Abstract
Background
With aging, daily physical activity (PA) becomes less frequent and more fragmented. Accumulation patterns of daily PA—including transitions from active-to-sedentary behaviors—may provide important insights into functional status in older, less active populations.
Methods
Participants of the Baltimore Longitudinal Study of Aging (n = 680, 50% male, aged 27–94 years) completed a clinical assessment and wore an Actiheart accelerometer. Transitions between active and sedentary states were modeled as a probability (Active-to-Sedentary Transition Probability [ASTP]) defined as the reciprocal of the average PA bout duration. Cross-sectional associations between ASTP and gait speed (m/s), fatigability (rating-of-perceived-exertion [RPE]), 400 m time (seconds), and expanded short physical performance battery score were modeled using linear and logistic regression, adjusted for chronic conditions. Further analyses explored the utility of ASTP over-and-above total daily PA.
Results
In continuous models, each 0.10-unit higher ASTP was associated slower gait (β = −0.06 m/s, SE = 0.01), higher fatigability (β = 0.60 RPE, SE = 0.12), slower 400 m time (β = 16.31 s, SE = 2.70), and lower functioning (β = −0.13 expanded short physical performance battery score, SE = 0.03; p < .001). In categorical analyses, those in the highest tertile of ASTP were >2 times more likely to have high fatigability (rating of perceived exertion ≥10), slow 400 m time (>300 seconds) and reduced functional performance (expanded short physical performance battery score < 3.07) than those in the lowest tertile (p < .01). Further analyses demonstrated ASTP provided additional insight into functional outcomes beyond total daily PA.
Conclusion
Fragmented daily PA—as measured by ASTP—is strongly linked with measures of health and functional status and may identify those at risk of high fatigability and reduced functional performance over and above traditional PA metrics.
Keywords: Fatigability, Physical activity, Sedentary, Accelerometer, Physical function
Adequate physical activity (PA) is important for maintaining physical function and quality of life with aging, and is a powerful predictor of healthy longevity (1–3). With aging, daily PA becomes less frequent and intense, and more fragmented, with shorter active bouts and longer bouts of sedentary or rest throughout the day (4–7). Previous studies linking PA with health outcomes in older adults have mainly focused on defining the health effects of total volume or intensity of PA (4,8,9) and, more recently, bouts of sedentary behavior (10–13), but the compensatory changes that mark the transition from active to sedentary states, and their relationship to health and functional status with aging, is not well understood.
Low and diminished levels of PA are considered indicative of frailty and related factors including fatigue, slow gait, disability, cognitive impairment, and increased mortality risk (10–13). Interventions aimed at changing such trajectories are most likely to be effective if implemented at an early stage in the process, when the degree of deterioration is relatively small. Accordingly, detailed, sensitive metrics detailing the manner in which daily PA is accumulated may provide critical insights into characteristics and trajectories of PA that act as early markers of worsening functional performance.
An early stage of functional decline may first be characterized by the progressive shortening of the activity bouts that can be tolerated without resting. For example, one individual may routinely achieve 20–30 minutes of walking per day accumulated mostly through a single bout of prolonged activity, whereas another may engage in multiple short active bouts throughout the day. The former activity profile may represent a healthy person who engages in volitional exercise and maintains relatively high capacity and endurance, whereas the latter profile may represent someone with diminished reserve who can only sustain activity for a few minutes at a time. Although both individuals may accrue similar amounts of active and sedentary time over a typical day or week, the number of transitions between active and sedentary states, or degree of activity fragmentation, differs dramatically. Extracting information on activity fragmentation from accelerometry data may thus provide a new and more informative criterion to identify individuals earlier in their transition from moderately active to largely sedentary (14,15).
The purpose of this article is to introduce an index of activity fragmentation, the Active-to-Sedentary Transition Probability (ASTP) (14,15), as an early marker of fragmented PA, diminished reserve capacity, and declining functional status in older adults. ASTP is easy to calculate, interpret, and translate across accelerometer devices and studies, which may improve cohesiveness across accelerometer-based research. Although similar fragmentation indices have been used to assess sleep quality (14,16,17), to the best of our knowledge, they have not been considered as an informative feature of daily PA in older adults. To provide proof of concept, we examined the association between ASTP and established measures of fatigability (18,19), endurance capacity (20,21), and functional performance (2,22) in well-functioning older adults participating in the Baltimore Longitudinal Study of Aging (BLSA). We hypothesized that (a) higher ASTP represents a more fragmented pattern of daily PA that is associated with higher levels of fatigability, lower endurance capacity, and poorer functional performance, and (b) ASTP is more strongly associated with function and endurance performance than total volume of PA in older adults.
Methods
Participants
The BLSA is a study of normative human aging, established in 1958 and conducted by the National Institute on Aging Intramural Research Program. A general description of the sample and enrollment procedures and criteria has been previously reported (23). Briefly, the BLSA is a continuously enrolled cohort with some targeted recruitment (eg, women, racial minorities) over its history of 60 years. All participants are community-dwelling volunteers who pass comprehensive health and functional screening evaluations and are free of major chronic conditions and cognitive and functional impairment at the time of enrollment. Once enrolled, participants are followed for life and undergo extensive testing every 1–4 years depending on age (<60 every 4 years, 60–79 every 2 years, ≥ 80 every year).
The sample for the current study consists of 680 men and women who underwent a physical examination, health history assessment, and functional testing during their clinic visit, and subsequently wore an accelerometer for 7 days between August 2007 and December 2015. Trained and certified technicians administered all assessments following standardized protocols. The internal review board of the National Institute of Environmental Health Sciences approved the study protocol and participants provided written informed consent.
