Abstract
Social reward plays a fundamental role in shaping human and animal behavior. The rewarding nature of many forms of social behavior including sexual behavior, parental behavior, and social play has been revealed using well-established procedures such as the conditioned place preference test. Many motivated social behaviors are regulated by the nonapeptides oxytocin (OT) and arginine vasopressin (AVP) through their actions in multiple brain structures. Interestingly, there are few data on whether OT or AVP might contribute to the rewarding properties of social interaction by their actions within brain structures that play a key role in reward mechanisms such as the ventral tegmental area (VTA). The goal of the present study was to investigate the role of OT and AVP in the VTA in regulating the reward-like properties of social interactions. Social interactions between two male hamsters reduced a spontaneous place avoidance in hamsters injected with saline control. Interestingly, however, OT and AVP injected into the VTA induced a significant two-fold reduction in place avoidance for the social interaction chamber when compared to control injections of vehicle. Finally, because OT and AVP can act on each other’s receptors to influence social behavior, we also injected highly selective OTR and V1aR agonists and antagonists to determine whether OT or AVP V1a receptors were responsible for mediating the effects of these neuropeptides on social reward. Our results not only demonstrated that OT and AVP activate OTRs and not V1aRs to mediate social reward, they also demonstrated that the activation of OT receptors in the VTA is essential for the expression of the rewarding properties of social interactions.
Keywords: Social interaction, Social behavior, Social salience, Social motivation, Conditioned place preference, Neuropeptides, Mesolimbic dopamine system, Dopamine
1. Introduction
The fact that many forms of social interaction have rewarding properties plays fundamental role in human and animal behavior. Indeed, the frequency with which groups of humans gather together illustrates the powerful rewarding properties of social interactions (Wagner et al., 2015). The neural mechanisms underlying social reward have been studied in a variety of species ranging from those that live in large social groups like rats to those that are less socially gregarious like Syrian hamsters (Mattson and Morrell, 2005; Meisel et al., 1996; Peartree et al., 2012; Trezza et al., 2011). The power of social reward is illustrated by the findings even in less gregarious species like hamsters that social interactions are not only rewarding for individuals that win competitive interactions, but also for those that lose as long as the loss is not too severe (Gil et al., 2013; Huhman, 2006). Indeed, social reward, along with social skills such as social recognition and social communication play a critical role in the development of adaptive social relationships in all mammalian species. The rewarding nature of social interactions even in species such as hamsters result in their ability to establish enduring social organizations including hierarchical dominant/subordinate relationships (Albers et al., 2002; Drickamer and Vandenbergh, 1973; Drickamer et al., 1973). Defining the mechanisms underlying social reward is not only critical for understanding the expression of adaptive social behavior, but also for revealing the dysfunctions in these mechanisms that contribute to the development of psychiatric disorders (Bora et al., 2009).
Oxytocin (OT) and arginine-vasopressin (AVP) are evolutionarily conserved mammalian neuropeptides that play essential roles in regulating a variety of motivated social behaviors including aggression, social recognition, parental behavior and social communication (Albers, 2012; Caldwell et al., 2008; Dumais and Veenema, 2015; Hammock, 2015; Ishak et al., 2011; Kelly and Goodson, 2014). The central effects of OT and AVP on social behavior are mediated primarily through the activation of OT receptors or AVP V1a receptors (Albers et al., 1986; Insel, 1992) as the expression of V1b receptors is restricted in the brain (Dhakar et al., 2013) and V2 receptors are not centrally expressed (Barberis et al., 1998). Many of the behavioral effects of OT and AVP are the result of their actions in structures that have been proposed to form a social behavior neural network (SBNN) (Newman, 1999). There is increasing evidence that the SBNN, composed of neural groups or “nodes” including, but not limited to, the extended amygdala, the bed nucleus of the stria terminalis (BNST), lateral septum (LS), periaqueductal gray (PAG), medial preoptic area (MPOA), ventromedial hypothalamus (VMH), and anterior hypothalamus (AH), plays a critical role in the expression of many different social behaviors (Albers, 2015; Crews, 1997; Goodson and Kingsbury, 2013).
It is likely that the expression of motivated social behaviors, like other motivated behaviors, also requires the activity of the mesolimbic dopamine (DA) system. The mesolimbic DA system is a network of reciprocally connected brain regions that are involved in determining the salience of stimuli, assigning their hedonic value, and initiating appropriate action (Caldwell and Albers, 2016; Love, 2014). There is also evidence that the mesolimbic DA system can play a critical role in at least some forms of social behavior (Aragona et al., 2003, 2006; Aragona and Wang, 2009; Curtis and Wang, 2005). While the SBNN and the mesolimbic DA system are distinct from one another, it has been proposed that they cooperate in the process of social decision making (O’Connell and Hofmann, 2011a,b). The mechanisms underlying the cooperation between these systems are not known but likely involve the actions of OT and AVP within the mesolimbic DA system.
The VTA is a key region in the mesolimbic DA system and provides many of the dopamine containing projections that innervate cortical and limbic structures that form the circuitry underlying motivation. The purpose of the present study was to test the hypothesis that activation of OT and/or V1a receptors in the VTA mediates the reward-like properties of social interaction in male Syrian hamsters. Because OT and AVP can influence social behavior by acting on each other’s receptors (Sala et al., 2011; Song et al., 2014) we also determined which receptors are responsible for inducing social reward by applying highly selective OT and AVP receptor agonists and antagonists.
2. Materials and methods
2.1. Animals
Adult male Syrian hamsters (Mesocricetus auratus), purchased from Charles River Laboratories Inc., Wilmington, MA, USA, were used in all experiments. The experimental hamsters were 10–12 weeks old, weighed 110–130 g, and were singly housed in poly-carbonate cages (23 × 43 × 20 cm) upon arrival to our vivarium. The stimulus hamsters were 8–10 weeks old, weighed 90–100 g, and were housed in a group of 4 per cage upon arrival. All hamsters were kept on a 14:10 light/dark cycle with food and water ad libitum. All experimental procedures were in accordance with the National Institutes of Health Guidelines for the Use of Animals and were approved by the Georgia State University Animal Care and Use Committee.
2.2. Surgery, and microinjections
2.2.1. Surgery
Seven days after arrival, hamsters were anesthetized with 5% isoflurane in an induction chamber and maintained with 3.75% isoflurane throughout all surgical procedures. A 4 mm, 26-gauge cannula guide was implanted unilaterally in each experimental hamster aimed at the VTA (−2.9 mm anterior to bregma, −0.6 mm from the midline, and −2.9 mm below dura) producing injection sites close to the midline. The guide was affixed to the skull using wound clips and Ortho-Jet dental acrylic (Lang Dental, Wheeling, IL, USA). Ketefen (1 ml/kg) was injected I.P. for analgesia and hamsters were monitored daily for 3 days and given additional ketefen as required.
