Abstract
Background:
There is no consistent association between individual histological lesions and composite scores in donor kidney biopsy and transplant outcomes.
Objective:
To evaluate which acute or chronic individual histological lesions and composite scores in donor kidney were associated with graft survival in the recipient.
Methods:
We investigated the association of individual histological lesions and 8 composite scoring systems in implantation biopsies of cadaveric (n=101) and living (n=29) kidneys with 5-year death-censored graft survival.
Results:
We found a high frequency of chronic lesions in donor kidneys, mostly associated with arteriosclerosis, and less dependent from donor age. Acute, chronic, and total Banff scores for post-transplant biopsies, chronic and total Banff scores for pre-implant biopsies, donor damage score and chronic damage score predicted death-censored graft loss. However, only chronic and total Banff-scores had significant effects in multivariate model. Chronic pre-implant and total post-transplant Banff scores demonstrated the highest area under the curve (AUC) of 0.722 and 0.717, respectively. Among individual lesions, glomerulosclerosis ≥20%, interstitial inflammation >0, arteriosclerosis =3, arteriolar hyalinosis >0, and interstitial fibrosis >0, assessed with Banff-grading criteria, were associated with lower allograft survival. We created the Donor Kidney Damage Index (DKDI), by summing regression coefficients for these lesions, which yielded the AUC of 0.747. When combined with retransplantation, cold ischemia time and acute rejection, DKDI, chronic pre-implant and total post-transplant Banff scores further improved their predictive accuracy, yielding AUCs of 0.842, 0.807, and 0.802, respectively.
Conclusion:
DKDI, chronic pre-implant and total post-transplant Banff scores alone and combined with clinical variables may facilitate decision making in post-transplant period.
Key Words: Implantation biopsy histology, Individual and composite histological scores, Kidney allograft survival
INTRODUCTION
Adiscrepancy between the number of patients on the waiting list for kidney transplantation and donor organs supply [1] fosters a wider use of kidneys from expanded criteria donors (ECD) [2-5], donors after cardiac death [6], donors with history of cardiovascular diseases (CVD) [3, 7], and older donors [1, 6]. However, there is evidence that graft survival after transplantation of higher risk donor kidneys is suboptimal [3, 4, 6]. Given an expanding pool of donors, many studies were performed on the association between baseline chronic histological features of deceased donor kidneys with short-term [8, 10, 14, 15] and long-term graft outcomes [3, 6-8, 10, 14, 15]. Glomerulosclerosis [7, 8, 14], vascular narrowing [2, 3, 10, 13, 16, 17] and interstitial fibrosis [2, 18] attract the most attention as predictive variables. However, there are no consistent association between individual lesions and transplant outcomes [2, 3, 10, 16, 18-22]. Some possible explanations for such inconsistent results are non-uniformity in grading histological lesions [12, 23, 24] or in defining graft outcomes [2, 7, 9, 14, 23]. Furthermore, studies vary in terms of patient selection [19, 25], and some results are not corrected for covariates [22]. It is also unclear whether acute lesions in the donor kidney can provide prognostic information, in addition to the chronic lesions [19, 21, 25, 26]. With reference to the complex kidney architecture, composite scoring systems have been proposed to integrate histopathological findings in different compartments [2, 7, 12, 15, 23, 24, 27-30]. A systematic review by Wang with co-workers [22] cites 15 scoring systems, which can be applied to procurement and implantation biopsies. However, their reliability is still a matter of debate [2, 7, 22]. The majority of results were obtained on selected deceased donor cohorts [28, 30] and less attention was paid to living donors [17, 25]. There is still no consensus on the prognostic significance of the pathologic findings in individual compartments of donor kidney biopsy compared to integrative assessment. Some authors suggested composite clinico-morphological scoring systems [7] that may improve prediction. Nevertheless, only few studies included post-transplant variables as covariates [10, 29]. Given a wide inequality in the prevalence of atherosclerosis and hypertension among different countries and ethnicities [31], it is possible that not all scales are equally well suited for all populations. Furthermore, Banff Consensus Criteria for pre-implant biopsies have only been recently published [32], therefore, their predictive values have not been examined in details yet.
Figure 3.
Association of death-censored kidney graft survival and selected individual histological lesions in intra-operative zero-hour biopsies (Kaplan-Meier estimates). A) Glomerulosclerosis; B) Arteriolar hyalinosis; C) Arteriosclerosis; D) Interstitial fibrosis; and E) Interstitial inflammation. P values are calculated with the log-rank test
The objective of this study was to evaluate which acute or chronic histological lesions and composite histological scores in donor kidney intraoperative biopsies alone or in combination with clinical variables were best associated with kidney graft survival over a 5-year period.
PATIENTS AND METHODS
Study Population and Design
We conducted a single-center, retrospective cohort study of 130 kidney transplant recipients. In April 2005, the practice to obtain intraoperative pre-implant and post-reperfusion biopsies was introduced at the Zaporizhzhia Transplant Center. Until December 2010, 165 consecutive patients received a single cadaveric or living kidney only transplant and 156 kidneys were biopsied, except for children under 13 years. After exclusion of nine grafts that failed within the first two weeks, seven patients without follow-up data, and 10 with inadequate biopsies, the remaining 130 patients and biopsies were included in the study (Table 1). Donor population included live donors (32.6%), ideal deceased donors (50%), ECD (7.9%), and non-heart-beating donors (9.0%). All recipients and donors were Caucasians. Recipients aged older than 16 years gave their written informed consents. Parental consent was obtained for all participants under 16 years. This research was carried out in accordance with the ethical standards laid down in the Declaration of Helsinki (2013 version) and the Declaration of Istanbul; it was approved by the local Ethical Review Board.
Table 1.