Usual gait speed was measured over a course of 6 m in an uncarpeted corridor. Participants stood with their feet behind a taped starting line and were asked to walk at a “normal comfortable pace.” After a command of “Go,” timing was initiated with the first foot-fall over the starting line and stopped after the first foot-fall over the finish line. Two timed trials were conducted to derive usual gait speed in meter per second; the faster of the two trials was used for analyses.
Endurance walking ability was assessed as time to complete the 400 m walk; a self-paced endurance walk test performed over a 20 m course and a validated measure of cardiorespiratory fitness (21). Participants were instructed to walk “as quickly as possible at a pace that can be maintained.” Standard encouragement was provided with each lap. Individual lap times and total time to complete were recorded in seconds (21,24).
Perceived fatigability was assessed immediately after a slow-paced 5 minute standardized treadmill walk (1.5 mph [0.67 m/s]; 0% grade) by asking participants to rate their perceived exertion using the Borg rating of perceived exertion (RPE) scale (range 6–20; 6 = no exertion at all, 9 = very light, 11 = light, 13 = somewhat hard, 20 = maximal exertion) (25). The speed of 0.67 m/s was selected because it is sufficiently low demand to minimize participant exclusion. Previous research has identified higher perceived fatigability as a risk factor for clinically meaningful decline in functional measures, independent of reported tiredness and low energy (18,19).
Physical Functioning was assessed using the Expanded Short Physical Performance Battery (ExSPPB), a composite measure of ability and time to complete 5 chair stands, 3 progressively harder standing balance poses (semi-tandem, full-tandem, and single-leg stand), timed 6 m walk at usual gait speed, and timed-narrow 6 m walk test (walking between 2 parallel lines separated by 20 cm). Ratio scores ranging from 0 to 1 were calculated for each component of the battery, where 1 represents the maximal performance observed for healthy older adults. Participants unable to complete a component were scored 0 for that component. Ratio scores from the four components were summed to obtain a continuous value ranging from 0 to 4, where participants with higher levels of physical performance receive a higher ExSPPB score (22).
Physical Activity (PA) was assessed using the Actiheart accelerometer (CamNtech, Cambridge, UK), a unidirectional chest-worn device that monitors heart rate and PA. On the last day of the BLSA clinic visit, participants were fitted with the Actiheart positioned horizontally at the chest at the third intercostal space using two standard electrocardiogram electrodes. Heart rate and accelerometry counts were measured in 1 minute epochs for the following 7 days in the free-living environment. Extra electrodes and specific placement instructions were provided, so participants could replace the device if needed. At the end of the 7 day period, the monitors were returned to the BLSA clinic via express mail. Actiheart data were downloaded using commercial software (Actiheart version 4.0.32) to derive activity counts in 1 minute epochs. Days with more than 5% of data missing were excluded from the analysis. For the remaining days, missing values were imputed as the average activity counts per minute during the same time period from the other complete days for each participant (4). A minimum of three valid days was required for inclusion in the analysis. Minute-level activity counts were averaged across all valid days to calculate the average counts per minute for every minute of the day (12:00 am–11:59 pm). A summary measure of total volume of activity was created for each participant and log-transformed (total log activity counts (TLAC)) due to the highly skewed distribution (4).
Active to Sedentary Transition Probability (ASTP) was calculated by quantifying each participant’s active and sedentary time on a minute-by-minute basis (12:00 am–11:59 pm) for each valid day. Visual examination of the distribution of activity counts showed 95% of participants had activity counts <10 counts per minute between the typical in-bed hours of 11:00 pm–5:00 am, as well as a consistently low heart rate indicative of a resting state (26). As a result, an active state was indicated when activity counts ≥10 counts/minute and a sedentary/sleep state was indicated when activity counts were <10 counts/minute (27). Bout length was defined as the number of consecutive minutes spent in either an active or sedentary state and a daily activity profile was created for each participant to detect alternating bouts of sedentary and active states. ASTP was defined as the probability of transitioning from an active to a sedentary state and calculated as the reciprocal of the average active bout duration (14,15). ASTP was calculated for each day and averaged across valid days to derive a single measure of ASTP for each participant.
Covariates
Height (meters) and weight (kilograms) were assessed in light clothing using a stadiometer and calibrated scale, respectively. Body mass index was calculated as kilograms per meter squared. Age, sex, race, smoking history, and history of cardiovascular disease, diabetes, hypertension, stroke, cancer, peripheral neuropathy, and lower extremity arthritis were extracted from a health history interview conducted by a nurse practitioner.
