Abstract
Background
Cerebral microbleeds (CMBs) are considered to be risk factors for cognitive dysfunction. The specific pathology and clinical manifestations of CMBs are different based on their locations. We investigated the association between CMBs at different locations and cognitive dysfunction and explored the potential underlying pathways in a rural Han Chinese population.
Methods
We used baseline data from 562 community-dwelling adults (55–65 years old) in the Taizhou Imaging Study between 2013 and 2015. All individuals underwent multimodal brain magnetic resonance imaging (MRI) and 444 subjects completed neuropsychological tests: the Mini-Mental Status Examination and the Montreal Cognitive Assessment. Multinomial logistic regression was used to estimate the association between CMBs and cognitive dysfunction. The volume of brain regions and white matter microstructure were analyzed using Freesurfer and tract-based spatial statistics, respectively.
Results
CMBs were detected in 104 individuals (18.5%) in our study. Multinomial logistic regression found deep/mixed CMBs were associated with global cognitive dysfunction (OR 3.52; 95% CI 1.21 to 10.26), whereas lobar CMBs (OR 1.76; 95% CI 0.56 to 5.53) were not. Quantification of multimodal brain MRI showed that deep/mixed CMBs were accompanied by decreased thalamic volume and loss of fractional anisotropy of bilateral anterior thalamic radiations.
Conclusion
Deep/mixed CMBs were associated with cognitive dysfunction in this Chinese cross-sectional study. Disruption of thalamocortical connectivity might be a potential pathway underlying this relationship.
Keywords: Cerebral microbleeds, Cognitive impairment, Multimodal imaging
Abbreviations: AD, Alzheimer's disease; ATR, anterior thalamic radiation; BPF, brain parenchymal fraction; CMB, cerebral microbleed; CSVD, cerebral small vessel disease; CVD, coronary vascular disease; DTI, diffusion tensor imaging; FA, fractional anisotropy; FLAIR, fluid attenuated inversion recovery; GLM, general linear model; GRE, gradient recalled echo; LAC, lacune; MCI, mild cognitive impairment; MMSE, Mini-Mental Status Examination; MoCA, Montreal Cognitive Assessment; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging; SWI, susceptibility weighted imaging; TBSS, tract-based spatial statistics; TFCE, threshold-free cluster enhancement; TGV, total gray volume; TIV, total intracranial volume; WMH, white matter hyperintensity
Highlights
-
•
Cerebral microbleeds (CMBs) are found in 18.5% of middle-aged Chinese population.
-
•
Deep/mixed CMBs, not lobar CMBs, are associated with cognitive dysfunction.
-
•
Atrophy and fiber connectivity disruption might be the underlying neural pathways.
1. Introduction
Cognitive dysfunction is a mental-health deficit that affects millions of people worldwide. The burden of cognitive dysfunction has increased in China with the aging of the population and lifestyle changes (Chan et al., 2013). Early identification of risk factors for cognitive dysfunction is necessary for prevention and treatment. Recently, cerebral microbleeds (CMBs), detected by susceptible magnetic resonance imaging (MRI) sequences, have been recognized as an independent risk factor for cognitive dysfunction with a high prevalence in the population (5–21%) (Smith et al., 2017). CMBs increase the risk of stroke, gait disturbance, depression, and mortality (Akoudad et al., 2015; de Laat et al., 2011; Xu et al., 2016; Romero et al., 2017a). Cross-sectional and prospective studies of mainly Western populations provide evidence that CMBs are associated with both mild cognitive impairment (MCI) and dementia (Zhang et al., 2018; Romero et al., 2017b).
The association between CMBs and cognitive dysfunction varies depending on the location of the CMBs. Some research shows lobar CMBs increase the risk of cognitive dysfunction (Chung et al., 2016), and there also is substantial research showing an association between deep CMBs and cognitive dysfunction (Ding et al., 2017). The underlying mechanisms by which CMBs in various locations induce cognitive dysfunction may differ based on different vasculopathy characteristics. Research indicates lobar CMBs have a pathological basis similar to Alzheimer's disease (AD) and they are more common in patients with AD (Yates et al., 2014). However, limited evidence precludes a hypothesis about deep CMBs. Thus, the pathway between deep CMBs and cognitive dysfunction requires more investigation.
In this study, we investigated the relationship between the location of CMBs and cognitive dysfunction in a rural Han Chinese population, and explored the potential neurodegenerative pathways of CMB-related cognitive dysfunction using multimodal imaging assessments.
2. Methods
2.1. Study population
The participants in this study were selected from the baseline population of the Taizhou Imaging Study (TIS). The TIS is an ongoing population-based, neuroimaging study in China that is nested within the Taizhou Longitudinal Study (TZL) (Wang et al., 2009). For the Phase I of the TIS, two villages (Hutou and Lubao) that had the highest TZL response rates were selected to be involved. The TIS inclusion criteria were being: (a) 55 to 65 years old; (b) Han Chinese who settled in Taizhou within the past 10 years and live there >9 months per year; (c) and able to participate in the MRI examination and complete the questionnaire. Individuals with a history of stroke, cancer, or other severe diseases were excluded from the study. A total of 636 individuals from the two villages met the inclusion criteria, 12 of whom were excluded because of stroke or cancer. Of the remaining 624 individuals, 562 (90.1%) agreed to participate in our study. The participants completed questionnaires, physical and laboratory examinations, brain MRI, and cognitive function assessments between March 2013 and January 2015. All the examinations and assessments for each subject were conducted on the same day. Supplementary Fig. 1 shows the procedure for selecting the study sample. The TIS was approved by the Ethics Committee of the School of Life Sciences, Fudan University, Shanghai, China (Institutional Review Board approval number 469). Written informed consent was obtained from each participant before data collection.
