Abstract
Background
Falls are associated with gait impairments in older adults (OA) and Parkinson’s disease (PD). Current approaches for evaluating falls risk are based on self-report or one-time assessment and may be suboptimal. Wearable technology allows gait to be measured continuously in free-living conditions. The aim of this study was to explore generic and specific associations in free-living gait in fallers and nonfallers with and without PD.
Methods
Two hundred and seventy-seven fallers (155 PD, 122 OA) who fell twice or more in the previous 6 months and 65 nonfallers (15 PD, 50 OA) were tested. Free-living gait was characterized as the volume, pattern, and variability of ambulatory bouts (Macro), and 14 discrete gait characteristics (Micro). Macro and Micro variables were quantified from free-living data collected using an accelerometer positioned on the low back for one week.
Results
Macro variables showed that fallers walked with shorter and less variable ambulatory bouts than nonfallers, independent of pathology. Micro variables within ambulatory bouts showed fallers walked with slower, shorter and less variable steps than nonfallers. Significant interactions showed disease specific differences in variability with PD fallers demonstrating greater variability (step length) and OA fallers less variability (step velocity) than their nonfaller counterparts (p < 0.004).
Conclusions
Common and disease-specific changes in free-living Macro and Micro gait highlight generic and selective targets for intervention depending on type of faller (OA-PD). Our findings support free-living monitoring to enhance assessment. Future work is needed to confirm the optimal battery of measures, sensitivity to change and value for fall prediction.
Keywords: Falls, Gait, Parkinsons, Wearable Technology
Falls are frequent among older adults (OA) and people with Parkinson’s disease (PD); approximately 30% of people over 65 years of age fall each year with the fall rate increasing with age and for people with PD to 60% (1,2). As the majority of falls occur while walking, gait impairments are commonly associated with and predict falls even in falls naive PD (1,3). Fall-related injuries cause loss of functional independence, poor quality of life and have associated costs to health of £1.7 billion per year in the United Kingdom alone (4), a figure estimated to rise due to increased longevity. Understanding falls risk and identifying key fall-related characteristics is critical to determine effective treatment and prevention strategies (3,5).
Clinical falls risk assessment is often based on questionnaires or one-time assessments of balance, gait and other falls risk factors. Due to their brief, subjective and sporadic nature, these approaches may not fully capture everyday falls risk and therefore may be suboptimal (1,6). Assessments based on recall such as falls diaries may be further compromised by cognitive impairment, thus limiting their utility. Falls also occur and may be precipitated by everyday activities and the environmental context, which is difficult to capture in a one-off assessment (7). It appears evident then that monitoring performance continuously during normal everyday activity may offer significant added benefits to understand falls risk and to enhance assessment of risk.
In this context, wearable technology (eg, accelerometers) is a valid and inexpensive tool to assess falls risk (8–12), walking activity, and gait impairment (13,14). Continuously monitoring activity during unsupervised and everyday activities (free-living) may provide an objective and more sensitive measure of falls risk than instrumented clinical-based assessments, being able to discriminate between fallers and nonfallers better than the clinical gait assessments (12). Free-living monitoring allows activity to be described by a broad framework that captures macro-structural characteristics (eg, volume, pattern, and variability of walking bouts) (referred to as Macro) and micro-structural characteristics that make up each walking bout (eg, spatial-temporal characteristics, gait stability outcomes, gait (a) symmetry outcomes, gait adaptability) (referred to as Micro or quality outcomes). Other models based on “quantity” (eg, volume) and “quality” (eg, endurance, variability, adaptability) measures of gait have also been shown to be promising in discriminating fall status in either PD or OA (10–12,15,16). To date, however, it is not clear if Macro and Micro characteristics of falls risk are similar in OA and PD or different. Comparing Macro and Micro gait characteristics with respect to falls risk and pathology may therefore be useful to highlight generic (ie, fallers/nonfallers) or disease-specific (PD fallers/OA fallers) differences. This nuanced understanding of falls risk could stress whether to target specific intervention across groups rather than a “one-size-fits-all” approach. Further work is therefore required to understand the nature of the relationship between free-living walking activity and falls risk to ultimately better inform strategies to reduce falls risk.
The aim of this study was to describe free-living walking activity taking a broad framework which encapsulated both Macro and Micro features of walking and to compare differences in fallers and nonfallers. Second, we wanted to establish generic (across all participants) and PD-specific associations between features of walking and a history of falls. Our primary hypothesis was that fallers would be less active and have more impaired gait with respect to nonfallers and that these differences would be more evident in people with PD compared to OA.
Methods
Participants
Fallers (F) were enrolled in the V-TIME study at five clinical centers across five countries (17). Participants were included in the study if they had fallen twice or more in the 6 months prior to assessment (17). Nonfallers (NF) were recruited from the Incidence of Cognitive Impairment in Cohorts with Longitudinal Evaluation-GAIT (ICICLE-GAIT) study, participants were included if they had not fallen for at least 18 months. ICICLE-GAIT is a collaborative study with ICICLE-PD, an incident cohort study (Incidence of Cognitive Impairment in Cohorts with Longitudinal Evaluation—Parkinson’s disease) conducted between June 2009 and December 2011 (18). PD participants were diagnosed with idiopathic PD according to the UK Parkinson’s Disease Brain Bank criteria and were excluded if they presented with significant cognitive impairment (Mini-Mental State Exam [MMSE] < 21 for V-TIME study and 24 for ICICLE-GAIT study (19)), psychiatric comorbidities, any neurological (other than PD), orthopaedic or cardiothoracic conditions that may have markedly affected their walking or safety during the testing sessions.
