Supplemental Digital Content is available in the text
Keywords: dementia, meta-analysis, osteoarthritis, systematic review
Abstract
Objective:
To investigate the possible association between osteoarthritis (OA) and the risk of dementia.
Methods:
Cohort, case-control, and cross-sectional studies were obtained from wide literature search up to 20 April 2018 from following electronic databases: PubMed, Embase, Cochrane, using the MeSH terms: “osteoarthritis” AND “dementia”. The literature search was then expanded to congress abstracts. After screening and selection of relevant studies by two investigators, data was extracted. Estimates were then calculated using a random-effect size model. Sensitivity-analysis was conducted for gender and age adjusted studies and pooled for studies with STROBE quality assessment score ≥75%. Publication bias was assessed by Funnel plot. Analyses were performed using Data Analysis and Statistical Software Version 14.2.
Results:
Nearly 1549 publication references were initially retrieved. Twenty-six publications were checked with full-text. Six observational studies with 388,252 individuals were included. OA was associated with a significantly increased risk for dementia (OR = 1.20; 95% confidence interval (CI), 1.03–1.39, I2 = 95.6%, P < .05). After pooling the studies with adjustment of age and gender, the risk increased (OR 1.36; 95% CI, 1.22–1.51, I2 = 75.6%, P < .0001). After pooling the study with a STROBE Quality score ≥75% the risk for dementia was slightly increased (OR 1.33; 95% CI, 1.17–1.5, I2 = 93.5%, p < 0.0001).
Conclusions:
There is an association between osteoarthritis and the risk of dementia. This meta-analysis does not provide causality. Further prospective cohort studies are needed to clarify, if knee-, hip-, or hand-OA are independent risk factors for Alzheimer's disease and vascular dementia.
1. Introduction
Osteoarthritis (OA) is a debilitating, multi-factorial joint disease which leads to pain and impaired function in the adult population. Its socioeconomic burden increased in the last century[1,2] and is expected to increase in the next decades.[3,4] OA was earlier considered to be a local cartilage problem caused by wear and tear. However, OA is currently broadly accepted as a whole joint disease, which includes synovitis and subchondral bone changes.[5] Bone and synovia are both highly vascularized tissues and can initiate inflammatory responses.[5–7] Beside local peripheral low-grade inflammation, it has been suggested that low-grade systemic inflammation could play a critical role in OA. This relationship has been discussed for patients with systemic inflammatory-associated diseases such as hypertension, obesity, dyslipidemia, and diabetes mellitus.[8–10] This concept of OA as a systemic-associated disease requires more supporting evidence.
A recent nation-wide study from Taiwan brought up that OA is also associated with increased risk for dementia.[11] Alzheimer's disease (AD) and vascular dementia are the most common sub forms of dementia.[12] Dementia is a progressive neurodegenerative brain disorder which leads to cognitive impairment (CIM), disability, and death in the elderly population. The prevalence of dementia is expected to increase rapidly over the next decades.[13] Dementia therefore will have a big impact on the burden of the society. It is of big importance to identify and address potential risk factors. Dementia and OA share similar risk factors, are both age-related and low-grade inflammatory-associated diseases.[5,7,14,15] However, the relationship between OA and dementia is not clear and only very limited data is available.
This is the first meta-analysis synthesizing all observational studies published in peer-reviewed journals up to April 20, 2018 with no lower limitation, investigating the association between OA and the risk of dementia.
2. Materials and methods
2.1. Literature search and inclusion criteria
This systemic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines[16] and slightly adjusted when necessary. Ethical approval was not necessary, because our meta-analysis is a synthesis of previous studies in which patient's approval has already been obtained. The literature search was conducted using following electronic databases; PubMed, Embase, Cochrane, using the MeSH terms: “osteoarthritis” AND “dementia” without any lower limit of time up to April 20, 2018. Congress abstract were searched in the archives of Osteoarthritis Research Society International (OARSI), American College of Rheumatology (ACR), and European League Against Rheumatism (EULAR).
The literature search was then extended to hand search and for screening of ongoing trials in the registry databases: Open-Grey and the WHO International Clinical Trials. The studies were restricted to human subjects and to studies with English title and abstract. Studies with full text in any translatable language were considered. Only those studies providing sufficient information for the calculation of the odds ratio (OR) of OA associated with the risk of dementia were included in this meta-analysis. Included were all types of observational studies (cohort studies, cross-sectional studies, and case-control studies). There was no restriction on the disease severity or localization (e.g., hip-, knee-, and hand-osteoarthritis were considered). Both radiographic and symptomatic OA were included. There was no restriction on sub-classification of dementia (e.g., AD and vascular dementia were included). The inclusion of the studies was decided according to the above-mentioned criteria by two investigators (AW and ShM). To qualify for inclusion both investigators had to reach agreement. A third investigator was involved in case of unsolved disagreement (CW).
