Abstract
Background and Aims
Heteroblasty is a non-reversible morphological change associated with life stage change and has been linked to predictable environmental variation. It is present in several clades from mediterranean-type climates, such as African Restionaceae (restios). These have heteroblastic shoots: juvenile shoots are thin, branched and sterile (sterile shoots); adult shoots are thicker and less branched, and bear inflorescences (reproductive shoots). Ten per cent of the restios retain juvenile-like, sterile shoots as adults (neoteny). We hypothesize (1) that the two shoot types differ in ecophysiological attributes, and (2) that these shoot types (and the neoteny) are associated with different environments.
Methods
We measured shoot mass per surface area (SMA), maximum photosynthetic capacity per biomass (Amass) and chlorenchyma to ground tissue ratio (CGR) of both shoot types in 14 restio species. We also calculated environmental niche overlap between neotenous and non-neotenous species using an improved multidimensional overlap function based on occurrence data, and linked shoot types with environments using a phylogenetic generalized linear model.
Key Results
Sterile shoots showed higher Amass, lower SMA and higher CGR than reproductive shoots. Neotenous and non-neotenous species overlapped ecologically less than expected by chance: neotenous species favoured more mesic, non-seasonal conditions.
Conclusions
We associate sterile shoot morphology with acquisitive ecophysiological strategies and reproductive shoots with conservative strategies. The heteroblastic switch optimizes carbon efficiency in the juvenile phase (by sterile shoots) in the mesic post-fire conditions. The adult shoots present a compromise between a more conservative strategy favourable under harsher conditions and reproductive success. Heteroblasty in seasonally arid, oligotrophic ecosystems with predictable, fire-driven shifts in water and nutrient availability might play a role in the success of restios and other species-rich lineages in mediterranean-type ecosystems. It may represent a previously unrecognized adaptation in mediterranean clades sharing similar conditions, contributing to their ecological and taxonomic dominance.
Keywords: Cape flora, dimorphism, diversity hotspot, fire disturbance, heterophylly, leaf economics spectrum, mediterranean ecosystem, multidimensional niche overlap, photosynthesis, trait–environment relationship, Restionaceae
INTRODUCTION
The economical spectrum of plants spans from acquisitive to conservative strategies (Pérez-Ramos et al., 2012; Reich, 2014). Acquisitive strategies are commonly associated with low-cost, rapid investment-returning, ephemeral structures, whereas conservative strategies are associated with robust, stress-resistant, persistent and slow investment-returning organs (Westoby and Wright, 2006). These strategies are associated with environmental conditions at the global (Reich et al., 1997, 1999) and local scale (McGill et al., 2006; Pérez-Ramos et al., 2012), with mesic environments supporting acquisitive strategies, and harsher conditions selecting for conservative strategies. Environmental conditions that change during the lifetime of individuals can select different traits at different life stages, resulting in selection for plants that are able to optimize their traits via phenotypic plasticity, polymorphism, or heteroblasty (Lloyd, 1984; Adler and Drake, 2008).
Heteroblasty, in contrast to phenotypic plasticity (including environmentally controlled heterophylly) or polymorphism, is an abrupt change in morphological traits linked to a particular life history stage (Goebel, 1889; Zotz et al., 2011). Furthermore, a heteroblastic phenotype switch is irreversibly pre-determined. Such a morphological switch can represent an adjustment mechanism to predictable changes in environmental conditions through the lifetime of individuals (Goebel, 1889; Zotz et al., 2011). Heteroblasty has been linked to predictable differences between the environments of juvenile and adult plants, such as the light gradient in forests (Bauer and Bauer, 1980), height-limited browsing (Day, 1998) or changes in water availability (Miller et al., 1995). However, the adaptive value of heteroblasty is not straightforward, and may differ between populations of the same species (Jordan et al., 2000; Climent et al., 2006). Despite the many reports and descriptions of heteroblastic species and groups from various ecosystems, there is still no general theory as to its advantages. Several species-rich and ecologically important groups occurring in mediterranean-type ecosystems have been reported as heteroblastic, including species of Eucalyptus L’Hér. (Rao, 1971) and Acacia Mill. (Pabón-Mora and González, 2012) in Australia, Juniperus L. in California (Miller et al., 1995), pines in the Mediterranean basin (Climent et al., 2006, 2013), and several species of the maquis (scrub) vegetation in Oceania, for example on New Caledonia (Mueller-Dombois and Fosberg, 1998; Burns and Dawson, 2006). The presence of heteroblasty in these groups could indicate that this trait emerged in response to the shared mediterranean-type climate.
The mediterranean-type climate is characterized by strong precipitation seasonality with dry summers, and fires on a decadal scale (Moreno and Oechel, 2012). Fires result, temporarily, in more nutrient-rich conditions with higher water availability by reduced water uptake from vegetation (Stock and Lewis, 1986; Mappin et al., 2003; Clemente et al., 2005; Parra and Moreno, 2017). Species in fire-prone environments are adapted to burning by resprouting (Verdú, 2000) and/or by having seeds that germinate after fire, with germination triggered by smoke (Brown et al., 2003; Crosti et al., 2006). Seedlings growing in post-fire conditions may thus benefit from the relatively better conditions. The progressive depletion of the fire-induced resources and the succession of the vegetation create a predictable temporal shift from an environment of low competition and high resource availability to one of higher competition and lower resource availability. The increasingly harsh conditions should lead to the selection of more conservative growth strategies. These short-term benefits of fertilization and the subsequently steep gradient of nutrient impoverishment (Bergh and Compton, 2015) might be more important in nutrient-poor conditions, in particular those of mediterranean-type climates in Australia and South Africa (Christensen, 1994).
The Cape Floristic Region (CFR), with its mediterranean-type climate, is a global diversity hotspot (Myers et al., 2000). The environmental conditions of the CFR vary both spatially and temporally, as a consequence of the topography, diverse geology and seasonality (Goldblatt and Manning, 2002), and the regular occurrence of fires (Kraaij and van Wilgen, 2014). The combination of spatial and temporal environmental variation in the CFR makes it suitable to study trait responses within individuals to changing environmental conditions through time (i.e. heteroblasty), and to test whether these responses are analogous to responses to spatial environmental variation. One of the ecologically dominant clades in the CFR is the 350 species-rich African Restionaceae (Restionoideae) (Briggs and Linder, 2009) (hereafter ‘restios’), all species of which appear to be heteroblastic (Linder et al., 1998; Linder and Caddick, 2001). Restios occur throughout the species-rich, heathy ‘fynbos’ vegetation (Rebelo et al., 2006). About 10 % of the species develop sterile shoots in their adult stage (Linder, 1990, 2013; Linder and Vlok, 1991) which can be interpreted as neoteny, i.e. retention of juvenile characters in the adult individuals (sensuMurphy et al., 2003; Brown et al., 2006).
Here we hypothesize that heteroblasty represents a response to temporal environmental shifts from relatively mesic post-fire conditions to harsher conditions in mature vegetation. Species with post-fire germination thus benefit from relatively mesic conditions with suitable acquisitive ecophysiology, and in less mesic conditions adjust by a switch to more conservative strategies. We test this hypothesis by comparing ecophysiological properties of the contrasting heteroblastic growth forms of restios, as an example of a heteroblastic mediterranean clade. We test (1) for a difference in surface area per biomass (a proxy for carbon investment), in photosynthetic capacity, and in the ratio of photosynthetic tissue versus ground tissue between juvenile and adult shoot types within individuals, in order to characterize the economic strategy of the shoots. We expect juveniles to present lower biomass per surface area, higher photosynthetic capacity per biomass, and relatively more photosynthetically active tissue compared to the adults, given the more favourable conditions following fire. Furthermore we test (2) whether acquisitive strategies are favoured in relatively more mesic conditions by comparing their niche differences between neotenous and non-neotenous species.
MATERIAL AND METHODS
Restios
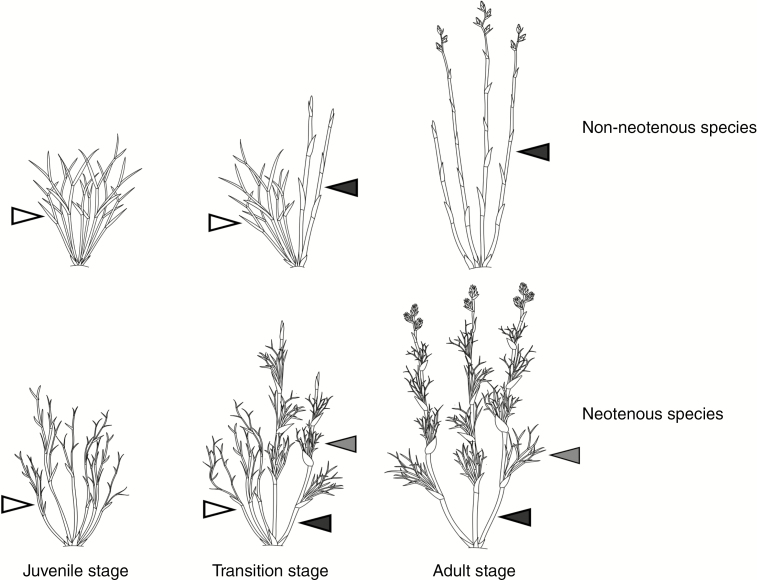
Restio leaves are highly reduced, and the shoots are the main photosynthetic organs. The juvenile plants have highly branched, thin sterile shoots, and the adult plants have unbranched or more sparsely branched and thicker, mostly erect shoots (Linder et al., 1998; Linder and Caddick, 2001) (Fig. 1). These shoots reach a greater height than the juvenile shoots, and bear an inflorescence in the first year, but remain green for several years after flowering (Linder and Caddick, 2001). Ten per cent of species in the adult stage have been reported to bear sterile shoots (morphologically similar to those on the juvenile plants) on the reproductive shoots (Linder, 1990, 2013; Linder and Vlok, 1991) (Fig. 1 and Supplementary Data Fig. S2). The retention of juvenile characteristics in adult plants is described as neoteny, such as seen in heteroblastic Acacia (Murphy et al., 2003; Brown et al., 2006).
