Abstract
Objectives
Grip strength and cognitive function reflect upper body muscle strength and mental capacities. Cross-sectional research has suggested that in old age these two processes are moderately to highly associated, and that an underlying common cause drives this association. Our aim was to synthesize and evaluate longitudinal research addressing whether changes in grip strength are associated with changes in cognitive function in healthy older adults.
Methods
We systematically reviewed English-language research investigating the longitudinal association between repeated measures of grip strength and of cognitive function in community-dwelling older adults to evaluate the extent to which the two indices decline concurrently. We used four search engines: Embase, PsychINFO, PubMed, and Web of Science.
Results
Of 459 unique citations, 6 met our full criteria: 4 studies reported a longitudinal association between rates of change in grip strength and cognitive function in older adults, 2 of which reported the magnitudes of these associations as ranging from low to moderate; 2 studies reported significant cross-sectional but not longitudinal associations among rates of change. All studies concluded that cognitive function and grip strength declined, on average, with increasing age, although with little to no evidence for longitudinal associations among rates of change.
Conclusions
Future research is urged to expand the study of physical and cognitive associations in old age using a within-person and multi-study integrative approach to evaluate the reliability of longitudinal results with greater emphasis on the magnitude of this association.
Systematic review registration number
CRD42016038544.
Keywords: Grip strength, Cognitive function, Longitudinal, Systematic review
As the aging process intensifies and starts to accelerate, individual differences in cognitive and physical function become more salient, with rates of change in cognitive and physical function potentially becoming more associated in older age (1).
Physical function, or strength, is indicative of overall health and has also been found, at cross-section, to be consistently associated with cognitive abilities. One of the main indicators of upper body muscle strength is grip strength. Being sensitive to age-related changes and changes in biological function, grip strength is not only an indicator of muscle strength but also of biological vitality (2). Grip strength is a commonly used measure of frailty in older adults that has been associated with morbidity, mortality, poor health, and loss of independence amongst other unfavorable outcomes (3). Grip strength has also been associated with cognitive aging, with and without cognitive impairment (4–7).
Most attempts to explain the association between physical and cognitive function fall into three categories: physical function directly affects changes in cognition (1,8); cognitive function directly affects changes in physical function (9); a third factor conjointly impacts both (6,7). The last explanation is possibly the most popular. The “common cause” (7) is predicated on the supposed existence of an underlying mechanism that drives the association between physical and cognitive function and their apparent simultaneous decline (7). Biological effects within the central nervous system, such as white matter integrity (4), and genetic influences, such as telomere length (10), have been proposed as possible underlying mechanisms. However, despite this tantalizing explanation, support for the common cause hypothesis stems mainly from between-person differences in cross-sectional designs (see Cloustan and colleagues (5) and Hofer and colleagues (11)), and rarely from within-person changes in longitudinal studies (5). Some studies have further suggested that this association may just be artefactual (12). Salthouse and colleagues (12) showed that controlling for between-person differences in age attenuated the association between physical and cognitive variables. Furthermore, when age was statistically adjusted, the association became nonsignificant or was greatly reduced. Given the number of confounds associated with cross-sectional designs for evaluating age-related associations (13,14), this is not to say that a relationship does not exist but that it is impossible to determine whether actual longitudinal associations in rates of within-person change exist from cross-sectional data alone.
Although there are some baseline versus baseline studies (15–18), and quite a few baseline versus changes studies (8,17–27), only a few studies have evaluated associations among rates of change (1,4,10,28–30). Change versus change studies provide evidence of whether, and the extent to which, these components change together over time and the magnitude of this association. As Hofer and Piccinin (31) argued, longitudinal studies are necessary to thoroughly examine multivariate associations among outcomes associated with the aging process. However, only a few studies, with differing methodologies, have examined the longitudinal association between changes in both grip strength and cognition. The rationale for this systematic review was to evaluate studies of simultaneous within-person change in cognition and grip strength. Thus, studies that report multivariate associations among rates of change are the focus of our systematic review.
Although our study has some overlap with the one presented by Clouston and colleagues (5), in our article, we systematically reviewed longitudinal studies that investigated the association between repeated measures of grip strength and of cognitive function in community-dwelling older adults to evaluate the extent to which these two indices decline concurrently. Initially, we aimed to include only studies that had at least two waves of follow-up following a baseline assessment (ie, three occasion’s total); however, due to a paucity of longitudinal reports in this area, we also included studies with only one follow-up assessment. Our focus was on the longitudinal association between individual differences in rate of change in both grip strength and cognitive function, in contrast to the association between grip and cognition at baseline (intercept). Our objectives were to outline the overall similarities and inconsistencies in the methodological and analytic approaches, as well as the reported findings across studies. We also discuss the limitations of the included studies, and provide suggestions for future research. To our knowledge, this is the first systematic review of the literature specifically addressing the longitudinal association between grip strength and cognitive function in older adults.
Methods
Protocol and Literature Search
We followed the protocol from the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) Statement (32). We performed a comprehensive literature search on the association between grip strength and cognitive function in longitudinal aging studies using Embase, PubMed, Web of Science, and PsychINFO. We performed three searches in: December 2015, May 2016, and April 2017. April 2017 is the publication date up to which all articles were included. We did not use date restrictions since we expected the majority of the reports to be from around the year 2000 onwards. The search strategy focused on three elements: grip strength, cognitive function, and older community-dwelling populations. The comprehensive list of search terms used in this review is included in Supplementary Appendix A.
Inclusion/Exclusion Criteria
In line with our rationale for the systematic review, studies were included that:
Used individual level data from ages 40 and older in community-dwelling samples;
Used objective measurements of both grip strength and cognitive function;
Analyzed longitudinal data (ie, two or more measurement occasions) on grip strength and cognition;
Reported original research in English;
Studies were excluded if:
They did not meet the above criteria;
They were intervention studies or trials.