2.2. Data extraction
The data extraction was conducted by two investigators individually (AW and ShM). The discrepancies in data extraction were all resolved by consensus. The following data was extracted from the included studies: author, year of publication, design, age of population, sample size, gender, definition of OA (International Classification of Diseases (ICD) codes, localization, radiographic or symptomatic), definition of dementia (ICD codes, Mini-Mental State Examination (MMSE), Modified Mini-Mental State (3MS) examination, AD or Vascular dementia, CIM), odds ratios (ORs), relative risk (RR), hazard risk (HR), standard mortality rate (SMR), and adjusted factors. The OR and the respective 95% confidence interval (CI) were directly extracted from the studies when available. OR were calculated from HR, RR, and SMR when OR was not available. OA was considered as the exposure factor. Dementia was considered as the outcome factor. The quality assessment of the included studies was estimated with the Checklist from Strengthening the Reporting of Observational Studies in Epidemiology (STROBE).[17] The score is expressed as the percentage of included numbers from the checklists.
2.3. Statistical analysis
RR, HR and SMR were used to calculate ORs and their 95% CI. The ORs and the calculated summary effects were then used to describe the risk of dementia when exposed to OA. To calculate the overall effect of the OR and their 95% CI, random-effect model was used, because the heterogeneity was high. The study heterogeneity was assessed using I2-test. I2 > 50% was considered as high heterogeneity. The ORs with their corresponding 95% CIs were illustrated with Forrest-plot graphic. OR > 1 with a P-value <.05 was stated as a statistical significant increased risk of dementia when exposed to OA. The publication bias was assessed by Funnel plot analysis. Analyses were performed using Data Analysis and Statistical Software (STATA) Version 14.2. All statistical tests were two sided and a P-value <.05 was considered as a statistically significant outcome. Sensitivity analysis was performed (refer for more details).
3. Results
3.1. Search strategy results
A total of 1549 publication references were initially retrieved using this search strategy. No studies were found in Open-Grey and the WHO International Clinical Trials, nor were any congress abstract received from OARSI, ACR, or EULAR. No further studies were obtained after extended hand search. After removal of 357 duplicates, 1192 publications were screened based on titles and abstracts and 26 publications were identified as potentially eligible. After checking the full text for detailed information and data extraction 6 out of 26 publications were included in this meta-analysis.[11,18–22] Three abstracts without full-text[23–25] were not included in this synthesis (Fig. 1). From this search strategy three cohort studies, two case control studies, and one cross section study was obtained (Table 1).
Figure 1.
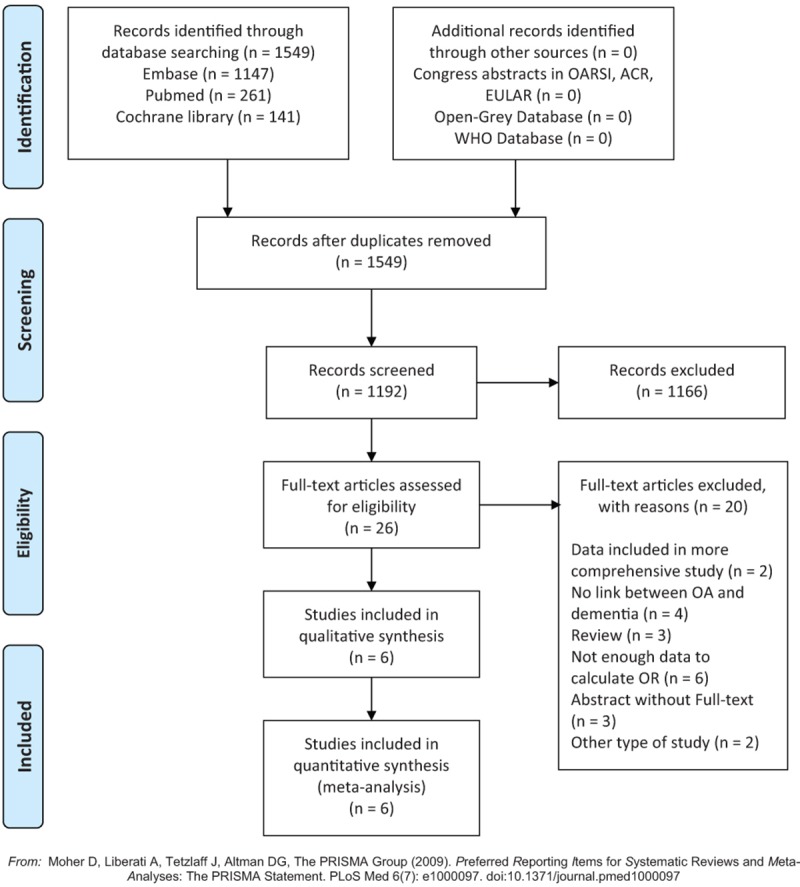
PRISMA flow-chart, result of applied search strategy.
