Abstract
Previous research indicates that decisions about when to eat in response to food cues in the environment are based on interoceptive energy states (i.e., hunger and fullness) and learning about and remembering prior eating experiences. However, this animal model has exclusively been tested on male rodents. Despite evidence that women are more susceptible to obesity and cognitive disorders associated with excess weight (e.g., Alzheimer’s disease) than men, the generality of these findings with males to females remains unknown. To address this gap, the current research investigated associative learning mechanisms involved in food intake control in females by training both males and females in a Pavlovian deprivation discrimination in which varying levels of food deprivation are trained with competitive external cues to signal reward. In Experiment 1, male and female rats showed similar performance in discriminating between 0 and 24h deprivation state /external cue compounds and in subsequent tests, confirming stimulus control by deprivation states. Experiment 2 assessed learning about more ecologically valid 0 and 4h deprivation states in competition with external cues in both males and females. With the low-level deprivation state parameters, females outperformed males in discriminative control by deprivation states, particularly on the contingency rewarded under satiation and not deprivation. While females showed an enhanced degree of energy state processing under some deprivation conditions, overall, these findings suggest similar mechanisms of learned appetitive control in both sexes.
Keywords: sex differences, satiety, energy regulation, appetitive, learning, obesity
1. Introduction
Much evidence has accumulated that links learning and memory processes to the control of energy intake and body weight (Attuquayefio & Stevenson, 2015; Davidson, Kanoski, Walls, & Jarrard, 2005; Francis & Stevenson, 2013; Higgs, 2016; Kanoski & Grill, 2015). However, despite many reports that sex is a fundamental variable to consider in the behavioral and neural bases for both energy regulation (Asarian & Geary, 2013; Palmer & Clegg, 2015) and associative learning (Dalla & Shors, 2009; Korol & Pisani, 2015; Koss & Frick, 2017), little research has assessed the possibility of sex differences in these interacting processes. The purpose of the present research is to investigate the role of sex in the learned control of eating and appetitive behavior.
We have proposed that decisions about when to eat and when to refrain from eating are based on the integration of information provided by internal energy states and external environmental stimuli (Davidson, Sample, & Swithers, 2014; Davidson, Tracy, Schier, & Swithers, 2014). Based on prior eating experiences, orosensory cues for food, as well as visual or olfactory cues related to food, are often followed by positive postingestive consequences. However, those same cues are also followed by nonrewarding or even aversive consequences on other occasions, such as after the end of a meal. In our view, the information provided by interoceptive satiety signals predicts when environmental food cues will not be followed by rewarding postingestive outcomes. Specifically, interoceptive signals corresponding to food satiety function as contextual cues that suppress appetitive behavior by inhibiting the capacity of food cues to retrieve the memory of the rewarding postingestive outcomes of intake.
Virtually all of the findings on which this model is based were obtained from studies using male rats. The present research compared male and female rats in two studies that assessed (a) the ability of interoceptive hunger and satiety cues to gain discriminative control over appetitive behavior and (b) the degree to which interoceptive cues can compete with external cues for the establishment of that discriminative control. Our previous research indicated that male rats could learn to use cues arising from 0 and 24-hour (h) food deprivation (Sample, Martin, Jones, Hargrave, & Davidson, 2015) and from 0 and 4h food deprivation (Sample, Jones, Hargrave, Jarrard, & Davidson, 2016) as discriminative stimuli for sucrose reward. Those studies also showed that interoceptive cues acquired strong stimulus control even in the face of competition from external cues that were trained as equally valid predictors of sucrose reinforcement. Whether or not female rats would exhibit comparable patterns of stimulus control has not yet been evaluated. The answer to this question will help to assess the degree to which our associative model of intake regulation extends to both sexes.
With this aim, Experiment 1 assessed both males and intact free-cycling females in a variation of what we have described as a compound internal deprivation state / external cue discrimination (Sample et al., 2015). In this study, we trained rats of both sexes under conditions in which cues arising from 24h food deprivation were presented concurrently with an auditory cue (e.g., white noise) to form a compound signal for the delivery of sucrose pellets, whereas cues arising from 0h food deprivation (i.e., access to food for 24 hours) were presented concurrently with a different auditory cue (e.g., tone) as a compound stimulus not followed by reinforcement. Thus, in this deprivation reinforced condition (referred to as Dep+), deprivation cues were trained with external cues as compound discriminative stimuli for sucrose. In a second training condition, both males and females were exposed to 0 and 24h deprivation states in compound with auditory cues, but, unlike the Dep+ group, sucrose delivery was only contingent on the presentation of auditory cues (e.g., tone+, white noise-), while the relationship between deprivation state and sucrose delivery varied noncontingently. Therefore, in this deprivation noncontingent condition (i.e., DepN) external cues were trained as relevant signals for sucrose pellets, while deprivation cues were established as irrelevant stimuli. When performance reached asymptote for both of these groups, external cues were removed to assess learning about the energy state contingency alone. Then, following a re-establishment of baseline performance with the training contingencies, the external cue contingency was presented in opposition to that of internal deprivation states. Presenting previously rewarded external cues with the nonrewarded internal cues (and vice versa) offered a comparison of the internal versus external locus of stimulus control over appetitive behavior.
Experiment 2 assessed females and males in learning about more ecologically valid, lower intensity food deprivation states that approximate free-feeding (i.e., meals every two hours) conditions. We compared males and females trained with compound discriminative stimuli in which 4h food deprivation and one auditory cue signaled sucrose pellets (Dep+), while 0h food deprivation and another auditory cue did not signal sucrose. In addition, some of our previous studies used the reversed deprivation contingency during training in which 0h food deprivation signaled sucrose and higher levels of food deprivation signaled nonreward (e.g., (Davidson et al., 2010; Sample et al., 2016). To make Experiment 2 comparable to this previous work, we substituted this reverse contingency (Sat+) for the DepN contingency used in Experiment 1. In other words, in addition to training rats with compounds in which 4h deprivation cues predicted sucrose delivery and 0h cues did not, we trained additional groups of male and female rats with compounds in which 0h food deprivation signaled sucrose and 4h food deprivation did not.
