Abstract
Dengue is one of the most common flavivirus infections which can manifest from common flu-like fever to fatal hemorrhagic complication. Epidemics of dengue return every year with peaks during the rainfall claiming substantial number of lives in the tropical and subtropical regions of the world. We present manifestations of dengue in patients who underwent neurosurgery in a tertiary referral center during such an epidemic. There were total four patients referred for neurosurgical intervention as sequelae to dengue coagulopathy. Among them, three had intracranial bleeds and one had spinal cord hematoma along with intracranial hemorrhages. This small series includes the youngest reported case of dengue coagulopathy with intracranial bleed and only the second case of spontaneous intraspinal hematoma sequelae to dengue hemorrhagic fever. The situations where patients contract dengue in a setting of neurosurgical intervention are grave. The margin of safety in the presence of dengue coagulopathy is narrow. The surgeon has to outweigh benefit against risk of surgery in each individual.
Keywords: Dengue, epidemic, neurosurgery
Introduction
Dengue virus is a single stranded RNA flavivirus belonging to the family of Arboviruses. The virus is transmitted to humans by the vector mosquitos of the Aedes spp. This disease has been recognized to be one among the fastest spreading arboviral disease by the WHO which estimates the number of cases being in the range of 50–100 million/year.[1] The incidence of dengue has increased 30 fold in the past 50 years. This virus has four known serotypes (1–4). Dengue outbreaks are very common in the setting of monsoon in India. In the current year (2017), India is witnessing one of the major outbreaks of this disease with the state of Kerala being hit the worst. Within Kerala, the district of Thiruvananthapuram had the highest toll claiming more than 100 lives. Dengue can have varying manifestations from simple flu-like symptoms to more severe forms such as dengue hemorrhagic fever and dengue shock syndrome. We present our experience which is unique series of four patients which include two postoperative neurosurgery patients contracting dengue hemorrhagic fever. The remaining two cases were direct sequelae to dengue coagulopathy which included a case of cerebellar infarct who underwent external ventricular drain (EVD) for obstructive hydrocephalus and a case of spontaneous intracranial hemorrhage with concomitant spinal cord hematoma.
Case Reports
Case 1
A 24-year-old female underwent transcranial surgery for pituitary macroadenoma. She underwent the right frontotemporal craniotomy. The lesion was approached through right subfrontal and pterional transsylvian corridor. Postoperatively, she was recuperating well in the neurosurgical intensive unit. She was fully conscious and oriented with a Glasgow Coma Scale (GCS) of 15/15. Computed tomography (CT) scan done at this juncture was uneventful with only postoperative changes [Figure 1]. She had mild fever which manifested on the 4th postoperative day which lasted for few days and subsided. All routine investigations were done which were equivocal and cultures were negative. After 2 days, we noticed a swelling over the craniotomy site which gradually increases by the 10th day to involve upper part of the face. A CT scan was repeated which showed subgaleal and extradural hematoma (EDH) beneath the craniotomy site with mild midline shift of 3 mm [Figure 2]. In view of the recent epidemic of Dengue in our region, we investigated her for NS1 antigen and Dengue IgM antibodies both of which turned out to be positive. Her platelet counts during this time were alarmingly low, 19,000/mm3. Further investigation revealed her activated partial thromboplastin time (APTT) was 59 s and international normalized ratio (INR) 1.3. Her blood group was “O” negative, and accordingly, she received multiple platelet concentrate transfusions without a notable rise in her platelet counts. By the 11th day her GCS deteriorated to 10/15 and there were multiple petechial hemorrhages all over the body. During this time, it was noticed that her left arm was swollen due to underlying hematoma at the venipuncture site. The radial pulse in her left arm disappeared due to ongoing compartment syndrome. She also started to develop anisocoria consequent to uncal herniation. Platelet count during this time was 40,000/mm3. In this life-threatening situation, it was decided to go ahead with emergency life and limb-saving surgery. She underwent craniotomy and evacuation of the EDH and fasciotomy of the left upper limb. Multiple transfusions of fresh blood, plasma, and platelets were given during the surgery as there was profuse bleeding from both sites. Hemostasis at the craniotomy site was achieved by the use of Floseal, a hemostatic matrix (Baxter Healthcare Inc.). After fasciotomy, the radial pulse in her hand reappeared and the wound was packed loosely with mop and dressing after achieving hemostasis; however, there was mild diffuse oozing from the dressing site.
Figure 1.
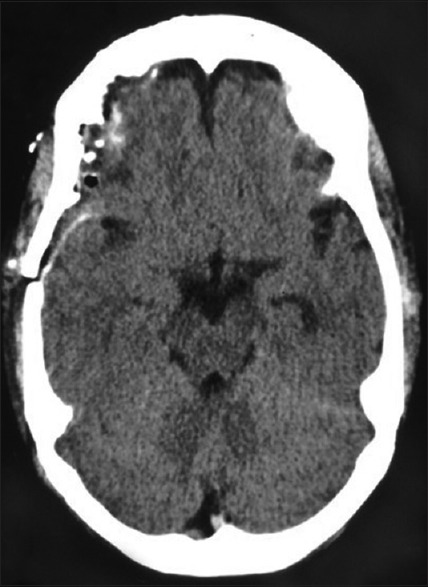
Computed tomography brain plain – postoperative picture of pituitary macroadenoma. Mild hemorrhages are noted below the craniotomy site
Figure 2.
