Abstract
Background
The Short Physical Performance Battery (SPPB) is advocated as a screening tool in geriatric care for predicting future disability. We aimed to identify the leg neuromuscular attributes to be targeted in rehabilitative care among older adults with poor SPPB scores.
Methods
Boston Rehabilitative Impairment Study of the Elderly (Boston RISE) participants (n = 430) underwent assessment of neuromuscular attributes (leg strength, leg velocity, trunk extensor endurance, knee flexion range of motion [ROM], ankle ROM, and foot sensation). Linear regression models examined association between each neuromuscular attribute and SPPB, adjusting for age, race, gender, comorbidity, body mass index, depression, cognition, and other neuromuscular attributes.
Results
Participants with 1 SD unit higher leg strength, leg velocity, and trunk extensor endurance had 0.52, 0.30, and 0.52 points higher SPPB total score. Participants with ankle ROM impairment and foot sensory loss had 0.43 and 0.57 lower SPPB total score compared with those without these. Leg strength and trunk extensor endurance were associated with balance; leg velocity, trunk extensor endurance, and ankle ROM were associated with gait speed; and strength, trunk extensor endurance, knee ROM, and feet sensation were associated with chair stand score. Neuromuscular attributes, along with covariates, explained 40.4% of the variance in the total SPPB score, a substantial increase over the 22.7% variance explained by covariates alone.
Conclusions
Neuromuscular attributes affect mobility performance in older patients as measured by the SPPB. Specific impairments are associated with poor performance in specific component scores. Assessment of the SPPB components and rehabilitation of associated impairments may help improve the functional performance among older adults.
Keywords: Functional performance, Rehabilitation, Gait, Balance, Disablement process
Functional performance measures are being increasingly recognized as excellent screening tools to assess risk for adverse health outcomes among older adults in clinical settings (1,2). Gait speed, for instance, is proposed as a sixth vital sign measure in primary care for older adults (3,4). However, there is evidence that other measures, such as the time taken to do a set number of chair rises (chair stand time), may provide important additional information regarding future health that is not captured by gait speed alone. For example, chair stand time was found to be a better predictor of injurious falls than gait speed (5). It has been proposed that measures such as chair stand time and standing balance may be able to discriminate disability risk within subgroups with similar gait speed (2). The Short Physical Performance Battery (SPPB) score captures lower extremity functional performance among older adults through the combined assessment of chair stand time, gait speed, and standing balance. The SPPB score is strongly predictive of important adverse health outcomes among older adults, including hospitalization, disability, and death (1,6). A recent meta-analysis demonstrated that SPPB scores have a dose–response relationship to mortality over the entire score range (7). All of these points make a strong case for the use of the SPPB as a comprehensive risk assessment tool. However, there is limited information on the underlying modifiable factors that are represented in SPPB performance. This is particularly relevant for rehabilitative care as those factors that underlie lower SPPB scores could be targeted within rehabilitative care as a means for reducing the risk for adverse health outcomes.
In a comprehensive analysis of neuromuscular attributes associated with late-life mobility, the Boston Rehabilitative Impairment Study of the Elderly (Boston RISE) identified a subset of five factors—leg strength, leg velocity, trunk extensor endurance, knee flexion range of motion (ROM), and ankle ROM—that predicted unfavorable self-reported mobility outcomes among older primary care patients (8,9). In addition, Boston RISE data have also demonstrated that foot sensory loss is an important factor in balance performance in this population (10). However, there is inadequate information on how these attributes affect and reflect in a lower limb functional performance measure such as the SPPB or its component measures. Because rehabilitative care enhances physical function by correcting the underlying neuromuscular impairments that limit the performance of functional tasks, this is important information potentially guiding the design and prescription for geriatric rehabilitative care.
The Boston RISE study provides a unique opportunity to examine the specific impairments associated with poor SPPB performance, given its comprehensive assessment of neuromuscular attributes and measurement of SPPB scores. In this study, we examined the association between neuromuscular attributes known to be important for mobility (leg strength, leg velocity, trunk extensor endurance, knee flexion ROM, ankle ROM, and feet sensation), with the SPPB total score and its component scores. We hypothesized that the attributes that were shown to be associated with self-reported mobility would also be associated with performance in the SPPB. We also estimated the proportion of variance in SPPB scores explained by the specified neuromuscular attributes to better understand the relative contribution the attributes made to SPPB performance.
