Abstract
Two aspects arise concerning the use of self-measured blood pressure monitoring to diagnose white-coat hypertension (WCH): the presence of target organ damage (TOD) and the normal cut-off threshold. This study aims to evaluate the cardiovascular risk of WCH according to different self-measured blood pressure normal cut-off thresholds and the influence of TOD at baseline.
In all, 678 patients were followed for 6.2 years; 223 normotensive patients, 271 patients with sustained hypertension (HT), and 184 with WCH. TOD was defined as: left ventricular hypertrophy according to ECG, albuminuria, or low estimated glomerular filtration rate. The risk for different cutting points of self-measured blood pressure (<135/85 mm Hg, <130/85 mm Hg, and <130/80 mm Hg) has been determined.
The patients with HT experienced an increase in cardiovascular risk and death higher than the normotensive patients (odds ratio [OR] 7.9, 95% confidence interval [CI] 3.8–16.2 for sustained HT; and OR 3.5, 95% CI 1.6–7.4 for WCH). This was observed for all the cut-off thresholds analyzed. In white-coat hypertensive patients (cut-off <135/85 mm Hg) with TOD, the risk was higher than in normotensive patients (OR 4.5; 95% CI 1.9–10.6). Using a self-monitoring blood pressure cut-off threshold of <130/80 mm Hg without TOD at baseline, the WCH cases exhibited no differences in risk to the normotensive patients (OR 2.0, 95% CI 0.5–7.7).
The decisions being taken for patients with WCH based on the presence of TOD and a self-administered home monitoring blood pressure measurement cut-off point probably lower than the one that is currently recommended.
Keywords: cardiovascular morbidity, cut-off threshold, self-monitoring home blood pressure, white-coat hypertension
1. Introduction
The diagnosis and treatment of hypertension (HT) is based on a precise and protocolized measuring of blood pressure (BP). BP measurements taken at the office, even in optimal conditions, have limitations attributable to the errors and biases of the observer, to environmental variables or to the reaction known as the “white-coat effect.”[1] BP measurements taken outside the office have a greater capacity for predicting cardiovascular (CV) risk than those taken at the office.[2,3] One of these measurement techniques used away from the office—the home self-monitoring of blood pressure (SMBP) measurement—occupies a prominent place, because it is easy, well-tolerated, reliable, and accessible for patients with HT.[4,5] However, 2 aspects in the use of this technique to diagnose “white-coat hypertension” (WCH) or “isolated office hypertension" are subject to discussion. The presence of target organ damage (TOD) confers a high CV risk, independent of the patient's HT phenotype, including WCH.[6] In this context, it seems important to take into account the cut-off threshold of normality to increase confidence in the diagnosis of the HT phenotype. It is accepted that the elevated cut-off threshold for SMBP is to have an average of several BP measurements higher than 135/85 mm Hg,[7] and a cut-off lower than 130/80 is considered as normal.[8] There is some evidence to suggest that a lower cut-off threshold is associated with a better evolution of TOD[9] and a better prognosis in CV morbidity and mortality.[10] In this sense, the cut-off definition of normality is important, because it is used to define the HT phenotype of each patient (normotension [NT], sustained HT [SHT], WCH, or masked HT). The aim of this study is to evaluate the CV risk of WCH phenotype according to different SMBP normal cut-off thresholds, and the influence of TOD at baseline, in relation to normotensive patient.
2. Patients and methods
2.1. Study population
In all, 696 patients aged between 15 and 75 years were recruited from 19 primary healthcare centers (14 in Girona and 5 in Barcelona [Catalonia, Spain], between 2003 and 2005). The study included patients with office HT (473 hypertensive patients; 283 with SHT and 190 with WCH), defined as an average of >139 mm Hg systolic BP and/or >89 mm Hg diastolic BP from at least 2 BP measurements per visit (taken at 2-minute intervals) on 3 consecutive days. The investigators were instructed to include all consecutive patients with HT who attended the office and met the inclusion criteria (systematic sampling). All subjects were newly diagnosed, had not received any antihypertensive treatment, and had no history of diabetes or CV disease. All patients with office HT (BP ≥140/90 mm Hg) were considered hypertensive regardless of the results of the SMBP. Clinical and treatment decisions were made according to the office BP and 2003 European Society of Hypertension—European Society of Cardiology.[11] All patients with hypertension (SHT or WCH) were advised to adopt lifestyle measures such as salt reduction, exercise, normocaloric, or hypocaloric (if body mass index [BMI] were ≥25 kg/m2) Mediterranean diet and smoking cessation. The use of the SMBP information was accepted in resistant HT, suspected “white coat effect,” and assessment of the response to treatment. We also recruited a subcohort of patients from the nonhypertensive general population who also used the primary care centers, including the first patient who was attended to subsequent to the hypertensive patient's visit recruitment until reaching the expected size (223 normotensive patients) (Fig. 1).
