participants | 1813 patients (mean age 64 years, 74% men) hospitalized with a primary acute MI |
| Interventions | multifactorial rehabilitation programs |
| Outcomes | "Mortality at 2 years. Secondary outcomes included mortality at 1 and 9 years, and cardiac outcomes, MI, hospitalization for heart disease, stroke, percutaneous transluminal coronary angioplasty, coronary artery bypass graft, health‐related quality of life (Short Form‐36) and psychological general well‐being (Psychological General Well‐Being [PGWB] scale) at 12 months." |
| Notes | We were unable to obtain the full text. We only found an extended abstract. |
Gao 2007.
| Methods | RCT |
| Participants | 368 post operative CABG patients |
| Interventions | health managment group |
| Outcomes | quality of life |
| Notes | Unclear if RCT was examined. We were unable to obtain the full text. |
Kellermann 1988.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | We found no abstract and were unable to obtain the full text. |
Korzeniowska‐Kubacka 2003.
| Methods | controlled trial (unclear if randomised) |
| Participants | 70 men with ischemic heart disease after MI and CABG |
| Interventions | systematical ambulatory rehabilitation over five years versus six months of physical training |
| Outcomes | RTW; QOL |
| Notes | We were unable to obtain the full text. |
Landrum 2000.
| Methods | unclear |
| Participants | patients with coronary artery disease |
| Interventions | traditional rehabilitation program veresus rehabiltation with additional stress management |
| Outcomes | cardiac events and days rehospitalized |
| Notes | We were unable to obtain this dissertation. |
Rangel de Donaldo 1994.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | We were unable to obtain this dissertation. |
CABG: coronary artery bypass grafting; MI: myocardial infarction; RTC: randomised controlled trial; RTW: return to work; QOL: quality of life
Characteristics of ongoing studies [ordered by study ID]
EXPERTIS.
| Trial name or title | Prevention of reduced employability with an expert system with telephone, motivational interviews supporting self‐management (EXPERTIS) |
| Methods | Cohort study; participants will be asked to fill out questionnaire when arriving at the rehabilitation clinic as well as to take part in 2 subsequent telephone interviews after 6 and 12 months |
| Participants | Insurants of the German Retirement Insurance (Deutsche Rentenversicherung) Oldenburg‐Bremen; age: 18‐85 years |
| Interventions | Medical rehabilitation |
| Outcomes | Primary: e.g. motivation for returning to work (time frame: when arriving at the rehabilitation clinic); change in motivation for returning to work at 6 and 12 months (time frame: after the rehabilitation treatment at 6 and 12 months); actual RTW (time frames: after the rehabilitation treatment at 6 and 12 months) |
| Starting date | October 2014 |
| Contact information | Responsible party: Prof. Dr. Sonia Lippke, Professor of Health Psychology, Jacobs University Bremen gGmbH |
| Notes |
LC‐REHAB.
| Trial name or title | Effect of learning and coping strategies in cardiac rehabilitation ‐ group study (LC‐REHAB) |
| Methods | RCT; 750 participants with data collection at baseline, just after rehabilitation and 3 months/years after rehabilitation |
| Participants | Patients 18‐60 years newly hospitalised with either ischaemic heart disease or heart failure |
| Interventions | Behavioral: learning and coping arm; other: control arm |
| Outcomes | Secondary: e.g. RTW (time frame: at baseline and after 1 year) |
| Starting date | November 30, 2010 |
| Contact information | Vibeke Lynggaard, Herning Hospital |
| Notes |
MILESTONE.
| Trial name or title | Revascularization in patients with non‐ST‐segment elevation acute coronary syndrome (NSTE‐ACS) with multivessel and/or unprotected left main coronary disease (MILESTONE) |
| Methods | RCT |
| Participants | Age: > 21 years |
| Interventions | Procedure 1: PCI; procedure 2: CABG |
| Outcomes | Secondary: e.g. RTW (time frame: peri‐hospital period, 1 month and 1 year after revascularisation procedure) |
| Starting date | June 2011 |
| Contact information | Professor Pawel E. Buszman, MD, PhD, FESC, FACC, FSCAI,, American Heart of Poland |
| Notes |
SATISFY‐SOS.
| Trial name or title | Systematic assessment and targeted improvement of services following yearlong surgical outcomes surveys (SATISFY‐SOS) |
| Methods | Cohort study; surveys by either email, mail or via a telephone interview at 30‐90 days and at 1 year post‐procedure |
| Participants | Surgical and procedural patients who require anaesthesia services; age: >18 years |
| Interventions | Several surgical procedures with anaesthesia services |
| Outcomes | Other: RTW (time frame: 1 year) |
| Starting date | July 2012 |
| Contact information | Sherry L McKinnon, AA (mckinnos@anest.wustl.edu); Michael S. Avidan, MBBCh, FCASA (avidanm@anest.wustl.edu) |
| Notes |
SUSTAINCSX.
| Trial name or title | SodiUm SeleniTe Administration IN Cardiac Surgery (SUSTAIN CSX®‐Trial) |
| Methods | Randomised, placebo‐controlled, double‐blind, multicentre trial; 1400 participants across 20 sites in Germany and Canada |
| Participants | Age: > 18 |
| Interventions | Perioperative supplementation in high‐risk cardiac surgical patients undergoing complicated open heart surgery; drug 1: sodium selenite; drug 2: placebo |
| Outcomes | Secondary: e.g. RTW (time frame: 6‐months) |
| Starting date | January 2015 |
| Contact information | Daren K Heyland, MD (dkh2@queensu.ca) |
| Notes |
WARRIOR.
| Trial name or title | Women's ischaemia trial to reduce events in non‐obstructive CAD (WARRIOR) |
| Methods | Multicenter, prospective, randomised, blinded outcome evaluation; 4422 participants |
| Participants | Symptomatic women patients with symptoms and/or signs of ischaemia but no obstructive CAD |
| Interventions | Experimental: intensive medical treatment (4 kinds of drugs, 2 behavioral interventions); active comparator: usual care (the same 2 behavioral interventions as in experimental group) |
| Outcomes | Secondary: time to RTW |
| Starting date | 9 February 2018 |
| Contact information | Trinity J Cromwell, RN (tcromwell@ufl.edu); Debra Landers (debra.landers@medicine.ufl.edu) |
| Notes |
CABG: coronary artery bypass graft; CAD: coronary artery disease; PCI: percutaneous coronary intervention; RCT: randomised controlled trial; RTW: return to work
Differences between protocol and review
Title
We changed the wording of the title from "Interventions to support return‐to‐work for patients with coronary heart disease" to "Interventions to support return to work for people with coronary heart disease" in agreement with Cochrane Work Coordinating Editor Jos Verbeek, following copy editor Denise Mitchell’s suggestion. The new formulation is more inclusive and, as such, it is better in line with general Cochrane principles.
Types of studies
Due to the difficulties of performing randomised controlled trials at workplaces, we originally intended to include controlled before‐after studies (CBAs). CBAs are non‐randomised studies with one group receiving the intervention and a control group, which does not. For a CBA study to have been included in this Cochrane review, data had to have been collected contemporaneously both at baseline and post‐intervention so that the timing of the study periods for the control and intervention groups are comparable. Although we found a large number of CBAs examining the effects of person‐directed interventions on return to work, none of the CBA studies that we identified used interventions conducted at workplaces. As CBA studies are more prone to bias than RCTs, and because the CBAs that we found did not contribute information on work‐directed interventions, we deviated from the published protocol and excluded CBAs from the review.
Selection of studies
Originally, two review authors (UE, UEW) were to independently screen titles and abstracts of all the studies identified as a result of the searching. Due to the length of time needed to complete the review, we had to update our searches. The titles and abstracts identified as a result of search updates were screened by other review authors (PH, AF, or JH).
Data synthesis
We pooled data from studies with similar interventions using Review Manager 5 software (Review Manager 2014), and not version 5.2 as was stated originally in the study protocol. We conducted the meta‐analysis of subgroups with Review Manager 5, not Stata® software, although we used Stata® software to conduct some sensitivity analyses and meta‐regression (Stata).
During the review process, we found that the heterogeneous reporting of occupational characteristics made it difficult to objectively establish which study populations could be considered as having participant populations with similar physically demanding occupational groups. Therefore, we created a definition for categorising studies into groups with similar physically demanding working conditions that was not a part of the original protocol. We defined physically demanding occupational groups as studies where a majority of study participants (more than 50%) worked in physically demanding employment, manual labour or were described as blue‐collar workers. If 50% or less of the study population worked in physically demanding employment, manual labour or were blue‐collar workers, we categorised these studies into the non‐physically demanding occupational group. We considered all other studies not reporting the characteristics of occupations before the incident CHD to have unknown physical demands.
Likewise, the immense variation in how studies reported baseline cardiovascular health made it necessary to create an objective framework for determining which studies could be considered to have study populations with similar CHD severity. We created this decision framework during the review process and it was not included in the original review protocol. We examined study exclusion criteria and the most commonly reported cardiovascular baseline characteristics in order to create a framework for identifying studies with similar distributions of CHD severity. We categorised study populations as having less severe CHD if the study reported:
-
excluding participants with one or more of the following:
heart failure or systolic dysfunction (left ventricular ejection fraction (LVEF) < 40%),
unstable or stable angina,
positive exercise stress test (i.e. ≥ 2 mm ST segment change, ischaemia) using treadmill or bicycle ergometer,
intracardiac defibrillator (ICD) or atrial fibrillation; or
the study reported that either less than 25% of the participant population had heart failure or the mean LVEF in the study population was more than 40% at baseline.
We considered that study populations had more severe CHD when: participants who had any or some of the above conditions were included or less than 25% of the participant population had heart failure or the mean LVEF in the study population was more than 40% at baseline. Where studies reported excluding participants based on some of the above conditions, a clinical occupational medical doctor specialised in occupational cardiology (JVD) examined the study to determine the categorisation. We categorised all other studies into a third category of unknown cardiovascular health or CHD severity where we could not determine the severity of CHD from the reported data.
Regarding the planned subgroup analysis and meta‐regression analysis, we did not perform meta‐regression analyses to relate the following study characteristics to their sizes of effect:
study population (age, gender, country),
length of follow‐up,
study date, and
physically demanding occupational groups or alternatively blue‐collar versus white‐collar workers.
Instead, when there were sufficient trials, we stratified all analyses according to length of follow‐up and conducted subgroup analyses to examine how the gender of the study populations, physically demanding occupational groups or CHD severity in the study population influenced the impact of the interventions. We performed meta‐regression analysis considering study date with the Stata package metareg (Stata), for outcomes where five or more trials were available, and ordered the studies in the forest‐plots according to their publication date to visually assess any change in effect over time.
'Summary of findings' tables
We planned to create a 'Summary of findings' table using the following outcomes: return to work, number of participants who were still at work after one year, number of participants still at work after five years, health‐related quality of life, and any adverse effects of interventions, if reported. We expanded the return‐to‐work outcomes to reflect the follow‐up times considered for each of the main comparisons (i.e. up to six months, between six months and one year, number of participants who were still at work after one year, number of participants still at work after five years) as well as the mean time until return to work.
Secondary outcomes
During the review process we encountered a number of studies that reported the number of participants who were still working after five years, so we added working after five years to the list of secondary outcomes.
Missing data
If numerical outcome data such as standard deviations (SDs) or correlation coefficients were missing, and could not be obtained from the study authors within six weeks of request, we calculated them from other available statistics such as P values and t‐scores according to the methods described in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011). In one case, we calculated the SD from the reported range and sample size using a formula for small studies where n ≤ 15 (Hozo 2005). Where only means and sample sizes were available, we imputed SDs from the pooled SD of the other studies in the same comparison group (Furukawa 2006).