2.2. MRI acquisition and assessment of cerebral small vessel diseases
All 562 participants underwent a brain MRI examination on the same 3.0T scanner (Magnetom Verio Tim scanner; Siemens, Erlangen, Germany) in Taizhou People's Hospital following a predetermined protocol. The set of MRI sequences included: T1-weighted; T2-weighted; fluid attenuated inversion recovery (FLAIR); T2⁎-weighted gradient recalled echo (GRE); proton density-weighted imaging (PDWI); perfusion-weighted imaging (PWI); diffusion tensor imaging (DTI); and time of flight (TOF) 3D magnetic resonance angiography (MRA). The specific parameters of each sequence are listed in Supplementary Table 1. Cerebral small vessel diseases (CSVDs) were diagnosed based on the Standards for Reporting Vascular Changes on Neuroimaging (STRIVE) criteria proposed in 2013 (Wardlaw et al., 2013). CMBs were defined as round- or ovoid-shaped local signal loss on the T2⁎-weighted GRE sequence. The diameters of the CMBs were usually 2–5 mm and generally no >10 mm. The CMBs were divided by location into three groups: lobar CMBs group, deep CMBs group and mixed CMBs group (with both lobar and deep CMBs). Lobar CMBs were located in cortices or white matter in subcortical and periventricular areas. Deep CMBs were located in deep areas, which included deep white matter (corpus callosum, internal, external and extreme capsule), deep nuclei (basal ganglia and thalamus), and infratentorial structures (brain stem and cerebellum). As mixed CMBs share similar characteristics with deep CMBs, according to the Rotterdam Scan Study (Poels et al., 2010), deep CMBs and mixed CMBs were merged into one group (deep/mixed CMBs) in this study. Lacunes (LAC) and white matter hyperintensities (WMHs) were also diagnosed according to the STRIVE criteria. Only moderate to severe WMHs (Fazekas score ≥ 2) were defined in this study.
Two experienced neurologists (M. Cui and Q. Yang) read and assessed the MRI images independently. Cases with disagreements about the assessments were rechecked by a senior neuroradiologist (W.J. Tang). The Kappa value for CMB identification was 0.752.
2.3. MRI quantitative analysis and post-processing
Original images were preprocessed using MRIConvert software (version 2.1.0). Freesurfer version 6.0.0 (http://surfer.nmr.mgh.harvard.edu/fswiki/) was used to evaluate the volume and thickness of brain regions. Data from 35 of the 562 participants (6.2%) were discarded because of motion artifacts and extreme outliers to maintain imaging quality control. The brain parenchymal fraction (BPF) was defined as the ratio of brain parenchymal volume to total intracranial volume (TIV). TIV, BPF, and total gray volume (TGV) were used to estimate global brain atrophy.
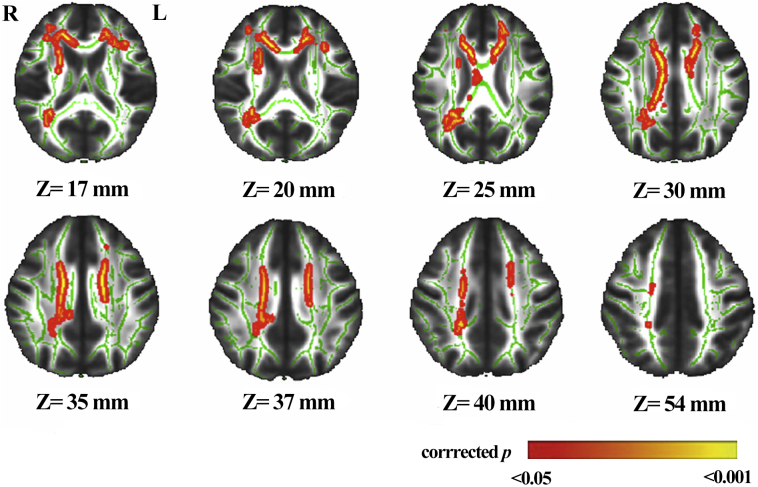
Diffusion images were processed using FMRIB Software Library (FSL) version 5.0 (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/), with the following steps: (1) Eddy Correct—correct eddy and head motion artifacts; (2) BET—extract brain tissue and generate individual brain masks; (3) DITFIT—fit diffusion tensor models to the raw data and create fractional anisotropy (FA) maps. Five participants in the deep/mixed CMBs group were excluded for poor imaging quality. Fifty age and sex exact-matched participants without CMBs were chosen as controls (1:1 matching). Tract-based spatial statistics (TBSS) were subsequently performed for voxel-wise analysis between the deep/mixed CMBs group (N = 50) and the no-CMBs group (N = 50). All FA images were aligned to the mask FMRIB58_FA, and then mapped into Montreal Neurological Institute (MNI) standard space using a non-linear transformation. The threshold was set at 0.2 to merge all FA images into a 4D skeletonized image. Apart from the inter-group analysis of FA values, which used the general linear model (GLM), threshold-free cluster enhancement (TFCE) was performed to correct the analysis with multiple iterations (N = 5000). Clusters with decreased FA values (P < .05) in the deep/mixed CMBs group, compared with the no-CMBs group, are shown as red-yellow in Fig. 1. Significant clusters were labeled by the Atlasquery Tool in FSL, based on the Johns Hopkins University white mater tractography atlas (Mori et al., 2002).
Fig. 1.
Clusters with decreased fractional anisotropy (FA) values in the deep/mixed cerebral microbleeds (CMBs) group compared to those without CMBs.
Abbreviations: L, left; R, right.
The result of contrast is overlaid on the mean FA skeleton (green) created by TBSS. The red–yellow color represents clusters with significant decreased FA in the deep/mixed CMBs group, compared with the no CMBs group (corrected P < .05).