Figure 1.
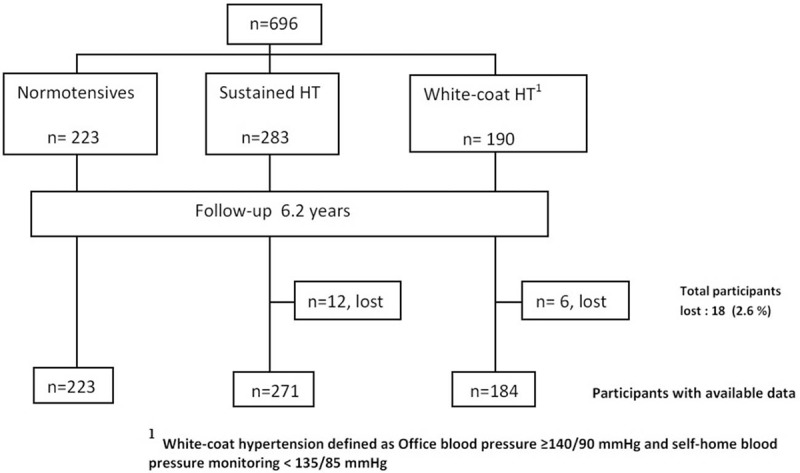
Flowchart of study cohort.
The exclusion criteria were as follows: the patient's inability (in the opinion of the health professional) to perform SMBP; diabetes mellitus; secondary hypertension; prior CV disease; advanced renal disease (glomerular filtration rate [GFR] <30 mL/min/1.73 m2 or urinary albumin excretion rate [UAER] ≥300 mg/g) or hepatic insufficiency; alcoholism or serious psychological illness; serious endocrine or hematological illness or other illness, or limitation that the physician considered to be a motive for exclusion; or lack of patient consent. This research is part of a study on self-monitoring of BP in isolated clinical hypertension (the VAMPAHICA study: a list of the VAMPAHICA study researchers is provided in the Supplementary Material available online).
All the patients or their legal representatives were asked to give informed consent. The study was approved by the Ethics Committee of the Girona Healthcare Institute (Catalonia, Spain)
2.2. Diagnosis of office hypertension
Nurses took an initial BP measurement with OMRON 705 CP or OMRON 705 IT monitors (HEM 759 E2 and HEM 759 E; Tokyo, Japan) using a cuff bladder adapted to the circumference of each patient's arm. International standard protocols were followed and all devices are calibrated annually. After 5 minutes of rest in a sitting position, 2 readings were taken at intervals of 2 minutes. If the difference between readings on the same day was >5 mm Hg, an additional measurement was taken. The BP value used in this study was the mean of all the measurements taken for each subject.
2.3. SMBP procedure
Each participant was instructed by an expert nurse in the steps required to obtain adequate readings. The process was then carried out correctly by the patient twice in the presence of the nurse. All the measurements were performed using OMRON 705 CP and OMRON 705 IT monitors and carried out with an armband adapted to the circumference of each patient's arm. Patients were also given written instructions. Over 3 working days, 2 readings were taken in the morning before breakfast and 2 more at night before dinner. These readings were taken at 2-minute intervals after sitting down for 5 minutes. Patients wrote the readings down on the form they had been given for this very purpose. To check the reliability of the data, they also provided the readings obtained through the monitor. The readings from the first day were discarded and not used for our calculations.
2.4. Hypertension phenotypes at baseline
2.4.1. Sustained hypertension
Patients with office BP ≥140/90 and SMBP ≥135/85 mm Hg or other cut-off thresholds evaluated (SMBP ≥135/85; ≥130/85; ≥130/80 mm Hg).
2.4.2. White-coat hypertension
Patients with office BP ≥140/90 mm Hg and SMBP <135/85 mm Hg or other cut-off thresholds evaluated (SMBP <135/85; <130/85; <130/80 mm Hg).
2.4.3. Normotensive patients
With office BP <140/90 mm Hg and SMBP <135/85 mm Hg, or other cut-off thresholds evaluated (SMBP <135/85; <130/85; <130/80 mm Hg). Patients with masked HT at baseline (office BP <140/90 mm Hg and SMBP ≥135/85 mm Hg) were discarded.
2.5. Initial study and follow-up
Initially, and then on a yearly basis, all hypertensive patients underwent an anamnesis and a physical examination, and had blood and urine sample analysis and an electrocardiogram of 12 standard derivations.
Normotensive patients initially had an anamnesis and a physical examination, performed an SMBP (to rule out masked hypertension), and then had a BP measurement taken in the surgery once a year.
