Abstract
Serine/threonine kinase Akt is a downstream effector of the PI3K pathway that is involved in many processes, including providing neuroprotection to stressed photoreceptor cells. Akt exists in three isoforms designated Akt1, Akt2, and Akt3. All of these isoforms are expressed in the retina. We previously reported that Akt2 knockout mice were susceptible to light stress-induced photoreceptor degeneration, whereas Akt1 deletion had no effect on the retina. We hypothesized that the phenotype of Akt2 knockout mice may be due to the inactivation of specific substrate(s) in the retina. Yeast two-hybrid screening of a bovine retinal cDNA library with Akt2 identified a multidomain protein, POSH (plenty of SH3s), that acts as a scaffold for the JNK pathway of neuronal death. Our results suggest a stable interaction between Akt2 and POSH. Previous studies show that overexpression of POSH leads to cell death. The cell death that we observed in Akt2 knockout mice could be due to the absence of inactivation of POSH-mediated JNK signaling in the retina.
XX.1. Introduction
Blindness and visual impairment are characteristic features of retinal degeneration, and apoptosis is the most common pathological phenotype. Survival factors suppress apoptosis in a transcription-independent manner by activating the serine/threonine kinase Akt, which then phosphorylates and inactivates components of the apoptotic machinery. Akt, the downstream effector of phosphoinositide 3-kinase (PI3K) and its activation, has been shown to promote cell survival. Akt exists in three isoforms designated Akt1, Akt2, and Akt3. These isoforms share more than 80% amino acid sequence homology, but respond differently to growth factor stimulation. Studies from our laboratory and others have shown the expression of all three isoforms in the retina and in photoreceptor cells (Li et al., 2008; Reiter et al., 2003). However, we demonstrated that Akt2 knockout mice exhibited a greater sensitivity to light stress-induced photoreceptor degeneration, whereas Akt1deletion had no effect on the retina (Li et al., 2007). The retinal degeneration phenotype of Akt2 was not rescued by the functional Akt1 and Akt3 in the retina, suggesting a non-redundant role of Akt2 in the retina. These observations led to the hypothesis that unique substrates of Akt2 in the retina may not be regulated by Akt1 and Akt3, or the phenotype of Akt2 knockout mice may be due to the inactivation of specific substrate(s). In the present study, we identified a specific Akt2 specific-substrate in the retina.
XX.2. Materials and Methods
XX.2.1. Plasmids and vectors
The bovine retinal cDNA library was kindly provided by Dr. Wolfgang Behr, University of Utah, Salt Lake City. The pLexA-ΔPH-Akt1, pLexA-ΔPH-Akt2, and pLexA-lamin were kindly provided by Dr. Anne Vojtek, University of Michigan, Ann Arbor. The mammalian expression constructs of hemagglutinin (HA)-tagged Akt1, Akt2, and Akt3 were kindly provided by Dr. Morris Birnbaum, University of Pennsylvania, Philadelphia.
XX.2.2. Yeast two-hybrid screen of the bovine retinal cDNA library
The yeast two-hybrid screen was performed in the L40 yeast strain using pLexA-ΔPH-Akt2 against a bovine retinal cDNA library (3.6 × 106) cloned in frame fusion with the GAL4 activation domain in the pGAD10. After transformation by the lithium acetate procedure (Rajala et al., 2004; Rajala and Chan, 2005), yeast were plated on a tryptophan-leucine-histidine-deficient medium plus 5 mM aminotriazole. Colonies growing in the absence of histidine were subsequently tested for β-galactosidase activity (Rajala et al., 2004). The plasmids of the library producing yeast colonies of a His+/LacZ+ phenotypes were isolated, and the cDNA inserts of these positive plasmids were sequenced.
XX.2.3. Cell lines and culture conditions
HEK 293T cells were maintained in DMEM medium containing 10% (v/v) FBS at 37°C. Approximately 2.5 × 105 cells were seeded in each 60-mm culture dish 12–18 h prior to transfection. Calcium phosphate-mediated DNA transfection was performed using each of the Akt1, Akt2, and Akt3 plasmids. Cells were harvested for experiments ~48 h post-transfection.
XX.2.4. GST pull-down assay
The cDNA encoding POSH encompassing the amino acids 447–550 were cloned into a pGEX bacterial expression vector, which contains a protein tag of glutathione S-transferase (GST). The pGEX-POSH construct or GST control vector was transformed into BL21 (DE3) cells, and the GST-POSH or GST was purified as described (Rajala et al., 2004). Pull-down experiments were carried out as described (Rajala et al., 2004), using 5 μg of GST-POSH fusion or GST protein that had been adsorbed onto a GST-Sepharose 4B matrix. HEK-293T cells expressing Akt1, Akt2, and Akt3 lysates were incubated with GST/GST-POSH proteins at 4°C for 1.5 h, with continuous stirring. The Sepharose beads were washed three times in 500 μl HNTG buffer [20 mM HEPES (pH 7.5), 150 mM NaCl, 0.1% Triton X-100, and 10% glycerol] and centrifuged at 5,000 rpm for 30–60 sec at 4°C. Bound proteins were eluted by boiling in 2X SDS sample buffer 5 min before 10% SDS-PAGE. After SDS-PAGE, the gels were subjected to immunoblot analysis with anti-HA and anti-GST antibodies.
