Abstract
BACKGROUND/OBJECTIVES:
Although the proportion of individuals ≥80 years (oldest old) in low-to-middle income countries is increasing rapidly, little is known about cardiovascular diseases (CVD) in this population, particularly in sub-Saharan Africa (SSA). The aim of this analysis is to characterize the CVD profile of these individuals in rural South Africa.
DESIGN:
First wave of a population-based longitudinal cohort.
SETTING:
Agincourt sub-district (Mpumalanga Province) in rural South Africa.
PARTICIPANTS:
Adult residents (sample size of 5,059 individuals).
MEASUREMENTS:
In-person interviews were conducted to obtain social, behavioral, economic and clinical data. Prevalence of hypertension (HTN), diabetes, dyslipidemia, increased waist-to-hip ratio, overweight/obesity, high risk hs-CRP, smoking, stroke, myocardial infarction, angina, and heart failure were compared among <65, 65–79, and ≥80 years participants. Associations between self-reported treatments and determinants of hypertension treatment in the oldest old were assessed using multivariable regression.
RESULTS:
Among 5,059 individuals included, 549 (10.8%) were oldest old and their CVD prevalence was 17.9% (stroke 3.8%, myocardial infarction 0.5%, angina 13.5% and heart failure 0.7%). HTN prevalence in this group was 73.8% and along with angina it increased across age groups (p<0.001), while overweight/obesity, dyslipidemia, and smoking prevalences (46.4%, 39.1%, and 3.1% respectively) decreased (p<0.001). HTN treatment was significantly associated with being 80 or over (OR 1.48; 95% CI: 1.14–1.92; p=0.003). Male sex (OR 0.73; 95%CI 0.66–0.88; p=0.001), being an immigrant (OR 0.80; 95%CI 0.65–0.98; p=0.033), higher social economic status (OR 1.28; 95%CI 1.06–1.53; p=0.009), and higher depression score (OR 1.12; 95%CI 1.05–1.19; p<0.001) were associated with HTN treatment among the oldest old.
CONCLUSION:
This is the first study to characterize the CVD profile of individuals aged ≥80 years in SSA and provides baseline data for comparison with future studies in this rapidly growing age group.
Keywords: sub-Saharan Africa, cardiovascular disease, hypertension, cohort studies
INTRODUCTION
The world’s population is ageing rapidly and the number of adults aged 65 and older is starting to outnumber young adults for the first time1. Although the focus for much research into health service delivery for ageing populations has been in high-income countries (HIC), attention is broadening to include lower and middle-income countries (LMIC). Middle-income country populations, in particular, are generally ageing at a much faster rate compared to high-income countries2. Subsequently, chronic diseases, including cardiovascular diseases (CVD), represent a major burden of morbidity and mortality in low-resource settings, including in sub-Saharan Africa (SSA).3–5
The fastest-growing segment of the total population is the oldest old – those aged 80 and over. Their global growth rate is twice that of those aged 65 and over and almost four times that for the total population. Worldwide, the oldest old population is projected to more than triple between 2015 and 2050, from 126.5 million to 446.6 million6. This increase will present a major challenge for health and social care systems if patterns in HIC are replicated in LMIC, with this age group having the highest burden of multi-morbidity, the greatest prevalence of frailty syndromes, and subsequently the greatest need for health and social care7.
Understanding patterns of disease in the oldest old segment of the population will help healthcare systems to predict future non-communicable disease (NCD) needs, in part by showing how disease burden might evolve as populations age. It will also assist in determining health and social service needs, along with effective services delivery. Yet, little consideration has been given to issues of old age and cardiovascular disease in South Africa, particularly for the oldest old, as reflected by the lack of literature on CVD and its risk factors in this growing segment of the population in SSA.
Driven by this lack of information, we assessed the oldest old participants from the HAALSI (Health and aging in Africa: Longitudinal Studies of INDEPTH communities) Study aiming to: compare cardiovascular risk factors and CVD prevalence’s between participants aged <65, 65–79, and ≥80 years (oldest old); identify associations between self-reported treatments and being oldest old; and explore predictors of CVD management in this age group.
METHODS
The HAALSI cohort is based in the Agincourt Health and socio-Demographic Surveillance System (HDSS) site, a sub-district of rural Mpumalanga Province comprising approximately 116,000 people living in 21,000 households and 31 villages in an area of ~450km2. The Agincourt HDSS covers a border region of rural South Africa adjacent to southern Mozambique8. A total of 5,890 persons were identified for recruitment from the HDSS database using random sampling based on the 2013 Agincourt census data. All adults aged 40 years and older who had permanently resided in the Agincourt sub-district for at least one year prior to the 2013 census update were eligible. The baseline wave of data collection for HAALSI was conducted in 2015, in a cohort of 5,059 men and women ≥40 years of age. Detailed descriptions of the cohort and data collection procedures have been published elsewhere 9–11 and in supplementary material.