Contributions of authors
Conceiving the protocol: UE, UEW
Designing the protocol: UE, UEW, JA, JVD, AS
Co‐ordinating the protocol: UE
Designing search strategies: UE, UEW
Writing the protocol: UE, UEW
Providing general advice on the protocol: JA, JVD, AS
Performing previous work that was the foundation of the current study: UE, UEW, JA, JVD, AS
Screening of Titles and Abstracts: UE, UEW, PH, AF, JH
Screening of Full‐Texts: UE, UEW, JA, PH, JH
Data Extraction: UEW, PH, JH
Quality Assessment (Risk of Bias): PH, JH
Planning the Sub‐Group Analyses: JVD, JH
Meta‐Analyses: JH
Sources of support
Internal sources
-
Institute and Policlinic for Occupational and Social Medicine, Medical Faculty Carl Gustav Carus, Technical University Dresden, Germany.
Support in form of salaries.
-
Federal Institute for Occupational Safety and Health (BAuA), Berlin, Germany.
Support in form of a salary and professional translation of documents.
External sources
No sources of support supplied
Declarations of interest
Janice Hegewald: None known.
Uta Wegewitz: I was employed at the Federal Institute for Risk Assessment (Germany) from 2007 to 2010. Currently I am employed by the Federal Institute for Occupational Safety and Health (BAuA) in Germany.
Ulrike Euler: I received payment for lectures in occupational epidemiology at the Berlin School of Public Health.
Jaap van Dijk: None known.
Jenny Adams: None known.
Alba Fishta: None known.
Philipp Heinrich: None known.
Andreas Seidler: I received payment for lectures in occupational epidemiology at the Berlin School of Public Health.
New
References
References to studies included in this review
Andersen 1981 {published data only}
- Andersen GS, Christiansen P, Madsen S, Schmidt G. Value of regular supervised physical training after acute myocardial infarction. Ugeskrift for Laeger 1981;143(45):2952‐5. [PubMed] [Google Scholar]
Andersson 2010 {published data only}
- Andersson A, Sundel KL, Undén AL, Schenck‐Gustafsson K, Eriksson I. A five‐year rehabilitation programme for younger women after a coronary event reduces the need for hospital care. Scandinavian Journal of Public Health 2010;38(6):566‐73. [DOI: 10.1177/1403494810377125] [DOI] [PubMed] [Google Scholar]
Bengtsson 1983 {published data only}
- Bengtsson K. Rehabilitation after myocardial infarction. A controlled study. Scandinavian Journal of Rehabilitation Medicine 1983;15(1):1‐9. [PubMed] [Google Scholar]
Bertie 1992 {published data only}
- Bertie J, King A, Reed N, Marshall AJ, Ricketts C. Benefits and weaknesses of a cardiac rehabilitation programme. Journal of the Royal College of Physicians of London 1992;26(2):147‐51. [PMC free article] [PubMed] [Google Scholar]
Bethell 1990 {published data only}
- Bethell HJ, Mullee MA. A controlled trial of community based coronary rehabilitation. British Heart Journal 1990;64:370‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Broadbent 2009 {published data only}
- Broadbent E, Ellis CJ, Thomas J, Gamble G, Petrie KJ. Further development of an illness perception intervention for myocardial infarction patients: a randomized controlled trial. Journal of Psychosomatic Research 2009;67(1):17‐23. [DOI] [PubMed] [Google Scholar]
Burgess 1987 {published data only}
- Burgess AW, Lerner DJ, D'Agostino RB, Vokonas PS, Hartman CR, Gaccione P. A randomized control trial of cardiac rehabilitation. Social Science and Medicine 1987;24(4):359‐70. [DOI] [PubMed] [Google Scholar]
Carson 1982 {published data only}
- Carson P, Phillips R, Lloyd M, Tucker H, Neophytou M, Buch NJ, et al. Exercise after myocardial infarction: a controlled trial. Journal of the Royal College of Physicians of London 1982;16(3):147‐51. [PMC free article] [PubMed] [Google Scholar]
Dugmore 1999 {published data only}
- Dugmore LD, Tipson RJ, Phillips MH, Flint EJ, Stentiford NH, Bone MF, et al. Changes in cardiorespiratory fitness, psychological wellbeing, quality of life, and vocational status following a 12 month cardiac exercise rehabilitation programme. Heart 1999;81(4):359‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Engblom 1997 {published data only}
- Engblom E, Hämäläinen H, Rönnemaa T, Vänttinen E. Cardiac rehabilitation and return to work after coronary artery bypass surgery. Quality of Life Research 1994;3(3):207‐13. [DOI] [PubMed] [Google Scholar]
- Engblom E, Korpilahti K, Hamalainen H, Ronnemaa T, Puukka P. Quality of life and return to work 5 years after coronary artery bypass surgery. Long‐term results of cardiac rehabilitation. Journal of Cardiopulmonary Rehabilitation 1997;17(1):29‐36. [DOI] [PubMed] [Google Scholar]
Erdman 1986 {published data only}
- Erdman RA, Duivenvoorden HJ. Psychologic evaluation of a cardiac rehabilitation program: a randomized clinical trial in patients with myocardial infarction. Journal of Cardiac Rehabilitation 1983;3(10):696‐704. [Google Scholar]
- Erdman RA, Duivenvoorden HJ, Verhage F. Predictability of beneficial effects in cardiac rehabilitation: a randomized clinical trial of psychosocial variables. Journal of Cardiopulmonary Rehabilitation 1986;6(6):206‐13. [Google Scholar]
Fielding 1980 {published data only}
- Fielding R. A note on behavioural treatment in the rehabilitation of myocardial infarction patients. British Journal of Social & Clinical Psychology 1980;19(2):157‐61. [DOI: 10.1111/j.2044-8260.1980.tb00942.x] [DOI] [PubMed] [Google Scholar]
Figueiras 2017 {published and unpublished data}
- Figueiras MJ, Maroco J, Monteiro R, Caeiro R, Dias Neto D. Randomized controlled trial of an intervention to change cardiac misconceptions in myocardial infarction patients. Psychology, Health & Medicine 2017;22(3):255‐65. [DOI] [PubMed] [Google Scholar]
Froelicher 1994 {published data only}
- Froelicher ES, Kee LL, Newton KM, Lindskog B, Livingston M. Return to work, sexual activity, and other activities after acute myocardial infarction. Heart & Lung 1994;23(5):423‐35. [PubMed] [Google Scholar]
- Ott CR, Sivarajan ES, Newton KM, Almes MJ, Bruce RA, Bergner M, et al. A controlled randomized study of early cardiac rehabilitation: the Sickness Impact Profile as an assessment tool. Heart & Lung 1983;12(2):162‐70. [PubMed] [Google Scholar]
Geissler 1979 {published data only}
- Geissler W, Gutschker A, Schaller K. Rehabilitation of myocardial infarction patients in the DDR. Medizin und Sport 1979;19(4‐6):143‐6. [Google Scholar]
Haerem 2000 {published data only}
- Haerem JW, Ronning EJ, Leidal R. Home access to hospital discharge information on audiotape reduces sick leave and readmissions in patients with first‐time myocardial infarction. Scandinavian Cardiovascular Journal 2000;34(2):219‐22. [DOI] [PubMed] [Google Scholar]
Hall 2002 {published data only}
- Hall JP, Wiseman VL, King MT, Ross DL, Kovoor P, Zecchin RP, et al. Economic evaluation of a randomised trial of early return to normal activities versus cardiac rehabilitation after acute myocardial infarction. Heart, Lung & Circulation 2002;11(1):10‐18. [DOI] [PubMed] [Google Scholar]
Hämäläinen 1991 {published data only}
- Hämäläinen H, Kallio V, Knuts LR, Arstila M, Aalto Setala L, Harmala V, et al. Community approach in rehabilitation and secondary prevention after acute myocardial infarction: results of a randomized clinical trial. Journal of Cardiopulmonary Rehabilitation 1991;11:221‐6. [Google Scholar]
Hanssen 2009 {published and unpublished data}
- Hanssen TA, Nordrehaug JE, Eide GE, Hanestad BR. Does a telephone follow‐up intervention for patients discharged with acute myocardial infarction have long‐term effects on health‐related quality of life? A randomised controlled trial. Journal of Clinical Nursing 2009;18(9):1334‐45. [DOI] [PubMed] [Google Scholar]
Higgins 2001 {published data only}
- Higgins HC, Hayes RL, McKenna KT. Rehabilitation outcomes following percutaneous coronary interventions (PCI). Patient Education and Counseling 2001;43(3):219‐30. [DOI] [PubMed] [Google Scholar]
Hofman‐Bang 1999 {published data only}
- Hofman‐Bang C, Lisspers J, Nordlander R, Nygren A, Sundin O, Ohman A, et al. Two‐year results of a controlled study of residential rehabilitation for patients treated with percutaneous transluminal coronary angioplasty. A randomized study of a multifactorial programme. European Heart Journal 1999;20(20):1465‐74. [DOI] [PubMed] [Google Scholar]
- Lisspers J, Sundin O, Hofman‐Bang C, Nordlander R, Nygren A, Ryden L, et al. Behavioral effects of a comprehensive, multifactorial program for lifestyle change after percutaneous transluminal coronary angioplasty: a prospective, randomized controlled study. Journal of Psychosomatic Research 1999;46(2):143‐54. [DOI] [PubMed] [Google Scholar]
Holmbäck 1994 {published data only}
- Holmbäck AM, Sawe U, Fagher B. Training after myocardial infarction: lack of long‐term effects on physical capacity and psychological variables. Archives of Physical Medicine and Rehabilitation 1994;75(5):551‐4. [PubMed] [Google Scholar]
Horlick 1984 {published data only}
- Horlick L, Cameron R, Firor W. The effects of education and group discussion in the post myocardial infarction patient. Journal of Psychosomatic Research 1984;28(6):485‐92. [DOI] [PubMed] [Google Scholar]
Lidell 1996 {published data only}
- Fridlund B, Pihlgren C, Wannestig LB. A supportive–educative caring rehabilitation programme; improvements of physical health after myocardial infarction. Journal of Clinical Nursing 1992;1(3):141‐6. [Google Scholar]
- Lidell E, Fridlund B. Long‐term effects of a comprehensive rehabilitation programme after myocardial infarction. Scandinavian Journal of Caring Sciences 1996;10(2):67‐74. [DOI] [PubMed] [Google Scholar]
Maeder 1977 {published data only}
- Bloch A, Maeder JP, Haissly JC, Felix J, Blackburn H. Early mobilization after myocardial infarction. A controlled study. American Journal of Cardiology 1974;34(2):152‐7. [DOI] [PubMed] [Google Scholar]
- Maeder JP, Bloch A. Early mobilization in the acute stage of myocardial infarction long term results]. Schweizerische Medizinische Wochenschrift 1977;107(16):566‐9. [PubMed] [Google Scholar]
Marra 1985 {published data only}
- Marra S, Paolillo V, Spadaccini F, Angelino PF. Long‐term follow‐up after a controlled randomized post‐myocardial infarction rehabilitation programme: effects on morbidity and mortality. European Heart Journal 1985;6(8):656‐63. [DOI] [PubMed] [Google Scholar]
- Spadaccini F, Marra S, Paolillo V, Bevilacqua R, Boncompagni F, Longo C, et al. Controlled study of the effects of physical rehabilitation after myocardial infarct. Preliminary results. Minerva Cardioangiologica 1979;27(5):305‐20. [PubMed] [Google Scholar]
Oldridge 1991 {published data only}
- Oldridge N, Guyatt G, Jones N, Crowe J, Singer J, Feeny D, et al. Effects on quality of life with comprehensive rehabilitation after acute myocardial infarction. American Journal of Cardiology 1991;67(13):1084‐9. [DOI] [PubMed] [Google Scholar]