Age and sex were recorded for each participant. The severity of PD motor symptoms was measured using the Hoehn and Yahr scale (20) and section III of the modified Movement Disorder Society version of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS (21)). For both studies, people with PD were assessed approximately 1 hour after their medication intake. V-TIME study testing took place at the five clinical sites (17). ICICLE-GAIT study testing took place at the Clinical Ageing Research Unit, Newcastle University. Both studies were conducted according to the declaration of Helsinki and were approved by local Ethics Committees (17,18). All participants signed an informed consent form prior to testing.
Free-Living Data Collection: Protocol
At the end of a laboratory testing session, participants were asked to wear a tri-axial accelerometer (Axivity AX3, York, UK) on the lower back for 1 week as detailed in previous work (22). The water-proof device was programmed to capture data for 7 days at 100 Hz (range ± 8 g); for more details, see Supplementary Methods.
Data Processing and Analysis
Data processing and variable extraction
Once the device was received, data were downloaded and segmented (per day) and individual ambulatory bouts (ABs) were extracted via MATLAB (23). Detailed data processing can be found in the Supplementary Methods.
Outcome measures were described according to a broad framework of Macro and Micro characteristics (24). Macro (behavioral outcomes) representing the volume of walking (total walking time per day, percentage [%] of walking time per day, number of bouts, and steps per day), mean AB length were generated based on the AB detected over the 7 days. In addition, a set of nonlinear descriptors were also derived: (i) pattern of ABs derived using a power-law distribution (alpha, α) based on a logarithmic scale from their density and length and (ii) the within AB variability (S2) estimated using a maximum likelihood technique (22,25,26).
Micro gait characteristics (n = 14 describing pace, rhythm, variability, asymmetry, and postural control) were also determined for each walking bout. Characteristics were selected based upon a model of gait validated both in OA and in people with PD (27,28). For further details on quantification of Micro outcomes, see Supplementary Methods.
Data considerations
All ABs with more than three steps (minimum bout length) were taken into account for the analysis (10,14,29,30) a threshold of 2.5 seconds was set for the maximum resting period between consecutive ABs (23). Each AB was considered individually to ensure robustness for the evaluation of the gait characteristics, to avoid sources of error in step detection, and facilitate the calculation of variability and asymmetry characteristics (13). Micro outcomes were evaluated for each AB and then averaged over the seven days; pooled 7-day data were used for quantifying Macro outcomes. No further threshold was applied to ABs length when evaluating Macro outcomes (all ABs greater than three steps were included) (11,12,23), while in agreement with previous work ABs >10 seconds were included into the analysis for the Micro outcomes (13,31).
Statistical analysis
Statistical analysis was carried out using SPSS v19 (IBM). Normality of data and homoscedasticity were tested with Shapiro-Wilk test and Levene’s Test of Equality of Variances respectively. Descriptive statistics were reported as means and standard deviations (SD). Clinical characteristics were described but not used in further analysis. Effect of pathology and falls history were examined using general linear modeling. Fall history (F vs NF) and pathology (OA vs PD) were entered as within-person factors. Age, sex, and BMI were included as covariates. When a Pathology × Fall History interaction was found, post-hoc secondary analysis was carried out using Tukey’s test. We used a threshold of p < .05 to guide statistical interpretation for the main effects, while a Bonferroni corrected threshold (p < .0083) was used accounting for the multiple comparisons (Fall Status × Pathology) of the post-hoc analysis. Further analysis of Macro outcomes was then repeated on walking bouts grouped by bout length: medium (ABs > 60 seconds) and long (Abs > 120 seconds) ABs to explore the impact of AB length on results.
Results
Two hundred and seventy-seven fallers (F: 122 Older Adult Fallers (OAF), 155 PD Fallers (PDF), age: 73.33 ± 6.78 years), together with 65 nonfallers (NF: 50 Older Adult Non-Fallers [OANF], 15 PD Non-Fallers [PDNF], age: 69.05 ± 7.67 years) were assessed. F were older (p < .001) and included proportionally less women (F: 42%, NF: 56%). Clinical and demographic characteristics are shown in Table 1.
Table 1.
