Abstract
Background
Environmental, social, geographical, and other factors could affect the distribution of intestinal parasites. Parasitic infections would impose on health and social problems like mal-absorption, diarrhea, impaired work capacity, and reduced growth rate. However, there is a scarcity of information regarding the prevalence of intestinal parasites and associated factors in the study area.
Methods
Institution based cross-sectional study was conducted among 310 study participants from April–May, 2017. Study participants were selected using a systematic random sampling technique. EPI Info version 7 and SPSS version 20 were used to enter and analyze the data. Both bivariate and multivariate logistic regression analyses were computed. In multivariate analysis, variables with P-value < 0.05 were considered statistically significant.
Results
In this study, the mean age of participants was 29.25 Months. The overall prevalence of intestinal parasites was 18.7% (95% CI = 14.4–23.3). Children who rarely feed fresh meal (AOR = 7.74, 95% CI: 1.61, 7.84), Children whose nails were sometimes trimmed (AOR = 3.41, 95% CI: 2.20–10.28), children who had no clean playing ground (AOR = 2.43, 95% CI: 1.25–5.18), and children who had open defecation of the family (AOR = 3.40, 95% CI: 1.27–10.86) were significantly associated with intestinal parasitic infections. Among the intestinal parasites, 31(53.5%) were G.lamblia (Giardia lamblia) and 21(36.2%) were E. histolytica/E. dispar/E. moshkovskii.
Conclusion
In this study, the prevalence of intestinal parasites was found low compared with the WHO annual or biannual population prevalence and treatment. However, strengthening of health education about food, personal and environmental hygiene of both children and mothers/guardians is crucial to limit the transmission. Besides, improving mothers/guardian awareness about the mode of intestinal parasites transmission is necessary.
Keywords: Intestinal parasite, Prevalence, Under-five children, Ethiopia
Background
Intestinal parasites primarily infect the gastrointestinal tract (GIT) but can live throughout the body. Helminths and protozoa are the two main types of intestinal parasites that live in the intestines [1].
Intestinal helminths and protozoan infections have been recognized as significant causes of illnesses and death worldwide [2]. These are among the most common human parasitic infections and have been associated with important morbidity and economic loss in endemic areas [3, 4]. Worldwide, more than one-sixth of the population is infected by intestinal parasites of which majority live in developing countries [5, 6]. Among intestinal parasitic infections, Ascariasis, hookworm, and Trichiuriasis are responsible for one billion, 900 million, and 500 million infections respectively, and cause significant morbidity and mortality [7].
The prevalence of intestinal parasites among under-five, preschool and school children were 17.7% in Riyadh, Saudi Arabia [8], 52.8% in an urban slum of Karachi, Pakistan [9], 19.6% in Zambia [10], and 30% in Khartoum, Sudan [11]. In Ethiopia its prevalence varies from area to area; in Wondo Genet 85.1% [12], Aynalem village, Tigray 48.1% [13], Debre Birhan referral Hospital 17.4% [14], Adare and millennium health center in Hawassa 26.6% [15], Wonji Shoa Sugar Estate 24.3% [16]. Intestinal parasitic infection accounts for a global health burden in developing countries mainly due to fecal contamination of water and food, climatic, environmental, and socio-cultural factors enhancing parasitic transmission [17–19]. In urbanized countries, protozoan parasites infection is in contrast to helminths. Amoebiasis is one of the most important reasons for death from parasitic diseases wide-reaching with its impact on people of developing countries [7].
The distribution of helminths and protozoan parasites are varied in different regions. In developing countries, enteric protozoa mainly Giardia intestinalis including G.lamblia (Giardia lamblia) and Entamoeba spp. were common in children. The systematic review and meta-analysis study on prevalence of gastrointestinal pathogens in developed and developing reviled Giardia intestinalis as the most frequently detected protozoa in developing regions, with the prevalence of 3.0 and 2.7% in South Asia and Sub-Saharan Africa respectively. The prevalence of Entamoeba spp. was 1.5% in Middle East, North Africa, and Sub-Saharan Africa. Cryptosporidium was 1.0 and 1.7% in the Middle East and North Africa, and in South Asia respectively. But Dientamoeba fragilis, was found in < 1% of cases in each region [20]. The prevalence of G.lamblia (Giardia lamblia) and Entamoeba histolytica were (8.5, 5.7%) 17.46, 0.87%), and (3, 2%) in Debre Birhan referral hospital, Southwestern Iran, and Taifg overnorate respectively [14, 21, 22].