As the reverse of the reinforced deprivation condition (Dep+), the reinforced satiated condition (Sat+) provides an alternative way, compared to the DepN group, to assess the role of contingency in deprivation discrimination performance. We expect that animals have ample extra-experimental experience (e.g., associations made in home cage) learning that interoceptive stimuli arising from low levels of hunger signal that food and eating will be followed by rewarding postingestive outcomes, whereas cues associated with satiation are not followed by those types of outcomes (i.e., Dep+ contingencies). However, animals would normally have no opportunity to learn the reverse contingency of satiation predicting reward. Moreover, the development of stimulus control with the satiated contingency would likely have to overcome any negative transfer based on extra-experimental learning involving the association of hunger, rather than satiation, with rewarding postingestive outcomes. Therefore, the emergence of discriminative performance by male and female rats trained with the 0h + and 4h – (Sat+) contingency provides an alternative test for the development of discriminative control by interoceptive food deprivation cues that could not be facilitated by positive transfer of extra-experimental learning. Accordingly, sex differences in learning the Sat+ contingency could reveal sex differences in extra-experimental learning about the opposite contingency.
2. Methods
2.1. Apparatus
All behavioral training took place in 8 identical operant chambers constructed of aluminum end walls and plexiglass sidewalls (59.7 × 34.3 × 26.35 cm) with stainless steel rod floors (.48 cm in diameter and 1.07 cm apart) (Lafayette Instruments, Lafayette, IN). At the end of one sidewall, an infrared photo transmitter and receiver were located on either side and immediately in front of a recessed food magazine. A computer infrared monitoring system recorded beam breaks required to gain entry into the food magazine. Reinforcers were 45 mg sucrose pellets (Research Diets, P.J. Noyes Company Inc., Lancaster, N.H.). The auditory stimuli that served as external cues were a 1500hz, 74 – 76 db tone and a 3 Hz white noise. External cue stimuli were counterbalanced across reward contingency and sex.
2.2. Experiment 1
2.2.1. Subjects
Subjects were 16 male and 16 female Sprague-Dawley rats approximately 65 days of age upon arrival from Envigo. Rats were housed individually in plastic Optirat cages with corncob bedding. Males and females were housed in separate colony rooms to promote estrous cycle synchrony (McClintock, 1984). Light: dark cycle was 12:12h with lights on at 1000h. Following acclimation to the animal colony on standard chow (LabDiet, Formula 5001) ad libitum, rats were placed on a daily alternating schedule of 0 and 24h food-deprivation. Rats received free access to water throughout the experiment. All procedures were approved by the American University Institutional Animal Care and Use Committee.
2.2.2. Procedures
After acclimating to the animal colony, both male and female rats were divided into two deprivation contingency groups matched on body weight (means = 339.63g and 339.75g for males and 234.13g and 234.13g for females). All rats were placed on a daily alternating schedule of food deprivation so that behavioral training took place following either 24 hours without food (24h) or 24 hours with access to food (0h). Food was removed (on 0h days, in preparation for 24h days) or replaced (on 24h days) immediately following behavioral testing, or at approximately 1030h on days without behavioral testing. One training session with one trial took place at approximately 1000h daily. Training sessions occurred approximately 5 times per week to prevent reinforcement on a single alternating schedule.
For each daily training session, rats were placed in the conditioning chamber for 6 min. Each session consisted of a 4 min presentation of a discrete auditory cue (i.e., tone or white noise), followed by the delivery of 5 sucrose pellets on the rewarded deprivation state / external cue contingency or no sucrose pellets on the non-rewarded contingency. Rats in Group Dep+ received sucrose reward under 24h food deprivation following the presentation of a tone, but not 0h deprivation and the presentation of white noise (see design in Table 1). External auditory stimuli were counterbalanced across deprivation contingency and sex. Group DepN received sucrose on approximately half of the training sessions occurring under 0h and half under 24h food deprivation. The pellet dispenser did not operate on non-rewarded trials. Following the delivery of sucrose or no sucrose, rats remained in the conditioning chamber for an additional two minutes before being returned to their home cages.
Table 1.
Experiment 1 design.
| Sex | Group | N | Compound | Dep Cue | Compound |
|---|---|---|---|---|---|
| Males | Dep+ | 8 | 24A +, 0B − |
24 +, 0 − | 24B − , 0A − |
| DepN | 8 | 0/24 A +, 0/24 B − | 24+/−, 0 +/− | 0/24 A −, 0/24 B − |
|
| Females | Dep+ | 8 | 24A +, 0B − |
24 +, 0 − | 24B − , 0A − |
| DepN | 8 | 0/24 A +, 0/24 B − | 24+/−, 0 +/− | 0/24 A −, 0/24 B − |
Note: Dep+ = received sucrose under 24h but no sucrose under 0h food deprivation; DepN = noncontingent deprivation state, receiving sucrose approximately half of the time under 0 and half under 24h food deprivation; A and B = counterbalanced external tone and white noise cues.
With one trial per session and one session per day, rewarded and nonrewarded trials occurred on separate days. In assessing behavioral performance and determining the duration of experimental phases, four sessions were combined to form a block of learning trials (two rewarded and two nonrewarded sessions to total four separate sessions). Compound cue training with the deprivation state / external cue stimuli consisted of 16 four-session blocks (i.e., 32 sessions under 0h deprivation and 32 sessions under 24h deprivation to total 64 sessions) (see Table 2 for experimental timeline). The day following the last session of training, external auditory cues were removed for one block (two 0h and two 24h sessions) to assess learning about deprivation states. To re-establish discriminative responding with the compound cue prior to the cue competition test, the deprivation state / external cue compound was reinstated for one block. The following day, all groups received the cue competition test, which occurred over two sessions (one each under 0 and 24h deprivation). For Group Dep+, the external cue contingency opposed the internal cue contingency so that the previously reinforced external cues were paired with the nonreinforced satiated deprivation state. Group DepN received the same deprivation state schedule. Sucrose reinforcement was not delivered in the cue competition test.
Table 2.
Experiment 1 timeline.

|
Note: A block was comprised of a total of four training separate sessions, two sessions under 0h and two sessions under 24h deprivation.
2.2.3. Statistical Analyses
The index of appetitive responding was the mean percent of 10s time bins in which the food magazine infrared beam was broken within the 4min period preceding sucrose delivery or no sucrose. With the exception of the cue competition test, appetitive responding was evaluated in blocks of four sessions (two under the rewarded and two under the nonrewarded contingency). Statistical comparisons of behavioral data were performed with repeated-measures analysis of variance (ANOVA). Sex and Contingency (Dep+ versus DepN) served as between-subjects factors and + / - (i.e., one block = two rewarded sessions / two nonrewarded sessions) as a within-subjects factor for ANOVAs on compound training, the internal cue test, and compound reinstatement. For the Cue Competition Test, Deprivation Level (0h or 24h) replaced +/- as the within-subjects factor and data were evaluated for two sessions, one under each deprivation level. A p-value < .05 was considered statistically significant.
