Abstract
Background
Escherichia coli is an important pathogen that causes diarrhea in both humans and animals. To determine the relationships between putative virulence factors and pathotypes or host taxa, many molecular studies on diarrhea-associated E. coli have been reported. However, little is known regarding genome-wide variation of E. coli from animal hosts. In this study, we performed whole genome sequencing of 127 E. coli isolates from sheep and swine with diarrhea in China. We compared isolates to explore the phylogenomic relatedness based on host origin. We explored the relationships of putative virulence factors across host taxa and pathotypes. Antimicrobial resistance was also tested.
Results
The E. coli genomes in this study were diverse with clear differences in the SNP, MLST, and O serotypes. Seven putative virulence factors (VFs) were prevalent (> 95%) across the isolates, including Hcp, csgC, dsdA, feoB, fepA, guaA, and malX. Sixteen putative VFs showed significantly different distributions (P < 0.05) in strains from sheep and swine and were primarily adhesion- and toxin-related genes. Some putative VFs were co-occurrent in some specific pathotypes and O serotypes. The distribution of 4525 accessory genes of the 127 strains significantly differed (P < 0.05) between isolates obtained from the two animal species. The 127 animal isolates sequenced in this study were each classified into one of five pathotypes: EAEC, ETEC, STEC, DAEC, and EPEC, with 66.9% of isolates belonging to EAEC. Analysis of stx subtypes and a minimum spanning tree based on MLST revealed that STEC isolates from sheep and EAEC isolates from sheep and swine have low potential to infect humans. Antibiotic resistance analysis showed that the E. coli isolates were highly resistant to ampicillin and doxycycline. Isolates from southeast China were more resistant to antibiotics than isolates from northwest China. Additionally, the plasmid-mediated colist in resistance gene mcr-1 was detected in 15 isolates, including 4 from sheep in Qinghai and 11 from swine in Jiangsu.
Conclusions
Our study provides insight into the genomes of E. coli isolated from animal sources. Distinguishable differences between swine and sheep isolates at the genomic level provides a baseline for future investigations of animal E. coli pathogens.
Electronic supplementary material
The online version of this article (10.1186/s12864-019-5588-2) contains supplementary material, which is available to authorized users.
Keywords: Escherichia coli, Genome, Putative virulence factor, Diarrhea, Animal isolates
Background
Escherichia coli is a part of the normal microflora of the human body, and also inhabits the intestinal tracts of other mammals. However, some E. coli pathotypes have acquired various putative virulence factors (VFs) from their environment. Pathogenic E. coli strains are classified as extraintestinal E. coli (ExPEC) or intestinal E. coli based on their ecological niche. Intestinal E. coli, also called diarrheagenic E. coli (DEC), can cause diarrhea in mammals; their classification is further broken down into six well-described categories: enteropathogenic E. coli (EPEC), shiga toxin-producing E. coli (STEC), enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC), enteroaggregative E. coli (EAEC), and diffuselyadherent E. coli (DAEC) [1].
Diarrhea is a majorcause of mortality and morbidity inhumans and young domestic animals all over the world, especially in developing countries. E. coli stands out as an important agent associated with acutediarrhea [2]. A large number of outbreaks of diarrhea among humans due to E. coli have been reported in different countries [2]. Postweaning diarrhea (PWD) which iscommonly associated with ETEC, is one of the most prevalent porcine diseases, accounting for substantial economic losses worldwide [3, 4]. Food- and water-borne transmission of pathogenic E. coli can occur in humans from domestic animals, especially from pigs, cattle and sheep [5]. Additionally, E. coli isolated from pigs, sheep and goats were found to be similar to strains from humans, suggesting that pigs and ruminants could be a potential source of infection in humans [6–8].
With the development of next-generation sequencing technologies, analysis of whole genome data from large numbers of clinically relevant bacterial isolates is now possible. However, most comparative genome analyses have focused on human isolates, and there is very little information regarding genome comparisons of large numbers of animal isolates. Here, we performed whole genome sequencing and comparative analysis of 127 intestinal E. coli isolates from animals with diarrhea in China. The goal of this study was to provide large-scale genomic data on E. coli from host animal species, and examine putative VFs across species, as well as antimicrobial resistance, in order to contribute to the understanding of E. coli from different hosts.
Results
Phylogenomic analysis
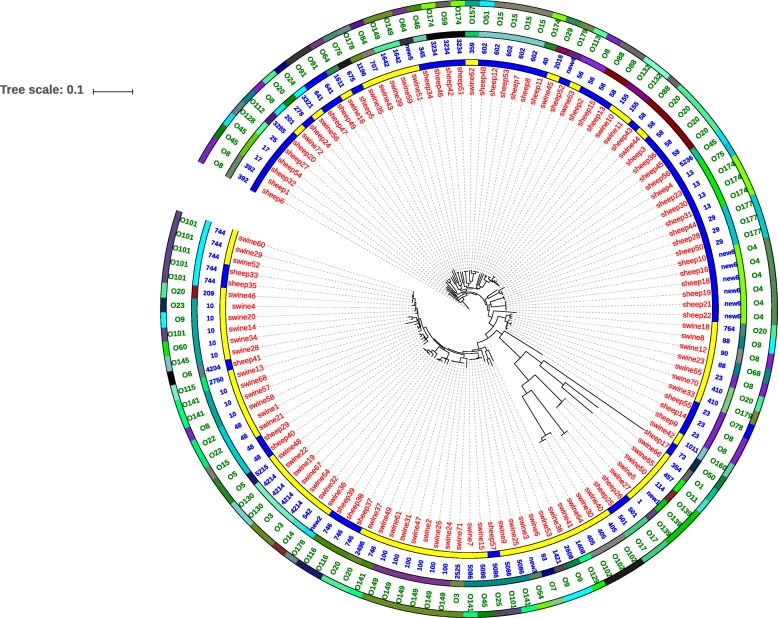
The 127 E. coli isolates from the two animal taxa (i.e. pigs and sheep) were extensively distributed across the phylogenetic tree, while there is no major clustering of host sources on the tree. The results of multilocus sequence typing (MLST) assay were largely concordant with the phylogenomic results (Fig. 1). The O antigens, such as O4,O5, O17, O22, O91, O102, O116, O130,O132, O139, and O177,corresponded with the phylogenomic results to a certain extent, however, some O antigens are interspersed in the tree, such as O3,O8, O9, O15, O20, O45, O64, O88, O101, O141, O149, O174 and O179 (Fig. 1). The genomes are diverse and they clearly have different SNP, MLST, and O serotypes. There is no obvious relationship between geographical distance and genome.
Fig. 1.