Petrie 2002 {published data only}
- Petrie KJ, Cameron L, Ellis CJ, Buick D, Weinman J. Changing illness perceptions after myocardial infarction: an early intervention randomized controlled trial. Psychosomatic Medicine 2002;64(4):580‐6. [DOI] [PubMed] [Google Scholar]
Pfund 2001 {published data only}
- Pfund A, Putz J, Wendland G, Theisson M, Aydin U, Hinzpeter B, et al. Coronary intervention and occupational rehabilitation‐‐a prospective, randomized intervention study. Zeitschrift fur Kardiologie 2001;90(9):655‐60. [DOI] [PubMed] [Google Scholar]
Picard 1989 {published data only}
- Dennis C, Houston‐Miller N, Schwartz RG, Ahn DK, Kraemer HC, Gossard D, et al. Early return to work after uncomplicated myocardial infarction. Results of a randomized trial. JAMA. 1988;260(2):214‐20. [PubMed] [Google Scholar]
- Picard MH, Dennis C, Schwartz RG, Ahn DK, Kraemer HC, Berger WE, et al. Cost‐benefit analysis of early return to work after uncomplicated acute myocardial infarction. American Journal of Cardiology 1989;63(18):1308‐14. [DOI] [PubMed] [Google Scholar]
Pilote 1992 {published data only}
- Pilote L, Thomas RJ, Dennis C, Goins P, Houston‐Miller N, Kraemer H, et al. Return to work after uncomplicated myocardial infarction: a trial of practice guidelines in the community. Annals of Internal Medicine 1992;117(5):383‐9. [DOI] [PubMed] [Google Scholar]
Pozen 1977 {published data only}
- Pozen MW, Stechmiller JA, Harris W. A nurse rehabilitator's impact on patients with myocardial infarction. Medical Care 1977;15(10):830‐837. [DOI] [PubMed] [Google Scholar]
PRECOR 1991 {published data only}
- P.RE.COR. Group. Comparison of a rehabilitation programme, a counselling programme and usual care after an acute myocardial infarction: results of a long‐term randomized trial. P.RE.COR. Group. European Heart Journal 1991;12(5):612‐16. [DOI] [PubMed] [Google Scholar]
Rahe 1979 {published data only}
- Rahe RH, Ward HW, Hayes V. Brief group therapy in myocardial infarction rehabilitation: three‐ to four‐year follow‐up of a controlled trial. Psychosomatic Medicine 1979;41(3):229‐42. [DOI] [PubMed] [Google Scholar]
Rivas 1988 {published data only}
- Rivas EE, Ponce dLA, Sin CC, Remirez dEA, Dueñas HA, Hernández CA. The influence of cardiac rehabilitation on reentering work after myocardial infarction. Revista Cubana de Cardiología y Cirugía Cardiovascular 1988;2:104‐117. [Google Scholar]
Stern 1983 {published data only}
- Stern MJ, Gorman PA, Kaslow L. The group counseling v exercise therapy study. A controlled intervention with subjects following myocardial infarction. Archives of Internal Medicine 1983;143(9):1719‐25. [PubMed] [Google Scholar]
Vermeulen 1988 {published data only}
- Vermeulen A. Ventricular ectopic activity during exercise testing in patients with myocardial infarction. The relation to severity of coronary artery disease and return to work. European Heart Journal 1988;9 Suppl L:95‐102. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Heyboer C, Lie KI. A comparative study of the effect of a rehabilitation program in patients with myocardial infarct. Nederlands Tijdschrift voor Geneeskunde 1978;122(45):1737‐41. [PubMed] [Google Scholar]
WHO 1983 {published data only}
- World Health Organization. Rehabilitation and comprehensive secondary prevention after acute myocardial infarction: report on a study. Rehabilitation and comprehensive secondary prevention after acute myocardial infarction: report on a study. Copenhagen: World Health Organization, 1983. [Google Scholar]
Worcester 1993 {published data only}
- Goble AJ, Hare DL, Macdonald PS, Foster EM, Bullen JF, Worcester MC. The effect of early exercise training on return to work after transmural acute myocardial infarction. Australian and New Zealand Journal of Medicine 1986;16:586. [Google Scholar]
- Worcester MC, Hare DL, Oliver RG, Reid MA, Goble AJ. Early programmes of high and low intensity exercise and quality of life after acute myocardial infarction. BMJ 1993;307(6914):1244‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ahlmark 1979 {published data only}
- Ahlmark G, Ahlberg G, Saetre H, Haglund I, Korsgren M. A controlled study of early discharge after uncomplicated myocardial infarction. Acta Medica Scandinavica 1979;206:87‐91. [DOI] [PubMed] [Google Scholar]
Al'khimovich 1990 {published data only}
- Al'khimovich VM, Bevzeliuk AA, Golubev VG, Laziuk DG, Nizovtsova LA, Rudina MD, et al. Possibilities of improving medical and socioeconomic effectiveness of the treatment of patients with myocardial infarct by physical training during posthospital stage of rehabilitation. Kardiologiia 1990;30(9):57‐61. [PubMed] [Google Scholar]
Ali 2018 {published data only}
- Ali S, Araujo Pio CS, Chaves GS, Britto R, Cribbie R, Grace SL. Psychosocial well‐being over the two years following cardiac rehabilitation initiation & association with heart‐health behaviors. General Hospital Psychiatry 2018;52:48‐57. [DOI] [PubMed] [Google Scholar]
Aronov 1991 {published data only}
- Aronov DM, Smirnov EI. Occupational therapy in the rehabilitation of myocardial infarct patients in the convalescence phase. Terapevticheskii Arkhiv 1991;63(9):72‐6. [PubMed] [Google Scholar]
Aronov 2006 {published data only}
- Aronov DM, Krasnitskii VB, Bubnova MG, Posdniakov IM, Ioseliani DV, Shchegol'kov AN, et al. Exercise in outpatient complex rehabilitation and secondary prophylaxis in patients with ischemic heart disease after acute coronary events (a cooperative trial in Russia). Terapevticheskii Arkhiv 2006;78(9):33‐8. [PubMed] [Google Scholar]
Bar 1992 {published data only}
- Bar FW, Hoppener P, Diederiks J, Vonken H, Bekkers J, Hoofd WV, et al. Cardiac rehabilitation contributes to the restoration of leisure and social activities after myocardial infarction. Journal of Cardiopulmonary Rehabilitation 1992;12:117‐25. [Google Scholar]
Ben‐Ari 1986 {published data only}
- Ben‐Ari E, Kellermann JJ, Fisman EZ. Benefits of long‐term physical training in patients after coronary artery bypass grafting ‐ A 58‐month follow‐up and comparison with a nontrained group. Journal of Cardiopulmonary Rehabilitation 1986;6:165‐70. [Google Scholar]
Bjarnason‐Wehrens 1999 {published data only}
- Bjarnason‐Wehrens B, Predel HG, Graf C, Rost R. Ambulatory cardiac phase II rehabilitation‐‐"the Cologne model"‐‐including 3‐year‐outcome after termination of rehabilitation. Herz 1999;24(Suppl 1):9‐23. [DOI] [PubMed] [Google Scholar]
Boszormenyi 1984 {published data only}
- Boszormenyi E, Ludwig G, Berenyi I, Molnar J, Mikes L, Ittzes L. Comparison of institutional and ambulatory methods of rehabilitation in myocardial infarct. Cor et Vasa 1984;26(2):105‐13. [PubMed] [Google Scholar]
Boulay 1983 {published data only}
- Boulay F, David P, Danchin N, Girard C, Bourassa MG. Rehabilitation and return to work of patients after aortocoronary bypass. Archives des Maladies du Coeur et des Vaisseaux 1983;76:1293‐301. [PubMed] [Google Scholar]
- Boulay FM, David PP, Bourassa MG. Strategies for improving the work status of patients after coronary artery bypass surgery. Circulation 1982;66:III43‐9. [PubMed] [Google Scholar]
Bounhoure 2014 {published data only}
- Bounhoure JP, Bousquet M. Cardiac rehabilitation: physiologic basis, beneficial effects and contraindications. Bulletin de l'Academie Nationale de Medecine 2014;198(3):491‐9. [PubMed] [Google Scholar]
Buchwalsky 2002 {published data only}
- Buchwalsky G, Buchwalsky R, Held K. Long‐term effects of rehabilitation of an outpatient "heart group". A case control study. Zeitschrift fur Kardiologie 2002;91(2):139‐46. [DOI] [PubMed] [Google Scholar]
Burns 2007 {published data only}
- Burns JW, Evon D. Common and specific process factors in cardiac rehabilitation: independent and interactive effects of the working alliance and self‐efficacy. Health Psychology 2007;26(6):684‐92. [DOI] [PubMed] [Google Scholar]
Carlsson 1998 {published data only}
- Carlsson R. Serum cholesterol, lifestyle, working capacity and quality of life in patients with coronary artery disease. Experiences from a hospital‐based secondary prevention programme. Scandinavian Cardiovascular Journal, Supplement 1998;50:1‐20. [DOI] [PubMed] [Google Scholar]
Cay 1981 {published data only}
- Cay EL, Philip AE, Stuckey NA. Ten years in cardiac rehabilitation. Psychiatria Fennica 1981;SUPPL:19‐31. [Google Scholar]
Christensen 2017 {published data only}
- Christensen AV, Ohlers AA, Zwisler AD, Svendsen JH, Berg SK. Employment status and sick leave after first‐time implantable cardioverter defibrillator implantation: results from the COPE‐ICD Trial. Journal of Cardiovascular Nursing 2017;32(5):448‐54. [DOI] [PubMed] [Google Scholar]
Danchin 1988 {published data only}
- Danchin N, Goepfert PC. Exercise training, cardiac rehabilitation and return to work in patients with coronary artery disease. European Heart Journal 1988;9(Suppl M):43‐6. [DOI] [PubMed] [Google Scholar]
David 2011 {published data only}
- David MA, Ioseliani DG, Krasnitsky VB, Sechenova EV, Bubnova MG. The use of a short‐term program of physical training for patients with IHD after percutaneous coronary interventions in the program of rehabilitation and secondary prevention. European Journal of Cardiovascular Prevention and Rehabilitation 2011;18(1):S15. [Google Scholar]
Davies 1991 {published data only}
- Davies SW, Greig CA. A controlled trial of community based coronary rehabilitation. British Heart Journal 1991;66(1):114‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dimopoulos 1999 {published data only}
- Dimopoulos VS, Antonakoudis CG, Tsoukas AS, Zouras CG, Raikos NS, Christakos SG. Significance of cardiac rehabilitation program participation on physical performance, return to work and prognosis of patients with acute myocardial infarction. Hellenic Journal of Cardiology 1999;40:311‐8. [Google Scholar]
Dominiak 2011 {published data only}
- Dominiak MT, Krawczyk M, Kurpesa M, Rechcinski T, Kasprzak JD. Early rehabilitation in acute coronary syndrome patients improves self‐assessing quality of life but not depression status. European Heart Journal 2011;32:388‐9. [Google Scholar]
Dorn 1999 {published data only}
- Dorn J, Naughton J, Imamura D, Trevisan M. Results of a multicenter randomized clinical trial of exercise and long‐term survival in myocardial infarction patients: the National Exercise and Heart Disease Project (NEHDP). Circulation 1999;100(17):1764‐9. [DOI] [PubMed] [Google Scholar]
Dorn 2001 {published data only}
- Dorn J, Naughton J, Imamura D, Trevisan M. Correlates of compliance in a randomized exercise trial in myocardial infarction patients. Medicine and Science in Sports and Exercise 2001;33(7):1081‐9. [DOI] [PubMed] [Google Scholar]
Dumont 1999 {published data only}
- Dumont S, Jobin J, Deshaies G, Trudel L, Chantale M. Rehabilitation and the socio‐occupational reintegration of workers who have had a myocardial infarct: a pilot study. Canadian Journal of Cardiology 1999;15:453‐61. [PubMed] [Google Scholar]