2.2.2. Microinjections
Hamsters were gently restrained and microinjections were given over the course of 1 min using an infusion pump (Harvard Apparatus), a 1 μl Hamilton syringe, and a 12 mm, 32-gauge microinjection needle. The volume of all microinjections was 250nl. Following the injection, the needle was left in the cannula guide for an additional minute to allow drug diffusion into the VTA. At the end of the experiment experimental hamsters were sacrificed by lethal injection of sodium pentobarbital (2 ml/kg) and were injected with 250nl ink to mark the injection sites (see cannula placements in Fig. 1).
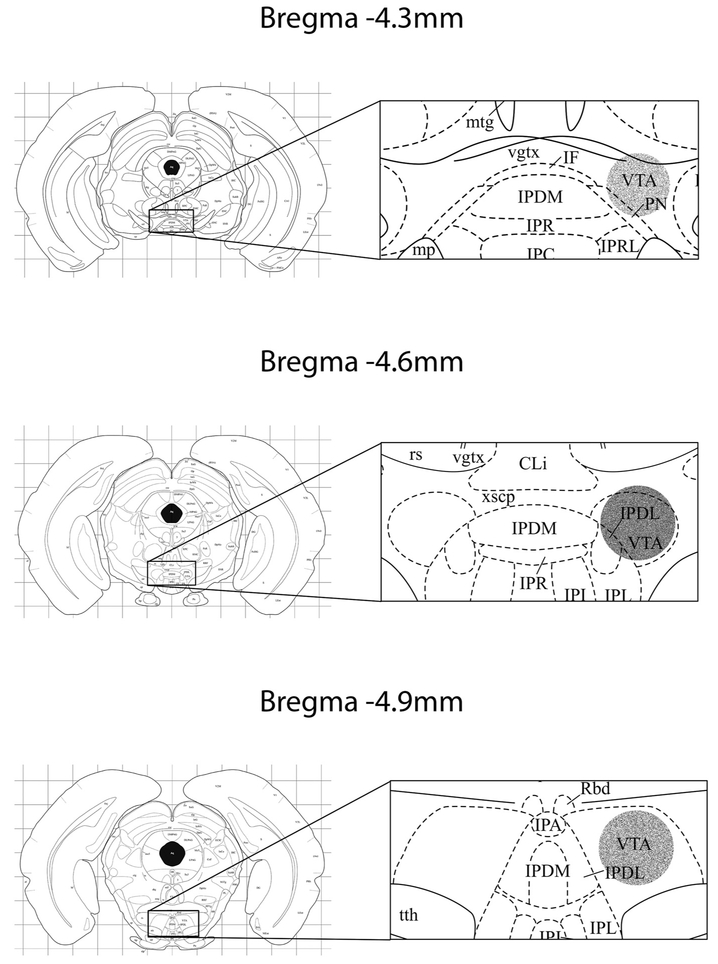
Fig. 1.
The sites of injection across all studies. Right panels are magnification of the target areas in the left panels. Circular areas in the left panels present the estimated area of diffusion. The density of the gray indicates the frequency of injection sites within that region. The diagram is modified from (Morin and Wood, 2001). Cli: caudal linear nucleus of raphe; IF: interfascicular nucleus; IPDL: interpeduncular nucleus, dorsolateral; IPDM: interpeduncular nucleus, dorsomedial; IPL: interpeduncular nucleus, lateral; IPR: interpeduncular nucleus, rostral; mtg: mammillotegmental tract; PN: paranigral nucleus; rs: rubrospinal tract; vgtx: ventral tegmental decussation; VTA: ventral tegmental area; xscp: decussation sup Cb ped.
2.3. Drugs
The following drugs were used: 9 μM, 90 μM OT (Bachem, CA, USA) and AVP (Fisher scientific, TX, USA); 27 μM [Thr4,Gly7]OT (OTAG, a highly selective OT receptor agonist (Lowbridge et al., 1977), a gift of Dr. Maurice Manning); 0.23 μM and 23 μM [Phe2]OVT (V1aAG, a highly selective V1a receptor agonist (Huguenin, 1964; Manning et al., 2012), a gift of Dr. Maurice Manning); 90 μM desGly-NH2-d(CH2)5[D-Tyr2,Thr4]OVT (OTA, a selective OTR antagonist (Manning et al., 1995), a gift of Dr. Maurice Manning) and 90 μM d(CH2)5[Tyr(Me)2]AVP (V1aA, a selective V1aR antagonist known as Manning Compound (Kruszynski et al., 1980), a gift of Dr. Maurice Manning). The concentrations of OT and AVP administered were based on the concentrations used in previous studies that were found effective in altering other social behavior in hamsters (Albers et al., 2006; Harmon et al., 2002; Song et al., 2014). The concentrations of the OT and AVP agonists used in these experiments (i.e., OT agonist: 27 μM [Thr4,Gly7]OT and V1a agonist: 0.23 μM [Phe2]OVT) were chosen based on dose-response studies of their efficacy in inducing social communication behavior in Syrian hamsters (Song et al., 2014) and their relative efficacies compared to OT and AVP in binding to OTR and V1aR in rats (Manning et al., 2012). The concentration of both OTR and V1aR antagonists were based on the concentration established in previous studies that was found to block social behavior in hamsters and rats (Albers et al., 1986; Nephew and Bridges, 2008). All controls were given a 250 nl injection of saline.
2.4. Behavioral testing
Male hamsters were tested using conditioned place preference (CPP) to examine whether social interaction with another male adult hamster could induce conditioned place preference, and if so, how much change in chamber preference would be induced by social interaction. Drugs of interest and saline were injected into the VTA and tested for their effects on the magnitude of change in chamber preference induced by social interaction, as a measure of the magnitude of social reward. The CPP apparatus consisted of a white chamber (61 cm × 46 cm × 38 cm), a black chamber (61 cm × 46 cm × 38 cm), and a transitional area (38 cm × 25 cm × 38 cm). Two different flooring materials, one black fiberglass mat and one cream-colored, non-slip rug mat, were used to serve additional contextual cues for the testing animals. Hamsters were given two 15 min pretests, five pairs of 10 min conditioning, and one 15 min post-test. In the pretest, hamsters were tested for their initial preference of the white and black chambers by allowing them to freely explore the whole apparatus for 15 min. The pretest was repeated again 24 h later and the average was used for data analysis. Immediately before each conditioning session, drugs of interest or saline were injected into the VTA; hamsters were then placed in the non-preferred chamber for social interactions (10 min) and in the empty preferred chamber for being alone (10 min) each day for 5 days. Hamsters went through one trial of social interaction and one trial of being alone with 1 h apart each day in a counter-balanced manner. The animals that received drugs always received the same drugs immediately prior to the two conditioning (social interaction and alone) sessions each day for 5 days. One day after the last conditioning, hamsters were tested again for their chamber preference in the post-test in the same way as in the pretests. Hamsters were not injected with drugs or saline during the pretest and the posttest. The apparatus was cleaned with 100% ethanol and the floor mats that were used to help hamsters discriminate chambers were changed between animals. All testing was video-taped from above (camera make: Panasonic-WVCP294) and scored later using the Observer (Noldus version 11.5, Lees-burg, VA) or a stopwatch by a trained experimenter unaware of the treatment conditions.
