Abstract
Background and Aims
Pyroloids, forest sub-shrubs of the Ericaceae family, are an important model for their mixotrophic nutrition, which mixes carbon from photosynthesis and from their mycorrhizal fungi. They have medical uses but are difficult to cultivate ex situ; in particular, their dust seeds contain undifferentiated, few-celled embryos, whose germination is normally fully supported by fungal partners. Their germination and early ontogenesis thus remain elusive.
Methods
An optimized in vitro cultivation system of five representatives from the subfamily Pyroloideae was developed to study the strength of seed dormancy and the effect of different media and conditions (including light, gibberellins and soluble saccharides) on germination. The obtained plants were analysed for morphological, anatomical and histochemical development.
Key Results
Thanks to this novel cultivation method, which breaks dormancy and achieved up to 100 % germination, leafy shoots were obtained in vitro for representatives of all pyroloid genera (Moneses, Orthilia, Pyrola and Chimaphila). In all cases, the first post-germination stage is an undifferentiated structure, from which a root meristem later emerges, well before formation of an adventive shoot.
Conclusions
This cultivation method can be used for further research or for ex situ conservation of pyroloid species. After strong seed dormancy is broken, the tiny globular embryo of pyroloids germinates into an intermediary zone, which is functionally convergent with the protocorm of other plants with dust seeds such as orchids. Like the orchid protocorm, this intermediary zone produces a single meristem: however, unlike orchids, which produce a shoot meristem, pyroloids first generate a root meristem.
Keywords: Pyrola, Monotropa, Ericaceae, seed germination, in vitro culture, Chimaphila, protocorm, orchid, convergent evolution, seed dormancy, mixotrophy, Moneses
INTRODUCTION
Mycorrhiza is a worldwide symbiosis of most plants (Smith and Read, 2008). Usually, plants provide photosynthates in exchange for fungal mineral nutrients (van der Heijden et al., 2015). However, mycorrhizal exchange may be less reciprocal, and perhaps exploitative, in mycoheterotrophic plants that derive carbon resources from their mycorrhizal fungi (Leake, 1994). Mycoheterotrophic plants evolved independently in various plant lineages (Merckx, 2013), and beyond full mycoheterotrophs, which are achlorophyllous, some green species display a mixed strategy, obtaining carbon from both mycorrhizal fungi and their own photosynthesis, so-called mixotrophy (Selosse and Roy, 2009). There are two non-exclusive mixotrophic conditions (Merckx, 2013): some species are mixotrophic at adulthood, while other species germinate in a mycoheterotrophic way, before switching to autotrophy or mixotrophy during ontogeny (such as orchids; Dearnaley et al., 2016; Selosse et al., 2016; Těšitel et al., 2018).
Little is known about the biology of mycoheterotrophic and mixotrophic plants, perhaps due to the difficulty of studying them (Merckx, 2013). The only cultivable group of mixotrophic plants is the orchid family, although many of them have so far escaped cultivation (Rasmussen, 1995). For this reason, orchids are the most explored mixotrophic models, but unfortunately it is largely unknown which results obtained for them can be generalized to other mixotrophic plants. Developing cultivation protocols for other mycoheterotrophic or mixotrophic plants is therefore urgently needed to understand general mechanisms connected with mycoheterotrophy.
Within Ericaceae, the subfamily Pyroloideae (hereafter pyroloids) comprises initial mycoheterotrophs that develop, depending on the species, into auto- or mixotrophic adults (Tedersoo et al., 2007; Matsuda et al., 2012; Hynson et al., 2013a), with the exception of one completely mycoheterotrophic species (Hynson and Bruns, 2009). This similarity to orchids is an evolutionary convergence (Tedersoo et al., 2007; Byng et al., 2016; Lallemand et al., 2016) and it is therefore interesting to compare adaptations of both groups to mycoheterotrophy. Pyroloids encompass about 40 sub-shrub species divided into four genera, mostly distributed in northern temperate and boreal ecosystems (Takahashi, 1993; Liu et al., 2010), and are of medicinal interest, mostly in Asia (e.g. Ma et al., 2014; Wang et al., 2014).
The seeds of pyroloids consist of oval, central living tissue surrounded by a coat of dead cells called a testa (Fürth, 1920; Christoph, 1921; Lück, 1941; Pyykkö, 1968; Takahashi, 1993). Despite the fact that the oval living part seems to be anatomically homogeneous, it is composed of a one-layered endosperm and triploid nutritive tissue of 40–50 cells surrounding a smaller embryo inside (Hofmeister, 1858; Fürth, 1920; Christoph, 1921; Pyykkö, 1968). A similar seed structure was observed in the related, mycoheterotrophic genus Monotropa, where the embryo consists of only 2–3 cells (Olson, 1993) to 5–9 cells (Goebel, 1887) in addition to the endosperm, although minute seeds evolved independently in this Ericaceae genus (Lallemand et al., 2016). The embryo of pyroloids is larger, with eight or 16 cells (Goebel, 1887) to 30 cells (Christoph, 1921). Similarly to Monotropa, the living part of the seed contains limited reserves of lipids and proteins (Fürth, 1920; Christoph, 1921; Lück, 1940). Other mycotrophic plants exhibit a similar seed structure, but, for example, orchids lack endosperm, and the only living part of the seed is undifferentiated globular embryo (Arditti and Ghani, 2000).
Post-germination development of such seeds in pyroloids with an undifferentiated embryo has attracted attention for a long time. First, researchers searched for seedlings in nature (Irmish, 1855; Velenovský, 1892, 1905; Fürth, 1920), albeit with limited success. They found only older seedlings, which revealed that a small root-like structure grows into an extensively branched structure which is formed before the first shoot emerges (Irmish, 1855; Velenovský, 1892; Fürth, 1920). In contrast, orchids, which also start mycoheterotrophic development from an undifferentiated embryo, first form a specific structure called a protocorm, from which shoots and roots later develop (Rasmussen, 1995; Dearnaley et al., 2016). This raised the following three questions. Are the structures observed in germinating pyroloids true roots? How does this root-like structure develop from a tiny undifferentiated embryo? What is its relationship to a protocorm?
Many attempts have been made to answer these questions, but with only ambiguous results to the date. Velenovský (1892, 1905) believed that this below-ground structure is ‘neither a root nor a stem’ and called it a ‘prokaulom’, while Christoph (1921) suggested that this structure is a root. Later, Lihnell (1942) and Copeland (1947) carried out the same anatomical analyses and found a typical root anatomical structure, where the radial vascular bundle is diarch, rarely triarch (i.e. has two or rarely three xylem strands). The middle layer of the root called the cortex is made of 3–4 (or rarely more) cell layers from which the innermost layer forms the endodermis (Lihnell, 1942; Copeland, 1947). The outermost root layer, the epidermis, is one layered and consists of isodiametric cells. Lateral branches grow from the outer layer of the stele (Lihnell, 1942).
The method of burying seed packets at natural sites yields pyroloid seedlings easily, but the anatomical structure of such seedlings has not been studied in detail. These structures, called ‘root-like structures’ (Hashimoto et al., 2012; Johansson and Eriksson, 2013), live heterotrophically below-ground for months, if not years (Lihnell, 1942; Hynson et al., 2013a; Johansson et al., 2017).
Other researchers tried to germinate pyroloid seeds in vitro axenically (Christoph, 1921; Lück, 1940; Lihnell, 1942) or symbiotically (Fürth, 1920; Lück, 1941; Lihnell, 1942). Germination was achieved, although the results were ‘mostly uneven, not very consistent’ (Lihnell, 1942) and the seedlings usually stopped growing very soon after germination (Christoph, 1921; Francke, 1934; Lück, 1940, 1941), sometimes being only a ‘few tenths of a mm long’ (Christoph, 1921). Similar tiny seedlings, which ceased growth, were obtained for Monotropa (Francke, 1934). The best results were achieved by Lihnell (1942) and yielded a few branched root-like seedlings of Pyrola rotundifolia.
In these studies, observing different pyroloids, a polarized cone-shaped structure resembling a root with large epidermal cells grows from the tiny globular embryo (Christoph, 1921; Lück, 1940, 1941; Lihnell, 1942). At this stage, the seedling breaks the testa and forms an approx. 1 mm long roll-shaped structure (Lihnell, 1942), whose central cells undergo elongation (Lück, 1941). At this point, growth usually ceases (Lück, 1940; Lihnell, 1942). Seedlings rarely grow further, producing a rod-shaped stage, where vessels start differentiation (Lihnell, 1942) and, soon after, a root cap. Then, the seedling becomes darker, possibly because of the accumulation of tannins (e.g. Holm, 1898; Christoph, 1921; Lück, 1940; Lihnell, 1942). Lihnell (1942) showed that such older seedlings have the structure of a root and their illumination does not lead to the formation of chlorophyll. Shoots were never produced in vitro.
Growth suddenly stopped in all in vitro experiments (Christoph, 1921; Lück, 1940, 1941; Lihnell, 1942), which raised the question of the ideal conditions for in vitro growth. Germination usually started 4–8 months after sowing (Lück, 1940, 1941; Lihnell, 1942) and it was difficult to keep the cultures moist for such a long period (Lihnell, 1942). It is also hard to say if a specific experiment was really axenic, as the authors themselves admitted (for example, Christoph, 1921, discussed whether worms were the reason for the failure of cultivation), and in symbiotic cultures it was not clear whether the fungus really formed symbiosis or not (Lihnell, 1942; indeed, sometimes ‘symbiotic’ seedlings grew away from the fungus). Moreover, in vitro cultivation media often contained substances of variable composition, such as potato extract, yeast extract, malt, peptone or even humus and soil extracts (Christoph, 1921; Lück, 1941; Lihnell, 1942). Previous results from in vitro cultures are therefore based on a few plants only, and do not indicate cultivation conditions that are ideal for more detailed observation.