2.3. Experiment 2
2.3.1. Subjects
Subjects were 16 male and 16 female Sprague-Dawley rats (Envigo), 90 days of age and weighing approximately 375 – 400g and 200 – 250g, respectively, upon arrival to the animal colony. Animal housing conditions were the same as those in Experiment 1. Light:dark cycle was 14:10h with lights on at 1430h. A change in light:dark cycle relative to Experiment 1 was necessitated by a change in the scheduling of animal husbandry for other studies in the animal holding area. All rats were maintained ad libitum on standard chow, except under the 4-hour deprivation condition. All procedures were approved by the American University Institutional Animal Care and Use Committee.
2.3.2. Procedures
Following one week of acclimation to the animal colony, male and female rats were divided into two deprivation contingency groups matched on body weight. In the same manner as Experiment 1, the deprivation schedule alternated between 0h and 4h deprivation states. Group Dep+ received sucrose under 4h food deprivation and following one external cue (i.e., tone) and did not receive sucrose under 0h deprivation with the other external cue (i.e., white noise) (see Table 3 for design). Group Sat+ received the opposite contingency, in which sucrose pellets were dispensed under 0h deprivation with white noise, but not 4h deprivation with tone. Auditory stimuli were counterbalanced across deprivation contingency and sex. As with Experiment 1, one daily session occurred immediately after lights on (1430h).
Table 3.
Experiment 2 design.
| Sex | Group | n | Compound | Dep Cue | Compound |
|---|---|---|---|---|---|
| Males | Sat+ | 8 | 0A +, 4B − |
0 +, 4 − | 0A +, 4B − |
| Dep+ | 8 | 4B +, 0A − | 4 +, 0 − | 4B +, 0A − |
|
| Females | Sat+ | 8 | 0A +, 4B − |
0 +, 4 − | 0A +, 4B − |
| Dep+ | 8 | 4B +, 0A − | 4 +, 0 − | 4B +, 0A − |
Note: Dep+ = received sucrose under 4 h but no sucrose under 0h food deprivation; Sat+ = received sucrose under 0h but not 4 h deprivation; A and B = counterbalanced external tone and white noise cues.
Following 10 four-session blocks (20 sessions under 0h and 20 sessions under 4h deprivation) of compound cue acquisition, external auditory cues were removed to probe learning about deprivation states alone (Table 4). This Deprivation Cue Probe Test occurred over one four-session block (two sessions under each deprivation level). Since rats did not exhibit significant discriminative responding by deprivation states in the deprivation cue probe test, compound cue acquisition was resumed for an additional 5 four-session blocks. Therefore, rats received a total of 15 four-session blocks of compound cue training (i.e., 40 sessions prior to and 20 sessions after the deprivation cue probe test).
Table 4.
Experiment 2 timeline.

|
Note: Two rewarded and two nonrewarded sessions constituted each block (i.e., two sessions under 0h and two sessions under 4h deprivation).
Following the completion of compound training and one day without behavioral training, external auditory stimuli were removed to assess learning about deprivation states alone. In this deprivation states alone period, rats received 16 four-session blocks of training (i.e., 32 sessions under 0h and 32 sessions under 4h deprivation). Finally, following one day without behavioral testing, external cues were re-introduced to reinstate the original compound discriminative stimuli (i.e., concurrent deprivation state and external auditory cue) for one block (two sessions under each deprivation level).
2.3.3. Statistical Analyses
The dependent measure was the percent of 10s time periods in which the food cup photobeam was interrupted in the 4min period preceding sucrose delivery. As in Experiment 1, behavioral data were evaluated by blocks of four sessions (i.e., one block = two sessions under 0h and two sessions under 4h deprivation). Compound cue acquisition was analyzed with repeated-measures ANOVA for Block (1 – 15), with Sex, Contingency (Dep + vs. Sat+), and External cue (reinforced with tone or white noise) as between-subjects factors, and Deprivation level (0 and 4h food deprivation) as a within-subjects factor. Post hoc Newman-Keuls tests were used as appropriate. To assess discriminative responding in the deprivation cue probe test, a separate ANOVA was conducted for this block (i.e., two reinforced and two nonreinforced sessions). An additional ANOVA was conducted to assess discriminative control in the presence and absence of external cues across four test periods, each spanning one four-session block (i.e., two sessions under 0h and two sessions under 4h deprivation conditions): terminal compound cue acquisition, initial deprivation cues alone, terminal deprivation cues alone, and reinstatement of the compound cue. For this analysis, Sex, Contingency, and External cue served as between-subjects factors, and Deprivation level served as a within-subjects factor. Post hoc Duncan’s tests were used to determine the basis of this interaction. Further, separate ANOVAs were performed on each of these four-session blocks to determine which experimental periods sex differences might be based on. Statistical significance level for all analyses was set to p < .05.
3. Results
3.1. Experiment 1
3.1.1. Training
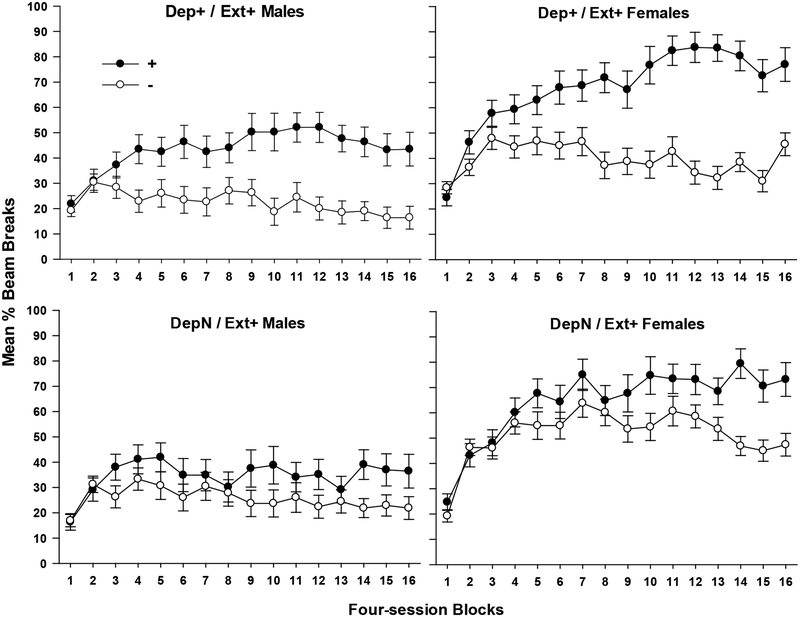
All groups learned to discriminate between rewarded and nonrewarded contingencies in compound training (see Figure 1). Females exhibited greater levels of appetitive responding than males, but the terminal magnitude of discrimination did not differ by sex. Confirming this pattern of responding, ANOVA on 16 blocks (each comprised of four sessions, two sessions under 0h and two under 4h deprivation) of acquisition yielded a significant +/ – × Block interaction, (F(15, 420) = 15.58, p < .001). Higher levels of responding by females yielded a main effect of Sex (F(1, 28) = 50.24, p < .001), which did not interact with +/ – (p > .30). ANOVA on the last block of training yielded a significant main effect of +/ – (F(1, 28) = 74.20, p < .001), which did not differ by Sex (p > .18) or Contingency (p > .12).