Fig. 1.
Schematic drawing of idealized Restionaceae plants in different life stages, showing shoot type differences of species without shoots in adult stage (top) and neotenous species (bottom). Black arrowheads indicate reproductive shoots, grey arrowheads show adult sterile shoots in neotenous species and white arrowheads show juvenile sterile shoots.
Data collection and analysis
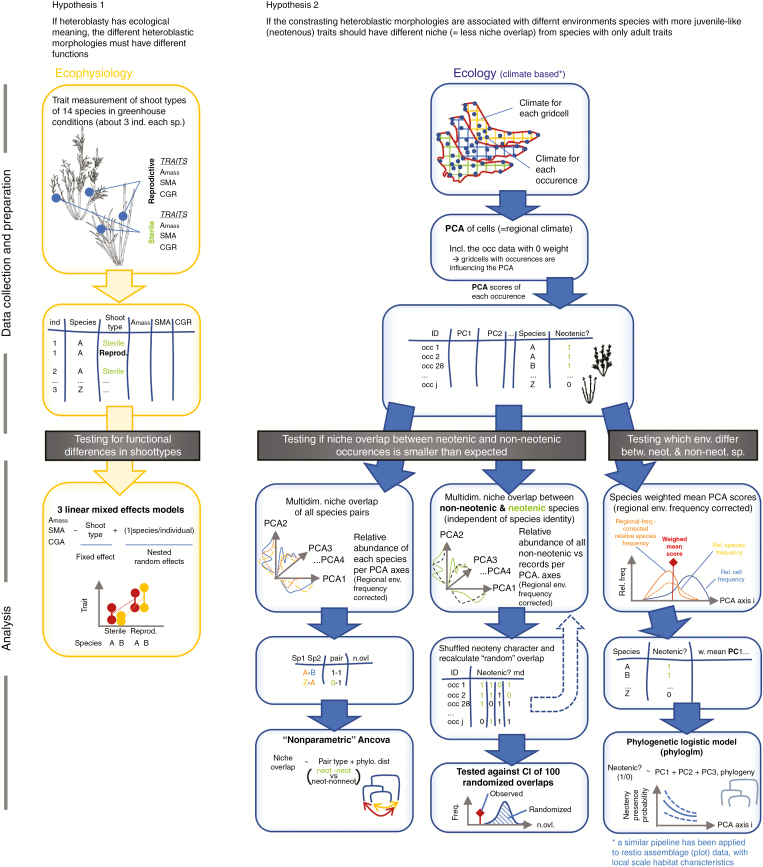
For all statistical analysis we used R version 3.4.3 (R Core Team, 2016). The methods pathway is summarized and shown in Fig. 2.
Fig. 2.
Flowchart detailing the methods employed in our analyses.
Ecophysiology – Plant material.
To assess ecophysiological differences between the two shoot types (Hypothesis 1) we selected 14 species representing the major groups of restios (Table 1). Twelve species were grown from seeds, collected from natural populations by Silverhill seeds (Kenilworth, Cape Town, South Africa), in the Botanical Garden of the University of Zurich. Three species were cultivated in the Kirstenbosch National Botanical Garden. One species was present in both Zurich and Cape Town. To compare juvenile and reproductive shoots we used plants in a transition life stage (Fig. 1) where the last juvenile (sterile) shoots were still present and the first reproductive shoots had already appeared. Depending on availability of plants in the transition stage we generally used three to five plants per species, apart from two exceptions with only two and one individual per species available, for measuring shoot biomass per surface area (SMA), photosynthetic capacity (Amass) and chlorenchyma to central tissue ratio (CGR) (Table 1).
Table 1.
Species of Restionaceae used for ecophysiological measurements with mean values of photosynthetic capacity (Amass, μmol g−1 s−m), shoot mass per surface area (SMA, mg mm−2) and the chlorenchyma to ground tissue ratio (CGR) with their standard deviation (s.d.) and number of individuals (n) as well as the location of the measurements taken, and voucher numbers
| Species | Shoot type | SMA | s.d. | A mass | s.d. | n | CGR | s.d. | n CGR | Locality | Voucher |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannomois grandis H.P.Linder | Reproductive | 0.304 ± 0.013 | 0.028 | 0.272 ± 0.053 | 0.118 | (5) | 0.125 ± 0.024 | 0.042 | (3) | Zurich | ME3001 |
| Sterile | 0.070 ± 0.007 | 0.015 | 1.004 ± 0.173 | 0.387 | 0.643 ± 0.098 | 0.170 | |||||
| Elegia capensis (Burm.f.) Schelpe | Reproductive | 0.077 ± 0.015 | 0.012 | 1.028 ± 0.225 | 0.318 | (2) | 0.240 ± 0.0512 | 0.089 | (3) | Zurich | ME3002 |
| Sterile | 0.043 ± 0.000 | 0.429 | 2.007 ± 0.287 | 0.406 | 1.133 ± 0.150 | 0.259 | |||||
| Elegia equisetacea (Mast.) Mast. | Reproductive | 0.062 ± 0.006 | 0.010 | 0.916 ± 0.103 | 0.179 | 0.390 ± 0.071 | 0.122 | (3) | Zurich | ME3011 | |
| Sterile | 0.041 ± 0.006 | 0.011 | 1.281 ± 0.437 | 0.759 | 1.219 ± 0.181 | 0.286 | |||||
| Elegia macrocarpa (Kunth) Moline and H.P.Linder | Reproductive | 0.145 ± 0.009 | 0.145 | 0.631 ± 0.246 | 0.427 | (3) | 0.221 ± 0.081 | 0.140 | (3) | Zurich | ME3003 |
| Sterile | 0.109 ± 0.007 | 0.115 | 0.889 ± 0.066 | 0.115 | 0.932 ± 0.141 | 0.244 | |||||
| Elegia persistens Mast. | Reproductive | 0.092 ± 0.013 | 0.022 | 0.333 ± 0.125 | 0.217 | (3) | Cape Town | ||||
| Sterile | 0.047 ± 0.005 | 0.009 | 0.849 ± 0.230 | 0.398 | |||||||
| Restio festuciformis Nees ex Mast. | Reproductive | 0.123* | 0.144* | (1) | 0.183 ± 0.074 | 0.129 | (3) | Zurich | ME3004 | ||
| Sterile | 0.054* | 0.405* | 0.542 ± 0.114 | 0.197 | |||||||
| Restio leptostachys Meyer | Reproductive | 0.099 ± 0.020 | 0.028 | 0.293 ± 0.015 | 0.021 | (2) | 0.301 ± 0.087 | 0.124 | (2) | Zurich | ME3005 |
| Sterile | 0.060 ± 0.012 | 0.017 | 0.654 ± 0.415 | 0.586 | 0.571 ± 0.099 | 0.479 | |||||
| Rhodocoma capensis Nees ex Steud. | Reproductive | 0.213 ± 0.038 | 0.094 | 0.213 ± 0.232 | 0.569 | (6) | 0.150 ± 0.050 | 0.080 | (7) | Zurich | ME3006 |
| Sterile | 0.041 ± 0.003 | 0.007 | 0.041 ± 0.231 | 0.565 | 1.160 ± 0.181 | 0.479 | |||||
| Rhodocoma gigantea (Kunth) H.P.Linder | Reproductive | 0.343 ± 0.055 | 0.095 | 0.231 ± 0.154 | 0.266 | (3) | 0.122 ± 0.024 | 0.053 | (4) | Zurich | ME3007 |
| Sterile | 0.041 ± 0.005 | 0.009 | 1.016 ± 0.284 | 0.493 | 0.590 ± 0.114 | 0.228 | |||||
| Thamnochortus bachmannii Mast. | Reproductive | 0.187 ± 0.016 | 0.036 | 0.688 ± 0.192 | 0.429 | (5) | 0.178 ± 0.024 | 0.333 | (2) | Zurich & Cape Town | ME3008 |
| Sterile | 0.064 ± 0.007 | 0.015 | 0.992 ± 0.299 | 0.668 | 0.985 ± 0.112 | 0.158 | |||||
| Thamnochortus cinereus. | Reproductive | 0.156 ± 0.020 | 0.035 | 0.491 ± 0.164 | 0.285 | 0.092 ± 0.016 | 0.028 | Zurich | ME3012 | ||
| Sterile | 0.034 ± 0.004 | 0.006 | 1.562 ± 0.248 | 0.430 | 1.381 ± 0.033 | 0.057 | |||||
| Thamnochortus insignis Mast. | Reproductive | 0.171 ± 0.005 | 0.009 | 0.190 ± 0.083 | 0.144 | (3) | Cape Town | ||||
| Sterile | 0.063 ± 0.008 | 0.014 | 0.164 ± 0.485 | 0.840 | |||||||
| Thamnochortus rigidus Esterhuysen | Reproductive | 0.257 ± 0.014 | 0.024 | 0.316 ± 0.064 | 0.111 | (3) | 0.312 ± 0.133 | 0.231 | (3) | Zurich | ME3009 |
| Sterile | 0.051 ± 0.004 | 0.006 | 0.946 ± 0.24 | 0.416 | 1.045 ± 0.093 | 0.160 | |||||
| Thamnochortus spicigerus (Thunb.) Spreng. | Reproductive | 0.234 ± 0.028 | 0.056 | 0.671 ± 0.119 | 0.238 | (4) | 0.261 ± 0.015 | 0.030 | (4) | Zurich | ME3010 |
| Sterile | 0.070 ± 0.008 | 0.016 | 1.153 ± 0.155 | 0.310 | 0.884 ± 0.030 | 0.060 |
*Single individual measurements.
