Abstract
Setting:
To reduce the risk of tuberculosis (TB) among individuals with HIV infection, WHO recommends at least six months of isoniazid preventive therapy (IPT). Completion of IPT remains a major challenge in resource-limited settings.
Objective:
We evaluated predictors of IPT completion in individuals newly diagnosed with HIV.
Design:
Predictors of IPT completion among adults newly diagnosed with HIV in rural Malawi were evaluated using a multilevel logistic regression model.
Results:
974 participants screened negative for active TB and were started on IPT, 732 (75%) of whom completed treatment. Only 1 IPT eligible individual refused treatment. Participants <25 years (compared to those >45 years, aOR: 0.33, 95% CI: 0.18–0.60) and men (compared to non-pregnant women, aOR: 0.57, 95% CI: 0.37–0.88) had lower odds of IPT completion.
Conclusion:
IPT provision at the time of an initial HIV diagnosis was highly acceptable in rural Malawi; three-quarters of those who initiated IPT successfully completed therapy. We observed lower odds of completion among men and female participants younger than 25 years old. Additional efforts may be needed to ensure IPT completion for men and young women who have been recently diagnosed with HIV.
Keywords: Tuberculosis, co-infection, IPT
INTRODUCTION:
Tuberculosis (TB) remains a leading cause of death among individuals living with HIV worldwide 1. While antiretroviral therapy (ART) reduces the risk of developing TB disease among HIV-infected individuals, the risk of TB activation is still higher among those with HIV than amongst their HIV-uninfected counterparts, especially in the first six months of ART 2. Treatment with isoniazid preventive therapy (IPT) has been shown to reduce the risk of TB disease in those with HIV above and beyond the effects of ART alone, and has also demonstrated a synergistic effect when administered concomitantly with ART 3,4. Recent evidence suggests that IPT may have a mortality benefit in this population as well 5,6.
Despite longstanding recommendations from the World Health Organization (WHO) that ≥6 months of IPT should be offered to all HIV-infected individuals in whom active TB disease is not suspected, global IPT coverage remains low 7. It is estimated that only 25% of the IPT-eligible individuals in HIV care are currently receiving IPT 8, and those who do initiate face barriers to treatment completion, including stigma, fear of side effects, poor relationships with healthcare providers, transport costs, and the challenge of taking medication in the absence of symptoms 9,10.
Providing IPT soon after an HIV diagnosis targets those at highest risk of TB disease in the period before and immediately after ART initiation (and who therefore stand to benefit the most from IPT) and is achievable in routine clinical settings. Identifying those individuals at greatest risk of non-adherence could inform targeted strategies to provide additional adherence support, which in turn may boost completion rates and reduce incident TB. While a small number of studies have evaluated predictors of IPT adherence in South Africa 9,11,12, few studies have been done in low-income settings. In an effort to inform strategies to improve IPT adherence, we sought to identify predictors of IPT completion under operational conditions among a cohort of recently diagnosed HIV patients in Malawi, a high TB/HIV burden country where IPT scale-up has achieved relatively higher coverage 13.
STUDY POPULATION AND METHODS:
Study Design
This analysis is nested in a larger randomized clinical trial of TB diagnosis among adults newly diagnosed with HIV in rural Malawi (CHEPETSA, clinicaltrials.gov #NCT01450085). Twelve clinics were randomized in three phases to one of two TB screening algorithms:symptom screening plus sputum smear microscopy, and symptom screening plus Xpert MTB/RIF (Cepheid, Inc.; Sunnyvale, CA). Individuals in both arms for whom active TB disease was excluded were prescribed six months of IPT. Data for this analysis were limited to participants recruited and started on IPT on or before July 1, 2014 from eight clinics participating in the first two phases of the trial.
Participants were eligible for the study if they were ≥18 years of age and were not taking IPT, on TB treatment, or receiving antiretroviral therapy (ART) in the study clinic at enrollment. Participants were not eligible to participate if they were unable to speak English or Chichewa, had hearing impairments, were a prisoner, or were unwilling/unable to give informed consent. All participants underwent TB symptom screening, and those who reported ≥1TB symptoms received same-day microbiological testing with either LED fluorescence microscopy or Xpert MTB/RIF.
