Older, overweight and obese adults with knee osteoarthritis, who lost weight and engaged in regular exercise viewed themselves as more capable in their ability to walk and function. Additionally, those with enhanced confidence were able to walk greater distances and experienced less pain.
Keywords: Self-efficacy, Exercise, Physical activity, Weight loss, Knee osteoarthritis
Abstract
Physical activity decreases the risk of osteoarthritis (OA)-related disability; however, pain and lack of confidence represent barriers for older adults with knee OA. The purpose of this study was to examine (a) the baseline associations among self-efficacy and physical activity, function, and pain; (b) longitudinal changes in self-efficacy; and (c) whether self-efficacy mediates treatment effects on clinical outcomes. The Intensive Diet and Exercise for Arthritis (IDEA) trial was a single-blind, randomized controlled 18-month study including 454 overweight/obese older adults (M age = 66 years) with knee OA. Participants were randomized to one of three interventions: exercise (E), diet-induced weight loss (D), or both (D+E). Self-efficacy for gait, balance, and walking duration were assessed at baseline, 6 months, and 18 months. Baseline associations were tested using Pearson correlations, and group least squares means were compared using mixed linear models at follow-up. Participants with higher self-efficacy reported significantly better physical function and less knee pain at baseline, walked farther (6-min walk), and were more physically active (all |r| > 0.12, all p < .01). Significant differences between groups were detected for all self-efficacy measures at 18 months; the D+E group reported significantly (all p < .005) higher self-efficacy for gait, walking duration, and balance compared with the D- or E-only groups. Self-efficacy significantly (p < .05) mediated treatment effects on physical function and pain at 18 months. A combined intervention of diet-induced weight loss and exercise is the treatment of choice to maximize self-efficacy, improve physical function, and reduce pain in overweight/obese adults with knee OA.
Implications.
Practice: Intentional physical activity and weight loss interventions that serve to enhance self-efficacy may lead to improved mobility, physical function, and pain management, making interventions designed to enhance self-efficacy essential in the clinical management of knee OA.
Policy: Given that exercise and weight reduction have Level 1 evidence as effective treatments for the management of knee OA, the development of self-efficacy should be integrated into treatment regimens for knee OA patients to maximize physical function and reduce pain.
Research: Future research is necessary to examine not only the feasibility of implementing strategies to positively affect self-efficacy in patient care and community-based settings, but also the sustainability of boosted self-efficacy on the road to the effective management of knee OA.
INTRODUCTION
Symptomatic knee osteoarthritis (OA) is the most common source of disability among older adults in the USA, affecting 12% of those aged 60 years and above [1, 2]. Older adults with knee OA report lower physical functioning and increased difficulty in performing activities of daily living compared with older adults without knee OA [2]. In addition to being a modifiable and independent risk factor in disease development, obesity can accelerate OA disease progression and contribute directly to disability [3, 4]. Thus, weight reduction is often prescribed to manage knee OA in obese and overweight adults [5].
Exercise is also a successful intervention for the management of knee OA in older adults [6], and the combination of exercise and weight reduction is even more effective than either one alone [7–10]. The Intensive Diet and Exercise for Arthritis (IDEA) trial demonstrated that a combined intervention of diet-induced intensive weight loss plus exercise resulted in significant clinical improvements in physical function and decreases in pain, knee compressive forces, and inflammation when compared with either weight reduction or exercise alone [8]. Despite the evidence to support weight loss and exercise as a viable management option for knee OA, adoption and adherence to dietary weight loss and exercise behaviors is complex and multifaceted [11].
Lifestyle behavior programs for older adults that have a strong theoretical framework are more likely to be successful [12]. Social cognitive theory (SCT) provides an important framework for understanding and modifying human behavior [13]. Self-efficacy, the belief in one’s ability to accomplish a task, plays a pivotal role in SCT and has gained considerable attention in knee OA research over the past decade [14]. In persons with knee OA, higher levels of mobility related self-efficacy are associated with higher levels of functioning [15–17] and lower levels of pain [18, 19].
Although these findings emphasize the importance of self-efficacy in the management of knee OA, less clear is the long-term effect of exercise and weight reduction on self-efficacy. In a small (n = 46) short-duration study, Baker et al. [20] demonstrated modest improvements in self-efficacy following a 4-month home-based strength training program. A handful of single arm studies showed similar results, with small to large effect sizes for self-efficacy after 8–12 weeks of structured exercise treatments [21–23] and one randomized clinical trial revealed that both aerobic and resistance training programs positively affect self-efficacy for stair climbing [24]. One long-term intervention demonstrated that 18 months of modest weight reduction (5%) coupled with exercise resulted in significant improvements in mobility related self-efficacy [15]. While the IDEA study showed that intensive weight loss (>10%) leads to significant clinical improvements [8], the chronic effects of exercise and more intensive weight loss on self-efficacy are unknown.