Whole genome phylogenetic tree of 127 E. coli isolates. Phylogenetic relationships of E. coli isolates based on core SNPs from whole genome sequencing. Isolates cultured from sheep and swine are represented in the inner ring in blue and yellow, respectively. The detailed host origin of each strain is described in red characters near the inner ring. The middle ring indicates groups of multilocus sequence typing (MLST) sequence types. Strains with the same ST types are denoted using the same color. Details regarding the ST type of each strain are described in blue near the middle ring. The outermost ring indicates O antigen groups. Strains with the same O serotypes are denoted using the same color. The detailed O serotype of every strain is described in green characters beside the outermost ring
In this study, serotype O101, O20, and O8 strains were present in both swine and sheep. O141, O149, O102, O9 and O139 were only present in swine, while O4, O174, O15, O177 and O88 were only present in sheep; most of these strains were EAEC. H21, H9 and H4 were highly distributed (≥ 5 isolates) in both swine and sheep isolates. H2, H8, H11and H31 were prevalence (≥ 5 isolates) in sheep isolates, while H5, H10 and H45 were prevalence (≥ 5 isolates) in swine isolates (Additional file 1: Table S1). Among seven O101 strains, five were O101:H9 (Additional file 1: Table S1). Sequence type (ST) 744 and ST5086 were distributed in both swine and sheep isolates. ST10, ST100, ST4214, and ST405 were distributed only in swine isolates, while ST602, ST13, ST29, ST3234 and a new ST (new6) were distributed only in sheep isolates.
Seven putative virulence genes are common
Among putative VFs, seven genes (Hcp, csgC, dsdA, feoB, fepA, guaA and malX) belonging to iron acquisition/transport systems and biosynthesis, were most prevalent (> 95%) across the isolates (Additional file 1: Table S2). fepA and malX were present in all strains. fepA encodes a ferric chelate receptor protein, which recognizes siderophore-ferric iron complexes and then transports iron into cells [9, 10]. malX encodes phosphotransferase system enzyme II, which recognizes glucose and maltose and facilitates the persistence of E. coli in the intestinal tract [11]. Unexpectedly, the two genes are also present in the genome of avirulent strain MG1655. In addition to these two, 27 putative VFs (Additional file 1: Table S3) were detected in the MG1655 genome.
Prevalence of adhesion- and toxin-related genes in strains isolated from swine and sheep
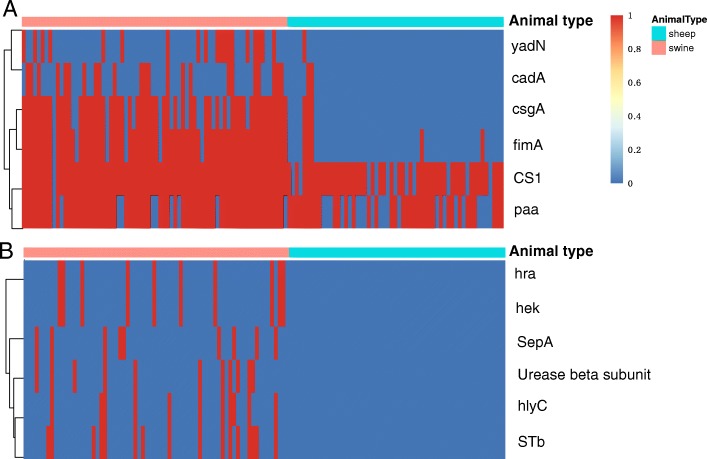
Swine and sheep E. coli harbored similar average numbers of putative VFs, with 33 and 30 putative VFs per isolate, respectively (Additional file 1: Table S4). Sixteen putative VFs showed significant differences in distribution between the swine and sheep isolates (P < 0.05, Additional file 1: Table S1). Of these 16 genes, five adhesion genes (CS1, csgA, fimA, paa and yadN) and one toxin gene (cadA) were more prevalent (P < 0.05) in swine isolates than in sheep isolates (Fig. 2A). Five toxin genes (hlyC, hra, hek, STb, and urease beta subunit) and one iron acquisition/transport gene (sepA) were found in 12.9 to 22.9% of the swine isolates but were absent from sheep isolates (P < 0.05;Fig. 2B). Putative VFs for adhesion, such as CS6 fimbrial subunits A and B, autotransporter protein EatA, and LT were prevalent in sheep isolates, but were rare in swine isolates (P < 0.05).
Fig. 2.
Presence of virulence genes in E. coli isolates. Red and green bars on the x-axis represent swine and sheep isolates, respectively. The bright red and dark blue regions represent the presence or absence of genes in a particular isolate, respectively. a) Significantly more virulence genes were found in swine isolates than in sheep isolates; b) virulence genes present in swine but absent from sheep isolates
The putative VF distribution of EAEC isolates was compared between swine and sheep. LT, eatA, and CS6 fimbrial subunits A and B were significantly more common (P < 0.05) in sheep EAEC isolates than in swine EAEC isolates, while csgA,CS1 and fimA were significantly more common (P < 0.05) in swine EAEC isolates than in sheep EAEC isolates (Additional file 1: Table S5). csgA and fimA were prevalent in swine STEC isolates, but were absent from sheep STEC isolates (Additional file 1: Table S6). Serotypes O8 and O20 were present with high frequency in both swine and sheep isolates. Hcp, ecpA, ecpB, ecpC, ecpD, ecpE and ecpR co-occurred in all O20 isolates from sheep but were absent from O20 isolates from swine; cadA,csgA, fimA, fimE, fimF, fimG, fimH, fimI and yadN co-occurred in all O20 isolates from swine but were absent from O20 isolates from sheep. csgC, dsdA, feoA, feoB, fliP, guaA and malX co-occurred in O20 isolates from swine and sheep. cadA, csgA, fimA, fimE, fimF, fimI and yadN co-occurred in all O8 isolates from swine but were absent from O8 isolates from sheep, while only flip was present in all O8 isolates from sheep but absent from O8 isolates from swine (Additional file 1: Table S7). Additional file 1: Table S8 lists the co-occurrence of putative VFs in high-frequency serotypes O4, O15,O101,O141, O149, and O174.
Stx subtyping
Twenty strains contained stx genes and were therefore classified as STEC. Among the 20 isolates, eight were from sheep and 12 were from swine (Additional file 1: Table S9). They were isolated from different areas, including Jiangsu, Beijing, Zhejiang, Shandong, Anhui and Qinghai. Eleven different O serotypes were identified among the 20 strains: O75(1/20), O76(1/20), O174 (4/20), O20(1/20), O128(1/20), O139(3/20), O149(2/20), O130(2/20), O141(2/20), O3(1/20), and O101(1/20). Twelve MLST were observed for the 20 STEC strains: ST13(4/20), ST4214 (4/20), ST100 (2/20), ST10 (2/20), ST675(1/20), ST278(1/20), ST40(1/20), ST25(1/20), ST5086(1/20), ST (1/20), ST114(1/20), and a novel ST (1/20). These strains contained four stx subtypes, stx1c, stx2c, stx2b, and stx2e. Among the sheep isolates, five were stx1c-positive, one was stx2b-positive, one was stx2c-positive, and one was stx1c + stx2b-positive. The stx subtype of all 12 swine isolates was stx2e. No putative EHEC VFs, including eaeA, hlyA, cnf1, and cnf2 [12, 13] were detected in STEC isolates in this study.