Espinosa 2004 {published data only}
- Espinosa Caliani S, Bravo Navas JC, Gomez‐Doblas JJ, Collantes Rivera R, Gonzalez Jimenez B, Martinez Lao M, et al. Postmyocardial infarction cardiac rehabilitation in low risk patients. Results with a coordinated program of cardiological and primary care. Revista Espanola de Cardiologia 2004;57(1):53‐9. [PubMed] [Google Scholar]
Fattirolli 1998 {published data only}
- Fattirolli F, Cartei A, Burgisser C, Mottino G, Lungo F, Oldridge N, et al. Aims, design and enrollment rate of the Cardiac Rehabilitation in Advanced Age (CR‐AGE) randomized, controlled trial. Aging (Milan, Italy) 1998;10(5):368‐76. [DOI] [PubMed] [Google Scholar]
Ferrario 2010 {published data only}
- Ferrario MM, Borchini R. The contribution of occupational medicine in cardiovascular prevention and return to work of cardiac patients. Giornale Italiano di Cardiologia 2010;11(5 Suppl 3):53S‐5S. [PubMed] [Google Scholar]
Follick 1988 {published data only}
- Follick MJ, Gorkin L, Smith TW, Capone RJ, Visco J, Stablein D. Quality of life post‐myocardial infarction: effects of a transtelephonic coronary intervention system. Health Psychology 1988;7(2):169‐82. [DOI] [PubMed] [Google Scholar]
Foster 1984 {published data only}
- Foster C, Pollock ML, Anholm JD, Squires RW, Ward A, Dymond DS, et al. Work capacity and left ventricular function during rehabilitation after myocardial revascularization surgery. Circulation 1984;69(4):748‐55. [DOI] [PubMed] [Google Scholar]
Fujita 1983 {published data only}
- Fujita Y, Hasegawa T, Niitani H. Study of the rehabilitation, prognosis and capacity to resume work of patients with myocardial infarction‐‐comparison of 4‐week and 8‐week rehabilitation programs. Japanese Circulation Journal 1983;47(6):696‐702. [DOI] [PubMed] [Google Scholar]
Gallagher 2003 {published data only}
- Gallagher R, McKinley S, Dracup K. Effects of a telephone counseling intervention on psychosocial adjustment in women following a cardiac event. Heart & Lung 2003;32(2):79‐87. [DOI] [PubMed] [Google Scholar]
Garrity 1973 {published data only}
- Garrity TF. Vocational adjustment after first myocardial infarction; comparative assessment of several variables suggested in the literature. Social Science and Medicine 1973;7(9):705‐17. [DOI] [PubMed] [Google Scholar]
Giannuzzi 1992 {published data only}
- Giannuzzi P, Temporelli PL, Tavazzi L, Corra U, Gattone M, Imparato A, et al. EAMI‐‐exercise training in anterior myocardial infarction: an ongoing multicenter randomized study. Preliminary results on left ventricular function and remodeling. The EAMI Study Group. Chest 1992;101(5 Suppl):315S‐21S. [PubMed] [Google Scholar]
Giannuzzi 1993 {published data only}
- Giannuzzi P, Tavazzi L, Temporelli PL, Corra U, Imparato A, Gattone M, et al. Long‐term physical training and left ventricular remodeling after anterior myocardial infarction: results of the Exercise in Anterior Myocardial Infarction (EAMI) trial. EAMI Study Group. Journal of the American College of Cardiology 1993;22(7):1821‐9. [DOI] [PubMed] [Google Scholar]
Giannuzzi 1997 {published data only}
- Giannuzzi P, Temporelli PL, Corra U, Gattone M, Giordano A, Tavazzi L. Attenuation of unfavorable remodeling by exercise training in postinfarction patients with left ventricular dysfunction: results of the Exercise in Left Ventricular Dysfunction (ELVD) trial. Circulation 1997;96(6):1790‐7. [DOI] [PubMed] [Google Scholar]
Goeminne 1989 {published data only}
- Goeminne HM, Faes K, Poelemans KM, Mersch C, Brutsaert DL. The effect of a cardiac rehabilitation program on return to work. Belgisch Archief / Archives Belges 1989;47:70‐2. [PubMed] [Google Scholar]
Grief 1995 {published data only}
- Grief H, Kreitler S, Kaplinsky E, Behar S. The effects of short‐term exercise on the cognitive orientation for health and adjustment in myocardial infarction patients. Behavioral Medicine 1995;21(2):75‐85. [DOI] [PubMed] [Google Scholar]
Griffo 1983 {published data only}
- Griffo R, Vecchio C, Cobelli F. Rehabilitation of women with recent myocardial infarction. Results and comparison with men. Archives des Maladies du Coeur et des Vaisseaux 1983;76(3):285‐93. [PubMed] [Google Scholar]
Groden 1967 {published data only}
- Groden BM. Return to work after myocardial infarction. Scottish Medical Journal 1967;12(9):297‐301. [DOI] [PubMed] [Google Scholar]
Gutschker 1977 {published data only}
- Gutschker A, Schaller K, Geissler W. Results of physical conditioning in patients with acute myocardial infarction over 65 years of age. Cardiology (Switzerland) 1977;62(2):135‐6. [Google Scholar]
Gysan 1999 {published data only}
- Gysan DB, Heinzler R, Schmidt K. Outcome of a four‐week ambulatory cardiac rehabilitation (phase II) on cardiovascular risk factors, physical fitness and occupational reintegration in patients after myocardial infarct, dilatation treatment and heart operation. Herz 1999;24(Suppl 1):44‐56. [DOI] [PubMed] [Google Scholar]
Gysan 2004 {published data only}
- Gysan DB, Latsch J, Bjarnason‐Wehrens B, Albus C, Falkowski G, Herold G, et al. The PreFord Study. A prospective cohort study to evaluate the risk of a cardiovascular event (overall‐collective) as well as a prospective, randomized, controlled, multicentre clinical intervention study (high‐risk‐collective) on primary prevention of cardiovascular diseases in the Ford Motor Company employees in Germany. Zeitschrift fur Kardiologie 2004;93(2):131‐6. [DOI] [PubMed] [Google Scholar]
Hakkila 1965 {published data only}
- Hakkila J, Rinne H. Rehabilitation of patients with myocardial infarction and patients after heart surgery. Finnish Institute of Occupational Health. Helsinki, 1965, issue No. 30:1‐9.
- Rinne H, Hakkila J. Vocational and Social Viewpoints on the Evaluation of Heart Patients for Rehabilitation. Finnish Institute of Occupational Health. Helsinki, 1965, issue No.31:1‐16.
Hare 1983 {published data only}
- Hare DL, Worcester M. The effects of early exercise training on outcome one year after myocardial infarction [abstract]. Australian and New Zealand Journal of Medicine 1983;13:413. [Google Scholar]
Haussler 1997 {published data only}
- Haussler B, Keck M. Improvement in occupational rehabilitation of myocardial infarct patients‐‐results of a model study in Rhineland‐Pfalz. Rehabilitation (Stuttg) 1997;36:106‐10. [PubMed] [Google Scholar]
- Keck M, Haussler B, Jacob M. Optimizing occupational reintegration/model trial for further development of inpatient rehabilitation. Zeitschrift fur Gastroenterologie 1996;34(Suppl 2):89‐92. [PubMed] [Google Scholar]
Havelkova 2010 {published data only}
- Havelkova A, Mifkova L, Pochmonova J, Anbais FH, Erajhi AA, Bartlova B, et al. Cardiovascular rehabilitation programme in men after acute myocardial infarction. Scripta Medica Facultatis Medicae Universitatis Brunensis Masarykianae 2010;83(2):92‐7. [Google Scholar]
Hedback 1993 {published data only}
- Hedback B, Perk J. 5‐year results of a comprehensive rehabilitation programme after myocardial infarction. European Heart Journal 1987;8(3):234‐42. [DOI] [PubMed] [Google Scholar]
- Hedback B, Perk J, Wodlin P. Long‐term reduction of cardiac mortality after myocardial infarction: 10‐year results of a comprehensive rehabilitation programme. European Heart Journal 1993;14:831‐5. [DOI] [PubMed] [Google Scholar]
Heller 1990 {published data only}
- Heller R, Blumchen G, Zurmann J, Jette M, Bannies H, Meiser M. Four‐week training of patients with large anterior wall infarct: comparison with a randomized untrained control group. Zeitschrift fur Kardiologie 1990;79(12):831‐6. [PubMed] [Google Scholar]
Heller 1993 {published data only}
- Heller RF, Knapp JC, Valenti LA, Dobson AJ. Secondary prevention after acute myocardial infarction. American Journal of Cardiology 1993;72(11):759‐62. [DOI] [PubMed] [Google Scholar]
Henritze 1989 {published data only}
- Henritze J, Brammell HL. Phase II cardiac wellness at the Adolph Coors Company. American Journal of Health Promotion, Vol.4, No.1, pages 25‐31, 6 references 1989. [DOI] [PubMed] [Google Scholar]
Hertzeanu 1993 {published data only}
- Hertzeanu HL, Shemesh J, Aron LA, Aron AL, Peleg E, Rosenthal T, et al. Ventricular arrhythmias in rehabilitated and nonrehabilitated post‐myocardial infarction patients with left ventricular dysfunction. American Journal of Cardiology 1993;71(1):24‐7. [DOI] [PubMed] [Google Scholar]
Huber 2014 {published data only}
- Huber D, Hoerschelmann N, Hoberg E, Karoff J, Karoff M, Kittel J. Vocational inpatient and post‐treatment proposals in cardiac rehabilitation patients (BERUNA): results of a randomized controlled trial. Rehabilitation (Stuttg) 2014;53(6):362‐8. [DOI] [PubMed] [Google Scholar]
Hui 2006 {published data only}
- Hui PN, Wan M, Chan WK, Yung PM. An evaluation of two behavioral rehabilitation programs, qigong versus progressive relaxation, in improving the quality of life in cardiac patients. Journal of Alternative and Complementary Medicine (New York, N.Y.) 2006;12(4):373‐8. [DOI] [PubMed] [Google Scholar]
Iacovino 1997 {published data only}
- Iacovino Vivian. A randomized comparison between a goal‐setting and a videotape and discussion intervention to improve return to work and quality of life among cardiac patients [Ph.D.]. Ottawa, Canada: University of Ottawa, 1997. [Google Scholar]
Isaaz 2010 {published data only}
- Isaaz K, Coudrot M, Sabry MH, Cerisier A, Lamaud M, Robin C, et al. Return to work after acute ST‐segment elevation myocardial infarction in the modern era of reperfusion by direct percutaneous coronary intervention. Archives of Cardiovascular Diseases 2010;103(5):310‐6. [DOI] [PubMed] [Google Scholar]
Jette 1991 {published data only}
- Jette M, Heller R, Landry F, Blumchen G. Randomized 4‐week exercise program in patients with impaired left ventricular function. Circulation 1991;84(4):1561‐7. [DOI] [PubMed] [Google Scholar]
Johnson 2014 {published data only}
- Johnson DA, Sacrinty MT, Gomadam PS, Mehta HJ, Brady MM, Douglas CJ, et al. Effect of early enrollment on outcomes in cardiac rehabilitation. American Journal of Cardiology 2014;114(12):1908‐11. [DOI] [PubMed] [Google Scholar]
Kadda 2015 {published data only}
- Kadda O, Kotanidou A, Manginas A, Stavridis G, Nanas S, Panagiotakos DB. Lifestyle intervention and one‐year prognosis of patients following open heart surgery: a randomised clinical trial. Journal of Clinical Nursing 2015;24(11‐12):1611‐21. [DOI] [PubMed] [Google Scholar]
Kagan‐Ponomarev 1994 {published data only}
- Kagan‐Ponomarev MY, Rubanovich AI, Chikvashvili DI, Vasyuk YA, Timonichev NV, Zakin AM, et al. Early discharge of patients after hospitalisation because of uncomplicated myocardial infarction. Cooperative study. Kardiologiya 1994;34:8‐14. [Google Scholar]
Kallio 1979 {published data only}
