Abstract
Clinical and experimental studies show that age-related decline in circulating insulin-like growth factor-1 (IGF-1) levels promotes the pathogenesis of intracerebral hemorrhages, which critically contribute to the development of vascular cognitive impairment and disability in older adults. Yet, the mechanisms by which IGF-1 deficiency compromises structural integrity of the cerebral vasculature are not completely understood. To determine the role of IGF-1 deficiency in pathological remodeling of middle cerebral arteries (MCAs), we compared alterations in vascular mechanics, morphology, and remodeling-related gene expression profile in mice with liver-specific knockdown of IGF-1 (Igf1f/f + TBG-Cre-AAV8) and control mice with or without hypertension induced by angiotensin-II treatment. We found that IGF-1 deficiency resulted in thinning of the media and decreased wall-to-lumen ratio in MCAs. MCAs of control mice exhibited structural adaptation to hypertension, manifested as a significant increase in wall thickness, vascular smooth muscle cell (VSMC) hypertrophy, decreased internal diameter and up-regulation of extracellular matrix (ECM)-related genes. IGF-1 deficiency impaired hypertension-induced adaptive media hypertrophy and dysregulated ECM remodeling, decreasing elastin content and attenuating adaptive changes in ECM-related gene expression. Thus, circulating IGF-1 plays a critical role in maintenance of the structural integrity of cerebral arteries. Alterations of VSMC phenotype and pathological remodeling of the arterial wall associated with age-related IGF-1 deficiency have important translational relevance for the pathogenesis of intracerebral hemorrhages and vascular cognitive impairment in elderly hypertensive patients.
Keywords: Vascular aging, Remodeling, Vascular smooth muscle cell, IGF-1, Neuroendocrine aging, Hypertension
There is strong evidence that the dramatic age-related decline in circulating levels of insulin-like growth factor 1 (IGF-1) contributes to vascular aging and promotes the development of vascular cognitive impairment (reviewed in (1)). IGF-1 is a pleiotropic growth factor that possesses multifaceted vasoprotective effects, including pro-angiogenic, anti-apoptotic, anti-oxidative, anti-inflammatory, anti-atherogenic and endothelial protective effects (1–6). Recent studies suggest that normal IGF-1 levels are also important to maintain the structural integrity of the cerebral microcirculation and that IGF-1 deficiency promotes the development of spontaneous intracerebral hemorrhages (ICHs) (6–8).
Larger ICHs account for over 13% of all strokes and often result in death or major disability. Small ICHs, known as “microhemorrhages” (diameter: <5 mm in humans) develop due to rupture of small intracerebral vessels (9). Their prevalence may reach 50% in older individuals at risk, which is considered of emerging importance as a contributing factor to the progressive impairment of neuronal function (10,11). Hypertension, which has an estimated prevalence of 66% in older adults (>65 years), is a critical risk factor for ICHs in older adults and there is strong evidence that aging exacerbates hypertension-induced ICHs both patients and experimental animals (9,12,13). Using mice with adult-onset isolated circulating IGF-1 deficiency we have recently demonstrated that low IGF-1 levels increase propensity for ICHs by exacerbating the effects of hypertension, mimicking the aging phenotype (6).
Resilience of the arterial wall to injury/rupture induced by mechanical stresses is determined by multiple factors, including the structure and function of vascular smooth muscle cells (VSMCs), the amount, composition, and organization/structure of the extracellular matrix (ECM), and adequate structural and functional adaptation to increased blood pressure. The critical role of ECM alterations in cardiovascular aging processes in general (14) and the pathogenesis of ICHs in particular (9) is widely recognized. Despite the growing evidence that IGF-1 deficiency promotes vascular injury/rupture and that IGF-1 in vitro regulates VSMC proliferation and ECM deposition, the pathophysiological link between circulating IGF-1 deficiency and structural alteration of cerebral arteries has never been studied.
The present study was designed to test the hypothesis that circulating IGF-1 deficiency promotes the development of a pro-fragility vascular phenotype, by dysregulating ECM composition and/or by promoting structural maladaptation to hypertension. To test our hypothesis, we used a novel murine model of aging: mice with isolated endocrine IGF-1 deficiency induced by adeno-associated viral knockdown of IGF-1 specifically in the liver using Cre-lox technology (Igf1f/f + TBG-Cre-AAV8) (6). We induced chronic hypertension in IGF-1 deficient mice and respective controls (by treatment with angiotensin II [Ang-II]) and compared biomechanical characteristics and structural remodeling and its molecular signature in middle cerebral arteries (MCAs).
Results
Circulating IGF-1 Levels and Blood Pressure Measurements
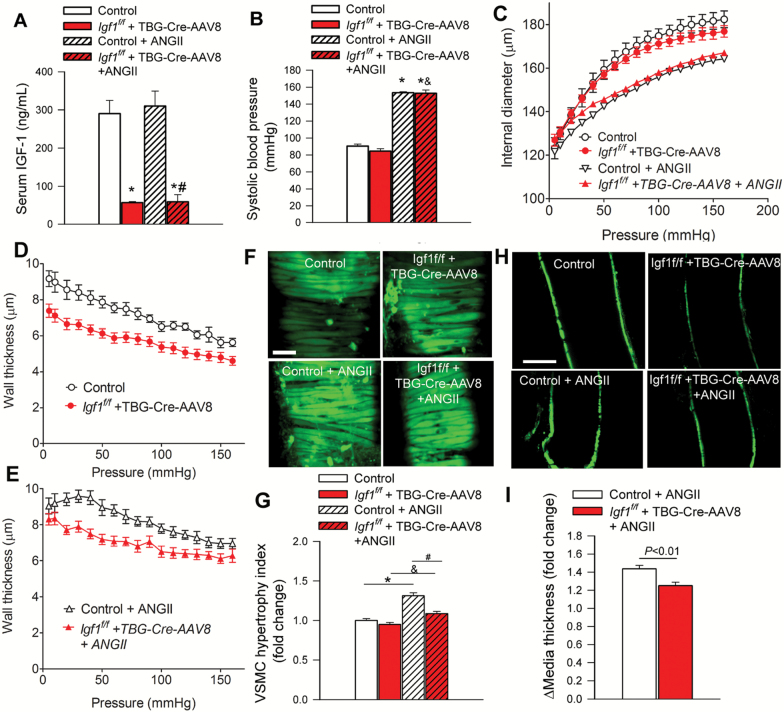
Mice receiving TBG-Cre-AAV8 had significantly lower serum IGF-1 levels compared with control mice receiving TBG-eGFP-AAV8 (Figure 1A) (3). Treatment with Ang II resulted in comparable increases in blood pressure in both IGF-1 deficient mice and their age-matched controls (Figure 1B).
Figure 1.
Circulating IGF-1 deficiency promotes medial atrophy in cerebral vessels. (A) Adeno-associated viral knockdown of hepatic Ifg1 (Igf1f/f + TBG-Cre-AAV8) significantly decreases the levels of circulating IGF-1 compared to control animals. (B) Treatment with angiotensin II elicited similar increases in systolic blood pressure in control and IGF-1 deficient mice. *p < .05 vs control normotensive. (C) Pressure-diameter curves of middle cerebral arteries (MCAs) from each experimental group under passive conditions. (D, E) Pressure-wall thickness curves of MCAs under passive conditions. Representative confocal micrographs of VSMCs in the medial layer of calcein-loaded MCAs, imaged in the longitudinal-circumferential plane (F, scale bar: 5 μm) and at midsection in the longitudinal plane (H, scale bar: 80 μm). Note that after removal of the adventitia the medial vascular smooth muscle cells (VSMCs) are predominantly stained. As an index for cellular hypertrophy the average diameter of the midsection of VSMCs were measured (G). (I) Bar graphs are summary data for hypertension-induced relative changes in media thickness measured at midsection of MCAs. Adaptive hypertrophy of the medial layer of MCAs in hypertensive control mice is evident, whereas this adaptive response is impaired in IGF-1 deficient mice. Data are mean ± SEM. (n = 11–27 for each data point) *p < .05 vs control; #p < .05 vs control+AngII; &p < .05 vs IGF-1 deficient. Differences between groups were established using a one-way ANOVA followed by Tukey’s post hoc tests.
