Abstract
Using multimodal neuroimaging methods, the current study was designed to examine the relationship between white matter microstructural integrity (WMI) and changes in prefrontal cortex (PFC) oxygenated hemoglobin (HbO2) during active walking in older adults. Consistent with neural inefficiency, we hypothesized that worse WMI would be associated with a greater increase in PFC HbO2 from single to dual-task walking in the context of worse or similar gait performance. Fifty-five cognitively healthy older adults (mean age = 74.76 years, 49% women) underwent diffusion tensor imaging (DTI) to derive a whole-brain measure of fractional anisotropy (FA) and functional Near Infrared Spectroscopy (fNIRS), which measured PFC HbO2 during walking tasks. Gait velocity was assessed using an instrumented walkway. A linear mixed effects model revealed that HbO2 levels increased from single to dual-task walking (P < 0.01) given the greater cognitive demands inherent in the latter condition. Moreover, WMI moderated the effect of dual tasking on PFC HbO2 (P < 0.05). Specifically, worse WMI was associated with a larger increase in PFC HbO2 levels from single to dual-task walking in the context of similar gait velocity. Results suggest that compromised WMI may be a mechanism underlying inefficient brain response to cognitive demands of locomotion.
Keywords: Brain Aging, Executive Function, Motor Activity, Translational
Decline in single- and dual-task gait is common in aging and is a reliable predictor of adverse outcomes that include falls, frailty, disability, transition to dementia, and mortality (1–4). Gait is considered a complex cognitive process involving executive functions that are subserved by the prefrontal cortex (PFC) (5,6). The executive demands of gait are often assessed with a well-validated walking paradigm (7,8), where individuals are required to walk at their normal pace under single- and dual-task walking while talking (DTW) conditions. Indeed, research using functional Near-Infrared Spectroscopy (fNIRS), a portable optical neuroimaging technology with capabilities for capturing hemodynamic activity during actual walking, demonstrated a robust increase in PFC oxygenation levels from single-task walking (STW) to DTW (9–14). These findings provide evidence for the key role of the PFC in cortical control of locomotion, notably under attention-demanding conditions. To some extent, increases in cortical activity may be an adaptive brain function to meet task demands, however it is widely held that more efficient neural processing utilizes fewer resources (15,16).
The brain is susceptible to structural and functional changes in normal aging that include degradation of microstructural white matter integrity (WMI) (17) and alterations in PFC activation (18,19). Evidence shows that poorer WMI is related to cognitive decline, particularly frontally-mediated executive processes (20–23) that are implicated in gait. Whole-brain fractional anisotropy (FA), a relative measure of microstructural WMI derived from diffusion tensor imaging (DTI), predicts greater variance in executive functioning compared to other measures of WMI (eg, mean diffusivity) (24). Degradation of WMI has also been linked to gait decline in aging, including slower gait velocity (25–27). Although there is evidence of association between WMI and higher-order cognitive functions, and WMI and gait impairment, the effect of WMI on task-related brain activation during real-time locomotion has not been directly examined. Hernandez et al. (28) found that patients with multiple sclerosis had greater increases in PFC oxygenated hemoglobin (HbO2) levels from normal walking to DTW compared to their healthy counterparts in the context of similar gait performance. This finding suggested that compromised WMI, as evidenced in patients with multiple sclerosis, may be a causal factor underlying inefficient brain activation during active walking. This study, however, did not directly assess WMI, thus limiting inferences about relationships between brain microstructure and functional activation.
Multimodal neuroimaging approaches combine structural and functional brain activation measures during information processing in the same individuals. This innovative combination allows us to investigate whether structural brain integrity might influence how efficiently the brain responds to task demands. Accumulating multimodal neuroimaging evidence shows that WMI is significantly associated with executive task-related cortical activation. Using functional magnetic resonance imaging (fMRI) to measure brain activity in older adults, Burzynska et al. (29) found a negative relationship between FA and brain activation whereby those with greater whole-brain FA showed smaller activation increases during an executive task relative to individuals with lower FA. Other researchers have demonstrated a similar negative relationship between FA and cortical activation as measured with fMRI (30–32). These results suggest WMI may be a structural brain moderator of altered neural efficiency.
Research of the neural substrate of gait has utilized single neuroimaging modalities that focus on either brain structure or function in isolation. Relations between brain structure and function during locomotion remain poorly understood. In fact, to date, there have been no published studies assessing WMI and functional activation during real-time locomotion in healthy older adults. The combination of DTI and fNIRS offers an opportunity to gain insight into how structural and functional brain systems interact in the context of locomotion. Specifically, an important question is whether WMI moderates PFC HbO2 levels during active walking. Based on multimodal imaging of other executive processes, it is conceivable that WMI is a predictor of cortical overactivation commonly observed during complex gait in older adults whereby less coherent wiring results in greater neural firing.