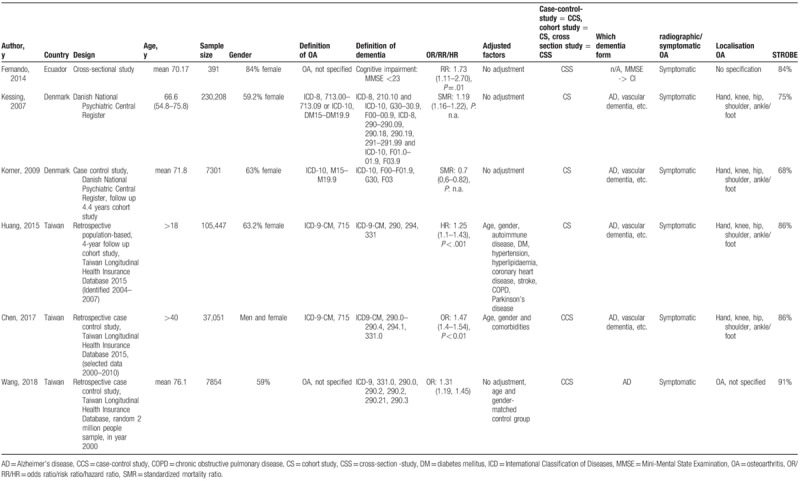
Table 1.
Basic characteristics of included studies.
3.2. Study characteristics
The 6 independently conducted observational studies included in the meta-analysis were published between 2007 and 2018 including a total of 388,252 individuals. The female rate was remarkably higher (59%–63.2% female) and very high in the study from Fernando et al (84%). Three studies were based in Asia, two in Europe, and one in South America. The 2 studies from Denmark and the 3 studies from Taiwan were conducted with data from national wide databases. The definition of OA is described in Table 1. Four studies defined OA with clinical ICD codes, including hand-, knee-, hip-, shoulder, and ankle/foot OA.[11,19–21] Two studies did not specify OA.[18,22] One study defined the investigation in dementia by CIM and defined it by MMSE <23.[18] One study defined dementia as AD using registered database.[22] Four studies used ICD codes including AD, vascular dementia, or other subforms.[11,19–21] Three studies adjusted for age and gender[11,20] or used age and gender matched control groups.[22] The median STROBE quality score was 81.7% (range 68%-91%). Five publications had a STROBE quality score ≥75%. The details of the study characteristics are shown in Table 1.
3.3. Osteoarthritis and the risk of dementia
Overall, OA was associated with a significantly increased risk for dementia (OR = 1.20; 95% CI, 1.03–1.39, I2 = 95.6%, p < .05) (Fig. 2). The overall estimate of OR increased after analysis using fixed effects model (OR 1.24; 95% CI, 1.21–1.26, p < .0001). The heterogeneity across the studies was very high (I2 = 95.6%, P < .0001). After exclusion of the study with a STROBE Quality score <75% the risk for dementia was slightly increased (OR 1.33; 95% CI, 1.17–1.5, I2 = 93.5%, P < .0001). After pooling the studies that reported adjustment for age and gender, the risk for dementia increased even more (OR 1.36; 95% CI, 1.22–1.51, I2 = 75.6%, P < 0.0001). For further sensitivity analysis, we analyzed the pooled estimates after removing different combinations of the studies which extracted their data from the same databases. Two patterns were seen. Pattern one (Korner 2009, Fernando 2014, including one Taiwanese study) showed a persistent increased risk of dementia (OR 1.22–1.37; 95% CI, (1.12–1.13)–(1.33–1.67), P < .001). Pattern two (Kessing 2007, Fernando 2014, including one Taiwanese study) showed a slightly increased risk of dementia without sufficient significant levels (OR 1.11–1.19; 95% CI, (0.67–0.69)–(1.8–1.88), P = .55–.66).