COG analysis of accessory genes
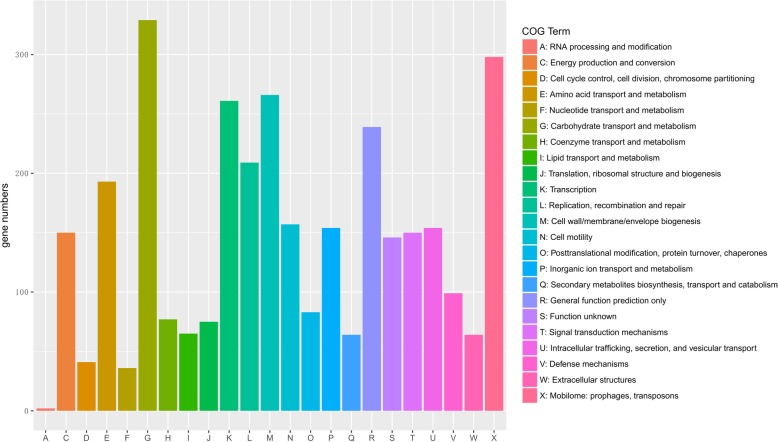
The accessory genomes of the 127 strains consisted of 28,724 genes. Among these accessory genes, 4525 were present with significant differences (P < 0.05) between swine and sheep isolates. The significantly different accessory genes between swine and sheep isolates covered 23 COGs, and were most enriched in carbohydrate transport and metabolism, followed by the mobilome (prophages and transposons), cell wall/membrane/envelope biogenesis, and transcription (Fig. 3).
Fig. 3.
COG of accessory genes. COG of accessory genes that significantly differed between swine and sheep isolates. Each COG contains 2–272 genes
The major pathotype is EAEC
The 127 animal isolates sequenced in this study were identified as belonging to five DEC categories: EAEC (66.9%, 85/127), ETEC (6.3%, 8/127), STEC (16.5%, 21/127), DAEC (6.3%, 8/127) and EPEC (3.9%, 5/127). EAEC has been reported as the most frequent or second most frequent DEC in humans [14, 15]. EAEC were also the most common DEC isolated from animals in this study, indicating that EAEC was the main pathogen of E. coli-caused animal diarrhea in China in recent years.
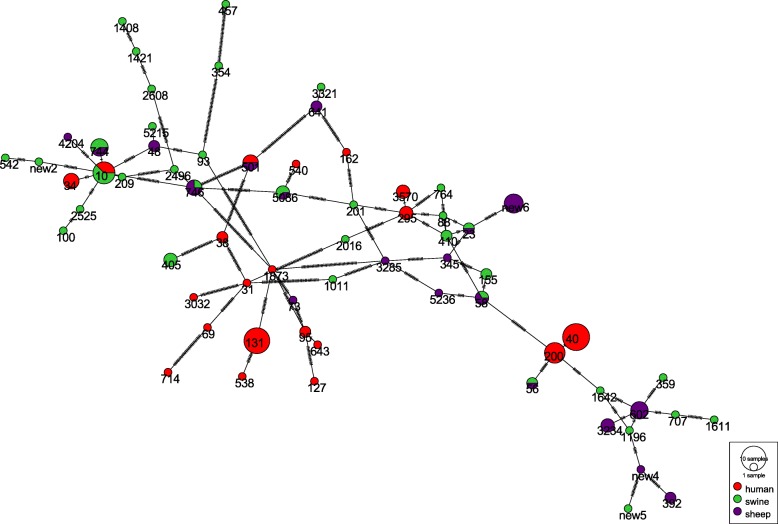
We constructed a minimum spanning tree containing 48 EAEC STs from our study and 20 human EAEC STs from Imuta’s report [16]. One ST (ST10) from swine and one ST (ST501) from sheep were clustered with the E. coli strains isolated from human clinical cases. Five ST (ST23, ST56, ST58, ST744, ST746, and ST5086) from swine were also observed in sheep. The remaining STs were only observed in one host (Fig. 4).
Fig. 4.
Minimum spanning tree of MLST types from different hosts. Minimum spanning network based on SNPs discovered in the collection of human, swine, and sheep isolates. Each circle corresponds to one MLST type; the circle size gives the proportion of isolates belonging to the MLST type. The color inside each circle represents the host, and indicates the proportion of isolates sampled in the different hosts. Each link between circles indicates one mutational event
Drug resistance is more severe in Southeast China
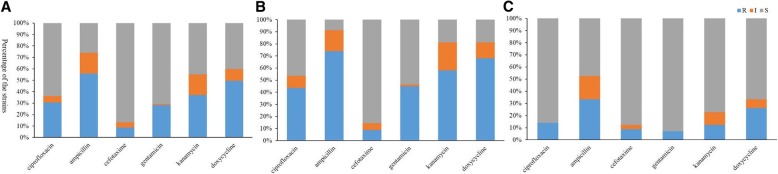
The resistance of the 127 E. coli strains to ciprofloxacin, ampicillin, cefotaxime, gentamycin, kanamycin, and doxycycline was tested (Additional file 1: Table S10). The majority of animal E. coli isolates (56%) were resistant to ampicillin, and 50% were resistant to doxycycline (Fig. 5A). Isolates from southeast China were more resistant to antibiotics than isolates from northwest China, with 74% and 68% of isolates from southeast China resistant to ampicillin and doxycycline, respectively (Fig. 5B);only 33% and 26% of isolates from northwest China were resistant to these antibiotics (Fig. 5C). This might be due to the greater use of antibiotics in southeast China [17, 18]. As all the sheep isolates are from northwest China and the swine isolates are from southeast China, it could be that swine are more likely to be treated with antibiotics than sheep.
Fig. 5.
Antibiotic resistance of E. coli isolated from animal species. Blue, orange, and gray columns denote resistant(R), intermediate(I), and susceptible (S) strains. (A) antibiotic resistance analysis of all 127 isolates from animals; (B) antibiotic-resistance of isolates from southeast China; (C) antibiotic-resistance of isolates from northwest China
A total of 109 antibiotic-resistance genes were predicted based on the CARD database. In this study, 38.6% of the isolates contained β-lactam-resistance genes, including CMY-2, TEM-1, OXA-1, OXA-10, CTX-M-55, TEM-116, CTX-M-65, TEM-95, and TEM-171. Tetracycline-resistance genes, including tetO, tetA, and tetD, were found in 29.1% of the isolates. Additionally, 57 antibiotic efflux pump-encoding genes were identified among the 127 isolates. Among the 109 antibiotic-resistance genes, 13 showed significant differences in distribution (P < 0.05) between isolates from southeast and northwest China, most of which were aminoglycoside- and sulfonamide-resistance genes (Additional file 2: Figure S1).
We detected the mcr-1 gene in 15 strains (Table 1). Four such strains were isolated from sheep in Qinghai, and the rest were isolated from swine in Jiangsu. All these strains were isolated between 2012 and 2014. Based on MLST, these strains belonged to ST 746 (4 strains), 4214 (4), 744 (3), 602 (1), 10 (1), 5215(1), and 457(1). The O serotypes of these strains were: O116 (2 strains), O130 (2), O101 (3), O3 (2), O141 (1), O20 (1), O51 (1), O145 (1),O5 (1), and O1 (1).
Table 1.