The goal of Experiment 1 was to determine whether microinjections of AVP and OT into the VTA could alter the magnitude of social reward. Hamsters were assigned to five groups and were injected with 90 μM AVP (n = 8), 9 μM AVP (n = 9), 90 μM OT (n = 6), 9 μM OT (n = 7) and saline (n = 10). In this experiment, we also measured the effects of OT and AVP in the VTA on social behavior including aggression, non-aggressive social investigation, self-grooming, and flank marking, immediately following drug administration during the social interaction conditioning. For detailed scoring criteria, see (Gutzler et al., 2010). The frequency of crossing the gates to both chambers during posttests was also scored to evaluate differences in the locomotive behavior after conditioning among the animals. After we found the enhancing effects of both OT and AVP in social reward in Experiment 1, Experiment 2 was conducted to test whether selective OTR and V1aR agonists (n = 6 and 7 respectively) could mimic the effects of OT and AVP in order to determine which receptor is involved in the regulation of social reward. Because there were no differences in the behavioral effects in controls injected with saline (n = 5) in this experiment and in Experiment 1, the data from saline controls were pooled for analyses. Experiment 3 determined whether antagonism of OTRs or V1aRs (n = 9 and 6 respectively) would reduce the rewarding aspects of social interaction, i.e., reduce the ability of social interaction to induce conditioned place preference. Because no difference was found in the saline control animals (n = 3) used in this experiment and in Experiments 1&2, the data were pooled together again for analyses.
2.5. Data analyses and statistics
All the data are presented as mean ± standard error of the mean. Difference in time spent in social interaction chamber was defined as time spent in the interaction chamber in the post-test minus time spent in that chamber in the pre-test. The preference score was defined as time in the social interaction chamber minus time in the alone chamber during either pre-test or post-test. SPSS V22 was used to analyze the data. Student t-test and Mann–Whitney test were used to detect differences between two animal groups depending on assumptions for parametric tests are met or not. One-way ANOVA test or Kruskal-Wallis test (for non-parametric test) was used to detect differences in the change of time spent in the social interaction chamber and change of chamber preference among animals injected with drugs of interest and saline control. Pearson correlation was used to examine the association between behaviors during the social interaction conditioning trials and magnitudes of increase in chamber preference scores or in time spent in the social interaction chamber. To determine effect sizes, eta squared, η2, for ANOVA, and Cohen’s d, d, for pair-wise comparisons and t-tests were calculated. All post-hoc comparisons were determined a priori (only treatment versus control would be analyzed); planned contrasts were performed following significant differences found in ANOVA tests and Mann–Whitney tests were performed following significant differences in Kruskal-Wallis tests. All tests were two tailed and differences were considered significant at p ≤ 0.05.
3. Results
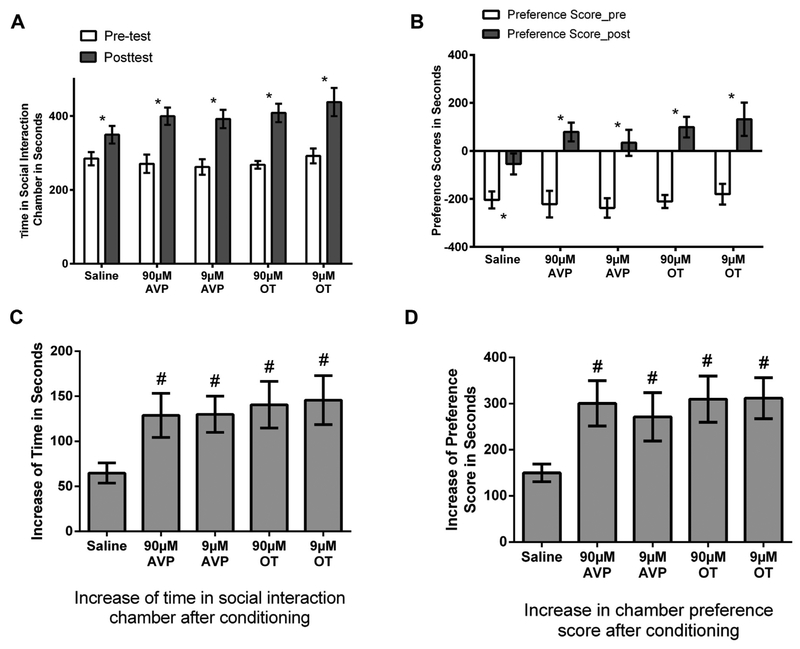
Experiment 1 tested whether OT or AVP injected into the VTA influences the magnitude of social reward. In the pretest all groups spent similar amounts of time in their non-preferred chamber (ANOVA, F(4,37) = 0.40, p > 0.05 Fig. 2A) and in their preferred chamber (data not shown). The chamber preference scores for all groups were also similar (ANOVA, F(4,37) = 0.26, p > 0.05 Fig. 2B). After 5 conditioning trials in the non-preferred chamber socially interacting within another hamster and 5 conditioning trials in the preferred chamber being alone, all hamsters had a significant increase in the time spent in the non-preferred chamber, i.e., the social-interaction chamber compared to pre-conditioning (paired t tests, saline: t(9) = 5.22, p < 0.01, d = 1.01, 90 μM AVP: t(7) = 5.25, p < 0.01, d = 1.89, 9 μM AVP: t(8) = 6.43, p < 0.01, d = 1.88, 90 μM OT: t(6) = 5.84, p < 0.01, d = 3.01, and 9 μM OT: t(7) = 5.62, p < 0.01, d = 1.67, Fig. 2A). The magnitudes of increase in the time in the social-interaction chamber were significantly affected by drug treatment (F(4,37) = 2.68, p < 0.05, η2 = 0.23, Fig. 2C), the hamsters injected with OT and AVP (at both doses) had a significantly greater increase in the time spent in the social interaction chamber compared to hamsters injected with saline (planned contrasts p < 0.05 for all; d = 1.14, 1.28, 1.44, and 1.38 for 90 μM AVP, 9 μM AVP, 90 μM OT, and 9 μM OT, respectively). Similarly, all hamsters had a significant increase in chamber preference scores (paired t tests, saline: t(9) = 6.47, p < 0.01, d = 1.24, 90 μM AVP: t(7) = 6.10, p < 0.01, d = 2.22, 9 μM AVP: t(8) = 5.17, p < 0.01, d = 1.88, 90 μM OT: t(6) = 6.67, p < 0.01, d = 3.53, and 9 μM OT: t(7) = 7.62, p < 0.01, d = 1.82, Fig. 2B) and the magnitudes of this increase were significantly affected by drug treatment (F(4,37) = 2.84, p < 0.05, η2 = 0.23, Fig. 2D), the hamsters injected with OT and AVP had a significantly greater increase compared to hamsters injected with saline (planned contrasts p < 0.05 for all; d = 1.35, 0.99, 1.58, and 1.67 for 90 μM AVP, 9 μM AVP, 90 μM OT, and 9 μM OT, respectively).