2.4. Assessment of cognitive function and vascular risk factors
The Mini-Mental Status Examination (MMSE) and the Montreal Cognitive Assessment (MoCA) were used to evaluate the global cognitive functioning of participants. As education strongly influences scores on these cognitive measures, the cut-off values of cognitive dysfunction on the MMSE and MoCA were adjusted according to Chinese population-based studies (Li et al., 2016; Lu et al., 2011). Participants who completed the cognitive evaluations were categorized into three groups according to the MMSE and MoCA cut-off values(Nasreddine et al., 2005): (a) Normal cognition—in the normal range of the MMSE and MoCA; (b) Mild global cognitive dysfunction—in the abnormal range of the MoCA and the normal range of the MMSE; and (c) Severe global cognitive dysfunction—in the abnormal range of the MMSE and MoCA (Supplementary Table 2). In our study, 109 participants involved between April 2014 and January 2015 did not complete the MoCA. When we compared the characteristics of the participants with and without MoCA scores, we found no significant difference between them except for hypertension and hyperlipidemia (Supplementary Table 3). As these risk factors were adjusted for in the statistical analyses, we assumed the remaining 453 participants with MoCA scores were representative of the entire study sample.
Blood pressure (BP) was measured in the sitting position after being seated for 10 min in a quiet room; BP was measured twice to obtain an average. Hypertension was defined as blood pressure ≥ 140/90 mmHg, a previous diagnosis of hypertension, or use of antihypertensive drugs. Serum fasting glucose, total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were assayed by the same automatic biochemical analyzer (TBA–40FR; TOSHIBA Corp., Tokyo, Japan). Diabetes was defined as a fasting plasma glucose level ≥ 7.0 mmol/L, a previous diagnosis of diabetes, or use of antidiabetic drugs. Hyperlipidemia was defined as a plasma level of total cholesterol ≥5.2 mmol/L, triglycerides ≥1.7 mmol/L, a previous diagnosis of hyperlipidemia, or use of lipid-lowering drugs.
2.5. Statistical analyses
All statistical analyses were performed using SPSS v19.0 (SPSS Inc., Armonk, NY, USA) or R software (Version 3.3.3). Continuous variables are presented as means and standard deviations (SDs) and compared using Student's t-test or ANOVA. Categorical variables are shown as frequencies (%) and compared using Pearson's chi-square test. The Kolmogorov-Smirnov test was used to evaluate the normality of continuous variables; no variable had to be transformed in this study. Multinomial logistic regression was used to estimate the odds ratios (OR) and 95% confidence intervals (CI) of CMBs and thalamic volume as risk factors for cognitive dysfunction, i.e., log(P(Yj)|P(Y1)) = α + β1x1 + βixi + ε, where Y1 = normal cognition, Yj = mild or severe cognitive dysfunction, x1 = CMBs in different locations or thalamic volume, and xi = covariates. The P-value for trend was derived from the Wald test in the ordinal logistic regression by treating global cognitive dysfunction as an ordinal variable. Multiple linear regression was used to assess the associations of brain volume and thickness with CMBs, using brain-region volumes and thickness as dependent variables, i.e., y = α + β1x1 + βixi + ε, where y = brain region volume or brain region thickness, x1 = CMBs in different locations, and xi = covariates. Poisson regression was used to estimate the net effect of thalamic volume on the presence of deep/mixed CMBs, i.e., log(E(Y|x1)) = α + β1x1 + βixi + ε, where y = thalamic volume, x1 = deep/mixed CMBs, and xi = covariates. The unit of brain region volume was defined as cm3. Different covariates were adjusted in different regression models, including age, sex, smoking (no/yes), drinking (no/yes), hypertension (no/yes), diabetes (no/yes), hyperlipidemia (no/yes), and TIV (see figure legends for details). As lacunes and WMHs were not significantly associated with cognitive dysfunction or brain region atrophy in this study, we therefore did not treat them as confounding variables in our analyses. Differences were considered to be statistically significant at P < .05.
3. Results
3.1. Characteristics of the study sample
The baseline characteristics of the 562 participants are described in Table 1. Their mean age (SD) was 59.3 (2.7) at the time of MRI acquisition and 303 (53.9%) participants were women. The participants had a median (IQR) 6 (0, 9) years of education and relatively low neuropsychological scores. The median scores (IQR) of the MMSE and MoCA were 27 (23, 29) and 19 (14, 23), respectively. CMBs were found in 104 (18.5%) participants. Among them, 55 (9.8%) were deep or mixed CMBs and 49 (8.7%) were strictly lobar CMBs. Compared to participants without CMBs, those with CMBs were more likely to be older, women, and have hypertension (P < .05). These associations differed by the CMB location. Only the prevalence of hypertension was significantly higher in the deep/mixed CMBs group, whereas lobar CMBs were significantly related to age and sex (P < .05). CMBs were strongly correlated with other types of CSVDs, such as LAC and WMHs, regardless of CMB location (P < .05). There was no significant association between CMBs and other risk factors.
Table 1.
