Abstract
Background
There is increasing evidence pointing to the important role of tumor necrosis factor-alpha (TNF-α), a key inflammatory and apoptotic mediator in urticarial inflammation. However, the role of the TNF-α system and Fas/Fas ligand (FasL) in the apoptosis-inducing pathways in chronic spontaneous urticaria (CSU), remain unclear.
Aim
To determine circulating concentrations of TNF-α, soluble TNF-α receptor type 1 and type 2 (sTNF-R1 and sTNF-R2, respectively) as well as soluble Fas (sFas) and FasL (sFasL) in CSU subjects.
Methods
Serum TNF-α, sTNF-R1, sTNF-R2, sFas, sFasL concentrations were measured using enzyme-linked immunosorbent assay in CSU subjects and in the healthy subjects.
Results
TNF-α concentrations were significantly higher in CSU subjects and moderate-to-severe CSU than in the controls, while there were no significant differences in TNF-α concentrations between subjects with mild CSU and the controls. sTNF-R1 and sTNF-R2 concentrations were significantly higher in all CSU and moderate-severe CSU subjects vs. the controls. Serum concentrations were also significantly higher in mild CSU vs. the controls, but not in moderate-severe CSU vs. mild CSU. No significant differences were observed in sFas and sFasL concentrations between CSU subjects and the healthy controls. Significant correlations were found between concentrations of TNF-α and its receptors, as well as sTNF-R1 and sTNF-R2, but not with the urticaria activity score (UAS). There was no relationship between TNF-α/sTNF-R1/sTNF-R2 and sFas/sFasL pathways in CSU.
Conclusions
CSU is associated with the activation of the TNF-α/receptors signaling pathway, marked by increased circulating concentrations of TNF-α, sTNF-R1 and sTNF-R2, which are related to each other in this disease. In contrast, the circulating sFas/FasL system is not up-regulated in CSU, and sFas/sFasL may not be a useful marker of the activity/severity of urticarial processes. Considering the lack of significant changes in sFas/sFasL (mainly reflecting systemic apoptosis) in CSU patients, it appears that elevated serum TNF-α concentrations are related to its pro-inflammatory function rather than an enhanced systemic apoptotic response in CSU.
Keywords: Chronic spontaneous urticaria, Tumor necrosis factor alpha (TNF-α), Soluble tumor necrosis factor receptor type 1 (sTNF-R1), Soluble tumor necrosis factor receptor type 2 (sTNF-R2), Soluble Fas (sFas), Soluble Fas ligand (sFasL)
Introduction
Chronic spontaneous urticaria (CSU) is associated with autoimmune processes and a systemic inflammatory response [1–3].
Tumor necrosis factor alpha (TNF-α) plays a central role in inflammatory processes, immune regulation and apoptosis. TNF-α exerts its action via cell-surface receptors. They exist in two cell-bound forms (TNF-Rs) type 1 (TNF-R1, p55) and 2 (TNF-R1, p75), as well as soluble forms (sTNF-Rs), sTNF-R1 and sTNF-R2, respectively. Binding to TNF-Rs, TNF-α activates the constitutive shedding processes of soluble receptors, that play an important role in regulating its in vivo activity [4–7]. High sTNF-Rs serum/plasma concentrations were found in infections and inflammatory diseases [8, 9]. Dysregulation of the TNF-α pathway is a key feature and pathogenic factor in various diseases, including sepsis, cancers, as well as autoimmune and inflammatory diseases [10]. Fas ligand (FasL CD95L), a membrane molecule, belonging to the TNF family, interacts with Fas (CD95/Apo-1)—a cell surface receptor that is regulated by their soluble forms sFasL and sFas, respectively [11, 12]. The Fas/FasL system is known as one of the most important apoptosis signaling pathways, but its non-apoptotic function is not yet fully understood. The Fas/FasL system is involved in the maintenance of immune homeostasis and its dysfunction is associated with immunological and inflammatory processes [11–14]. The role of TNF family molecules involved in inflammation and apoptosis in CSU has not yet been fully understood [15, 16]. Therefore, it seems important to determine circulating concentrations of TNF-α and their soluble receptors, as well as the sFas/sFasL system in individuals with CSU.
Materials and methods
The study involved 17 men and 41 women with active CSU [median age 39 (range 21–45 years)]. The control group consisted of 22 healthy people who were matched for age and sex with CSU subjects; recruited from the hospital staff [17].
The subjects were part of a study regarding inflammatory reaction in chronic urticaria, the protocol and clinical examination of which have been described elsewhere [17, 18]. CSU subjects were not on active treatments. Antihistamines have been discontinued for at least 4 days. Treatment with cyclosporine and glucocorticoids has not been used for the period of at least 2 months preceding the study. Cyclosporine was discontinued only in the case of ineffectiveness or adverse reactions. In addition, none of these subjects had previous omalizumab treatment. Subjects were included in the study if the symptoms lasted longer than 6 months. The mean CSU duration was 30.5 months (range 7–62 months).
All subjects gave written informed consent and the Bioethics Committee of the Medical University of Silesia approved the study.