Study Selection and Data Extraction
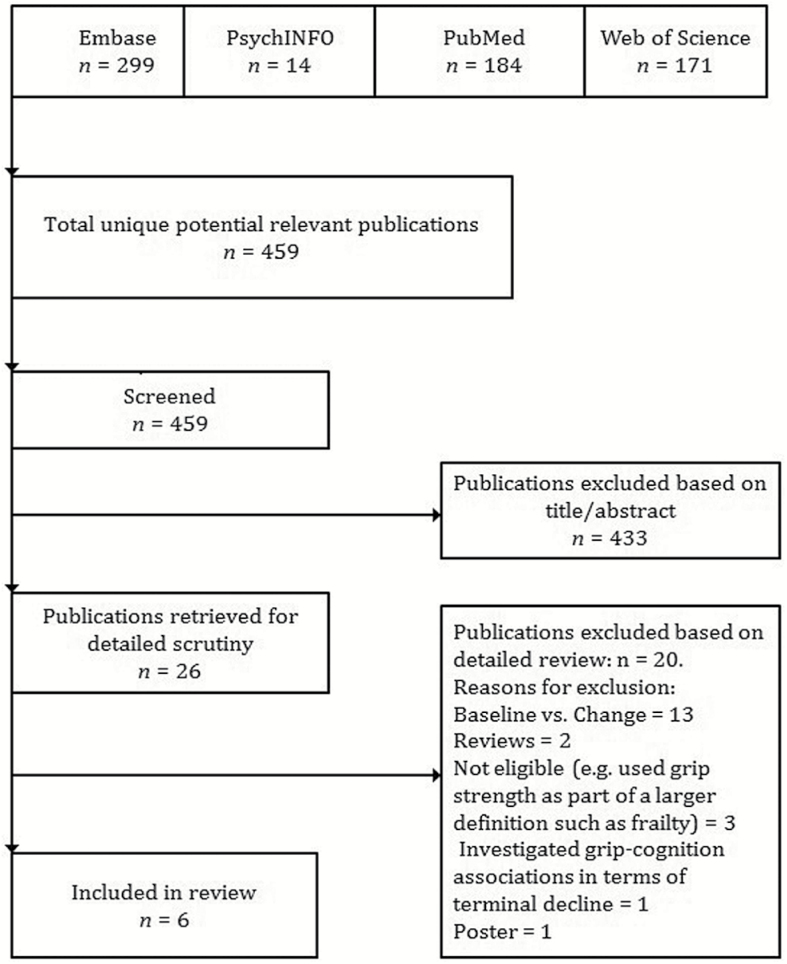
Two authors (A.R.Z. and A.R.) followed a common data collection instrument to independently review and extract information from each study. First, the authors screened the titles of 459 unique citations, and identified 26 candidate citations for detailed scrutiny based on title/abstract. The authors then reviewed the full text of the 26 citations, extracting basic eligibility information and study characteristics such as measurement of grip strength and cognitive function, waves of data collection, sample characteristics, and outcome measures. Since this systematic review focused on changes in cognition and grip strength in relation to each other over time, studies reporting baseline effects on within person change were not reviewed. Six citations (1,4,10,28–30) met full eligibility criteria. These studies were determined to be sufficiently methodologically rigorous (33). Of the excluded studies (n = 20), 13 modeled the association between baseline performance on one variable and within person change in another variable (9,18,19,21,26,27,34–41); only one addressed grip strength in relation to physical disability (42); one used grip strength as part of a “biological vitality” component (2); one applied latent growth modeling to study the slope of grip strength within and across cognitive change groups (43), one was a poster (44), and three were review articles on (i) the relationship between physical and cognitive function (5), (ii) a life course approach to physical function (45), and (iii) a life course approach to healthy aging and frailty (46). Figure 1 illustrates a summary of the study selection.
Figure 1.
Flow diagram of articles selected for the systematic review on the longitudinal association between grip strength and cognitive function in community-dwelling population samples aged ≥40.
Results
Systematic Review of Longitudinal Studies
Study and sample characteristics
Six citations derived from four different longitudinal studies met the full systematic review inclusion criteria: Christensen and colleagues (4); Christensen and colleagues (30); Deary and colleagues (10); MacDonald and colleagues (28); Ritchie and colleagues (29); and Sternäng and colleagues (1). Table 1 provides more information on the demographic characteristics and measures of these studies.
Table 1.
Summary of Study Sample and Characteristics of Studies Included in the Systematic Review of Longitudinal Associations Between Change in Grip Strength and Changes in Cognitive Function
| First Author, Year | Study | Sample Characteristics | Geographic Region | No. of Participants | Waves of Data | % Male | Baseline Mean Age (range) | Grip Strength Measures, Number of Trials, Handedness, Mean or Maximum Measures | Neurocognitive Battery |
|---|---|---|---|---|---|---|---|---|---|
| Christensen, 2000 (4) | CLS | Population | Australia | 425 | 2 | 48.5 | 75.8 (70–93) | Smedley hand dynamometer; 4 trials on each hand; average | Memory: Word recognition, Recall of three items, Address Recall. Crystallized IQ: Vocabulary and Similarities (WAIS-R); National Adult Reading Test. Speed of processing: Simple and choice reaction time. |
| Christensen, 2004 (30) | CLS | Population | Australia | 887 | 3 | 48.5 | 75.8 (70–93) | Smedley hand dynamometer; 4 trials on each hand; mean of the average of left and right | Memory: Word Recognition, Recall of three items, Address Recall. Speed: The Symbol Letter Modalities Test (SLMT). Reaction Time: Simple Reaction Time. |
| Deary, 2011 (10) | LBC 1921 | Population | Scotland | 207 | 3 | 42.5 | 79.1 (N/A)* | Jamar Hydraulic Hand Dynamometer; 3 trials on dominant hand; best score | Reasoning: Raven’s Standard progressive matrices. |
| MacDonald, 2011 (28) | VLS | Convenience population | Canada | 1043 | 6 | 34.1 | 73.6 (55–85) | Smedley hand dynamometer; 2 trials on dominant hand; best score | Fluid reasoning: letter series task. Working memory: computation span task. Episodic memory: word recall task. Semantic memory: fact recall task. Crystallized ability: 54-iten recognition vocabulary measures. |
| Ritchie, 2016 (29) | LBC 1936 | Population | Scotland | 1091 | 3 | 50.2 | 69.5 (N/A)* | North Coast Hydraulic Hand Dynamometer; 3 trials on dominant hand; best score. | Premorbid IQ/General intelligence: Moray House Test No.12. Fluid intelligence (gf): Matrix Reasoning, Block Design, Digit Span Backwards, Letter-Number Sequencing. Psychophysical speed: Inspection time. |
| Sternäng, 2015 (1) | SATSA | Swedish twin registry | Sweden | 708 | 6 | 43.9 | 64.4 (40–86) | Collins hand grip dynamometer 3 trials on each hand; best score | Verbal ability: Information Subtest from WAIS-R, Synonyms, and Analogies; Spatial ability: Figure Logic, Block Design, and Card Rotation; Processing speed: Symbol Digit, and Figure Identification; Memory: Digit Span, and Thurstone’s Picture Memory Task. |
Note: CLS = Canberra Longitudinal Study; LBC = Lothian Birth Cohort; VLS = Victoria Longitudinal Study; SATSA = Swedish Adoption/Twin Study of Aging.