- Kallio V, Hämäläinen H, Hakkila J, Luurila OJ. Reduction in sudden deaths by a multifactorial intervention programme after acute myocardial infarction. Lancet 1979;2:1091‐4. [DOI] [PubMed] [Google Scholar]
Kamath 2012 {published data only}
- Kamath SV, Burt V, Goolsby M, Thomas L. A comparison of cardiac rehabilitation outcomes based on exercise sessions attended. Journal of Cardiopulmonary Rehabilitation and Prevention 2012;32(4):236. [Google Scholar]
Karoff 2000b {published data only}
- Karoff M. Individual outcome‐oriented cardiologic rehabilitation treatment. MMW Fortschritte der Medizin 2000;20(142):179‐82. [PubMed] [Google Scholar]
- Karoff M. Rehabilitation with the "Ennepetal model". Herz 1999;24(Suppl 1):67‐72. [DOI] [PubMed] [Google Scholar]
- Karoff M, Roseler S. Flexibility of cardiologic rehabilitation exemplified by the Konigsfeld (Ennepetal) model. Versicherungsmedizin 1997;49(1):14‐9. [PubMed] [Google Scholar]
- Karoff M, Roseler S, Lorenz C, Kittel J. Intensified after‐care‐‐a method for improving occupational reintegration after myocardial infarct and/or bypass operation. Zeitschrift fur Kardiologie 2000;89:423‐33. [DOI] [PubMed] [Google Scholar]
- Roseler S, Schwartz FW, Karoff M. Evaluation of the effectiveness of an ambulatory after‐care rehabilitation program. Gesundheitswesen (Bundesverband der Arzte des Offentlichen Gesundheitsdienstes (Germany)) 1997;59(4):236‐41. [PubMed] [Google Scholar]
Kelbaek 1981 {published data only}
- Kelbaek H, Eskildsen P, Hansen PF, Godtfredsen J. Physical training after acute myocardial infarction. Ugeskrift for Laeger 1981;143(45):2949‐51. [PubMed] [Google Scholar]
Kellermann 1968 {published data only}
- Kellermann JJ, Modan B, Levy M, Feldman S, Kariv I. Return to work after myocardial infarction. Comparative study of rehabilitated and nonrehabilitated patients. Geriatrics 1968;23(3):151‐6. [PubMed] [Google Scholar]
Kellermann 1975 {published data only}
- Kellermann JJ. Rehabilitation of patients with coronary heart disease. Progress in Cardiovascular Diseases 1975;17(4):303‐28. [DOI] [PubMed] [Google Scholar]
Kittel 2008 {published data only}
- Kittel J, Karoff M. Improvement of worklife participation through vocationally oriented cardiac rehabilitation? Findings of a randomized control group study. Rehabilitation (Stuttg) 2008;47:14‐22. [DOI] [PubMed] [Google Scholar]
Kokutsov 1990 {published data only}
- Kokutsov A, Petev L. The effect of combined rehabilitation on the physical work capacity of patients who have had a myocardial infarct. Vutreshni Bolesti 1990;29:41‐5. [PubMed] [Google Scholar]
Korzeniowska‐Kubacka 2004 {published data only}
- Korzeniowska‐Kubacka I, Piotrowicz R. Influence of exercise training on physical capacity, lipid profile and return to work of women after myocardial infarction. Folia Cardiologica 2004;11:719‐25. [Google Scholar]
Korzeniowska‐Kubacka 2015 {published data only}
- Korzeniowska‐Kubacka I, Bilinska M, Dobraszkiewicz‐Wasilewska B, Piotrowicz R. Hybrid model of cardiac rehabilitation in men and women after myocardial infarction. Cardiology Journal 2015;22(2):212‐8. [DOI] [PubMed] [Google Scholar]
Kovoor 2006 {published data only}
- Kovoor P, Lee AK, Carrozzi F, Wiseman V, Byth K, Zecchin R, et al. Return to full normal activities including work at two weeks after acute myocardial infarction. American Journal of Cardiology 2006;97(7):952‐8. [DOI] [PubMed] [Google Scholar]
Krasemann 1979 {published data only}
- Krasemann EO. Organized myocardial infarct rehabilitation. Fortschritte der Medizin 1979;97(43):1987‐90. [PubMed] [Google Scholar]
Kushnir 1976 {published data only}
- Kushnir B, Fox KM, Tomlinson IW, Aber Clive P. The effect of a pre‐discharge consultation on the resumption of work, sexual activity, and driving following acute myocardial infarction. Scandinavian Journal of Rehabilitation Medicine 1976;8:155‐9. [Google Scholar]
Laaksovirta 1985 {published data only}
- Laaksovirta S, Kallio V. Implications of social factors in cardiac rehabilitation. Public Health in Europe 1985;NO. 24:55‐71. [Google Scholar]
Lamberti 2016 {published data only}
- Lamberti M, Ratti G, Gerardi D, Capogrosso C, Ricciardi G, Fulgione C, et al. Work‐related outcome after acute coronary syndrome: implications of complex cardiac rehabilitation in occupational medicine. International Journal of Occupational Medicine and Environmental Health 2016;29(4):649‐57. [DOI] [PubMed] [Google Scholar]
Lamm 1982 {published data only}
- Lamm G, Denolin H, Dorossiev D, Pisa Z. Rehabilitation and secondary prevention of patients after acute myocardial infarction. WHO collaborative study. Advances in Cardiology 1982;31:107‐11. [DOI] [PubMed] [Google Scholar]
Langosch 1982 {published data only}
- Langosch W, Seer P, Brodner G, Kallinke D, Kulick B, Heim F. Behavior therapy with coronary heart disease patients: results of a comparative study. Journal of Psychosomatic Research 1982;26(5):475‐84. [DOI] [PubMed] [Google Scholar]
Lautamaki 2017 {published data only}
- Lautamaki A, Gunn JM, Airaksinen KE, Biancari F, Kajander OA, Anttila V, et al. Permanent work disability in patients ≤50 years old after percutaneous coronary intervention and coronary artery bypass grafting (the CRAGS study). European Heart Journal ‐ Quality of Care and Clinical Outcomes 2017;3(2):101‐6. [DOI] [PubMed] [Google Scholar]
Lear 2002 {published data only}
- Lear SA, Ignaszewski A, Linden W, Brozic A, Kiess M, Spinelli JJ, et al. A randomized controlled trial of an extensive lifestyle management intervention (ELMI) following cardiac rehabilitation: study design and baseline data. Current Controlled Trials in Cardiovascular Medicine 2002;3(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2004 {published data only}
- Li H, Guo L, Sun JZ, Feng JZ, Wang P, Wu GL, et al. Effect of exercise therapy on the quality of life in patients after successful percutaneous transluminal coronary angioplasty. Chinese Journal of Clinical Rehabilitation 2004;8(9):1601‐3. [Google Scholar]
Liang 1988 {published data only}
- Liang MT, Garcia MD, McAllister L. Effects of an exercise and stress management program on cardiac patients' psychosocial and vocational status: preliminary study. Journal of the American Osteopathic Association 1988;88(10):1209‐18. [PubMed] [Google Scholar]
Lie 2009 {published data only}
- Lie I, Arnesen H, Sandvik L, Hamilton G, Bunch EH. Health‐related quality of life after coronary artery bypass grafting. The impact of a randomised controlled home‐based intervention program. Quality of Life Research 2009;18(2):201‐7. [DOI] [PubMed] [Google Scholar]
Lisspers 1999 {published data only}
- Lisspers J, Hofman‐Bang C, Nordlander R, Ryden L, Sundin O, Ohman A, et al. Multifactorial evaluation of a program for lifestyle behavior change in rehabilitation and secondary prevention of coronary artery disease. Scandinavian Cardiovascular Journal 1999;33(1):9‐16. [DOI] [PubMed] [Google Scholar]
Liu 1997 {published data only}
- Liu LZ, Zhang ZJ. The ability on return to work and the effect of prevention and cure in coronary heart disease patients by training of exercise prescription. Chinese Journal of Gerontology 1997;17:12‐3. [Google Scholar]
Maeland 1987 {published data only}
- Maeland JG, Havik OE. The effects of an in‐hospital educational programme for myocardial infarction patients. Scandinavian Journal of Rehabilitation Medicine 1987;19(2):57‐65. [PubMed] [Google Scholar]
Maeland 1989 {published data only}
- Maeland JG, Havik OE. After the myocardial infarction. A medical and psychological study with special emphasis on perceived illness. Scandinavian Journal of Rehabilitation Medicine. Supplement 1989;22:1‐87. [PubMed] [Google Scholar]
Marchionni 1994 {published data only}
- Marchionni N, Fattirolli F, Valoti P, Baldasseroni L, Burgisser C, Ferrucci L, et al. Improved exercise tolerance by cardiac rehabilitation after myocardial infarction in the elderly: results of a preliminary, controlled study. Aging (Milan, Italy) 1994;6(3):175‐80. [DOI] [PubMed] [Google Scholar]
Maroto 1996 {published data only}
- Maroto Montero JM, Pable Zarzosa C, Morales Duran MD, Artigao Ramirez R. Heart rehabilitation. Cost‐effectiveness analysis. Revista Espanola de Cardiologia 1996;49(10):753‐8. [PubMed] [Google Scholar]
Mayou 1981 {published data only}
- Mayou R, MacMahon D, Sleight P, Florencio MJ. Early rehabilitation after myocardial infarction. Lancet 1981;2:1399‐402. [DOI] [PubMed] [Google Scholar]
Miller 1988 {published data only}
- Miller P, Wikoff R, McMahon M, Garrett MJ, Ringel K. Influence of a nursing intervention on regimen adherence and societal adjustments postmyocardial infarction. Nurse Researcher 1988;37(5):297‐302. [PubMed] [Google Scholar]
Mirmohammadi 2014 {published data only}
- Mirmohammadi SJ, Sadr‐Bafghi SM, Mehrparvar AH, Gharavi M, Davari MH, Bahaloo M, et al. Evaluation of the return to work and its duration after myocardial infarction. ARYA Atherosclerosis 2014;10(3):137‐40. [PMC free article] [PubMed] [Google Scholar]
Mital 1999 {published data only}
- Mital A, Shrey DE, Govindaraju M. Job simulated phase 2 cardiac rehabilitation training program, part B: effects of training. International Journal of Industrial Ergonomics 1999. [Google Scholar]
- Mital A, Shrey DE, Govindaraju M, Broderick TM, Colon‐Brown K, Gustin BW. Job‐simulated phase II cardiac rehabilitation training program. International Journal of Industrial Ergonomics 1999;24:531‐43. [Google Scholar]
Mital 2000 {published data only}
- Mital A, Shrey DE, Govindaraju M, Broderick TM, Colon‐Brown K, Gustin BW. Accelerating the return to work (RTW) chances of coronary heart disease (CHD) patients: part 1‐‐development and validation of a training programme. Disability and Rehabilitation 2000;22:604‐20. [DOI] [PubMed] [Google Scholar]
Mulcahy 1971 {published data only}
- Mulcahy R, Hickey N. The rehabilitation of patients with coronary heart disease: a comparison of the return to work experience of National Health Insurance patients with coronary heart disease and of a group of coronary patients subjected to a specific rehabilitation programme. Journal of the Irish Medical Association 1971;60(422):541‐5. [PubMed] [Google Scholar]
Nelson 1994 {published data only}
- Nelson DV, Baer PE, Cleveland SE, Revel KF, Montero AC. Six‐month follow‐up of stress management training versus cardiac education during hospitalization for acute myocardial infarction. Journal of Cardiopulmonary Rehabilitation 1994;14(6):384‐90. [Google Scholar]
Ng 2000 {published data only}
- Ng JY, Tam SF. Effect of exercise‐based cardiac rehabilitation on mobility and self‐esteem of persons after cardiac surgery. Perceptual and Motor Skills 2000;91(1):107‐14. [DOI] [PubMed] [Google Scholar]
Nikolaeva 1986 {published data only}
