Abstract
Background and Aims
In seed plants, stomata regulate CO2 acquisition and water relations via transpiration, while minimizing water loss. Walls of guard cells are strong yet flexible because they open and close the pore by changing shape over the substomatal cavity. Pectins are necessary for wall flexibility and proper stomata functioning. This study investigates the differences in pectin composition in guard cells of two taxa that represent key lineages of plants with stomata: Arabidopsis, an angiosperm with diurnal stomatal activity, and Phaeoceros, a bryophyte that lacks active stomatal movement.
Methods
Using immunolocalization techniques in transmission electron microscopy, this study describes and compares the localization of pectin molecule epitopes essential to stomata function in guard cell walls of Arabidopsis and Phaeoceros.
Key Results
In Arabidopsis, unesterified homogalacturonans very strongly localize throughout guard cell walls and are interspersed with arabinan pectins, while methyl-esterified homogalacturonans are restricted to the exterior of the wall, the ledges and the junction with adjacent epidermal cells. In contrast, arabinans are absent in Phaeoceros, and both unesterified and methyl-esterified homogalacturonans localize throughout guard cell walls.
Conclusions
Arabinans and unesterified homogalacturonans are required for wall flexibility, which is consistent with active regulation of pore opening in Arabidopsis stomata. In contrast, the lack of arabinans and high levels of methyl-esterified homogalacturonans in guard cell walls of Phaeoceros are congruent with the inability of hornwort stomata to open and close with environmental change. Comparisons across groups demonstrate that variations in guard cell wall composition reflect different physiological activity of stomata in land plants.
Keywords: Arabidopsis, arabinans, guard cell, hornwort, cell walls, immunolocalization, stomata, pectins, Phaeoceros
INTRODUCTION
Guard cells in tracheophytes have to endure substantial deformation in order to open and close the pore (Franks and Farquhar, 2007). Consequently, guard cell walls are highly specialized in composition and construction, reflecting the necessity for durability, strength and flexibility. Cellulose and hemicelluloses form the main scaffolding of plant cell walls, while pectins determine porosity, thickness and flexibility of the walls (Cosgrove, 2005). In guard cell walls, pectins are critical for active regulation of stomatal movement because they enable guard cells to change shape. In particular, the interactions between homogalacturonan (HG) and arabinan side chains of rhamnogalacturonan-I (RG-I) are key to the ability of tracheophyte stomata to respond to environmental and endogenous cues (Jones et al., 2003, 2005).
Homogalacturonans are the most abundant pectins in plant cell walls. These linear chains of galacturonic acid behave differently based on the degree and pattern of methyl-esterification, which determine the mechanical properties of cell walls, i.e. strengthening or loosening of wall polysaccharide interconnections (Caffall and Mohnen, 2009, Levesque-Tremblay et al., 2015). Highly methyl-esterified HG is characteristic of growing walls, which remain loose, while unesterified HG forms stable, tight gels by crosslinking through Ca2+ bonds (Verhertbruggen et al., 2009a; Wolf et al., 2009). De-methyl-esterification of guard cell walls is necessary for normal stomata function (Amsbury et al., 2016). Rhamnogalacturonan-I pectins have a linear backbone adorned with side chains of arabinans or galactans. In angiosperms, degradation of arabinans in guard cell walls renders stomata unable to open or close, an effect that can be reversed by removing HG pectins (Jones et al., 2003, 2005). It is proposed that the arabinan branches of RG-I prevent unesterified HG from bonding and consequently maintain the flexibility of cell walls. In the moss Funaria, guard cell walls contain abundant unesterified HG and arabinans when young and flexible, but developmental changes that include wall thickening and a decrease in total pectin content prevent movement in mature stomata (Merced and Renzaglia 2014).