The current study was designed to examine the moderating effect of whole-brain microstructural WMI on PFC HbO2 levels, as assessed with fNIRS, under STW and DTW conditions in non-demented, community-dwelling older adults. Consistent with neural inefficiency (15,16), we hypothesized that worse WMI, as indicated by lower whole-brain FA, would be associated with a larger increase in PFC HbO2 levels from STW to DTW. To complement our understanding of brain structure–function relationships, a well-validated behavioral measure of walking, gait velocity, was evaluated and controlled for in our analyses.
Methods
Study Population
Participants were right-handed, non-demented community-dwelling older adults, who were enrolled in the Central Control of Mobility and Aging (CCMA) study. CCMA aims to identify cognitive and brain predictors of mobility. Details concerning study procedures have been described previously (6). CCMA recruits adults older than 65 years who reside in Westchester, NY. Exclusion criteria included: inability to speak or understand English; visual or auditory loss; inability to walk independently; recent hospitalization for a condition that affects mobility; residence in a nursing home; and diagnosis of a serious or acute illness, psychiatric condition, or neurodegenerative disease. During an initial phone interview, prospective participants were screened for dementia using a validated instrument (33). Exclusion criteria specific to MRI participation included left handedness, presence of a neurological gait disorder, and standard contraindications for MRI (eg, claustrophobia, surgically implanted metal devices). Written informed consent, approved by the university’s Institutional Review Board, was obtained from all participants.
Measures
Walking protocol
As described previously (6,9), participants completed two walking conditions: STW and DTW. In the STW condition, participants were asked to walk around an electronic walkway (see Zenometrics section below) at their normal pace for three consecutive loops, which consisted of six straight walks and five left-sided turns with demarcated start and end points. In the DTW condition, participants were asked to walk three consecutive loops at their normal pace while concurrently reciting alternate letters of the alphabet aloud starting with the letter “B”. Participants were instructed to pay equal attention to walking and the secondary verbal task, with explanation that tasks were to be prioritized equally. These test conditions were counterbalanced. Gait assessment was conducted in a quiet room. Participants completed the tasks with the fNIRS sensor attached to their forehead and they did not use any assistive devices while completing the walking tasks. Participants were instructed to wear comfortable footwear.
Quantitative gait assessment
ProKinetics Movement Analysis Software (PKMAS) was used to calculate gait velocity from footfalls measured on a 4 × 20 foot Zeno electronic walkway (Zenometrics, LLC: Peekskill, NY). Gait velocity (cm/s) was calculated by dividing the distance traveled by the time elapsed from commencement to conclusion of the three consecutive loops. Split-half intra-class correlations (ICC) for stride velocity in STW and DTW were greater than 0.95 revealing excellent internal consistency (9).
Magnetic resonance imaging
Magnetic resonance imaging was performed on a 3T Philips scanner (Achieva TX, Philips Medical Systems, Best, The Netherlands) with a 32-channel head coil. DTI data was acquired using a single-shot, spin echo EPI sequence with TE/TR—65/10000 ms, voxel size 3 mm isotropic, max b-factor = 800, 32 non-collinear directions, and SENSE acceleration factor 2.8. B0 field map images (gradient echo, TE/delta TE/TR = 2.4/2.3/20 ms, voxel size 4 mm isotropic) were collected to correct for EPI-related distortion. High-resolution T1-weighted images (MPRAGE—TE/TR/TI = 4.6/9.9/900 ms, voxel size 1 mm isotropic) were acquired to provide anatomical images for registration and segmentation of white matter.
Image preprocessing
DTI raw images were inspected for artifact, and were corrected for eddy currents and motion. DTI and high resolution T1-weighted structural images were brain extracted using BET from the FSL FMRIB Structural Toolkit (34). T1-weighted images were segmented into grey, white and CSF components using FAST from FSL’s FMRIB’s Automated Segmentation Tool (35), and whole-brain white matter masks were then generated by thresholding the white matter output at 50% and eroding with a three-pixel kernel to avoid the grey–white interface. DTI-derived maps of FA were obtained using the dtifit function from the FSL FMRIB Diffusion Toolkit (36), followed by distortion correction using the B0 field maps. Finally, whole-brain FA measures were obtained in T1 space by registering the FA maps to the subject’s T1 image (using FSL’s FLIRT package (37), six degrees of freedom) and multiplying the FA maps by the white matter mask, and calculating mean FA over the white matter mask.