- Nikolaeva LF, Karpova GD, Rubanovich AI. Effect of stepwise rehabilitation of patients with various types of myocardial infarct on their work capacity and disability and means of increasing the social efficacy of rehabilitation measures (cooperative study). Kardiologiia 1986;26:71‐8. [PubMed] [Google Scholar]
Nikrahan 2016 {published data only}
- Nikrahan GR, Laferton JA, Asgari K, Kalantari M, Abedi MR, Etesampour A, et al. Effects of positive psychology interventions on risk biomarkers in coronary patients: a randomized, wait‐list controlled pilot trial. Psychosomatics: Journal of Consultation and Liaison Psychiatry 2016;57(4):359‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ohm 1987 {published data only}
- Ohm D. Entspannungstraining und Hypnose bei Patienten mit koronarer Herzkrankheit in der stationären Rehabilitation. 4. Regensburg: Roderer, 1987. [Google Scholar]
Palatsi 1976 {published data only}
- Palatsi I. Feasibility of physical training after myocardial infarction and its effect on return to work, morbidity and mortality. Acta Medica Scandinavica. Supplementum 1976;599:7‐84. [PubMed] [Google Scholar]
Pegus 2002 {published data only}
- Pegus C, Bazzarre TL, Brown JS, Menzin J. Effect of the Heart At Work program on awareness of risk factors, self‐efficacy, and health behaviors. Journal of Occupational and Environmental Medicine 2002;44(3):228‐36. [DOI] [PubMed] [Google Scholar]
Petrie 1996 {published data only}
- Petrie KJ, Weinman J, Sharpe N, Buckley J. Role of patients' view of their illness in predicting return to work and functioning after myocardial infarction: longitudinal study. BMJ 1996;312(7040):1191‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pierson 2001 {published data only}
- Pierson LM, Herbert WG, Norton HJ, Kiebzak GM, Griffith P, Fedor JM, et al. Effects of combined aerobic and resistance training versus aerobic training alone in cardiac rehabilitation. Journal of Cardiopulmonary Rehabilitation 2001;21(2):101‐10. [DOI] [PubMed] [Google Scholar]
Pitscheider 1995 {published data only}
- Pitscheider W, Erlicher A, Zammarchi A, Crepaz R, Romeo C, Oberhollenzer R, et al. Left ventricular remodelling at 3 months from a first transmural infarct: the effect of physical activity and of the patency of the necrotic artery on changes in volume and segmental kinetics. Giornale Italiano di Cardiologia 1995;25(4):421‐31. [PubMed] [Google Scholar]
Price 2005 {published data only}
- Price J, Landry M, Rolfe D, Delos‐Reyes F, Groff L, Sternberg L. Women's cardiac rehabilitation: improving access using principles of women's health. The Canadian Journal of Cardiovascular Nursing 2005;15(3):32‐41. [PubMed] [Google Scholar]
Rakowska 2015 {published data only}
- Rakowska JM. Brief strategic therapy in first myocardial infarction patients with increased levels of stress: a randomized clinical trial. Anxiety, Stress, and Coping 2015;28(6):687‐705. [DOI] [PubMed] [Google Scholar]
Rauscha 1988 {published data only}
- Rauscha F, Muller C, Kiss H, Mlczoch J, Schuster J, Weber H, et al. Return to work following myocardial infarct. Wiener Klinische Wochenschrift 1988;100(18):605‐10. [PubMed] [Google Scholar]
Redfern 2007 {published data only}
- Redfern J, Ellis ER, Briffa T, Freedman SB. High risk‐factor level and low risk‐factor knowledge in patients not accessing cardiac rehabilitation after acute coronary syndrome. Medical Journal of Australia 2007;186(1):21‐5. [DOI] [PubMed] [Google Scholar]
Reid 2012 {published data only}
- Reid RD, Morrin LI, Beaton LJ, Papadakis S, Kocourek J, McDonnell L, et al. Randomized trial of an internet‐based computer‐tailored expert system for physical activity in patients with heart disease. European Journal of Preventive Cardiology 2012;19(6):1357‐64. [DOI] [PubMed] [Google Scholar]
Roviaro 1984 {published data only}
- Roviaro S, Holmes DS, Holmsten RD. Influence of a cardiac rehabilitation program on the cardiovascular, psychological, and social functioning of cardiac patients. Journal of Behavioral Medicine 1984;7(1):61‐81. [DOI] [PubMed] [Google Scholar]
Rudnicki 1977 {published data only}
- Rudnicki S, Tylka J, Barylak J, Czarniak S. Social, family and professional adjustment of patients after myocardial infarction during the period of a 4 year ambulatory rehabilitation. Cardiology (Switzerland) 1977;62(2):no pagination. [Google Scholar]
Rugulies 2003 {published data only}
- Rugulies R, Krasemann EO, Siegrist J. Evaluation of physician‐accompanied vacation trips for heart patients ("heart vacation trips"). Participants' satisfaction and long term impact on health related quality of life. Rehabilitation (Stuttg) 2003;42(4):211‐7. [DOI] [PubMed] [Google Scholar]
Salonen 1980 {published data only}
- Salonen JT, Puska P. A community programme for rehabilitation and secondary prevention for patients with acute myocardial infarction as part of a comprehensive community programme for control of cardiovascular diseases (North Karelia Project). Scandinavian Journal of Rehabilitation Medicine 1980;12(1):33‐42. [PubMed] [Google Scholar]
Salvetti 2008 {published data only}
- Salvetti XM, Filho JA, Servantes DM, Paola AA. How much do the benefits cost? Effects of a home‐based training programme on cardiovascular fitness, quality of life, programme cost and adherence for patients with coronary disease. Clinical Rehabilitation 2008;22(10‐11):987‐96. [DOI] [PubMed] [Google Scholar]
Saner 1999 {published data only}
- Saner H, Saner B, Staubli R. Initial experiences with a comprehensive ambulatory rehabilitation program for heart patients. Herz 1999;24(Suppl 1):80‐7. [DOI] [PubMed] [Google Scholar]
Sanne 1973 {published data only}
- Sanne H. Exercise tolerance and physical training of non‐selected patients after myocardial infarction. Acta Medica Scandinavica. Supplementum 1973;551:1‐124. [PubMed] [Google Scholar]
Schaller 1977 {published data only}
- Schaller K. Effectiveness of rehabilitation of patients with acute myocardial infarction measured by return to work. Results of a randomized long term study. Cardiology (Switzerland) 1977;62(2):150. [Google Scholar]
Schiller 1976 {published data only}
- Schiller E, Baker J. Return to work after a myocardial infarction: evaluation of planned rehabilitation and of a predictive rating scale. Medical Journal of Australia 1976;1(23):859‐62. [PubMed] [Google Scholar]
Schlierf 1995 {published data only}
- Schlierf G, Schuler G, Hambrecht R, Niebauer J, Hauer K, Vogel G, et al. Treatment of coronary heart disease by diet and exercise. Journal of Cardiovascular Pharmacology 1995;25(Suppl 4):S32‐4. [PubMed] [Google Scholar]
Schuster 1995 {published data only}
- Schuster PM, Wright C, Tomich P. Gender differences in the outcomes of participants in home programs compared to those in structured cardiac rehabilitation programs. Rehabilitation Nursing 1995;20(2):93‐101. [DOI] [PubMed] [Google Scholar]
Schwartze 1991 {published data only}
- Schwartze D. Long‐term results of rehabilitation of heart infarct patients in heart groups. Experience from the Halle/Saale city district. Zeitschrift fur die Gesamte Innere Medizin und Ihre Grenzgebiete 1991;46(1‐2):27‐31. [PubMed] [Google Scholar]
Shapiro 1972 {published data only}
- Shapiro S, Weinblatt E, Frank CW. Return to work after first myocardial infarction. Archives of Environmental Health 1972;24(1):17‐26. [DOI] [PubMed] [Google Scholar]
Shrey 2000 {published data only}
- Shrey DE, Mital A. Accelerating the return to work (RTW) chances of coronary heart disease (CHD) patients: Part 2ƒ_"development and validation of a vocational rehabilitation programme. Disability and Rehabilitation: An International, Multidisciplinary Journal 2000;22(13‐14):621‐6. [DOI] [PubMed] [Google Scholar]
Sieber 1986 {published data only}
- Sieber R, Rothlin ME, Senning A. Vocational rehabilitation after an aortocoronary bypass operation. Schweizerische Medizinische Wochenschrift 1986;116(25):838‐45. [PubMed] [Google Scholar]
Siggeirsdottir 2016 {published data only}
- Siggeirsdottir K, Brynjolfsdottir RD, Haraldsson SO, Vidar S, Gudmundsson EG, Brynjolfsson JH. Determinants of outcome of vocational rehabilitation. Work 2016;55(3):577‐83. [DOI] [PubMed] [Google Scholar]
Simchen 2001 {published data only}
- Simchen E, Naveh I, Zitser‐Gurevich Y, Brown D, Galai N. Is participation in cardiac rehabilitation programs associated with better quality of life and return to work after coronary artery bypass operations? The Israeli CABG Study. Israel Medical Association Journal 2001;3(6):399‐403. [PubMed] [Google Scholar]
Sledzevskaia 1994 {published data only}
- Sledzevskaia IK, Il'iash MG, Golovkov IZ, Karbovnichaia NM. The results of the rehabilitative treatment of patients who have had a myocardial infarct complicated by a block of the right branch of the atrioventricular bundle. Likars'ka Sprava / Ministerstvo Okhorony Zdorov'ia Ukrainy 1994, (3‐4):98‐100. [PubMed] [Google Scholar]
Smirnov 1989 {published data only}
- Smirnov EK. Effect of occupational therapy on the psychosocial status of patients with myocardial infarction at the second stage of rehabilitation. Kardiologiia 1989;29(4):88‐91. [PubMed] [Google Scholar]
Speiser 1982 {published data only}
- Speiser K, Pfluger N, Rothlin M. Occupational rehabilitation following aorto‐coronary bypass operations. Schweizerische Medizinische Wochenschrift 1982;112(31‐32):1095‐8. [PubMed] [Google Scholar]
Steinacker 2011 {published data only}
- Steinacker JM, Liu Y, Muche R, Koenig W, Hahmann H, Imhof A, et al. Long term effects of comprehensive cardiac rehabilitation in an inpatient and outpatient setting. Swiss Medical Weekly 2011;140:w13141. [DOI: 10.4414/smw.2010.13141] [DOI] [PubMed] [Google Scholar]
Stepanova 1975 {published data only}
- Stepanova TA. Rehabilitation of patients with acute uncomplicated myocardial infarct according to 3 1/2‐week program. Kardiologiia 1975;15(9):40‐6. [PubMed] [Google Scholar]
Sturchio 2012 {published data only}
- Sturchio A, Gianni A, Campana B, Genua M, Storti M, Iasi G, et al. Coronary artery risk management programme (CARIMAP) delivered by a rehabilitation day‐hospital: impact on patients with coronary artery disease. Journal of Cardiopulmonary Rehabilitation and Prevention 2012;32(6):386‐93. [DOI] [PubMed] [Google Scholar]
Sundin 1994 {published data only}
- Sundin O, Ohman A, Burell G, Palm T, Strom G. Psychophysiological effects of cardiac rehabilitation in post‐myocardial infarction patients. International Journal of Behavioral Medicine 1994;1(1):55‐75. [DOI] [PubMed] [Google Scholar]
Szalewska 2015a {published data only}
- Szalewska D, Niedoszytko P, Gierat‐Haponiuk K. The impact of professional status on the effects of and adherence to the outpatient followed by home‐based telemonitored cardiac rehabilitation in patients referred by a social insurance institution. International Journal of Occupational Medicine and Environmental Health 2015;28(4):761‐70. [DOI] [PubMed] [Google Scholar]
Szalewska 2015b {published data only}