All participants in whom active TB disease had been excluded were screened for IPT eligibility 14. Symptomatic participants who tested negative for TB disease became eligible for IPT if/when their symptoms resolved. Participants who had known liver disease, reported excessive alcohol consumption (>2 times per week, or 3+ drinks per drinking day), or had a history of epilepsy, kidney failure, or severe peripheral neuropathy were considered ineligible; all others were offered IPT. Eligible participants were prescribed six months of IPT, dispensed in monthly or semi-monthly intervals during routine clinic visits. The number of pills dispensed at each visit was documented in the participant’s study file and used to track adherence. ART was initiated after enrollment per the Malawian Ministry of Health’s contemporary HIV guidelines 14; eligibility for ART included WHO Stage 3 or 4 disease, CD4+ T-cell count <350 cells/mm3, or pregnancy/breastfeeding.
Study Procedures
Study participants who were eligible for IPT were offered 300 mg of isoniazid daily, as well as 25 mg of pyridoxine daily, for six months. Up to 180 doses of IPT were dispensed during visits routine clinical visits. Participants were followed-up during visits for HIV-related care every 1–3 months, until one year of follow-up was attained.
Participants receiving IPT also underwent safety monitoring by study nurses to track potential adverse reactions per local guidelines 14. Before initiating IPT, participants received counseling regarding the potential side effects, and were advised to seek treatment immediately if they experienced any of those symptoms. Individuals reporting symptoms of hepatotoxicity underwent blood draws to test bilirubin and transaminase levels.
Participants completed a demographic and clinical history questionnaire at baseline, as well as at each follow-up visit. Questionnaires were completed by study staff, and included questions about TB symptoms, possible IPT-related adverse events, ART status, pregnancy, and the number of isoniazid pills dispensed to date. The presence of TB symptoms was assessed at enrollment and at each subsequent follow-up visit.
Statistical Methods
Our primary outcome was completion of IPT, which was defined as receipt of ≥ 150 doses of isoniazid. Non-completion was defined receiving <150 doses of IPT. We examined univariate associations with IPT completion using chi-square or Fisher’s exact tests for dichotomous exposures, and Wilcoxon-Mann-Whitney tests for continuous exposures. We constructed a multilevel logistic regression model to explore the relationship between IPT completion and individual- and clinic-level predictors including concomitant receipt of ART, age, pregnancy status, IPT side-effects, and alcohol use. Concomitant ART exposure was defined as any self-reported receipt of ART drugs beginning either before, or within 7 days after, IPT initiation. We incorporated a random intercept to account for clustering at the clinic level. Variables that reached a significance of p<0.1 in the univariate analysis, or that were considered to be of epidemiological significance a priori, were included in the multivariate model. Variables in the model were checked for co-linearity using variation inflation factors, and evaluated for interaction. Additionally, we performed a time-to-treatment-discontinuation survival analysis, stratified by key exposure variables identified in the analysis above. Time to treatment-discontinuation was defined as days from IPT initiation to the date of the clinic visit where the participant received his/her last IPT dosages. All analyses were performed in Stata 12 (Stata Corp., College Station, USA).
Ethical Considerations
This study was approved by the Malawi University College of Medicine Research Ethics Committee and the Johns Hopkins University School of Medicine Institutional Review Board. All study participants provided individual written informed consent before study participation.
RESULTS:
A total of 1,359 participants were enrolled (Figure 1) across 8 study clinics, for an average of 122 participants per clinic (range: 47–199). During screening, 504 (37%) participants reported ≥1symptoms of TB and received microbiological testing for active TB disease. Of those tested, 27 (5%) had active TB and were thus ineligible for IPT. Of the 477 symptomatic individuals testing negative for TB disease (i.e., initially ineligible for IPT), 253 (53%) returned for subsequent IPT eligibility screening. Altogether 1,106 participants were screened for IPT eligibility (253 symptomatic TB-negative participants, and 853 asymptomatic participants). Of these, 95 (9%) failed to meet the IPT eligibility criteria, and 984 (89%) were started on preventive therapy by the pre-specified analysis close date. Only one IPT-eligible individual in our study refused to initiate preventive therapy. Ten participants were subsequently excluded from analysis because they were missing IPT outcome data.