XX.3. Results
XX.3.1. Identification of POSH as a substrate of Akt2
The bovine retinal cDNA library was screened against Akt2, and identified POSH as one of the interacting proteins. We isolated HIS3 and LacZ positive clones, and the majority of the clones represent the protein POSH. The multidomain protein POSH acts as a scaffold for the JNK pathway of neuronal death (Xu et al., 2003).
XX.3.2. POSH interacts specifically with Akt2
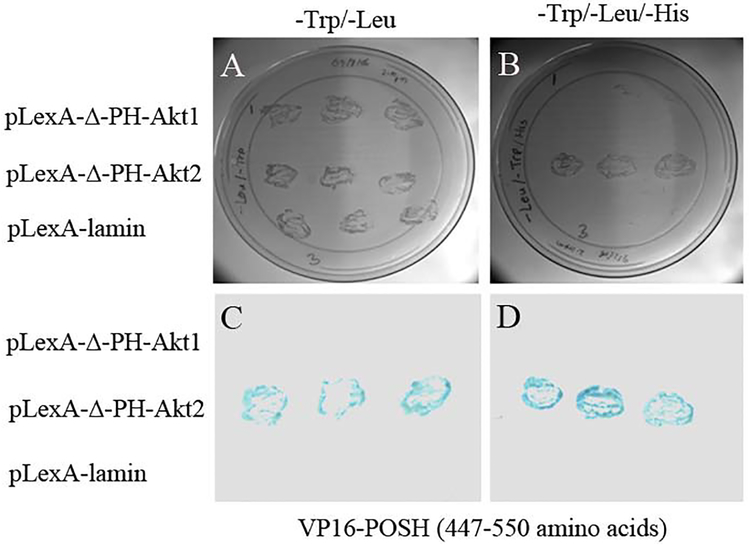
Yeast transformations were performed by the lithium acetate method, as described elsewhere (Rajala and Chan, 2005). Briefly, 0.5 μg of each plasmid of pLexA-ΔPH-Akt1, pLexA-ΔPH-Akt2, or pLexA-lamin and VP16-POSH (amino acids 447–550) was used to simultaneously transform the L40 yeast strain that harbors both His and LacZ reporters under the control of LexA binding sites. Transformants were plated on media lacking tryptophan and leucine, or tryptophan, leucine, and histidine plus 5 mM aminotriazole. Growth on media lacking tryptophan and leucine secured the presence of both plasmids independently of protein-protein interactions, and further eliminated the possibility of false negatives. Selection on media lacking tryptophan, leucine, and histidine plus 5 mM aminotriazole was used to detect potential interactions. Cells surviving on these plates were further screened for the LacZ phenotype by filter assay (Rajala et al., 2004). Lifted colonies were scored for LacZ phenotypes by detection of a blue color in the presence of 5-bromo-4-chloro-3-indolyl β-D-galactosidase after incubation at room temperature for 4 h. Our results showed specific interaction between Akt2 and POSH (Fig. 1). In this assay, we did not observe any interaction between Akt1 and POSH or laminin and POSH.
Fig. XX.1. Interactions of Akt2 with POSH in the yeast two-hybrid assay.
Yeast plasmid of pLexA-ΔPH-Akt1, pLexA-ΔPH-Akt2, or pLexA-lamin and VP16-POSH was used to simultaneously transform the L40 yeast strain that harbors both His and LacZ reporters under the control of LexA binding sites. Transformants were plated on media lacking tryptophan and leucine (A), or tryptophan, leucine, and histidine plus 5 mM aminotriazole (B). Cells surviving on these plates were further screened for the LacZ phenotype by filter assay (C, D). Lifted colonies were scored for LacZ phenotypes by detection of a blue color in the presence of 5-bromo-4-chloro-3-indolyl β-D-galactosidase after incubation at room temperature for 2-to-4 h.
We further examined the specific interaction between Akt2 and POSH in vitro using GST pull-down assays. The results clearly suggest a stable interaction between Akt2 and POSH. We observed no interaction of POSH with Akt1 or Akt3 (Fig. 2).
Fig. XX.2. GST pull-down experiments.
Individual HA-tagged Akt isoforms were expressed in HEK-293T mammalian cells. Expressed proteins were incubated with GST or GST-POSH, and then GST pull-down assays were carried out. The bound proteins were washed and subjected to immunoblot analysis with anti-HA (A) and anti-GST (B, C) antibodies.