Characteristics of participants with and without cerebral microbleeds.
| No CMBs (N = 458) | CMBs (N = 104) |
||
|---|---|---|---|
| Deep/Mixed (N = 55) | Lobar (N = 49) | ||
| Demographic characteristics | |||
| Age, mean ± SD, years | 59.1 ± 2.7 | 59.8 ± 2.6 | 60.1 ± 2.6⁎ |
| Female, n (%) | 237 (51.7) | 33 (60.0) | 33 (67.3)⁎ |
| Education, median (IQR), years | 6 (0, 9) | 4 (0, 9) | 4 (0, 9) |
| Risk factors | |||
| Smoking#, n (%) | 190 (41.5) | 20 (36.4) | 14 (28.6) |
| Drinking#, n (%) | 151 (33.0) | 16 (29.1) | 13 (26.5) |
| Hypertension, n (%) | 240 (52.4) | 38 (69.1)⁎ | 32 (65.3) |
| Diabetes, n (%) | 69 (15.1) | 8 (14.5) | 3 (6.1) |
| Hyperlipidemia, n (%) | 247 (53.9) | 36 (65.5) | 25 (51.0) |
| CVD, n (%) | 11 (2.4) | 1 (1.8) | 2 (4.1) |
| Cognitive function | |||
| MMSE#, median (IQR) | 27 (24, 29) | 27 (20, 29) | 26 (22, 29) |
| MoCA#, median (IQR) | 19 (14, 23) | 17 (11, 23) | 18 (12, 22) |
| CSVD markers | |||
| WMHs, n (%) | 7 (1.5) | 35 (63.6)⁎ | 18 (36.7)⁎ |
| LAC, n (%) | 65 (14.2) | 50 (90.9)⁎ | 35 (71.4)⁎ |
| Brain volume | |||
| TIV#, mean ± SD, cm3 | 1495.7 ± 158.0 | 1475.5 ± 191.4 | 1492.4 ± 117.4 |
| TGV#, mean ± SD, cm3 | 545.2 ± 63.8 | 549.7 ± 59.1 | 539.5 ± 72.1 |
| BPF#, median (IQR), % | 73.2 (70.9, 75.8) | 73.3 (71.8, 75.5) | 73.9 (71.8,75.9) |
Abbreviations: BPF, brain parenchymal fraction; CMBs, cerebral microbleeds; CSVD, cerebral small vessel disease; CVD, coronary vascular disease; IQR, interquartile range; LAC, lacune; MMSE, Mini-Mental Status Examination; MoCA, Montreal Cognitive Assessment; TGV, total gray volume; TIV, total intracranial volume; WMHs, white matter hyperintensities.
Significant difference between participants with and without CMBs (P < .05).
10 subjects had missing data for smoking and drinking status, nine for MMSE, 109 for MoCA, and nine for TIV, TGV and BPF.
3.2. CMBs and global cognitive dysfunction
Based on the education-adjusted cut-off values of the MMSE and MoCA, 127 (28.6%) participants had “normal cognition,” 256 (57.7%) had “mild cognitive dysfunction,” and 61 (13.7%) had “severe cognitive dysfunction”. The distribution of different locations of CMBs among the three cognitive groups is shown in Supplementary Table 4. In Table 2, the logistic regression revealed CMBs, especially deep/mixed CMBs were significantly associated with severe cognitive dysfunction (Model 1 and Model 2). Participants with deep/mixed CMBs exhibited an increased risk of severe cognitive dysfunction compared to participants without CMBs (Ptrend = 0.012). However, there was no significant association between either type of CMBs and mild cognitive dysfunction. Moreover, no significant association was found between lobar CMBs and global cognitive dysfunction in either of the models.
Table 2.
Association between cerebral microbleeds and global cognitive dysfunction.
| Global Cognitive Dysfunction |
P for trend | ||||
|---|---|---|---|---|---|
| Normal |
Mild |
Severe |
|||
| (N = 127) | (N = 256) | (N = 61) | |||
| CMBs | Model 1 | 1.0 (ref) | 1.79 (0.95–3.36) | 2.77 (1.23–6.20)⁎ | 0.009 |
| Model 2 | 1.0 (ref) | 1.60 (0.84–3.05) | 2.51 (1.10–5.73)⁎ | 0.017 | |
| Deep/Mixed | Model 1 | 1.0 (ref) | 2.40 (1.02–5.66) | 3.67 (1.28–10.47)⁎ | 0.009 |
| Model 2 | 1.0 (ref) | 2.16 (0.90–5.18) | 3.52 (1.21–10.26)⁎ | 0.012 | |
| Lobar | Model 1 | 1.0 (ref) | 1.22 (0.51–2.92) | 2.01 (0.65–6.19) | 0.251 |
| Model 2 | 1.0 (ref) | 1.10 (0.45–2.66) | 1.76 (0.56–5.53) | 0.363 | |
All results are presented as OR with 95%CI.
Model 1 was adjusted for age and sex; Model 2 was additionally adjusted for smoking, drinking, hypertension, diabetes, and hyperlipidemia based on Model 1.
P < .05.
3.3. CMBs and brain region volumes
Brain atrophy was a major finding of the brain MRI for cognitive dysfunction. Previous studies have demonstrated that several brain regions are atrophied in AD patients with cerebral amyloid angiopathy-related CMBs (Samuraki et al., 2015). Although we found no correlation between global brain atrophy (TIV, TGV, and BPF) and CMBs (Table 1), we further examined the relationships between CMBs and specific brain-region volumes (Table 3). The presence of CMBs (both deep/mixed and lobar) was significantly associated with smaller thalamic volume in the linear regression models, adjusting for age, sex, smoking, drinking, hypertension, diabetes, hyperlipidemia, and TIV. Strictly lobar CMBs were associated with decreased volumes in AD signature regions, including the hippocampus, thalamus, and amygdala. However, the volume and thickness of the main cortical lobes were not significantly decreased in the CMBs groups, regardless of CMB location (Table 3 and Supplementary Table 5). Moreover, decreased thalamic volume was associated with an increased probability of the presence of deep/mixed CMBs in the Poisson regression model (Supplementary Fig. 2). We also found decreased thalamic volume was correlated with severe cognitive dysfunction (Supplementary Table 6). The effects of thalamic volume on cognitive dysfunction were presented in different conditions (with and without CMBs) in Supplementary Table 7.
Table 3.