Transmission electron microscopy combined with immunolocalization is a powerful tool to examine the fine structure and arrangement of molecular components in cells. Such studies are advancing our understanding of the structure, function and evolution of the plant cell wall. Although guard cell wall constituents have been shown to be critical to stomatal function (Jones et al., 2003, 2005; Amsbury et al., 2016), the spatiotemporal localization of cell wall polymers has not been examined in critical taxa, including angiosperms and hornworts. Hornworts and mosses are the only bryophyte groups that produce stomata, but stomata in bryophytes occur on sporangia, not leaves as in tracheophytes. In mosses, stomata are able to open and close the stomatal pore for a limited period following capsule expansion but are immovable in mature capsules (Garner and Paolillo, 1973; Chater et al., 2011; Merced and Renzaglia, 2014, 2017). Recent studies have demonstrated that hornwort stomata are unresponsive to environmental cues following the initial development of the pore (Renzaglia et al., 2017; Pressel et al., 2018).
The present study compares the ultrastructural localization of pectins in guard cell walls of Arabidopsis, which has fully functional stomata, with those of a hornwort that lacks active control of stomatal pores. Mature guard cell walls were probed in Arabidopsis, while pectins were examined in developing and mature guard cells of Phaeoceros for comparison with early and late stages of the moss Funaria (Merced and Renzaglia, 2014). Pectins were localized using monoclonal antibodies to five pectin molecule epitopes, with emphasis on those involved in stomata function, namely HG and arabinan RG-I. The study addresses the fundamental question: how do the ultrastructure and pectin localization of guard cell walls of an angiosperm with fully functional stomata compare to those of a hornwort that lacks physiological control of stomata? We hypothesized that cell wall components necessary for reversible bending of guard cells are abundant and specific in location in Arabidopsis stomata, and absent or scarce throughout development in Phaeoceros.
MATERIALS AND METHODS
The protocols used followed those in Merced and Renzaglia (2014) and were as follows. Phaeoceros carolinianus were collected from Maricao, Puerto Rico, and sporophytes were harvested and cut into sections at 2-mm intervals from the gametophyte upward. Leaves of 2-month-old wild-type Arabidopsis thaliana Columbia ecotype (WT CS23084) grown at 23 °C in a 16-h photoperiod in a growth chamber were cut into 1-cm squares. Specimens were fixed in 2 % glutaraldehyde in 0.05M NaPO4buffer for 1 h at room temperature, then overnight at 20 °C. Afterwards, specimens were rinsed three times for 30 min in 0.05M NaPO4 buffer and postfixed for 20 min in 1 % OsO4 in 0.05M NaPO4 buffer, followed by three 10-min rinses in distilled water, and then dehydrated in a graded ethanol series ending with three immersions in 100 % ethanol. Slow infiltration of specimens in LR White resin (London Resin Company, Berkshire, UK) was done over 4 d by increasing the percentage of resin with respect to ethanol. Plant material was placed in moulds with fresh resin and cured for 2 d at 65 °C. Semithin sections (250–750 nm) were mounted on glass slides and stained with 1.5 % toluidine blue in distilled water to monitor for stomata using light microscopy. Thin sections (60–90 nm) were collected on nickel grids and dried for 1–3 h at room temperature.
Immunogold localization of pectin epitopes was done with five primary monoclonal antibodies: LM19 (unesterified HG); LM20 (esterified HG); LM5 (galactan RG-I) (tested only in Arabidopsis); LM6 (arabinan RG-I); and LM13 (linear arabinan RG-I) (Plant Probes, University of Leeds, UK). One control that excluded incubation of the primary antibody and two or three treatments was made for each antibody on three to five stomata for each species. Grids were placed in 2 % bovine serum albumin (BSA) in 0.02 m phosphate-buffered saline solution, pH 7.2 (PBS), overnight at 4 °C in a humid chamber. Treatments were transferred to primary antibody (diluted 1:20 in 2 % BSA/PBS) for 3 h while controls were left in buffer. Treatment and control grids were rinsed four times for 3 min in 2 % BSA/PBS, then incubated for 30 min in gold-conjugated (10 nm) IgG goat anti-rat secondary antibody (Sigma–Aldrich, St Louis, MO, USA) diluted 1:20 in 2 % BSA/PBS. Grids were then rinsed four times for 3–5 min with PBS, followed by distilled/deionized, autoclaved, filtered water, and dried at room temperature. Grids were observed unstained with a Hitachi H7650 transmission electron microscope at 60 kV.