Figure 1:

Screening and IPT Initiation, * 224 individuals who had TB symptoms at enrollment and received microbiological testing for TB disease were not able to be screened for IPT eligibility, either because their TB symptoms did not resolve, or they did not present for additional follow-up
Participants in our study were majority female (n=650, 67%) and had a median age of 33 (interquartile range [IQR]: 27–40). Male participants were significantly older than their female counterparts (38.1 vs. 32.4 years, p<0.001). Most participants reported being in “Good” or “Fair” health at enrollment (n=788, 81%), and were classified as WHO HIV Stage I or II (n=794, 82%) at the time of their HIV diagnosis (Table 1). Individuals in our sample completed a median of 6 (IQR: 4–7) routine clinical follow-up visits during the study period. Concomitant ART and IPT initiation was relatively uncommon in our cohort (n=152, 16%), though the majority of participants reported initiating ART at some point during the IPT treatment period (n=670, 69%).
Table 1:
Patient-level clinical and demographic characteristics, by IPT completion status
| Baseline Variables | Completed IPT (N=732) |
Did Not Complete IPT (N=242) |
Total (N=974) |
P-value |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Age Category | ||||
| 18–25 | 132 (18.0) | 72 (29.8) | 204 (20.9) | 0.001 |
| 26–35 | 296 (40.4) | 87 (36.0) | 383 (39.3) | |
| 36–45 | 204 (27.9) | 58 (24.0) | 262 (26.9) | |
| >45 | 100 (13.7) | 25 (10.3) | 125 (12.8) | |
| Sex/Pregnancy Status | ||||
| Pregnant female | 151 (20.6) | 60 (28.4) | 211 (21.7) | 0.133 |
| Non-pregnant female | 343 (46.9) | 96 (39.7) | 439 (45.1) | |
| Male | 238 (32.5) | 86 (35.5) | 324 (33.3) | |
| Self-Reported Health | ||||
| Excellent | 100 (13.7) | 31 (12.8) | 131 (13.5) | 0.827 |
| Good | 368 (50.3) | 125 (51.7) | 493 (50.6) | |
| Fair | 225 (30.7) | 70 (28.9) | 295 (30.3) | |
| Poor | 39 (5.3) | 16 (6.6) | 55 (5.7) | |
| WHO HIV Stage* | ||||
| 1 or 2 | 599 (82.4) | 195 (81.3) | 794 (82.1) | 0.689 |
| 3 or 4 | 128 (17.6) | 45 (18.8) | 173 (17.9) | |
| Previous History of TB | 10 (1.4) | 3 (1.2) | 13 (1.3) | 1.0‡ |
| Any TB Symptoms at Enrollment | 164 (22.4) | 40 (16.5) | 204 (20.9) | 0.052 |
| Concurrent ART Exposure | 122 (16.7) | 30 (12.4) | 152 (15.6) | 0.113 |
| Eligible for ART at Enrollment | 355 (48.8) | 117 (48.8) | 472 (48.8) | 0.983 |
| Number of Follow-Up Visits (Median, IQR) | 6 (5–7) | 1 (0–3) | 6 (4–7) | <0.001 |
| Experienced IPT Side-Effects during Follow-Up | 6 (0.8) | 3 (1.7) | 9 (1.0) | 0.389‡ |
| Hospitalized during Follow-Up | 36 (4.9) | 7 (3.9) | 43 (4.7) | 0.696‡ |
| Experienced an IPT interruption of >2 months | 52 (7.1) | 30 (12.5) | 82 (8.4) | 0.010 |
| Current/Former Smoker | 112 (15.3) | 42 (17.4) | 154 (15.8) | 0.448 |
| Any Alcohol Use | 147 (20.1) | 57 (23.6) | 204 (20.9) | 0.250 |
P-value obtained from a two sample Wilcoxon rank-sum test
P-value obtained from a Fisher’s exact test
7 individuals were missing WHO stage classification
5 individuals were missing ART eligibility because of missing CD4 cell counts and missing WHO stage
The majority of participants initiating IPT completed treatment (n=732/974, 75%). Most non-completers stopped IPT due to treatment cessation/failure to return for follow-up (n=198, 82%). Individuals completing IPT received a median of 177 doses (IQR: 167–200), while non-completers received a median of 58 doses (IQR: 28–139). Compared to non-completers, those who completed IPT were older (median age 34 vs. 31 years, p<0.001), had attended more follow-up visits (median 6 vs. 1, p<0.001), and were less likely to have experienced a prolonged treatment interruption prior to ending therapy (7% vs. 13%, p=0.01). Rates of completion were highest among non-pregnant women above the age of 25 (82%, 95% CI: 78–86%), and lowest among young women, whether pregnant (64%, 95% CI: 54–74%) or not (63%, 95% CI: 53–73%) (Figure 2).