Methods
We conducted a cross-sectional analysis of baseline data from participants in Boston RISE. The Boston RISE is a longitudinal cohort study of 430 primary care patients aged 65 years and older recruited from nine large primary care practices located across the greater Boston area from December 2009 to January 2012. Study methods have been described in detail previously (11). Eligibility included difficulty or task modification with walking one-half mile and/or climbing one flight of stairs (12), absence of moderate or severe dementia (Mini–Mental State Examination score < 18) (13), or absence of severe mobility limitation (SPPB score < 4) (1). Targeted recruitment was used to approximate ethnic/racial representation of older adults residing within a 10-mile radius of the study clinic. Methods were approved by the Spaulding Rehabilitation Hospital Institutional Review Board, and written consent was obtained from all participants.
Neuromuscular Impairments
Strength was measured in each leg by determining the one-repetition maximum for each leg with a Keiser pneumatic leg press machine using a previously published protocol (14). The maximum value observed on either side was recorded as the peak leg strength. Peak power was recorded as the highest recorded power out of five trials performed with each leg at 40% and 70% of the one-repetition maximum. Peak leg velocity was calculated by dividing peak power by the graphically displayed force at peak power recorded during the testing. Trunk extensor muscle endurance (15) was measured with the participant lying prone on a specialized plinth positioned 45° from vertical with feet fixed in position on a footplate and the body supported below the waist by the table. The participant maintained their trunk in a neutral position within the sagittal plane in line with their pelvis and legs for as long as possible up to 2.5 minutes with arms across the chest. The time for which the position was maintained was recorded in seconds. Passive ROM of the knee and ankle were measured with a goniometer (16). Ankle ROM was considered to be impaired if there was inability to dorsiflex past 90° or plantar flex past 110° in either leg. Foot sensation was measured over the dorsum of the big toe using the Semmes–Weinstein monofilament test employing the standard clinical 10- and 1.4-g monofilaments (17). Foot sensation was considered to be impaired if the participant could not perceive three out of four touches from both the monofilaments on both sides. Sensory loss measured using this method has been found to be a predictor of mobility disability in the Health ABC Study (18). Both impaired ankle ROM and sensory loss were dichotomized as being present or absent.
The Short Physical Performance Battery
The SPPB is composed of three components: progressive standing balance, usual pace walking speed, and a five-repetition chair stand test (1). For the standing balance test, participants are asked to maintain their balance for 10 seconds with their legs in side-by-side, semi-tandem, and full-tandem positions. For walking speed, participants walk at their usual pace over a 4-m walking course; time was recorded; and the best performance of two walks was considered for calculation of walk speed. In the chair stand test, participants are asked to stand up and down five times, as quickly as they can with their hands folded across their chest. The test is scored using time to complete the test in seconds. Using established cut points, scores for each component are grouped from 0 to 4 and summed to create a total score between 0 and 12, with higher scores indicating better performance (1).
Covariates
Age, gender, and race were self-reported. The comorbidity index was assessed by a comorbidity questionnaire developed and validated by Sangha and colleagues (19) and included heart disease, high blood pressure, lung disease, diabetes, ulcer or stomach disease, kidney disease, liver disease, anemia or other blood diseases, cancer, depression, osteoarthritis or degenerative arthritis, back pain, and rheumatoid arthritis. Weight and height were measured using standardized techniques, and body mass index was calculated. The Digit Symbol Substitution Test (DSST), which measures attention, processing speed, and executive function, was used to assess cognitive ability (13). Depressive symptoms were measured using the Patient Health Questionnaire-9 (PHQ) (20).
Statistical Analysis
Leg strength and trunk extensor endurance were normalized for body weight. Missing values of neuromuscular attributes, namely, leg strength (10%), leg velocity (11.4%), trunk extensor endurance (5.8%), knee flexion ROM (1.2%), ankle ROM impairment (1.9%), and sensory loss (0.2%), were imputed using multiple imputations. Pearson correlation coefficients were computed to examine correlations between neuromuscular attributes. We tested differences between mean values of neuromuscular attributes across SPPB total scores using generalized linear models (F test); chi-square tests were used for categorical variables. Multivariable linear regression models were used to examine the association between neuromuscular attributes and the SPPB total score as well as SPPB component scores. To facilitate comparison of the effect sizes of different neuromuscular attributes on SPPB scores, we standardized the continuous measures by subtracting the mean and dividing by their SD. Models were adjusted for other neuromuscular attributes as well as confounders chosen on the basis of their known association with neuromuscular attributes and mobility outcomes. These included age, race, gender, comorbidity score, body mass index, pain score, PHQ score, and the DSST score. Collinearity among predictor variables was examined by estimating the variance inflation factor.