Donors and recipients demographics. Values are mean±SD, n (%) or median (IQR)
| Recipient characteristics (n=130) | Transplantation from deceased donor (n=101) | Transplantation from living donor (n=29) | p value |
|---|---|---|---|
| Age (yrs) | 40±11 | 26±11 | <0.001 |
| Males | 65 (64.4) | 16 (55.2) | 0.368 |
| Cause of ESRD: | |||
| Glomerulonephritis | 80 (79.2) | 18 (62.07) | 0.059 |
| Congenital urological anomaly | 5 (5.0) | 4 (13.79) | 0.098 |
| Polycystic kidney disease | 7 (6.9) | 2 (6.90) | 0.995 |
| Metabolic/Diabetic nephropathy | 5 (5.0) | 1 (3.45) | 0.734 |
| Pyelonephritis/interstitial nephritis | 2 (2.0) | 3 (10.34) | 0.039 |
| Hereditary | 1 (1.0) | 1 (3.45) | 0.343 |
| Vascular | 1 (1.0) | 0 (0) | 0.591 |
| Treated hypertension (without space before) | 69 (68.3) | 20 (70.0) | 0.947 |
| BMI (kg/m2) | 24.2±4.3 | 21.9±3.8 | 0.020 |
| Dialysis duration (months) | 30 (12–48) | 10 (5–20) | <0.001 |
| HD vs. PD | 96 (95.0)/5 (5.0) | 26 (89.7)/3 (10.2) | 0.287 |
| Second warm ischemia time (min) | 22.8±9.1 | 22.4±4.9 | 0.819 |
| Previous transplants | 4 (3.1) | 0 | 0.276 |
| Donor characteristics (n=89) | Cadaver (n=60) | Living (n=29) | |
| Males | 48 (80) | 9 (31) | <0.001 |
| Age, years | 39±11 | 46±10 | <0.001 |
| Cause of death: | |||
| Stroke | 29 (48) | ||
| Cranial trauma | 24 (40) | ||
| Polytrauma | 3 (5) | ||
| Brain tumor | 3 (5) | ||
| Anoxia | 1 (2) | ||
| DCD | 8 (13) | ||
| ECD | 7 (12) | ||
| Cold ischemia time, hours | 15.1±4.1 | 1.2±0.4 | <0.001 |
ESRD: end-stage renal disease; BMI: body mass index; HD: hemodialysis; PD: peritoneal dialysis; DCD: donors after cardiac death; ECD: expanded-criteria donors
Histological Scoring
Needle (14–18G) pre-implant and post-reperfusion biopsies were obtained. The tissue was fixed in 10% buffered formalin and embedded in paraffin. For light microscopy, 3–4-µm-thick sections were stained with H&E, PAS, and Masson’s trichrome. Pathologic examination was carried out by two renal pathologists (TN and AT) according to Banff 1997 criteria [24], as the biopsies arrived. Then, all biopsies were rescored by one pathologist (AT) who was blind to demographic and post-transplant course data. In such a way, the following criteria were applied: 1) Banff criteria for post-transplant biopsies [24, 33], 2) Banff criteria for pre-implant biopsies [32], 3) Remuzzi criteria [12], and 4) Cosyns criteria [23].
Banff grading approach and related scores
Briefly, individual acute and chronic lesions in all kidney compartments were evaluated with post-transplant Banff score, as described elsewhere [24, 33]. Interstitial inflammation (I), tubulitis (T), glomerulitis (G), PTC-capillaritis (PTC), arteritis (V), interstitial fibrosis (IF), tubular atrophy (TA), arteriolar hyalinosis (AH), arteriosclerosis (AS), glomerular basement membrane thickening (BM), and mesangial matrix increase (MM) were scored on a 0–3 scale. Glomerulosclerosis (GS) was assessed as a percentage of glomeruli with global sclerosis. Based on the estimation of individual variables, we calculated the chronic post-transplant Banff score as a sum of scores for BM, MM, TA, IF, AS, AH, and GS%×3 [2, 6]. We derived acute post-transplant Banff score by adding scores for G, I, T, V, and PTC. Eventually, we computed the total post-transplant Banff score. Banff-criteria for pre-implant biopsies also adopt a four-point (0, 1, 2, 3) scale, and suggest evaluation of I score, glomeruli thrombi (GT) and acute tubular injury (ATI) among acute lesions, and TA, IF, AS, AH, and GS among chronic lesions. ATI was scored separately for pre-implant and post-reperfusion biopsies, whereas for scoring all the rest variables biopsies were combined. The sum of scores for individual variables yielded the composite scores such as chronic pre-implant Banff score (TA, IF, AS, AH, GS%×3), acute pre-implant Banff score (I, ATI, GT), and total pre-implant Banff score. Furthermore, we calculated previously published composite histological scores, such as chronic allograft damage index (CADI) [27], donor damage score (DDS) [28], chronic damage score (CDS) [15], and interstitial fibrosis and fibrous thickening score (CIV) [2]. These scores grade the lesions as 0–3, based on Banff-criteria [24, 33], except for GS, which is scored semi-quantitatively.
Remuzzi grading approach and score
Criteria of Remuzzi [12] include evaluation of the percentage of global GS, TA, IF, and vascular narrowing, each scored from 0–3. The Remuzzi score was calculated by adding GS, TA, IF, and vascular narrowing scores.
Cosyns grading approach and scores
Acute and chronic changes in all kidney compartments were also graded from 1–3, using definitions suggested by Cosyns and co-authors [23]. Evaluation of acute lesions included GT, tubular epithelial cell degeneration and vacuolization, and interstitial edema. Chronic lesions included global GS, TA, AS, AH, IF, and I scores. Based on the estimation of individual variables by Cosyns qualifier, we calculated acute lesion index (ALI) as the sum of GT, tubular epithelial cell degeneration, tubular epithelial cell vacuolization, and interstitial edema scores; chronic lesion index (CLI) was derived by adding GS, TA, I, IF, AH, AS scores, and total score, i.e., the sum of ALI and CLI.
Clinical Risk Factors and Outcomes Examined
The analysis was performed 60 months later after the last transplantation was done in the study population. Donor and recipient characteristics (Table 1) and transplant outcomes were extracted from patients’ records and outpatients’ cards. Post-transplant data retrieved were limited to the initial graft function—immediate or delayed (DGF)—and the number and time of acute rejection (AR) episodes or pyelonephritis, and time of graft failure (defined as return to dialysis therapy). The end-point of the study was 5-year graft survival. All recipients received triple maintenance immunosuppressive therapy consisting of calcineurin-inhibitor, mycophenolate mofetil and steroid. Each patient enrolled in this study was followed for five years until death, return to dialysis or until December 2015.
Statistical Analysis
Normally distributed data are presented as mean±SD; their means were compared with Student’s t test. Continuous nonparametric data are expressed as median (IQR); their comparison was made by Mann-Whitney U test. Proportions are expressed as percentages and compared with χ2 test. The relationships between the individual histologic lesions and donor age were assessed using principal component analysis (PCA). To determine the predictive value of the clinical and histological predictors of worse graft survival, a forward stepwise multivariate Cox regression analysis was performed. Death-censored graft survival was calculated by the Kaplan-Meier method. The groups were compared with the log-rank test. We used rounded coefficients obtained in Cox regression for individual variables to create a new composite score. We calculated the area under the receiver operating characteristic (ROC) curves (AUC) to assess predictive accuracy of composite scoring systems for graft failure. AUCs were compared with DeLong-test. SPSS® for Windows® ver 19.0 (SPSS Inc, Chicago, IL, USA) and Medcalc V.14.8.1 (MedCalc Software bvba, Ostend, Belgium) were used for statistical analyses. A p value <0.05 was considered statistically significant.