Ethiopia has one of the lowest qualities of drinking water supply and latrine coverage in the world (https://en.wikipedia.org/wiki/Water_supply_and_sanitation_in_Ethiopia, [23–25]). The distribution and prevalence of various species of intestinal parasites differ from region to region because of several environmental, social and geographical and other factors. Mal-absorption, diarrhea, impaired work capacity, and reduced growth rate due to intestinal parasitic infections constitute important health and social problems. These infections are more prevalent among the poor segments of the population and intimately linked with low economic level, poor personal and environmental sanitation, and overcrowding, limited access to clean water, tropical climate and low altitude [26–29]. However, there is scarcity of information regarding the prevalence of intestinal parasites and associated factors in the study area. Therefore, this study aimed to assess the magnitude of intestinal parasite infection and its associated factors among under-five children attending in Woreta Health Center, Northwest Ethiopia.
Methods
Study design and period
An institution-based cross-sectional study was conducted from April–May, 2017.
Study area
The study was conducted in Woreta health center at Woreta town. Woreta town is the capital town of Fogera district located in South Gondar zone, Amhara region, east of Lake Tana and south of Addis Zemen and 614 km northeast from Addis Ababa, the capital city of Ethiopia. Woreta is home of 42,595 inhabitants. Fogera has been known for its flat and low land. The altitude of the district ranged between 1750 and 2100 m above sea level. The mean annual rainfall of the woreda is 1216.3 mm and ranges from 1103 to 1336 mm. the climatic condition of Woreta town is tropical savanna climate with 20.3 c0 average temperature. The population in the town mainly lived with trade work, while the rural population lived with crop production and by raring chattels. There are two major rivers that are of great economic importance to the district and to the region which found in short distance to Woreta town. These rivers are mainly used for irrigation during the dry season for the production of horticultural crops, mainly vegetables. Some farmers also use water pumps to produce vegetables, cereals, and pulses.
Woreta health center provides health service for the town as well as a catchment area, the district. An average, 50 children were attending the under-five clinic per day.
Source population
All under five children who attended Woreta health center.
Study population
All under-five children who attended Woreta Health Center the during data collection period.
Inclusion criteria
Under-five children attended in under- five clinic at Woreta health center during data collection.
Exclusion criteria
Under-five children who started anti-parasitic drug/s and those who were critically ill.
Sample size determination and sampling procedure
The sample size was calculated using a single population proportion formula ((n = [(Zα/2)2 × P (1-P)]/D2) (n = sample size, Zα = level of confidence, P = estimated proportion/prevalence of the population, D = tolerated margin of error) with the assumption of a 95% level of confidence, 5% margin of error, taking prevalence of 26.6% from the study conducted in Hawssa, Ethiopia [15] and with a 5% non response rate; the final sample size was 310. Study participants were selected using systematic random sampling and every third child was interviewed based on their order of arrival.
Data collection tool and procedure
A questionnaire regarding the explanatory variables was prepared by intensively reviewing local and international literature. The questionnaire was first developed in English and translated to Amharic, the local language, and then retranslated back to English for analysis to ensure the consistency. A pre-test was administered on 16 under-five children at Addis Zemen health center, northwest Ethiopia. Some amendment was made on the tool after the pre-test. One BSc graduate nurse for supervision and collection of explanatory variables and two well-experienced laboratory technologists for stool specimen diagnosis were employed. The data collection technique was a face to face interview with their mothers/guardians by using a structured questionnaire using the Amharic language.
Stool sample collection and examination
Single, 5 g of fresh stool specimens were collected from study participants in clean, labeled stool cups then direct wet mount technique was used. Kato-Katz thick smear method using delivering a plug of 50 mg of stool was processed in a portion of the sample and the residual was placed in vials having 10% formalin. Samples were qualitatively examined on the spot for hookworm ova and other intestinal helminthic infections.
Quantitative examination of the Kato-Katz slides for helminthiasis (except for hookworms) was done in the laboratory within one week of stool collection. But the Kato-Katz preparation was read immediately for hookworm parasite. Stool specimens placed in vials were also qualitatively examined in the laboratory for strongyloidiasis and protozoan parasites by the formol-ether concentration method. To ensure the quality of the investigation, the two readers read the slides independently and their readings were compared. Discordant were immediately resolved with a discussion of each other and in consultation with other experts.
Data quality control technique
The questionnaires were prepared in English version then translate into the local language, Amharic for the interview and then to ensure the consistency, it was translated back to English. The tool was pretested one week prior to the actual data collection date. One day training was given for data collectors and supervisors about the questionnaire and data collection technique.
Operational definition
Intestinal parasites: are parasites that can infect gastrointestinal tracts of the human body.
Parasite infection: intestinal parasite infection/positive result confirmed by laboratory stool examination.
Utensils: any materials/ handheld tools used to store/carrying, cook, and feed food.
Clean playing ground: surfaces with no visible dust, dirt, mud or contaminating particles on which children climb, slide, crawl, push, pull, swing and contact for playing purpose.
