Abstract
Background and Aims
It has been reported that low temperatures (LTs) and the plant hormone abscisic acid (ABA) induce the expression of CBF/DREB1 transcription factors in vegetative tissues and seedlings of Vitis vinifera and Vitis riparia and that foliar applications of ABA to V. vinifera increase the freezing tolerance or cold-hardiness of dormant buds. However, the combined effect of ABA and LTs on the expression of CBF/DREB1 transcription factors and on the acquisition of freezing tolerance in dormant grapevine buds has not been investigated. The objective of this study was to analyse the combined effect of ABA and LT treatments on the expression of CBF/DREB transcription factors and the acquisition of freezing tolerance.
Methods
In vitro experiments with single-bud cuttings of grapevines were used to analyse the effect of ABA, ABA + LT and LT on the expression of CBF/DREB transcription factors, dehydrin and antioxidant genes, the acquisition of freezing tolerance and the endogenous content of ABA. Gene expression analysis was performed by quantitative real-time PCR and freezing tolerance was determined by measuring the low-temperature exotherm by differential thermal analysis. ABA levels were determined by gas chromatography coupled to an electron capture detector.
Key Results
The LT treatment and exogenous application of ABA to grapevine dormant buds increased the expression of the CBF/DREB1 transcription factors VvCBF2, VvCBF3, VvCBF4 and VvCBF6. The joint application of LT and ABA produced a huge increase in the expression of these transcription factors, which was greater than the sum of the increases produced by them individually, which indicates the existence of a synergistic effect between ABA and LT on the activation of these transcription factors. This synergic effect was also observed on the increase in bud cold-hardiness and on the expression of antioxidant and dehydrin genes.
Conclusions
The synergy between ABA and LT on the expression of CBF/DREB1 transcription factors VvCBF2, VvCBF3, VvCBF4 and VvCBF6 plays a key role in cold acclimatization of grapevine buds. The results highlight the importance of the combination of stimuli in the improvement of genetic and physiological responses and help us to understand the adaption of plants to complex environments.
Keywords: Abscisic acid, cold-hardiness, CBF/DREB transcription factors, dehydrins, antioxidant genes, grapevine buds, low temperatures
INTRODUCTION
Vitis vinifera is one of the world’s most important fruit crops (This et al., 2006). It is cultivated mainly in temperate and semi-arid climates that can sometimes experience freezing and subfreezing temperatures, which can cause significant damage to the plants. As deciduous fruit trees, Vitis spp. acquire freezing tolerance or cold-hardiness before the arrival of winter, once the buds have entered endodormancy (Rubio et al., 2016). Freezing tolerance is part of the cold acclimatization process that buds undergo when exposed to low, non-freezing temperatures. Cold acclimatization is a complex trait involving multiple biochemical and physiological changes and results from the induced expression or repression of a battery of regulatory and functional genes (Fennell, 2014; Wisniewski et al., 2014). Among the regulatory genes, those encoding transcription factors play an important role in plant stress responses, acting as coordinators of stress signals and orchestrating the expression of functional genes (Singh et al., 2002; Wang et al., 2016). It is well known that the C-repeat (CRT)-binding factor/dehydration-responsive element (DRE) binding protein 1 (CBF/DREB1) transcription factors are involved in improving cold resistance (Stockinger et al., 1997; Chew and Halliday, 2011). The CBF/DREB1 proteins bind to the CRT/dehydration responsive element (CRT/DRE) of target cold-responsive genes and promote freezing tolerance (Thomashow, 2010; Theocharis et al., 2012). The most well-documented cold- and CBF-regulated genes are dehydrins, a group of late embryogenesis abundant proteins (LEAs) that contain the CRT/DRE regulatory element in their promoter. Several studies have shown an increased abundance of dehydrin transcripts and proteins accumulating in woody plant buds and bark during induction of dormancy and cold acclimatization (Wisniewski et al., 2014; Rubio et al., 2016). Analysis of the CBF/DREB1 pathway in grapevines has identified four CBF genes in Vitis vinifera and Vitis riparia that are upregulated in response to low temperature (LT) and abscisic acid (ABA) (Xiao et al., 2006, 2008). Thus, an increase in CBF3 and CBF4 expression was observed in the leaves of V. riparia and V. vinifera after 1–2 d at 4 °C (Xiao et al., 2006, 2008), contrasting with the quick cold induction observed in the case of CBF1 and CBF2 (Xiao et al., 2006). Overexpression of VvCBF4 in V. vinifera “Freedom” improved freezing survival and reduced freezing-induced electrolyte leakage by up to 2 °C in non-cold acclimated buds (Tillett et al., 2012). Likewise, overexpression of CBF transcription factors from V. vinifera “Koshu” (Takuhara et al., 2011) or V. riparia (Siddiqua and Nassuth, 2011) in Arabidopsis improved the freezing tolerance of transgenic plants. A total of seven CBF/DREB1 genes were cloned and sequenced from V. riparia and the less frost-tolerant V. vinifera (Carlow et al., 2017). Amino acid sequence comparison and phylogenetic analysis showed two different groups of Vitis CBFs. One group contained CBF1, CBF2, CBF3 and CBF8 and the other group contained CBF4, CBF5 and CBF6 (Carlow et al., 2017). However, it was reported that CBF1 (Xiao et al., 2006) was not found in the 12X V. vinifera genome database (Wisniewski et al., 2014; Vázquez-Hernandez et al., 2017). A careful analysis of the data indicates that two codes entered into the NCBI database by Xiao et al. (2006), AY390372 and AY390376, correspond to the same gene, GIDVvT00031496001, in the Vitis genome database. In addition, this gene has been designated as VvCBF2 in the work of Karimi et al. (2015) and Zandkarimi et al. (2015). So, in fact there are six CBF genes in the 12X Vitis genome database, which are shown in Table 1. In the present study we have analysed the effect of LT, ABA and LT + ABA on bud cold-hardiness and on the expression of CBF/DREB, dehydrin and antioxidant genes in grapevine dormant buds. The results clearly showed that bud cold-hardiness, CBF/DREB1 genes and expression of putative target genes of CBF/DREB1 were synergistically induced by combined treatment with ABA and LT.
Table 1.
