Abstract
Ischemic heart disease (IHD) is caused by the narrowing of arteries that work to provide blood, nutrients, and oxygen to the myocardial tissue. The worldwide epidemic of IHD urgently requires innovative treatments despite the significant advances in medical, interventional and surgical therapies for this disease. Angiogenesis is a physiological and pathophysiological process that initiates vascular growth from pre-existing blood vessels in response to a lack of oxygen. This process occurs naturally over time and has encouraged researchers and clinicians to investigate the outcomes of accelerating or enhancing this angiogenic response as an alternative IHD therapy. Therapeutic angiogenesis has been shown to revascularize ischemic heart tissue, reduce the progression of tissue infarction and evade the need for invasive surgical procedures or tissue/organ transplants. Several approaches, including the use of proteins, genes, stem/progenitor cells and various combinations, have been employed to promote angiogenesis. While clinical trials for these approaches are ongoing, microvesicles and exosomes have recently been investigated as a cell-free approach to stimulate angiogenesis and may circumvent limitations of using viable cells. This review summarizes the approaches to accomplish therapeutic angiogenesis for IHD by highlighting the advances and challenges that addresses the applicability of a potential pro-angiogenic medicine.
Introduction
Angiogenesis is a fundamental process in both physiology and pathophysiology. It is an essential mechanism in fetal development, reproduction, wound healing, and cancer growth and metastasis. The formation and development of new blood vessels is also a potentially important remedy to pathologically impaired or absent blood flow to and from vital organs and tissues. A functional vascular system is essential for the proper maintenance of tissues and organs by delivering oxygen and nutrients, removing waste, and trafficking the transport of immune cells. The interruption of adequate blood flow can have devastating consequences.
Insufficient blood flow to the myocardium (coronary insufficiency or myocardial ischemia) is a major public health problem and a leading cause of death worldwide; ischemic heart disease (IHD) kills over 370,000 people annually in the United States [1]. The primary pathophysiological cause of IHD is atherosclerosis, a gradual blockage in blood flow causing poor oxygenation of the heart tissue that is coupled with endothelial dysfunction and age-related decline in angiogenic response [2]. Coronary atherosclerosis causes blockage of the coronary arteries, leading to myocardial ischemia, myocardial infarction (MI), and ischemic cardiomyopathy. Current medical interventions for IHD include pharmacologic therapy (antiplatelet drugs, β-blockers, or statins) for disease stabilization and reduction of acute events (e.g., myocardial infarction or sudden death) or immediate restoration of the blood supply via surgical treatment, such as coronary artery bypass graft (CABG) surgery and percutaneous coronary intervention (PCI), two of the most common revascularization procedures performed worldwide [3, 4]. However, a significant number of patients are clinically refractory to medical therapy and are ineligible to receive either PCI or CABG due to the patient’s clinical condition or to technological limitations of currently available therapies [2, 4, 5]. As a result, the development of advanced and targeted pro-angiogenic strategies for high-risk patients is urgently needed. The proposed methods to combat IHD include the delivery of protein(s), gene(s), cell(s) or, more recently, extracellular vesicles to induce vessel growth (Figure 1). In this review, we discuss the current approaches to revascularization therapy using therapeutic angiogenesis, the advances in research, the caveats, and the potential in clinical applications.
Figure 1.
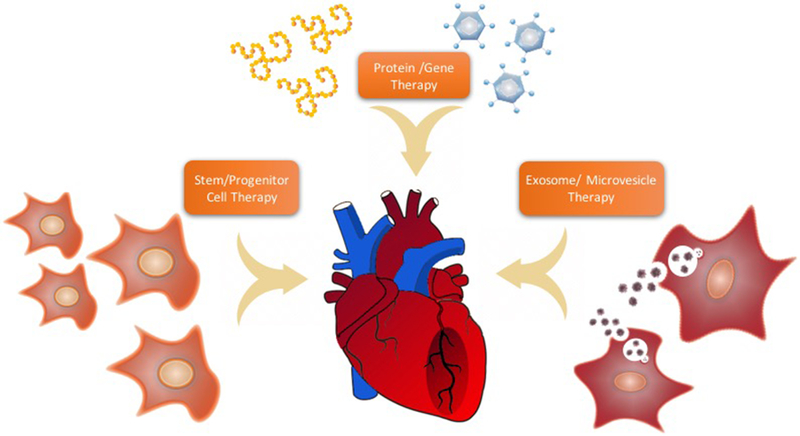
Proposed methods to combat ischemic heart disease (IHD)
Concept of Therapeutic Angiogenesis
Fundamentally, angiogenesis is the formation of new vasculature from pre-existing vessels. Therapeutic angiogenesis involves exogenously administering an agent that stimulates the postnatal growth of new blood vessels to restore circulation to the tissue. Angiogenesis can be induced in several human and animal models of inflammation [6], peripheral artery disease [7], and ischemic heart disease [8] for vascular restoration. Due to the complex nature of this process, the induction of angiogenesis to revascularize ischemic tissue must be extensively coordinated and controlled to prevent critical adverse events, such as uncontrolled angiogenesis and leaky and immature vessel formation. Normally by adulthood, blood vessels are maintained in a state of quiescence but will deviate from this state, especially in coronary atherosclerosis, when there is a reduction of oxygen to the tissue. Under normal conditions, capillaries are stabilized by “classic” factors (Notch1, angiopoietins, and thrombospondin) that are balanced by pro-angiogenic cytokines such as vascular endothelial growth factors (VEGF), fibroblast growth factors (FGF), and platelet-derived growth factor (PDGF) within the circulation [9]. The angiogenic mechanism, in physiological and pathological states, can be broken down into three essential steps: (1) activation, (2) proliferation, migration, and sprouting of existing endothelial cells (ECs) and (3) vessel stabilization by pericytes. Angiogenic cytokines can bind directly to receptors or indirectly initiate the activation on the ECs, activating downstream signaling pathways to develop new capillary-sized blood vessels [10]. Homeostasis is then re-established through the influence of stabilizing signals from surrounding ECs, pericytes, and stromal cells. It should be mentioned that the importance of pro-angiogenic factors is more clearly understood when they are genetically knocked down, which reliably produces embryonic lethality [11].
Vascular growth can be mediated by two additional processes: arteriogenesis and vasculogenesis. Arteriogenesis is the remodeling of collateral vessels to bypass a blockage to support distal portions of the limb or organ [12]. By producing larger vessels with an established tunica media, arteriogenesis can restore up to 30–40% of basal blood flow in critical stenosis, which is effective for tissue restoration and survival. However, this mechanism is reduced in activity due to aging and disease states [13]. Vasculogenesis is the de novo formation of vessels that establishes the primary vascular plexus. This is a hallmark mechanism that occurs when angioblasts accompanied by hematopoietic progenitor cells proliferate, migrate, and associate to form primitive vessels [14]. Although more prominently seen in early development, tissue ischemia can trigger postnatal vasculogenesis, which is mediated via the mobilization of endothelial progenitor cells (EPCs). These EPCs will then infiltrate the site of injury and can either differentiate into mature ECs or regulate preexisting ECs via paracrine/juxtacrine signaling [15], which makes EPCs a favorable candidate for therapeutic studies. Therefore, angiogenesis, arteriogenesis, and vasculogenesis are mechanisms that have been investigated as methods to stimulate therapeutic angiogenesis.
Protein/Gene Therapy
The use of proteins or genes to stimulate angiogenesis at the cellular level has been a well-established approach for researchers [16]. Protein therapy is performed by the intravenous, intra-arterial, intramuscular, or intramyocardial injection of a recombinant angiopeptide. The benefits of this method include a quantifiable biologic effect and the easy feasibility of reconstituting the purified protein in a buffer for an “off-the-shelf” treatment. To date, several agents can be administered to promote vascular growth. Among these factors, VEGF is the most important for the development and differentiation of the vascular network, with favorable preclinical evidence showing significantly increased perfusion, improved tissue metabolism, improved cardiac function, and cardiac protection [17]. However, the promising potential of such growth factors has not yielded much clinical success. The principal limitation of proteins is the short half-life of exogenous proteins in target tissue, which reduces the therapeutic benefit. Therefore, this method could be improved by the sustained expression of the angiopeptide.