Given that exercise and weight reduction have Level 1 evidence as effective treatments for the management of knee OA [10], identifying potential mechanisms underlying treatment effects on clinical outcomes is critical [25]. As lowered self-efficacy represents a barrier to healthy lifestyle behaviors [26], the IDEA study employed a social cognitive framework for the exercise and diet-induced weight loss interventions to maximize the utility of self-efficacy beliefs in the behavior change process. The objectives of the current study were to examine the associations among self-efficacy and physical activity, function and pain at baseline, to examine changes in self-efficacy over the course of the 18-month interventions in the IDEA trial, and to determine whether changes in self-efficacy mediate the relationship between treatment effects and key clinical outcomes at 18 months. Weight loss and physical activity interventions designed to positively affect self-efficacy may ultimately empower older adults with knee OA to more effectively improve function and manage pain.
METHODS
Design
Complete details of the IDEA study design and main outcomes have been reported elsewhere [8, 27]. After providing informed consent in writing, participants were randomized into one of three groups: diet-induced weight loss (D), exercise (E), or diet-induced weight loss plus exercise (D+E). Participants in the D group were asked to lose at least 10% of baseline body weight, primarily through caloric restriction, by initially replacing two meals with provided meal replacement shakes, with the third meal based on individually tailored meal plans and recipes. Nutrition counseling was provided by trained staff members and tapered over time. For the first 6 months, participants attended one individual session and three group sessions per month, and for Months 7 through 18, participants attended biweekly group sessions and an individual session every 2 months.
Participants in the E group engaged in group-based, interventionist-supervised exercise sessions consisting of aerobic walking (15 min), strength training (20 min), a second aerobic phase (15 min), and cool-down (10 min) for 1 hr, 3 days a week for 18 months. To maximize interventionist interaction and as a safety measure, exercisers were placed in a small group that accessed the facility at a regularly scheduled time. Weight loss instructions were not given to the E group. D+E participants received both interventions. To minimize the number of sessions attended, dietary counseling was conducted prior to or after the exercise sessions.
Behavioral framework
Originally developed by Bandura [13, 28], SCT provided the theoretical framework for implementation of the diet-induced weight loss and exercise interventions. The interventions were tailored to each participant and delivered in a group setting led by trained interventionists providing the structure and social support indicative to behavior change. Interventionists met regularly to track participant progress, to identify those that had difficulty with adherence, and to develop strategies with participants to overcome obstacles. Self-efficacy beliefs are acquired through mastery experiences, vicarious experiences, verbal persuasion, and the interpretation of physiological cues and psychological states [13]. The exercise and diet-induced weight loss interventions in IDEA were designed to present numerous opportunities to bolster self-efficacy. Table 1 presents some of the strategies used within the interventions to increase self-efficacy over time.
Table 1.
Sample of behavioral strategies
| Mastery experience | ● Interventions were tailored to the participant’s ability and gradually increased over time ● Periodic individual updates on the participant’s overall progress (i.e., progress charts) ● Use of logs reflecting achievements in diet and exercise |
| Vicarious experience | ● Participants were assigned to a group where they attended diet counseling or exercise sessions with other older, overweight and obese individuals with knee osteoarthritis |
| Verbal persuasion | ● Built-in pattern of frequent contact that demonstrated personal interest in the participant ● Interventions were supervised by trained interventionists who encouraged participants ● Interventionists helped participants create measurable and attainable goals |
| Physiological cues/psychological states | ● Interventionists helped participants interpret physiological responses to starting an exercise program (i.e., pain, soreness) ● In the exercise intervention groups participants were shown how to monitor heart rate and complete activity logs ● In the diet intervention groups participants received specific counseling in adapting to a lower calorie diet ● Interventionists talked with patients about their stress and anxiety for making lifestyle changes |
Participants
Community-dwelling, overweight, and obese older adults, age 55 or older, were recruited using mass mailings, newspaper advertisements, community centers, and physician referrals from 2006 to 2009. The study sample included 454 adults who met the following eligibility requirements: (a) Grade II–III (mild to moderate) radiographic tibiofemoral OA or tibiofemoral plus patellofemoral OA of one or both knees with pain on most days of the week; (b) 27.0 ≤ BMI ≤ 41 kg/m2; (c) a sedentary lifestyle, defined as less than 30 min of formal exercise per week; and (d) lived within 50 miles of the research site. Participants were excluded if (a) they were unwilling or unable to commit to the 18-month intervention, including the willingness to modify diet; (b) had a significant comorbid disease that prevented safe participation in an exercise program; or (c) had significant cognitive impairment or depression.
Measures
All measurements were taken at baseline, 6 months, and 18 months. Data collection was made by research staff blinded to group assignment, and all participants, regardless of group assignment, received the same directions and set of questionnaires.
Physical Activity Scale for the Elderly (PASE)
To account for baseline levels of physical activity and changes incurred throughout the intervention, participants were asked to recall their occupational, household, and leisure activities over the past 7 days. This self-reported estimation of physical activity is valid and reliable in this population [29].
Six-Minute Walk Test (6MWT)
The 6MWT is a practical measure of functional capacity that has been used extensively in older adults with knee OA [30]. Participants were instructed to walk as far as possible in 6 min on an established, indoor course. No encouragement was given during the test and the final score was recorded in meters.