List of putative Vitis vinifera CBF/DREB1 genes
| Gene | NCBI* | Vitis genome† | CHROMOSOME | Reference |
|---|---|---|---|---|
| VvCBF1 | AY390372 | GIDVvT00031496001 | - | Xiao et al., 2006 |
| VvCBF2 | AY390376 | GIDVvT00031496001 | 6 | Xiao et al., 2006 |
| VvCBF3 | AY390375 | GIDVvT00031494001 | 6 | Xiao et al., 2006 |
| VvCBF4 | DQ497624 | GIDVvT00040836001 | 16 | Xiao et al., 2008 |
| VvCBF5 | KX197193 | GIDVvT00040120001 | 19 | Carlow et al., 2017 |
| VvCBF6 | KX197195 | GIDVvT00041492001 | 2 | Carlow et al., 2017 |
| VvCBF8 | KX197197 | GIDVvT00007002001 | 8 | Carlow et al 2017 |
*NCBI: https://www.ncbi.nlm.nih.gov.
† Vitis genome 12X.v2: https://urgi.versailles.inra.fr/species/Vitis.
MATERIALS AND METHODS
Plant material and treatments
Canes were taken from 10-year-old Vitis vinifera ‘Thompson Seedless’ grown at the experimental station of the Chilean National Institute of Agricultural Research (INIA, La Platina) located in the Maipo valley (33°34′ S). All experiments were carried out with grapevine dormant buds collected on 23 April and 14 May (Vergara and Pérez, 2010; Rubio et al., 2016). For bud cold-hardiness and gene expression analysis, detached canes each carrying ten buds at positions 5–14 were collected from the vineyard and transferred to the laboratory and excised in single-bud cuttings. The cuttings were mounted on propylene sheets and floated in tap water in a plastic container (Supplementary Data Fig. S1) and exposed to the following treatments: (1) LT: buds were placed in a refrigerator at 4 °C in the dark; (2) ABA treatment: buds were sprayed with 100 µm ABA solution and kept in the dark at 14 °C in a growth chamber; (3) LT + ABA treatment: buds were sprayed with 100 µm ABA solution and placed in the refrigerator at 4 °C in the dark. ABA solution was prepared from 2.6 mg of ABA (Sigma–Aldrich, St Louis, MO, USA) dissolved in 1 mL of deionized water with 0.02 % Tween 20 (Sigma–Aldrich, St Louis, MO, USA). Single-node cuttings were sprayed on the bud side with the ABA solution or with water containing 0.02 % Tween-20 until runoff. To determine bud cold-hardiness and endogenous ABA content, samples collected on 14 May were harvested 0, 3, 7, 12 and 16 d after treatment. For gene expression analysis, samples were harvested after 1 and 2 weeks. To analyse the effect of ABA concentration on bud cold-hardiness, cuttings collected on 29 April were sprayed with 50, 100, 200 or 400 µm ABA solution and placed in the refrigerator at 4 °C in the dark for 1 week. To test whether hydrogen cyanamide (HC), a dormancy-breaking compound that degrades endogenous ABA in grapevine buds (Zheng et al., 2015; Vergara et al., 2017), affects bud cold-hardiness, two groups of 30 single-bud cuttings, each harvested on 23 April, were sprayed with 200 µm ABA solution and with 200 µm ABA plus 2.5 % (w/v) HC (Sigma–Aldrich, St Louis, MO, USA), respectively. After the treatments, both groups were placed in the refrigerator at 4 °C in the dark and bud cold-hardiness was determined after 1 week.
Bud cold-hardiness
Bud cold-hardiness was measured in grapevine dormant buds by low temperature exotherm (LTE) detection using differential thermal analysis following the method of Mills et al. (2006). The differential thermal analysis was performed with a Kryoscan (Supplementary Data Fig. S2), a freezing and data acquisition device that uses Peltier elements for the cooling and detection modules (Badulescu and Ernst, 2006). Signals were recorded every 2 s, and a decrease in temperature of 4 °C h−1 starting at 10 °C and ending at −30 °C was programmed (Mills et al., 2006). Generally, two peaks were observed, one corresponded to the high-temperature exotherm (HTE), which was assigned to the freezing point of extracellular (apoplast) water, which is non-lethal (Burke et al., 1976), and the other corresponded to the LTE, which was assigned to the freezing point of intracellular water, which is lethal (Burke et al., 1976). Because lethal damage to the grapevine buds occurs at temperatures equal to or lower than the LTE, the LTE value is considered the lowest temperature that the grapevine bud can resist without damage, and therefore is a measure of its cold-hardiness (Pierquet and Stushnoff, 1980; Mills et al., 2006; Ferguson et al., 2011). Each value corresponds to the average of 20 biological replicates of single buds.
ABA determinations
Fresh plant material (ten buds per harvest time) was washed with cold water before grounded with liquid nitrogen. The samples were extracted in a shaker for 1 h at 4 °C and for 10 min by ultra-sonication with 3 mL of 80 % methanol containing 1 % acetic acid and 3 ng of 2,3,5-triiodobenzoic acid (TIBA) as an internal standard (Sigma–Aldrich, USA). The extracts were centrifuged at 3000 g for 10 min, and the supernatant was filtered through glass wool and a Sep-Pack C18 cartridge (Waters, Milford, MA, USA) that had been prewashed with 5 mL of 80 % methanol. The procedure was repeated twice, and the filtrate was evaporated to dryness. After evaporation, the residue was dissolved in 1.2 mL of ethyl acetate and 1.2 mL of 0.5 m KH2PO4 pH 3.0 was added. The mixture was agitated with a vortex and centrifuged at 3000 g for 3 min. After centrifugation, the ethyl acetate layer was removed and the aqueous phase was extracted twice with 1.2 mL of ethyl acetate. The collected ethyl acetate layers were evaporated to dryness. The dry sample was dissolved in 1 mL of ethyl acetate, and an ABA derivative was formed by reaction with pentafluorobenzyl bromide (PFB) (Sigma–Aldrich, USA), which allowed highly sensitive detection by an electron capture detector (Michler et al., 1986). A Shimadzu gas chromatograph (model GC-2014) equipped with an electron capture detector (ECD-2014, Shimadzu, Kyoto, Japan) and computer integrator was used for ABA determinations. A CBP1 capillary column (25 m × 0.25 mm internal diameter) with helium as the carrier gas at a flux of 1.5 mL min−1 was used. The temperature of the column was initially 80 °C and after 1 min was raised to 270 °C at a rate of 20 °C min−1 and maintained there for 5 min. The injector was operated in the splitless mode at 225 °C, and the temperature of the detector was 300 °C. A calibration curve for ABA–PFB derivative was constructed.