IGF-1 Deficiency Alters Arterial Morphology, Vascular Mechanics, and Impairs Structural Adaptation of MCAs to Hypertension
Changes in the passive internal diameter and wall thickness of MCAs derived from each experimental group as a function of intraluminal pressure are depicted in Figure 1C–E. The most conspicuous effect of circulating IGF-1 deficiency on arterial morphology in normotensive mice was a significant decrease in wall thickness of MCAs (Figure 1D). We found that MCAs of control mice exhibited structural adaptation to hypertension, manifested as a significant increase in wall thickness (Figure 1E), decreased internal diameter (Figure 1C) and consequential increases of wall-to-lumen ratios (not shown). Imaging of the calcein loaded VSMCs in the arterial wall (Figure 1F and H) revealed that increase in wall thickness was, at least in part, due to medial VSMC hypertrophy (Figure 1G and I). Diameters in MCAs during maximal dilatation were also smaller in hypertensive IGF-1 deficient mice than in normotensive IGF-1 deficient mice at all levels of intraluminal pressure (Figure 1C). Wall thickness of MCAs isolated from hypertensive IGF-1 deficient mice was significantly smaller than wall thickness of MCAs derived from hypertensive control mice (Figure 1E). Hypertension-induced adaptive hypertrophy of medial VSMCs was significantly reduced in MCAs derived from IGF-1 deficient mice (Figure 1G and I).
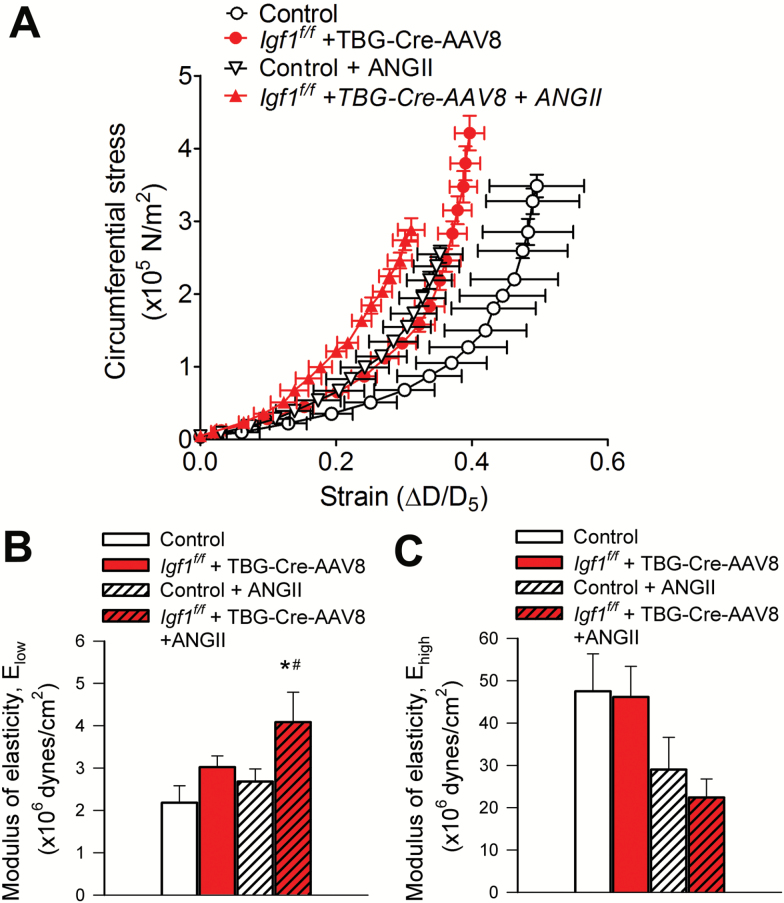
The stress-strain curve in MCAs of hypertensive control mice was shifted to the left of the curve obtained in MCAs of normotensive control mice (Figure 2A) indicating stiffening of cerebral arteries. IGF-1 deficiency itself was associated with a leftward shift and an increased level of circumferential stress in the stress–strain relationship of the MCAs (Figure 2A). Infusion of Ang-II to IGF-1 deficient mice resulted in a further shift to the left of the stress–strain curve of MCAs (Figure 2A), indicating the presence of exacerbated vessel stiffness. Elow, which reflects the contribution of elastin to vascular elastic behavior, tended to decease in MCAs from normotensive IGF-1 deficient mice, but the difference did not reach statistical significance. Elow was significantly increased in MCAs of hypertensive IGF-1 deficient mice, indicating decreased elasticity (Figure 2B). Elow in MCAs from hypertensive control mice did not differ statistically from that in normotensive control vessels. Ehigh was not statistically different in the four experimental groups (Figure 2C).
Figure 2.
IGF-1 deficiency reduces elasticity of cerebral arteries. (A) Strain–stress relationship curves of middle cerebral arteries (MCAs) isolated from normotensive control, hypertensive control (control+Ang II) and normotensive IGF-1 deficient (Igf1f/f + TBG-Cre-AAV8) and hypertensive IGF-1 deficient mice (Igf1f/f +TBG-Cre-AAV8 + AngII) under passive conditions at different intravascular pressures. (B) Elastic moduli of MCAs under passive conditions. Elow and Ehigh represent the slope of the linear regressions in the lower and the higher range of intravascular pressures, respectively. At low pressures elastin, at high pressures collagen dominate the elastic behavior of the arteries. Higher values indicate increased arterial stiffness. Data are means ± SEM (n =6–11 for each data point). *p < .05 vs control; #p < .05 vs control+AngII. Differences between groups were established using a one-way ANOVA followed by Tukey’s post hoc tests.
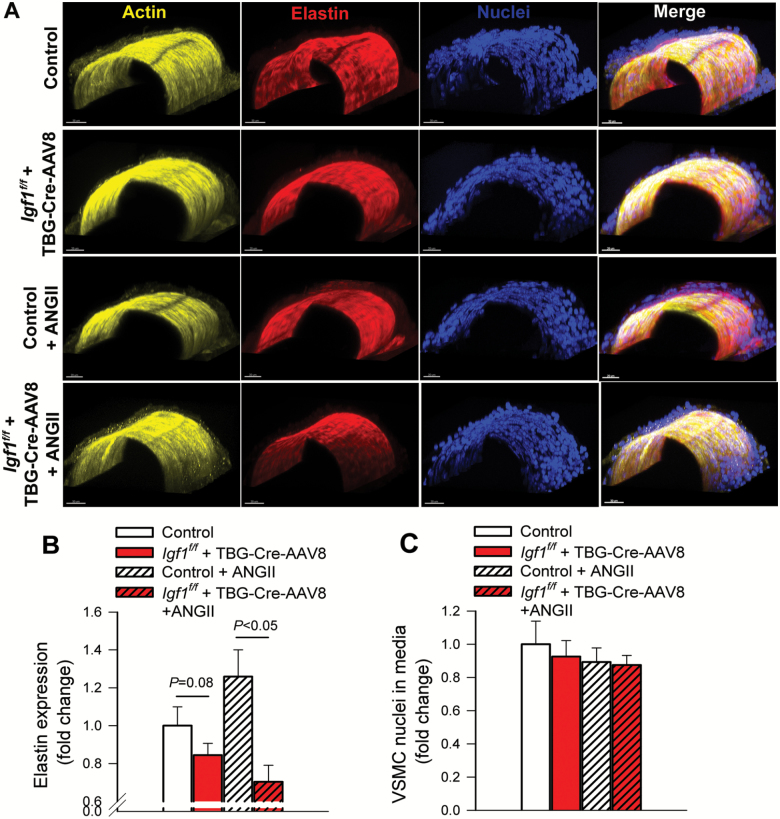
Confocal and multi-photon microscopy was used to further assess the composition of the vascular wall in MCAs (Figure 3A). These experiments revealed a trend for decreased elastin content in the wall of MCAs with IGF-1 deficiency, which became more prominent after induction of hypertension (Figure 3B). There was no significant change in the number of medial VSMC nuclei either with hypertension or with IGF-1 deficiency (Figure 3C).