Ecophysiology – Shoot biomass per surface area.
As the main photosynthetic organ in restios is the shoot, we measured SMA as an equivalent to the commonly used leaf mass per area (LMA) (Ávila-Lovera et al., 2017). We collected three to five shoot segments of juvenile and adult shoots per individual and scanned the projected area using an Epson Perfection 750Pro flatbed scanner with transparency mode and a resolution of 300 dpi. The projected area of the samples was measured and surface area was computed using ImageJ 2.0 with the IJ-Rhizo macro (Pierret et al., 2013) and automatic settings. The software computes the surface area using diameter and total length, assuming a cylindrical shape. The scanned plant samples were dried for at least 72 h at 60 °C and weighed. SMA was then calculated by dividing biomass by its respective surface area.
Ecophysiology – Photosynthetic measurements.
We measured maximum photosynthetic rate for both shoot types using a Licor 6400XT Photosynthesis System (Lincoln, NB, USA) (hereafter 6400XT). All measurements were taken in climate chambers at 23 °C, 50 % humidity and about 95 µE m−2 s−1 light intensity. The sensor head of the 6400XT was set to 400 ppm CO2, 23 °C air temperature, and air humidity was kept within the range of 50–56 %. Measurements were taken at 2000 µE m−2 s−1 light intensity after visually checking for acclimatization to the highest light setting, when photosynthetic rate stopped rising (which occurred within 10 min). We programmed the 6400XT to measure photosynthetic rate for 20 s taking a measurement each second. The 6400XT is primarily designed for large leaves, and consequently air-tight sealing of the chamber for round shoots is difficult. We compensated for this by sealing the chamber with putty-like adhesives and tested for its tightness by watching for CO2 spikes when blowing air around the chamber prior to the measurements. We corrected the photosynthetic rates by the estimated half surface area of each sample, because only one side of the inserted shoot is exposed to the light source in the XT6400 measurement chamber. Area and mass for the photosynthesis samples was measured in the same way as described for the SMA measurements.
Ecophysiology – Anatomy.
To explore anatomical differences between juvenile and adult shoots, we fixed juvenile and adult shoots of two to four individuals of each species in 70 % EtOH (Table 1). These were hand sectioned and mounted in Hoyer’s medium (Anderson, 1954) according to the recipe of Coiro and Truernit (2017), photographed under fluorescence illumination using a Zeiss Axiocam HRs digital camera fitted with a Zeiss HF fluorescence filter. We measured the area of chlorenchyma and central ground tissue, which was taken to include all tissues inside the chlorenchyma, using the Circle Points method implemented in Zeiss AxioVision Version 4.8.2. We calculated the CGR of each sample.
Ecophysiology – Comparison between juvenile and adult sterile shoots in neotenous species.
To test the assumption that neotenic adult sterile shoots are indeed functionally equivalent to juvenile sterile shoots, we used three neotenous species, each represented by three individuals in the transition stage (Elegia equisetaceae, Rhodocoma gigantea and Thamnochortus cinereus). We measured SMA, Amass and CGR of adult sterile shoots, juvenile sterile shoots and reproductive shoots. The assumption of functional analogy comes from the visual similarity in architecture and general morphology and has also been described in other heteroblastic species (Murphy et al., 2003; Brown et al., 2006).
Ecophysiology – Statistical analysis.
We tested for differences in SMA, Amass and CGR with linear mixed effects models. SMA, Amass and CGR were each used as response variables, predicted by shoot type as an explanatory variable (fixed effect) considering individual and species (nested random effects). We compared these models with models including the presence or absence of adult sterile shoots characteristic of the species (neotenous vs. non-neotenous) as a second fixed effect explanatory variable (but removing species identity from the random effects as neoteny is species-specific) to test if there are differences between neotenous and non-neotenous species.
The hypothesis that juvenile and neotenous shoots are functionally equivalent was tested by calculating the pairwise Euclidean distance in the trait-space between and within the shoot type samples within each of the three species. We were not interested in differences between species. This resulted in a data matrix of paired comparisons of shoot type for each species and the trait dissimilarity (distance) of each paired comparison. We then tested if the trait distance between neotenous and reproductive shoots is larger than the distance between neotenous and sterile shoots. We applied a linear mixed effects model of distance as the response variable predicted and the compared shoot types (e.g. ‘juvenile–reproductive’ comparison, ‘juvenile–neotenic’ comparison, ‘juvenile–juvenile’ comparison) as explanatory variable (fixed effect) and species identity as the random effect.
Ecology – Data acquisition.
To test whether there is a niche difference between neotenous and non-neotenous species (Hypothesis 2) we scored all studied species for the presence of adult sterile shoots, based on Linder (2013). We used georeferenced occurrence data from Restionaceae assemblage plot data and herbarium records from Bolus and Compton Herbaria, assembled by H.P.L.; H.P.L. checked each record for accuracy (Fig S10).
We excluded all species that were not included in the phylogeny (Fig. S3) or had fewer than five occurrence records from the analysis. We selected annual precipitation, precipitation seasonality, precipitation in the driest month, temperature in the coldest month and in the warmest month, and temperature isothermality to reflect the climate, as these cover the range of climate variation in the CFR (Fig. S10). For each record, the environmental data were extracted from the Chelsa climate model (Karger et al., 2017). Chelsa models provide data with a grid size of 0.5 arcminutes (roughly 1 km at the equator). The regional environment was defined by a polygon surrounding the occurrence locations of all restios. We extracted environmental data for all grid cells within that polygon from the environmental models (Fig. S10)
We tested for spatial correlation between occurrences of neotenous and non-neotenous species, by first calculating the pairwise geographical distances between all individuals’ locations (Euclidean distance, function dist in the stats package). We then calculated the Pearson correlation between the distance and the pairwise trait comparison matrix. An extremely small correlation of 0.0518 showed that there is no relevant spatial pattern.
Ecology – Niche overlap.
We performed a principal components analysis (PCA) based on the climate data extracted from all raster grid cells of the defined region and predicted PCA scores for all species occurrences in that grid cell-based (regional climate) PCA space. We used an amended method of Broennimann et al. (2012) to compute the niche overlap between neotenous and non-neotenous species based on the climatic conditions represented by the PCA scores associated with the occurrence points. We calculated Schoener’s D niche overlap (Schoener, 1965) metric. The method presented by Broennimann et al. (2012) allows a comparison of binned probability densities along two environmental dimensions (here PCA axes), corrected for the regional environment abundances of two subjects (here the neotenous vs. the non-neotenous occurrences), to calculate overlap metrics. We extended the method to allow the use of more than two dimensions (see File S1 and Fig. S1 for details of the niche overlap calculation and method). We then constructed a null distribution of random overlaps under the assumption that neoteny state is random, by shuffling the neoteny state and computing the niche overlap between the two randomly assigned groups in 999 generated datasets. If the observed Schoener’s D fell outside the upper 95 % quantile of the null distribution, we interpreted the observed niche overlap as significantly greater than the overlaps of random neotenic or non-neotenic assignment to species’ occurrences.