*Sample is a single age cohort.
Overall, the samples had relatively moderate numbers of participants, ranging from 207 in the Lothian Birth Cohort 1921 [LBC 1921 (10)] to 1,043 in the Victoria Longitudinal Study (VLS). The majority of participants were female in all studies, with a mean age spanning 65 in the Swedish Adoption/Twin Study of Aging [SATSA (1)] to 79 in the LBC1921 (10). Three reports were from two longitudinal studies based in Europe (1,10,29); two reports were from one longitudinal study in Australia (4,30), and one was from Canada (28). Average years of formal education were only reported in two studies: participants in the VLS had a mean of 15 years of education (28), while the CLS sample mean was 12 years (4).
Follow-up ranged from two waves (4) to six waves (1,28). In the LBC1921 (10), 550 individuals participated at baseline, 319 at Wave 2, and 206 at Wave 3; while in the LBC 1936 (29) 1,091 individuals participated at baseline, 866 returned at a second wave, and 679 returned for a third wave. The VLS (28) had 1,043 participants at baseline, but included no information regarding follow-up. In SATSA (1), the authors used six waves of data representing information over up to 20 years of follow up; a total of 708 participants had at least one follow up on grip strength and had no dementia. In Christensen and colleagues (30), 780 (of 897) CLS participants had complete cognitive data at Wave 1, 483 participants had complete data and 599 had partial data at Wave 2, and 294 participants had complete data while 367 had partial data at Wave 3. Finally, in Christensen and colleagues (4), of 897 CLS participants, only 426 participants had data on the full cognitive battery and grip strength across the two available waves. Participants with dementia were excluded in four studies (1,10,28,29); in Christensen and colleagues’ participants were not screened for cognitive impairment (4,30).
Each report used one type of grip strength instrument: three used the Smedley hand dynamometer (4,28,30), one the Jamar Hydraulic dynamometer (10); one the North Coast Hydraulic Hand Dynamometer (29); and one the Collins handgrip dynamometer (1). Although each study applied slightly different procedures to collect grip strength data, the differences were minor. All studies measured grip strength in kilograms, and four used the best score out of a number of trials; Deary and colleagues (10) and Ritchie and colleagues (29), used the best of three from the dominant hand, MacDonald and colleagues (28) used the best of two from the dominant hand, and Sternäng and colleagues (1) used best of six (three on each hand). Christensen and colleagues (4,30) applied four trials; it seems that they used the mean score; however, it was not clear whether they applied the mean of all four trials or just the mean of the two dominant hand trials.
All but one study (10) included multiple measures of cognition: Deary and colleagues (10) used the Raven’s Standard Progressive Matrices since their objective was to study reasoning in association with grip strength. The other studies included multiple cognitive tests that were classified into various cognitive constructs that included memory (1,4,28,30), reaction time and processing speed (1,4,29,30), crystallized IQ (4,28,29), general intelligence (29), fluid intelligence (gf) (28–30), and spatial and verbal ability (1). Despite the focus on similar domains, different tests were employed in each study (detailed in Table 1).
Analytic approaches
Each of the six reports used a different analytic method. Supplementary Table 1 provides a summary of the statistical methods, specific data adjustments, and main results of each study, while Table 2 provides a summary of the main finding of each paper, that is, the longitudinal association between change in grip strength and change in cognitive function. The two most common analytic approaches were growth curve models and latent modeling techniques.
Table 2.
Brief Summary of Analytic Methods and Main Results of Studies Included in the Systematic Review of Longitudinal Associations Between Change in Grip Strength and Changes in Cognitive Function
| First Author, Year | Statistical Method | Main Result |
|---|---|---|
| Christensen, 2000 (4) | Repeated measures ANOVA and latent change models | Changes in grip strength, processing speed, and memory “moved together.” Correlations among changes between grip strength and cognitive function ranged from 0.23 to 0.45 in magnitude. |
| Christensen, 2004 (30) | Latent growth models, growth curve analysis | Changes in grip strength and changes in cognitive function were present, ranging from r = .31 for Memory, r = .45 for SLMT, and r = .47 for SRT in magnitude. |
| Deary, 2011 (10) | Bivariate growth curve model | Changes in grip strength and changes in reasoning measures did not correlate. |
| MacDonald, 2011 (28) | Multi-level linear mixed growth models and time co-variation models | Decline in cognitive function shared significant time-varying associations with declines in grip strength. |
| Sternäng, 2015 (1) | Latent class analysis; linear and quadratic growth curve models and time co-variation models | Grip strength across time did not predict any cognitive decline before age 65; however, it predicted cognitive decline in all cognitive domains for over age 65 (p < .05). |
| Ritchie, 2016 (29) | Multivariate growth curve modeling | Changes in grip strength were not correlated with changes in cognitive function. |
Christensen and colleagues (4) examined whether changes in sensory disability, reaction time, and grip strength were associated with changes in memory and crystallized IQ. They used repeated measures ANOVA to examine within subject changes in cognition and grip strength over 3.5 years. Participants were stratified by age group (<75, 76–79, 80–84, ≥85) at their second wave to examine age trends. The authors then applied latent change models [ie, models estimating latent change scores (47)] to examine strength of the associations between grip strength and both memory and crystallized IQ using latent factors for memory at time 1, memory at time 2, crystallized IQ at time 1, crystallized IQ at time 2, change in memory, and change in crystallized IQ in relation to grip strength and change in grip strength. Age and sex were adjusted for in all analyses.