Possible seed dormancy was also discussed. Christoph (1921) noticed that it is difficult to soak pyroloid seeds in water, indicating impermeability of the seed coat. Seeds disinfected with calcium hypochlorite solution showed the best germination after the longest bleaching time (15 and 30 min; Lihnell, 1942). Lihnell (1942) and Lück (1941) suggested that some ‘water-soluble substances’ have an inhibitory effect on germination, and Harley (1959) hypothesized that some substances could be removed by soaking in solution. Although dormancy in minute seeds may seem unexpected, long bleaching of seeds enhances germination in many orchids (e.g. Burgeff, 1936; Rasmussen, 1992, 1995) probably because hypochlorite solutions have a high pH and strong oxidative effects on a wide range of compounds, which could break impermeable seed coats (Arditti, 1967; Rasmussen, 1995; Zeng et al., 2014). In some hardly germinating orchid species, pre-treatment with a weak H2SO4 solution enhances germination, probably ensuring stronger degradation of seed coats (e.g. Malmgren, 1993; Ponert et al., 2013; Malmgren and Nyström, 2018). However, the mechanisms are unclear in orchids, and strong differences exist between species. Dormancy clearly requires further study in pyroloid seeds.
To summarize, no reliable protocol for in vitro germination of pyroloids exists and their early ontogenetic development remains elusive. We therefore sought to develop an efficient protocol for in vitro culture. We successfully report early steps of the post-germination development from undifferentiated embryo to leafy plant, in terms of storage compounds, and morphological and anatomical development, which enabled us clearly to answer the long-standing above-mentioned questions about the nature of root-like structures of pyroloid seedlings, their development from a tiny undifferentiated embryo and their relationship to a protocorm.
MATERIALS AND METHODS
Plant material
We selected five European species as representatives of all four genera of the subfamily Pyroloideae (Pyrola media, Pyrola minor, Orthilia secunda, Moneses uniflora and Chimaphilla umbellata; Supplementary Data Table S1). To compare these with another subfamily of Ericaceae where minute seeds and mycoheterotrophic germination independently evolved (Freudenstein et al., 2016; Lallemand et al., 2016), we used Monotropa uniflora from the Monotropoideae (Supplementary Data Table S1). Ripe capsules were collected, dried at room temperature, and extracted seeds were stored in the dark and in dry conditions at +4 °C (Supplementary Data Table S1).
Cultivation media
To find a suitable cultivation medium for germination and growth, we tested nine media originally designed for orchid in vitro culture (Supplementary Data Table S2). All media contained 0.7 % agar (w/v, Sigma-Aldrich) and 1–3 % sucrose (Supplementary Data Table S2). After the pH was adjusted to 5.8 using NaOH, media were autoclaved at 144 kPa, 121 °C (Tuttnauer 2540 EK-N) for 20 min and poured into 5 cm plastic Petri dishes, unless otherwise indicated. Medium Knudson C with activated charcoal (Sigma-Aldrich) was used for all subsequent experiments, unless otherwise indicated. Activated charcoal is used to improve germination and growth of orchids and slow-growing tissue cultures generally, perhaps due to its ability to adsorb toxic products of plant metabolism (van Waes, 1985; Thomas, 2008). To test the effect of different soluble saccharides that could mimic the carbon provided by the fungi in natural situations, sucrose was excluded or replaced with the monosaccharide 100 mm glucose or the disaccharide 50 mm sucrose or trehalose. To test the effect of gibberellins, 0.01, 0.1 or 1 mg L–1 GA3 (Sigma-Aldrich) was added to the medium before autoclaving.
Seed disinfection and sowing
Seeds were disinfected in 5 mL syringes and sown as a suspension in sterile deionized water as described previously (Ponert et al., 2011, 2013), but the application times of disinfection solutions of H2SO4 and Ca(OCl)2 were modified in a fully factorial design to find the proper seed treatment. All disinfection treatments were pre-incubated in 70 % ethanol for 5 min, washed three times with deionized water (<0.2 μm cm–1), treated with 2 % H2SO4 for 10 min or not, treated with Ca(OCl)2 solution for 5, 10 or 15 min, and finally washed three times with sterile deionized water. Ca(OCl)2 solution was prepared by dissolving 20 g of chlorinated lime (Kittfort, Czech Republic) in 100 mL of deionized water, filtering through filter paper and adding a drop of Tween-20. For all subsequent experiments, we selected the most efficient treatment: 70 % ethanol for 5 min, washed three times with deionized water, 2 % H2SO4 for 10 min, Ca(OCl)2 solution for 10 min, washed three times with sterile deionized water. Seven Petri dishes sealed with air-permeable foil (Parafilm M) were prepared for each experimental treatment.
Cultivation conditions
Because seeds ripen late in the season, all cultures were incubated in the dark at 4 °C for 3 months after sowing to simulate winter, and then transferred to the dark at 20 °C, except for experiments where the effect of light or the cold stratification period was tested (see below). Cultures were observed every 2 weeks using a Krüss, MSZ 5400 Stereo Zoom Microscope (magnification ×40) and an Olympus Provis AX70 microscope for higher magnification. The germination rate was counted three times after the end of cold stratification, at 30, 60 and 90 d. The last count (the third; after 90 d at 20 °C) was used to determine the total germination rate, because no further germination was observed. To count the germination rate, seeds without an embryo or with an obviously undeveloped embryo were excluded and well-developed seeds with broken testa were regarded as germinated seeds (Supplementary Data Fig. S1). To estimate seedling size in selected experiments, the total length of all branches of each seedling was measured from pictures taken with a Nikon D7000 + Micro Nikkor 55/2.8 using ImageJ 1.6.0_24 software.
To study the establishment of shoots in detail, we used approx. 3–5 mm long seedlings of P. minor pre-cultivated on Knudson C medium (Supplementary Data Table S2) for 60 d after transfer to 20 °C. Each seedling (n = 22) was transplanted to an individual 9 cm Petri dish with BM-1 medium (Supplementary Data Table S2). Pictures of plants were taken every 10 d. Plants with established shoots were continuously collected for anatomical analyses.
Plants with growing etiolated shoots were transferred to light (16 h light/8 h dark) and cultivated for the next month to produce green leafy shoots. Plants with green shoots were deflasked, washed with water and potted in a mixture of coarse expanded perlite, fine pumice gravel, fine pine bark and loamy soil (1:1:1:2) in clay pots. Pots were sealed in polyethylene bags to keep air humidity high and were kept on a windowsill at 25 °C in moderate light.
Anatomical analysis
Plant material (seeds and seedlings of P. minor) was fixed in 4 % formaldehyde in phosphate buffer (0.1 m, pH 7.1). Selected samples were embedded in paraplast after dehydration using an ethanol–butanol series (for details, see Soukup and Tylová, 2014). Sections (10 μm) were prepared using a Leica 2155 microtome and collected on microscope slides coated with alum gelatine adhesive. Cryosections were prepared on a Shandon cryomicrotome. Hand sections were prepared on a Leica hand microtome. For whole-mount preparations, samples were gradually equilibrated in 65 % glycerol and mounted in NaI-based clearing solution of high refractive index (Soukup and Tylová, 2014). Histochemical tests involved staining with safranin O (2 h incubation) and Fast Green FCF (2 min). Lipids were detected with Sudan Red 7B (1 h) according to Brundrett et al. (1991). Proteins were stained with Ponceau 2R in 2 % acetic acid (10 min) and Azur II (10 s) according to Gutmann et al. (1996). Detection of starch involved staining with Lugol solution. Observations were made with an Olympus BX51 microscope (Olympus Corp., Tokyo, Japan) equipped with an Apogee U4000 digital camera (Apogee Imaging Systems, Inc., Roseville, CA, USA) or with a Zeiss LSM 880 confocal microscope.
Endogenous starch HPLC analysis
To confirm the presence of starch in seedlings, we characterized the endogenous saccharide spectrum of selected pyroloids. Six-month-old (including the period of cold stratification) seedlings of Moneses uniflora, O. secunda and P. minor (n = 3 for each species) cultivated on BM-1 medium were collected in liquid nitrogen. Soluble carbohydrates were extracted following the protocol of Kubeš et al. (2014).
The pellets left after soluble saccharide extraction were carefully washed with Milli-Q ultrapure water (sonicated in 1 mL of water for 15 min, centrifuged at 14 000 rpm for 15 min and the supernatant removed) and used for starch analysis. Starch was enzymatically degraded by α-amylase (Sigma-Aldrich) and amyloglucosidase (Sigma-Aldrich) following the protocol of Steinbachova-Vojtiskova et al. (2006), and the glucose content was quantified with the HPLC system described above, except for the use of an IEX Ca form 8 μm column in this case.
Data analysis
Statistical analyses were performed using statistical software R 3.2.3 (R Core Team, 2015). The normality of data was tested using the Shapiro–Wilk test (Shapiro and Wilk, 1965) and homogeneity of variances was tested using the Bartlett test (Bartlett, 1937). Differences between the measurements were statistically tested with analysis of variance (ANOVA), followed by the Tukey–Kramer test (Kramer, 1956) for data with a normal distribution and the Kruskal–Wallis test (Kruskal and Wallis, 1952), followed by pairwise comparisons using Wilcoxon’s rank-sum test for data that did not have normal distribution. To compare the effects of different disinfectants on seed germination, we used two-way ANOVA, followed by a Tukey–Kramer test.