Figure 3.
IGF-1 deficiency reduces elastin content in cerebral arteries. (A–E) Representative confocal images of middle cerebral arteries (MCAs) isolated from normotensive control, hypertensive control (control+Ang II) and normotensive IGF-1 deficient (Igf1f/f + TBG-Cre-AAV8) and hypertensive IGF-1 deficient mice (Igf1f/f +TBG-Cre-AAV8 + AngII; see Methods for details) showing F-actin (yellow), elastin (red) and nuclei (blue). Scale bar: 20 μm. (B) IGF-1 deficiency is associated with a significant reduction in arterial elastin content. (C) Vascular smooth muscle cell number, represented by nuclei contained within the medial layer of MCAs. Data are means ± SEM (n = 5–7 for each data point).
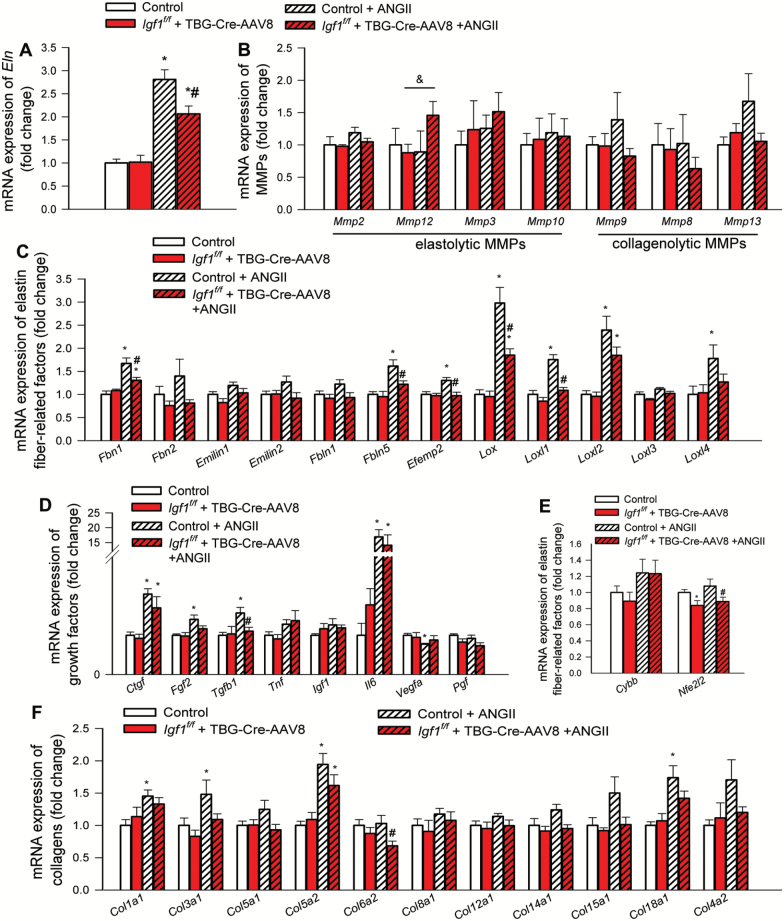
IGF-1 Deficiency Impairs Hypertension-Induced Adaptive Changes in ECM-Related Vascular Gene Expression Profile
Expression of genes involved in regulation of synthesis, assembly and degradation of ECM components and remodeling processes was determined by qPCR. We found that in MCAs IGF-1 deficiency significantly inhibited hypertension-induced changes in mRNA expression of Eln (Figure 4A), factors regulating elastin fiber assembly and remodeling and ECM crosslinking (Figure 4C), growth factors that regulate vascular remodeling processes (Figure 4D) and collagens (Figure 4F), whereas it did not exert marked effects on MMP expression (Figure 4B). Among factors involved in vascular redox regulation we found that IGF-1 deficiency tended to down-regulate expression of the antioxidative transcription factor Nrf2 (Nfe2l2) (Figure 4E).
Figure 4.
IGF-1 deficiency impairs hypertension-induced changes in gene expression signature associated with vascular remodeling. qPCR data showing mRNA expression of (A) elastin (Eln), (B) elastolytic and collagenolytic matrix metalloproteinases (MMPs), (C) factors regulating elastin fiber assembly and remodeling (fibrillin-1 [Fbn1], fibrillin-1 [Fbn2], Emilin1, Emilin2, fibulin-1 [Fbln1], fibulin-5 [Fbln5] and fibulin-4 [Efemp2]) and ECM crosslinking (the lysyl oxidases Lox, Loxl1, Loxl2, Loxl3, Loxl4), (D) growth factors and cytokines that regulate vascular remodeling processes, (E) factors involved in vascular redox regulation and ROS-mediated MMP activation, including the NADPH oxidase subunit NOX2 (Cybb) and the antioxidative transcription factor Nrf2 (Nfe2l2), and (F) collagens in middle cerebral arteries isolated from normotensive control, hypertensive control (control+Ang II) and normotensive IGF-1 deficient (Igf1f/f + TBG-Cre-AAV8) and hypertensive IGF-1 deficient mice (Igf1f/f +TBG-Cre-AAV8 + AngII; see Methods for details). Data are mean ± SEM (n = 6 for each data point), *p < .05 vs control,#p < .05 vs control+AngII. Differences between different groups were established using a one-way ANOVA followed by Tukey’s post hoc tests.
Discussion
The results of this study suggest that circulating IGF-1 deficiency results in weakening and thinning of the cerebral arterial wall and promotes structural maladaptation of these vessels to hypertension, due to media hypotrophy, decreased elasticity, and dysregulation of ECM components.
Circulating IGF-1 levels significantly decline with age in humans, which is thought to promote vascular aging processes (1). Our findings provide new evidence that IGF-1 deficiency contributes to remodeling and structural weakening of mouse cerebral arteries, mimicking many aspects of vascular aging. With advanced age in cerebral arteries the wall thickness/wall-to-lumen ratio and vascular distensibility are decreased, whereas wall circumferential stress is increased, which are associated with a leftward shift in the stress–strain relationship of cerebral arteries (15). This is the first study to demonstrate that IGF-1 deficiency per se results in aging-like alterations in the morphology and the mechanical properties of cerebral arteries. Previous studies confirm that IGF-1 levels significantly correlate with aortic strain and aorta stiffness in humans (16) and that aorta stiffness also increases in mouse models of IGF-1 deficiency, including the “Little” mouse (Ghrhrlit/lit, generated by a missense mutation in the hypothalamic GH-releasing hormone receptor) (17).