Association between different locations of cerebral microbleeds and brain region volumes.
| CMBs vs. no CMBs (94 vs. 433) | Deep/Mixed CMBs vs. no CMBs (50 vs. 433) | Lobar CMBs vs. no CMBs (44 vs. 433) | ||
|---|---|---|---|---|
| Subcortical | ||||
| Hippocampus | Model 1 | −0.030 (0.062) | 0.039 (0.080) | −0.091 (0.085)⁎ |
| Model 2 | −0.029 (0.064) | 0.040 (0.083) | −0.091 (0.086)⁎ | |
| Thalamus | Model 1 | −0.103 (0.105)⁎ | −0.068 (0.134)⁎ | −0.092 (0.144)⁎ |
| Model 2 | −0.105 (0.108)⁎ | −0.070 (0.138)⁎ | −0.094 (0.146)⁎ | |
| Amygdala | Model 1 | −0.029 (0.033) | 0.028 (0.043) | −0.076 (0.046)⁎ |
| Model 2 | −0.032 (0.034) | 0.031 (0.044) | −0.080 (0.047)⁎ | |
| Accumbens | Model 1 | 0.027 (0.015) | 0.059 (0.019) | −0.080 (0.019) |
| Model 2 | 0.020 (0.015) | 0.047 (0.020) | −0.023 (0.019) | |
| Cortical | ||||
| Frontal | Model 1 | −0.019 (2.056) | 0.019 (2.650) | −0.049 (2.900) |
| Model 2 | −0.011 (2.109) | 0.024 (2.731) | −0.039 (2.931) | |
| Parietal | Model 1 | −0.015 (1.686) | 0.015 (2.194) | −0.037 (2.353) |
| Model 2 | −0.005 (1.736) | 0.022 (2.275) | −0.029 (2.390) | |
| Temporal | Model 1 | −0.018 (1.763) | 0.028 (2.276) | −0.054 (2.481) |
| Model 2 | −0.008 (1.812) | 0.038 (2.350) | −0.047 (2.514) | |
| Occipital | Model 1 | −0.016 (0.847) | 0.007 (1.102) | −0.030 (1.184) |
| Model 2 | −0.003 (0.872) | 0.015 (1.142) | −0.017 (1.201) | |
All results are presented as standardized coefficients β (SE).
Model 1 was adjusted for age, sex and TIV; Model 2 was additionally adjusted for smoking, drinking, hypertension, diabetes, and hyperlipidemia based on Model 1.
P < .05.
3.4. Deep/mixed CMBs and white matter fiber tract integrity
Decreased integrity of white matter fiber tracts is another contributing factor to cognitive dysfunction. To explore the underlying association between deep/mixed CMBs and global cognitive dysfunction, we used TBSS to compare the integrity of the white matter fiber tracts of participants with no CMBs to those with deep/mixed CMBs (Fig. 1). The FA value of bilateral anterior thalamic radiations (ATR) was significantly decreased in the deep/mixed CMBs group, compared with the no-CMBs group, after adjusting for the covariates (P < .05) (Table 4). In addition, the right superior longitudinal fasciculus was observed with small voxels; the P-value was not statistically significant, which suggests limited clinical significance (Table 4).
Table 4.
Locations of white matter tracts with significantly decreased fractional anisotropy values in participants with deep/mixed cerebral microbleeds (CMBs) compared to those without CMBs.
| Cluster index | Location | R/L | MNI |
Voxels | P value | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| 1 | Anterior thalamic radiation | R | 14 | −9 | 32 | 4426 | 0.02 |
| 2 | Anterior thalamic radiation | L | −17 | 40 | −6 | 2227 | 0.02 |
| 3 | Superior longitudinal fasciculus | R | −19 | 27 | 32 | 10 | 0.05 |
| 4 | Anterior thalamic radiation | L | −31 | 38 | 8 | 1 | 0.05 |
Abbreviations: MNI, Montreal Neurological Institute; L, left; R, right.
4. Discussion
This study assessed the relationship between CMBs at different locations and global cognitive dysfunction. We found CMBs, particularly deep/mixed CMBs, were associated with cognitive dysfunction. Moreover, deep/mixed CMBs had a significant relationship with decreased thalamic volume and decreased FA values of anterior thalamic radiations. These findings have the potential to illuminate the mechanism underlying the association between deep/mixed CMBs and cognitive dysfunction.
The prevalence of CMBs was 18.5% (9.8% for deep/mixed CMBs and 8.7% for lobar CMBs in our sample of rural Han Chinese [age 55–65 years]). In another population with similar age range (60–69 years) from the Rotterdam Study, the prevalence of CMBs was 16.8% (Poels et al., 2010). In general, the prevalence of CMBs in our study was higher than that found in Caucasian samples (Framingham Study = 8.8%; AGES-Reykjavik Study = 11.1%; the Atahualpa Project = 11%) (Romero et al., 2014; Sveinbjornsdottir et al., 2008; Del Brutto et al., 2015). Differences in race-ethnicity, MRI parameters, and diagnostic criteria may contribute to this heterogeneity.
The clinical features and pathological characteristics of CMBs vary depending on their location. CMBs in deep or mixed regions mostly result from hypertensive vasculopathy, whereas lobar CMBs are related to cerebral amyloid angiopathy. Our results demonstrated that deep/mixed CMBs, rather than strictly lobar CMBs, were related to cognitive dysfunction, which corresponds with the findings of previous studies. A cross-sectional Japanese study of a health-screening sample without neurological disorders showed that CMB-related global cognitive dysfunction was more common in persons with deep CMBs (Yakushiji et al., 2012). Another cross-sectional study on an elderly sample with increased vascular risk revealed infratentorial CMBs were associated with impaired memory and executive function (van Es et al., 2011). Similar results were found by longitudinal research on Western populations. A recent report from the AGES–Reykjavik Study found a causal relationship between multiple deep/mixed CMBs (≥2) and decline in global cognitive function (Ding et al., 2017). However, several studies suggest lobar CMBs are related to cognitive dysfunction (Chung et al., 2016; Poels et al., 2012), but this relationship was not observed in our study. This negative result might be due to the relatively small sample size. Unlike deep CMBs, β-amyloid deposition is the basic pathological characteristic of lobar CMBs. Lobar CMBs in our study were associated with decreased volume in AD-related regions (i.e., the hippocampus, thalamus, and amygdala), which is in line with the results of the Atherosclerosis Risk in Communities (ARIC) study (Graff-Radford et al., 2017).