Data processing and analysis
The data were checked for its completeness and coded manually before data entry. EPI Info version 7 and SPSS version 20 were used to enter and analyze the data. Descriptive statistics; including frequency, mean and standard deviations, were used to describe the data. Bivariate logistic regression analysis was carried out and variables having P-value of ≤0.2 were entered into multivariate logistic regression for final analysis.
Results
Socio-demographic characteristics of the participants
In this study, 15 children were excluded from the study by the exclusion criteria. A total of 310 under-five year children were included in the study with a response rate of 100%. The mean age of participants was 29.25 Months. More than half of the respondents 158(51%) were females. Majority of mothers/guardian religion 285 (91.9%) were Orthodox Christian and 250 (80.6%) were from Amhara ethnicity (Table 1).
Table 1.
Socio-demographic characteristics of under-five children/mother/guardian in Woreta Health Center, Northwest Ethiopia, 2017 (n = 310)
| Variables | Frequency | Percent (%) |
|---|---|---|
| Sex | ||
| Male | 152 | 49.0 |
| Female | 158 | 51.0 |
| Age | ||
| < 6 months | 26 | 8.4 |
| 6 month- 11 month | 50 | 16.1 |
| 12–23 months | 63 | 20.3 |
| 24–59 months | 171 | 55.2 |
| Family Residence | ||
| Urban | 166 | 53.5 |
| Rural | 144 | 46.5 |
| Religion of mother/guardian | ||
| Orthodox | 285 | 91.9 |
| Muslim | 25 | 8.1 |
| Ethnicity of mother/guardian | ||
| Amhara | 250 | 80.6 |
| Tigrie | 60 | 19.4 |
| Occupation of mother/guardian | ||
| Government | 71 | 22.9 |
| Housewife | 121 | 39 |
| Merchant | 72 | 23.2 |
| Farmer | 46 | 14.8 |
| Mother/guardian educational back ground | ||
| Unable to read and writing | 79 | 25.48 |
| Able to read and writing | 69 | 22.3 |
| Grade 1–8 | 68 | 21.9 |
| Grade 9–12 | 32 | 10.3 |
| Certified and above | 62 | 20 |
| Monthly family income | ||
| < 1000 Et Birr | 67 | 21.6 |
| 2000–3000 Et Birr | 152 | 49 |
| > 3000 Et Birr | 91 | 29.4 |
Characteristics of food, personal and environmental hygiene
In this study, the majority 282 (91.2%) of participants were had clean dinning utensils. More than half 166 (53.5%) and two-thirds 210 (67.7%) of mothers/caregivers wash their hand sometimes after toileting and trim their child’s nails when grown respectively. Only 178 (57.4%) of mothers/caregivers had tap water as a source of drinking water and 55 (17.7%) of mothers/caregivers knew both contaminated food and water as a mode of transmission of intestinal parasites (Table 2).
Table 2.
Characteristics of food, personal and environmental hygiene of participants at Woreta Health Center, Northwest Ethiopia, 2017 (n = 310)
| Variable | Frequency | Percent (%) |
|---|---|---|
| Are dinning utensils Clean | ||
| Yes | 282 | 91.0 |
| No | 28 | 9.0 |
| Do you wash your hand after toilet before touching your child | ||
| Always | 144 | 46.5 |
| Sometimes | 166 | 53.5 |
| Does your child eat unwashed fruits and vegetables | ||
| Always | 22 | 7.1 |
| Sometimes | 90 | 29.0 |
| Never | 154 | 63.9 |
| Your child meal | ||
| Always fresh | 98 | 31.6 |
| Sometimes fresh | 183 | 59.0 |
| Rarely fresh | 29 | 9.4 |
| Do you trim your child’s nails when grown | ||
| Always | 100 | 32.3 |
| Sometimes | 210 | 67.7 |
| Does your child take other food before the age of six month | ||
| No | 286 | 92.3 |
| Yes | 24 | 7.7 |
| Your child playing ground | ||
| Not clean | 110 | 35.5 |
| Clean | 200 | 64.5 |
| What is your source of drinking water | ||
| Tap water | 178 | 57.4 |
| Stream water | 132 | 42.6 |
| Type of your toilet | ||
| Open defecation | 138 | 44.5 |
| Public | 74 | 23.9 |
| Private | 98 | 31.6 |
| Knowledge of mode of transmission | ||
| Contaminated food | 124 | 40 |
| Contaminated water | 131 | 42.3 |
| Both | 55 | 17.7 |
Prevalence of intestinal parasitic infections
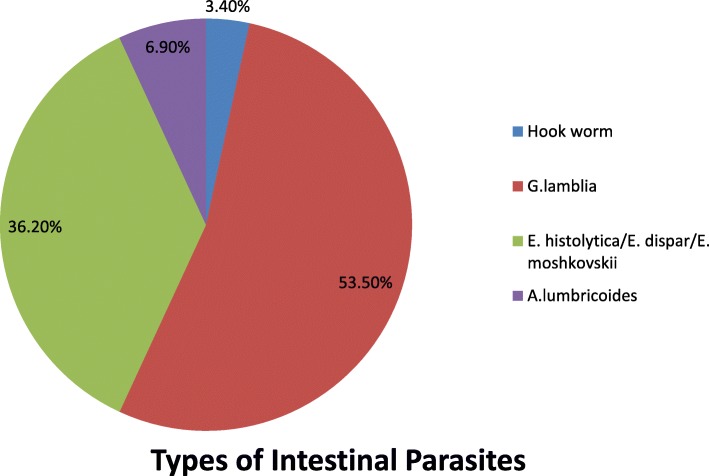
The overall prevalence of intestinal parasitic infections among under-five children was 18.70% (95% confidence interval (CI) =14.4–23.3). Among the four identified intestinal parasites; the predominant were 31(53.5%) G.lamblia (Giardia lamblia) and 21(36.2%) E. histolytica/E. dispar/E. moshkovskii (Fig. 1).