Fig. 2.
(A) All groups of hamsters increased the time spent in the social interaction chamber (SIC) in the posttest compared to the pretest. (B) All hamsters increased the preference scores (time spent in the SIC minus time spent in the alone chamber) for the SIC after conditioning. C. Increase of the time spent in the SIC in the post-test compared to the pretest. Hamsters injected with AVP and OT at both doses had a larger increase after conditioning compared with saline animals. (D) Hamsters injected with OT and AVP had a larger increase in preference scores after conditioning compared to saline animals. Note: * indicates a difference in the post-test compared with the pretest within each group (p < 0.05). # indicates a difference compared with saline (p < 0.05).
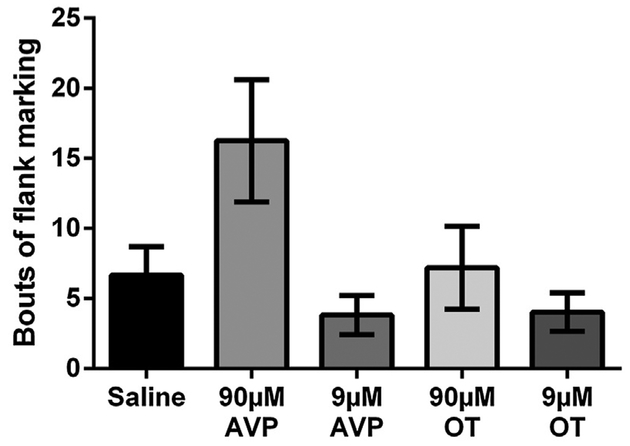
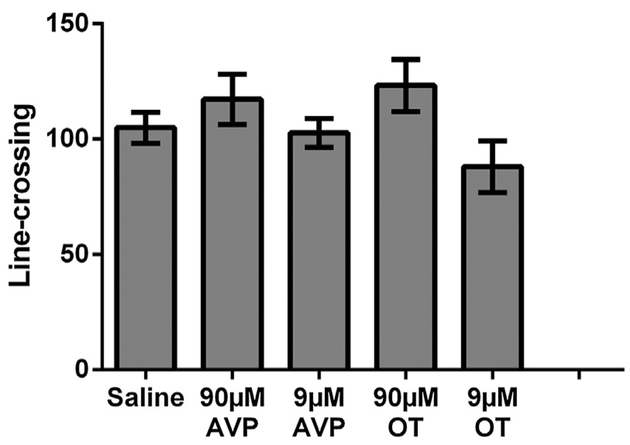
Because OT and AVP were injected into the VTA just before hamsters were placed in the chamber for each conditioning session, we also measured social behavior during the session to examine whether OT or AVP in the VTA affects the expression of social behaviors including aggression, social investigation, self-grooming, and flank marking. All groups exhibited similar levels of aggression (F(4,37) = 0.206, p = 0.93, Fig. 3A) and did not differ in the duration of non-social behavior (F(4,37) = 1.91, p = 0.13). There were, however, significant differences in the duration of non-aggressive social behavior (F(4,37) = 2.80, p < 0.05, η2 = 0.23), in the duration of self-grooming (Krusakal-Wallis test, H(4) = 15.10, p < 0.01), and a trending difference in the frequency of flank marking (Krusakal-Wallis test, H(4) = 8.48, p = 0.075). Post-hoc planned contrasts indicated that hamsters injected with 90 μM AVP trended to spend less time engaged in social behavior than controls injected with saline (p = 0.075, d = 0.74). The same group of hamsters also spent more time self-grooming (Mann-Whitney test, U = 15.00, p < 0.05) and trended to flank mark more than controls injected with saline (Mann-Whitney test, U = 18.50, p = 0.055, Fig. 3B). However, there were no significant differences between any other drug injected groups (i.e. 9 μM AVP, 90 μM or 9 μM OT) and controls injected with saline in the duration of social behavior, self-grooming, or the frequency of flank marking. None of the Pearson correlation tests revealed any significant correlations (aggression vs increase of chamber preference scores, r = 0.15, p > 0.05; aggression vs increase of time in social interaction chamber, r = 0.17, p > 0.05; non-aggressive social behavior vs increase of chamber preference scores, r = 0.08, p > 0.05; non-aggressive social behavior vs increase of time in social interaction chamber, r = 0.11, p > 0.05;). There was no significant difference among the 5 groups of hamsters in the frequencies of crossing the gates to both chambers (F(4,37) = 2.06, p > 0.05, Fig. 4).
Fig. 3.
Effects of OT and AVP injected into the ventral tegmental area on social behavior during conditioning. Hamsters in all groups had similar levels of aggression and nonsocial behavior. The group injected with 90 μM AVP had significantly higher levels of self-grooming than controls. Note: # indicates a significant difference compared to controls (p < 0.05).
Fig. 4.
Locomotor activity as measured by the amount of line-crossing during the post-test. No significant between group differences (p > 0.05) were observed in line-crossing.