Cardiovascular risk factors definitions
Hypertension was defined as a mean systolic blood pressure >140 mmHg or mean diastolic blood pressure > 90 mmHg12 or self-reported treatment. Diabetes was defined as a self-reported history, or a fasting blood glucose level (FBG) > 7.0 mmol/L, or a random blood glucose level (RBG) > 11.1 mmol/L13 or self-reported treatment. Dyslipidemia was defined as a self-reported history, or measured total cholesterol level > 5 mmol/L, or low-density lipoprotein (LDL) > 3 mmol/L, or high-density lipoprotein (HDL) < 1.2 mmol/L, or triglyceride > 1.7 mmol/L14 or self-reported treatment. Waist-to-hip ratios (WHR) were classified as high if > 0.90 for men and > 0.85 for women15. Body Mass Index (BMI) in kg/m2 was used to categorize subjects as overweight (BMI ≥25kg/m2) using World Health Organization (WHO) cutoffs16. The hs-CRP was categorized as low (< 1 mg/L), intermediate (1–3 mg/L), or high (> 3 mg/L) risk 17, 18. Smoking was defined by self-reported current smoking status.
Cardiovascular diseases (CVD) definitions
CVD events were assessed using self-reported history of myocardial infarction (MI), stroke, heart failure (HF), and angina (defined by self-report or applying the globally validated Rose criteria questionnaire) 19. CVD was defined as any report of stroke, myocardial infarction, angina, or heart failure.
Age stratification
Individuals were stratified into three age groups: <65 years, 65–79 years and ≥80 years old (oldest old) to assess the impact of age on CVD prevalence and risk factors.
Other covariates
HIV-positive status was defined as a self-reported history of being informed of the condition by a health professional or a positive result on assay analysis. Socioeconomic status (SES) is a composite, constructed variable incorporating measures of traditional and modern wealth, using methodology created for Demographic Health Surveys (DHS) and divided into 5 quintiles. The first 3 quintiles were classified as low SES and the last 2 quintiles as high SES 20, 21. Immigrants, mostly Mozambican, were defined as participants born outside South Africa. Illiteracy was defined as self-reported inability to read or write. Depression was scored administering the Center for Epidemiological Studies Depression Scale (CESD-8) of 0–8, with higher scores indicating higher burdens of depressive symptoms22. The total number of social contacts was assessed by self-report, based on past six months’ contacts, ranging from 0–7, and reported as a continuous variable.
Analyses
All analyses were conducted using STATA ® V14 software (STATAcorp, Texas, USA). Normally distributed continuous variables were expressed in mean values (±standard deviations), while categorical variables were expressed as absolute numbers and percentages. The Wilcoxon signed-rank test was used to compare the variables across age categories and results are presented as p-value for trends. A multivariable logistic regression model was built to identify which cardiovascular disease/risk factor treatments were associated with being 80 or over. The model was adjusted for hypertension, diabetes, dyslipidemia. smoking, overweight/obesity, increased waist-hip ratio, high risk hs-CRP, angina, myocardial infarction, stroke, heart failure and HIV status. A second model was built to identify variables associated with receiving hypertension treatment among the oldest old, not focusing on age interactions since just elderly over 80 years were include in the model. Predictors used in the regression model included those previously assessed in this cohort (sex, HIV-status, SES, immigration status, illiteracy) 23, along with depressive symptoms (CESD-8) and total number of social contacts. The model was adjusted for diabetes, dyslipidemia, smoking, overweight/obesity, increased waist-hip ratio, high risk hs-CRP, angina, MI, stroke and heart failure. The results are presented using odds ratios and 95% confidence intervals. An Alpha-level = 0.05 was used to determine statistical significance in all analyses.
RESULTS
Out of the 5,890 persons identified for recruitment, those who were alive and residing in the study area, 85.9% (5,059) agreed to be interviewed, 7.3% (430) refused to participate, 6.0% (354) could not be located, and 0.8% (47) were unable to participate. The population characteristics of the oldest old compared to those aged 65–79 and those younger than 65 are shown in Table 1. The number of oldest old participants was 549 (10.8%). Mean systolic blood pressure increased, while diastolic blood pressure and body mass index decreased with increasing age category. The proportion of males and HIV positive participants among the oldest old was lower when compared to the other age groups.
Table 1.