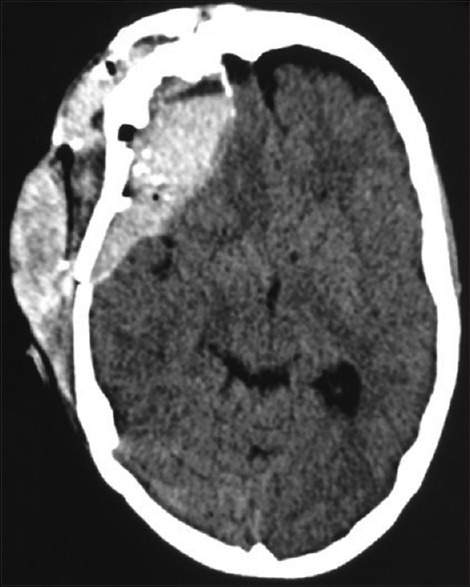
Computed tomography brain plain – shows hematoma in the subcutaneous plain as well as in the left frontal extradural region causing mass effect and midline shift
After surgery, she regained consciousness to a GCS of 13/15 and her platelet count showed mild increase to 50,000/mm3. A repeat CT scan was taken which showed recollection of EDH [Figure 3]. Since she was conscious and alert we decided to wait and watch. But again, after 2 days she deteriorated in her consciousness and a repeat CT revealed the size of the EDH had further increased with mass effect on the brain [Figure 4]. She underwent emergency surgery during which there was diffuse ooze from the underlying dura. A meticulous hemostasis was achieved with bipolar cautery and surgical. She was given multiple platelet concentrate transfusions during this time. Postoperatively, she improved in her consciousness to a GCS of 12/15 and platelet counts rose to 65,000/mm3. A repeat CT scan was take after 3 days of second reexploration which showed that there was no further bleeding [Figure 5]. The fasciotomy site wound which had diffuse oozing also stopped by this time.
Figure 3.
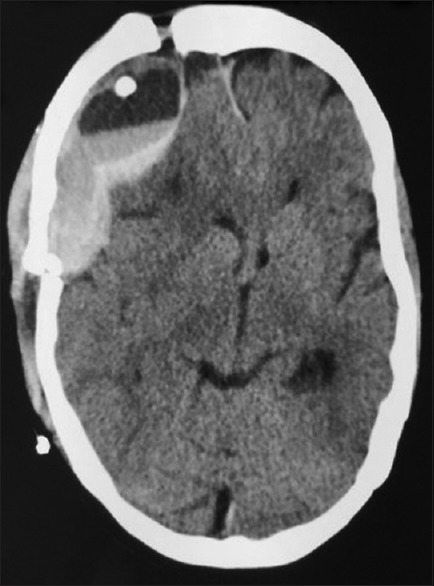
Computed tomography brain plain – after the first reexploration showing recollection of extradural hematoma in the left frontal extradural region causing mass effect and midline shift
Figure 4.
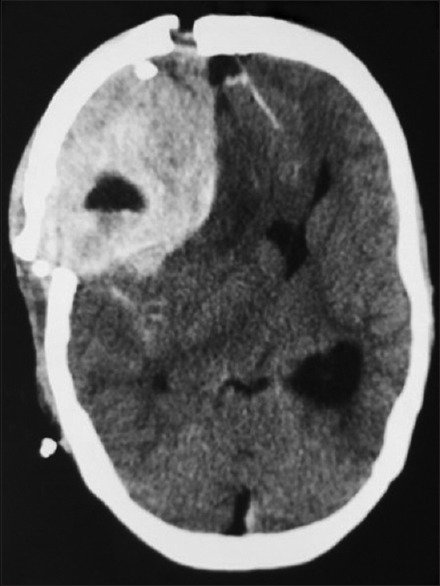
Computed tomography brain plain – showing further increase in the size of extradural hematoma in the left frontal extradural region causing severe mass effect and midline shift
Figure 5.
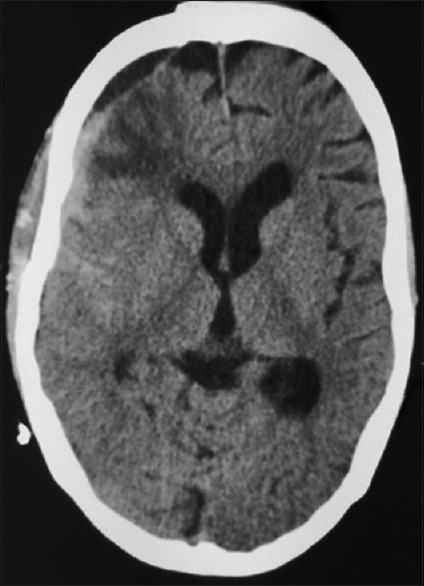
Computed tomography brain plain after second reexploration showing significant decrease in the collection at the extradural site. The mass effect and midline shift has been resolved
After the second resurgery, she developed chest infection and went into adult respiratory distress syndrome (ARDS). She was transferred to the critical care unit and was weaned off from the ventilator gradually. She recovered well from the ARDS and was discharged after 4 weeks with a Glasgow outcome scale of 5/5.