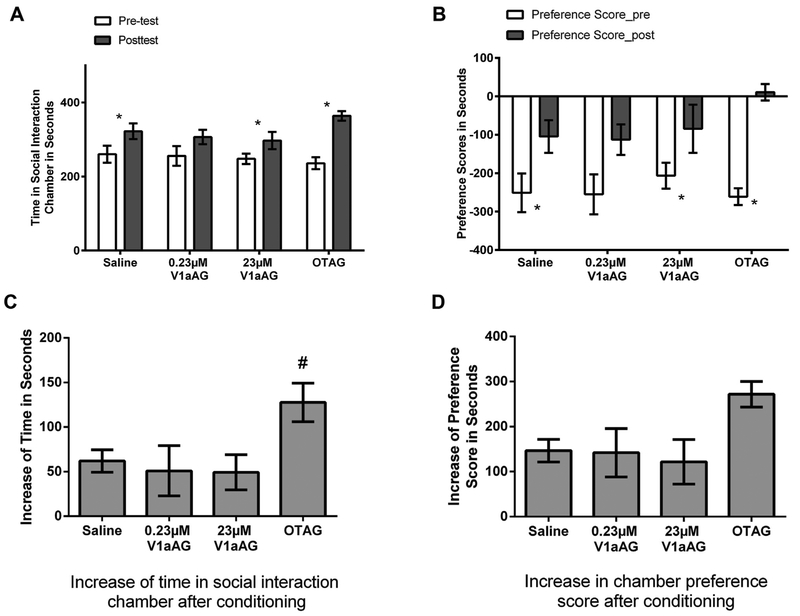
Experiment 2 investigated whether highly selective OT and V1a receptor agonists could mimic the effects of OT and AVP on social reward. In the pretests there were no between group differences in the time spent in the non-preferred chamber (F(3,35) = 0.09, p > 0.05, Fig. 5A) or in their chamber preference scores (F(3,35) = 0.20, p > 0.05, Fig. 5B). After conditioning in the non-preferred chamber with social interaction and in the preferred chamber being alone, hamsters injected with saline, the OTR agonist, and the 23 μM V1aR agonist spent significantly more time in the social-interaction chamber in the posttest versus in the pretest (saline: t(14) = 5.116, p < 0.001, d = 0.76; OTAG: t(7) = 6.54, p < 0.001, d = 2.92, 23 μM V1aAG: t(7) = 2.68, p < 0.05, d = 0.97, Fig. 5A) and all hamsters increased chamber preference scores (saline: t(14) = 6.00, p < 0.001, d = 0.84; OTAG: t(7) = 9.45, p < 0.001, d = 3.14, 0.23 μM V1aAG: t(7) = 2.83, p < 0.05, d = 1.16, 23 μM V1aAG: t(7) = 2.62, p < 0.05, d = 0.91, Fig. 5B). The magnitudes of increase in the time in the social-interaction chamber were significantly affected by drug treatment (F(3,35) = 3.06, p < 0.05, η2 = 0.21, Fig. 5C). Hamsters injected with the OTR agonist had a greater increase than those injected with the V1aR agonist or saline in the time spent in the social-interaction chamber (p < 0.05 for all; d = 1.19, 1.09, and 1.38 for OTR agonist compared with saline, 0.23 μM V1aAG, and 23 μM V1aAG, respectively). There was a difference in the increase of chamber preference scores among all groups that approached but did not reach statistical significance (F(3,35) = 2.52, p = 0.074, Fig. 5D).
Fig. 5.
(A) Time spent in the social interaction chamber (SIC) in the pretest and the post-test. All hamsters except those injected with the 0.23 μM V1a agonist (V1aAG) increased the time spent in the SIC in the posttest vs in the pretest. (B) Preference scores (time spent in the SIC minus time spent in the alone chamber) for the SIC in the pretest and the post-test. All hamsters except those injected with the 0.23 μM V1a agonist (V1aAG) increased the preference scores for the SIC in the posttest vs in the pretest. (C) Increase of time spent in the SIC in the post-test vs in the pretest. Hamsters injected with OTAG had a larger increase after conditioning compared with saline animals. D. Increase of the preference scores for the SIC in the post-test vs in the pretest. Note: * indicates a difference in the post-test compared with the pretest within each group (p < 0.05). # indicates a difference compared with saline (p < 0.05). V1aAG: V1aR agonist; OTAG: OTR agonist.
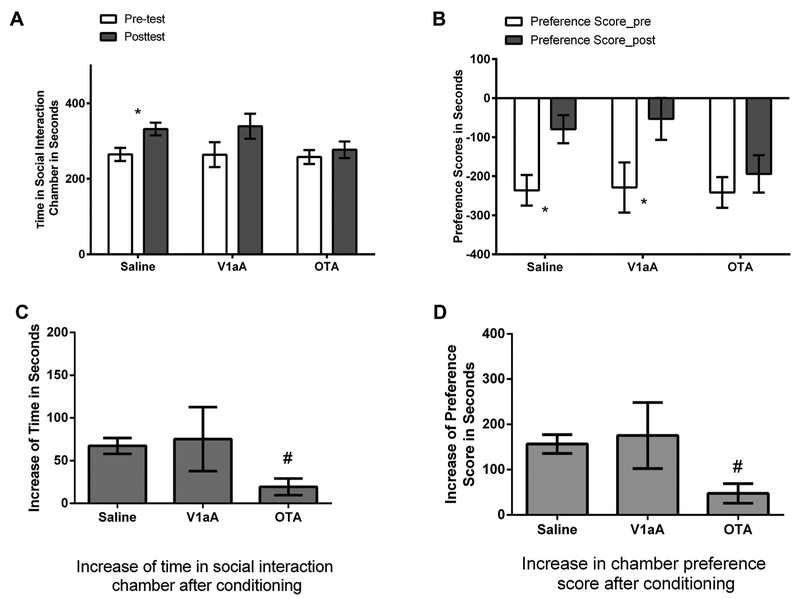
The aim of Experiment 3 was to test the hypothesis that activation of either OT or V1a receptors is necessary for the rewarding properties of social interaction. All groups spent similar amounts of time in their social interaction chamber in the pretest (F(2,32) = 0.031, p > 0.05, Fig. 6A) or in their chamber preference scores (F(2,32) = 0.01, p > 0.05, Fig. 6B). Hamsters injected with saline spent significantly more time in the social-interaction chamber after conditioning (t(19) = 7.47, p < 0.001, d = 0.90, Fig. 6A), and the same animals and those injected with V1aA increased their chamber preference scores (saline: t(19) = 7.72, p < 0.001, d = 0.96; V1aA: t(5) = 2.64, p < 0.05, d = 1.32, Fig. 6B). Hamsters injected with the OTR antagonist, however, did not spend significantly more time in the social interaction chamber after conditioning (t(8) = 2.09, p > 0.05, Fig. 6A). The magnitudes of increase in the time spent in the social-interaction chamber and in chamber preference scores were significantly affected by drug treatment (time in social-interaction chamber: Kruskal-Wallis test, H(2) = 7.19, p < 0.05, Fig. 6C; chamber preference scores: ANOVA test, F(2,32) = 4.40, p < 0.05, η2 = 0.22, Fig. 6D). The change of time in the social-interaction chamber or in chamber preference scores in hamsters injected with the OTR antagonist after conditioning was significantly smaller than those of hamsters injected with saline (Mann-Whitney test, U = 28.00, p < 0.01 for time in social interaction chamber, Fig. 6C; d = 1.41, p < 0.01 for chamber preference scores, Fig. 6D). There was no difference in the magnitude of the increase in the time spent in the social-interaction chamber or in the change of chamber preference scores between hamsters injected with the V1aR antagonist and those with saline (Mann-Whitney test, U = 28.00, p > 0.05 for increase in time spent in social-interaction chamber; planned contrast, p < 0.05 for change in chamber preference scores).