RESULTS
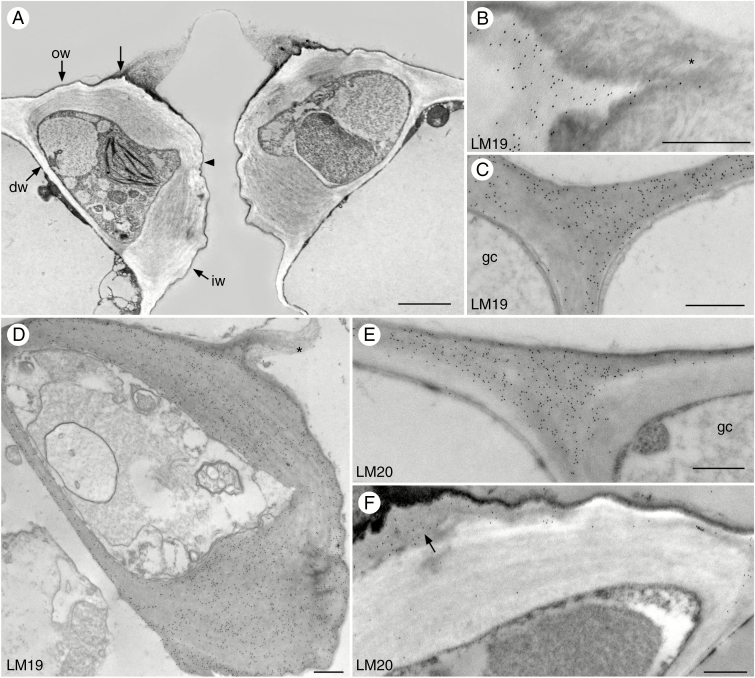
Stomata of Arabidopsis leaves are smaller than epidermal cells and have highly thickened walls and dense cytoplasm (Fig. 1). In cross-section, guard cell outer walls are thinner near the dorsal walls and expand at the outer ledge where they join the ventral walls (Fig. 1A). The ventral walls are thinnest at the midsection and the inner walls are very thick (Fig. 1A). Except for a slight differentiation of the more electron-lucent outer primary wall, the walls are not conspicuously layered but are striated. Inner ledges are absent and outer ledges are small with long electron-opaque extensions of amorphous material containing irregular plates similar in density to cuticle or waxes (Fig. 1B, D).
Fig. 1.
Immunolocalization of homogalacturonan pectin epitopes in guard cell walls of Arabidopsis. Black round dots are colloidal gold labels attached to the specific pectin antibody. (A) Cross-section of guard cells showing outer walls (ow), outer ledges (unlabelled arrow), inner walls (iw) and ventral walls which are thinner at midsection (arrowhead). Dorsal walls (dw) are thin and abut epidermal cells. (B) The outer ledge labels with the LM19 antibody and the electron-dense cuticular extension (*) is unlabelled. (C) LM19 antibody labels guard cell (gc) walls and adjacent epidermal cell walls. (D) LM19 label is evenly distributed over the guard cell walls. The outer ledge is extended with cuticle-like material (*). (E) LM20 antibody strongly labels the junction of guard cell (gc) and adjacent epidermal cell wall. (F) LM20 antibody labels the outside wall of the guard cell and outer ledges (arrow), but there is less labelling in the electron-lucent internal thick wall. Scale bars: (A) = 2 µm; (B–F) = 500 nm.
Labelling of unesterified HG (LM19) is very strong and widespread in Arabidopsis guard cell walls and wall ledges but does not extend into the cuticular projections (Fig. 1B–D). Labelling for LM19 is of similar high intensity in guard cell walls and epidermal cells (Fig. 1C). In contrast, esterified HG (LM20) labelling is strong towards the exterior of the guard cell walls, the first wall layer to be deposited, but weak in the expanded internal wall layer (Fig. 1E). This pectin epitope seems to be restricted to the oldest region of the guard cell walls and is very strongly labelled or strongly localized in the junction with the adjacent epidermal cells (Fig. 1F).