Statistical Analysis
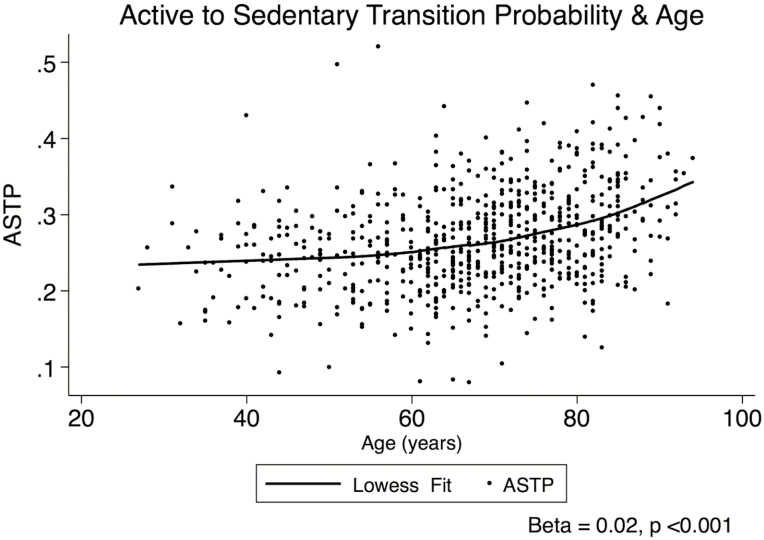
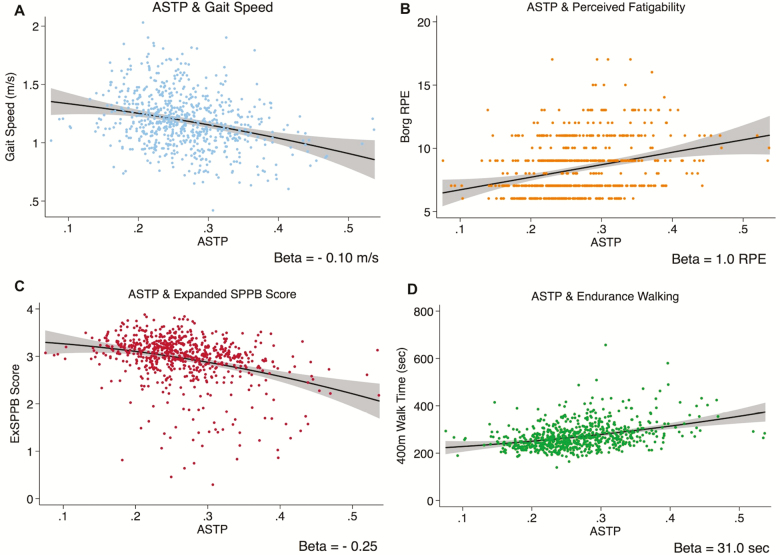
Exploratory diagnostics using histograms and normal Q-Q plots indicated that ASTP was normally distributed and was modeled as a continuous variable. Differences in participant characteristics and comorbidities by tertiles of ASTP were evaluated using chi-square tests for categorical variables and analysis of variance with Tukey’s honest significance test (HSD) for continuous variables. Scatterplots between ASTP and age, and between ASTP and each of the outcome variables (gait speed, time to walk 400 m, perceived fatigability, and the ExSPPB), were used to assess the direction and magnitude of the associations between variables (Figures 1 and 2). On the basis of the appearance of these plots, the association between ASTP and each of the outcomes was modeled continuously using multiple linear regression models, adjusted for demographics, height, weight, and history of chronic conditions. To provide clinical relevance, logistic regression models were added to assess odds of reduced functional performance by tertile of ASTP. Slow gait was defined as <1.0 m/s (28), high fatigability was defined as a RPE ≥10 (18,19), and low endurance performance was defined as taking >5 minutes to complete the 400 m walk (24). Cut points for reduced functional performance using the ExSPPB are not well defined; thus, histograms were used for data exploration, highlighting a break in the data at just below 3.07. Statistical significance was determined using two-tailed hypothesis testing with an alpha level of 0.05. All analyses were performed using R version 3.3.3 and Stata MP version 14.
Figure 1.
Univariate scatterplot showing the association between Active-to-Sedentary Transition Probability (ASTP) and age (n = 680) in the Baltimore Longitudinal Study of Aging.
Figure 2.
Scatterplots, fitted lines, and 95% confidence interval depicting the univariate association between Active-to-Sedentary Transition Probability (ATSP) and (A) usual gait speed (meters per second), (B) perceived fatigability (Borg rating of perceived exertion [RPE]), (C) expanded short physical performance battery score, and (D) time to walk 400 m (seconds; p < .001 for all).
Results
Participant characteristics are summarized in Table 1, overall and by tertiles of ASTP. The overall mean age of the study sample was 67.9 (± 13.2) years (range 27–94). Participants were high functioning with low comorbidity prevalence. The most prevalent comorbidities included lower extremity arthritis pain (32.9%), history of cancer (10.4%), and generally well-controlled hypertension (39.9%); all other chronic conditions were infrequent, with a prevalence of <10%. ASTP averaged 0.27 (±0.06) overall, ranging from 0.20 (±0.03) in the lowest ASTP tertile to 0.34 (± 0.05) in the highest ASTP tertile (p < .001). Participants in the highest ASTP tertile were more likely to be older and male, and to have a higher body mass index, history of smoking, cardiovascular disease, hypertension, stroke, and cancer (p < .03 for all). They also had slower gait speed, lower ExSPPB score, slower endurance walking time, higher perceived fatigability, lower total daily PA counts, and lower amounts of moderate-to-vigorous and light-intensity PA (p < .001 for all).
Table 1.