Fig. 6.
(A) Time spent in the social interaction chamber (SIC) in the pretest and post-test. (B) Preference scores (time spent in the SIC minus time spent in the alone chamber) for the SIC in the pretest and post-test. (C) Increase of time spent in the SIC in the post-test vs in the pretest. Hamsters injected with the selective OT antagonist (OTA) had a smaller increase after conditioning compared with saline animals. (D) Hamsters injected with OTA had a smaller increase in the preference scores after conditioning compared with saline controls. Note: * indicates a difference in the post-test compared with the pretest (p < 0.05). # indicates a difference compared with saline (p < 0.05). V1aA: V1aR antagonist; OTA: OTR antagonist.
Histological analysis of the sites of injection revealed most placements were found midway along the rostral-caudal axis of the VTA. The sites where the microinjection needle tips were located are shown in Fig. 1. Eight animals were excluded for data analysis because of poor cannula placements revealed by histological analysis.
4. Discussion
Our data support the hypothesis that activation of OT receptors in the VTA is necessary for the reward-like properties of social interactions. Although both OT and AVP can enhance social reward by their actions within the VTA, the results obtained with highly selective OTR and V1aR agonists and antagonists indicate that these neuropeptides influence social reward by acting on OT and not V1a receptors. More specifically, compared to saline controls, the highly selective OTR agonist, but not the highly selective V1aR agonist further increased the time spent in the social-interaction associated chamber after conditioning. While the concentrations of the OTR and V1aR selective agonists used in these experiments were chosen based on their efficacy in behavioral studies in hamsters it remains possible, although unlikely, that higher concentrations of the V1a agonist could have also increased social reward (Song et al., 2014). Most importantly, the highly selective OTR antagonist, but not the highly selective V1aR antagonist significantly reduced the increase of time spent in the social-interaction associated chamber after conditioning compared to saline control. These data indicate that activation of OT receptors in a key element of the mesolimbic DA system, the VTA, plays an essential role in mediating the reward-like properties of social interactions.
Although OT and AVP clearly activate their own receptors, there are an increasing number of examples of the ability of OT and AVP to cross-activate each other’s receptors in the brain. Previous studies have shown that OT can modulate social behaviors such as social communication by acting on V1a receptors and not OT receptors (Sala et al., 2011; Song et al., 2014) and that AVP can enhance social recognition of adult conspecifics by acting on OT receptors and not V1a receptors (Song et al., 2016). The present data are yet another example of cross-activation between OT and AVP that can occur in the regulation of social behavior. The absence of selectivity between OT and AVP receptors in rodents suggest that a functional distinction between OT and AVP may be overstated. Of course, it remains important to demonstrate that endogenously released OT and AVP cross-activate each other’s receptors in functionally significant ways.
The reward-like properties of OT and/or AVP in the VTA did not appear to be an indirect effect of altering other social behaviors such as aggressive behavior. Levels of aggression following injections of OT and AVP into the VTA were similar to those seen following saline injection and aggression did not correlate with the magnitude of social reward in OT and AVP injected animals. Also, it seems unlikely that OT injected into the VTA altered anxiety because we have found that introcerebralventricular injections of OT did not alter the time spent in an OT-associated chamber (or preference for that chamber) in male Syrian hamsters when administered without social interaction. One possibility is that the rewarding properties of OT are dependent on individual’s social experience and/or the social context. Social experience and social context can interact to dramatically alter the effects of OT on the expression of social behavior (Harmon et al., 2002). It has also been suggested that OT might amplify the rewarding properties of social interactions perhaps by priming social reward rather than providing the reward itself (McGregor and Bowen, 2012).
While the VTA has long been recognized to be involved in DA-related reward mechanisms (Love, 2014), the present data now indicate that OT and possibly AVP also play an essential role in the rewarding properties of social interactions through the activation of OT receptors in the VTA. The mechanisms underlying the regulation of social reward likely includes the nucleus accumbens (NAcc), another site within the mesolimbic DA system that receives efferent DA projections from the VTA. Indeed, injection of OT into the VTA has been found to increase DA levels in the NAcc in male and lactating female rats (Melis et al., 2007). There is also recent evidence that OT receptors in the NAcc are involved in mediating the rewarding properties of social interactions. Inactivation of OT receptors in the NAcc, particularly those located in the presynaptic serotonin neurons were found to reduce the rewarding properties of social interactions among male mice (Dolen et al., 2013). As a result, activation of OT receptors within multiple sites of the mesolimbic DA system may be necessary to provide the linkage between the social and motivational elements that underlie the rewarding properties of social interactions.
There is a limited but growing body of evidence that OT and AVP can act within the mesolimbic DA system to influence the expression of social behaviors (Baskerville and Douglas, 2010; Caldwell and Albers, 2016; Lim and Young, 2004; Numan and Young, 2015; Young et al., 2011). For example, rat maternal behavior is inhibited by the injection of OT antagonists in the VTA (Pedersen et al., 1994). Studies in humans employing functional magnetic resonance imaging indicate that intranasal OT significantly enhances VTA activation in response to both positive and negative social cues (Groppe et al., 2013; Scheele et al., 2013). Another example comes from studies of pair bonding in voles. Although it was originally thought that OT was responsible for the formation of partner preferences in female prairie voles and that AVP was responsible for the formation of partner preferences in males, more recent studies indicate that blocking OTRs in the NAcc inhibits the formation of partner preferences in both males and females (Keebaugh, et. al., unpublished cited in Numan and Young (2015), Young et al. (2001).
The important role of OT receptor activation in the mesolimbic DA system for social reward indicates that OT and DA interactions will likely be an important target for the development of strategies to treat psychiatric disorders and drug addiction (Groppe et al., 2013). Although still in their infancy pre-clinical and clinical studies suggest that OT administration may improve some aspects of social functioning in disorders characterized by social dysfunction (e.g., autism spectrum disorder and schizophrenia) and provide a potential mechanisms with which to inhibit drug self-administration (Cochran et al., 2013; McGregor and Bowen, 2012; Young and Barrett, 2015).