Clinical and Demographic Characteristics for Older Adult Non-Fallers (OANF), Older Adult Fallers (OAF), participants with Parkinson’s Disease Nonfallers (PDNF) and Participants with Parkinson’s Disease Fallers (PDF)
| Characteristics | OANF (n = 50) Mean (SD) | OAF (n = 122) Mean (SD) | PDNF (n = 15) Mean (SD) | PDF (n = 155) Mean (SD) |
p
OANF vs OAF |
p*
OANF vs PDNF |
p**
OANF vs PDF |
p***
OAF vs PDNF |
p
ǂ
OAF vs PDF |
p
§
PDNF vs PDF |
|---|---|---|---|---|---|---|---|---|---|---|
| Female (n, %) | 23, 46% | 95, 77.9% | 4, 26.7% | 59, 38.1% | <.001 | .183 | .319 | <.001 | <.001 | .383 |
| Age (years) | 70.40 (6.88) | 75.58 (6.32) | 64.54 (8.64) | 71.55 (6.44) | <.001 | .014 | .700 | <.001 | <.001 | .001 |
| BMI (kg/m2) | 28.30 (4.23) | 26.28 (4.29) | 28.45 (4.91) | 25.75 (3.69) | .017 | .999 | .001 | .204 | .709 | .067 |
| MMSE | 28.40 (1.74) | 28.52 (1.36) | 28.87 (1.60) | 28.04 (1.72) | .974 | .760 | .554 | .859 | .105 | .235 |
| Hoehn & Yahr (HY) stage (%) | - | - | HY 2—100% | HY 2—48.05% HY 2.5—11.69% HY 3—40.26% |
- | - | - | - | - | .310 |
| MDS-UPDRS III | - | - | 28.60 (5.65) | 31.42 (13.17) | - | - | - | - | - | <.001 |
| Freezing of gait (%, Score) | - | - | 13.3%, 7.71 (9.03) | 43.2%, 2.13 (5.64) | - | - | - | - | - | .002 |
| FES-I (16–64) | - | 28.68 (7.68) | - | 35.46 (12.00) | - | - | - | - | <.001 | - |
| ABCs (0–100%) | 91.02 (11.66) | - | 85.64 (15.73) | - | - | .185 | - | - | - | - |
Note: Significant p values (p < .05) are shown in bold.
*, **, ***, ‡, and § P-values in bold indicate p < 0.05.
ABCs = Activities specific balance confidence scale; BMI = Body mass index; FES-I = Falls Efficacy Scale; MDS-UPDRS III = Movement Disorder Society Unified Parkinson’s Disease Rating Scale part III; MMSE = Mini-Mental State Examination.
Macro Gait Characteristics
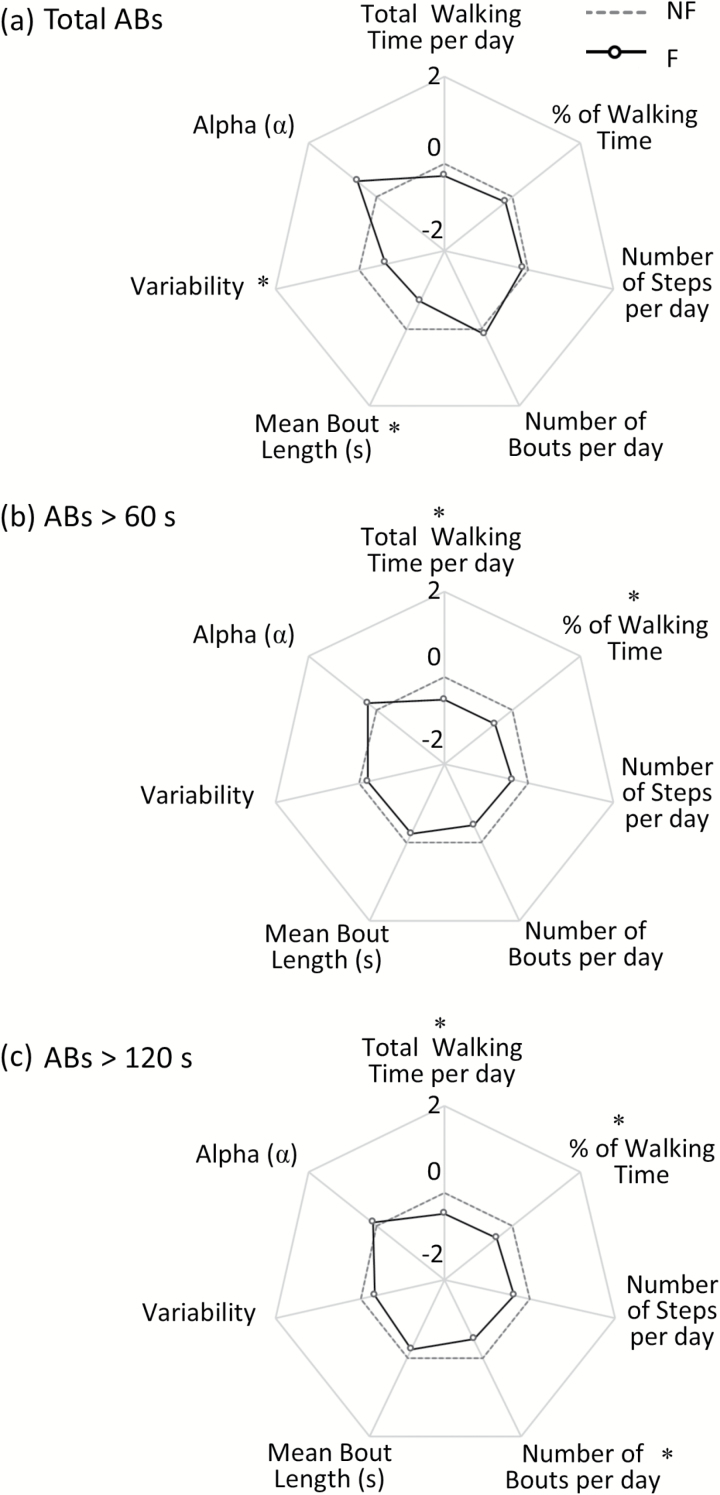
Mean bout length and variability were related to fall history where F walked with shorter and less variable walking bouts (lower S2) (Figure 1a). Volume of walking bouts (eg, total walking time per day, % of walking time per day, total number of steps and bouts per day) was not related to fall history.