Figure 1.
This graph depicts the mean ± S.E.M. percent magazine entries during the 4min period preceding sucrose delivery for rewarded (+) and nonrewarded (-) four-session blocks during acquisition. Males and females received either a predictive deprivation and external cue contingency (Group Dep+ / Ext+, top panels) or a predictive external cue but noncontingent deprivation state (DepN / Ext+, bottom panels).
3.1.2. Deprivation Cue Test
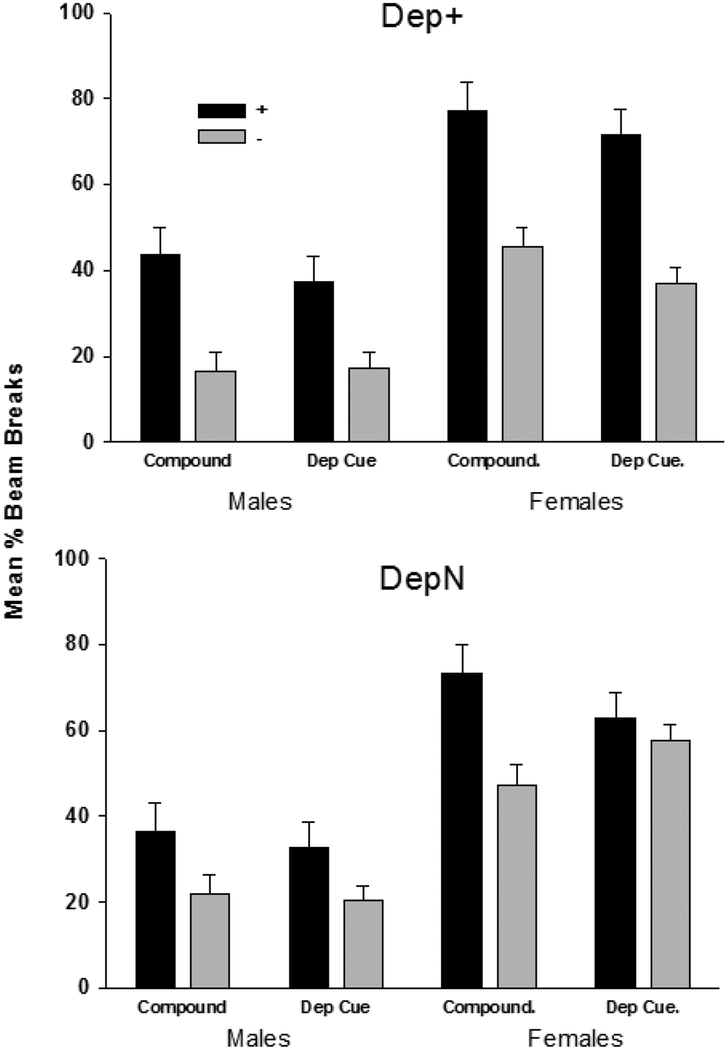
When external cues were excluded from the schedule, Dep+ groups of both sexes maintained significant discrimination based solely on deprivation cues, while DepN groups did not (see Figure 2). Consistent with this pattern of results, ANOVA on the deprivation cue test showed a +/ – × Contingency interaction, F(1, 28) = 10.83, p < .01. This interaction trended towards a difference by Sex, but this did not achieve significance, F(1, 28) = 3.79, p = .062. As with training, a main effect of Sex confirmed greater levels of responding in females compared to males, F(1, 28) = 58.06, p < .001. No other interactions were significant.
Figure 2.
Mean ± S.E.M. percent magazine entries during the 4min period preceding sucrose delivery for rewarded (+) and nonrewarded (-) four-session blocks of terminal compound acquisition (i.e., Compound) and deprivation states alone (i.e., Dep. Cue) for Deprivation Contingent (top panel) and Deprivation Noncontingent (Dep N; bottom panel) males (left panels) and females (right panels).
3.1.3. Cue Competition Test
Prior to the Cue Competition Test, the original training contingencies for Dep+ and DepN groups were reinstated (left panel of Figure 3). On the last two sessions (one each under 0h and 24h deprivation) of retraining, all groups showed similar levels of discriminative responding. Confirming this, ANOVA yielded a main effect of +/ – , F(1, 28) = 93.41, p < .001, which did not differ by Sex (p > .06) or Contingency (p > .91).
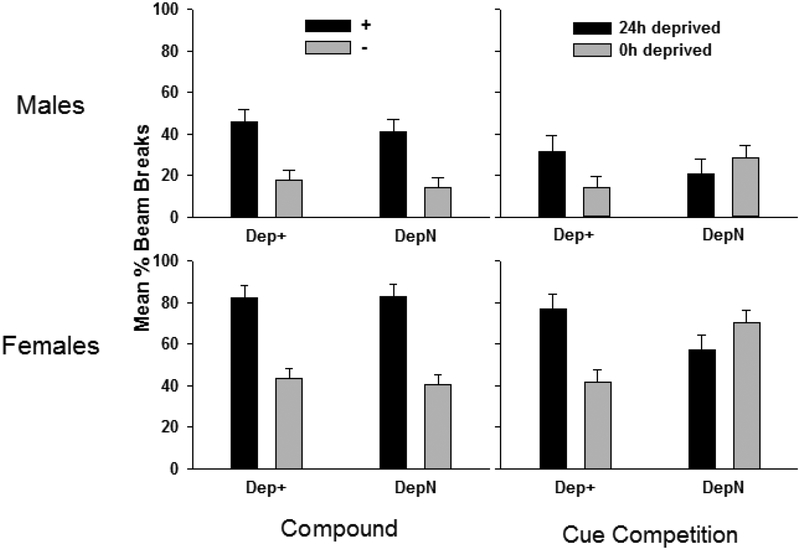
Figure 3.
Mean ± S.E.M. percent magazine entries during the 4min period preceding sucrose during compound re-training (left panels) and the Cue Competition Test (right panels), in which previously reinforced external cues were paired with previously reinforced deprivation states. Cue competition test sessions were not reinforced with sucrose.