5. Conclusion
Activation of OT receptors within the VTA is necessary for social interactions to be rewarding. Both OT and AVP can enhance the rewarding properties of social interactions by acting on OT receptors and not V1aRs within the VTA.
Acknowledgements
We would like to thank Dr. Maurice Manning for his generous gifts of the OT and AVP receptor agonists and antagonists used in this study.
Funding
This work was supported by NSF grant IOS-0923301, NIH grant MH110212 to HEA and funds from the Brains and Behavior Program at Georgia State University.
Abbreviations:
- AH
anterior hypothalamus
- AVP
arginine vasopressin
- BNST
the bed nucleus of the stria terminalis
- CPP
conditioned place preference
- DA
dopamine
- LS
lateral septum
- MPOA
medial preoptic area
- NAcc
nucleus accumbens
- OTR
oxytocin receptor
- OT
oxytocin
- PAG
periaqueductal gray
- SBNN
social behavior neural network
- V1aR
vasopressin 1a receptor
- VMH
ventromedial nucleus of the hypothalamus
- VTA
ventral tegmental area
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
References
- Albers HE, Pollock J, Simmons WH, Ferris CF, 1986. A V1-like receptor mediates vasopressin-induced flank marking behavior in hamster hypothalamus. J. Neurosci 6, 2085–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers HE, Huhman K, Meisel RL, 2002. Hormonal basis of social conflict and communication In: Pfaff D, Arnold AP, Etgen A, Fahrbach SE, Rubin RT (Eds.), Hormones, Brain and Behavior. Academic Press, Amsterdam, pp. 393–433. [Google Scholar]
- Albers HE, Dean A, Karom MC, Smith D, Huhman KL, 2006. Role of V1a vasopressin receptors in the control of aggression in Syrian hamsters. Brain Res. 1073–1074, 425–430. [DOI] [PubMed] [Google Scholar]
- Albers HE, 2012. The regulation of social recognition, social communication and aggression: vasopressin in the social behavior neural network. Horm. Behav 61, 283–292. [DOI] [PubMed] [Google Scholar]
- Albers HE, 2015. Species, sex and individual differences in the vasotocin/vasopressin system: relationship to neurochemical signaling in the social behavior neural network. Front. Neuroendocrinol 36, 49–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Wang Z, 2009. Dopamine regulation of social choice in a monogamous rodent species. Front. Behav. Neurosci 3, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Curtis JT, Stephan FK, Wang Z, 2003. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J. Neurosci 23, 3483–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z, 2006. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat. Neurosci 9, 133–139. [DOI] [PubMed] [Google Scholar]
- Barberis C, Mouillac B, Durroux T, 1998. Structural bases of vasopressin/oxytocin receptor function. J. Endocrinol 156, 223–229. [DOI] [PubMed] [Google Scholar]
- Baskerville TA, Douglas AJ, 2010. Dopamine and oxytocin interactions underlying behaviors: potential contributions to behavioral disorders. CNS Neurosci. Ther 16, e92–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Yucel M, Allen NB, 2009. Neurobiology of human affiliative behaviour: implications for psychiatric disorders. Curr. Opin. Psychiatry 22, 320–325. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Albers HE, 2016. Oxytocin, vasopressin, and the motivational forces that drive social behaviors. Curr. Top. Behav. Neurosci (27), 51–103. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Lee HJ, Macbeth AH, Young WS 3rd, 2008. Vasopressin: behavioral roles of an original neuropeptide. Prog. Neurobiol 84, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran DM, Fallon D, Hill M, Frazier JA, 2013. The role of oxytocin in psychiatric disorders: a review of biological and therapeutic research findings. Harv. Rev. Psychiatry 21, 219–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D, 1997. Species diversity and the evolution of behavioral controlling mechanisms. Ann. N. Y. Acad. Sci 807, 1–21. [DOI] [PubMed] [Google Scholar]
- Curtis JT, Wang Z, 2005. Ventral tegmental area involvement in pair bonding in male prairie voles. Physiol. Behav 86, 338–346. [DOI] [PubMed] [Google Scholar]
- Dhakar MB, Stevenson EL, Caldwell HK, 2013. Oxytocin, vasopressin, and their interplay with gonadal steroids In: Choleris E, Pfaff DW, Kavaliers M (Eds.), Oxytocin, Vasopressin and Related Peptides in the Regulation of Behavior. Cambridge University Press, pp. 3–26. [Google Scholar]
- Dolen G, Darvishzadeh A, Huang KW, Malenka RC, 2013. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501, 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drickamer LC, Vandenbergh JG, 1973. Predictors of social dominance in the adult female golden hamster (Mesocricetus auratus). Anim. Behav 21, 564–570. [DOI] [PubMed] [Google Scholar]
- Drickamer LC, Vandenbergh JG, Colby DR, 1973. Predictors of dominance in the male golden hamster (Mesocricetus auratus). Anim. Behav 21, 557–563. [DOI] [PubMed] [Google Scholar]
- Dumais KM, Veenema AH, 2015. Vasopressin and oxytocin receptor systems in the brain: sex differences and sex-specific regulation of social behavior. Front. Neuroendocrinol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil M, Nguyen NT, McDonald M, Albers HE, 2013. Social reward: interactions with social status, social communication, aggression, and associated neural activation in the ventral tegmental area. Eur. J. Neurosci 38, 2308–2318. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Kingsbury MA, 2013. What’s in a name? Considerations of homologies and nomenclature for vertebrate social behavior networks. Horm. Behav 64, 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppe SE, Gossen A, Rademacher L, Hahn A, Westphal L, Grunder G, Spreckelmeyer KN, 2013. Oxytocin influences processing of socially relevant cues in the ventral tegmental area of the human brain. Biol. Psychiatry 74, 172–179. [DOI] [PubMed] [Google Scholar]
- Gutzler SJ, Karom M, Erwin WD, Albers HE, 2010. Arginine-vasopressin and the regulation of aggression in female Syrian hamsters (Mesocricetus auratus). Eur. J. Neurosci 31, 1655–1663. [DOI] [PubMed] [Google Scholar]
- Hammock EA, 2015. Developmental perspectives on oxytocin and vasopressin. Neuropsychopharmacology 40, 24–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon AC, Huhman KL, Moore TO, Albers HE, 2002. Oxytocin inhibits aggression in female Syrian hamsters. J. Neuroendocrinol 14, 963–969. [DOI] [PubMed] [Google Scholar]
- Huguenin R, 1964. Synthe’se de la Phe2-Orn8-ocytocine, deux analogues de la vasopressine douse d’une activiteí pressorique selective. Helv. Chim. Acta 47, 1934–1941. [Google Scholar]
- Huhman KL, 2006. Social conflict models: can they inform us about human psychopathology? Horm. Behav 50, 640–646. [DOI] [PubMed] [Google Scholar]