Christensen and colleagues (30) used growth curve models to examine initial level and rate of change using memory, speed, reaction time, grip strength, and sensory disability across three waves of follow-up. The authors adjusted for age, sex, education, the National Adult Reading Test, and APOE.
Sternäng and colleagues (1) ran growth curve models based on six waves of follow-up, with grip strength as a time-varying covariate and cognitive function as the outcome. Age was treated as a basis of the models; the authors applied a linear 2-spline model with age 65 as the turning point. This model was then reversed so that the various cognitive domains were the covariates, and grip strength the outcome. Sex was adjusted for in all models. The authors also tested mediation and moderator models using covariates of height, socioeconomic status (SES), smoking, and chronic disease.
Similar to Sternäng and colleagues (1), MacDonald and colleagues (28) used linear mixed models of chronological time in study, and including biological time indices as time co-varying covariates. They first examined whether within person cognitive function and grip strength displayed significant longitudinal changes. All analyses were adjusted for sex and age.
Ritchie and colleagues (29) used bivariate latent growth curve models to estimate the correlation between changes in grip strength and changes in fluid ability (ie, slope–slope correlation) in addition to intercept–intercept, and intercept–slope correlations. All analyses were adjusted for childhood IQ, sex, and age at the time of testing.
Deary and colleagues (10) used a bivariate latent growth curve model with Raven’s Standard progressive matrices and grip strength as outcome variables measured on three occasions, at ages 79, 83, and 87, to find out whether a reciprocal association exists among intercepts and slopes of grip and cognition. Covariates included childhood IQ, sex, height, self-reported mood state, the highest occupation-based SES, smoking status, units of alcohol consumed per week, history of cardiovascular disease, hypertension, and/or diabetes. Models were not adjusted for age since all participants were born in the same year.
Summary of results
Longitudinally, all studies reported declines in average levels of both grip strength and cognitive function with increasing age.
All six studies first analyzed the association between grip strength and cognition at baseline. Deary and colleagues (10) reported a significant association between baseline (age 79) grip strength and reasoning as determined by scores on the Raven’s Standard Progressive Matrices; they continued to find this association at subsequent waves, that is, at ages 83 and 87 at cross-section; however, when they studied decline, the slopes of reasoning and the slopes of grip strength were not significantly correlated. Similarly, Ritchie and colleagues (29) reported significant correlations between baseline grip strength and baseline fluid intelligence, as well as significant correlations between these two functions at waves 2 and 3. A cross-sectional association was not found between grip strength and memory, as measured by Word Recognition, Recall of Three Items, and Address Recall in Christensen and colleagues (4). However, in their subsequent study (31), they found significant associations at baseline between grip strength and Memory, Simple Reaction Task (SRT), and Symbol Letter Modalities Test (SMLT). Sternäng and colleagues reported an association between grip strength at baseline and verbal and spatial ability, but not with processing speed and memory at age 65.
Three studies (4,10,29) looked at whether grip strength intercept predicts decline in cognitive function; Deary and colleagues (10) and Ritchie and colleagues (29) further investigated whether cognition intercept predicted decline in grip strength. In both Deary and colleagues (10) and Ritchie and colleagues (29) neither intercept of grip nor of cognition reliably predicted the slope of the other. In Christensen and colleagues (4), grip strength intercept did not predict future changes in memory performance or crystallized IQ.
Sternäng and colleagues (1) compared two models in two directions: “grip strength to cognition” and “cognition to grip strength.” They found a better model fit for the predictive direction “cognition to grip strength,” however, the association for memory was not significant, and the authors focused on the alternative direction (ie, “grip strength to cognition”). In their models, grip strength slope did not predict decline in cognition in any of the domains before age 65; however, it was associated with all four domains (verbal ability, spatial ability, processing speed, and memory) after age 65. Similarly, Sternäng and colleagues (1) reported that slope of grip strength predicted decline in verbal ability, spatial ability, processing speed, and memory for individuals over the age of 65 but not for those under age 65. Although the authors mentioned that results indicated a small but stable relationship between decreasing grip strength and decreasing cognitive performance after 65 years of age, they did not report the magnitude of these associations.
The most common reported finding was that grip strength and cognitive function “changed together over time” (30) “moved together” (4), “travelled together” (28), or “declined in parallel” (10). However, in both Deary and colleagues (10) and Ritchie and colleagues (29), slope of cognition and slope of grip strength were not significantly correlated, even though grip and cognition were associated at cross-section with every wave (ie, cognition at Wave 1 by grip strength at Wave 1, cognition at Wave 2 by grip strength at Wave 2, and cognition at Wave 3 by grip strength at Wave 3). Christensen and colleagues’ (4) two-wave change in cognition across waves was associated with two-wave change in grip strength, and this association held with more follow-up data (31). In both studies, Christensen and colleagues (4,30) reported that the associations between change in grip strength and change in cognitive performance were low to moderate in magnitude, ranging from 0.31 to 0.47 (30) and from 0.23 to 0.45 (4).
MacDonald and colleagues (28) used time-varying covariates to evaluate whether change in cognition is systematically associated with change in grip strength. They reported that decline in all cognitive measures studied, including fluid reasoning, episodic memory, semantic memory, and crystallized ability shared significant time-varying associations with grip strength; however, they did not report the magnitude of the association. Upon entering grip strength in the model, the developmental time effect was reduced for each of the cognitive tests.
In summary, when compared to baseline associations, changes in grip strength and changes in cognitive function are not consistent across studies. Since longitudinal change is the aim of our systematic review, we discuss those in more detail below.
Discussion
In this review, we systematically presented the results of literature that used longitudinal data to examine the relationship between change in cognitive function and change in grip strength in older adults. Although we started with a large pool of studies, most reported cross-sectional associations or baseline associations with change, with only a few meeting the inclusion criteria.