Bud water content
For determinations of bud water content, canes collected on 12 and 19 June from ‘Thompson Seedless’ grapevines grown in the Maipo valley were excised in single-bud cuttings. Four groups of seven single-bud cuttings each were separated; two were treated with water and two with 200 µm ABA. Of the groups treated with water, one was maintained at 14–15 °C in the growth chamber and the other at 4–5 °C in a refrigerator for 1 week; the same was done with the ABA-treated groups. After treatments, the buds were excised and weighed before and after placing in an oven at 70 °C for 24 h. Water content was expressed as a percentage of fresh weight (FW).
RNA purification and cDNA synthesis
For gene expression analysis, total RNA was isolated and purified from grapevine buds (0.5 g FW) of V. vinifera ‘Thompson Seedless’. In all cases, total RNA was extracted and purified using a modification of the method of Chang et al. (1993), as described by Noriega et al. (2007). DNA was removed by treatment with RNase-free DNase (1 U µg−1) (Thermo Scientific, USA) at 37 °C for 30 min. First-strand cDNA synthesis was performed using the Superscript® II RT system (Invitrogen, CA, USA). A 1-µg aliquot of purified RNA with 1 µL of oligo(dT)12–18 (0.5 µg µL−1) was used as a primer and 1 µL of dNTP mix (10 mm) was used for cDNA synthesis. RNA quality and quantity were reassessed with a Qubit 2.0 fluorometer (Invitrogen, USA).
Quantitative real-time PCR
Quantitative real-time PCR (RT-qPCR) was carried out in an Eco Real-Time PCR system (Illumina, San Diego, USA) using KAPA SYBR FAST (KK 4602) qPCR Master Mix (2×). Design of specific primers for VvCBF genes was carried out using the primer3 program (Rozen and Skaletsky, 2000). Dehydrin primers were taken from Rubio et al. (2016) and primers for antioxidant genes were taken from Vergara et al. (2012). cDNA was amplified under the following conditions: denaturation at 94 °C for 2 min and 40 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 45 s. Relative changes in gene expression levels were determined using the 2-ΔΔCT method (Livak and Schmittgen, 2001). Each reaction was performed in at least three biological replicates, each with three technical replicates. VvUBIQUITIN and VvACTIN were used as a reference genes for normalization.
Statistical analysis
Differences between treatments were analysed by ANOVA, and multiple comparison analysis was carried out using Dunnett’s test.
RESULTS
ABA and LT synergistically upregulated the expression of VvCBF transcription factors in grapevine dormant buds
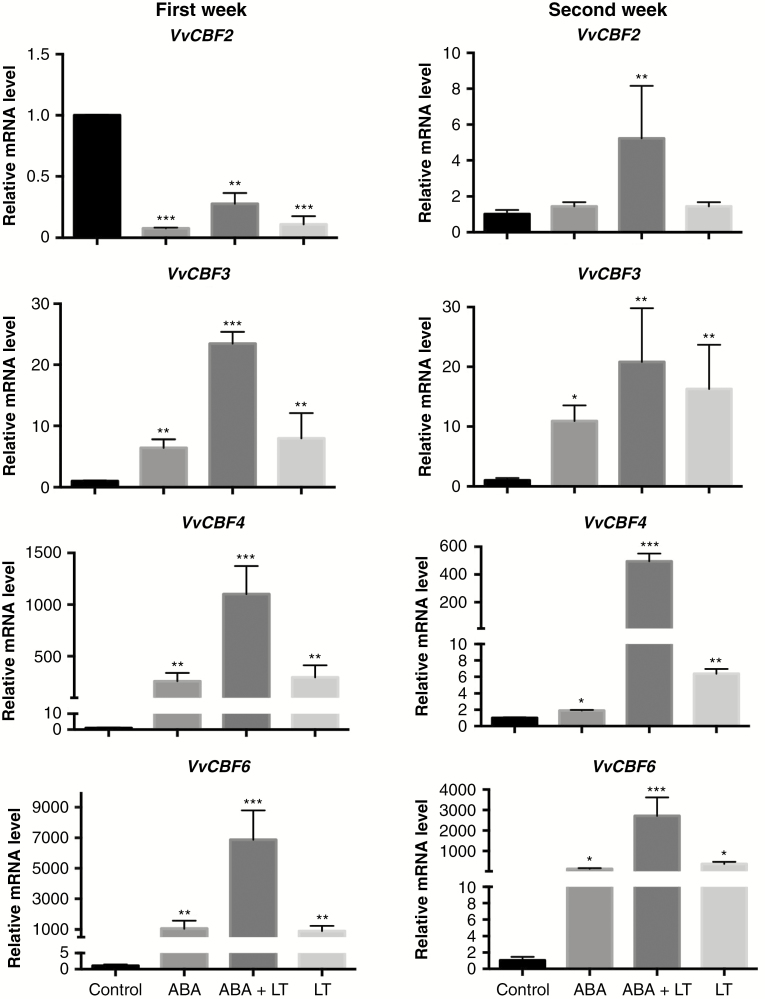
The effects of LT (4 °C in the dark), ABA (100 μm) and LT + ABA on the expression of VvCBF genes were studied by RT-qPCR in grapevine dormant buds after 1 and 2 weeks of treatment. All the treatments increased the expression of the VvCBF genes, both in the first and in the second week post-treatment, except for the VvCBF2 gene, whose expression decreased after the first week of treatment (Fig. 1). Joint application of LTs + ABA produced a greater increase in the expression of VvCBF genes than the application of either stimulus separately (Fig. 1). It was also observed that the effect of ABA + LT on the expression of the VvCBF4 and VvCBF6 genes decreased after the second week of treatment in relation to the first week, while the expression of VvCBF2 increased and the expression of VvCBF3 did not change (Fig. 1).
Fig. 1.
ABA and low temperature (LT) synergistically induced the expression of CBF/DREB1 transcription factors in grapevine dormant buds. Effect of LT, ABA and ABA + LT on the expression of VvCBF2, VvCBF3, VvCBF4 and VvCBF6 in grapevine dormant buds collected on 14 May after 1 and 2 weeks of treatment. Transcript levels were determined by RT-qPCR and normalized against VvUBIQUITIN and VvACTIN. Values are averages of three biological replicates each with three technical repetitions. Bars represent ± s.d. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 (Dunnett’s multiple comparison test).