The association between deep/mixed CMBs and cognition has been confirmed by numerous studies, but the underlying mechanisms remain unclear. Hypertensive arteriopathy is thought to induce neurodegenerative diseases by decreasing blood flow and impairing vascular autoregulation (Yarchoan et al., 2012). Thus, deep/mixed CMBs are likely to be downstream lesions of cerebral angiopathy and affect cognitive function together with widespread cerebral small vascular diseases. However, some research shows deep or mixed CMBs in specific regions can strategically impair particular cognitive domains (van Es et al., 2011; Roddy et al., 2016). One reasonable hypothesis is that CMBs cause cognitive dysfunction by damaging surrounding structures or significant connective fibers (Werring et al., 2004). Our results support this hypothesis in that we found deep/mixed CMBs were associated with both decreased thalamic volume and damaged thalamocortical tracts, which represents local necrosis resulting from nearby CMBs. Given our findings, it is reasonable to suppose the thalamus is involved in deep/mixed CMB-related cognitive dysfunction.
The thalamus is an important structure regulating sensory and motor function, cognition, and consciousness throughout the entire projection system. Thalamic lesions (e.g., atrophy, infarcts, and trauma) can lead to cognitive dysfunction in several domains (Little et al., 2010; Danet et al., 2015). The Epidemiology of Dementia in Singapore (EDIS) study found smaller thalamic volume was significantly associated with lower cognitive functioning in non-demented, community-dwelling individuals (Hilal et al., 2015). A subcortical atrophy pattern (including the thalamus, striatum and cerebellum) appears to be involved in the network decline of memory and executive function in late-onset AD patients (Zhang et al., 2016). This relationship was also identified in our study. Moreover, the integrity of functional connectivity and microstructure within the thalamus is significant for normal cognitive functioning (Fama and Sullivan, 2015), which will be detailed in the following discussion.
Changes in white matter microstructure are considered to be pathological manifestation secondary to gray matter atrophy in relation to cognitive dysfunction. Compared with conventional MRI, DTI can detect abnormal signals (i.e., anisotropy decrease) arising from disruptions in white matter microstructure in the early disease stage. Many studies have reported abnormal DTI parameters in various brain regions among patients with mild cognitive impairment (MCI) and AD (Zhou et al., 2008; Liu et al., 2011). A prospective study of the disruption of white matter microstructure found DTI parameters predicted cognitive decline and the future progression of dementia (Zeestraten et al., 2017). Recent research has found vascular angiopathy contributes to this process. Cross-sectional studies found DTI measures were significantly related to global cognitive function in patients with sporadic CSVDs or cerebral autosomal dominance with subcortical infarcts and leukoencephalopathy (O'Sullivan, 2004; Molko et al., 2002). Compared to MCI patients without CSVDs, patients with MCI and CSVDs have decreased FA values in the genu of the corpus callosum, the bilateral internal and external capsule, and periventricular white matter regions (Papma et al., 2014). Our study found the FA values of bilateral anterior thalamic radiations in the deep/mixed CMBs group were lower than those of participants without CMBs, indicating damage to the integrity of thalamocortical connectivity. ATR, a major thalamic white matter tract, which projects to the hippocampus through connections between anterior thalamic nuclei and the anterior cingulate cortex, contributes to long-term memory storage. Moreover, ATR-connected mediodorsal thalamic nuclei are linked to the prefrontal cortex, which controls executive functions. Our findings suggest that deep/mixed CMBs might be associated with cognitive dysfunction by destroying the surrounding ATR, which is consistent with the viewpoint described earlier.
In this study, we highlight the role of thalamocortical connectivity in deep/mixed CMB-associated cognitive dysfunction, which provides a new direction for the study of CSVDs. Multimodal MRI assessments of brain volume and fiber tract integrity may help us understand the associations among CMBs, thalamic lesions, and cognitive dysfunction. Several limitations of our study need to be mentioned. First, the sequence we used to identify CMBs was not sensitive enough for evaluating quite small lesions. Considering this limitation, a susceptibility weighted imaging (SWI) sequence with higher resolution (0.7 × 0.7 × 1.5 mm) was used in our following research. Second, the study participants were selected using the TIS baseline data. As causal inferences cannot be drawn from cross-sectional designs, we cannot conclude there are causal relationships among CMBs, the thalamus, and cognition. We are currently expanding the TIS and following all the participants longitudinally to establish solid associations between CMBs, MCI, and dementia. Third, the measures of cognitive function we used have limitations. Given the high sensitivity and specificity of the MMSE and MoCA, they provide reasonable estimates of global cognitive function; however, more specific neuropsychological measures of different cognitive domains are needed in future studies. And finally, our study focused on a population 55 to 65 years old, so most of the sample was middle-aged and had a low prevalence of age-related chronic diseases. This choice of age-range represents a compromise: the participants were old enough to exhibit pre-dementia, yet young enough for initial assessments to be made before the incipient disease had a material impact. In general, the TIS provides an invaluable resource for exploring environmental and genetic risk factors for stroke and dementia by measuring numerous variables (including brain MRI, carotid artery ultrasound, and cognitive function) for each participant. As the time frame of the study is extended, we expect to discover evidence-based causal relationships over decades.