The concentrations of studied parameters in serum samples were measured by the enzyme-linked immunosorbent assay (ELISA) using commercially available kits according to manufacturer’s detailed instructions: for Tumor Necrosis Factor alpha (TNF-α)—Quantikine ELISA Human TNF-α kit, for Fas—Quantikine ELISA Human Fas/TNFRSF6 kit, for Fas Ligand (FasL)—Quantikine ELISA Human Fas Ligand/TNFSF6 kit, for soluble TNF receptor I (sTNF RI)—Quantikine ELISA Human sTNF RI/TNFRSF1A kit and for soluble TNF receptor II (sTNF RII)—Quantikine ELISA Human TNF RII/TNFRSF1B kit (all kits from R&D Systems, MN, USA). The coefficients of variance for intra-assay and inter-assay were below 8% and 10%, respectively.
The obtained results were presented using basic parameters of descriptive statistics. Normal distribution of data was verified using Shapiro–Wilk’s test.
A comparison of two independent groups (CSU subjects as compared to the control group) was performed using the non-parametric U Mann–Whitney test. Independent data between the three groups (moderate-severe CSU vs. mild CSU vs. controls) were compared using the ANOVA rang Kruskal–Wallis’ test and appropriate post hoc tests. The Spearman’s rank test was used for correlations. The value of p < 0.05 was considered statistically significant. The calculations were performed with STATISTICA for Windows 10.0 software (StatSoft, Cracow, Poland).
Results
Serum concentrations of TNF-α and its receptors
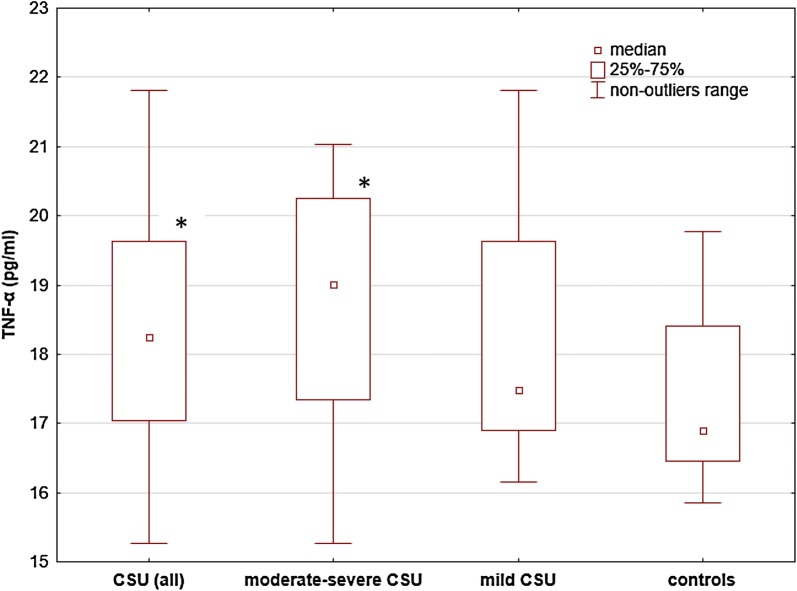
TNF-α concentrations were significantly higher in CSU subjects and moderate-to-severe CSU than in the controls [median and IQR 18.25 (17.04–19.62) and 19.01 (17.34–20.24) vs 16.89 (16.45–18.40) pg/ml; p < 0.05] (Fig. 1), while there were no significant differences in TNF-α concentrations between subjects with mild [median and IQR 17.49 (16.90–19.62) pg/ml] and moderate-to-severe CSU [median and IQR 19.01 (17.34–20.24) pg/ml] (Fig. 1). Similarly, there were no significant differences in TNF-α concentrations between mild CSU subjects and the controls (Fig. 1).
Fig. 1.