Although all reports suggested that cognitive function and grip strength declined together, results were mixed. Deary and colleagues (10) reported that although cognitive function (reasoning) and grip strength were associated at every wave of testing, individual differences in rate of change were not associated, which does not support the common-cause hypothesis. Similarly, Ritchie and colleagues (29) failed to find associations between declines in cognitive function and declines in grip strength. Deary and colleagues (16) and Ritchie and colleagues (29) were the only studies that focused solely on showing the difference between grip and cognition both declining on average, exhibiting associations among between-person differences at each wave, while being unassociated in terms of rates of change over time. Christensen and colleagues (4), on the other hand, reported that the slopes of cognition (speed and memory) and grip strength were correlated over time; however, this analysis included only two waves of data, relied on age as the time metric, and accounted for age differences at baseline by dividing participants into four groups (≤75, 76–79, 80–84, ≥85). Sternäng and colleagues (1) reported an association between grip strength changes and changes in verbal ability, spatial ability, processing speed, and memory, but only in individuals older than 65 years; none of these associations were significant in those under 65, implying that something more underlying and widespread, such as life changes, retirement, or accelerated aging, takes place during the later time period that is not present before. MacDonald and colleagues (28) also found that poor grip strength and cognitive function in the domains of executive function, episodic memory, semantic memory, and crystallized ability were associated longitudinally. However, participants’ age in this sample ranged from 55 to 85, which contradicts Sternäng and colleagues (1) on an age-specific association. Sternäng and colleagues’ (1) results were also contradicted by Deary and colleagues (10), where all participants in the LBC1921 data set are over the age of 79 and by Ritchie and colleagues (29), where all participants in the LBC1936 are over the age of 70. However, individuals aged 79+ years may have truncated variances in the cognitive and grip strength slopes (10), and this single-age sample does not contain the cross-sectional age differences that may be responsible for the associations reported in some studies (1,37). These studies also used very different cognitive tests.
While Deary and colleagues (10) only evaluated reasoning (Raven’s Progressive Matrices), Ritchie and colleagues (29) included measures of Matrix Reasoning, Block Design, Digit Span Backwards, and Letter-Number Sequencing as indicators of “fluid” cognition, and Inspection Time to measure processing speed; Sternäng and colleagues (1) included verbal ability, spatial ability, processing speed, and memory. The two other studies with similar results to Sternäng and colleagues (1) focused on similar cognitive domains: Christensen and colleagues (4) considered memory, speed of processing, and crystallized IQ, while MacDonald and colleagues (28) studied fluid reasoning, episodic memory, semantic memory, and crystallized ability. Neither included the Raven’s Progressive Matrices to study reasoning in association with grip strength. Although this suggests that the association between grip strength and cognition is domain-specific, it seems unlikely that grip strength is associated with most cognitive domains but not with reasoning. Different measures of fluid cognition are not substantially different and it is improbable that physical strength would be associated with some but not all measures of fluid cognitive ability.
Christensen and colleagues (4) suggest that, rather than one single common cause, several processes, such as white matter changes and telomere shortening, may operate together. They also suggest that associations such as between grip strength and processing speed may reflect specific motor skills rather than anything more underlying. Other authors propose central nervous system changes, white matter integrity, and processes operating at the cellular level as possible mechanisms (1,6). Deary and colleagues (10) do not exclude the possibility of shared genetic influence as a potential mechanism for the correlations at cross-section. Sternäng and colleagues (1) further state that they would not exclude the possibility of grip strength influencing cognition or vice-versa, or possibly a combination of factors. Another possibility, more applicable in the context of incipient dementia, is that reduced understanding of the test instructions for the physical function measure may introduce an association between changes in physical and cognitive function.
Lastly, these studies differed in number of participants, rates of attrition, and follow-ups. The LBC1921 sample was the smallest but had two follow-ups (no longitudinal association) (10); the LBC1936 was larger at baseline with 1,091 participants and also had two follow-ups (also no longitudinal association) (29). Christensen and colleagues used the same study (the CLS) for two different reports, the first (4) had only one follow-up (and despite this, they reported a longitudinal association), and the second (48) had two follow-ups and a larger number of participants (n = 887), and associations over time were also reported. MacDonald and colleagues (28) and Sternäng and colleagues (1), each had five waves of follow-up with larger sample sizes and wider age-ranges (both reported significant associations) with MacDonald and colleagues reporting time-varying associations and Sternäng and colleagues reporting significant associations only in age 65+ age group; they also had the largest number of participants, which may have affected the power of the study and thus the results.
Two separate noteworthy studies
In our search, we also came across two studies (43,49) that did not meet the criteria of our systematic review because one was an intervention study (43) and the other investigated grip strength and cognitive function in relation to mortality (49). However, we briefly mention them here because they report on longitudinal cognitive change and grip strength. In the first, Lin and colleagues (43) estimated a mixture model to derive trajectory groups of cognitive change in speed of processing tasks; their objective was to study group differences as opposed to individual level associations. They applied bivariate latent class analysis to jointly model lab-based (Useful Field of View) and real world-based (Road Sign Test, and timed Instrumental Activities of Daily Living) speed of processing tasks to identify trajectory groups. They identified four distinct groups displaying different trajectories of speed of processing. Compared with participants in the stable groups (Class 4, reference group), participants in the very poor (Class 1), poor (Class 2), and moderately declining (Class 3) groups also declined in grip strength at 2.15, 1.22, and 0.57 units per visit. Although these results do not directly provide an estimate of the association between grip strength and cognition, they do support the possible existence of one, with poorer cognitive groups showing greater declines in grip strength. In the second study, Praetorius Björk and colleagues (49) applied bivariate growth curve models using linear and quadratic change to investigate whether cognitive function and grip strength decline conjointly before death. Praetorius Björk and colleagues (49) reported baseline associations between all six cognitive domains (semantic memory, episodic memory, spatial ability, motor and perceptual speed, short-term memory, and working memory) and grip strength. The authors (49) also reported that association between rates of change in cognition and grip strength was stronger before death (as opposed to chronological age).