HAALSI population characteristics by age groups, HAALSI Cohort, Agincourt sub-district, South Africa 2015.
| n | <65 years | 65 – 79 years | ≥80 years | p-value* | |
|---|---|---|---|---|---|
| n | 5,059 | 3,081 (60.9%) | 1,429 (28.3%) | 549 (10.8%) | |
| Male Sex | 5,059 | 1,422 (46.2%) | 709 (49.6%) | 214 (39.0%) | 0.157 |
| Age (years) | 5,059 | 53.09 (±7.13) | 71.27 (±4.17) | 85.48 (±4.63) | <0.001 |
| Weight (kg) | 4,794 | 73.70 (±17.95) | 70.84 (±16.52) | 63.33 (±15.93) | <0.001 |
| Height (m) | 4,694 | 1.64 (±0.09) | 1.62 (±0.09) | 1.58 (±0.09) | <0.001 |
| Body Mass Index (kg/m2) | 4,689 | 27.56 (±7.25) | 27.17 (±6.19) | 25.46 (±6.04) | <0.001 |
| Waist Circumference (cm) | 4,758 | 92.33 (±15.26) | 93.66 (±14.71) | 89.09 (±14.54) | 0.281 |
| Waist-Hip Ratio | 4,728 | 0.90 (±0.08) | 0.92 (±0.08) | 0.91 (±0.08) | <0.001 |
| Average SBP (mmHg) | 4,895 | 135.26 (±22.10) | 141.22 (±23.70) | 144.99 (±26.53) | <0.001 |
| Average DBP (mmHg) | 4,895 | 83.79 (±12.56) | 80.36 (±12.27) | 77.47 (±12.88) | <0.001 |
| Glucose (mmol/L) | 4,626 | 6.48 (±2.98) | 6.98 (±3.50) | 6.92 (±3.17) | <0.001 |
| Total Cholesterol (mmol/L) | 4,196 | 4.17 (±1.26) | 4.34 (±1.24) | 4.30 (±1.32) | <0.001 |
| HDL-cholesterol (mmol/L) | 4,234 | 1.55 (±0.56) | 1.59 (±0.51) | 1.62 (±0.53) | <0.001 |
| Triglycerides (mmol/L) | 4,223 | 1.74 (±0.93) | 1.77 (±0.98) | 1.82 (±4.00) | 0.076 |
| LDL-cholesterol (mmol/L) | 3,841 | 2.09 (±1.77) | 2.17 (±0.99) | 2.13 (±0.95) | 0.002 |
| High sensitivity CRP (mg/L) | 4,302 | 3.26 (±3.04) | 3.28 (±2.91) | 3.22 (±3.29) | 0.798 |
| HIV positive | 5,043 | 943 (30.7%) | 177 (12.4%) | 14 (2.6%) | <0.001 |
Data given as mean (±SD) or n (%)
p-value for trend across the 3 categories; α = 0.05
Cardiovascular risk factors and cardiovascular disease (CVD) prevalence by age groups are presented in Table 2 and Figure 1, respectively. Hypertension prevalence increased at older ages, while dyslipidemia, overweight/obesity and smoking decreased. Prevalence of any CVD and of angina (individually) increased with age. For stroke the highest prevalence was found in the 65–79 years age group.
Table 2:
Cardiovascular risk factors prevalence by age groups, HAALSI Cohort, Agincourt sub-district, South Africa 2015.
| Cardiovascular Risk Factors | <65 years | 65 – 79 years | ≥80 years | p-value* | |||
|---|---|---|---|---|---|---|---|
| Total | n (%) | Total | n (%) | Total | n (%) | ||
| Hypertension | 3,001 | 1,556 (51.8%) | 1,408 | 939 (66.7%) | 527 | 389 (73.8%) | <0.001 |
| Diabetes | 3,080 | 282 (9.2%) | 1,429 | 210 (14.7%) | 549 | 67 (12.2%) | <0.001 |
| Dyslipidemia | 2,559 | 1,174 (45.9%) | 1,248 | 507 (40.6%) | 440 | 172 (39.1%) | <0.001 |
| Overweight/obesity | 2,923 | 1,735 (59.4 %) | 1,318 | 769 (58.3 %) | 448 | 208 (46.4 %) | <0.001 |
| Increased Waist-hip Ratio | 2,919 | 1,912 (65.5%) | 1,343 | 964 (71.8%) | 467 | 334 (71.5%) | <0.001 |
| Smoker | 3,081 | 370 (12.0%) | 1,429 | 73 (5.1%) | 549 | 17 (3.1%) | <0.001 |
| High Risk hs-CRP | 2,607 | 987 (37.9%) | 1,247 | 521 (41.8%) | 448 | 144 (32.1 %) | 0.519 |
Hypertension = Systolic Blood Pressure ≥ 140 mmHg or Diastolic Blood Pressure ≥ 90 mmHg or Self-reported treatment.
Diabetes = Fasting Blood Glucose (FBG) ≥ 7.0 mmol/L or Random Blood Glucose (RBG) ≥ 11.1 mmol/L or Self-reported use of medication.
Dyslipidemia = Total cholesterol ≥ 5 mmol/L or low-density lipoprotein (LDL) > 3 mmol/L or high-density lipoprotein (HDL) < 1.2 mmol/L or Triglycerides > 1.7 mmol/L or Self-reported treatment.