In hopes of prolonging the effects of angiogenic cytokines, much attention has been directed to the stimulation of the major genetic regulators of angiogenesis. To do this, administration of a nonviral or viral vector delivery system allows for the consistent replication of the gene responsible for the angiopeptide. The most widely accepted nonviral system includes recombinant plasmid DNA, which suffers from low transfection efficiency, leading to minimal therapeutic benefits. Therefore, empty viral vectors, including adeno-, adeno-associated, and retroviruses, which can transduce postmitotic cells for long-lasting protein expression, represent a major advance in this field of research. Clinical trial evidence for the transfection efficacy of empty viral vectors was found in the Kuopio Angiogenesis Trial, which studied the intramyocardial injection of Ad-VEGF165 versus plasmid DNA-VEGF165 during percutaneous coronary angioplasty. The administration of Ad-VEGF165 allowed a higher degree of myocardial perfusion [18]. Recently, adeno-associated viral vectors encoding human VEGF-transduced pectineus muscular flaps increased the formation of new tissue through the induction of angiogenesis, which can be used as a strategy for heart tissue regeneration [19]. However, the use of viral vectors as a delivery method of angiopeptides also presents a risk of immune attack and still needs to be further optimized for dose, delivery routes, and administration. Clinical trials, such as the Randomized Evaluation of VEGF for Angiogenesis (REVASC) trial [20], Ripa, et al. [21], and the Angiogenic Gene Therapy (AGENT) trial [22], produced minimal results, which may be because virtually all clinical trials have been carried out as monotherapy and could be improved with combinatorial therapy.
Combination methods employing the concurrent use of two or more proteins, genes, or a combination of both protein and genes have been studied to serve as a more effective yet stable way to promote vascular growth. VEGF has had highly debatable success for therapeutic angiogenesis; therefore, Bouïs D et al. decided to use a combination plasmid encoding both VEGF and PDGF and revealed that it had inducing effects on endothelial migration and tube formation [23]. Additionally, implementing this paired approach, researchers observed that simultaneous delivery of FGF-2 and PDGF-β significantly improved the formation of collateral networks and blood perfusion in rat and rabbit ischemic hind limb models [24]. Combinations of VEGF and FGF [25], HGF and placental-derived growth factor (PlGF) [26], and bone morphogenetic proteins-2 with FGF-2/VEGF [27] as well as many others have provided convincing preclinical evidence for vascular growth and warrant future studies in clinical trials (Table 1). Future directions may include testing the efficacy and finding the optimal dosage of these growth factors in combination, which may lead to more clinically relevant outcomes.
Table 1:
Summary of Protein, Gene, and Combinational Therapy
| Therapy | Functional Role | Reference |
|---|---|---|
| VEGF | Stimulator of angiogenesis and lymphangiogenesis | [9] |
| HGF | Stimulates cell proliferation | |
| PlGF | Stimulates angiogenesis and regulates cellular proliferation | |
| Ad-VEGF | Kupio Angiogenesis Trial: Ad-VEGF165 increased myocardial perfusion compared to plasmid DNA-VEGF165 Randomized Evaluation of VEGF for Angiogenesis (REVASC): Exercise time to 1 mm ST-depression differed significantly at week 26 compared to control |
[18, 20] |
| AAV-VEGF | Enhanced tissue formation and significantly increased number of arterioles | [19] |
| Plasmid VEGF | Mobilization of progenitor cells from bone marrow were seen once G-CSF was administered in conjunction to plasmid VEGF165 | [21] |
| Ad-FGF4 | Angiogenic Gene Therapy (AGENT): Ad-FGF4 was administered and had an acceptable safety profile with a trend toward anti-ischemic effect | [22] |
| Plasmid VEGF + PDGF | Induced endothelial cell migration and tube formation | [23] |
| FGF-2 + PDGF-ββ | Stimulated collateral arteriogenesis, significant increase in vascularization and improvement in blood flow | [24] |
| VEGF-A + FGF-2 | Stimulates cell recruitment and formation of functional neovasculature in vivo | [25] |
| HGF + PlGF | Induced angiogenesis | [26] |
| BMP-2 + FGF + VEGF | Promoted angiogenesis with lower concentrations needed of each factor | [27] |
Stem/Progenitor Cell Therapy
In the last decade, stem cell transplantation to induce angiogenesis to repair the ischemic myocardium has emerged as an innovative alternative to traditional methods of gene or protein stimulation. This technique transplants viable cells into the myocardium to provide a regulated source of secreted growth factors and cytokines beneficial to the overall goal of reducing the amount of cardiomyocyte loss and improving the heart’s vascular network. Stem and progenitor cells possess beneficial qualities such as self-renewal, high differentiation capacity, colocalization with vessel components, and proliferation capability, making them ideal inducers or components of vascular growth. Stem and progenitor cells derived from various cell populations have shown therapeutic benefit in boosting angiogenesis as well as restoring ischemic heart tissue function. The various cell types seen in these types of investigational studies include the following: EPCs, bone marrow mononuclear cells (BM-MNCs), bone marrow mesenchymal stem cells (BM-MSCs), cardiac stem cells, adipose-derived stem cells (ASCs), induced pluripotent stem cells (iPSCs), and embryonic stem cells (ESCs). EPCs correspond to a subtype of stem cells that mobilize from the BM to circulation when stimulated by an angiogenic inducer. Once in circulation, they are recruited by the injured endothelium and differentiate into mature ECs. BM cells are multipotent and have the capacity to undergo two processes of differentiation: BM-MNCs and BM-MSCs. The former can form cells of hematopoietic cell origin such as blood cells. The latter, however, may produce highly specialized cells such as ASC and osteoblasts. BM-derived cell types are favorable candidates because they have the potential to improve heart function, differentiate into cardiomyocytes, or secrete angiogenic factors based on the vascular need. Cardiac stem cells are multipotent, self-renewing, and capable of differentiating into cardiomyocytes, smooth muscle cells, and ECs. Unfortunately, these cells are found at a very low density in the adult heart, which limits their use for clinical trials. iPSCs have been recently discovered as an autologous cell source that can circumvent the shortage of stem cell quantities in adults. These cells are reprogrammed adult cells mainly using the lentiviral vector transduction of the Oct-4, Sox2, C-Myc and Klf4 transcription factors. ESCs are pluripotent stem cells that are derived from the inner cell mass of the blastocyst. There is limited translational relevance of ESCs due to the ethical concerns arising from embryo destruction. To date, most clinical trials have focused on the use of BM-MSCs for their therapeutic potential. MSCs are multipotent stromal cells that have the capability to differentiate into various adult cell types. Unlike other adult stem cells, they appear to escape allorecognition by the immune system. This characteristic makes them useful for many preclinical models for cardiac repair and therapeutic angiogenesis [28, 29]. Furthermore, BM-derived stem cells have been pushed into early-phase clinical trials [30]. A pilot study of cell-based therapies was introduced by Asahara et al., who observed that EPCs, isolated from human peripheral blood, contributed to vessel growth in ischemic tissues. This angiogenic potential has been replicated in clinical studies using cardiac progenitor cells [31, 32] and ASCs [33]. However, excitement about EPCs and their phenotype, origin, and mechanism has been dampened because of unresolved questions, despite results demonstrating improved neovascularization in animal models of ischemia [34]. It is widely accepted that current EPC nomenclature is describes two distinct subpopulations: endothelial colony forming cells and myeloid angiogenic cells [35]. Thus, the specific phenotype, mechanism, and biological function provides a clear indication of the way they accomplish angiogenesis. Currently, there are several Phase III/IV clinical trials using a variety of different cell types that display the clinically relevant outcomes using cytotherapy based on the improvement of cardiac function (Table 2). Adult BM cells were examined by meta-analysis from patients who suffered from IHD and this systematic review concluded that these cells had positive and considerable therapeutic benefits [36]. In a randomized study, BM-MSCs cells were tested for safety in patients who have had an acute myocardial infarction. The study revealed that there was no treatment-related toxicity during the intracoronary administration of the cells [37]. Similarly, intramyocardial injection of autologous BM-MNCs were evaluated for the long-term effect of this treatment plan. The results from that study, which were similar to others [38], indicated that intramyocardial transplantation was safe and improved survival and clinical symptoms with chronic ischemic disease [39]. The intracoronary administration of BM-derived cells at 4 months showed significant improvement in left ventricular ejection fraction and after 1 year was associated with a reduction in myocardial infarction and clinical endpoint of death [40]. The CD133+ BM-derived cell population has been of interest due to their clinical involvement in restoring myocardial tissue viability and induction of angiogenesis [41]. Results from the Cardio133 trial, intramyocardial transplantation of CD133+ BM-derived stem cells in patients with chronic ischemic heart disease showed minimal improvements in scar size and regional perfusion [42]. CD34+ cells have also been investigated for their therapeutic benefits for IHD. In a phase III, randomized, double-blinded study called the RENEW study, intramyocardial injection of CD34+ cells improved angina frequency and exercise tolerance [43]. Moreover, EPC-capturing stents have also been utilized to counter the angiographic outcomes in percutaneous coronary intervention. The number of circulating EPCs were increased by 2-fold [44]. Along with the success, there are still some caveats that overwhelm this type of therapeutic angiogenesis, which includes the development of robust approaches for cell characterization, uniform isolation and/or maintenance, survival, prevention of microembolism, and optimal delivery strategies. It is estimated that 40–75% of injected cells will succumb to cell death within the first three days [45], especially in cases where multiple cell types will be replaced and restored because long-term functional integration remains a challenge for effective clinical improvement [46]. Currently, noninvasive ultrasound targeted microbubble destruction technology aims to improve the efficacy of transplanted cells [47]. Additionally, cell sheets—an attached mono- or multilayer xenon-free graft that is made of viable cells with their appropriate extracellular matrix—may also circumvent poor survival of cells for therapeutic interventions [48]. The use of iPSC-derived cells, although not currently used in clinical trials for the treatment of IHD, have shown positive preclinical evidence. These fully reprogrammed adult cells can be differentiated to a bona fide cell type, typically by a differentiation cocktail expressing factors for the specialized cell type. Moreover, iPSC-derived cells have been shown to rescue tissues from ischemia by the induction of angiogenesis [49]. The oncogenic cocktail of transcription factors continues to invite much controversy. The epigenetic memory of iPSC-derived cells makes them high-risk for tumorigenic activity and is often a testable measure that occurs when demonstrating the regenerative ability of these cells. Alternatively, induced vascular progenitor cells (iVPCs), which are partially reprogrammed vascular ECs, have been proposed to counter iPSC usage. IVPCs have been shown to enhance coronary collateral flow in a repetitive ischemia rat model and do not present a risk of tumorigenesis, which was confirmed in a 47-day tissue harvest study of injected iVPCs and iPSCs [50]. These cells present higher promise for vascular tissue engineering using cell-based approaches for cardiac regeneration and ischemic rescue. In conclusion, optimizing cell sources is a critical factor for the clinical success of this approach.