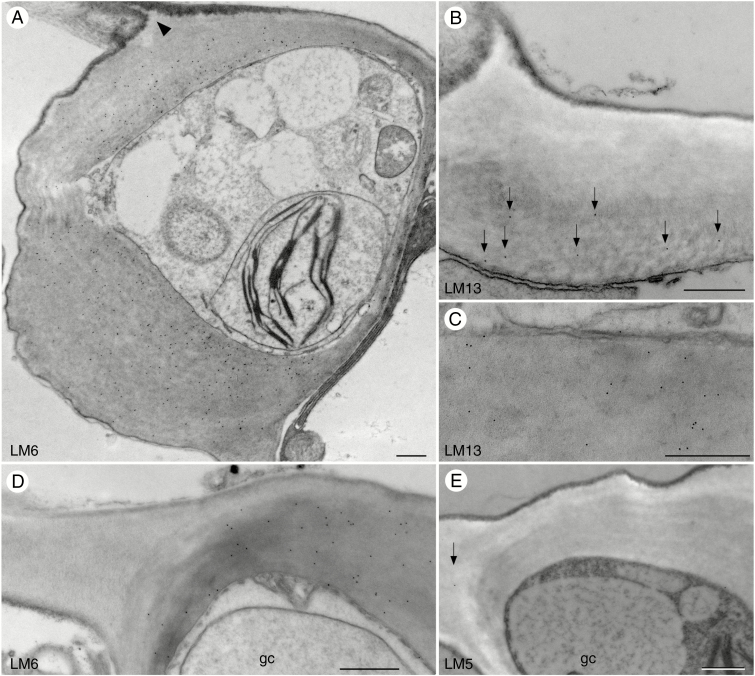
Monoclonal antibodies LM6 and LM13 recognize arabinan side chains of RG-I but exhibit slightly different localizations in Arabidopsis guard cell walls. LM6 strongly localizes throughout the thickened guard cell walls of Arabidopsis (Fig. 2A), while LM13 weakly localizes at the interior wall layer (Fig. 2B, C). Both antibodies are absent in the walls of epidermal cells adjacent to stomata (Fig. 2D) and have weak but consistent localization in other epidermal cell walls. Galactan chains of RG-I recognized by LM5 (Jones et al., 1997) are absent in guard cell walls and rare in epidermal cells of Arabidopsis (Fig. 2E).
Fig. 2.
Immunolocalization of rhamnogalacturonan-I pectin epitopes in guard cell walls of Arabidopsis. Black round dots in images are colloidal gold labels attached to the specific pectin antibody. (A) LM6 antibody strongly localizes in guard cell walls, especially in the interior, but not towards the ledges (arrowhead). (B) Sparse labelling of the LM13 antibody (arrows) towards the interior of the guard cell outer wall. (C) Guard cell walls label weakly with LM13 antibody. (D) LM6 antibody recognizes the thick interior of guard cell (gc) walls and is absent in epidermal cell walls. (E) Labelling with LM5 antibody is absent in guard cell (gc) walls and low in epidermal cell walls (arrow). Scale bars = 500 nm.