Figure 2.
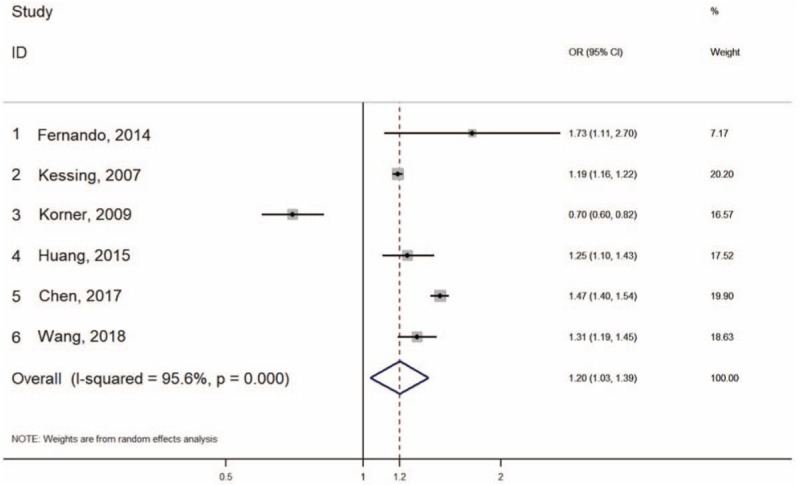
Forrest plot for risk of dementia when OA is diagnosed.
There was a moderate asymmetrical distribution in the funnel plot for the studies presenting the association between OA and dementia (Fig. 3).
Figure 3.
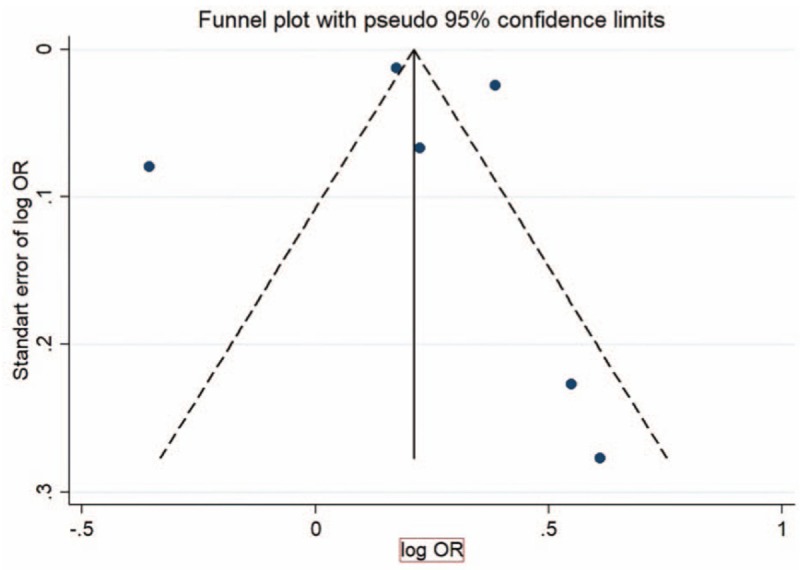
Funnel plot showing association of OA and dementia.
4. Discussion
This study investigated the potential relationships between OA and the risk of dementia by conducting a systemic review and meta-analysis. The result of our meta-analysis shows an association between OA and increased risk for dementia. The risk slightly increased after pooling for those that adjusted for age and gender, and also after pooling the studies with a STROBE quality score above-equal 75%. Our results provide mounting evidence that OA is not only a local disease but also a systemic-associated disease.
The relationship for the association between OA and increased risk for dementia remains unclear. First, we need to consider possible bias. There are several possible confounders in the relationship between OA and dementia. Firstly, the use of non-steroidal-anti-inflammatory drugs (NSAIDs) is known to increase hypertension and cardiovascular risk, which are both recognized risk factors for dementia and CIM.[12] Second, the population of the included studies contain more female and elderly patients. Female gender and age are not only risk factors for OA but also for dementia. Obesity was not documented in the selected studies but could also be a typical population-based confounder. Our pooled analysis for studies that adjusted for age and gender showed an increased risk for dementia in OA population. Third, in elderly population we also need to consider the increased prevalence of age-associated common diseases and syndromes such as hypertension, cardiovascular diseases, and insulin resistance. They potentially could bias our demonstrated association between OA and risk for dementia (Fig. 4), as they are well recognized risk factors for dementia.[12] Particularly vascular co-morbidities need to be considered as confounders, because they are highly prevalent in OA population.[26] Two out of six studies included in this meta-analysis adjusted for comorbidities and showed an increased risk for dementia in OA population.[11,20] Another OA population-specific characteristic is physical inactivity when lower limbs are affected. Physical inactivity is broadly accepted to increase systemic inflammation and could therefore play an indirect role for the association between OA and the risk of dementia.[27]
Figure 4.