ABA and LT synergistically induced cold-hardiness in grapevine dormant buds
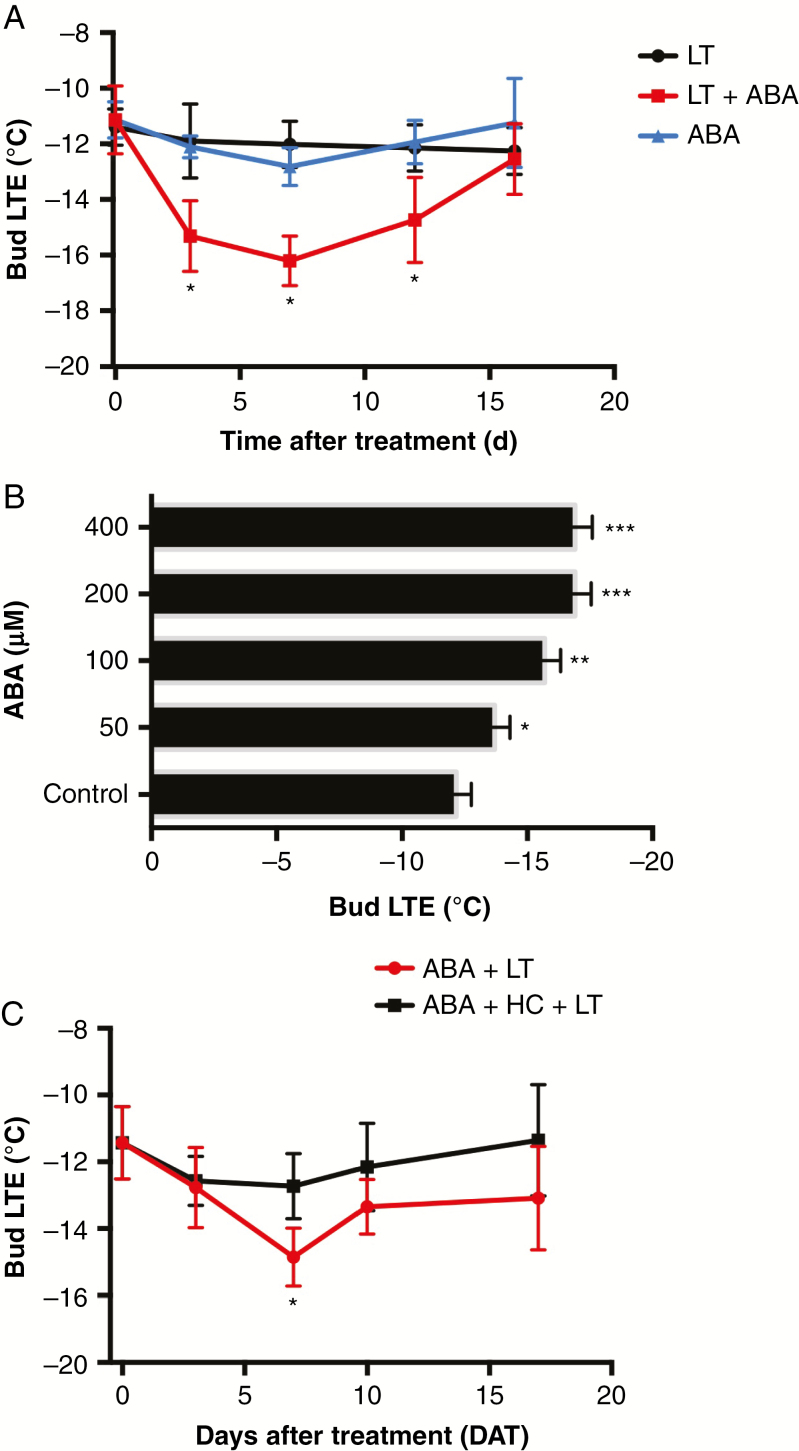
The effects of LT, ABA and LT + ABA on bud cold-hardiness were studied in grapevine dormant buds by measuring their LTE by differential thermal analysis. The samples were analysed after 0, 3, 7, 12 and 16 d of treatment. In the samples treated with ABA, the LTE values decreased until the first week and thereafter recovered. However, when ABA and LT were applied together, a strong decrease in LTE was detected after 3 d of treatment, a minimum was reached after 7 d, and then LTE values recovered to reach values close to the initial value (Fig. 2A). To test whether the synergy between ABA and LT with respect to LTE depends on ABA concentration, single-bud cuttings were treated with 50, 100, 200 and 400 µm ABA solutions, and the LTE was determined after 1 week of exposure to LT (Fig. 2B). The results showed a consistent decrease in LTE values with increasing ABA concentration; however, beyond 200 µm ABA the LTE value remained constant, indicating a saturation effect. Because the dormancy-breaking compound HC catabolizes ABA in grapevine buds (Zheng et al., 2015; Vergara et al., 2017), we tested whether this compound would abolish the synergy between ABA and LT with respect to bud cold-hardiness. The results showed that after 1 week of exposure to LT, HC significantly increased the LTE value, indicating a reduction in its bud cold-hardiness (Fig. 2C).
Fig. 2.
ABA and LT synergistically enhanced bud cold-hardiness in grapevine dormant buds. (A) Effects of LT, ABA and ABA + LT on the low thermal exotherm (LTE) of grapevine dormant buds collected on 14 May were determined by differential thermal analysis after 0, 3, 7, 12 and 16 d of treatment. Values are averages for 20 buds and bars represent ± s.d. *P ≤ 0.05 (Dunnett’s multiple comparison test). (B) Effect of ABA concentration on the LTE of grapevine buds collected on 23 April after 1 week of LT in the dark. Values are averages for 20 buds and bars represent ± s.d. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 (Dunnett’s multiple comparison test). (C) Effect of hydrogen cyanamide (HC) on the LTE of grapevine dormant buds treated with ABA + LT. The buds were collected on 23 April. Values are the average of 20 buds and bars represent ± s.d. *P ≤ 0.05 (Dunnett’s multiple comparison test).