RESULTS
Effect of seed disinfection
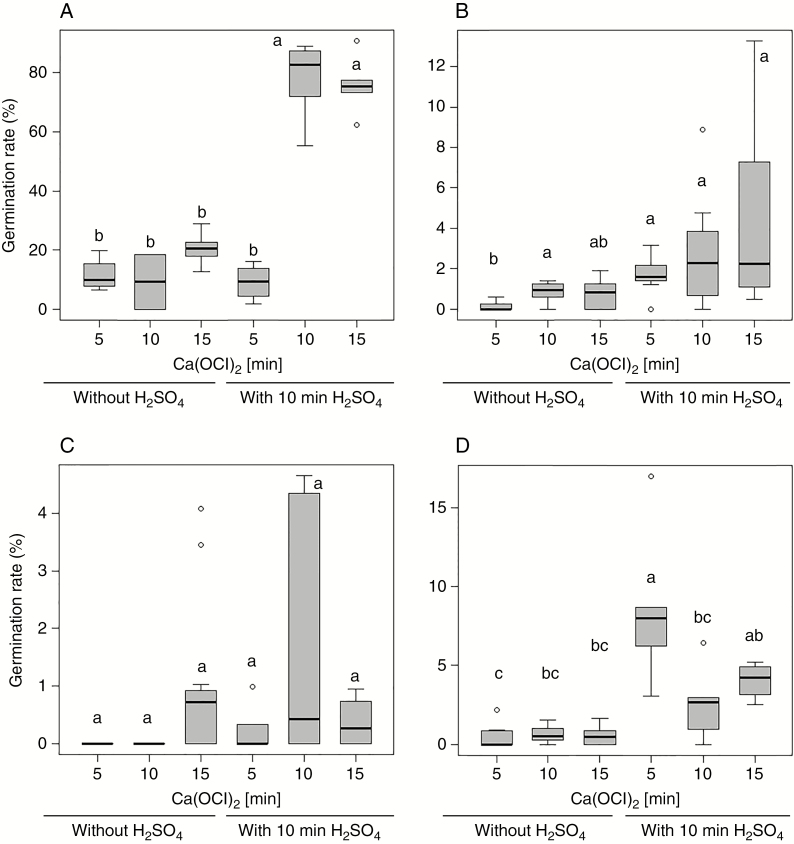
It turned out to be impossible to sow non-disinfected seeds in vitro because of overwhelming contamination (data not shown). The highest germination rate of all tested species was reached after H2SO4 treatment and the effect of H2SO4 was significant in all tested species (P. minor, F1,24 = 193.96, P = 5.4 × 10–13; Moneses uniflora, F1,40 = 8.23, P = 0.0065; O. secunda, F1,23 = 24.32, P = 5.5 × 10–5) except for C. umbellata (F1,28 = 0.06, P = 0.8; Fig. 1). The effect of Ca(OCl)2 was significant in P. minor (F2,24 = 57.42, P = 7.1 × 10–10) and O. secunda (F1,23 = 3.79, P = 0.038), but the optimal duration of disinfection differed between these taxa. Longer incubation in Ca(OCl)2 strongly promoted germination of P. minor, but slightly inhibited germination of O. secunda (Fig. 1). Moneses uniflora germination was also higher after longer incubation in Ca(OCl)2, especially after the pre-treatment with H2SO4 (Fig. 1B), but the effect was not significant (F2,40 = 2.13, P = 0.13). The interaction between the effects of H2SO4 and Ca(OCl)2 was significant for P. minor (F2,24 = 35.97, P = 6.0 × 10–8) and for O. secunda (F1,23 = 4.05, P = 0.031) only.
Fig. 1.
Effect of different disinfection treatments on seed germination of (A) Pyrola minor, (B) Moneses uniflora, (C) Chimaphila umbellata and (D) Orthilia secunda. In vitro cultivation on Knudson C medium was evaluated 3 months after the cold stratification. Median values are given (n = 7). Different letters indicate a significant difference according to the ANOVA followed by Tukey HSD post-hoc test for multiple comparison.
Effect of different media
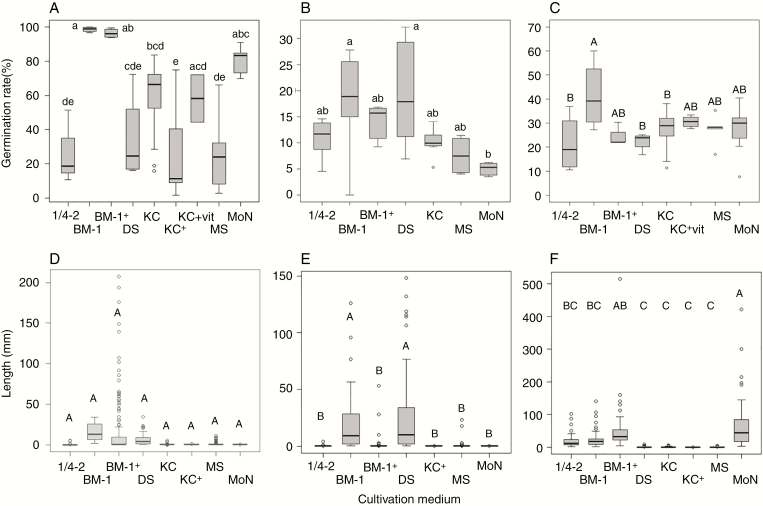
Optimal media differed between species (Fig. 2A–C). Generally, the highest germination rates were observed on the media BM-1 (with or without activated charcoal), DS and MoN (Fig. 2; Supplementary Data Fig. S2). Media 1/4-2, Knudson C and Murashige and Skoog (MS) allowed slightly lower germination generally (Fig. 2; Supplementary Data Fig. S2). Surprisingly, few seedlings of C. umbellata germinated on Knudson C medium [χ2(7) = 8.45, P = 0.29; Supplementary Data Fig. S3B]. The best medium for germination of P. minor was BM-1 (with or without activated charcoal), with a germination rate of almost 100 % [χ2(8) = 32.59, P = 7.29 × 10–5; Fig. 2A]. For Moneses uniflora, the highest germination rates were achieved on media BM-1 and DS [χ2(5) = 7.34, P = 0.20; Fig. 2B]. Orthilia secunda germinated on all media tested; however, after longer incubation, the highest germination rate was achieved on BM-1 medium without activated charcoal (F7,41 = 3.21, P = 0.0083; Fig. 2C).
Fig. 2.
Effect of different media on germination of (A) Pyrola minor, (B) Moneses uniflora and (C) Orthilia secunda, and growth of (D) Pyrola minor, (E) Moneses uniflora and (F) Orthilia secunda. Both germination and the total length of seedlings were measured 3 months after cold stratification. Different letters indicate a significant difference. Results of Tukey HSD post-hoc test for multiple comparisons are indicated by upper case letters and results of pairwise Wilcoxon rank sum test by lower case letters.
Media also differed in their suitability for seedling growth in a more or less similar way (Fig. 2D–F). We were unable to compare the effects of the different media on C. umbellata growth because few seedlings developed on Knudson C medium. The largest seedlings of P. minor developed on BM-1 medium (with or without activated charcoal) and on DS medium, although the differences between treatments were not significant (F7,16 = 0.984, P = 0.48; Fig. 2D). The largest seedlings of Moneses uniflora developed on the media DS and BM-1 without activated charcoal (F6,15 = 21.76, P =1.32 × 10–6; Fig. 2E). The largest seedlings of O. secunda developed on MoN medium followed by BM-1 medium with activated charcoal (F7,17 = 14.54, P = 1.19 × 10–5; Fig. 2F).
Effect of light
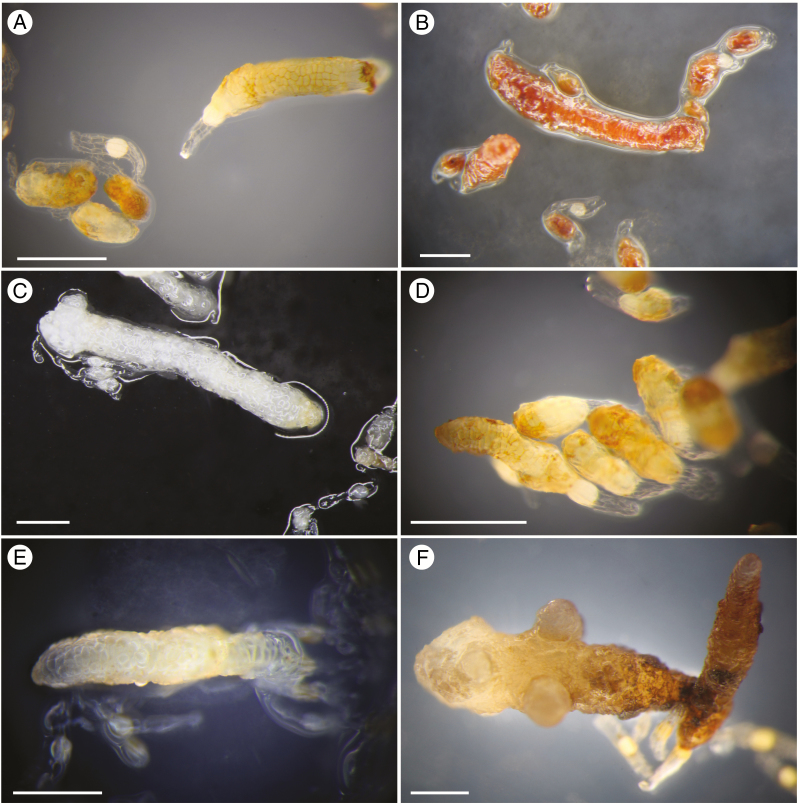
The germination of both tested species (P. minor and O. secunda) was inhibited by light (16/8 h photoperiod; Supplementary Data Fig. S4A, B). For O. secunda, this difference was significant [Supplementary Data Fig. S4B; χ2(1) = 5.4; P = 0.02 in the second month; only one plate in the third month because light cultures became contaminated]. For P. minor, a similarly small proportion of seeds germinated after the first month in both treatments, and further germination was only slightly lower in the light (differences not significant; F1,8 = 0.058, P = 0.82; Supplementary Data Fig. S4A). However, after another 6 months of cultivation, seedlings in light did not grow, while the seedlings in the dark grew and produced many branches, and even some buds and first shoots (data not shown). Interestingly, seedlings cultivated in light displayed a reddish colour (Fig. 3B).
Fig. 3.
Representatives of in vitro grown seedlings of species used in this study. (A) Pyrola minor cultivated in the dark, (B) Pyrola minor cultivated in the light, and (C) Moneses uniflora, (D) Orthilia secunda, (E) Chimaphila umbellata and (F) Monotropa uniflora cultivated in the dark. Scale bars = 0.5 mm.
Effect of gibberellic acid and selected soluble saccharides
We found little effect of GA3 on germination of the tested species, namely P. minor (Supplementary Data Fig. S4C, D) and O. secunda (Supplementary Data Fig. S4E, F). For O. secunda, there was no difference in germination rate between GA3 concentrations on both media tested (Supplementary Data Fig. S4E, F), except after the first month on Knudson C (higher germination on the highest GA3 concentration; F3,15 = 7.94, P = 0.002; Supplementary Data Fig. S4F). For P. minor, there was a weak stimulatory effect after the first month on BM-1 medium at the highest (1 mg L–1) GA3 concentration [χ2(3) = 4.719, P = 0.002; Supplementary Data Fig. S4C].