Case 2
A 1-year old female child was referred to us with fever of 1-week duration and altered sensorium. She was initially assessed by the emergency paediatrician. She was drowsy with a GCS of 9/15. A CT scan was advised which revealed bilateral cerebellar infarcts and patchy hypodensities in the left middle cerebral artery territory [Figures 6 and 7]. There was dilatation of the ventricles suggestive of obstructive hydrocephalus. She was referred to us for urgent intervention. After evaluating her clinically, placement of external ventricular drain was planned as an emergency measure to relieve the obstructive hydrocephalus. Preoperative investigations revealed she had low platelet count(30,000/mm3) and also showed positive reaction to dengue NS1 antigen. Her INR was 1.4 and APTT was 45 s. Multiple platelet transfusions were given with a modest rise in platelet counts to 50,000/mm3. An EVD was placed over the right Kocher's point to relieve the hydrocephalus after which the child became conscious to a GCS of 11/15. She started to localize for pain. After 1 week of placing the EVD, we were planning to convert it into a permanent ventriculoperitoneal shunt but her platelet counts never reached a crescendo to ensure a safe neurosurgical intervention. After 10 days when her platelets improved to 95,000/mm3, the EVD was clamped overnight. As there was no deterioration in her consciousness, the EVD was removed after draining a moderate amount of cerebrospinal fluid (CSF). Unfortunately, within 15 min of this, there was sudden worsening of her neurological status to decerebrate posturing with anisocoria. The EVD was immediately reinserted along the previous track. An urgent CT scan was taken which revealed a large intracerebral hematoma in the opposite cerebral hemisphere [Figure 8]. She went into profound hypotension and her pupils became nonreactive following this. She was started on hemodynamic supports with dual vasopressors but succumbed to a cardiac arrest on the next day.
Figure 6.
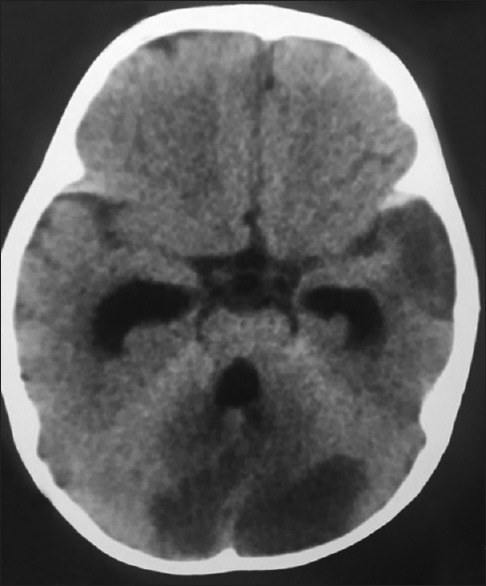
Computed tomography brain plain – showing bilateral infarcts involving the cerebellum, there is patchy infarct involving the right temporal lobe. The temporal horns of the lateral ventricle as well as the fourth ventricle are dilated
Figure 7.
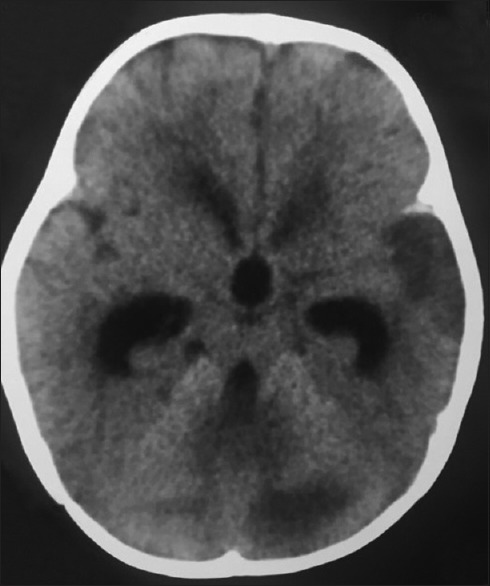
Computed tomography brain plain – showing rounding of the third ventricle and dilatation of the frontal and temporal horns of the lateral ventricle due to obstructive hydrocephalus
Figure 8.
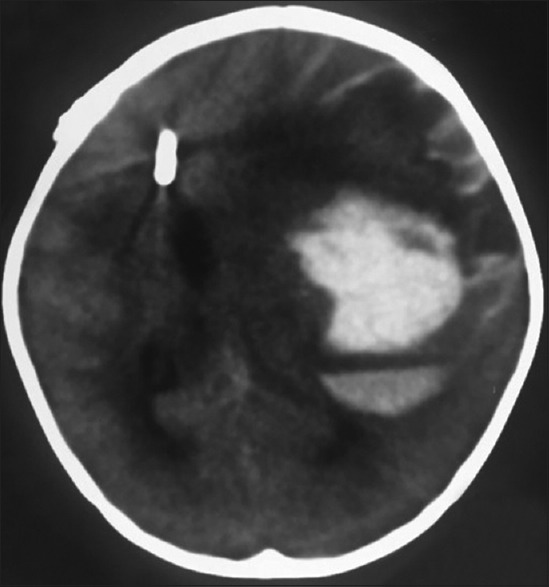
Computed tomography brain plain – showing the external ventricular drain in situ with intracerebral hematoma in the left frontoparietal region with severe mass effect and midline shift
Case 3
A 75-year-old female had a history of fall at home after which she became unconscious and was brought to our hospital emergency. After resuscitation, a CT scan was taken which showed a right temporal lobe hematoma [Figure 9]. She underwent craniotomy and evacuation of hematoma after which she regained consciousness. She had an uneventful postoperative period until the 2nd week. Postoperative CT scan showed no fresh bleeding [Figure 10]. She was set to be discharged when she developed mild fever and altered sensorium. She was transferred to Intensive care unit and evaluated. Investigations revealed she had thrombocytopenia (63,000/mm3) which prompted us to evaluate her for dengue. She was positive for NS1 antigen and IgM antibody. Her INR was 1.3 and APTT was 40 s. She was administered platelet concentrates without a notable rise in her thrombocytopenia. After 4 days, her consciousness further deteriorated and chest X-rays revealed she had severe chest infection [Figure 11a and b]. She was ventilated as she further developed respiratory failure sequelae to ARDS. Throughout her illness, her platelet counts never recovered to normal levels despite several platelet transfusions. Finally, she succumbed to her disease after a prolonged period of ventilation.