Figure 2:
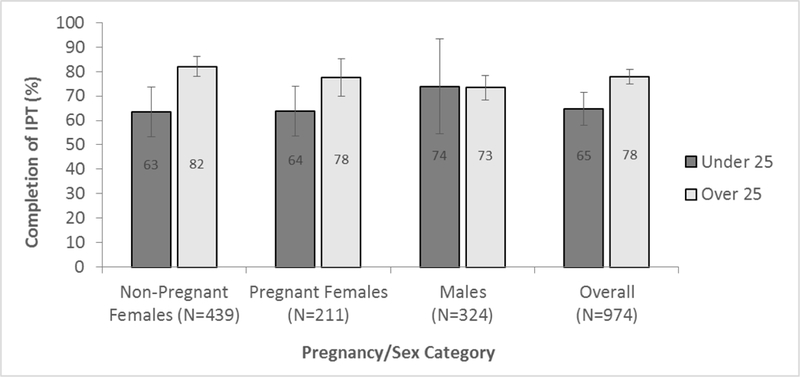
IPT Completion Rates by Age Category and Sex/Pregnancy Status, Error bars indicate 95% Confidence Intervals around estimates. Isoniazid Preventive Therapy (IPT) completion was higher in the older age category, and lowest among young women, regardless of pregnancy status.
Multilevel Model & Survival Analysis
After multivariate adjustment, age less than 25 (compared to age >45, aOR: 0.33, 95% CI: 0.18–0.60) and male sex (compared to non-pregnant females: aOR: 0.57, 95% CI: 0.37–0.88) were significantly associated with worse IPT completion rates. Each five-year increase in age was associated with 1.3 times greater odds of IPT completion among women (p<0.001) compared to only 1.1 times the odds (p=0.107) among men on univariate analysis, but this age-sex interaction was not statistically significant in our multivariate model (p>0.05, data not shown). Concomitant exposure to ART, self-reported alcohol use, being a current or former smoker, or reporting any TB symptoms at baseline were not significantly associated with IPT completion in our model. Survival analysis of time-to-IPT-discontinuation illustrates the higher probability of treatment non-completion among participants younger than 25 years of age compared to their older counterparts (Figure 3), a difference that was statistically significant (Log-rank test p-value: 0.001).