In agreement with previous studies (18), we found that hypertension in control mice induces adaptive hypertrophic remodeling in cerebral arteries, with a reduction in internal diameter, as well as media hypertrophy and changes in the expression of ECM components. Hypertension in humans also often results in hypertophic remodeling with increased media-to-lumen ratio of cerebral vessels (19). Media hypertrophy and ECM remodeling are adaptive processes that reduce tensile stress, protecting the integrity of the vascular wall in hypertension. In addition, the aforementioned structural changes also exert protective effects on the downstream cerebral microvessels by increasing segmental hydrodynamic resistance, preventing the penetration of high pressure to the vulnerable distal portion of the cerebral vascular tree. Adaptive remodeling results in a leftward shift in the stress–strain relationship of cerebral arteries from hypertensive control mice, similar to vessels of spontaneously hypertensive rats (20,21). The stress–strain curve is also shifted leftward in cerebral arteries from patients with essential hypertension (19), indicating vessel stiffening. Previous studies demonstrate that structural changes of cerebral arteries are also associated with functional adaptation of these vessels to higher systemic blood pressure (22). In particular, in hypertension the myogenic constriction of cerebral arteries is enhanced and the range of cerebrovascular autoregulation is extended, which contribute to protection of the cerebral microcirculation from high pressure (22). There is strong evidence that aging impairs both structural and functional adaptation of cerebral arteries to hypertension (12,22). In particular, aging compromises vascular resilience by altering both media hypertrophy and ECM remodeling (22). The available evidence suggests that aging results in loss of elasticity due to reduced elastin deposition and/or increased elastin fragmentation that occurs due to increased expression/activity of MMPs (12,23). Aging also impairs autoregulatory protection in the brain, exacerbating hypertension-induced cerebrovascular injury (12,22,24). It has been proposed that the aforementioned aging-induced structural and functional cerebrovascular alterations promote the genesis of ICHs in hypertensive older individuals (9).
Several lines of evidence support the concept that structural and functional adaptation of cerebral arteries to hypertension is dysfunctional in mice with IGF-1 deficiency, mimicking the aging phenotype. First, our present findings demonstrate that hypertension-induced adaptive changes in cerebral arterial morphology and vascular mechanics are impaired in IGF-1 deficient mice. Second, previous studies demonstrate that hypertension-induced hypertrophic remodeling in penetrating arterioles is also impaired in IGF-1 deficiency (6). Third, our results show that IGF-1 deficiency impairs adaptive changes in vascular elastin content and expression of ECM components and factors that regulate ECM assembly, crosslinking, and remodeling. Fourth, previous studies demonstrate that in hypertensive IGF-1 deficient mice autoregulation is markedly disrupted, and their MCAs do not show adaptive increases in myogenic constriction (3).
Adaptive changes in the medial smooth muscle layer and the ECM play key roles in maintaining arterial wall resilience, preventing injury and rupture of the arterial wall. Our findings have important translational relevance for the pathogenesis of multiple diseases that compromise the integrity of the arterial wall. Larger ICHs are the second most common causes of strokes, whose incidence significantly increases with age and which have a high case fatality (13). Despite significant advance in prevention of ischemic strokes, the incidence of ICHs did not decrease over the past decades (13). Furthermore, advanced imaging methods can detect small ICHs (cerebral microhemorrhages) in close to 50% of high risk older populations, which predict cognitive decline and subsequent larger ICHs (9).
Previous studies (6–8) and our present findings underscore the likely pathogenic role of age-related IGF-1 deficiency in loss of arterial resilience and the pathogenesis of ICHs. There is strong clinical (25,26) and experimental (12) evidence that aging and hypertension synergistically interact to promote the development of ICHs. We recently demonstrated that similar to aging, IGF-1 deficiency also renders the cerebral vessels significantly more vulnerable to high pressure-induced rupture, exacerbating ICHs (6). Dysregulation of elastin is likely a critical mechanism involved in the pathogenesis of ICHs, as decreases in elastin content in cerebral vessels after treatment with elastase results in formation of cerebral aneurysms and promotes development of ICHs (27).
In IGF-1 deficiency aortic stiffening increases pulse pressure and pathological arterial remodeling and loss of autoregulatory protection likely act synergistically to allow high pulsatile pressure to penetrate the vulnerable distal portion of the arterial tree, facilitating development of small ICHs (3,6,28). Further translational studies are evidently needed to determine whether in older adults and in animal models of aging and IGF-1 deficiency pathological remodeling of both larger and smaller cerebral arteries can be reversed and incidence of larger ICHs and microhemorrhages can be decreased by interventions that increase circulating IGF-1 levels. In addition, our current findings may also have relevance to the pathogenesis of cerebral aneurysms, which should be established by future studies. ECM secretion by VSMCs also plays a critical role in the formation of the protective fibrous cap in atherosclerotic lesions. Our findings are consistent with the concept that by modulating VSMC phenotype and promoting synthesis of ECM components IGF-1 may stabilize the fibrous cap (29). We predict that interventions that increase IGF-1 levels in older patients may confer multifaceted vasoprotective effects, improving structure and function of both smaller and larger cerebral arteries. These effects are synergistic with the beneficial effects of IGF-1 on cerebral blood flow regulation and blood brain barrier function suggested by previous pre-clinical studies (2,3,5).
The IGF-1 dependent mechanisms responsible for altered VSMC phenotype, pathological remodeling of ECM and maladaptation to the altered hemodynamic environment in hypertension are likely multifaceted. There is strong evidence that that IGF-1 regulates VSMC growth and proliferation (30) and regulates synthesis of elastin (31,32), collagens (33) and other ECM components. These effects on ECM are critical for vessel structure. For example, a study using seven inbred rat strains demonstrated that there is a significant correlation between circulating IGF-1 levels and ECM composition in the aorta (34). Long-term GH treatment in aged rats, which significantly increases circulating IGF-1, was also reported to increase media thickness and restore ECM content of the aorta (35). Further, treatment with IGF-1 was also shown to stabilize atherosclerotic plaques by promoting VSMC proliferation and ECM deposition, preventing intraplaque bleedings (33,36).
There is also extensive evidence that IGF-1 modulates mechanosensitive gene expression in VSMCs, regulating adaptive responses to cyclic stretch, which mimics hypertension. For example, stretch up-regulates expression of IGF-1 receptor (37) rendering cells more sensitive to IGF-1 and blocking IGF-1 signaling attenuates the protective proliferative effects of mechanical stretch (38,39). Mechanisms underlying IGF-1 mediated stretch based signaling and its role in remodeling of the ECM are incompletely understood, although PI3K/AKT signaling and Sirt2 have been clearly implicated (39,40).
Previous studies also suggest a critical role for increased hypertension-induced ROS production and free radical-mediated activation of MMPs in VSMCs in the pathogenesis of ICHs (6,12). Activated MMPs degrade components of the ECM, promoting localized weakening of the vascular wall. Although IGF-1 deficiency does not appear to be associated with marked changes in vascular expression of MMPs, our recent studies demonstrate that IGF-1 deficiency exacerbates hypertension-induced MMP activation in the wall of cerebral arteries. Oxidative stress-induced MMP activation (41) likely contributes to the decreased elastin content, pathologic remodeling, and increased vascular fragility (6) observed in IGF-1 deficient mice. The mechanisms underlying exacerbated hypertension-induced vascular oxidative stress in aging involve up-regulation of NADPH oxidases, increased mitochondrial ROS production and deficiency of Nrf2-driven antioxidant defense mechanisms (12,42–44). Importantly, attenuation of ROS production by these sources prevents ICHs in aged mice (12) and other studies have shown that IGF-1 can block oxidative stress-induced cell death (45). The available evidence suggest that IGF-1 deficiency impairs the same pathways involved in redox regulation in the vascular wall similar to aging, including mtROS production and the Nrf2-dependent antioxidant defense system (4,46). Thus, future preclinical studies are warranted to determine the protective effects of interventions that attenuate oxidative stress on ECM homeostasis, vascular resilience, and the development of ICHs in models of IGF-1 deficiency as well.