Strains containing the mcr-1 gene
| Strain | Host | Collection year | Geographical location | ST type | O antigen | Pathotype |
|---|---|---|---|---|---|---|
| sheep37 | sheep | 2013 | China:Qinghai | 746 | O20 | EAEC |
| sheep38 | sheep | 2013 | China:Qinghai | 746 | O116 | EAEC |
| sheep39 | sheep | 2013 | China:Qinghai | 746 | O116 | EAEC |
| sheep48 | sheep | 2013 | China:Qinghai | 602 | O51 | EAEC |
| swine19 | swine | 2014 | China:Jiangsu | 4214 | O130 | STEC |
| swine22 | swine | 2014 | China:Jiangsu | 4214 | O130 | STEC |
| swine28 | swine | 2012 | China:Jiangsu | 10 | O145 | EAEC |
| swine29 | swine | 2012 | China:Jiangsu | 744 | O101 | EAEC |
| swine48 | swine | 2012 | China:Jiangsu | 5215 | O5 | EAEC |
| swine49 | swine | 2012 | China:Jiangsu | 746 | O141 | EAEC |
| swine52 | swine | 2012 | China:Jiangsu | 744 | O101 | EAEC |
| swine54 | swine | 2012 | China:Jiangsu | 4214 | O3 | STEC |
| swine60 | swine | 2012 | China | 744 | O101 | EAEC |
| swine65 | swine | 2012 | China:Jiangsu | 457 | O11 | EAEC |
| swine67 | swine | 2012 | China:Jiangsu | 4214 | O3 | STEC |
Discussion
With the development of high-throughput sequencing, the genomes of large numbers of organisms have been sequenced, especially well-characterized microorganisms, such as E. coli. However, most genome sequencing of E. coli focuses on human isolates, and little is known regarding genome variation in animal isolates. In this study, whole genomes of 127 E. coli isolates, collected from two types of domestic animal, were sequenced and comparative genomic analysis was performed.
We did not find a phylogenetic relationship between host source and E. coli isolates, which supports previous studies [19, 20]. This suggests that E. coli maintains an infection mechanism without strict host restriction. In this study, MLST was largely concordant with the phylogenomic results.
VFs play an important role in the infection process of E. coli strains, and virulent strains have higher VFs distributions than commensals. However, known VFs of the strains in this study are limited. We used VFs in the VFDB as putative VFs in this study, which covers experimentally demonstrated VFs from 24 genera of medically important bacterial pathogens and several predicted VFs from complete genomes [21]. Putative VF distribution exhibited different patterns across the two host species investigated. Six putative VFs, hlyC, hra, hek, STb, urease beta subunit, and sepA, were widely distributed in swine isolates, but absent from sheep isolates. HlyC and STb were highly correlated with ETEC [22, 23], and all ETEC strains in this study were isolated from swine; thus, hlyC and STb were absent from sheep isolates. Stx is a typical toxin of STEC, which disrupts protein synthesis and kills impaired endothelial or epithelial cells by cleaving ribosomal RNA [1]. Stx can be classified into two subgroups, stx1 and stx2. There are three stx1 subtypes (stx1a, stx1c, and stx1d) and seven stx2 subtypes (stx2a, stx2b, stx2c, stx2d, stx2e, stx2f, and stx2g) [24]. The different stx types and subtypes may be associated with differences in STEC pathogenicity. STEC carrying stx1a, stx2a, stx2c, and stx2d are associated with severe clinical symptoms, while STEC carrying stx1c and stx2b are mainly associated with diarrheal disease [25]. Stx2e has been reported in association with ED in pigs and is probably not pathogenic to humans [26]. Stx2a, stx2c and stx2d have been recognized in relation to severe STEC infections in humans [27, 28]. Sheep isolates were more likely to carry stx1c and stx2b subtypes, while the 12 STEC isolates from swine were positive only for stx2e. These results corresponded to previous reports [29] and suggest that these STEC isolates had low potential to infect humans.
Zhu et al. [30] reported that ompA, fimH, vat, traT, and iutA were highly prevalent (> 60%) in swine ExPEC strains isolated in China, and fimH was the most prevalent (81.2%) adhesion factor. Similarly in our study, fimH and traT had a high prevalence (> 60%) in swine isolates, and fimH was present in 87.1% of swine isolates, suggesting that fimH and traT were prevalent both in ExPEC and intestinal E. coli. In this study, ompA was excluded as it was found in 199 E. coli genomes in the NCBI GenBank database (Additional file 1: Table S11); this was in agreement with the report of Zhu et al., in which ompA was observed in all ExPEC isolates. Notably, iutA was present at 60.9 and 14.3% in ExPEC and intestinal E. coli, respectively. Vat was present at 65.6% and 0% in ExPEC and intestinal E. coli, respectively, indicating that iutA and vat could be used to distinguish putative VFs between ExPEC and intestinal E. coli from pigs.
Serotypes O139,O141, O149, O9 and O102 were present with high frequency in swine isolates but absent from sheep isolates, corresponding with previous reports that O139 and O149 were prevalent serotypes and the main cause of ED in swine [31, 32].O101, O20 and O8 were present in both swine and sheep isolates, while O4, O174, O15,O177 and O88 were prevalent in sheep isolates but absent from swine isolates, indicating that these O serotypes had host preference. Based on the results of VFs distribution in O20 and O8 between swine and sheep isolates (Additional file 1: Table S7), we speculate that the VFs distribution also has a host preference even within certain serotypes. H21, H9 and H4 were widely distributed (≥ 5 isolates) in both swine and sheep isolates and five strains were O101:H9, indicating that H9 is prevalence is E.coli strains, which agreed with previous report [33].
In recent years, drug resistance has become a serious problem, which has attracted increasing attention from researchers. As the incidence of carbapenem-resistant Enterobacteriaceae increased worldwide, polymyxins have been adopted as the last line of defense against Gram-negative bacterial infections [34]. Resistance to polymyxins mainly depends on modification of lipopolysaccharide (LPS), which is often chromosomally mediated [35]. The mcr-1 gene, which was first reported by Liu [34], is a plasmid-mediated colistin resistance mechanism. After Liu’s report, a series of mcr-1 distribution surveys have been published. mcr-1 was reported with a high frequency in E. coli isolates from pig (24.1%) and chicken (14.0%) farms [36] in China. In this study, mcr-1 was present in 15.7% and 7.0% of swine and sheep isolates, respectively. All the mcr-1-positive strains were tested for antibiotic resistance to polymyxin. Ten strains were resistant to polymyxin and five (sheep37, swine19, swine29, swine60, and swine67) showed intermediate resistance to polymyxin (data not shown). The results of antibiotic resistance tests showed that isolates from southeast China were more resistant to antibiotics; this might due to the antibiotic is greater used in southeast China. As most of the sheep came from one province (Qinghai) whereas the swine came from other regions, the antibiotic resistance differences may also be because swine are more likely to be treated with antibiotics than sheep.
Conclusions
In this study, comparative genomic analysis was performed for 127 E. coli isolates from swine, and sheep with diarrhea. To differentiate between E. coli strains obtained from different hosts, various aspects of these E. coli isolates, including putative VFs, accessory genes, antibiotic resistance, MLST, O serotypes, pathotypes, and phylogenomic trees were analyzed. No specific putative VFs were found to be completely present or absent in isolates from any one animal group. However, some putative VFs co-occurred in some specific pathotypes and O serotypes. The frequency of some VFs and accessory genes present in swine and sheep isolates differed significantly. We have described the genomic profiles of intestinal E. coli isolates from different animals with diarrhea, which will provide a baseline for future research into DEC.