For the gait speed component alone, we used gait speed measured in meters per second instead of the component score. For the chair stand component, we opted to use the score instead of chair stand time to facilitate categorization of participants who attempted but could not stand without using arms or failed to complete the test. For the balance component score, we combined scores 0 and 1 into a single category, as only four people had a score of 0. As sensitivity analyses, we developed ordinal regression models for the component scores and compared results with those from the linear regression models. Coefficient of determination (R2) values from multiple linear regression models were used to examine the proportion of variance in outcomes explained by six attributes; models were compared for fit using the Akaike information criterion, a likelihood-based statistic appropriate for comparison of non-nested models that impose a penalty for increased model complexity. Model estimates were generated using SAS software version 9.4 (SAS Institute, Inc., Cary, NC).
Results
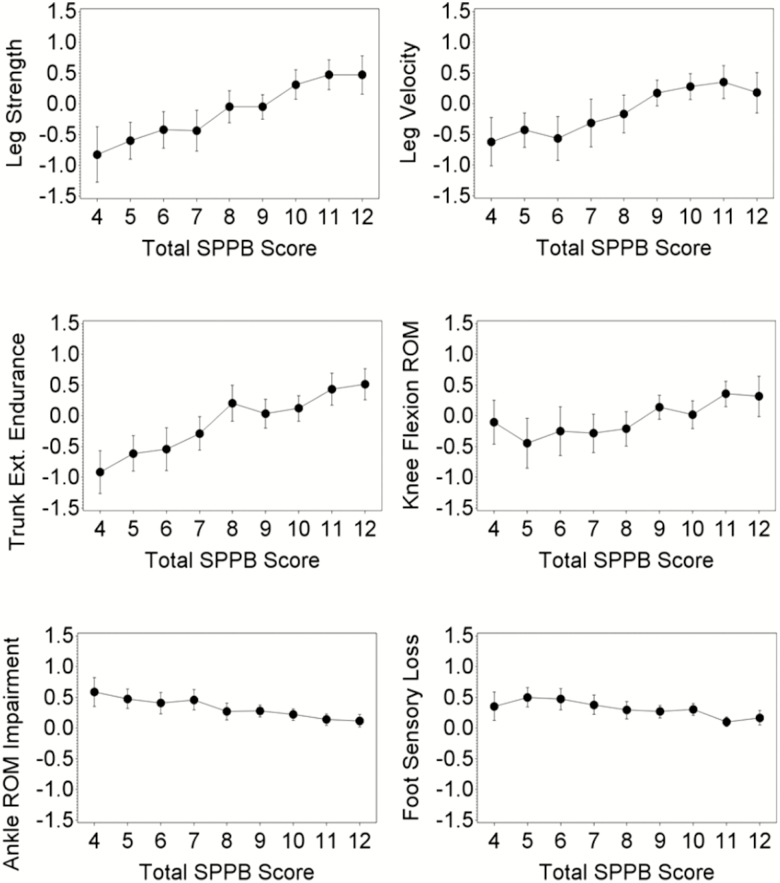
Boston RISE participants had an average age of 77 years, 68% (n = 291) were women, and 17% (n = 75) were non-White. The age, sex, and racial distributions are consistent with the 2004 U.S. Census for older adults living in the recruitment area (11). The baseline characteristics of participants according to SPPB total scores are given in Table 1. Figure 1A–E demonstrates the relationships between leg strength, leg velocity, trunk extensor endurance, knee flexion ROM, ankle ROM Impairment, and sensory loss in the lower extremity with the SPPB total score. There were overall significant differences (p < .0001) in each of the neuromuscular attribute measures, across SPPB total scores.
Table 1.