Figure 3:
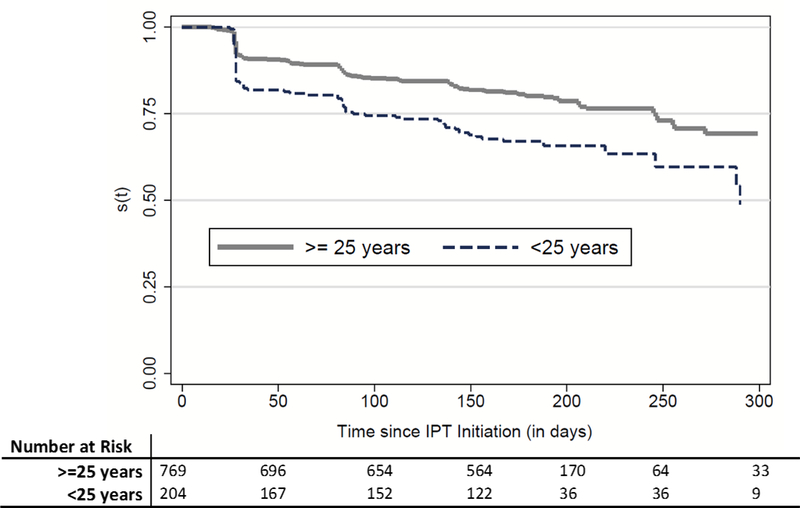
IPT Non-Completion Survival Curve, Time to non-completion of Isoniazid Preventive Therapy Stratified by Age, Shown are the Kaplan-Meier curves describing the time from IPT initiation to the date at which the participant either completed IPT (received the last dose), or was censored (due to treatment default, death , refusal of further treatment, IPT stopped by doctor, side effects, TB diagnosis, or transfer out of the clinic area), according to whether participants were older or younger than 25 years of age.
DISCUSSION:
We found high rates of IPT completion in this cohort of adults newly diagnosed with HIV in rural Malawi. Our results indicate that IPT completion among newly diagnosed HIV patients can be reasonably high under operational conditions in rural, low-income settings. Given that over 99% of the IPT-eligible participants in our study initiated preventive therapy, it appears that IPT eligibility screening at the time of an initial HIV diagnosis was acceptable to patients. In our study population, older non-pregnant women had the highest odds of IPT completion. While concomitant receipt of ART was not significantly associated with increased odds of IPT completion, most of our study participants did initiate ART during the preventive treatment period. Our findings suggest that point-of-HIV-diagnosis TB screening and IPT initiation may be an effective strategy to reduce the TB burden in individuals newly diagnosed with HIV, especially in the high-risk period immediately preceding and following ART initiation.
Our findings are supported by other findings from the published literature. Earlier studies conducted in high TB/HIV burden settings 15–19 have found similarly high rates of IPT completion. A previous meta-analysis on interventions to improve IPT adherence also suggests that TB/HIV integration may boost IPT completion rates 20. These findings support the conclusion that the initial HIV diagnosis represents an opportunity to screen HIV-infected individuals for TB, to initiate IPT for those who are eligible, and to further integrate HIV/TB programs.
While overall IPT completion was high in our study, several sub-groups had significantly lower rates. We found that individuals (especially pregnant women) who were less than 25 years old were significantly less likely to complete IPT than their older counterparts. Previous work from Tanzania 18 and Uganda 21 also found that younger age was associated with an increased risk of treatment non-completion among HIV-infected individuals on IPT. Younger participants in our study tended to be healthier at baseline, and were less likely to have initiated ART during follow-up, potentially making it harder to adhere to IPT without the support of ongoing clinic visits to obtain antiretroviral drugs. Younger individuals who returned for at least one clinic visit after initiating preventive therapy had IPT completion rates similar to their older counterparts. Interventions designed to decrease early drop-out, and which address the particular barriers to treatment adherence among younger patients, are needed to prevent non-completion in this group.
While the IPT completion rates among pregnant women observed in this study were similar to rates observed in other studies conducted in low-resource settings 22, compared to their non-pregnant counterparts, women diagnosed with HIV through antenatal care still had decreased odds of IPT completion (though this did not reach statistical significance after adjustment). WHO guidelines for the treatment of tuberculosis recommend the integration of TB prevention and treatment into existing antenatal care and prevention of mother-to-child HIV transmission programs 23. However, integration remains a challenge in many settings, and low rates of IPT initiation among pregnant women still persist 24,25, again highlighting the need for adherence interventions tailored to this group.