Table 2:
Outcomes of Phase III/IV Cell-based Therapy Clinical Trials for Ischemic Heart Disease
| Cell Type | Cell Qty. | Delivery Route | # of patients/follow-up | Outcomes | Trial | Ref. |
|---|---|---|---|---|---|---|
| BM-derived MSCs | 1 × 106 cells/kg |
IC | 80 patients; 6 months | Improved LVEF, no reported treatment-related toxicity or adverse CVD events | NCT01392105 | [38] |
| BM-derived Stem Cells | --- | IC | 68 patients; 6 months | Improved diastolic function | NCT00363324 | [39] |
| BM Mononuclear Cells | 41 ± 16 × 106 cells | IM | 250 patients; 12 months | Improved CCSAC, LVEF, stress score, and survival | NCT00841958 | [40] |
| BM-derived Progenitor Cells | 236 ± 174 × 106 cells | IC | 204 patients; 4,12, 60 months | Improved LVEF, maximal vascular conductance capacity, and reduced ventricular remodeling and occurrence of major adverse CVD events | NCT00279175 | [41] |
| BM-derived CD133+ and Mononuclear Cells | --- | IM |
90 patients; 6 months | Improved myocardial viability, cardiac function, and perfusion of infarcted myocardium | NCT01167751 | [42] |
| BM-derived CD133+ Cells | 1.5 × 106 cells | IM | 60 patients; 6 months | Feasible and safe, Improved LVEF and perfusion | NCT00462774 | [43] |
| CD34+ Cells | 1 × 105 cells/kg | IM | 291 patients; 12 months | Improved angina frequency and exercise tolerance | NCT01508910 | [44] |
| Endocardial Progenitor Cells | EPC – capture stents | --- | 60 patients; 6 and 12 months | Increased number and mobilization of circulating EPCs and lower restenosis rate | NCT00494247 | [45] |
Combinational Protein/Gene and Stem/Progenitor Cell Therapy
The combinational method using proteins, genes, and cells has been investigated to enhance the effects observed with monotherapy strategies. The coinjection of BM-MSCs with adeno-associated viral vector of VEGF into the ischemic myocardium was shown to increase the cardiac function and survival of transplanted cells [51]. Moreover, MSCs transfected with adenoviral vector encoding hypoxia inducible factor-α (HIF-1α) and co-transplantation of MSCs and adenoviral vector expressing HIF-1α were both studied after intramyocardial injection in a rat myocardial infarction model and showed significantly improved cardiac function in the peri-infarcted region [52]. The use of this technique has encouraged researchers to investigate dual therapy to further enhance results for cardiac regeneration and improvement post ischemic attack. Before transplantation into IHD patients, interventional cells can be exposed to hypoxia to mimic the ischemic environment in the host which causes the upregulation of pro-angiogenic and pro-survival factors and therefore equips the cells to be resistant to cell death under the deprived conditions. Additionally, the dual use of stem cells and proteins has had clinical success. Duan et al. showed that treatment of myocardial ischemia with BM-MSCs overexpressing hepatocyte growth factor increased capillary density and reduced the area of ischemia [53]. Moreover, the combination of cardiovascular disease pharmaceutics with additional growth factors has also been recognized as an alternative strategy for cardiovascular improvement. Statin and stromal cell-derived factor-1 additively promoted angiogenesis by enhancing progenitor cell mobilization and incorporation into new vessels in ischemic hindlimb mouse models [54]. New techniques to enhance gene transfection have been a critical goal for cell-based therapies. Nonviral-based gene modification of adult stem cells is currently being developed to evade the immune response against bacterial proteins. Minicircle plasmid DNA technology uses supercoiled DNA molecules for nonviral gene transfer. Bandara et al. used this technique to transfer endothelial nitric oxide synthase into BM-MSCs, which enhanced the angiogenic response [55]. The combinational method may prove to enhance biological responses, which can translate to beneficial clinical outcomes.
Microvesicle/Exosome Therapy
Studies have proposed that the beneficial properties of transplanted stem cells are mainly carried out by paracrine effects rather than cell differentiation. Therefore, many researchers are interested in the secreted factors released from transplanted cells [56]. In addition to growth factors and chemokines, the exploratory field of extracellular vesicles (EVs) is quickly gaining much attention as a therapeutic strategy for pro-angiogenic treatment. EVs are widely used to refer to the heterogeneous classes of small membrane-enclosed vesicles, which include exosomes, microvesicles, ectosomes, budding vesicles, shedding vesicles and apoptotic bodies [57]. These vesicles are released from the surface of different cell types and can be isolated from bodily fluids such as milk, sweat, semen and urine [58]. Exosomes are double-membraned nanovesicles that range 30–100 nm in diameter that can transfer lipids, proteins, mRNA, and microRNAs that have regulatory effects on the genetic and epigenetic processes in recipient cells [59–61]. Microvesicles (MVs) originate from the budding of the plasma membrane and are characteristically larger than exosomes [62, 63]. For this review, discussion will be limited to exosomes and MVs, and it will be specific regarding the nomenclature and function mentioned in the cited literature.
The intracellular exchange of exosomes has been shown to stimulate pro-angiogenic, proliferative, anti-apoptotic and anti-inflammatory signaling cascades in cardiovascular disease [64]. MicroRNAs retain strong stability and express tissue-specific patterns and are enriched in exosomes to induce cardiac repair and the reduction of fibrosis in ischemic myocardium models [65]. Our research has also reported that the transfer of miRNA-31 encapsulated in MVs from human ASCs promotes vascular EC tube formation and migration which are indicative of an angiogenic response [66]. Moreover, cells that participate in cardiac tissue repair are of interest as cell source candidates to isolate extracellular vesicles from. Epicardial epithelial to mesenchymal transition (EMT), is a key step in heart development that occurs at a higher rate following myocardial infarction. In a study by Foglio et al., exosomes released into the pericardial fluid by epicardial cells induced epicardial EMT arteriogenesis, and reduced cardiomyocyte apoptosis [67]. Using a proteomic approach, they were able to identify clusterin, a heterodimeric secreted glycoprotein of 75 kDa, as a key component encapsulated within pericardial fluid exosomes. Similarly, exosomes isolated from human pericardial fluid have also been shown to be enriched with cardiovascular-expressed microRNAs and promote vessel formation [68]. Thus, the exchange of protein and microRNAs within exosomes has been a method to explore the mechanistic approach that occurs once exosomes are internalized into recipient cells [69]. Overall, exosomes from MSCs [70–75], cardiac progenitor cells [76–80], embryonic stem cells [81, 82], and human pericardial fluid [67] have all been shown to improve cardiac function by increasing capillary density, among other factors (Table 3). Cell-derived exosomes also evade limitations of using cell-based therapies, home to specific tissue, and serve as a novel drug delivery mechanism, with their bilipid membranes aiding in the protection of biologically active cargo allowing a longer half-life in patients [83].