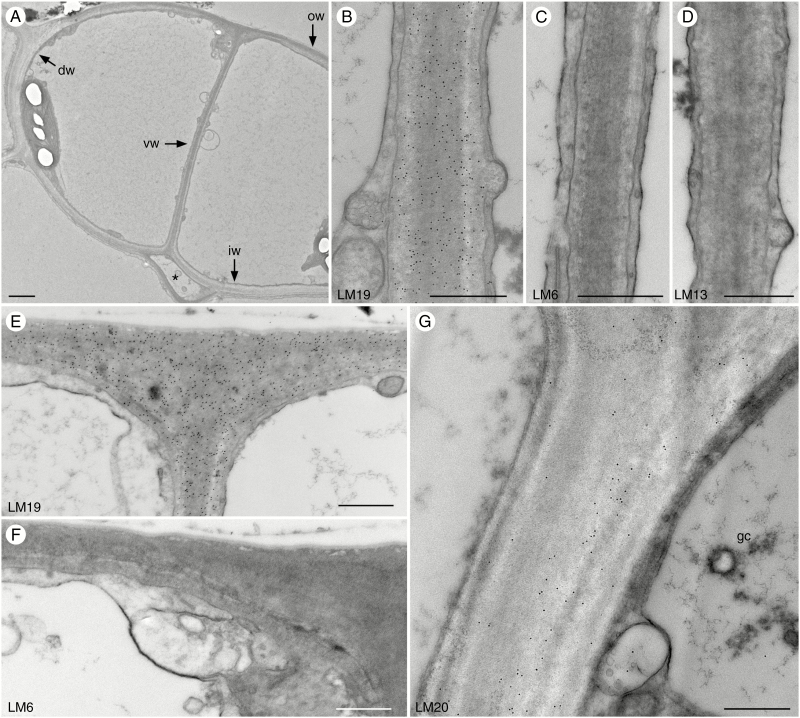
Phaeoceros stomata are large and guard cell walls are thin before pore opening (Fig. 3A). The substomatal cavity starts to form before the ventral walls separate to form the pore (Fig. 3A). Labelling of ventral guard cell walls is very strong for unesterified HG labelled with LM19 (Fig. 3B), but no labelling was detected for arabinans using LM6 and LM13 antibodies (Fig. 3C, D) at this stage. This pattern is similar for all other guard cell walls, which label very strongly for unesterified HG (Fig. 3E) but do not label for arabinans (Fig. 3F). Labelling for methyl-esterified HG is absent in outer, ventral and inner walls, and only dorsal walls labelled for LM20 in pre-opened stomata (Fig. 3G).
Fig. 3.
Immunolocalization of pectin epitopes in young guard cell walls of Phaeoceros. Black round dots in images are colloidal gold labels attached to the specific pectin antibody. dw, dorsal wall; iw, inner wall; ow, outer wall. (A) Guard cell walls in pre-opened stomata are thin. The substomatal cavity (*) develops before the pore forms along the ventral wall (vw). (B–D) Ventral walls of pre-opened stomata. (B) LM19 antibody labels ventral walls strongly and evenly. (C) No labelling for LM6 antibody was detected. (D) No labelling was detected for LM13 antibody. (E) LM19 antibody strongly recognizes outer walls. (F) No labelling was detected for LM6 antibody in outer walls. (G) LM20 antibody only labels the dorsal wall of guard cells (gc). Scale bars: (A) = 2 µm; (B–G) = 500 nm.
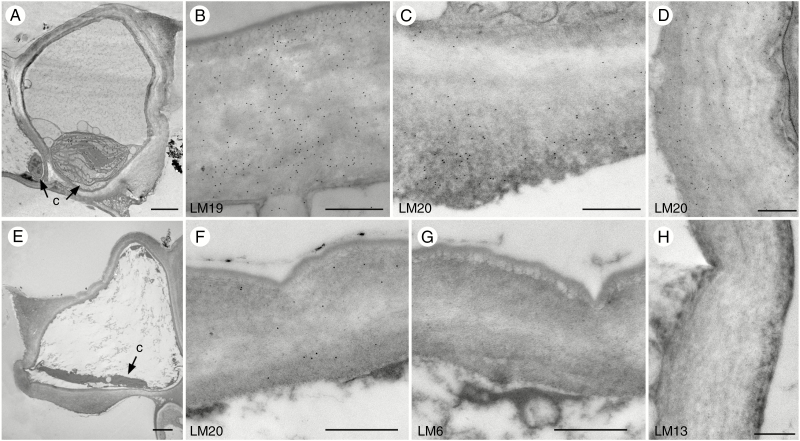
After pore opening, walls are differentially thickened, with thin outer and ventral walls and thick inner walls (Fig. 4A) (Renzaglia et al., 2017). The outer ledge is prominent, while no inner ledge extends from the thickened inner wall. Labelling for LM19 remains very strong (Fig. 4B) and all guard cell walls show strong labelling for LM20 (Fig. 4C, D). In older parts of the sporophyte, guard cells begin to collapse as the protoplast degrades (Fig. 4E). Labelling of LM19 (not shown) and LM20 remains strong when guard cells begin to collapse (Fig. 4F). No labelling for LM6 and LM13 was found in any of the guard cell walls throughout development (Fig. 4G, H). As in younger guard cells, unesterified HG and methyl-esterified HG are the primary pectins in mature and collapsed stomata walls of Phaeoceros, while arabinan side chains of RG-I are absent.