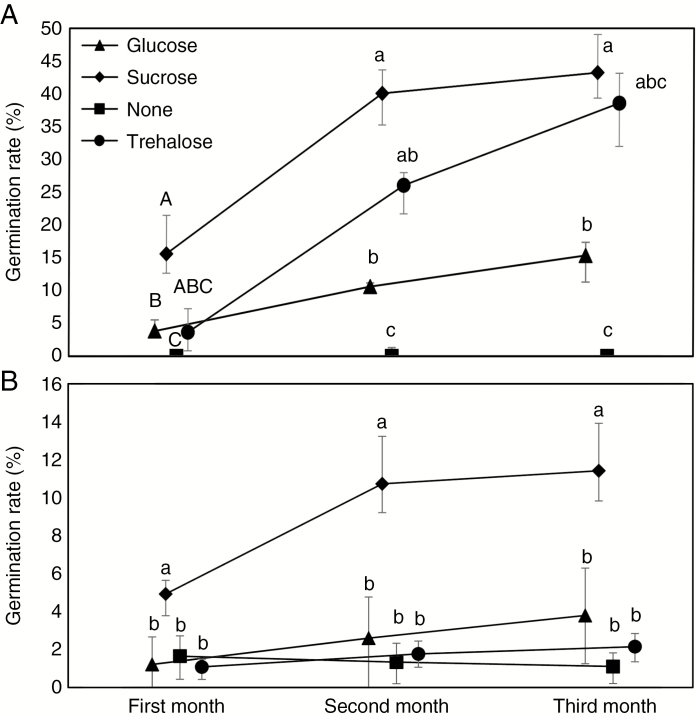
Tested saccharides stimulated germination of seeds of both tested species. Pyrola minor was stimulated by all saccharides, with sucrose being the best [χ2(3) = 20.57, P = 0.0001; Fig. 4A], and the seeds cultivated without soluble saccharides germinated very rarely. Orthilia secunda reached higher germination rates only on sucrose [χ2(3) = 16.34, P = 0.001; Fig. 4B], while glucose and trehalose hardly enhanced germination. Seedling size was similarly affected by saccharides, with all saccharides acting on P. minor (Supplementary Data Figs S5A and S6A; F3,14 = 27.11, P = 4.32 × 10–6) and sucrose only on O. secunda (Supplementary Data Figs S5B and S6B; F3,16 = 9.57, P = 0.00074). After three additional months of cultivation, seedlings from all saccharide-supplemented treatments produced at least a few long roots, while those from saccharide-free cultures did not, indicating that trehalose and glucose were also partially utilized. Moreover, after cultivation for 1 year, the individual seedlings on media with glucose and trehalose showed significant growth (Supplementary Data Fig. S7).
Fig. 4.
Effect of selected soluble saccharides on germination of Pyrola minor (A) and Orthilia secunda (B). In vitro cultivation on Knudson C medium where sucrose was replaced by saccharide is indicated. Different letters indicate a significant difference. Results of Tukey HSD post-hoc test for multiple comparisons are indicated by upper case letters and results of pairwise Wilcoxon rank sum test by lower case letters; bars indicate the first and third quantiles. Pairwise comparisons were calculated separately for each month.
Ontogenesis of seedlings
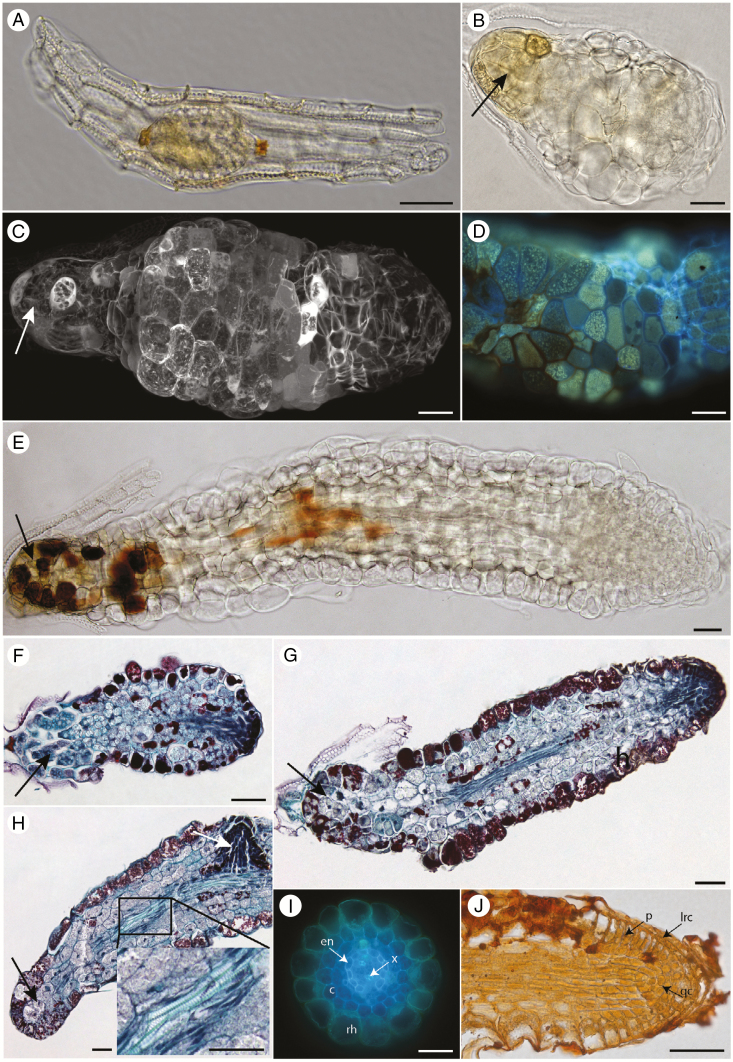
All species tested produced elongated seedlings of similar morphology (Fig. 3), irrespective of the medium. We used P. minor as a model species to analyse seedling development in detail. Like other pyroloids, P. minor has a minute seed with a few-celled embryo surrounded by a cellular endosperm enclosed in a single-layered transparent testa with pitted cell walls (Fig. 5A). Histochemical tests indicate storage of lipids (Supplementary Data Fig. S8C, E) and proteins (Supplementary Data Fig. S8B, D) but not starch (Supplementary Data Fig. S8A) in mature seeds. When germinated aseptically on media supplemented with soluble carbohydrate, germination starts with enlargement of the embryo at the micropylar pole (Fig. 5B) and with the formation of an apical meristematic area (in seedlings <0.5 mm long; Fig. 5F) that later forms into root meristem (Fig. 5G). The transition from embryo into growing root is fast, but gradual. Less organized tissue is produced during a short initial phase to form an ‘intermediary zone’ (Fig. 5C–H) that exhibits a heterogeneous surface cell pattern (Fig. 5D) and differentiates vascular tissues in its central part (Fig. 5G, H). In seedlings >0.5 mm long, typical root organization is achieved within the growing apex (Fig. 5G). The root produced includes a radial vascular bundle devoid of pith (mostly diarch), exarch xylem with protoxylem close to the pericycle, suberized endodermis, and a root cap (Fig. 5I, J; Supplementary Data Fig. S8I, L).
Fig. 5.
Germination and early development of Pyrola minor. (A) A mature seed with globular embryo. (B) Germination starts with enlargement of the embryo (arrows indicate the former embryo). (C) A seedling develops further into a root through the less organized intermediary zone with a very heterogenic surface cell pattern. (D) Detail of the cell surface pattern of the intermediary zone. (E) A seedling 1–1.5 mm in size with an already established root meristem. (F) The meristematic zone is established very early after germination. (G) A seedling <1 mm in size with an established meristem and differentiated vascular tissues in the central part of the intermediary zone close to the former embryo. (H) A seedling approx. 3 mm in size with an already established lateral root primordium (white arrow) and differentiated vascular tissues (in detail). (I) The root structure is obvious at 1.5 cm from the position of the former embryo in a 3 cm long seedling. Abbreviations: en, endodermis; x, protoxylem vessels of the radial vascular bundle; c, cortex; rh, rhizodermis. (J) The root tip of a 3 cm long seedling. Abbreviations: lrc, lateral root cap; p, protoderm; qc, quiescent centre. (A–E) whole mounts, DIC; (C) whole mount, confocal image; (D) whole mount, UV; (F–H) paraplast sections, Safranin O + Fast Green FCF; (I) hand section, UV; (J), paraplast section, Lugol + Orange G. Black arrows indicate the position of the former embryo; scale bars = 50 μm.
Seedling establishment on sucrose-amended media is accompanied by gradual disappearance of lipids (Supplementary Data Fig. S8C, F, J) and accumulation of starch (Supplementary Data Fig. S8F, H, K). Starch grains are located in cortical cells (Supplementary Data Fig. S8H, I, K, L). High starch content was confirmed by HPLC analyses (Supplementary Data Table S3). Conversely, germination on media lacking carbohydrates terminated in the very initial phase, with very limited enlargement of the embryo even after 11 months of cultivation (Supplementary Data Fig. S8G).