Functional near-infrared spectroscopy
Acquisition and processing procedures have been described previously (9,38). The current study used the fNIRS Imager 1000 (fNIRS Devices, LLC, Potomac, MD) to measure changes in PFC HbO2 levels during STW and DTW. HbO2 levels were chosen over deoxygenated hemoglobin (Hb) levels for better signal-to-noise ratio, in particular superior reliability and sensitivity to locomotion-related cerebral blood flow (39). The fNIRS device utilizes a flexible circuit board containing four light-emitting diodes at peak wavelengths of 730, 805, and 850 nm, and 10 photoreceptors (light source and detectors are 2.5cm apart). The device was placed on participants’ forehead using a standard procedure based on landmarks from the international 10–20 system, and was fixed with two elasticized bands to prevent movement.
Hemodynamic signal extraction
fNIRS data was preprocessed by a separate researcher who was not involved in data collection for this experiment. Once artifacts were removed by visual inspection for movement artifacts, saturation and dark current levels and by applying a low-pass filter with cut-off frequency set at 0.14 Hz for respiration or other high frequency interference, the modified Beer–Lambert law with a constant differential pathlength factor (DPF) of 6 was used to transform raw intensity measurements of 730 and 850nm to a HbO2 and Hb signal for each of the 16 optodes. Noise (saturation or dark current levels) was observed in 14 percent of the data that were subsequently excluded. Prior to each walking task, a baseline measure of HbO2 was collected from participants by asking them to remain still in a standing position for 10-s with fixated gaze while counting silently. Baseline measures were adjusted to a mean value of zero and used to quantify change from an individualized HbO2 baseline for each walking condition. Using E-Prime 2.0 software (Psychology Software Tools, Inc.) on a separate computer, synchronized triggers were sent to the fNIRS and PKMAS systems to optimize acquisition and extraction of task-related hemodynamic changes. Additionally, in post-processing a time-synchronization method used the first recorded foot fall on the walkway and the end of the sixth and final straight walk as time stamps to determine fNIRS data extraction points. From this extracted data, average HbO2 levels in each optode were used for comparison between STW and DTW. Internal consistency of HbO2 measurements, determined by split-half ICCs within each task, was excellent for STW (0.830) and DTW (0.849) (9).
Additional measures
Factors that have been reported to, or may plausibly influence brain structure or function, were also included in analyses. These factors include participant’s age, sex, level of cognitive functioning as measured by the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (40), and a global health status (GHS) measure. Participant’s GHS was obtained from dichotomous ratings (ie, presence or absence) of 10 health conditions: diabetes; chronic heart failure; arthritis; hypertension; depression; stroke; Parkinson’s disease; chronic obstructive pulmonary disease; angina; and myocardial infarction (41).
Statistical Analysis
First, an unadjusted linear-mixed effects (LME) model, taking into account the 16 optodes and correlations across repeated measures, was used to confirm the expected increase in PFC HbO2 levels from STW to DTW. Then a paired-samples t-test was used to confirm the expected decline in gait velocity from STW to DTW.
A fully adjusted LME model was used to determine the effect of walking condition (STW vs DTW), FA, and their interaction on PFC HbO2 levels. Walking condition was entered as the two-level repeated within-subject factor and FA as the continuous between-subject factor. An interaction term of walking condition-by-FA value was included to examine whether FA moderated the change in HbO2 levels from single- to dual-task walking. Covariates included gait velocity, age, sex, RBANS total score, and GHS score. SPSS statistical software package, (version 24; SPSS, Inc., Chicago, IL) was used for all statistical analyses.
Sensitivity analyses
Given that subtle changes in WMI can be detected in as little as six months in the elderly (42), we examined whether the length of time interval between MRI and fNIRS data collection influenced the moderating effect of FA on the change in HbO2 levels from STW to DTW. Additionally, FA was dichotomized using the median (low FA vs high FA) to provide a visual depiction of the FA by task interaction. While the current study focused on changes in PFC HbO2 in repsonse to walking and as related to WMI, Hb levels across walking tasks were also assessed to confirm task-dependent hemodynamic changes.
Results
A total of 55 non-demented participants (mean age in years = 74.76 ± 4.97; %female = 49.1) were included in this study. The low mean disease comorbidity score (GHS = 1.44 ± 1.07) confirmed the relatively healthy nature of the sample. The mean RBANS total score (91.89 ± 11.06) was indicative of average overall cognitive function. Preliminary analyses of the data revealed one outlier whose gait velocity data was excluded from further analyses. Sample characteristics of the 55 participants, as well as mean values for PFC activation, whole-brain FA, and gait velocity are presented in Table 1.
Table 1.