In the Cue Competition Test, in which external cues were paired with the opposite deprivation cue with which they had previously been paired, deprivation states predicted appetitive responding for deprivation contingent groups, but not for deprivation noncontingent groups (right panel of Figure 3). Statistically confirming this, ANOVA yielded a Deprivation Level (0h versus 24h) × Contingency interaction, F(1, 28) = 26.31, p < .001. Males and females showed similar patterns of appetitive responding, which was supported by the absence of an interaction between Sex and any other factor. Females exhibited higher levels of appetitive responding, as shown by a main effect of Sex, F(1, 28) = 46.24, p < .001. Levels of appetitive responding were higher under the 24h deprivation condition compared to the 0h condition. Confirming this, ANOVA yielded a main effect of Deprivation Level, F(1, 28) = 4.92, p < .05.
3.1.4. Discussion
Results from Experiment 1 demonstrated that, like males, free-cycling female rats learn to use food deprivation states as discriminative cues for sucrose, even when trained alongside competing external food cues. Both sexes showed discriminative responding to a compound deprivation state / external cue contingency during training. When external cues were removed (deprivation cue test) or when the external cue contingency was reversed to oppose the deprivation state contingency (cue competition test), both males and females trained with reinforcement contingent on food deprivation maintained discriminative responding based on those deprivation states. Further, in the absence of predictive external cues, Group DepN rats of both sexes did not show differential appetitive responding based on deprivation state. Matched on food deprivation conditions, the absence of discrimination for Group DepN in the deprivation cue and cue competition tests challenges the notion that Group Dep+ was merely responding more when food-deprived than sated. In other words, the pattern of results indicates that differential appetitive responding reflects associative learning about interoceptive states rather than some motivational difference in food deprivation.
Results from Experiment 1 showed that 24 and 0h deprivation states acquire robust associative control over appetitive behavior in both male and female rats. These results confirm our previous findings in males, which showed that external cues do not overshadow learning about 0 and 24h food deprivation states (Sample et al., 2015). While females showed elevated levels of appetitive responding, the magnitude of discrimination by deprivation states alone or in compound with external cues did not differ significantly across sex. These findings suggest similarities in the learning mechanisms underlying the control of appetitive behavior between internal and external cues in female and male rats.
3.2. Experiment 2
3.2.1. Compound Training
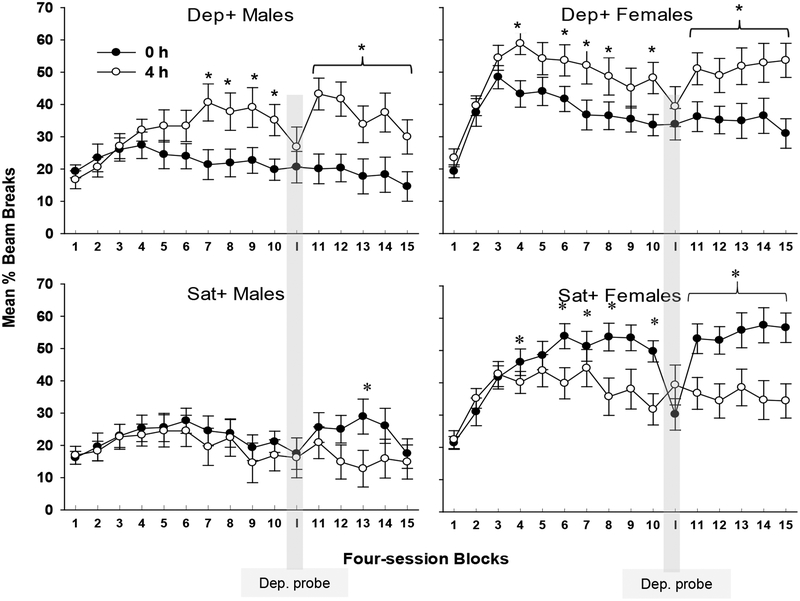
Group Dep+ males and females learned the deprivation state/external cue compound discrimination. Group Sat+ females showed sustained discriminative responding with the compound cue, while Sat+ males showed a transient difference under 0 versus 4h deprivation conditions that did not persist to terminal performance (see Figure 4). Across compound acquisition, females showed overall greater levels of appetitive responding. ANOVA on all 15 blocks of compound acquisition (i.e., without the deprivation cue probe test block) yielded a Deprivation level × Contingency × Block interaction (F(14, 336) = 13.36, p < .001). Though this interaction did not differ by Sex (p > .09), a main effect of Sex (F(1, 24) = 30.00, p < .001) confirmed higher levels of responding in females. Since discrimination across deprivation levels differed by Contingency, we then analyzed acquisition for the Dep+ and Sat+ contingency groups separately. For Group Sat+, there was a Sex × Deprivation level × Block interaction, F(14, 168) = 2.70, p < .01. Post hoc Newman-Keuls tests revealed that males only responded more under 0h compared to 4h deprivation on Block 13, while females showed discriminative responding on Blocks 4, 6–8, and 10–15. This interaction was not significant for Group Dep+ (p > .15).
Figure 4.
This graph shows the mean ± S.E.M. percent magazine entries during the 4min period preceding sucrose delivery for training with compound deprivation state/external cue discriminative stimuli for Dep+ and Sat+ males and females. Following 10 four-session blocks of training with the compound, external cues were removed in the deprivation cue probe test (“Dep. Probe”) highlighted in gray. On the next block, training with the compound cue resumed, so that external cues were again present in Blocks 11–15.
3.2.2. Deprivation Cue Probe Test
Discriminative responding emerged earlier in compound cue training for females, which had not previously been trained with low level 0 and 4h deprivation states, compared to previous work in males (Sample et al., 2016). To assess whether deprivation states, as opposed to external cues or the compound discriminative stimuli, held discriminative control after 40 sessions (20 sessions under each contingency), external cues were removed to probe learning about deprivation states alone. None of the groups showed discriminative control by deprivation states during the deprivation cue probe test. Confirming this, ANOVA on this four-session block (two sessions under each deprivation level) did not yield a Deprivation level × Contingency interaction (p > .65). A main effect of Sex, F(1, 28) = 7.43, p < .05, confirmed higher levels of responding by females than males. A main effect of Deprivation Level, F(1, 28) = 5.12, p < .05, showed that responding was greater under 4h compared to 0h deprivation. The lack of discriminative responding in the deprivation cue probe test indicated that 40 sessions of compound cue training were insufficient for rats to learn a 0 and 4h deprivation state contingency. Therefore, compound cue training was resumed for 20 additional sessions, until performance reached asymptote, so that deprivation state learning could again be assessed with the removal of external cues in the deprivation cues alone period.