Moreover, to account for phylogenetic relatedness between species, we calculated species pairwise niche overlaps. We then tested if niche overlap between neotenous species is smaller (neotenous species sharing more similar conditions) than niche overlap between neotenous and non-neotenous species. We used non-parametric analysis of covariance (ANCOVA), implemented in the fANCOVA package with function T.aov (Wang, 2010), using niche overlap as the response variable, the pair’s neoteny states as the explanatory categorical variable (both species neotenous vs. a neotenous and non-neotenous pair) and phylogenetic distance as a numerical explanatory variable. Phylogenetic distances were calculated with the function cophenetic (Paradis et al., 2004) in the package ape using the dated restio phylogeny from Bouchenak-Khelladi and Linder (2017).
Ecology – Phylogenetic logistic model.
Besides niche overlap comparisons, we tested which environmental conditions may be favoured by neotenous or non-neotenous species. Based on the regional climatic PCA, we calculated mean ordination scores for each species, weighted by their relative frequency along PCA axes, which were corrected by the relative frequency of grid cells along the PCA axis – thus, rare occurrences in conditions that are generally rare in that region have the same impact on the species mean as many occurrences in common climatic conditions. Details of the PCA are given in Fig. S5 and Tables S6–8, and the regional environment–frequency weighted-mean calculation is presented in File S2. We tested environmental effects using a phylogenetic generalized linear model (pglm) with logit link function, using the binary neoteny state (neotenous = 1, non-neotenous = 0) as the response variable, predicted by the climatic conditions represented as the mean PCA scores used as explanatory variables. We used the first three PCA axes, explaining 84.3 % of the regional variation. The model predicts a probability of presence of neotenous species (which can be understood as the probability of non-neotenic species equal to 1 − the probability of neotenous species) that theoretically translates directly into expected neotenous to non-neotenous species proportions under given environmental conditions (PCA scores).
We applied the phyloglm function implemented in the phylolm package (Ho and Ane, 2014) with the IG10 method that optimizes a generalized estimating equation (GEE) approximation to the penalized likelihood of the logistic regression function. The phyloglm function incorporates the framework developed by Ives and Garland (2010). Pglms are generalized linear models implemented with a logit link function for binary response variables, and account for non-independence of closely related species, thus reducing inflated Type I errors occurring using standard methods in cases of phylogenetic conservatism (Ives and Garland, 2014). The applied method uses a correlation matrix defined by a matrix formulation of the phylogenetic tree and the rate of state transitions of the species by simulated character evolution over the phylogeny. Higher transition rates make it more likely that the phylogenetic conservatism in the trait breaks, and thus can be understood as the trait being more phylogenetically independent in such a case (Ives and Garland, 2010). For phylogenetic correction we used pruned trees with all species that were included in the regional climate PCA scores dataset.
We also analysed a local plot-based environmental dataset including simple moisture and soil information (Table S1) in a similar way as described using the grid cell-based climatic date for more local-scale effects (see File S3 for details about the plot-based analysis).
Furthermore, we ran two validation tests (1) to evaluate the potential of any significant results being stochastic artefacts by randomizing the neotenous species and repeating the models 999 times comparing the models’ z-values to the observed model’s z-value; and (2) to analyse the impact of phylogenetic uncertainty by rerunning the models on 1000 randomly sampled trees from the set of post-burnin phylogenies.
RESULTS
Ecophysiology
Shoot mass per area.
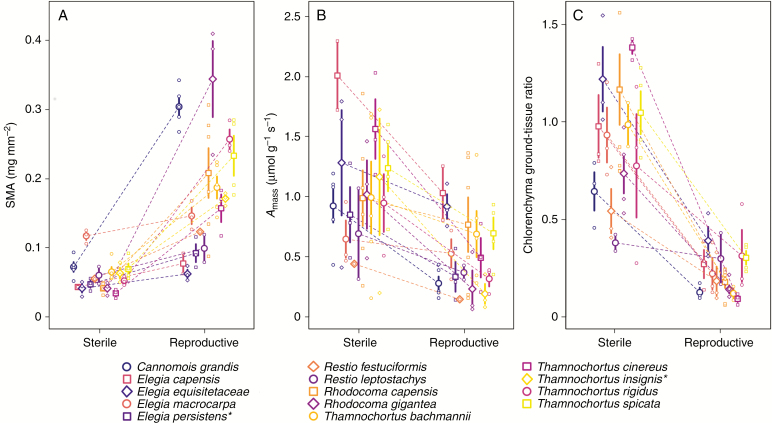
The reproductive shoots have a significantly higher SMA than juvenile sterile shoots in our sampled species (P < 0.001, marginal R2 = 0.540, conditional R2 = 0.699, Fig. 3A, Table 1 and Supplementary Data Table S1). Differences between shoot types are species-specific and range from a subtle difference of 0.02 mg mm−2 in Elegia macrocarpa to large differences such as in Rhodocoma gigantea with 0.3 mg mm−2 difference. These reflect species-specific variation in differences in shoot types, but cannot be explained by the species being neotenous or non-neotenous when using the model including species’ neoteny characterization (Pshoot type < 0.001, Pneoteny = 0.928, marginal R2 = 0.552, conditional R2 = 0.680).
Fig. 3.
Species pairwise comparison of sterile and reproductive shoots in (A) shoot mass per area (SMA), (B) maximum photosynthetic capacity(Amass) and (C) chlorenchyma to central ground tissue ratio. Large points with bar show the species’ shoot type-specific mean traits with standard error, while small points represent measurement data; dashed lines link species’ sterile and reproductive shoot trait means; asterisks (*) indicate species which were not included in the chlorenchyma – ground tissue ratio comparison.
Maximum photosynthetic capacity per biomass.
The reproductive shoots have a lower Amass compared to the juvenile sterile shoots (P < 0.001, marginal R2 = 0.209, conditional R2 = 0.438, Fig. 3B, Table 1 and Supplementary Data Table S2) in all cases. The differences are species-specific, but the variation is not explained by species being neotenous or non-neotenous according to the model including neoteny character of the species (Pshoot type < 0.001, Pneoteny = 0.457, marginal R2 = 0.194, conditional R2 = 0.446). The highest differences of 0.9 µmol g−1 s−1 were observed in Elegia capensis and Thamnochortus insignis and the lowest difference of 0.25 µmol g−1 s−1 in Restio festuciformis and Elegia persistens.
Shoot anatomy.
The general restio shoot anatomy agrees with previous reports (Linder, 1984). Generally, sterile shoots have a higher CGR than reproductive shoots (P < 0.001, marginal R2 = 0.641, conditional R2 = 0.707, Fig. 3C, Table 1 and Supplementary Data Table S3). The ground tissue in the reproductive shoots has a diameter up to five times larger than in the sterile shoots, but neotenous species do not behave significantly differently from non-neotenous species (Pshoot type < 0.001, Pneoteny = 0.404, marginal R2 = 0.645, conditional R2 = 0.712).
Neotenic adult sterile shoots.
The trait distance between adult sterile shoots and reproductive shoots is significantly than that between adult sterile shoots and juvenile sterile shoots in the trait space defined by using SMA, Amass and CGR (P < 0.05, Table 2, Supplementary Data Table S4).
Table 2.
Model coefficients of selected variables in contrast to distance between adult sterile (neotenous) shoots and sterile (juvenile) shoots of the linear mixed effects model: trait distance within species ~ shoot types compared with nested random effects of individual in species
| Estimate | s.e. | d.f. | t-value | P-value | |
|---|---|---|---|---|---|
| Intercept (= Dneot – sterile) | 0.944 | 0.084 | 25.2 | 11.303 | <0.001 |
| D neot – repr | +0.263 | 0.116 | 109 | 2.279 | <0.05 |
| D sterile – repr | +0.411 | 0.116 | 109 | 3.554 | <0.001 |
s.e., standard error; d.f., degrees of freedom; D, Euclidean distance; neot, adult sterile (neotenous) shoots; sterile, juvenile sterile shoots; repr, reproductive shoots.
Ecology
Niche overlap.
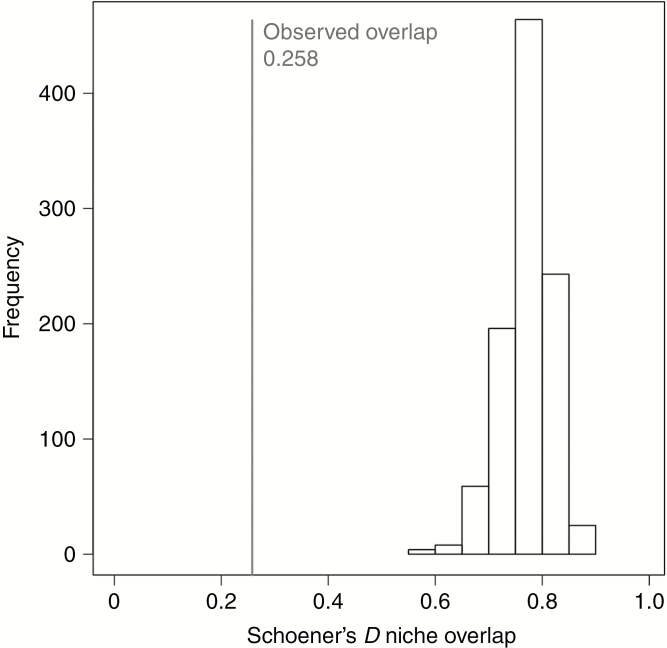
Schoener’s D overlap between neotenous and non-neotenous species, calculated for the first three PC axes, was lower than the overlap distribution of the randomized trait groups (P < 0.001, Fig. 5). In the pairwise comparison the niche overlap between neotenous and non-neotenous species is significantly smaller (P < 0.05) than the within-trait (both neotenous, or both non-neotenous species compared) overlaps when testing with a non-parametric ANCOVA which included the phylogenetic distance.