Figure 9.
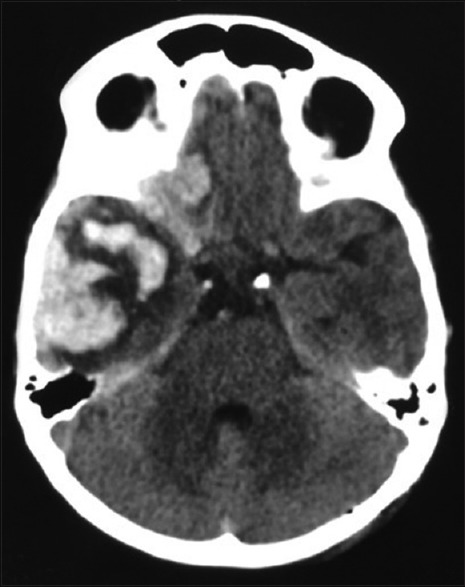
Computed tomography brain plain – preoperative image showing hematoma in the right temporal and frontal regions with mass effect on the brain
Figure 10.
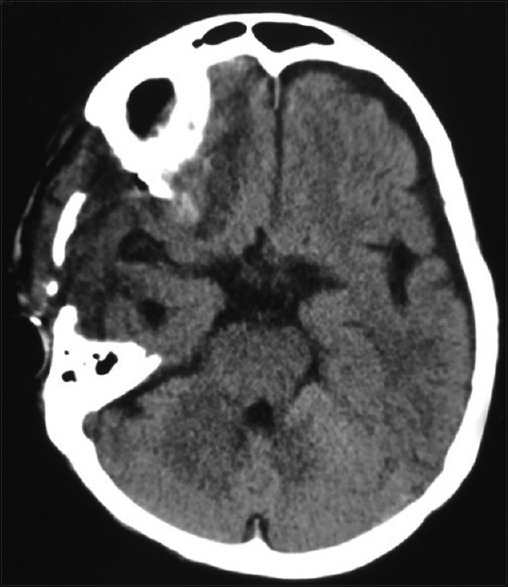
Computed tomography brain plain – postoperative image showing resolution of the hematoma in the temporal lobe along with postoperative changes
Figure 11.
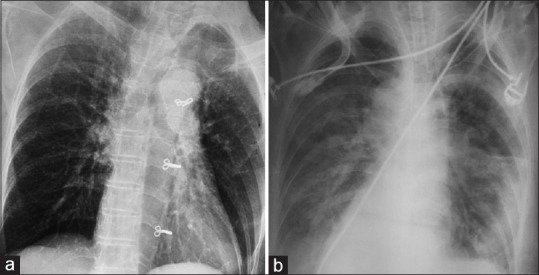
(a) Preoperative chest X-ray posteroanterior view showing clear lung fields. (b) Bilateral lung infiltrates in the setting of adult respiratory distress syndrome
Case 4
A-82-year-old otherwise healthy male had a history of fever of 1 week. He was not a diabetic or hypertensive. There was no history of trauma. After few days, he developed headache with vomiting which was associated with low backache. This was followed with gradual onset of weakness of lower limbs and urinary incontinence. The weakness worsened over the next few days to flaccid paralysis in both lower limbs. He was taken to a local hospital from where he was referred to us. On examination, he was conscious but not oriented. There were patchy ecchymotic patches on his sacral region. GCS was E4V4M5 along with Grade 0 power of lower limbs. There was no perception of any sensations below the trunk. There was normal power in the upper limbs. Investigations revealed he had severe thrombocytopenia (platelet count 20,000/mm3) which prompted further evaluation for dengue in view of ongoing epidemic. He was positive for dengue IgM antibodies. His INR was 1.3 and APTT was 45 s. Emergency CT scan of the brain along with magnetic resonance imaging (MRI) of the spine was advised. CT scan revealed diffuse patchy subarachnoid hemorrhages involving both cerebral hemispheres along with intraventricular bleed [Figure 12a and b]. MRI spine showed he had subdural and intramedullary hematoma involving the thoracolumbar region. The spinal cord was distended from T11 to L1 regions due to underlying hematoma [Figures 13 and 14]. It was decided to perform emergency decompression of the spinal cord, but his platelet counts were too low to perform a safe surgery. He was given multiple platelet transfusions after which thrombocytopenia slowly recovered partially to more than one lac over a period of 4 days after admission. He was taken up for surgery during which, a laminectomy of the T11, T12, and L1 vertebrae was performed. The cord was seen swollen with underlying bluish blush of the hematoma [Figure 15]. The dura was opened to visualize the underlying subdural clot which was removed slowly with biopsy forceps [Figure 16]. The hematoma was seen to enter the substance of the spinal cord and was splaying the nerve roots. Thorough irrigation with saline was given and the hematoma was evacuated in piecemeal. At the end of the surgery, the cord was seen pulsating after which a meticulous dural closure was achieved. He regained consciousness after surgery but the power in his lower limbs did not improve.