Overweight/obesity = Body mass index ≥ 25kg/m2
Increased Waist-hip ratio = Waist-Hip > 0.90 (men) or > 0.85 (women).
hs-CRP = high sensitivity C - reactive protein > 3mg/L
p-value for trend across the 3 categories; α = 0.05
Figure 1. Cardiovascular disease prevalence by age groups in HAALSI Cohort, Agincourt sub-district, South Africa 2015.
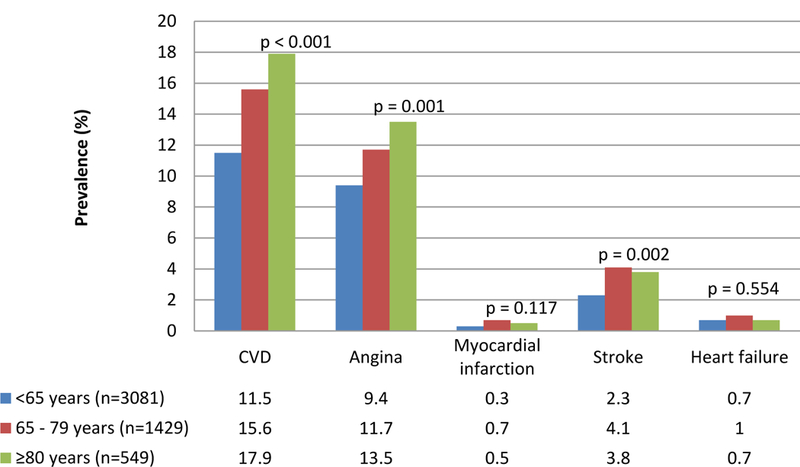
Cardiovascular disease = angina or myocardial infarction or stroke or heart failure
Angina = Self-reported or Rose Criteria
Myocardial infarction = Self-reported
Stroke = Self-reported
Heart failure = Self-reported
p-value for trend across the 3 categories; α = 0.05
The distribution of treatment for CVD and cardiovascular risk factors is shown in Table 3. The only statistically significant difference in treatment rates between the three age groups was found for hypertension (p < 0.001) and treatment rates were highest among those 65–79 years.
Table 3:
Self-reported treatment of cardiovascular diseases and risk factors by age groups, HAALSI Cohort, Agincourt sub-district, South Africa 2015
| Self-Reported treatment of Cardiovascular diseases and risk factors | <65 years | 65 – 79 years | ≥80 years | p-value* | |||
|---|---|---|---|---|---|---|---|
| Total | n (%) | Total | n (%) | Total | n (%) | ||
| Hypertension | 1,556 | 671 (43.1%) | 939 | 550 (58.6%) | 389 | 213 (54.8%) | <0.001 |
| Diabetes | 282 | 122 (43.3%) | 210 | 97 (46.2%) | 67 | 30 (44.8%) | 0.810 |
| Dyslipidemia | 1,174 | 7 (0.6%) | 507 | 5 (1.0%) | 172 | 1 (0.6%) | 0.670 |
| Angina | 289 | 12 (4.2%) | 167 | 14 (8.4%) | 74 | 6 (8.1%) | 0.140 |
| Myocardial infarction | 9 | 6 (67%) | 10 | 9 (90%) | 3 | 3 (100%) | 0.290 |
| Stroke | 70 | 21 (30%) | 58 | 24 (41%) | 21 | 8 (38%) | 0.390 |
| Heart Failure | 21 | 8 (38%) | 14 | 7 (50%) | 4 | 1 (25 %) | 0.620 |
Hypertension = Systolic Blood Pressure ≥ 140 mmHg or Diastolic Blood Pressure ≥ 90 mmHg or Self-reported treatment.
Diabetes = Fasting Blood Glucose (FBG) ≥ 7.0 mmol/L or Random Blood Glucose (RBG) > 11.1 mmol/L or Self-reported treatment.
Dyslipidemia = Total cholesterol > 5 mmol/L or low-density lipoprotein (LDL) > 3 mmol/L or high-density lipoprotein (HDL) < 1.2 mmol/L or Triglycerides > 1.7 mmol/L or Self-reported treatment.
Angina = Self-reported or Rose Criteria
Myocardial infarction = Self-reported
Stroke = Self-reported
Heart failure = Self-reported
p-value for trend across the 3 categories; α = 0.05
To identify cardiovascular disease/risk factor treatments independently associated with being 80 or over, we conducted a multivariable regression. This showed that only treatment for hypertension was independently associated with being oldest old (OR = 1.48; 95% CI 1.14 – 1.92; p = 0.003), while diabetes, dyslipidemia, angina, myocardial infarction, stroke, and heart failure treatments were not associated (Table S1 - supplementary material).