TNF-α concentrations in subjects with chronic spontaneous urticaria (CSU) and the healthy subject. TNF-α concentrations were significantly higher in CSU subjects and moderate-to-severe CSU than in the controls (p < 0.05). There were no significant differences between mild and moderate-severe CSU, and between mild CSU and the controls
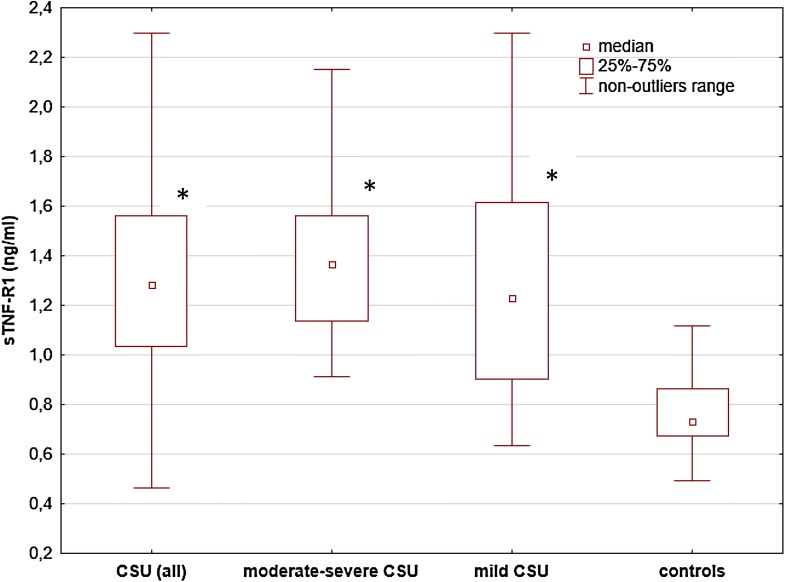
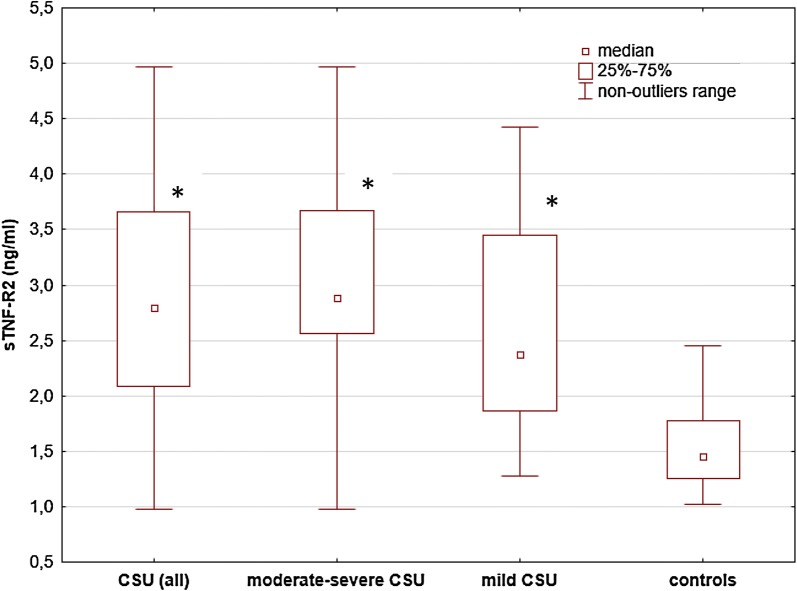
sTNF-R1 and sTNF-R2 concentrations [median and IQR 1.28 (1.03–1.56) and 2.79 (2.09–3.66) ng/ml] were significantly higher in CSU subjects as compared with the healthy subjects [median and IQR 0.73 (0.67–0.86) and 1.45 (1.26–1.77) ng/ml, respectively; p < 0.00001].
Serum concentrations of sTNF-R1 and sTNF-R2 were significantly higher in moderate-to-severe CSU vs. the controls [median and IQR 1.37 (1.14–1.56) and 2.89 (2.56–3.67) vs. 0.73 (0.67–0.86) and 1.45 (1.26–1.77) ng/ml, respectively; p < 0.00001], but not vs. mild CSU subjects [1.23 (0.9–1.62) and 2.38 (1.87–3.45) ng/ml, p > 0.05] (Figs. 2, 3).
Fig. 2.
sTNF-R1 concentrations in serum of subjects with chronic spontaneous urticaria (CSU) and the healthy subject. sTNF-R1 concentrations were significantly higher in all CSU and moderate-severe CSU subjects vs. the controls (p < 0.00001). The concentrations were also significantly higher in mild CSU vs. the controls (p < 0.0001), but not in moderate-severe CSU vs. mild CSU (p > 0.05)
Fig. 3.
sTNF-R2 concentrations in serum of subjects with chronic spontaneous urticaria (CSU) and the healthy subject. sTNF-R2 concentrations were significantly higher in CSU and moderate-severe CSU subjects vs. the controls (p < 0.00001). The concentrations were also significantly higher in mild CSU vs. the controls (p < 0.0001), but not in moderate-severe CSU vs. mild CSU (p > 0.05)
Similarly, sTNF-R1 and sTNF-R2 concentrations were also significantly higher in mild CSU than in the healthy subjects [median and IQR 1.23 (0.9–1.62) and 2.38 (1.87–3.45) vs. 0.73 (0.67–0.86) and 1.45 (1.26–1.77) ng/ml, respectively; p < 0.0001] (Figs. 2, 3).
Serum concentrations of sFas and sFasL
No significant differences were observed in sFas and sFasL concentrations between all CSU, mild, moderate-to-severe CSU subjects and the controls [median and IQR 15.96 (12.29–21.61) and 87.59 (54.64–106.6), 17.09 (14.33–24.15) and 76.78 (45.23–97.03), 13.79 (10.20–21.50) and 102.03 (67.76–114.88) vs. 12.78 (10.3–19.26) and 75.71 (53.76–91.58) ng/ml and pg/ml, respectively; p > 0.05).
Serum C reactive protein (CRP) concentrations
CRP concentrations were significantly higher in all CSU subjects, mild, and moderate-severe CSU as compared to the controls as well as between the groups (data not shown, published earlier) [17].