Fig. 5.
Histogram of Schoener’s D niche overlaps between the neotenous and non-neotenous species groups of the 999 randomized data sets with shuffled species characterization as neotenous or non-neotenous in contrast to the observed niche overlap measures in grey.
Phylogenetic logistic models.
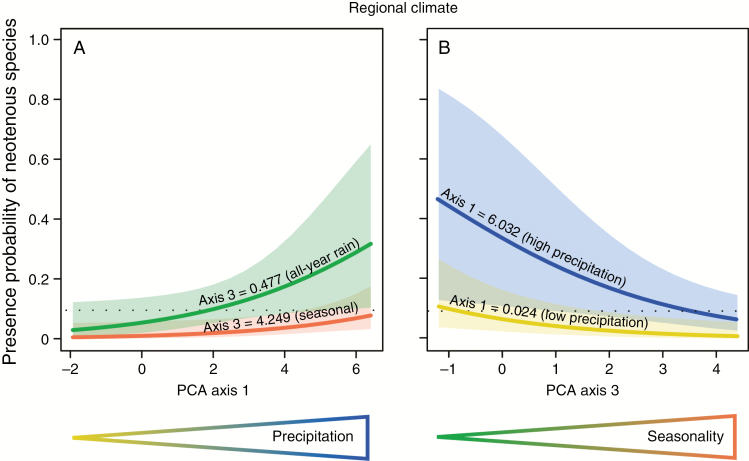
The phylogenetic logistic model shows a significant relationship between PCA axes 1 (positively correlated with annual precipitation and precipitation in the driest month, explaining 40.8 % of the total variance, Tables S6–8) and 3 (negatively correlated with rainfall seasonality and positively correlated with summer drought, explaining 13.3 % of the total variance, Tables S6–8) of the regional climate PCA, and the probability of the presence of neotenous species (PCA axis 1: P < 0.05; PCA axis 2: P < 0.01, Fig. S4, Table S5). The model predicts a higher probability of neotenous species at lower values of PCA axes 1 and 3. Generally, the probability of the presence of neotenous species is low when seasonality is very high regardless of the amount of annual precipitation, and similarly when annual precipitation is low regardless of seasonality (Fig. 6 and Supplementary Data Fig. S3). The plot-based principal coordinates analysis (PCoA) (Fig. S5, Tables S9–11) also shows an increased probability of the presence of neotenous species with increasing humidity and soil fertility (P < 0.05, Fig. S2, Table S9).
Fig. 6.
Multivariate phylogenetic logistic models predicting the probability of the presence of neotenous restio species along climatic gradients represented by PCA axes based on grid-based climatic models. (A) Axis 1 correlates with precipitation and (B) axis 3 correlates with precipitation seasonality. The two curves in each graph reflect the 90th (coloured curves) and 10th (black curves) percentile values of the second significant predictor of the model. The shaded area represents the 95 % confidence intervals. The dotted line represents the expected probability of the presence of neotenous species under the null model. The non-significant predictor (axis 2) was fixed at its median.
The randomization test of the trait groups shows that the z-values for the effect of PCA axes 1 and 3 are lower than the upper 95th percentile of the randomization test results (Fig. S6), showing that neotenous species are not distributed randomly across the climatic variation. P-value distributions of the topological uncertainty analysis are given in Fig. S8 for both axes, showing the robustness of the results. Similar results were obtained using the plot-based PCoA (Figs S7 and S9).
DISCUSSION
Here we show that heteroblasty in restios represents an ecophysiological transition: juvenile sterile shoots have a lower SMA and a higher Amass than reproductive shoots, due to differences in the ratio of photosynthetically active and structural tissue (Figs 3 and 4). Moreover, the niche overlap between neotenous restios with adult sterile shoots and non-neotenous species is smaller than expected from random comparisons between groups of species, indicating niche differences between neotenous and non-neotenous species. Neotenous species are also more likely to be found in wetter conditions (higher annual precipitation, less seasonality, more ground moisture) and on more fertile soils (derived from shales, shale-bands or tillite, and with loamy or clayey texture) than non-neotenous species (Supplementary Data File S3 and Fig. S3). This inference corroborates our expectation that heteroblasty in mediterranean species is advantageous in habitats that experience strong environmental changes through the life cycle of the individual plants.
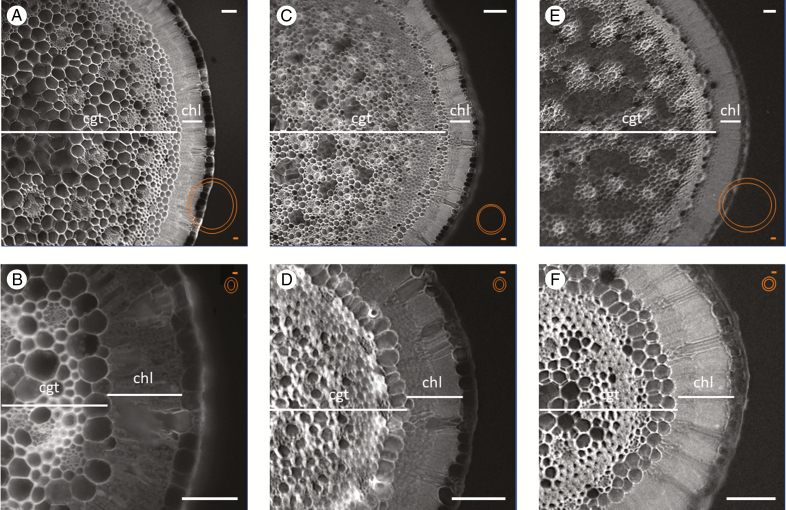
Fig. 4.
Anatomy of restio shoots. The top row shows reproductive shoots and the bottom row sterile shoots, scaled to show equal shoot radius. In orange, the dimensions are visualized with equal scales. A, B, Elegia capensis; C, D, Rhodocoma capensis; E, F, Thamnochortus spicigerus. White scale bar = 100 µm; orange scale bar = 300 µm. cgt, central ground tissue; chl, chlorenchyma.
Physiological difference of sterile and reproductive shoots
We interpret the different SMA and photosynthesis per biomass between the two shoot types as representing contrasting ecophysiological strategies. On the one hand, sterile shoots are cheap in terms of biomass per surface area, a proxy for carbon investment in leaves (Williams et al., 1989) and green stems (Ávila-Lovera et al., 2017). With higher photosynthesis rates per invested carbon, they return the invested carbon much faster than reproductive shoots. On the other hand, reproductive shoots have a slower, more conservative carbon return rate, but structurally allow for greater plant height, probably to facilitate pollination by increased pollen transfer distance (Niklas, 1985), and placing inflorescences in better wind conditions (Rosenberg et al., 1983), as well as reducing the filtration effect of the vegetation (Levin and Kerster, 1974). The combination of these factors may have a large impact at even small height differences (Handel, 1976). Taller plants may also be more effective dispersers (Thomson et al., 2018). Thus, the sterile shoot morphology may be optimal for photosynthesis, while the reproductive shoot morphology may constitute a compromise between photosynthesis, pollination, and dispersal biology. This pattern is driven largely by an allometric relationships between stem diameter and proportion of green tissue to structural tissue, representing a trade-off between photosynthesis and structural support (Boyce, 2008). The pattern we find fits the general model of the leaf economics spectrum, and its underlying anatomical basis of leaf mass per area (John et al., 2017), a key trait in the leaf economics spectrum.
Our finding of a higher SMA and lower Amass in sterile shoots compared to reproductive shoots is based on measurements of plants growing in a glasshouse. We are aware that our measurements of photosynthetic rates may not represent true values under natural conditions. Nonetheless, they allowed us to compare shoot types within and between species, as conditions were kept constant for all plants, and sterile and reproductive shoots were measured sequentially on the same individual. We focused our analyses on photosynthetic rates under intense light, reflecting maximum rates, where measurement noise, overestimation due to gasket CO2 diffusion (Pons and Welschen, 2002) or internal CO2 transport should have the least impact. In some species, and particularly for sterile shoots, we found large variance between individuals. Other than biological variability, several factors could have impacted this pattern: (1) the difficulty of using the 6400XT with multiple round samples with low surface area; (2) low readings that result in a higher impact of leaks and noise; (3) the age of the shoots, which may play a role in photosynthetic responses (Makino et al., 1985); or (4) whole plant responses we did not account for, such as time of day (Dodd et al., 2005) when we placed the species in the growth chambers for measuring. Nevertheless, the general trend fits those found in SMA and CGR, and follows our expectation: we therefore believe that the differences in Amass between juvenile and reproductive stages are real.