Baseline Characteristics of 430 Boston RISE Participants According to SPPB Total Score
| Variable | SPPB Total Score | Overall | ||
|---|---|---|---|---|
| 4–6 | 7–9 | 10–12 | ||
| Age, mean (SD) | 79.3 (0.8) | 76.9 (0.6) | 75.0 (0.5) | 76.6 (0.3) |
| Women, N (%) | 62 (69.7) | 101 (64.3) | 128 (69.6) | 291 (67.7) |
| Non-White, N (%) | 20 (22.5) | 24 (15.3) | 31 (16.9) | 75 (17.4) |
| BMI, mean (SD) | 29.3 (0.7) | 29.7 (0.5) | 29.5 (0.5) | 29.5 (0.3) |
| Comorbidity score, mean (SD) | 4.6 (0.2) | 4.3 (0.2) | 3.5 (0.1) | 4.0 (0.1) |
| PHQ score, mean (SD) | 2.2 (0.5) | 1.0 (0.2) | 1.2 (0.2) | 1.3 (0.2) |
| DSST score, mean (SD) | 29.7 (1.1) | 36.3 (0.8) | 39.4 (0.8) | 36.3 (0.5) |
| Leg strength, N/kg, mean (SD) | 7.8 (0.3) | 9.0 (0.2) | 10.4 (0.2) | 9.3 (0.1) |
| Leg velocity, m/s, mean (SD) | 0.9 (0.03) | 1.0 (0.02) | 1.1 (0.02) | 1.0 (.01) |
| Trunk extensor endurance, s, mean (SD) | 0.7 (0.1) | 1.3 (0.1) | 1.6 (0.1) | 1.3 (0.04) |
| Knee flexion ROM, degrees, mean (SD) | 120.2 (1.7) | 123.8 (1.0) | 127.1 (1.0) | 124.5 (0.7) |
| Ankle ROM impairment, N (%) | 42 (47.2) | 50 (31.9) | 31 (16.9) | 123 (28.6) |
| Foot sensory loss, N (%) | 41 (46.1) | 47 (29.9) | 38 (20.7) | 126 (29.3) |
Note: BMI = body mass index; DSST = Digit Symbol Substitution Test; PHQ = Patient Health Questionnaire-9; RISE = Rehabilitative Impairment Study of the Elderly; ROM = range of motion; SPPB = Short Physical Performance Battery.
Figure 1.
Standardized means (with 95% confidence limits) of (A) leg strength, (B) leg velocity, (C) trunk extensor endurance, (D) knee flexion range of motion and prevalence (with 95% confidence limits) of (E) ankle range of motion impairment, and (F) foot sensory loss, across SPPB scores among 430 participants in Boston Rehabilitative Impairment Study of the Elderly (Boston RISE).
Ordinal regression models gave results similar to the linear regression models, so we present results from linear regression here. Neuromuscular attributes were only weakly correlated (Supplementary Table 1) with Pearson correlation coefficients ranging from .03 to .39. There was also no evidence of multicollinearity in any of the models, and therefore, all neuromuscular attributes were included together in all the models. With adjustment for all covariates, as well as other neuromuscular attributes, the strong association between each neuromuscular attribute and the SPPB total score persisted except in the case of knee flexion ROM (Table 2). In addition, there were strong associations between each attribute and the component scores of the SPPB after adjusting for other attributes and covariates. Of all attributes, trunk extensor endurance was most strongly and consistently associated with all outcomes. Specifically, 1 SD unit greater trunk extensor endurance time was associated with 0.18 points higher balance score, 0.22 points higher chair stand score, 0.03 m/s faster gait speed, and 0.52 points higher SPPB total score. Leg strength, greater by 1 SD unit, was associated with 0.16 points higher balance score, 0.35 points higher chair stand score, and 0.58 points higher SPPB total score. One SD unit higher velocity was associated with 0.05 m/s higher gait speed and 0.30 points higher SPPB total score. Knee flexion ROM was not associated with the SPPB total score, but each unit increase in knee flexion ROM was associated with 0.14 points higher chair stand score. Participants with ankle ROM impairment had 0.07 m/s slower gait speed and 0.43 points lower SPPB total score compared with those without ankle ROM impairment. Participants with sensory loss were found to have 0.28 points lower chair stand score, 0.22 points lower balance score (borderline statistical significance), and 0.57 points lower total score compared with those without sensory loss.
Table 2.
Coefficients and p Values From Linear Regression Modelsa Examining Association Between Standardized Neuromuscular Attributes and Lower Limb Function Among 430 Boston RISE Participants
| SPPB Balance Score | Gait Speed | SPPB Chair Stand Score | SPPB Total Score | |||||
|---|---|---|---|---|---|---|---|---|
| Estimate (SD) | p Value | Estimate (SD) | p Value | Estimate (SD) | p Value | Estimate (SD) | p Value | |
| Leg strength | 0.16 (0.06) | .01 | 0.02 (0.01) | .15 | 0.35 | <.0001 | 0.58 (0.12) | <.0001 |
| Leg velocity | 0.10 (0.06) | .10 | 0.05 (0.01) | <.0001 | 0.08 | .24 | 0.30 (0.12) | .01 |
| Trunk extensor endurance | 0.18 (0.06) | .002 | 0.03 (0.01) | .0004 | 0.22 | .003 | 0.52 (0.10) | <.0001 |
| Knee flexion ROM | −0.01 (0.06) | .80 | 0.02 (0.01) | .12 | 0.14 | .03 | 0.16 (0.11) | .13 |
| Ankle ROM impairment | −0.10 (0.11) | .36 | −0.07 (0.02) | .0003 | −0.12 | .32 | −0.43 (0.21) | .04 |
| Foot sensory loss | −0.22 (0.11) | .05 | −0.03 (0.02) | .09 | −0.28 | .02 | −0.57 (0.21) | .01 |
Note: RISE = Rehabilitative Impairment Study of the Elderly; ROM = range of motion; SPPB = Short Physical Performance Battery.