Correlations
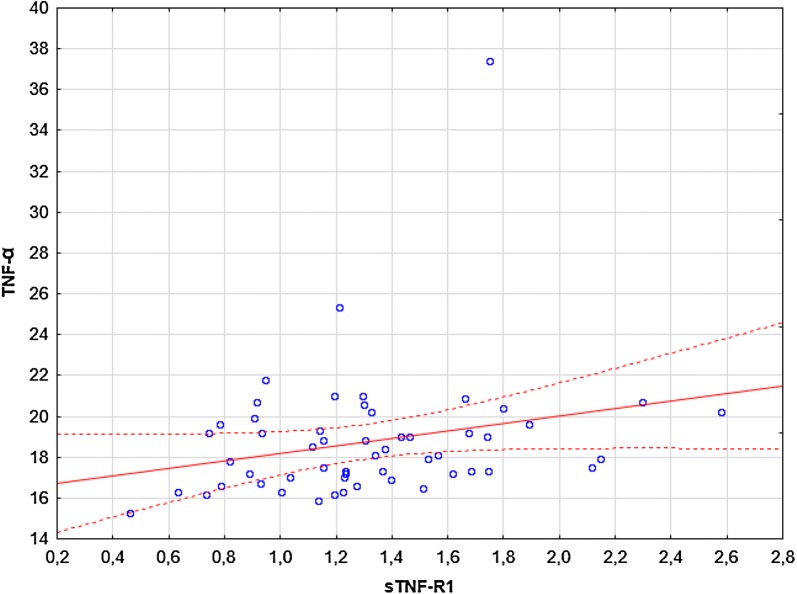
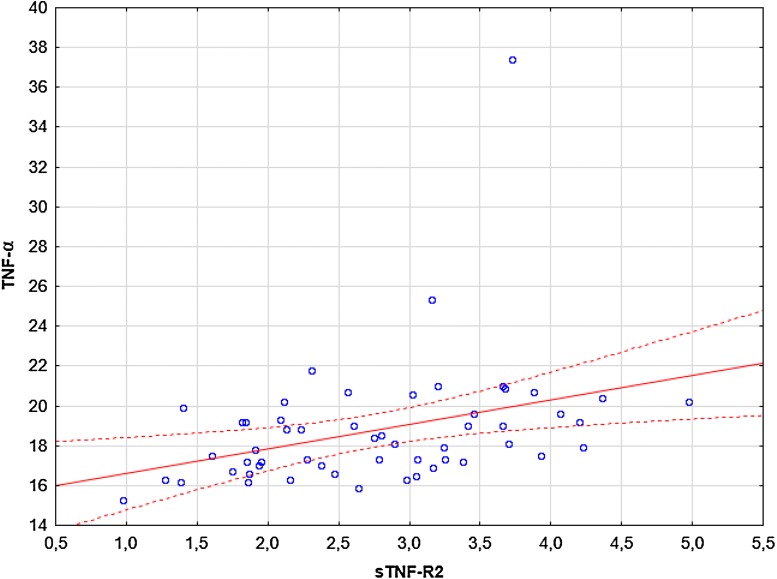
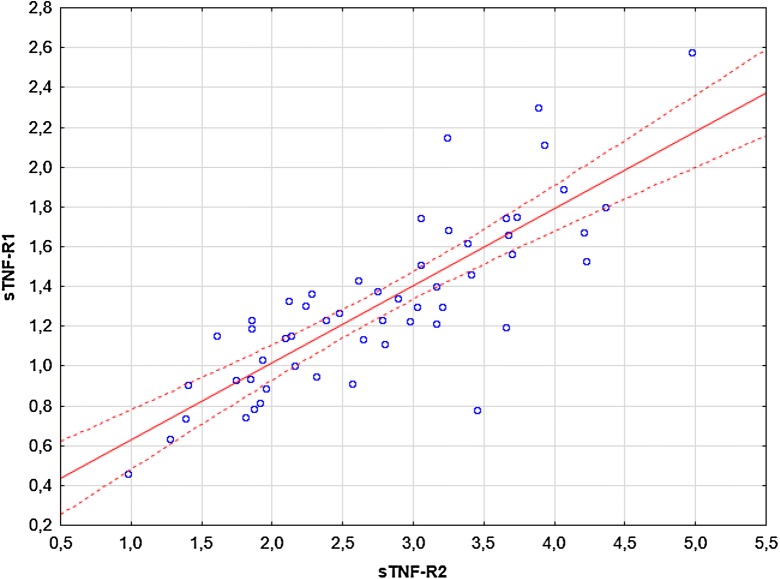
Significant correlations were observed between: (i) TNF-α and sTNF-R1 (R = 0.28, p = 0.036; Fig. 4), (ii) TNF-α and sTNF-R2 (R = 0.44, p = 0.00078; Fig. 5), (iii) sTNF-R1 and sTNF-R2 (R = 0.79, p = 0.00000; Fig. 6). No significant correlations were observed between: (i) CRP and TNF-α, sTNF-R1, sTNF-R2, sFas, sFasL, (ii) sFas and sFasL, (iii) sFas/sFasL and TNF-α/sTNF-R1/sTNF-R2 (data not shown). There were no significant correlations between UAS4 and TNF-α, sTNF-R1, sTNF-R2, sFas, sFasL (R = 0.26, p = 0.052; R = 0.22, p = 0.09; R = 0.2, p = 0.13; R = -0.16, p = 0.22; R = 0.14, p = 0.29, respectively).