RESULTS
Recipients and Donors Pretransplant Characteristics
The demographics and clinical characteristics of recipients and donors are shown in Table 1. The majority of patients received their first allograft (96.9%) from a deceased donor (77.7%). Most of deceased donors died of stroke or head injury; males prevailed in this cohort. Deceased donors were also younger; the cold ischemia time was significantly longer than in living donors. Fourteen (10.8%) recipients included in the study received kidneys from donors after cardiac arrest; nine (6.9%) received kidneys from ECD.
Biopsy Data
Acute lesions
Table 2 shows the distribution of the histopathological lesions. Interstitial inflammation was graded I1 in four (3.1%) patients. ATI of grade 1 was observed in 29 (24.4%) of pre-implant biopsies and grade 2 in 90 (75.6%) of pre-implant biopsies (Fig 1). ATI of grade 1 was noted in 9 (8.0%) and grade 2 in 104 (92.0%) of post-reperfusion biopsies. The severity of ATI in pre-implant biopsies was significantly higher in cadaver kidneys. The severity of ATI in post-reperfusion biopsies increased compared to pre-implant ones (p=0.006), but the difference was significant (p=0.012) only for cadaver kidneys. The mean±SD number of glomeruli per patient was 13.8±6.5. Glomerulitis was graded as G1 in 14 (10.8%) patients and G2 in 12 (9.2%). The frequencies of GT score 1, 2, and 3 were 21.5%, 12.3%, and 6.9%, respectively (Fig 1). The GT score was significantly higher in cadaver biopsies.
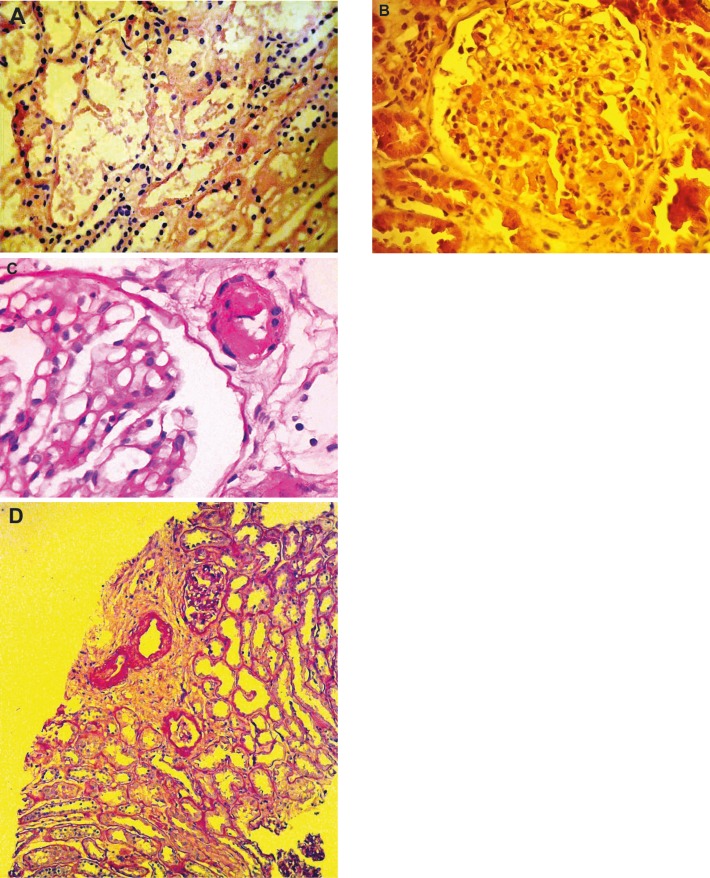
Figure 1.
Histopathological findings in implantation kidney biopsies. A) Focal acute tubular necrosis, H&E staining (original magnification ×400); B) Glomerular thrombi, H&E (original magnification ×400); C) Arteriolar hyalinosis, PAS staining (original magnification ×400); D) Arteriosclerosis, focal interstitial fibrosis, and acute tubular injury, PAS staining (original magnification ×200).
Table 2.
Biopsy characteristics evaluated with Banff-grading criteria
| Histological lesions | Deceased-donor kidney (n=101) |
Live-donor kidney (n=29) |
p value | ||||
|---|---|---|---|---|---|---|---|
| Score>0 n (%) |
Mean±SD | Range | Score>0 n (%) | Mean±SD | Range | ||
| I score | 3 (3.0) | 0.03±0.17 | 0–1 | 1 (3) | 0.03±0.19 | 0–1 | 0.969 |
| ATI pre-implant score | 95 (100.0) | 1.84±0.37 | 1–2 | 24 (100) | 1.54±0.51 | 1–2 | 0.026 |
| ATI post-reperfusion score | 95 (100.0) | 1.96±0.21 | 1–2 | 18 (100) | 1.72±0.46 | 1–2 | 0.121 |
| G score | 22 (21.8) | 0.20±0.49 | 0–2 | 4 (14) | 0.21±0.56 | 0–2 | 0.903 |
| GT score | 46 (45.5) | 0.77±0.99 | 0–3 | 7 (24) | 0.31±0.66 | 0–3 | 0.040 |
| PTC score | 14 (13.9) | 0.14±0.35 | 0–1 | 3 (10) | 0.10±0.31 | 0–1 | 0.777 |
| IF score | 70 (69.3) | 0.72±0.55 | 0–2 | 10 (35) | 0.45±0.57 | 0–2 | 0.035 |
| TA score | 86 (85.1) | 0.92±0.44 | 0–2 | 24 (83) | 0.83±0.47 | 0–2 | 0.487 |
| BM score | 6 (5.9) | 0.06±0.24 | 0–1 | 1 (3) | 0.03±0.19 | 0–1 | 0.841 |
| MM score | 8 (7.9) | 0.10±0.36 | 0–2 | 3 (10) | 0.10±0.31 | 0–1 | 0.859 |
| Global GS, % | 31 (30.7) | 3.78±7.19 | 0–38.5 | 6 (21) | 1.37±2.90 | 0–10 | 0.267 |
| AS score | 64 (63.4) | 0.97±0.94 | 0–3 | 12 (41) | 0.55±0.74 | 0–2 | 0.042 |
| AH score | 42 (41.6) | 0.76±1.02 | 0–3 | 11 (38) | 0.59±0.91 | 0–3 | 0.546 |
ATI: acute tubular injury; G: glomerulitis; GT: glomeruli thrombi; I: interstitial inflammation; PTC: peritubular capillaritis; AS: arteriosclerosis; AH: arteriolar hyalinosis; BM: glomerular basement membrane thickening; GS: glomerulosclerosis; IF: interstitial fibrosis; MM: mesangial matrix increase; TA: tubular atrophy
Chronic lesions
Twenty-two (16.9%) patients had GS 1%–10%, nine (6.9%) had GS 11%–19%, and six (4.6%) had GS ≥20%. The mean±SD number of arterial cross-sections per patient was 2.5±1.3. AS (Fig 1) of grade 1 was found in 47 (36.2%) biopsies, grade 2 in 20 (15.4%), and grade 3 in 9 (6.9%) biopsies. AS score was significantly higher in cadaver biopsies. AH (Fig 1) of grade 1 was found in 23 (17.7%) biopsies, grade 2 in 20 (15.4%), and grade 3 in 10 (7.7%) biopsies. We found IF (Fig 1) of grade 1 in 74 (56.9%) and grade 2 in 6 (4.6%) biopsies. There were no cases with IF >50%. IF was significantly higher in cadaver kidneys. We observed grade 1 of TA in 103 (79.2%) and grade 2 in 7 (5.4%) biopsies. Nine (6.9%) patients had MM increase of grade 1, two (1.5%) had grade 2 MM increase. Glomerular basement membrane thickening was graded as 1 in 7 (5.4%) patients. We observed no differences of any lesion between donor after cardiac death and heart-beating donors, as well as between ECD and standard-criteria donors.