aAll models were adjusted for age, race, gender, Katz comorbidity score, body mass index, Patient Health Questionnaire-9 score (depression), Digit Symbol Substitution Test, and other neuromuscular attributes.
On examining the relative contribution of the neuromuscular attributes to mobility performance, we found that these had a substantial impact on the variance of the SPPB total and component scores (Table 3). Neuromuscular attributes, along with covariates, explained 40.4% of the variance in the total SPPB score, a substantial increase over the variance explained by demographic and health characteristics alone (variance = 22.7% without neuromuscular attributes in the model). Neuromuscular attributes also explained additional variance in each of the SPPB component scores. Neuromuscular attributes and the covariates in the models explained 26.8% of the variance in SPPB balance score, 44.7% of the variance in gait speed, and 23.9% of the variance in chair stand time.
Table 3.
Additional Variance in SPPB Performance Explained by Neuromuscular Impairments
| Domains | Independent Variables | Percent Variance Explained (R2) | AIC |
|---|---|---|---|
| SPPB total score | Covariatesa | 22.7 | 607.3 |
| Covariates + neuromuscular impairmentsb | 40.4 | 507.6 | |
| SPPB balance score | Covariates | 20.1 | 3.5 |
| Covariates + neuromuscular impairments | 26.8 | −22.2 | |
| Gait speed | Covariates | 31.9 | −1,471.6 |
| Covariates + neuromuscular impairments | 44.7 | −1,549.5 | |
| SPPB chair stand score | Covariates | 8.3 | 135.6 |
| Covariates + neuromuscular impairments | 23.9 | 67.0 |
Note: AIC = Akaike information criterion; SPPB = Short Physical Performance Battery.
aCovariates included age, race, gender, Katz comorbidity score, body mass index, Patient Health Questionnaire-9 score (depression), and Digit Symbol Substitution Test score. bLeg strength, leg velocity, trunk extensor endurance, knee flexion range of motion, presence/absence of ankle range of motion limitation, and sensory loss.
Discussion
Our study has demonstrated that leg strength, leg velocity, trunk extensor endurance, ankle ROM impairment, and foot sensory loss independently affect the total SPPB score. The results also revealed that specific neuromuscular attributes affect different components of the SPPB, indicating that poor component scores reflect distinct impairments. In particular, trunk extensor muscle endurance and leg strength, both of which can be targeted and modified by exercise, were found to be important for two or more aspects of the SPPB.
The chair stand test or the sit to stand test has been commonly utilized as a measure of lower limb function in older adults; however, the lower limb neuromuscular attributes that contribute to performance in this test has not been well delineated. In a study of 26 residents of a long-term care institution, leg extensor power (which combines leg strength and velocity) was found to be correlated with chair stand speed (21). Subsequently, more studies have confirmed the strong association between quadriceps strength or power and chair stand time (22,23). However, it was also found that leg strength measures explained less than 50% of the variance in chair stand time in a study of average to high performing sexagenarian women, questioning its use as a proxy measure for leg strength (24). Lord and colleagues demonstrated that multiple psychological, sensorimotor, and balance-related factors are associated with chair stand performance after controlling for multiple leg strength measures (22); joint ROM and trunk extensor endurance were not evaluated in this study. Our study has demonstrated that trunk extensor endurance, foot sensation, and knee ROM are important factors in chair stand performance and could be key factors to evaluate and restore along with leg strength in rehabilitating an older adult with difficulty rising from a chair.
Although gait speed has been a variable of great interest as a functional performance marker among older adults, an understanding of mechanisms that underlie slow gait speed has not been clear. Leg strength, in particular, has demonstrated mixed results in its association with gait speed. Some cross-sectional studies have shown significant association (25), with some indicating a nonlinear relationship between leg strength and gait speed, whereby the association is strongest among adults with less strength (26,27) Longitudinal studies have also demonstrated that change in leg strength is associated with change in gait speed (28,29). Nonetheless, other studies have not demonstrated this association in longitudinal analysis (25,30). Our study provides evidence that leg velocity rather than leg strength is an important factor in gait speed, albeit using a cross-sectional analysis. Spinal alignment is another attribute that has been shown to be associated with gait speed (31). This may be due to weaker trunk extensors, which was shown to be important for gait speed in our study.