Western Ontario McMasters Universities Osteoarthritis Index (WOMAC)
The WOMAC was used to measure self-reported pain and physical function [31]. Specific to knee and hip OA, the WOMAC asks participants about the pain and difficulty experienced during normal ambulatory activities using a scale from 0 (none) to 4 (extreme). Higher scores indicate greater pain and poorer function.
Self-efficacy measurements
Three measures of self-efficacy were assessed: the activities-specific balance confidence scale, a walking efficacy for duration scale, and the gait efficacy scale. All scales asked participants to rate their perceived ability to complete a task using a scale ranging from 0% (not at all confident) to 100% (completely confident), in increments of 10. Each scale was scored by computing the average of all items with total scores ranging from 0 to 100. Higher scores indicate greater self-efficacy beliefs.
Activities-specific balance confidence
Aging is associated with declines in balance, and knee OA compounds the problem [32]. This measure of balance efficacy is comprised of 16 items that ask participants to rate their confidence in performing common daily tasks that require balance and stability [33].
Gait self-efficacy scale
Walking rarely takes place in the absence of obstacles. Participants were given the gait efficacy scale that included common gait obstacles such as ascending and descending stairs [34].
Walking efficacy for duration
To gauge perception in the ability to walk at increasingly challenging distances, a walking efficacy scale presented participants with the statement “I believe that I can walk for 5 min at a moderately fast pace without stopping.” The scale progressed from 5 to 40 min, in increments of 5 min. This scale was constructed using guidelines recommended by Bandura [35].
Statistical analysis
Descriptive statistics of baseline measures were computed using means and standard deviations for continuous measures and frequencies and percentages for discrete measures. Pearson correlation coefficients were used to test for significant associations among measures of self-efficacy and the independent variables at baseline. Treatment effects were assessed using contrast statements from mixed linear models fit using treatment group, visit month (6 or 18), and the treatment by month interaction, adjusted for baseline values of the outcome, baseline BMI, and gender. The primary outcome was the overall treatment effect at 18 months, as well as the pairwise group comparison when the main 18-month treatment effect was significant. Visit-specific group comparisons were deemed significant at the 0.05 level, and post hoc pairwise comparisons were conducted at a Bonferroni-adjusted 0.0167 level of significance. Effect sizes were calculated using pairwise comparison means relative to baseline standard deviation. Mediation analyses were conducted to determine if self-efficacy variables mediate the relationship between the treatment effects (D+E vs. E only; D+E vs. D only) and key clinical outcomes (6MWT, gait speed, WOMAC pain, and WOMAC function) [36, 37]. A significant mediating relationship was determined based on Sobel’s test where p < .05. All analyses were performed using SAS v9.4 (SAS Institute, Cary, NC).
RESULTS
Participant characteristics
Participants (n = 454; female = 72%) were older adults (age, M = 66 years) with symptomatic and radiographic knee OA. As seen in Table 2, participants reported at baseline mild to moderate pain (WOMAC pain, M = 6.5), some difficulty with daily functioning (WOMAC function, M = 24.2), low physical activity levels (PASE, M = 114.2), low levels of functional capacity as evidenced by distance on the 6MWT (M = 474 meters), and moderate levels of self-efficacy: balance efficacy (M = 78.1), gait efficacy (M = 51.9), and efficacy for walking duration (M = 77.1).
Table 2.
Demographic and clinical mean (SD) characteristics of the study participants at baseline
| Overall N = 454 |
D-only N = 152 |
E-only N = 150 |
D+E N = 152 |
|||||
|---|---|---|---|---|---|---|---|---|
| Age (years) | 66.0 | (6.0) | 65.8 | (6.2) | 65.5 | (6.4) | 65.4 | (6.0) |
| Female n (%) | 325.0 | (72.0) | 108.0 | (71.0) | 108.0 | (72.0) | 109.0 | (72.0) |
| Non-Whites n (%) | 85.0 | (19.0) | 25.0 | (16.0) | 30.0 | (20.0) | 30.0 | (20.0) |
| BMI (kg/m2) | 33.6 | (3.7) | 33.7 | (3.8) | 33.5 | (3.7) | 33.6 | (3.7) |
| WOMAC function (0–68) | 24.2 | (10.9) | 24.8 | (10.4) | 23.1 | (10.3) | 24.6 | (11.7) |
| WOMAC pain (0–20) | 6.5 | (3.1) | 6.6 | (3.0) | 6.1 | (2.9) | 6.7 | (3.4) |
| Balance efficacy (0–100) | 78.1 | (18.5) | 76.1 | (19.5) | 80.5 | (16.6) | 77.7 | (19.1) |
| Duration efficacy (0–100) | 51.9 | (28.8) | 52.7 | (28.4) | 56.3 | (28.5) | 46.7 | (28.8) |
| Gait efficacy (0–100) | 77.1 | (22.4) | 74.7 | (22.9) | 79.4 | (21.0) | 77.3 | (23.0) |
| PASE | 114.2 | (52.3) | 115.3 | (53.2) | 110.8 | (47.7) | 116.6 | (55.8) |
| Six-min walk distance (meters) | 473.8 | (86.7) | 475.4 | (81.7) | 479.7 | (89.9) | 466.5 | (85.4) |
PASE Physical Activity Scale for the Elderly; WOMAC Western Ontario McMasters Universities Osteoarthritis Index.