Post-transplant Course and Graft Survival
Eighteen recipients of deceased-donor graft (17.8%) and one recipients of living-donor graft (3.5%) experienced DGF (p=0.053). AR in the course of follow-up was diagnosed in 20 (19.8%) recipients of deceased-donor graft and in two (6.9%) of living-donor graft (p=0.102). Pyelonephritis occurred in 22 (21.8%) recipients of deceased-donor graft and in 11 (37.9%) of living-donor graft (p=0.078). During the follow-up period, 16 grafts failed. Five-year death-censored graft survival rate was 87.7%. The death-censored graft survival rate was not significantly different between living and cadaver grafts (93.1% vs. 86.1%, p=0.315), as well as between grafts from donor after cardiac death and heart-beating donors, and from ECD vs. standard criteria donors (data are not shown).
Predictors of Post-transplant Course and Graft Survival
Clinical predictors
In univariate analysis of clinical only model, death-censored graft loss was significantly predicted by previous transplants (HR=6.17, 95% CI: 1.39–27.35, p=0.017), every six hours of cold ischemia (HR=1.77, 95% CI: 1.02–3.06, p=0.041) and by AR (HR=7.87, 95% CI: 2.92–21.21, p<0.001). In ROC-analysis, combination of regraft-status, cold ischemia time (CIT) and AR yielded an AUC (0.735, 95%CI: 0.577–0.893) significantly (p=0.002) different from 0.5, corresponding to a non-informative test.
Composite histological predictors
Chronic composite histologic scores, such as chronic pre-implant Banff score (HR=1.02, 95% CI: 1.00–1.03, p=0.013), chronic post-transplant Banff score (HR=1.02, 95% CI: 1.00–1.03, p=0.016), DDS (HR=1.20, 95% CI: 1.03–1.40, p=0.019), and CDS (HR=1.41, 95% CI: 1.02–1.94, p=0.037) significantly predicted graft loss in univariate analysis. Furthermore, higher scores were associated with worse graft survival (Fig 2). The effects of Remuzzi and CIV scores, CADI, CLI, and summation of ALI and CLI were not significant even in univariate analysis (data not shown). Acute post-transplant Banff score was the only acute score that significantly predicted death-censored graft loss (HR=1.79, 95% CI: 1.05–3.06, p=0.033; insignificant associations are not shown). The association of higher score with lower graft survival was confirmed by Kaplan-Meier analysis (Fig 2). The total pre-implant Banff score (HR=1.02, 95% CI: 1.00–1.03, p=0.012) and total post-transplant Banff score (HR=1.02, 95% CI: 1.00–1.03, p=0.013) significantly predicted death-censored graft loss in unadjusted model. After controlling for clinical predictors, only chronic (HR=1.02, 95% CI: 1.00–1.04, p=0.025) and total (HR=1.02, 95% CI: 1.00–1.04, p=0.020) post-transplant Banff scores as well as chronic (HR=1.02, 95% CI: 1.00–1.04, p=0.019) and total (HR=1.02, 95% CI: 1.00–1.04, p=0.018) pre-implant Banff scores held up association with graft failure.
Figure 2.
Association of death-censored kidney graft survival and selected composite histological scores in intra-operative zero-hour biopsies (Kaplan-Meier estimates). A) Chronic pre-implant Banff score; B) Chronic post-transplant Banff score; C) Donor damage score; D) Chronic damage score; E) Acute post-transplant Banff score; and F) Donor Kidney Damage Index. P values are calculated with the log-rank test
Individual histological predictors
We tested the ability of individual components of composite histological scores to predict graft loss. We found that only I>0 (HR=14.58, 95% CI: 4.06–52.33, p<0.001), percentage of global GS (HR=1.06, 95% CI: 1.01–1.11, p=0.016), AS=3 (HR=5.12, 95% CI: 1.43–18.22, p=0.012), IF>0 (HR=4.67, 95% CI: 1.06–20.57, p=0.041), and AH>0 (HR=3.19, 95% CI: 1.09–9.34, p=0.034), all assessed with Banff-grading criteria, were associated with an increased risk of graft failure (insignificant associations are not shown). To simplify the evaluation of donor biopsies, we transformed glomerulosclerosis into a dichotomous variable with cut-off values of 5%, 10%, and 20%. Each of these transformations was analyzed by forward stepwise multivariate regression analysis, including I, AS, AH, and I as covariates, and the best prediction of graft loss was obtained at a cut-off of 20% (data not shown). GS≥20% significantly predicted death-censored graft loss in univariate analysis: HR=5.31, 95% CI: 1.51–18.67, p=0.009. Graft survival rates were significantly reduced if the score exceeded threshold for any of these individual components (Fig 3). Three of four patients with the I score>0 received kidney from deceased donors after death from cerebrovascular accident. However, the fourth patient received a living graft. Three of them (75%) lost grafts during follow-up. Two recipients of cadaver kidney (both from single donor) lost their grafts due to chronic active antibody-mediated rejection at seven and nine months; one living-donor graft was lost because of pyelonephritis complicated by sepsis (after one month).
In order to get insight into etiology of histological lesions in donor kidneys and taking into account the established superiority of Banff-qualifier, we decided to run PCA for individual lesions, graded with Banff-criteria. We included donor age as a variable as it was weakly associated with arteriolar hyalinosis (Spearman’s r = 0.281, p=0.001). Table 3 highlights the extracted communalities and values of factor loadings as a result of the pattern matrix. PCA revealed three components underlying histological lesions in donor kidney: 1) closely clustering chronic lesions (TA, AH, IF, and GS) associating with donor AS, which demonstrated the highest communalities in PCA; 2) acute lesions (I, G, and PTC); and 3) chronic glomerular lesions (MM and BM), associating with donor age. The PCA revealed a satisfactory percentage (44.4%) of total variance explained by the three factors, wherein, the first, second and third components accounted for 22.7%, 12.1% and 9.6% of the total variance, respectively.