Correlation between ABA content and cold-hardiness in grapevine dormant buds treated with ABA + LT
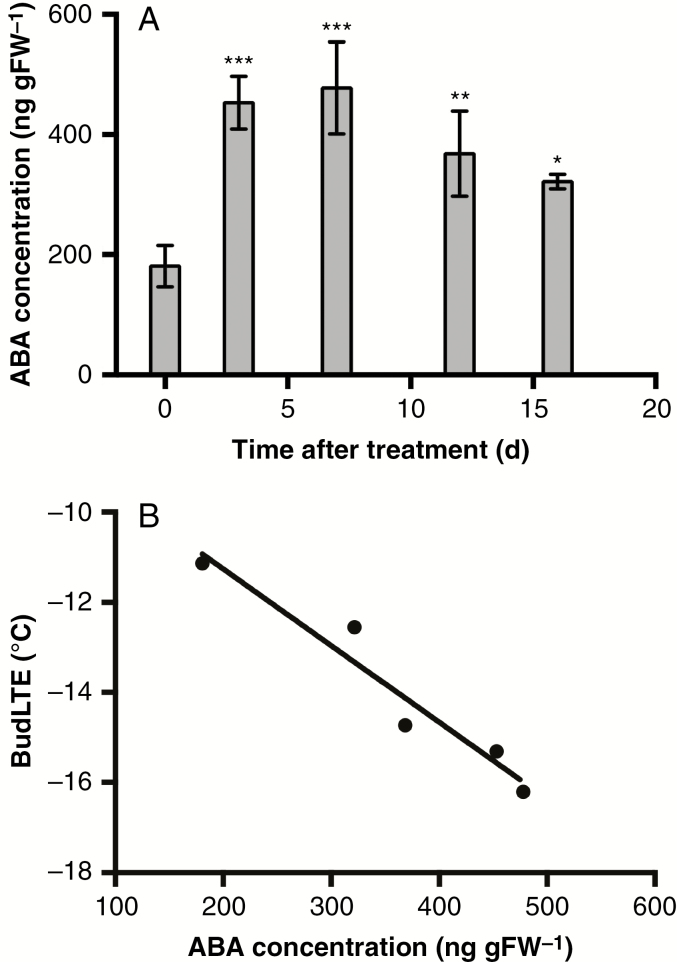
To analyse whether the recovery of bud cold-hardiness (LTE) after the first weeks of treatment with ABA + LT is due to a reduction in endogenous ABA content, we determined the content of ABA in grapevine dormant buds treated with ABA + LT after 0, 3, 7, 12 and 16 d of treatment (Fig. 3A), and the data were plotted against the LTE values determined at the same treatment intervals (Fig. 3B). The results showed that endogenous ABA content increased in grapevine dormant buds during the first week of treatment, and thereafter decreased (Fig. 3A). A negative correlation with r2 = 0.935 was obtained when bud LTE values were plotted against the content of endogenous ABA (Fig. 3B).
Fig. 3.
Correlation between the endogenous content of ABA and the cold-hardiness of grapevine dormant buds after ABA + LT treatment. (A) ABA was determined in grapevine buds collected on 14 May after 0, 3, 7, 12 and 16 d of ABA + LT treatment by gas chromatography using an electron capture detector. Values are the average of three biological replicates and bars represent ± s.d. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 (Dunnett’s multiple comparison test). (B) Correlation of bud LTE (using values obtained in Fig. 2) with endogenous ABA concentration. gFW, grams fresh weight.
ABA + LT synergistically induced dehydration in grapevine dormant buds
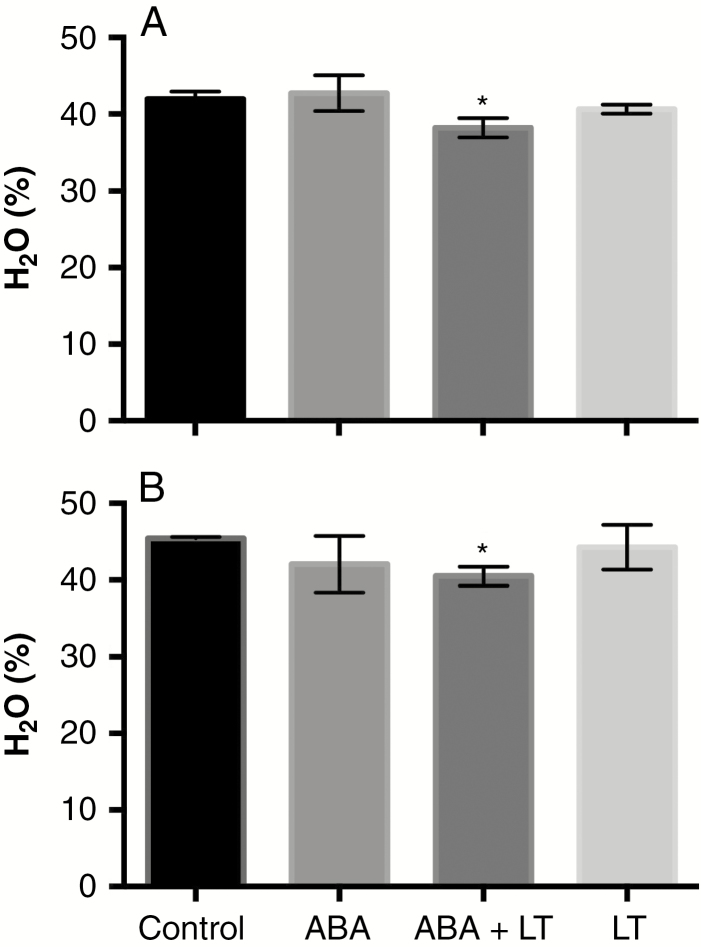
The effects of LT, ABA and ABA + LTs on bud water content of ‘Thompson Seedless’ grapevines were analysed at two collection dates. One week after the combined application of ABA + LT, a significant reduction in the water content of the buds was observed for material collected at the two dates, while the other treatments showed no differences with respect to the control buds (Fig. 4).
Fig. 4.
ABA and LT synergistically increased dehydration in grapevine dormant buds. Effect of LT, ABA and ABA + LT on bud water content (% FW) in ‘Thompson Seedless’ buds collected on (A) 12 June and (B) 19 June. Bars represent ± s.d. *P ≤ 0.05 (Dunnett’s multiple comparison test).
ABA and LT synergistically induced expression of dehydrins in grapevine dormant buds
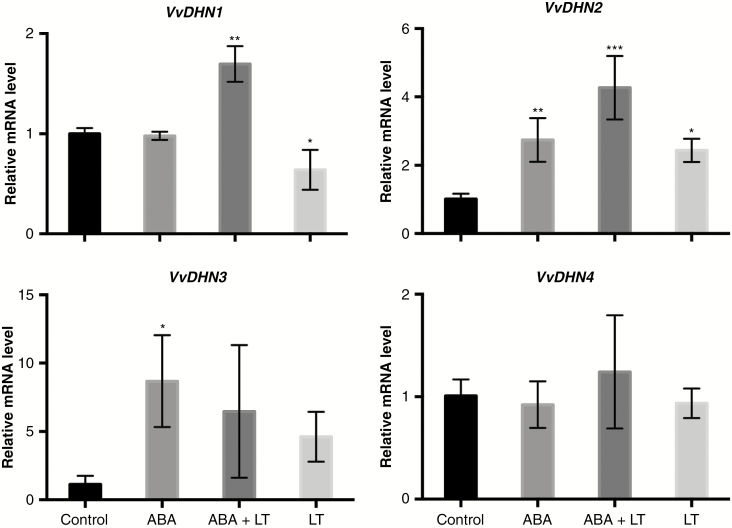
The effects of LT, ABA and LT + ABA on the expression of VvDHN genes in grapevine dormant buds were analysed. One week after the combined application of ABA and LT, only VvDHN1 and VvDHN2 expression levels were increased synergistically (Fig. 5). The expression of VvDHN4 was induced neither by ABA nor by LT and no synergism was observed between these stimuli (Fig. 5).