Adherence and retention
Of the 454 participants randomized, 88% (n = 399) completed the study by returning for 18-month follow-up testing. Adherence to exercise sessions in the E group was 66% for the first 6 months and 54% over 18 months, 70% and 58% respectively for D+E. Diet session adherence was 61% in the D only group and 63% for D+E.
Baseline associations between self-efficacy and physical activity, function, and pain
Participants who reported more physical activity at baseline walked farther on the 6MWT (r = .19, p < .001) and had higher levels of self-efficacy (r = .12–.17, p < .05). Furthermore, self-efficacy was inversely related to age, in that older participants were less confident in their ability to walk and navigate obstacles than their younger counterparts (r = −.14 to −.23, p < .001). WOMAC function and pain subscales displayed a moderate negative relationship (r = −.26 to −.20, p < .0001) with all self-efficacy measures indicating that participants who reported greater self-efficacy prior to randomization had significantly better function and less pain. In addition, greater efficacy at baseline was associated with greater 6MWT distance (r = .37–.51, p < .001).
Effects of the interventions on self-efficacy
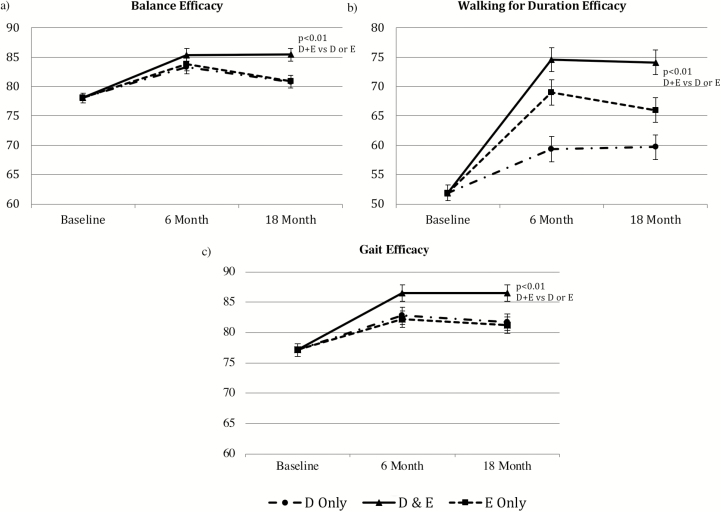
Results of the mixed models revealed significant differences between groups for all three self-efficacy measures at 18 months (p < .003). As depicted in Fig. 1, the group that received a combined intervention of intensive dietary weight loss and exercise reported significantly higher self-efficacy as measured by the balance efficacy, gait efficacy, and walking efficacy for duration scales when compared with the D or E groups (p < .005). Although the effect sizes of D+E compared with E were small for these three scales (d = 0.24–0.28), D+E was considerably higher than D for walking efficacy (d = 0.50) (Table 3).
Fig 1.
Adjusted means for self-efficacy measures for the three intervention groups across the 18-month intervention period as measured by the (a) Activities-Specific Balance Confidence Scale, (b) Walking for Duration Efficacy Scale, and (c) Gait Efficacy Scale. All 6 and 18 month means are adjusted for baseline values, baseline body mass index (BMI), and gender. Error bars are SE. Total scores range from 0 to 100 for all measures.
Table 3.
Treatment effects on self-efficacy from mixed linear models
| 6 months Mean (95% CI) |
18 months Mean (95% CI) |
Change at 18 months (95% CI) |
18 Months | ||
|---|---|---|---|---|---|
| p value | Effect size | ||||
| Balance efficacy | |||||
| E | 83.76 (81.58 to 85.94) | 80.90 (78.77 to 83.03) | 1.89 (−0.24 to 4.02) | .0019 | – |
| D | 83.26 (81.08 to 85.44) | 80.82 (78.69 to 82.95) | 1.81 (−0.32 to 3.94) | −0.00 | |
| D+E | 85.39 (83.30 to 87.48) | 85.44 (83.32 to 87.56) | 6.43 (4.31 to 8.55) | 0.25 | |
| Duration efficacy | |||||
| E | 69.03 (64.81 to 73.24) | 65.97 (61.81 to 70.12) | 13.56 (9.40 to 17.72) | <0.0001 | – |
| D | 59.30 (55.07 to 63.54) | 59.69 (55.51 to 63.87) | 7.29 (3.10 to 11.47) | −0.22 | |
| D+E | 74.59 (70.50 to 78.68) | 74.10 (69.99 to 78.22) | 21.70 (17.59 to 25.81) | 0.28 | |
| Gait efficacy | |||||
| E | 82.17 (79.47 to 84.88) | 81.18 (78.51 to 83.84) | 3.12 (0.46 to 5.79) | 0.0039 | – |
| D | 82.26 (79.50 to 85.02) | 81.01 (78.32 to 83.71) | 2.96 (0.27 to 5.65) | −0.01 | |
| D+E | 86.46 (83.84 to 89.09) | 86.49 (83.83 to 89.14) | 8.43 (5.78 to 11.09) | 0.24 | |
All 6- and 18-month means are adjusted for baseline values, baseline body mass index, and gender.