2.6. Primary endpoint
The composite endpoint was: coronary artery disease (ICD-10 code: I20-I25), stroke (ICD-10 code: I61-I66), transitory ischemic accident (ICD-10 code: G45.9), symptomatic peripheral arterial disease (ICD-10 code: I70.2, I73.9), heart failure (ICD-10 code: I11.0. I13.0, I13.2, I50.1, I50.9), atrial fibrillation (ICD-10 code: I49.0), retinal arterial or venous thrombosis (ICD-10 code: H34), and death from all cause. The data on CV events and death from all cause were obtained from the data collection notebook of each patient and were checked in the electronic medical record of each patient using the ICD-10 codes registered and the evaluation from the clinical reports and hospital admissions.
2.7. Other variables
Other variables measured were: GFR by Chronic Kidney Disease Epidemiology Collaboration,[12] left ventricular hypertrophy (LVH) (electrocardiography criteria as per Cornell and/or as per Sokolow–Lyon), and UAER was determined by measuring the ratio of albumin to creatinine (milligrams of albumin per gram creatinine) in the first morning urine. A reactive strip was used for testing the urine, and if the test was positive, the urine was then analyzed and the anomaly was treated (ie, urinary infection); microalbuminuria was tested for again after 15 days. A diagnosis was made when at least 2 out of 3 tests were positive.
2.8. Target organ damage
The presence of any of the following at baseline was considered as TOD: LVH by electrocardiogram, UAER between 30 AND 299 mg/g, or GFR between 30 and 59 mL/min/1.73 m2.
2.9. Sample size
The sample size was calculated for the comparison between 3 groups (SHT, WCH vs normotension). The event rate in the normotension group was estimated at 0.058 and the estimated prevalence of WCH was 30%. With these data, accepting an alpha risk of 0.05 and a beta risk of 0.2, 458 patients with HT (263 with SHT and 222 with WCH) and 333 normotensive subjects are needed to identify risk differences greater or equal to 0.1, assuming a 20% loss of participants. With a sample size of 678 (223 normotensive and 455 hypertensive—271 SHT and 184 WCH) and an alpha risk of 5%, assuming bilateral contrast, the statistical power to compare SHT and WCH versus NT, is superior to 80% in all the cut-off points studied due to the fact that the incidence of CV events in the group of normotensive subjects was lower than estimated (0.045) and that the lost participants was lower than expected (2.6%).
2.10. Statistical analysis
All the variables were summarized, stratifying between normotensives, sustained hypertension, and WCH by the SMBP cut-off at baseline (SMBP <135/85; <130/85; <130/80 mm Hg). The summary was made both at baseline and during follow-up. Here, we considered the final value minus the baseline value for the qualitative variables, and the mean values of the variable during follow-up (except baseline) minus the baseline for the quantitative variables. The quantitative variables were summarized by mean and standard deviation, median, and first and third quartiles. The median and the interquartile range have been calculated in the case that the distribution was not normal. The qualitative variables were summarized by proportions.
The relationship between 6-year CV morbidity, total mortality, and the SMBP cut-off point at baseline were adjusted in a logistic regression including, where possible, the confounders observed: age, sex, smoker, angiotensin-converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB) antihypertensive treatment during follow-up (model 1), all antihypertensive treatment during follow-up (model 2), and statin treatment during follow-up, and also systolic and diastolic BP, BMI, fasting glucose, high-density lipoprotein-cholesterol and low-density lipoprotein-cholesterol (in all of these cases, the mean follow-up minus the baseline value). Residual confounding was controlled by introducing a random effect, which collected the individual heterogeneity (ie, factors specific to the individual that do not vary in time), into the logistic regression.
The same logistic regression was estimated by stratifying the presence of TOD at baseline. All analyses were made with the free software R (version 3.4.2),[13] made available through the INLA package.[14,15]
3. Results
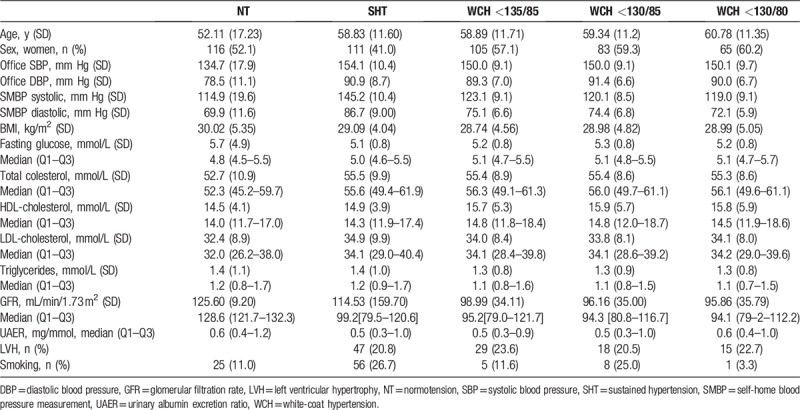
IN all, 678 patients were followed for an average of 6.2 years (SD 3.9). There were 223 normotensive patients (52.1% women), 271 who had sustained hypertension (41% women) and 184 with WCH criteria (57.1% women). Eighteen participants (3.8% of hypertensive patients) were lost during the follow-up (Fig. 1). Table 1 shows the characteristics of each subcohort, and also those corresponding to the different SMBP cut-off points for WCH. Twenty-two normotensive patients (10%) evolved into SHT in an average time of 64.6 months. No differences were observed for the baseline prevalence of LVH.