Figure 12.
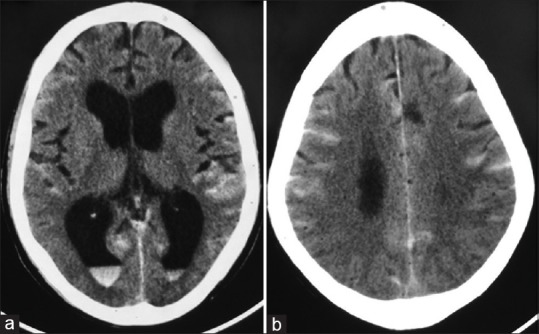
(a and b) Computed tomography brain plain – showing diffuse Subarachnoid hemorrhage along with intraventricular bleed
Figure 13.
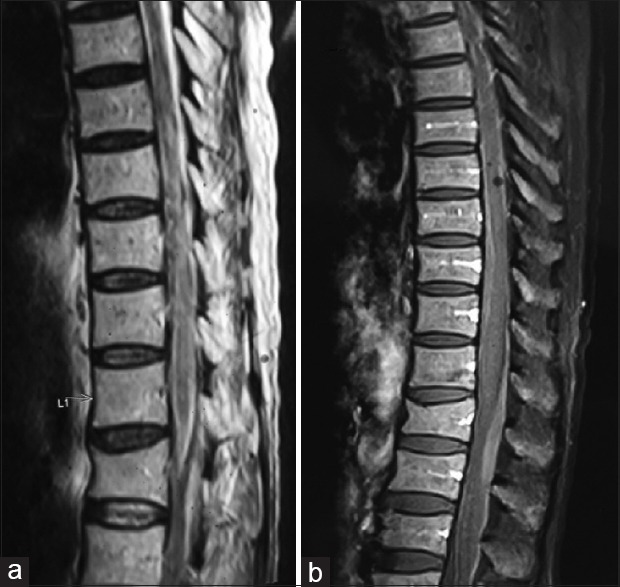
(a) Magnetic resonance imaging T1-weighted sagittal image showing diffuse hypointense signals with in the parenchyma of the spinal cord of T11–L1. The cord is also seen expanded in this region. (b) Contrast image showing no contrast enhancement of the lesion
Figure 14.
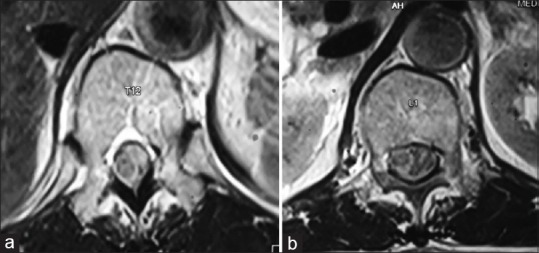
(a) Magnetic resonance imaging T1-weighted axial image at the level of T12 vertebra showing isointense lesion in the spinal canal arising from the left side pushing the cord toward the right. (b) T1-weighted axial image at the level of L1 vertebra showing the isointense lesion arising from the left side of the spinal cord with mass effect
Figure 15.
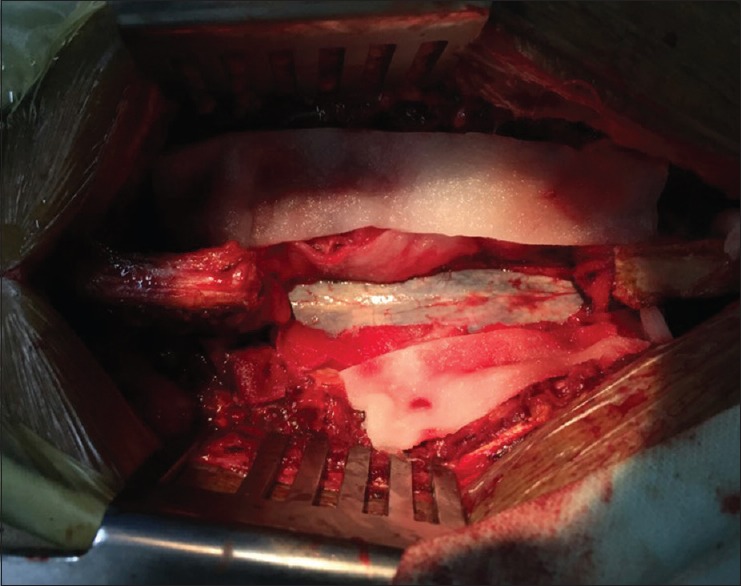
Intraoperative image-showing T12 and L1 laminectomy. The spinal cord is seen expanded at the region of the T12 with underlying bluish blush of the hematoma
Figure 16.