3.2.3. Post-Compound Cue Training
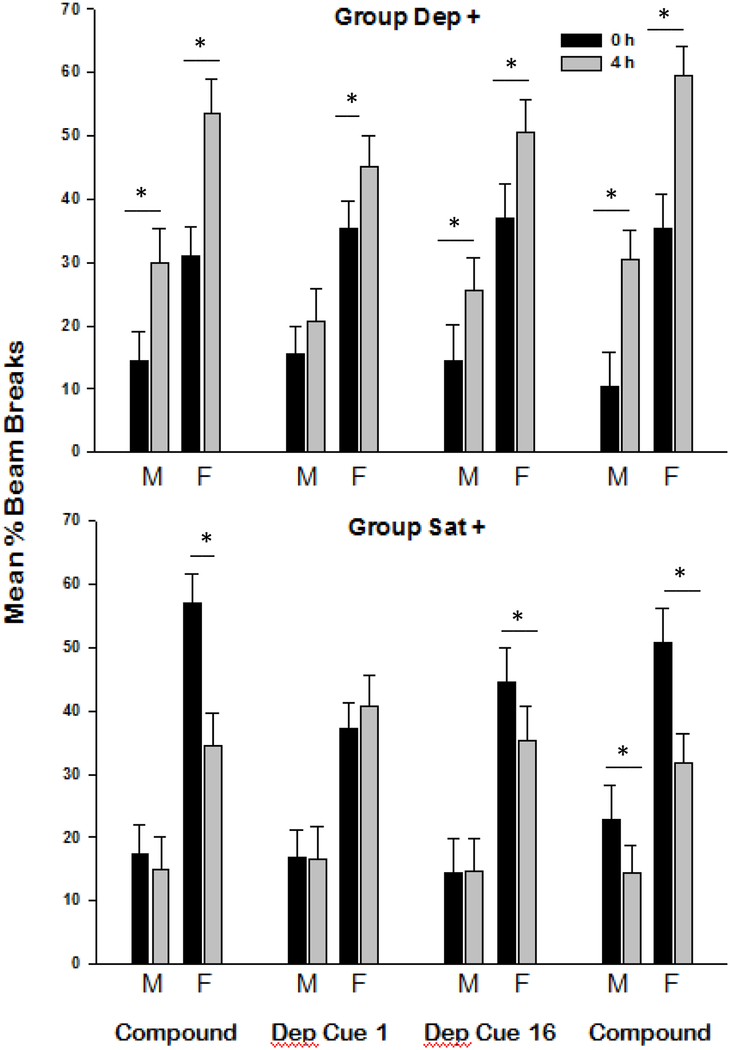
Figure 5 shows discriminative responding based on Deprivation level and Contingency for males and females in Groups Dep+ (upper panel) and Sat+ (lower panel) on the last block of compound cue training, the first and last block of training with deprivation cues alone, and the final reinstatement of the original compound cue. Summarizing the data across all periods shown in that figure, females appear to demonstrate better discrimination between their reinforced and nonreinforced deprivation states than males, and rats that were trained with the Dep+ contingency tended to show superior discrimination performance than rats trained with the Sat+ contingency.
Figure 5.
Mean ± S.E.M. percent magazine entries for each learning period for Dep+ (above) and Sat+ (Below) males (M) and females (F). Periods are as follows: last block of compound cue acquisition, the first and last block of deprivation cues alone (Dep Cue 1 and Dep Cue 16), and reinstatement of the deprivation state/ external cue compound; *indicates p < .05 for post hoc pairwise comparisons.
An omnibus ANOVA comparing responding as a function of Sex, Deprivation level, Contingency, and Period yielded a Sex × Deprivation level × Contingency × Period interaction, F(3, 72) = 2.77, p < .05. Further, ANOVA yielded a Deprivation level × Contingency interaction, confirming that Group Dep+ showed greater discriminative responding overall than Group Sat+, F(1, 28) = 64.27, p < .001. Post-hoc Duncan tests were used to identify significant differences in responding due to Deprivation level for each Sex and Contingency within each Period. At the end compound cue training, both males and females in Group Dep+ exhibited significant discrimination by Deprivation level, whereas only females in Group Sat+ discriminated significantly (ps < .05). A sex difference was also observed in the first block of testing with deprivation cues alone, with only females in Group Dep+ exhibiting significant discrimination (p < .05) based on Deprivation level when external cues were removed. Differences in responding based on Deprivation level were not significant for either sex in Group Sat+. However, on the final block with deprivation cues alone, both sexes in Group Dep+ showed discriminative responding based on Deprivation level, whereas discrimination based on Deprivation level was significant only for female rats in Group Sat+ (ps < .05). Finally, both sexes in Group Dep+ maintained significant discriminative control when the original deprivation state / external cue compound was reinstated. In addition, for the first time, males in Group Sat+ joined the Sat+ females in achieving significant discriminative responding.
3.2.4. Discussion
Results from Experiment 2 indicate that learning about low intensity deprivation state information differs by sex. Males and females were trained to use low levels of food deprivation and discrete auditory stimuli as compound discriminative cues for sucrose. Dep+ males and females learned to discriminate between the compound deprivation state/external cue stimuli, but discriminative responding emerged more rapidly for females. Receiving the opposite contingency, Group Sat+ females acquired the compound discrimination, but Group Sat+ males failed to solve the compound discrimination even after extensive training. Since females had never been trained with this paradigm, we removed auditory stimuli in a preliminary probe test of deprivation state learning. None of the groups maintained significant discrimination with deprivation states alone at this early time point, which is consistent with the duration of acquisition of 0h / 24h deprivation discrimination of males in Experiment 1 and previous work with 0h / 4h deprivation states in males (Sample et al., 2016). Since these results indicated insufficient learning about low-level deprivation states at this earlier time point, training with the compound discriminative stimuli was resumed.
Following the completion of compound cue training, external cues were removed to evaluate learning about deprivation states alone. Immediately after this removal of external cues, Group Dep+ females maintained discriminative control based on food deprivation states. Group Dep+ males lost discrimination when external cues were first removed, but were able to achieve discriminative control with additional training of deprivation states alone. While neither Group Sat+ males nor females showed discriminative control with the initial removal of external cues, Group Sat+ females solved the discrimination by the end of the deprivation cues alone period. When external cues were re-introduced to assess retention of the compound discriminative stimuli, all groups discriminated based on the compound cue, with Group Sat+ males showed discriminative control for the first time throughout all phases. With the exception of the preliminary deprivation cue probe test, Group Dep+ females maintained discriminative control, regardless of the presence of external cues, through all phases. In addition to a lack of discriminative control in the deprivation cue probe test, Experiment 2 males showed weaker discriminative control by deprivation states trained in compound with external cues.