Participant Characteristics Stratified by Tertiles of ASTP
| Lowest Tertile (n = 227) | Middle Tertile (n = 227) | Highest Tertile (n = 226) | p-value for trend | ||||
|---|---|---|---|---|---|---|---|
| Mean/No. | SD (%) | Mean/No. | SD (%) | Mean/No. | SD (%) | ||
| Age | 64.25 | 12.84 | 66.11 | 13.43 | 73.42 | 11.61 | <.001 |
| Male sex | 98 | (43.17) | 111 | (48.90) | 132 | (58.41) | .005 |
| Body mass index (kg/m2)† | 26.51 | 4.14 | 27.02 | 4.42 | 27.95 | 5.06 | .003 |
| Non-white | 67 | (29.52) | 76 | (33.48) | 79 | (34.96) | .442 |
| Ever smoked‡ | 92 | (40.53) | 71 | (31.28) | 98 | (43.36) | .022 |
| Cardiovascular disease§ | 15 | (6.61) | 18 | (7.93) | 34 | (15.04) | .005 |
| Diabetes|| | 13 | (5.73) | 16 | (7.05) | 17 | (7.52) | .733 |
| Hypertension¶ | 78 | (34.36) | 78 | (34.36) | 115 | (50.88) | <.001 |
| Stroke# | 1 | (0.44) | 5 | (2.20) | 11 | (4.87) | .010 |
| Cancer†† | 22 | (9.69) | 16 | (7.05) | 33 | (14.60) | .029 |
| Peripheral neuropathy‡‡ | 21 | (9.25) | 17 | (7.49) | 20 | (8.85) | .780 |
| Lower extremity arthritis§§ | 67 | (29.51) | 69 | (30.40) | 88 | (38.94) | .062 |
| Gait speed (m/s)|||| | 1.26 | 0.23 | 1.21 | 0.22 | 1.09 | 0.22 | <.001 |
| Expanded SPPB score¶¶ | 3.10 | 0.38 | 2.99 | 0.53 | 2.74 | 0.58 | <.001 |
| 400 m walk time (seconds)## | 249.26 | 37.28 | 264.84 | 51.51 | 295.14 | 65.65 | <.001 |
| Perceived Fatigability (RPE)††† | 7.73 | 1.83 | 8.15 | 1.88 | 9.20 | 2.47 | <.001 |
| Total daily activity counts‡‡‡ | 53,009.04 | 25,578.54 | 35,114.73 | 13,698.46 | 21,675.93 | 11,309.85 | <.001 |
| MVPA§§§ | 79.56 | 74.42 | 61.89 | 57.26 | 52.55 | 64.42 | <.001 |
| LiPA|||||| | 245.66 | 92.66 | 208.67 | 90.24 | 176.34 | 81.41 | <.001 |
| ASTP¶¶¶ | 0.20 | 0.03 | 0.26 | 0.01 | 0.34 | 0.05 | <.001 |
Note: ASTP = Active-to-Sedentary Transition Probability; LiPA = light intensity physical activity; MVPA = Moderate to vigorous physical activity; RPE = rating of perceived exertion; SPPB = Short Physical Performance Battery.
†Weight in kilograms divided by height in meters squared.
‡Self-reported ever smoked on a regular basis.
§Self-reported diagnosis of heart disease or cardiac surgery, including myocardial infarction, congestive heart failure, angina pectoris, coronary artery bypass graft, and angioplasty.
||Self-reported diagnosis of and current medication for diabetes.
¶Self-reported diagnosis of and treatment with antihypertensive drugs.
#Self-reported history of stroke.
††Self-reported history of cancer.
‡‡Self-reported diagnosis of peripheral neuropathy or nerve damage in the lower legs, feet, or hands.
§§Self-reported diagnosis of arthritis of the knees, hips, or feet.
||||Usual gait speed in meters per second.
¶¶Score on the expanded version of the short physical performance battery.
##Time (seconds) to walk 400 m at a fast pace.
†††Rating of perceived exertion after 5 minutes of slow treadmill walking (0.67 m/s, 0% grade).
‡‡‡Average activity counts per day as measured by accelerometry.
§§§Minutes per day spent in moderate or greater intensity activities.
||||||Minutes per day spent in light intensity activities.
¶¶¶Active to Sedentary Transition Probability.
The univariate associations between ASTP and age, and ASTP and each of the functional outcomes, are shown in Figures 1 and 2. ASTP was positively associated with age, with a 0.02-unit higher ASTP for each 1 year increase in age (p < .001, Figure 1). Higher ASTP was also associated with poorer functional performance; for each 0.10-unit higher ASTP, gait speed was 0.10 m/s slower, perceived fatigability was 1.0 RPE higher, ExSPPB score was 0.30 units lower, and 400 m walk time was 31.0 seconds slower (p < .001 for all, Figure 2).
In fully adjusted models, these results were attenuated, but remained significant. For each 0.10-unit higher ASTP, usual gait speed was 0.06 m/s slower (model A), perceived fatigability was 0.61 RPE higher (model B), 400 m walk time was 16.31 seconds slower (model C), and the ExSPPB score was 0.13 units lower (model D; p < .001 for all; Table 2, row [i]). Model fit statistics for continuous analyses indicate the strongest fit for model C (endurance walking), followed by models D (ExSPPB), A (gait speed), and B (perceived fatigability), respectively (Table 2, row [i]). Sensitivity analyses revealed that removing the overnight hours (11 pm–5 am) from the analysis did not alter the results. In addition, although the association between age and ASTP appeared to be slightly nonlinear at older ages (Figure 1), additional analyses to examine interactions between ASTP and age, as well as nonlinear trends did not alter the results.
Table 2.