Limitations of the Study
A number of important limitations of the present study need to be considered. First, data on protein expression of the investigated ECM-related target genes should be obtained in future studies. For example, dysregulation of fibrillin-1 at the protein level should be demonstrated. Fibrillin-1 is a large, ECM glycoprotein that serves as a structural component of elastin-associated extracellular microfibrils, which provide force bearing structural support in elastic arteries. Importantly, in mice underexpressing fibrillin-1 inflammation-mediated elastolysis and functional collapse of the elastic lamellae in the aortic wall were reported, which associated with aneurysm formation (47). Second, IGF-1 is a potent survival factor for VSMCs, thus it would be also informative to assess apoptosis in cerebral arteries. Third, the presence of numerous interchain cross-links is essential for the mechanical stability of collagen and elastin fibers. The pathophysiological importance of lysyl oxidase-derived cross-linking was established from animal studies in which lysyl oxidase was inhibited pharmacologically. This results in decreased cross-linking of collagen and elastin which associates with increased occurrence of aortic aneurysms (48).). Dysregulation of mRNA expression of Fibrillin-1 is also potentially interesting. Because IGF-1 deficiency appears to have a major effect on expression of lysyl oxidases, in future studies it would be interesting to elucidate the functional consequences of these findings.
In conclusion, our results add to the growing evidence that circulating IGF-1 contributes to the maintenance of the structural integrity of cerebral arteries (Figure 5). Our present and previous findings suggest that phenotypic changes in VSMCs induced by IGF-1 deficiency result in weakening and thinning of the cerebral arterial wall and promote structural and functional maladaptation of these vessels to hypertension, due to media hypotrophy, dysregulation of ECM components, increased oxidative stress-mediated MMP activation, and myogenic autoregulatory dysfunction. Alterations of VSMC phenotype and pathological remodeling of the arterial wall associated with age-related IGF-1 deficiency have important translational relevance for the pathogenesis of ICHs and vascular cognitive impairment in elderly hypertensive patients. The evidence available from preclinical studies (1), point to potential benefits of interventions preventing/reversing aging-induced reduction of circulating IGF-1 levels and promoting cerebrovascular health for prevention of ICHs and cognitive decline in older adults.
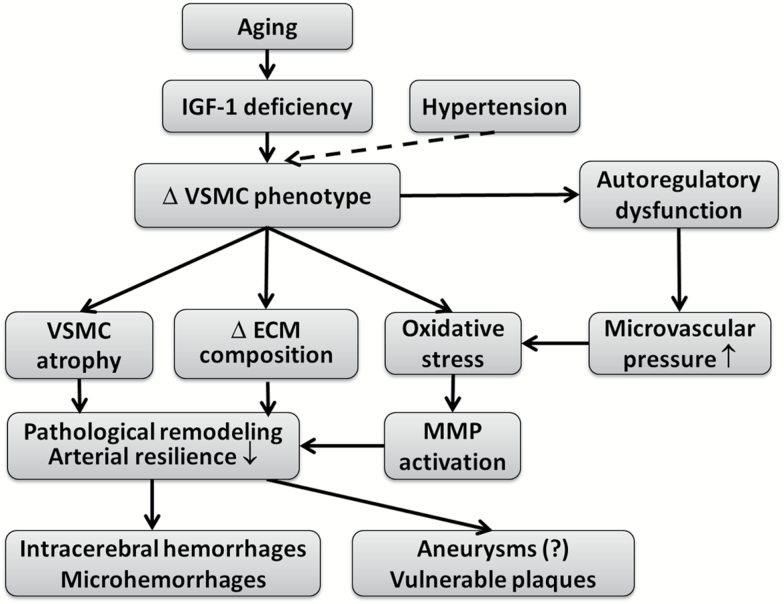
Figure 5.
Proposed scheme depicting the mechanisms by which age-related IGF-1 deficiency may promote pathological remodeling of cerebral arteries, reducing vascular resilience, and exacerbating the pathogenesis of intracerebral hemorrhages.
Experimental Procedures
All procedures were approved by and followed the guidelines of the Institutional Animal Care and Use Committee of the University of Oklahoma HSC.
Induction of Adult-Onset IGF-1 Deficiency in Mice
Male mice homozygous for a floxed exon 4 of the Igf1 gene (Igf1f/f; in a C57BL/6 background) were purchased from Jackson Laboratories. These mice have the entirety of exon 4 of the Igf1 gene flanked by loxP sites, which allows for genomic excision of this exon when exposed to Cre recombinase. Transcripts of the altered Igf1 gene yield a protein upon translation that fails to bind the IGF-1 receptor. Circulating IGF-1 is produced in the liver. Circulating IGF-1 deficiency was induced in Igf1f/f mice by adeno-associated virus (AAV8)-mediated expression of Cre recombinase in the liver at 4 months of age, as reported (3,6). In order to avoid dwarfism knockdown of IGF-1 was achieved in adult animals (body mass at 10 months of age; control: 29 ± 1 g, IGF-1 deficient: 27 ± 0.5 g). The AAV8 vector was purchased from the University of Pennsylvania Viral Vector Core (Penn Vector Core, Philadelphia, PA; http://www.med.upenn.edu/gtp/vectorcore). Although AAV8 is effective at transducing multiple tissues, the use of thyroxine-binding globulin (TBG) promoter allows for the restriction of expression to hepatocytes. Validation of the model has been published (3). At 4 months of age, Igf1f/f mice were randomly assigned to two groups and were administered approximately 1.3×1010 viral particles of AAV8-TBG-Cre or AAV8-TBG-eGFP via retro-orbital injection, as described (3). Animals were housed in the Rodent Barrier Facility at OUHSC under specific pathogen-free barrier conditions, on a 12 h light/12 h dark cycle, with access to standard rodent chow (Purina Mills, Richmond, IN) and water ad libitum.
Measurement of Serum IGF-1 Levels
IGF-1 concentration in the serum samples was measured by ELISA (R&D Systems, Minneapolis, MN) as reported (3).
Induction of Hypertension
To study the effects of IGF-1 deficiency on hypertension-induced vascular remodeling we used the previously well-characterized mouse model of Ang-II-dependent hypertension (3,22). Briefly, in 10 months old male IGF-1 deficient mice (Igf1f/f + TBG-Cre-AAV, n = 60) and respective age-matched control mice (Igf1f/f + TBG-eGFP-AAV8, n = 60) hypertension was induced by administration Ang-II (s.c. via osmotic mini-pumps [Alzet Model 2006, Durect Co, Cupertino, CA]). Pumps were filled either with saline (vehicle) or Ang II solutions (Sigma) that delivered (subcutaneously) 1 µg/min/kg of Ang II for 28 days thus generating four experimental groups: (a) Igf1f/f + TBG-Cre-AAV8 + Ang II, (b) Igf1f/f + TBG-Cre-AAV8 + vehicle, (c) Igf1f/f + TBG-eGFP-AAV8 + Ang II, and (d) Igf1f/f + TBG-eGFP-AAV8 + vehicle. Since aging is associated with increased activity of the vascular renin–angiotensin system and Ang II-dependent hypertension is common among older individuals, Ang II-dependent hypertension is a clinically highly relevant model to study aging-related cerebrovascular alterations (22). Further, there is strong evidence that the in addition to elevated pressure, the renin–angiotensin system is a critical determinant of vascular remodeling during chronic hypertension (49–51). Blood pressure of the animals was recorded before the treatment and at the end of the treatment period using the tail-cuff method, as described (12).