All groups significantly increased in balance, gait, and walking efficacy for duration at 6 months (p < .003). At 18 months, D+E remained significantly increased in these three measures of self-efficacy when compared with baseline (p < .0001). The D- and E-only groups remained significantly increased in self-efficacy as measured by the gait efficacy scale (p < .03) and walking efficacy for duration scale (p < .001).
Mediation of treatment effects on physical function and pain
The mediation analyses revealed that self-efficacy significantly (Sobel p < .05) mediated the effects of D+E on the primary clinical outcomes. Change in walking self-efficacy significantly mediates the relationship between D+E versus E [and D+E vs. D] and 6MWT, gait speed, WOMAC function, and WOMAC pain (Table 4). Change in balance efficacy significantly mediated the relationship between D+E versus E [and D+E vs. D] and WOMAC function, and between D+E versus E and WOMAC pain. As gait self-efficacy only mediated WOMAC function for D+E versus E (p < .05) and D versus E had no significant mediators with the outcomes, they were not included in the table for ease of interpretation.
Table 4.
Mediation analyses between treatment assignment and physical function and pain
| Outcome | Predictor | Mediator | Indirect effect | Direct effect | Ratio of indirect to direct | Sobel test p value |
|---|---|---|---|---|---|---|
| Change in 6MWT (m) | D+E versus E | aDuration Chg | 0.067 | 0.099 | 0.672 | .0353* |
| bBalance Chg | 0.012 | 0.132 | 0.092 | .5436 | ||
| D+E versus D | Duration Chg | 0.087 | 0.237 | 0.365 | .0196* | |
| Balance Chg | 0.026 | 0.268 | 0.095 | .1508 | ||
| Change in gait speed (m/s) | D+E versus E | Duration Chg | 0.085 | 0.082 | 1.030 | .0083* |
| Balance Chg | 0.041 | 0.102 | 0.396 | .0629 | ||
| D+E versus D | Duration Chg | 0.072 | 0.053 | 1.372 | .0338* | |
| Balance Chg | 0.004 | 0.093 | 0.043 | .7447 | ||
| Change in WOMAC function | D+E versus E | Duration Chg | −0.111 | −0.144 | 0.767 | .0033* |
| Balance Chg | −0.072 | −0.178 | 0.405 | .0187* | ||
| D+E versus D | Duration Chg | −0.175 | −0.049 | 3.603 | .0003* | |
| Balance Chg | −0.095 | −0.091 | 1.038 | .0356* | ||
| Change in WOMAC pain | D+E versus E | Duration Chg | −0.082 | −0.127 | 0.646 | .0170* |
| Balance Chg | −0.040 | −0.169 | 0.239 | .1066 | ||
| D+E versus D | Duration Chg | −0.146 | −0.097 | 1.507 | .0028* | |
| Balance Chg | −0.071 | −0.146 | 0.485 | .0483* |
All predictors and outcomes are standardized. All models adjusted for baseline BMI, gender, and baseline values of the outcome. 6MWT Six-Minute Walk Test; WOMAC Western Ontario McMasters Universities Osteoarthritis Index.
aDuration Chg: Self-efficacy for walking duration.
bBalance Chg: Balance efficacy.
*p < .05.
DISCUSSION
The present study identified the relationships among self-efficacy and physical activity, function, and pain at baseline; determined the effects of intensive weight loss (≥10% of baseline body weight) and exercise on self-efficacy over time; and examined whether change in self-efficacy mediated treatment effects on function and pain in overweight and obese older adults with knee OA. Although all groups experienced longitudinal improvements in self-efficacy, an intervention of diet-induced weight loss combined with exercise (D+E) was significantly better for increasing self-efficacy than either exercise (E) or diet-induced weight loss alone (D) at 18-month follow-up. As previously reported [8], the D+E group also had less pain, better function, and faster walking speed when compared with the other treatment arms. Furthermore, the treatment effects of D+E versus E or D on these clinical outcomes were mediated by changes in self-efficacy over the course of the trial, such that the D+E intervention had a significant impact on physical function and pain partly because of changes in self-efficacy. These collective findings suggest that a combined intervention of diet-induced weight loss and exercise is the treatment of choice to maximize both psychological process-related outcomes and patient-centered clinical outcomes for older, overweight, and obese adults with knee OA.
A closer examination of the change in self-efficacy reveals that gait, walking duration, and balance efficacy significantly improved at 6 months in D+E and showed no regression toward baseline values even after 18 months. Gains in self-efficacy in the first 6 months reflect the transitioning of a sedentary population to exercise [38]; the subsequent lack of regression in the D+E group speaks to the strength of the combined intervention. It is interesting to note that the D group also experienced significant increases in gait efficacy and walking efficacy for duration without having the benefit of an exercise intervention. This finding, combined with the significantly greater increase in self-efficacy evidenced by the D+E group, corroborates Garver’s [39] observation that weight status in older adults with knee OA directly affects walking self-efficacy and walking performance.