Fig. 4.
Immunolocalization of pectin epitopes in mature and collapsed guard cell walls of Phaeoceros. Black round dots in images are colloidal gold labels attached to the specific pectin antibody. (A–D) Stomata with fully developed guard cell walls and open pores. (A) Walls of opened stomata are differentially thickened. Compared with epidermal cells, guard cells contain larger chloroplasts (c) with pyrenoids. (B) LM19 antibody recognizes outer guard cell walls. (C) LM20 antibody strongly labels inner guard cell walls. (D) Ventral walls label with LM20 antibody. (E–H) Dead and collapsing stomata with degraded chloroplasts (c). (E) Outer and ventral walls bend when guard cells begin to collapse. (F) LM20 antibody recognizes the outer guard cell walls. (G) No labelling in guard cell walls was found using LM6 antibody, as shown in the outer wall. (H) No labelling in guard cell walls was found using LM13 antibody, as shown in the ventral wall. Scale bars: (A, E) = 2 µm; (B–D), (F–H) = 500 nm.
DISCUSSION
Stomata of all land plants are superficially similar, with a pair of guard cells and a pore that opens to a substomatal cavity connected to a network of air spaces. The developmental ultrastructural of stomata is alike in many respects in Arabidopsis (Zhao and Sack, 1999) and hornworts (Renzaglia et al., 2017). In both, the substomatal cavity develops prior to wall thickening and pore formation. Guard cell walls thicken differentially to form an outer ledge and thickened inner walls. Guard cells contain dense cytoplasm and well-differentiated chloroplasts compared with epidermal cells in both taxa. Following cell wall development, hornwort guard cells uniquely undergo protoplasmic degradation and collapse, while those in Arabidopsis are physiologically active and remain so when fully developed. In contrast to seed plants, experimental evidence demonstrates that hornwort stomata do not close in response to abscisic acid (ABA) or darkness, and the slight reduction in pore size in response to desiccation and plasmolysis is likely the result of non-osmotic water loss (Pressel et al. 2018). These differences are reflected in pectin composition in guard cell walls in these two disparate taxa. We demonstrate that mature hornwort guard cell walls do not label for the two arabinan epitopes found in Arabidopsis, and a higher level of methyl-esterification of HG was detected compared with the angiosperm.
Guard cell walls of Arabidopsis are rich in unesterified HG (LM19) but less so in methyl-esterified HG (LM20). Methyl-esterified HG localizes only towards the external part of the walls, which is deposited early in development, and in dorsal walls at the junction with adjacent epidermal cells. Deposition of the thick inner wall involves HG, which is secreted as methyl-esterified and de-methyl-esterified within the wall. The pattern of de-methyl-esterification is regulated to modify wall properties by exposing bonding sites that form stable structures with other HGs, thus imparting strength to walls (Caffall and Mohnen, 2009; Wolf et al., 2009; Sénéchal et al., 2014). The pectin methyl-esterase PME6 is believed to be responsible for de-methyl-esterification of HG in guard cell walls of Arabidopsis. PME6 is highly expressed in guard cells and when it is mutated stomatal walls have higher levels of methyl-esterification and limited movement (Amsbury et al., 2016). Mature Phaeoceros guard cell walls contain abundant unesterified and methyl-esterified HG in the inner wall, suggesting that removal of methyl-ester groups of HG is not complete and the walls are less flexible.
The structure and occurrence of RG-I are highly dynamic and variable; arabinans and galactan side chains may occur differentially in different tissues and developmental stages, or may have a variable location in the same cell (Jones et al., 1997; Verhertbruggen et al., 2009b; Willats et al., 2001). Galactan side chains, as recognized by the LM5 antibody, are absent in Arabidopsis guard cell walls, a finding that is consistent with incubation of Commelina communis leaves in galactanase with no effect on stomata function (Jones et al., 2003). Galactans are posited to be responsible for increasing the firmness of tissues (Willats et al., 2001) and this could be why they do not occur in guard cell walls. Labelling of arabinans in Arabidopsis guard cell walls was strong; in particular, the LM6 epitope was more abundant than LM13. Arabinan-rich polymers have been experimentally shown to be necessary for proper stomata function by providing flexibility to cell walls and ensuring wall integrity (Jones et al. 2003, 2005; Moore et al., 2012).