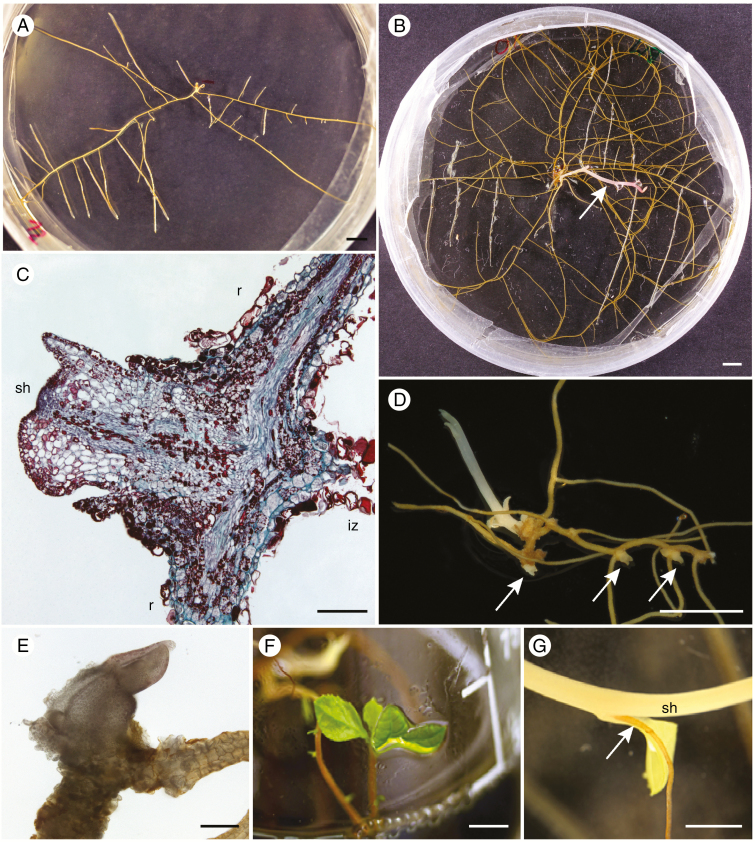
The emerging root started to branch very early and produced an extensive root system (Fig. 6A), which initiates the first shoot bud much later (Fig. 6B). To observe the timing and positioning of the emergence of the first bud, we transferred seedlings individually (n = 16) to new Petri dishes soon after germination on sucrose-amended BM-1 media. Seedlings produced their first buds at different times, starting 35 d after transplantation, and seven out of 16 plants did not sprout after 6 months. The first shoot always sprouted a few millimetres away from the original position of the embryo, often at the nearest root branching (eight out of nine plants; Fig. 6C–E). Very often additional buds appeared approx. 1–2 months after the first one, emerging along the root axis at sites of branching (Fig. 6D). One individual displayed seven sprouts after 6 months. The buds grew quickly into leafy shoots which became green when transferred to the light (Fig. 6F, G). Seedlings left in a small Petri dish with other seedlings grew much more slowly and did not form buds till the end of the experiment. We also successfully germinated branching Monotropa uniflora (n = 3; Supplementary Data Fig. S9A on BM-1 with activated charcoal; Supplementary Data Fig. S3A) from which one plant produced multiple buds within a dense nest-like root cluster after 1 year of cultivation (Supplementary Data Fig. S9B).
Fig. 6.
Transition to shoot formation in P. minor seedlings. (A) Nine-month-old seedling with an established root system prior to the onset of shoot growth. (B) Nine-month-old seedling with an emerging shoot (arrow). (C) The shoot bud emerges at the first root branching site close to the position of the former embryo (r, root; sh, shoot bud; iz, intermediary zone connecting the former embryo; x, xylem), section stained with Safranin O and Fast Green FCF. (D) Later, additional shoot buds emerge along the root axis (arrows). (E) Detail of the first shoot bud, whole mount preparation. (F) Fully established in vitro plant. (G) Adventitious roots emerging at the stem node (sh, shoot). Scale bars = 0.5 cm, except (C) and (E) = 200 μm.
In summary, germination was successful to the stage of green leaves in P. minor (Fig. 6F, G) and also in C. umbellata, O. secunda, Moneses uniflora and P. media (not shown). The seedlings of P. minor were successfully transferred to ex vitro conditions, and cultivated for the next 6 months (not shown).
DISCUSSION
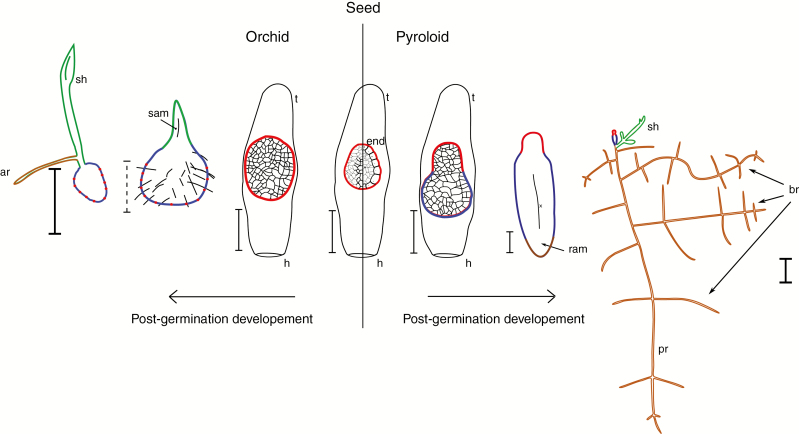
We developed an efficient protocol for in vitro axenic sowing of pyroloids, which enabled us to germinate successfully representatives of all pyroloid genera and a related mycoheterotroph Monotropa uniflora. This protocol allowed us to overcome problems with seed germination and seedling growth cessation that were previously reported. Germination is probably driven mostly by physical dormancy because gibberellins had little effect on germination, while intensive pre-treatment (chemical scarification) of seeds greatly improved germination. This reliable protocol allowed us to produce seedlings in great number and to describe ontogenesis clearly, from early germination to leafy plantlets. In all cases, a tiny undifferentiated embryo produces an intermediary zone, which subsequently establishes a first root. Adventitious shoots grow later from this and secondary roots (Fig. 7, right). Our results suggest convergence in mixotrophic plants with dust-like seeds in regressive evolution leading to a single meristem that builds mycorrhizal tissue at germination.
Fig. 7.
Comparison of germination and subsequent development of orchids (left) and pyroloids (right). The embryo is highlighted in red, the protocorm in blue, shoots in green and roots in brown. Abbreviations: a, adventive root; br, branching roots; e, embryo; end, endosperm; h, hilum; iz, intermediary zone; p, protocorm; ram, root meristem; sh, shoot; sam, shoot meristem; t, testa; x, xylem. The thin bar is 100 μm, the dotted bar is 1 mm and the thick bar is 0.5 cm.
Germination conditions
Gibberellic acid (GA3), which greatly stimulates germination of many plant species (Shu et al., 2016), had little effect on pyroloid germination. A strikingly similar situation occurs in minute orchid seeds, where gibberellins usually have little effect on seed germination (Arditti, 1967; Rasmussen, 1995), despite occasional reports of inhibition (Van Waes and Debergh, 1986) or stimulation (Pedroza-Manrique et al., 2005; Pierce and Cerabolini, 2011). Low sensitivity to gibberellins may be a general feature of dust-like seeds. The situation in orchids is sometimes explained by the absence of endosperm (Arditti and Ghani, 2000; Yeung, 2017), because the stimulation of germination by gibberellins is mostly connected to the induction of the expression of genes encoding enzymes hydrolysing the endosperm (Groot and Karssen, 1987; Groot et al., 1988; Schuurink et al., 1992; Leubner-Metzger et al., 1996). Yet, since pyroloid seeds have a one-layered endosperm (Hofmeister, 1858; Fürth, 1920; Christoph, 1921; Pyykkö, 1968), the low sensitivity to gibberellins of dust-like seeds may not be associated with the absence of endosperm but with other characteristics yet to be clarified.
The strong inhibitory effect of light on germination could serve as a protection against germination on the soil surface, where mycorrhizal fungi may not be present. A similar mechanism of light inhibition is well known in many terrestrial species of orchids (Arditti, 1967, 2008; Rasmussen, 1995). We also found a strong effect of disinfection on seed germination. More intensive seed disinfection stimulated germination, and longer incubation in calcium hypochlorite solution after sulphuric acid treatment was often most effective. Similarly, Lihnell (1942) achieved the highest germination rate after the longest incubation in calcium hypochlorite solution, which he was unable to explain. Since these solutions also act corrosively to destroy the impermeable coats of dust-like seeds (e.g. Arditti, 1967; Rasmussen, 1995; Zeng et al., 2014), we propose that strong physical dormancy due to the testa explains the long pre-germination period and the low germination rates observed in situ (Hashimoto et al., 2012; Johansson and Eriksson, 2013; Hynson et al., 2013b; Johansson et al., 2017). After proper chemical scarification, we reached 100 % and 99 % germination for P. minor and O. secunda seeds, respectively, which is much higher than observed in situ (Hashimoto et al., 2012; Hynson et al., 2013b) or in vitro (Lück, 1940; Lihnell, 1942) in different species of the same genera. It is hard to discuss possible actions of disinfection agents on seeds in detail, because the chemical composition of pyroloid seeds is unknown. In orchids, seed coats seems to consist of lignin, lipids and other uncharacterized compounds (Barsberg et al., 2013, 2018). Ethanol is a good solvent for many lipids and wax compounds, and it usually makes seeds less hydrophobic and more accessible for subsequent treatment with aqueous solutions. Lignin is highly sensitive to the oxidative effect of hypochlorite solutions, so we could expect disintegration of the seed coat during hypochlorite treatment. The effect of H2SO4 is hard to guess, but we could expect the existence of some other compound sensitive to acid hydrolysis, but not to alkaline hydrolysis.
Chimaphila umbellata and Moneses uniflora germinated at significantly lower rates compared with P. minor and O. secunda in our experiments. Since these two genera form a clade separate from that encompassing Pyrola and Orthilia (Freudenstein et al., 2016; Lallemand et al., 2016), some additional dormancy mechanism may occur in this clade, which remains to be identified. In soils, dormancy, added to photoinhibition, may increase the ability of the plant to wait for suitable fungi required for germination.
Our in vitro methods provided seedlings in great number and shed light on the early development of pyroloids, with replicates for dissection to an extent that has never been reached before, allowing a clear discussion of early ontogenesis of pyroloids – assuming, of course, that our artificial, non-symbiotic conditions do not alter ontogeny, as supported below.
Cultivation protocol
Based on our results, we propose a cultivation protocol that would allow germination of all pyroloids generally. This procedure uniquely combines different techniques used previously in orchid culture to satisfy the needs of pyroloids. Seed disinfection implies: 70 % ethanol for 5 min, washing three times with deionized water, then 2 % H2SO4 for 10 min, followed by Ca(OCl)2 solution for 10 min and washing three times with sterile deionized water. The best cultivation medium is BM-1, but DS and MoN also work well. For cultivation conditions, we recommend 4 °C for 3 months after sowing, and subsequently 20 °C, in the dark. Seedlings can be transferred to the new medium when they are approx. 5 mm long. When shoots start to grow, seedlings should be transferred to the light and, at that time, it is better to place them separately into 100 mL cultivation jars.