A second multivariable regression was conducted using hypertension treatment as the outcome (Table 4). Men (OR = 0.73; 95% CI 0.60 – 0.88; p = 0.001) and immigrants (OR = 0.80; 95% CI 0.65 – 0.98; p = 0.033) were found to have a statistically significant inverse association with hypertension treatment among the oldest old, while those with higher SES (OR = 1.28; 95% CI 1.06 – 1.53; p = 0.009) and higher depressive symptom score (OR = 1.12; 95% CI 1.05 – 1.19; p < 0.001) had a statistically significant positive association.
Table 4.
Variables associated with hypertension treatment in the oldest old (≥ 80 years), HAALSI Cohort, Agincourt sub-district, South Africa 2015.
| ≥ 80 years Reporting
Hypertension treatment |
||
|---|---|---|
| Variable | Odds Ratio (95% Confidence Interval) | p-value |
| Male sex | 0.73 (0.60 – 0.88) | 0.001 |
| HIV positive | 1.08 (0.86 – 1.36) | 0.498 |
| High social economic status | 1.28 (1.06 – 1.53) | 0.009 |
| Immigrant | 0.80 (0.65 – 0.98) | 0.033 |
| Illiterate | 1.19 (0.98 – 1.44) | 0.075 |
| Depression score | 1.12 (1.05 – 1.19) | <0.001 |
| Total number of social contacts | 1.00 (0.95 – 1.06) | 0.916 |
Model adjusted for diabetes, dyslipidemia, smoking, overweight/obesity, increased waist-hip ratio, high risk hs-CRP, angina, myocardial infarction, stroke and heart failure.
Discussion
There was a higher proportion of participants aged ≥ 80 years in this baseline wave of the HAALSI study compared to the African continent pattern1. These oldest old participants had a higher percentage of females and HIV-negative participants, compared to other age groups. Hypertension and angina prevalence increased with age, while dyslipidemia, overweight/obesity and tobacco smoking decreased. The only cardiovascular condition for which treatment was independently associated with being oldest old was hypertension, with males and immigrants having an inverse association with hypertension treatment in the oldest old subjects (ie being less likely to receive treatment), while higher SES and higher depression scores were positively associated with hypertension treatment.
Assessing CVD in older people from South Africa might seem to be a paradoxical undertaking since life expectancy is 57.2 years in the country 24. However, life expectancy in the region is increasing very rapidly, and this epidemiological transition will lead to an enormous growth in both the proportion of the population reaching old age and huge increases in absolute numbers of those surviving into old age. It is therefore necessary to understand the burden of disease in old age to allow health systems to plan for this transition at both the country and continental levels25. As expected, the oldest old were more likely to be female1 and to have the lowest percentage of HIV-positive participants when compared to the overall cohort26. This finding can be explained not only by lower infection rates among older participants (who would have been 60 years or older in the mid-1990s when HIV transmission increased rapidly in the heterosexual population), but also by a reduced life expectancy of the HIV positive participants27, with a low probability of living to 80. The survivors therefore represent a cohort with relatively less HIV infection.
The blood pressure patterns found in this study are also consistent with data on older people from observational28 and intervention studies29. This age group usually displays higher levels of systolic blood pressure and lower levels of diastolic blood pressure, attributable to arterial stiffening and increased pulse wave velocity. Higher hypertension prevalence in high income countries, particularly isolated systolic hypertension 30, is well described in the oldest old. The 73.8% prevalence of hypertension in the oldest old in our cohort is similar to results from high income countries 31, 32 and shows a similar increase with age consistent with the pathophysiology of hypertension described above 31. We also found that the percentage of participants receiving hypertension treatment was higher among the oldest old when compared to the rest of the population. This result is consistent with a previous analysis of this population that focused on hypertension management and showed a positive association between being older and receiving hypertension treatment 23. Hypertension has been well established to be strongly associated with a decrease in life expectancy 33 and treatment of carefully selected people aged 80 and over can reduce cardiovascular events and even all-cause mortality29.
CVD defined here as any report of angina, myocardial infarction (MI), stroke, or heart failure (HF) was more common in the oldest old. This result was mainly attributed to the high percentage of angina in this age group when compared to the overall population, rather than a consistently high percentage of all types of CVD. This may be due to information bias resulting from self-report. It has been shown that assessing CVD in the oldest old by self-report underestimates the true prevalence of conditions34; in high-income countries, the prevalence of chronic HF is 5 to 10 times higher than we report here and previous MI is similarly more common in high income countries35, 36. The only exception is angina, a condition that has previously shown reliable agreement between self-report by the oldest old and physician report of CVD 34. It is important to note however that angina was defined by both self-report and Rose criteria, and may be overestimated in this population. This provides another possible explanation for the marked difference between the prevalence of angina and that of MI.