Adjusted Association Between ATSP and Measures of Physical Function (n = 680)
| Outcome (N = 680) | Gait Speed (m/s) (A) | Perceived Fatigability (RPE) (B) | Time to Walk 400 m (seconds) (C) | Expanded SPPB Score (D) |
|---|---|---|---|---|
| (i) Continuous analysis: Coefficient (SE) | ||||
| Independent variable | ||||
| ASTP (per 0.1 unit) | −0.06*** (0.01) | 0.60*** (0.12) | 16.31*** (2.70) | −0.13*** (0.03) |
| Model R2/Adjusted R2 | .27/.25 | .23/.22 | .45/.44 | .32/.31 |
| (ii) Categorical analysis: odds ratios (95% CI) | ||||
| Independent Variable | Slow Gait (<1.0 m/s) | High Perceived Fatigability (RPE ≥10) | Poor Endurance Performance (>300 s) | Low Functional Performance (<3.07) |
| Lowest tertile | Ref | Ref | Ref | Ref |
| Middle tertile | 1.04 (0.59–1.83) | 1.45 (0.85–2.48) | 2.04* (1.07–3.90) | 1.15 (0.73–1.80) |
| Highest tertile | 1.50 (0.88–2.58) | 2.18* (1.30–3.65) | 2.48** (1.35–4.56) | 2.13** (1.32–3.43) |
Note: Rows show multivariable regression models assessing the (i) continuous and (ii) categorical associations between ASTP and (A) usual gait speed (m/s), (B) perceived fatigability (RPE), (C) time to walk 400 m (seconds), and (D) expanded SPPB score. All models are adjusted for age, sex, race, smoking history, height, weight, and history of cardiovascular disease, stroke, peripheral neuropathy, hypertension, diabetes, cancer, and lower extremity arthritis pain. ASTP = Active-to-Sedentary Transition Probability.
*p < .05, **p < .01, ***p < .001.
To provide clinical relevance, the odds of reduced performance in each of the functional outcomes were modeled comparing tertiles of ASTP, using the lowest tertile as the reference group (Table 2, row [ii]). In fully adjusted models, there was a clear, graded association between tertiles of ASTP and each of the functional performance measures. Those in the middle ASTP tertile were more than twice as likely to have slow endurance performance (OR = 2.04 [1.07–3.90]) than those in the lowest ASTP tertile. Those in the highest ASTP tertile were more than twice as likely to have high perceived fatigability (OR = 2.18 [1.30–3.65]), poor endurance performance (OR = 2.48 [1.35–4.56]), and lower functional performance (OR = 2.13 [1.32–3.43; Table 2, row ii]). Although the relationship between ASTP and risk of slow gait did not reach statistical significance, further sensitivity analyses suggested a hierarchical association between ASTP and gait speed, with slowed gait speed (<1.0 m/s) commencing at an ASTP threshold of ≥0.40.
To understand whether ASTP is informative above and beyond a traditional measure of total volume of PA (TLAC) (4,8,29), we compared four separate models for each of the functional measures, using different combinations of ASTP and TLAC as independent variables: (a) TLAC only, (b) ASTP only, (c) both TLAC and ASTP, and (d) both TLAC and a volume-adjusted measure of ASTP to account for differences in PA that are not captured by volume alone (Table 3). To calculate volume-adjusted ASTP, we regressed ASTP on TLAC and used the regression residuals to define volume-adjusted ASTP.
Table 3.
Comparative Analysis of the Strength of the Associations Between ASTP and Total Volume of Physical Activity (TLAC) With (A) Usual Gait Speed (m/s), (B) Perceived Fatigability (Borg RPE), (C) Time to Walk 400 m (seconds), and (D) Expanded SPPB score
| Outcome | (A) Gait Speed (m/s) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||
| Std. β | SE | p | Std. β | SE | p | Std. β | SE | p | Std. β | SE | p | |
| TLAC | 0.11 | 0.04 | .004 | −0.05 | 0.06 | .37 | 0.11 | 0.04 | <.01 | |||
| ASTP | −0.17 | 0.04 | <.001 | −0.21 | 0.06 | <.001 | ||||||
| Adjusted ASTP | −0.13 | 0.03 | <.001 | |||||||||
| (B) Perceived Fatigability (RPE) | ||||||||||||
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||
| Std. β | SE | p | Std. β | SE | p | Std. β | SE | p | Std. β | SE | p | |
| TLAC | −0.16 | 0.04 | <.001 | −0.05 | 0.06 | .37 | −0.16 | 0.04 | <.001 | |||
| ASTP | 0.18 | 0.04 | <.001 | 0.14 | 0.06 | .01 | ||||||
| Adjusted ASTP | 0.09 | 0.04 | .01 | |||||||||
| (C) Time to Walk 400 m (seconds) | ||||||||||||
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||
| Std. β | SE | p | Std. β | SE | p | Std. β | SE | p | Std. β | SE | p | |
| TLAC | −0.16 | 0.03 | <.001 | −0.04 | 0.05 | 0.48 | −0.16 | 0.03 | <.001 | |||
| ASTP | 0.19 | 0.03 | <.001 | 0.17 | 0.05 | <.001 | ||||||
| Adjusted ASTP | 0.10 | 0.03 | <.001 | |||||||||
| (D) Expanded SPPB Score | ||||||||||||
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||
| Std. β | SE | p | Std. β | SE | p | Std. β | SE | p | Std. β | SE | p | |
| TLAC | 0.13 | 0.04 | <.001 | 0.02 | 0.05 | 0.67 | 0.13 | 0.04 | <.001 | |||
| ASTP | −0.16 | 0.04 | <.001 | −0.14 | 0.05 | <.01 | ||||||
| Adjusted ASTP | −0.09 | 0.03 | <.01 | |||||||||
Note: Model 1 includes TLAC, Model 2 includes ASTP, Model 3 includes TLAC and ASTP, and Model 4 includes TLAC and ASTP adjusted for total volume. All models are adjusted for age, sex, race, smoking history, height, weight, and history of cardiovascular disease, stroke, peripheral neuropathy, hypertension, diabetes, cancer, and lower extremity arthritis pain. ASTP = Active-to-Sedentary Transition Probability; RPE = rating of perceived exertion; SPPB = Short Physical Performance Battery; TLAC = total log activity counts.