Table 3.
Communalities and the three factor solution of the principal component analysis
| Individual lesions | Communalities | Components |
||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| IF score | 0.614 | 0.786 | ||
| TA score | 0.614 | 0.783 | ||
| AS score | 0.622 | 0.669 | 0.386 | |
| AH score | 0.513 | 0.547 | 0.398 | |
| GS (%) | 0.407 | 0.538 | ||
| GT score | 0.190 | 0.424 | ||
| ATI score | 0.278 | 0.395 | ||
| I score | 0.595 | 0.767 | ||
| PTC score | 0.535 | 0.684 | ||
| G score | 0.180 | 0.324 | ||
| BM score | 0.397 | 0.575 | ||
| MM score | 0.198 | 0.350 | ||
| Donor age | 0.624 | 0.762 | ||
ATI: acute tubular injury; G: glomerulitis; GT: glomeruli thrombi; I: interstitial inflammation; PTC: peritubular capillaritis; AS: arteriosclerosis; AH: arteriolar hyalinosis; BM: glomerular basement membrane thickening; GS: glomerulosclerosis; IF: interstitial fibrosis; MM: mesangial matrix increase; TA: tubular atrophy
Development of the Pathologic Scoring System
Considering GS, AS, AH, IF, and I scores, we generated a new pathological scoring system. For this purpose, HRs for each of these variable were rounded to the nearest integer, and were summed up to yield the Donor Kidney Damage Index (DKDI) = GS≥20% (0 or 5) + I>0 (0 or 15) + AS=3 (0 or 5) + AH>0 (0 or 3) + IF>0 (0 or 5). A Cox multivariate analysis showed the DKDI was significantly associated with graft loss after adjustment for clinical variables. For every point increase in DKDI, the relative risk for graft loss was increased by 15% (HR=1.15, 95% CI: 1.09–1.22, p<0.001). Survival curves for three levels of DKDI are shown in Figure 2. Five-year death-censored graft survival for kidneys with DKDI <3 was 100%, whereas graft survival for kidneys with index from 3–10 was 86.6% (p=0.015). If DKDI exceeded 10, the survival was 58.3% (p<0.001).
Clinical utility of pathologic scoring systems
Then ROC analysis was applied to test the predictive accuracy of those composite scales, which showed significant influence on graft loss in univariate Cox analysis. All scales except acute post-transplant Banff and CDS scores yielded AUCs, which significantly differed from 0.5 (Table 4), when predicting 5-year graft loss. However, DDS score had only poor predictive ability (AUCs<0.7). Chronic pre-implant Banff score demonstrated an AUC of 0.722, indicating fair predictive power, and was followed by total post-transplant Banff score. Importantly, total pre-implant Banff score and chronic post-transplant Banff score were not significantly different from chronic pre-implant and total post-transplant Banff scores, respectively. The DKDI yielded an AUC of 0.747, which was higher than AUCs for any other significant scores, though not significantly. The sensitivity and specificity for graft loss were calculated for different cut-off points of DKDI. The optimal cut-off point was set at 10, at which the DKDI has a specificity of 93.9% for the diagnosis of graft failure. We tested further whether a combination of clinical predictors of graft loss with DKDI, chronic pre-implant and total post-transplant Banff scores could enhance predictive performance of clinical and histopathological scores alone. All tested models (clinical only, histopathologicals only, and clinico-histopathologicals) allowed to predict allograft loss (Table 4). Models that included clinical and histopathological parameters yielded non-significantly higher AUCs than clinical and histopathological models alone. Combination of DKDI with clinical variables resulted in an AUC of 0.842 in predicting graft loss, which indicated good discriminating ability. This AUC showed non-significantly higher predictive power than combination of chronic pre-implant or total post-transplant Banff scores with clinical variables.
Table 4.
Areas under the receiver operating characteristic curve (AUC) for composite clinical, histopathological and clinico-histopathological scales in predicting graft loss
| Composite scores | AUC (95% CI) | p value |
|---|---|---|
| Composite clinical score | 0.735 (0.577–0.893) | 0.002 |
| Acute post-transplant Banff score | 0.579 (0.417–0.741) | 0.306 |
| Chronic post-transplant Banff score | 0.704 (0.570–0.838) | 0.008 |
| Total post-transplant Banff score | 0.717 (0.589–0.846) | 0.005 |
| Chronic pre-implant Banff score | 0.722 (0.598–0.845) | 0.004 |
| Total pre-implant Banff score | 0.714 (0.582–0.845) | 0.006 |
| Donor Damage score | 0.691 (0.571–0.812) | 0.013 |
| Chronic Damage score | 0.646 (0.510–0.783) | 0.058 |
| Donor Kidney Damage Index | 0.747 (0.632–0.863) | 0.001 |
| Total post-transplant Banff score+regraft+CIT+AR | 0.802 (0.661–0.942) | <0.001 |
| Chronic pre-implant Banff score+regraft+CIT+AR | 0.807 (0.672–0.942) | <0.001 |
| Donor Kidney Damage Index+regraft+CIT+AR | 0.842 (0.732–0.951) | <0.001 |
CIT: cold ischemia time; AR: acute rejection
DISCUSSION
This study was conducted to assist clinicians with prognostic significance of the pathologic findings in implantation biopsies for survival of kidney allografts. Firstly, although over 80% of donors were standard-criteria deceased and living donors, many had significant histopathology, indicating that clinical assessment of donors in our population per se might be insufficient. The most common chronic lesions were tubular atrophy (84%), interstitial fibrosis (62%), and glomerulosclerosis (29%). As such, tubular atrophy and interstitial fibrosis were mostly of mild degree, as determined by Banff scores. However, vascular pathology was more advanced—we observed severe arteriosclerosis and arteriolar hyalinosis in 6.9% and 7.7% of biopsies, respectively. PCA revealed a three-component model underlying histological lesions in donor kidney. The first component, comprising chronic lesions such as TA, AH, IF, GS, and AS but not donor age, could explain the largest amount of variance observed. The inspection of the communalities showed that this component was associated with donor arteriosclerosis. Therefore, we considered arteriosclerosis of intrarenal arteries a main determinant of chronic lesions in donors’ kidneys, which is supported by literature [34, 35]. The observed frequencies of chronic tubulointerstitial and vascular lesions in our donor population were higher than those reported by other authors [7, 25, 26]. However, previously published results were mostly obtained on the donor cohorts with low cardiovascular risk. Atherosclerosis-related vascular pathology has high prevalence and cause mortality in earlier ages in Eastern Europe [31]. Early development of atherosclerosis can also explain why calendar age of donor in our population was not associated with arteriosclerosis and why it did not predict allograft loss. However, in western population mild to moderate arteriosclerosis in kidneys of younger deceased [9] or live donors [17] also presents more commonly than it is generally appreciated. Our finding allowed us to suggest that procurement biopsies might be relevant for all kidney donors, especially in populations with high cardiovascular risk, which needs further study.