Descriptive Statistics of Demographic Information, WMI, PFC HbO2 Levels and Gait Velocity (N = 55)
| Total Sample | Low WMI (n = 27) | Med-High WMI (n = 28) | |
|---|---|---|---|
| Age (range 65–88 years) | 74.76 (±4.97) | 75.67 (±5.83) | 73.89 (±3.88) |
| Gender (%female) | 49.1 | 44.4 | 53.6 |
| Education | 15.73 (±3.82) | 13.65 (±3.56) | 16.58 (±3.69) |
| Global health status score | 1.44 (±1.07) | 1.48 (±1.16) | 1.39 (±0.99) |
| RBANS total | 91.89 (±11.06) | 91.33 (±11.09) | 92.43 (±11.21) |
| Gait velocity—STW (cm/s) | 69.79 (±15.47) | 68.02 (±11.67) | 71.50 (±18.48) |
| Gait velocity—DTW (cm/s) | 60.47 (±12.62) | 60.06 (±11.76) | 60.86 (±13.62) |
| HbO2—STW (μM units) | 0.39 (±0.97) | 0.34 (±1.11) | 0.44 (± 0.81) |
| HbO2—DTW (μM units) | 0.90 (±1.54) | 1.09 (±1.61) | 0.71 (±1.45) |
| Whole-brain FA | 0.34 (±0.02) | 0.33 (±0.01) | 0.36 (±0.01) |
Note: DTW = dual-task walk; FA = fractional anisotropy; Global Health Status score potential range from 0 to 10; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status; STW = single-task walk.
Unadjusted analysis revealed that average HbO2 levels increased from STW to DTW (estimate = −0.57, 95% confidence interval [CI] = −0.68 to −0.46, P < 0.001). There was a main effect of walking condition on gait velocity such that velocity was decreased during DTW relative to STW; t (54) = 5.53, p < .001. There was a medium, positive correlation between our predictor variable, whole-brain FA, and STW gait velocity (r = .30, p = .024). The correlation between whole-brain FA and DTW gait velocity was not significant (r = .18, p = .183).
A fully adjusted LME model was used to examine the effects of walking condition, FA, and their interaction on PFC HbO2 levels. Results showed a significant main effect of walking condition (p = .009), indicating that PFC HbO2 levels significantly increased from STW to DTW. There was no significant main effect of FA (p = .370). There was, however, a significant task-by-FA interaction (p = .035), indicating that FA moderated the change in PFC HbO2 levels from STW to DTW, with lower FA associated with a greater change in PFC HbO2 from STW to DTW (see Table 2).
Table 2.
Linear Mixed-Effects Model Examining the Effects of Task, Whole-Brain WMI, and Their Interaction on PFC Oxygenation (N = 55)
| Variable | Estimate | t | 95%CI | p-value |
|---|---|---|---|---|
| STW versus DTW | −2.79 | −2.63 | [−4.88, −0.70] | .009 |
| FA | −4.75 | −0.90 | [−15.23, 5.72] | .370 |
| FA * task | 6.46 | 2.12 | [0.46, 12.47] | .035 |
| STW velocity | −0.01 | −0.70 | [−0.01, 0.01] | .485 |
| Age | 0.01 | 0.19 | [−0.02, 0.03] | .846 |
| Sex | −0.05 | −0.44 | [−0.28, 0.18] | .658 |
| GHS | −0.01 | −0.24 | [−0.13, 0.10] | .810 |
| RBANS total | 0.01 | 0.27 | [−0.01, 0.01] | .790 |
Note: DTW = dual-task walk; FA = fractional anisotropy; Global Health Status score potential range from 0 to 10; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status; STW = single-task walk.
Sensitivity analyses
The results reported here were based on a sample with significant range of time (0–347 days) between the acquisition of fNIRS and MRI data. Sensitivity analysis controlling for the time between fNIRS and MRI data collection was performed; the moderation effect of FA on the change in PFC HbO2 levels from STW to DTW remained significant (estimate = 6.25, 95% CI = 0.19 to 12.31, p = .043). The moderating effect remained significant and appeared stronger when considering only participants with less than 6-months between DTI and fNIRS data collection (n = 48; estimates = 16.92, 95% CI = 10.79 to 23.05, p < .001).