Figure 1.
Radar plot illustrating the free-living Macro gait characteristics for Fallers (F), compared to Non Fallers (NF), evaluated in free-living conditions for total ambulatory bouts (ABs > three steps, panel a), ABs > 60 s (panel b), and ABs > 120 s (panel c). The central dotted line represents NF data, deviation from zero along the axis radiating from the center of the plot represents how many standard deviations the F differ from NF (range: ± 2 SD, z score based on NF means and SD). * represents significant differences between F and NF (effect of Fall History) (p values < .05).
When exploring differences based on walking bout length, a different picture emerged. ABs > 60 seconds represented less than 10% of the total amount of ABs, and volume of walking (based on total walking time per day and % of walking time per day) was significantly less in F. Longer ABs (>120 seconds) represented less than 2% of the total amount of ABs, and once again volume of walking (based on total walking time per day, % of walking time per day and in addition number of bouts per day) was significantly less in F (Figure 1b and c).
There were no interactions between fall history and pathology for any of the outcomes (Supplementary Table 2), indicating that Macro based outcomes respond in a similar manner irrespective of PD.
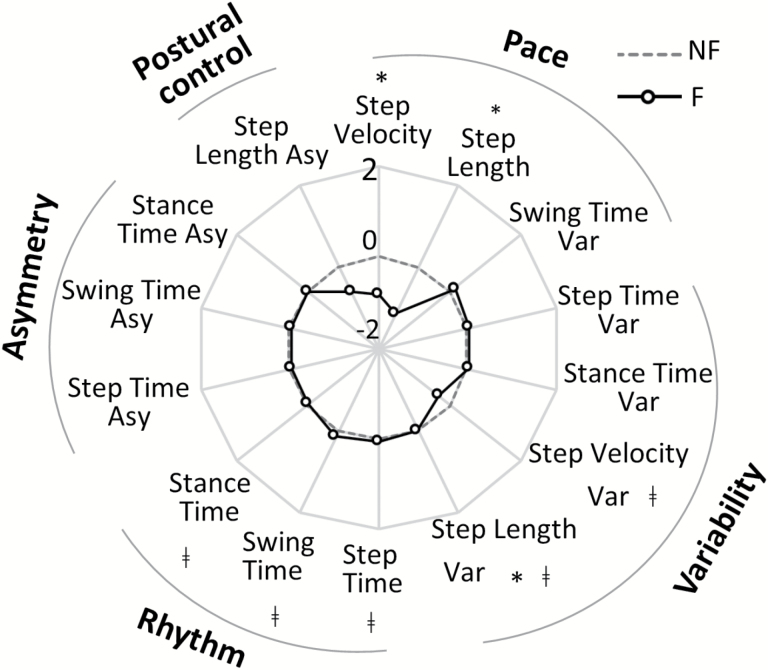
Micro Gait Characteristics
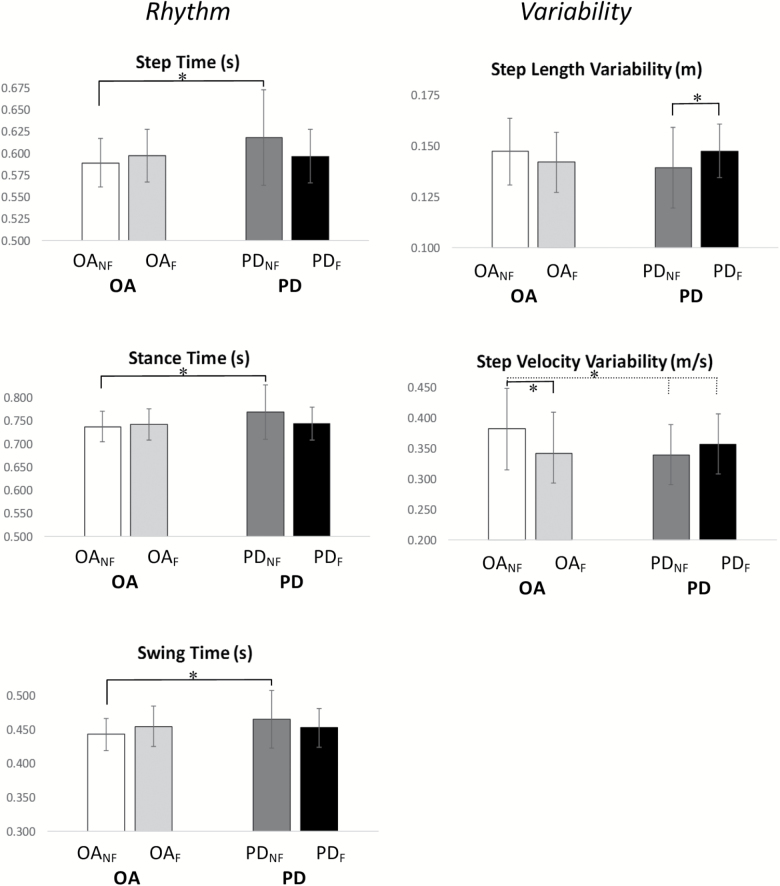
Characteristics relating to pace (step velocity, step length) and variability (step length variability) were significantly different between F and NF. F walked with reduced velocity and shorter, less variable steps (Supplementary Table 3, Figure 2). A significant interaction was found for fall history and PD in rhythm (step time, swing time, stance time) and variability (step length variability and step velocity variability) characteristics (Supplementary Table 3, Figure 2). PDNF had a slower step time, swing time, and stance time compared to OANF (p < .004, Figure 3). Although nonsignificant, OAF tended to walk at a slower cadence (higher step time, swing time, and stance time) compared to OANF. In contrast, PDF had a quicker time on all of these characteristics compared to PDNF, indicated a faster cadence overall (Figure 3). Variability characteristics (step length and step velocity) also showed significant interactions effects. Post-hoc analysis showed increased step length variability for PDF compared to PDNF (p = .004) in contrast to OAF who had reduced step velocity variability compared to OANF (p < .001) (Figure 3).