Methods
Bacterial strains
A total of 127 E. coli strains were isolated from sheep (n = 57) and swine (n = 70) with diarrhea from 1972 to 2013 in China. Additional file 1: Table S1 lists the sources and location of all isolates.
Library construction and DNA sequencing
Genomic DNA was extracted using a Bacterial DNA Kit (Omega Bio-Tek, America). The genomic DNA was fragmented by ultrasonication, and library preparation was performed using Illumina TruSeq DNA Sample Prep Kits (Illumina, San Diego, CA, USA). Paired-end sequencing was performed on an Illumina HiSeq 2000 system.
Bioinformatic analysis
Low quality reads were filtered using the DynamicTrim Perl script within SolexaQA [37]. Raw reads were assembled using SOAPdenovo, a genome assembler developed specifically for next-generation short-read sequences [38]. SOAP GapCloser was used to scaffold the contigs after genome assembly and assembled sequences were annotated using Prokka [39]. Trees were constructed by maximum likelihood (ML) using the core SNPs detected by kSNP3.0 with a k-mer size of 21 based on concatenated genome sequence data. Trees were visualized using FigTree v1.4.2 (2 (http://tree.bio.ed.ac.uk/ so/ software/figtree/). Roary [40] is a high-speed standalone pan-genome pipeline, which uses annotated assemblies in the GFF3 format created by Prokka and calculates the pan genome. The functions of predicted protein-coding genes were then annotated through comparisons with the COG database. Minimum Spanning Networks of EAEC strains were constructed by PopART software [41]. Seven conserved housekeeping genes (adk, fumC, gyrB, icd, mdh, purA and recA) were blast according to the protocol of the E. coli MLST database (https://pubmlst.org/data/) [42].
Pathotype detection
Pathotypes of E. coli strains were identified by PCR or sequence alignment. Multiplex PCR was performed as previously described to detect the pathotype of the strains [43]. An isolate was identified as: STEC if positive for the gene coding the stx gene; ETEC if positive for genes coding for heat-stable enterotoxin or heat-labile enterotoxin; EPEC if positive for the gene coding for the outer membrane protein intimin; EAEC if positive for the gene coding for transporter protein Aat; EIEC if positive for the gene coding for the invasion protein IpaH; and DAEC if positive for the gene coding for an accessory protein with a function in F1845 fimbriae production.
Antimicrobial susceptibility testing
Antibiotic resistance was determined using the agar dilution method according to Clinical and Laboratory Standards Institute (CLSI) guidelines. The following antibiotics were tested: ciprofloxacin, ampicillin, cefotaxime, gentamycin, kanamycin, and doxycycline. The reference strain E. coli ATCC 25922 was used as the positive control. The antibiotic-resistance database (CARD, https://card.mcmaster.ca/) [44] was used to predicted antibiotic-resistance genes. The Evalue was set to <1e–5, while the hit coverage was at least 90%.
Analysis of putative virulence genes
E. coli putative VF reference sequences (Additional file 3: Dataset S1) were collected from the Virulence Factor Database [21] and previous studies [1, 45]. Isolates from this study were compared to putative VF reference sequences using BLAST and each putative VF was considered matched with the query DNA sequence by > 60% sequence identity and > 50% aligned length coverage. The assembled sequences of 199 E. coli genomes (Additional file 1: Table S11) from the NCBI GenBank database were annotated using Prokka [39]. The accessory genes of isolates in this study were identified by comparing the genomes to the core genes of the 199 E. coli genomes. The stx subtype was considered matched with the reference sequence in GenBank database by > 99% sequence identity and > 99% aligned length coverage. Statistically significant differences in presence/absence patterns for each putative virulence gene were determined using Fisher’s Exact Test with the Bonferroni correction.
Serotype determination
O serotypes were determined by blasting genome sequences from this study against sequences of 184 O antigen biosynthesis gene clusters as previously reported [46]. H serotype of the strains by using SeroTypefinder (https://cge.cbs.dtu.dk/services/SerotypeFinder/). Strains lacking matched sequences were detected by classical serum agglutination tests.
Accession nos
All sequences from the 127 E. coli isolates were entered in NCBI and accession numbers for each sample are listed in Additional file 1: Table S12.
Additional files
Table S1. Strains information. Host, collection year, geographical location, sample type, MLST type, O antigen, pathotype, number of scaffolds, and average scaffold size of the 127 isolates used in this study. Table S2. Percentage of strain type. Isolates from this study were compared to putative virulence factor (VF) reference sequences using BLAST, and each putative VF was considered matched with a query sequence if the sequence identity was > 60% and the aligned length coverage was > 50%. The proportion of putative VF in distinct groups was included. Fisher’s exact test was used to determine significant differences of each putative VF between two different groups. P-values were adjusted using Bonferroni correction. Table S3. Putative VF in MG1655. Putative VF detected in the E. coli MG1655 genome. Table S4. Average putative VF numbers of each strain type. This table contains the numbers of putativeVF in each strain and the average numbers of putative VF in the two strain types. Table S5. Percentage of VFs in EAEC strains. Isolates from this study were compared to putative virulence factor (VF) reference sequences using BLAST, and each putative VF was considered matched with a query sequence if the sequence identity was > 60% and the aligned length coverage was > 50%. The proportion of putative VFs in distinct groups is included. Fisher’s exact test was used to determine significant differences in each putative VF between two different groups. P-values were adjusted using Bonferroni correction. Table S6. Percentage of VFs in STEC strains. Isolates from this study were compared to putative virulence factor (VF) reference sequences using BLAST, and each putative VF was considered matched with a query sequence if the sequence identity was > 60% and the aligned length coverage was > 50%. The proportion of putative VFs in distinct groups is included. Fisher’s exact test was used to determine significant differences in each putative VF between two different groups. P-values were adjusted using Bonferroni correction. Table S7. Distribution of VFs in O20 and O8 isolates. Isolates from this study were compared to putative virulence factor (VF) reference sequences using BLAST, and each putative VF was considered matched with a query sequence if the sequence identity was > 60% and the aligned length coverage was > 50%. Table S8. Distribution of putative virulence factors in different O serotypes. Isolates from this study were compared to putative virulence factor (VF) reference sequences using BLAST, and each putative VF was considered matched with a query sequence if the sequence identity was > 60% and the aligned length coverage was > 50%. Table S9. Stx subtype information. This table lists the stx subtypes as well as host, collection year, geographical location, sample type, MLST type, and O antigen of 20 STEC strains in this study. Table S10. Antibiotic resistance of strains. The antibiotic resistance to six drugs (ciprofloxacin, ampicillin, cefotaxime, gentamicin, kanamycin, and doxycycline) of the 127 isolates in this study. Table S11. Reference E. coli genomes. The putative VF reference sequences in this study were collected from the Virulence Factor Database and from previous studies, and the core genes of 199 E.coli genomes in the NCBI GenBank database were then removed. This table contains the name and accession numbers of the 199E.coli genomes. Table S12. Accession numbers of the strains. All genome data from this study were submitted to the NCBI GenBank database. This table contains the accession numbers of the genomes of all 127 E. coli isolates. (PDF 1165 kb)
Figure S1. Presence of antibiotic-resistance genes in E. coli isolates. Red and green bars on the x-axis represent a geographical location in southeast and northwest China, respectively. The bright red and dark blue regions represent the presence or absence of genes in a particular isolate, respectively. This figure shows that significantly more antibiotic resistance genes were found in isolates from southeast than northwest China. (PDF 46 kb)
Dataset S1. putative VF reference sequences. This table contains putative VF reference sequences used in this study. All putative VFs were classified according to their function. (XLSX 163 kb)
Acknowledgements
Not applicable.