Determination of Arterial Structural and Mechanical Characteristics
On day 28 post-implantation mice were decapitated, the brains were removed and segments of the MCAs were isolated using microsurgery instruments for functional studies, as reported (3,22). In brief, segments of MCAs were mounted onto two glass micropipettes in an organ chamber in oxygenated (21% O2, 5% CO2, 75% N2) Ca2+ free Krebs’ buffer (pH ~7.4; at 37°C). Inflow and outflow pressures were controlled and measured by a pressure servo-control system (Living Systems Instrumentation, Burlington, VE). Vessels were studied at in situ length. The absence of leaks was verified by observing no changes in intraluminal pressure over 3 min upon turning off the pressure servo-control system.
To study the structural and mechanical characteristics of the arteriolar wall, pressure–diameter curves were obtained under passive conditions (Ca2+-free Krebs’ solution plus 10–6 mol/L nimodipine and 2mmol/L EGTA). Internal diameter and wall (left and right) thicknesses were recorded in response changes in intraluminal pressure (from 5 and 160 mmHg) using a custom-built videomicroscope system as reported (3,22). To assess media thickness and VSMC hypertrophy, the VSMCs within the wall of the MCAs were loaded with fluorescent dye calcein AM (20 µM; in the organ bath for 30 min). The vessels were imaged (at 70 mmHg) using a Leica SP2 MP confocal laser scanning microscope.
Circumferential strain, circumferential stress, and Young’s modulus of elasticity were all calculated using the morphometric data obtained. Vascular remodeling and VSMC hypertrophy were assessed as changes in the passive internal diameter of the MCAs and the dimensions of the VSMCs, respectively.
The circumferential strain was calculated as the difference between the intraluminal diameter obtained under passive conditions at each intraluminal pressure level (Dp) and the diameter at the lowest pressure tested (D5mmHg) divided by the diameter at lowest pressure as described (52):
Circumferential stress is the force per unit area exerted by the arterial wall in opposition to the distending pressure. Circumferential stress was calculated by using the formula for thin-walled vessels, where P is the intraluminal pressure, and τ is wall thickness (52):
The Young’s modulus of elasticity provides information about arterial stiffness. Blood vessels exhibit an overall non-linear stress–strain behavior, which can be separated in two portions depending on the level of intravascular pressure in order to separate the elastic modulus dominated by loading mechanics of elastic fibers and that dominated by collagen fiber mechanics (52). We chose the first three values in the lower rage of intravascular pressures to provide information on the low Young’s modulus of elasticity (Elow). At low pressures, elastin dominates the elastic behavior of the artery. At higher pressures, the dominant element is collagen and we chose the last three values of intraluminal pressure range to determine the high Young modulus of elasticity (Ehigh). Elow and Ehigh represent the slope of the linear regressions fitted onto the stress-strain curves corresponding to these pressure ranges.
Confocal/Multiphoton Microscopy Imaging of MCAs
To assess changes in elastin content and cellularity within the medial layer in MCAs, in separate experiments cannulated MCAs were fixed in 4% paraformaldehyde, while pressurized at 70 mmHg for 1 h. The vessels were rinsed twice in phosphate-buffered saline (PBS) and once in 0.1 M Glycine for 5 min each time. Cannulated vessels were flushed with 1 mL PBS to rinse their lumen and permeabilized via incubation in 0.5% Triton X-100 for 20 min. Vessels were washed twice in PBS and incubated for 1 h in 0.5 μg/mL 4′,6-diamidino-2-phenylindole (DAPI), 0.2 μM Alexa Fluor 633 Hydrazide (Molecular Probes) and 0.02 μM Alexa Fluor 546 phalloidin (Molecular Probes) in PBS. After being washed three times in PBS, vessels were imaged using a Leica SP5 confocal/multiphoton microscope with a 63×/1.2 numerical aperture water objective. Alexa Fluor 633, to image elastin, was excited with a 633 nm HeNe laser. Alexa Fluor 546 phalloidin, to image F-actin components, was excited with a 543 nm HeNe laser. DAPI, to image nuclei, was excited with a multi-photon laser at 720 nm. Images were processed, and all channels were quantified to determine the total volume (number of expressed voxels) occupied by VSMC nuclei, elastin and actin as previously described (53) with a MATLAB algorithm available upon request.
Determination of Expression Changes in Vascular Resilience-Related Genes by qPCR
Using text mining approaches, we recently identified important genes relevant for the pathogenesis of ICHs and structural integrity of the vasculature (6). In the present study, we used a custom-designed microfluidic card-based TaqMan qPCR arrays and a Stratagene MX3000 platform to assess mRNA expression of elastin (Eln), elastolytic and collagenolytic matrix metalloproteinases (MMPs), factors regulating elastin fiber assembly and remodeling (fibrillin-1 [Fbn1], fibrillin-1 [Fbn2], Emilin1, Emilin2, fibulin-1 [Fbln1], fibulin-5 [Fbln5] and fibulin-4 [Efemp2]) and ECM crosslinking (the lysyl oxidases Lox, Loxl1, Loxl2, Loxl3, Loxl4), growth factors and cytokines that regulate vascular remodeling processes, factors involved in vascular redox regulation and ROS-mediated MMP activation, including the NADPH oxidase subunit NOX2 (Cybb) and the antioxidative transcription factor Nrf2 (Nfe2l2) and collagens in MCAs isolated from normotensive control, hypertensive control and normotensive IGF-1 deficient and hypertensive IGF-1 deficient mice. Total RNA was isolated with a Mini RNA Isolation Kit (Zymo Research, Orange, CA) and was reverse transcribed using Superscript III RT (Invitrogen). Quantification was performed using the ΔΔCq method. The relative quantities of the reference genes Hprt, Ywhaz, B2m, Gapdh, Actb, and S18 were determined and a normalization factor was calculated based on the geometric mean.
Statistical Analysis
An a priori power analysis was performed to ensure 80% or greater power for the primary outcome measures, considering the findings of previous studies (6) and pilot experiments. Differences between groups were established using a one-way ANOVA followed by Tukey’s post hoc tests. p < .05 was considered significant. Data are expressed as mean ± SEM. Statistical analyses were conducted using Prism 5 software (GraphPad).
Funding
This work was supported by grants from the American Heart Association (to ST, MNVA), the National Institute on Aging (R01-AG055395 to ZU, R01-AG047879 to AC; R01-AG038747), the National Institute of Neurological Disorders and Stroke (NINDS; R01-NS056218 to AC, R01-NS100782 to ZU), the National Center for Complementary and Alternative Medicine (R01-AT006526 to ZU), the National Heart, Lung and Blood Institute (NHLBI, R01-HL-088105 to LAML), the NIA-supported Geroscience Training Program in Oklahoma (T32AG052363), the NIA-supported Oklahoma Nathan Shock Center (to ZU and AC; 3P30AG050911-02S1), NIH-supported Oklahoma Shared Clinical and Translational Resources (to AY, NIGMS U54GM104938), College of Medicine Alumni Association (to AY), the Hungarian Academy of Sciences Bolyai Research Scholarship BO/00634/15, the PTE AOK-KA 3/2016 04.01/F, NKFI-FK123798 and UNKP-17-4-I-PTE-7 (to PT), the Oklahoma Center for the Advancement of Science and Technology (to AC, ZU, AY), the Presbyterian Health Foundation (to ZU, AC, AY), the EU-funded Hungarian grant EFOP-3.6.1-16-2016-00008, and the Reynolds Foundation (to ZU and AC).