Fig. 4.
The correlation between TNF-α and sTNF-R1 concentrations in CSU subjects. The blue data points represent the values of the variables tested for individual observations. Solid red lines representing regression lines and broken red lines represent 95% confidence intervals
Fig. 5.
The correlation between TNF-α and sTNF-R2 concentrations in CSU subjects. The blue data points represent the values of the variables tested for individual observations. Solid red lines representing regression lines and broken red lines represent 95% confidence intervals
Fig. 6.
The correlation between sTNF-R1 and sTNF-R2 concentrations in CSU subjects. The blue data points represent the values of the variables tested for individual observations. Solid red lines representing regression lines and broken red lines represent 95% confidence intervals
Discussion
In patients with different types of urticaria, increased TNF-α activity was detected in skin biopsies [19]. In addition, it has been suggested that TNF-α promoter polymorphisms may induce upregulation of TNF-α [20]. In our study, serum TNF-α concentrations were significantly higher in all CSU and moderate-to-severe CSU than in the control group, but not in mild CSU. These results confirm previous observations and suggest the role of the TNF-α system in the pathogenesis of CSU [15, 16].
TNF-α is one of four key cytokines (IL-1, IL-6 and IL-8) involved in the activation of the acute phase response. We have previously found a significant correlation between increased IL-6, IL-8 and CRP concentrations in CSU subjects [3, 21]. In the present study, there was no significant correlation between TNF-α and CRP, which may indicate that TNF-α does not play a significant role in the production of CRP in CSU.
However, circulatory TNF-α is unstable, therefore to get a more precise insight into TNF-α/receptors system activity, both TNF-α and its soluble receptors, which maintain a more stable form should be measured. The soluble TNF-α receptors are more useful for monitoring the activity of the TNF-α system activity [7, 22]. The importance of soluble TNF-α receptors in CSU is unknown.
In our study, the concentrations of sTNF-R1 and sTNF-R2 were significantly higher in all CSU subjects, moderate-to-severe CSU, and mild CSU as compared with the healthy group.
However, no significant differences in these values were observed between subjects with moderate-to-severe and mild CSU. There was no correlation between TNF-α sTNF-R1, sTNF-R2 and UAS in CSU.
It has been suggested that circulating concentrations of sTNF-Rs may reflect the state of activation of the TNF-α/receptors system [7, 22]. Therefore, our results may indicate that CSU is associated with increased activity of the TNF-α/receptors system. In addition increased circulating concentrations of sTNF-R1 and sTNF-R1 may reflect the systemic activation of an immune-inflammatory response in CSU, rather than their increased release form the urticarial lesion into circulation. It has been demonstrated that circulating levels of sTNF-R1 and sTNF-R2 are strongly correlated with the levels of such receptors spontaneously released by isolated peripheral blood T cells in vitro in rheumatoid arthritis [23].
Binding of TNF-α to TNF-Rs activates the constitutive shedding process of soluble receptors, which plays an important role in regulating its in vivo activity [6, 22]. sTNF-Rs may modulate TNF-α activity likely in two different manners depending on their concentrations [24].
In the negative feedback mechanism, high concentrations of TNF-R act as inhibitors/antagonists of TNF-α activity, preventing its binding to membrane-bound TNF-R, maintaining homeostasis and protecting against overwhelming immunological-inflammatory activation [6, 22, 25].
On the other hand, it has been shown that sTNF-Rs at low/intermediate concentrations may preserve and enhance some of the effects of TNF-α by stabilizing its structure in complexes and prolonging its half-life [22, 25].
We observed significant correlations between concentrations of TNF-α and its receptors as well as, sTNF-R1 and sTNF-R2 in CSU.
The open question is whether the increased release of sTNF-Rs in CSU is sufficient to neutralize TNF-α activity to prevent over-response of the TNF/receptors system, systemically and locally at sites of inflammation. One the one hand, we speculate that the higher release of TNF-α in CSU stimulates shedding of sTNF-Rs into the circulation, probably leading to neutralization of TNF-α activity and attenuation/limitation of the inflammatory response, similarly as suggested in other immune-inflammatory diseases [22, 26]. However, it has also been found that their concentration may be insufficient to neutralize the excessive TNF-α response in some chronic inflammatory diseases [9]. Therefore, insufficiently raised circulating concentration of sTNR-Rs might even enhance the effect of TNF-α in urticarial inflammation and systemic inflammatory response by stabilizing its activity and prolonging its function.
However, the source of circulating forms in CSU is still unknown. In contrast to widely expressed TNF-R1, TNF-R2 is expressed typically by immune cells [5, 27], thus an increase in circulating sTNF-R2 concentration may indirectly indicate immune activation in CSU.