Funding
This work was supported by the Natural Science Foundation of Jiangsu Province (BK20180075); the National Basic Research Program of China (2015CB554203); the Fundamental Research Funds for the Central Universities (KYZ201326), and the Fund of Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions. The funding body had no role in the design of the study, data collection, analysis, interpretation of data, or writing the manuscript.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article (and its additional files). The accession numbers of 127 draft genomes are listed in Additional file 1(Table S12) .
Abbreviations
- CLSI
Clinical and laboratory standards institute
- COG
Cluster of orthologous groups of proteins
- DAEC
diffuselyadherent E. coli
- DEC
diarrheagenic E. coli
- E.coli
Escherichia coli
- EAEC
enteroaggregative E. coli
- ED
edema disease
- EHEC
enterohemorrhagic E. coli
- EIEC
enteroinvasive E. coli
- EPEC
enteropathogenic E. coli
- ETEC
enterotoxigenic E. coli
- ExPEC
extraintestinal E. coli
- LPS
lipopolysaccharide
- MLST
multilocus sequence typing
- PWD
Postweaning diarrhea
- ST
sequence type
- STEC
Shigatoxin-producing E. coli
- VFs
virulence factors
Authors’ contributions
JD conceived the study; DL, LM and JW performed the antimicrobial susceptibility testing and pathotype detection; FL, XZand JR performed the library construction, and DNA sequencing; SG,YL and GY performed the phylogenomic and accessory gene analysis; FT performed most of the bioinformatic analysis, and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Authors’ information
F T: associate professor of College of Veterinary Medicine, Nanjing Agricultural University. J W: master student of College of Veterinary Medicine, Nanjing Agricultural University. D L: doctoral student of College of Veterinary Medicine, Nanjing Agricultural University. Song Gao: professor of College of Veterinary Medicine, Yangzhou University. J R: associate research fellow of College of Veterinary Medicine, Nanjing Agricultural University.
L M: research fellow of Qinghai Academy of veterinary Medicine and Animal Science,Qinghai University. F L: staff of Institute of Microbiology, Chinese Academy of Sciences. X Z: postdoctor of College of Veterinary Medicine, Nanjing Agricultural University. G Y: professor of College of Animal Science and Technology, ShiheziUniversity. Y L: associate research fellow of Qinghai Academy of veterinary Medicine and Animal Science,Qinghai University. J D: professor of College of Veterinary Medicine, Nanjing Agricultural University.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fang Tang, Email: tfalice@126.com.
Juanfang Wang, Email: wangjuanfang_s@163.com.
Dezhi Li, Email: 593397758@qq.com.
Song Gao, Email: gsong@yzu.edu.cn.
Jianluan Ren, Email: dyyrjl@njau.edu.cn.
Liqing Ma, Email: maliq67@hotmail.com.
Fei Liu, Email: liuf@im.ac.cn.
Xiangkai Zhuge, Email: zgxiangkai@163.com.
Genqiang Yan, Email: ygq58@shzu.edu.cn.
Yan Lu, Email: Luyan0971@163.com.
Jianjun Dai, Phone: +86(0) 25-84396566, Email: daijianjun@njau.edu.cn.
References
- 1.Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2(2):123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 2.Canizalez-Roman A, Flores-Villasenor HM, Gonzalez-Nunez E, Velazquez-Roman J, Vidal JE, Muro-Amador S, Alapizco-Castro G, Diaz-Quinonez JA, Leon-Sicairos N. Surveillance of Diarrheagenic Escherichia coli strains isolated from diarrhea cases from children. Adults and Elderly at Northwest of Mexico Frontiers in microbiology. 2016;7:1924. [DOI] [PMC free article] [PubMed]
- 3.Han W, Liu B, Cao B, Beutin L, Kruger U, Liu H, Li Y, Liu Y, Feng L, Wang L. DNA microarray-based identification of serogroups and virulence gene patterns of Escherichia coli isolates associated with porcine postweaning diarrhea and edema disease. Appl Environ Microbiol. 2007;73(12):4082–4088. doi: 10.1128/AEM.01820-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng D, Sun H, Xu J, Gao S. PCR detection of virulence factor genes in Escherichia coli isolates from weaned piglets with edema disease and/or diarrhea in China. Vet Microbiol. 2006;115(4):320–328. doi: 10.1016/j.vetmic.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Chandran A, Mazumder A. Prevalence of diarrhea-associated virulence genes and genetic diversity in Escherichia coli isolates from fecal material of various animal hosts. Appl Environ Microbiol. 2013;79(23):7371–7380. doi: 10.1128/AEM.02653-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maluta RP, Fairbrother JM, Stella AE, Rigobelo EC, Martinez R, de Avila FA. Potentially pathogenic Escherichia coli in healthy, pasture-raised sheep on farms and at the abattoir in Brazil. Vet Microbiol. 2014;169(1–2):89–95. doi: 10.1016/j.vetmic.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Orden JA, Ruiz-Santa-Quiteria JA, Blanco M, Blanco JE, Mora A, Cid D, Gonzalez EA, Blanco J, de la Fuente R. Prevalence and characterization of Vero cytotoxin-producing Escherichia coli isolated from diarrhoeic and healthy sheep and goats. Epidemiol Infect. 2003;130(2):313–321. doi: 10.1017/S0950268802008154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osman KM, Mustafa AM, Elhariri M, Abdelhamed GS. The distribution of Escherichia coli serovars, virulence genes, gene association and combinations and virulence genes encoding serotypes in pathogenic E. coli recovered from diarrhoeic calves, sheep and goat. Transbound Emerg Dis. 2013;60(1):69–78. doi: 10.1111/j.1865-1682.2012.01319.x. [DOI] [PubMed] [Google Scholar]
- 9.Jalal MA, van der Helm D. Purification and crystallization of ferric enterobactin receptor protein, FepA, from the outer membranes of Escherichia coli UT5600/pBB2. FEBS Lett. 1989;243(2):366–370. doi: 10.1016/0014-5793(89)80163-2. [DOI] [PubMed] [Google Scholar]
- 10.Hagelueken G, Duthie FG, Florin N, Schubert E, Schiemann O. Expression, purification and spin labelling of the ferrous iron transporter FeoB from Escherichia coli BL21 for EPR studies. Protein Expr Purif. 2015;114:30–36. doi: 10.1016/j.pep.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Ostblom A, Adlerberth I, Wold AE, Nowrouzian FL. Pathogenicity island markers, virulence determinants malX and usp, and the capacity of Escherichia coli to persist in infants' commensal microbiotas. Appl Environ Microbiol. 2011;77(7):2303–2308. doi: 10.1128/AEM.02405-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh KH, Jung SM, Shin E, Chung GT, Seong WK, Cho SH. Comparison of Enterohemorrhagic Escherichia coli (EHEC) O157 and EHEC non-O157 isolates from patients with diarrhea in Korea. Jpn J Infect Dis. 2017;70(3):320–322. doi: 10.7883/yoken.JJID.2016.178. [DOI] [PubMed] [Google Scholar]