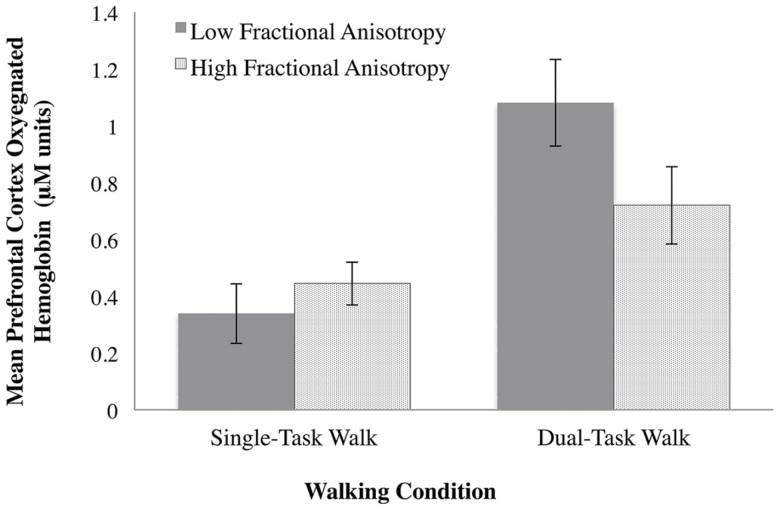
FA was used as a continuous variable in all statistical analyses. However, to visually depict the task by FA interaction, another LME model was executed using FA as a binary variable based on the median, comparing low FA subjects versus high FA subjects. The moderating effect of FA on the change in HbO2 levels from STW to DTW remained significant (estimate = −0.36, 95% CI = −0.58 to −0.13, p = .002; see Figure 1).
Figure 1.
Mean prefrontal cortex (PFC) oxygenated hemoglobin (HbO2) during single-task walk (STW) and dual-task walk (DTW) according to whole-brain fractional anisotropy (FA; low FA vs high FA).
Unadjusted analysis demonstrated that average Hb levels decreased from STW (mean = 0.23 ± 1.49) to DTW (mean = −0.18 ± 1.66; estimate = 0.40, 95% CI = −0.28 to 0.52, p < .001) in the opposite direction of HbO2 changes across walking tasks.
Discussion
Consistent with the extant literature, we found an increase in PFC HbO2 levels and a reduction in gait velocity from STW to DTW in healthy older adults. Using DTI and fNIRS, the current study was specifically designed to examine the moderating effect of microstructural WMI on the change in PFC HbO2 levels, assessed during active walking, from single- to dual-task conditions. We have found that individuals with lower overall FA showed a greater increase in PFC HbO2 levels from STW to DTW compared to those with higher FA. This finding suggests that poor WMI may underlie neural inefficiency during active walking under experimental conditions that manipulate cognitive demands. The positive association between whole-brain FA and gait velocity further supports this interpretation. That is, the behavioral context wherein a greater increase in PFC HbO2 levels across walking conditions among individuals with lower FA whose gait velocity was slower is consistent with current conceptualizations of brain inefficiency models (15). Moreover, it is noteworthy that the moderating effect of FA on the change in HbO2 levels remained significant when controlling for age and gait velocity. Daselaar et al. (32) aptly describe this phenomenon as “less wiring, more firing”.
The directionality of the moderating effect of whole-brain FA on the change in PFC HbO2 levels from single to DTW is consistent with negative structure–function relationships demonstrated in other studies focusing on executive processes using fMRI (29–32). Our findings extend these results by showing the negative structure–function relationship exists during real-time locomotion. Our results are also consistent with studies using fNIRS in subclinical and clinical samples of older adults with conditions associated with WMI declines (ie, individuals with slow gait, multiple sclerosis, and neurological gait abnormalities), which demonstrated an inefficient increase in PFC activity in response to DTW (11,28,38). Our replication of these results in a neurologically healthy older sample with normal gait, suggests the moderating effect of FA on cortical activity may precede the onset of clinical syndromes.
The moderating effect of FA on HbO2 levels across walking tasks when controlling for gait velocity suggests that larger increases in PFC activity from STW to DTW for those with poorer structural connections might not have been futile. These findings might also be consistent with neural compensation theory, which posits that overactivation coupled with preserved behavioral performance, in our case gait velocity, represents a compensatory effort to maintain performance in light of declining brain capacity and function (43–45). In our case, it appears poorer structural integrity is related to a brain that is working harder to successfully meet demands, albeit inefficiently relative to a brain consuming less energy.
In this sample, there was a significant range of time between acquisition of fNIRS and MRI data. Sensitivity analyses considering only participants with less than 6 months between imaging data collection replicated the moderating effect of FA on the change in PFC activation from STW to DTW and appeared stronger in comparison to the entire sample. The difference in strength but not directionality of the association between FA and the change in PFC activation levels during walking may be attributed to subtle changes in WMI that have been documented in as little as 6 months in the elderly (42). Our findings suggest that the time interval between acquisition of structural and functional neuroimaging data should be minimized in order to better characterize complex interactions of brain systems vis-à-vis gait and other cognitive functions. Moreover, longitudinal research tracking changes in both WMI and functional activation will be important.