Development of pyroloid seedlings
The dust seed structure with an undifferentiated globular embryo is typical for many mycoheterotrophic plants (Leake, 1994) and plants with mycoheterotrophic early development (Dearnaley et al., 2016). Such dust seeds have limited reserves, the storage compounds of which are mostly unknown. In pyroloids, previous studies found storage lipids and proteins in mature seeds (Fürth, 1920; Christoph, 1921; Lück, 1940). Similarly, ripe seeds of orchids usually contain lipids and proteins (Harrison, 1977; Manning and Van Staden, 1987; Rasmussen, 1990; Richardson et al., 1992; Yam et al., 2002; Li et al., 2016), and few orchid species display starch in mature embryos (Tian and Wang, 1985; Guo and Xu, 1990; Yeung and Law, 1992). Our histochemical tests confirmed the presence of storage lipids and proteins, but the absence of starch in P. minor seeds, thus further amplifying the convergence with orchids.
During germination, lipids and storage proteins were utilized, and seedlings shifted to use starch as the main storage compound, as confirmed by histochemistry and HPLC. This switch to starch had already been observed in several pyroloids (Christoph, 1921; Lück, 1940; Lihnell, 1942) and is common among orchids (Manning and Van Staden, 1987; Rasmussen, 1990; Richardson et al., 1992). The transition from lipid and protein reserves in seeds to starch in seedlings therefore also convergently characterizes mycoheterotrophic germination.
Mycoheterotrophic pyroloid seedlings grow in vitro as branching roots for a long time before the first green shoot emerges. Such non-green seedlings grow below-ground in nature very slowly, for an even longer time (Hashimoto et al., 2012; Johansson and Eriksson, 2013; Hynson et al., 2013b; Johansson et al., 2017). Seedling growth was also promoted by more complex cultivation media (e.g. BM-1, compared with Knudson C or MS), which could indicate dependency on some other organic compounds provided in nature by fungi. Complex media are also beneficial for many mixotrophic orchids (Rasmussen, 1995; Arditti, 2008).
Post-germination development differs from that of the orchids despite some parallels. Orchids form a protocorm during germination whose enlargement establishes a shoot meristem on its anterior pole (Burgeff, 1936; Leroux et al., 1997; Yeung, 2017). In pyroloids, germination starts with enlargement of the embryo at the suspensor pole, which develops into a small elongated structure growing apically – the ‘cone stadium’ of Lück (1941) and Lihnell (1942). Soon after, the apical region establishes a root meristem and grows into a typical root. Although the transition is pretty fast, a short region (approx. 0.5 mm) close to the former embryo exhibits a slightly different internal structure (Fig. 7), which is why we call this part the ‘intermediary zone’ (see discussion below).
The first root grows and establishes lateral roots to develop an extensively branched root system as also observed in situ (Irmish, 1855; Velenovsky, 1892, 1905; Bobrov, 2009; Hashimoto et al., 2012). For a long time, it was unclear whether these structures are true roots (see Goebel, 1900; Lihnell, 1942). Velenovský (1892, 1905) suggested that these are ‘neither root nor stem’. We clearly show a typical root organization (including the root cap, the endogenous origin of lateral roots and radial vascular bundle; Fig. 5H–J), as concluded in earlier anatomical studies (Lihnell, 1942; Copeland, 1947). The above-mentioned discussions probably arose because of insufficient anatomical examination and confusion between the intermediary zone and the true roots. As in the observations of Velenovský (1905) and Lihnell (1942) and of adult roots (Hynson, 2009), the roots observed had no root hairs. Our data clearly show that the first shoot sprouts from a small section between the original position of the embryo and the first root branching site. Shortly after, other adventitious shoots usually emerge from other parts of the root system. Such root sprouting also exists in adult pyroloids in natural conditions (Copeland, 1947; Klimešová, 2007).
Is the intermediary zone a protocorm?
Typical plant embryos develop from both radicle (basal pole establishing the primary root) and plumule (apical pole establishing the primary shoot) poles, while in pyroloids or orchids one embryo pole does not grow: the primary shoot or the primary root, respectively, is completely missing (Fig. 7). Other mycoheterotrophic species with dust seeds that have been investigated to date, Afrothismia hydra (Burmanniaceae; Imhof and Sainge, 2008) and the genus Voyria (Gentianaceae; Imhof, 2010; Imhof et al., 2013), also germinate by a single, basal (radicle) embryo pole. This independent loss of one meristematic embryo pole in plants with dust-like seeds indicates some general selection pressure in mycoheterotrophic germinations perhaps for several non-exclusive reasons: (1) the limited resources available cannot support two growth sites (Imhof and Sainge, 2008); and/or (2) the absence of a requirement for shoots and roots in mycoheterotrophy, since a single organ for interaction with the fungus is sufficient; and/or (3) the later mycorrhizal organ makes one embryo pole unable to differentiate into a meristem. We therefore propose that transformation of one embryo pole into mycorrhizal tissue is a necessary evolutionary step enabling reduction of seed reserves to a minimum of the production of a true dust-like seed structure.
It could be argued that plants cultivated in vitro exhibit different development from plants in nature. However, the seedlings observed by us fully fit those observed in situ (Irmish, 1855; Velenovsky, 1892, 1905; Hashimoto et al., 2012; Hynson et al., 2013a; Johansson and Eriksson, 2013). In particular, the drawings and photographs in vitro by Christoph (1921), Lück (1940, 1941) and Lihnell (1942) reveal similar seedlings.
How should we classify the structure that develops between the undifferentiated globular embryo and the typical root? It starts with polarized growth at the suspensor pole of the embryo and continues with cell divisions in an emerging meristematic area at its apical part. Later, it becomes thick, with extremely heterogeneous large epidermal cells and a broad central area of vascular tissues, but without a typical root structure. We therefore called it the intermediary zone here. In previous studies, it was named a ‘root-like structure’ (Hashimoto et al., 2012), a ‘procaulom’ (Velenovsky, 1892, 1905) or a ‘protosoma’ (Bobrov, 2004, 2009, 2014). We believe that previous studies did not explore the anatomical structure in detail (an analysis now allowed by the number of seedlings provided by our cultivation methods) and therefore did not distinguish between the intermediary zone and the true roots. Moreover, previous studies analysed seedlings that were already much larger.
Orchids also display an intermediary transition stage between the globular embryo and the first shoot meristem that is larger than the pyroloid intermediary zone and is called a protocorm (Rasmussen, 1995; Yeung, 2017) because it precedes formation of the typical plant cormus (i.e. the first shoot or root). Since the intermediary zone of pyroloids also precedes the cormus (here, the first root), we also suggest use of the term protocorm for the intermediary zone of pyroloids. The term protocorm was first used for post-embryonic stages of clubmosses (Treub, 1890), which also form a mycoheterotrophic structure appearing before formation of the first shoot and root. This term was later transferred to orchids (Bernard, 1909) and it is thus historically not orchid limited, but is actually providing a name for a convergently evolved structure. Recently, protocorm was even used in the obligately parasitic Rafflesiaceae, for an endophytic structure developing from proembryonic endophytic tissue before the formation of the flower-bearing shoot (Nikolov et al., 2014; Nikolov and Davis, 2017). Interestingly, Harley (1959) also suggested the term protocorm for seedlings of Monotropa, which are closely related to pyroloids. The pyroloid protocorm is significantly smaller and grows on the opposite embryo pole compared with orchids, but this transitory structure precedes the typical cormus in both types of mycoheterotrophic development. Investigations of the early ontogenetic development of other unrelated mycoheterotrophic plants with dust seeds (e.g. Burmaniaceae, Gentianaceae and Triuridaceae; Eriksson and Kainulainen, 2011) are pending. Scarce studies have revealed that Afrothismia hydra (Burmanniaceae; Imhof and Sainge, 2008) and Voyria spp. (Gentianaceae; Imhof, 2010, 2013) produce a root as the first organ, but nothing is known about initiation of the root from the globular embryo in these plants.
Conclusion
We used a unique combination of cultivation techniques, which for the first time allowed us to develop an efficient cultivation protocol to germinate and grow leafy plants of representatives from all pyroloid genera. Our methods for pyroloid cultivation may be used for conservation purposes and for physiological investigations, since pyroloid mixotrophic nutrition in adulthood is currently of considerable interest (e.g. Lallemand et al., 2017). The cultures obtained allow fine analyses of germination, with the transition from lipid and protein storage in seeds to starch accumulation, based on saccharides from the environment. Seedlings form first roots, before adventitious sprouting. The first root emerges from an intermediary zone (Fig. 7), which we suggest should be called a protocorm, due to its functional and developmental similarity to protocorms in other plants. Our data further support the many convergent traits in mycoheterotrophic germination and early development (especially the existence of a single meristem) in plants with dust seeds.
SUPPLEMENTARY DATA
Supplementary Data are available online at https://academic.oup.com/aob and consist of the following. Table S1: seed sources. Table S2: composition of cultivation media used in this study. Table S3: endogenous starch content revealed by HPLC analysis. Figure S1: higher and lower magnification showing a pyroloid seed with a well-developed embryo, sole seed coat, seeds with an undeveloped embryo and different stages of seedlings. Figure S2: effect of different media on germination rate of Pyrola minor, Moneses uniflora and Orthilia secunda seeds cultivated in vitro, 1, 2 and 3 month(s) after cold stratification. Figure S3: effect of different media on germination rate of Monotropa uniflora and Chimaphila umbellata seeds cultivated in vitro for 3 months after cold stratification. Figure S4: effect of light on seed germination of Pyrola minor and Orthilia secunda and effect of GA3 on seed germination of Pyrola minor cultivated in vitro on BM-1 or Knudson C medium and Orthilia secunda on BM-1 or Knudson C medium. Figure S5: effect of selected soluble saccharides on growth of Pyrola minor and Orthilia secunda. Figure S6: effect of selected soluble saccharides on growth of Pyrola minor and Orthilia secunda. Figure S7: 1-year-old seedlings of Pyrola minor cultivated in vitro on Knudson C medium with glucose, sucrose or trehalose as the sole saccharide source or without any soluble saccharide. Figure S8: Pyrola minor storage compounds. Figure S9: Monotropa uniflora seedlings grown in vitro.
ACKNOWLEDGEMENTS
We thank Montreal Botanical Garden for sending us seeds of C. umbellata and M. uniflora, Yvetta Šefrnová and Marek Širl for help with the anatomical study, Radim Hédl and Jitka Klimešová for help with the old literature, and Karel Fajmon and Michal Štefánek for help with sampling localities. We also thank the editor Margreth Sauter and two anonymous reviewers for useful comments. M.-A Selosse’s research is funded by the Fondation de France (funds from the Fondation Ars Cuttoli & Paul Appell) in Paris and by the 2015/18/A/NZ8/00149 grant funded by National Science Centre in Gdansk (Poland). This work was supported by a grant of the Czech Ministry of Education, Youth and Sports [grant no. LO1417] and by the Grant Agency of Charles University [grant no. 468318]. Confocal microscopy was performed in the Laboratory of Confocal and Fluorescence Microscopy co-financed by the European Regional Development Fund and the state budget of the Czech Republic [grant no. CZ.1.05/4.1.00/16.0347, CZ.2.16/3.1.00/21515].