Since hypertension is the key risk factor for stroke, surprisingly few strokes were reported in our study. The higher prevalence in the 65–79 years age group may indicate that stroke is underdiagnosed as the population gets older or, alternately, while stroke incidence is high, survival after stroke is very short, resulting in a low prevalence37.
Our findings suggest a healthy survivor effect for some risk factors, represented here by the lower prevalence of such risk factors in the oldest old members of the cohort. Smoking and dyslipidemia for instance, were less prevalent with increasing age. For diabetes, prevalence peaked in the 65–79 years age group but was lower among the oldest old. These results reinforce the need to examine measured, rather than extrapolated prevalence in the oldest old. Further work is required to tease out whether these findings are due to truly lower incidence, or to differences in the impact of risk factors on the oldest old.
After multivariable adjustment for multiple risk factors, being male and an immigrant were previously reported as being associated with worse hypertension management in this population23, and this is consistent with our findings in this analysis. Higher SES was found to be directly associated to hypertension treatment in this age group, and this result is also consistent with previous findings for this cohort 23. Depression score and its association with hypertension have not been previously assessed in this cohort, and we are unaware of any studies that have examined this association in very old people. Prior reports have demonstrated that depressive symptoms are associated with inadequate blood pressure control and complications of hypertension 38, 39 across all ages. Since our results showed that higher depression scores are positively associated with hypertension treatment in this age group, further studies are needed to determine how age is related to higher level of depressive symptoms and high blood pressure management.
Despite not being able to assess causality with this baseline data, the innovative aspect of characterizing cardiovascular risk factors/diseases in oldest old persons living in a region where an epidemiologic transition is occurring 40 is an important contribution. Our results will serve as baseline for future population-based studies in this age group, and for a better understanding of aging in all SSA. The age profile, poverty, poor infrastructure and patchy healthcare in the Agincourt area make the results generalizable to many other low-resource areas of SSA.
The self-reported nature of disease diagnosis is a potential limitation of our study. This approach can result in incorrect estimations of prevalence, especially when dealing with chronic asymptomatic conditions such as hypertension and dyslipidemia. The use of biomarkers and echocardiograms (piloted during the baseline phase) will provide more accurate estimations.
Another potential limitation is the lack of information about past and current treatment regimens for participants. Such information, which will be collected in the follow-up phase through already established linkage to clinic registers, will provide more accurate data to inform CVD management in this age group.
The assessment of CVD/risk factors prevalence in this age group provides previously undocumented baseline data for South Africa and also provides data to guide health care systems and policy development for effective clinical management in this rapidly growing population living far removed from more resourced public health sector infrastructure. This is the first study to characterize the cardiovascular risk factors and CVD profile of oldest old citizens in SSA. Our results provide baseline data for comparison with both planned follow up of this cohort and future studies in this rapidly growing age group from the region.
Supplementary Material
IMPACT STATEMENT.
We certify that this work is novel.
This is the first study to characterize the cardiovascular risk factors and cardiovascular diseases profile of oldest old (≥80 years) citizens in Sub-Saharan Africa. Our results provide baseline data for comparison with both planned follow up of this cohort and future studies in this rapidly growing age group from the region.
ACKNOWLEDGEMENTS
Sincere thanks to the Agincourt Unit field staff, quality checkers and data team who toiled long and hard to ensure the quality data for this analysis. Thanks also to the Unit research management, community engagement and administration teams: your efforts are essential. Further thanks to Mark Collinson and Carren Ginsburg for advice on conceptualizing the health effects of migration. The Division of Clinical Pharmacology Laboratory at the University of Cape Town, South Africa is supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Numbers UM1 AI068634, UM1 AI068636 and UM1 AI106701, U01 AI068632, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (NIMH) [AI068632].
SPONSOR’S ROLE
The HAALSI study, funded by the National Institute on Aging (P01 AG041710), is nested within the Agincourt Health and Demographic Surveillance System site, funded by the University of the Witwatersrand and Medical Research Council, South Africa, and the Wellcome Trust, UK (058893/A/99A; 069683/Z/02/Z; 085477/Z08/Z). The HAALSI study is a collaboration between the Harvard Center for Population and Development Studies from the Harvard T.H. Chan School of Public Health, the MRC/Wits Rural Public Health and Health Transitions Research Unit from the School of Public Health at the University of the Witwatersrand in South Africa, and the INDEPTH Network in Accra, Ghana.
Sponsors were not involved in the design, methods, subject recruitment, data collections, analysis and preparation of paper.
Footnotes
CONFLICT OF INTEREST
No financial interests or connections, direct or indirect, or other situations that might raise the question of bias in the work reported of the conclusions, implications, or opinions stated--including pertinent commercial or other sources of funding for the individual author(s) or for the associated department(s) or organization(s), personal relationships, or direct academic competition need to be disclosed.