Fig. 1.
Types of intestinal parasites among under-five children attending at Woreta Health Center, Northwest Ethiopia, 2017 (n = 58)
Factors associated with intestinal parasitic infections
Multivariate logistic regression analysis showed that child food freshness, regular trimming fingernails, children playground cleanliness, and the family use of toilet were significantly associated with intestinal parasitic infection.
Children who were rarely fed fresh meal were 7.74 (AOR = 7.74; 95% CI: 1.61, 7.84) more likely to be infected by intestinal parasites than children feed always a fresh meal. Children whose nails were trimmed sometimes were 3.41(AOR = 3.41; 95% CI: 2.20–10.28) more likely to be infected by intestinal parasites than children whose nails were cut always. Those children who have not clean playing ground were 2.43 times (AOR = 2.43, 95% CI: 1.25–5.18) more likely to be infected by intestinal parasites than children who had clean playing ground. Besides, children who had open defecation of the family were 3.4 times (AOR = 3.40, 95% CI: 1.27–10.86) less likely infected by intestinal parasites than children who had a family open defecation. However, there is no observed significant association between the dependent variable and age of children and other socio-demographic variables (Table 3).
Table 3.
Bivariate and multivariate analysis for the prevalence of intestinal parasites under-five children attending Woreta Health Center, Northwest Ethiopia, 2017 (n = 310)
| Variables | Parasite infection | COR (95% CI) | AOR (95% CI) | |
|---|---|---|---|---|
| Positive/Yes | Negative/No | |||
| Child Age | ||||
| < 6 months | 6 | 20 | 1 | 1 |
| 6–11 months | 6 | 44 | 0.45 (0.65–4.5) | 0.92 (0.40–4.96) |
| 12–23 months | 8 | 55 | 0.48 (0.27–1.44) | 0.54 (0.51–1.42) |
| 23–59 months | 38 | 133 | 0.95 (0.68–3.57) | 1.74 (0.41–4.22) |
| Family residence | ||||
| Urban | 21 | 145 | 1 | 1 |
| Rural | 37 | 107 | 2.40 (1.12–6.10)* | 2.64 (0.51–4.11) |
| Mother/Guardian Occupation | ||||
| Government | 10 | 61 | 1 | 1 |
| Housewife | 34 | 87 | 2.33 (1.07–5.07)* | 1.94 (0.37–10.15) |
| Merchant | 8 | 64 | 0.76 (0.28–2.06) | 0.55 (0.26–1.83) |
| Farmer | 6 | 4 | 0.96 (0.32–2.87) | 1.40 (0.36–5.34) |
| Mother/guardian educational status | ||||
| Unable to read and write | 24 | 55 | 1 | 1 |
| Able to read and write | 13 | 56 | 2.77 (1.14–6.73)* | 0.87 (0.34–2.59) |
| Grade 1–8 | 8 | 60 | 1.58 (0.60–4.08) | 0.80 (0.15–4.26) |
| Grade 9–12 | 5 | 27 | 0.90 (0.32–2.56) | 1.08 (0.22–7.27) |
| Certified and above | 8 | 54 | 1.56 (0.49–4.96) | 0.74 (0.03–17.52) |
| Family income | ||||
| < 1000 Et Birr | 10 | 57 | 1.12 (0.29–3.99 | 1.98 (0.66–4.23) |
| 2000–3000 Et Birr | 37 | 115 | 2.08 (0.47–2.88) | 3.48 (0.98–4.69) |
| > 3000 Et Birr | 11 | 71 | 1 | 1 |
| Are dinning utensils clean | ||||
| Yes | 49 | 233 | 1 | 1 |
| No | 9 | 19 | 2.25 (0.96–5.27) | 1.88 (0.66–5.38) |
| Do you wash your hand after toilet before touching your child | ||||
| Always | 17 | 127 | 1 | |
| Sometimes | 41 | 125 | 2.46 (1.01–3.23)* | 3.30 (0.92–4.27) |
| Does your child eat unwashed fruits and vegetables | ||||
| Always | 8 | 14 | 4.71 (1.71–12.97) | 2.31 (0.70–7.58) |
| Sometimes | 34 | 56 | 2.65 (1.39–5.05) | 1.72 (0.81–3.77) |
| Never | 16 | 138 | 1 | 1 |
| Your child meal | ||||
| Always fresh | 5 | 93 | 1 | 1 |
| Sometimes fresh | 45 | 138 | 6.07 (0.56–4.07) | 6.69 (0.16–3.56) |
| Rarely fresh | 8 | 21 | 7.09 (1.35–8.13)* | 7.74 (1.61–7.84)** |
| Do you trim your child’s nails when grown | ||||
| Always | 7 | 93 | 1 | 1 |
| Sometimes | 51 | 159 | 4.26 (2.50–11.21)* | 3.41 (2.20–10.28)** |