Limitations, Clinical Implications, and Future Work
Outcome level
A minor limitation of our systematic review is that we included a study with only one follow-up (5), permitting computation of a difference score and not model-based longitudinal change. However, this study’s results were consistent with results from other included studies (1,28). Another limitation is that we only considered studies in English; it is possible that we may have missed studies in other languages.
Study level
A general criticism of the literature that applies here too is that any association that is treated the same, that is, without estimating its magnitude, permits statements stating presence of associations even when these associations are quite weak. For example, a correlation coefficient of .23 is different from a correlation coefficient of .74, and should not be treated the same. If such estimates are not reported, the interpretation of any association remains challenging.
Clinical implications
Hand grip strength is a marker of biological aging; it is a simple measurement that is correlated with total body muscle strength, ability to carry out activities of daily living, quality of life, subsequent frailty, and has been demonstrated to be a sensitive indicator of disability, morbidity, and mortality in older adults (35). Studying potential causal influences of developmental change, and which reflect the integrity of underlying biological processes, such as grip strength, and that consequently affect or are affected by brain structural and cognitive changes may help in further understanding underlying pathways that become manifested clinically.
Implications for future research
Grip strength is an indicator of biological function that reflects the functional capacity of underlying pathways, declines with age, is objectively measured, defines fitness and health conditions, and is associated with specific outcomes (28). Other markers, such as lung function and gait velocity, also reflect physical capacity. Alternative time-metrics, such as time-to-death (49), or even time-to-dementia, may be worth exploring and may give more insightful results than the ones that we currently present. We also urge future research to pay particular attention to the types of cognitive measures used. Future work should explore all possibilities that include bidirectional associations and underlying third factors that drive these associations. With state of the art methods in studying the brain, research should also incorporate potential causal markers of developmental change that reflect underlying biological and cognitive processes that affect or are affected by structural and functional changes in the brain. We lastly offer one methodological suggestion for future work. A wide variety of techniques can be used to analyze whether, and how, these processes are related, and each of these methods may answer somewhat different questions and provide somewhat different answers. Researchers should consider the sensitivity of the questions they are interested in answering to these different methods. Whichever method researchers pursue in future research, we strongly suggest collaboration across studies to use and build on approaches of coordinated multi-study integrative analysis, and to achieve comparable cross-study results. We realize that this method may be time-consuming; however, it is a rigorous, powerful, and worthwhile method to study specific associations across independent longitudinal cohort studies, particularly when identical measures are not available. Existing examples of these include the Integrative Analysis of the Longitudinal Studies of Aging and Dementia (IALSA) network (31), the Healthy Aging Across the Lifecourse (HALCyon) research programme (50) and the Cohort Studies of Memory in an International Consortium (COSMIC) (51) to name a few. Such consortiums harmonize datasets and have results simultaneously available within one large, though not necessarily pooled, study. Although these networks may address similar research questions, they offer different perspectives, for example, a general cognitive aging perspective (IALSA), a life course perspective (HALCyon) or a pathological (eg, dementia onset) perspective (COSMIC). This approach fulfills the National Institutes of Health requirements for rigor and reproducibility, as well as facilitates measurement harmonization in an effort to replicate longitudinal research using similar analyses and methodological approaches (48). Other innovative methods such as home-based assessments (52) or more recent approaches, such as intensive repeated-measures designs (53) may further offer a glimpse into day-to-day life, including, for example, whether poor grip strength is associated with more variable cognitive function throughout several assessments during the course of one day. In combination, results from such different methods would offer a more complete picture, which may address longstanding research gaps on the physical (specifically, grip strength in this study) and cognitive association.
Conclusion
The main conclusion derived from this systematic review is that despite the different analytic techniques and inconsistencies across results, all studies concluded that although cognitive function and grip strength decline on average in later life, their declines are not necessarily associated. In order to further examine the conclusions from the studies described in this article, and build a stronger body of evidence, a rigorous evaluation of multiple studies using a coordinated modeling approach should be undertaken.
Funding
This work was supported by the National Institute on Aging of the National Institutes of Health (NIH; P01AG043362 and K01AG054700); by the Einstein Aging Study (PO1 AG03949) from the National Institutes on Aging program; the National Institutes of Health CTSA (1UL1TR001073) from the National Center for Advancing Translational Sciences (NCATS), the Sylvia and Leonard Marx Foundation, and the Czap Foundation. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Supplementary Material
Acknowledgments
We thank Karen Sorensen the reference librarian at the Albert Einstein College of Medicine for her help with the literature search.
Conflict of Interest
None declared.