In Models 1 and 2, TLAC and ASTP were associated with each of the functional measures in their respective models (Table 3, p < .01 for all). However, the magnitude of the standardized β coefficients were consistently stronger for ASTP (Table 3; Model 2: β = −0.17 for gait speed, β = 0.18 for perceived fatigability, β = 0.19 for 400 m time, and β = −0.16 for ExSPPB score, p < .001 for all) than for TLAC (Table 3, Model 1: β = 0.11 for gait speed, β = −0.16 for perceived fatigability, β = −0.16 for 400 m time, and β = 0.13 for ExSPPB score, p < .01 for all) across all functional measures. In Model 3, when both TLAC and ASTP were included in the models, only ASTP remained significant. Finally, in Model 4 both TLAC and volume-adjusted ASTP were significant (p < .01) but the magnitude of the TLAC coefficient remained unchanged from Model 1. Together, these results demonstrate that ASTP provides additional information when modeling the association between PA and functional measures, independent of total volume of PA.
Discussion
Use of accelerometers in scientific and clinical research presents new opportunities to understand how patterns of free-living PA may act as early markers of diminished endurance, greater fatigability, and poorer lower-extremity function, enhancing traditional PA research and its translational value (29). The current results suggest that fragmented daily activity, as defined by the probability of transitioning from an active to a sedentary state (ASTP), is strongly linked with established measures of health and functional status, and that this measure represents a feature of daily PA that cannot be explained by total volume of activity alone. Combined with greater odds of reduced functional performance among those with higher ASTP, these findings support the hypothesis that daily PA becomes more fragmented even among well-functioning older adults, and that quantifying ASTP may assist with identifying individuals earlier in their declining functional trajectory.
Although accelerometers have become quite common in aging-related studies, interpretation of the data is often problematic, with few analytical methods specific to older adults who have lower aerobic capacity, slower speeds, and altered patterns of movement than younger adults (27,29). Moreover, a lack of cohesiveness between studies and across devices has made it difficult to compare results across study populations (29,30). Recent advances in accelerometry methodology have improved discriminatory power (4,27,29,31–37), but more accurate analytical techniques to enhance data interpretation in older populations are needed to better understand the depth and clinical meaning of this highly complex data, beyond traditional approaches that define volume and intensity of daily activities.
Recent interest in the accumulation of sedentary or active bouts shows promise in augmenting and advancing PA research, and understanding the deleterious effects of prolonged sedentary behaviors (5,10,11,38,39) The current findings extend the literature on bouts of activity and inactivity by demonstrating the utility of a straightforward method of defining and quantifying both active and sedentary behaviors throughout the day in a real-world setting by calculating a probability that focuses on the number and duration of transitions from active to sedentary behaviors. As a transition probability, ASTP captures properties of the tendency to stay in active behaviors that cannot be captured by either the number of active breaks or the average active bout. Moreover, our results suggest ASTP provides information above and beyond a single measure of PA volume in older adults, as total daily PA declines and sustained bouts of PA become shorter.
Traditional laboratory- or clinical-based measures of function are often limited by ceiling effects and/or lack a thorough picture of functional ability outside of the lab (22,29). Accelerometer-derived measures collected in free-living settings complement clinical measurements and can provide a more comprehensive assessment of real-world activities, and the manner in which they are accumulated, thus establishing more accurate risk assessments for the disablement process. In the multivariable analyses (Table 2, row i), the higher model fit parameters for models (C) endurance walking and (D) ExSPPB suggest that higher ASTP is more strongly associated with higher-order measures of endurance and function, and thus may first become apparent at the higher end of the functional spectrum. Further, differences in ASTP become apparent before gait speed reaches clinically slow thresholds (40). Together these results suggest that patterns of activity may provide earlier insights into functional status than traditional clinical measures. However, the BLSA is a healthy aging population, thus limiting our ability to detect the sensitivity of ASTP in lower functioning individuals. Further validation of ASTP in poorer functioning populations is warranted, as well as replication in longitudinal data sets, to validate discriminatory power and predictive ability.
A significant advantage of ASTP is that it can be translated across accelerometer devices and placements (eg, chest, waist) after defining a device-specific threshold of movement that is consistent with sedentary behavior (eg, 10 counts/min, 100 counts/min). Using hip-worn accelerometer data from 3,401 participants aged 50 years and older in the National Health and Nutrition Examination Survey 2003–2006 cohorts, Di and colleagues found that a higher ASTP was associated with a greater risk of mortality after 6.4 years of follow-up (adjusted hazard ratio [aHR]: 1.40 [1.23–1.58]) (14). Median ASTP values among National Health and Nutrition Examination Survey participants aged 50 years and older were comparable to those in the current study, suggesting good replicability across studies and accelerometer placements (Supplementary Figure 1). Further research is warranted to test the utility of ASTP in wrist-worn devices, as well as other placements.
Although ASTP captures the probability of more fragmented behaviors, it is limited in its ability to assess intensity. In the current study, we focused on the transition from active to sedentary behaviors using a threshold of 10 counts/min. Researchers wishing to focus on transitions between activities of higher intensity (eg, transitioning from moderate to light intensity activities) would need to define the population and device-specific threshold of movement associated with moderate intensity activity and recalculate the probability accordingly. Given that many older adults do not engage in moderate or greater intensity activity as defined by traditional cut point thresholds, we chose to focus the current analysis on transitioning from activity of any intensity to a sedentary state to provide a more comprehensive picture of daily active and sedentary behaviors. Further application of this methodology to defining higher level transitions may provide additional insights into patterns of activity that represent various aspects of declining health including aerobic capacity, metabolic function, and muscle strength (41, 42).