Table 1.
Baseline characteristics of cohort.
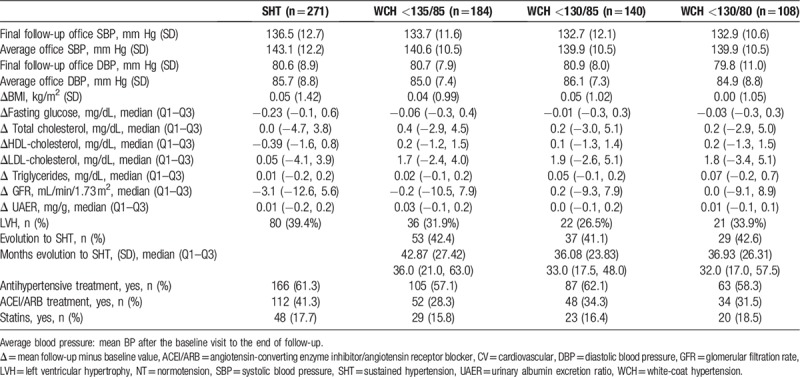
During the follow-up of the cohort, a reduction in office BP was observed in all patients. BP reduction at the end of follow-up was similar for all groups (−17.6/−10.3 mm Hg), and with WCH at different SMBP cut-off thresholds (<135/85 mm Hg: −16.3/−8.6 mm Hg; <130/85 mm Hg: −17.3/−10.5 mm Hg; <130/80 mm Hg: −17.2/−10.2 mm Hg). No differences were observed between the SHT and WCH groups in the percentage of antihypertensive drugs, although the WCH group had been prescribed fewer ACEIs and/or less ARB therapy. Neither were there any differences observed between the groups for prescription of statins. The percentage of patients with WCH who progressed to SHT was around 40% and without any differences in the different cut-off points defining WCH. However, patients with WCH at the <130/80 mm Hg cut-off point, evolved to SHT 6 months slower than those whose cut-off point was <135/85 mm Hg (Table 2). On average, CV events for patients with WCH (11.4%) were among the normotensive patients (4.0%, data not shown) and those with SHT (25.1%). There was only 1 death (0.4%).
Table 2.
Evolution of the cohort during 6 years of follow- up.
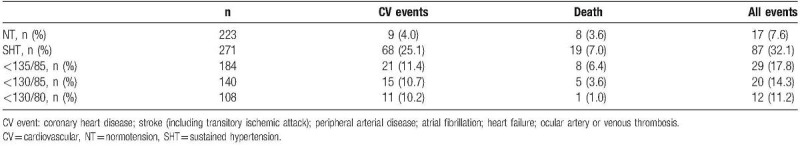
There were 35 deaths from all cause (5.1%). Table 3 shows the distribution of the 98 nonfatal CV events (14.4%) from the entire cohort throughout the follow-up. The 98 nonfatal CV events were distributed as follows: coronary heart disease, 54 events (7.9%); stroke (including transitory ischemic attack), 9 events (1.3%); peripheral arterial disease, 11 events (1.6%); atrial fibrillation, 9 events (1.3%); heart failure, 9 events (0.6%); and ocular arterial or venous thrombosis, 11 events (1.6%).
Table 3.
Cardiovascular events and death for normotension, sustained hypertension, and white-coat hypertension (for each SMBP cut-off).
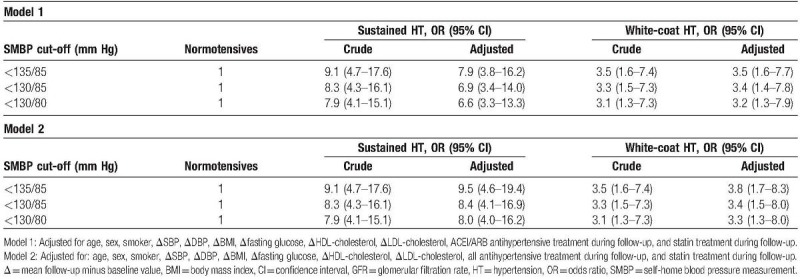
Patients with hypertension (SHT or WCH) had a significantly higher risk of CV disease and all-cause mortality than normotensive patients did (odds ratio [OR] 7.9, 95% confidence interval [CI] 3.8–16.2 for SHT; and OR 3.5, 95% CI 1.6–7.4 for WCH), even after adjusting for confounding variables such as differences in antihypertensive treatment or BP reduction. This higher risk was observed for all the WCH defining cut-off thresholds analysed. The CV risk of WCH is approximately half that of the SHT; however, both present a risk significantly higher than that of the normotensive ones (Table 4). The results are similar for the 2 adjustment models (adjusted for the use of ACEI/ARB or for all antihypertensive treatment).