Heteroblasty as an adaptive attribute
We find that neotenous species tend to be found in different ecological conditions than non-neotenous species, suggesting that the two morphologies may have an adaptive importance. Environmental gradients, mostly studied on a spatial scale, are associated with trait changes usually resulting from changes in species composition (Pérez-Ramos et al., 2012). Generally, mesic conditions are linked to fast economic strategies, and harsher conditions to slow resource turnover (Reich et al., 1999; Wright et al., 2004). Our results, showing the retention of sterile shoots with their fast economics strategies in the neotenous restios under more mesic conditions, are consistent with these predictions.
Succession is commonly characterized by a shift in communities’ plant traits, mediated by a temporal species turnover (Vile et al., 2006) or trait adjustment by species. In the early stages of succession, high growth rates and fast resource turnover may be advantageous, whereas in later stages, resource efficiency, reproduction and stress resistance gain importance (Huston and Smith, 1987). Traits associated with these strategies (Wright et al., 2004; Pérez-Ramos et al., 2012; Pierce et al., 2016) should change accordingly, possibly driven by increasing competition. The implication is that the same species must be adapted to different, successionally separated, selective regimes.
Fire results in an increase of both total nitrogen and ammonium nitrogen just after the fire, with an increase in nitrogen in the form of nitrate due to rapid nitrification of the ammonium which lasts for 9^months after the fire (Stock and Lewis, 1986). Fire also increases the concentration of resin-extractable phosphorus for up to 4–6 months after the fire, with a later mineralization phase which lasts months (Brown and Mitchell, 1986). Moreover, the removal of vegetation by fire reduces evaporation of the soil-water for up to 2 years after fire (Scott and van Wyk, 1992; Mappin et al., 2003; Clemente et al., 2005; Parra and Moreno, 2017), as well as reducing below-ground competition for water, which has been shown to have a significant negative effect on the growth of restio seedlings (Silvertown et al., 2012).
Resource availability declines in the years after fire, and below-ground competition for water and nutrients increases, thus aggravating the effects of summer drought, and leading to a harsher environment. We argue that heteroblasty in restios is an adjustment mechanism providing individuals with appropriate traits for these contrasting conditions. The high-nutrient, low below-ground competition conditions with higher water availability early in the post-fire succession may select for the fast-economics strategy of sterile shoots, enabling the plants to invest more carbon in their rooting systems and rhizomes. This allows them to use the increased soil nitrogen available in the year after the fire by storing such nitrogen in the rhizome (Stock et al., 1987), as well as to reach deeper and wetter soil layers before competition and summer drought reduces available water in shallow soil horizons. The decline in water availability 2 years after fire, due to the increase in vegetation cover, and the exhaustion of the stored nutrients could promote the switch to a more conservative maintenance strategy, which increases stress resistance ability.
The production of organs with higher mass per area in larger, older plants contrasts with the expected allometric relationships between age and LMA in poalean monocots, where leaves on younger plants tend to present higher LMA than leaves on older plants (reviewed by Poorter et al., 2009). The switch in shoot type in restios is also associated with the switch to reproduction, and so one might expect that the production of more robust shoots might be simply driven by the structural needs of bearing inflorescences (Fig. 7). However, the complete loss of juvenile shoots (and their retention in neotenous species growing in more mesic conditions) suggests that the switch to reproductive investment is not the only cause for such a heteroblastic strategy. Moreover, reproductive shoots flower only in their first year but are maintained after flowering for years (Linder and Caddick, 2001). The reproductive shoots in restios could represent a morphological adaptation that combines the advantages of a conservative economic strategy and the structural needs of reproduction.
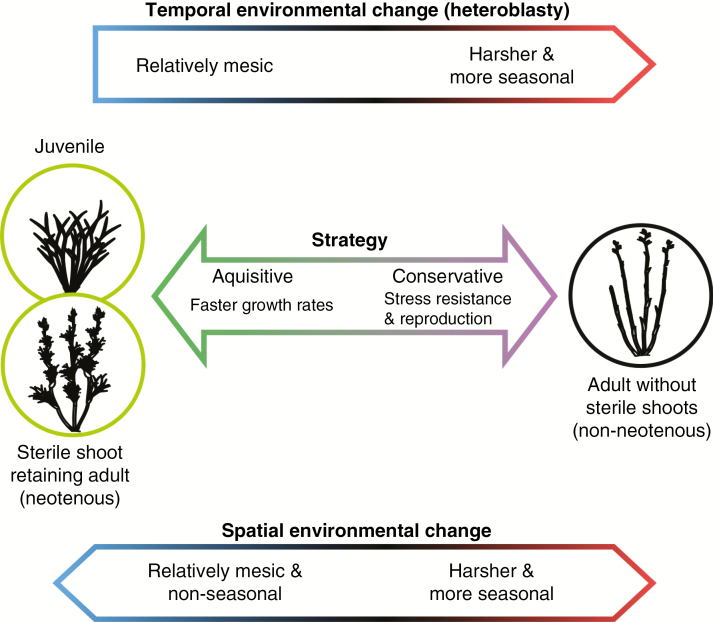
Fig. 7.
Conceptual figure presenting the main results of the heteroblastic change (top) and species differences (bottom) in terms of sterile shoots linked to environmental conditions.
The success of restios in the Cape flora may be partially due to their heteroblasty. With heteroblasty, plants can optimize photosynthetic efficiency in the juvenile phase, releasing carbon that can be invested in the rapid development of the rooting system, allowing the juveniles to survive the summer droughts and giving them a competitive edge in nutrient acquisition in the severely oligotrophic soils. In the adult stage, the shift in morphology to more robust, taller and more persistent shoots is functionally equivalent to being conservative with available resources and improving reproductive success. Similar ecophysiological strategy shifts from acquisitive to conservative have been shown in other heteroblastic groups, such as the Australian acacias (Morris et al., 2011), Mediterranean pines (Climent et al., 2006, 2013) and Juniperus (Miller et al., 1995), and might also apply to heteroblastic members of Eucalyptus and Australian Proteaceae. All these groups occur in seasonally dry, relatively infertile conditions with fires on a decadal scale. These fires lead to a sudden nutrient flush, followed by a decay of the nutrient addition and moisture availability together with an increase in below-ground competition during the post-fire succession. If germination is fire-triggered (so that the seedlings are found in the post-fire environment), then each plant is exposed to relatively benign conditions as a seedling, and much harsher conditions as an established plant. For heteroblasty to evolve and to persist as an advantageous mechanism, it may require long-term stable systems with periodic environmental enhancements and successive decay.
Heteroblasty as an inflexible mechanism in contrast to plasticity might cause heteroblastic species to go extinct or become displaced when the conditions for which the heteroblastic trait is advantageous disappear. However, in the restios, heteroblasty seems to have evolved at least in the common ancestor of all South African species. Therefore, heteroblasty might represent an important background trait (sensuBouchenak-Khelladi et al., 2015): that is, a character that evolved before the radiation of the restios and helped to trigger the increase in diversification when the group encountered mediterranean environmental conditions. Also, in Juniperus, heteroblasty potentially evolved well before the origin of Juniperus sect. Sabina, as heteroblasty is present in other Cupressaceae close to Juniperus (Eckenwalder, 2009). The variation in the advantages of heteroblasty under differing environmental conditions in Eucalyptus (Jordan et al., 2000) provides further evidence that heteroblasty might be selected and evolve under one condition but prove be successful in other conditions as well.
We conclude that heteroblasty might present a previously unrecognized adaptation to fire-driven supra-annual flushes of nutrients and groundwater typical of mediterranean-type ecosystems.
DATA ACCESSIBILITY
Data and R scripts are available from the Figshare Digital Repository (https://figshare.com/s/56e2cb0c742629c2cf95).