- Szalewska D, Zielinski P, Tomaszewski J, Kusiak‐Kaczmarek M, Lepska L, Gierat‐Haponiuk K, et al. Effects of outpatient followed by home‐based telemonitored cardiac rehabilitation in patients with coronary artery disease. Kardiologia Polska 2015;73(11):1101‐7. [DOI] [PubMed] [Google Scholar]
Tarasov 1998 {published data only}
- Tarasov NI, Barbarash OL, Berns SA, Anikin BS. Clinical and occupational prognosis in myocardial infarction patients which were on different activation regimens and stayed in hospital for different time. Klinicheskaia Meditsina 1998;76(1):54‐7. [PubMed] [Google Scholar]
Toms 2003 {published data only}
- Toms LV, O'Neill ME, Gardner A. Long‐term risk factor control after a cardiac rehabilitation programme. Australian Critical Care 2003;16(1):24‐8. [DOI] [PubMed] [Google Scholar]
Tooth 1998 {published data only}
- Tooth LR, McKenna KT, Maas F. Pre‐admission education/counselling for patients undergoing coronary angioplasty: impact on knowledge and risk factors. Australian and New Zealand Journal of Public Health 1998;22(5):583‐8. [DOI] [PubMed] [Google Scholar]
Van der Peijl 2004 {published data only}
- Peijl ID, Vliet Vlieland TP, Versteegh MI, Lok JJ, Munneke M, Dion RA. Exercise therapy after coronary artery bypass graft surgery: a randomized comparison of a high and low frequency exercise therapy program. Annals of Thoracic Surgery 2004;77(5):1535‐41. [DOI] [PubMed] [Google Scholar]
Van Dixhoorn 1989 {published data only}
- Dixhoorn J, Duivenvoorden HJ, Staal HA, Pool J. Physical training and relaxation therapy in cardiac rehabilitation assessed through a composite criterion for training outcome. American Heart Journal 1989;118:545‐52. [DOI] [PubMed] [Google Scholar]
Varvaro 2000 {published data only}
- Varvaro FF. Family role and work adaptation in MI women. Clinical Nursing Research 2000;9(3):339‐51. [DOI] [PubMed] [Google Scholar]
Velasco 1982 {published data only}
- Velasco JA, Tormo V. Influence of duration of cardiac rehabilitation on myocardial infarction patients. Journal of Cardiac Rehabilitation 1982;2(3):243‐6. [Google Scholar]
Vibulchai 2016 {published data only}
- Vibulchai N, Thanasilp S, Preechawong S. Randomized controlled trial of a self‐efficacy enhancement program for the cardiac rehabilitation of Thai patients with myocardial infarction. Nursing & Health Sciences 2016;18(2):188‐95. [DOI] [PubMed] [Google Scholar]
Wallach 1969 {published data only}
- Wallach L, Berant N, Dreyfuss F. Return to work after a first myocardial infarction. Harefuah 1969;77(6):224‐7. [PubMed] [Google Scholar]
Wieslander 2005 {published data only}
- Wieslander I, Baigi A, Turesson C, Fridlund B. Women's social support and social network after their first myocardial infarction; a 4‐year follow‐up with focus on cardiac rehabilitation. European Journal of Cardiovascular Nursing 2005;4(4):278‐85. [DOI] [PubMed] [Google Scholar]
Yonezawa 2009 {published data only}
- Yonezawa R, Masuda T, Matsunaga A, Takahashi Y, Saitoh M, Ishii A, et al. Effects of phase II cardiac rehabilitation on job stress and health‐related quality of life after return to work in middle‐aged patients with acute myocardial infarction. International Heart Journal 2009;50(3):279‐90. [DOI] [PubMed] [Google Scholar]
Yoshida 1999 {published data only}
- Yoshida T, Kohzuki M, Yoshida K, Hiwatari M, Kamimoto M, Yamamoto C, et al. Physical and psychological improvements after phase II cardiac rehabilitation in patients with myocardial infarction. Nursing & Health Sciences 1999;1(3):163‐70. [DOI] [PubMed] [Google Scholar]
Yu 2003 {published data only}
- Yu CM, Li LSW, Ho HH, Lau CP. Long‐term changes in exercise capacity, quality of life, body anthropometry, and lipid profiles after a cardiac rehabilitation program in obese patients with coronary heart disease. American Journal of Cardiology 2003;91(3):321‐5. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Franklin 2012 {published data only}
- Franklin BA. Multifactorial cardiac rehabilitation did not reduce mortality or morbidity after acute myocardial infarction. Annals of Internal Medicine 2012;157(2):JC2‐11. [DOI] [PubMed] [Google Scholar]
Gao 2007 {published data only}
- Gao WG, Hu DY, Ma WL, Tang CZ, Li J, Hasimu B, et al. Effect of health management on the rehabilitation of patients undergoing coronary artery bypass graft. Journal of Clinical Rehabilitative Tissue Engineering Research 2007;11(25):4874‐8. [Google Scholar]
Kellermann 1988 {published data only}
- Kellermann JJ. Return to work after CABG in Israel. Rehabilitacia Supplementum 1988;21(36‐37):10‐2. [Google Scholar]
Korzeniowska‐Kubacka 2003 {published data only}
- Korzeniowska‐Kubacka I, Kowalska M, Tylka J, Piotrowicz R. Psychosomatic indicators of quality of life in cardiac patients assessed in the course of short and long‐term rehabilitation. Polski Przeglad Kardiologiczny 2003;5(2):157‐61. [Google Scholar]
Landrum 2000 {published data only}
- Landrum DR. A comparison of the effects of cardiac rehabilitation programs on coping levels and outcome measures in cardiac patients. A comparison of the effects of cardiac rehabilitation programs on coping levels and outcome measures in cardiac patients [PhD Thesis]. Austin (USA): The University of Texas at Austin, 2000. [Google Scholar]
Rangel de Donaldo 1994 {published data only}
- Rangel de Donaldo VE, Velasquez Hincapi A. Impact of cardiac rehabilitation program to return to work and the improve of quality of life in patients with coronary artery disease [Thesis] [Impacto de la rehabilitación cardiovascular en el tiempo de reintegro laboral y en la calidad de vida de los trabajadores que consultaron por cardiopatía isquémica]. Impact of cardiac rehabilitation program to return to work and the improve of quality of life in patients with coronary artery disease [Thesis]. Bogota (Columbia): Universidad El Bosque, Facultad de Medicina, 1994. [Google Scholar]
References to ongoing studies
EXPERTIS {published data only}
- NCT02415075. Prevention of Reduced Employability With an Expert System With Telephone, Motivational Interviews Supporting Self‐management (EXPERTIS). clinicaltrials.gov/ct2/show/NCT02415075 (first received 14 April 2015).
LC‐REHAB {published data only}
- NCT01668394. Effect of Learning and Coping Strategies in Cardiac Rehabilitation ‐ Group Study (LC‐REHAB). clinicaltrials.gov/ct2/show/NCT01668394 (first received 20 August 2012).
MILESTONE {published data only}
- NCT01311323. Revascularization in Patients With Non‐ST‐Segment Elevation Acute Coronary Syndrome (NSTE‐ACS) With Multivessel and/or Unprotected Left Main Coronary Disease (MILESTONE). clinicaltrials.gov/ct2/show/NCT01311323 (first received 9 March 2011).
SATISFY‐SOS {published data only}
- NCT02032030. Systematic Assessment and Targeted Improvement of Services Following Yearlong Surgical Outcomes Surveys (SATISFY‐SOS). clinicaltrials.gov/ct2/show/NCT02032030 (first received 09 January 2014).
SUSTAINCSX {published data only}
- NCT02002247. SodiUm SeleniTe Adminstration IN Cardiac Surgery (SUSTAIN CSX®‐Trial). (SUSTAINCSX). clinicaltrials.gov/ct2/show/NCT02002247 (first received 05 December 2013).
WARRIOR {published data only}
- NCT03417388. Women's IschemiA TRial to Reduce Events In Non‐ObstRuctive CAD (WARRIOR). clinicaltrials.gov/ct2/show/NCT03417388 (first received 31 January 2018).
Additional references
Adams 2009
- Adams J, Roberts J, Simms K, Cheng D, Hartman J, Bartlett C. Measurement of functional capacity requirements to aid in development of an occupation‐specific rehabilitation training program to help firefighters with cardiac disease safely return to work. American Journal of Cardiology 2009;103:762‐5. [DOI] [PubMed] [Google Scholar]
Adams 2010
- Adams J, Schneider J, Hubbard M, McCullough‐Shock T, Cheng D, Simms K, et al. Measurement of functional capacity requirements of police officers to aid in development of an occupation‐specific cardiac rehabilitation training program. Baylor University Medical Center Proceedings 2010;23(1):7‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2016
- Anderson L, Thompson DR, Oldridge N, Zwisler AD, Rees K, Martin N, et al. Exercise‐based cardiac rehabilitation for coronary heart disease. Cochrane Database of Systematic Reviews 2016, Issue 1. [CENTRAL: CD001800; DOI: 10.1002/14651858.CD001800.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2017a
- Anderson L, Brown JP, Clark AM, Dalal H, Rossau HK, Bridges C, et al. Patient education in the management of coronary heart disease. Cochrane Database of Systematic Reviews 2017, Issue 6. [CENTRAL: CD008895; DOI: 10.1002/14651858.CD008895.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2017b
- Anderson L, Sharp GA, Norton RJ, Dalal H, Dean SG, Jolly K, et al. Home‐based versus centre‐based cardiac rehabilitation. Cochrane Database of Systematic Reviews 2017, Issue 6. [CENTRAL: CD007130; DOI: 10.1002/14651858.CD007130.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bethge 2016
- Bethge M. Effects of graded return‐to‐work: a propensity‐score‐matched analysis. Scandinavian Journal of Work, Environment & Health 2016;42(4):273‐9. [DOI: 10.5271/sjweh.3562] [DOI] [PubMed] [Google Scholar]
Bhattacharyya 2007
- Bhattacharyya MR, Perkins‐Porras L, Whitehead DL, Steptoe A. Psychological and clinical predictors of return to work after acute coronary syndrome. European Heart Journal 2007;28(2):160‐5. [DOI] [PubMed] [Google Scholar]
Blair 2014
- Blair J, Volpe M, Aggarwal B. Challenges, needs, and experiences of recently hospitalized cardiac patients and their informal caregivers. Journal of Cardiovascular Nursing 2014;29(1):29‐37. [DOI: 10.1097/JCN.0b013e3182784123] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowling 1995
- Bowling A. What things are important in people's lives? A survey of the public's judgements to inform scales of health related quality of life. Social Science & Medicine 1995;41(10):1447‐62. [DOI] [PubMed] [Google Scholar]
Brown 2011
- Brown JP, Clark AM, Dalal H, Welch K, Taylor RS. Patient education in the management of coronary heart disease. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD008895.pub2] [DOI] [PubMed] [Google Scholar]
Bürger 2011
- Bürger W, Streibelt M. Who benefits from stepwise occupational reintegration provided under the statutory pension insurance scheme? [Wer profitiert von Stufenweiser Wiedereingliederung in Trägerschaft der gesetzlichen Rentenversicherung?]. Rehabilitation (Stuttg) 2011;50(3):178‐85. [DOI] [PubMed] [Google Scholar]
Cancelliere 2016
- Cancelliere C, Donovan J, Stochkendahl MJ, Biscardi M, Ammendolia C, Myburgh C, et al. Factors affecting return to work after injury or illness: best evidence synthesis of systematic reviews. Chiropractic & Manual Therapies 2016;24(1):32. [DOI: 10.1186/s12998-016-0113-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation. Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation, accessed 01 March 2019.