Table 4.
Six years’ cardiovascular morbidity and total mortality according to SMBP cut-off at baseline.
When the risk of CV disease or all-cause mortality was analyzed based on its presence or absence at the TOD baseline, it was observed (Table 5 and Fig. 2) that in WCH patients with TOD, the risk is significantly higher than that of the normotensive patients (OR 4.5; 95% CI 1.9–10.6, P < .05) and lower to those with SHT (OR 7.9, 95% CI 3.8–16.2, P < .05). Only WCH defined by the SMBP cut-off threshold <130/80 mm Hg without TOD at baseline had a risk without significant differences in regard to the normotensive patients (OR 2.0, 95% CI 0.5–7.7, P = ns), whereas the higher SMBP cut-off points to define WCH (<130/85 and <135/85 mm Hg) showed a higher risk (OR 3.1, 95% CI 1.0–9.2, P < .05, and OR 2.5, 95% CI 0.9–6.9, P < .05, respectively). The results are similar for the 2 adjustment models (adjusted for the use of ACEI/ARB or for all antihypertensive treatment).
Table 5.
Six years’ cardiovascular morbidity and total mortality according to SMBP cut-off and target organ disease at baseline.
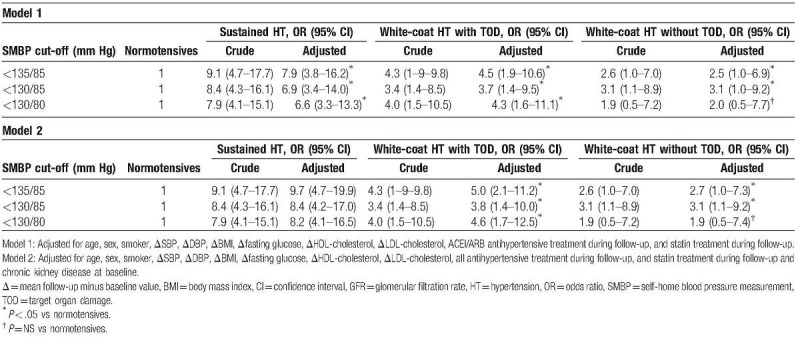
Figure 2.
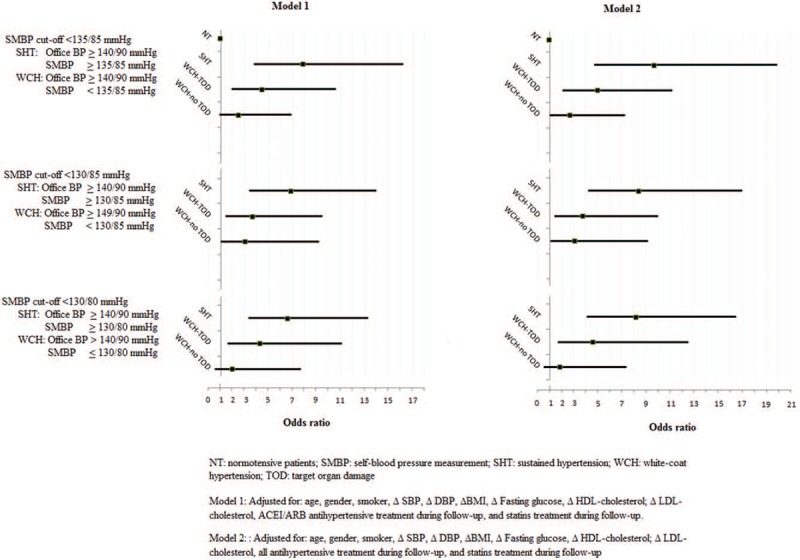
Cardiovascular risk and all-cause mortality according to SMBP cut-off threshold and target organ damage.