Figure 2.
Radar plot illustrating the free-living Micro gait characteristics for Non Fallers (NF) and Fallers (F) evaluated in free-living conditions for ambulatory bouts > 10 s. The central dotted line represents NF data, deviation from zero along the axis radiating from the center of the plot represents how many standard deviations (range: ± 2 SD, z score based on NF means and SD) the F differ from NF. * represents significant differences between F and NF (effect of Fall History), ǂ represents Fall History × Pathology interactions (p values < .05). (Var: Variability, Asy: Asymmetry).
Figure 3.
Post-hoc analysis results for interactions found in free-living Micro gait characteristics for Older Adult Fallers (OAF, in light grey), Non Fallers (OANF, in white), people with Parkinson’s disease Fallers (PDF, in black) and Non Faller (PDNF, in dark gray) evaluated in free-living conditions for ambulatory bouts > 10s. Error bars represent standard deviations. * represents post-hoc significant differences (p values < .0083).
Discussion
We quantified gait using a framework that captured Macro and Micro gait characteristics measured during free-living with a wearable accelerometer worn for 1 week and compared findings with respect to falls risk and pathology. We found an association between falls history, activity pattern, and variability of walking bouts (Macro outcomes) regardless of pathology. In contrast, discrete Micro gait characteristics were not only different with respect to falls status but also revealed generic and PD specific associations between gait impairment and a history of falls. Together, these findings highlight generic differences and disease-specific differences in macro and micro characteristics that inform a nuanced understanding of falls risk and intervention across groups.
Macro Characteristics
Our findings partly support our primary hypothesis that fallers would be less active than nonfallers, irrespective of pathology. We found that fallers were as active as nonfallers, irrespective of pathology, when considering the total amount of activity and our findings concur with others (12,15). However, when taking bout length into account a different picture emerged. Fallers spent less time walking during bouts over 1 minute, and even less during bouts of 2 minutes. These findings are in agreement with previous studies (32,33).
Differences were also observed in the pattern and variability of all walking bouts. We found that fallers had a greater number of shorter walking bouts, in agreement with previous work reporting a higher short-walk exposure for fallers (12). Fallers also had less variability in walking bout duration. This may reflect restricted engagement in sustained walking bouts. Contrasting results have been reported for measures representing walking bout variability with reports of increased (16) or decreased variability (34) in fallers when compared to nonfallers. Comparison across studies however is difficult due to different methodological approaches and metrics used for describing across bout variability. Our findings that changes in Macro characteristics were similar for OA and PD fallers not only extend previous work, they also suggest that these may be fundamental features of falls risk.
Agreement with previous work validates the veracity of our findings while at the same time raising interesting questions about the relationship between activity levels and fall risk/exposure (33). Falls often occur when individuals are engaged in dynamic activities such as walking (5,7,33), and therefore it is often assumed that individuals reduce overall exposure to falls risk by becoming less active. The data, however, suggest that the relationship of fall risk and activity is more complex and influenced by duration of walking bouts, particularly longer duration bouts (33). Differences observed in patterns of walking through a reduction in longer walking bouts may be due to compensatory change to reduce risk, possibly by reducing duration of walking bouts either by limiting access to the community or exercise. Alternatively, these changes may be related to fundamental features of falls risk. Reduction in variability of walking bout length in fallers may also be due to changes in patterns of walking behaviors indicating reduced confidence and a less varying walking “routine.” We performed further analysis to support this hypothesis and found that falls efficacy scale (FES-I) scores were negatively correlated with Macro variability (r < −.149), showing that fallers who were less confident (higher FES-I score) also had a less variable walking pattern (lower variability). Compensatory strategies or higher attentional load (eg, dual task) required for walking during free-living conditions may also play a role in modifying Macro level outcomes. At present, this is unclear and further work is required to understand the relationship with activity and falls more fully.
Either way, the relationship of reduced activity, health comorbidity (such as cardiovascular disease and diabetes) and function is an important consideration. A reduction in sustained bouts of walking is problematic given the putative positive benefits of activity and the subtle, insidious nature of inactivity on health and disease burden. Interventions should aim to find a balance of maintaining activity while minimizing falls risk, as well as the need to understand the relationship between these characteristics (35).