LITERATURE CITED
- Arditti J. 1967. Factors affecting the germination of orchid seeds. Botanical Review 33: 1–97. [Google Scholar]
- Arditti J. 2008. Micropropagation of orchids, Vol. 1, 2nd edn. Chichester, UK: John Wiley and Sons. [Google Scholar]
- Arditti J, Ghani A. 2000. Numerical and physical properties of orchid seeds and their biological implications. New Phytologist 145: 367–421. [DOI] [PubMed] [Google Scholar]
- Bartlett M. 1937. Properties of sufficiency and statistical tests. Proceedings of the Royal Society A: Mathematical and Physical Sciences 160: 268–282. [Google Scholar]
- Bernard N. 1909. L’évolution dans la symbiose. Les Orchideés et leurs champignons commensaux. Annales des Sciences Naturelles Serie (Botanique) 9: 1–196. [Google Scholar]
- Barsberg S, Rasmussen HN, Kodahl N. 2013. Composition of Cypripedium calceolus (Orchidaceae) seeds analyzed by attenuated total reflectance IR spectroscopy: in search of understanding longevity in the ground. American Journal of Botany 100: 2066–2073. [DOI] [PubMed] [Google Scholar]
- Barsberg ST, Lee YI, Rasmussen HN. 2018. Development of C-lignin with G/S-lignin and lipids in orchid seed coats – an unexpected diversity exposed by ATR-FT-IR spectroscopy. Seed Science Research 28: 41–51. [Google Scholar]
- Bobrov J. 2004. On the early stages of ontogenesis of Europaean Pyrolaceae species [in Russian]. Botaniceskij Zhurnal 89: 1342–1351. [Google Scholar]
- Bobrov J. 2009. Grushankovye Rossii. Thesis, Vyatka State University of Humanities, Kirov, Russia. [Google Scholar]
- Bobrov J. 2014. Zhiznennaya forma Moneses uniflora (Pyroloideae, Ericaceae). Vektor nauki Toľyattinskogo gosudarstvennogo universiteta 29: 21–29. [Google Scholar]
- Brundrett MC, Kendrick B, Peterson CA. 1991. Efficient lipid staining in plant material with Sudan Red 7B or Fluoral Yellow 088 in polyethylene glycol–glycerol. Biotechnic & Histochemistry 66: 111–116. [DOI] [PubMed] [Google Scholar]
- Burgeff H. 1936. Samenkeimung der Orchideen und Entwicklung ihrer Keimpflanzen. Jena, Germany: Verlag von Gustav Fisher. [Google Scholar]
- Byng JW, Chase MW, CHristenhusz MJM, et al. 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society 181: 1–20. [Google Scholar]
- Christoph H. 1921. Untersuchungen über mykotrophen Verhältnisse der ‘Ericales’ und die Keimung von Pirolaceen. Beihefte Botanisches Centralblatt 38: 115–157. [Google Scholar]
- Copeland HF. 1947. Observations on the structure and classification of the Pyroleae. Madroño 9: 65–102. [Google Scholar]
- Dearnley JDW, Perotto D, Selosse M-A. 2016. Structure and development of orchid mycorrhizas, In: Martin, F, ed. Molecular mycorrhizal symbiosis. Berlin Heidelberg: Springer, 63–86. [Google Scholar]
- Eriksson O, Kainulainen K. 2011. The evolutionary ecology of dust seeds. Perspectives in Plant Ecology, Evolution and Systematics 13: 73–87. [Google Scholar]
- Francke H-L. 1934. Beiträge zur Kenntnis der Mykorrhiza von Monotropa hypopitys. Flora 129: 1–52. [Google Scholar]
- Freudenstein JV, Broe MB, Feldenkris ER. 2016. Phylogenetic relationships at the base of ericaceae: implications for vegetative and mycorrhizal evolution. Taxon 65: 794–804. [Google Scholar]
- Fürth P. 1920. Zur Biologie und Mikrochemie einiger Pirola-Arten. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften in Wien, Mathematish-Naturwissenschaftlige Klasse, Abteilung I 129: 559–587. [Google Scholar]
- Goebel K. 1887. Outlines of classification and special morphology of plants. Oxford: Clarendon Press. [Google Scholar]
- Goebel K. 1900. Organographie der Pflanzen Insbesondere der Archegoniaten und Samenpflanzen. Zweiter Teil. II Heft:Pteridophyten und Samenpflanzen. Jena, Germany: Verlag von Gustav Fischer. [Google Scholar]
- Groot SPC, Karssen CMC. 1987. Gibberellins regulate seed germination in tomato by endosperm weakening: a study with gibberellin-deficient mutants. Planta 171: 525–531. [DOI] [PubMed] [Google Scholar]
- Groot S, Kieliszewska-Rokicka B, Vermeer E, Karssen CM. 1988. Gibberellin-induced hydrolysis of endosperm cell walls in gibberellin-deficient tomato seeds prior to radicle protrusion. Planta 174: 500–504. [DOI] [PubMed] [Google Scholar]
- Guo S, Xu J. 1990. Studies on the changes of cell ultrastructure in the course of seed germination of Bletilla striata under fungus infection conditions. Acta Botanica Sinica 32: 594–598. [Google Scholar]
- Gutmann M, von Aderkas P, Label P, Lelu MA. 1996. Effects of abscisic acid on somatic embryo maturation of hybrid larch. Journal of Experimental Botany 47: 1905–1979. [Google Scholar]
- Harley JL. 1959. The biology of mycorrhiza. London: Leonard Hill. [Google Scholar]
- Harrison CR. 1977. Ultrastructural and histochemical changes during the germination of Cattleya aurantiaca (Orchidaceae). Botanical Gazette 138: 41–45. [Google Scholar]
- Hashimoto Y, Fukukawa S, Kunishi A, et al. 2012. Mycoheterotrophic germination of Pyrola asarifolia dust seeds reveals convergences with germination in orchids. New Phytologist 195: 620–630. [DOI] [PubMed] [Google Scholar]
- van der Heijden MGA, Martin FM, Selosse M-A, Sanders IR. 2015. Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytologist 205: 1406–1423. [DOI] [PubMed] [Google Scholar]
- Hofmeister W. 1858. Neuere Beobachtungen über Embryobildung der Phanerogamen. Jahrbücher für Wissenschaftliche Botanik 1: 82–188. [Google Scholar]
- Holm T. 1898. Pyrola aphylla: a morphological study. Botanical Gazette 25: 246–254. [Google Scholar]
- Hynson NA, Bruns TD. 2009. Evidence of a myco-heterotroph in the plant family Ericaceae that lacks mycorrhizal specificity. Proceedings of the Royal Society B: Biological Sciences 276: 4053–4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynson NA, Madsen TP, Selosse M-A, et al. 2013. a The physiological ecology of mycoheterotrophy In: Merckx, VSFT, ed. Mycoheterotrophy: the biology of plants living on fungi. New York: Springer New York, 297–342. [Google Scholar]
- Hynson NA, Weiß M, Preiss K, Gebauer G, Treseder KK. 2013b Fungal host specificity is not a bottleneck for the germination of Pyroleae species (Ericaceae) in a Bavarian forest. Molecular Ecology 22: 1473–1481. [DOI] [PubMed] [Google Scholar]
- Imhof S. 2010. Are monocots particularly suited to develop mycoheterotrophy? In: Seberg O, Petersen G, Barfod A, Davis JI, eds. Diversity, phylogeny, and evolution in the monocotyledons. Aarhus, Denmark: Aarhus University Press, 11–23. [Google Scholar]
- Imhof S, Sainge MN. 2008. Ontogeny of the mycoheterotrophic species Afrothismia hydra (Burmanniaceae). Botanical Journal of the Linnean Society 157: 31–36. [Google Scholar]
- Imhof S, Massicotte H, Melville LH, Peterson R. 2013. Subterranean morphology and mycorrhizal structures In: Merckx, VSFT, ed. Mycoheterotrophy: the biology of plants living on fungi. New York: Springer, 157–214. [Google Scholar]
- Irmish T. 1855. Bemerkungen über einige Pflanzen der deutschen Flora. Flora 13: 625–638. [Google Scholar]
- Johansson VA, Bahram M, Tedersoo L, Kõljalg U, Eriksson O. 2017. Specificity of fungal associations of Pyroleae and Monotropa hypopitys during germination and seedling development. Molecular Ecology 26: 2591–2604. [DOI] [PubMed] [Google Scholar]
- Johansson VA, Eriksson O. 2013. Recruitment limitation, germination of dust seeds, and early development of underground seedlings in six Pyroleae species. Botany 91: 17–24. [Google Scholar]
- Klimešová J. 2007. Root-sprouting in myco-heterotrophic plants: prepackaged symbioses or overcoming meristem limitation?New Phytologist 173: 8–10. [DOI] [PubMed] [Google Scholar]
- Knudson L. 1922. Nonsymbiotic germination of orchid seeds. Botanical Gazette 73: 1–25. [Google Scholar]
- Kramer C. 1956. Extension of multiple range tests to group means with unequal numbers of replications. Biometrics 12: 307–310. [Google Scholar]
- Kruskal WH, Wallis WA. 1952. Use of ranks in one-criterion variance analysis. Journal of the American Statistical Association 47: 583–621. [Google Scholar]
- Kubeš M, Drážná N, Konrádová H, Lipavská H. 2014. Robust carbohydrate dynamics based on sucrose resynthesis in developing Norway spruce somatic embryos at variable sugar supply. In Vitro Cellular and Developmental Biology 50: 45–57. [Google Scholar]
- Lallemand F, Gaudeul M, Lambourdière J, Matsuda Y, Hashimoto Y, Selosse MA. 2016. The elusive predisposition to mycoheterotrophy in Ericaceae. New Phytologist 212: 314–319. [DOI] [PubMed] [Google Scholar]
- Leake J. 1994. Tansley Review No. 69. The biology of myco‐heterotrophic (‘saprophytic’) plants. New Phytologist 127: 171–216. [DOI] [PubMed] [Google Scholar]
- Leroux G, Barabé D, Vieth J. 1997. Morphogenesis of the protocorm of Cypripedium acaule (Orchidaceae). Plant Systematics and Evolution 205: 53–72. [Google Scholar]