Collectively, these results show that females exhibited enhanced learning about deprivation states compared to males. Sex differences were more pronounced for the Sat+ contingency. Group Sat+ females showed discrimination not only with the compound cue, but also without the presence of external cues in the last block of the deprivation cues alone period. In contrast, Sat+ males never achieved discriminative control with deprivation states alone. This failure of males to learn the Sat+ contingency at low levels of food deprivation departs from prior deprivation discrimination findings from our lab. Recently, Sample et al. (2016) found that males can learn an approximately free-feeding 0+ / 4- (i.e., Sat+, Dep-) contingency, but discriminative responding was modest even after extensive training. Further, this previous study did not train competing external cues as additional predictors of sucrose.
4. General Discussion
Experiments 1 and 2 were the first to show that female rats can use food deprivation states as discriminative cues for sucrose. As we have previously reported in males (Sample et al., 2015), Experiment 1 showed that females learn to use 24 and 0h food-deprivation states to predict appetitive outcomes when those energy states are trained in compound with highly valid external cues. Matching the Dep+ group in deprivation state regimen but not learned contingency, the DepN group excluded the possibility that discrimination was based solely on the effects of food deprivation or satiation on performance. When we manipulated internal and external cue contingencies to be in opposition, rats of both sexes trained with a predictive deprivation state contingency relied on energy states to predict sucrose, while groups trained with noncontingent deprivation states could not. Extending these findings to more ecologically relevant parameters, Experiment 2 showed that 0 and 4h deprivation states gained associative control over appetitive behavior in females, who outperformed males in using free-feeding levels of food deprivation to signal sucrose. Therefore, while findings from Experiment 1 did not show evidence for sex differences in appetitive control by deprivation states and external cues, Experiment 2 indicated enhanced associative learning about lower intensity food deprivation and satiety states in females.
These different patterns of results were not surprising given the different deprivation contingencies across experiments. The lower stimulus intensity of 4h food deprivation might make it more difficult to learn about, and discriminate from 0h deprivation, compared to 24h deprivation. While operationalized with the absence of food for 4 hours or access to food for 24 hours, the animal determines its own meal pattern and thus deprivation state (e.g., with meals typically occurring every two hours, discrimination stimuli could range from 0–2 and 4–6 h). While this is the most ecologically relevant way to examine appetitive control, this variability may make discrimination more sensitive to disruption, as may have occurred with the weaker discriminative control by males in Experiment 2. However, these properties also make this a particularly sensitive index of learning about deprivation states.
Additionally, we obtained different results in males and females across the contingencies reinforced under deprivation (Dep+) compared to the reversed contingency reinforced under satiation (Sat+). In our theoretical framework (Davidson, Sample, et al., 2014), animals arrive in the lab with prior experience associating free-feeding levels of food deprivation with positive post-ingestive outcomes when they eat. These extra-experimental eating associations would be expected to confer positive transfer to experimental learning about the Dep+ contingency and negative transfer to the Sat+ contingency. Indeed, male rats typically learn the Dep+ contingency more readily than the Sat+ contingency. Females’ enhanced performance on the satiated contingency in Experiment 2 suggests greater negative transfer of extra-experimental learning about deprivation states for males than females.
The current findings are consistent with previously reported sex effects in associative learning about appetitive outcomes. Females acquire Pavlovian conditioned responding to a discrete food cue more rapidly than males, though these differences do not appear to persist after asymptote is reached (Hammerslag & Gulley, 2014; Pitchers et al., 2015). Nonmnemonic performance factors may contribute to these sex effects. Females are generally more active than males, as reflected by increased food cup approach (Dalla & Shors, 2009). An additional possibility is that the sucrose reinforcer has differential salience for males versus females. In line with these non-mnemonic factors, females had higher levels of appetitive responding throughout Experiments 1 and 2. However, this greater overall responding would not be expected to produce the pattern of differences in the magnitude of discriminative control shown in Experiment 2.
Results from Experiment 2 suggest that females learn about deprivation state information, particularly satiety cues, better than males. These findings are supported by prior evidence for sex differences in the regulation of feeding, which are largely mediated by estradiol (Asarian & Geary, 2006, 2013). Since the females in the current research were intact and free cycling, estradiol would exert both tonic and phasic inhibition of food intake (Eckel, 2004). Estradiol suppresses food intake by both enhancing the potency of anorectic satiety signals (e.g., leptin, (Clegg, Brown, Woods, & Benoit, 2006)) and weakening the potency of orexigenic feeding signals (e.g., ghrelin, (Clegg et al., 2007)) (see (Asarian & Geary, 2006; Mauvais-Jarvis, Clegg, & Hevener, 2013) for reviews). For instance, estradiol augments the intake suppressing effects of CCK, a satiety signal released when food enters the gut. This enhanced sensitivity to short-term satiety signals appears to work through altered neural processing of CCK (Geary, Trace, McEwen, & Smith, 1994). Females’ greater sensitivity to the effects of anorectic signals is consistent with their ability to associate appetitive outcomes with the satiated contingency, as opposed to males, in Experiment 2.
Recent work connecting sex-specific processing of feeding signals with alterations in appetitive responding to food cues implicates deprivation state processing. Richard et al. (Richard, Anderberg, Lopez-Ferreras, Olandersson, & Skibicka, 2016) found sex-dependent effects of glucagon-like peptide-1 (GLP-1) agonist Exendin-4, a favorable therapeutic target for weight loss (Kanoski, Hayes, & Skibicka, 2016), on progressive ratio responding for sucrose. Food satiation potentiated the effects of central Ex-4 in suppressing appetitive food seeking in females, but not male rats. Whether this differential appetitive responding to satiety hormones that comprise the interoceptive milieu reflects differences in salience or utilization remains unclear.
5. Conclusions
Based on the model that interoceptive energy states suppress the ability of food cues to elicit food consumption (Davidson, Sample, et al., 2014), associative learning about deprivation states provides a useful framework for understanding the development of overeating and obesity. Previous work has demonstrated how environmental factors, such as maintenance on a Westernized diet high in fat and sugar, can alter these deprivation state learning mechanisms and interfere with hippocampal-dependent cognitive function ((Hargrave, Jones, & Davidson, 2016; Kanoski & Davidson, 2011; Sample et al., 2016; Sample et al., 2015)). While these associative processes have been scrutinized in male rats, the current research extends this investigation to females for the first time. The results of the present experiments demonstrate that deprivation states exert control over appetitive behavior, even in competition with external cues, in females. Further, these results suggest that sex differences in energy state processing may emerge with more ecologically relevant, low level deprivation state parameters, in which females show enhanced learning about deprivation states, particularly satiety cues. The pattern of results suggests that this female performance benefit is one of degree in the magnitude of the discrimination rather than type of learning. Overall, these findings suggest that males and females rely on similar associative mechanisms to predict appetitive outcomes, supporting a shared model of energy regulation for both sexes.