Micro Gait Characteristics
As hypothesized, gait impairment was more evident in fallers who walked with a slower gait and shorter step length compared to nonfallers. Our findings agree with previously published work in free-living gait (12,16). However, of more interest were the interactions in select Micro characteristics (related to rhythm and variability) indicating a different response in PDF and OAF compared to their nonfalling counterparts. For example, variability of step velocity and step length showed an interesting pattern with respect to pathology and falls status with OA fallers typically showing reduced variability in these characteristics and PD fallers increased variability compared to their nonfalling counterparts. Although to date no studies have compared OA and PD fallers, independent analysis of these groups in free-living studies lends support to our findings. For example, previous reports have shown that PD fallers have increased variability (represented by width of dominant frequency) to PD nonfallers (16). Studies of OA fallers have reported both higher and lower variability compared to nonfallers depending on the outcome measure. For example, when considering the amplitude and slope of the dominant frequency (measures of variability of the “quality” of walking), OA fallers were significantly more variable in the vertical axis but had less variability in the mediolateral axis (15). Others reported lower (although nonsignificant) between-walk variability (“adaptability”) (12) but higher within-walk variability or mode variability (10).
The intrinsic meaning of these differences is still unclear. Variability measured in the laboratory or clinic is typically greater in fallers compared to nonfallers (34) and has been reported to be predictive of future falls (36,37). For micro level variability in free-living, the picture is not as clear but it seems that it is influenced by the environment or context (38). Possible explanations for this dichotomy could be related to the different type metrics (and therefore methods) used to describe variability (eg, frequency based, within-walk variability, between-walk adaptability, etc.) which may indicate different constructs. Moreover, while some studies focused on steady-state walking for evaluating variability, in the current approach we included also short bouts of walking. The influence of “embedded” dual-task nature of real-life on walking poses additional challenges and ability to adapt, and our findings raise the possibility of a different adaption and control strategy in OA compared to PD. Whether they reflect compensatory adaptations or primary pathological disturbances in gait underpinning falls is as yet unclear. These selective differences however, suggest the need for strategies dependant on type of faller (OA or PD) targeting specific micro gait characteristics (eg, increase variability for PD and decrease for OA) in order to reduce falls risk.
Consistent with previous results (15,16,39), our findings corroborate the suggestion that “variability” measures may represent different constructs. Higher variability in Macro outcomes (“behavior”) may be “good”—representing ability to engage and adapt in a wider variety of walking activities. While higher variability in Micro outcomes may be “good” or “bad” representing either compensatory adaptions to minimize risk (eg, in OA) or impaired control and inability to minimize risk (eg, in people with PD).
Clinical Implications
Similar to what has been reported when investigating differences between fallers and nonfallers both in PD and OA (10,11,16), we found that Micro outcomes seem to be more sensitive than Macro to identify selective Faller × Pathology “type” dependant differences (eg, OAF and PDF). However, both contribute to a bigger picture that suggests group specific and generic features as targets for intervention development.
Limitations
This study informs understanding of the association between walking activity quantified via a range of Macro and Micro outcomes and falls history, however further work is required to identify the merits of this exploratory analysis especially in a larger sample of nonfallers. We acknowledge that use of different studies (V-TIME an ICICLE-GAIT) for populations of fallers and nonfallers and the limited number of PD fallers may affect generalizability of the results. Only one model of gait including specific Macro and Micro gait characteristics was included in this work, in the future other reported models and outcomes should be considered to identify best measure (or combination of measures) for falls risk detection. In addition, examination of other pathologies with fall history will allow us to determine whether free-living Macro and Micro outcomes can be a selective tool for identification of “pathology-dependant” falls risk. Future work is also needed to examine the effect of merging short ABs, turning and freezing of gait on results, and to confirm if Macro and Micro outcomes can predict falls in order to provide an insight into falls risk for guiding clinical decision-making.
Conclusions
We found common and disease-specific changes in Macro and Micro gait characteristics that highlight generic and selective targets for intervention in OAs and PD fallers allowing a more nuanced approach to falls intervention development. Macro outcomes seem to be associated with fall history regardless of pathology, while Micro outcomes seem to be a more sensitive outcome for detecting disease specific falls risk. Our findings support a role for free-living monitoring and the use of wearable technology to enhance assessment and understanding of falls risk. Future work is needed to confirm the optimal battery of measures and to fully understand the relationship of walking in the real-world and falls risk, especially its prognostic utility to enhance clinical decision-making and intervention development.
Funding
The work was supported by the V-Time project, which is a European Union 7th Framework Programme (FP7) under the Health theme (FP7 - 278169). The ICICLE-GAIT study was supported by Parkinson’s UK (J-0802, G-1301) and by the NIHR Newcastle Biomedical Research Centre. S.D.D. is supported by the Newcastle Biomedical Research Unit (BRU) based at Newcastle upon Tyne and Newcastle University. The work was also supported by the NIHR/Wellcome Trust Clinical Research Facility (CRF) infrastructure at Newcastle upon Tyne Hospitals NHS Foundation Trust. All opinions are those of the authors and not the funders.
Supplementary Material
Acknowledgments
The authors would like to thank all the V-TIME participants and all the members of the V-TIME project who have provided assistance with data collection. The authors would also like to thank Mrs Dadirayi Mhiripiri, Drs Rosie Morris and Philip Brown for their assistance with data collection for the ICICLE-GAIT study.