5. Conclusion
Deep/mixed CMBs were associated with global cognitive dysfunction in a rural Han Chinese population (age 55–65 years), which might have resulted from disruption of thalamic connectivity. A longitudinal cohort study using a larger sample size is warranted to confirm our findings.
Acknowledgments
Acknowledgments
We thank all the individuals who participated in this study and appreciate the special contributions of statistical analyses and MRI post-processing by Dr. Zhenqiu Liu, Dr. Fei Dai, Dr. Lei Wei and Dr. He Wang.
Funding
This work was supported by the National Key Research and Development Program of China (grant numbers: 2017YFC0907002, 2017YFC0907501/4, 2017YFC0907201); the International S&T Cooperation Program of China (grant number: 2014DFA32830); the Key Research and Development Plans of Jiangsu Province, China (grant number: BE2016726); the Key Basic Research Grants from Science and Technology Commission of the Shanghai Municipality (grant number: 16JC1400500); and the Shanghai Municipal Science and Technology Major Project (grant number: 2017SHZDZX01). Except data collection, none of these funding sources were involved in the study design, data analysis, or interpretation, or the writing of the report and the decision to submit the article for publication.
Declarations of interest
None.
Ethics approval
The study was approved by the Ethics Committee of the School of Life Sciences, Fudan University, Shanghai, China (Institutional Review Board approval number 469).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101749.
Contributor Information
Mei Cui, Email: cuimei@fudan.edu.cn.
Xingdong Chen, Email: xingdongchen@fudan.edu.cn.
Appendix A. Supplementary data
Supplementary material
References
- Akoudad S., Portegies M.L., Koudstaal P.J. Cerebral microbleeds are associated with an increased risk of stroke: the Rotterdam study. Circulation. 2015;132:509–516. doi: 10.1161/CIRCULATIONAHA.115.016261. [DOI] [PubMed] [Google Scholar]
- Chan K.Y., Wang W., Wu J.J. Epidemiology of Alzheimer's disease and other forms of dementia in China, 1990-2010: a systematic review and analysis. Lancet (London, England) 2013;381:2016–2023. doi: 10.1016/S0140-6736(13)60221-4. [DOI] [PubMed] [Google Scholar]
- Chung C.P., Chou K.H., Chen W.T. Strictly lobar cerebral microbleeds are associated with cognitive impairment. Stroke. 2016;47:2497–2502. doi: 10.1161/STROKEAHA.116.014166. [DOI] [PubMed] [Google Scholar]
- Danet L., Barbeau E.J., Eustache P. Thalamic amnesia after infarct: the role of the mammillothalamic tract and mediodorsal nucleus. Neurology. 2015;85:2107–2115. doi: 10.1212/WNL.0000000000002226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Brutto V.J., Zambrano M., Mera R.M., Del Brutto O.H. Population-based study of cerebral microbleeds in stroke-free older adults living in rural Ecuador: the Atahualpa project. Stroke. 2015;46:1984–1986. doi: 10.1161/STROKEAHA.115.009594. [DOI] [PubMed] [Google Scholar]
- Ding J., Sigurðsson S., Jónsson P.V. Space and location of cerebral microbleeds, cognitive decline, and dementia in the community. Neurology. 2017;88:2089–2097. doi: 10.1212/WNL.0000000000003983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es A.C., van der Grond J., de Craen A.J. Cerebral microbleeds and cognitive functioning in the PROSPER study. Neurology. 2011;77:1446–1452. doi: 10.1212/WNL.0b013e318232ab1d. [DOI] [PubMed] [Google Scholar]
- Fama R., Sullivan E.V. Thalamic structures and associated cognitive functions: relations with age and aging. Neurosci. Biobehav. Rev. 2015;54:29–37. doi: 10.1016/j.neubiorev.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Radford J., Simino J., Kantarci K. Neuroimaging correlates of cerebral microbleeds: the ARIC study (atherosclerosis risk in communities) Stroke. 2017;48:2964–2972. doi: 10.1161/STROKEAHA.117.018336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilal S., Amin S.M., Venketasubramanian N. Subcortical atrophy in cognitive impairment and dementia. J. Alzheimers Dis. 2015;48:813–823. doi: 10.3233/JAD-150473. [DOI] [PubMed] [Google Scholar]
- de Laat K.F., van den Berg H.A., van Norden A.G., Gons R.A., Olde Rikkert M.G., de Leeuw F.E. Microbleeds are independently related to gait disturbances in elderly individuals with cerebral small vessel disease. Stroke. 2011;42:494–497. doi: 10.1161/STROKEAHA.110.596122. [DOI] [PubMed] [Google Scholar]
- Li H., Jia J., Yang Z. Mini-mental state examination in elderly Chinese: a population-based normative study. J. Alzheimers Dis. 2016;53:487–496. doi: 10.3233/JAD-160119. [DOI] [PubMed] [Google Scholar]
- Little D.M., Kraus M.F., Joseph J. Thalamic integrity underlies executive dysfunction in traumatic brain injury. Neurology. 2010;74:558–564. doi: 10.1212/WNL.0b013e3181cff5d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Spulber G., Lehtimaki K.K. Diffusion tensor imaging and tract-based spatial statistics in Alzheimer's disease and mild cognitive impairment. Neurobiol. Aging. 2011;32:1558–1571. doi: 10.1016/j.neurobiolaging.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Lu J., Li D., Li F. Montreal cognitive assessment in detecting cognitive impairment in Chinese elderly individuals: a population-based study. J. Geriatr. Psychiatry Neurol. 2011;24:184–190. doi: 10.1177/0891988711422528. [DOI] [PubMed] [Google Scholar]
- Molko N., Pappata S., Mangin J.F. Monitoring disease progression in CADASIL with diffusion magnetic resonance imaging: a study with whole brain histogram analysis. Stroke. 2002;33:2902–2908. doi: 10.1161/01.str.0000041681.25514.22. [DOI] [PubMed] [Google Scholar]