In conclusion, our findings suggest that modeling patterns of daily activity as the probability of transitioning from an active to a sedentary state using accelerometry data—ASTP—is highly associated with clinical measures of physical function in older adults, and may better represent functional abilities than a summary measure of total volume of daily PA. ASTP is transferrable across accelerometer devices, and thus may provide opportunities for future pooled analyses of accelerometry data, enhancing PA research in older populations to include cumulative patterns of daily activities. More research on ASTP, its association with clinical outcomes, and longitudinal predictive value is warranted to further elucidate patterns of PA that may be informative to preserving functional health with aging.
Funding
This research was supported by the Intramural Research Program of the National Institute on Aging of the National Institutes of Health. This work was supported by R21AG053198, P30AG021334, U01AG057545, and R01AG050507 from the National Institute on Aging. A.P.S. agreed to serve as a consultant to Awarables, Inc. in support of a National Institutes of Health grant.
Conflict of Interest
The authors have no financial conflicts of interest to disclose. J.A.S., E.M.S., A.P.S., and L.F. serve on the editorial board of JGMS.
Supplementary Material
References
- 1. Ferrucci L, Cooper R, Shardell M, Simonsick EM, Schrack JA, Kuh D. Age-related change in mobility: perspectives from life course epidemiology and geroscience. J Gerontol Biol Sci Med Sci. 2016;71:1184–1194. doi: 10.1093/gerona/glw043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schrack JA, Zipunnikov V, Goldsmith J, et al. Assessing the “physical cliff”: detailed quantification of age-related differences in daily patterns of physical activity. J Gerontol A Biol Sci Med Sci. 2014;69:973–979. doi: 10.1093/gerona/glt199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shiroma EJ, Freedson PS, Trost SG, Lee IM. Patterns of accelerometer-assessed sedentary behavior in older women. JAMA. 2013;310:2562–2563. doi: 10.1001/jama.2013.278896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wanigatunga AA, Tudor-Locke C, Axtell RS, et al. Effects of a long-term physical activity program on activity patterns in older adults. Med Sci Sports Exerc. 2017;49:2167–2175. doi: 10.1249/MSS.0000000000001340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yerrakalva D, Cooper AJ, Westgate K, et al. The descriptive epidemiology of the diurnal profile of bouts and breaks in sedentary time in older English adults. Int J Epidemiol. 2017;46:1871–1881. doi: 10.1093/ije/dyx123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Varma VR, Dey D, Leroux A, et al. Total volume of physical activity: TAC, TLAC or TAC(λ). Prev Med. 2018;106:233–235. doi: 10.1016/j.ypmed.2017.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bassett DR, Troiano RP, McClain JJ, Wolff DL. Accelerometer-based physical activity: total volume per day and standardized measures. Med Sci Sports Exerc. 2015;47:833–838. doi: 10.1249/MSS.0000000000000468 [DOI] [PubMed] [Google Scholar]
- 10. Diaz KM, Howard VJ, Hutto B, et al. Patterns of sedentary behavior and mortality in U.S. middle-aged and older adults: a national cohort study. Ann Intern Med. 2017;167:465–475. doi: 10.7326/M17-0212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diaz K, Howard V, Hutto B, et al. Patterns of sedentary behavior in US middle-age and older adults: the REGARDS study. Med Sci Sports Exerc. 2016;48:430–438. doi: 10.1249/MSS.0000000000000792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wanigatunga AA, Ambrosius WT, Rejeski WJ, et al. Association between structured physical activity and sedentary time in older adults. JAMA. 2017;318:297–299. doi: 10.1001/jama.2017.7203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sardinha LB, Santos DA, Silva AM, Baptista F, Owen N. Breaking-up sedentary time is associated with physical function in older adults. J Gerontol A Biol Sci Med Sci. 2015;70:119–124. doi: 10.1093/gerona/glu193 [DOI] [PubMed] [Google Scholar]
- 14.Di J, Leroux A, Urbanek J, Varadhan R, Spira A, Schrack J, Zipunnikov V. Patterns of sedentary and active time accumulation are associated with mortality in US adults: The NHANES study. bioRxiv. 2017. p.182337. doi:10.1101/182337
- 15. Wanigatunga AA, Gresham GK, Kuo PL, Martinez-Amezcua P, Zipunnikov V, Dy SM, Simonsick EM, Ferrucci L, Schrack JA Contrasting characteristics of daily physical activity in older adults by cancer history. Cancer. 2018. doi: 10.1002/cncr.31745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep fragmentation and the risk of incident Alzheimer’s disease and cognitive decline in older persons. Sleep. 2013;36:1027–1032. doi: 10.5665/sleep.2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lambiase M, Gabriel K, Kuller L, Matthews K. Temporal relationships between physical activity and sleep in older women. Med Sci Sports Exerc. 2013;45:2362–2368. doi: 10.1249/MSS.0b013e31829e4cea [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simonsick EM, Schrack JA, Glynn NW, Ferrucci L. Assessing fatigability in mobility-intact older adults. J Am Geriatr Soc. 2014;62:347–351. doi: 10.1111/jgs.12638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simonsick EM, Glynn NW, Jerome GJ, Shardell M, Schrack JA, Ferrucci L. Fatigued, but not frail: perceived fatigability as a marker of impending decline in mobility-intact older adults. J Am Geriatr Soc. 2016;64:1287–1292. doi: 10.1111/jgs.14138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simonsick EM, Montgomery PS, Newman AB, Bauer DC, Harris T. Measuring fitness in healthy older adults: the health ABC long distance corridor walk. J Am Geriatr Soc. 2001;49:1544–1548. doi: 10.1046/j.1532-5415.2001.4911247.x [DOI] [PubMed] [Google Scholar]