Conflict of Interest
None reported.
References
- 1. Allen NE, Schwarzel AK, Canning CG. Recurrent falls in Parkinson’s disease: A systematic review. Parkinsons Dis. 2013;2013:906274. doi:10.1155/2013/906274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peel NM. Epidemiology of falls in older age. Can J Aging. 2011;30:7–19. doi:10.1017/S071498081000070X [DOI] [PubMed] [Google Scholar]
- 3. Lord S, Galna B, Yarnall AJ, Coleman S, Burn D, Rochester L. Predicting first fall in newly diagnosed Parkinson’s disease: Insights from a fall-naïve cohort. Mov Disord. 2016;31:1829–1836. doi:10.1002/mds.26742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Age UK. Falls in the Over 65s Cost NHS £4.6 Million a Day Press Release. 2010. http://www.ageuk.org.uk/latest-press/archive/falls-over-65s-cost-nhs/.
- 5. Ross A, Yarnall AJ, Rochester L, Lord S. A novel approach to falls classification in Parkinson’s disease: Development of the fall-related activity classification (FRAC). Physiotherapy. 2016;103:459–464. doi:10.1016/j.physio.2016.08.002 [DOI] [PubMed] [Google Scholar]
- 6. Cho CY, Kamen G. Detecting balance deficits in frequent fallers using clinical and quantitative evaluation tools. J Am Geriatr Soc. 1998;46:426–430. [DOI] [PubMed] [Google Scholar]
- 7. Lamont RM, Morris ME, Menz HB, McGinley JL, Brauer SG. Falls in people with Parkinson’s disease: A prospective comparison of community and home-based falls. Gait Posture. 2017;55:62–67. doi:10.1016/j.gaitpost.2017.04.005 [DOI] [PubMed] [Google Scholar]
- 8. Del Din S, Godfrey A, Mazzà C, Lord S, Rochester L. Free-living monitoring of Parkinson’s disease: Lessons from the field. Mov Disord. 2016;31:1293–1313. doi:10.1002/mds.26718 [DOI] [PubMed] [Google Scholar]
- 9. Maetzler W, Domingos J, Srulijes K, Ferreira JJ, Bloem BR. Quantitative wearable sensors for objective assessment of Parkinson’s disease. Mov Disord. 2013;28:1628–1637. doi:10.1002/mds.25628 [DOI] [PubMed] [Google Scholar]
- 10. Brodie MA, Lord SR, Coppens MJ, Annegarn J, Delbaere K. Eight-week remote monitoring using a freely worn device reveals unstable gait patterns in older fallers. IEEE Trans Biomed Eng. 2015;62:2588–2594. doi:10.1109/TBME.2015.2433935 [DOI] [PubMed] [Google Scholar]
- 11. Brodie MA, Okubo Y, Annegarn J, Wieching R, Lord SR, Delbaere K. Disentangling the health benefits of walking from increased exposure to falls in older people using remote gait monitoring and multi- dimensional analysis. Physiol Meas. 2017;38:45–62. doi:10.1088/1361- 6579/38/1/45 [DOI] [PubMed] [Google Scholar]
- 12. Brodie MA, Coppens MJ, Ejupi A, et al. Comparison between clinical gait and daily-life gait assessments of fall risk in older people. Geriatr Gerontol Int. 2017;17:2274–2282. doi:10.1111/ggi.12979 [DOI] [PubMed] [Google Scholar]
- 13. Del Din S, Godfrey A, Galna B, Lord S, Rochester L. Free-living gait characteristics in ageing and Parkinson’s disease: Impact of environment and ambulatory bout length. J Neuroeng Rehabil. 2016;13:46. doi:10.1186/s12984-016-0154-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brodie MA, Coppens MJ, Lord SR, et al. Wearable pendant device monitoring using new wavelet-based methods shows daily life and laboratory gaits are different. Med Biol Eng Comput. 2016;54:663–674. doi:10.1007/s11517-015-1357-9 [DOI] [PubMed] [Google Scholar]
- 15. Weiss A, Brozgol M, Dorfman M, et al. Does the evaluation of gait quality during daily life provide insight into fall risk? A novel approach using 3-day accelerometer recordings. Neurorehabil Neural Repair. 2013;27:742–752. doi:10.1177/1545968313491004 [DOI] [PubMed] [Google Scholar]
- 16. Weiss A, Herman T, Giladi N, Hausdorff JM. Objective assessment of fall risk in Parkinson’s disease using a body-fixed sensor worn for 3 days. PLoS One. 2014;9:e96675. doi:10.1371/journal.pone.0096675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mirelman A, Rochester L, Maidan I, et al. Addition of a non-immersive virtual reality component to treadmill training to reduce fall risk in older adults (V-TIME): A randomised controlled trial. Lancet. 2016;388:1170–1182. doi:10.1016/S0140-6736(16)31325-3 [DOI] [PubMed] [Google Scholar]
- 18. Yarnall AJ, Breen DP, Duncan GW, et al. ICICLE-PD Study Group Characterizing mild cognitive impairment in incident Parkinson disease: The ICICLE-PD study. Neurology. 2014;82:308–316. doi:10.1212/WNL.0000000000000066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi:10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 20. Hoehn MM, Yahr MD. Parkinsonism: Onset, progression and mortality. Neurology. 1967;17:427–442. doi:10.1212/WNL.17.5.427 [DOI] [PubMed] [Google Scholar]