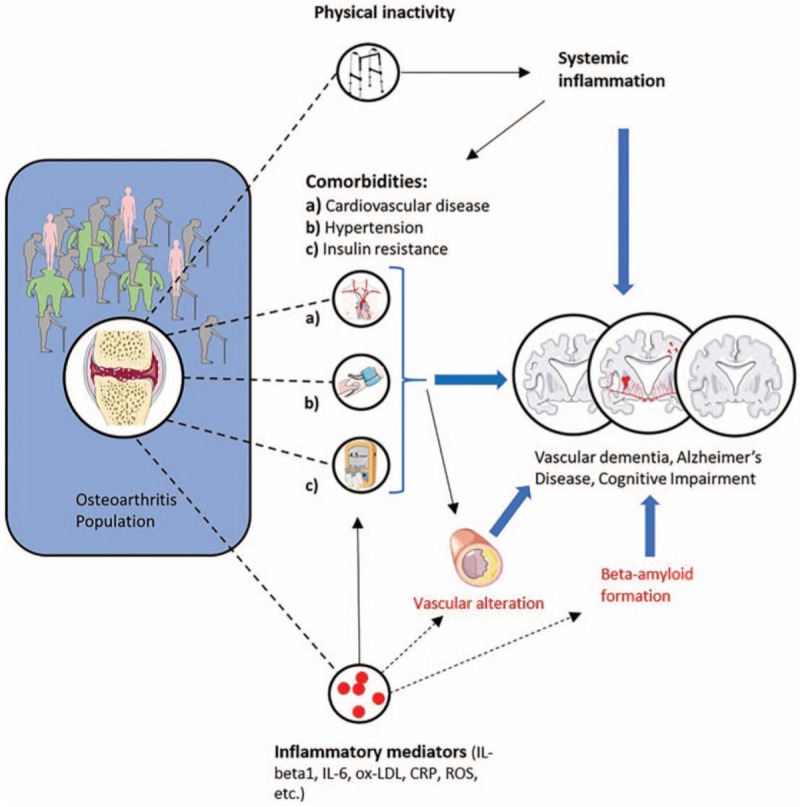
Possible association between osteoarthritis and increased risk for dementia. Frequent age-related comorbidities and physical inactivity in osteoarthritis patients increase risk for Alzheimer's disease, vascular dementia, and cognitive impairment. Local inflammatory mediators can reach the brain through the blood-circulation and directly lead to accelerated beta-amyloid plaques in Alzheimer's disease. Local inflammatory mediators could accelerate vascular alteration and lead to vascular dementia.
A possible explanation for direct role of OA to increase the risk of dementia and CIM could be through local inflammatory cytokines. An experimental OA animal model supports this view: interleukin-1-beta (IL-1beta) was proposed to be one key mediator to mediate neuroinflammation and promote amyloid-beta-plaques deposition, which is a hallmark of AD[28] (Fig. 4). Beta amyloid is a neurotoxin which potentially induces neuronal loss[29] and also potentially enhances plaque formation in cerebral arteries and promote vascular dementia and CIM[30] (Fig. 4). There is a distinct possibility that local cytokines are released from hypertrophic cartilage, sclerotic subchondral bone, or inflamed synovium in OA joints and reach the brain tissue through the blood circulation and lead to neuroinflammation and cerebrovascular alteration. Different possible shared inflammatory mechanism has been reviewed recently. C-reactive protein (CRP), interleukin-6 (IL-6), oxidized LDL (ox-LDL), and reactive oxygen species (ROS) has been proposed as potential key players.[31]
This systemic review and meta-analysis does not claim causal relationship between OA and the pathogenesis of dementia or CIM. However, it points out the importance of further investigations in OA and its associated co-morbidities. Despite our wide literature search, only a few relevant publications were found. There is clearly a lack of studies investigating in this matter.