Table 3:
The Therapeutic Angiogenic Effects of Microvesicles/Exosomes in Myocardial Infarction Animal Models
| Exosome Donor Cell | Exosome Recipient Species | Delivery Route | Outcome | Ref. |
|---|---|---|---|---|
| Embryonic Stem Cells | Mouse | IM | Improved LV contractility and function, increased neovascularization, myocyte proliferation and survival post MI | [64] |
| Cardiac Progenitor Cells | Rat | IM | Reduced fibrosis and hypertrophy, increased vascularization and cardiomyocyte proliferation | [66] |
| Pericardial Fluid | Mouse | IM | increased protective effect on cardiomyocytes, number of epicardial cells, arteriolar length density, improved cardiac function and lowered apoptotic rates in the peri-infarct heart | [68] |
| CXCR4-Modified Mesenchymal Stem Cells | Rat | IM | Reduced infarct size and fibrosis and increased angiogenesis | [71] |
| Akt-Modified Mesenchymal Stem Cells | Rat | TV | Improved cardiac function and increased blood vessel formation | [72] |
| Mesenchymal Stem Cells | Rat | IM | Reduced infarct size, enhanced blood flow recovery, and improved cardiac systolic/diastolic performance by increasing angiogenesis | [73] |
| Bone Marrow Mesenchymal Stem Cells | Rat | IM | Reduced infarct size, preserved cardiac performance, and increased capillary density | [74] |
| Mesenchymal Stem Cells | Rat | IM | Increased endothelial cell differentiation, neovascularization, and reduced fibrosis | [75] |
| Mesenchymal Stem Cells | Mouse | IM | Increased angiogenesis and reduced cardiac fibrosis | [76] |
| Cardiac Progenitor Cells | Rat | IM | Improved LVEF, Increased cardiomyocyte survival and angiogenesis | [77] |
| Cardiosphere-derived Cells | Rat | IM | Reduced infarct size, and Increased global pump function, VEGF and SDF1 secretion and vessel density | [78] |
| Cardiac Progenitor Cells | Rat | IM | Improved cardiac function by increasing angiogenesis, and reduced fibrosis | [79] |
| Cardiosphere-derived Cells | Porcine | IM | Reduced infarct size, fibrosis, and increased vessel density | [80] |
| Embryonic Stem Cell-derived Cardiovascular Progenitors | Mouse | PCI | Reduced LVESV and EDV, infarct size, and improved cardiac function | [81] |
Scientists have recognized exosomes as natural nanocarriers for use as advanced drug delivery systems. Recently, hybrid exosomes have been engineered by fusing their membranes with liposomes. Liposomes are vesicles with a simple lipid bilayer typically conjugated to polyethylene glycol to aid in immune invasion. The change in lipid composition with the concurrent properties already contained within the exosomes had increased cellular uptake of exosomes [84]. One of the most widely accepted approaches for therapeutic agent loading involves transfecting exosome-producing cells to overexpress angiogenic genes [85]. Direct loading of isolated exosomes by incubating them with the genetic material of interest has also been reported [86]. Additionally, transfecting cells with an expression construct encoding a fusion protein containing the ligand of interest attached to an endogenous exosome membrane protein, such as MFGE8 and LAMP2B, has also been used to aid in exosome targeting [87, 88]. Furthermore, pretreatment of cells with activators to augment cell recruitment and survival in the ischemic target area and/or the improvement of cell functions, such as their paracrine ability to release proangiogenic factors and vasoactive molecules, has also been rigorously investigated. By ischemic preconditioning of donor cells, it has been shown to be a powerful approach to enhance cell survival [89] and regeneration of stem cells [90]. Several studies have shown an increased release of EVs in response to hypoxia as well as acidosis and oxidative stress [91]. Moreover, one study found that when glioblastoma multiforme cells were grown in hypoxic compared to normoxic environments, these vesicles were potent inducers of angiogenesis ex vivo and in vitro [92]. Cell-preconditioning has an additive effect by promoting survival factors and inducing angiogenesis, which may prove beneficial for IHD.
Mechanistic studies still warrant further investigation, and functional studies of EVs in human biological fluids are still lacking. Such studies are important to characterize the relevance of exosome-based communication in human pathophysiology. Standardization of exosome and microvesicle isolation techniques are required to establish retention of the pure vesicles desired; to date, there are various isolation methods that complicate using this therapy [93]. Additionally, due to its novelty within the literature, this approach has not been moved to clinical trials as a pro-angiogenic. However, clinical trials using exosome administration in humans has been tested for cancer immunotherapy, the safety of the treatment has been established [94], and there are several completed registered NIH clinical trials using exosomes as diagnostic agents [95].
Engineered Exosome Therapy
Another critical finding in the field has shown that cells senesce with age, resulting in a decline of their ability to release exosomes [96]. To ensure stable production of exosomes, synthesizing artificial vesicles is a way to bypass this caveat. Synthetic exosomes can be prepared from amphiphilic linear block copolymers. There are several methods to produce these vesicles, including solvent exchange, film rehydration, electroformation, and double emulsion methods. Artificial exosomes covalently attach with HSP70 peptides and could inhibit the death of cardiomyocytes induced by hypoxia [97]. Engineered exosomes with antibodies, peptides, or carbohydrates have been used to aid in the targeting of specific tissues or cells. This method could decrease the minimum concentration of artificial exosome dose and reduce adverse effects. Moreover, synthetic exosomes or nanoparticles have emerged as a groundbreaking development for fast diagnosis, molecule delivery, and tissue engineering. Nanoparticles range in diameter from 1–100 nm and are artificial replicas of the exosomal phenotype. Nanoparticles can be directly loaded to contain pro-angiogenic peptides or can be used as nonviral gene vectors to establish genetically engineered stem cells for in vivo cardiac repair [98]. Nanoparticles can be synthesized by two general approaches: top-down or bottom-up. Top-down approaches typically involve imposing a structure on a flat surface and adding or removing thin layers of materials. The bottom-up approach uses molecular chemistry to form nanostructures. Overall, size plays an important role and can be advantageous to medicine, as nanoparticles can cross the blood–brain barrier. However, nanoparticles are still subjected to immunosurveillance, which can lead to their degradation or clearance from the bloodstream. In conclusion, engineered exosomes could be a promising method to induce therapeutic angiogenesis and allow for mass production without the need for immortalized cells.
Summary and Conclusion
Overall, protein, gene, cell, and exosome/microvesicle therapies have all contributed to the forward progress of pro-angiogenic treatment moving further down the pipeline to cardiovascular therapeutics. The vehicle that will stimulate the therapeutic angiogenic effect is of major importance to this field. Initially, protein and gene therapy approaches were implemented due to their vast foundational knowledge and the ease of being able to pharmaceutically insert these factors into medicinal constructs, whereupon administration allows for relatively easy testing to see if there is any clinical value. However, the results observed in preclinical studies are not the same as those seen in Phase III clinical studies [99]. In that regard, gene/protein therapy is still being observed as a potentially wise choice and is feasible for off-the-shelf availability. The use of stem/progenitor cells from various sources is attractive due to isolation efficiency and in vivo success. However, there is a need to investigate the optimal cell source and regulation techniques to ensure cell viability, differentiation, localization to target sites and a decreased risk of tumorigenesis. The use of multiple proteins/genes or proteins/genes with concurrent use of stem/progenitor cells are useful alternatives to monotherapy. In this way, it targets two hurdles: (1) efficacy in integration into host vessels and (2) aiding in cell survival and transplantation, thus emulating a more comprehensive therapeutic angiogenesis model. Moreover, the use of exosomes/microvesicles, the most recent of the three approaches, is a very striking method relative to conventional methods. These small microparticles have the advantage of their size and contain a content that can elicit a response from the target cells. This method evades the limitations of the other approaches but must be studied vigorously before clinical trials are initiated.