4. Discussion
The main results show that the presence of TOD in patients with WCH means the risk of experiencing a CV incident in 6 years and the risk of death is significantly higher than for normotensive patients, and that this risk is independent of the SMBP cut-off threshold used for the WCH diagnosis. There is no normal cut-off threshold for SMBP in which all-cause death and nonfatal CV events are different from those for patients with sustained hypertension when the presence of TOD has not been previously discarded. However, in patients with WCH without TOD at the time of inclusion, the risk of CV disease or death is not significantly superior to that of normotensive patients when the cut-off threshold for diagnosing this phenotype is <130/80 mm Hg. There is evidence that, at the time of diagnosis, patients with WCH have a higher prevalence of TOD in relation to normotensive patients and a prevalence of intermediate or similar to that of patients with SHT,[16,17] albeit that other studies find no differences.[18,19] It is possible that this is associated with an evolution towards established CV disease or death. If the presence of TOD is not evaluated during the diagnosis of WCH, the ensuing antihypertensive treatment may be less intensive and favor the evolution of the TOD to an established CV disease. It is likely that published results, which are discordant with the prognosis of WCH, are partly due to differences in the basal prevalence of TOD and in the treatment and monitoring of patients with WCH.
To date, it would appear that no research on the prognostic value of the WCH defined by SMBP has been carried out as all the studies that have been published are based on ambulatory BP monitoring. Typically, the WCH phenotype is considered a benign condition at mid-term.[20] In recent years, Verdecchia et al[21] showed that the long-term CV risk for WCH patients is mid-way between normotensive and SHT patients, or even identical to SHT patients, and concluded that WCH is not a benign condition. In a recent review, Mancia et al[22] showed that WCH is associated with an increased prevalence of metabolic risk factors, an increase in TOD, a higher percentage of type 2 diabetes and evolution to LVH, and, ultimately, an increase in CV morbidity and mortality. One of the reasons for this is the threshold cut-offs used to define WCH are not discriminatory enough between WCH and SHT. Indeed, the cut-off threshold to define normal SMBP has been the subject of research in recent years. The first study that proposed a threshold of normality for SMBP from total mortality data was the Ohasama study[23] that showed that the threshold of <137/84 mm Hg significantly distinguishes the incidence of total mortality in the cohort. The Pressione arteriosa monitorate e lloro associazoni study cohort[24] data not only show the SMBP <122.5/76 mm Hg cut-off threshold as being optimal for discriminating CV mortality, but also show that the <135/83 mm Hg cut-off point for SMBP has an independent prognostic value of CV mortality, even once adjusted for office or ambulatory BP monitoring. The short-term results from a cohort of patients with HT showed that patients with an SMBP <130/80 mm Hg have a significantly better evolution of TOD than those with values higher than this threshold.[9] In the same sense, the results from an international cohort[10] comprising the general population show that the normality of an SMBP cut-off threshold should be less than 130/85, which is different from what is currently proposed. In our study, the cut-off of normality for SMBP is similar to the study of the international cohort, albeit only for hypertensive patients who do not have TOD at baseline. It is likely that the results from the international cohort that find differences in risk according to the cut-off threshold for the entire sample are because the cohort is from a general population (hypertensive or not) with a lower prevalence of TOD. In this sense, a 130/80 mm Hg SMBP corresponds to an office BP measurement of 130/80 mm Hg in accordance with the recent American Heart Association document on high BP that recommends this threshold to define hypertension.[25]
The strengths of our study are as follows. First, the participating cohort consists of patients with hypertension who had not received any antihypertensive treatment at the time of inclusion. Second, a cohort of normotensive participants was followed as a reference. Third, in the follow-up data collected on BP, analysis, antihypertensive treatment, and other risk factors such as dyslipidemia could act as confusing variables. When performing studies on out-of-office BP measurements,[26,27] these very conditions have been indicated as being important.
This study also has some limitations. First, because of its multicenter character, the laboratory tests were carried out in 2 different places, which is likely to have influenced the results for some of the kidney function or lipids parameters. However, both laboratories involved are subject to ISO standards and use standardized techniques to determine creatinine. Second, although all the initial parameters have been arranged, at the end of the follow-up, the intermediate values are not available in some cases. This meant that exposure time to CV risk factors during the monitoring period could not be included in the analysis. Third, the primary endpoint composed of a broad spectrum of CV disease, with cardiac involvement (HF, atrial fibrillation), macrovascular disease (coronary heart disease, stroke/transitory ischemic attack/peripheral arterial disease), and microvascular disease (arterial or venous thrombosis of the retina). The limited number of events in each subgroup did not allow a stratified analysis for the different CV events. Fourth, although all patients were advised to adopt lifestyle changes, no data are available about the degree of adherence to them. However, some final variable changes related to lifestyle (weight, fasting glucose, cholesterol profile, and triglycerides) do not differ among the subgroups, so it can be assumed that the adherence of the lifestyle modifications was similar in all of them. Finally, decisions about the antihypertensive treatment were made based on the office BP measurements. Patients with WCH were treated in a similar percentage to those with SHT, although with fewer combinations of ACEI and ARB. The results could have been different if patients with WCH had not received treatment. However, the results have been adjusted for the use of renin-angiotensin system blockers and did not change significantly once the adjustment had been made.