De Vries 2018
- Vries H, Fishta A, Weikert B, Rodriguez Sanchez A, Wegewitz U. Determinants of sickness absence and return to work among employees with common mental disorders: a scoping review. Journal of Occupational Rehabilitation 2018;28(3):393‐417. [DOI: 10.1007/s10926-017-9730-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
DeBusk 1983
- DeBusk RF, Kraemer HC, Nash E, Berger WE 3rd, Lew H. Stepwise risk stratification soon after acute myocardial infarction. American Journal of Cardiology 1983;52(10):1161‐6. [PUBMED: 6650403] [DOI] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG (editors) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Den Bakker 2018
- Bakker CM, Anema JR, Zaman Agnm, Vet HC, Sharp L, Angenete E, et al. Prognostic factors for return to work and work disability among colorectal cancer survivors; a systematic review. PLoS One 2018;13(8):e0200720. [DOI: 10.1371/journal.pone.0200720] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dickens 2006
- Dickens CM, McGowan L, Percival C, Tomenson B, Cotter L, Heagerty A, et al. Contribution of depression and anxiety to impaired health‐related quality of life following first myocardial infarction. British Journal of Psychiatry 2006;189:367‐72. [DOI] [PubMed] [Google Scholar]
Downs 1998
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. Journal of Epidemiology and Community Health 1998;52(6):377‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dreyer 2016
- Dreyer RP, Xu X, Zhang W, Du X, Strait KM, Bierlein M, et al. Return to work after acute myocardial infarction: comparison between young women and men. Circulation. Cardiovascular Quality and Outcomes 2016;9(2 Suppl 1):S45‐52. [PUBMED: 26908859] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiabane 2012
- Fiabane E, Argentero P, Calsamiglia G, Candura SM, Giorgi I, Scafa F, et al. Does job satisfaction predict early return to work after coronary angioplasty or cardiac surgery?. International Archives of Occupational and Environmental Health. 2012 Jun 9 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7‐10. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 15 January 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Gragnano 2018
- Gragnano A, Negrini A, Miglioretti M, Corbiere M. Common psychosocial factors predicting return to work after common mental disorders, cardiovascular diseases, and cancers: a review of reviews supporting a cross‐disease approach. Journal of Occupational Rehabilitation 2018;28(2):215‐31. [DOI: 10.1007/s10926-017-9714-1] [DOI] [PubMed] [Google Scholar]
Heran 2011
- Heran BS, Chen JM, Ebrahim S, Moxham T, Oldridge N, Rees K, et al. Exercise‐based cardiac rehabilitation for coronary heart disease. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD001800.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 6: Searching for studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hämäläinen 2004
- Hämäläinen H, Mäki J, Virta L, Keskimäki I, Mähönen M, Moltchanov V, et al. Return to work after first myocardial infarction in 1991‐1996 in Finland. European Journal of Public Health 2004;14(4):350‐3. [DOI] [PubMed] [Google Scholar]
Järvholm 2012
- Järvholm B. How should methods for return to work be evaluated?. Scandinavian Journal of Work, Environment & Health 2012;38(2):89‐91. [DOI] [PubMed] [Google Scholar]
Karoff 2000a
- Karoff M, Röseler S, Lorenz CH, Kittel J. Interdisciplinary support program (INA) for patients discharged from cardiac rehabilitation‐ program to improve return to work rates after myocardial infarction and/or coronary artery bypass surgery. Zeitschrift für Kardiologie 2000;89:423‐33. [DOI] [PubMed] [Google Scholar]
Kivimäki 2005
- Kivimäki M, Head J, Ferrie JE, Hemingway H, Shipley MJ, Vahtera J, et al. Working while ill as a risk factor for serious coronary events: the Whitehall II Study. American Journal of Public Health 2005;95(1):98‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leal 2006
- Leal J, Luengo‐Fernandez R, Gray A, Petersen S, Rayner M. Economic burden of cardiovascular diseases in the enlarged European Union. European Heart Journal 2006;27(13):1610‐9. [PUBMED: 16495286] [DOI] [PubMed] [Google Scholar]
Mital 2004
- Mital A, Desai A, Mital A. Return to work after a coronary event. Journal of Cardiopulmonary Rehabilitation 2004;24(6):365‐73. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group Prisma. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2013
- National Institute for Health and Care Excellence (NICE). Myocardial infarction: cardiac rehabilitation and prevention of further cardiovascular disease. Clinical guideline [CG172]. www.nice.org.uk/guidance/cg172/ November 2013 (accessed prior to 01 March 2019). [PubMed]
NICE 2015
- National Institute for Health and Care Excellence (NICE). Secondary prevention after a myocardial infarction [QS99]. www.nice.org.uk/guidance/qs99 2015 (accessed prior to 01 March 2019).
NVVC 2011
- Committee for cardiovascular prevention and cardiac rehabilitation. The Dutch Multidisciplinary Guideline for Cardiac Rehabilitation [Multidisciplinaire richtlijn Hartrevalidatie 2011]. NVVC. Utrecht 2011.
O'Brien 2017
- O'Brien L, Wallace S, Romero L. Effect of psychosocial and vocational interventions on return‐to‐work rates post‐acute myocardial infarction: a systematic review. Journal of Cardiopulmonary Rehabilitation and Prevention 2018;38(4):215‐23. [DOI] [PubMed] [Google Scholar]
O'Neil 2010
- O'Neil A, Sanderson K, Oldenburg B. Depression as a predictor of work resumption following myocardial infarction (MI): a review of recent research evidence. Health and Quality of Life Outcomes 2010;8:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perk 2004
- Perk J, Alexanderson K. Swedish Council on Technology Assessment in Health Care (SBU). Chapter 8. Sick leave due to coronary artery disease or stroke. Scandinavian Journal of Public Health. Supplement 2004;63:181‐206. [PUBMED: 15513657] [DOI] [PubMed] [Google Scholar]
Perk 2012
- Perk J, Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) [Erratum in Eur Heart J. 2012 Sep;33(17):2126.]. European Heart Journal 2012;33(13):1635‐701. [DOI] [PubMed] [Google Scholar]
Piepoli 2014
- Piepoli MF, Corra U, Adamopoulos S, Benzer W, Bjarnason‐Wehrens B, Cupples M, et al. Secondary prevention in the clinical management of patients with cardiovascular diseases. Core components, standards and outcome measures for referral and delivery: a policy statement from the Cardiac Rehabilitation Section of the European Association for Cardiovascular Prevention & Rehabilitation. Endorsed by the Committee for Practice Guidelines of the European Society of Cardiology. European Journal of Preventive Cardiology 2014;21(6):664‐81. [DOI: 10.1177/2047487312449597] [DOI] [PubMed] [Google Scholar]
Price 2016
- Price KJ, Gordon BA, Bird SR, Benson AC. A review of guidelines for cardiac rehabilitation exercise programmes: is there an international consensus?. European Journal of Preventive Cardiology 2016;23(16):1715‐33. [PUBMED: 27353128] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhangen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richards 2017
- Richards SH, Anderson L, Jenkinson CE, Whalley B, Rees K, Davies P, et al. Psychological interventions for coronary heart disease. Cochrane Database of Systematic Reviews 2017, (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2017
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. on behalf of the Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Standards of Reporting Trials Group 1994
- Standards of Reporting Trials Group. A proposal for structured reporting of randomized controlled trials. The Standards of Reporting Trials Group. JAMA 1994;272(24):1926‐31. [PubMed] [Google Scholar]
Stata [Computer program]
- StataCorp. Statistics/Data Analysis. StataCorp, 11.2.
Sterne 2017
- Sterne JA, Egger M, Moher D, Boutron I (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Sunamura 2017
- Sunamura M, Ter Hoeve N, Geleijnse ML, Steenaard RV, Berg‐Emons HJ, Boersma H, et al. Cardiac rehabilitation in patients who underwent primary percutaneous coronary intervention for acute myocardial infarction: determinants of programme participation and completion. Netherlands Heart Journal 2017;25(11):618‐28. [PUBMED: 28917025] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thompson 2003
- Thompson DR, Yu CM. Quality of life in patients with coronary heart disease‐I: assessment tools. Health and Quality of Life Outcomes 2003;1:42. [PUBMED: 14505492] [DOI] [PMC free article] [PubMed] [Google Scholar]
Verbeek 2006
- Verbeek JH. How can doctors help their patients to return to work?. PLoS Medicine 2006;3(3):e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vooijs 2015
- Vooijs M, Leensen MC, Hoving JL, Daams JG, Wind H, Frings‐Dresen MH. Disease‐generic factors of work participation of workers with a chronic disease: a systematic review. International Archives of Occupational and Environmental Health 2015;88(8):1015‐29. [DOI: 10.1007/s00420-015-1025-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 2018
- Walsh A, Kitko L, Hupcey J. The experiences of younger individuals living with heart failure. Journal of Cardiovascular Nursing 2018;33(6):E9‐E16. [DOI: 10.1097/JCN.0000000000000525] [DOI] [PubMed] [Google Scholar]
Wenger 1995
- Wenger NK, Froelicher ES, Smith LK, Ades PA, Berra K, Blumenthal JA, et al. Cardiac rehabilitation as secondary prevention. Agency for Health Care Policy and Research and National Heart, Lung, and Blood Institute. Clinical Practice Guideline. Quick Reference Guide for Clinicians 1995, (17):1‐23. [PubMed] [Google Scholar]
Wenger 2008
- Wenger NK. Current status of cardiac rehabilitation. Journal of the American College of Cardiology 2008;51(17):1619‐31. [DOI: 10.1016/j.jacc.2008.01.030.] [DOI] [PubMed] [Google Scholar]
Whalley 2011
- Whalley B, Rees K, Davies P, Bennett P, Ebrahim S, Liu Z, et al. Psychological interventions for coronary heart disease. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD002902.pub3] [DOI] [PubMed] [Google Scholar]
WHO 1993
- WHO. Rehabilitation after cardiovascular diseases, with special emphasis on developing countries. Report of a WHO Expert Committee. World Health Organisation Technical Report Series 1993; Vol. 831:1‐122. [PubMed]
WHO 2012
- World Health Organization (WHO). Cardiovascular diseases (CVDs), Fact sheet No 317. www.who.int/en/news‐room/fact‐sheets/detail/cardiovascular‐diseases‐(cvds) (accessed prior to 01 March 2019).
WHO 2018a
- World Health Organization (WHO). Global health estimates 2016: deaths by cause, age, sex, by country and by region, 2000‐2016. www.who.int/healthinfo/global_burden_disease/estimates/en/ 2018 (accessed prior to 01 March 2019).
WHO 2018b
- World Health Organization (WHO). Global health estimates 2016: disease burden by cause, age, sex, by country and by region, 2000‐2016. www.who.int/healthinfo/global_burden_disease/estimates/en/index1.html 2018 (accessed prior to 01 March 2019).