OA and dementia are both age-associated diseases and because we are facing a growing elderly population, we will face a big burden on our society caused by both diseases. Besides increasing health costs, it has been shown that patients with hip and knee osteoarthritis were found to have a higher risk of death compared to the normal population and the mortality was pronounced with causes of death for dementia.[32] Walking disability has been proposed to be one of the major risk factor in this population.
Another problem within the OA and dementia cohort is that patients with dementia or CIM often be underdiagnosed, because the objectivation of pain and other symptoms are more difficult.[33]
In the literature search we found that OA was often used as a control group, when association between a specific disease and dementia was investigated.[19,21,34,35] The authors legitimize using OA as a control group by claiming that OA does not affect the brain's integrity.[36–38] The result of our meta-analysis suggests that this might not be the case and that the use of OA as a control group could be misleading. This is supported by a clinical study that demonstrated that half of the OA patients were in a depressive state.[39]
This meta-analysis provides a wide search for the very rear data on the link between OA and dementia. However, this study had several limitations. First, due to the lack of published data, we were not able to do subgroup-analyses for different OA localizations. Knee OA might have a different effect than hand OA. Only one abstract found investigating specifically in radiologic knee OA and showed a positive association of increased risk of CIM.[23] The same limitation counts for dementia subgroups like AD, vascular dementia, or cognitive impairment. This study therefore provides an explorative overview between OA and dementia. To further clarify the relationships between OA and dementia, we suggest that the subgroups of dementia and primary joint-specific OA should be carefully investigated in. Prospective studies with clear disease definition could provide evidence for the increased risk and could further explore the causality. Second, we included only six studies in this meta-analysis and excluded 3 abstracts without full-text. Within these six studies the two studies from Denmark and the three studies from Taiwan, although independently conducted, they used the same databases. There is probably an overlap of data between those studies, as they also used overlapping time periods for the data extraction. In our sensitivity analysis, we can see that our result was altered in two patterns. After removing Korner 2009 and two studies from Taiwan, our result was only slightly affected, whereas the statistical significance of the summarized pooled OR was not sufficiently reached when removing Kessing 2007 and two studies from Taiwan. It suggests that the studies from Taiwan might push towards increased risk, whereas the studies from Denmark use very different data from the register. Given the fact that the number of patients in the Korner 2009 study was very small and no adjustment of gender and age was provided, the result from Korner 2009 should be assessed very carefully. Third, the included studies are observational studies and do not clarify if the association is causal and do not elucidate possible underlying mechanism.
In summary, this meta-analysis supports mounting evidence that OA is a systemic associated disease. There is an association between OA and risk of dementia. However, it does not provide causality, nor can we state that the risk of dementia can be improved by treating OA. Further prospective cohort studies are needed to clarify whether OA is an independent risk factor for dementia.
Author contributions
AW takes responsibility for the integrity of the work as a whole, from inception to the finished manuscript.
Conception and design: AW, CW, ShM.
Collection and assembly of data: AW, ShM.
Analysis and interpretation of the data: AW, CW, ShM, FB, JS.
Drafting and final approval of the manuscript: AW, CW, ShM, FB, JS, YH, YPZ.
Conceptualization: Adrian Weber, Shing hung Mak, Chunyi Wen.
Data curation: Adrian Weber, Shing hung Mak, Chunyi Wen.
Formal analysis: Adrian Weber, Shing hung Mak, Chunyi Wen.
Funding acquisition: Chunyi Wen.
Investigation: Adrian Weber, Shing hung Mak, Chunyi Wen.
Methodology: Adrian Weber, Shing hung Mak, Francis Berenbaum, Jérémie Sellam, Yong-Ping Zheng, Chunyi Wen.
Project administration: Adrian Weber, Shing hung Mak, Chunyi Wen.
Resources: Adrian Weber, Shing hung Mak, Chunyi Wen.
Software: Adrian Weber, Shing hung Mak, Chunyi Wen.
Supervision: Adrian Weber, Shing hung Mak, Chunyi Wen.
Validation: Adrian Weber, Shing hung Mak, Francis Berenbaum, Jérémie Sellam, Yong-Ping Zheng, Yifan Han, Chunyi Wen.
Visualization: Adrian Weber, Shing hung Mak, Chunyi Wen.
Writing – original draft: Adrian Weber, Shing hung Mak, Francis Berenbaum, Jérémie Sellam, Yong-Ping Zheng, Chunyi Wen.
Writing – review & editing: Adrian Weber, Shing hung Mak, Francis Berenbaum, Jérémie Sellam, Yong-Ping Zheng, Yifan Han, Chunyi Wen.
