Abstract
Rationale:
Paraneoplastic dermatomyositis (DM) is an inflammatory disease of the connective tissue caused by immunologic events in the presence of malignant tumors, which are typically related to ovarian, pancreatic, stomach, and colon cancer. Traditional treatment of paraneoplastic DM includes combination therapy for the underlying malignancy with systemic steroids.
Patient concerns:
A 41-year-old woman presented with facial erythema and myalgia of the extremities.
Diagnosis:
The patient was diagnosed with DM associated with a fallopian-tube carcinoma.
Interventions:
The cancer staging surgery was performed via muilt-port laparoscope and administered 6 cycles of adjuvant chemotherapy with paclitaxel (210 mg) and carboplatin (600 mg) right ovary and the left fallopian tube were removed laparoscopically.
Outcomes:
The DM healed spontaneously without the use of general glucocorticoids after the cancer staging surgery. During the 9-month follow-up, no recurrence of DM or neoplasm was observed.
Lessons:
This case highlights the fact that paraneoplastic DM can heal spontaneously after therapy for the underlying neoplasm, thereby avoiding the use of systemic steroids and their side effects. Moreover, DM can be an initial symptom for gynecological cancer such as fallopian-tube cancer. Thus, if DM is refractory to standard treatment, gynecological neoplasms should be considered.
Keywords: dermatomyositis, fallopian-tube carcinoma, general glucocorticoids
1. Introduction
Dermatomyositis (DM) is a rare, autoimmune, idiopathic, inflammatory myopathy involving the skin and muscles. It presents with a spectrum of manifestations and has an incidence of 0.5 to 0.89 per 100,000 in the general population.[1] In 18% to 32% of patients, especially those who are 40 years or older, DM is closely associated with underlying neoplasms, usually of the ovary, breast, lung, pancreas, stomach, colon, rectum, and non-Hodgkin lymphomas, presenting as paraneoplastic symptoms.[2,3] However, its association with fallopian-tube carcinoma is rare. The traditional treatment for paraneoplastic DM involves a high dose of glucocorticoids along with management of the malignancy.[4,5] Here we report an interesting case wherein DM was the first manifestation of a fallopian-tube carcinoma, which healed spontaneously without the use of general glucocorticoids following the malignancy staging surgery.
2. Case presentation
The patient was a 41-year-old multiparous woman in good health. Her medical history included a cesarean section and a laparoscopic right fallopian-tubectomy for an ectopic pregnancy. Her mother had died of ovarian cancer. She had a 2-month history of facial erythema, progressive soreness in the upper and lower limbs, and asthenia for roughly 1 month. The significant findings of the physical examinations were violaceous erythemas on the malar, dorsal, and the knuckles. Her laboratory examinations revealed negative autoimmune disease profiles, creatine kinase (CK): 303 U/L (normal 30–135 U/L), and CK-MB: 4.37 μg/L (normal 0–3.38 μg/L). After being diagnosed with DM, she was treated with Plaquenil (2 g, ivgtt, bid), loratadine (10 mg, oral, qd), ketotifen (1 mg, oral, bid), and topical hydrocortisone (bid). One week later, the symptoms persisted, indicating that DM was refractory to treatment, which was considered a paraneoplastic sign. A whole body enhanced computed tomography (CT) revealed a 2.5 × 2.9 × 2.9 cm mass in the right ovary. The level of tumor marker carbohydrate antigen-125 (CA-125) was 48.2 U/mL (normal <35 U/mL), while alpha-fetoprotein (AFP) and carbohydrate antigen-19–9 (CA19–9) levels were normal. Based on these findings, laparoscopy was performed. The laparoscopic examination revealed a solid mass in the right ovary as well as a hard, thickened left fallopian tube with atresia fimbria. The right ovary and the left tube were removed. Examination of the fast-frozen section showed an adenocarcinoma, and staging surgery was performed. After the staging surgery, the DM healed spontaneously without the use of general glucocorticoids. The final histopathology indicated a high-grade serous adenocarcinoma in the left tube (Fig. 1A and B). Immunohistochemistry revealed CK7++, CA125+, ER+, P16++, P53+++ (Fig. 1C), WT-1++, and Ki67 positivity in 80% of the cells and absence of CK20 and PR. The neoplasm FIGO staging was IIIc. The patient was administered 6 cycles of chemotherapy with paclitaxel (210 mg) and carboplatin (600 mg) and was followed-up every 3 months to survey both the malignancy and DM. During the 9 months of follow up there was no recurrence of either the DM or the neoplasm.
Figure 1.