- Mori S., Kaufmann W.E., Davatzikos C. Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magn. Reson. Med. 2002;47:215–223. doi: 10.1002/mrm.10074. [DOI] [PubMed] [Google Scholar]
- Nasreddine Z.S., Phillips N.A., Bédirian V. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- O'Sullivan M. Diffusion tensor MRI correlates with executive dysfunction in patients with ischaemic leukoaraiosis. J. Neurol. Neurosurg. Psychiatry. 2004;75:441–447. doi: 10.1136/jnnp.2003.014910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papma J.M., de Groot M., de Koning I. Cerebral small vessel disease affects white matter microstructure in mild cognitive impairment. Hum. Brain Mapp. 2014;35:2836–2851. doi: 10.1002/hbm.22370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poels M.M., Vernooij M.W., Ikram M.A. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke. 2010;41:S103–S106. doi: 10.1161/STROKEAHA.110.595181. [DOI] [PubMed] [Google Scholar]
- Poels M.M., Ikram M.A., van der Lugt A. Cerebral microbleeds are associated with worse cognitive function: the Rotterdam Scan Study. Neurology. 2012;78:326–333. doi: 10.1212/WNL.0b013e3182452928. [DOI] [PubMed] [Google Scholar]
- Roddy E., Sear K., Felton E. Presence of cerebral microbleeds is associated with worse executive function in pediatric brain tumor survivors. Neuro-oncology. 2016;18:1548–1558. doi: 10.1093/neuonc/now163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero J.R., Preis S.R., Beiser A. Risk factors, stroke prevention treatments, and prevalence of cerebral microbleeds in the Framingham Heart Study. Stroke. 2014;45:1492–1494. doi: 10.1161/STROKEAHA.114.004130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero J.R., Preis S.R., Beiser A. Cerebral microbleeds as predictors of mortality: the Framingham Heart Study. Stroke. 2017;48:781–783. doi: 10.1161/STROKEAHA.116.015354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero J.R., Beiser A., Himali J.J., Shoamanesh A., DeCarli C., Seshadri S. Cerebral microbleeds and risk of incident dementia: the Framingham Heart Study. Neurobiol. Aging. 2017;54:94–99. doi: 10.1016/j.neurobiolaging.2017.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuraki M., Matsunari I., Yoshita M. Cerebral amyloid Angiopathy-related microbleeds correlate with glucose metabolism and brain volume in Alzheimer's disease. J. Alzheimers Dis. 2015;48:517–528. doi: 10.3233/JAD-150274. [DOI] [PubMed] [Google Scholar]
- Smith E.E., Saposnik G., Biessels G.J. Prevention of stroke in patients with silent cerebrovascular disease: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48 doi: 10.1161/STR.0000000000000116. e44-e71. [DOI] [PubMed] [Google Scholar]
- Sveinbjornsdottir S., Sigurdsson S., Aspelund T. Cerebral microbleeds in the population based AGES-Reykjavik study: prevalence and location. J. Neurol. Neurosurg. Psychiatry. 2008;79:1002–1006. doi: 10.1136/jnnp.2007.121913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Lu M., Qian J. Rationales, design and recruitment of the Taizhou Longitudinal Study. BMC Public Health. 2009;9 doi: 10.1186/1471-2458-9-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw J.M., Smith E.E., Biessels G.J. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werring D.J., Frazer D.W., Coward L.J. Cognitive dysfunction in patients with cerebral microbleeds on T2*-weighted gradient-echo MRI. Brain. 2004;127:2265–2275. doi: 10.1093/brain/awh253. [DOI] [PubMed] [Google Scholar]
- Xu X., Chan Q.L., Hilal S. Cerebral microbleeds and neuropsychiatric symptoms in an elderly Asian cohort. J. Neurol. Neurosurg. Psychiatry. 2016;88:7–11. doi: 10.1136/jnnp-2016-313271. [DOI] [PubMed] [Google Scholar]
- Yakushiji Y., Noguchi T., Hara M. Distributional impact of brain microbleeds on global cognitive function in adults without neurological disorder. Stroke. 2012;43:1800–1805. doi: 10.1161/STROKEAHA.111.647065. [DOI] [PubMed] [Google Scholar]
- Yarchoan M., Xie S.X., Kling M.A. Cerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementias. Brain. 2012;135:3749–3756. doi: 10.1093/brain/aws271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates P.A., Desmond P.M., Phal P.M. Incidence of cerebral microbleeds in preclinical Alzheimer disease. Neurology. 2014;82:1266–1273. doi: 10.1212/WNL.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeestraten E.A., Lawrence A.J., Lambert C. Change in multimodal MRI markers predicts dementia risk in cerebral small vessel disease. Neurology. 2017;89:1869–1876. doi: 10.1212/WNL.0000000000004594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Mormino E.C., Sun N., Sperling R.A., Sabuncu M.R., Yeo B.T. Bayesian model reveals latent atrophy factors with dissociable cognitive trajectories in Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E6535–E6544. doi: 10.1073/pnas.1611073113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Liu L., Sun H. Cerebral microbleeds are associated with mild cognitive impairment in patients with hypertension. J. Am. Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.008453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Dougherty J.H., Jr., Hubner K.F., Bai B., Cannon R.L., Hutson R.K. Abnormal connectivity in the posterior cingulate and hippocampus in early Alzheimer's disease and mild cognitive impairment. Alzheimers Dement. 2008;4:265–270. doi: 10.1016/j.jalz.2008.04.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material