Supplementary Material
Footnotes
Abbreviations: 3 MS = Modified Mini-Mental State, ACR = American College of Rheumatology, AD = Alzheimer's disease, CI = confidence interval, CIM = cognitive impairment, CRP = C-reactive protein, EULAR = European League Against Rheumatism, HR = hazard risk, ICD = International Classification of Diseases, IL-1beta = interleukin-1-beta, IL-6 = interleukin-6, MMSE = Mini-Mental State Examination, NSAID's = non-steroidal-anti-inflammatory drugs, OA = osteoarthritis, OARSI = Osteoarthritis Research Society International, OR = odds ratio, ox-LDL = oxidized LDL, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, ROS = reactive oxygen species, RR = relative risk, SMR = standard mortality rate, STROBE = Observational Studies in Epidemiology.
AW, ShM and CW contributed equally to this work.
This work was supported by Shenzhen Basic Research Program (JCYJ20160331141459373), Guangdong-Hong Kong Technology Cooperation Funding Scheme (GHP/012/16GD), and Research Grants Council of Hong Kong (15101014).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Ackerman IN, Bohensky MA, de Steiger R, et al. Substantial rise in the lifetime risk of primary total knee replacement surgery for osteoarthritis from 2003 to 2013: an international, population-level analysis. Osteoarthritis Cartilage 2017;25:455–61. [DOI] [PubMed] [Google Scholar]
- [2].Ackerman IN, Zomer E, Gilmartin-Thomas JF, et al. Forecasting the future burden of opioids for osteoarthritis. Osteoarthritis Cartilage 2018;26:350–5. [DOI] [PubMed] [Google Scholar]
- [3].Sharif B, Kopec J, Bansback N, et al. Projecting the direct cost burden of osteoarthritis in Canada using a microsimulation model. Osteoarthritis Cartilage 2015;23:1654–63. [DOI] [PubMed] [Google Scholar]
- [4].Palazzo C, Nguyen C, Lefevre-Colau MM, et al. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med 2016;59:134–8. [DOI] [PubMed] [Google Scholar]
- [5].Hügle T, Geurts J. What drives osteoarthritis?—synovial versus subchondral bone pathology. Rheumatology (Oxford) 2017;56:1461–71. [DOI] [PubMed] [Google Scholar]
- [6].Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage 2013;21:16–21. [DOI] [PubMed] [Google Scholar]
- [7].Geurts J, Patel A, Hirschmann MT, et al. Elevated marrow inflammatory cells and osteoclasts in subchondral osteosclerosis in human knee osteoarthritis. J Orthop Res 2016;34:262–9. [DOI] [PubMed] [Google Scholar]
- [8].Baudart P, Louati K, Marcelli C, et al. Association between osteoarthritis and dyslipidaemia: a systematic literature review and meta-analysis. RMD Open 2017;3:e000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhang YM, Wang J, Liu XG. Association between hypertension and risk of knee osteoarthritis: a meta-analysis of observational studies. Medicine (Baltimore) 2017;96:e7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Frasca D, Blomberg BB, Paganelli R. Aging, obesity, and inflammatory age-related diseases. Front Immunol 2017;8:1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chen KT, Chen YC, Fan YH, et al. Rheumatic diseases are associated with a higher risk of dementia: a nation-wide, population-based, case-control study. Int J Rheum Dis 2018;21:373–80. [DOI] [PubMed] [Google Scholar]
- [12].Gorelick PB, Counts SE, Nyenhuis D. Vascular cognitive impairment and dementia. Biochim Biophys Acta 2016;1862:860–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wortmann M. Dementia: a global health priority—highlights from an ADI and World Health Organization report. Alzheimers Res Ther 2012;4:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sarker MR, Franks SF. Efficacy of curcumin for age-associated cognitive decline: a narrative review of preclinical and clinical studies. Geroscience 2018;40:73–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cunningham C, Hennessy E. Co-morbidity and systemic inflammation as drivers of cognitive decline: new experimental models adopting a broader paradigm in dementia research. Alzheimers Res Ther 2015;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–9. [DOI] [PubMed] [Google Scholar]