Few studies have examined the impact of combined weight loss and exercise on self-efficacy in older adults with knee OA. In the Arthritis, Diet, and Activity Promotion Trial (ADAPT), Focht et al. [15] demonstrated a significant increase in mobility-related self-efficacy in a combined intervention of modest weight loss (5% of body weight) and exercise when compared with a healthy education control group in a sample of sedentary, overweight, and obese older adults with knee OA. Somers et al. [40] found that combining behavioral weight management with pain-coping skills significantly increased self-efficacy over a 12-week long intervention in overweight and obese adults with knee OA when compared with receiving pain-coping training only, behavioral weight loss only, or a standard of care control group. Taken together, these findings suggest that nonpharmacologic interventions seeking to affect self-efficacy while improving the lives of those with knee OA should focus on exercise and weight loss.
At baseline, participants reporting higher self-efficacy had better function, less pain, and higher levels of physical activity participation. Self-reported function via the WOMAC was comparable with other knee OA cohorts [7, 41]. Prospective studies have shown that baseline self-efficacy is protective against future functional decline [16], and among older adults with poor cardiovascular health, Brawley and colleagues [25] found that walking self-efficacy at 6 months mediated the impact of a weight loss and physical activity intervention on 400-meter walk time at 18 months. Although few studies have tested the mediational role played by self-efficacy changes in older adults with knee OA, group-mediated physical activity intervention changes in self-regulatory and mobility-related self-efficacy were correlated with changes in mobility at 3 and 12 months [42]; both knee pain and stair climb self-efficacy also mediate stair climb time in aerobic and resistance training exercise interventions [24]. These investigators identified the need for larger randomized controlled trials with more power to examine a broader range of clinically meaningful outcomes. The present study meets this call for larger clinical trials to test the theoretical mechanisms in behavioral interventions, and the findings clearly demonstrate that self-efficacy mediated the effects that the intervention had on physical function and pain suggesting that the development of self-efficacy should be integrated into treatment regimens for knee OA patients.
Limitations
This study has several limitations. Weight self-efficacy is a predictor of successful weight loss and can be positively affected in older adults with knee OA [40]. As weight loss and diet self-efficacy were not assessed in the IDEA study, it is uncertain what effect the combined intervention had on the beliefs of participants in their ability to lose and maintain weight. In addition, the delivery of the intervention changed at 6 months. Participants receiving the diet-induced weight loss intervention tapered contact as follows: from Months 1 through 6, one individual session and three group sessions per month, and from Months 7 through 18, biweekly group sessions and an individual session every 2 months. Participants receiving the exercise intervention were given the opportunity to exercise at home some or all of the time starting at 6 months. While few participants chose to exercise at home, the reduction in contact hours with intervention staff could have possibly dampened the effect of the intervention on self-efficacy.
Implications of the findings
Intentional physical activity and weight loss interventions that serve to enhance self-efficacy may lead to improved mobility, physical function, and pain management, making interventions designed to enhance self-efficacy essential in the clinical management of knee OA. Interventions built on SCT that focus on equipping knee OA patients with the tools to manage their disease through weight loss and exercise might very well be affordable alternatives to current pharmacologic and surgical management regimes. On an individual level, knee OA patients that engage in diet-induced weight loss and participate in exercise experience increased function, less pain, and enhanced quality of life [8, 43]. Mastery in these domains that are habitually difficult for overweight and obese older adults with knee OA may largely explain why participants in the D+E intervention reported the greatest gains in self-efficacy at study end.
CONCLUSION
Overall, these findings suggest that a combined intervention of exercise and diet-induced weight loss designed within a social cognitive framework improves self-efficacy in overweight and obese adults with knee OA and that these changes in self-efficacy underlie the effects of behavioral interventions on important clinical outcomes. As Hermsen [44] suggests, the contributions made by social cognitive constructs, and in particular self-efficacy, to physical function is essential to take into consideration in the future clinical management of patients with joint pain and comorbidity. In knee OA, along with many other comorbid conditions, control beliefs play a key role in disability and quality of life and should be targeted as potential mechanisms for important health outcomes [8, 43, 45]. Future research is necessary to examine not only the feasibility of implementing strategies to positively affect self-efficacy in patient care and community-based settings, but also the sustainability of boosted self-efficacy on the road to the effective management of knee OA.
Acknowledgments
This study is registered at www.clinicaltrials.gov (No. NCT00381290). We thank the Intensive Diet and Exercise for Arthritis (IDEA) research staff and the IDEA participants for their important contributions. This work was supported by grants from the National Institutes of Health: R01 AR052528-01 from the National Institute of Arthritis and Musculoskeletal and Skin Disease, P30-AG21332 from the National Institute of Aging, M01-RR07122 from the National Center for Research Resources, and by General Nutrition Centers.
Compliance with Ethical Standards
Primary Data: These findings have not been previously published. This manuscript is not being simultaneously submitted elsewhere. Data reflecting the main outcomes of the IDEA trial were reported in Messier SP, Mihalko SL, Legault C, Miller GD, Nicklas BJ, DeVita P, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee OA: the IDEA randomized clinical trial. JAMA. 2013;310(12):1263–73. The authors have full control of all primary data and agree to allow the journal to review data if requested.