Acknowledgments
This research was supported by Grant R01HD028792 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development and Grant R01DK110412 from the National Institutes of Diabetes and Digestive and Kidney Diseases. Some of the work reported in this manuscript was performed as part of completing Ph.D. requirements by Camille Sample at American University, Washington D.C. We thank Alexandra Olson for assistance with data collection
References
- Asarian L, & Geary N (2006). Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci, 361(1471), 1251–1263. doi: 10.1098/rstb.2006.1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asarian L, & Geary N (2013). Sex differences in the physiology of eating. Am J Physiol Regul Integr Comp Physiol, 305(11), R1215–1267. doi: 10.1152/ajpregu.00446.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attuquayefio T, & Stevenson RJ (2015). A systematic review of longer-term dietary interventions on human cognitive function: Emerging patterns and future directions. Appetite, 95, 554–570. doi: 10.1016/j.appet.2015.08.023 [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Woods SC, & Benoit SC (2006). Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes, 55(4), 978–987. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, … Geary N (2007). Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes, 56(4), 1051–1058. doi: 10.2337/db06-0015 [DOI] [PubMed] [Google Scholar]
- Dalla C, & Shors TJ (2009). Sex differences in learning processes of classical and operant conditioning. Physiol Behav, 97(2), 229–238. doi: 10.1016/j.physbeh.2009.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Kanoski SE, Chan K, Clegg DJ, Benoit SC, & Jarrard LE (2010). Hippocampal lesions impair retention of discriminative responding based on energy state cues. Behav Neurosci, 124(1), 97–105. doi: 10.1037/a0018402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Kanoski SE, Walls EK, & Jarrard LE (2005). Memory inhibition and energy regulation. Physiol Behav, 86(5), 731–746. doi: 10.1016/j.physbeh.2005.09.004 [DOI] [PubMed] [Google Scholar]
- Davidson TL, Sample CH, & Swithers SE (2014). An application of Pavlovian principles to the problems of obesity and cognitive decline. Neurobiol Learn Mem, 108, 172–184. doi: 10.1016/j.nlm.2013.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Tracy AL, Schier LA, & Swithers SE (2014). A view of obesity as a learning and memory disorder. J Exp Psychol Anim Learn Cogn, 40(3), 261–279. doi: 10.1037/xan0000029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel LA (2004). Estradiol: a rhythmic, inhibitory, indirect control of meal size. Physiol Behav, 82(1), 35–41. doi: 10.1016/j.physbeh.2004.04.023 [DOI] [PubMed] [Google Scholar]
- Francis H, & Stevenson R (2013). The longer-term impacts of Western diet on human cognition and the brain. Appetite, 63, 119–128. doi: 10.1016/j.appet.2012.12.018 [DOI] [PubMed] [Google Scholar]
- Geary N, Trace D, McEwen B, & Smith GP (1994). Cyclic estradiol replacement increases the satiety effect of CCK-8 in ovariectomized rats. Physiol Behav, 56(2), 281–289. [DOI] [PubMed] [Google Scholar]
- Hammerslag LR, & Gulley JM (2014). Age and sex differences in reward behavior in adolescent and adult rats. Dev Psychobiol, 56(4), 611–621. doi: 10.1002/dev.21127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrave SL, Jones S, & Davidson TL (2016). The Outward Spiral: A vicious cycle model of obesity and cognitive dysfunction. Curr Opin Behav Sci, 9, 40–46. doi: 10.1016/j.cobeha.2015.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs S (2016). Cognitive processing of food rewards. Appetite, 104, 10–17. doi: 10.1016/j.appet.2015.10.003 [DOI] [PubMed] [Google Scholar]
- Kanoski SE, & Davidson TL (2011). Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav, 103(1), 59–68. doi: 10.1016/j.physbeh.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, & Grill HJ (2015). Hippocampus Contributions to Food Intake Control: Mnemonic, Neuroanatomical, and Endocrine Mechanisms. Biol Psychiatry. doi: 10.1016/j.biopsych.2015.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Hayes MR, & Skibicka KP (2016). GLP-1 and weight loss: unraveling the diverse neural circuitry. Am J Physiol Regul Integr Comp Physiol, 310(10), R885–895. doi: 10.1152/ajpregu.00520.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korol DL, & Pisani SL (2015). Estrogens and cognition: Friends or foes?: An evaluation of the opposing effects of estrogens on learning and memory. Horm Behav, 74, 105–115. doi: 10.1016/j.yhbeh.2015.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss WA, & Frick KM (2017). Sex differences in hippocampal function. J Neurosci Res, 95(1–2), 539–562. doi: 10.1002/jnr.23864 [DOI] [PubMed] [Google Scholar]
- Mauvais-Jarvis F, Clegg DJ, & Hevener AL (2013). The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev, 34(3), 309–338. doi: 10.1210/er.2012-1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock MK (1984). Estrous synchrony: modulation of ovarian cycle length by female pheromones. Physiol Behav, 32(5), 701–705. [DOI] [PubMed] [Google Scholar]
- Palmer BF, & Clegg DJ (2015). The sexual dimorphism of obesity. Mol Cell Endocrinol, 402, 113–119. doi: 10.1016/j.mce.2014.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchers KK, Flagel SB, O’Donnell EG, Woods LC, Sarter M, & Robinson TE (2015). Individual variation in the propensity to attribute incentive salience to a food cue: influence of sex. Behav Brain Res, 278, 462–469. doi: 10.1016/j.bbr.2014.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JE, Anderberg RH, Lopez-Ferreras L, Olandersson K, & Skibicka KP (2016). Sex and estrogens alter the action of glucagon-like peptide-1 on reward. Biol Sex Differ, 7, 6. doi: 10.1186/s13293-016-0059-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample CH, Jones S, Hargrave SL, Jarrard LE, & Davidson TL (2016). Western diet and the weakening of the interoceptive stimulus control of appetitive behavior. Behav Brain Res, 312, 219–230. doi: 10.1016/j.bbr.2016.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample CH, Martin AA, Jones S, Hargrave SL, & Davidson TL (2015). Western-style diet impairs stimulus control by food deprivation state cues: Implications for obesogenic environments. Appetite, 93, 13–23. doi: 10.1016/j.appet.2015.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]