Funding Information:
This work was supported, in whole or in part, by NIH grants SC1HL134212 and P50HL117929 to D.L.
Abbreviations:
- AMI
acute myocardial infarction
- ASC
adipose-derived stem cell
- BM
bone marrow
- CABG
coronary artery bypass grafting
- CCSAC
Canadian Cardiovascular Society Angina Classification
- EC
endothelial cells
- EDV
end diastolic volume
- EMT
epithelial to mesenchymal transition
- FGF
fibroblast growth factor
- EPC
endothelial progenitor cell
- EV
extracellular vesicles
- HIF-1α
hypoxia inducible factor-1α
- IC
intracoronary infusion
- IHD
ischemic heart disease
- IM
intramyocardial injection
- IPSC
induced pluripotent stem cell
- IVPC
induced vascular progenitor cell
- LVEF
left ventricle ejection fraction
- LVESV
left ventricle end systolic volume
- MSC
mesenchymal stem cells
- MV
microvesicle
- PCI
percutaneous injection
- PlGF
placental-derived growth factor
- PDGF
platelet-derived growth factor
- SDF1
stromal cell-derived factor 1
- TV
tail vein
- VEGF
vascular endothelial growth factor
Footnotes
Conflicts of Interest
None
Contributor Information
Takerra Johnson, Morehouse School of Medicine, Cardiovascular Research Institute, 720 Westview Drive SW, Atlanta, Georgia 30310, USA, tjohnson@msm.edu.
Lina Zhao, Morehouse School of Medicine, Cardiovascular Research Institute, 720 Westview Drive SW, Atlanta, Georgia 30310, USA, lzhao@msm.edu.
Gygeria Manuel, Spelman College, Department of Biochemistry, 350 Spelman Lane, Atlanta, Georgia 30314, USA, gmanuel@scmail.spelman.edu.
Herman Taylor, Morehouse School of Medicine, Cardiovascular Research Institute, 720 Westview Drive SW, Atlanta, Georgia 30310, USA, htaylor@msm.edu.
Dong Liu, Morehouse School of Medicine, Cardiovascular Research Institute, Department of Physiology, 720 Westview Drive SW, Atlanta, Georgia 30310, USA, dliu@msm.edu.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, et al. (2017) Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 135: e146–e603. DOI 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dragneva G, Korpisalo P, Yla-Herttuala S (2013) Promoting blood vessel growth in ischemic diseases: challenges in translating preclinical potential into clinical success. Dis Model Mech 6: 312–322. DOI 10.1242/dmm.010413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X-L, Zhu Q-Q, Yang J-J, Chen Y-H, Li Y, Zhu S-H, Xie J, Wang L, Kang L-N, Xu B (2017) Percutaneous intervention versus coronary artery bypass graft surgery in left main coronary artery stenosis: a systematic review and meta-analysis. BMC Medicine 15: 84 DOI 10.1186/s12916-017-0853-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez AE, Pavlovsky H, Del Pozo JF (2016) Understanding the Outcome of Randomized Trials with Drug-Eluting Stents and Coronary Artery Bypass Graft in Patients with Multivessel Disease: A Review of a 25-Year Journey. Clinical Medicine Insights Cardiology 10: 195–199. DOI 10.4137/CMC.S40645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Stahle E, Feldman TE, van den Brand M, Bass EJ, et al. (2009) Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 360: 961–972. DOI 10.1056/NEJMoa0804626 [DOI] [PubMed] [Google Scholar]
- 6.Folkman J, Shing Y (1992) Angiogenesis. Journal of Biological Chemistry 267: 10931–10934 [PubMed] [Google Scholar]
- 7.Shimamura M, Nakagami H, Taniyama Y, Morishita R (2014) Gene therapy for peripheral arterial disease. Expert Opinion on Biological Therapy 14: 1175–1184. DOI 10.1517/14712598.2014.912272 [DOI] [PubMed] [Google Scholar]
- 8.Gibson CM, Ryan K, Sparano A, Moynihan JL, Rizzo M, Kelley M, Marble SJ, Laham R, Simons M, McClusky TR, et al. (1999) Angiographic methods to assess human coronary angiogenesis. American Heart Journal 137: 169–179. DOI 10.1016/s0002-8703(99)70473-4 [DOI] [PubMed] [Google Scholar]
- 9.Ribatti D, Conconi MT, Nussdorfer GG (2007) Nonclassic Endogenous Novel Regulators of Angiogenesis. Pharmacological Reviews 59: 185–205. DOI 10.1124/pr.59.2.3 [DOI] [PubMed] [Google Scholar]
- 10.Conway EM, Collen D, Carmeliet P (2001) Molecular mechanisms of blood vessel growth. Cardiovascular Research 49: 507 DOI 10.1016/S0008-6363(00)00281-9 [DOI] [PubMed] [Google Scholar]
- 11.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, et al. (1996) Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380: 435–439. DOI 10.1038/380435a0 [DOI] [PubMed] [Google Scholar]
- 12.Persson AB, Buschmann IR (2011) Vascular Growth in Health and Disease. Front Mol Neurosci 4: 14 DOI 10.3389/fnmol.2011.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meier P, Seiler C (2014) The coronary collateral circulation--past, present and future. Curr Cardiol Rev 10: 1 DOI 10.2174/1573403X113099990004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel-Hett S, D’Amore PA (2011) Signal transduction in vasculogenesis and developmental angiogenesis. Int J Dev Biol 55: 353–363. DOI 10.1387/ijdb.103213sp [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balaji S, King A, Crombleholme TM, Keswani SG (2013) The Role of Endothelial Progenitor Cells in Postnatal Vasculogenesis: Implications for Therapeutic Neovascularization and Wound Healing. Adv Wound Care (New Rochelle) 2: 283–295. DOI 10.1089/wound.2012.0398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rissanen TT, Yla-Herttuala S (2007) Current Status of Cardiovascular Gene Therapy. Mol Ther 15: 1233–1247. DOI 10.1038/sj.mt.6300175 [DOI] [PubMed] [Google Scholar]
- 17.Zhu H, Jiang X, Li X, Hu M, Wan W, Wen Y, He Y, Zheng X (2016) Intramyocardial delivery of VEGF165 via a novel biodegradable hydrogel induces angiogenesis and improves cardiac function after rat myocardial infarction. Heart and Vessels 31: 963–975. DOI 10.1007/s00380-015-0710-0 [DOI] [PubMed] [Google Scholar]
- 18.Hedman M, Hartikainen J, Syvanne M, Stjernvall J, Hedman A, Kivela A, Vanninen E, Mussalo H, Kauppila E, Simula S, et al. (2003) Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT). Circulation 107: 2677–2683. DOI 10.1161/01.CIR.0000070540.80780.92 [DOI] [PubMed] [Google Scholar]
- 19.Moimas S, Manasseri B, Cuccia G, Stagno d’Alcontres F, Geuna S, Pattarini L, Zentilin L, Giacca M, Colonna MR (2015) AAV vector encoding human VEGF165-transduced pectineus muscular flaps increase the formation of new tissue through induction of angiogenesis in an in vivo chamber for tissue engineering: A technique to enhance tissue and vessels in microsurgically engineered tissue. J Tissue Eng 6: 2041731415611717 DOI 10.1177/2041731415611717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart DJ, Hilton JD, Arnold JMO, Gregoire J, Rivard A, Archer SL, Charbonneau F, Cohen E, Curtis M, Buller CE, et al. (2006) Angiogenic gene therapy in patients with nonrevascularizable ischemic heart disease: a phase 2 randomized, controlled trial of AdVEGF121 (AdVEGF121) versus maximum medical treatment. Gene Ther 13: 1503–1511. DOI 10.1038/sj.gt.3302802 [DOI] [PubMed] [Google Scholar]
- 21.Ripa RS, Wang Y, Jorgensen E, Johnsen HE, Hesse B, Kastrup J (2006) Intramyocardial injection of vascular endothelial growth factor-A165 plasmid followed by granulocyte-colony stimulating factor to induce angiogenesis in patients with severe chronic ischaemic heart disease. Eur Heart J 27: 1785–1792. DOI 10.1093/eurheartj/ehl117 [DOI] [PubMed] [Google Scholar]