Conflicts of Interest: Ali Guermazi is a president of Boston Imaging Core Lab, LLC, and a consultant to Genzyme, AstraZeneca, TissueGene, Merck Serono, and OrthoTrophix. Felix Eckstein is CEO/CMO and co-owner of Chondrometrics GmbH. He provides consulting services to MerckSerono, Samumed, Abbvie, Bioclinica, Tissuegene, and Servier, and has prepared education content/lectures for Medtronic. Shannon Mihalko, Phillip Cox, Daniel Beavers, Gary Miller, Barbara Nicklas, Mary Lyles, David Hunter, Richard Loeser, Paul DeVita, and Stephen Messier declare they have no conflict of interest.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
References
- 1. Lawrence RC, Felson DT, Helmick CG et al. ; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991–94. J Rheumatol. 2006;33(11):2271–2279. [PubMed] [Google Scholar]
- 3. Manek NJ, Hart D, Spector TD, MacGregor AJ. The association of body mass index and osteoarthritis of the knee joint: an examination of genetic and environmental influences. Arthritis Rheum. 2003;48(4):1024–1029. [DOI] [PubMed] [Google Scholar]
- 4. Niu J, Zhang YQ, Torner J et al. Is obesity a risk factor for progressive radiographic knee osteoarthritis?Arthritis Rheum. 2009;61(3):329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hochberg MC, Altman RD, April KT et al. ; American College of Rheumatology. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64(4):465–474. [DOI] [PubMed] [Google Scholar]
- 6. Ettinger WH Jr, Burns R, Messier SP et al. A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis. The Fitness Arthritis and Seniors Trial (FAST). JAMA. 1997;277(1):25–31. [PubMed] [Google Scholar]
- 7. Messier SP, Loeser RF, Miller GD et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50(5):1501–1510. [DOI] [PubMed] [Google Scholar]
- 8. Messier SP, Mihalko SL, Legault C et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310(12):1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang W, Moskowitz RW, Nuki G et al. OARSI recommendations for the management of hip and knee osteoarthritis, part I: critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthritis Cartilage. 2007;15(9):981–1000. [DOI] [PubMed] [Google Scholar]
- 10. Zhang W, Moskowitz RW, Nuki G et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16(2):137–162. [DOI] [PubMed] [Google Scholar]
- 11. Mihalko SL, Brenes GA, Farmer DF, Katula JA, Balkrishnan R, Bowen DJ. Challenges and innovations in enhancing adherence. Control Clin Trials. 2004;25(5):447–457. [DOI] [PubMed] [Google Scholar]
- 12. McAuley E, Courneya KS, Rudolph DL, Lox CL. Enhancing exercise adherence in middle-aged males and females. Prev Med. 1994;23(4):498–506. [DOI] [PubMed] [Google Scholar]
- 13. Bandura A. Self-Efficacy : The Exercise of Control. New York: W.H. Freeman; 1997. [Google Scholar]
- 14. Marks R. Self-efficacy and its application in the treatment of knee osteoarthritis: a critical review. Rheumatol Reports. 2012;4(1). doi:10.4081/rr.2012.e10. [Google Scholar]
- 15. Focht BC, Rejeski WJ, Ambrosius WT, Katula JA, Messier SP. Exercise, self-efficacy, and mobility performance in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005;53(5):659–665. [DOI] [PubMed] [Google Scholar]
- 16. Sharma L, Cahue S, Song J, Hayes K, Pai YC, Dunlop D. Physical functioning over three years in knee osteoarthritis: role of psychosocial, local mechanical, and neuromuscular factors. Arthritis Rheum. 2003;48(12):3359–3370. [DOI] [PubMed] [Google Scholar]
- 17. Rejeski WJ, Mihalko SL. Physical activity and quality of life in older adults. j Gerontol a Biol Sci Med Sci. 2001;56 (Spec No 2):23–35. [DOI] [PubMed] [Google Scholar]
- 18. Wright LJ, Zautra AJ, Going S. Adaptation to early knee osteoarthritis: the role of risk, resilience, and disease severity on pain and physical functioning. Ann Behav Med. 2008;36(1):70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Somers TJ, Keefe FJ, Pells JJ et al. Pain catastrophizing and pain-related fear in osteoarthritis patients: relationships to pain and disability. J Pain Symptom Manage. 2009;37(5):863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baker KR, Nelson ME, Felson DT, Layne JE, Sarno R, Roubenoff R. The efficacy of home based progressive strength training in older adults with knee osteoarthritis: a randomized controlled trial. J Rheumatol. 2001;28(7):1655–1665. [PubMed] [Google Scholar]
- 21. Brooks MA, Beaulieu JE, Severson HH et al. Web-based therapeutic exercise resource center as a treatment for knee osteoarthritis: a prospective cohort pilot study. BMC Musculoskelet Disord. 2014;15:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lamb SE, Toye F, Barker KL. Chronic disease management programme in people with severe knee osteoarthritis: efficacy and moderators of response. Clin Rehabil. 2008;22(2):169–178. [DOI] [PubMed] [Google Scholar]
- 23. Sullivan T, Allegrante JP, Peterson MG, Kovar PA, MacKenzie CR. One-year follow-up of patients with osteoarthritis of the knee who participated in a program of supervised fitness walking and supportive patient education. Arthritis Care Res. 1998;11(4):228–233. [DOI] [PubMed] [Google Scholar]