| Does your child take food other than breast milk before the age of six month | ||||
| Yes | 51 | 235 | 0.53 (0.22–1.36) | 1.28 (0.37–4.67) |
| No | 7 | 17 | 1 | 1 |
| Your child playing Ground | ||||
| Not clean | 32 | 78 | 2.75 (1.53–4.95)* | 2.43 (1.25–5.18)** |
| Clean | 26 | 174 | 1 | 1 |
| What is your source of drinking water | ||||
| Tap water | 24 | 154 | 1 | 1 |
| Stream | 34 | 98 | 2.23 (1.24–3.26)* | 2.55 (0.65–5.27) |
| Type of your toilet | ||||
| Open defecation | 22 | 116 | 0.51 (0.22–0.67)* | 3.40 (1.27–10.86)** |
| Public | 5 | 69 | 0.16 (0.06–0.43) | 3.83 (1.25–10.60) |
| Private | 31 | 67 | 1 | 1 |
| Knowledge about the mode of transmission | ||||
| Contaminated food | 27 | 97 | 1 | 1 |
| Contaminated water | 23 | 108 | 1.64 (0.69–3.87) | 1.41 (0.45–2.37) |
| Both | 8 | 47 | 1.25 (0.52–2.99) | 1.02 (0.35–2.95) |
*Variables those were significant during bivariate logistic analysis at P value ≤ 0.05
**Variables that were found to have a significant association in multivariate analysis at p-value ≤ 0.05
Discussion
It is of paramount importance to know the distribution and extent of intestinal parasitic infection in a given community to devise successful preventive and therapeutic interventions. This study assessed the prevalence as well as factors associated with intestinal parasite infection. The overall prevalence of intestinal parasitic infections among under-five children was 18.70% (95% confidence interval (CI) =14.4–23.3 with 31(53.5%) G.lamblia and 21(36.2%) E. histolytica/E. dispar/E. moshkovskii the most prevalent species.
The finding of this study was in-line with what was found in Wonji Shoa 24.3% and Addis Ababa 14.9% [16, 30]. This similarity could be due to the fact that both studies used similar study design and selection of participants.
But the current study showed a lower prevalence of intestinal parasite infection as compared to those studies conducted in Cuba 45.2% [31], Egypt 47.3% [32], Kenya 25.6% [12], Addis Ababa 27.5%, Wondogenet 85.1% [33]. This could be because of in the current study all children attending the health facility were included while in the study conducted in Kenya the investigators considered children with the diarrheal disease for the study which could increase the prevalence in the latter. In the case of a study in Cuba, investigators took three different samples from each participant and conducted three separate tests and this may increase the likelihood of the diagnosis of at least one intestinal parasitosis while in the case of Egyptian study it was conducted among squatter settlement which has no adequate infrastructure such as clean water source which also can increase the probability of acquiring parasitic infections.
In this study, it was shown that child food freshness, regular trimming fingernails, children playground cleanliness, and the use of toilet were significantly associated with intestinal parasitic infection.
Eating rarely fresh food was found to be a risk to intestinal parasites compared to those who were feed always fresh. This is because of the fact that storage of cooked food for longer period gives rise to a proliferation of bacteria and other parasites [34]. Irregular trimming of fingernails of children was also significantly associated with intestinal parasitosis. This result is supported by similar studies conducted in Ethiopia [35]. This could be attributed to the removal of accumulated dirt containing eggs of parasites on fingernails up on trimming [36]. For example, a study in Egypt found E. vermicularis and H. nana eggs in 2% of the fingernail clippings [37].