The balance between TNF- activity and sTNF-Rs determines the course of immune activation and inflammatory response, and its dysregulation may play a role in susceptibility and resistance to various diseases [4], including sepsis, cancers, as well as autoimmune and inflammatory diseases [10].
In addition, persistent expression of the nonsheddable form of TNF-R1 has been shown to increase susceptibility to hyperreactivity and inflammatory response in mice [4].
The TNF-R1 mutation may result in a decrease in receptor cleavage and a reduction in the shedding of potentially antagonistic sTNR-1 and is associated with certain autoinflammatory syndromes called TNF-R1-associated periodic syndromes [28].
It has been suggested that the Fas/FasL system may have pro-inflammatory activity [29, 30]. The relationship between sFas/sFasL and the activity and severity inflammatory processes has been demonstrated [31, 32].
In contrast to other immune-inflammatory diseases, CSU is not associated with up-regulation of circulating soluble Fas/FasL system. No significant differences were observed in sFas and sFasL concentrations between CSU subjects and the control group.
We only measured circulating concentrations of sFas and sFasL, which may be less important than their local levels at the site of inflammation [33].
Our results do not exclude the importance of the Fas/FasL system and its soluble forms in urticarial inflammation. It seems, however, that concentrations of soluble apoptotic factors in serum/plasma would not be useful indicators of the activity/severity of urticarial inflammation.
TNF-α/receptors and Fas/FasL systems are two key cell death-inducing systems in one apoptotic pathway [11]. Considering the lack of significant changes in circulating sFas/sFasL in CSU subjects, it seems that increased plasma concentrations of TNF-α are related to its pro-inflammatory function rather than the enhanced systemic apoptopic response in CSU subjects.
The use of TNF-alpha inhibitors beyond therapeutic indications may be effective in a significant proportion of patients with refractory chronic urticaria, also previously unsuccessfully treated with omalizumab (anti-IgE) [34, 35].
Sand et al. observed complete or almost complete response in 60% and a partial response in a further 15% of subjects with refractory chronic urticaria during therapy with TNF‐alpha inhibitors (adalimumab or etanercept) [35].
Taking into consideration our and previous results, inhibiting the TNF-α system activity as a therapy for refractory chronic urticaria unresponsive to omalizumab and cyclosporine or associated with unacceptable drug side effects seems to be interesting for further investigations.
Nevertheless, our study has some limitations, therefore we can not prove the existence of any causal relationship. We have not studied the correlation between TNFα/sTNF-Rs and the effectiveness of anti-inflammatory or anti-IgE medications.
Conclusions
CSU is associated with the activation of the TNF-α signaling pathway, marked by increased circulating concentrations of sTNF-R1 and sTNF-R2, probably as part of the systemic inflammatory response in the disease. TNF-α and its soluble receptors are related to each other in CSU. In contrast, the circulating soluble Fas/FasL system that mainly reflects systemic apoptosis is not upregulated in CSU patients. There is no relationship between TNF-α/sTNF-R1/sTNF-R2 and sFas/sFasL pathways in the disease.
Authors’ contributions
RG: designed the study, collected samples, provided clinical data, contributed to the lab and data analysis and interpretation as well as wrote the manuscript. ADB: contributed to the lab and statistical data analysis, interpretation. AKZ: conceived and reviewed the study. All authors read and approved the final manuscript.
Acknowledgements
This study was supported by the Committee for Scientific Research (KNW-1-041/K/6/K); publication of this manuscript leads to no conflict of interest.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Consent to publish
Not applicable.
Ethics approval and consent to participate
The Ethics Committee of the Medical University of Silesia approved of the study and written, informed consent was obtained from all the subjects participating.