Compared to non-pregnant women, men also had significantly lower rates of IPT completion in our analysis. Numerous studies on HIV diagnosis and treatment in sub-Saharan Africa have noted men’s lower uptake of health-facility-based services [24] (and resulting poorer health outcomes 26), due at least in part on gender norms and difficulty visiting a clinic during working hours 27. These findings are supported by the significantly older age and worse general health reported by men in our study, which may reflect later initial HIV diagnoses. In addition to efforts to diagnose HIV-infected men earlier in the disease course, HIV and TB-related health outcomes for men may be improved by strategies such as community-based delivery of IPT, which could reduce barriers to preventive treatment adherence and boost completion rates in this part of the population.
As with any observational study, this research has limitations. Measurement of IPT completion status was based on the number of IPT doses dispensed during routine study visits rather than any objective measure (e.g., urine metabolites). Nevertheless, self-reported and/or pill count adherence correlates reasonably well with other measures of adherence 28–30, and from a pragmatic perspective, it is unlikely that adherence will be measured in any other fashion in the field. While we captured the variables that are most likely to be programmatically accessible, we were unable to measure all potential predictors of adherence, including clinic-level variables that might have exerted strong influence. We were also unable to comprehensively capture outcomes, including IPT-related adverse events, among participants who were lost to follow up. Future research could more fully evaluate a wider range of associations with IPT adherence, and could also consider study of potential interventions to improve adherence in those at greatest risk of non-completion. Additional studies should also consider the impact and cost-effectiveness of concurrent IPT and ART initiation as test-and-start continues to be scaled-up globally.
Table 2:
Univariate and Multivariate Models for IPT Completion*
| Baseline Variables | Unadjusted | Adjusted** | ||
|---|---|---|---|---|
| OR | 95% CI | aOR | 95% CI | |
| Age Category | ||||
| 18–25 | 0.37 | (0.21–0.64) | 0.33 | (0.18–0.60) |
| 26–35 | 0.80 | (0.48–1.35) | 0.78 | (0.46–1.33) |
| 36–45 | 0.84 | (0.49–1.45) | 0.85 | (0.49–1.47) |
| >45 | Ref | Ref | Ref | Ref |
| Sex/Pregnancy Status | ||||
| Female, not pregnant | Ref | Ref | Ref | Ref |
| Female, pregnant | 0.66 | (0.44–0.98) | 0.86 | (0.56–1.31) |
| Male | 0.69 | (0.49–0.99) | 0.57 | (0.37–0.88) |
| Any TB Symptoms at Enrollment | 1.25 | (0.82–1.89) | 1.09 | (0.55–2.19) |
| Concurrent ART Exposure | 1.27 | (0.80–2.00) | 1.08 | (0.70–1.72) |
| Any Alcohol Use | 0.96 | (0.67–1.39) | 1.09 | (0.70–1.72) |
| Current/Former Smoker | 0.90 | (0.60–1.36) | 1.01 | (0.62–1.63) |
Multilevel logistic models of IPT completion with clinic-level random intercept
Model adjusted for age (over/under 25 years), sex/pregnancy status, the presence of TB symptoms at enrollment, concurrent ART exposure, any alcohol use, and current/former smoking status.
Acknowledgements
Author KML performed data cleaning and analysis, as well as manuscript writing and editing. MK assisted with data quality assurance, data cleaning and analysis, and manuscript editing. GLB managed the study and participated in manuscript editing. LGN assisted with study implementation, data quality assurance, and manuscript preparation. AN participated in study implementation, data quality assurance, and manuscript editing. SM provided technical inputs and assisted with manuscript editing. ELB, REC, and DWD designed the study, performed study oversight, provided technical inputs during data analysis, and assisted in manuscript preparation.