Acknowledgments
Author contribution: GF, LAML, AC and ZU designed research; GF, FIRP, TK, ST, PT, MNVA, AY, PT, ZU, AC performed experiments; GF, FIRP, LAML, ST, PT, AY, SMC, PB, ZU, AC analyzed and interpreted data; GF, AC and ZU wrote the manuscript, TK, FIRP, ST, MNVA, AY, PB, PT, SMC, and LAML revised the article.
Conflict of interest
None declared.
References
- 1. Sonntag WE, Deak F, Ashpole N, et al. Insulin-like growth factor-1 in CNS and cerebrovascular aging. Front Aging Neurosci. 2013;5:27. doi:10.3389/fnagi.2013.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Toth P, Tarantini S, Ashpole NM, et al. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell. 2015;14:1034–1044. doi:10.1111/acel.12372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Toth P, Tucsek Z, Tarantini S, et al. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab. 2014;34:1887–1897. doi:10.1038/jcbfm.2014.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bailey-Downs LC, Mitschelen M, Sosnowska D, et al. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:313–329. doi:10.1093/gerona/glr164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tarantini S, Tucsek Z, Valcarcel-Ares MN, et al. Circulating IGF-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the mouse hippocampus and retrosplenial cortex: implications for cerebromicrovascular and brain aging. Age (Dordr). 2016;38:273–289. doi:10.1007/s11357-016-9931-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, et al. Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype. Aging Cell. 2017;16:469–479. doi:10.1111/acel.12583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sonntag WE, Carter CS, Ikeno Y, et al. Adult-onset growth hormone and insulin-like growth factor I deficiency reduces neoplastic disease, modifies age-related pathology, and increases life span. Endocrinology. 2005;146:2920–2932. doi:10.1210/en.2005-0058 [DOI] [PubMed] [Google Scholar]
- 8. Kuramoto K, Tahara S, Sasaki T, et al. Spontaneous dwarf rat: a novel model for aging research. Geriatr Gerontol Int. 2010;10:94–101. doi:10.1111/j.1447-0594.2009.00559.x [DOI] [PubMed] [Google Scholar]
- 9. Ungvari Z, Tarantini S, Kirkpatrick AC, Csiszar A, Prodan CI. Cerebral microhemorrhages: mechanisms, consequences, and prevention. Am J Physiol Heart Circ Physiol. 2017;312:H1128–H1143. doi:10.1152/ajpheart.00780.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Akoudad S, Wolters FJ, Viswanathan A, et al. Association of cerebral microbleeds with cognitive decline and dementia. JAMA Neurol. 2016;73:934–943. doi:10.1001/jamaneurol.2016.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Poels MM, Ikram MA, van der Lugt A, et al. Cerebral microbleeds are associated with worse cognitive function: the Rotterdam Scan Study. Neurology. 2012;78:326–333. doi:10.1212/WNL.0b013e3182452928 [DOI] [PubMed] [Google Scholar]
- 12. Toth P, Tarantini S, Springo Z, et al. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: role of resveratrol treatment in vasoprotection. Aging Cell. 2015;14:400–408. doi:10.1111/acel.12315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176. doi:10.1016/S1474-4422(09)70340-0 [DOI] [PubMed] [Google Scholar]
- 14. Meschiari CA, Ero OK, Pan H, Finkel T, Lindsey ML. The impact of aging on cardiac extracellular matrix. Geroscience. 2017;39:7–18. doi:10.1007/s11357-017-9959-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Diaz-Otero JM, Garver H, Fink GD, Jackson WF, Dorrance AM. Aging is associated with changes to the biomechanical properties of the posterior cerebral artery and parenchymal arterioles. Am J Physiol Heart Circ Physiol. 2016;310:H365–H375. doi:10.1152/ajpheart.00562.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hopkins KD, Lehmann ED, Gosling RG, Parker JR, Sonksen PH. Biochemical correlates of aortic distensibility in vivo in normal subjects. Clin Sci (Lond). 1993;84:593–597. doi:10.1042/cs0840593 [DOI] [PubMed] [Google Scholar]
- 17. Reddy AK, Hartley CJ, Pham TT, Darlington G, Entman ML, Taffet GE. Young little mice express a premature cardiovascular aging phenotype. J Gerontol A Biol Sci Med Sci. 2014;69:152–159. doi:10.1093/gerona/glt055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baumbach GL, Heistad DD. Remodeling of cerebral arterioles in chronic hypertension. Hypertension. 1989;13:968–972. [DOI] [PubMed] [Google Scholar]
- 19. Rizzoni D, De Ciuceis C, Porteri E, et al. Altered structure of small cerebral arteries in patients with essential hypertension. J Hypertens. 2009;27:838–845. doi:10.1097/HJH.0b013e32832401ea [DOI] [PubMed] [Google Scholar]
- 20. Izzard AS, Graham D, Burnham MP, Heerkens EH, Dominiczak AF, Heagerty AM. Myogenic and structural properties of cerebral arteries from the stroke-prone spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol. 2003;285:H1489–H1494. doi:10.1152/ajpheart.00352.2003 [DOI] [PubMed] [Google Scholar]
- 21. Izzard AS, Horton S, Heerkens EH, Shaw L, Heagerty AM. Middle cerebral artery structure and distensibility during developing and established phases of hypertension in the spontaneously hypertensive rat. J Hypertens. 2006;24:875–880. doi:10.1097/01.hjh.0000222757.54111.06 [DOI] [PubMed] [Google Scholar]
- 22. Toth P, Tucsek Z, Sosnowska D, et al. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab. 2013;33:1732–1742. doi:10.1038/jcbfm.2013.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duca L, Blaise S, Romier B, et al. Matrix ageing and vascular impacts: focus on elastin fragmentation. Cardiovasc Res. 2016;110:298–308. doi:10.1093/cvr/cvw061 [DOI] [PubMed] [Google Scholar]
- 24. Toth P, Csiszar A, Tucsek Z, et al. Role of 20-HETE, TRPC channels, and BKCa in dysregulation of pressure-induced Ca2+ signaling and myogenic constriction of cerebral arteries in aged hypertensive mice. Am J Physiol Heart Circ Physiol. 2013;305:H1698–H1708. doi:10.1152/ajpheart.00377.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Poels MM, Ikram MA, van der Lugt A, et al. Incidence of cerebral microbleeds in the general population: the Rotterdam Scan Study. Stroke. 2011;42:656–661. doi:10.1161/STROKEAHA.110.607184 [DOI] [PubMed] [Google Scholar]
- 26. Kato H, Izumiyama M, Izumiyama K, Takahashi A, Itoyama Y. Silent cerebral microbleeds on T2*-weighted MRI: correlation with stroke subtype, stroke recurrence, and leukoaraiosis. Stroke. 2002;33:1536–1540. doi:10.1161/01.STR.0000018012.65108.86 [DOI] [PubMed] [Google Scholar]
- 27. Hasan DM, Starke RM, Gu H, et al. Smooth muscle peroxisome proliferator-activated receptor gamma plays a critical role in formation and rupture of cerebral aneurysms in mice in vivo. Hypertension. 2015;66:211–220. doi:10.1161/HYPERTENSIONAHA.115.05332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smeda JS. Cerebral vascular changes associated with hemorrhagic stroke in hypertension. Can J Physiol Pharmacol. 1992;70:552–564. [DOI] [PubMed] [Google Scholar]