The main results from our work show that the presence of TOD at the time of HT diagnosis is associated with an increased risk in all-cause mortality and CV disease, independent of the HT phenotype. In all the different SMBP cut-off thresholds evaluated, patients with WCH have lower risk as those with SHT, although this is much higher when compared with normotensive patients. There is no safe SMBP cut-off point for diagnosing WCH if the presence or absence of TOD is not evaluated as well. However, our results show that the best SMBP cut-off point for WCH without TOD is below 130/80 mm Hg. This threshold coincides with the 2010 ESH Practice Guidelines for home BP monitoring.[11] These patients have not differences in CV morbidity and total mortality in 6 years as NTs do. These data are in favor of the initial detection of TOD in patients with HT. Furthermore, the WCH phenotype is not benign if its presence has not been ruled out previously. Hypertensive patients without TOD at baseline and with an SMBP <130/80 mm Hg have a risk of death and/or CV disease at 6 years, which is not significantly superior to that of the normotensive patients. The results of this study are in favor of making decisions for patients with WCH based on the presence or absence of TOD and an SMBP cut-off of normality discretely lower than the one currently recommended. According to these results, we propose running a clinical trial to determine if untreated WCHs with an SMBP <130/80 mm Hg and without TOD, have a similar evolution when compared to those receiving treatment or to normotensive patients.
Acknowledgments
Our thanks to all the researchers from the 19 primary healthcare centers who carried out the fieldwork (see supplementary online list).
Author contributions
Conceptualization: Gabriel Coll-de-Tuero, Joan Bayó-Llibre, Carla Reyes-Negre.
Data curation: Marc Saez, Antonio Rodriguez-Poncelas, Joan Bayó-Llibre, Marta Beltran-Vilella, Antoni Dalfó-Baqué, Mª Antonia Barceló-Rado.
Formal analysis: Marc Saez, Mª Antonia Barceló-Rado.
Funding acquisition: Gabriel Coll-de-Tuero, Marc Saez, Joan Bayó-Llibre, Mª Antonia Barceló-Rado.
Investigation: Gabriel Coll-de-Tuero, Marc Saez.
Methodology: Gabriel Coll-de-Tuero.
Project administration: Gabriel Coll-de-Tuero.
Resources: Gabriel Coll-de-Tuero, Joan Bayó-Llibre, Marta Beltran-Vilella.
Software: Marc Saez.
Supervision: Antonio Rodriguez-Poncelas.
Validation: Mª Antonia Barceló-Rado.
Writing – original draft: Gabriel Coll-de-Tuero, Marc Saez, Carla Reyes-Negre.
Writing – review & editing: Marc Saez, Antonio Rodriguez-Poncelas, Joan Bayó-Llibre, Marta Beltran-Vilella, Antoni Dalfó-Baqué, Mª Antonia Barceló-Rado.
Gabriel Coll-de-Tuero orcid: 0000-0001-7578-9569.
Footnotes
Abbreviations: ACEI = angiotensin-converting enzyme inhibitor, ARB = angiotensin receptor blocker, BMI = body mass index, BP = blood pressure, CV = cardiovascular, ESH = European Society of Hypertension, GFR = glomerular filtration rate, HT = hypertension, LVH = left ventricular hypertrophy, NT = normotension, SHT = sustained hypertension, SMBP = self-monitoring blood pressure, TOD = target organ damage, UAER = urinary albumin excretion rate, WCH = white-coat hypertension.
Funding: This work was made possible through the grants from the the Instituto de Salud Carlos III, Spanish Ministry of Economy, Industry and Competitiveness (FIS PI03/0436, FIS PI07/0140, FIS PI11/01145), University of Girona (MPCUdG2016 and GdRCompetUdG2017), and a Catalan Academy of Medical Sciences (L’Acadèmia), (06/2018).
The authors report no conflicts of interest.