Fig. 5.
ABA and LT synergistically induced the expression of dehydrins in grapevine dormant buds. The effects of LT, ABA and ABA + LT on dehydrin gene (VvDHN) expression in ‘Thompson Seedless’ grapevine buds collected on 23 April. Transcript levels were determined 1 week after treatment by RT-qPCR and normalized against VvUBIQUITIN and VvACTIN. Values are averages of three biological replicates each with three technical repetitions. Bars represent ± s.d. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 (Dunnett’s multiple comparison test).
ABA and LT synergistically induced expression of antioxidant genes in grapevine dormant buds
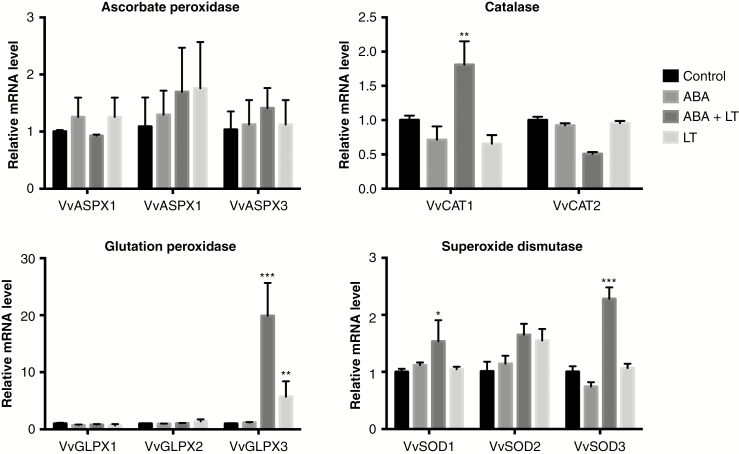
The expression of three paralogues of ascorbate peroxidase (VvAPX), two of catalase (VvCAT), three of glutathione peroxidase (GLPX) and three of superoxide dismutase (VvSOD) were studied in grapevine dormant buds treated with ABA, LT and ABA + LT after 1 week of treatment (Fig. 6). When they were applied together, ABA and LTs synergistically increased the expression of VvCAT1, VvGLPX3, VvSOD1 and VvSOD3. The different treatments did not significantly modify the expression of any of the VvASPX transcripts (Fig. 6).
Fig. 6.
ABA and LT synergistically induced the expression of antioxidant genes in grapevine dormant buds. Effect of LT, ABA and ABA + LT on the expression of VvAPX, VvCAT, VvGPX and VvSOD genes in ‘Thompson Seedless’ grapevine buds collected on 23 April. Transcript levels were determined 1 week after treatment by RT-qPCR and normalized against VvUBIQUITIN and VvACTIN. Values are averages of three biological replicates each with three technical repetitions. Bars represent ± s.d. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 (Dunnett’s multiple comparison test).
DISCUSSION
The CBF/DREB1 transcription factors, a subfamily of the APETALA2/ETHYLENE RESPONSE FACTOR (AP27/ERF), have been characterized as a regulatory hub in freezing tolerance in herbaceous plant systems (Thomashow, 2010; Theocharis et al., 2012). Functional studies of CBF genes in woody plants have indicated that their regulation and impact on abiotic stress are more complex than in herbaceous plants (Wisniewski et al., 2014). In grapevine leaves and seedlings, Xiao et al. (2006, 2008) reported that ABA and LT upregulated the expression of CBF/DREB1 genes. In the present study we found that LT and ABA treatments induced the expression of VvCBF transcription factors in grapevine dormant buds in a way similar to that in leaves and seedlings. However, when stimuli were applied together an increase in the expression of VvCBF genes was observed that was greater than the increase produced by them when applied individually. These results clearly indicate the existence of a synergistic induction of VvCBF transcription factors by the combined application of ABA and LT, a phenomenon that was not described in grapevine vegetative tissues (Xiao et al., 2006). In the grapevine, as in other temperate fruit trees, the dormant bud can develop a much greater degree of freezing tolerance than vegetative tissues, such as leaves and seedlings. Thus, while green tissues cannot withstand temperatures below 4 °C, the dormant grapevine bud can withstand temperatures of −25°C or lower (Mullins, 1992). Therefore, it is plausible that the high expression level of VvCBF genes in grapevine dormant buds could be associated with high freezing tolerance, and a mechanistic relationship could exist between the two variables. The fact that the joint application of ABA and LT also synergistically increased bud cold-hardiness, and that in the buds treated with ABA + LT the expression of the VvCBF genes and bud cold-hardiness followed the same trend, supports the hypothesis that the level of expression of the VvCBF genes and the cold-hardiness of grapevine dormant buds are related. Comparison between V. riparia and V. vinifera showed that V. riparia, which is endemic to cold regions, had a higher expression level for all the VvCFB genes analysed than V. vinifera (Karimi et al., 2015), suggesting that the abundance of VvCBF transcripts might be related to the degree of cold-hardiness. Interestingly, the expression of VvCBF2 was inversely related to the expression of VvCBF4 and VvCBF6 after the treatments. It has been reported that AtCBF2 is a negative regulator of AtCBF1 and AtCBF3 expression and plays a central role in stress tolerance in Arabidopsis, suggesting that repression of AtCBF2 would induce the expression of AtCBF1 and AtCBF3 (Novillo et al., 2004).
Foliar applications of ABA to grapevines increase the cold-hardiness of dormant buds (Zhang and Dami, 2012). Here we showed that in single-bud cuttings of grapevines the combined application of ABA and LT synergistically increased bud cold-hardiness in a manner dependent on the ABA concentration. However, the effect was transient, since bud cold-hardiness started to decrease after 1 week of treatment. Moreover, the endogenous content of ABA also decreased after the first week and correlated with bud cold-hardiness, and HC, which degrades ABA in grapevine buds (Zheng et al., 2015; Vergara et al., 2017), abolished the synergistic effect of ABA + LT on bud cold-hardiness. All these results confirmed the hypothesis that ABA plays a crucial role in strengthening the effect of LT in the acquisition of cold-hardiness in grapevine dormant buds. Interestingly, it has been reported that under natural conditions the level of ABA increases in grapevine dormant buds just before the seasonal fall in temperature (Or et al., 2000), suggesting that ABA plays a crucial role in the acquisition of cold-hardiness in grapevine dormant buds.