Funding
This study was supported by a research grant from the Committee for Scientific Research. The publication is a part of the doctoral thesis by Ryszard Grzanka. M.D, which commenced on 18.05.2017 at the Medical University of Silesia.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- CSU
chronic spontaneous urticaria
- TNF-α
tumor necrosis factor alpha
- TNF-R1
tumor necrosis factor receptor type 1
- TNF-R2
tumor necrosis factor receptor type 2
- sTNF-Rs
soluble tumor necrosis factor receptors
- sTNF-R1
soluble tumor necrosis factor receptor type 1
- sTNF-R2
soluble tumor necrosis factor receptor type 2
- FasL
Fas ligand
- UAS
urticaria activity score
- ELISA
enzyme-linked immunosorbent assay
- CRP
C reactive protein
Contributor Information
R. Grzanka, Email: ryszard.grzanka@gmail.com
A. Damasiewicz-Bodzek, Email: olabodzek@interia.pl
A. Kasperska-Zajac, Phone: (+48) 323732323, Email: alakasperska@gmail.com, Email: alicjakasperska@gmail.com
References
- 1.Kolkhir P, Church MK, Weller K, Metz M, Schmetzer O, Maurer M. Autoimmune chronic spontaneous urticaria: what we know and what we do not know. J Allergy Clin Immunol. 2017;139:1772–1781. doi: 10.1016/j.jaci.2016.08.050. [DOI] [PubMed] [Google Scholar]
- 2.Kasperska-Zajac A. Acute-phase response in chronic urticaria. J Eur Acad Dermatol Venereol. 2012;26:665–672. doi: 10.1111/j.1468-3083.2011.04366.x. [DOI] [PubMed] [Google Scholar]
- 3.Kasperska-Zajac A, Grzanka A, Machura E, Mazur B, Misiolek M, Czecior E, Kasperski J, Jochem J. Analysis of procalcitonin and CRP concentrations in serum of patients with chronic spontaneous urticaria. Inflamm Res. 2013;62:309–312. doi: 10.1007/s00011-012-0580-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xanthoulea S, Pasparakis M, Kousteni S, Brakebusch C, Wallach D, Bauer J, Lassmann H, Kollias G. Tumor necrosis factor (TNF) receptor shedding controls thresholds of innate immune activation that balance opposing TNF functions in infectious and inflammatory diseases. J Exp Med. 2004;200:367–376. doi: 10.1084/jem.20040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 6.Pinckard JK, Sheehan KC, Arthur CD, Schreiber RD. Constitutive shedding of both p55 and p75 murine TNF receptors in vivo. J Immunol. 1997;158:3869–3873. [PubMed] [Google Scholar]
- 7.Diez-Ruiz A, Tilz GP, Zangerle R, Baier-Bitterlich G, Wachter H, Fuchs D. Soluble receptors for tumour necrosis factor in clinical laboratory diagnosis. Eur J Haematol. 1995;54:1–8. doi: 10.1111/j.1600-0609.1995.tb01618.x. [DOI] [PubMed] [Google Scholar]
- 8.Schröder J, Stüber F, Gallati H, Schade FU, Kremer B. Pattern of soluble TNF receptors I and II in sepsis. Infection. 1995;23:143–148. doi: 10.1007/BF01793854. [DOI] [PubMed] [Google Scholar]
- 9.Cope AP, Aderka D, Doherty M, Engelmann H, Gibbons D, Jones AC, Brennan FM, Maini RN, Wallach D, Feldmann M. Increased levels of soluble tumor necrosis factor receptors in the sera and synovial fluid of patients with rheumatic diseases. Arthritis Rheum. 1992;35:1160–1169. doi: 10.1002/art.1780351008. [DOI] [PubMed] [Google Scholar]
- 10.Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;31(296):1634–1635. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- 11.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/S0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 12.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 13.Wajant H, Pfizenmaier K, Scheurich P. Non-apoptotic Fas signaling. Cytokine Growth Factor Rev. 2003;14:53–66. doi: 10.1016/S1359-6101(02)00072-2. [DOI] [PubMed] [Google Scholar]
- 14.Nagata S. A death factor–the other side of the coin. Behring Inst Mitt. 1996;97:1–11. [PubMed] [Google Scholar]
- 15.Trinh HK, Pham DL, Ban GY, Lee HY, Park HS, Ye YM. Altered systemic adipokines in patients with chronic urticaria. Int Arch Allergy Immunol. 2016;171:102–110. doi: 10.1159/000452626. [DOI] [PubMed] [Google Scholar]
- 16.Atwa MA, Emara AS, Youssef N, Bayoumy NM. Serum concentration of IL-17, IL-23 and TNF-α among patients with chronic spontaneous urticaria: association with disease activity and autologous serum skin test. J Eur Acad Dermatol Venereol. 2014;28:469–474. doi: 10.1111/jdv.12124. [DOI] [PubMed] [Google Scholar]
- 17.Grzanka R, Damasiewicz-Bodzek A, Kasperska-Zajac A. Interplay between acute phase response and coagulation/fibrinolysis in chronic spontaneous urticaria. Allergy Asthma Clin Immunol. 2018;14:27. doi: 10.1186/s13223-018-0255-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuberbier T, et al. The EAACI/GA2LEN/EDF/WAO Guideline for the Definition, Classification, Diagnosis and Management of Urticaria. The 2017 Revision and Update. . Allergy. 2018 doi: 10.1111/all.13397. [DOI] [PubMed] [Google Scholar]
- 19.Hermes B, Prochazka AK, Haas N, Jurgovsky K, Sticherling M, Henz BM. Upregulation of TNF-alpha and IL-3 expression in lesional and uninvolved skin in different types of urticaria. J Allergy Clin Immunol. 1999;103:307–314. doi: 10.1016/S0091-6749(99)70506-3. [DOI] [PubMed] [Google Scholar]