- 22.Grines CL, Watkins MW, Mahmarian JJ, Iskandrian AE, Rade JJ, Marrott P, Pratt C, Kleiman N (2003) A randomized, double-blind, placebo-controlled trial of Ad5FGF-4 gene therapy and its effect on myocardial perfusion in patients with stable angina. Journal of the American College of Cardiology 42: 1339–1347. DOI 10.1016/S0735-1097(03)00988-4 [DOI] [PubMed] [Google Scholar]
- 23.Bellu AR, Rotz MG, Bouis D, Mulder NH, Dam W, Weel Vv, Koolwijk P, Quax PHA, Haisma HJ, Hosper GAP (2004) 153. VEGF associated with TP to refine angiogenesis in gene therapy. Mol Ther 9: S58–S59. DOI 10.1016/j.ymthe.2004.06.076 [DOI] [Google Scholar]
- 24.Cao R, Brakenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E, Leboulch P, Cao Y (2003) Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med 9: 604–613. DOI 10.1038/nm848 [DOI] [PubMed] [Google Scholar]
- 25.Kano MR, Morishita Y, Iwata C, Iwasaka S, Watabe T, Ouchi Y, Miyazono K, Miyazawa K (2005) VEGF-A and FGF-2 synergistically promote neoangiogenesis through enhancement of endogenous PDGF-B-PDGFRbeta signaling. J Cell Sci 118: 3759–3768. DOI 10.1242/jcs.02483 [DOI] [PubMed] [Google Scholar]
- 26.Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, Nagy JA, Hooper A, Priller J, De Klerck B, et al. (2002) Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med 8: 831–840. DOI 10.1038/nm731 [DOI] [PubMed] [Google Scholar]
- 27.Bai Y, Leng Y, Yin G, Pu X, Huang Z, Liao X, Chen X, Yao Y (2014) Effects of combinations of BMP-2 with FGF-2 and/or VEGF on HUVECs angiogenesis in vitro and CAM angiogenesis in vivo. Cell and Tissue Research 356: 109–121. DOI 10.1007/s00441-013-1781-9 [DOI] [PubMed] [Google Scholar]
- 28.Boyle AJ, McNiece IK, Hare JM (2010) Mesenchymal stem cell therapy for cardiac repair. Methods Mol Biol 660: 65–84. DOI 10.1007/978-1-60761-705-1_5 [DOI] [PubMed] [Google Scholar]
- 29.Lee EJ, Park HW, Jeon HJ, Kim HS, Chang MS (2013) Potentiated therapeutic angiogenesis by primed human mesenchymal stem cells in a mouse model of hindlimb ischemia. Regenerative Med 8: 283–293. DOI 10.2217/rme.13.17 [DOI] [PubMed] [Google Scholar]
- 30.Fisher SA, Brunskill SJ, Doree C, Mathur A, Taggart DP, Martin-Rendon E (2014) Stem cell therapy for chronic ischaemic heart disease and congestive heart failure. Cochrane Database Syst Rev: CD007888. DOI 10.1002/14651858.CD007888.pub2 [DOI] [PubMed] [Google Scholar]
- 31.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM (1997) Isolation of Putative Progenitor Endothelial Cells for Angiogenesis. Science 275: 964 DOI 10.1126/science.275.5302.964 [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Tian S, Lei I, Liu L, Ma P, Wang Z (2017) Transplantation of multipotent Isl1+ cardiac progenitor cells preserves infarcted heart function in mice. American Journal of Translational Research 9: 1530–1542. DOI 10.1186/s13287-017-0675-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao L, Johnson T, Liu D (2017) Therapeutic angiogenesis of adipose-derived stem cells for ischemic diseases. Stem Cell Research & Therapy 8: 125 DOI 10.1186/s13287-017-0578-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fadini GP, Losordo D, Dimmeler S (2012) Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res 110: 624–637. DOI 10.1161/CIRCRESAHA.111.243386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medina RJ, Barber CL, Sabatier F, Dignat-George F, Melero-Martin JM, Khosrotehrani K, Ohneda O, Randi AM, Chan JKY, Yamaguchi T, et al. (2017) Endothelial Progenitors: A Consensus Statement on Nomenclature. Stem Cells Transl Med 6: 1316–1320. DOI 10.1002/sctm.16-0360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeevanantham V, Butler M, Saad A, Abdel-Latif A, Zuba-Surma EK, Dawn B (2012) Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation 126: 551–568. DOI 10.1161/CIRCULATIONAHA.111.086074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JW, Lee SH, Youn YJ, Ahn MS, Kim JY, Yoo BS, Yoon J, Kwon W, Hong IS, Lee K, et al. (2014) A randomized, open-label, multicenter trial for the safety and efficacy of adult mesenchymal stem cells after acute myocardial infarction. J Korean Med Sci 29: 23–31. DOI 10.3346/jkms.2014.29.1.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miettinen JA, Salonen RJ, Niemela M, Kervinen K, Saily M, Koistinen P, Savolainen ER, Ukkonen H, Pietila M, Airaksinen KE, et al. (2010) Effects of intracoronary infusion of bone marrow-derived stem cells on pulmonary artery pressure and diastolic function after myocardial infarction. Int J Cardiol 145: 631–633. DOI 10.1016/j.ijcard.2010.09.064 [DOI] [PubMed] [Google Scholar]
- 39.Pokushalov E, Romanov A, Chernyavsky A, Larionov P, Terekhov I, Artyomenko S, Poveshenko O, Kliver E, Shirokova N, Karaskov A, et al. (2010) Efficiency of intramyocardial injections of autologous bone marrow mononuclear cells in patients with ischemic heart failure: a randomized study. J Cardiovasc Transl Res 3: 160–168. DOI 10.1007/s12265-009-9123-8 [DOI] [PubMed] [Google Scholar]
- 40.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, et al. (2006) Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med 355: 1210–1221. DOI 10.1056/NEJMoa060186 [DOI] [PubMed] [Google Scholar]
- 41.Ahmadi H, Baharvand H, Ashtiani SK, Soleimani M, Sadeghian H, Ardekani JM, Mehrjerdi NZ, Kouhkan A, Namiri M, Madani-Civi M, et al. (2007) Safety analysis and improved cardiac function following local autologous transplantation of CD133(+) enriched bone marrow cells after myocardial infarction. Curr Neurovasc Res 4: 153–160. DOI 10.2174/156720207781387141 [DOI] [PubMed] [Google Scholar]
- 42.Nasseri BA, Ebell W, Dandel M, Kukucka M, Gebker R, Doltra A, Knosalla C, Choi YH, Hetzer R, Stamm C (2014) Autologous CD133+ bone marrow cells and bypass grafting for regeneration of ischaemic myocardium: the Cardio133 trial. Eur Heart J 35: 1263–1274. DOI 10.1093/eurheartj/ehu007 [DOI] [PubMed] [Google Scholar]
- 43.Povsic TJ, Junge C, Nada A, Schatz RA, Harrington RA, Davidson CJ, Fortuin FD, Kereiakes DJ, Mendelsohn FO, Sherman W, et al. (2013) A phase 3, randomized, double-blinded, active-controlled, unblinded standard of care study assessing the efficacy and safety of intramyocardial autologous CD34+ cell administration in patients with refractory angina: design of the RENEW study. Am Heart J 165: 854–861 e852. DOI 10.1016/j.ahj.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 44.Wojakowski W, Pyrlik A, Krol M, Buszman P, Ochala A, Milewski K, Smolka G, Kawecki D, Rudnik A, Pawlowski T, et al. (2013) Circulating endothelial progenitor cells are inversely correlated with in-stent restenosis in patients with non-ST-segment elevation acute coronary syndromes treated with EPC-capture stents (JACK-EPC trial). Minerva Cardioangiol 61: 301–311 [PubMed] [Google Scholar]