We showed that allograft loss within five years in univariate analysis was significantly predicted by such lesions as I, IF, AS, AH, and GS. The peculiarity of our study was emphasizes on acute lesions. The second component, revealed by PCA, consisted of interstitial inflammation, glomerulitis, and PTC-capillaritis; the highest communality belonged to interstitial inflammation. Such inflammatory lesions in cadaver kidneys are most probably related to brain death, which is known to initiate an inflammatory state of the graft [36, 37]. Our study, in which brain-dead donors constitute the majority, highlighted the presence of I-lesions in 3.1% of biopsies. The observed frequency of I-lesions was lower than that reported earlier for zero-hour biopsies of cadaveric kidneys [21, 26]. Interstitial inflammation, which was observed in zero-hour biopsies of live donor kidney, and reported earlier by other authors [25], could reflect pre-existing subclinical kidney inflammatory pathology. Two recipients, who received cadaveric grafts with I-score >0, lost their grafts because of chronic active antibody-mediated rejection, whereas one living-donor graft was lost because of pyelonephritis. These findings allowed us to speculate that considerable negative impact of interstitial inflammatory infiltrate in zero-hour biopsies on graft survival involves alloantigen-dependent and -independent mechanisms. The ability of intragraft passenger leucocytes to predispose to rejection [36, 38] and thus to contribute to graft loss [39], has been shown earlier by several authors. Importantly, I-lesion in post-transplant biopsies is regarded as important determinant of kidney allograft function and survival [40]. However, the amount of interstitial inflammation is not often reported when evaluating donor biopsies [11]. Although inflammation was not frequent in our donors cohort, the observed strong negative impact of I-lesion on graft survival underlined the necessity of zero-hour biopsy examination for the presence of inflammatory infiltrate. Glomerulosclerosis, as the most-studied kidney biopsy finding, was reported by many authors [8, 14, 22] to be associated with graft loss. However, the predictive accuracy of the percentage of glomerulosclerosis alone was insufficient in our study. After verification of different cutoff values for this parameter, we found that a cut-off value of glomerulosclerosis of ≥20% better predicted the graft loss, as it was suggested previously by many authors [8, 11, 14]. Most of earlier studies did not observe independent impact of donor interstitial fibrosis on graft survival [10, 18]. However, interstitial fibrosis was found to be associated with worse long-term function in one study [18]. One explanation for these discrepancies is poorer reproducibility associated with semiquantitative evaluation of interstitial fibrosis [41] compared with the assessment of glomerulosclerosis or arteriosclerosis [42]. We also found association between intrarenal vasculature injury and graft loss. Only severe arteriosclerosis predicted graft loss, whereas it was predicted by any extent of arteriolar hyalinosis. This result emphasized the importance of donor vascular pathology and chronic ischemia as an antigen-independent risk factor for kidney allograft survival, which was supported by several authors [3, 10, 13, 16]. Some negative results [19-21] can be attributed to shorter follow-up [20] or the application of different grading systems [19-21], or to the use of wedge biopsies [21], where arteries were under-represented [17].
Although several authors [19, 21] found no association between donor kidney vascular lesions and survival, they reported the importance of such lesions for long-term graft function. Our results also suggested that kidneys with mild to moderate arteriosclerosis could be safely used. This could maximize the yield of the existing donor pool and potentially narrow the indication for dual kidney transplantation. Given observed associations of lesions in each kidney compartment with worse outcome, we believe that any lesions in isolation should not be used for prediction of graft survival. This conclusion is in line with Banff consensus recommendations, which suggest that changes in all kidney anatomic compartments require attention [32].
Next, we assessed the association between previously published composite histopathological scores and kidney graft survival. Taking into account the established effect of interstitial inflammation, we also decided to assess predictive effect of acute and chronic components of those schemas separately. Since prediction of allograft survival heavily depends on pre- and post-transplant clinical events, regraft status, CIT and AR were included into analyses as covariates. Among acute scales, only post-transplant Banff scale comprising I-lesion was associated with graft loss and only before adjustment for confounders. Among chronic schemas, only higher chronic and total post-transplant Banff scores, as well as chronic and total pre-implant Banff scores independently predicted death-censored graft loss within five years in our patient population; the total scores were no better than only chronic respective scores. Several studies reported an association between various histopathological scoring systems and graft failure. As in our study, the most frequently used scoring systems relied on Banff qualifiers [2, 7, 15, 27, 28, 30]. Given that only a few of existing schemas allowed predicting allograft loss in our study and their poor to fair predictive accuracy, we decided to develop a new composite score relying on actual HR for variables that were significantly associated with the risk of graft failure in univariate analyses (GS, IF, AS, AH, and I). Taking into account AUCs, DKDI, chronic pre-implant and total post-transplant Banff scores were non-significantly superior to other scales. We found that DKDI predicted graft outcome independently of the other donor characteristics with an increase in relative risk of 15% for graft loss for every point increase. When DKDI increased from the first level (0–2 points) to the second (3–10 points), graft survival decreased by 13%; with an increase of DKDI to the third level, the survival was reduced by 42%. This new scale could predict the greatest and sharp decrease in survival with increasing scores.
In addition, we tried to evaluate the predictive accuracy of the composite scores for graft survival. Chronic and total pre-implant and post-transplant Banff scores provided fair predictive accuracy. However, DKDI was non-significantly superior. At a cut-off value of >10, the DKDI has 93.9% specificity for the diagnosis of graft failure. Either the clinical model or the composite histopathological models per se, yielded fair AUCs. However, considering clinical model together with DKDI or with chronic pre-implant or total post-transplant Banff scores, the predictive accuracy improved from fair to good. Although the results of the current study were far from definitive, we hypothesize that they might have further clinical applications. They might be used not only for estimation of graft survival but also for post-transplant clinical decision-making to obtain superior long-term outcomes. Given negative impact of AR, steroid withdrawal or CNI minimization might not be indicated in recipients of kidneys with high DKDI, as well as with high chronic pre-implant or total post-transplant Banff scores. Also, if such a compromised kidney has been transplanted, caution is needed regarding urinary tract infections. Here we only reported the prognostic significance of histopathological findings. Nevertheless, chronic pre-implant or total post-transplant Banff scores, as well as the newly created DKDI, might also be used to identify kidneys that could be accepted or discarded for transplantation. If such scores would be used for procurement biopsies, kidneys with higher scores would require shorter CIT and might not be recommended for re-transplantation.