- 21. Simonsick EM, Fan E, Fleg JL. Estimating cardiorespiratory fitness in well-functioning older adults: treadmill validation of the long distance corridor walk. J Am Geriatr Soc. 2006;54:127–132. doi: 10.1111/j.1532-5415.2005.00530.x [DOI] [PubMed] [Google Scholar]
- 22. Simonsick EM, Newman AB, Nevitt MC, et al. ; Health ABC Study Group. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the health ABC study. J Gerontol A Biol Sci Med Sci. 2001;56:M644–M649. [DOI] [PubMed] [Google Scholar]
- 23. Stone JL, Norris AH. Activities and attitudes of participants in the Baltimore longitudinal study. J Gerontol. 1966;21:575–580. [DOI] [PubMed] [Google Scholar]
- 24. Simonsick EM, Newman AB, Visser M, et al. ; Health, Aging and Body Composition Study. Mobility limitation in self-described well-functioning older adults: importance of endurance walk testing. J Gerontol A Biol Sci Med Sci. 2008;63:841–847.doi:63/8/841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Borg G. Psychophysical scaling with applications in physical work and the perception of exertion. Scand J Work Environ Health. 1990;16(suppl 1):55–58. [DOI] [PubMed] [Google Scholar]
- 26. Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn. 1957;35:307–315. [PubMed] [Google Scholar]
- 27. Schrack JA, Leroux A, Fleg JL, et al. Using heart rate and accelerometry to define quantity and intensity of physical activity in older adults. J Gerontol A Biol Sci Med Sci. 2018;73:668–675. doi: 10.1093/gerona/gly029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people–results from the health, aging and body composition study. J Am Geriatr Soc. 2005;53:1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x [DOI] [PubMed] [Google Scholar]
- 29. Schrack JA, Cooper R, Koster A, et al. Assessing daily physical activity in older adults: unraveling the complexity of monitors, measures, and methods. J Gerontol A Biol Sci Med Sci. 2016;71:1039–1048. doi: 10.1093/gerona/glw026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shiroma EJ, Schrack JA, Harris TB. Accelerating accelerometer research in aging. J Gerontol A Biol Sci Med Sci. 2018;73:619–621. doi: 10.1093/gerona/gly033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Varma VR, Dey D, Leroux A, et al. Re-evaluating the effect of age on physical activity over the lifespan. Prev Med. 2017;101:102–108. doi: 10.1016/j.ypmed.2017.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rejeski WJ, Marsh AP, Brubaker PH, et al. ; LIFE Study Investigators. Analysis and interpretation of accelerometry data in older adults: the LIFE study. J Gerontol A Biol Sci Med Sci. 2016;71:521–528. doi: 10.1093/gerona/glv204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rejeski WJ, Walkup MP, Fielding RA, et al. ; LIFE Study Investigators. Evaluating accelerometry thresholds for detecting changes in levels of moderate physical activity and resulting major mobility disability. J Gerontol A Biol Sci Med Sci. 2018;73:660–667. doi: 10.1093/gerona/glx132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Corbett DB, Valiani V, Knaggs JD, Manini TM. Evaluating walking intensity with hip-worn accelerometers in elders. Med Sci Sports Exerc. 2016;48:2216–2221. doi: 10.1249/MSS.0000000000001018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schrack J, Zipunnikov V, Crainiceanu C. Electronic devices and applications to track physical activity. JAMA. 2015;313:2079–2080. doi: 10.1001/jama.2015.3877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schrack JA, Zipunnikov V, Goldsmith J, Bandeen-Roche K, Crainiceanu CM, Ferrucci L. Estimating energy expenditure from heart rate in older adults: a case for calibration. PLoS One. 2014;9:e93520. doi: 10.1371/journal.pone.0093520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goldsmith J, Zipunnikov V, Schrack J. Generalized multilevel function-on-scalar regression and principal component analysis. Biometrics. 2015;71:344–353. doi: 10.1111/biom.12278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Clarke J, Janssen I. Sporadic and bouted physical activity and the metabolic syndrome in adults. Med Sci Sports Exerc. 2014;46:76–83. doi: 10.1249/MSS.0b013e31829f83a0 [DOI] [PubMed] [Google Scholar]
- 39. Holman RM, Carson V, Janssen I. Does the fractionalization of daily physical activity (sporadic vs. bouts) impact cardiometabolic risk factors in children and youth?PLoS One. 2011;6:e25733. doi: 10.1371/journal.pone.0025733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cummings SR, Studenski S, Ferrucci L. A diagnosis of dismobility–giving mobility clinical visibility: a mobility working group recommendation. JAMA. 2014;311:2061–2062. doi: 10.1001/jama.2014.3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schrack JA, Simonsick EM, Ferrucci L. The energetic pathway to mobility loss: an emerging new framework for longitudinal studies on aging. J Am Geriatr Soc. 2010;58(suppl 2):S329–S336. doi: 10.1111/j.1532-5415.2010.02913.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yates FE, Benton LA. Loss of integration and resiliency with age: a dissipative destruction. Compr Physiol. 2011. doi:10.1002/cphy.cp110122 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.