Children who had unclean playground were found to be at an increased rate of having intestinal parasites (AOR = 2.43, 95% CI: 1.25–5.18). This is due to the presence of microorganisms and eggs of parasites in dirty surfaces. This is supported by a study which concluded that inadequate sanitation and hygiene behavior are associated with soil-transmitted helminths and intestinal protozoa infections [38]. On the other hand, children who had open defecation of the family were 3.4 times more likely to harbor intestinal parasites (AOR = 3.40, 95% CI: 1.27–10.86). This finding is in line with a cluster randomized controlled trial conducted in India that showed the inversely proportional relationship between improved latrine use and intestinal parasitosis prevalence [39] and another study conducted in Côte d’Ivoire [38].
As to the limitation; Even though, molecular assays and other techniques could best estimate the prevalence of intestinal parasites and differentiating different specious of ameabiasis, in this study the microscopy technique was used to determine the prevalence.
Conclusion
In this study, the prevalence of intestinal parasites was found lower than WHO annual or biannual population treatment level. Child food freshness, regular trimming fingernails, Children playground cleanliness, and family use of toilet were significantly associated with intestinal parasitic infection. Thus, strengthening of health education about food, personal and environmental hygiene of both children and mothers/guardians is crucial. Besides, improving mothers/guardian awareness about the mode of intestinal parasites transmission is necessary.
Acknowledgments
Authors would like to express their gratitude to the University of Gondar College of Medicine and Health Science, School of Nursing Research and Ethical Review committee for the approval of the ethical clearance. The authors would like to thank data collectors and supervisors for their commitment and the study participants for their valuable information.
Funding
The authors received no specific funding for this work.
Availability of data and materials
Raw data would not be provided for the reason of protecting participant confidentiality. But, the summary data are available in the main document.
Abbreviations
- A.lumbricoides
Ascaris Lumbericoides
- AOR
Adjusted odds ratio
- CI
Confidence interval
- COR
Crude odds ratio
- G.lamblia
Giardia lamblia
- GIT
Gastrointestinal tract
- IP
Intestinal parasites
- SPSS
a Statistical package for social sciences
- WHO
World Health Organization
Authors’ contributions
HSM wrote the proposal, participated in data collection, analyzed the data and drafted the manuscript. DTE approved the proposal with revisions, participated in data collection, analysis and revised subsequent drafts of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by University of Gondar College of Medicine and Health Sciences School of nursing Research and Ethical Review Committee. Permission was obtained from the office of the health center head. Each study participant’s mother/guardian was informed about the purpose, method, expected benefit, and risk of the study. Mothers/guardians were also informed about their full right not to participate their children in the study or withdraw from the study at any time, without repercussion to the services they seek. Written informed consent was obtained from study participants’ mothers/guardians and anonymity were employed to maintain confidentiality. Those children who were positive for intestinal parasites were linked to the health center to be treated.
Consent for publication
Not applicable.
Competing interests
The authors declared that they have no any competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Habtamu Sewunet Mekonnen, Email: habtsew@ymail.com.
Daniale Tekelia Ekubagewargies, Email: dtekle16@gmail.com.
References
- 1.WHO . prevention and control of Schistomiasis and soil-transmitted helminthiasis Geneva. 2002. [Google Scholar]
- 2.Peter J, Hotez PJB, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118(4):1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enrique Chacon-Cruz. Intestinal Protozoal Diseases. Medscape. 2017. https://emedicine.medscape.com/article/999282-overview.