The present study had some limitations. It was a single-center retrospective study carried out on a small group of patients from population with high cardiovascular risk. However, it should be mentioned that the tendency to observe moderate and severe arteriosclerosis was also reported for populations with low to moderate cardiovascular risk. The limited number of cases and rarity of some pathology did not allow us to validate results internally. Therefore, it would be desirable to evaluate the reproducibility of the DKDI on a larger-scale external cohort from populations with low to moderate cardiovascular risk. For the vast majority of cadaveric donors, anamnestic and laboratory data were not available. Consequently, having data of complete donor examination, some results might be refined. For the few cases with interstitial inflammation in our study sample, we suggest further studies on samples with higher frequencies of this lesion to figure out its association with graft outcomes.
In conclusion, integral evaluation of chronic lesions in all kidney compartments enhanced by evaluating of interstitial inflammation provided a fair accuracy in the prediction of graft outcome. A new composite histological scoring system, the DKDI, relying on regression coefficients for significant individual predictors, such as interstitial inflammation, interstitial fibrosis, arteriosclerosis, arteriolar hyalinosis and glomerulosclerosis, along with total pre-implant Banff and total post-transplant Banff scores and their chronic components provided fair predictive accuracy for worse graft survival. DKDI, chronic pre-implant Banff score, and total post-transplant Banff score in conjunction with some clinical variables such as regraft status, CIT and AR predicted graft loss with good accuracy and may facilitate decision making in post-transplant period.
ACKNOWLEDGMENTS
Alexandra S. Troyan, MD, is acknowledged for proofreading the English of the manuscript.
CONFLICTS OF INTEREST:
None declared.
FINANCIAL SUPPORT:
This study was supported by grants from Ukrainian Ministry of Health 0111U006184 and 0114U002440.
References
- 1.Saran R, Robinson B, Abbott KC, et al. US renal data system 2016 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2017;69(Suppl 1):S1–S688. doi: 10.1053/j.ajkd.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balaz P, Rokosny S, Wohlfahrtova M, et al. Identification of expanded-criteria donor kidney grafts at lower risk of delayed graft function. Transplantation. 2013;96:633–8. doi: 10.1097/TP.0b013e31829d9225. [DOI] [PubMed] [Google Scholar]
- 3.Kayler LK, Mohanka R, Basu A, et al. Correlation of histologic findings on preimplant biopsy with kidney graft survival. Transpl Int. 2008;21:892–8. doi: 10.1111/j.1432-2277.2008.00681.x. [DOI] [PubMed] [Google Scholar]
- 4.Schold JD, Kaplan B, Baliga RS, Meier-Kriesche HU. The broad spectrum of quality in deceased donor kidneys. Am J Transplant. 2005;5:757–65. doi: 10.1111/j.1600-6143.2005.00770.x. [DOI] [PubMed] [Google Scholar]
- 5.Carta P, Zanazzi M, Caroti L, et al. Impact of the pre-transplant histological score on 3-year graft outcomes of kidneys from marginal donors: a single-centre study. Nephrol Dial Transplant. 2013;28:2637–44. doi: 10.1093/ndt/gft292. [DOI] [PubMed] [Google Scholar]
- 6.Snoeijs MG, Buurman WA, Christiaans MH, et al. Histological assessment of preimplantation biopsies may improve selection of kidneys from old donors after cardiac death. Am J Transplant. 2008;8:1844–51. doi: 10.1111/j.1600-6143.2008.02318.x. [DOI] [PubMed] [Google Scholar]
- 7.De Vusser K, Lerut E, Kuypers D, et al. The predictive value of kidney allograft baseline biopsies for long-term graft survival. J Am Soc Nephrol. 2013;24:1913–23. doi: 10.1681/ASN.2012111081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaber LW, Moore LW, Alloway RR, et al. Glomerulosclerosis as a determinant of posttransplant function of older donor renal allografts. Transplantation. 1995;60:334–9. doi: 10.1097/00007890-199508270-00006. [DOI] [PubMed] [Google Scholar]
- 9.Randhawa PS, Minervini MI, Lombardero M, et al. Biopsy of marginal donor kidneys: correlation of histologic findings with graft dysfunction. Transplantation. 2000;69:1352–7. doi: 10.1097/00007890-200004150-00024. [DOI] [PubMed] [Google Scholar]
- 10.Cockfield SM, Moore RB, Todd G, et al. The prognostic utility of deceased donor implantation biopsy in determining function and graft survival after kidney transplantation. Transplantation. 2010;89:559–66. doi: 10.1097/TP.0b013e3181ca7e9b. [DOI] [PubMed] [Google Scholar]
- 11.Kasiske BL, Stewart DE, Bista BR, et al. The role of procurement biopsies in acceptance decisions for kidneys retrieved for transplant. Clin J Am Soc Nephrol. 2014;9:562–71. doi: 10.2215/CJN.07610713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Remuzzi G, Grinyò J, Ruggenenti P, et al. Early experience with dual kidney transplantation in adults using expanded donor criteria. J Am Soc Nephrol. 1999;10:2591–8. doi: 10.1681/ASN.V10122591. [DOI] [PubMed] [Google Scholar]
- 13.Bosmans JL, Woestenburg A, Ysebaert DK, et al. Fibrous intimal thickening at implantation as a risk factor for the outcome of cadaveric renal allografts. Transplantation. 2000;69:2388–94. doi: 10.1097/00007890-200006150-00030. [DOI] [PubMed] [Google Scholar]
- 14.Cicciarelli J, Cho Y, Mateo R, et al. Renal biopsy donor group: the influence of glomerulosclerosis on transplant outcomes. Transplant Proc. 2005;37:712–3. doi: 10.1016/j.transproceed.2004.12.108. [DOI] [PubMed] [Google Scholar]
- 15.Lopes JA, Moreso F, Riera L, et al. Evaluation of pre-implantation kidney biopsies: comparison of Banff criteria to a morphometric approach. Kidney Int. 2005;67:1595–1600. doi: 10.1111/j.1523-1755.2005.00241.x. [DOI] [PubMed] [Google Scholar]
- 16.Pokorná E, Vítko S, Chadimová M, Schück O. Adverse effect of donor arteriolosclerosis on graft outcome after renal transplantation. Nephrol Dial Transplant. 2000;15:705–10. doi: 10.1093/ndt/15.5.705. [DOI] [PubMed] [Google Scholar]