ETHICS APPROVAL
The study received ethical approvals from the Ethics Committees of three Institutions directly involved in the project: University of the Witwatersrand Human Research Ethics Committee (ref M141159), the Harvard T.H. Chan School of Public Health, Office of Human Research Administration (ref C13-1608-02) and the Mpumalanga Provincial Research and Ethics Committee (approved on 22nd October 2014). All individuals who agreed to participate signed a consent form or, if not able to sign their name, were asked to have a literate witness sign and date the informed consent on their behalf.
REFERENCES
- [1].United Nations Department of Economic and Social Affairs, Population Division, (2015). World Population Ageing 2015 (ST/ESA/SER.A/390).
- [2].Aboderin IA, Beard JR. Older people’s health in sub-Saharan Africa. Lancet (London, England) 2015;385: e9–e11. [DOI] [PubMed] [Google Scholar]
- [3].Yach D, Hawkes C, Gould CL, Hofman KJ. The global burden of chronic diseases: overcoming impediments to prevention and control. JAMA 2004;291: 2616–2622. [DOI] [PubMed] [Google Scholar]
- [4].Kabudula CW, Tollman S, Mee P, et al. Two decades of mortality change in rural northeast South Africa. Glob Health Action 2014;7: 25596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang H, Dwyer-Lindgren L, Lofgren KT, et al. Age-specific and sex-specific mortality in 187 countries, 1970–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet (London, England) 2012;380: 2071–2094. [DOI] [PubMed] [Google Scholar]
- [6].Wan He DG, Paul Kowal. An Aging World: 2015 In: Bureau USC, ed. International Population Reports: U.S. Government Publishing Office, 2016. [Google Scholar]
- [7].Zeng Y, Feng Q, Hesketh T, Christensen K, Vaupel JW. Survival, disabilities in activities of daily living, and physical and cognitive functioning among the oldest-old in China: a cohort study. Lancet (London, England) 2017;389: 1619–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gaziano TA, Abrahams-Gessel S, Gomez-Olive FX, et al. Cardiometabolic risk in a population of older adults with multiple co-morbidities in rural south africa: the HAALSI (Health and Aging in Africa: longitudinal studies of INDEPTH communities) study. BMC Public Health 2017;17: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gomez-Olive FX, Montana L, Wagner RG, et al. Cohort Profile: Health and Ageing in Africa: a Longitudinal Study of an INDEPTH Community in South Africa (HAALSI). Int J Epidemiol 2018. [DOI] [PMC free article] [PubMed]
- [10].Reiger S, Jardim TV, Abrahams-Gessel S, et al. Awareness, treatment, and control of dyslipidemia in rural South Africa: The HAALSI (Health and Aging in Africa: A Longitudinal Study of an INDEPTH Community in South Africa) study. PLoS One 2017;12: e0187347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jardim TV, Reiger S, Abrahams-Gessel S, et al. Disparities in Management of Cardiovascular Disease in Rural South Africa: Data From the HAALSI Study (Health and Aging in Africa: Longitudinal Studies of International Network for the Demographic Evaluation of Populations and Their Health Communities). Circulation Cardiovascular quality and outcomes 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the Jnc 7 Report.[Comment][Erratum Appears in Jama. 2003 Jul 9;290(2):197]. JAMA 2003;289: 2560–2572. [DOI] [PubMed] [Google Scholar]
- [13].American Diabetes Association. Standards of medical care in diabetes−−2013. Diabetes Care 2013;36 Suppl 1: S11–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shisana O LD, Rehle T, Simbayi L, Zuma K, Dhansay A, Reddy P, Parker W, Hoosain E, Naidoo P, Hongoro C, Mchiza Z, Steyn NP, Dwane N, Makoae M, Maluleke T, Ramlagan S, Zungu N, Evans MG, Jacobs L, Faber M, & SANHANES-1 Team (2013) South African National Health and Nutrition Examination Survey (SANHANES-1) Cape Town, South Africa: Human Sciences Research Council and MRC, 2013. [Google Scholar]
- [15].WHO Expert Consultation. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation. GENEVA, 8–11 DECEMBER 2008 2011.
- [16].Obesity: preventing and managing the global epidemic. Report of a WHO consultation World Health Organization technical report series; 2000;894. [PubMed] [Google Scholar]
- [17].Buckley DI, Fu R, Freeman M, Rogers K, Helfand M. C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the U.S. Preventive Services Task Force. Ann Intern Med 2009;151: 483–495. [DOI] [PubMed] [Google Scholar]
- [18].Windgassen EB, Funtowicz L, Lunsford TN, Harris LA, Mulvagh SL. C-reactive protein and high-sensitivity C-reactive protein: an update for clinicians. Postgrad Med 2011;123: 114–119. [DOI] [PubMed] [Google Scholar]
- [19].Achterberg S, Soedamah-Muthu SS, Cramer MJ, Kappelle LJ, van der Graaf Y, Algra A. Prognostic value of the Rose questionnaire: a validation with future coronary events in the SMART study. Eur J Prev Cardiol 2012;19: 5–14. [DOI] [PubMed] [Google Scholar]
- [20].Rutstein SO, Johnson K, MEASURE OM. The DHS wealth index: ORC Macro, MEASURE DHS, 2004.