- Insel TR, 1992. Oxytocin-a neuropeptide for affiliation: evidence from behavioral, receptor autoradiographic, and comparative studies. Psychoneuroendocrinology 17, 3–35. [DOI] [PubMed] [Google Scholar]
- Ishak WW, Kahloon M, Fakhry H, 2011. Oxytocin role in enhancing well-being: a literature review. J. Affect. Disord 130, 1–9. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Goodson JL, 2014. Social functions of individual vasopressin-oxytocin cell groups in vertebrates: what do we really know? Front. Neuroendocrinol 35, 512–529. [DOI] [PubMed] [Google Scholar]
- Kruszynski M, Lammek B, Manning M, Seto J, Haldar J, Sawyer WH, 1980. [1-beta-Mercapto-beta,beta-cyclopentamethylenepropionic acid),2-(O-methyl)tyrosine]argine-vasopressin and [1-beta-mercapto-beta,beta-cyclopentamethylenepropionic acid)]argine-vasopressine, two highly potent antagonists of the vasopressor response to arginine-vasopressin. J. Med. Chem 23, 364–368. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ, 2004. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience 125, 35–45. [DOI] [PubMed] [Google Scholar]
- Love TM, 2014. Oxytocin, motivation and the role of dopamine. Pharmacol. Biochem. Behav 119, 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowbridge J, Manning M, Haldar J, Sawyer WH, 1977. Synthesis and some pharmacological properties of [4-threonine, 7-glycine]oxytocin, [1-(L-2-hydroxy-3-mercaptopropanoic acid), 4-threonine, 7-glycine]oxytocin (hydroxy[Thr4, Gly7]oxytocin), and [7-Glycine]oxytocin, peptides with high oxytocic-antidiuretic selectivity. J. Med. Chem 20, 120–123. [DOI] [PubMed] [Google Scholar]
- Manning M, Miteva K, Pancheva S, Stoev S, Wo NC, Chan WY, 1995. Design and synthesis of highly selective in vitro and in vivo uterine receptor antagonists of oxytocin: comparisons with Atosiban. Int. J. Pept. Protein Res 46, 244–252. [DOI] [PubMed] [Google Scholar]
- Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, Durroux T, Mouillac B, Corbani M, Guillon G, 2012. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J. Neuroendocrinol 24, 609–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson BJ, Morrell JI, 2005. Preference for cocaine- versus pup-associated cues differentially activates neurons expressing either Fos or cocaine- and amphetamine-regulated transcript in lactating, maternal rodents. Neuroscience 135, 315–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor IS, Bowen MT, 2012. Breaking the loop: oxytocin as a potential treatment for drug addiction. Horm. Behav 61, 331–339. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Joppa MA, Rowe RK, 1996. Dopamine receptor antagonists attenuate conditioned place preference following sexual behavior in female Syrian hamsters. Eur. J. Pharmacol 309, 21–24. [DOI] [PubMed] [Google Scholar]
- Melis MR, Melis T, Cocco C, Succu S, Sanna F, Pillolla G, Boi A, Ferri GL, Argiolas A, 2007. Oxytocin injected into the ventral tegmental area induces penile erection and increases extracellular dopamine in the nucleus accumbens and paraventricular nucleus of the hypothalamus of male rats. Eur. J. Neurosci 26, 1026–1035. [DOI] [PubMed] [Google Scholar]
- Morin L, Wood RI, 2001. A Stereotaxic Atlas of the Brain of the Golden Hamster. Academic Press. [Google Scholar]
- Nephew BC, Bridges RS, 2008. Central actions of arginine vasopressin and a V1a receptor antagonist on maternal aggression, maternal behavior, and grooming in lactating rats. Pharmacol. Biochem. Behav 91, 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SW, 1999. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann. N. Y. Acad. Sci 877, 242–257. [DOI] [PubMed] [Google Scholar]
- Numan M, Young LJ, 2015. Neural mechanisms of mother-infant bonding and pair bonding: similarities, differences, and broader implications. Horm. Behav [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell LA, Hofmann HA, 2011a. Genes, hormones, and circuits: an integrative approach to study the evolution of social behavior. Front. Neuroendocrinol 32, 320–335. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, Hofmann HA, 2011b. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J. Comp. Neurol 519, 3599–3639. [DOI] [PubMed] [Google Scholar]
- Peartree NA, Hood LE, Thiel KJ, Sanabria F, Pentkowski NS, Chandler KN, Neisewander JL, 2012. Limited physical contact through a mesh barrier is sufficient for social reward-conditioned place preference in adolescent male rats. Physiol. Behav 105, 749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA, 1994. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav. Neurosci 108, 1163–1171. [DOI] [PubMed] [Google Scholar]
- Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, Finardi A, Donzelli A, Pattini L, Rubino T, Parolaro D, Nishimori K, Parenti M, Chini B, 2011. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol. Psychiatry 69, 875–882. [DOI] [PubMed] [Google Scholar]
- Scheele D, Wille A, Kendrick KM, Stoffel-Wagner B, Becker B, Gunturkun O, Maier W, Hurlemann R, 2013. Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proc. Natl. Acad. Sci. U. S. A 110, 20308–20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, McCann KE, McNeill J.K.t., Larkin TE 2nd, Huhman KL, Albers HE, 2014. Oxytocin induces social communication by activating arginine-vasopressin V1a receptors and not oxytocin receptors. Psychoneuroendocrinology 50c, 14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Larkin TE 2nd, O’Malley M, Albers HE, 2016. Oxytocin (OT) and arginine-vasopressin (AVP) act on OT receptors and not AVP V1a receptors to enhance social recognition in adult Syrian hamsters (Mesocricetus auratus). Horm. Behav 81, 20–27. [DOI] [PubMed] [Google Scholar]
- Trezza V, Campolongo P, Vanderschuren LJ, 2011. Evaluating the rewarding nature of social interactions in laboratory animals. Dev. Cogn. Neurosci 1, 444–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U, Galli L, Schott BH, Wold A, van der Schalk J, Manstead AS, Scherer K, Walter H, 2015. Beautiful friendship: social sharing of emotions improves subjective feelings and activates the neural reward circuitry. Soc. Cogn. Affect. Neurosci 10, 801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Barrett CE, 2015. Neuroscience. Can oxytocin treat autism? Science 347, 825–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR, 2001. Cellular mechanisms of social attachment. Horm. Behav 40, 133–138. [DOI] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Liu Y, Wang Z, 2011. The neurobiology of pair bonding: insights from a socially monogamous rodent. Front. Neuroendocrinol 32, 53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]