In contrast to Arabidopsis and moss, no arabinans were detected in hornworts using LM6 or LM13. The overall composition of pectins did not change throughout development, except for an increase in methyl-esterified HG pectins in the highly thickened walls. Since arabinans are essential for stomata opening and closing, the absence of this pectin in hornworts is consistent with the inability to reversibly bend, as required for active regulation of the stomatal aperture, and suggests that the walls are less flexible. The lack of detectable arabinans and high levels of methyl-esterified HG support the finding of Pressel et al. (2018) that hornwort stomata are not able to actively open and close. These results also support the gradual acquisition of stomata control through the evolutionary history of these structures (Merced and Renzaglia, 2017; Sussmilch et al., 2017; Pressel et al., 2018).
Modifications in cell wall architecture and composition may reflect taxonomic variability, functional differences, adaptations to the environment or combinations of the three. Taxon-specific patterns of cellulose crystallinity and lignin, phenolic and pectin (as determined by ruthenium red staining) composition in guard cells were described in kidney-shaped stomata of ferns and dumbbell-shaped stomata of grasses and other angiosperms (Shtein et al., 2017). In angiosperms, pectins are important for the proper function of stomata, which are able to open and close for the entire lifespan of the organ on which they reside. Although Shtein et al. (2017) demonstrated that total pectin content in ferns is less than in angiosperms, the pectin composition remains to be tested. With physiologically active stomata, it is likely that arabinan-containing pectins are present in ferns and lycophytes. Localization of pectins in Arabidopsis is similar to that in the moss Funaria at the stage when stomata can move (Garner and Paolillo, 1973; Merced and Renzaglia, 2014). In contrast, pectin localization in Phaeoceros remains relatively constant over development, supporting the finding that, contrary to moss stomata, hornwort stomata are not able to move at any stage of development. The structure and function of bryophyte stomata are inherently linked to their existence on a sporangium that is fated to dry, dehisce and release spores (Ligrone et al., 2012; Merced and Renzaglia, 2017; Renzaglia et al., 2017). Modifications in pectin composition of guard cell walls, in particular arabinan side chains of RG-I pectins and regulation of the esterification status of HG pectins, likely reflect independent adaptations in response to environmental and physiological pressures to perform divergent functions. For example, arabinans are found in turgid pseudostomatal walls of Sphagnum, and these cells collapse but do not bend, nor do they form a pore (Merced, 2015). Further immunochemical analyses on key lineages with stomata, especially lycophytes, ferns and gymnosperms, are necessary to assess the degree to which guard cell wall polymers are conserved across land plants and whether or not pectin composition universally reflects functional differences.
ACKNOWLEDGEMENTS
We thank Dr Matt Geisler for providing Arabidopsis plants. This work was supported by the National Science Foundation (DUE 0638722 to K.S.R).