It has been hypothesized that in grapevines ABA enhances bud cold-hardiness by inducing bud-dehydration (Zhang and Dami, 2012). Our results support this hypothesis, since the combined application of ABA and LT synergistically enhanced bud dehydration and bud cold-hardiness. Moreover, it is well known that VvCBF genes are upregulated by dehydration (Yamaguchi-Shinozaki and Shinozaki, 2006). Therefore, the synergistic increase in bud dehydration caused by ABA + LT could be responsible for the increased expression of VvCBF genes and for the enhancement of bud cold-hardiness. Dehydrins, a subgroup of LEAs, are among the most commonly observed proteins that accumulate in plants in response to LT and environmental factors leading to cell dehydration. The presence of DRE/CRT motifs in the promoter of several cold-regulated dehydrins suggests that they play a role in CBF/DREB-mediated signalling pathway (Gilmour et al., 2004). A small family of four dehydrins genes have been identified in V. vinifera and their expression has been analysed (Yang et al., 2012). In this study we found that the expression of VvDHN1 and VvDHN2, which possess a DRE cis-regulatory element in their promoter (Yang et al., 2012), were synergistically induced by the combined application of ABA and LT, suggesting that they are regulated by VvCBF transcription factors and play a role in the cold acclimatization process of grapevine dormant buds. Recently, it has been shown that VvCBF4 transcription factor binds to the promoter of VvDHN2 (Vázquez-Hernandez et al., 2017). Although VvDHN3 gene expression was induced by ABA, no synergistic effect was observed after combined application of both stimuli, and VvDHN4 was induced neither by ABA nor by LT.
It is important to note that not all changes in gene expression that occur during the cold acclimatization process are directly related to freezing tolerance (Wisniewsky et al., 2014). Many of the biochemical and gene expression changes have to do with tissue recovery after cold stress events (Meitha et al., 2015). Plants regulate the availability of oxygen and its metabolism during key transitions, including the transition from a quiescent state to an active growth state (Considine and Foyer, 2014). In this regard, the increase in the expression of antioxidant genes is an important aspect of the cold-acclimatization response. In this study we showed that two genes coding for superoxide dismutase (VvSOD1, VvSOD3), one gene for glutathione peroxidase (VvGPX3) and one for catalase (VvCAT1) showed synergistically induced expression through the combined application of ABA and LT. These results suggest that the VvCBF transcription factors can control the expression of genes related to all aspects of the cold-acclimatization process in grapevine dormant buds.
Conclusions
The huge increase in the expression of VvCBF/DREB1 transcription factors in grapevine dormant buds as a result of the combined effect of ABA and LT, and its relationship to the increase in bud dehydration and bud cold-hardiness, together with an increase in the expression of dehydrins and antioxidant genes, highlight the importance of these transcription factors and ABA in the process of cold acclimatization in grapevine dormant buds. Additionally, the results help us to understand why, under field conditions, there is a great increase in the endogenous content of ABA in the grapevine dormant bud at the beginning of autumn, when temperatures start to drop.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: single-bud cuttings of ‘Thompson Seedless’ grapevines mounted on a propylene sheet and floated in tap water in a plastic container. Figure S2: the Kryoscan, a freezing and data acquisition device that uses Peltier elements for the cooling and detection modules.
ACKNOWLEDGEMENTS
The financial support of Vicerrectoria de Investigación y Desarrollo (VID) of the University of Chile through the program Enlaze-2017 is gratefully acknowledged.
LITERATURE CITED
- Badulescu R, Ernst M. 2006. Changes of temperature exotherms and soluble sugar in grapevine (Vitis vinifera L) buds during winter. Journal of Applied Botany and Food Quality 80: 165–170. [Google Scholar]
- Burke MJ, Gusta LV, Quamme HA, Weiser CJ, Li PH. 1976. Freezing and injury in plants. Annual Review of Plant Physiology 27: 507–528. [Google Scholar]
- Carlow CE, Faultless JT, Lee C, Siddiqua M, Edge A, Nassuth A. 2017. Nuclear localization and transactivation by Vitis CBF transcription factors are regulated by combinations of conserved amino acid domains. Plant Physiology and Biochemistry 118: 306–319. [DOI] [PubMed] [Google Scholar]
- Chang S, Puryear J, Cairney J. 1993. A simple and efficient method for isolating RNA from pine trees. Plant Molecular Biology Report 11: 113–116. [Google Scholar]
- Chew YH, Halliday K. 2011. A stress-free walk from Arabidopsis to crops. Current Opinion in Biotechnology 2: 281–286. [DOI] [PubMed] [Google Scholar]
- Considine MJ, Foyer CH. 2014. Redox regulation of plant development. Antioxidants & Redox Signaling 21: 1305–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennell A. 2014. Genomic and functional genomics of winter low temperatures tolerance in temperate fruit crops. Critical Reviews in Plant Sciences 32: 125–140. [Google Scholar]
- Ferguson JC, Tarara JM, Mills LJ, Grove GG, Keller M. 2011. Dynamic thermal time model of cold hardiness for dormant grapevine buds. Annals of Botany 107: 389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour SJ, Fowler SG, Thomashow MF. 2004. Arabidopsis transcriptional activators CBF1, CBF2, and CBF3 have matching functional activities. Plant Molecular Biology 54: 767–781. [DOI] [PubMed] [Google Scholar]
- Karimi M, Rbadi A, Mousavi AA, Salami SA, Zarei A. 2015. Comparison of CBF1, CBF2, CBF3 and CBF4 expression in some grapevine cultivars and species under cold stress. Scientia Horticulturae 197: 521–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Meitha K, Konnerup D, Colmer TD, Considine JA, Foyer CH, Considine MJ. 2015. Spatio-temporal relief from hypoxia and production of reactive oxygen species during bud burst in grapevine (Vitis vinifera). Annals of Botany 116: 703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michler CH, Lineberger RD, Chism GW. 1986. A highly sensitive method for quantitative determination of abscisic acid. Plant Physiology 82: 600–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills LJ, Ferguson JC, Keller M. 2006. Cold-hardiness evaluation of grapevine buds and cane tissues. American Journal of Enology and Viticulture 57: 194–200. [Google Scholar]