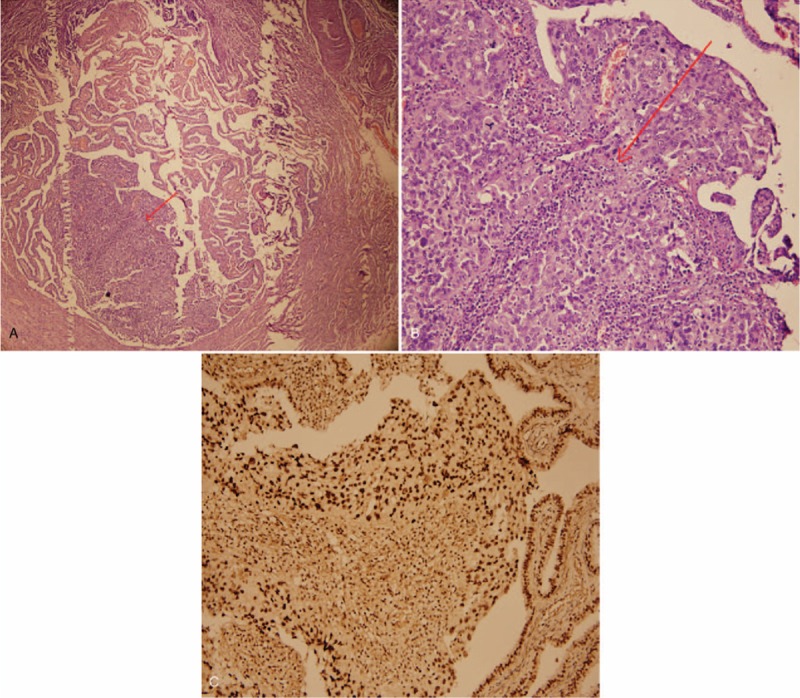
Histopathological findings. A: High-grade serous adenocarcinoma in the lumen of the left fallopian tube (arrow, under 40× magnification). B: Carcinoma under 200× magnification. C: Immunostaining positive for P53 in the carcinoma and the adjacent tubal epithelium (under 200× magnification).
3. Discussion
DM is a rare, idiopathic, inflammatory myopathy that with heliotrope rashes, typically involving the malar, forehead, and back, as well as the violaceous papules over the knuckles and symmetric proximal muscle weakness. Elevated CK, which is released from damaged muscle, is common but not required for diagnosis.[6] Other myositis-specific autoantibodies including anti-Mi-2, anti-Jo-1, anti-U1-RNP, and anti-155/140 are associated with DM.[7] Anti-p155/140 increases the risk of an underlying malignancy by 18-fold and some reports have shown that 71% of patients with this antibody develop cancer.[8] Incidence of DM shows a bimodal distribution, typically occurring as either juvenile or adult DM. Very few cases of juvenile DM associated with cancer have been published, as it is not typically a paraneoplastic disease. In contrast, women who are 40 years or older and diagnosed with DM should be assessed for underlying malignancies since 15% to 25% of adult DM cases involve paraneoplastic conditions.[9,10] In particular, when DM does not overlap with other collagen vascular diseases, such as polymyositis or scleroderma, lacks auto-antibodies, and is refractory to treatment, it is often associated with an underlying malignancy.[11]
Paraneoplastic DM can predate, coincide with, or develop after the diagnosis of cancer. The risk of cancer is highest within the first year of DM diagnosis and drops thereafter. After being diagnosed with DM, patients are advised to undergo screening for malignancy during the first 3 years, with the exception of ovarian cancer, which may present during the next 5 years. Therefore, DM patients should be followed-up for the underlying malignancy, even if primary screening is negative. It is also advisable that screening is repeated after 3 to 6 months.[12,13]
The mechanism of DM is that of a microangiopathy, characterized by complement deposition in the microvasculature of the muscle and antibody-mediated capillary destruction. However, the pathogenesis of DM in malignancy is poorly understood, but it is thought to be caused by an altered immune system. Traditional treatment for paraneoplastic DM includes combination therapy for the underlying malignancy with systemic steroids.[4,5] There is a report of DM symptom regression with definitive treatment of the underlying malignancy.[4] In most cases, the recurrence of muscle weakness and skin rashes should be assessed because they may be associated with the relapse of malignancy.
According to previous reports, as many as 15% to 25% of DM cases are associated with an occult neoplasm, one of the most common ones being ovarian (13.3–26%).[2,5] The association with fallopian-tube carcinoma is rare. However, recent reports have suggested serous tubal intraepithelial carcinoma as the precursor for serous ovarian or peritoneal carcinoma with a P53 signature.[14,15] Our case met the clinical features of paraneoplastic DM, which healed spontaneously when definitive treatment of the underlying malignancy was administered. However, this case has some limitations, including the lack of a skin or muscle biopsy as well as the lack of testing to establish the presence/absence of the autoantibodies anti-p155/140.