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. File S1: Extension of the niche overlap method of Broennimann et al. (2012). Figure S1: Framework for calculating the species niches from multidimensional data, using multiple single dimension kernel density estimations or applying a multivariate kernel density estimation. Figure S2: Pairwise correlation matrix of two-dimensional niche overlap measures derived from the different niche computation methods. File S2: Calculation of species-weighted mean environments. File S3: Model of abundance of neotenous species in local habitat conditions. Table S1: Habitat data descriptors used as categorical variables together with their ordered states used to calculate a Gower distance to compute the plot-based habitat PCoA. Figure S3: Multivariate phylogenetic logistic models predicting the probability of presence of retaining restio species along local environmental gradients. Figure S4: Phylogenetic tree of all Restionaceae species included in our study. Figure S5: Correlation matrix plots of PCA axes with environmental variables and PCoA axes with the ordered habitat conditions. Figure S6: Histograms of z-values of phylogenetic logistic models of predicting retaining restio occurrence probabilities by regional climate PCA axes. Figure S7: Histograms of z-values of phylogenetic logistic models of predicting retaining restio occurrence probabilities by local habitat PCoA axes. Figure S8: Histograms of probability values for the phylogenetic logistic model’s null hypothesis (no climatic effect on the presence of retaining species) being true using 1000 randomly sampled post-burnin trees for phylogenetic correction. Figure S9: Histograms of probability values (P-values) for the phylogenetic logistic model’s null hypothesis (no local environment effect on the presence of retaining species) being true using 1000 randomly sampled post-burnin trees for phylogenetic correction. Figure S10: Occurrence points in the Cape region with polygon defining the region. Table S2: Model coefficients of the linear mixed effects model (SMA ~ shoot type) with nested random effects of individual in species. Table S3: Model coefficients of the linear mixed effects model (Amass ~ shoot type) with nested random effects of individual in species. Table S4: Model coefficients of the linear mixed effects model (CSR ~ shoot type) with nested random effects of individual in species. Table S5: Post hoc pairwise differences of least squares means (population means) table of the model trait distance within species ~ shoot types compared with nested random effects of individual in species. Table S6: Model coefficients of the phylogenetic logistic model (presence of adult sterile shoots ~ PCA axis 1 + PCA axis 2 + PCA axis 3, using IG10 method). Table S7: Regional climate PCA variable loadings. Table S8: Correlation matrix of regional climate PCA axes and climatic variables. Table S9: Regional climate PCA axes’ eigenvalues and explained variance. Table S10: Model coefficients of the phylogenetic logistic model (presence of adult sterile shoots ~ PCoA axis 1 + … + PCoA axis 5, using IG10 method). Table S11: Local habitat PCoA axis eigenvalues and explained variance. Table S12: Correlation matrix of PCoA axis with ordinal scored habitat variables.
ACKNOWLEDGEMENTS
We thank the members of the Linder Lab and Anna Weigand for reviewing early manuscript versions and lively discussions that improved the work, René Stalder for cultivating our plants, the ScienceCloud service of S3IT, University of Zurich, for computational support and Cape Nature for collecting permits. We also thank Ben Shipley, Kent Holsinger and two anonymous reviewers for helpful comments that greatly improved the manuscript. This work was supported by the Swiss National Science Foundation [grant number 31003A_152982 to H.P.L.] and the Claraz Schenkung. The authors have no conflict of interests to declare.
LITERATURE CITED
- Adler PB, Drake JM. 2008. Environmental variation, stochastic extinction, and competitive coexistence. American Naturalist 172: E186–E195. [DOI] [PubMed] [Google Scholar]
- Anderson LE. 1954. Hoyer’s solution as a rapid permanent mounting medium for bryophytes. The Bryologist 57: 242–244. [Google Scholar]
- Ávila-Lovera E, Zerpa AJ, Santiago LS. 2017. Stem photosynthesis and hydraulics are coordinated in desert plant species. New Phytologist 216: 1119–1129. [DOI] [PubMed] [Google Scholar]
- Bauer H, Bauer U. 1980. Photosynthesis in leaves of the juvenile and adult phase of ivy (Hedera helix). Physiologia Plantarum 49: 366–372. [Google Scholar]
- Bergh EW, Compton JS. 2015. A one-year post-fire record of macronutrient cycling in a mountain sandstone fynbos ecosystem, South Africa. South African Journal of Botany 97: 48–58. [Google Scholar]
- Bouchenak-Khelladi Y, Linder HP. 2017. Frequent and parallel habitat transitions as driver of unbounded radiations in the Cape flora. Evolution 71: 2548–2561. [DOI] [PubMed] [Google Scholar]
- Bouchenak‐Khelladi Y, Onstein RE, Xing Y, Schwery O, Linder HP. 2015. On the complexity of triggering evolutionary radiations. New Phytologist 207: 313–326. [DOI] [PubMed] [Google Scholar]
- Boyce CK. 2008. How green was Cooksonia? The importance of size in understanding the early evolution of physiology in the vascular plant lineage. Paleobiology 34: 179–194. [Google Scholar]
- Briggs B, Linder HP. 2009. A new subfamilial and tribal classification of Restionaceae (Poales). Telopea 12: 333–345. [Google Scholar]
- Broennimann O, Fitzpatrick MC, Pearman PB, et al. 2012. Measuring ecological niche overlap from occurrence and spatial environmental data. Global Ecology and Biogeography 21: 481–497. [Google Scholar]
- Brown G, Mitchell DT. 1986. Influence of fire on the soil phosphorus status in sand plain lowland fynbos, south-western Cape. South African Journal of Botany 52: 67–72. [Google Scholar]
- Brown GK, Ariati SR, Murphy DJ, Miller JTH, Ladiges PY. 2006. Bipinnate acacias (Acacia subg. Phyllodineae sect. Botrycephalae) of eastern Australia are polyphyletic based on DNA sequence data. Australian Systematic Botany 19: 315–326. [Google Scholar]
- Brown NAC, van Staden J, Daws MI, Johnson T, van Wyk AE. 2003. Patterns in the seed germination response to smoke in plants from the Cape Floristic Region, South Africa. South African Journal of Botany 69: 514–525. [Google Scholar]
- Burns KC, Dawson JW. 2006. A morphological comparison of leaf heteroblasty between New Caledonia and New Zealand. New Zealand Journal of Botany 44: 387–396. [Google Scholar]
- Christensen NL. 1994. The effects of fire on physical and chemical properties of soils in Mediterranean-Climate shrublands. In: Moreno JM, Oechel WC, eds. Ecological Studies. The role of fire in mediterranean-type ecosystems. New York: Springer, 79–95. [Google Scholar]
- Clemente AS, Rego FC, Correia OA. 2005. Growth, water relations and photosynthesis of seedlings and resprouts after fire. Acta Oecologica 27: 233–243. [Google Scholar]
- Climent J, Chambel MR, López R, Mutke S, Alía R, Gil L. 2006. Population divergence for heteroblasty in the Canary Island pine (Pinus canariensis, Pinaceae). American Journal of Botany 93: 840–848. [DOI] [PubMed] [Google Scholar]
- Climent J, Dantas AK, Alia R, Majada J. 2013. Clonal variation for shoot ontogenetic heteroblasty in maritime pine (Pinus pinaster Ait.). Trees 27: 1813–1819. [Google Scholar]
- Coiro M, Truernit E. 2017. Xylem characterization using improved pseudo-Schiff propidium iodide staining of whole mount samples and confocal laser-scanning microscopy. In: Methods in molecular biology. Xylem. New York: Humana Press, 127–132. [DOI] [PubMed] [Google Scholar]
- Crosti R, Ladd PG, Dixon KW, Piotto B. 2006. Post-fire germination: the effect of smoke on seeds of selected species from the central Mediterranean basin. Forest Ecology and Management 221: 306–312. [Google Scholar]
- Day JS. 1998. Light conditions and the evolution of heteroblasty (and the divaricate form) in New Zealand. New Zealand Journal of Ecology 22: 43–54. [Google Scholar]
- Dodd AN, Salathia N, Hall A, et al. 2005. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309: 630–633. [DOI] [PubMed] [Google Scholar]
- Eckenwalder JE. 2009. Conifers of the world: the complete reference. Portland: Timber Press. [Google Scholar]
- Goebel K. 1889. Über die Jugendzustände der Pflanzen. Flora 72: 1–45. [Google Scholar]
- Goldblatt P, Manning JC. 2002. Plant diversity of the Cape Region of Southern Africa. Annals of the Missouri Botanical Garden 89: 281–302. [Google Scholar]
- Handel SN. 1976. Restricted pollen flow of two woodland herbs determined by neutron-activation analysis. Nature 260: 422–423. [Google Scholar]
- Ho LST, Ane C. 2014. A linear-time algorithm for Gaussian and non-Gaussian trait evolution models. Systematic Biology 63: 397–408. [DOI] [PubMed] [Google Scholar]
- Huston M, Smith T. 1987. Plant succession: Life history and competition. The American Naturalist 130: 168–198. [Google Scholar]
- Ives AR, Garland T. 2010. Phylogenetic logistic regression for binary dependent variables. Systematic Biology 59: 9–26. [DOI] [PubMed] [Google Scholar]