- Leubner-Metzger G, Fründt C, Meins F. 1996. Effects of gibberellins, darkness and osmotica on endosperm rupture and class I β-1,3-glucanase induction in tobacco seed germination. Planta 199: 282–288. [Google Scholar]
- Li Y-Y, Chen X-M, Guo S-X, Lee Y-I. 2016. Embryology of two mycoheterotrophic orchid species, Gastrodia elata and Gastrodia nantoensis: ovule and embryo development. Botanical Studies 57: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lihnell D. 1942. Keimungsversuche mit Pyrolasamen. Symbolae Botanicae Upsalienses 6: 1–37. [Google Scholar]
- Liu Z-W, Zhou J, Liu E-D, Peng H. 2010. A molecular phylogeny and a new classification of Pyrola (Pyroleae, Ericaceae). Taxon 59: 1690–1700. [Google Scholar]
- Lück R. 1940. Zur Biologie der heimischen Pirola-arten. Schriften der Physikalisch-Ökonomischen Gesellschaft zu Königsberg 71: 300–334. [Google Scholar]
- Lück R. 1941. Zur keimung der heimischen Pirola-Arten. Flora 135: 1–5. [Google Scholar]
- Ma W-D, Zou Y-P, Wang P, et al. 2014. Chimaphilin induces apoptosis in human breast cancer MCF-7 cells through a ROS-mediated mitochondrial pathway. Food and Chemical Toxicology 70: 1–8. [DOI] [PubMed] [Google Scholar]
- Malmgren S. 1993. Asymbiotisk fröförökning i stor skala av Anacamptis, Ophrys, Orchis och andra orkidéer med runda rotknölar, Svensk Botanisk Tidskrift 87: 221–234. [Google Scholar]
- Malmgren S, Nyström H. 2018. Orchid propagation. http://www.lidaforsgarden.com/orchids/engelsk.htm [Google Scholar]
- Manning J, Van Staden J. 1987. The development and mobilisation of seed reserves in some African orchids. Australian Journal of Botany 35: 343–353. [Google Scholar]
- Matsuda Y, Shimizu S, Mori M, Ito S-I, Selosse M-A. 2012. Seasonal and environmental changes of mycorrhizal associations and heterotrophy levels in mixotrophic Pyrola japonica (Ericaceae) growing under different light environments. American Journal of Botany 99: 1177–1188. [DOI] [PubMed] [Google Scholar]
- Merckx VSFT. 2013. Mycoheterotrophy: the biology of plants living on fungi. New York: Springer. [Google Scholar]
- Murashige T, Skoog F. 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum 15: 473–497. [Google Scholar]
- Nikolov LA, Davis CC. 2017. The big, the bad, and the beautiful: biology of the world’s largest flowers. Journal of Systematics and Evolution 55: 516–524. [Google Scholar]
- Nikolov LA, Staedler YM, Manickam S, et al. 2014. Floral structure and development in Rafflesiaceae with emphasis on their exceptional gynoecia. American Journal of Botany 101: 225–243. [DOI] [PubMed] [Google Scholar]
- Olson AR. 1993. Patterns of embryo and endosperm formation in Monotropa hypopitys (Monotropaceae) from North America and Western Sweden. American Journal of Botany 80: 839–846. [Google Scholar]
- Pedroza-Manrique J, Fernandez-Lizarazo C, Suarez-Silva A. 2005. Evaluation of the effect of three growth regulators in the germination of Comparettia falcata seeds under in vitro conditions. In Vitro Cellular & Developmental Biology - Plant 41: 838–843. [Google Scholar]
- Pierce S, Cerabolini BE. 2011. Asymbiotic germination of the White Mountain Orchid (Pseudorchis albida) from immature seed on media enriched with complex organics or phytohormones. Seed Science and Technology 39: 199–203. [Google Scholar]
- Ponert J, Vosolsobě S, Kmecová K, Lipavská H. 2011. European orchid cultivation – from seed to mature plant. European Journal of Environmental Sciences 1: 95–107. [Google Scholar]
- Ponert J, Figura T, Vosolsobě S, Lipavská H, Vohník M, Jersáková J. 2013. Asymbiotic germination of mature seeds and protocorm development of Pseudorchis albida (Orchidaceae) are inhibited by nitrates even at extremely low concentrations. Canadian Journal of Botany 91: 662–670. [Google Scholar]
- Pyykkö M. 1968. Embryological and anatomical studies on Finnish species of the Pyrolaceae. Annales Botanici Fennici 5: 153–165. [Google Scholar]
- R Core Team 2015. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Rasmussen HN. 1990. Cell differentiation and mycorrhizal infection in Dactylorhiza majalis (Rchb. f.) Hunt & Summerh. (Orchidaceae) during germination in vitro. New Phytologist 116: 137–147. [Google Scholar]
- Rasmussen HN. 1992. Seed dormancy patterns in Epipactis palustris (Orchidaceae): requirements for germination and establishment of mycorrhiza. Physiologia Plantarum 86: 161–167. [Google Scholar]
- Rasmussen HN. 1995. Terrestrial orchids: from seed to mycotrophic plant. Cambridge: Cambridge University Press. [Google Scholar]
- Richardson KA, Peterson RL, Currah RS. 1992. Seed reserves and early symbiotic protocorm development of Platanthera hyperborea (Orchidaceae). Canadian Journal of Botany 70: 291–300. [Google Scholar]
- Selosse MA, Roy M. 2009. Green plants that feed on fungi: facts and questions about mixotrophy. Trends in Plant Science 14: 64–70. [DOI] [PubMed] [Google Scholar]
- Selosse M-A, Bocayuva MF, Kasuya MCM, Courty PE. 2016. Mixotrophy in mycorrhizal plants: extracting carbon from mycorrhizal networks. In: Martin, F, ed. Molecular mycorrhizal symbiosis. Chichester, UK: John Wiley and Sons, 451–471. [Google Scholar]
- Shapiro SS, Wilk MB. 1965. An analysis of variance test for normality (complete samples). Biometrika 52: 591–611. [Google Scholar]
- Shu K, Liu X, Xie Q, He Z. 2016. Two faces of one seed: hormonal regulation of dormancy and germination. Molecular Plant 9: 34–45. [DOI] [PubMed] [Google Scholar]
- Schuurink R, Sedee N, Wang M. 1992. Dormancy of the barley grain is correlated with gibberellic acid responsiveness of the isolated aleurone layer. Plant Physiology 100: 1834–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Read D. 2008. Mycorrhizal symbiosis. London: Academic Press. [Google Scholar]
- Soukup A, Tylová E. 2014. Essential methods of plant sample preparation for light microscopy. Methods im Molecular Biology 1080: 1–23. [DOI] [PubMed] [Google Scholar]
- Steinbachová-Vojtíšková L, Tylová E, Soukup A, et al. 2006. Influence of nutrient supply on growth, carbohydrate, and nitrogen metabolic relations in Typha angustifolia. Environmental and Experimental Botany 57: 246–257. [Google Scholar]
- Takahashi H. 1993. Seed morphology and its systematic implications in Pyroloideae (Ericaceae). International Journal of Plant Sciences 154: 175–186. [Google Scholar]
- Tedersoo L, Pellet P, Kõljalg U, Selosse MA. 2007. Parallel evolutionary paths to mycoheterotrophy in understorey Ericaceae and Orchidaceae: ecological evidence for mixotrophy in Pyroleae. Oecologia 151: 206–217. [DOI] [PubMed] [Google Scholar]
- Těšitel J, Těšitelová T, Minasiewicz J, Selosse M-A. 2018. Mixotrophy in land plants: why to stay green?Trends in Plant Science 23: 656–659. [DOI] [PubMed] [Google Scholar]
- Thomas TD. 2008. The role of activated charcoal in plant tissue culture. Biotechnology Advances 26: 618–631. [DOI] [PubMed] [Google Scholar]
- Tian M, Wang F. 1985. In vitro seed germination and developmental morphology of seedling in Cymbidium ensifolium. Acta Botanica Sinica 27: 455–459. [Google Scholar]
- Treub M. 1890. Études sur les Lycopodiaceès. Annales du Jardin Botanique de Buitenzorg 1: 23–27. [Google Scholar]
- Velenovský J. 1892. O biologii a morfologii rodu ‘Monesis’. Rozpravy Královskéčeské společnosti nauk, řada, matematicko-přírodovědná 11: 147–159. [Google Scholar]
- Velenovský J. 1905. Über die Keimpflanzen der Pirolaceen. Bulletin International de l’Académie des Science de Bohéme 14: 6–12. [Google Scholar]
- van Waes JM. 1985. Effect of activated charcoal on in vitro propagation of Western European orchids. Acta Horticulturae 212: 131–138. [Google Scholar]
- van Waes JM, Debergh PC. 1986. In vitro germination of some Western European orchids. Physiologia Plantarum 67: 253–261. [Google Scholar]
- Wang D, He F, Lv Z, Li D. 2014. Phytochemical composition, antioxidant activity and HPLC fingerprinting profiles of three Pyrola species from different regions. PLoS One 9: e96329. doi: 10.1371/journal.pone. 0096329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam TW, Yeung EC, Ye X-L, Zee S-Y, Arditti J. 2002. Orchid embryos. In: Kull T, Arditti J, eds. Orchid biology: reviews and perspectives, VIII. Dordrecht: Kluwer Academic Publisher, 287–385. [Google Scholar]
- Yeung EC. 2017. A perspective on orchid seed and protocorm development. Botanical Studies 58: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung EC, Law SK. 1992. Embryology of Calypso bulbosa. II. Embryo development. Canadian Journal of Botany 70: 461–468. [Google Scholar]
- Zeng S, Zhang Y, Teixeira da Silva JA, Wu K, Zhang J, Duan J. 2014. Seed biology and in vitro seed germination of Cypripedium. Critical Reviews in Biotechnology 34: 358–371. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.