Leg strength (32), trunk muscle strength (33), and foot sensation (34) have been shown to influence balance performance among older adults in separate studies. Our study consolidates and confirms the independent role of these individual neuromuscular factors—specifically leg strength, trunk extensor muscle endurance, and foot sensation—in analyses that adjusted for each other. In another study using data from Boston RISE, Thomas and colleagues demonstrated that trunk extensor muscle endurance, sensory loss, and leg velocity are important determinants of the Frailty and Injuries: Cooperative Studies of Intervention Techniques (FICSIT)-4 balance score (10). The FICSIT-4 includes the SPPB balance test components and the more complex unipedal stance, which may explain the difference in the determinants.
Our findings have important implications in rehabilitation interventions for mobility-limited older adults. A systematic review that evaluated the role of progressive resistance strength training for improving physical function among older adults identified that resistance training resulted in a large positive effect on muscle strength, moderate to large effect on chair stand time, and a modest impact on gait speed (35). This aligns with our findings that strength is associated with chair stand capacity but not with gait speed. For improving gait, therefore, rehabilitation providers may need to focus on improving leg velocity (through training that emphasizes leg power), trunk extensor muscle endurance, and ankle ROM rather than leg strength. In fact, there is evidence that power training is better than strength training for improving functional performance (36) especially among older adults with poor leg velocity (37) or leg strength (38). Our findings thus provide valuable clues for developing and testing rehabilitative care interventions for mobility-limited older adults. It is to be noted, however, that mobility may not necessarily be restored purely by rehabilitative interventions that address neuromuscular impairments. Sensory deficits as well as social and environmental barriers may exist and may need to be addressed (39). Chronic conditions need to be managed clinically. Also, although exercise has been showed to improve mobility performance in community-dwelling older adults (40), approaches are needed that can address the range of impairments contributing to poor mobility in older adults.
Among the study limitations is that our design was cross-sectional, so we cannot confirm temporality of the observed relationships. The study sample included only older patients with known mobility problems and was not population based because participants were recruited from a single health care system. Hence, these findings may not be generalizable to diverse populations or general community populations. We acknowledge that other leg strength and velocity measures that were not measured in our study may be important for certain measures such as gait speed. Among our study strengths, we examined several neuromuscular attributes and their roles in a relatively large sample of primary care patients.
Conclusions
Neuromuscular attributes are strong independent factors that may contribute to mobility performance in older patients as measured by the SPPB. Specific impairments are associated with poor performance in specific component scores. Assessment of the SPPB and rehabilitation of associated impairments may help improve functional performance and thereby function and mobility in day-to-day living among older adults.
Funding
This study was supported by the National Institute on Aging (R01 AG032052-03); the Eunice Kennedy Shriver National Institute of Child Health and Human Development (1K24HD070966-01 to J.B.); and the National Center for Research Resources in a grant to the Harvard Clinical and Translational Science Center (1 UL1 RR025758-01). MEJ was supported by the National Institute on Disability, Independent Living and Rehabilitation Research through its Advanced Rehabilitation Research and Training Fellowship program.
Supplementary Material
Acknowledgments
An abstract was accepted, and an oral presentation was given on the results from these data at the Gerontological Society of America Annual Meeting in New Orleans, LA, in November 2016.
Conflict of Interest
None reported.