XX.3.3. Localization of mixed-lineage protein kinase 3 (MLK3) in the retina
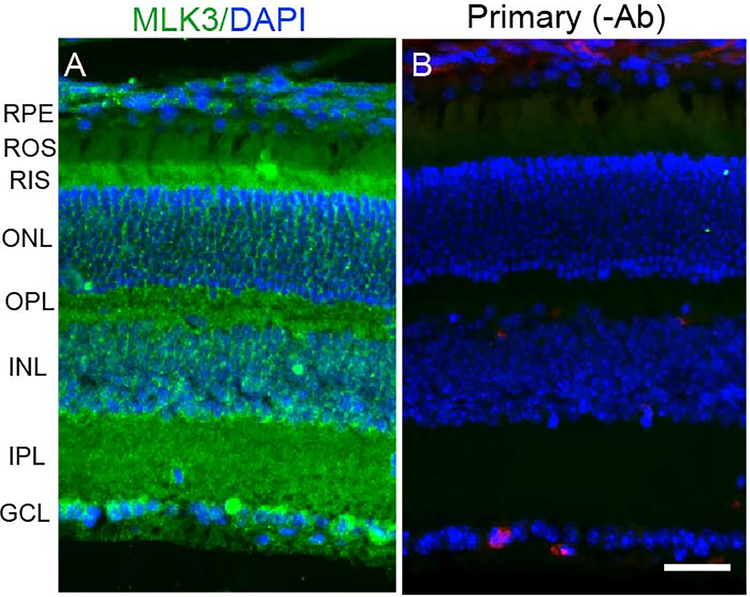
Immunolocalization studies suggest that MLK3 is predominantly localized to rod inner segments, ganglion cells layers, outer and inner plexiform layers of the retina (Fig. 3A). The results indicate the expression of MLK3 in the retina.
Fig. XX.3. Immunofluorescence analysis of MLK3 in the retina.
Prefer-fixed sections of mouse retinas were stained for MLK3 (A) and DAPI (A, B) and the immunofluorescence was analyzed by epifluorescence. Panel B represent the omission of MLK3 antibody. RPE, retinal pigment epithelium; ROS, rod outer segments; RIS, rod inner segments; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bar 50 μm.
XX.4. Discussion
Our earlier studies showed that Akt2 has non-redundant functions in the retina. We hypothesize that this may be mediated through specific down-stream target of Akt2. In the present study, we identified POSH as an Akt2 substrate. The interaction between POSH and Akt2 has also been observed outside the retina (Figueroa et al., 2003; Xu et al., 2003). POSH is an adapter protein with no catalytic function. Previous studies have demonstrated that POSH is involved in the JNK signaling pathway (Xu et al., 2003). Overexpression of POSH in cells has been shown to induce cell death (Lyons et al., 2007). A complex containing POSH, mixed-lineage protein kinase 3 (MLK3), and JNK was reported to trigger cell death. Activated Akt2 has been shown to phosphorylate MLK3, which results in the dissociation of MLK3 from the POSH and JNK complex and results in cell survival. The same signaling pathway might be occurring in the retina, as Akt2 knockout mice have increased susceptibility to light-induced photoreceptor degeneration. We found the expression of MLK3 (Fig. 3), and POSH (data not shown) in the retina. Further studies are required to examine the regulation of POSH by Akt2, and to understand the functional roles of JNK, MLK3, and POSH in the retina.
Acknowledgments:
This study was supported by grants from the National Institutes of Health (EY00871, and NEI Core grant EY021725) and an unrestricted grant from Research to Prevent Blindness, Inc. to the Department of Ophthalmology. The technical help of Dr. Ashok K. Dilly is appreciated. The authors acknowledge Ms. Kathy J. Kyler, Staff Editor, University of Oklahoma Health Sciences Center, for editing this manuscript.
References
- Figueroa C, Tarras S, Taylor J et al. (2003) Akt2 negatively regulates assembly of the POSH MLK-JNK signaling complex. J Biol Chem 278:47922–47927. [DOI] [PubMed] [Google Scholar]
- Li G, Anderson RE, Tomita H et al. (2007) Nonredundant role of Akt2 for neuroprotection of rod photoreceptor cells from light-induced cell death. J Neurosci 27:203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Rajala A, Wiechmann AF et al. (2008) Activation and membrane binding of retinal protein kinase Balpha/Akt1 is regulated through light-dependent generation of phosphoinositides. J Neurochem 107:1382–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons TR, Thorburn J, Ryan PW et al. (2007) Regulation of the Pro-apoptotic scaffolding protein POSH by Akt. J Biol Chem 282:21987–21997. [DOI] [PubMed] [Google Scholar]
- Rajala RV, Chan MD (2005) Identification of a NPXY Motif in Growth Factor Receptor-Bound Protein 14 (Grb14) and Its Interaction with the Phosphotyrosine-Binding (PTB) Domain of IRS-1. Biochemistry 44:7929–7935. [DOI] [PubMed] [Google Scholar]
- Rajala RV, McClellan ME, Chan MD et al. (2004) Interaction of the Retinal Insulin Receptor beta-Subunit with the P85 Subunit of Phosphoinositide 3-Kinase. Biochemistry 43:5637–5650. [DOI] [PubMed] [Google Scholar]
- Reiter CE, Sandirasegarane L, Wolpert EB et al. (2003) Characterization of insulin signaling in rat retina in vivo and ex vivo. Am J Physiol Endocrinol Metab 285:E763–E774. [DOI] [PubMed] [Google Scholar]
- Xu Z, Kukekov NV, Greene LA (2003) POSH acts as a scaffold for a multiprotein complex that mediates JNK activation in apoptosis. EMBO J 22:252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]