- 13.Bai X, Zhao A, Lan R, Xin Y, Xie H, Meng Q, Jin D, Yu B, Sun H, Lu S, et al. Shiga toxin-producing Escherichia coli in yaks (Bos grunniens) from the Qinghai-Tibetan plateau, China. PloS one. 2013;8(6):e65537. doi: 10.1371/journal.pone.0065537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patzi-Vargas S, Zaidi MB, Perez-Martinez I, Leon-Cen M, Michel-Ayala A, Chaussabel D, Estrada-Garcia T. Diarrheagenic Escherichia coli carrying supplementary virulence genes are an important cause of moderate to severe diarrhoeal disease in Mexico. PLoS Negl Trop Dis. 2015;9(3):e0003510. doi: 10.1371/journal.pntd.0003510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ochoa TJ, Ecker L, Barletta F, Mispireta ML, Gil AI, Contreras C, Molina M, Amemiya I, Verastegui H, Hall ER, et al. Age-related susceptibility to infection with diarrheagenic Escherichia coli among infants from Periurban areas in Lima, Peru. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;49(11):1694–1702. doi: 10.1086/648069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imuta N, Ooka T, Seto K, Kawahara R, Koriyama T, Kojyo T, Iguchi A, Tokuda K, Kawamura H, Yoshiie K, et al. Phylogenetic analysis of Enteroaggregative Escherichia coli (EAEC) isolates from Japan reveals emergence of CTX-M-14-producing EAEC O25:H4 clones related to sequence type 131. J Clin Microbiol. 2016;54(8):2128–2134. doi: 10.1128/JCM.00711-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bu Q, Wang B, Huang J, Liu K, Deng S, Wang Y, Yu G. Estimating the use of antibiotics for humans across China. Chemosphere. 2016;144:1384–1390. doi: 10.1016/j.chemosphere.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Zhang QQ, Ying GG, Pan CG, Liu YS, Zhao JL. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: source analysis, multimedia modeling, and linkage to bacterial resistance. Environmental science & technology. 2015;49(11):6772–6782. doi: 10.1021/acs.est.5b00729. [DOI] [PubMed] [Google Scholar]
- 19.Murinda SE, Nguyen LT, Landers TL, Draughon FA, Mathew AG, Hogan JS, Smith KL, Hancock DD, Oliver SP. Comparison of Escherichia coli isolates from humans, food, and farm and companion animals for presence of Shiga toxin-producing E. Coli virulence markers. Foodborne Pathog Dis. 2004;1(3):178–184. doi: 10.1089/fpd.2004.1.178. [DOI] [PubMed] [Google Scholar]
- 20.Leimbach A, Hacker J, Dobrindt U. E. Coli as an all-rounder: the thin line between commensalism and pathogenicity. Curr Top Microbiol Immunol. 2013;358:3–32. doi: 10.1007/82_2012_303. [DOI] [PubMed] [Google Scholar]
- 21.Chen L, Xiong Z, Sun L, Yang J, Jin Q. VFDB 2012 update: toward the genetic diversity and molecular evolution of bacterial virulence factors. Nucleic Acids Res. 2012;40(Database issue):D641–D645. doi: 10.1093/nar/gkr989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandhu KS, Clarke RC, Gyles CL. Virulence markers in Shiga toxin-producing Escherichia coli isolated from cattle. Canadian journal of veterinary research = Revue canadienne de recherche veterinaire. 1999;63(3):177–184. [PMC free article] [PubMed] [Google Scholar]
- 23.Shin SJ, Chang YF, Timour M, Lauderdale TL, Lein DH. Hybridization of clinical Escherichia coli isolates from calves and piglets in New York state with gene probes for enterotoxins (STaP, STb, LT), Shiga-like toxins (SLT-1, SLT-II) and adhesion factors (K88, K99, F41, 987P) Vet Microbiol. 1994;38(3):217–225. doi: 10.1016/0378-1135(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 24.Scheutz F, Teel LD, Beutin L, Pierard D, Buvens G, Karch H, Mellmann A, Caprioli A, Tozzoli R, Morabito S, et al. Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing Stx nomenclature. J Clin Microbiol. 2012;50(9):2951–2963. doi: 10.1128/JCM.00860-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jelacic JK, Damrow T, Chen GS, Jelacic S, Bielaszewska M, Ciol M, Carvalho HM, Melton-Celsa AR, O'Brien AD, Tarr PI. Shiga toxin-producing Escherichia coli in Montana: bacterial genotypes and clinical profiles. J Infect Dis. 2003;188(5):719–729. doi: 10.1086/376999. [DOI] [PubMed] [Google Scholar]
- 26.Beutin L, Kruger U, Krause G, Miko A, Martin A, Strauch E. Evaluation of major types of Shiga toxin 2E-producing Escherichia coli bacteria present in food, pigs, and the environment as potential pathogens for humans. Appl Environ Microbiol. 2008;74(15):4806–4816. doi: 10.1128/AEM.00623-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vu-Khac H, Cornick NA. Prevalence and genetic profiles of Shiga toxin-producing Escherichia coli strains isolated from buffaloes, cattle, and goats in Central Vietnam. Vet Microbiol. 2008;126(4):356–363. doi: 10.1016/j.vetmic.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 28.McFarland N, Bundle N, Jenkins C, Godbole G, Mikhail A, Dallman T, O’Connor C, Mccarthy N, O’Connell E, Treacy J et al: Recurrent seasonal outbreak of an emerging serotype of Shiga toxin-producing Escherichia coli (STEC O55:H7 Stx2a) in the south west of England, July 2014 to September 2015. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin 2017, 22(36). [DOI] [PMC free article] [PubMed]
- 29.Bai X, Wang H, Xin Y, Wei R, Tang X, Zhao A, Sun H, Zhang W, Wang Y, Xu Y, et al. Prevalence and characteristics of Shiga toxin-producing Escherichia coli isolated from retail raw meats in China. Int J Food Microbiol. 2015;200:31–38. doi: 10.1016/j.ijfoodmicro.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 30.Zhu Y, Dong W, Ma J, Yuan L, Hejair HM, Pan Z, Liu G, Yao H. Characterization and virulence clustering analysis of extraintestinal pathogenic Escherichia coli isolated from swine in China. BMC Vet Res. 2017;13(1):94. doi: 10.1186/s12917-017-0975-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brand P, Gobeli S, Perreten V. Pathotyping and antibiotic resistance of porcine enterovirulent Escherichia coli strains from Switzerland (2014-2015) Schweizer Archiv fur Tierheilkunde. 2017;159(7):373–380. doi: 10.17236/sat00120. [DOI] [PubMed] [Google Scholar]
- 32.Helgerson AF, Sharma V, Dow AM, Schroeder R, Post K, Cornick NA. Edema disease caused by a clone of Escherichia coli O147. J Clin Microbiol. 2006;44(9):3074–3077. doi: 10.1128/JCM.00617-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Contrepois M, Bertin Y, Pohl P, Picard B, Girardeau JP. A study of relationships among F17 a producing enterotoxigenic and non-enterotoxigenic Escherichia coli strains isolated from diarrheic calves. Vet Microbiol. 1998;64(1):75–81. doi: 10.1016/S0378-1135(98)00253-3. [DOI] [PubMed] [Google Scholar]
- 34.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16(2):161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 35.Olaitan AO, Morand S, Rolain JM. Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Front Microbiol. 2014;5:643. doi: 10.3389/fmicb.2014.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang X, Yu L, Chen X, Zhi C, Yao X, Liu Y, Wu S, Guo Z, Yi L, Zeng Z, et al. High prevalence of Colistin resistance and mcr-1 gene in Escherichia coli isolated from food animals in China. Front Microbiol. 2017;8:562. doi: 10.3389/fmicb.2017.00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cox MP, Peterson DA, Biggs PJ. SolexaQA: at-a-glance quality assessment of Illumina second-generation sequencing data. BMC bioinformatics. 2010;11:485. doi: 10.1186/1471-2105-11-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo R, Liu B, Xie Y, Li Z, Huang W, Yuan J, He G, Chen Y, Pan Q, Liu Y, et al. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. Gigascience. 1(1):18. [DOI] [PMC free article] [PubMed]