References
- 1. Guralnik JM, Simonsick EM, Ferrucci L, et al. . A Short Physical Performance Battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi:10.1093/geronj/49.2.M85 [DOI] [PubMed] [Google Scholar]
- 2. Studenski S, Perera S, Wallace D, et al. . Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. doi:10.1046/j.1532-5415.2003.51104.x [DOI] [PubMed] [Google Scholar]
- 3. Middleton A, Fritz SL, Lusardi M. Walking speed: the functional vital sign. J Aging Phys Act. 2015;23:314–322. doi:10.1123/japa.2013-0236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign”. J Geriatr Phys Ther. 2009;32:46–49. doi:10.1519/00139143-200932020-00002 [PubMed] [Google Scholar]
- 5. Ward RE, Leveille SG, Beauchamp MK, et al. . Functional performance as a predictor of injurious falls in older adults. J Am Geriatr Soc. 2015;63:315–320. doi:10.1111/jgs.13203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guralnik JM, Ferrucci L, Pieper CF, et al. . Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the Short Physical Performance Battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi:10.1093/gerona/55.4.M221 [DOI] [PubMed] [Google Scholar]
- 7. Pavasini R, Guralnik J, Brown JC, et al. . Short Physical Performance Battery and all-cause mortality: systematic review and meta-analysis. BMC Med. 2016;14:215. doi:10.1186/s12916-016-0763-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ward RE, Beauchamp MK, Latham NK, et al. . Neuromuscular impairments contributing to persistently poor and declining lower-extremity mobility among older adults: new findings informing geriatric rehabilitation. Arch Phys Med Rehabil. 2016;97:1316–1322. doi:10.1016/j.apmr.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beauchamp MK, Jette AM, Ni P, et al. . Leg and trunk impairments predict participation in life roles in older adults: results from Boston RISE. J Gerontol A Biol Sci Med Sci. 2016;71:663–669. doi:10.1093/gerona/glv157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thomas JC, Odonkor C, Griffith L, et al. . Reconceptualizing balance: attributes associated with balance performance. Exp Gerontol. 2014;57:218–223. doi:10.1016/j.exger.2014.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holt NE, Percac-Lima S, Kurlinski LA, et al. . The Boston Rehabilitative Impairment Study of the Elderly: a description of methods. Arch Phys Med Rehabil. 2013;94:347–355. doi:10.1016/j.apmr.2012.08.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fried LP, Bandeen-Roche K, Chaves PH, Johnson BA. Preclinical mobility disability predicts incident mobility disability in older women. J Gerontol A Biol Sci Med Sci. 2000;55:M43–M52. doi:10.1093/gerona/55.1.M43 [DOI] [PubMed] [Google Scholar]
- 13. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi:10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 14. Callahan D, Phillips E, Carabello R, Frontera WR, Fielding RA. Assessment of lower extremity muscle power in functionally-limited elders. Aging Clin Exp Res. 2007;19:194–199. doi:10.1007/BF03324689 [DOI] [PubMed] [Google Scholar]
- 15. Suri P, Kiely DK, Leveille SG, Frontera WR, Bean JF. Trunk muscle attributes are associated with balance and mobility in older adults: a pilot study. PM R. 2009;1:916–924. doi:10.1016/j.pmrj.2009.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Watkins MA, Riddle DL, Lamb RL, Personius WJ. Reliability of goniometric measurements and visual estimates of knee range of motion obtained in a clinical setting. Phys Ther. 1991;71:90–96; discussion 96–97. doi:10.1093/ptj/71.2.90 [DOI] [PubMed] [Google Scholar]
- 17. Olaleye D, Perkins BA, Bril V. Evaluation of three screening tests and a risk assessment model for diagnosing peripheral neuropathy in the diabetes clinic. Diabetes Res Clin Pract. 2001;54:115–128. doi:10.1016/S0168-8227(01)00278-9 [DOI] [PubMed] [Google Scholar]
- 18. Ward RE, Boudreau RM, Caserotti P, et al. . Sensory and motor peripheral nerve function and incident mobility disability. J Am Geriatr Soc. 2014;62:2273–2279. doi:10.1111/jgs.13152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The self-administered comorbidity questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156–163. doi:10.1002/art.10993 [DOI] [PubMed] [Google Scholar]
- 20. Zuithoff NP, Vergouwe Y, King M, et al. . The Patient Health Questionnaire-9 for detection of major depressive disorder in primary care: consequences of current thresholds in a cross-sectional study. BMC Fam Pract. 2010;11:98. doi:10.1186/1471-2296-11-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bassey EJ, Fiatarone MA, O’Neill EF, Kelly M, Evans WJ, Lipsitz LA. Leg extensor power and functional performance in very old men and women. Clin Sci (Lond). 1992;82:321–327. doi:10.1042/cs0820321 [DOI] [PubMed] [Google Scholar]