- 17.Haas M, Segev DL, Racusen LC, et al. Arteriosclerosis in Kidneys from Healthy Live Donors: Comparison of Wedge and Needle Core Perioperative Biopsies. Arch Pathol Lab Med. 2008;132:37–42. doi: 10.5858/2008-132-37-AIKFHL. [DOI] [PubMed] [Google Scholar]
- 18.Sulikowski T, Tejchman K, Ziętek Z, et al. Histopathologic evaluation of pretransplantation biopsy as a factor influencing graft function after kidney transplantation in 3-year observation. Transplant Proc. 2010;42:3375–81. doi: 10.1016/j.transproceed.2010.08.060. [DOI] [PubMed] [Google Scholar]
- 19.Oda A, Morozumi K, Uchida K. Histological factors of 1-h biopsy influencing the delayed renal function and outcome in cadaveric renal allografts. Clin Transplant. 1999;13(Suppl 1):6–12. [PubMed] [Google Scholar]
- 20.Minakawa R, Tydén G, Lindholm B, Reinholt FP. Donor kidney vasculopathy: impact on outcome in kidney transplantation. Transpl Immunol. 1996;4:309–12. doi: 10.1016/s0966-3274(96)80052-9. [DOI] [PubMed] [Google Scholar]
- 21.Szánya J, Szakály P, Magyarlaki T, et al. Predictive morphological findings in “zero-hour” biopsies of renal allografts. Acta Chir Hung. 1997;36:346–8. [PubMed] [Google Scholar]
- 22.Wang CJ, Wetmore JB, Crary GS, Kasiske BL. The Donor Kidney Biopsy and Its Implications in Predicting Graft Outcomes: A Systematic Review. Am J Transplant. 2015;15:1903–14. doi: 10.1111/ajt.13213. [DOI] [PubMed] [Google Scholar]
- 23.Cosyns JP, Malaise J, Hanique G, et al. Lesions in donor kidneys: nature, incidence, and influence on graft function. Transpl Int. 1998;11:22–7. doi: 10.1007/s001470050097. [DOI] [PubMed] [Google Scholar]
- 24.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–3. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 25.Lee AL, Kim YS, Lim BJ, et al. The impact of time-zero biopsy on early graft outcomes after living donor kidney transplantation. Transplant Proc. 2013;45:2937–40. doi: 10.1016/j.transproceed.2013.08.081. [DOI] [PubMed] [Google Scholar]
- 26.Lee AL, Huh KH, Lee SH, et al. Significance of Time-Zero Biopsy for Graft Renal Function After Deceased Donor Kidney Transplantation. Transplant Proc. 2016;48:2656–62. doi: 10.1016/j.transproceed.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 27.Kahu J, Kyllönen L, Räisänen-Sokolowski A, Salmela K. Donor risk score and baseline biopsy CADI value predict kidney graft outcome. Clin Transplant. 2011;25:E276–83. doi: 10.1111/j.1399-0012.2011.01401.x. [DOI] [PubMed] [Google Scholar]
- 28.Azancot MA, Moreso F, Salcedo M, et al. The reproducibility and predictive value on outcome of renal biopsies from expanded criteria donors. Kidney Int. 2014;85:1161–8. doi: 10.1038/ki.2013.461. [DOI] [PubMed] [Google Scholar]
- 29.Howie AJ, Ferreira MA, Lipkin GW, Adu D. Measurement of chronic damage in the donor kidney and graft survival. Transplantation. 2004;77:1058–65. doi: 10.1097/01.tp.0000120177.44144ff. [DOI] [PubMed] [Google Scholar]
- 30.Hofer J, Regele H, Böhmig GA, et al. Pre-implant biopsy predicts outcome of single-kidney transplantation independent of clinical donor variables. Transplantation. 2014;97:426–32. doi: 10.1097/01.tp.0000437428.12356.4a. [DOI] [PubMed] [Google Scholar]
- 31.Barquera S, Pedroza-Tobías A, Medina C, et al. Global Overview of the Epidemiology of Atherosclerotic Cardiovascular Disease. Arch Med Res. 2015;46:328–8. doi: 10.1016/j.arcmed.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Liapis H, Gaut JP, Klein C, et al. Banff Working Group Banff Histopathological Consensus Criteria for Preimplantation Kidney Biopsies. Am J Transplant. 2017;17:140–50. doi: 10.1111/ajt.13929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solez K, Colvin RB, Racusen LC, et al. Banff 07 Classification of Renal Allograft Pathology: Updates and Future Directions. Am J Transplant. 2008;8:753–60. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 34.Adamczak M, Wiecek A. Ischemic nephropathy - pathogenesis and treatment. Nefrologia. 2012;32:432–8. doi: 10.3265/Nefrologia.pre2012.Apr.11472. [DOI] [PubMed] [Google Scholar]
- 35.Iwakiri T, Sato Y, Matsuura Y, et al. Association between renal vasculature changes and generalized atherosclerosis: an autopsy survey. J Atheroscler Thromb. 2014;21:99–107. doi: 10.5551/jat.19869. [DOI] [PubMed] [Google Scholar]
- 36.de Vries DK, Lindeman JH, Ringers J, et al. Donor brain death predisposes human kidney grafts to a proinflammatory reaction after transplantation. Am J Transplant. 2011;11:1064–70. doi: 10.1111/j.1600-6143.2011.03466.x. [DOI] [PubMed] [Google Scholar]
- 37.Nagareda T, Kinoshita Y, Tanaka A, et al. Clinicopathology of kidneys from brain-dead patients treated with vasopressin and epinephrine. Kidney Int. 1993;43:1363–70. doi: 10.1038/ki.1993.192. [DOI] [PubMed] [Google Scholar]
- 38.Kim YS, Lim CS, Kim S, et al. Cadaveric renal allograft at the time of implantation has the similar immunological features with the rejecting allograft. Transplantation. 2000;70:1080–5. doi: 10.1097/00007890-200010150-00015. [DOI] [PubMed] [Google Scholar]
- 39.Bodonyi-Kovacs G, Putheti P, Marino M, et al. Gene expression profiling of the donor kidney at the time of transplantation predicts clinical outcomes 2 years after transplantation. Hum Immunol. 2010;71:451–5. doi: 10.1016/j.humimm.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gago M, Cornell LD, Kremers WK, et al. Kidney allograft inflammation and fibrosis, causes and consequences. Am J Transplan. 2012;12:1199–1207. doi: 10.1111/j.1600-6143.2011.03911.x. [DOI] [PubMed] [Google Scholar]
- 41.Farris AB, Chan S, Climenhaga J, et al. Banff fibrosis study: multicenter visual assessment and computerized analysis of interstitial fibrosis in kidney biopsies. Am J Transplant. 2014;14:897–907. doi: 10.1111/ajt.12641. [DOI] [PubMed] [Google Scholar]
- 42.Furness PN, Taub N. Convergence of European Renal Transplant Pathology Assessment Procedures (CERTPAP) Project International variation in the interpretation of renal transplant biopsies: report of the CERTPAP Project. Kidney Int. 2001;60:1998–2012. doi: 10.1046/j.1523-1755.2001.00030.x. [DOI] [PubMed] [Google Scholar]