References
- 1. Sternäng O, Reynolds CA, Finkel D, Ernsth-Bravell M, Pedersen NL, Dahl Aslan AK. Grip strength and cognitive abilities: associations in old age. J Gerontol B Psychol Sci Soc Sci. 2016;71:841–848. doi:10.1093/geronb/gbv017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. MacDonald SW, Dixon RA, Cohen AL, Hazlitt JE. Biological age and 12-year cognitive change in older adults: findings from the Victoria Longitudinal Study. Gerontology. 2004;50:64–81. doi:10.1159/000075557 [DOI] [PubMed] [Google Scholar]
- 3. Cooper R, Kuh D, Hardy R; Mortality Review Group; FALCon and HALCyon Study Teams Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Christensen H, Korten AE, Mackinnon AJ, Jorm AF, Henderson AS, Rodgers B. Are changes in sensory disability, reaction time, and grip strength associated with changes in memory and crystallized intelligence? A longitudinal analysis in an elderly community sample. Gerontology. 2000;46:276–292. doi:10.1159/000022172 [DOI] [PubMed] [Google Scholar]
- 5. Clouston SA, Brewster P, Kuh D, et al. . The dynamic relationship between physical function and cognition in longitudinal aging cohorts. Epidemiol Rev. 2013;35:33–50. doi:10.1093/epirev/mxs004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Christensen H, Mackinnon AJ, Korten A, Jorm AF. The “common cause hypothesis” of cognitive aging: evidence for not only a common factor but also specific associations of age with vision and grip strength in a cross-sectional analysis. Psychol Aging. 2001;16:588–599. [DOI] [PubMed] [Google Scholar]
- 7. Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: a strong connection. Psychol Aging. 1994;9:339–355. [DOI] [PubMed] [Google Scholar]
- 8. Auyeung TW, Lee JS, Kwok T, Woo J. Physical frailty predicts future cognitive decline—a four-year prospective study in 2737 cognitively normal older adults. J Nutr Health Aging. 2011;15:690–694. [DOI] [PubMed] [Google Scholar]
- 9. Deary IJ, Whalley LJ, Batty GD, Starr JM. Physical fitness and lifetime cognitive change. Neurology. 2006;67:1195–1200. doi:10.1212/ 01.wnl.0000238520.06958.6a [DOI] [PubMed] [Google Scholar]
- 10. Deary IJ, Johnson W, Gow AJ, et al. . Losing one’s grip: a bivariate growth curve model of grip strength and nonverbal reasoning from age 79 to 87 years in the Lothian Birth Cohort 1921. J Gerontol B Psychol Sci Soc Sci. 2011;66:699–707. doi:10.1093/geronb/gbr059 [DOI] [PubMed] [Google Scholar]
- 11. Hofer SM, Berg S, Era P. Evaluating the interdependence of aging-related changes in visual and auditory acuity, balance, and cognitive functioning. Psychol Aging. 2003;18:285–305. [DOI] [PubMed] [Google Scholar]
- 12. Salthouse TA, Hambrick DZ, McGuthry KE. Shared age-related influences on cognitive and noncognitive variables. Psychol Aging. 1998;13:486–500. [DOI] [PubMed] [Google Scholar]
- 13. Hofer SM, Flaherty BP, Hoffman L. Cross-sectional analysis of time-dependent data: mean-induced association in age-heterogeneous samples and an alternative method based on sequential narrow age-cohort samples. Multivariate Behav Res. 2006;41:165–187. doi:10.1207/s15327906mbr4102_4 [DOI] [PubMed] [Google Scholar]
- 14. Hofer SM, Sliwinski MJ. Understanding ageing. An evaluation of research designs for assessing the interdependence of ageing-related changes. Gerontology. 2001;47:341–352. doi:10.1159/000052825 [DOI] [PubMed] [Google Scholar]
- 15. Anstey KJ, Smith GA. Interrelationships among biological markers of aging, health, activity, acculturation, and cognitive performance in late adulthood. Psychol Aging. 1999;14:605–618. [DOI] [PubMed] [Google Scholar]
- 16. Takata Y, Ansai T, Soh I, et al. . Physical fitness and cognitive function in an 85-year-old community-dwelling population. Gerontology. 2008;54:354–360. doi:10.1159/000129757 [DOI] [PubMed] [Google Scholar]
- 17. Camargo E, Beiser A, Tan Z, et al. . Walking speed, handgrip strength and risk of dementia and stroke: the Framingham Offspring Study. Neurology. 2012;78. [Google Scholar]
- 18. Aichberger MC, Busch MA, Reischies FM, Strohle A, Heinz A, Rapp MA.. Effect of physical inactivity on cognitive performance after 2.5 years of follow-up: longitudinal results from the Survey of Health, Ageing, and Retirement (SHARE). GeroPsych. 2010;23:7–15. [Google Scholar]
- 19. Alfaro-Acha A, Al Snih S, Raji MA, Kuo YF, Markides KS, Ottenbacher KJ. Handgrip strength and cognitive decline in older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2006;61:859–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Auyeung TW, Lee SW, Leung J, Kwok T, Woo J. Age-associated decline of muscle mass, grip strength and gait speed: a 4-year longitudinal study of 3018 community-dwelling older Chinese. Geriatr Gerontol Int. 2014;14:76–84. doi:10.1111/ggi.12213 [DOI] [PubMed] [Google Scholar]
- 21. Taekema DG, Gussekloo J, Maier AB, Westendorp RG, de Craen AJ. Handgrip strength as a predictor of functional, psychological and social health. A prospective population-based study among the oldest old. Age Ageing. 2010;39:331–337. doi:10.1093/ageing/afq022 [DOI] [PubMed] [Google Scholar]
- 22. Raji M, Snih SA, Ostir GA, Markides KS, Ottenbacher KJ.. Poor cognition predicts subsequent risk of frailty in older Mexican Americans. J Am Geriatr Soc. 2010;58:S102. [Google Scholar]
- 23. Buchman AS, Wilson RS, Boyle PA, Bienias JL, Bennett DA. Grip strength and the risk of incident Alzheimer’s disease. Neuroepidemiology. 2007;29:66–73. doi:10.1159/000109498 [DOI] [PubMed] [Google Scholar]
- 24. Charles LE, Burchfiel CM, Fekedulegn D, et al. . Occupational and other risk factors for hand-grip strength: the Honolulu-Asia Aging Study. Occup Environ Med. 2006;63:820–827. doi:10.1136/oem.2006.027813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tabbarah M, Crimmins EM, Seeman TE. The relationship between cognitive and physical performance: MacArthur Studies of Successful Aging. J Gerontol A Biol Sci Med Sci. 2002;57:M228–M235. [DOI] [PubMed] [Google Scholar]
- 26. Stessman J, Rottenberg Y, Fischer M, Hammerman-Rozenberg A, Jacobs JM. Handgrip strength in old and very old adults: mood, cognition, function, and mortality. J Am Geriatr Soc. 2017;65:526–532. doi:10.1111/jgs.14509 [DOI] [PubMed] [Google Scholar]
- 27. Stijntjes M, Aartsen MJ, Taekema DG, et al. . Temporal relationship between cognitive and physical performance in middle-aged to oldest old people. J Gerontol A Biol Sci Med Sci. 2017;72:662–668. doi:10.1093/gerona/glw133 [DOI] [PubMed] [Google Scholar]