LITERATURE CITED
- Amsbury S, Hunt L, Elhaddad N, et al. 2016. Stomatal function requires pectin de-methyl-esterification of the guard cell wall. Current Biology 26: 2899–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffall KH, Mohnen D. 2009. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydrate Research 344: 1879–1900. [DOI] [PubMed] [Google Scholar]
- Chater C, Kamisugi Y, Movahedi M, et al. 2011. Regulatory mechanism controlling stomatal behavior conserved across 400 million years of land plant evolution. Current Biology 21: 1025–1029. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. 2005. Growth of the plant cell wall. Nature Reviews Molecular Cell Biology 6: 850–861. [DOI] [PubMed] [Google Scholar]
- Franks PJ, Farquhar GD. 2007. The mechanical diversity of stomata and its significance in gas exchange control. Plant Physiology 143: 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner DLB, Paolillo DJ. 1973. On the functioning of stomates in Funaria. Bryologist 76: 423–427. [Google Scholar]
- Jones L, Seymour GB, Knox JP. 1997. Localization of pectic galactan in tomato cell walls using a monoclonal antibody specific to (1→4)-β-D-galactan. Plant Physiology 113: 1405–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L, Milne JL, Ashford D, McQueen-Mason SJ. 2003. Cell wall arabinan is essential for guard cell function. Proceedings of the National Academy of Sciences of the USA 100: 11783–11788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L, Milne JL, Ashford D, McCann MC, McQueen-Mason SJ. 2005. A conserved functional role of pectic polymers in stomatal guard cells from a range of plant species. Planta 221: 255–264. [DOI] [PubMed] [Google Scholar]
- Levesque-Tremblay G, Pelloux J, Braybrook SA, Müller K. 2015. Tuning of pectin methylesterification: consequences for cell wall biomechanics and development. Planta 242: 791–811. [DOI] [PubMed] [Google Scholar]
- Ligrone R, Duckett JG, Renzaglia KS. 2012. The origin of the sporophyte shoot in land plants: a bryological perspective. Annals of Botany 110: 935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merced A. 2015. Novel insights on the structure and composition of pseudostomata of Sphagnum (Sphagnaceae). American Journal of Botany 102: 329–335. [DOI] [PubMed] [Google Scholar]
- Merced A, Renzaglia K. 2014. Developmental changes in guard cell wall structure and pectin composition in the moss Funaria: implications for function and evolution of stomata. Annals of Botany 114: 1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merced A, Renzaglia KS. 2017. Structure, function and evolution of stomata from a bryological perspective. Bryophyte Diversity and Evolution 39: 7–20. [Google Scholar]
- Moore JP, Nguema-Ona EE, Vicre-Gibouin M, et al. 2012. Arabinose-rich polymers as an evolutionary strategy to plasticize resurrection plant cell walls against desiccation. Planta 237: 739–754. [DOI] [PubMed] [Google Scholar]
- Pressel S, Renzaglia KS, Duckett JG. 2018. Hornwort stomata do not respond actively to exogenous and environmental cues. Annals of Botany 122: 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzaglia KS, Villareal JC, Piatkowski BT, Lucas JR, Merced A. 2017. Hornwort stomata: architecture and fate shared with 400-million-year-old fossil plants without leaves. Plant Physiology 174: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sénéchal F, Wattier C, Rusterucci C, Pelloux J. 2014. Homogalacturonan-modifying enzymes: structure, expression, and roles in plants. Journal of Experimental Botany 65: 5125–5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtein I, Shelef Y, Marom Z, et al. 2017. Stomatal cell wall composition: distinctive structural patterns associated with different phylogenetic groups. Annals of Botany 119: 1021–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussmilch FC, Brodribb TJ, McAdam SAM. 2017. What are the evolutionary origins of stomatal responses to abscisic acid (ABA) in land plants?Journal of Integrative Plant Biology 59: 240–260. [DOI] [PubMed] [Google Scholar]
- Verhertbruggen Y, Marcus SE, Haeger A, Ordaz-Ortiz JJ, Knox JP. 2009a An extended set of monoclonal antibodies to pectic homogalacturonan. Carbohydrate Research 344: 1858–1862. [DOI] [PubMed] [Google Scholar]
- Verhertbruggen Y, Marcus SE, Haeger A, et al. 2009. b Developmental complexity of arabinan polysaccharides and their processing in plant cell walls. Plant Journal 59: 413–425. [DOI] [PubMed] [Google Scholar]
- Wolf S, Mouille G, Pelloux J. 2009. Homogalacturonan methyl-esterification and plant development. Molecular Plant 2: 851–860. [DOI] [PubMed] [Google Scholar]
- Willats WG, McCartney L, Mackie W, Knox JP. 2001. Pectin: cell biology and prospects for functional analysis. Plant Molecular Biology 47: 9–27 [PubMed] [Google Scholar]
- Zhao L, Sack FD. 1999. Ultrastructure of stomatal development in Arabidopsis (Brassicaceae) leaves. American Journal of Botany 86: 929–939. [PubMed] [Google Scholar]