A key strength of the current study is the novel combination of DTI and fNIRS allowing investigation of the effects of WMI on brain activation during real-time locomotion as opposed to common multimodal neuroimaging research that has been limited to examining imagined gait conditions due to requirement that participants lie motionless during data acquisition (46,47). A number of limitations of the current study, however, should be considered. Given the relatively healthy nature and normal overall cognitive functioning of this sample, the generalizability of these findings to populations with cognitive, neurological, or medical impairments must be determined with future studies. Previous research by our group has demonstrated increased PFC activation during DTW relative to both walking and cognitive single tasks (10,14). Given the small sample size and focus on the brain substrate of gait in the current study, we restricted this investigation to two walking conditions to the exclusion of a cognitive single-task condition (ie, talking). In addition to the PFC, other cortical regions are implicated in locomotion but were not assessed with our fNIRS system. Nonetheless, executive functions supported by the PFC are particularly vulnerable to age-related neurocognitive changes and is of consequent to adverse outcomes and functional independence in the elderly. While we controlled for overall cognitive status in this sample, it will be important for future research to assess whether cognitive functions, executive functions in particular, influence the moderating effects reported herein. It will also be important to include spatiotemporal gait variables beyond velocity to further expand understanding of relationships between WMI, PFC activation, and gait. The fNIRS system in the current study did not include short source-detector channels to account for the influence of extracerebral signals (eg, blood flow in skin or connective tissue). Given this study was a comparison of PFC HbO2 across walking tasks, which were counterbalanced, it is expected that any confounding effects of extracerebral signals would appear across both tasks, thus not influence our results or conclusions. It is also possible that age and changes in WMI influenced fNIRS optical features such as the DPF. Currently, it is unknown whether and how WMI may influence optical features and this requires further investigation. While formulas have been devised to account for the effect of age on DPF (48), such formulas require further examination in older populations (age 70+). Furthermore, the current study did not utilize advanced filtering (eg, wavelet or spline) to account for movement artifact. We note, however, that of the data eliminated, over 90% were due to saturation or dark current levels in the measurements, which were detected and removed by algorithmic filtering and visual inspection of the data conducted by one of the authors (MI), who was blind to experimental condition and not involved in statistical analyses. Examination of PFC activation was based solely on HbO2 levels due to evidence of better signal-to-noise ratio, in particular superior reliability and sensitivity to locomotion-related cerebral blood flow, relative to Hb (39). Nonetheless, sensitivity analyses demonstrated an expected task effect in Hb with a significant reduction in Hb from STW to DTW, further supporting our interpretation reported herein regarding changes in hemodynamic activity in response to an increasingly demanding walking task rather than systemic changes.
Despite a number of limitations, this study provides necessary initial evidence of structural brain substrate of neural efficiency during locomotion, unconstrained by specific tract or region of interest. Although it will be important for future multimodal imaging research to investigate region- and tract-specific effects of microstructural integrity on brain activation during locomotion, apparent age-related white matter changes in tracts or regions may, in fact, represent a global aging brain effect (49). Certainly, others have found a high degree of shared variance among white matter tracts (50).
Conclusion
Individuals with lower whole-brain FA demonstrated an inefficient neural response in the PFC to the increased demands of dual-task gait after controlling for gait velocity. These findings have significance for cognitive aging theories as they pertain to the central control of gait.
Funding
This work was supported by the National Institute on Aging grants (R01AG036921, R01AG044007).
Conflicts of Interest
Dr. Izzetoglu has a very minor share in the company that manufactures the fNIRS device used in this study. All other authors have no conflicts of interest to report in relation to the current article.
References