- Mullins MG, Bouquet A, Williams LE. 1992. Biology of the grapevine. Cambridge: Cambridge University Press. [Google Scholar]
- Noriega X, Burgos B, Pérez FJ. 2007. Short-day photoperiod triggers and low temperatures increase expression of peroxidase RNA transcripts and basic peroxidase isoenzyme activity in grapevine-buds. Phytochemistry 68: 1376–1383. [DOI] [PubMed] [Google Scholar]
- Novillo F, Alonso JM, Ecker JR, Salinas J. 2004. CBF2/DREB1C is a negative regulator of CBF1/DREB1B and CBF3/DRE1A expression and plays a central role in stress tolerance in Arabidopsis. Proceedings of the National Academy of Sciences of the USA 101: 3985–3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Or E, Belausov E, Popilevsky I, Ben Tal Y. 2000. Changes in endogenous ABA level in relation to the dormancy cycle in grape-vines grown in a hot climate. Journal of Horticultural Science and Biotechnology 75: 190–194. [Google Scholar]
- Pierquet P, Stushnoff C. 1980. Relationship of low temperature exotherms to cold injury in Vitis riparia Michx. American Journal of Enology and Viticulture 31: 1–6. [Google Scholar]
- Rozen S, Skaletsky H. 2000. Primer3 on the WWW for general users and for biologist programmers. Methods in Molecular Biology 132: 365–386. [DOI] [PubMed] [Google Scholar]
- Rubio S, Dantas D, Bressan-Smith R, Pérez FJ. 2016. Relationship between endodormancy and cold-hardiness in grapevine buds. Journal of Plant Growth Regulation 35: 266–275. [Google Scholar]
- Siddiqua M, Nassuth A. 2011. Vitis CBF1 and Vitis CBF4 differ in their effect on Arabidopsis abiotic stress tolerance, development and gene expression. Plant Cell & Environment 34: 1345–1359. [DOI] [PubMed] [Google Scholar]
- Singh KB, Foley RC, Oñate-Sánchez L. 2002. Transcription factors in plant defense and stress responses. Current Opinion in Plant Biology 5: 430–436. [DOI] [PubMed] [Google Scholar]
- Stockinger EJ, Gilmour SJ, Thomashow MF. 1997. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proceedings of the National Academy of Sciences of the USA 94: 1035–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuhara Y, Kobayashi M, Suzuki S. 2011. Low-temperature-induced transcription factors in grapevine enhance cold tolerance in transgenic Arabidopsis plants. Journal of Plant Physiology 168: 967–975. [DOI] [PubMed] [Google Scholar]
- Theocharis A, Clement C, Barka EA. 2012. Physiological and molecular changes in plants grown at low temperatures. Planta 235: 1091–1105. [DOI] [PubMed] [Google Scholar]
- Tillett RL, Wheatley MD, Tattersall EAR, Schlauch RA, Cramer GR, Cushman JC. 2012. The Vitis vinifera C-repeat binding protein 4 (VvCBF4) transcriptional factor enhances freezing tolerance in wine grape. Plant Biotechnology Journal 10: 105–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- This P, Lacombe T, Thomas M. 2006. Historical origins and genetic diversity of wine grapes. Trends in Genetics 22: 511–519. [DOI] [PubMed] [Google Scholar]
- Thomashow MF. 2010. Molecular basis of plant cold acclimation: insights gained from studying the CBF cold response pathway. Plant Physiology 154: 571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Hernandez M, Romero I, Escribano MI, Merodio C, Sanchez-Ballesta MT. 2017. Deciphering the role of CBF/DREB transcription factors and dehydrins in maintaining the quality of table grapes cv. Autumn Royal treated with high CO2 levels and stored at 0°C. Frontiers in Plant Science 8: 1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara R, Pérez FJ. 2010. Similarities between natural and chemically induced bud-endodormancy release in grapevine Vitis vinifera L. Scientia Horticulturae 125: 648–653. [Google Scholar]
- Vergara R, Parada F, Rubio S, Pérez FJ. 2012. Hypoxia induces H2O2 production and activates antioxidant defense system in grapevine buds through mediation of H2O2 and ethylene. Journal of Experimental Botany 3: 4123–4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara R, Noriega X, Aravena KS, Prieto H, Pérez FJ. 2017. ABA represses the expression of cell cycle genes and may modulate the development of endodormancy in grapevine buds. Frontiers in Plant Science 8: 812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Shao H, Tang X. 2016. Recent advances in utilizing transcription factors to improve plant abiotic stress tolerance by transgenic technology. Frontiers in Plant Science 7: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski M, Nassuth A, Teulières C, Marque C, Rowland J, Cao P, Brown A. 2014. Genomics of cold hardiness in woody plants. Critical Reviews in Plant Science 33: 92–124. [Google Scholar]
- Xiao H, Siddiqua M, Braybrook S, Nassuth A. 2006. Three grape CBF/DREB1 genes respond to low temperature, drought and abscisic acid. Plant Cell & Environment 29: 1410–1421. [DOI] [PubMed] [Google Scholar]
- Xiao H, Tattersall EAR, Siddiqua M, Cramer GR, Nassuth A. 2008. CBF4 is a unique member of the CBF transcription factor family of Vitis vinifera and Vitis riparia. Plant Cell & Environment 31: 1–10. [DOI] [PubMed] [Google Scholar]
- Yang Y, He M, Zhu Z, et al. . 2012. Identification of dehydrin gene family from grapevine species and analysis of their responsiveness to various forms of abiotic and biotic stress. BMC Plant Biology 12: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Schinozaki K, Shinozaki K. 2006. Transcriptional regulatory networks in cellular response and tolerance to dehydration and cold stress. Annual Review of Plant Biology 57: 781–803. [DOI] [PubMed] [Google Scholar]
- Zandkarimi H, Ebadi A, Salami SA, Alizade H, Baisakh N. 2015. Analyzing the expression profile of AREB/ABF and DREB/CBF genes under drought and salinity stresses in grape (Vitis vinifera L.). PLoS ONE 10: e0134288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Dami IE. 2012. Foliar application of abscisic acid increases freezing tolerance of field-grown Vitis vinifera Cabernet Franc grapevines. American Journal of Enology and Viticulture 63: 377–384. [Google Scholar]
- Zheng C, Halaly T, Acheampong AK, et al. . 2015. Abscisic acid (ABA) regulates grape bud dormancy, and dormancy release stimuli may act through modification of ABA metabolism. Journal of Experimental Botany 66: 1527–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.