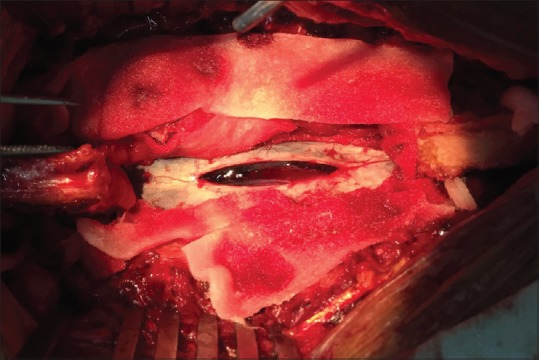
Intraoperative image – showing T12 and L1 laminectomy. The dura is opened, and the bulging hematoma is seen within the rent in the arachnoid
Discussion
Manifestations of dengue can be variable in individuals. Neurological presentations can vary from more common encephalitis or meningitis to uncommon life-threatening stroke syndromes. The complications of dengue have been well documented in patients who have undergone gastro,[2,3] urological,[4] cardiac,[5] and gynecological surgery.[6] In this unique short series, we present the cases of dengue infection in neurosurgery patients in the setting of an ongoing epidemic. Spontaneous intracranial bleeds are among the most common neurosurgical emergencies related to dengue reported in the literature.[7,8,9,10,11,12] The deleterious effects it has on the brain is without doubt. The management of such complications is a conundrum, as surgery in dengue coagulopathy is detrimental. A surgeon has to weigh risk and outcome of surgery on each patient and choose what is best for them.
Our first patient had compartment syndrome of the left upper limb in addition to intracranial bleed. Compartment syndromes in dengue are rare phenomenon, and such instances have been reported earlier involving both upper limb[13,14] as well as lower limb.[15] Altered coagulation, which leads to the formation of expanding hematoma secondary to venepuncture has been cited the most common etiology. Similarly, the cause of the hematoma in our patient was due to the cubital venepuncture. This could have been avoided, if only the disease had been detected earlier. The recurrent intracranial bleeding requiring two surgeries in this patient was due to precariously low platelet counts precluding hemostasis. However, after balancing options of waiting for the resolution of coagulopathy against the threat of impending gangrene in her limb and death due to uncal herniation, the decision was taken for emergency surgery. The good outcome in this patient justifies the risk we took in performing concomitant emergency fasciotomy along with EDH evacuation.
Stroke in dengue is uncommon. Both ischemic and hemorrhagic strokes have been reported in dengue.[16,17,18,19,20] Although cerebellar hemorrhages have been described earlier,[18,19] ischemia of the cerebellum has never been reported. Our patient is the first case of dengue-related bilateral infarct of the cerebellum and the youngest patient reported so far in the literature. The cause of brain infarcts in dengue is secondary to dengue vasculitis,[17] dengue-related coagulopathy, and vascular thrombosis.[21] Our second case, had obstructive hydrocephalus due to edema and closure of the fourth ventricular outlet. She might have developed intracranial bleed in the opposite hemisphere after the insertion the EVD. However, it did not result neurological deterioration because the intracranial pressure was well balanced as a result of CSF drainage by EVD. The removal of this might have altered this balance and caused a sudden rise in intracranial pressure leading to decompensated coning in her. We had not anticipated another intracranial adverse event as the child was clinically improving; hence, post-EVD CT scan was not done. However, now we feel, it is imperative to perform CT scans after even minor intracranial procedure in patients with dengue coagulopathy.
Our third patient had recovered well after the craniotomy and evacuation of hematoma and was fit to be discharged when she contracted dengue. We were anticipating an intracranial bleed in her as her condition worsened un presence of thrombocytopenia; however, CT scan did not show any hematoma. Finally, she succumbed to her disease following ARDS. The respiratory complications of dengue are grave and are well documented in the literature.[22,23,24,25,26,27] The onset of ARDS is directly associated with the severity of dengue. The presence of dengue virus in the lungs lead to capillary leak and resultant hemoconcentration and hypoalbuminemia can lead to pleural effusion.[26] The capillary leak will finally lead to severe hypoxemic respiratory failure secondary to pulmonary edema.[27] The occurrence of nosocomial infection is well documented in literature. Inoculation of the dengue virus in health-care workers by needle stick injuries leading to the disease have been reported earlier.[28,29] However, in our patient, there was no such history of needlestick injury. She might have contracted the disease by aerosol transmission which has been documented earlier.[30] Health-care nurses as well as patient care providers should be on high vigil during epidemics of dengue to prevent nosocomial infection. Mortality is more likely in females with advanced age.[31]
The last patient in our series had paraplegia secondary to spinal bleed in the convalescence period of dengue. There are few case reports available in the literature describing spontaneous epidural hematoma developing secondary to dengue coagulopathy;[12,32,33] however, the incidence of spontaneous intraspinal hematoma has been reported only once.[34] Our patient had concurrent intraspinal and intracranial bleed secondary to dengue coagulopathy which has never been reported before. The delay in diagnosis, late referral, advanced age, and time taken to achieve an optimum platelet count to conclude a safe surgery lead to the poor outcome in our patient. We did anticipate poor recovery following surgery but some form of late recovery has been reported following traumatic spinal cord injury.[35] Ours was a case of spontaneous spinal cord bleed without traumatic disruptive forces involved in trauma which would shear the neural structures; hence, we wanted to facilitate a milieu in which recovery would be accentuated if any.