- 1. Verghese J, Holtzer R, Lipton RB, Wang C. Mobility stress test approach to predicting frailty, disability, and mortality in high-functioning older adults. J Am Geriatr Soc. 2012;60:1901–1905. doi: 10.1111/j.1532-5415.2012.04145.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Montero-Odasso MM, Sarquis-Adamson Y, Speechley M, et al. Association of dual-task gait with incident dementia in mild cognitive impairment: results from the gait and brain study. JAMA Neurol. 2017;74:857–865. doi: 10.1001/jamaneurol.2017.0643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc. 2012;60:2127–2136. doi: 10.1111/j.1532-5415.2012.04209.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008;23:329–42; quiz 472. doi: 10.1002/mds.21720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holtzer R, Wang C, Verghese J. Performance variance on walking while talking tasks: theory, findings, and clinical implications. Age (Dordr). 2014;36:373–381. doi: 10.1007/s11357-013-9570-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coppin AK, Shumway-Cook A, Saczynski JS, et al. Association of executive function and performance of dual-task physical tests among older adults: analyses from the InChianti study. Age Ageing. 2006;35:619–624. doi: 10.1093/ageing/afl107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Holtzer R, Verghese J, Xue X, Lipton RB. Cognitive processes related to gait velocity: results from the Einstein Aging Study. Neuropsychology. 2006;20:215–223. doi: 10.1037/0894-4105.20.2.215 [DOI] [PubMed] [Google Scholar]
- 9. Holtzer R, Mahoney JR, Izzetoglu M, Wang C, England S, Verghese J. Online fronto-cortical control of simple and attention-demanding locomotion in humans. Neuroimage. 2015;112:152–159. doi: 10.1016/j.neuroimage.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holtzer R, Mahoney JR, Izzetoglu M, Izzetoglu K, Onaral B, Verghese J. fNIRS study of walking and walking while talking in young and old individuals. J Gerontol A Biol Sci Med Sci. 2011;66:879–887. doi: 10.1093/gerona/glr068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen M, Pillemer S, England S, Izzetoglu M, Mahoney JR, Holtzer R. Neural correlates of obstacle negotiation in older adults: an fNIRS study. Gait Posture. 2017;58:130–135. doi: 10.1016/j.gaitpost.2017.07.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mirelman A, Maidan I, Bernad-Elazari H, et al. Increased frontal brain activation during walking while dual tasking: an fNIRS study in healthy young adults. J Neuroeng Rehabil. 2014;11:85. doi: 10.1186/1743-0003-11-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holtzer R, Yuan J, Verghese J, Mahoney JR, Izzetoglu M, Wang C. Interactions of subjective and objective measures of fatigue defined in the context of brain control of locomotion. J Gerontol A Biol Sci Med Sci. 2017;72:417–423. doi: 10.1093/gerona/glw167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu CF, Liu YC, Yang YR, Wu YT, Wang RY. Maintaining gait performance by cortical activation during dual-task interference: a functional near-infrared spectroscopy study. PLoS One. 2015;10:e0129390. doi: 10.1371/journal.pone.0129390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Neubauer AC, Fink A. Intelligence and neural efficiency. Neurosci Biobehav Rev. 2009;33:1004–1023. doi: 10.1016/j.neubiorev.2009.04.001 [DOI] [PubMed] [Google Scholar]
- 16. Haier RJ, Siegel BV, Nuechterlein KH, et al. Cortical glucose metabolic rate correlates of abstract reasoning and attention studied with positron emission tomography. Intelligence. 1988;12:199–217. doi: 10.1016/0160-2896(88)90016-5 [DOI] [Google Scholar]
- 17. Madden DJ, Bennett IJ, Song AW. Cerebral white matter integrity and cognitive aging: contributions from diffusion tensor imaging. Neuropsychol Rev. 2009;19:415–435. doi: 10.1007/s11065-009-9113-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nyberg L, Salami A, Andersson M, et al. Longitudinal evidence for diminished frontal cortex function in aging. Proc Natl Acad Sci USA. 2010;107:22682–22686. doi: 10.1073/pnas.1012651108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rajah MN, D’Esposito M. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–1983. doi: 10.1093/brain/awh608 [DOI] [PubMed] [Google Scholar]
- 20. Davis SW, Dennis NA, Buchler NG, White LE, Madden DJ, Cabeza R. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage. 2009;46:530–541. doi: 10.1016/j.neuroimage.2009.01.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen NK, Song AW. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim Biophys Acta. 2012;1822:386–400. doi: 10.1016/j.bbadis.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gold BT, Powell DK, Xuan L, Jicha GA, Smith CD. Age-related slowing of task switching is associated with decreased integrity of frontoparietal white matter. Neurobiol Aging. 2010;31:512–522. doi: 10.1016/j.neurobiolaging.2008.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zahr NM, Rohlfing T, Pfefferbaum A, Sullivan EV. Problem solving, working memory, and motor correlates of association and commissural fiber bundles in normal aging: a quantitative fiber tracking study. Neuroimage. 2009;44:1050–1062. doi: 10.1016/j.neuroimage.2008.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schiavone F, Charlton RA, Barrick TR, Morris RG, Markus HS. Imaging age-related cognitive decline: a comparison of diffusion tensor and magnetization transfer MRI. J Magn Reson Imaging. 2009;29:23–30. doi: 10.1002/jmri.21572 [DOI] [PubMed] [Google Scholar]