- 45.Aguado BA, Mulyasasmita W, Su J, Lampe KJ, Heilshorn SC (2012) Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng Part A 18: 806–815. DOI 10.1089/ten.TEA.2011.0391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hou L, Kim JJ, Woo YJ, Huang NF (2016) Stem cell-based therapies to promote angiogenesis in ischemic cardiovascular disease. American Journal of Physiology - Heart and Circulatory Physiology 310: H455 DOI 10.1152/ajpheart.00726.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liao YY, Chen ZY, Wang YX, Lin Y, Yang F, Zhou QL (2014) New Progress in Angiogenesis Therapy of Cardiovascular Disease by Ultrasound Targeted Microbubble Destruction. Biomed Res Int 2014: 872984 DOI 10.1155/2014/872984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee S, Valmikinathan CM, Byun J, Kim S, Lee G, Mokarram N, Pai SB, Um E, Bellamkonda RV, Yoon YS (2015) Enhanced therapeutic neovascularization by CD31-expressing cells and embryonic stem cell-derived endothelial cells engineered with chitosan hydrogel containing VEGF-releasing microtubes. Biomaterials 63: 158–167. DOI 10.1016/j.biomaterials.2015.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirschi KK, Li S, Roy K (2014) Induced Pluripotent Stem Cells for Regenerative Medicine. Annu Rev Biomed Eng 16: 277–294. DOI 10.1146/annurev-bioeng-071813-105108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yin L, Ohanyan V, Fen Pung Y, DeLucia A, Bailey E, Enrick M, Stevanov K, Kolz CL, Guarini G, Chilian WM (2012) Induction of Vascular Progenitor Cells From Endothelial Cells Stimulates Coronary Collateral Growth. Circulation Research 110: 241–252. DOI 10.1161/CIRCRESAHA.111.250126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pons J, Huang Y, Takagawa J, Arakawa-Hoyt J, Ye J, Grossman W, Kan YW, Su H (2009) Combining angiogenic gene and stem cell therapies for myocardial infarction. The Journal of Gene Medicine 11: 743–753. DOI 10.1002/jgm.1362 [DOI] [PubMed] [Google Scholar]
- 52.Huang B, Qian J, Ma J, Huang Z, Shen Y, Chen X, Sun A, Ge J, Chen H (2014) Myocardial transfection of hypoxia-inducible factor-1a and co-transplantation of mesenchymal stem cells enhance cardiac repair in rats with experimental myocardial infarction. Stem Cell Res Ther 5: 22–22. DOI 10.1186/scrt410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duan HF, Wu CT, Wu DL, Lu Y, Liu HJ, Ha XQ, Zhang QW, Wang H, Jia XX, Wang LS (2003) Treatment of Myocardial Ischemia with Bone Marrow-Derived Mesenchymal Stem Cells Overexpressing Hepatocyte Growth Factor. Mol Ther 8: 467–474. DOI 10.1016/S1525-0016(03)00186-2 [DOI] [PubMed] [Google Scholar]
- 54.Shao H, Tan Y, Eton D, Yang Z, Uberti MG, Li S, Schulick A, Yu H (2008) Statin and Stromal Cell-Derived Factor-1 Additively Promote Angiogenesis by Enhancement of Progenitor Cells Incorporation into New Vessels. STEM CELLS 26: 1376–1384. DOI 10.1634/stemcells.2007-0785 [DOI] [PubMed] [Google Scholar]
- 55.Bandara N, Gurusinghe S, Chen H, Chen S, Wang L-x, Lim SY, Strappe P (2016) Minicircle DNA-mediated endothelial nitric oxide synthase gene transfer enhances angiogenic responses of bone marrow-derived mesenchymal stem cells. Stem Cell Research & Therapy 7: 48 DOI 10.1186/s13287-016-0307-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jung J-H, Fu X, Yang PC (2017) Exosomes Generated From iPSC-Derivatives. New Direction for Stem Cell Therapy in Human Heart Diseases 120: 407–417. DOI 10.1161/circresaha.116.309307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, et al. (2014) Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles 3: 26913 DOI 10.3402/jev.v3.26913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lawson C, Vicencio JM, Yellon DM, Davidson SM (2016) Microvesicles and exosomes: new players in metabolic and cardiovascular disease. Journal of Endocrinology 228: R57–R71. DOI 10.1530/JOE-15-0201 [DOI] [PubMed] [Google Scholar]
- 59.Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L (2010) Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int 78: 838–848. DOI 10.1038/ki.2010.278 [DOI] [PubMed] [Google Scholar]
- 60.Zhu H, Fan GC (2011) Extracellular/circulating microRNAs and their potential role in cardiovascular disease. Am J Cardiovasc Dis 1: 138–149. DOI 10.1016/j.als.2016.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harp D, Driss A, Mehrabi S, Chowdhury I, Xu W, Liu D, Garcia-Barrio M, Taylor RN, Gold B, Jefferson S, et al. (2016) Exosomes derived from endometriotic stromal cells have enhanced angiogenic effects in vitro. Cell Tissue Res 365: 187–196. DOI 10.1007/s00441-016-2358-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vickers KC, Remaley AT (2012) Lipid-based carriers of microRNAs and intercellular communication. Curr Opin Lipidol 23: 91–97. DOI 10.1097/MOL.0b013e328350a425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thery C, Ostrowski M, Segura E (2009) Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 9: 581–593. DOI 10.1038/nri2567 [DOI] [PubMed] [Google Scholar]
- 64.Li X, Chen C, Wei L, Li Q, Niu X, Xu Y, Wang Y, Zhao J (2016) Exosomes derived from endothelial progenitor cells attenuate vascular repair and accelerate reendothelialization by enhancing endothelial function. Cytotherapy 18: 253–262. DOI 10.1016/j.jcyt.2015.11.009 [DOI] [PubMed] [Google Scholar]
- 65.Gray WD, French KM, Ghosh-Choudhary S, Maxwell JT, Brown ME, Platt MO, Searles CD, Davis ME (2015) Identification of Therapeutic Covariant MicroRNA Clusters in Hypoxia-Treated Cardiac Progenitor Cell Exosomes Using Systems Biology. Circulation Research 116: 255–263. DOI 10.1161/CIRCRESAHA.116.304360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang T, Jones TM, Naddell C, Bacanamwo M, Calvert JW, Thompson WE, Bond VC, Chen YE, Liu D (2016) Adipose-Derived Stem Cells Induce Angiogenesis via Microvesicle Transport of miRNA-31. Stem Cells Translational Medicine 5: 440–450. DOI 10.5966/sctm.2015-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Foglio E, Puddighinu G, Fasanaro P, D’Arcangelo D, Perrone GA, Mocini D, Campanella C, Coppola L, Logozzi M, Azzarito T, et al. (2015) Exosomal clusterin, identified in the pericardial fluid, improves myocardial performance following MI through epicardial activation, enhanced arteriogenesis and reduced apoptosis. International Journal of Cardiology 197: 333–347. DOI 10.1016/j.ijcard.2015.06.008 [DOI] [PubMed] [Google Scholar]
- 68.Beltrami C, Besnier M, Shantikumar S, Shearn AIU, Rajakaruna C, Laftah A, Sessa F, Spinetti G, Petretto E, Angelini GD, et al. (2017) Human Pericardial Fluid Contains Exosomes Enriched with Cardiovascular-Expressed MicroRNAs and Promotes Therapeutic Angiogenesis. Molecular Therapy 25: 679–693. DOI 10.1016/j.ymthe.2016.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Atienzar-Aroca S, Flores-Bellver M, Serrano-Heras G, Martinez-Gil N, Barcia JM, Aparicio S, Perez-Cremades D, Garcia-Verdugo JM, Diaz-Llopis M, Romero FJ, et al. (2016) Oxidative stress in retinal pigment epithelium cells increases exosome secretion and promotes angiogenesis in endothelial cells. J Cell Mol Med 20: 1457–1466. DOI 10.1111/jcmm.12834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kang K, Ma R, Cai W, Huang W, Paul C, Liang J, Wang Y, Zhao T, Kim HW, Xu M, et al. (2015) Exosomes Secreted from CXCR4 Overexpressing Mesenchymal Stem Cells Promote Cardioprotection via Akt Signaling Pathway following Myocardial Infarction. Stem Cells International 2015: 659890 DOI 10.1155/2015/659890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma J, Zhao Y, Sun L, Sun X, Zhao X, Sun X, Qian H, Xu W, Zhu W (2017) Exosomes Derived from Akt-Modified Human Umbilical Cord Mesenchymal Stem Cells Improve Cardiac Regeneration and Promote Angiogenesis via Activating Platelet-Derived Growth Factor D. Stem Cells Transl Med 6: 51–59. DOI 10.5966/sctm.2016-0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H (2014) Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med (Berl) 92: 387–397. DOI 10.1007/s00109-013-1110-5 [DOI] [PubMed] [Google Scholar]
- 73.Teng X, Chen L, Chen W, Yang J, Yang Z, Shen Z (2015) Mesenchymal Stem Cell-Derived Exosomes Improve the Microenvironment of Infarcted Myocardium Contributing to Angiogenesis and Anti-Inflammation. Cellular Physiology and Biochemistry 37: 2415–2424. DOI 10.1159/000438594 [DOI] [PubMed] [Google Scholar]