- 4.WHO/Department of Communicable Disease Prevention Control and Eradication. Prevention and control of intestinal parasitic infections: WHO Technical Report Series N° 749. 1987. https://www.who.int/neglected_diseases/resources/who_trs_749/en/. [PubMed]
- 5.WHO . Soil-transmitted helminth infections. fact sheet. 2017. [Google Scholar]
- 6.Michael O, Harhay JH, Piero L. Olliaro. Epidemiology and control of human gastrointestinal parasites in children. Expert Rev Anti Infect Ther. 2010;8(2):219–234. doi: 10.1586/eri.09.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desta Haftu ND, Agedew E. Prevalence and determinant factors of intestinal parasites among school children in Arba Minch town, southern Ethiopia. Am J Health Res. 2014;2(5):247–254. doi: 10.11648/j.ajhr.20140205.15. [DOI] [Google Scholar]
- 8.WA AL-M. Assessment the prevalance of intestinal parasities and associated risk factors among preschool children in Riyadh. Saudi Arabia Resj parastology. 2015;10(1):31–41. [Google Scholar]
- 9.Mehraj V, Hatcher J, Akhtar S, Rafique G, Beg MA. Prevalence and Factors Associated with Intestinal Parasitic Infection among Children in an Urban Slum of Karachi. pLoS ONE. 2008;3(11):e3680. doi: 10.1371/journal.pone.0003680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamukwamba Mwale SS. Intestinal infestations in under-five children in Zambia. International Journal of MCH and AIDS. 2015;4(2):40–46. [PMC free article] [PubMed] [Google Scholar]
- 11.Abd Elhafiz MA. Muhajir KH, Zeehaida Mohamed an, Azam Afifi Abdel Aal. Prevalence of intestinal parasitic infection among children in Al-kalakla, Khartoum, Sudan. World Appl Sci J. 2017;35(2):219–222. [Google Scholar]
- 12.Liza A. Nyantekyi ML, Mulugeta Belay, Konjit Tadesse, Kebreten Manaye, Chanda Macias, Berhanu Erko. Intestinal parasitic infections among under-five children and maternal awareness about the infections in Shesha Kekele, Wondo Genet, Southern Ethiopia. Ethiop J Health Dev. 2010;24(3):185-190.
- 13.Asfaw ST, Giotom L. Malnutrition and enteric parasitoses among under-fife children in Aynalem Village. Tigray EthiopJHealth Dev. 2000;14(1):67–75. [Google Scholar]
- 14.Zemene T, Shiferaw MB. revalence of intestinal parasitic infections in children under the age of 5 years attending the Debre Birhan referral hospital, North Shoa, Ethiopia. BMC Res Notes. 2018;11(1):58. doi: 10.1186/s13104-018-3166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulatu G, Zeynudin A, Zemene E, Debalke S, Beyene G. Intestinal parasitic infections among children under five years of age presenting with diarrhoeal diseases to two public health facilities in Hawassa, South Ethiopia. Infect Dis Poverty. 2015;4:49. doi: 10.1186/s40249-015-0081-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.G/hiwot Y, Degarege A, Erko B. Prevalence of Intestinal Parasitic Infections among Children under Five Years of Age with Emphasis on Schistosoma mansoni in Wonji Shoa Sugar Estate, Ethiopia. pLoS ONE. 2014;9(10):e109793. doi: 10.1371/journal.pone.0109793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamed Kiani AH, Salehi R, Azargashb E. Distribution and risk factors associated with intestinal parasite infections among children with gastrointestinal disorders. Gastroenterol Hepatol Bed Bench. 2016;9(1):S80–SS7. [PMC free article] [PubMed] [Google Scholar]
- 18.Forson AO, Arthur I, Olu-Taiwo M, Glover KK, Pappoe-Ashong PJ, Ayeh-Kumi PF. Intestinal parasitic infections and risk factors: a cross-sectional survey of some school children in a suburb in Accra, Ghana. BMC Res Notes. 2017;10:485. doi: 10.1186/s13104-017-2802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bahmani P, Maleki A, Sadeghi S, Shahmoradi B, Ghahremani E. Prevalence of Intestinal Protozoa Infections and Associated Risk Factors among Schoolchildren in Sanandaj City, Iran. Iran J Parasitol. 2017;12(1):108–116. [PMC free article] [PubMed] [Google Scholar]
- 20.Fletcher SM, ML ML, Ellis JT. Prevalence of gastrointestinal pathogens in developed and developing countries: systematic review and meta-analysis. J Public Health Res. 2013;2(e9):42–53. doi: 10.4081/jphr.2013.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarkari B, Hosseini G, Motazedian MH, Fararouei M, Moshfe A. Prevalence and risk factors of intestinal protozoan infections: a population-based study in rural areas of Boyer-Ahmad district, Southwestern Iran. BMC Infect Dis. 2016;16:703. doi: 10.1186/s12879-016-2047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ismail KA. Prevalence of intestinal parasitic infection among school children in Taif. Insights Biomed. 2018;3(2):10. [Google Scholar]
- 23.Kumie A, Ali A. An overview of environmental health status in Ethiopia with particular emphasis to its organization, drinking water and sanitation: A literature survey. EthiopJHealth Dev. 2005;19(2):89. [Google Scholar]
- 24.Adank M, Butterworth J, Godfrey S, Abera M. Looking beyond headline indicators: water and sanitation services in small towns in Ethiopia. J Water, Sanitation Hyg Dev. 2016;6(3):435–446. doi: 10.2166/washdev.2016.034. [DOI] [Google Scholar]
- 25.Drinking water, sanitation and hygiene in Ethiopia. https://www.wearewater.org/en/drinking-water-sanitation-and-hygiene-in-ethiopia_253215.