- 24. Rejeski WJ, Ettinger WH Jr, Martin K, Morgan T. Treating disability in knee osteoarthritis with exercise therapy: a central role for self-efficacy and pain. Arthritis Care Res. 1998;11(2):94–101. [DOI] [PubMed] [Google Scholar]
- 25. Brawley L, Rejeski WJ, Gaukstern JE, Ambrosius WT. Social cognitive changes following weight loss and physical activity interventions in obese, older adults in poor cardiovascular health. Ann Behav Med. 2012;44(3):353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McAuley E, Szabo A, Gothe N, Olson EA. Self-efficacy: implications for physical activity, function, and functional limitations in older adults. Am J Lifestyle Med. 2011;5(4):1–15. doi:10.1177/1559827610392704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Messier SP, Legault C, Mihalko S et al. The Intensive Diet and Exercise for Arthritis (IDEA) trial: design and rationale. bmc Musculoskelet Disord. 2009;10:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bandura A. Social Foundations of Thought and Action : A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- 29. Martin KA, Rejeski WJ, Miller ME, James MK, Ettinger WH Jr, Messier SP. Validation of the PASE in older adults with knee pain and physical disability. Med Sci Sports Exerc. 1999;31(5):627–633. [DOI] [PubMed] [Google Scholar]
- 30. Bennell K, Dobson F, Hinman R. Measures of physical performance assessments: self-paced walk test (SPWT), stair climb test (SCT), six-minute walk test (6MWT), chair stand test (CST), timed up & go (TUG), sock test, lift and carry test (LCT), and car task. Arthritis Care Res. 2011;63(suppl 11):350–370. doi:10.1002/acr.20538. [DOI] [PubMed] [Google Scholar]
- 31. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 32. Nguyen US, Felson DT, Niu J et al. The impact of knee instability with and without buckling on balance confidence, fear of falling and physical function: the Multicenter Osteoarthritis Study. Osteoarthritis Cartilage. 2014;22(4):527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Powell LE, Myers AM. The activities-specific balance confidence (ABC) scale. j Gerontol a Biol Sci Med Sci. 1995;50A(1):M28–M34. [DOI] [PubMed] [Google Scholar]
- 34. McAuley E, Mihalko SL, Rosengren K. Self-efficacy and balance correlates of fear of falling in the elderly. J Aging Phys Act. 1997;5(4):329–340. [Google Scholar]
- 35. Bandura A. Guide for constructing self-efficacy scales. In: Pajares F, Urdan TC, eds. Self-Efficacy Beliefs of Adolescents. Adolescence and education. Greenwich, Conn: Information Age Publishing; 2006:307–337. [Google Scholar]
- 36. Sobel E. Asymptotic Confidence Intervals for Indirect Effects in Structural Equation Models. Sociol Methodol. 1982;13(1982):290–312. [Google Scholar]
- 37. Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. [DOI] [PubMed] [Google Scholar]
- 38. McAuley E, Katula J, Mihalko SL et al. Mode of physical activity and self-efficacy in older adults: a latent growth curve analysis. J Gerontol b Psychol Sci Soc Sci. 1999;54(5):P283–P292. [DOI] [PubMed] [Google Scholar]
- 39. Garver MJ, Focht BC, Dials J et al. Weight status and differences in mobility performance, pain symptoms, and physical activity in older, knee osteoarthritis patients. Arthritis. 2014;2014:375909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Somers TJ, Blumenthal JA, Guilak F et al. Pain coping skills training and lifestyle behavioral weight management in patients with knee osteoarthritis: a randomized controlled study. Pain. 2012;153(6):1199–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pisters MF, Veenhof C, van Dijk GM, Dekker J; CARPA Study Group Avoidance of activity and limitations in activities in patients with osteoarthritis of the hip or knee: a 5 year follow-up study on the mediating role of reduced muscle strength. Osteoarthritis Cartilage. 2014;22(2):171–177. [DOI] [PubMed] [Google Scholar]
- 42. Focht BC, Garver MJ, Devor ST et al. Group-mediated physical activity promotion and mobility in sedentary patients with knee osteoarthritis: results from the IMPACT-pilot trial. J Rheumatol. 2014;41(10):2068–2077. [DOI] [PubMed] [Google Scholar]
- 43. Rejeski WJ, Focht BC, Messier SP, Morgan T, Pahor M, Penninx B. Obese, older adults with knee osteoarthritis: weight loss, exercise, and quality of life. Health Psychol. 2002;21(5):419–426. [DOI] [PubMed] [Google Scholar]
- 44. Hermsen LAH, van der Wouden JC, Leone SS, Smalbrugge M, van der Horst HE, Dekker J. The longitudinal association of cognitive appraisals and coping strategies with physical functioning in older adults with joint pain and comorbidity: a cohort study. bmc Geriatr. 2016;16:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lorig KR, Sobel DS, Ritter PL, Laurent D, Hobbs M. Effect of a self-management program on patients with chronic disease. Eff Clin Pract. 2001;4(6):256–262. [PubMed] [Google Scholar]