These four cases of dengue-related neurosurgical emergencies highlight the importance of screening every suspicious case in a setting of ongoing epidemic. All our patients except one required emergency surgery which could not be done in the presence of coagulopathy. The reason for coagulopathy in dengue is multifactorial. The thrombocytopenia and dysfunctional platelets are secondary to decreased production from the bone marrow and increased destruction during the interplay between the platelets and the endothelial cells infected with the virus.[36] The other mechanism which play a significant role is the depletion of the coagulation factors which result in abnormalities of the coagulation cascade.[37] Platelets counts of at least 100,000/mm3 are required for safe neurosurgery as per literature.[38] However, our patients who required intervention were deteriorating rapidly and we could not wait for the platelets to get normalized. There have been small case series with good outcome in patients with low platelet count undergoing neurosurgery; however, it has been limited to minor burr hole evacuation.[39] Along similar thought process, we postulate that it is better to be on the safer side in such situations and proceed for surgery under cover of platelet transfusions and deal with the complications later.
Conclusion
The outcome of dengue-related neurosurgical emergencies depends on many factors. Early diagnosis is the key to good outcome before surgery. There should be a high index of suspicion and screening for dengue in a setting of ongoing epidemic. In the presence of coagulopathy, the choice of surgery against conservative treatment has to be decided on individual basis in each patient.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Cucunawangsih, Lugito NPH. Trends of dengue disease epidemiology. Virology (Auckl) 2017;8:1178122X17695836. doi: 10.1177/1178122X17695836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dan D, King K, Seetahal S, Naraynsingh V, Hariharan S. Portal vein thrombosis following laparoscopic cholecystectomy complicated by dengue viral infection: A case report. J Med Case Rep. 2011;5:126. doi: 10.1186/1752-1947-5-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh P, Kumar S, Chandra A. Dengue fever outbreak in surgical patients: Diagnostic challenges and outcome impact, 2014. Open Access J Surg. 2017;3:2–4. [Google Scholar]
- 4.Kumar S, Pushkarna A, Ganesamoni R, Nanjappa B. Dengue hemorrhagic fever as a rare cause of bleeding following percutaneous nephrolithotomy. Urol Res. 2012;40:177–9. doi: 10.1007/s00240-011-0394-6. [DOI] [PubMed] [Google Scholar]
- 5.Rawat SK, Mehta Y, Juneja R, Trehan N. Dengue fever in a patient recovering from coronary artery bypass grafting. Ann Card Anaesth. 2011;14:155–6. doi: 10.4103/0971-9784.81575. [DOI] [PubMed] [Google Scholar]
- 6.Tan FL, Loh DL, Prabhakaran K. Case report dengue haemorrhagic fever after living donor renal transplantation. Nephrol Dial Transplant. 2005;20:447–8. doi: 10.1093/ndt/gfh601. [DOI] [PubMed] [Google Scholar]
- 7.Kumar R, Prakash O, Sharma B. Dengue hemorrhagic fever: Acute subdural hematoma. Pediatr Neurosurg. 2008;44:490–2. doi: 10.1159/000180305. [DOI] [PubMed] [Google Scholar]
- 8.Bhargava A, Banakar BF, Pujar GS, Shubhkaran K, Jangid H. Dengue hemorrhagic fever: A rare cause of pituitary apoplexy. Neurol India. 2014;62:92–3. doi: 10.4103/0028-3886.128350. [DOI] [PubMed] [Google Scholar]
- 9.Sam JE, Gee TS, Wahab NA. Fatal intracranial hemorrhage in a patient with severe dengue fever. AJNS. 2018;13:56–8. doi: 10.4103/1793-5482.185056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sam JE, Gee TS, Nasser AW. Deadly intracranial bleed in patients with dengue fever: A series of nine patients and review of literature. J Neurosci Rural Pract. 2017;7:1–6. doi: 10.4103/0976-3147.182777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sunder S, Prasad R. Case report acute subdural hematoma in a patient with dengue – Case report and review of literature. Apollo Med. 2008;5:125–9. [Google Scholar]
- 12.Gautam S, Meena RK, Meena SC, Gautam B. Retrospective analysis of prognostic factors in dengue infected patients with intracranial bleed. Surg Neurol Int. 2016;7:S935–9. doi: 10.4103/2152-7806.195229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bandyopadhyay D, Mondal P, Samui S, Bishnu S, Manna S. Acute compartment syndrome of upper limb as an unusual complication of dengue hemorrhagic fever. N Am J Med Sci. 2012;4:667–8. doi: 10.4103/1947-2714.104326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khoo C, Chu G, Rosaida MS, Chidambaram SK. Dengue fever with compartment syndrome of the right arm. J R Coll Physicians Edinb. 2016;46:241–3. doi: 10.4997/JRCPE.2016.406. [DOI] [PubMed] [Google Scholar]
- 15.Remalayam B, Viswanathan S, Chitrapadi S, Karanth N, Iqbal N, Muthu V. Double trouble in dengue. Asian Pac J Trop Dis. 2012;1808:10–2. [Google Scholar]