References
- 1.Saraceni V, King BS, Cavalcante SC, Golub JE, Lauria LM, Moulton LH, et al. Tuberculosis as primary cause of death among AIDS cases in Rio de Janeiro, Brazil. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2008. July;12(7):769–72. [PMC free article] [PubMed] [Google Scholar]
- 2.Lawn SD, Harries AD, Meintjes G, Getahun H, Havlir DV, Wood R. Reducing deaths from tuberculosis in antiretroviral treatment programmes in sub-Saharan Africa. AIDS Lond Engl. 2012. November 13;26(17):2121–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Golub JE, Pronyk P, Mohapi L, Thsabangu N, Moshabela M, Struthers H, et al. Isoniazid preventive therapy, HAART and tuberculosis risk in HIV-infected adults in South Africa: a prospective cohort. AIDS Lond Engl. 2009. March 13;23(5):631–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golub JE, Saraceni V, Cavalcante SC, Pacheco AG, Moulton LH, King BS, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS Lond Engl. 2007. July 11;21(11):1441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.TEMPRANO ANRS 12136 Study Group, Danel C, Moh R, Gabillard D, Badje A, Le Carrou J, et al. A Trial of Early Antiretrovirals and Isoniazid Preventive Therapy in Africa. N Engl J Med. 2015. August 27;373(9):808–22. [DOI] [PubMed] [Google Scholar]
- 6.Badje AD, Moh R, Gabillard D, Guehi C, Kabran M, Menan H, et al. Six-Month IPT Reduces Mortality Independently of ART in African Adults with High CD4. In Seattle, Washington; 2017 [cited 2017 Jun 30]. p. Abstract 78. Available from: http://www.croiconference.org/sessions/six-month-ipt-reduces-mortality-independently-art-african-adults-high-cd4 [Google Scholar]
- 7.World Health Organization, UNAIDS. Policy statement on preventive therapy against tuberculosis in people living with HIV [Internet]. Geneva, Switzerland: WHO, UNAIDS; 1998. February [cited 2015 Mar 25] p. 1–20. Available from: http://whqlibdoc.who.int/hq/1998/WHO_TB_98.255.pdf [Google Scholar]
- 8.World Health Organization. The Three I’s for TB/HIV: Isoniazid preventive therapy (IPT) [Internet]. Geneva, Switzerland: WHO; 2015. [cited 2015 Oct 24]. (HIV/AIDS). Available from: http://www.who.int/hiv/topics/tb/3is_ipt/en/ [Google Scholar]
- 9.Rowe KA, Makhubele B, Hargreaves JR, Porter JD, Hausler HP, Pronyk PM. Adherence to TB preventive therapy for HIV-positive patients in rural South Africa: implications for antiretroviral delivery in resource-poor settings? Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2005. March;9(3):263–9. [PubMed] [Google Scholar]
- 10.Makanjuola T, Taddese HB, Booth A. Factors Associated with Adherence to Treatment with Isoniazid for the Prevention of Tuberculosis amongst People Living with HIV/AIDS: A Systematic Review of Qualitative Data: e87166. PLoS One [Internet]. 2014. February [cited 2015 Oct 24];9(2). Available from: http://search.proquest.com.proxy1.library.jhu.edu/docview/1494055323/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohammed A, Myer L, Ehrlich R, Wood R, Cilliers F, Maartens G. Randomised controlled trial of isoniazid preventive therapy in South African adults with advanced HIV disease. Int J Tuberc Lung Dis. 2007. October 1;11(10):1114–20. [PubMed] [Google Scholar]
- 12.Szakacs TA, Wilson D, Cameron DW, Clark M, Kocheleff P, Muller FJ, et al. Adherence with isoniazid for prevention of tuberculosis among HIV-infected adults in South Africa. BMC Infect Dis. 2006. June 13;6(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Global Tuberculosis Report 2014 [Internet]. Geneva, Switzerland: WHO; 2014. [cited 2015 Mar 5] p. 1–171. Available from: http://www.who.int/tb/publications/global_report/en/ [Google Scholar]
- 14.Ministry of Health, Malawi. Clinical Management of HIV in Children and Adults [Internet]. Malawi: Ministry of Health; 2011. Available from: http://www.who.int/hiv/pub/guidelines/malawi_art.pdf [Google Scholar]
- 15.Munseri PJ, Talbot EA, Mtei L, Fordham von Reyn C. Completion of isoniazid preventive therapy among HIV-infected patients in Tanzania. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2008. September;12(9):1037–41. [PubMed] [Google Scholar]