- 74.Zhang Z, Yang J, Yan W, Li Y, Shen Z, Asahara T (2016) Pretreatment of Cardiac Stem Cells With Exosomes Derived From Mesenchymal Stem Cells Enhances Myocardial Repair. Journal of the American Heart Association: Cardiovascular and Cerebrovascular Disease 5: e002856 DOI 10.1161/JAHA.115.002856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang N, Chen C, Yang D, Liao Q, Luo H, Wang X, Zhou F, Yang X, Yang J, Zeng C, et al. (2017) Mesenchymal stem cells-derived extracellular vesicles, via miR-210, improve infarcted cardiac function by promotion of angiogenesis. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1863: 2085–2092. DOI 10.1016/j.bbadis.2017.02.023 [DOI] [PubMed] [Google Scholar]
- 76.Izarra A, Moscoso I, Levent E, Cañón S, Cerrada I, Díez-Juan A, Blanca V, Núñez-Gil I-J, Valiente I, Ruíz-Sauri A, et al. (2014) miR-133a Enhances the Protective Capacity of Cardiac Progenitors Cells after Myocardial Infarction. Stem Cell Reports 3: 1029–1042. DOI 10.1016/j.stemcr.2014.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM, Torre T, Siclari F, Moccetti T, Vassalli G (2014) Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovascular Research 103: 530–541. DOI 10.1093/cvr/cvu167 [DOI] [PubMed] [Google Scholar]
- 78.Tseliou E, Fouad J, Reich H, Slipczuk L, de Couto G, Aminzadeh M, Middleton R, Valle J, Weixin L, Marbán E (2015) Exosomes from cardiac stem cells amplify their own bioactivity by converting fibroblasts to therapeutic cells. Journal of the American College of Cardiology 66: 599–611. DOI 10.1016/j.jacc.2015.05.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Agarwal U, George A, Bhutani S, Ghosh-Choudhary S, Maxwell JT, Brown ME, Mehta Y, Platt MO, Liang Y, Sahoo S, et al. (2017) Experimental, Systems, and Computational Approaches to Understanding the MicroRNA-Mediated Reparative Potential of Cardiac Progenitor Cell-Derived Exosomes From Pediatric Patients. Circ Res 120: 701–712. DOI 10.1161/CIRCRESAHA.116.309935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gallet R, Dawkins J, Valle J, Simsolo E, de Couto G, Middleton R, Tseliou E, Luthringer D, Kreke M, Smith RR, et al. (2017) Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. European Heart Journal 38: 201–211. DOI 10.1093/eurheartj/ehw240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P, Mackie AR, Vaughan E, Garikipati VNS, Benedict C, et al. (2015) Embryonic Stem Cell-Derived Exosomes Promote Endogenous Repair Mechanisms and Enhance Cardiac Function Following Myocardial Infarction. Circulation research 117: 52–64. DOI 10.1161/CIRCRESAHA.117.305990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kervadec A, Bellamy V, El Harane N, Arakélian L, Vanneaux V, Cacciapuoti I, Nemetalla H, Périer M-C, Toeg HD, Richart A, et al. (2016) Cardiovascular progenitor–derived extracellular vesicles recapitulate the beneficial effects of their parent cells in the treatment of chronic heart failure. The Journal of Heart and Lung Transplantation 35: 795–807. DOI 10.1016/j.healun.2016.01.013 [DOI] [PubMed] [Google Scholar]
- 83.Xiong Y, Zhang Y, Mahmood A, Chopp M (2015) Investigational agents for treatment of traumatic brain injury. Expert Opin Investig Drugs 24: 743–760. DOI 10.1517/13543784.2015.1021919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sato YT, Umezaki K, Sawada S, Mukai Sa, Sasaki Y, Harada N, Shiku H, Akiyoshi K (2016) Engineering hybrid exosomes by membrane fusion with liposomes. Scientific Reports 6: 21933 DOI 10.1038/srep21933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kanada M, Bachmann MH, Hardy JW, Frimannson DO, Bronsart L, Wang A, Sylvester MD, Schmidt TL, Kaspar RL, Butte MJ, et al. (2015) Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proceedings of the National Academy of Sciences 112: E1433–E1442. DOI 10.1073/pnas.1418401112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Johnsen KB, Gudbergsson JM, Skov MN, Pilgaard L, Moos T, Duroux M (2014) A comprehensive overview of exosomes as drug delivery vehicles — Endogenous nanocarriers for targeted cancer therapy. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 1846: 75–87. DOI 10.1016/j.bbcan.2014.04.005 [DOI] [PubMed] [Google Scholar]
- 87.Xitong D, Xiaorong Z (2016) Targeted therapeutic delivery using engineered exosomes and its applications in cardiovascular diseases. Gene 575: 377–384. DOI 10.1016/j.gene.2015.08.067 [DOI] [PubMed] [Google Scholar]
- 88.El-Andaloussi S, Lee Y, Lakhal-Littleton S, Li J, Seow Y, Gardiner C, Alvarez-Erviti L, Sargent IL, Wood MJA (2012) Exosome-mediated delivery of siRNA in vitro and in vivo. Nat Protocols 7: 2112–2126. DOI 10.1038/nprot.2012.131 [DOI] [PubMed] [Google Scholar]
- 89.Won Kim H, Haider HK, Jiang S, Ashraf M (2009) Ischemic Preconditioning Augments Survival of Stem Cells via miR-210 Expression by Targeting Caspase-8-associated Protein 2. J Biol Chem 284: 33161–33168. DOI 10.1074/jbc.M109.020925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leroux L, Descamps B, Tojais NF, Seguy B, Oses P, Moreau C, Daret D, Ivanovic Z, Boiron JM, Lamaziere JM, et al. (2010) Hypoxia preconditioned mesenchymal stem cells improve vascular and skeletal muscle fiber regeneration after ischemia through a Wnt4-dependent pathway. Mol Ther 18: 1545–1552. DOI 10.1038/mt.2010.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kucharzewska P, Belting M (2013) Emerging roles of extracellular vesicles in the adaptive response of tumour cells to microenvironmental stress. J Extracell Vesicles 2: 10 DOI 10.3402/jev.v2i0.20304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringner M, Morgelin M, Bourseau-Guilmain E, Bengzon J, Belting M (2013) Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A 110: 7312–7317. DOI 10.1073/pnas.1220998110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Witwer KW, Buzas EI, Bemis LT, Bora A, Lasser C, Lotvall J, Nolte-’t Hoen EN, Piper MG, Sivaraman S, Skog J, et al. (2013) Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 2 DOI 10.3402/jev.v2i0.20360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, et al. (2005) Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med 3: 10 DOI 10.1186/1479-5876-3-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Conlan RS, Pisano S, Oliveira MI, Ferrari M, Mendes Pinto I Exosomes as Reconfigurable Therapeutic Systems. Trends in Molecular Medicine 23: 636–650. DOI 10.1016/j.molmed.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen TS, Arslan F, Yin Y, Tan SS, Lai RC, Choo ABH, Padmanabhan J, Lee CN, de Kleijn DPV, Lim SK (2011) Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. Journal of Translational Medicine 9: 47–47. DOI 10.1186/1479-5876-9-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Radenkovic D, Arjun S, Poma A, Nyberg S, Battaglia B, Yellon DM, Davidson S (2016) 162 Polymersomes Functionalized with HSP70 – Novel, Synthetic Cardioprotective Nanovesicles. Heart 102: A115–A115. DOI 10.1136/heartjnl-2016-309890.162 [DOI] [Google Scholar]
- 98.Zhu K, Li J, Wang Y, Lai H, Wang C (2016) Nanoparticles-Assisted Stem Cell Therapy for Ischemic Heart Disease. Stem Cells International 2016: 1384658 DOI 10.1155/2016/1384658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chu H, Wang Y (2012) Therapeutic angiogenesis: controlled delivery of angiogenic factors. Ther Deliv 3: 693–714. DOI 10.4155/tde.12.50 [DOI] [PMC free article] [PubMed] [Google Scholar]