- 16.Liou LM, Lan SH, Lai CL. Dengue fever with ischemic stroke: A case report. Neurologist. 2008;14:40–2. doi: 10.1097/NRL.0b013e3180d0a391. [DOI] [PubMed] [Google Scholar]
- 17.Nanda SK, Jayalakshmi S, Mohandas S. Pediatric ischemic stroke due to dengue vasculitis. Pediatr Neurol. 2014;51:570–2. doi: 10.1016/j.pediatrneurol.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 18.Vargas-Sánchez A, Chiquete E, Gutiérrez-Plascencia P, Castañeda-Moreno V, Alfaro-Castellanos D, Paredes-Casillas P, et al. Cerebellar hemorrhage in a patient during the convalescent phase of dengue fever. J Stroke. 2014;16:202–4. doi: 10.5853/jos.2014.16.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathew S, Pandian JD. Stroke in patients with dengue. J Stroke Cerebrovasc Dis. 2010;19:253–6. doi: 10.1016/j.jstrokecerebrovasdis.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Verma R, Sahu R, Singh AS, Atam V. Dengue infection presenting as ischemic stroke: An uncommon neurological manifestation. Neurol India. 2013;61:317–8. doi: 10.4103/0028-3886.115083. [DOI] [PubMed] [Google Scholar]
- 21.Murthy JM. Neurological complication of dengue infection. Neurol India. 2010;58:581–4. doi: 10.4103/0028-3886.68654. [DOI] [PubMed] [Google Scholar]
- 22.Kumar N, Gadpayle AK, Trisal D. Atypical respiratory complications of dengue fever. Asian Pac J Trop Med. 2013;6:839–40. doi: 10.1016/S1995-7645(13)60149-2. [DOI] [PubMed] [Google Scholar]
- 23.Mahajan SN, Rathi NP, Acharya S. ARDS in dengue infection – A case series. Indian J Med Healthcare. 2013;2:235–37. [Google Scholar]
- 24.Sam SS, Omar SF, Teoh BT, Abd-Jamil J, AbuBakar S. Review of dengue hemorrhagic fever fatal cases seen among adults: A retrospective study. PLoS Negl Trop Dis. 2013;7:e2194. doi: 10.1371/journal.pntd.0002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belagavi AC, Sunil H, Sudhir U, Kempegowda P. Adult respiratory distress syndrome in dengue – A case report. Al Ameen J Med Sci. 2011;4:405–7. [Google Scholar]
- 26.Mohamed NA, El-Raoof EA, Ibraheem HA. Respiratory manifestations of dengue fever in Taiz-Yemen. Egypt J Chest Dis Tuberc. 2013;62:319–23. [Google Scholar]
- 27.Devarajan TV, Prashant PS, Mani AK, Victor SM, Shabeena Khan P. Dengue with ARDS. J Indian Acad Clin Med. 2008;9:146–9. [Google Scholar]
- 28.Wagner D, de With K, Huzly D, Hufert F, Weidmann M, Breisinger S, et al. Nosocomial acquisition of dengue. Emerg Infect Dis. 2004;10:1872–3. doi: 10.3201/eid1010.031037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohnishi K. Needle-stick dengue virus infection in a health-care worker at a Japanese hospital. J Occup Health. 2015;57:482–3. doi: 10.1539/joh.14-0224-CS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta V, Bhoi S, Goel A, Admane S. Nosocomial dengue in health-care workers. Lancet. 2008;371:299. doi: 10.1016/S0140-6736(08)60158-0. [DOI] [PubMed] [Google Scholar]
- 31.Wang CC, Liu SF, Liao SC, Lee IK, Liu JW, Lin AS, et al. Acute respiratory failure in adult patients with dengue virus infection. Am J Trop Med Hyg. 2007;77:151–8. [PubMed] [Google Scholar]
- 32.Fong CY, Hlaing CS, Tay CG, Kadir KA, Goh KJ, Ong LC, et al. Longitudinal extensive transverse myelitis with cervical epidural haematoma following dengue virus infection. Eur J Paediatr Neurol. 2016;20:449–53. doi: 10.1016/j.ejpn.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Verma SP, Himanshu D, Tripathi AK, Vaish AK, Jain N. An atypical case of dengue haemorrhagic fever presenting as quadriparesis due to compressive myelopathy. BMJ Case Rep 2011. 2011:pii: bcr1020103421. doi: 10.1136/bcr.10.2010.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaur J, Virk JS, Paul BS, Saggar K. Dengue fever presenting as cauda equina syndrome. BMJ Case Rep 2017. 2017:pii: bcr-2017-221251. doi: 10.1136/bcr-2017-221251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waters RL, Adkins RH, Yakura JS, Sie I. Motor and sensory recovery following complete tetraplegia. Arch Phys Med Rehabil. 1993;74:242–7. [PubMed] [Google Scholar]
- 36.Funahara Y, Ogawa K, Fujita N, Okuno Y. Three possible triggers to induce thrombocytopenia in dengue virus infection. Southeast Asian J Trop Med Public Health. 1987;18:351–5. [PubMed] [Google Scholar]
- 37.Chuansumrit A, Chaiyaratana W. Hemostatic derangement in dengue hemorrhagic fever. Thromb Res. 2014;133:10–6. doi: 10.1016/j.thromres.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 38.Ali N, Usman M, Syed N, Khurshid M. Haemorrhagic manifestations and utility of haematological parameters in dengue fever: A tertiary care centre experience at Karachi. Scand J Infect Dis. 2007;39:1025–8. doi: 10.1080/00365540701411492. [DOI] [PubMed] [Google Scholar]
- 39.Abdelfatah M. Management of chronic subdural hematoma in patients with intractable thrombocytopenia. Turk Neurosurg. 2016 doi: 10.5137/1019-5149.JTN.18825-16.1. doi: 10.5137/10195149JTN18825-16.1 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