- [18].Fernando EA, Carlos AO, Richard AA, et al. Cognitive decline and risk of dementia, a reality for Ecuador. Study of risk factors in a group of retired patients of IESS in Cuenca in 2013. Rev Ecuat Neurol 2014;23:12–7. [Google Scholar]
- [19].Kessing LV, Lopez AG, Andersen PK, et al. No increased risk of developing Alzheimer disease in patients with glaucoma. J Glaucoma 2007;16:47–51. [DOI] [PubMed] [Google Scholar]
- [20].Huang SW, Wang WT, Chou LC, et al. Osteoarthritis increases the risk of dementia: a nationwide cohort study in Taiwan. Sci Rep 2015;5:10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kørner A, Lopez AG, Lauritzen L, et al. Acute and transient psychosis in old age and the subsequent risk of dementia: a nationwide register-based study. Geriatr Gerontol Int 2009;9:62–8. [DOI] [PubMed] [Google Scholar]
- [22].Wang JH, Wu YJ, Tee BL, et al. Medical comorbidity in Alzheimer's disease: a nested case-control study. J Alzheimers Dis 2018;63:773–81. [DOI] [PubMed] [Google Scholar]
- [23].Yoshimura N, Muraki S, Oka H, et al. Osteoarthritis, osteoporosis and cognitive impairment: The Research on Osteoarthritis/Osteoporosis Against Disability (ROAD) study. Bone 2009;44:S415. [Google Scholar]
- [24].Malone D, Mucha L, McLaughlin T, et al. Increased risk of serious comorbidities in a cohort of AD patients compared to a similar non-AD cohort. Alzheimers Dement 2009;5:283. [Google Scholar]
- [25].Gujar B, Gruber-Baldini A, Baumgarten M, et al. Older adults with osteoarthritis do not have an increased risk of cognitive impairment. Arthritis Rheum 2014;66:S564. [Google Scholar]
- [26].Calvet J, Orellana C, Larrosa M, et al. High prevalence of cardiovascular co-morbidities in patients with symptomatic knee or hand osteoarthritis. Scand J Rheumatol 2016;45:41–4. [DOI] [PubMed] [Google Scholar]
- [27].Straub RH. The brain and immune system prompt energy shortage in chronic inflammation and ageing. Nat Rev Rheumatol 2017;13:743–51. [DOI] [PubMed] [Google Scholar]
- [28].Kyrkanides S, Tallents RH, Miller JNH, et al. Osteoarthritis accelerates and exacerbates Alzheimer's disease pathology in mice. J Neuroinflammation 2011;8:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chen GF, Xu TH, Yan Y, et al. Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacol Sin 2017;38:1205–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hecht M, Krämer LM, von Arnim CAF, et al. Capillary cerebral amyloid angiopathy in Alzheimer's disease: association with allocortical/hippocampal microinfarcts and cognitive decline. Acta Neuropathol 2018;135:681–94. [DOI] [PubMed] [Google Scholar]
- [31].Al-Khazraji BK, Appleton CT, Beier F, et al. Osteoarthritis, cerebrovascular dysfunction and the common denominator of inflammation: a narrative review. Osteoarthritis Cartilage 2018;26:462–70. [DOI] [PubMed] [Google Scholar]
- [32].Nuesch E, Dieppe P, Reichenbach S, et al. All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. BMJ 2011;342:d1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cornali C, Franzoni S, Gatti S, et al. Diagnosis of chronic pain caused by osteoarthritis and prescription of analgesics in patients with cognitive impairment. J Am Med Dir Assoc 2006;7:1–5. [DOI] [PubMed] [Google Scholar]
- [34].Kessing LV, Nilsson FM. Increased risk of developing dementia in patients with major affective disorders compared to patients with other medical illnesses. J Affect Disord 2003;73:261–9. [DOI] [PubMed] [Google Scholar]
- [35].Preuss UW, Watzke S, Choi JH. Diagnostic correlates of Alzheimer dementia in a U.S. Nationwide inpatient sample. Am J Geriatr Psychiatry 2010;18:821–9. [DOI] [PubMed] [Google Scholar]
- [36].Gabriel SE, Crowson CS, O’Fallon WM. Comorbidity in arthritis. J Rheumatol 1999;26:2475–9. [PubMed] [Google Scholar]
- [37].Cohen J. A power primer. Psychol Bull 1992;112:155–9. [DOI] [PubMed] [Google Scholar]
- [38].Hendrie HC. Epidemiology of dementia and Alzheimer's disease. Am J Geriatr Psychiatry 1998;6Suppl 1:S3–18. [DOI] [PubMed] [Google Scholar]
- [39].Shimura Y, Kurosawa H, Tsuchiya M, et al. Serum interleukin 6 levels are associated with depressive state of the patients with knee osteoarthritis irrespective of disease severity. Clin Rheumatol 2017;36:2781–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