In summary, integrating the findings of this case with the existing literature, there are some lessons to be learned. First, it is possible for paraneoplastic DM to heal spontaneously when the associated malignancy is treated. Therefore, the use of systemic steroids and their side effects may be avoided in some cases. Second, dermatologists and gynecologists should screen for underlying gynecologic malignancies if a female patient, 40 years or older, is diagnosed with DM, particularly in high-risk cases, as a third of paraneoplastic DM cases are associated with ovaries. The involvement of the ovary or fallopian-tube carcinoma does not usually manifest in the early stages, and therefore staging is an important factor for prognosis. Finally, in general, DM responds well to steroids. However, if it is refractory, the patient should be screened for an occult malignancy.
Acknowledgments
The authors would like to acknowledge the patient for allowing this case to be published.
Author contributions
Conceptualization: Zheng Ying.
Writing – original draft: Chen Lin.
Writing – review & editing: Chen Sijing.
Chen Lin orcid: 0000-0001-8317-3836.
Chen Sijing orcid: 0000-0003-2852-188X.
Zheng Ying orcid: 0000-0002-9008-9525.
Footnotes
Abbreviations: CA = carbohydrate antigen, CK = creatine kinase, CT = computed tomography, DM = dermatomyositis, FIGO = Federation International of Gynecology and Obstetrics.
Informed written consent was obtained from the patient for publication of this case report and accompanying images.
No financial support.
The authors declare no conflicts of interest.
References
- [1].Robinson AB, Reed AM. Clinical features, pathogenesis and treatment of juvenile and adult dermatomyositis. Nat Rev Rheumatol 2011;7:664–75. [DOI] [PubMed] [Google Scholar]
- [2].Sigurgeirsson B, Lindelof B, Edhag O, et al. Risk of cancer in patients with dermatomyositis or polymyositis. A population-based study. N Engl J Med 1992;326:363–7. [DOI] [PubMed] [Google Scholar]
- [3].Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet 2001;357:96–100. [DOI] [PubMed] [Google Scholar]
- [4].Orth T, Galle PR, Mayet WJ. Severe dermatomyositis associated with lymph node recurrence of an endometrial carcinoma. J Clin Rheumatol 1999;5:41–2. [DOI] [PubMed] [Google Scholar]
- [5].Requena C, Alfaro A, Traves V, et al. Paraneoplastic dermatomyositis: a study of 12 cases. Actas Dermosifiliogr 2014;105:675–82. [DOI] [PubMed] [Google Scholar]
- [6].Mammen AL. Autoimmune myopathies: autoantibodies, phenotypes and pathogenesis. Nat Rev Neurol 2011;7:343–54. [DOI] [PubMed] [Google Scholar]
- [7].Wolff M, Mancuso C, Lal K, et al. Paraneoplastic dermatomyositis with cutaneous and myopathic disease responsive to adrenocorticotropic hormone therapy. J Clin Aesthet Dermatol 2017;10:57–62. [PMC free article] [PubMed] [Google Scholar]
- [8].Selva-O’Callaghan A, Trallero-Araguas E, Grau-Junyent JM, et al. Malignancy and myositis: novel autoantibodies and new insights. Curr Opin Rheumatol 2010;22:627–32. [DOI] [PubMed] [Google Scholar]
- [9].Stockton D, Doherty VR, Brewster DH. Risk of cancer in patients with dermatomyositis or polymyositis, and follow-up implications: a Scottish population-based cohort study. Br J Cancer 2001;85:41–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ungprasert P, Leeaphorn N, Hosiriluck N, et al. Clinical features of inflammatory myopathies and their association with malignancy: a systematic review in asian population. ISRN Rheumatol 2013;2013:509354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Scheinfeld N. A review of the cutaneous paraneoplastic associations and metastatic presentations of ovarian carcinoma. Clin Exp Dermatol 2008;33:10–5. [DOI] [PubMed] [Google Scholar]
- [12].Arshad I, Barton D. Dermatomyositis as a paraneoplastic phenomenon in ovarian cancer. BMJ Case Rep 2016;2016:bcr2016215463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Callen JP, Wortmann RL. Dermatomyositis. Clin Dermatol 2006;24:363–73. [DOI] [PubMed] [Google Scholar]
- [14].Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol 2010;34:433–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kurman RJ, Shih Ie M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm. Hum Pathol 2011;42:918–31. [DOI] [PMC free article] [PubMed] [Google Scholar]