- 39.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30(14):2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 40.Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, Holden MT, Fookes M, Falush D, Keane JA, Parkhill J. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics. 2015;31(22):3691–3693. doi: 10.1093/bioinformatics/btv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leigh JW, Bryant D. PopART: full-feature software for haplotype network construction. Methods in Ecology & Evolution. 2015;6(9):1110–1116. doi: 10.1111/2041-210X.12410. [DOI] [Google Scholar]
- 42.Shabana II, Zaraket H, Suzuki H. Molecular studies on diarrhea-associated Escherichia coli isolated from humans and animals in Egypt. Vet Microbiol. 2013;167(3–4):532–539. doi: 10.1016/j.vetmic.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 43.Tau NP, Meidany P, Smith AM, Sooka A, Keddy KH. Group for enteric R, meningeal disease surveillance in south a: Escherichia coli O104 associated with human diarrhea, South Africa, 2004-2011. Emerg Infect Dis. 2012;18(8):1314–1317. doi: 10.3201/eid1808.111616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McArthur AG, Waglechner N, Nizam F, Yan A, Azad MA, Baylay AJ, Bhullar K, Canova MJ, De Pascale G, Ejim L, et al. The comprehensive antibiotic resistance database. Antimicrob Agents Chemother. 2013;57(7):3348–3357. doi: 10.1128/AAC.00419-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Croxen MA, Finlay BB. Molecular mechanisms of Escherichia coli pathogenicity. Nat Rev Microbiol. 2010;8(1):26–38. doi: 10.1038/nrmicro2265. [DOI] [PubMed] [Google Scholar]
- 46.Iguchi A, Iyoda S, Kikuchi T, Ogura Y, Katsura K, Ohnishi M, Hayashi T, Thomson NR. A complete view of the genetic diversity of the Escherichia coli O-antigen biosynthesis gene cluster. DNA research : an international journal for rapid publication of reports on genes and genomes. 2015;22(1):101–107. doi: 10.1093/dnares/dsu043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Strains information. Host, collection year, geographical location, sample type, MLST type, O antigen, pathotype, number of scaffolds, and average scaffold size of the 127 isolates used in this study. Table S2. Percentage of strain type. Isolates from this study were compared to putative virulence factor (VF) reference sequences using BLAST, and each putative VF was considered matched with a query sequence if the sequence identity was > 60% and the aligned length coverage was > 50%. The proportion of putative VF in distinct groups was included. Fisher’s exact test was used to determine significant differences of each putative VF between two different groups. P-values were adjusted using Bonferroni correction. Table S3. Putative VF in MG1655. Putative VF detected in the E. coli MG1655 genome. Table S4. Average putative VF numbers of each strain type. This table contains the numbers of putativeVF in each strain and the average numbers of putative VF in the two strain types. Table S5. Percentage of VFs in EAEC strains. Isolates from this study were compared to putative virulence factor (VF) reference sequences using BLAST, and each putative VF was considered matched with a query sequence if the sequence identity was > 60% and the aligned length coverage was > 50%. The proportion of putative VFs in distinct groups is included. Fisher’s exact test was used to determine significant differences in each putative VF between two different groups. P-values were adjusted using Bonferroni correction. Table S6. Percentage of VFs in STEC strains. Isolates from this study were compared to putative virulence factor (VF) reference sequences using BLAST, and each putative VF was considered matched with a query sequence if the sequence identity was > 60% and the aligned length coverage was > 50%. The proportion of putative VFs in distinct groups is included. Fisher’s exact test was used to determine significant differences in each putative VF between two different groups. P-values were adjusted using Bonferroni correction. Table S7. Distribution of VFs in O20 and O8 isolates. Isolates from this study were compared to putative virulence factor (VF) reference sequences using BLAST, and each putative VF was considered matched with a query sequence if the sequence identity was > 60% and the aligned length coverage was > 50%. Table S8. Distribution of putative virulence factors in different O serotypes. Isolates from this study were compared to putative virulence factor (VF) reference sequences using BLAST, and each putative VF was considered matched with a query sequence if the sequence identity was > 60% and the aligned length coverage was > 50%. Table S9. Stx subtype information. This table lists the stx subtypes as well as host, collection year, geographical location, sample type, MLST type, and O antigen of 20 STEC strains in this study. Table S10. Antibiotic resistance of strains. The antibiotic resistance to six drugs (ciprofloxacin, ampicillin, cefotaxime, gentamicin, kanamycin, and doxycycline) of the 127 isolates in this study. Table S11. Reference E. coli genomes. The putative VF reference sequences in this study were collected from the Virulence Factor Database and from previous studies, and the core genes of 199 E.coli genomes in the NCBI GenBank database were then removed. This table contains the name and accession numbers of the 199E.coli genomes. Table S12. Accession numbers of the strains. All genome data from this study were submitted to the NCBI GenBank database. This table contains the accession numbers of the genomes of all 127 E. coli isolates. (PDF 1165 kb)
Figure S1. Presence of antibiotic-resistance genes in E. coli isolates. Red and green bars on the x-axis represent a geographical location in southeast and northwest China, respectively. The bright red and dark blue regions represent the presence or absence of genes in a particular isolate, respectively. This figure shows that significantly more antibiotic resistance genes were found in isolates from southeast than northwest China. (PDF 46 kb)
Dataset S1. putative VF reference sequences. This table contains putative VF reference sequences used in this study. All putative VFs were classified according to their function. (XLSX 163 kb)
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article (and its additional files). The accession numbers of 127 draft genomes are listed in Additional file 1(Table S12) .