- 16.Oni T, Tsekela R, Kwaza B, Manjezi L, Bangani N, Wilkinson KA, et al. A Recent HIV Diagnosis Is Associated with Non-Completion of Isoniazid Preventive Therapy in an HIV-Infected Cohort in Cape Town. PLoS ONE. 2012. December 20;7(12):e52489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durovni B, Cavalcante SC, Saraceni V, Vellozo V, Israel G, King BS, et al. The implementation of isoniazid preventive therapy in HIV clinics: the experience from the TB/HIV in Rio (THRio) Study. AIDS Lond Engl. 2010. November;24(Suppl 5):S49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shayo GA, Moshiro C, Aboud S, Bakari M, Mugusi FM. Acceptability and adherence to Isoniazid preventive therapy in HIV-infected patients clinically screened for latent tuberculosis in Dar es Salaam, Tanzania. BMC Infect Dis. 2015. August 26;15(1):368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berhe M, Demissie M, Tesfaye G, Berhe M, Demissie M, Tesfaye G. Isoniazid Preventive Therapy Adherence and Associated Factors among HIV Positive Patients in Addis Ababa, Ethiopia, Isoniazid Preventive Therapy Adherence and Associated Factors among HIV Positive Patients in Addis Ababa, Ethiopia. Adv Epidemiol Adv Epidemiol. 2014. July 22;2014, 2014:e230587. [Google Scholar]
- 20.Adams LV, Talbot EA, Odato K, Blunt H, Steingart KR. Interventions to improve delivery of isoniazid preventive therapy: an overview of systematic reviews. BMC Infect Dis. 2014;14:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Namuwenge PM, Mukonzo JK, Kiwanuka N, Wanyenze R, Byaruhanga R, Bissell K, et al. Loss to follow up from isoniazid preventive therapy among adults attending HIV voluntary counseling and testing sites in Uganda. Trans R Soc Trop Med Hyg. 2012. February 1;106(2):84–9. [DOI] [PubMed] [Google Scholar]
- 22.Tiam A, Machekano R, Gounder CR, Maama-Maime LBM, Ntene-Sealiete K, Sahu M, et al. Preventing tuberculosis among HIV-infected pregnant women in Lesotho: the case for rolling out active case finding and isoniazid preventive therapy. J Acquir Immune Defic Syndr 1999. 2014. September 1;67(1):e5–11. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Treatment of Tuberculosis: Guidelines for national programmes--Forth Edition. Geneva, Switzerland: WHO; 2009. [Google Scholar]
- 24.Uwimana J, Jackson D. Integration of tuberculosis and prevention of mother-to-child transmission of HIV programmes in South Africa. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2013. October;17(10):1285–90. [DOI] [PubMed] [Google Scholar]
- 25.Peters JA, Heunis C, Kigozi G, Osoba T, van der Walt M. Integration of TB-HIV services at an ANC facility in Frances Baard District, Northern Cape, South Africa. Public Health Action. 2015. March 21;5(1):30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bor J, Rosen S, Chimbindi N, Haber N, Herbst K, Mutevedzi T, et al. Mass HIV Treatment and Sex Disparities in Life Expectancy: Demographic Surveillance in Rural South Africa. PLoS Med. 2015. November 24;12(11):e1001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mane P, Aggleton P. Gender and HIV/AIDS: What Do Men have to Do with it? Curr Sociol. 2001. November 1;49(6):23–37. [Google Scholar]
- 28.Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, Maartens G. Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Ann Intern Med. 2007. April 17;146(8):564–73. [DOI] [PubMed] [Google Scholar]
- 29.Nachega JB, Hislop M, Dowdy DW, Lo M, Omer SB, Regensberg L, et al. Adherence to highly active antiretroviral therapy assessed by pharmacy claims predicts survival in HIV-infected South African adults. J Acquir Immune Defic Syndr 1999. 2006. September;43(1):78–84. [DOI] [PubMed] [Google Scholar]
- 30.Fallab-Stubi CL, Zellweger JP, Sauty A, Uldry C, Iorillo D, Burnier M. Electronic monitoring of adherence to treatment in the preventive chemotherapy of tuberculosis. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 1998. July;2(7):525–30. [PubMed] [Google Scholar]