- 22. Lord SR, Murray SM, Chapman K, Munro B, Tiedemann A. Sit-to-stand performance depends on sensation, speed, balance, and psychological status in addition to strength in older people. J Gerontol A Biol Sci Med Sci. 2002;57:M539–M543. doi:10.1093/gerona/57.8.M539 [DOI] [PubMed] [Google Scholar]
- 23. Hardy R, Cooper R, Shah I, Harridge S, Guralnik J, Kuh D. Is chair rise performance a useful measure of leg power?Aging Clin Exp Res. 2010;22:412–418. doi:10.1007/BF03324942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McCarthy EK, Horvat MA, Holtsberg PA, Wisenbaker JM. Repeated chair stands as a measure of lower limb strength in sexagenarian women. J Gerontol A Biol Sci Med Sci. 2004;59:1207–1212. [DOI] [PubMed] [Google Scholar]
- 25. Buchner DM, Cress ME, Esselman PC, et al. . Factors associated with changes in gait speed in older adults. J Gerontol A Biol Sci Med Sci. 1996;51:M297–M302. doi:10.1093/gerona/59.11.1207 [DOI] [PubMed] [Google Scholar]
- 26. Ferrucci L, Guralnik JM, Buchner D, et al. . Departures from linearity in the relationship between measures of muscular strength and physical performance of the lower extremities: the Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci. 1997;52:M275–M285. doi:10.1093/gerona/52A.5.M275 [DOI] [PubMed] [Google Scholar]
- 27. Buchner DM, Larson EB, Wagner EH, Koepsell TD, de Lateur BJ. Evidence for a non-linear relationship between leg strength and gait speed. Age Ageing. 1996;25:386–391. doi:10.1093/ageing/25.5.386 [DOI] [PubMed] [Google Scholar]
- 28. Francis P, Mc Cormack W, Toomey C, et al. . Twelve weeks’ progressive resistance training combined with protein supplementation beyond habitual intakes increases upper leg lean tissue mass, muscle strength and extended gait speed in healthy older women. Biogerontology. 2017;18(6): 881–891. doi:10.1007/s10522-016-9671-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Purser JL, Pieper CF, Poole C, Morey M. Trajectories of leg strength and gait speed among sedentary older adults: longitudinal pattern of dose response. J Gerontol A Biol Sci Med Sci. 2003;58:M1125–M1134. doi:10.1093/gerona/58.12.M1125 [DOI] [PubMed] [Google Scholar]
- 30. Uematsu A, Tsuchiya K, Kadono N, et al. . A behavioral mechanism of how increases in leg strength improve old adults’ gait speed. PLoS One. 2014;9:e110350. doi:10.1371/journal.pone.0110350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miyazaki J, Murata S, Horie J, Uematsu A, Hortobagyi T, Suzuki S. Lumbar lordosis angle (LLA) and leg strength predict walking ability in elderly males. Arch Gerontol Geriatr. 2013;56:141–147. doi:10.1016/j.archger.2012.09.004 [DOI] [PubMed] [Google Scholar]
- 32. Mayson DJ, Kiely DK, LaRose SI, Bean JF. Leg strength or velocity of movement: which is more influential on the balance of mobility limited elders?Am J Phys Med Rehabil. 2008;87:969–976. doi:10.1097/PHM.0b013e31818dfee5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Granacher U, Gollhofer A, Hortobagyi T, Kressig RW, Muehlbauer T. The importance of trunk muscle strength for balance, functional performance, and fall prevention in seniors: a systematic review. Sports Med. 2013;43:627–641. doi:10.1007/s40279-013-0041-1 [DOI] [PubMed] [Google Scholar]
- 34. Menz HB, Morris ME, Lord SR. Foot and ankle characteristics associated with impaired balance and functional ability in older people. J Gerontol A Biol Sci Med Sci. 2005;60:1546–1552. doi:10.1093/gerona/60.12.1546 [DOI] [PubMed] [Google Scholar]
- 35. Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. The Cochrane database of systematic reviews. 2009(3):Cd002759. doi:10.1002/14651858.CD002759.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miszko TA, Cress ME, Slade JM, Covey CJ, Agrawal SK, Doerr CE. Effect of strength and power training on physical function in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2003;58:171–175. doi:10.1093/gerona/58.2.M171 [DOI] [PubMed] [Google Scholar]
- 37. Bean JF, Kiely DK, LaRose S, O’Neill E, Goldstein R, Frontera WR. Increased velocity exercise specific to task training versus the National Institute on Aging’s strength training program: changes in limb power and mobility. J Gerontol A Biol Sci Med Sci. 2009;64:983–991. doi:10.1093/gerona/glp056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bean JF, Beauchamp MK, Ni M. Targeted exercise training to optimize leg power, leg speed, and mobility in older adults. J Am Geriatr Soc. 2016;64:2608–2609. doi:10.1111/jgs.14397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rantanen T. Promoting mobility in older people. J Prev Med Public Health. 2013;46(suppl 1):S50–S54. doi:10.3961/jpmph.2013.46.S.S50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pahor M, Guralnik JM, Ambrosius WT, et al. . Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311:2387–2396. doi:10.1001/jama.2014.5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.