- 26.Dudlová A, Juriš P, Jurišová S, Jarčuška P, Krčméry V. Epidemiology and geographical distribution of gastrointestinal parasitic infection in humans in Slovakia. HELMINTHOLOGIA. 2016;53(4):309–317. doi: 10.1515/helmin-2016-0035. [DOI] [Google Scholar]
- 27.Faria CP, Zanini GM, Dias GS, da Silva S, de Freitas MB, Almendra R, Santana P, Sousa MD. Geospatial distribution of intestinal parasitic infections in Rio de Janeiro (Brazil) and its association with social determinants. PLoS Negl Trop Dis. 2017;11(3):e0005445. doi: 10.1371/journal.pntd.0005445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen NL, Gelaye B, Aboset N, Kumie A, Williams MA, Berhane Y. Intestinal parasitic infection and nutritional status among school children in Angolela. Ethiopia J Prev Med Hyg. 2012;53(3):157–164. [PMC free article] [PubMed] [Google Scholar]
- 29.Curval LG, França AO, Fernandes HJ, Mendes RP, de Carvalho LR, Higa MG, Ferreira EC, Dorval MEC. Prevalence of intestinal parasites among inmates in Midwest Brazil. PLoS One. 2017;12(9):e0182248. doi: 10.1371/journal.pone.0182248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adamu H, Endeshaw T, Teka T, Kifle A, Petros B. The prevalence of intestinal parasites in paediatric diarrhoeal and non-diarrhoeal patients in Addis Ababa hospitals, with special emphasis on opportunistic parasitic infections and with insight into the demographic and socio-economic factors. Ethiop. J. Health Dev. 2006;20(1):39–46. doi: 10.4314/ejhd.v20i1.10010. [DOI] [Google Scholar]
- 31.Cañete R, Díaz MM, García RA, Lau´d Martinez PM, Ponce FM. Intestinal Parasites in Children from a Day Care Centre in Matanzas City, Cuba. PLoS ONE. 2012;7(12):e51394. 10.1371/journal.pone.0051394. [DOI] [PMC free article] [PubMed]
- 32.Mahfouz AA, el-Morshedy H, Farghaly A, Khalil A. Ecological Determinants of Intestinal Parasitic Infections Among Pre-school Children in an Urban Squatter Settlement of Egypt. Journal of Tropical Pediatrics. 1997;43(6):341-4. 10.1093/tropej/43.6.341. [DOI] [PubMed]
- 33.Intestinal parasitic infections among under-five children and maternal awareness about the infections in Shesha Kekele, Wondo Genet, Southern Ethiopia | Nyantekyi | Ethiopian J Health Develop [Internet]. [cited 2018 Mar 5]. Available from: https://www.ajol.info/index.php/ejhd/article/view/68383.
- 34.Mølbak K, Højlyng N, Jepsen S, Gaarslev K. Bacterial contamination of stored water and stored food: A potential source of diarrhoeal disease in West Africa. Epidemiol Infect. 1989;102(2):309-16. 10.1017/S0950268800029988. [DOI] [PMC free article] [PubMed]
- 35.Mahmud MA, Spigt M, Bezabih AM, Pavon IL, Dinant G-J, Velasco RB. Efficacy of Handwashing with Soap and Nail Clipping on Intestinal Parasitic Infections in School-Aged Children: A Factorial Cluster Randomized Controlled Trial. PLOS Med. 2015;12(6):e1001837. doi: 10.1371/journal.pmed.1001837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mirisho R, Neizer ML, Sarfo B. Prevalence of Intestinal Helminths Infestation in Children Attending Princess Marie Louise Children’s Hospital in Accra, Ghana. Journal of Parasitology Research. 2017;2017:7. Available from: 10.1155/2017/8524985. [DOI] [PMC free article] [PubMed]
- 37.El-Nadi NAF, Omran EK, Ahmed NS, Fadel EF. Current status of intestinal parasites among elementary school children in Sohag, Egypt. J Adv Parasitol. 2017;4(2):33-40.
- 38.Schmidlin T, Hürlimann E, Silué KD, Yapi RB, Houngbedji C, Kouadio BA, et al. Effects of Hygiene and Defecation Behavior on Helminths and Intestinal Protozoa Infections in Taabo, Côte d’Ivoire. PLOS ONE. 2013;8(6):e65722. doi: 10.1371/journal.pone.0065722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patil SR, Arnold BF, Salvatore AL, Briceno B, Ganguly S, Colford JM, Jr, et al. The Effect of India’s Total Sanitation Campaign on Defecation Behaviors and Child Health in Rural Madhya Pradesh: A Cluster Randomized Controlled Trial. PLOS Med. 2014;11(8):e1001709. doi: 10.1371/journal.pmed.1001709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data would not be provided for the reason of protecting participant confidentiality. But, the summary data are available in the main document.