- Ives AR, Garland T. 2014. Phylogenetic regression for binary dependent variables In: Garamszegi, LZ, ed. Modern phylogenetic comparative methods and their application in evolutionary biology. Berlin: Springer, 231–261. [Google Scholar]
- John GP, Scoffoni C, Buckley TN, Villar R, Poorter H, Sack L. 2017. The anatomical and compositional basis of leaf mass per area. Ecology Letters 20: 412–425. [DOI] [PubMed] [Google Scholar]
- Jordan GJ, Potts BM, Chalmers P, Wiltshire RJE. 2000. Quantitative genetic evidence that the timing of vegetative phase change in Eucalyptus globulus ssp. globulus is an adaptive trait. Australian Journal of Botany 48: 561–567. [Google Scholar]
- Karger DN, Conrad O, Böhner J, et al. 2017. Climatologies at high resolution for the earth’s land surface areas. Scientific Data 4: sdata2017122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraaij T, van Wilgen B. 2014. Drivers, ecology, and management of fire in fynbos In: Allsopp N, Colville JF, Verboom GA, eds. Fynbos: ecology, evolution, and conservation of a megadiverse region. New York: Oxford University Press, 47–72. [Google Scholar]
- Levin DA, Kerster HW. 1974. Gene flow in seed plants In: Evolutionary biology. Boston: Springer, 139–220. [Google Scholar]
- Linder HP. 1984. A phylogenetic classification of the genera of the African Restionaceae. Bothalia 15: 11–76. [Google Scholar]
- Linder HP. 1990. A morphological study on the Thamnochortus erectus complex (Restionaceae). South African Journal of Botany 56: 443–449. [Google Scholar]
- Linder HP. 2013. Delta database to Restionaceae species. Available at: restionaceae.e-monocot.org/node/1. [Google Scholar]
- Linder HP, Caddick LR. 2001. Restionaceae seedlings: morphology, anatomy and systematic implications. Feddes Repertorium 112: 59–80. [Google Scholar]
- Linder HP, Vlok JH. 1991. The morphology, taxonomy and evolution of Rhodocoma (Restionaceae). Plant Systematics and Evolution 175: 139–160. [Google Scholar]
- Linder HP, Briggs BG, Johnson L a. S. 1998. Restionaceae. In: The families and genera of vascular plants. Flowering plants · Monocotyledons. Berlin: Springer, 425–445. [Google Scholar]
- Lloyd DG. 1984. Variation strategies of plants in heterogeneous environments. Biological Journal of the Linnean Society 21: 357–385. [Google Scholar]
- Makino A, Mae T, Ohira K. 1985. Photosynthesis and ribulose-1,5-bisphosphate carboxylase/oxygenase in rice leaves from emergence through senescence. Quantitative analysis by carboxylation/oxygenation and regeneration of ribulose 1,5-bisphosphate. Planta 166: 414–420. [DOI] [PubMed] [Google Scholar]
- Mappin KA, Pate JS, Bell TL. 2003. Productivity and water relations of burnt and long-unburnt semi-arid shrubland in Western Australia. Plant and Soil 257: 321–340. [Google Scholar]
- McGill BJ, Enquist BJ, Weiher E, Westoby M. 2006. Rebuilding community ecology from functional traits. Trends in Ecology & Evolution 21: 178–185. [DOI] [PubMed] [Google Scholar]
- Miller PM, Eddleman LE, Miller JM. 1995. Juniperus occidentalis juvenile foliage: advantages and disadvantages for a stress-tolerant, invasive conifer. Canadian Journal of Forest Research 25: 470–479. [Google Scholar]
- Moreno JM, Oechel WC. 2012. The role of fire in mediterranean-type ecosystems. Berlin: Springer Science & Business Media. [Google Scholar]
- Morris TL, Esler KJ, Barger NN, Jacobs SM, Cramer MD. 2011. Ecophysiological traits associated with the competitive ability of invasive Australian acacias. Diversity and Distributions 17: 898–910. [Google Scholar]
- Mueller-Dombois D, Fosberg FR. 1998. Vegetation of the tropical Pacific islands. New York: Springer. [Google Scholar]
- Murphy DJ, Miller JT, Bayer RJ, Ladiges PY. 2003. Molecular phylogeny of Acacia subgenus Phyllodineae (Mimosoideae : Leguminosae) based on DNA sequences of the internal transcribed spacer region. Australian Systematic Botany 16: 19–26. [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853–858. [DOI] [PubMed] [Google Scholar]
- Niklas KJ. 1985. The aerodynamics of wind pollination. The Botanical Review 51: 328. [Google Scholar]
- Pabón-Mora N, González F. 2012. Leaf development, metamorphic heteroblasty and heterophylly in Berberis s. l. (Berberidaceae). The Botanical Review 78: 463–489. [Google Scholar]
- Paradis E, Claude J, Strimmer K. 2004. APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics 20: 289–290. [DOI] [PubMed] [Google Scholar]
- Parra A, Moreno JM. 2017. Post-fire environments are favourable for plant functioning of seeder and resprouter Mediterranean shrubs, even under drought. The New Phytologist 214: 1118–1131. [DOI] [PubMed] [Google Scholar]
- Pérez-Ramos IM, Roumet C, Cruz P, Blanchard A, Autran P, Garnier E. 2012. Evidence for a ‘plant community economics spectrum’ driven by nutrient and water limitations in a Mediterranean rangeland of southern France. Journal of Ecology 100: 1315–1327. [Google Scholar]
- Pierce S, Negreiros D, Cerabolini BEL, et al. 2016. A global method for calculating plant CSR ecological strategies applied across biomes world-wide. Functional Ecology 31: 444–457. [Google Scholar]
- Pierret A, Gonkhamdee S, Jourdan C, Maeght J-L. 2013. IJ_Rhizo: an open-source software to measure scanned images of root samples. Plant and Soil 373: 531–539. [Google Scholar]
- Pons TL, Welschen RAM. 2002. Overestimation of respiration rates in commercially available clamp-on leaf chambers. Complications with measurement of net photosynthesis. Plant, Cell & Environment 25: 1367–1372. [Google Scholar]
- Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R. 2009. Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytologist 182: 565–588. [DOI] [PubMed] [Google Scholar]
- R Core Team 2016. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Rao CV. 1971. Proteaceae. New Delhi: Council of Scientific & Industrial Research. [Google Scholar]
- Rebelo AG, Boucher C, Helme N, Mucina L. 2006. Fynbos biome. In: Mucina L, Rutherford MC, eds. The vegetation of South Africa, Lesotho and Swaziland. Pretoria: South African National Biodiversity Institute, 53–219. [Google Scholar]
- Reich PB. 2014. The world-wide ‘fast–slow’ plant economics spectrum: a traits manifesto. Journal of Ecology 102: 275–301. [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS. 1997. From tropics to tundra: Global convergence in plant functioning. Proceedings of the National Academy of Sciences, USA 94: 13730–13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich PB, Ellsworth DS, Walters MB, et al. 1999. Generality of leaf trait relationships: a test across six biomes. Ecology 80: 1955–1969. [Google Scholar]
- Rosenberg NJ, Blad BL, Verma SB. 1983. Microclimate: the biological environment. New York: Wiley-Interscience. [Google Scholar]
- Schoener TW. 1965. The evolution of bill size differences among sympatric congeneric species of birds. Evolution 19: 189–213. [Google Scholar]
- Scott DF, van Wyk DB. 1992. The effects of fire on soil water repellency, catchment sediment yields and streamflow In: Ecological studies. Fire in South African mountain fynbos. Berlin: Springer, 216–239. [Google Scholar]
- Silvertown J, Araya YN, Linder HP, Gowing DJ. 2012. Experimental investigation of the origin of fynbos plant community structure after fire. Annals of Botany 110: 1377–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock WD, Lewis OAM. 1986. Soil nitrogen and the role of fire as a mineralizing agent in a South African coastal Fynbos ecosystem. Journal of Ecology 74: 317–328. [Google Scholar]
- Stock WD, Sommerville JEM, Lewis OAM. 1987. Seasonal allocation of dry mass and nitrogen in a fynbos endemic Restionaceae species Thamnochortus punctatus Pill. Oecologia 72: 315–320. [DOI] [PubMed] [Google Scholar]
- Thomson FJ, Letten AD, Tamme R, Edwards W, Moles AT. 2018. Can dispersal investment explain why tall plant species achieve longer dispersal distances than short plant species?New Phytologist 217: 407–415. [DOI] [PubMed] [Google Scholar]
- Verdú M. 2000. Ecological and evolutionary differences between Mediterranean seeders and resprouters. Journal of Vegetation Science 11: 265–268. [Google Scholar]
- Vile D, Shipley B, Garnier E. 2006. A structural equation model to integrate changes in functional strategies during old-field succession. Ecology 87: 504–517. [DOI] [PubMed] [Google Scholar]
- Wang X-F. 2010. fANCOVA: Nonparametric Analysis of Covariance. R package version 0.5-1. https://CRAN.R-project.org/package=fANCOVA. [Google Scholar]
- Westoby M, Wright IJ. 2006. Land-plant ecology on the basis of functional traits. Trends in Ecology & Evolution 21: 261–268. [DOI] [PubMed] [Google Scholar]
- Williams K, Field CB, Mooney HA. 1989. Relationships among leaf construction cost, leaf longevity, and light environment in rain-forest plants of the genus Piper. The American Naturalist 133: 198–211. [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. 2004. The worldwide leaf economics spectrum. Nature 428: 821–827. [DOI] [PubMed] [Google Scholar]
- Zotz G, Wilhelm K, Becker A. 2011. Heteroblasty—A review. Botanical Review 77: 109–151. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and R scripts are available from the Figshare Digital Repository (https://figshare.com/s/56e2cb0c742629c2cf95).