References
- [1].Pickering TG, Daichi Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med 2006;354:2368–74. [DOI] [PubMed] [Google Scholar]
- [2].Niiranen TJ, Hänninen MR, Johansson J, et al. Home measured blood pressure is a stronger predictor of cardiovascular risk than office blood pressure. Hypertension 2010;55:1346–50. [DOI] [PubMed] [Google Scholar]
- [3].de la Sierra A, Banegas JR, Segura J, et al. Ambulatory blood pressure monitoring and development of cardiovascular events in high-risk patients included in the Spanish ABPM registry: the CARDIORISC Event study. J Hypertens 2012;30:713–9. [DOI] [PubMed] [Google Scholar]
- [4].Patel B, Turban S, Anderson C, et al. A comparison of web sites used to manage and present home blood pressure readings. J Clin Hypertens (Greenwich) 2010;12:389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fukunaga H, Ohkubo T, Kobayashi M, et al. Cost-effectiveness of the introduction of home blood pressure measurement in patients with office hypertension. J Hypertens 2008;26:685–95. [DOI] [PubMed] [Google Scholar]
- [6].Pickering TG. Should white coat hypertension be treated? J Clin Hypertens (Greenwich) 2005;7:550–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].ESH/ESC Task Force for the Management of Arterial Hypertension. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens 2013;31:1925–1938. [DOI] [PubMed] [Google Scholar]
- [8].Parati G, Stergiou GS, Asmar R, et al. 2010 ESH practice guidelines for home BP monitoring. J Hum Hypertens 2010;24:779–85. [DOI] [PubMed] [Google Scholar]
- [9].Coll-de-Tuero G, Saez M, Roca-Saumell C, et al. Evolution of target organ damage by different values of self-blood pressure measurement in untreated hypertensive patients. Am J Hypertens 2012;25:1256–63. [DOI] [PubMed] [Google Scholar]
- [10].Niiranen TJ, Asayama K, Thijs L, et al. Outcome-Driven Thresholds for Home Blood Pressure Measurement. International Database of Home blood pressure in relation to Cardiovascular Outcome. Hypertension 2013;61:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Guidelines Committee. 2003 European Society of Hypertension–European Society of Cardiology guidelines for the management of arterial Hypertension. J Hypertens 2003;21:1011–1053. [DOI] [PubMed] [Google Scholar]
- [12].Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rue H, Martino S, Chopin N. Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations (with discussion). J R Stat Soc Series B 2009;71:319–92. [Google Scholar]
- [14].R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; 2017. Available at: https://www.R-project.org/ Accessed August 16, 2017. [Google Scholar]
- [15].R INLA project; 2017. Available at: http://www.r-inla.org/home Accessed August 16, 2017. [Google Scholar]
- [16].Julius S, Mejia A, Jones K, et al. White-coat” versus “sustained” borderline hypertension in Tecumseh, Michigan. Hypertension 1990;16:617–23. [DOI] [PubMed] [Google Scholar]
- [17].Palatini P, Mormino P, Santonastaso M, et al. Target-organ damage in stage I hypertensive subjects with white coat and sustained hypertension. Results from the HARVEST study. Hypertension 1998;31:57–63. [DOI] [PubMed] [Google Scholar]
- [18].Sega R, Trocino G, Lanzarotti A, et al. Alterations of cardiac structure in patients with isolated office, ambulatory, or home hypertension: Data from the general population (Pressione Arteriose Monitorate E Loro Associazioni [PAMELA] Study). Circulation 2001;104:1385–92. [DOI] [PubMed] [Google Scholar]
- [19].Kotsis V, Stabouli S, Toumanidis S, et al. Target organ damage in “white coat hypertension” and “masked hypertension”. Am J Hypertens 2008;21:393–9. [DOI] [PubMed] [Google Scholar]
- [20].Verdecchia P, Porcellati C, Schillaci G, et al. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension 1994;24:793–801. [DOI] [PubMed] [Google Scholar]
- [21].Verdecchia P, Reboldi P, Angeli F, et al. Short- and long-term incidence of stroke in white-coat hypertension. Hypertension 2005;45:203–8. [DOI] [PubMed] [Google Scholar]
- [22].Mancia G, Bombelli M, Cuspidi C, et al. Cardiovascular risk associated with white-coat hypertension: pro side of the argument. Hypertension 2017;70:668–75. [DOI] [PubMed] [Google Scholar]
- [23].Tsuji I, Imai Y, Nagai K, et al. Proposal of reference values for home blood pressure measurement: prognostic criteria based on a prospective observation of the general population in Ohasama, Japan. Am J Hypertens 1997;10:409–18. [PubMed] [Google Scholar]
- [24].Sega R, Facchetti R, Bombelli M, et al. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population. Follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation 2005;111:1777–83. [DOI] [PubMed] [Google Scholar]
- [25].ACC/AHA/AAPA/ABC/ACPM/AGS] /APhA /ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2017;71; e13–e115. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- [26].Mancia G, Facchetti R, Bombelli M, et al. Long-term risk of mortality associated with selective and combined elevation in office, home, and ambulatory blood pressure. Hypertension 2006;47:846–53. [DOI] [PubMed] [Google Scholar]
- [27].Verdecchia P, Angeli F, A. Staessen JA. Compared with whom? Addressing the prognostic value of ambulatory blood pressure categories. Hypertension 2006;47:820–1. [DOI] [PubMed] [Google Scholar]