- 25. Bhadelia RA, Price LL, Tedesco KL, et al. Diffusion tensor imaging, white matter lesions, the corpus callosum, and gait in the elderly. Stroke. 2009;40:3816–3820. doi: 10.1161/STROKEAHA.109.564765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Callisaya ML, Beare R, Phan TG, et al. Brain structural change and gait decline: a longitudinal population-based study. J Am Geriatr Soc. 2013;61:1074–1079. doi: 10.1111/jgs.12331 [DOI] [PubMed] [Google Scholar]
- 27. Bruijn SM, Van Impe A, Duysens J, Swinnen SP. White matter microstructural organization and gait stability in older adults. Front Aging Neurosci. 2014;6:104. doi: 10.3389/fnagi.2014.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hernandez ME, Holtzer R, Chaparro G, et al. Brain activation changes during locomotion in middle-aged to older adults with multiple sclerosis. J Neurol Sci. 2016;370:277–283. doi: 10.1016/j.jns.2016.10.002 [DOI] [PubMed] [Google Scholar]
- 29. Burzynska AZ, Garrett DD, Preuschhof C, et al. A scaffold for efficiency in the human brain. J Neurosci. 2013;33:17150–17159. doi: 10.1523/JNEUROSCI.1426-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhu Z, Johnson NF, Kim C, Gold BT. Reduced frontal cortex efficiency is associated with lower white matter integrity in aging. Cereb Cortex. 2015;25:138–146. doi: 10.1093/cercor/bht212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Persson J, Nyberg L, Lind J, et al. Structure-function correlates of cognitive decline in aging. Cereb Cortex. 2006;16:907–915. doi: 10.1093/cercor/bhj036 [DOI] [PubMed] [Google Scholar]
- 32. Daselaar SM, Iyengar V, Davis SW, Eklund K, Hayes SM, Cabeza RE. Less wiring, more firing: low-performing older adults compensate for impaired white matter with greater neural activity. Cereb Cortex. 2015;25:983–990. doi: 10.1093/cercor/bht289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65:559–564. doi: 10.1212/01.wnl.0000172958.95282.2a [DOI] [PubMed] [Google Scholar]
- 34. Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424 [DOI] [PubMed] [Google Scholar]
- 36. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- 37. Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1006/nimg.2002.1132 [DOI] [PubMed] [Google Scholar]
- 38. Holtzer R, Verghese J, Allali G, Izzetoglu M, Wang C, Mahoney JR. Neurological gait abnormalities moderate the functional brain signature of the posture first hypothesis. Brain Topogr. 2016;29:334–343. doi: 10.1007/s10548-015-0465-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leff DR, Orihuela-Espina F, Elwell CE, et al. Assessment of the cerebral cortex during motor task behaviours in adults: a systematic review of functional near infrared spectroscopy (fNIRS) studies. Neuroimage. 2011;54:2922–2936. doi: 10.1016/j.neuroimage.2010.10.058 [DOI] [PubMed] [Google Scholar]
- 40. Thaler NS, O’Rourke JJ, Scott JG, Duff K, Mold J, Adams RL. Longitudinal stability of RBANS profiles in a geriatric community-dwelling sample. Clin Neuropsychol. 2014;28:269–280. doi: 10.1080/13854046.2014.884243 [DOI] [PubMed] [Google Scholar]
- 41. Holtzer R, Verghese J, Wang C, Hall CB, Lipton RB. Within-person across-neuropsychological test variability and incident dementia. JAMA. 2008;300:823–830. doi: 10.1001/jama.300.7.823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Burzynska AZ, Jiao Y, Knecht AM, et al. White matter integrity declined over 6-months, but dance intervention improved integrity of the fornix of older adults. Front Aging Neurosci. 2017;9:59. doi: 10.3389/fnagi.2017.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Sci. 2008;17:177–182. doi: 10.1111/j.1467-8721.2008.00570.x [DOI] [Google Scholar]
- 45. Hamacher D, Herold F, Wiegel P, Hamacher D, Schega L. Brain activity during walking: a systematic review. Neurosci Biobehav Rev. 2015;57:310–327. doi: 10.1016/j.neubiorev.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 46. Bürki CN, Bridenbaugh SA, Reinhardt J, Stippich C, Kressig RW, Blatow M. Imaging gait analysis: an fMRI dual task study. Brain Behav. 2017;7:e00724. doi: 10.1002/brb3.724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Blumen HM, Holtzer R, Brown LL, Gazes Y, Verghese J. Behavioral and neural correlates of imagined walking and walking-while-talking in the elderly. Hum Brain Mapp. 2014;35:4090–4104. doi: 10.1002/hbm.22461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Scholkmann F, Wolf M. General equation for the differential pathlength factor of the frontal human head depending on wavelength and age. J Biomed Opt. 2013;18:105004. doi: 10.1117/1.JBO.18.10.105004 [DOI] [PubMed] [Google Scholar]
- 49. Bennett IJ, Madden DJ. Disconnected aging: cerebral white matter integrity and age-related differences in cognition. Neuroscience. 2014;276:187–205. doi: 10.1016/j.neuroscience.2013.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Penke L, Deary IJ. Some guidelines for structural equation modelling in cognitive neuroscience: the case of Charlton et al. ’s study on white matter integrity and cognitive ageing. Neurobiol Aging. 2010;31:1656–60; discussion 1561. doi: 10.1016/j.neurobiolaging.2009.10.019 [DOI] [PubMed] [Google Scholar]