- 28. MacDonald SW, DeCarlo CA, Dixon RA. Linking biological and cognitive aging: toward improving characterizations of developmental time. J Gerontol B Psychol Sci Soc Sci. 2011;66:i59–i70. doi:10.1093/geronb/gbr039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ritchie SJ, Tucker-Drob EM, Starr JM, Deary IJ.. Do cognitive and physical functions age in concert from age 70 to 76? Evidence from the Lothian Birth Cohort 1936. Span J Psychol. 2016;19:12. [DOI] [PubMed] [Google Scholar]
- 30. Christensen H, Mackinnon A, Jorm AF, et al. . The Canberra Longitudinal Study: design, aims, methodology, outcomes and recent empirical investigations. Aging Neuropsychol Cogn. 2004;11:169–195. [Google Scholar]
- 31. Hofer SM, Piccinin AM. Integrative data analysis through coordination of measurement and analysis protocol across independent longitudinal studies. Psychol Methods. 2009;14:150–164. doi:10.1037/a0015566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi:10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang LJ, Chang KW, Chung KC. Methodologically rigorous clinical research. Plast Reconstr Surg. 2012;129:979e–988e. doi:10.1097/PRS.0b013e31824eccb7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gallucci M, Mazzuco S, Ongaro F, et al. . Body mass index, lifestyles, physical performance and cognitive decline: the “Treviso Longeva (TRELONG)” study. J Nutr Health Aging. 2013;17:378–384. doi:10.1007/s12603-012-0397-1 [DOI] [PubMed] [Google Scholar]
- 35. Kuh D, Cooper R, Hardy R, Guralnik J, Richards M; Musculoskeletal Study Team Lifetime cognitive performance is associated with midlife physical performance in a prospective national birth cohort study. Psychosom Med. 2009;71:38–48. doi:10.1097/PSY.0b013e31818a1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Raji MA, Kuo YF, Snih SA, Markides KS, Peek MK, Ottenbacher KJ. Cognitive status, muscle strength, and subsequent disability in older Mexican Americans. J Am Geriatr Soc. 2005;53:1462–1468. doi:10.1111/j.1532-5415.2005.53457.x [DOI] [PubMed] [Google Scholar]
- 37. Sattler C, Erickson KI, Toro P, Schröder J. Physical fitness as a protective factor for cognitive impairment in a prospective population-based study in Germany. J Alzheimers Dis. 2011;26:709–718. doi:10.3233/JAD-2011-110548 [DOI] [PubMed] [Google Scholar]
- 38. Starr JM, Deary IJ, Macintyre S. Associations with successful ageing in the “Healthy Old People in Edinburgh” cohort: being well, fit and healthy. Aging Clin Exp Res. 2003;15:336–342. [DOI] [PubMed] [Google Scholar]
- 39. Taekema DG, Ling CH, Kurrle SE, et al. . Temporal relationship between handgrip strength and cognitive performance in oldest old people. Age Ageing. 2012;41:506–512. doi:10.1093/ageing/afs013 [DOI] [PubMed] [Google Scholar]
- 40. Dolcos S, MacDonald SWS, Braslavsky A, Camicioli R, Dixon RA. Mild cognitive impairment is associated with selected functional markers: integrating concurrent, longitudinal, and stability effects. Neuropsychology. 2012;26:209–223. doi:10.1037/a0026760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Herbert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2012 census. Neurology. 2013;80:1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hebert LE, Scherr PA, McCann JJ, Bienias JL, Evans DA. Change in direct measures of physical performance among persons with Alzheimer’s disease. Aging Ment Health. 2008;12:729–734. doi:10.1080/ 13607860802154390 [DOI] [PubMed] [Google Scholar]
- 43. Lin F, Chen DG, Vance D, Mapstone M. Trajectories of combined laboratory- and real world-based speed of processing in community-dwelling older adults. J Gerontol B Psychol Sci Soc Sci. 2013;68:364–373. doi:10.1093/geronb/gbs075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen T, Narazaki K, Chen S, Haeuchi Y, Kumagai S.. The dynamic association between physical and cognitive functions in nondemented community-dwelling older adults: a 2-year longitudinal analysis. Alzheimers Dement. 2016;12:P412. [Google Scholar]
- 45. Kuh D. A life course approach to physical capability: findings from the HALCyon research programme. BMC Proc. 2013;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kuh D; New Dynamics of Ageing (NDA) Preparatory Network A life course approach to healthy aging, frailty, and capability. J Gerontol A Biol Sci Med Sci. 2007;62:717–721. [DOI] [PubMed] [Google Scholar]
- 47. McArdle JJ, Nesselroade JR.. Using Multivariate Data to Structure Developmental Change, in Life-Span Developmental Psychology: Methodological Contributions. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1994:223–267. [Google Scholar]
- 48. Hofer SM, Piccinin AM. Toward an integrative science of life-span development and aging. J Gerontol B Psychol Sci Soc Sci. 2010;65B:269–278. doi:10.1093/geronb/gbq017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Praetorius Björk M, Johansson B, Hassing LB. I forgot when I lost my grip-strong associations between cognition and grip strength in level of performance and change across time in relation to impending death. Neurobiol Aging. 2016;38:68–72. doi:10.1016/ j.neurobiolaging.2015.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kuh D, Cooper R, Richards M, Gale C, von Zglinicki T, Guralnik J. A life course approach to healthy ageing: the HALCyon programme. Public Health. 2012;126:193–195. doi:10.1016/j.puhe.2012.01.025 [DOI] [PubMed] [Google Scholar]
- 51. Sachdev PS, Lipnicki DM, Kochan NA, et al. ; COSMIC COSMIC (Cohort Studies of Memory in an International Consortium): an international consortium to identify risk and protective factors and biomarkers of cognitive ageing and dementia in diverse ethnic and sociocultural groups. BMC Neurol. 2013;13:165. doi:10.1186/1471-2377-13-165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sano M, Egelko S, Ferris S, et al. . Pilot study to show the feasibility of a multicenter trial of home-based assessment of people over 75 years old. Alzheimer Dis Assoc Disord. 2010;24:256–263. doi:10.1097/WAD.0b013e3181d7109f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bielak AAM, Mogle J, Sliwinski MJ. What did you do today? Variability in daily activities is related to variability in daily cognitive performance. J Gerontol B Psychol Sci Soc Sci. 2017:1–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.