- 21. Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society UPDRS Revision Task Force Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–2170. doi:10.1002/mds.22340 [DOI] [PubMed] [Google Scholar]
- 22. Godfrey A, Lord S, Galna B, Mathers JC, Burn DJ, Rochester L. The association between retirement and age on physical activity in older adults. Age Ageing. 2014;43:386–393. doi:10.1093/ageing/aft168 [DOI] [PubMed] [Google Scholar]
- 23. Hickey A, Del Din S, Rochester L, Godfrey A. Detecting free-living steps and walking bouts: Validating an algorithm for macro gait analysis. Physiol Meas. 2017;38:N1–N15. doi:10.1088/1361-6579/38/1/N1 [DOI] [PubMed] [Google Scholar]
- 24. Lord S, Galna B, Rochester L. Moving forward on gait measurement: Toward a more refined approach. Mov Disord. 2013;28:1534–1543. doi:10.1002/mds.25545 [DOI] [PubMed] [Google Scholar]
- 25. Lord S, Godfrey A, Galna B, Mhiripiri D, Burn D, Rochester L. Ambulatory activity in incident Parkinson’s: More than meets the eye?J Neurol. 2013;260:2964–2972. doi:10.1007/s00415-013-7037-5 [DOI] [PubMed] [Google Scholar]
- 26. Lara J, O’Brien N, Godfrey A, et al. Pilot randomised controlled trial of a web-based intervention to promote healthy eating, physical activity and meaningful social connections compared with usual care control in people of retirement age recruited from workplaces. PLoS One. 2016;11:e0159703. doi:10.1371/journal.pone.0159703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Godfrey A, Del Din S, Barry G, Mathers JC, Rochester L. Instrumenting gait with an accelerometer: A system and algorithm examination. Med Eng Phys. 2015;37:400–407. doi:10.1016/j.medengphy.2015.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Del Din S, Godfrey A, Rochester L. Validation of an accelerometer to quantify a comprehensive battery of gait characteristics in healthy older adults and Parkinson’s disease: Toward clinical and at home use. IEEE J Biomed Health Inform. 2016;20:838–847. doi:10.1109/JBHI.2015.2419317 [DOI] [PubMed] [Google Scholar]
- 29. de Bruin ED, Najafi B, Murer K, Uebelhart D, Aminian K. Quantification of everyday motor function in a geriatric population. J Rehabil Res Dev. 2007;44:417–428. doi:10.1682/JRRD.2006.01.0003 [DOI] [PubMed] [Google Scholar]
- 30. Schwenk M, Hauer K, Zieschang T, Englert S, Mohler J, Najafi B. Sensor-derived physical activity parameters can predict future falls in people with dementia. Gerontology. 2014;60:483–492. doi:10.1159/000363136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Schooten KS, Pijnappels M, Rispens SM, Elders PJ, Lips P, van Dieën JH. Ambulatory fall-risk assessment: Amount and quality of daily-life gait predict falls in older adults. J Gerontol A Biol Sci Med Sci. 2015;70:608–615. doi:10.1093/gerona/glu225 [DOI] [PubMed] [Google Scholar]
- 32. Hiorth YH, Larsen JP, Lode K, et al. Impact of falls on physical activity in people with Parkinson’s disease. J Parkinsons Dis. 2016;6:175–182. doi:10.3233/JPD-150640 [DOI] [PubMed] [Google Scholar]
- 33. Mactier K, Lord S, Godfrey A, Burn D, Rochester L. The relationship between real world ambulatory activity and falls in incident Parkinson’s disease: Influence of classification scheme. Parkinsonism Relat Disord. 2015;21:236–242. doi:10.1016/j.parkreldis.2014.12.014 [DOI] [PubMed] [Google Scholar]
- 34. Howcroft J, Kofman J, Lemaire ED, McIlroy WE. Analysis of dual-task elderly gait in fallers and non-fallers using wearable sensors. J Biomech. 2016;49:992–1001. doi:10.1016/j.jbiomech.2016.01.015 [DOI] [PubMed] [Google Scholar]
- 35. Ahlskog JE. Does vigorous exercise have a neuroprotective effect in Parkinson disease?Neurology. 2011;77:288–294. doi:10.1212/WNL.0b013e318225ab66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mirelman A, Herman T, Brozgol M, et al. Executive function and falls in older adults: New findings from a five-year prospective study link fall risk to cognition. PLoS One. 2012;7:e40297. doi:10.1371/journal.pone.0040297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: A 1-year prospective study. Arch Phys Med Rehabil. 2001;82:1050–1056. doi:10.1053/apmr.2001.24893 [DOI] [PubMed] [Google Scholar]
- 38. Rispens SM, Van Dieën JH, Van Schooten KS, et al. Fall-related gait characteristics on the treadmill and in daily life. J Neuroeng Rehabil. 2016;13:12. doi:10.1186/s12984-016-0118-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moe-Nilssen R, Aaslund MK, Hodt-Billington C, Helbostad JL. Gait variability measures may represent different constructs. Gait Posture. 2010;32:98–101. doi:10.1016/j.gaitpost.2010.03.019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.