- [21].Rutstein SO. Steps to constructing the new DHS Wealth Index Available from: http://dhsprogram.com/programming/wealth%20index/Steps_to_constructing_the_new_DHS_Wealth_Index.pdf [Date Accessed: March 18, 2016], 2014.
- [22].Missinne S, Vandeviver C, Van de Velde S, Bracke P. Measurement equivalence of the CES-D 8 depression-scale among the ageing population in eleven European countries. Social science research 2014;46: 38–47. [DOI] [PubMed] [Google Scholar]
- [23].Jardim TV, Reiger S, Abrahams-Gessel S, et al. Hypertension management in a population of older adults in rural South Africa. Journal of Hypertension 2017;35: 1283–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Keates AK, Mocumbi AO, Ntsekhe M, Sliwa K, Stewart S. Cardiovascular disease in Africa: epidemiological profile and challenges. Nature Reviews Cardiology 2017;14: 273. [DOI] [PubMed] [Google Scholar]
- [25].Yusuf S, Reddy S, Stephanie O, Sonia A Global Burden of Cardiovascular Diseases Part I: General Considerations, the Epidemiologic Transisiton, Risk Factors, and the Impact of Urbanization. Circulation 2001;104. [DOI] [PubMed] [Google Scholar]
- [26].World Health Organization. Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations: World Health Organization, 2014. [PubMed] [Google Scholar]
- [27].Munthree C, Maharaj P. Growing Old in the Era of a High Prevalence of HIV/AIDS: The Impact of AIDS on Older Men and Women in KwaZulu-Natal, South Africa. Research on Aging 2010;32: 155–174. [Google Scholar]
- [28].Peters R, Beckett N, McCormack T, Fagard R, Fletcher A, Bulpitt C. Treating hypertension in the very elderly—benefits, risks, and future directions, a focus on the hypertension in the very elderly trial. European Heart Journal 2014;35: 1712–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Beckett NS, Peters R, Fletcher AE, et al. Treatment of Hypertension in Patients 80 Years of Age or Older. New England Journal of Medicine 2008;358: 1887–1898. [DOI] [PubMed] [Google Scholar]
- [30].Ogihara T, Saruta T, Rakugi H, et al. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension 2010;56: 196–202. [DOI] [PubMed] [Google Scholar]
- [31].Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension. European Heart Journal 2013;34: 2159. [DOI] [PubMed] [Google Scholar]
- [32].Leung AA, Nerenberg K, Daskalopoulou SS, et al. Hypertension Canada’s 2016 Canadian Hypertension Education Program Guidelines for Blood Pressure Measurement, Diagnosis, Assessment of Risk, Prevention, and Treatment of Hypertension. The Canadian journal of cardiology 2016;32: 569–588. [DOI] [PubMed] [Google Scholar]
- [33].Franco OH, Peeters A, Bonneux L, de Laet C. Blood Pressure in Adulthood and Life Expectancy With Cardiovascular Disease in Men and Women. Hypertension 2005;46: 280. [DOI] [PubMed] [Google Scholar]
- [34].Andersen-Ranberg K, Fjederholt KT, Madzak A, Nybo M, Jeune B. Cardiovascular diseases are largely underreported in Danish centenarians. Age and ageing 2013;42: 249–253. [DOI] [PubMed] [Google Scholar]
- [35].McDonagh TA, Morrison CE, Lawrence A, et al. Symptomatic and asymptomatic left-ventricular systolic dysfunction in an urban population. Lancet (London, England) 1997;350: 829–833. [DOI] [PubMed] [Google Scholar]
- [36].Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population Trends in the Incidence and Outcomes of Acute Myocardial Infarction. New England Journal of Medicine 2010;362: 2155–2165. [DOI] [PubMed] [Google Scholar]
- [37].Feigin VL, Krishnamurthi R, Parmar P, et al. UPDATE ON THE GLOBAL BURDEN OF ISCHAEMIC AND HAEMORRHAGIC STROKE IN 1990–2013: THE GBD 2013 STUDY. Neuroepidemiology 2015;45: 161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Krousel-Wood MA, Frohlich ED. Hypertension and Depression: Co-existing Barriers to Medication Adherence. Journal of clinical hypertension (Greenwich, Conn) 2010;12: 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Scalco AZ, Scalco MZ, Azul JB, Lotufo Neto F. Hypertension and depression. Clinics (Sao Paulo, Brazil) 2005;60: 241–250. [DOI] [PubMed] [Google Scholar]
- [40].Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS medicine 2006;3: e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