- 20.Tavakol M, Amirzargar AA, Movahedi M, Aryan Z, Bidoki AZ, Gharagozlou M, Aghamohammadi A, Nabavi M, Ahmadvand A, Behniafard N, Heidari K, Soltani S, Rezaei N. Interleukin-6 and tumor necrosis factor-alpha gene polymorphisms in chronic idiopathic urticaria. Allergol Immunopathol (Madr). 2014;42:533–538. doi: 10.1016/j.aller.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Kasperska-Zajac A, Grzanka A, Damasiewicz-Bodzek A, Bieniek K, Skrzypulec-Frankel A, Stencel-Gabriel K, Sikora-Zydek A. Elevated plasma IL-8 concentration is related to severity of systemic inflammation in chronic spontaneous urticaria. J Biol Regul Homeost Agents. 2017;31:957–961. [PubMed] [Google Scholar]
- 22.Aderka D. The potential biological and clinical significance of the soluble tumor necrosis factor receptors. Cytokine Growth Factor Rev. 1996;7:231–240. doi: 10.1016/S1359-6101(96)00026-3. [DOI] [PubMed] [Google Scholar]
- 23.Glossop JR, Dawes PT, Nixon NB, Mattey DL. Polymorphism in the tumour necrosis factor receptor II gene is associated with circulating levels of soluble tumour necrosis factor receptors in rheumatoid arthritis. Arthritis Res Ther. 2005;7:R1227–R1234. doi: 10.1186/ar1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohler KM, Torrance DS, Smith CA, Goodwin RG, Stremler KE, Fung VP, Madani H, Widmer MB. Soluble tumor necrosis factor (TNF) receptors are effective therapeutic agents in lethal endotoxemia and function simultaneously as both TNF carriers and TNF antagonists. J Immunol. 1993;151:1548–1561. [PubMed] [Google Scholar]
- 25.Aderka D, Engelmann H, Maor Y, Brakebusch C, Wallach D. Stabilization of the bioactivity of tumor necrosis factor by its soluble receptors. J Exp Med. 1992;175:323–329. doi: 10.1084/jem.175.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Zee KJ, Kohno T, Fischer E, Rock CS, Moldawer LL, Lowry SF. Tumor necrosis factor soluble receptors circulate during experimental and clinical inflammation and can protect against excessive tumor necrosis factor alpha in vitro and in vivo. Proc Natl Acad Sci USA. 1992;89:4845–4849. doi: 10.1073/pnas.89.11.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tartaglia LA, Weber RF, Figari IS, Reynolds C, Palladino MA, Jr, Goeddel DV. The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc Natl Acad Sci USA. 1991;88:9292–9296. doi: 10.1073/pnas.88.20.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDermott MF, Aksentijevich I, Galon J, McDermott EM, Ogunkolade BW, Centola M, Mansfield E, Gadina M, Karenko L, Pettersson T, McCarthy J, Frucht DM, Aringer M, Torosyan Y, Teppo AM, Wilson M, Karaarslan HM, Wan Y, Todd I, Wood G, Schlimgen R, Kumarajeewa TR, Cooper SM, Vella JP, Amos CI, Mulley J, Quane KA, Molloy MG, Ranki A, Powell RJ, Hitman GA, O’Shea JJ, Kastner DL. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell. 1999;97:133–144. doi: 10.1016/S0092-8674(00)80721-7. [DOI] [PubMed] [Google Scholar]
- 29.Adly AA, Ismail EA, Andrawes NG, Mahmoud MM, Eladawy R. Soluble Fas/FasL ratio as a marker of vasculopathy in children and adolescents with sickle cell disease. Cytokine. 2016;79:52–58. doi: 10.1016/j.cyto.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 30.Ricker LJ, Altara R, Goezinne F, Hendrikse F, Kijlstra A, La Heij EC. Soluble apoptotic factors and adhesion molecules in rhegmatogenous retinal detachment. Invest Ophthalmol Vis Sci. 2011;52:4256–4262. doi: 10.1167/iovs.10-6892. [DOI] [PubMed] [Google Scholar]
- 31.Myśliwiec H, Baran A, Myśliwiec P, Górska M, Flisiak I. Upregulation of the sFas/sFasL system in psoriatic patients. Adv Med Sci. 2015;60:64–68. doi: 10.1016/j.advms.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Kuwano K, Maeyama T, Inoshima I, Ninomiya K, Hagimoto N, Yoshimi M, Fujita M, Nakamura N, Shirakawa K, Hara N. Increased circulating levels of soluble Fas ligand are correlated with disease activity in patients with fibrosing lung diseases. Respirology. 2002;7:15–21. doi: 10.1046/j.1440-1843.2002.00369.x. [DOI] [PubMed] [Google Scholar]
- 33.Kuwano K, Hagimoto N, Kawasaki M, Nakamura N, Shirakawa K, Maeyama T, Hara N. Expression of FasL and Fas protein and their soluble form in patients with hypersensitivity pneumonitis. Int Arch Allergy Immunol. 2000;122:209–215. doi: 10.1159/000024399. [DOI] [PubMed] [Google Scholar]
- 34.Sand FL, Thomsen SF. Off-label use of TNF-alpha inhibitors in a dermatological university department: retrospective evaluation of 118 patients. Dermatol Ther. 2015;28:158–165. doi: 10.1111/dth.12222. [DOI] [PubMed] [Google Scholar]
- 35.Sand FL, Thomsen SF. TNF-alpha Inhibitors for Chronic Urticaria: experience in 20 Patients. J Allergy (Cairo). 2013;2013:130905. doi: 10.1155/2013/130905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.