- 29. Higashi Y, Sukhanov S, Anwar A, Shai SY, Delafontaine P. Aging, atherosclerosis, and IGF-1. J Gerontol A Biol Sci Med Sci. 2012;67:626–639. doi:10.1093/gerona/gls102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen Y, Capron L, Magnusson JO, Wallby LA, Arnqvist HJ. Insulin-like growth factor-1 stimulates vascular smooth muscle cell proliferation in rat aorta in vivo. Growth Horm IGF Res. 1998;8:299–303. doi:10.1016/S1096-6374(98)80125-1 [DOI] [PubMed] [Google Scholar]
- 31. Badesch DB, Lee PD, Parks WC, Stenmark KR. Insulin-like growth factor I stimulates elastin synthesis by bovine pulmonary arterial smooth muscle cells. Biochem Biophys Res Commun. 1989;160:382–387. doi:0006-291X(89)91667-7 [DOI] [PubMed] [Google Scholar]
- 32. Wolfe BL, Rich CB, Goud HD, et al. Insulin-like growth factor-I regulates transcription of the elastin gene. J Biol Chem. 1993;268:12418–12426. [PubMed] [Google Scholar]
- 33. von der Thüsen JH, Borensztajn KS, Moimas S, et al. IGF-1 has plaque-stabilizing effects in atherosclerosis by altering vascular smooth muscle cell phenotype. Am J Pathol. 2011;178:924–934. doi:10.1016/j.ajpath.2010.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Behmoaras J, Osborne-Pellegrin M, Gauguier D, Jacob MP. Characteristics of the aortic elastic network and related phenotypes in seven inbred rat strains. Am J Physiol Heart Circ Physiol. 2005;288:H769–H777. doi:10.1152/ajpheart.00544.2004 [DOI] [PubMed] [Google Scholar]
- 35. Brüel A, Oxlund H, Nyengaard JR. Growth hormone increases the total number of cardiac myocyte nuclei in young rats but not in old rats. Mech Ageing Dev. 2002;123:1353–1362. doi:S0047637401004092 [DOI] [PubMed] [Google Scholar]
- 36. Shai SY, Sukhanov S, Higashi Y, Vaughn C, Kelly J, Delafontaine P. Smooth muscle cell-specific insulin-like growth factor-1 overexpression in Apoe-/- mice does not alter atherosclerotic plaque burden but increases features of plaque stability. Arterioscler Thromb Vasc Biol. 2010;30:1916–1924. doi:10.1161/ATVBAHA.110.210831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu G, Hitomi H, Hosomi N, et al. Mechanical stretch augments insulin-induced vascular smooth muscle cell proliferation by insulin-like growth factor-1 receptor. Exp Cell Res. 2011;317:2420–2428. doi:10.1016/j.yexcr.2011.07.016 [DOI] [PubMed] [Google Scholar]
- 38. Standley PR, Obards TJ, Martina CL. Cyclic stretch regulates autocrine IGF-I in vascular smooth muscle cells: implications in vascular hyperplasia. Am J Physiol. 1999;276:E697–E705. doi:10.1152/ajpendo.1999.276.4.E697 [DOI] [PubMed] [Google Scholar]
- 39. Cheng J, Du J. Mechanical stretch simulates proliferation of venous smooth muscle cells through activation of the insulin-like growth factor-1 receptor. Arterioscler Thromb Vasc Biol. 2007;27:1744–1751. doi:10.1161/ATVBAHA.107.147371 [DOI] [PubMed] [Google Scholar]
- 40. Wang L, Han Y, Shen Y, et al. Endothelial insulin-like growth factor-1 modulates proliferation and phenotype of smooth muscle cells induced by low shear stress. Ann Biomed Eng. 2014;42:776–786. doi:10.1007/s10439-013-0957-5 [DOI] [PubMed] [Google Scholar]
- 41. Wakisaka Y, Chu Y, Miller JD, Rosenberg GA, Heistad DD. Critical role for copper/zinc-superoxide dismutase in preventing spontaneous intracerebral hemorrhage during acute and chronic hypertension in mice. Stroke. 2010;41:790–797. doi:10.1161/STROKEAHA.109.569616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ungvari Z, Bailey-Downs L, Gautam T, et al. Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-{kappa}B activation in the nonhuman primate Macaca mulatta. J Gerontol A Biol Sci Med Sci. 2011;66:866–875. doi:10.1093/gerona/glr092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ungvari Z, Bailey-Downs L, Sosnowska D, et al. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Am J Physiol Heart Circ Physiol. 2011;301:H363–H372. doi:10.1152/ajpheart.01134.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Springo Z, Tarantini S, Toth P, et al. Aging exacerbates pressure-induced mitochondrial oxidative stress in mouse cerebral arteries. J Gerontol A Biol Sci Med Sci. 2015;70:1355–1359. doi:10.1093/gerona/glu244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li Y, Higashi Y, Itabe H, Song YH, Du J, Delafontaine P. Insulin-like growth factor-1 receptor activation inhibits oxidized LDL-induced cytochrome C release and apoptosis via the phosphatidylinositol 3 kinase/Akt signaling pathway. Arterioscler Thromb Vasc Biol. 2003;23:2178–2184. doi:10.1161/01.ATV.0000099788.31333.DB [DOI] [PubMed] [Google Scholar]
- 46. Csiszar A, Labinskyy N, Perez V, et al. Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am J Physiol Heart Circ Physiol. 2008;295:H1882–H1894. doi:10.1152/ajpheart.412.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pereira L, Lee SY, Gayraud B, et al. Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc Natl Acad Sci USA. 1999;96:3819–3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Martínez-Revelles S, García-Redondo AB, Avendaño MS, et al. Lysyl oxidase induces vascular oxidative stress and contributes to arterial stiffness and abnormal elastin structure in hypertension: role of p38MAPK. Antioxid Redox Signal. 2017;27:379–397. doi:10.1089/ars.2016.6642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Baumbach GL, Sigmund CD, Faraci FM. Cerebral arteriolar structure in mice overexpressing human renin and angiotensinogen. Hypertension. 2003;41:50–55. [DOI] [PubMed] [Google Scholar]
- 50. Chillon JM, Baumbach GL. Effects of an angiotensin-converting enzyme inhibitor and a beta-blocker on cerebral arterioles in rats. Hypertension. 1999;33:856–861. doi:10.1161/01.HYP.33.3.856 [DOI] [PubMed] [Google Scholar]
- 51. Hajdu MA, Heistad DD, Baumbach GL. Effects of antihypertensive therapy on mechanics of cerebral arterioles in rats. Hypertension. 1991;17:308–316. doi:10.1161/01.HYP.17.3.308 [DOI] [PubMed] [Google Scholar]
- 52. Pennington KA, Ramirez-Perez FI, Pollock KE, et al. Maternal hyperleptinemia is associated with male offspring’s altered vascular function and structure in mice. PLoS One. 2016;11:e0155377. doi:10.1371/journal.pone.0155377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bender SB, Castorena-Gonzalez JA, Garro M, et al. Regional variation in arterial stiffening and dysfunction in western diet-induced obesity. Am J Physiol Heart Circ Physiol. 2015;309:H574–H582. doi:10.1152/ajpheart.00155.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]